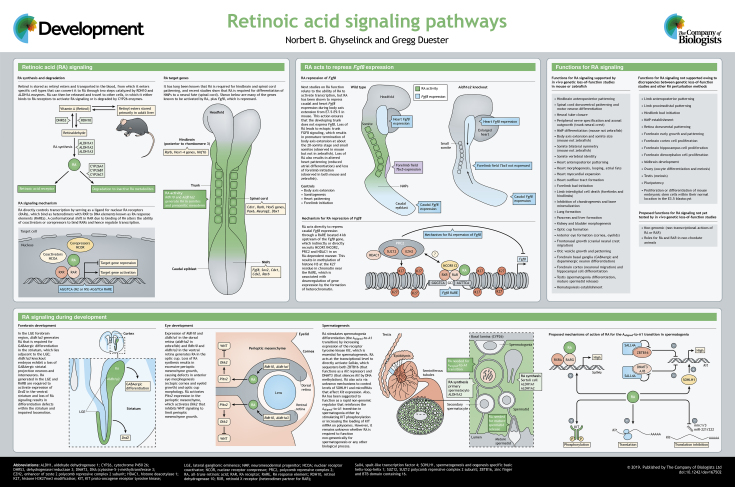
ABSTRACT
Retinoic acid (RA), a metabolite of retinol (vitamin A), functions as a ligand for nuclear RA receptors (RARs) that regulate development of chordate animals. RA-RARs can activate or repress transcription of key developmental genes. Genetic studies in mouse and zebrafish embryos that are deficient in RA-generating enzymes or RARs have been instrumental in identifying RA functions, revealing that RA signaling regulates development of many organs and tissues, including the body axis, spinal cord, forelimbs, heart, eye and reproductive tract. An understanding of the normal functions of RA signaling during development will guide efforts for use of RA as a therapeutic agent to improve human health. Here, we provide an overview of RA signaling and highlight its key functions during development.
KEY WORDS: Development, Genetic loss of function, Retinoic acid, Signaling
Summary: This Development at a Glance article highlights how retinoic acid and its nuclear receptors regulate organ development, which may guide efforts for its use as a therapeutic agent to improve human health.
Introduction
Retinoic acid (RA) is derived from retinol (vitamin A) as a metabolic product. RA exists in several isomeric forms including all-trans-RA, 9-cis-RA and 13-cis-RA; however, all-trans-RA is the primary ligand during development (Cunningham and Duester, 2015). Early studies uncovered the roles of RA during embryogenesis by subjecting mammalian or avian embryos to vitamin A deficiency, revealing that retinol (and thus likely RA) is essential for development of many organs including the hindbrain, spinal cord, forelimb buds, skeleton, heart, eye, pancreas, lung and genitourinary tract (Clagett-Dame and DeLuca, 2002). Subsequent studies have shown that RA is essential for embryonic development of chordate animals (Marlétaz et al., 2006). Although nuclear receptors that are similar to RA receptors (RARs) might exist in some non-chordate animals (Handberg-Thorsager et al., 2018), there is no conclusive evidence showing that RA is required for development of non-chordates.
In addition to vitamin A deficiency, genetic studies are essential for identifying RA-dependent processes, as discrepancies between genetic loss-of-function and pharmacological manipulation of RA signaling has made identifying the specific developmental processes that require RA a challenge (Rhinn and Dolle, 2012; Cunningham and Duester, 2015). Drug studies might abnormally affect expression of RA-dependent genes because exogenous RA or RAR antagonists are typically provided at higher concentrations (∼1000-fold) than endogenous RA levels (Horton and Maden, 1995). Thus, this article focuses on the essential components of the RA signaling pathway and the required functions of RA that have been identified by in vivo genetic loss-of-function studies in mouse and zebrafish embryos.
Regulation of RA signaling
RA metabolism
RA is produced from retinol in two steps. In both mouse and zebrafish embryos, RA synthesis is initiated by retinol dehydrogenase-10 (RDH10) that produces retinaldehyde (Metzler and Sandell, 2016). To prevent excessive RA synthesis, reverse conversion of retinaldehyde back to retinol is facilitated by at least one enzyme in vivo – DHRS3 (Feng et al., 2010; Billings et al., 2013) – that interacts with RDH10 to regulate RA synthesis (Belyaeva et al., 2017). In the second step, retinaldehyde is converted to RA by three retinaldehyde dehydrogenases in mice: ALDH1A1, ALDH1A2, and ALDH1A3 (also known as RALDH1, RALDH2, and RALDH3) (Cunningham and Duester, 2015). In zebrafish, which lack an ALDH1A1 ortholog (Cañestro et al., 2009), Aldh1a2 and Aldh1a3 produce RA. ALDH8A1 (RALDH4) was originally proposed to be involved in RA synthesis, but further studies do not support this role (Teletin et al., 2019). Instead, ALDH8A1 functions in tryptophan degradation (Davis et al., 2018). Conversion of retinaldehyde to RA is irreversible, although RA is rapidly degraded by P450 family enzymes (CYP26A1, CYP26B1 and CYP26C1), resulting in a short (∼1 h) half-life (Hernandez et al., 2007; Pennimpede et al., 2010).
RA regulates transcription through RA receptors
RA functions as a ligand for nuclear RARs. Mouse possess three RARs (RARA, RARB, RARG), which are required for many developmental processes (Lohnes et al., 1994; Mendelsohn et al., 1994). Zebrafish possess two homologs of RARA (encoded by raraa and rarab) and two homologs of RARG (encoded by rarga and rargb), but lack an RARB ortholog (Linville et al., 2009). These RARs bind target genes as a heterodimer complex with retinoid X receptors (RXRA, RXRB or RXRG) at a DNA sequence known as the RA response element (RARE) (Kastner et al., 1995). RARE binding recruits either nuclear receptor coactivators (NCOA) or nuclear receptor corepressors (NCOR), thus directly activating or repressing transcription (Cunningham and Duester, 2015). RAR chromatin immunoprecipitation studies have reported 13,000-15,000 potential RAREs in the mouse genome (Moutier et al., 2012). However, most of these RAREs might not be required for development, owing to weak affinity to RARs or having locations within the genome that are not normally able to control nearby genes (Cunningham et al., 2018).
RA is required for early neural differentiation
RA controls hindbrain anteroposterior patterning
In mouse, RA synthesis begins at embryonic day (E)7.5 with expression of Rdh10 and Aldh1a2 in the presomitic mesoderm; secreted RA then diffuses into the developing spinal cord and hindbrain as far as rhombomere 3 (r3) (Sirbu et al., 2005). Here, RA target genes include the 3′-Hox genes (Hox1-4 groups) essential for rhombomere formation and identity in vertebrates (Krumlauf, 1993; Maden et al., 1996; Niederreither et al., 2000; Begemann et al., 2001). RA directly regulates Hoxb1 through two RAREs that activate expression in r4, but repress expression in r3 and r5 (Marshall et al., 1994; Studer et al., 1994). RA also activates Hnf1b (Vhnf1) in posterior hindbrain and spinal cord to prevent Hoxb1 from being expressed posterior to r4 (Hernandez et al., 2004; Sirbu et al., 2005). Although RA activation of Hoxb1 is consistent with RA stimulating recruitment of coactivators, it is less clear how RA represses Hoxb1 transcription; this topic is further addressed below with respect to RA repression of Fgf8.
RA directs differentiation of neuromesodermal progenitors
Bipotential neuromesodermal progenitors (NMPs) undergo balanced differentiation to either spinal cord neuroectoderm or presomitic mesoderm in both mouse and zebrafish (Wilson et al., 2009; Kondoh and Takemoto, 2012; Henrique et al., 2015; Kimelman, 2016). Although RA is not required for the establishment of NMPs (Cunningham et al., 2016), loss of RA in mouse or chick results in altered differentiation; decreased Sox1/Sox2-expressing neural progenitors and increased Tbx6-expressing mesodermal progenitors (Cunningham et al., 2015). Zebrafish, however, do not require RA for NMP differentiation (Berenguer et al., 2018).
When neural progenitors emerge from the caudal epiblast or tailbud during body axis extension, they are exposed to somite-derived RA and Sonic hedgehog (SHH) generated in the floor plate. Both activate Pax6 and Olig2 in ventral spinal cord progenitors to stimulate motor neuron fate (Diez del Corral et al., 2003; Novitch et al., 2003; Molotkova et al., 2005; England et al., 2011). In the spinal cord, RA also activates Pax6 indirectly through Neurog2 (Ribes et al., 2008), and activates Cdx1 to repress hindbrain fate and specify the hindbrain/spinal cord boundary (Skromne et al., 2007; Sturgeon et al., 2011).
This understanding has been translated for in vitro differentiation of embryonic stem (ES) cells or induced pluripotent stem cells to motor neurons. Although RA is not required for the early differentiation events of ES cells in vivo, mouse and human ES cells exposed to both RA and SHH at specific time points form motor neurons at high efficiency (Wichterle et al., 2002).
RA repression of Fgf8 plays a permissive role in mesoderm development
RA controls body axis extension and somitogenesis in amniotes
In mouse, the early phase of body axis extension is directed by a population of trunk NMPs that generate trunk somites, whereas the later phase is directed by a population of tail NMPs that generate tail somites (Steventon and Martinez Arias, 2017). In Aldh1a2−/− embryos (that completely lack RA activity) trunk somites are approximately half the normal size, suggesting that RA is required for trunk somitogenesis; however, RA is not required for tail somitogenesis (Cunningham et al., 2011). Treatment with FGF inhibitor SU5402 rescues trunk somite size, suggesting that RA functions to repress caudal Fgf8, which interferes with somitogenesis when expressed too far anteriorly (Cunningham et al., 2015). Consistent with these observations, mouse and chick require RA repression of caudal Fgf8 for bilateral somite symmetry (Vermot et al., 2005; Vermot and Pourquié, 2005; Sirbu and Duester, 2006).
Zebrafish require NMPs for tail development; however, formation of the zebrafish trunk uses gastrulation convergence and extension rather than trunk NMPs (Steventon and Martinez Arias, 2017). Therefore, RA is not required for repression of caudal fgf8a at any stage in zebrafish body axis extension (Sorrell and Waxman, 2011), nor for regulating somite size (Begemann et al., 2001; Berenguer et al., 2018; Simsek and Özbudak, 2018). Overall, differences in gastrulation indicate that the mouse requires RA for caudal Fgf8 repression between the 1-25 somite stages, whereas zebrafish do not. Evidently, RA-mediated control of vertebrate body axis extension was co-opted by higher vertebrates when trunk NMPs evolved, perhaps in amniotes.
The mechanism of Fgf8 repression has been studied in detail during mouse body-axis extension. A RARE upstream of Fgf8 (conserved in amniotes but not zebrafish) is required for caudal Fgf8 repression (Kumar and Duester, 2014; Kumar et al., 2016). The Fgf8 RARE recruits nuclear receptor corepressors NCOR1 and NCOR2 (SMRT), plus polycomb repressive complex 2 (PRC2), and stimulates deposition of the repressive H3K27me3 chromatin mark, all in an RA-dependent manner (Kumar and Duester, 2014; Kumar et al., 2016). These observations provide the first in vivo evidence that RA repression can function directly on a gene through a RARE, and also show that NCOR can function ligand-dependently, in contrast to previous in vitro studies (Xu et al., 1999).
RA control of heart anteroposterior patterning
In vertebrates, loss of RA causes a dilated heart tube that fails to properly loop and form chambers along the anteroposterior axis (Dersch and Zile, 1993; Niederreither et al., 2001; Hochgreb et al., 2003; Keegan et al., 2005). FGF signaling is needed to establish ventricular identity (Pradhan et al., 2017); therefore, RA limits the expansion of anterior ventricular progenitors by repression of Fgf8 in the posterior region of the developing heart, where the atria develop (Ryckebusch et al., 2008; Sirbu et al., 2008; Sorrell and Waxman, 2011). Thus, RA has a similar role to that in body axis extension.
RA is required for forelimb initiation
RA entering the limb buds from the trunk was originally proposed to activate proximal limb markers to control limb patterning, with Cyp26b1 expressed distally preventing further RA activation; however this model is not supported by genetic studies that eliminate RA synthesis in mouse and zebrafish (Cunningham et al., 2013). Recently, polycomb repressive complex 1 (PRC1) has been reported to repress proximal limb markers such as Meis1/2 in the distal limb – a function that is perturbed by excess RA (Yakushiji-Kaminatsui et al., 2018). Thus, RA is not required instructively to activate Meis1/2 in the proximal limb for proximodistal patterning. However, degradation of distal RA by CYP26B1 is required permissively for proximodistal patterning. Without degradation, RA prevents PRC1 repression of Meis1/2, which leads to distal expression.
In contrast, RA is required for initiation of the forelimb bud (but not hindlimb bud) in mouse (Zhao et al., 2009; Cunningham et al., 2013) and the pectoral fin bud in zebrafish (Begemann et al., 2001; Grandel et al., 2002). Comparison of mouse Aldh1a2 and Rdh10 RA synthesis mutants (Zhao et al., 2009; Cunningham et al., 2013) revealed that RA repression of both caudal and cardiac Fgf8 domains is needed for Tbx5 activation – the earliest known marker in the forelimb bud. Other studies propose that RA might directly activate Tbx5 via a RARE located in intron 2 (Nishimoto et al., 2015), however subsequent enhancer knockout experiments showed that this RARE is not required (Cunningham et al., 2018). Thus, the most parsimonious model is that RA permits forelimb Tbx5 expression by repressing Fgf8, which allows another factor to activate Tbx5.
Function of RA during eye development
Expression of Aldh1a1 in the dorsal retina (aldh1a2 in zebrafish) and Aldh1a3 in the ventral retina generates RA in the optic cup (Matt et al., 2005; Molotkov et al., 2006; Cañestro et al., 2009). Loss of RA synthesis results in excessive perioptic mesenchyme growth, causing defects in anterior eye morphogenesis (ectopic cornea and eyelid growth) and optic cup morphology (Matt et al., 2005; Molotkov et al., 2006; Bohnsack et al., 2012). RA activates Pitx2 expression in the perioptic mesenchyme, which activates Dkk2 that inhibits WNT signaling to limit perioptic mesenchyme growth (Kumar and Duester, 2010).
Recently, it was shown that RA generated in the mouse retina activates Sox9 in retinal pigment epithelia, which stimulates secretion of vascular endothelial growth factor (VEGF) and encourages blood vessel growth in the choroid (Goto et al., 2018). Although RA is not required for retinal differentiation, excess RA causes retinal defects: loss of gdf6a in zebrafish results in ectopic expression of aldh1a3 in the dorsal eye and premature retina differentiation (Valdivia et al., 2016). In chick, Cyp26a1 and Cyp26c1 are expressed in the fovea – a region of high visual acuity located between the Aldh1a1 and Aldh1a3 expression domains. Here, RA is degraded to allow expression of Fgf8, which stimulates fovea patterning (da Silva and Cepko, 2017).
Function of RA in forebrain development
GABAergic differentiation in basal ganglia
In the mouse lateral ganglionic eminence (LGE) forebrain region, Aldh1a3 generates RA that is required for GABAergic differentiation in the striatum, which lies adjacent to the LGE; Aldh1a3 knockout embryos exhibit a loss of GABAergic striatal projection neurons and interneurons (Chatzi et al., 2011). RA treatment of ES cells results in differentiation to GABAergic neurons, potentially providing cells for regenerative medicine applications (Shin et al., 2012).
Dopaminergic differentiation in the striatum
Knockout of murine Rarb results in reduced expression of dopamine receptor D2 (Drd2) in the ventral striatum, which leads to impaired locomotion (Krezel et al., 1998). Supporting this, Aldh1a3 knockout mouse embryos have differentiation defects within the striatum, including loss of Drd2 expression (Molotkova et al., 2007).
Neuronal migration between cortical layers
RA generated by RDH10 and ALDH1A2 in the meninges surrounding the forebrain was proposed to be needed for expansion of cortical neuron progenitors (Siegenthaler et al., 2009); however, further genetic studies have not supported this conclusion (Chatzi et al., 2011). Studies with a meninges-specific Aldh1a2 conditional knockout confirmed that RA loss does not affect cortical neuron progenitor expansion, but RA is required to control cell migration and specification of cortical layers (Haushalter et al., 2017).
Function of RA during spermatogenesis
There are three classes of spermatogonia: stem (responsible for renewal), undifferentiated spermatogonia (termed ‘Aaligned’, which expand the pool of progenitors), and differentiating spermatogonia (including ‘A1’, which are committed towards spermatogenesis). RA is required early for differentiation of Aaligned spermatogonia into A1 spermatogonia (Aaligned-to-A1 transition) and then later for mature spermatid release (reviewed by de Rooij, 2001) (Hogarth and Griswold, 2010; Mark et al., 2015; Busada and Geyer, 2016; Yoshida, 2018). Expression pattern analyses have revealed that multiple cell types within the testis are involved in setting up and transducing the RA signal (reviewed by Hogarth and Griswold, 2010). Importantly, a catabolic barrier employing CYP26 prevents external RA prematurely stimulating the Aaligned-to-A1 transition, unless a large dose of RA is administered (reviewed by Teletin et al., 2017).
RA produced by Sertoli cells initiates the first round of Aaligned-to-A1 transitions in spermatogonia
RA signaling for spermatogenesis initiates at postnatal day 3 in mouse, when the first round of Aaligned-to-A1 transitions occurs (Snyder et al., 2010). Current evidence indicates that RA originates from Sertoli cells; genetic ablations of Aldh1a1, Aldh1a2, Aldh1a3 or Rdh10 in Sertoli cells (Raverdeau et al., 2012; Tong et al., 2013), or treatment of neonatal mice with the ALDH1A inhibitor WIN 18446 (Hogarth et al., 2013), results in accumulation of undifferentiated spermatogonia. Importantly, a single shot of RA can restore the initial transitions in Rdh10-deficient, Aldh1a-deficient or WIN 18,446-treated mice, in which spermatogenesis resumes unimpeded for months. Thus, once the first Aaligned-to-A1 transition has occurred in spermatogonia, RA-synthesizing activity in Sertoli cells becomes dispensable for spermatogenesis.
RA produced by spermatocytes and Sertoli cells acts redundantly to maintain spermatogenesis
ALDH1A generates RA in spermatocytes, but is fully dispensable for spermatogenesis (Beedle et al., 2019; Teletin et al., 2019). Initially, this suggested that ALDH1A-independent sources of RA could compensate for the loss of Aldh1a2 in spermatocytes (Beedle et al., 2019). However, analysis of compound mutant mice demonstrates that ALDH1A-dependent activities account for all of the RA required for spermatogenesis, and that the Sertoli and spermatocyte sources of RA exert redundant functions in spermatogenesis maintenance (Teletin et al., 2019). This finding precludes models where spermatocyte-derived RA is the only source for maintaining spermatogenic waves (Sugimoto et al., 2012) and stimulating mature spermatid release (Endo et al., 2017). In fact, RA generated by Sertoli cells is sufficient for both processes.
RA is dispensable for meiosis initiation
Previous studies suggested that RA is required for initiation of meiosis, which is observed a few days after the Aaligned-to-A1 transition in spermatogonia (Raverdeau et al., 2012; Evans et al., 2014). Also, it has been proposed that periodic production of RA in the seminiferous epithelium coordinates Aaligned-to-A1 transitions in spermatogonia, meiosis initiation, spermiogenesis initiation and mature spermatid release (Endo et al., 2017). However, when spermatogonia in Aldh1a1/2/3-deficient mutants are stimulated to undergo the Aaligned-to-A1 transition in response to a single dose of RA, initiation of meiosis is observed a few days later in the absence of RA. Thus, germ cells express canonical meiosis markers (including Stra8 and Rec8) after the administered RA has been cleared, well before meiosis initiates (Teletin et al., 2019). One possible explanation for this finding is that the concentration of RA needed for initiation of meiosis is extremely low compared to that required for the Aaligned-to-A1 transition. However, the most parsimonious model is that RA is not required for male meiosis. Supporting this latter model, meiosis occurs normally in male mice lacking all RAR genes in germ cells (Gely-Pernot et al., 2015), plus female meiosis occurs normally in Aldh1a2;Aldh1a3 double mutants that lack RA activity in the fetal ovary (Kumar et al., 2011).
RA-activated RAR in Sertoli cells control spermiogenesis and spermiation
It has been proposed that RA from pachytene spermatocytes is required for initiation of spermiogenesis, i.e. differentiation of spermatocytes into mature spermatids (Endo et al., 2017). Opposing this, no spermiogenesis defect is observed when all ALDH1A-dependent activities, RAR or RXR are missing in germ cells (Gely-Pernot et al., 2015; Teletin et al., 2019). Conversely, spermiogenesis defects are observed when just RARA is missing in Sertoli cells (Vernet et al., 2006) or in mice treated with low doses of pan-RAR antagonists (Chung et al., 2011). Thus, spermiogenesis relies exclusively on events controlled by RA-activated RARA in Sertoli cells.
Spermiation (i.e. the release of mature spermatids) is impaired in mutants lacking ALDH1A activity in Sertoli cells, but not in germ cells (Raverdeau et al., 2012; Teletin et al., 2019). This indicates that the Sertoli cell-derived source of RA is sufficient for spermiation and contradicts the view that spermatocyte-derived sources of RA are specifically required for this process (Endo et al., 2017). As mice lacking either RAR or RXR in Sertoli cells (Vernet et al., 2006) and wild-type mice treated with a pan-RAR antagonist (Chung et al., 2011) also display spermiation defects, it is proposed that RA-activated RAR/RXR, cell-autonomously controls mechanisms in the Sertoli cell for spermatid release (reviewed by Mark et al., 2015).
Downstream effectors of RA signaling during spermatogenesis
The Aaligned-to-A1 transition in spermatogonia is stimulated by increased expression of the receptor tyrosine kinase Kit, which is repressed by ZBTB16 in the absence of RA. RA-bound RARG/RXRA heterodimers directly activate Sall4a expression (Gely-Pernot et al., 2015), SALL4A then sequesters ZBTB16 and relieves ZBTB16-dependent repression of Kit transcription (Hobbs et al., 2012). Alternatively, SALL4A can promote the ‘epigenetic shift’ that is required for the Aaligned-to-A1 transition (Yang et al., 2012). RA is also proposed to increase the level of SOHLH1, which can increase Kit expression by displacing ZBTB16 (Barrios et al., 2012). However, SOHLH1 is not a direct target of RA; RAREs are not found in this gene (Moutier et al., 2012) and RA treatment does not upregulate expression (Gely-Pernot et al., 2015). In parallel, RA can further increase KIT protein levels by decreasing the level of microRNAs, such as Mirc1/3 and miR-221/222, which prevent KIT mRNA translation (Tong et al., 2012; Yang et al., 2013) – although RAREs have not been identified in these microRNA genes (Moutier et al., 2012). Lastly, RA has been suggested to reinforce the Aaligned-to-A1 transition in spermatogonia by increasing the loading of KIT mRNA on polysomes (Busada et al., 2015) or stimulating KIT phosphorylation (Pellegrini et al., 2008). However, it remains unknown whether RA is required to function non-genomically for spermatogenesis or any other biological process.
Perspectives for future studies
In addition to the RA functions described above, there are many additional functions for RA that are supported by in vivo genetic loss-of-function studies in mouse or zebrafish. Future studies are needed to identify the key genes regulated by RA signaling in specific tissues at specific times in development, and to decipher the transcriptional mechanisms used by RA and RARs to activate or repress genes. Genome-wide studies have identified thousands of RAREs (Moutier et al., 2012), but future studies (including DNA element knockouts) are needed to identify which RAREs are required to regulate specific genes to allow developmental processes to occur (Duester, 2019). In particular, although loss of RA results in upregulation and downregulation of many genes, not much has been reported on mechanisms of RA-dependent repression other than RA repression of caudal Fgf8 in mouse. Future studies are needed to determine how some RAREs act as enhancers, whereas others act as silencers.
Although many advances have been made in understanding RA signaling, a reproducibility crisis for RA signaling is evident (Duester, 2017). Several previously proposed functions for RA are not supported by in vivo genetic loss-of-function studies. The reproducibility crisis is often due to a lack of concurrence between drug treatment studies and genetic studies in animals, or between cell line studies and in vivo studies. Also, some studies that use morpholinos for loss of function or dominant-negative RARs for genetic gain of function do not coincide with genetic loss of function for determining RA functions required for normal development. All of these methods can contribute to understanding the mechanism of RA action, but in vivo genetic loss-of-function studies are essential for determining whether RA is required in the first place. Thus, future studies are needed to bring consensus on identifying the developmental processes that require RA and those that do not, by having support from both genetic and molecular approaches. Such knowledge will provide valuable basic insight into how RA controls development and guide efforts for effective use of RA as a therapeutic agent for regenerative medicine applications.
Acknowledgements
A special thanks to Pierre Chambon for championing the early genetic efforts that led to uncovering many of the functions of RA in vivo. We also thank present and former members of our laboratories for their dedicated genetic studies leading to many new insights on RA function.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
This work was funded by the National Institutes of Health grant R01 AR067731 to G.D., and grants from the Centre National de la Recherche Scientifique, Institut National de la Santé et de la Recherche Médicale, Université de Strasbourg, Agence Nationale de la Recherche (ANR-10-BLAN-1239, ANR-10-LABX-0030-INRT, 13-BSV6-0003, 13-BSV2-0017) and from the Marie Curie Intra-European Fellowships for Career Development (FP7-PEOPLE-IEF-2012-331687 to N.G.). Deposited in PMC for release after 12 months.
Development at a Glance
A high-resolution version of the poster is available for downloading in the online version of this article at http://dev.biologists.org/content/146/13/dev167502/F1.poster.jpg
References
- Barrios F., Filipponi D., Campolo F., Gori M., Bramucci F., Pellegrini M., Ottolenghi S., Rossi P., Jannini E. A. and Dolci S. (2012). SOHLH1 and SOHLH2 control Kit expression during postnatal male germ cell development. J. Cell Sci. 125, 1455-1464. 10.1242/jcs.092593 [DOI] [PubMed] [Google Scholar]
- Beedle M.-T., Stevison F., Zhong G., Topping T., Hogarth C., Isoherranen N. and Griswold M. D. (2019). Sources of all-trans retinal oxidation independent of the aldehyde dehydrogenase 1A isozymes exist in the postnatal testis. Biol. Reprod. 100, 547-560. 10.1093/biolre/ioy200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begemann G., Schilling T. F., Rauch G. J., Geisler R. and Ingham P. W. (2001). The zebrafish neckless mutation reveals a requirement for raldh2 in mesodermal signals that pattern the hindbrain. Development 128, 3081-3094. [DOI] [PubMed] [Google Scholar]
- Belyaeva O. V., Adams M. K., Wu L. and Kedishvili N. Y. (2017). The antagonistically bifunctional retinoid oxidoreductase complex is required for maintenance of all-trans-retinoic acid homeostasis. J. Biol. Chem. 292, 5884-5897. 10.1074/jbc.M117.776914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenguer M., Lancman J. J., Cunningham T. J., Dong P. D. S. and Duester G. (2018). Mouse but not zebrafish requires retinoic acid for control of neuromesodermal progenitors and body axis extension. Dev. Biol. 441, 127-131. 10.1016/j.ydbio.2018.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billings S. E., Pierzchalski K., Butler Tjaden N. E., Pang X.-Y., Trainor P. A., Kane M. A. and Moise A. R. (2013). The retinaldehyde reductase DHRS3 is essential for preventing the formation of excess retinoic acid during embryonic development. FASEB J. 27, 4877-4889. 10.1096/fj.13-227967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnsack B. L., Kasprick D. S., Kish P. E., Goldman D. and Kahana A. (2012). A zebrafish model of Axenfeld-Rieger syndrome reveals that pitx2 regulation by retinoic acid is essential for ocular and craniofacial development. Invest. Ophthalmol. Vis. Sci. 53, 7-22. 10.1167/iovs.11-8494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busada J. T. and Geyer C. B. (2016). The role of Retinoic Acid (RA) in spermatogonial differentiation. Biol. Reprod. 94, 10 10.1095/biolreprod.115.135145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busada J. T., Chappell V. A., Niedenberger B. A., Kaye E. P., Keiper B. D., Hogarth C. A. and Geyer C. B. (2015). Retinoic acid regulates Kit translation during spermatogonial differentiation in the mouse. Dev. Biol. 397, 140-149. 10.1016/j.ydbio.2014.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cañestro C., Catchen J. M., Rodríguez-Marí A., Yokoi H. and Postlethwait J. H. (2009). Consequences of lineage-specific gene loss on functional evolution of surviving paralogs: ALDH1A and retinoic acid signaling in vertebrate genomes. PLoS Genet. 5, e1000496 10.1371/journal.pgen.1000496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzi C., Brade T. and Duester G. (2011). Retinoic acid functions as a key GABAergic differentiation signal in the basal ganglia. PLoS Biol. 9, e1000609 10.1371/journal.pbio.1000609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S. S. W., Wang X., Roberts S. S., Griffey S. M., Reczek P. R. and Wolgemuth D. J. (2011). Oral administration of a retinoic Acid receptor antagonist reversibly inhibits spermatogenesis in mice. Endocrinology 152, 2492-2502. 10.1210/en.2010-0941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clagett-Dame M. and DeLuca H. F. (2002). The role of vitamin A in mammalian reproduction and embryonic development. Annu. Rev. Nutr. 22, 347-381. 10.1146/annurev.nutr.22.010402.102745E [DOI] [PubMed] [Google Scholar]
- Cunningham T. J. and Duester G. (2015). Mechanisms of retinoic acid signalling and its roles in organ and limb development. Nature Rev. Mol. Cell Biol. 16, 110-123. 10.1038/nrm3932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham T. J., Zhao X. and Duester G. (2011). Uncoupling of retinoic acid signaling from tailbud development before termination of body axis extension. Genesis 49, 776-783. 10.1002/dvg.20763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham T. J., Zhao X., Sandell L. L., Evans S. M., Trainor P. A. and Duester G. (2013). Antagonism between retinoic acid and fibroblast growth factor signaling during limb development. Cell Rep. 3, 1503-1511. 10.1016/j.celrep.2013.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham T. J., Brade T., Sandell L. L., Lewandoski M., Trainor P. A., Colas A., Mercola M. and Duester G. (2015). Retinoic acid activity in undifferentiated neural progenitors Is sufficient to fulfill Its role in restricting Fgf8 expression for somitogenesis. PLoS ONE 10, e0137894 10.1371/journal.pone.0137894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham T. J., Colas A. and Duester G. (2016). Early molecular events during retinoic acid induced differentiation of neuromesodermal progenitors. Biol. Open 5, 1821-1833. 10.1242/bio.020891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham T. J., Lancman J. J., Berenguer M., Dong P. D. S. and Duester G. (2018). Genomic knockout of two presumed forelimb Tbx5 enhancers reveals they are nonessential for limb development. Cell Rep. 23, 3146-3151. 10.1016/j.celrep.2018.05.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva S. and Cepko C. L. (2017). Fgf8 expression and degradation of retinoic acid are required for patterning a high-acuity area in the retina. Dev. Cell 42, 68-81.e6. 10.1016/j.devcel.2017.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis I., Yang Y., Wherritt D. and Liu A. (2018). Reassignment of the human aldehyde dehydrogenase ALDH8A1 (ALDH12) to the kynurenine pathway in tryptophan catabolism. J. Biol. Chem. 293, 9594-9603. 10.1074/jbc.RA118.003320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rooij D. G. (2001). Proliferation and differentiation of spermatogonial stem cells. Reproduction 121, 347-354. 10.1530/rep.0.1210347 [DOI] [PubMed] [Google Scholar]
- Dersch H. and Zile M. H. (1993). Induction of normal cardiovascular development in the vitamin A-deprived quail embryo by natural retinoids. Dev. Biol. 160, 424-433. 10.1006/dbio.1993.1318 [DOI] [PubMed] [Google Scholar]
- Diez del Corral R., Olivera-Martinez I., Goriely A., Gale E., Maden M. and Storey K. (2003). Opposing FGF and retinoid pathways control ventral neural pattern, neuronal differentiation, and segmentation during body axis extension. Neuron 40, 65-79. 10.1016/S0896-6273(03)00565-8 [DOI] [PubMed] [Google Scholar]
- Duester G. (2017). Retinoic acid's reproducible future. Science 358, 15 10.1126/science.aar6752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duester G. (2019). Knocking out enhancers to enhance epigenetic research. Trends Genet. 35, 89 10.1016/j.tig.2018.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo T., Freinkman E., de Rooij D. G. and Page D. C. (2017). Periodic production of retinoic acid by meiotic and somatic cells coordinates four transitions in mouse spermatogenesis. Proc. Natl. Acad. Sci. USA 114, E10132-E10141. 10.1073/pnas.1710837114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- England S., Batista M. F., Mich J. K., Chen J. K. and Lewis K. E. (2011). Roles of Hedgehog pathway components and retinoic acid signalling in specifying zebrafish ventral spinal cord neurons. Development 138, 5121-5134. 10.1242/dev.066159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E., Hogarth C., Mitchell D. and Griswold M. (2014). Riding the spermatogenic wave: profiling gene expression within neonatal germ and sertoli cells during a synchronized initial wave of spermatogenesis in mice. Biol. Reprod. 90, 108 10.1095/biolreprod.114.118034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L., Hernandez R. E., Waxman J. S., Yelon D. and Moens C. B. (2010). Dhrs3a regulates retinoic acid biosynthesis through a feedback inhibition mechanism. Dev. Biol. 338, 1-14. 10.1016/j.ydbio.2009.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gely-Pernot A., Raverdeau M., Teletin M., Vernet N., Féret B., Klopfenstein M., Dennefeld C., Davidson I., Benoit G., Mark M. et al. (2015). Retinoic acid receptors control spermatogonia cell-fate and induce expression of the SALL4A transcription factor. PLoS Genet. 11, e1005501 10.1371/journal.pgen.1005501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto S., Onishi A., Misaki K., Yonemura S., Sugita S., Ito H., Ohigashi Y., Ema M., Sakaguchi H., Nishida K. et al. (2018). Neural retina-specific Aldh1a1 controls dorsal choroidal vascular development via Sox9 expression in retinal pigment epithelial cells. eLife 7, e32358 10.7554/eLife.32358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandel H., Lun K., Rauch G. J., Rhinn M., Piotrowski T., Houart C., Sordino P., Küchler A. M., Schulte-Merker S., Geisler R. et al. (2002). Retinoic acid signalling in the zebrafish embryo is necessary during pre-segmentation stages to pattern the anterior-posterior axis of the CNS and to induce a pectoral fin bud. Development 129, 2851-2865. [DOI] [PubMed] [Google Scholar]
- Handberg-Thorsager M., Gutierrez-Mazariegos J., Arold S. T., Kumar Nadendla E., Bertucci P. Y., Germain P., Tomancak P., Pierzchalski K., Jones J. W., Albalat R. et al. (2018). The ancestral retinoic acid receptor was a low-affinity sensor triggering neuronal differentiation. Sci. Adv. 4, eaao1261 10.1126/sciadv.aao1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haushalter C., Schuhbaur B., Dollé P. and Rhinn M. (2017). Meningeal retinoic acid contributes to neocortical lamination and radial migration during mouse brain development. Biol. Open 6, 148-160. 10.1242/bio.021063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrique D., Abranches E., Verrier L. and Storey K. G. (2015). Neuromesodermal progenitors and the making of the spinal cord. Development 142, 2864-2875. 10.1242/dev.119768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez R. E., Rikhof H. A., Bachmann R. and Moens C. B. (2004). vhnf1 integrates global RA patterning and local FGF signals to direct posterior hindbrain development in zebrafish. Development 131, 4511-4520. 10.1242/dev.01297 [DOI] [PubMed] [Google Scholar]
- Hernandez R. E., Putzke A. P., Myers J. P., Margaretha L. and Moens C. B. (2007). Cyp26 enzymes generate the retinoic acid response pattern necessary for hindbrain development. Development 134, 177-187. 10.1242/dev.02706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs R. M., Fagoonee S., Papa A., Webster K., Altruda F., Nishinakamura R., Chai L. and Pandolfi P. P. (2012). Functional antagonism between Sall4 and Plzf defines germline progenitors. Cell Stem Cell 10, 284-298. 10.1016/j.stem.2012.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochgreb T., Linhares V. L., Menezes D. C., Sampaio A. C., Yan C. Y. I., Cardoso W. V., Rosenthal N. and Xavier-Neto J. (2003). A caudorostral wave of RALDH2 conveys anteroposterior information to the cardiac field. Development 130, 5363-5374. 10.1242/dev.00750 [DOI] [PubMed] [Google Scholar]
- Hogarth C. A. and Griswold M. D. (2010). The key role of vitamin A in spermatogenesis. J. Clin. Invest. 120, 956-962. 10.1172/JCI41303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth C. A., Evanoff R., Mitchell D., Kent T., Small C., Amory J. K. and Griswold M. D. (2013). Turning a spermatogenic wave into a tsunami: synchronizing murine spermatogenesis using WIN 18,446. Biol. Reprod. 88, 40 10.1095/biolreprod.112.105346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton C. and Maden M. (1995). Endogenous distribution of retinoids during normal development and teratogenesis in the mouse embryo. Dev. Dyn. 202, 312-323. 10.1002/aja.1002020310 [DOI] [PubMed] [Google Scholar]
- Kastner P., Mark M. and Chambon P. (1995). Nonsteroid nuclear receptors: what are genetic studies telling us about their role in real life? Cell 83, 859-869. 10.1016/0092-8674(95)90202-3 [DOI] [PubMed] [Google Scholar]
- Keegan B. R., Feldman J. L., Begemann G., Ingham P. W. and Yelon D. (2005). Retinoic acid signaling restricts the cardiac progenitor pool. Science 307, 247-249. 10.1126/science.1101573 [DOI] [PubMed] [Google Scholar]
- Kimelman D. (2016). Tales of tails (and trunks): forming the posterior body in vertebrate embryos. Curr. Top. Dev. Biol. 116, 517-536. 10.1016/bs.ctdb.2015.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondoh H. and Takemoto T. (2012). Axial stem cells deriving both posterior neural and mesodermal tissues during gastrulation. Curr. Opin. Genet. Dev. 22, 374-380. 10.1016/j.gde.2012.03.006 [DOI] [PubMed] [Google Scholar]
- Krezel W., Ghyselinck N., Samad T. A., Dupé V., Kastner P., Borrelli E. and Chambon P. (1998). Impaired locomotion and dopamine signaling in retinoid receptor mutant mice. Science 279, 863-867. 10.1126/science.279.5352.863 [DOI] [PubMed] [Google Scholar]
- Krumlauf R. (1993). Hox genes and pattern formation in the branchial region of the vertebrate head. Trends Genet. 9, 106-112. 10.1016/0168-9525(93)90203-T [DOI] [PubMed] [Google Scholar]
- Kumar S. and Duester G. (2010). Retinoic acid signaling in perioptic mesenchyme represses Wnt signaling via induction of Pitx2 and Dkk2. Dev. Biol. 340, 67-74. 10.1016/j.ydbio.2010.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S. and Duester G. (2014). Retinoic acid controls body axis extension by directly repressing Fgf8 transcription. Development 141, 2972-2977. 10.1242/dev.112367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Chatzi C., Brade T., Cunningham T. J., Zhao X. and Duester G. (2011). Sex-specific timing of meiotic initiation is regulated by Cyp26b1 independent of retinoic acid signalling. Nat. Commun. 2, 151 10.1038/ncomms1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Cunningham T. J. and Duester G. (2016). Nuclear receptor corepressors Ncor1 and Ncor2 (Smrt) are required for retinoic acid-dependent repression of Fgf8 during somitogenesis. Dev. Biol. 418, 204-215. 10.1016/j.ydbio.2016.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linville A., Radtke K., Waxman J. S., Yelon D. and Schilling T. F. (2009). Combinatorial roles for zebrafish retinoic acid receptors in the hindbrain, limbs and pharyngeal arches. Dev. Biol. 325, 60-70. 10.1016/j.ydbio.2008.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohnes D., Mark M., Mendelsohn C., Dollé P., Dierich A., Gorry P., Gansmuller A. and Chambon P. (1994). Function of the retinoic acid receptors (RARs) during development. (I) Craniofacial and skeletal abnormalities in RAR double mutants. Development 120, 2723-2748. [DOI] [PubMed] [Google Scholar]
- Maden M., Gale E., Kostetskii I. and Zile M. H. (1996). Vitamin A-deficient quail embryos have half a hindbrain and other neural defects. Curr. Biol. 6, 417-426. 10.1016/S0960-9822(02)00509-2 [DOI] [PubMed] [Google Scholar]
- Mark M., Teletin M., Vernet N. and Ghyselinck N. B. (2015). Role of retinoic acid receptor (RAR) signaling in post-natal male germ cell differentiation. Biochim. Biophys. Acta 1849, 84-93. 10.1016/j.bbagrm.2014.05.019 [DOI] [PubMed] [Google Scholar]
- Marlétaz F., Holland L. Z., Laudet V. and Schubert M. (2006). Retinoic acid signaling and the evolution of chordates. Int. J. Biol. Sci. 2, 38-47. 10.7150/ijbs.2.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall H., Studer M., Pöpperl H., Aparicio S., Kuroiwa A., Brenner S. and Krumlauf R. (1994). A conserved retinoic acid response element required for early expression of the homeobox gene Hoxb-1. Nature 370, 567-571. 10.1038/370567a0 [DOI] [PubMed] [Google Scholar]
- Matt N., Dupé V., Garnier J.-M., Dennefeld C., Chambon P., Mark M. and Ghyselinck N. B. (2005). Retinoic acid-dependent eye morphogenesis is orchestrated by neural crest cells. Development 132, 4789-4800. 10.1242/dev.02031 [DOI] [PubMed] [Google Scholar]
- Mendelsohn C., Lohnes D., Décimo D., Lufkin T., LeMeur M., Chambon P. and Mark M. (1994). Function of the retinoic acid receptors (RARs) during development. (II) Multiple abnormalities at various stages of organogenesis in RAR double mutants. Development 120, 2749-2771. [DOI] [PubMed] [Google Scholar]
- Metzler M. A. and Sandell L. L. (2016). Enzymatic metabolism of vitamin A in developing vertebrate embryos. Nutrients 8, 15 10.3390/nu8120812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molotkova N., Molotkov A., Sirbu I. O. and Duester G. (2005). Requirement of mesodermal retinoic acid generated by Raldh2 for posterior neural transformation. Mech. Dev. 122, 145-155. 10.1016/j.mod.2004.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molotkov A., Molotkova N. and Duester G. (2006). Retinoic acid guides eye morphogenetic movements via paracrine signaling but is unnecessary for retinal dorsoventral patterning. Development 133, 1901-1910. 10.1242/dev.02328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molotkova N., Molotkov A. and Duester G. (2007). Role of retinoic acid during forebrain development begins late when Raldh3 generates retinoic acid in the ventral subventricular zone. Dev. Biol. 303, 601-610. 10.1016/j.ydbio.2006.11.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutier E., Ye T., Choukrallah M.-A., Urban S., Osz J., Chatagnon A., Delacroix L., Langer D., Rochel N., Moras D. et al. (2012). Retinoic acid receptors recognize the mouse genome through binding elements with diverse spacing and topology. J. Biol. Chem. 287, 26328-26341. 10.1074/jbc.M112.361790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederreither K., Vermot J., Schuhbaur B., Chambon P. and Dollé P. (2000). Retinoic acid synthesis and hindbrain patterning in the mouse embryo. Development 127, 75-85. [DOI] [PubMed] [Google Scholar]
- Niederreither K., Vermot J., Messaddeq N., Schuhbaur B., Chambon P. and Dollé P. (2001). Embryonic retinoic acid synthesis is essential for heart morphogenesis in the mouse. Development 128, 1019-1031. [DOI] [PubMed] [Google Scholar]
- Nishimoto S., Wilde S. M., Wood S. and Logan M. P. O. (2015). RA acts in a coherent feed-forward mechanism with Tbx5 to control limb bud induction and initiation. Cell Rep. 12, 879-891. 10.1016/j.celrep.2015.06.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novitch B. G., Wichterle H., Jessell T. M. and Sockanathan S. (2003). A requirement for retinoic acid-mediated transcriptional activation in ventral neural patterning and motor neuron specification. Neuron 40, 81-95. 10.1016/j.neuron.2003.08.006 [DOI] [PubMed] [Google Scholar]
- Pellegrini M., Filipponi D., Gori M., Barrios F., Lolicato F., Grimaldi P., Rossi P., Jannini E. A., Geremia R. and Dolci S. (2008). ATRA and KL promote differentiation toward the meiotic program of male germ cells. Cell Cycle 7, 3878-3888. 10.4161/cc.7.24.7262 [DOI] [PubMed] [Google Scholar]
- Pennimpede T., Cameron D. A., MacLean G. A., Li H., Abu-Abed S. and Petkovich M. (2010). The role of CYP26 enzymes in defining appropriate retinoic acid exposure during embryogenesis. Birth Defects Res. 88, 883-894. 10.1002/bdra.20709 [DOI] [PubMed] [Google Scholar]
- Pradhan A., Zeng X.-X. I., Sidhwani P., Marques S. R., George V., Targoff K. L., Chi N. C. and Yelon D. (2017). FGF signaling enforces cardiac chamber identity in the developing ventricle. Development 144, 1328-1338. 10.1242/dev.143719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raverdeau M., Gely-Pernot A., Feret B., Dennefeld C., Benoit G., Davidson I., Chambon P., Mark M. and Ghyselinck N. B. (2012). Retinoic acid induces Sertoli cell paracrine signals for spermatogonia differentiation but cell autonomously drives spermatocyte meiosis. Proc. Natl. Acad. Sci. USA 109, 16582-16587. 10.1073/pnas.1214936109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhinn M. and Dolle P. (2012). Retinoic acid signalling during development. Development 139, 843-858. 10.1242/dev.065938 [DOI] [PubMed] [Google Scholar]
- Ribes V., Stutzmann F., Bianchetti L., Guillemot F., Dollé P. and Le Roux I. (2008). Combinatorial signalling controls Neurogenin2 expression at the onset of spinal neurogenesis. Dev. Biol. 321, 470-481. 10.1016/j.ydbio.2008.06.003 [DOI] [PubMed] [Google Scholar]
- Ryckebusch L., Wang Z., Bertrand N., Lin S.-C., Chi X., Schwartz R., Zaffran S. and Niederreither K. (2008). Retinoic acid deficiency alters second heart field formation. Proc. Natl. Acad. Sci. USA 105, 2913-2918. 10.1073/pnas.0712344105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin E., Palmer M. J., Li M. and Fricker R. A. (2012). GABAergic neurons from mouse embryonic stem cells possess functional properties of striatal neurons in vitro, and develop into striatal neurons in vivo in a mouse model of Huntington's disease. Stem Cell Rev. 8, 513-531. 10.1007/s12015-011-9290-2 [DOI] [PubMed] [Google Scholar]
- Siegenthaler J. A., Ashique A. M., Zarbalis K., Patterson K. P., Hecht J. H., Kane M. A., Folias A. E., Choe Y., May S. R., Kume T. et al. (2009). Retinoic acid from the meninges regulates cortical neuron generation. Cell 139, 597-609. 10.1016/j.cell.2009.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simsek M. F. and Özbudak E. M. (2018). Spatial fold change of FGF signaling encodes positional information for segmental determination in zebrafish. Cell Rep. 24, 66-78.e8. 10.1016/j.celrep.2018.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirbu I. O. and Duester G. (2006). Retinoic-acid signalling in node ectoderm and posterior neural plate directs left-right patterning of somitic mesoderm. Nature Cell Biol. 8, 271-277. 10.1038/ncb1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirbu I. O., Gresh L., Barra J. and Duester G. (2005). Shifting boundaries of retinoic acid activity control hindbrain segmental gene expression. Development 132, 2611-2622. 10.1242/dev.01845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirbu I. O., Zhao X. and Duester G. (2008). Retinoic acid controls heart anteroposterior patterning by down-regulating Isl1 through the Fgf8 pathway. Dev. Dyn. 237, 1627-1635. 10.1002/dvdy.21570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skromne I., Thorsen D., Hale M., Prince V. E. and Ho R. K. (2007). Repression of the hindbrain developmental program by Cdx factors is required for the specification of the vertebrate spinal cord. Development 134, 2147-2158. 10.1242/dev.002980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder E. M., Small C. and Griswold M. D. (2010). Retinoic Acid availability drives the asynchronous initiation of spermatogonial differentiation in the mouse. Biol. Reprod. 83, 783-790. 10.1095/biolreprod.110.085811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrell M. R. J. and Waxman J. S. (2011). Restraint of Fgf8 signaling by retinoic acid signaling is required for proper heart and forelimb formation. Dev. Biol. 358, 44-55. 10.1016/j.ydbio.2011.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steventon B. and Martinez Arias A. (2017). Evo-engineering and the cellular and molecular origins of the vertebrate spinal cord. Dev. Biol. 432, 3-13. 10.1016/j.ydbio.2017.01.021 [DOI] [PubMed] [Google Scholar]
- Studer M., Pöpperl H., Marshall H., Kuroiwa A. and Krumlauf R. (1994). Role of a conserved retinoic acid response element in rhombomere restriction of Hoxb-1. Science 265, 1728-1732. 10.1126/science.7916164 [DOI] [PubMed] [Google Scholar]
- Sturgeon K., Kaneko T., Biemann M., Gauthier A., Chawengsaksophak K. and Cordes S. P. (2011). Cdx1 refines positional identity of the vertebrate hindbrain by directly repressing Mafb expression. Development 138, 65-74. 10.1242/dev.058727 [DOI] [PubMed] [Google Scholar]
- Sugimoto R., Nabeshima Y.-I. and Yoshida S. (2012). Retinoic acid metabolism links the periodical differentiation of germ cells with the cycle of Sertoli cells in mouse seminiferous epithelium. Mech. Dev. 128, 610-624. 10.1016/j.mod.2011.12.003 [DOI] [PubMed] [Google Scholar]
- Teletin M., Vernet N., Ghyselinck N. B. and Mark M. (2017). Roles of retinoic acid in germ cell differentiation. Curr. Top. Dev. Biol. 125, 191-225. 10.1016/bs.ctdb.2016.11.013 [DOI] [PubMed] [Google Scholar]
- Teletin M., Vernet N., Yu J., Klopfenstein M., Jones J. W., Feret B., Kane M. A., Ghyselinck N. B. and Mark M. (2019). Two functionally redundant sources of retinoic acid secure spermatogonia differentiation in the seminiferous epithelium. Development 146, dev170225 10.1242/dev.170225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong M.-H., Mitchell D. A., McGowan S. D., Evanoff R. and Griswold M. D. (2012). Two miRNA clusters, Mir-17-92 (Mirc1) and Mir-106b-25 (Mirc3), are involved in the regulation of spermatogonial differentiation in mice. Biol. Reprod. 86, 72 10.1095/biolreprod.111.096313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong M.-H., Yang Q.-E., Davis J. C. and Griswold M. D. (2013). Retinol dehydrogenase 10 is indispensible for spermatogenesis in juvenile males. Proc. Natl. Acad. Sci. USA 110, 543-548. 10.1073/pnas.1214883110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdivia L. E., Lamb D. B., Horner W., Wierzbicki C., Tafessu A., Williams A. M., Gestri G., Krasnow A. M., Vleeshouwer-Neumann T. S., Givens M. K. et al. (2016). Antagonism between Gdf6a and retinoic acid pathways controls timing of retinal neurogenesis and growth of the eye in zebrafish. Development 143, 1087-1098. 10.1242/dev.130922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermot J. and Pourquié O. (2005). Retinoic acid coordinates somitogenesis and left-right patterning in vertebrate embryos. Nature 435, 215-220. 10.1038/nature03488 [DOI] [PubMed] [Google Scholar]
- Vermot J., Llamas J. G., Fraulob V., Niederreither K., Chambon P. and Dollé P. (2005). Retinoic acid controls the bilateral symmetry of somite formation in the mouse embryo. Science 308, 563-566. 10.1126/science.1108363 [DOI] [PubMed] [Google Scholar]
- Vernet N., Dennefeld C., Guillou F., Chambon P., Ghyselinck N. B. and Mark M. (2006). Prepubertal testis development relies on retinoic acid but not rexinoid receptors in Sertoli cells. EMBO J. 25, 5816-5825. 10.1038/sj.emboj.7601447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichterle H., Lieberam I., Porter J. A. and Jessell T. M. (2002). Directed differentiation of embryonic stem cells into motor neurons. Cell 110, 385-397. 10.1016/S0092-8674(02)00835-8 [DOI] [PubMed] [Google Scholar]
- Wilson V., Olivera-Martinez I. and Storey K. G. (2009). Stem cells, signals and vertebrate body axis extension. Development 136, 1591-1604. 10.1242/dev.021246 [DOI] [PubMed] [Google Scholar]
- Xu L., Glass C. K. and Rosenfeld M. G. (1999). Coactivator and corepressor complexes in nuclear receptor function. Curr. Opin. Genet. Dev. 9, 140-147. 10.1016/S0959-437X(99)80021-5 [DOI] [PubMed] [Google Scholar]
- Yakushiji-Kaminatsui N., Kondo T., Hironaka K.-I., Sharif J., Endo T. A., Nakayama M., Masui O., Koseki Y., Kondo K., Ohara O. et al. (2018). Variant PRC1 competes with retinoic acid-related signals to repress Meis2 in the mouse distal forelimb bud. Development 145, dev166348 10.1242/dev.166348 [DOI] [PubMed] [Google Scholar]
- Yang J., Corsello T. R. and Ma Y. (2012). Stem cell gene SALL4 suppresses transcription through recruitment of DNA methyltransferases. J. Biol. Chem. 287, 1996-2005. 10.1074/jbc.M111.308734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q.-E., Racicot K. E., Kaucher A. V., Oatley M. J. and Oatley J. M. (2013). MicroRNAs 221 and 222 regulate the undifferentiated state in mammalian male germ cells. Development 140, 280-290. 10.1242/dev.087403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S. (2018). Open niche regulation of mouse spermatogenic stem cells. Dev. Growth Differ. 60, 542-552. 10.1111/dgd.12574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Sirbu I. O., Mic F. A., Molotkova N., Molotkov A., Kumar S. and Duester G. (2009). Retinoic acid promotes limb induction through effects on body axis extension but is unnecessary for limb patterning. Curr. Biol. 19, 1050-1057. 10.1016/j.cub.2009.04.059 [DOI] [PMC free article] [PubMed] [Google Scholar]