Abstract
Dysfunctional regulation of mood and emotion is a key component of major depressive disorder and leads to sustained negative feelings. Using functional MRI (fMRI), we investigated the temporal dynamics of emotion regulation in patients with major depressive disorder and in healthy controls, testing for acute and sustained neural effects of active emotion regulation. Moderately depressed individuals (n = 17) and never-depressed healthy control subjects (n = 17) underwent fMRI during performance of an active cognitive emotion regulation task while viewing emotionally arousing pictures. In a second task, completed 15 min later, subjects were presented with the same stimuli in a passive viewing task. Whole-brain analyses and connectivity measures were used to determine acute and sustained effects of emotion regulation on brain activation and coupling between regions. On the group level, patients were able to downregulate negative emotions and corresponding amygdala activation, but this ability decreased with increasing symptom severity. Moreover, only healthy control subjects showed a sustained regulation effect in the amygdala after a 15 min delay, whereas depressed patients did not. Finally, patients exhibited diminished prefrontal activation and reduced prefrontolimbic coupling during active regulation. Although emotion regulation capacity in medicated depressive patients appears to be preserved depending on symptom severity, the effect is not sustained. Correlational analyses provide evidence that this diminished sustained-regulation effect might be related to reduced prefrontal activation during regulation.
Introduction
Major depressive disorder (MDD) is the leading cause of years lived with disability (World Health Organization, 2001). A dysfunction in the neural circuitry supporting adaptive regulation, including regions of the prefrontal cortex and amygdala, may play a decisive role in vulnerability to depression (Davidson et al., 2002; Drevets, 2003; Campbell-Sills and Barlow, 2007). Although a number of studies have investigated automatic regulation of mood and emotion (Phillips et al., 2008), research on cognitive emotion regulation in depression has only recently been launched (Beauregard et al., 2006; Johnstone et al., 2007).
In healthy controls, cognitive emotion regulation using a reappraisal strategy is effective in reducing negative feelings and corresponding physiological responses in the amygdala (Ochsner and Gross, 2005; Phillips et al., 2008). Regulation of negative affect involves the medial and lateral prefrontal and the parietal cortex (Ochsner and Gross, 2005; Phillips et al., 2008). These regions may support processes involved in regulating inner states to achieve desired outcomes (Phillips et al., 2008). Recently, we showed that the effects of voluntary emotion regulation by detachment, a specific reappraisal strategy, extend beyond the period of active regulation, demonstrating a sustained regulation effect on the amygdala in a subsequent passive viewing task (Walter et al., 2009).
The main brain areas involved in cognitive emotion regulation, i.e., amygdala and regions of the prefrontal cortex, appear to be dysfunctional in depression. Neuroimaging studies have found elevated baseline activity of the amygdala associated with symptom severity (Drevets, 1999; Drevets et al., 2002), as well as heightened amygdala responsivity to affective stimuli (Sheline et al., 2001; Hamilton and Gotlib, 2008). Decreases in blood flow and glucose metabolism, as well as ineffective functioning, have been reported for the dorsolateral prefrontal cortex (DLPFC) (Elliott et al., 1997; Davidson et al., 2002; Siegle et al., 2007; Vasic et al., 2007; Walter et al., 2007). DLPFC hypoactivation may result in a reduced regulatory influence on amygdala activation in response to an emotional challenge, which might be expressed phenomenologically as perseveration of negative affect and rumination (Davidson et al., 2002).
Cognitive emotion regulation in depression has been investigated with functional MRI (fMRI) only recently. It has been found that patients with MDD experienced more difficulty in regulating sad feelings and showed heightened regulatory activation in dorsal anterior cingulate cortex (ACC) (Beauregard et al., 2006). In another study, patients exhibited right prefrontal activation and a positive correlation between ventromedial prefrontal cortex (VMPFC) and amygdala during reappraisal, whereas controls show an inverse pattern (Johnstone et al., 2007). However, neither study reported any activation of the amygdala in response to emotional stimuli nor a direct downregulation effect on the amygdala for either the control or the depressed group. In the present study, we aimed to investigate the effects of active emotion regulation by detachment in patients with MDD, using a task that fosters a reliable regulation effect in the amygdala of healthy controls. Moreover, following results of our previous studies (Walter et al., 2009; Schardt et al., 2010), we explored sustained neural effects of emotion regulation on subsequent passive emotional stimulation. Prior work has shown dysfunctional on-line regulation of emotion in depression, but the question of how enduring regulatory effects are in MDD has yet to be addressed. We hypothesized that patients exhibit a dysfunction in active reappraisal by detachment that would be associated with diminished coupling between amygdala and prefrontal cortex and a consecutively reduced sustained downregulation effect (Schardt et al., 2010).
Materials and Methods
Participants
Seventeen right-handed inpatients (eight females, 43.5 ±10.9 years old) with MDD were recruited in the Department of Psychiatry and Psychotherapy at the University of Frankfurt am Main. All patients were diagnosed according to DSM-IV criteria and subjects with concurrent Axis I disorders were excluded. Psychopathology was assessed with the Beck Depression Inventory (BDI), the 21-item Hamilton Depression Scale (HAMD), the Clinical Global Impression Scale, and the State Trait Anxiety Inventory (Table 1). Subjects were additionally given tests for memory, attention, and executive function to rule out attentional deficits or deficits in executive function that might influence emotion regulation (Table 1). All patients were mild to moderately depressed (HAMD, 18.5 ±4.4; BDI, 25.4 ±13.4; depressive episodes, 4.1 ± 3.2) and all but one were on medication. Four patients were treated with a serotonin reuptake inhibitor (SSRI), three with a serotonin norepinephrine reuptake inhibitor (SSNRI), and two with a tricyclic antidepressant agent (TCA). One patient was medicated with a combination of SSRI and TCA; two with SSNRI and TCA; one with SSNRI, TCA, and lithium; and one with SSNRI and a neuroleptic agent. Two patients received benzodiazepines (≤10 mg of diazepam equivalents/d) in addition to stable medication.
Table 1.
Demographic and clinical characteristics of patients with major depression and control subjects
| Patients with MDD (n = 17) | Healthy controls (n = 17) | p value | |
|---|---|---|---|
| Age (years) | 43.53 (10.9) | 43.9 (10.1) | 0.9 |
| Education (years) | 11.7 | 11.9 | 0.7 |
| Number of depressive episodes | 4.1 (3.2) | N.A. | N.A. |
| HAMD score | 18.5 (4.4) | N.A. | N.A. |
| BDI | 25.4 (13.4) | 3.6 (3.1) | <0.001 |
| CGI | 4.4 (0.7) | N.A. | N.A |
| STAI-T | 56.4 (10.7) | 33.5 (5.7) | <0.001 |
| STAI-S (I) | 50.4 (12.0) | 33.1 (6.3) | <0.001 |
| STAI-S (II) | 47.5 (8.8) | 31.8 (7.6) | <0.001 |
| Attention | 77.2 (19) | 80.1 (10.9) | 0.58 |
| Episodic Memory | 12.8 (2.2) | 13.4 (1.8) | 0.46 |
| WM | 8.0 (2.3) | 8.8 (2.0) | 0.26 |
| ToL | 9.0 (1.8) | 8.4 (2.2) | 0.39 |
STAI-T, State Trait Anxiety Inventory Trait; STAI-S (I), State Trait Anxiety Inventory State (before scanning); STAI-S (II), State Trait Anxiety Inventory State (after scanning); CGI, Clinical Global Impression Scale; Attention, sustained attention test of the Neurobat7 Battery, percentage of correct answers; Episodic Memory, Verbal memory test of the Neurobat7 Battery (www.neurobat.de), number of correctly recalled items; WM, working memory, number of correct answers; ToL, Tower of London, problems solved in minimum; N.A., not applicable.
Values given as mean (standard deviation).
Seventeen healthy control participants (eight females, 43.9 ± 10.1 years old) without any history of neurological or psychiatric disorder or use of psychotropic medication were recruited from the community and were matched for gender, age, and education (Table 1).
This study was approved by the ethics committee of the University of Frankfurt am Main. After complete description of the study to the subjects, written informed consent was obtained.
Experimental procedure
The study was composed of two tasks. During task 1 (active regulation), subjects were presented with 60 pictures of negative or neutral content taken from the International Affective Picture System and matched for content of faces, scenery, food, and nature [mean valence (V) and arousal (A) values: negative no regulation, V = 2.7, A = 5.4; negative regulation, V = 2.8, A = 5.4; neutral no regulation, V = 5.7, A = 3.4; neutral regulation, V = 5.7, A = 3.2). Subjects were instructed to either look at the pictures and permit all upcoming emotions or to cognitively regulate their emotions by taking the position of a neutral observer. More specifically, subjects were instructed to “Look at the following picture directly but try to take the position of a detached observer, thinking about the present picture in a neutral way” for the regulation condition or “Look at the following picture directly and permit feeling your emotions” for the no-regulation condition. The instruction during scanning was given by presenting a cue word for 2 s stating either “permit” or “regulate.” Pictures were presented for 8 s each. After picture presentation, subjects were instructed to not regulate any more and relax. Duration of this relaxation period was 20 s. Trials were presented in pseudorandomized order. Task 1 was performed in two consecutive sessions of 15 min each and was followed by the acquisition of structural images.
Approximately 15 min after end of task 1, we tested for a sustained regulation effect in task 2 (passive viewing). Participants were instructed to just look at the 60 pictures again, which were presented for only 1 s each in a newly pseudorandomized order. Thus, we minimized intentional emotion regulation efforts. Intertrial interval was 3 s with a variable jitter within ±1 repetition time.
Since it has been shown that even the linguistic evaluation of emotional stimuli can significantly reduce amygdala activation (Hariri et al., 2003), and because trial-by-trial ratings of valence and regulation successfully tap into processes that are not related to emotion regulation, per se, but, for example, secondary self-reflection and recall of feelings during actual regulation, and thus might induce brain activation that is distinct from those related to emotion regulation, we intentionally refrained from a trial-by-trial rating of emotional intensity and evaluated regulation success after the scanning session. A control experiment conducted previously in an independent sample (n = 10) using a trial-by-trial rating confirmed the success of emotion regulation in our procedure (Walter et al., 2009).
Data acquisition
Imaging was performed on a 3 Tesla Siemens Allegra scanner. Functional images were taken with an echo planar imaging sequence. In task 1, whole brain coverage was obtained with 33 axially tilted slices [slice thickness, 3 + 0.75 mm gap; field of view (FOV), 192 mm; repetition time, 2 s; echo time, 35 ms; 64 × 64 matrix, flip angle, 80°]. In task 2, we used a shorter repetition time (1.5 s) due to a rapid event-related design. One volume thus consisted of 23 slices covering the brain from the temporal poles to the superior parietal cortex and thus in each case included the amygdala and the prefrontal and the parietal cortices (slice thickness, 3 + 0.75 mm gap; FOV, 192 mm; echo time, 35; 64 × 64 matrix, flip angle, 80°).
Data analysis
Data preprocessing.
Data preprocessing and statistical analyses for both tasks were performed with SPM 5 (http://www.fil.ion.ucl.ac.uk/spm/software/spm5/). Individual functional images were corrected for motion by realignment to the first volume of each session. All images were spatially normalized (2 × 2 × 2 mm) to an echo planar image in MNI space and spatially smoothed with an 8 mm full width at half maximum isotropic Gaussian kernel. For each trial, the variance of each voxel was estimated according to the general linear model. Intrinsic autocorrelations were accounted for by an autoregressive model of first order and low-frequency drifts were removed via high pass filter.
Categorical analysis.
The first-level regression model in both tasks consisted of a set of four regressors (nonregulated negative, nonregulated neutral, regulated negative, and regulated neutral) modeled as an event with a duration of 8 s and convolved with the hemodynamic response function, and six regressors describing residual motion. For task 1, instruction was modeled additionally as an event of 2 s. In a second-level random effects group analysis, individual regionally specific effects of conditions for each subject were compared using a full factorial design (ANOVA) with valence, regulation condition, and group as factors resulting in a t statistic for every voxel. The significance threshold was set to p < 0.05, family-wise error corrected for multiple comparisons across the whole brain, or for multiple comparisons within an anatomically defined region of interest (ROI) (amygdala, DLPFC) provided by the Wake Forest University PickAtlas (www.fmri.wfubmc.edu). For regression analyses, individual peak voxel data were extracted from the respective contrast and region and analyzed externally using SPSS Statistics 17.0.
Psychophysiological interaction analysis.
To assess coupling between regions, we used a 2 × 2 factorial design and estimated a psychophysiological interaction (PPI) analysis (Friston et al., 1997). We extracted the subject-specific time course of activity in the amygdala ROI with an 8-mm-radial sphere centered at the voxel displaying peak activity. We then calculated the product of this activation time course with the interaction term of the regulation > no-regulation trials to create the psychophysiological interaction term. PPI analyses were performed for each ROI (left and right amygdala) in each subject and then entered into a random effects group analysis using a one-sample t test for each group separately and a two-sample t test for group comparison. For PPI analyses, we used a significance threshold of p < 0.001 uncorrected at the voxel level and p < 0.05 corrected at the cluster level.
Results
Behavioral results
First, affective valence of the pictures did not differ between groups (1 = very unpleasant, 9 = very pleasant; negative: controls, 2.91; patients, 2.69, p = 0.47; neutral: controls, 6.21; patients, 5.90, p = 0.11). All subjects succeeded in regulating their emotions according to a semistructured postscanning interview. General success was rated 6.09 (1 = not successful, 9 = very successful; controls, 6.35; patients, 5.82; p = 0.4). Additionally, subjects had to rate the regulation success for each individual picture of the negative regulation condition with no significant group difference (controls, 5.43; patients, 5.28; p = 0.8). The helpfulness of the instructed technique during the experiment was rated 6.24 (1 = not helpful, 9 = very helpful; controls, 6.76; patients, 6.12; p = 0.2). Neuropsychological tests of attention, memory, and executive function revealed no differences between groups (Table 1).
Functional MRI results
Task 1 (active regulation)
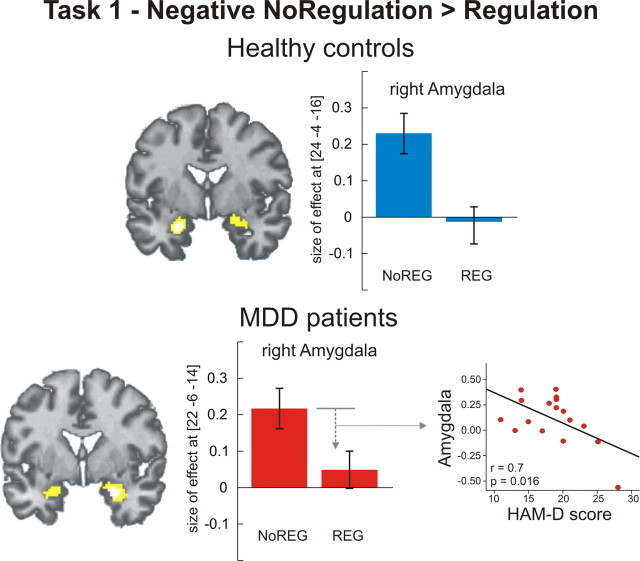
No difference was observed between groups for the contrast negative > neutral and no regulation negative > neutral. Both groups showed bilateral activation of the amygdala in response to negative pictures (Table 2). This effect was significantly reduced during regulation (negative regulation > no regulation), with no difference between groups (Fig. 1). In patients, a regression analysis revealed that downregulation of the right amygdala correlated negatively with individual HAMD scores (r = 0.7, p = 0.016, two-tailed), i.e., the higher the individual symptom severity, the less effective amygdala downregulation (Fig. 1).
Table 2.
Regional brain activation
| Region | x | y | z | Z | |
|---|---|---|---|---|---|
| Regional brain activation during task 1 (active regulation) | |||||
| Negative > neutral | |||||
| Healthy control subjects | |||||
| Amygdala | R | 24 | −4 | −16 | 4.26† |
| L | −24 | −4 | −22 | 4.88 | |
| Ventral striatum | R | 8 | 0 | −2 | 6.87 |
| Dorsomedial prefrontal cortex | L | −6 | 54 | 24 | 6.83 |
| Ventrolateral prefrontal cortex | L | −42 | 22 | −20 | 6.59 |
| Fusiform gyrus | R | 45 | −56 | −20 | 5.73 |
| L | −42 | −50 | −22 | 5.87 | |
| MDD patients | |||||
| Amygdala | R | 24 | 0 | −22 | 5.30 |
| L | −20 | −4 | −20 | 4.87 | |
| Dorsomedial prefrontal cortex | 0 | 60 | 22 | 6.82 | |
| Ventrolateral prefrontal cortex | R | 28 | 20 | −24 | 6.51 |
| L | −36 | 22 | −20 | 5.58 | |
| Dorsolateral prefrontal cortex | R | 58 | 26 | 28 | 6.39 |
| Fusiform gyrus | R | 44 | −56 | −20 | 7.62 |
| L | −40 | −60 | −16 | 8.00 | |
| Regulation > no regulation | |||||
| Healthy control subjects | |||||
| Dorsolateral prefrontal cortex | R | 40 | 26 | 46 | 5.85 |
| 42 | 44 | 24 | 5.79 | ||
| Inferior parietal cortex | R | 60 | −56 | 38 | 7.40 |
| MDD patients | |||||
| Inferior parietal cortex | R | 62 | −48 | 32 | 6.45 |
| L | −58 | −36 | 40 | 5.67 | |
| No regulation negative > neutral | |||||
| Healthy controls | |||||
| Amygdala | R | 24 | −4 | −16 | 3.69† |
| L | −22 | −4 | −22 | 3.27† | |
| Dorsomedial prefrontal cortex | 4 | 38 | 56 | 4.77 | |
| Dorsolateral prefrontal cortex | R | 54 | 22 | 30 | 4.80 |
| Fusiform gyrus | R | 48 | −72 | −18 | 5.18 |
| L | −38 | −60 | −14 | 4.80 | |
| MDD patients | |||||
| Amygdala | R | 24 | −2 | −22 | 3.98† |
| L | −30 | −4 | −16 | 3.89† | |
| Dorsomedial prefrontal cortex | 0 | 54 | 32 | 4.83 | |
| Dorsolateral prefrontal cortex | R | 56 | 24 | 30 | 5.35 |
| Fusiform gyrus | R | 40 | −66 | −18 | 6.26 |
| L | −40 | −60 | −16 | 6.21 | |
| Negative no regulation > regulation | |||||
| Healthy control subjects | |||||
| Amygdala | R | 24 | −4 | −16 | 4.46† |
| L | −24 | −8 | −16 | 3.99† | |
| Ventral striatum | R | 8 | 0 | −4 | 5.25 |
| Hippocampus | R | 24 | −22 | −14 | 5.90 |
| L | 20 | −36 | −12 | 5.33 | |
| Lingual gyrus | R | 2 | −90 | −6 | 7.73 |
| L | −6 | −94 | −6 | 7.73 | |
| MDD patients | |||||
| Amygdala | R | 22 | −6 | −14 | 3.37† |
| L | −22 | −8 | −16 | 3.21† | |
| Hippocampus | R | 16 | −28 | −12 | 5.03 |
| Lingual gyrus | R | 28 | −94 | −2 | 6.13 |
| L | −20 | −98 | 0 | 6.08 | |
| Negative regulation > no regulation | |||||
| Healthy control subjects | |||||
| Dorsolateral prefrontal cortex | R | 42 | 44 | 24 | 5.15 |
| 42 | 32 | 40 | 5.08 | ||
| 26 | 6 | 52 | 4.79 | ||
| Inferior parietal cortex | R | 60 | −46 | 30 | 6.93 |
| MDD patients | |||||
| Inferior parietal cortex | R | 62 | −48 | 30 | 5.53 |
| L | −60 | −36 | 38 | 5.31 | |
| Healthy control subjects > MDD patients | |||||
| Dorsolateral prefrontal cortex | R | 36 | 44 | 28 | 3.55† |
| Negative > Neutral (Regulation > No Regulation) | |||||
| Healthy control subjects | |||||
| Inferior parietal/superior temporal cortex | R | 48 | −62 | 16 | 5.75 |
| L | −54 | −60 | 16 | 5.22 | |
| MDD patients | |||||
| Inferior parietal/superior temporal cortex | R | 52 | −60 | 12 | 5.65 |
| Regional brain activation during task 2 (passive viewing) | |||||
| Negative > neutral | |||||
| Healthy control subjects | |||||
| Amygdala | R | 20 | 0 | −24 | 4.71 |
| L | −20 | −2 | −26 | 2.97† | |
| MDD patients | |||||
| Amygdala | R | 20 | −4 | −16 | 3.06† |
| L | −26 | −2 | −20 | 4.14† | |
| Inferior frontal gyrus | L | −58 | 16 | 14 | 4.92 |
| Fusiform gyrus | R | 48 | −48 | −26 | 6.20 |
| L | −44 | −68 | −20 | 6.77 | |
| Negative no regulation > regulation | |||||
| Healthy control subjects | |||||
| Amygdala | L | −22 | −4 | −22 | 3.88† |
| R | 22 | 2 | −20 | 3.47† | |
| Healthy control subjects > MDD patients | |||||
| Amygdala | R | 22 | 2 | −20 | 3.26† |
| L | −18 | −6 | −18 | 2.85† | |
| Negative > neutral (regulation > no regulation) | |||||
| Healthy control subjects | |||||
| Amygdala | R | 24 | 0 | −14 | 3.61† |
| L | −24 | −6 | −14 | 3.19† | |
| Healthy control subjects > MDD patients | |||||
| Amygdala | R | 22 | −2 | −20 | 3.24† |
| L | −22 | −6 | −20 | 3.01† |
R, Right; L, left; x, y, z, respective MNI coordinates of peak voxel activation; Z, Z value; all results p < 0.05 family-wise error corrected for multiple comparisons across whole brain;
†p < 0.05 family-wise error corrected for anatomical a priori ROI.
Figure 1.
Regional brain activation during active regulation (task 1). Healthy controls and MDD patients showed significantly reduced bilateral amygdala activation during regulation of negative emotion (p < 0.05 family-wise error corrected for ROI). The amount of downregulation in MDD patients depended on symptom severity: the higher the scores in the HAMD scale, the less the downregulation effect in the right amygdala (r = 0.7, p = 0.016, two-tailed). Bar plots indicate size of the effect at the maximum activated voxel in the amygdala for the contrast negative no regulation > negative regulation. Note: plots were depicted only for the right amygdala, the same pattern was observed for the left amygdala. NoREG, No regulation condition; REG, Regulation condition.
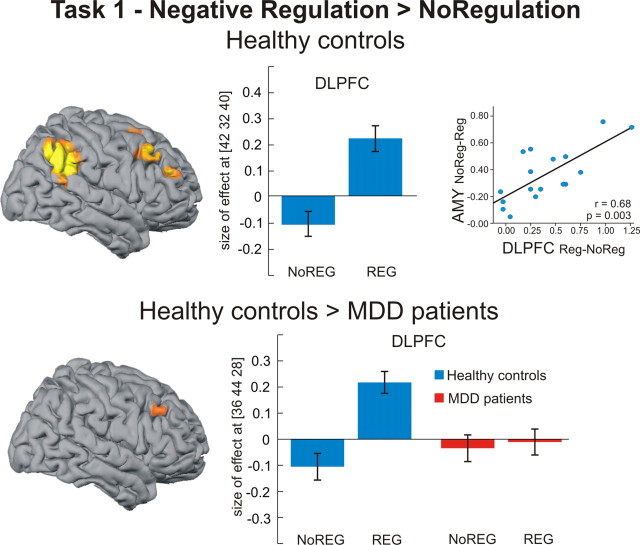
During negative regulation (negative regulation > no regulation), we observed activation of a right hemispheric prefrontoparietal network in healthy controls, comprising right DLPFC and inferior parietal cortex (IPL). In the patient group, we observed activation of the right IPL but, relative to control subjects, patients showed a significantly diminished response in right DLPFC (Fig. 2).
Figure 2.
Regional brain activation during active regulation (task 1). Healthy controls exhibited significant activation of the DLPFC and inferior parietal cortex during active regulation of negative emotions (p < 0.05, family-wise error corrected for whole brain). The activation increase in the right DLPFC was positively correlated with the amount of downregulation in the right amygdala (r = 0.68, p = 0.003, two-tailed). Compared with healthy controls, MDD patients showed reduced DLPFC activation during regulation, as seen in a significant group-by-regulation interaction (p < 0.05, family-wise error corrected for ROI). Bar plots indicate size of effect at the maximum activated voxel in right DLPFC for the contrast negative regulation > no regulation (healthy controls) and the group-by-regulation contrast [healthy controls (regulation > no regulation) > MDD patients (regulation > no regulation)]. AMY, Amygdala; NoREG, no regulation condition; REG, regulation condition.
A regression analysis on the extracted individual data for the contrast negative regulation > no regulation (DLPFC) with the contrast negative no regulation > regulation (amygdala left and right) revealed a significant positive correlation between activation increase in right DLPFC and activation decrease in the right amygdala (r = 0.68, p = 0.003, two-tailed) (Fig. 2). The same analysis was applied to individual data in the IPL with a significant correlation between IPL and right amygdala in healthy controls (r = 0.77, p = 0.0003, two-tailed) and MDD patients (r = 0.67, p = 0.003, two-tailed).
Psychophysiological interaction (task 1)
The PPI analysis revealed that, in healthy controls, blood oxygenation level-dependent (BOLD) responses in the left amygdala showed increased coupling during regulation with the ventromedial prefrontal cortex (x = 6, y = 50, z = −2, Z = 3.60), posterior cingulate gyrus (x = 8, y = −30, z = 40, Z = 4.71), right IPL (x = 58, y = −36, z = 40, Z = 3.88), and right DLPFC (x = 24, y = 28, z = 48, Z = 4.41) (Fig. 3). BOLD responses in the right amygdala show a similar coupling pattern but at a less stringent statistical level (p < 0.005). In the patient group, no significant coupling of the amygdala with other brain regions was observed. Direct group comparison revealed significantly reduced coupling between left amygdala and right DLPFC in patients compared with controls (x = 30, y = 28, z = 48, Z = 3.53) (Fig. 3).
Figure 3.
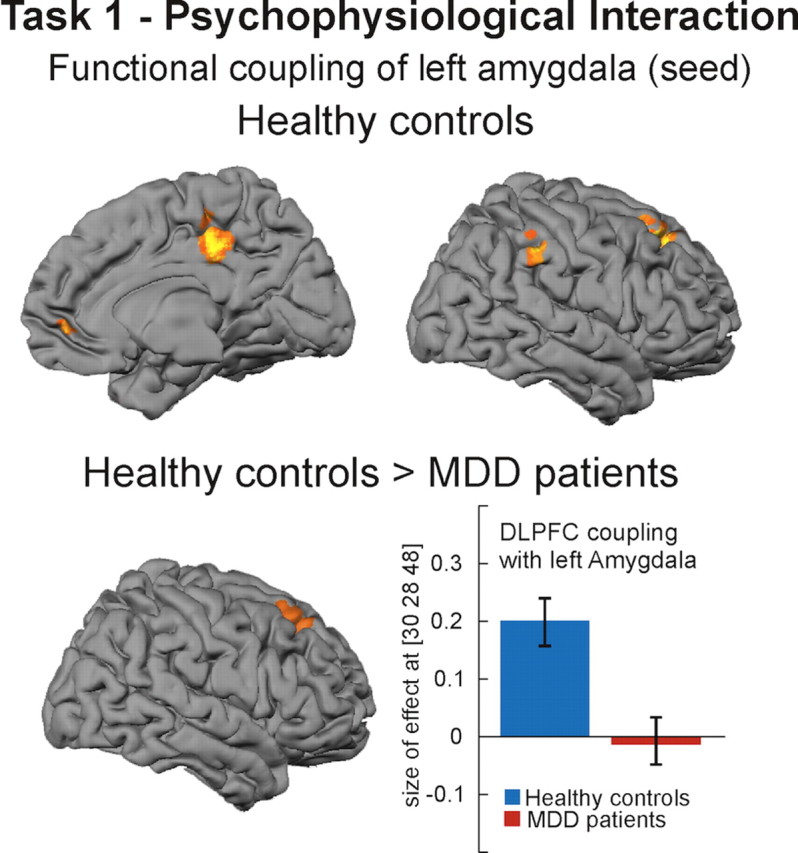
Results of psychophysiological interaction analysis (task 1). Healthy controls showed increased coupling between left amygdala (seed) and right DLPFC, inferior parietal cortex, posterior cingulate gyrus, and ventromedial prefrontal cortex (p < 0.001 uncorrected at voxel level, p < 0.05 corrected at the cluster level). Compared with healthy controls, MDD patients showed significantly reduced coupling between amygdala and DLPFC. Bar plots indicate size of effect at the voxel showing the maximum coupling effect.
Task 2 (passive viewing)
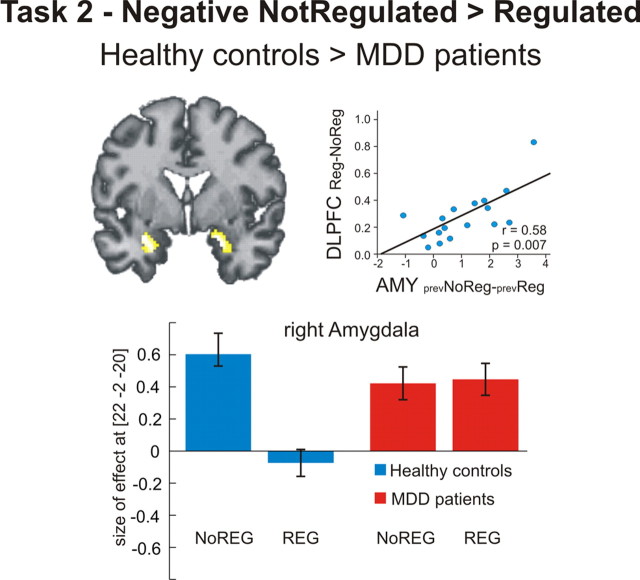
Presentation of negative pictures (negative > neutral) elicited a significant activation of bilateral amygdala in both groups (Table 2). In the control group, this effect was present only for negative pictures that were formerly not regulated (negative no regulation > regulation), whereas formerly regulated negative pictures exhibited a clear sustained downregulation effect on amygdala activation, reflected in a significant interaction of valence and former regulation [negative > neutral (regulation > no regulation)] (Table 2). The patient group did not exhibit any sustained amygdala downregulation effect, resulting in a significant interaction of former regulation and group [healthy controls > MDD subjects (negative no regulation > regulation)] (Fig. 4).
Figure 4.
Regional brain activation during passive viewing (task 2). Healthy controls exhibited a significant sustained regulation effect in the bilateral amygdala. (Note that there was no activation in DLPFC or IPL during task 2 even when the threshold was lowered to an uncorrected p < 0.05). The amount of this sustained regulation effect in the amygdala was positively correlated with DLPFC activation during active regulation in task 1 (r = 0.058, p = 0.007, two-tailed). Compared with healthy controls, MDD patients did not show a sustained regulation effect in the amygdala, as seen in a significant group-by-regulation interaction (p < 0.05 family-wise error corrected for ROI). Bar plots indicate size of the effect at the maximum activated voxel in the right amygdala for the group by regulation contrast [healthy controls (no regulation > regulation) > MDD patients (no regulation > regulation)]. AMY, Amygdala; NoREG, no regulation during task 1; REG, regulation during task 1; prev, previous.
We performed a regression analysis on the extracted individual data for the contrast negative regulation > no regulation (DLPFC) in task 1 with the contrast negative no regulation > regulation (amygdala left and right) in task 2. We found a significant positive correlation between activation increase in right DLPFC during task 1 and activation decrease in the right (r = 0.58, p = 0.007, two-tailed) and left (r = 0.56, p = 0.009, two-tailed) amygdala during task 2 in the control group (Fig. 4).
Discussion
The goal of the present study was to investigate the neural bases of emotion regulation by detachment and its temporal dynamics in patients with MDD. Using two tasks, one with the instruction to regulate feelings upon negative visual scenes and a subsequent task using passive viewing of the same scenes, we were able to not only examine the ability to actively regulate emotions but also to examine the persistence of successful regulation.
Effects of active emotion regulation
Behaviorally, regulation success was similar between subjects with MDD and healthy controls. However, subjective ratings might be unreliable as they could be due to social desirability effects. Neurally, amygdala reactivity upon negative stimulation did not differ between groups and—crucially—both groups showed a significant downregulation effect in the amygdala. We regard this neural effect as a proxy for regulation success, although it cannot be directly equated with emotion regulation (Fig. 1). Empirical evidence suggests that depressive patients exhibit increased and relatively unmodulated amygdala activity during emotional stimulation without being asked to regulate at all (Drevets, 1999; Sheline et al., 2001; Siegle et al., 2002, 2007) and that this effect decreases with antidepressant medication (Brody et al., 1999; Sheline et al., 2001; Fu et al., 2004). That we did not observe increased amygdala activation upon negative stimulation in patients may thus be a consequence of medication. To date, there is no study directly showing that patients with MDD are not able to downregulate amygdala activation (Ochsner et al., 2004; but see Johnstone et al., 2007). The downregulation effect shown in our patient sample could be either due to a preserved ability to actively regulate amygdala activation, to antidepressant medication, or to (medication-related) partial remission. As our patient sample still exhibited depressive symptomatology, we could test for the influence of symptom severity on the emotion regulation capacity. We found a significant negative correlation between HAMD scores and the amount of amygdala downregulation (Fig. 1). In a recent study, we found a similar influence of symptom severity on amygdala reactivity during expectation of negative scenes (Abler et al., 2007). We can thus conclude that patients under stable antidepressant medication were able to modulate amygdala activation, but less so if they were more severely depressed. It remains an open question whether this modulatory effect can be observed in unmedicated patients and patients with HAMD scores >20.
In healthy controls, the observed downregulation effect in the amygdala was accompanied by increased activation in a right frontoparietal network, showing a significant correlation between the amount of regulatory increase in DLPFC and IPL activation and the extent of amygdala decrease. As we told our subjects to take the position of a detached observer, IPL activation can be interpreted as reflecting the direction of spatial attention and the transformation of environmental coordinates (Colby and Goldberg, 1999). Actually, the inferior parietal/superior temporal cortex was specifically active during regulation of negative pictures in both groups (Table 2). This region has been implicated in switching from an egocentric to an allocentric perspective (Vogeley and Fink, 2003) in shifts of spatial attention and saliency attribution (Husain and Nachev, 2007) and self-monitoring (Schnell et al., 2007).
Depressive patients showed a similar pattern in right IPL, but no regulation-dependent increase in right DLPFC activation (Fig. 2). Interestingly, a recent study on emotion regulation in patients with borderline personality disorder that used a similar strategy reported no differences between groups in IPL activation during regulation but showed reduced activation of the ACC, a region of cognitive control, in the patient group (Koenigsberg et al., 2009).
Moreover, in a PPI analysis that allows inference as to whether region-to-region coactivation changes significantly as a function of task, healthy subjects showed increased coupling of the amygdala with VMPFC, posterior cingulate cortex, right DLPFC, and right IPL (Fig. 3), a pattern that has been observed in a recent study with healthy controls using a similar analysis method (Banks et al., 2007). Compared with controls, depressive patients show significantly reduced coupling of the amygdala with right DLPFC (Fig. 3), suggesting a reduction of DLPFC–amygdala coupling as a function of task.
Findings from both resting state PET and task-related fMRI studies have provided evidence for decreased prefrontal activation in depression (Mayberg et al., 1999; Siegle et al., 2007; Vasic et al., 2009). There is some evidence that, in contrast to resting state (Mayberg et al., 1999; Goldapple et al., 2004), task-related prefrontal activation is less susceptible to antidepressant medication, with studies showing, for example, reduced prefrontal connectivity during working memory in medicated, partially remitted patients (Vasic et al., 2009). A recent study reporting reduced activation of the DLPFC in response to maternal criticism in remitted MDD (Hooley et al., 2009) supports the assumption that diminished prefrontal activation in response to a challenge might be a sign of vulnerability to depression and does not necessarily disappear with symptom recovery or medication. Recently, it has been demonstrated that lesions of the lateral PFC, in contrast to medial PFC lesions, increase the vulnerability to MDD (Koenigs et al., 2008). Our results were further supported by a study investigating the effects of antidepressant treatment on corticolimbic connectivity (Anand et al., 2005). Antidepressant medication increased corticolimbic connectivity during rest and during exposure to positive and neutral pictures, but remained decreased during exposure to negative pictures (Anand et al., 2005). We conclude that diminished DLPFC activation during emotion regulation in MDD might be a trait effect (Beck et al., 1979; Ingram et al., 1983). The implication of this will be discussed further below.
In a recent study, unmedicated depressive patients exhibited increased activation in the right DLPFC during reappraisal of negative emotions (Johnstone et al., 2007). Moreover, compared with healthy controls showing a negative correlation between VMPFC and amygdala during reappraisal, patients showed an inverse pattern, i.e., a positive correlation (Johnstone et al., 2007). Although these diverging results might be due to medication, another reason for the lack of a direct main effect of strategy in the amygdala may relate to differences in the regulatory strategies used. Whereas Johnstone et al. (2007) have emphasized positive-generating and negative-blunting reappraisals, our reappraisal strategy used detachment, i.e., taking the position of a detached observer, and no enhancement of positive feelings (which also might involve amygdala activation). It has been proposed that the right DLPFC plays a specific role in reappraisal by detachment (Kalisch et al., 2006) and most studies investigating detachment found right lateralized DLPFC activation (Beauregard et al., 2001; Levesque et al., 2003; Kalisch et al., 2005, 2006; Eippert et al., 2007; Koenigsberg et al., 2009). Furthermore, it has been argued that the longer the duration of the reappraisal process, the more the cognitive processes underlying reappraisal involve monitoring processes that are subserved by right DLPFC activation (Kalisch, 2009). Our results were further supported by a recent study in patients with social anxiety disorder (SAD). Although able to behaviorally regulate emotions upon social threat stimuli using a cognitive-linguistic strategy, patients with SAD exhibited diminished DLPFC activation during active regulation compared with healthy controls (Goldin et al., 2009). The hypothesized trait effect might therefore be a sign of mood and anxiety disorders in general. If patients with mood and anxiety disorders are able to regulate their emotions for the respective moment, but show diminished activation in a region crucially involved in downregulation of the amygdala, the functional implications of this diminished DLPFC activation is an open question. A preliminary answer might be given by the results of our second task.
Sustained effects of emotion regulation during subsequent passive viewing
Fifteen minutes after active emotion regulation, subjects were presented with the same stimuli again, now with the instruction to passively watch the respective pictures. In healthy controls, we found a sustained downregulation effect in the amygdala bilaterally, i.e., although subjects did not voluntarily regulate their feelings, passive viewing of formerly regulated scenes was accompanied by diminished activation in the amygdala (Fig. 4) (Walter et al., 2009). Our results were supported by recent evidence showing that detaching from a depressive experience, in contrast to other strategies like using a self-immersed perspective or a distraction technique, leads to reduced depressive affect and fewer recurring depressive thoughts 7 d after the experimental manipulation in healthy subjects (Kross and Ayduk, 2008).
In contrast, patients exhibited no sustained regulation effect in the amygdala (Fig. 4). It might be that subjects, once having realized the beneficial effects of regulation in task 1, choose to use a detached perspective that might work better on pictures already regulated previously, and that patients were less able to do so (e.g., because of avolition or anhedonia). However, postexperimental interviews show that subjects report not having actively regulated during task 2. Moreover, we did not observe DLPFC or IPL activation during task 2, even when lowering the threshold to an uncorrected level of p < 0.05, which we would expect if subjects take a detached perspective. Therefore, we conclude that although patients were able to actively detach from negative feelings and associated physiological signs, this effect seemed to be short-lived. We hypothesize that this diminished sustained regulation effect is due to the observed reduced DLPFC activation during active regulation in task 1. This assumption is supported by our finding that in controls the extent of the sustained downregulation effect in the amygdala was positively correlated with the amount of DLPFC activation during regulation. The stronger the regulation effect in DLPFC during task 1, the stronger the downregulation effect during subsequent passive viewing (Fig. 4). We assume that sustained regulation success is mediated by the DLPFC and hypothesize that one mechanism might be through prefrontolimbic coupling. However, as we did not observe a correlation between the amount of prefrontolimbic coupling in task 1 and the amount of sustained amygdala downregulation during task 2, this suggestion is only tentative.
It has been assumed that one of the core symptoms of depression, rumination, and perseveration of negative affect results from dysfunctional corticolimbic coupling (Davidson et al., 2002). Here, diminished coupling between DLPFC, a region crucially involved in the implementation of associations between circumstances and new behavior (Miller and Cohen, 2001), and amygdala might hamper longer lasting learning effects, e.g., remodeling of stimulus-response associations or changing the meaning of the stimulus, that would make it no longer necessary to mobilize resources of effortful control, resulting in a sustained regulation effect (Walter et al., 2009; Schardt et al., 2010).
Recently, DeRubeis et al. (2008) proposed a theory of the effects of antidepressant treatment and cognitive behavioral therapy (CBT) on brain activation. Although CBT and antidepressant medication exhibit comparable short-term effects (DeRubeis et al., 2005), patients who had had former treatment of CBT were protected against depression relapse in a similar way as patients with continued antidepressant medication, but better than patients with discontinued medication (Hollon et al., 2005). The authors suggest that antidepressant medication might target limbic brain regions directly rather than relying on the inhibitory function of the PFC. As the goal of CBT in depression relies on replacing automatic emotional reactivity with more controlled processing by identifying negative thoughts and beliefs and increasing the ability to distance oneself from these negative beliefs, CBT might bolster prefrontal inhibitory control, helping to dampen automatic limbic reactions (DeRubeis et al., 2008). This might initiate learning mechanisms that help remodeling stimulus-response associations or stimulus meaning. It would therefore be of great interest whether patients successfully treated with CBT exhibit a sustained regulation effect in the amygdala.
In summary, we show that although emotion regulation capacity in medicated depressive patients appears to be preserved depending on symptom severity, this effect is only short-lived. We provide evidence that the lack of a sustained regulation effect is associated with reduced DLPFC activation during active regulation. Further research should evaluate the modulatory effects of different forms of psychotherapy on sustained regulation effects in patients with MDD.
Footnotes
This study was supported by the Volkswagen Foundation (Grants II/80777 and II/84051) and the German Research Foundation (Grant WA-1539/2-1).
References
- Abler B, Erk S, Herwig U, Walter H. Anticipation of aversive stimuli activates extended amygdala in unipolar depression. J Psychiatr Res. 2007;41:511–522. doi: 10.1016/j.jpsychires.2006.07.020. [DOI] [PubMed] [Google Scholar]
- Anand A, Li Y, Wang Y, Wu J, Gao S, Bukhari L, Mathews VP, Kalnin A, Lowe MJ. Antidepressant effect on connectivity of the mood-regulating circuit: an FMRI study. Neuropsychopharmacology. 2005;30:1334–1344. doi: 10.1038/sj.npp.1300725. [DOI] [PubMed] [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala-frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci. 2007;2:303–312. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauregard M, Lévesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. J Neurosci. 2001;21(RC165) doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauregard M, Paquette V, Lévesque J. Dysfunction in the neural circuitry of emotional self-regulation in major depressive disorder. Neuroreport. 2006;17:843–846. doi: 10.1097/01.wnr.0000220132.32091.9f. [DOI] [PubMed] [Google Scholar]
- Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive therapy of depression. New York: Guilford; 1979. [Google Scholar]
- Brody AL, Saxena S, Silverman DH, Alborzian S, Fairbanks LA, Phelps ME, Huang SC, Wu HM, Maidment K, Baxter LR., Jr Brain metabolic changes in major depressive disorder from pre- to post-treatment with paroxetine. Psychiatry Res. 1999;91:127–139. doi: 10.1016/s0925-4927(99)00034-7. [DOI] [PubMed] [Google Scholar]
- Campbell-Sills L, Barlow DH. Incorporating emotion regulation into conceptualizations and treatments of anxiety and mood disorders. In: Gross JJ, editor. Handbook of emotion regulation. New York: Guilford; 2007. pp. 542–559. [Google Scholar]
- Colby CL, Goldberg ME. Space and attention in parietal cortex. Annu Rev Neurosci. 1999;22:319–349. doi: 10.1146/annurev.neuro.22.1.319. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: perspectives from affective neuroscience. Annu Rev Psychol. 2002;53:545–574. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- DeRubeis RJ, Hollon SD, Amsterdam JD, Shelton RC, Young PR, Salomon RM, O'Reardon JP, Lovett ML, Gladis MM, Brown LL, Gallop R. Cognitive therapy vs medications in the treatment of moderate to severe depression. Arch Gen Psychiatry. 2005;62:409–416. doi: 10.1001/archpsyc.62.4.409. [DOI] [PubMed] [Google Scholar]
- DeRubeis RJ, Siegle GJ, Hollon SD. Cognitive therapy versus medication for depression: treatment outcomes and neural mechanisms. Nat Rev Neurosci. 2008;9:788–796. doi: 10.1038/nrn2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC. Prefrontal cortical-amygdalar metabolism in major depression. Ann N Y Acad Sci. 1999;877:614–637. doi: 10.1111/j.1749-6632.1999.tb09292.x. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging abnormalities in the amygdala in mood disorders. Ann N Y Acad Sci. 2003;985:420–444. doi: 10.1111/j.1749-6632.2003.tb07098.x. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Bardgett ME, Reich T, Todd RD, Raichle ME. Glucose metabolism in the amygdala in depression: relationship to diagnostic subtype and plasma cortisol levels. Pharmacol Biochem Behav. 2002;71:431–447. doi: 10.1016/s0091-3057(01)00687-6. [DOI] [PubMed] [Google Scholar]
- Eippert F, Veit R, Weiskopf N, Erb M, Birbaumer N, Anders S. Regulation of emotional responses elicited by threat-related stimuli. Hum Brain Mapp. 2007;28:409–423. doi: 10.1002/hbm.20291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Baker SC, Rogers RD, O'Leary DA, Paykel ES, Frith CD, Dolan RJ, Sahakian BJ. Prefrontal dysfunction in depressed patients performing a complex planning task: a study using positron emission tomography. Psychol Med. 1997;27:931–942. doi: 10.1017/s0033291797005187. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Fu CH, Williams SC, Cleare AJ, Brammer MJ, Walsh ND, Kim J, Andrew CM, Pich EM, Williams PM, Reed LJ, Mitterschiffthaler MT, Suckling J, Bullmore ET. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Arch Gen Psychiatry. 2004;61:877–889. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- Goldapple K, Segal Z, Garson C, Lau M, Bieling P, Kennedy S, Mayberg H. Modulation of cortical-limbic pathways in major depression: treatment-specific effects of cognitive behavior therapy. Arch Gen Psychiatry. 2004;61:34–41. doi: 10.1001/archpsyc.61.1.34. [DOI] [PubMed] [Google Scholar]
- Goldin PR, Manber T, Hakimi S, Canli T, Gross JJ. Neural bases of social anxiety disorder: emotional reactivity and cognitive regulation during social and physical threat. Arch Gen Psychiatry. 2009;66:170–180. doi: 10.1001/archgenpsychiatry.2008.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Gotlib IH. Neural substrates of increased memory sensitivity for negative stimuli in major depression. Biol Psychiatry. 2008;63:1155–1162. doi: 10.1016/j.biopsych.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR. Neocortical modulation of the amygdala response to fearful stimuli. Biol Psychiatry. 2003;53:494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- Hollon SD, DeRubeis RJ, Shelton RC, Amsterdam JD, Salomon RM, O'Reardon JP, Lovett ML, Young PR, Haman KL, Freeman BB, Gallop R. Prevention of relapse following cognitive therapy vs medications in moderate to severe depression. Arch Gen Psychiatry. 2005;62:417–422. doi: 10.1001/archpsyc.62.4.417. [DOI] [PubMed] [Google Scholar]
- Hooley JM, Gruber SA, Parker HA, Guillaumot J, Rogowska J, Yurgelun-Todd DA. Cortico-limbic response to personally challenging emotional stimuli after complete recovery from depression. Psychiatry Res. 2009;172:83–91. doi: 10.1016/j.pscychresns.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Husain M, Nachev P. Space and the parietal cortex. Trends Cogn Sci. 2007;11:30–36. doi: 10.1016/j.tics.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram RE, Miranda J, Segal ZV. Cognitive vulnerability to depression. New York: Guilford; 1983. [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch R. The functional neuroanatomy of reappraisal: time matters. Neurosci Biobehav Rev. 2009;33:1215–1226. doi: 10.1016/j.neubiorev.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Wiech K, Critchley HD, Seymour B, O'Doherty JP, Oakley DA, Allen P, Dolan RJ. Anxiety reduction through detachment: subjective, physiological, and neural effects. J Cogn Neurosci. 2005;17:874–883. doi: 10.1162/0898929054021184. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Wiech K, Herrmann K, Dolan RJ. Neural correlates of self-distraction from anxiety and a process model of cognitive emotion regulation. J Cogn Neurosci. 2006;18:1266–1276. doi: 10.1162/jocn.2006.18.8.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Huey ED, Calamia M, Raymont V, Tranel D, Grafman J. Distinct regions of prefrontal cortex mediate resistance and vulnerability to depression. J Neurosci. 2008;28:12341–12348. doi: 10.1523/JNEUROSCI.2324-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsberg HW, Fan J, Ochsner KN, Liu X, Guise KG, Pizzarello S, Dorantes C, Guerreri S, Tecuta L, Goodman M, New A, Siever LJ. Neural correlates of the use of psychological distancing to regulate responses to negative social cues: a study of patients with borderline personality disorder. Biol Psychiatry. 2009;66:854–863. doi: 10.1016/j.biopsych.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kross E, Ayduk O. Facilitating adaptive emotional analysis: distinguishing distanced-analysis of depressive experiences from immersed-analysis and distraction. Pers Soc Psychol Bull. 2008;34:924–938. doi: 10.1177/0146167208315938. [DOI] [PubMed] [Google Scholar]
- Lévesque J, Eugène F, Joanette Y, Paquette V, Mensour B, Beaudoin G, Leroux JM, Bourgouin P, Beauregard M. Neural circuitry underlying voluntary suppression of sadness. Biol Psychiatry. 2003;53:502–510. doi: 10.1016/s0006-3223(02)01817-6. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Robertson ER, Ray RD, Cooper JC, Gabrieli JD, Gross JJ, Gotlib IH. An fMRI investigation of the cognitive regulation of emotion in depression. Soc Neurosci Abstr. 2004;30 669.8. [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13:833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schardt DM, Erk S, Nüsser C, Nöthen MM, Cichon S, Rietschel M, Treutlein J, Goschke T, Walter H. Volition diminishes genetically mediated amygdala hyperreactivity. Neuroimage. 2010;53:943–951. doi: 10.1016/j.neuroimage.2009.11.078. [DOI] [PubMed] [Google Scholar]
- Schnell K, Heekeren K, Schnitker R, Daumann J, Weber J, Hesselmann V, Möller-Hartmann W, Thron A, Gouzoulis-Mayfrank E. An fMRI approach to particularize the frontoparietal network for visuomotor action monitoring: detection of incongruence between test subjects' actions and resulting perceptions. Neuroimage. 2007;34:332–341. doi: 10.1016/j.neuroimage.2006.08.027. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can't shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol Psychiatry. 2002;51:693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biol Psychiatry. 2007;61:198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Vasic N, Wolf RC, Walter H. [Executive functions in patients with depression: the role of prefrontal activation] Nervenarzt. 2007;78:628–636. doi: 10.1007/s00115-006-2240-6. [DOI] [PubMed] [Google Scholar]
- Vasic N, Walter H, Sambataro F, Wolf RC. Aberrant functional connectivity of dorsolateral prefrontal and cingulate networks in patients with major depression during working memory processing. Psychol Med. 2009;39:977–987. doi: 10.1017/S0033291708004443. [DOI] [PubMed] [Google Scholar]
- Vogeley K, Fink GR. Neural correlates of the first-person-perspective. Trends Cogn Sci. 2003;7:38–42. doi: 10.1016/s1364-6613(02)00003-7. [DOI] [PubMed] [Google Scholar]
- Walter H, Wolf RC, Spitzer M, Vasic N. Increased left prefrontal activation in patients with unipolar depression: an event-related, parametric, performance-controlled fMRI study. J Affect Disord. 2007;101:175–185. doi: 10.1016/j.jad.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Walter H, von Kalckreuth A, Schardt D, Stephan A, Goschke T, Erk S. The temporal dynamics of voluntary emotion regulation. PLoS One. 2009;4:e6727. doi: 10.1371/journal.pone.0006726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. WHO report 2001: mental health: new understanding, new hope. Geneva: World Health Organization; 2001. Chapter 2: burden of mental and behavioral disorders. [Google Scholar]