Abstract
Long-term memory formation is believed to involve alterations of synaptic efficacy. It has been shown that GluR1-containing AMPA receptors are inserted into synapses following stimuli leading to plasticity and that GluR2/GluR3-containing receptors replace existing synaptic AMPA receptors continuously and may act to maintain synaptic efficacy. Maintaining GluR2/GluR3 receptors level in synapse requires interactions of N-ethylmaleimide-sensitive factor (NSF) with GluR2. To assess possible roles of NSF-GluR2 interaction in rat lateral amygdala (LA) in fear memory formation we used a specific GluR2-NSF interaction inhibitory peptide (pep-R845A). This inhibitory peptide, composed of a modified NSF binding site of GluR2, was previously shown to interact specifically with NSF and to affect AMPA-mediated synaptic efficacy. The inhibitory peptide was linked to a TAT peptide (TAT-pep-R845A) to facilitate internalization into LA cells. Infusion of the TAT-pep-R845A inhibitory peptide into LA 30 min before fear conditioning led to a significant impairment of long-term fear memory formation. In contrast, the control TAT peptide alone had no effect on fear memory. Injection of TAT-pep-R845A peptide into LA had no effect on short-term fear memory. In addition, the inhibitory peptide had no effect on memory retrieval when injected into LA 30 min before fear memory test. Furthermore, maintenance of memory was not impaired when the peptide was injected 24 h after fear conditioning and fear memory was tested 48 h afterward. These results show that GluR2-NSF interaction in LA is necessary for fear memory consolidation but not retrieval or persistence.
Introduction
Changes in synaptic efficacy are believed to underlie the formation of long-term memory (Hebb, 1949; Bliss and Collingridge, 1993; Martin et al., 2000; Kandel, 2001). Studies have shown that trafficking into or removal from the synapse of AMPA-type glutamate receptors mediate changes in synaptic efficacy [e.g., long-term potentiation (LTP) and long-term depression] (Barry and Ziff, 2002; Song and Huganir, 2002; Kessels and Malinow, 2009) and memory formation (Rumpel et al., 2005; Yeh et al., 2006). Synaptic activity leading to increase in synaptic strength (e.g., LTP) drives GluR1-containing AMPA receptors into the synapse (Hayashi et al., 2000). GluR2/GluR3-containing AMPA receptors constitutively replace synaptic AMPA receptors keeping the synaptic strength constant (Shi et al., 2001). The replacement of synaptic AMPA receptors by GluR2/GluR3 subunits requires the interaction of GluR2 with NSF (Shi et al., 2001). NSF-GluR2 interaction was also shown to be important to maintain AMPA-mediated transmission at the synapse (Nishimune et al., 1998; Song et al., 1998; Lüscher et al., 1999; Lüthi et al., 1999; Lee et al., 2002). Stabilizing synaptic strength by GluR2/GluR3 in an NSF-mediated manner could serve as a molecular mechanism for memory consolidation and retention.
In the present study we aimed to further explore the roles of NSF-GluR2 interaction in memory formation. Toward that end, we have used the fear conditioning paradigm where an association is formed between a neutral conditioned stimulus (CS), such as a tone, and an aversive unconditioned stimulus (US), typically a mild footshock (Fanselow and LeDoux, 1999; LeDoux, 2000; Davis and Whalen, 2001; Sah et al., 2003; Maren, 2005). The putative site of fear conditioning memory, the lateral nucleus of the amygdala (LA), has been identified (Fanselow and LeDoux, 1999; Schafe et al., 2001; Rodrigues et al., 2004; Maren, 2005). It has been shown that fear conditioning drives GluR1-containing AMPA receptors into synapses in LA neurons (Rumpel et al., 2005; Yeh et al., 2006). Moreover, fear conditioning is impaired if AMPA insertion is blocked (Rumpel et al., 2005). These results and the observation that GluR1 could be replaced by GluR2/GluR3-containing receptors in an NSF-interaction-dependent manner (Shi et al., 2001) led us to hypothesize that NSF-GluR2 interaction is important for the consolidation and retention of long-term fear memory in LA.
With the aim of studying the roles of NSF-GluR2 interactions in LA in fear memory formation, we used a well established NSF-GluR2 interaction disrupting peptide (pep-R845A). This blocking peptide, which contains part of the C-terminal of GluR2 (with one amino acid replacement for effective binding), binds NSF specifically, but not AP2 that has an overlapping binding site on GluR2 (Lee et al., 2002). By using the peptide it was shown that blocking NSF-GluR2 interaction affects synaptic, but not extrasynaptic, AMPA receptor responses. Microinfusion of the peptide into hippocampal neurons led to gradual rundown of EPSC amplitude in CA1 pyramidal cells over a time scale of minutes (Lee et al., 2002). This peptide was further used to block specifically the interaction of GluR2 and NSF in cerebellum-Purkinje neurons (Kakegawa and Yuzaki, 2005), granule-Pukinje cells synapses (Steinberg et al., 2004) and Stellate cells (Gardner et al., 2005). In these studies the peptide caused gradual rundown in EPSCs over minutes and interfered with induction of synaptic plasticity. Cumulatively, these results suggest that NSF-GluR2 interaction is essential for GluR2 maintenance in the synapse either by its stabilization or incorporation from extrasynaptic membrane sites.
In our study we further used the specific NSF-GluR2 disrupting peptide to elucidate the roles of NSF-GluR2 interaction in fear memory formation in LA.
Materials and Methods
Animals.
Male Sprague Dawley rats (250–300 g), were used in the study (Harlan Laboratories). Rats were housed separately at 22 ± 2°C in a 12 h light/dark cycle, with free access to food and water. Behavioral experiments were approved by the University of Haifa Institutional Committee for animal experiments in accordance with National Institutes of Health guidelines.
Fear conditioning.
Fear conditioning took place in a Plexiglas rodent conditioning chamber with a metal grid floor. Rats were habituated to the training chamber for 1 d. Animals were presented with five pairings of a tone for 40 s as the CS (5 kHz, 80 dB) that was coterminate with a foot shocks as the US (0.5 s, 1.5 mA). The intertrial interval (ITI) was random with average of 180 s. Rat groups were tested 1 h after training for short-term memory or 24 h after training for long-term memory in a different chamber with different context and Formica floor, to diminish the effect of context. Animals were presented with 5 tones (40 s, 5 kHz, 80 dB) with average ITI of 180 s. Behavior was recorded and the video images were transferred to a computer equipped with an analysis program. The percentage of changed pixels between two adjacent 1 s images was used as a measure of activity.
Surgical procedures.
Rats were anesthetized with equithesin (0.45 ml/100 g) (2.12% w/v MgSO4, 10% v/v ethanol, 39.1% v/v propylene glycol, 0.98% w/v sodium pentobarbital, and 4.2% w/v chloral hydrate) and restrained in a stereotaxic apparatus (Stoelting). Guide stainless-steel cannulas (23 gauge) were implanted bilaterally 1.5 mm above the LA [LA coordinates are in reference to bregma: anteroposterior (AP), −3.0; lateral (L) ±5.3; and dorsoventral (DV), −8.0) or the central nucleus of the amygdala (CE) (coordinates are in reference to bregma: AP, −2.3; L, ±4.0–4.4; DV −8.2). Rats were given antibiotics (Pen and Strep, Norbrook) and Calmagine (Vetoquinol) for analgesia on surgery day and on the following day. Rats were given 7 d for recovery before behavioral training.
Microinjection.
The stylus was removed from the guide cannula and a 28 gauge injection cannula, extending 1.5 mm from the tip of the guide cannula aimed to the LA or CE, was carefully placed. The injection cannula was connected via PE20 tubing, back filled with saline with a small air bubble separating the saline from the peptide solution, to a 10 μl Hamilton micro-syringe, driven by a microinjection pump (CMA/100, Carnegie Medicin; or PHD 2000, Harvard Apparatus). Solution was injected at a rate of 0.5 μl/min. Total volume injected per amygdala was 0.5 μl. TAT-pep-R845A peptide (Biotin-YGRKKRRQRRRKAMKVAKNPQ, Anaspec) and TAT control peptide (Biotin-YGRKKRRQRRR; Anaspec) were dissolved in saline at concentration of 50 μg/μl. We conjugated the pep-R845A to TAT to facilitate its entrance into cells (Schwarze et al., 1999) and to biotin for detection. Following injection, the injection cannula was left for an additional 1 min before withdrawal to minimize dragging of injected liquid along the injection track.
Histology.
After behavior was completed rats were decapitated and the brains were quickly removed, placed on dry ice and stored at −80°C until use. Brains were sliced (60 μm) and stained with methylene blue. Cannula placements were verified. Only rats with cannula tips at or within the boundaries of the LA were included in the data analysis.
Immunohistochemistry.
Rats were anesthetized using 1 ml/100 g sodium pentobarbital and perfused intracardially with PBS followed by 4% paraformaldehyde in PBS using a peristaltic pump (Ismatec REGLO Digital, IDEX). After perfusion, rats were decapitated and the brains removed and placed for postfixation in 30% sucrose in PBS for 48 h at 4°C. Brains were frozen and sliced at thickness of 40 μm. Slices were blocked with 20% normal goat serum (NGS) and 0.5% Triton X-100 in PBS for 1 h. Blocking was removed and slices were subjected to anti-GluR2 antibody (1:500; #AB1768, Millipore Bioscience Research Reagents) and anti-NSF antibody (1:500; #612272, BD Biosciences) in 2% NGS in PBS and incubated overnight at room temperature. Slices were washed thrice 5 min each with PBS and incubated with secondary antibodies (anti-mouse Alexa Fluor 488 1:200 for NSF and anti-rabbit rhodamine 1:200 for GluR2, Invitrogen) for 1 h at room temperature. Afterward slices were washed thrice with PBS and mounted on slides. Photographs were taken using a Bio-Rad Radiance 2100 laser-scanning confocal microscope equipped with Nikon microscope (Eclipse E600). Images were analyzed using LaserSharp 2000 software (Zeiss).
Peptides localization in brain.
Rats were anesthetized 30 min following microinjection with TAT-pep-R845A or TAT peptides and perfused as described above. After postfixation (see above) brains were frozen and sliced (40 μm). Slices were incubated with PBS for 1 h, followed by 1 h incubation with streptavidin-Alexa Fluor 568 (1:2000; S-11226, Invitrogen) in PBS at room temperature to detect the biotin-labeled peptide. Slices were washed thrice with PBS. The third wash contained Hoechst dye (1:5000, #33258, Invitrogen) in PBS for nucleus staining. Slices were loaded on slides and examined using a Bio-Rad confocal microscope.
Statistics.
Repeated-measures analysis using the generalized estimating equations (GEE) approach was performed (Zeger and Liang, 1986). GEE was used instead of ANOVA since, in some cases, the assumptions of equality of covariance matrix and of multivariate normality of residuals of our data were not met. The GEE approach is especially robust to misspecification of variance/covariance structure. Furthermore, GEE assumes that the correlations among measures across time are not of direct interest, and focuses on the comparison of groups across time. Statistical analysis was done using the SPSS 15.0 software.
Results
GluR2 and NSF proteins colocalize in lateral amygdala neurons
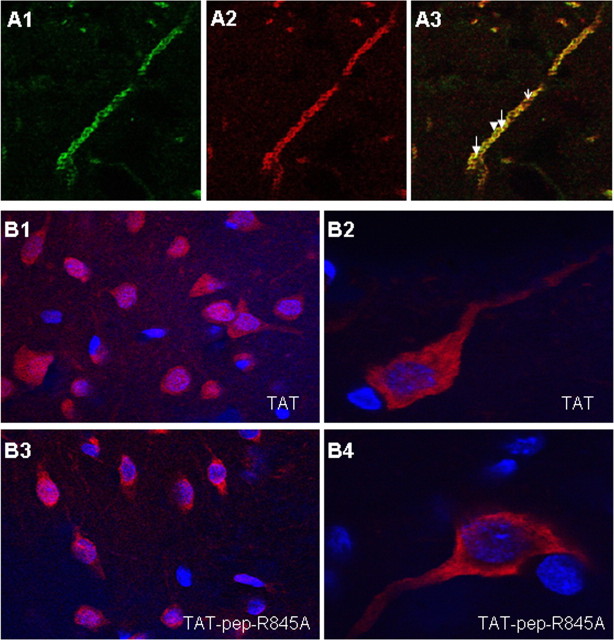
We hypothesize that the interaction of GluR2 and NSF is essential for fear conditioning memory formation in LA. A prerequisite for the involvement of GluR2-NSF interaction in LA in fear memory formation is the colocalization of these proteins in LA cells. We therefore performed double-labeling immunohistochemistry to detect possible colocalization of NSF and GluR2 in LA. The immunohistochemical experiments revealed that NSF and GluR2 colocalized in neurons in LA. Figure 1A1–A3 shows a representative example of NSF and GluR2 colocalization in a neuronal dendrite in LA (n = 3). There was no complete overlapping of the proteins in these neurons. The labeling was specific as omitting the primary antibodies abolished the signal.
Figure 1.
A1–A3, Double immunohistochemistry experiment for NSF (green) and GluR2 (red) in lateral amygdala dendrite (n = 3). NSF labeling (A1) and GluR2 labeling (A2) colocalize in LA dendrite (yellow-arrows, A3). Not all NSF and GluR2 proteins colocalize (A3, NSF alone, arrowhead; GluR2, open arrow). B1–B4, Microinjection of TAT or TAT-pep-R845A peptides leads to internalization of peptides into LA cells. Biotin-labeled TAT (B1, B2; n = 4) or TAT-pep-R845A (B3, B4; n = 5) were microinjected into the LA (50 μg/μl, 0.5 μl per LA). Thirty minutes after injection the animals were perfused, the brains were removed sliced and subjected to streptavidin conjugated to Alexa Fluor 568 (red) and to nuclear Hoechst (blue) staining. Cells were labeled with the streptavidin conjugated to Alexa Fluor 568 indicating that both peptides were inserted into neurons. Higher magnification of TAT (B2)- or TAT-pep-R845A (B4)-labeled cell shows equal distribution of peptides in soma and dendrites.
NSF-GluR2 interaction disrupting peptide impairs long-term fear conditioning memory formation
To study the roles of NSF-GluR2 interaction in fear conditioning memory formation in LA we used the specific NSF-GluR2 interaction blocking peptide (pep-R845A conjugated to TAT). Microinjection of TAT (n = 4) or TAT-pep-R845A (n = 5) peptides into LA led to their internalization into cells 30 min later (Fig. 1B1,B3). Higher-magnification observation of neurons showed equal distribution of the peptides in neuronal soma and dendrites (Fig. 1B2,B4).
The effect of TAT-pep-R845A on long-term fear conditioning memory formation was tested. Microinjection of TAT-pep-R845A (n = 13) into LA significantly impaired fear conditioning LTM when compared with TAT peptide (n = 5) or saline-microinjected animals (n = 12) (χ2(1) = 9.078, p < 0.004; χ2(1) = 6.846, p < 0.01, respectively) (Fig. 2A) (see Fig. 4 for cannula placements). The TAT and saline rat groups were not significantly different (χ2(1) = 0.396, p > 0.5). The treatment × tone trial interaction was not significant (χ2(6) = 7.679, p > 0.2) indicating that the rate of changes in fear responses along the trials was similar in all groups. The saline and TAT-pep-R845A-microinjected animals were reconditioned drug-free a month later and were tested 24 h afterward. There was no significant difference between the groups (χ2(1) = 1.419, p > 0.2) indicating that the peptide did not cause any permanent damage to the LA.
Figure 2.
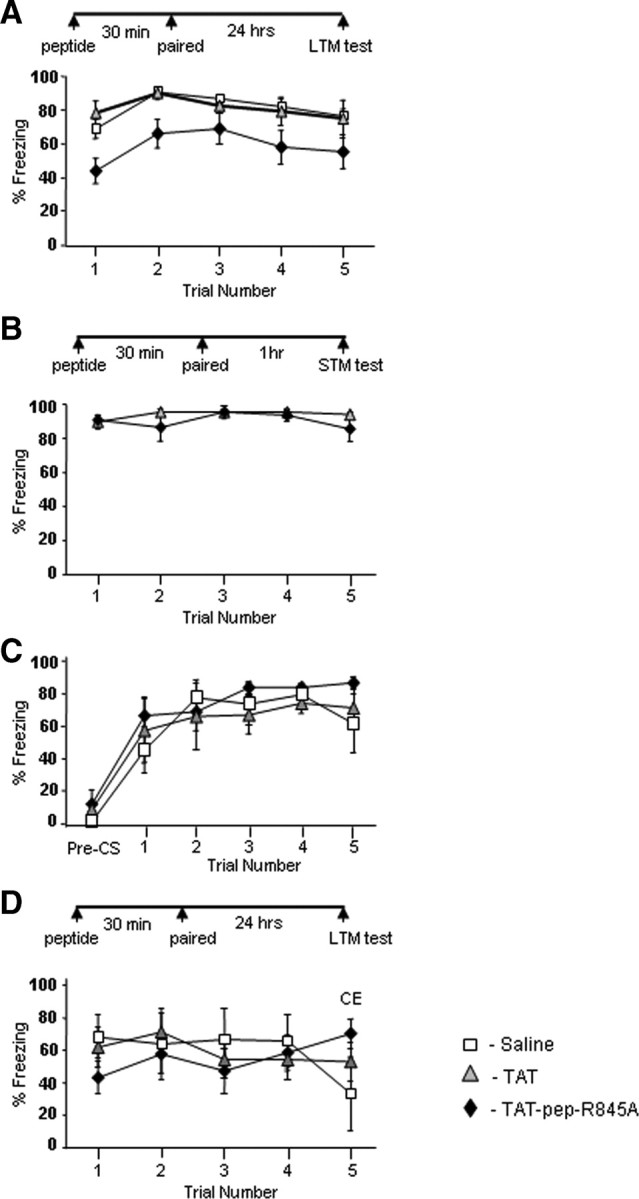
Microinjection of TAT-pep-R845A into LA before training impairs long-term, but not short-term, fear conditioning memory formation. A, TAT-pep-R845A peptide (50 μg/μl, 0.5 μl per LA n = 13), TAT peptide (50 μg/μl, 0.5 μl per LA n = 5), or 0.5 μl saline per LA (n = 12) were injected 30 min before fear conditioning. Microinjection of the TAT-pep-R845A peptide significantly impaired fear memory formation tested 24 h after training when compared with saline (p < 0.01) or TAT (p < 0.004). B, TAT-pep-R845A peptide (n = 4) or TAT (n = 4) (0.5 μl per LA at 50 μg/μl) were injected into LA 30 min before fear conditioning. Microinjection of TAT-pep-R845A had no effect on fear memory formation tested 1 h after training when compared with TAT (p > 0.4). C, Pretraining or postshock freezing in rats microinjected with TAT-pep-R845A (n = 6); TAT (n = 4); or saline (n = 5). Freezing before the training (pre-CS) or postshock during training was not affected by the treatment (Pre-CS, p > 0.1; postshock, p > 0.9). D, TAT-pep-R845A peptide (50 μg/μl, 0.5 μl per CE n = 8), TAT peptide (50 μg/μl, 0.5 μl per CE n = 7) or saline (n = 4) were injected into CE 30 min before fear conditioning. Microinjection of the TAT-pep-R845A 30 min before fear conditioning training had no effect on long term fear memory formation tested 24 h later when compared with the TAT-microinjected group (χ2(1) = 2.233, p > 0.1) or saline (χ2(1) = 2.938, p > 0.08).
Figure 4.
Cannula placements. A, B, Cannula tip placements from rats injected with saline, TAT peptide or TAT-pep-R845A peptide before training tested for LTM (A) or STM (B). C–E, Cannula tip placements from rats injected with TAT peptide or TAT-pep-R845A peptide immediately after training (C), before LTM test (D) or 24 h after training tested 48 h later (E). F, Cannula tip placements from rats injected with TAT peptide, TAT-pep-R845A peptide or saline into CE before training and tested for LTM. G, H, Photomicrograph of injecting cannula trace LA (G) or CE (H). I, Schematic drawing of amygdala nuclei.
Freezing before the training (pre-CS) or postshock during training was not affected by the treatment (Pre-CS, χ2(2) = 4.3, p > 0.1; postshock, χ2(2) = 0.189, p > 0.9; Figure 2C) [TAT-pep-R845A (n = 6); TAT (n = 4); or saline (n = 5)]. The treatment × tone trial interaction for postshock freezing was not significant (χ2(2) = 1.013, p > 0.6). These results indicate that the peptides do not affect freezing per se, foot shock sensitivity and US processing in the LA.
Together, the results above show that microinjection of TAT-pep-R845A peptide into LA impairs the ability to form long-term fear conditioning memory.
To evaluate the anatomical specificity of the peptides we aimed the microinjection of the TAT-pep-R845A (n = 8), TAT (n = 9) or saline (n = 4) into the central nucleus of the amygdala. Infusion of the TAT-pep-R845A 30 min before fear conditioning training had no effect on long term fear memory formation tested 24 h later when compared with the TAT-microinjected group (χ2(1) = 2.233, p > 0.1) or saline (χ2(1) = 2.938, p > 0.08) (Fig. 2D).
NSF-GluR2 interaction interfering peptide has no effect on short-term fear conditioning memory formation
To study whether the GluR2 and NSF interaction is needed for short-term memory (STM) formation, we microinjected TAT-pep-R845A inhibitory peptide into LA 30 min before fear conditioning and tested 1 h after training. Figure 2B shows that fear STM was not affected by TAT-pep-R845A peptide (n = 4) when compared with animals microinjected with TAT peptide (n = 4) (χ2(1) = 0.469, p > 0.4). The treatment × tone trial interaction was not significant (χ2(4) = 4.592, p > 0.3) suggesting that the rate of changes in fear responses along the trials was similar in all rat groups. The aforementioned results show that NSF binding to GluR2 is essential for consolidation of fear conditioning STM into LTM and not for fear conditioning acquisition.
Post-training injection of TAT-pep-R845A peptide has no effect on fear memory formation
To investigate the time window when NSF-GluR2 interaction is important for fear conditioning memory consolidation we injected TAT-pep-R845A immediately post-training and studied the effects on LTM. Figure 3A shows that post-training injection of TAT-pep-R845A (n = 6) into LA had no significant effect on fear LTM formation when compared with TAT-injected control animals (n = 5) (χ2(1) = 2.065, p > 0.1). The treatment × tone trial interaction was not significant (χ2(4) = 8.529, p > 0.07) (cannula placements are shown in Fig. 4). These results indicate that NSF-GluR2 interaction is essential during learning or immediately afterward, but not minutes later, for fear memory consolidation.
Figure 3.
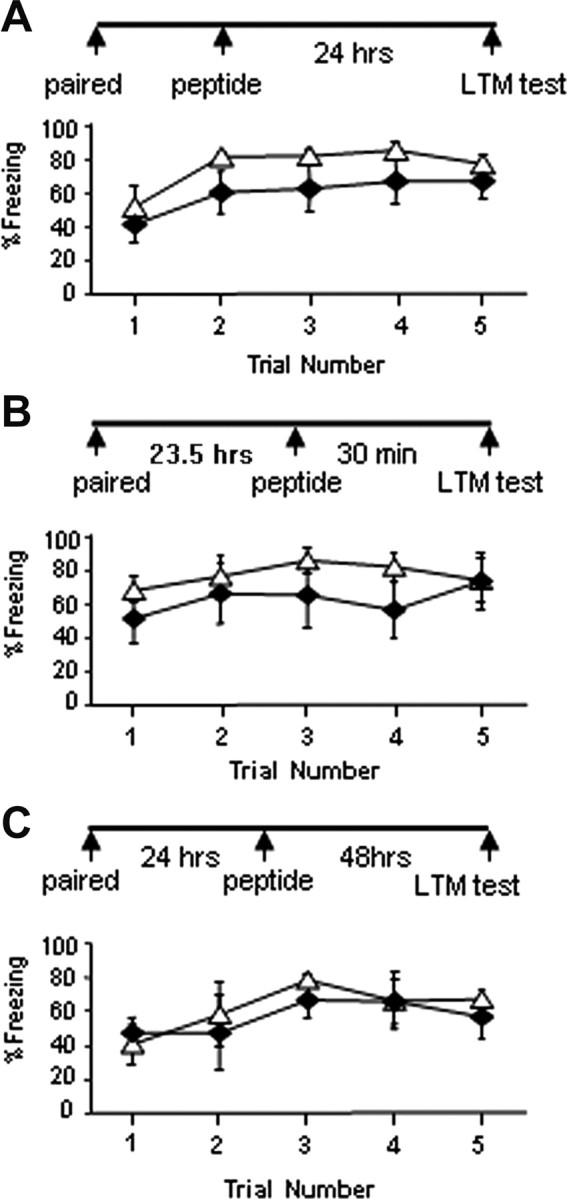
Injection of TAT-pep-R845A peptide into LA immediately after training, 30 min before test, or 24 h post-training has no effect on long-term fear conditioning memory formation, retrieval, or maintenance. A, Rats were injected into LA with TAT-pep-R845A (n = 6) or TAT (n = 5) peptides (50 μg/μl), 0.5 μl per LA) immediately after fear conditioning training and tested for fear memory formation 24 h later. Rats microinjected with TAT-pep-R845A were not significantly different in long-term fear memory from TAT-injected rats (p > 0.1). B, TAT-pep-R845A (n = 5) or TAT (n = 5) peptides (50 μg/μl, 0.5 μl per LA) were injected into LA 30 min before fear conditioning memory test. There was no significant effect of the TAT-pep-R845A peptide when compared with the TAT peptide (p > 0.3). C, TAT-pep-R845A (n = 3) or TAT (n = 4) peptides (50 μg/μl, 0.5 μl per LA) were injected into LA 24 h after fear conditioning training. The animals were tested 48 h afterward. There was no significant effect of the TAT-pep-R845A peptide when compared with the TAT peptide (p > 0.4).
Fear conditioning memory retrieval is not affected by NSF-GluR2 inhibitory peptide
We microinjected the TAT-pep-R845A peptide 30 min before LTM test and studied its effects on retrieval of memory. Figure 3B shows that the TAT-pep-R845A peptide (n = 5) had no significant effect on fear memory compared with TAT peptide (n = 5) alone when injected before memory test (χ2(1) = 0.79, p > 0.3). The treatment × tone trial interaction was not significant (χ2(2) = 4.102, p > 0.1). These results indicate that TAT-pep-R845A inhibitory peptide in LA has no effect on fear memory retrieval.
NSF-GluR2 inhibitory peptide has no effect on fear conditioning memory maintenance
We studied the possible effects of NSF-GluR2 interaction on maintenance of fear conditioning memory. We microinjected TAT-pep-R8445A 24 h after fear conditioning and tested 48 h later for fear memory. The TAT-pep-R845A peptide (n = 3) had no effect on fear memory formation when compared with TAT peptide (n = 4) (χ2(1) = 0.582, p > 0.4; Fig. 3C). The treatment × tone trial interaction was not significant (χ2(3) = 5.138, p > 0.1). These results show that NSF-GuR2 interactions are not necessary for fear memory maintenance in LA.
Discussion
Previous studies have shown that GluR2 interacts with NSF in neurons and that this interaction is needed to stabilize GluR2-containing AMPA receptors level in synapse (Nishimune et al., 1998; Osten et al., 1998; Song et al., 1998; Lüscher et al., 1999; Lüthi et al., 1999; Lee et al., 2002). In this study we show that this interaction is essential for fear conditioning memory consolidation, but not acquisition, retrieval or maintenance in LA.
Microinjection of TAT-conjugated NSF-GluR2 interaction disrupting peptide (pep-R845A) into LA before fear conditioning impaired long-term fear memory (LTM) but not short-term memory (STM) formation. These results suggest that NSF-GluR2 interaction is needed for the consolidation of short-term memory into long-term fear memory. These observations also show that NSF-GluR2 interaction is not needed for fear memory acquisition. Furthermore, interfering with NSF-GluR2 binding had no effect on synaptic transmission during learning which is essential for fear memory acquisition (Muller et al., 1997; Wilensky et al., 1999). It is noteworthy that infusion of the NSF-GluR2 interaction blocking peptide into hippocampal or cerebellar neurons-induced rundown in EPSC amplitude over minutes (Lee et al., 2002; Steinberg et al., 2004; Gardner et al., 2005; Kakegawa and Yuzaki, 2005). These results suggest that LTM requires proper expression of GluR2-containing AMPA receptor in synapses, mediated and stabilized by NSF-GluR2 interaction whereas STM formation might be mediated by other molecular mechanisms. One possibility is that acquisition and STM are mediated by GluR1 insertion into synapses (Rumpel et al., 2005) whereas memory consolidation is mediated by incorporation of GluR2-containing AMPA receptors into these synapses for the replacement of synaptic AMPA receptors, an event mediated by NSF-GluR2 interaction (Shi et al., 2001).
TAT-pep-R845A peptide affected long-term fear memory formation when microinjected into LA but not into the adjacent nucleus the CE. Although the CE is required for fear conditioning memory formation (Wilensky et al., 2006) the interruption of NSF-GluR2 interaction affected fear memory only when the blocking peptide was injected into the LA. Other areas adjacent to LA may also be affected by the peptide as its diffusion is not controlled. It would be of interest to further study the role of this interaction in other brain regions located within the auditory pathway and involved in fear memory formation.
The role of NSF-GluR2 interaction in memory consolidation is consistent with the observation that NSF is important for GluR2/GluR3 incorporation and stabilization in the synapse, a process that can stabilize long-term changes in synaptic efficacy (Shi et al., 2001). We show in the present study that GluR2-NSF interaction is important during or immediately after learning only because microinjection of the NSF-GluR2 interaction blocking peptide into LA after fear conditioning had no effect on fear LTM. Although there is a nonsignificant trend for reduced freezing in the TAT-pep-R845A peptide during trials 2–4, the first trial and the last trials are similar in both rat groups indicating that long-term memory retrieval and its extinction are also not different. This finding implies that NSF-GluR2 interaction is important for early stages of memory consolidation whereas other interacting proteins may become involved in stabilizing AMPA receptors in the synapse at later stages of memory consolidation.
Consistent with the aforementioned observation is the result showing that TAT-pep-R845A peptide had no effect on memory maintenance as injection of the peptide 24 h after training did not impair fear memory tested 48 h after the injection. It was shown that GluR2 is involved in fear memory maintenance and that this process is regulated by PKMζ that maintains long-term memories by blocking a GluR2-dependent pathway involved in the receptor removal (Migues et al., 2010). In hippocampal slices NSF is involved in mediating the persistence of LTP (Yao et al., 2008). Injection of the NSF-GluR2 disrupting peptide (pep2m, intracerebroventricularly) was also shown to impair stress-induced facilitation of spatial LTM (Conboy and Sandi, 2010).
By using a well established and specific NSF-GluR2 interaction disrupting peptide (Lee et al., 2002; Steinberg et al., 2004; Gardner et al., 2005; Kakegawa and Yuzaki, 2005) we show that NSF-GluR2 interaction has a key role in fear memory consolidation but not acquisition, maintenance and retrieval. These results are consistent with the observation that NSF is needed for incorporation of GluR2-containing AMPA receptors into the synapse to stabilize synaptic currents. Other interacting proteins may be involved in later events leading to maintenance of GluR2-containing AMPA receptors in the synapse for fear memory persistence.
Footnotes
This research was supported in part by the Israel Science Foundation.
References
- Barry MF, Ziff EB. Receptor trafficking and the plasticity of excitatory synapses. Curr Opin Neurobiol. 2002;12:279–286. doi: 10.1016/s0959-4388(02)00329-x. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Conboy L, Sandi C. Stress at learning facilitates memory formation by regulating AMPA receptor trafficking through a glucocorticoid action. Neuropsychopharmacology. 2010;35:674–685. doi: 10.1038/npp.2009.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, LeDoux JE. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron. 1999;23:229–232. doi: 10.1016/s0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- Gardner SM, Takamiya K, Xia J, Suh JG, Johnson R, Yu S, Huganir RL. Calcium-permeable AMPA receptor plasticity is mediated by subunit-specific interactions with PICK1 and NSF. Neuron. 2005;45:903–915. doi: 10.1016/j.neuron.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- Hebb DO. New York: Wiley; 1949. The organization of behavior: a neuropsychological theory. [Google Scholar]
- Kakegawa W, Yuzaki M. A mechanism underlying AMPA receptor trafficking during cerebellar long-term potentiation. Proc Natl Acad Sci U S A. 2005;102:17846–17851. doi: 10.1073/pnas.0508910102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61:340–350. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lee SH, Liu L, Wang YT, Sheng M. Clathrin adaptor AP2 and NSF interact with overlapping sites of GluR2 and play distinct roles in AMPA receptor trafficking and hippocampal LTD. Neuron. 2002;36:661–674. doi: 10.1016/s0896-6273(02)01024-3. [DOI] [PubMed] [Google Scholar]
- Lüscher C, Xia H, Beattie EC, Carroll RC, von Zastrow M, Malenka RC, Nicoll RA. Role of AMPA receptor cycling in synaptic transmission and plasticity. Neuron. 1999;24:649–658. doi: 10.1016/s0896-6273(00)81119-8. [DOI] [PubMed] [Google Scholar]
- Lüthi A, Chittajallu R, Duprat F, Palmer MJ, Benke TA, Kidd FL, Henley JM, Isaac JT, Collingridge GL. Hippocampal LTD expression involves a pool of AMPARs regulated by the NSF-GluR2 interaction. Neuron. 1999;24:389–399. doi: 10.1016/s0896-6273(00)80852-1. [DOI] [PubMed] [Google Scholar]
- Maren S. Synaptic mechanisms of associative memory in the amygdala. Neuron. 2005;47:783–786. doi: 10.1016/j.neuron.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- Migues PV, Hardt O, Wu DC, Gamache K, Sacktor TC, Wang YT, Nader K. PKMzeta maintains memories by regulating GluR2-dependent AMPA receptor trafficking. Nat Neurosci. 2010;13:630–634. doi: 10.1038/nn.2531. [DOI] [PubMed] [Google Scholar]
- Muller J, Corodimas KP, Fridel Z, LeDoux JE. Functional inactivation of the lateral and basal nuclei of the amygdala by muscimol infusion prevents fear conditioning to an explicit conditioned stimulus and to contextual stimuli. Behav Neurosci. 1997;111:683–691. doi: 10.1037//0735-7044.111.4.683. [DOI] [PubMed] [Google Scholar]
- Nishimune A, Isaac JT, Molnar E, Noel J, Nash SR, Tagaya M, Collingridge GL, Nakanishi S, Henley JM. NSF binding to GluR2 regulates synaptic transmission. Neuron. 1998;21:87–97. doi: 10.1016/s0896-6273(00)80517-6. [DOI] [PubMed] [Google Scholar]
- Osten P, Srivastava S, Inman GJ, Vilim FS, Khatri L, Lee LM, States BA, Einheber S, Milner TA, Hanson PI, Ziff EB. The AMPA receptor GluR2 C terminus can mediate a reversible, ATP-dependent interaction with NSF and alpha- and beta-SNAPs. Neuron. 1998;21:99–110. doi: 10.1016/s0896-6273(00)80518-8. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, Schafe GE, LeDoux JE. Molecular mechanisms underlying emotional learning and memory in the lateral amygdala. Neuron. 2004;44:75–91. doi: 10.1016/j.neuron.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Rumpel S, LeDoux J, Zador A, Malinow R. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;308:83–88. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Schafe GE, Nader K, Blair HT, LeDoux JE. Memory consolidation of Pavlovian fear conditioning: a cellular and molecular perspective. Trends Neurosci. 2001;24:540–546. doi: 10.1016/s0166-2236(00)01969-x. [DOI] [PubMed] [Google Scholar]
- Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 1999;285:1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- Shi S, Hayashi Y, Esteban JA, Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell. 2001;105:331–343. doi: 10.1016/s0092-8674(01)00321-x. [DOI] [PubMed] [Google Scholar]
- Song I, Huganir RL. Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci. 2002;25:578–588. doi: 10.1016/s0166-2236(02)02270-1. [DOI] [PubMed] [Google Scholar]
- Song I, Kamboj S, Xia J, Dong H, Liao D, Huganir RL. Interaction of the N-ethylmaleimide-sensitive factor with AMPA receptors. Neuron. 1998;21:393–400. doi: 10.1016/s0896-6273(00)80548-6. [DOI] [PubMed] [Google Scholar]
- Steinberg JP, Huganir RL, Linden DJ. N-ethylmaleimide-sensitive factor is required for the synaptic incorporation and removal of AMPA receptors during cerebellar long-term depression. Proc Natl Acad Sci U S A. 2004;101:18212–18216. doi: 10.1073/pnas.0408278102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, LeDoux JE. Functional inactivation of the amygdala before but not after auditory fear conditioning prevents memory formation. J Neurosci. 1999;19:RC48. doi: 10.1523/JNEUROSCI.19-24-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J Neurosci. 2006;26:12387–12396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Kelly MT, Sajikumar S, Serrano P, Tian D, Bergold PJ, Frey JU, Sacktor TC. PKM zeta maintains late long-term potentiation by N-ethylmaleimide-sensitive factor/GluR2-dependent trafficking of postsynaptic AMPA receptors. J Neurosci. 2008;28:7820–7827. doi: 10.1523/JNEUROSCI.0223-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh SH, Mao SC, Lin HC, Gean PW. Synaptic expression of glutamate receptor after encoding of fear memory in the rat amygdala. Mol Pharmacol. 2006;69:299–308. doi: 10.1124/mol.105.017194. [DOI] [PubMed] [Google Scholar]
- Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]