Abstract
Prenatal exposure to infection is a significant environmental risk factor in the development of schizophrenia and related disorders. Recent evidence indicates that disruption of functional and structural dopaminergic development may be at the core of the developmental neuropathology associated with psychosis-related abnormalities induced by prenatal exposure to infection. Using a mouse model of prenatal immune challenge by the viral mimic polyriboinosinic-polyribocytidilic acid, the present study critically evaluated this hypothesis by longitudinally monitoring the effects of maternal immune challenge during pregnancy on structural and functional dopaminergic development in the offspring from fetal to adult stages of life. Our study shows that prenatal immune challenge leads to dopaminergic maldevelopment starting as early as in the fetal stages of life and produces a set of postnatal dopaminergic abnormalities that is dependent on postnatal maturational processes. Furthermore, our longitudinal investigations reveal a striking developmental correspondence between the ontogeny of specific dopaminergic neuropathology and the postnatal onset of distinct forms of dopamine-dependent functional abnormalities implicated in schizophrenia. Prenatal immune activation thus appears to be a significant environmental risk factor for primary defects in normal dopaminergic development and facilitates the expression of postnatal dopamine dysfunctions involved in the precipitation of psychosis-related behavior. Early interventions targeting the developing dopamine system may open new avenues for a successful attenuation or even prevention of psychotic disorders following neurodevelopmental disruption of dopamine functions.
Introduction
Exposure to infection during critical periods of prenatal life is a notable environmental risk factor in the later development of schizophrenia and related psychotic disorders (Fatemi, 2005; Brown, 2006). Since this association is not limited to a single infectious pathogen, it has been suggested that factors common to the immune response to diverse infectious agents may be the critical mediators of the link between prenatal infection and enhanced risk of neurodevelopmental disturbances. According to one prevalent hypothesis, infection-induced elevation of proinflammatory cytokines in the maternal host and eventually in the fetal brain may be one of the critical neuroimmunological factors precipitating enhanced risk of schizophrenia and related disorders in the offspring (Gilmore and Jarskog, 1997; Meyer et al., 2009b). Numerous experimental investigations in rats and mice provide direct support for this hypothesis by demonstrating the appearance of long-lasting brain and behavioral dysfunctions relevant to schizophrenia following prenatal exposure to cytokine-stimulating agents such as lipopolysaccharide (LPS) and polyriboinosinic-polyribocytidilic acid (Poly-I:C), or to specific proinflammatory cytokines (for recent review, see Meyer et al., 2007, 2009a,b; Patterson, 2009).
Many of the prenatal infection-induced behavioral and pharmacological abnormalities in adult rats and mice appear to be closely associated with imbalances in the central dopamine system (Borrell et al., 2002; Zuckerman et al., 2003; Fortier et al., 2004; Meyer et al., 2005, 2008b; Ozawa et al., 2006). Abnormalities in the central dopamine system have also long been recognized to play a major role in the pathophysiology of schizophrenia (Laruelle, 1998; Di Forti et al., 2007; Guillin et al., 2007; Howes et al., 2007). According to recent theoretical accounts, multiple genetic and environmental risk factors may converge in a final common pathway of abnormal dopamine functions that ultimately facilitate the expression of psychotic disturbances (Howes and Kapur, 2009). In accord with this theoretical framework, prenatal exposure to immune challenge may be one of the critical environmental risk factors for abnormal dopaminergic development relevant to the etiopathology of schizophrenia and related psychotic disorders (Meyer et al., 2008a; Meyer and Feldon, 2009).
However, direct evidence for this hypothesis is relatively sparse thus far; and the ontogenetic mechanisms underlying the induction of adult dopaminergic dysfunctions following prenatal immune activation remain elusive. In the present study, we therefore addressed these issues by testing the hypothesis whether prenatal immune challenge may induce multiple changes in dopaminergic development. This was achieved by longitudinally monitoring the effects of prenatal immune activation on dopaminergic structures and functions from the fetal to adult stages of life. We used a well established mouse model of prenatal immune challenge by the viral mimic Poly-I:C, which is known to capture a wide spectrum of schizophrenia-related brain and behavioral dysfunctions associated with dopaminergic imbalances in adulthood (e.g., Zuckerman et al., 2003; Meyer et al., 2005, 2008b; Ozawa et al., 2006). This experimental model system thus offers an excellent opportunity to explore primary prenatal immune activation effects on dopaminergic maldevelopment and to identify putative developmental correlations between specific dopamine-related structural and functional brain abnormalities relevant to schizophrenia.
Materials and Methods
Animals.
C57BL/6J mice were used throughout the study. Female and male breeders were obtained from our in-house specific-pathogen-free colony at the age of 10–14 weeks. Breeding began after 2 weeks of acclimatization to the new animal holding room, which was a temperature- and humidity-controlled (21 ± 1°C, 55 ± 5%) holding facility under a reversed light–dark cycle (lights off: 8:00 A.M. to 8:00 P.M.). All animals had ad libitum access to food (Kliba 3430, Klibamühlen) and water. All procedures described in the present study had been previously approved by the Cantonal Veterinarian's Office of Zurich and are in agreement with the principles of laboratory animal care in the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication No. 86-23, revised 1985). All efforts were made to minimize the number of animals used and their suffering.
Maternal viral-like immune activation during pregnancy.
For the purpose of the maternal immunological manipulations during pregnancy, female mice were subjected to a timed mating procedure as described previously (Meyer et al., 2005). Pregnant dams on gestation day 9 (GD9) received either a single injection of Poly-I:C (potassium salt; Sigma-Aldrich) or vehicle. The gestational stage (i.e., GD9) was selected based on our previous findings (Meyer et al., 2005, 2008a,b). GD9 in mice corresponds approximately to the first trimester of human pregnancy with respect to developmental biology and percentage of gestation (Clancy et al., 2001). Poly-I:C (5 mg/kg) was dissolved in sterile pyrogen-free 0.9% NaCl (= vehicle) solution to yield a final concentration of 1 mg/ml and was administered via the intravenous route at the tail vein under mild physical constraint (see below). The dose of Poly-I:C was chosen based on our previous studies in C57BL/6 mice (Meyer et al., 2005, 2008a,b). All solutions were freshly prepared at the day of administration and injected with a volume of 5 ml/kg. All animals were returned to their home cages immediately after the injection procedure.
For the purpose of the intravenous injections, each animal's tail was first bathed in 40°C water to better visualize and dilate the tail veins. Subsequently, the animals were mildly restrained using a nonrestrictive Plexiglas restrainer (561-RC, Plas Labs) and the substance was injected either at the right or left tail vein. The success of the injection was verified according to three main criteria: (1) resistance of the injection (this refers to the fact that an incomplete hit of the vein prevents the intravenous administration of the full volume of substance), (2) temporary replacement of blood by the injected substance (this refers to the fact that a successful injection at the tail vein can be visualized by the temporary replacement of blood by the injected substance), and (3) appearance of a blood drop following retraction of the needle (this refers to the fact that following successful intravenous injection, a blood drop appears at the injection site following retraction of the needle). In addition to these criteria, we have previously verified the effectiveness of intravenous Poly-I:C administration in mice in terms of the elicited cytokine responses in maternal and fetal tissue (Meyer et al., 2005, 2006).
Collection of fresh brain specimen.
Brain specimens of offspring of Poly-I:C- and saline-treated mothers were collected on GD19 and postnatal day 35 (PND35) and PND70 to capture the fetal, peripubertal, and adult stage of development. Peripubertal (PND35) and adult (PND70) stages were defined based on the gradual attainment of sexual maturity and age-specific behavioral discontinuities from younger to older animals (for rats, Spear, 2000; for mice, Koshibu et al., 2004). These two developmental stages are identical to those included in our earlier studies (Meyer et al., 2008b) and approximately correspond to periods between 11 and 16 years and from 20 years onward, respectively, in humans.
For the purpose of collecting fetal brain samples, pregnant dams were killed by decapitation, and the fetuses were immediately removed from the uteri. The fetal head regions were separated from the fetal bodies and immediately frozen on powdered dry ice. The fetal specimens were then stored at −80°C until further processing (see below). Fetuses were derived from six mothers in each treatment condition (Poly-I:C or vehicle). This served to rule out the possibility that putative differences between Poly-I:C- and vehicle-exposed fetuses may stem from individual differences in prenatal development.
For the collection of peripubertal and adult brain samples, offspring were weaned and sexed on PND24. Littermates of the same sex were caged separately. All animals were maintained under ad libitum food and water diet and kept in a temperature- and humidity-controlled animal vivarium under a 12:12 h reversed light–dark cycle as described above. For the collection of fresh brain tissue, offspring were killed by decapitation on PND35 or PND70. The brains were quickly removed from the skull, immediately frozen on powdered dry ice, and stored at −80°C until further processing (see below). Each experimental group on PND35 and PND70 consisted of offspring derived from multiple independent litters (at least six in each treatment condition). Brain samples of both male and female offspring were collected and subjected to immunohistochemical analyses (see below). All animals were experimentally naive at the time of killing to avoid possible confounds from previous testing.
One of the main goals of the present study was to seek evidence for the hypothesis that prenatal immune activation may lead to long-lasting dopaminergic dysfunctions by interfering with early fetal dopaminergic development. Therefore, fetal brain tissue was the critical starting point for our longitudinal neuroanatomical investigations across the fetal to the adult stage of life. Perfusion of fetal mice was technically not reliable. Therefore, we adopted the same procedures for the evaluation of fetal dopaminergic markers as published before (Meyer et al., 2008a) and thus used nonperfused fetal brain tissue. To be consistent in tissue preparation across all developmental stages, we used identical procedures for our immunohistochemical studies in all prenatal and postnatal subjects, i.e., decapitation without prior perfusion. In a second step, however, we repeated parts of the immunohistochemical analyses using perfused brain tissue to confirm the results obtained in the initial investigations conducted on nonperfused tissue (see below).
Collection of perfused brain specimen.
We performed additional immunohistochemical analyses of dopaminergic markers on perfused adult brain specimen so as to confirm the prenatal Poly-I:C-induced neuroanatomical alterations using standard immunohistochemistry procedures using perfused brain samples. For this purpose, we focused on the dopaminergic phenotypes observed in adult (PND70) Poly-I:C relative to control offspring because this developmental stage corresponds to our endpoint phenotype. Adult offspring of Poly-I:C- and saline-treated mothers were anesthetized with an overdose of Nembutal (Abbott Laboratories) and perfused transcardially with 0.9% NaCl, followed by 4% phosphate-buffered paraformaldehyde solution containing 15% picric acid. The dissected brains were postfixed in the same fixative for 6 h and processed for antigen retrieval involving overnight incubation in citric acid buffer (pH = 4.5) followed by a 90 s microwave treatment at 480 W. The brains were then cryoprotected using 30% sucrose in PBS, frozen with powdered dry ice, and stored at −80°C until further processing.
Immunohistochemistry.
Nonperfused frozen brain samples were cut coronally at 20 μm thickness using a cryostat (HM 500, Microm, Global Medical Instrumentation). Six serial sections were collected, dried at room temperature, and stored at −80°C until further processing. Perfused brain samples were cut coronally at 30 μm thickness from frozen blocks with a sliding microtome. Six series of sections were collected, rinsed in PBS, and stored at −20°C in antifreeze solution until further processing. For immunostaining of nonperfused brain sections, the slices were fixed in 4% paraformaldehyde for 3 h, rinsed three times for 5 min in PBS, and blocked for 1 h in 10% normal goat serum (NGS), in 0.3% Triton X-100 in PBS. For immunohistochemical staining of perfused brain sections, the slices were rinsed three times for 10 min in PBS, and blocking was done in PBS, 0.3% Triton X-100, 5% normal serum for 1 h at room temperature. The following primary antibodies were used: rabbit anti-tyrosine hydroxylase (TH; Santa Cruz Biotechnology; diluted: 1:500), rat anti-dopamine transporter (DAT; Millipore Bioscience Research Reagents; diluted 1:250), rabbit anti-dopamine D1 receptor (D1R; Millipore Bioscience Research Reagents; diluted 1:250), rabbit anti-dopamine D2 receptor (D2R; Millipore Bioscience Research Reagents; diluted 1:500), and rabbit anti-Nurr1 (Santa Cruz Biotechnology; diluted: 1:50).
For immunostaining of nonperfused brain sections, all antibodies were diluted in PBS containing 2% NGS and 0.3% Triton X-100 and incubated overnight at room temperature. After three washes with PBS (5 min each), the sections were incubated at room temperature for 2 h with the biotinylated secondary antibodies diluted 1:500 in PBS containing 2% NGS and 0.3% Triton X-100. The sections were washed again three times for 5 min in PBS and incubated with Vectastain ABC-Kit (Vector Laboratories) diluted in PBS containing 0.3% Triton X-100 for 2 h. After three rinses in Tris–HCl 0.1 m, pH 7.4, the sections were stained with 0.05% 3,3-diaminobenzidine, 0.024% H2O2, and 0.4% NiCl for 5–10 min, rinsed again three times in Tris–HCl 0.1 m, pH 7.4, dehydrated, and coverslipped with Eukitt (Kindler).
For immunostaining of perfused brain sections, all antibodies were diluted in PBS containing 0.3% Triton X-100 and 2% normal serum, and the sections were incubated free floating overnight at room temperature. After three washes with PBS (10 min each), the sections were incubated for 1 h with the biotinylated secondary antibodies diluted 1:500 in PBS containing 2% NGS and 0.3% Triton X-100. Sections were washed again three times for 10 min in PBS and incubated with Vectastain Kit (Vector Laboratories) diluted in PBS for 1 h. After three rinses in Tris–HCl 0.1 m, pH 7.4, the sections were stained with 1.25% 3,3-diaminobenzidine and 0.08% H2O2 for 10–15 min, rinsed again four times in PBS, dehydrated, and coverslipped with Eukitt (Kindler).
The chosen immunohistochemical methods have been proven to be a reliable procedure to detect and quantify midbrain TH- and DAT-positive cells in fetal brain tissue (Meyer et al., 2008a). Furthermore, they also proved to be an efficient way to stain and quantify TH, DAT, D1R, DR2, and Nurr1 protein in striatal and/or midbrain regions of peripubertal and adult subjects (see Figs. 1–7).
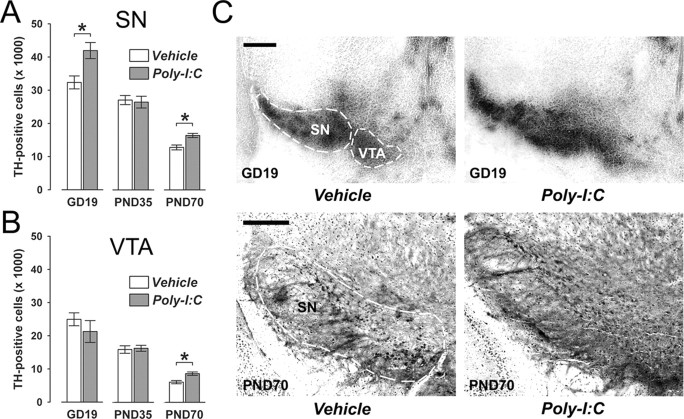
Figure 1.
Age-dependent alterations in midbrain dopamine cell numbers following prenatal immune challenge. Pregnant mice were exposed to the viral mimic Poly-I:C or vehicle treatment, and the effects on midbrain dopamine cell numbers were investigated in the resulting offspring at the fetal (GD19), peripubertal (PND35), and adult (PND70) stages of development using stereological estimations of TH-positive cells on serial coronal brain sections. A, Maternal Poly-I:C treatment significantly increased the number of TH-positive cells in the fetal SN relative to maternal vehicle treatment. At the peripubertal stage of development, prenatally immune challenged and control offspring did not differ in the number of TH-positive neurons in the SN. However, a significant increase in the number of SN TH-positive cells was noticeable in adult offspring born to Poly-I:C-challenged mothers in comparison with age-matched control offspring. *p < 0.05, based on Fisher's LSD post hoc group comparisons of GD19 or PND70 specimen following the presence of a significant two-way interaction in the initial 2 × 3 (prenatal treatment × age) ANOVA (F(2,63) = 4.94, p < 0.05). B, Prenatal Poly-I:C exposure led to a significant increase in TH-positive cells in the VTA specifically in adult (but not peripubertal or fetal) offspring compared with adult offspring of control mothers. *p < 0.05, based on Fisher's LSD post hoc group comparisons of PND70 specimen following the presence of a significant two-way interaction in the initial 2 × 3 (prenatal treatment × age) ANOVA (F(2,63) = 3.62, p < 0.05). C, Representative images of coronal brain sections of fetal (GD19) and adult (PND70) offspring derived from vehicle- or Poly-I:C-treated mothers stained for TH protein by immunohistochemistry. The images show TH cell stainings in the fetal SN and VTA, and in the adult SN. For all developmental stages, TH-positive cells in the midbrain were clearly identifiable by the appearance of darkly stained cell bodies. Scale bars, 250 μm. All values in A and B are means ± SEM. The numbers of offspring included in the analyses were N(GD19-vehicle) = 12, N(GD19-Poly-I:C) = 11, N(PND35-vehicle) = 12, N(PND35-Poly-I:C) = 11, N(PND70-vehicle) = 11, N(PND70-Poly-I:C) = 12.
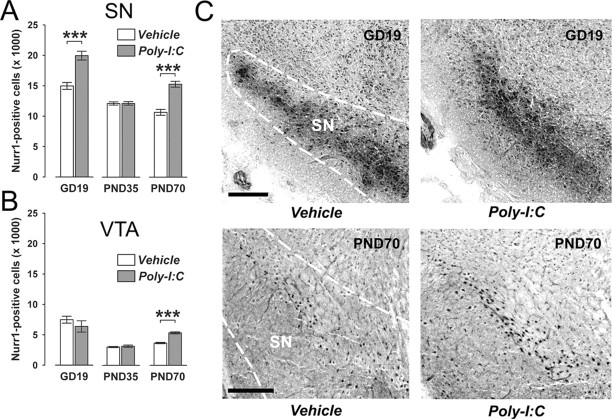
Figure 2.
Age-dependent alterations in mesencephalic expression of the orphan nuclear transcription factor Nurr1 following prenatal immune challenge. Pregnant mice were exposed to the viral mimic Poly-I:C or vehicle treatment, and the effects on Nurr1 protein expression in midbrain structures were investigated in the resulting offspring at the fetal (GD19), peripubertal (PND35), and adult (PND70) stages of development using stereological estimations of Nurr1-positive cells on serial coronal brain sections. A, Maternal Poly-I:C treatment significantly increased the number of Nurr1-positive cells in the fetal SN relative to maternal vehicle treatment. At the peripubertal stage of development, prenatally immune challenged and control offspring did not differ in the number of Nurr1-positive neurons in the SN region. However, a significant increase in the number of SN Nurr1-positive cells was noticeable in adult offspring born to Poly-I:C-challenged mothers in comparison with age-matched control offspring. ***p < 0.001, based on Fisher's LSD post hoc group comparisons of GD19 or PND70 specimen following the presence of a significant two-way interaction in the initial 2 × 3 (prenatal treatment × age) ANOVA (F(2,63) = 17.38, p < 0.001). B, Prenatal Poly-I:C exposure led to a significant increase in Nurr1-positive cells in the VTA specifically in adult (but not peripubertal or fetal) offspring compared with adult offspring of control mothers. ***p < 0.001, based on Fisher's LSD post hoc group comparisons of PND70 specimen following the presence of a significant two-way interaction in the initial 2 × 3 (prenatal treatment × age) ANOVA (F(2,63) = 4.06, p < 0.05). C, Representative images of coronal brain sections of fetal (GD19) and adult (PND70) offspring derived from vehicle- or Poly-I:C-treated mothers stained for Nurr1 protein by immunohistochemistry. The images show Nurr1 cell stainings in the fetal SN and VTA, and in the adult SN. For all developmental stages, Nurr1-positive cells in the midbrain were clearly identifiable by the appearance of darkly stained cell bodies. Scale bars, 250 μm. All values in A and B are means ± SEM. The numbers of offspring included in the analyses were N(GD19-vehicle) = 12, N(GD19-Poly-I:C) = 11, N(PND35-vehicle) = 12, N(PND35-Poly-I:C) = 11, N(PND70-vehicle) = 11, N(PND70-Poly-I:C) = 12.
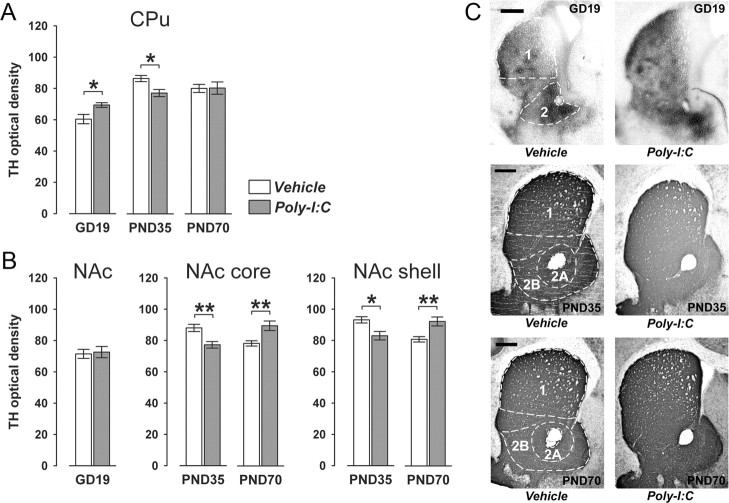
Figure 3.
Age- and region-specific alterations in striatal TH expression following prenatal immune challenge. Pregnant mice were exposed to the viral mimic Poly-I:C or vehicle treatment, and the effects on striatal TH expression were investigated in the resulting offspring at the fetal (GD19), peripubertal (PND35), and adult (PND70) stages of development using optical densitometry of immunohistochemically stained coronal brain sections. A, Maternal Poly-I:C treatment significantly increased TH immunoreactivity in the caudate putamen region of the fetal striatum relative to maternal vehicle treatment. However, at the peripubertal stage of development, offspring born to Poly-I:C-treated mothers displayed a significant reduction in TH immunoreactivity in the CPu relative to age-matched control offspring, but no significant group differences in CPu TH immunoreactivity were present at adult age. *p < 0.05, based on Fisher's LSD post hoc group comparisons of GD19 or PND35 specimen following the presence of a significant two-way interaction in the initial 2 × 3 (prenatal treatment × age) ANOVA (F(2,63) = 6.11, p < 0.01). B, Prenatal Poly-I:C exposure did not significantly influence TH immunoreactivity in the NAc region of the fetal striatum relative to maternal vehicle treatment. However, the prenatal immunological manipulation significantly decreased TH immunoreactivity in both NAc core and shell at the peripubertal stage of development, but it significantly increased NAc core and shell TH immunoreactivity in adult offspring relative to age-matched control offspring. *p < 0.05 and **p < 0.01, based on Fisher's LSD post hoc group comparisons of PND35 or PND70 specimen following the presence of a significant two-way interaction in the initial 2 × 2 (prenatal treatment × postnatal age) ANOVA (NAc core: F(1,42) = 14.79, p < 0.001; NAc shell: F(1,42) = 15.52, p < 0.05). C, Representative images of coronal brain sections of fetal (GD19), peripubertal (PND35), and adult (PND70) offspring derived from vehicle- or Poly-I:C-treated mothers stained for TH by immunohistochemistry. 1, CPu; 2, NAc; 2A, NAc core; 2B, NAc shell. Scale bars, 500 μm. All values in A and B are means ± SEM. The numbers of offspring included in the analyses were N(GD19-vehicle) = 12, N(GD19-Poly-I:C) = 11, N(PND35-vehicle) = 12, N(PND35-Poly-I:C) = 11, N(PND70-vehicle) = 11, N(PND70-Poly-I:C) = 12.
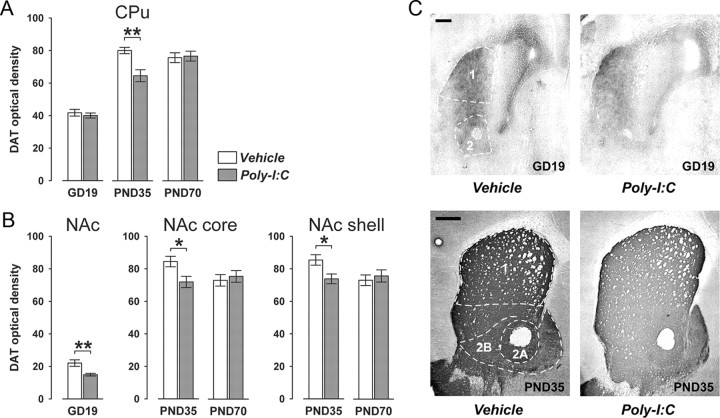
Figure 4.
Age- and region-specific reduction in striatal DAT expression following prenatal immune challenge. Pregnant mice were exposed to the viral mimic Poly-I:C or vehicle treatment, and the effects on striatal DAT expression were investigated in the resulting offspring at the fetal (GD19), peripubertal (PND35), and adult (PND70) stages of development using optical densitometry of immunohistochemically stained coronal brain sections. A, Offspring born to Poly-I:C-treated mothers displayed a significant decrease in DAT immunoreactivity in the CPu specifically at peripubertal age in comparison with age-matched offspring born to vehicle-treated control mothers. **p < 0.01, based on Fisher's LSD post hoc group comparison of PND35 specimen following the presence of a significant two-way interaction in the initial 2 × 3 (prenatal treatment × age) ANOVA (F(2,63) = 5.60, p < 0.01). B, Prenatal Poly-I:C exposure also led to a significant reduction in DAT immunoreactivity in the fetal NAc region compared with fetuses derived from control mothers. *p < 0.05, based on one-way ANOVA with the between-subjects factor of prenatal treatment ANOVA (F(1,21) = 10.36, p < 0.01). In addition, peripubertal (but not adult) offspring born to Poly-I:C-treated mothers showed a significant reduction in DAT immunoreactivity in both the NAc core and shell subregions relative to peripubertal control offspring. *p < 0.01, based on Fisher's LSD post hoc group comparison of PND35 specimen following the presence of a significant two-way interaction in the initial 2 × 2 (prenatal treatment × postnatal age) ANOVA (NAc core: F(1,42) = 4.72, p < 0.05; NAc shell: F(1,42) = 4.56, p < 0.05). C, Representative images of coronal brain sections of fetal (GD19) and peripubertal (PND35) offspring derived from vehicle- or Poly-I:C-treated mothers stained for DAT by immunohistochemistry. 1, CPu; 2, NAc; 2A, NAc core; 2B, NAc shell. Scale bars, 500 μm. All values in A and B are means ± SEM. The numbers of offspring included in the analyses were N(GD19-vehicle) = 12, N(GD19-Poly-I:C) = 11, N(PND35-vehicle) = 12, N(PND35-Poly-I:C) = 11, N(PND70-vehicle) = 11, N(PND70-Poly-I:C) = 12.
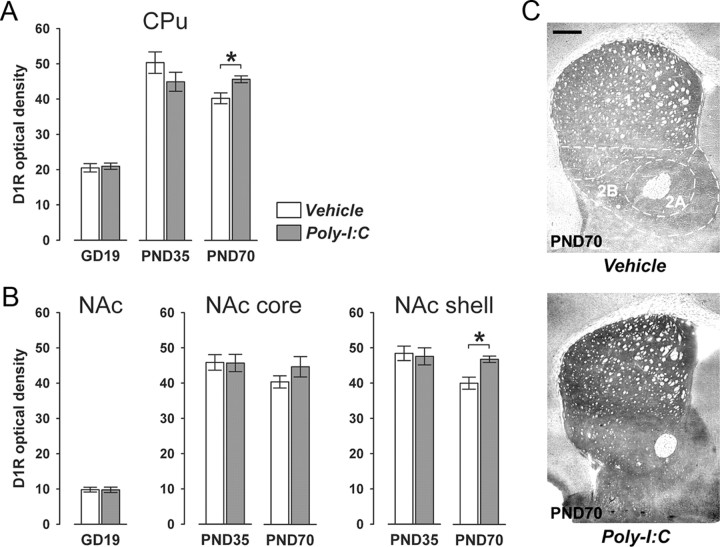
Figure 5.
Postpubertal onset of altered D1R expression in striatal regions following prenatal immune challenge. Pregnant mice were exposed to the viral mimic Poly-I:C or vehicle treatment, and the effects on striatal D1R expression were studied in the resulting offspring at the fetal (GD19), peripubertal (PND35), and adult (PND70) stages of development using optical densitometry of immunohistochemically stained coronal brain sections. A, Offspring born to Poly-I:C-treated mothers displayed a significant increase in D1R immunoreactivity in the CPu specifically at adult age in comparison with adult offspring born to vehicle-treated control mothers. *p < 0.05, based on Fisher's LSD post hoc group comparison of PND70 specimen following the presence of a significant two-way interaction in the initial 2 × 3 (prenatal treatment × age) ANOVA (F(2,63) = 3.94, p < 0.05). B, Prenatal Poly-I:C exposure also significantly increased D1R immunoreactivity in the NAc shell but not in the core subregion in the adult offspring relative to adult control offspring. *p < 0.05, based on Fisher's post hoc group comparison of PND70 specimen following the presence of a significant two-way interaction in the initial 2 × 2 (prenatal treatment × postnatal age) ANOVA (F(1,42) = 3.31, p < 0.05). C, Representative images of coronal brain sections of adult (PND70) offspring born to vehicle- or Poly-I:C-treated mothers stained for D1R by immunohistochemistry. 1, CPu; 2A, NAc core; 2B, NAc shell. Scale bar, 500 μm. All values in A and B are means ± SEM. The numbers of offspring included in the analyses were N(GD19-vehicle) = 12, N(GD19-Poly-I:C) = 11, N(PND35-vehicle) = 12, N(PND35-Poly-I:C) = 11, N(PND70-vehicle) = 11, N(PND70-Poly-I:C) = 12.
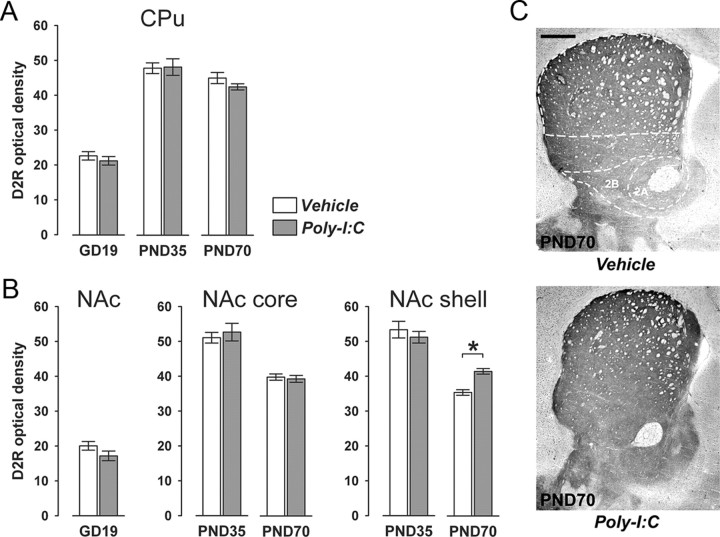
Figure 6.
Postpubertal onset of altered D2R expression in striatal regions following prenatal immune challenge. Pregnant mice were exposed to the viral mimic Poly-I:C or vehicle treatment, and the effects on striatal D2R expression were studied in the resulting offspring at the fetal (GD19), peripubertal (PND35), and adult (PND70) stages of development using optical densitometry of immunohistochemically stained coronal brain sections. A, Offspring born to Poly-I:C-treated mothers did not display any significant differences in D2R immunoreactivity in the CPu at the fetal, peripubertal, or adult stage of development compared with age-matched offspring born to vehicle-treated control mothers. B, However, prenatal Poly-I:C exposure significantly increased D2R immunoreactivity specifically in the NAc shell but not in the core subregion in the adult offspring relative to adult control offspring. *p < 0.05, based on Fisher's LSD post hoc group comparison of PND70 specimen following the presence of a significant two-way interaction in the initial 2 × 2 (prenatal treatment × postnatal age) ANOVA (F(1,42) = 4.28, p < 0.05). C, Representative images of coronal brain sections of adult (PND70) offspring born to vehicle- or Poly-I:C-treated mothers stained for D2R by immunohistochemistry. 1, CPu; 2A, NAc core; 2B, NAc shell. Scale bar, 500 μm. All values in A and B are means ± SEM. The numbers of offspring included in the analyses were N(GD19-vehicle) = 12, N(GD19-Poly-I:C) = 11, N(PND35-vehicle) = 12, N(PND35-Poly-I:C) = 11, N(PND70-vehicle) = 11, N(PND70-Poly-I:C) = 12.
Figure 7.
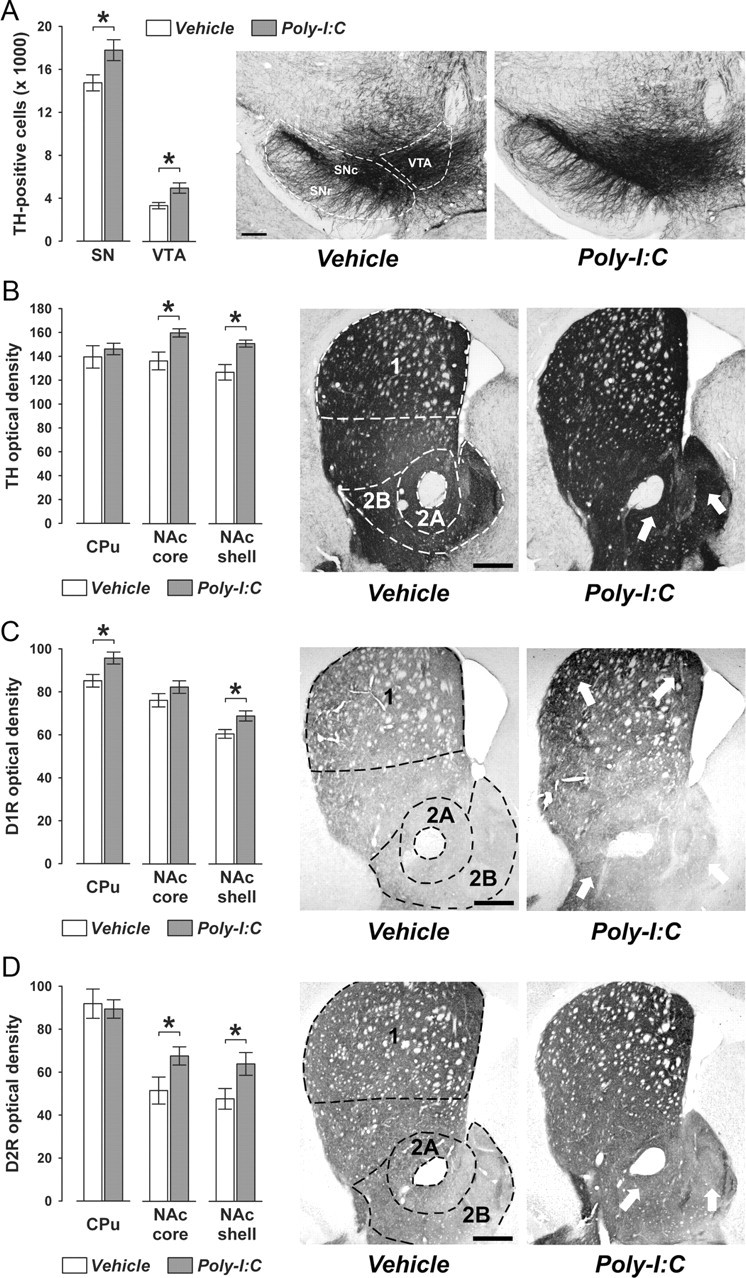
Confirmation of the adult dopaminergic phenotype in perfused adult brain samples. Pregnant mice were exposed to the viral mimic Poly-I:C or vehicle treatment, and immunoreactivities of TH, DAT, D1R, and D2R were assessed in the adult (PND70) offspring according to standard procedures using perfused brain samples. A, Prenatal Poly-I:C exposure led to a significant increase in the number of TH-positive cells in the SN (including both SNc and SNr) and the VTA compared with prenatal vehicle treatment. B, Offspring born to Poly-I:C-treated mothers displayed enhanced TH immunoreactivity specifically in the nucleus accumbens core (NAc core) and shell (NAc shell) subregions (indicated by the white arrows) but not in the CPu. C, Prenatal Poly-I:C exposure led to a significant increase in D1R immunoreactivity in the CPu and NAc core regions of the striatum (indicated by the white arrows) relative to prenatal control treatment. D, Offspring born to Poly-I:C-exposed mothers displayed enhanced D2R immunoreactivity specifically in the NAc core NAc shell subregions (indicated by the white arrows) but not in the CPu. All values are means ± SEM. *p < 0.05, based on independent Student's t tests (two tailed). For all analyses, the numbers of offspring included in the analyses were N(vehicle) = 8, N(Poly-I:C) = 7. 1, CPu; 2A, NAc core; 2B, NAc shell. Scale bars, 250 μm.
Stereological estimation of TH- and Nurr1-positive midbrain neurons.
The numbers of midbrain TH- and Nurr1-positive neurons were quantified in the two relevant midbrain areas, namely in the ventral tegmental area (VTA) and substantia nigra (SN). Even though the vast majority of dopaminergic projections to the dorsal striatum stem from dopaminergic cells located in the substantia nigra pars compacta (SNc), we also included quantification of dopaminergic cells located in the substantia nigra pars reticulata (SNr). Our rationale to include both SNc and SNr dopaminergic cells in our analyses was to rule out the possibility that potential group differences in the primary dopaminergic region of the SN (i.e., the SNc) may simply reflect a difference in distribution across the distinct SN regions rather than a genuine change in the total number of dopaminergic cells in the SN.
The numbers of midbrain TH- and Nurr1-positive neurons in the left brain hemisphere were determined by unbiased stereological estimations using the optical fractionator method (Gundersen et al., 1988). With the aid of the image analysis computer software Stereo Investigator (version 6.50.1; Microbrightfield), every section of a one-in-six series was measured, resulting in an average of four to six sections per GD19 brain sample and in an average of six to eight sections per PND35 and PND70 brain sample. For fetal (GD19) brain samples, the following sampling parameters were used: (1) a fixed counting frame with a width of 25 μm and a length of 25 μm; and (2) a sampling grid size of 150 × 110 μm. For prepubertal (PND35) and adult (PND70) brain samples, the following sampling parameters were used: (1) a fixed counting frame with a width of 40 μm and a length of 40 μm; and (2) a sampling grid size of 150 × 110 μm. The counting frames were placed randomly at the intersections of the grid within the outlined structure of interest by the software. The cells were counted following the unbiased sampling rule using the 40× oil lens [numerical aperture (NA), 1.3] and included in the measurement when they came into focus within the optical dissector (Howard and Reed, 2005).
Optical densitometry of dopaminergic markers in striatal regions.
Quantification of the immunoreactivity for TH, DAT, D1R, and D2R in dorsal and ventral striatal regions of the left brain hemisphere was achieved by means of optical densitometry using ImageJ software (NIH). Optical densitometry was chosen because these dopaminergic markers are highly enriched at synaptic sites in the areas of interest (i.e., dorsal and ventral striatal regions). Digital images were acquired at a magnification of 2.5× (NA 0.075) using a digital camera (Axiocam MRc5, Zeiss) mounted on a Zeiss Axioplan microscope. Exposure times were set so that pixel brightness was never saturated. Pixel brightness was measured in the respective areas of the right brain hemisphere. In addition, pixel brightness was measured in the corpus callosum as background area. The background-corrected optical densities were averaged per brain region and animal. Six to 8 coronal brain sections per animal were analyzed in GD19 specimens, and 8 to 10 sections per animal were analyzed in PND35 and PND70 specimens. All immunohistochemical preparations of postnatal brain samples were quantified in the dorsal striatum [caudate putamen (CPu)], nucleus accumbens core (NAc core), and nucleus accumbens shell (NAc shell). Since the NAc is not yet fully matured into core and shell subregions at the late fetal stage (GD19), no further subdivisions were made in the densitometric analyses of dopaminergic markers in the fetal NAc (see below).
Delineation of fetal, peripubertal, and adult brain regions.
The areas of interest in fetal brain specimen encompassed the fetal CPu, NAc, SN, and VTA at GD19. Each of these regions was delineated according to the work of Paxinos et al. (2007). The analyses conducted in the fetal NAc region included sections ranging from +2.07 to +2.43 mm from the rostral fetal pole. The dorsal border of the fetal NAc lined the ventral side of the fetal CPu, while its ventral border followed the dorsal side of the ventral pallidum. Its lateral border was adjacent to the piriform cortex, while the medial one followed the neuroepithelium. The analyses conducted in the fetal CPu region also included sections ranging from +2.07 to +2.43 mm from the rostral fetal pole. For the delineation of the ventral border of the fetal CPu, a horizontal line was drawn slightly above the anterior commissure. The lateral border and the lateral part of the dorsal border of the fetal CPu were adjacent to the medial border of the external capsule, whereas the medial side and the medial part of the dorsal border of the fetal CPu lined the lateral side of the neuroepithelium. The analyses conducted in the fetal SN and VTA regions also included sections ranging from +3.48 to +3.84 mm from the rostral fetal pole. The dorsal border of the fetal SN followed the ventral border of the parabrachial nucleus, while the ventral border of the fetal SN lined the dorsal side of the cerebral peduncle. On the other hand, the dorsal border of the fetal VTA followed the ventral side of the mammillotegmental tract, and its ventral border was adjacent to the dorsal border of the cerebral peduncle. The lateral side of the VTA lined the medial border of the parabrachial pigmented nucleus, and its medial border followed the lateral side of the rostral linear nucleus raphe.
The areas of interest in peripubertal (PND35) and adult (PND70) brain specimens encompassed CPu, NAc shell and core, SN, and VTA. Each of these regions was delineated according to the work by Franklin and Paxinos (2008). The analyses conducted in the NAc core included sections ranging from bregma +1.94 to +0.74 mm. The dorsal border of the NAc core lined the ventral side of the CPu, while its ventral border followed the dorsal side of the NAc shell. The lateral border of the NAc core lined the medial border of the intermediate endopiriform claustrum, and its medial border was adjacent to the lateral side of the NAc shell. The analyses conducted in the NAc shell included sections ranging from bregma +1.94 to +0.86 mm. The dorsal side of the NAc shell followed the ventral border of the NAc core, except for the most rostral section (bregma +1.94 mm), in which it lined the ventral border of the CPu. The ventral side of the NAc shell lined the dorsal border of the ventral pallidum. The lateral border of the NAc shell was adjacent to the medial side of the NAc Core, and the medial NAc shell border followed the lateral border of the lateral septal nucleus. The analyses conducted in the CPu included sections ranging from bregma +1.94 to +0.14 mm. The dorsal border of the CPu lined the ventral side of the forceps minor of the corpus callosum for rostral slices (i.e., from bregma +1.94 to +1.34 mm), and the ventral side of the external capsule for more caudal sections (i.e., from bregma +1.18 to +0.14 mm). The ventral border of the CPu lined the dorsal border of the NAc core for rostral slices (i.e., from bregma +1.94 to +1.70 mm). For more caudal regions of the CPu (i.e., from bregma +1.54 to +0.14 mm), a horizontal line was drawn at the tip of the lateral ventricle to delineate its ventral border. The lateral and medial borders of the CPu were always adjacent to the medial border of the lateral part of the forceps minor of the corpus callosum, and to the lateral border of the medial part of the forceps minor of the corpus callosum, respectively. The analyses conducted in the peripubertal and adult SN and VTA regions included sections ranging from bregma −2.80 to −3.16 mm. The dorsal border of the SN lined the ventral border of the parabrachial pigmented nucleus, and its ventral side was adjacent to the dorsal border of the cerebral peduncle. Hence, the analyses of TH- and Nurr1-positive cells in the peripubertal and adult SN region took into account cells of both the SNc and the SNr. The area of interest (i.e., SNc and SNr) was extended laterally but did not include cells of the substantia nigra pars lateralis. The medial border of the SN was defined by a vertical line passing through the medial tip of the cerebral peduncle to exclude cells located in the VTA. For the delineation of the dorsal border of the VTA, a horizontal line was drawn through the mammillotegmental tract. The ventral border of the VTA lined the dorsal side of the retromamillary decussation for rostral sections (i.e., from bregma −2.80 to −2.92 mm) and of the mamillary peduncle for the more caudal slices (i.e., from bregma −3.08 to −3.16 mm). The lateral border of the VTA always followed the medial side of the parabrachial pigmented nucleus. The medial border of the VTA was defined by a vertical line passing through the middle of the lateral–medial mamillary nucleus for sections ranging from bregma −2.80 to −2.92 mm, and by the lateral border of the fasciculus retroflexus for sections ranging from bregma −3.08 to −3.16 mm.
Spontaneous and amphetamine-induced locomotor activity.
Spontaneous and d-amphetamine sulfate (AMPH)-induced locomotor activity was assessed in four identical open-field arenas (40 × 40 × 35 cm high) made of wood and painted white as described before (Meyer et al., 2005, 2008b). They were located in a testing room under dim diffused lighting (∼35 lux as measured in the center of the arenas). A digital camera was mounted directly above the four arenas. Images were captured at a rate of 5 Hz and transmitted to a PC running the Ethovision (Noldus) tracking system.
To acclimatize the animals to the open field, they were placed in the center of the arena and allowed to explore freely for 20 min. At the end of this time period, the animals were removed from the apparatus and injected with sterile 0.9% NaCl (saline) solution. They were then immediately returned to the same arenas and allowed to explore for another 20 min. Subsequently, the animals were briefly removed from the apparatus once more, administered with AMPH, and returned to the same arenas again. The locomotor responses to the acute drug challenge were then monitored for a period of 80 min. AMPH (Sigma-Aldrich) was dissolved in isotonic 0.9% NaCl solution to achieve the desired concentration for injection. AMPH was administered via the intraperitoneal route at a dose of 2.5 mg/kg. This dose was selected based on our previous studies in C57BL/6 mice (Meyer et al., 2005, 2008b). The selected dose of AMPH (2.5 mg/kg, i.p.) does not produce any ceiling effects on locomotor activity enhancement (Meyer et al., 2005, 2008b). This readily facilitates the assessment of modulatory effects of the prenatal immunological manipulation on AMPH-induced changes in locomotor activity. The volume of injection was 5 ml/kg. The AMPH solutions were freshly prepared on the day of testing.
Spontaneous and drug-induced locomotor activity was assessed when the animals reached the peripubertal (PND35) or adult (PND70) stage of development. Each experimental group on PND35 and PND70 consisted of offspring derived from multiple independent litters (at least six in each prenatal treatment condition) and included male and female subjects. Testing of peripubertal and adult animals was conducted in two separate cohorts of drug-naive offspring to avoid repeated drug exposure and was performed in the dark phase of the light–dark cycle.
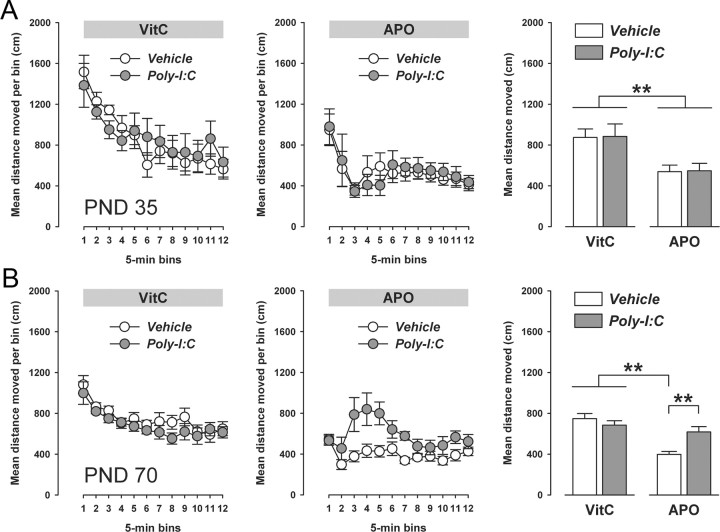
Apomorphine-induced locomotor activity and stereotypy.
Apomorphine-induced locomotor activity and stereotypy were assessed in a transparent Plexiglas cylinder (30 cm in diameter; 40 cm high), whose inner wall was wrapped with metal wire mesh (1 mm thick with 8 × 8 mm squares). A transparent Plexiglas wall (without wire mesh) was used as the cylinder top to prevent the animals from escaping. The floor of the cylinder was covered with sawdust, which was changed after each test run. Wire mesh and sawdust embedding were used to allow the expression of (stereotyped) climbing and sniffing, respectively. The cylinder was located in a testing room under dim diffused lighting (∼35 lux as measured in the center of the cylinder). A digital camera was mounted directly above cylinder, which transmitted images (captured at a rate of 5 Hz) to a PC running the Ethovision (Noldus) tracking system for the purpose of assessment of horizontal locomotor activity. An additional digital video camera was mounted perpendicular to the cylinder to record vertical movements and behaviors (see below). The output of the perpendicular camera was transmitted to a PC running the Windows XP operating system and the WINTV-2000 program for data acquisition.
The animals were injected with vehicle (vitamin C solution, VitC) or apomorphine HCl (APO) solution and then gently placed in the center of the cylinder. They were then allowed to explore freely for 60 min, during which horizontal and vertical movements and behaviors were recorded by the two cameras. APO (Sigma-Aldrich) was dissolved in 1% sterile ascorbic acid (VitC, pH 3.2) to achieve the desired dose of 0.75 mg/kg selected based on our previous studies in C57BL/6 mice (Singer et al., 2009). The selected dose of APO (0.75 mg/kg, i.p.) does not produce any floor effects on locomotor activity depression (Singer et al., 2009). This readily facilitates the assessment of modulatory effects of the prenatal immunological manipulation on APO-induced changes in locomotor activity. APO and vehicle (VitC) solutions were freshly prepared on the day of testing and administered via the subcutaneous route with an injection volume of 5 ml/kg.
For each animal, horizontal locomotor activity was measured automatically with the aid of the Ethovision tracking system. Stereotypic behaviors were scored manually by the analysis of video tapes and included the following four categories: climbing (defined as the event when the animal had all four feet off the bottom of the cage, i.e., when it was positioned on the cylinder wire mesh), rearing (defined as the event when the animal removed both forepaws from the floor of the cage and assumed a vertical or nearly vertical position), licking/grooming, and sniffing/gnawing. Behavioral scores were obtained every 3 min for 30 s. Hence, a total of 20 observations were equally distributed across the test period of 60 min. Scores (expressed in absolute time) obtained at each time point were added to attain a total score for each behavioral category. All scores were obtained and analyzed under blind conditions.
APO-induced locomotor activity and stereotypy was assessed in peripubertal (PND35) and adult (PND70) offspring born to Poly-I:C- or saline-treated mothers. Each experimental group on PND35 and PND70 consisted of offspring derived from multiple independent litters (at least six in each prenatal treatment condition) and included male and female subjects. Testing of peripubertal and adult offspring was conducted in two separate cohorts of drug-naive offspring to avoid repeated drug exposure and was performed in the dark phase of the light–dark cycle.
Prepulse inhibition of the acoustic startle reflex.
The prepulse inhibition (PPI) test was conducted using four startle chambers for mice (San Diego Instruments). The test apparatus has been fully described previously (Meyer et al., 2005). In the demonstration of PPI of the acoustic startle reflex (ASR), subjects were presented with a series of discrete trials comprising a mixture of four trial types. These included pulse-alone trials, prepulse-plus-pulse trials, prepulse-alone trials, and no-stimulus trials in which no discrete stimulus other than the constant background noise was presented. The pulse and prepulse stimuli used were in the form of a sudden elevation in broadband white noise level (sustaining for 40 and 20 ms, respectively) from the background (65 dBA), with a rise time of 0.2–1.0 ms. In all, three different intensities of pulse (100, 110, and 120 dBA) and three intensities of prepulse (71, 77, and 83 dBA, which corresponded to 6, 12 and 18 dBA above background, respectively) were used. The stimulus onset asynchrony of the prepulse and pulse stimuli on all prepulse-plus-pulse trials was 100 ms (onset to onset).
A session began with the animals being placed into the Plexiglas enclosure. They were acclimatized to the apparatus for 2 min before the first trial began. The first six trials consisted of six startle-alone trials, comprising two trials of each of the three possible pulse intensities. These trials served to habituate and stabilize the animals' startle response and were not included in the analysis. Subsequently, the animals were presented with 10 blocks of discrete test trials. Each block consisted of the following: three pulse-alone trials (100, 110, or 120 dBA), three prepulse-alone trials (+6, +12, or +18 dBA above background), nine possible combinations of prepulse-plus-pulse trials (3 levels of prepulse × 3 levels of prepulse), and one no-stimulus trial. The 16 discrete trials within each block were presented in a pseudorandom order, with a variable intertrial interval of a mean of 15 s (ranging from 10 to 20 s). For each of the three pulse intensities (100, 110, or 120 dBA), PPI was indexed by percentage inhibition of the startle response obtained in the pulse-alone trials by the following expression: 100% × (1 − [mean reactivity on prepulse-plus-pulse trials/mean reactivity on pulse-alone trials]), for each subject, and at each of the three possible prepulse intensities (+6, +12, or +18 dBA above background).
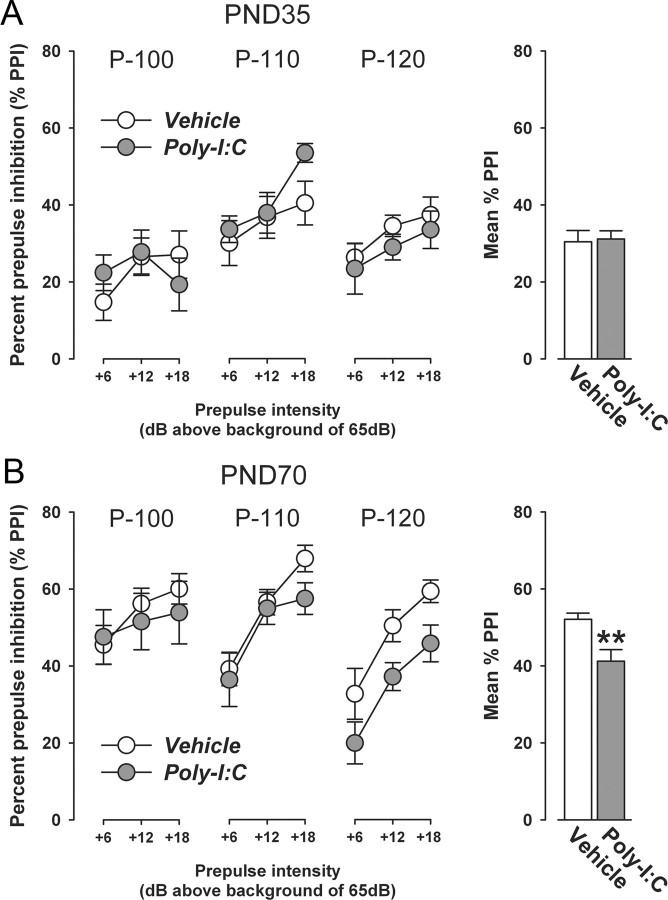
Testing of PPI was conducted in peripubertal (PND35) and adult (PND70) offspring born to Poly-I:C- or vehicle-treated mothers. Again, each experimental group on PND35 and PND70 consisted of offspring derived from multiple independent litters (at least six in each prenatal treatment condition) and included male and female subjects. PPI testing of peripubertal and adult subjects was conducted in two separate cohorts of experimentally naive offspring to avoid possible confounds arising from peripubertal testing. All PPI tests were performed in the dark phase of the light–dark cycle.
Drug-induced modification of prepulse inhibition.
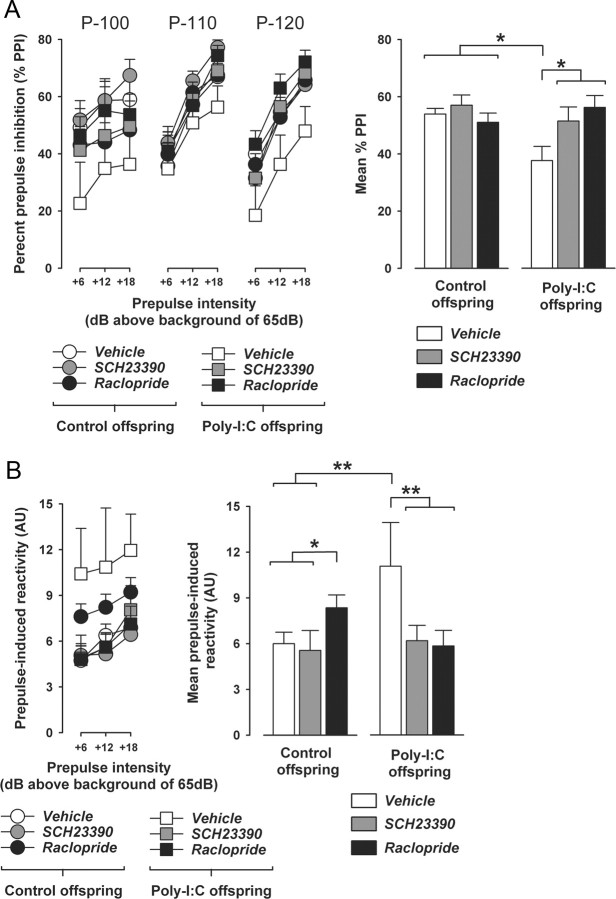
To gauge dose-dependent effects of selective pharmacological stimulation of D1R or D2R on PPI in adulthood, adult (PND70) male C57BL/6 mice without any prenatal manipulation were subjected to acute treatment with the selective D1R agonist SKF38393 at a dose of 0.3, 1, or 3 mg/kg, or with the selective D2R-like agonist quinpirole at a dose of 1, 5, or 10 mg/kg. SKF38393 hydrochloride (Sigma-Aldrich) and quinpirole hydrochloride (Sigma-Aldrich) were dissolved in isotonic 0.9% NaCl solution to obtain the desired concentrations and were injected intraperitoneally with an injection volume of 5 ml/kg 10 min before PPI testing. Control animals received corresponding vehicle (NaCl) pretreatment. A total of 64 mice (N = 8 per group) were used in these dose–response studies. Each mouse was tested only once so that repeated drug exposures were avoided. The apparatus and procedures used for the PPI test were identical to the ones described above.
Following the dose–response studies involving SKF38393 or quinpirole pretreatment, an additional cohort of experimentally naive offspring born to Poly-I:C- or vehicle-exposed mothers were then used for the evaluation of drug effects on PPI in adulthood (PND70). Both prenatal treatment groups consisted of offspring derived from multiple independent litters (at least six in each prenatal treatment condition) and included male and female subjects. We evaluated the effects of acute treatment with the preferential D1R antagonist SCH23390 or the preferential D2R receptor antagonist raclopride relative to corresponding vehicle treatment. Animals were treated with SCH23390 (1 mg/kg, s.c.), raclopride (3 mg/kg, i.p.), or vehicle (isotonic 0.9% NaCl solution) 10 min before commencement of the PPI test. The doses and pretreatment intervals of SCH23390 and raclopride were based on previous studies showing that these drugs are efficient in antagonizing the PPI-disrupting effects of acute APO treatment in C57BL/6 mice (Ralph-Williams et al., 2003) and in normalizing PPI deficits in DAT knock-out mice (Ralph et al., 2001), respectively. Half of the animals assigned to the vehicle group received subcutaneous injections of NaCl solution, and the other half received intraperitoneal injections to account for the different injection routes for SCH23390 (subcutaneous) and raclopride (intraperitoneal). The volume of injection was 5 ml/kg for all solutions. The apparatus and procedures used for the PPI test were identical to the ones described above.
Statistical analyses.
All data were analyzed using parametric ANOVA, followed by Fisher's least significant difference (LSD) post hoc comparisons or restricted ANOVAS whenever appropriate. Statistical significance was set at p < 0.05. All statistical analyses were performed using the statistical software StatView (version 5.0) implemented on a PC running the Windows XP operating system.
All immunohistochemical and stereological data were analyzed under blind conditions. For each dopaminergic marker and developmental stage investigated, preliminary statistical analyses were conducted to assess possible sex-dependent effects. These analyses provided no evidence for significant interactions between sex and prenatal treatment. Therefore, data from male and female offspring were collapsed to enhance statistical power. Stereological counts of TH- and Nurr1-positive cells in the SN and VTA regions of the midbrain were analyzed using 2 × 3 (prenatal treatment × developmental age) ANOVAs. Optical densitometric measures of TH, DAT, D1R, and D2R immunoreactivity in the CPu region of the striatum were also analyzed using 2 × 3 (prenatal treatment × developmental age) ANOVAs. Since NAc core and shell subregions were not readily distinguishable in fetal brain specimen, we analyzed the optical densitometric measures of TH, DAT, D1R, and D2R immunoreactivity in the ventral striatal regions separately for fetal and postnatal (peripubertal and adult) offspring. Hence, TH, DAT, D1R, and D2R immunoreactivities in the total fetal NAc region were analyzed using a one-way ANOVA with the between-subjects factor of prenatal treatment. TH, DAT, D1R, and D2R immunoreactivities in the NAc core and NAc shell of peripubertal and adult offspring were analyzed using 2 × 2 (prenatal treatment × postnatal age) ANOVAs.
In the test of AMPH sensitivity, locomotor activity (indexed by the distance traveled in the open field) was expressed as a function of 5 min bins and analyzed using a 2 × 4 (prenatal treatment × bins) repeated-measures ANOVA for the acclimatization and saline treatment phases, and by a 2 × 16 (prenatal treatment × bins) repeated-measures ANOVA for the AMPH treatment phase. Basal and amphetamine-induced activities were separately analyzed for peripubertal (PND35) and adult (PND70) offspring. In the test of APO sensitivity, locomotor activity (indexed by the horizontal distance traveled in wire mesh cylinder) was expressed as a function of 5 min bins and analyzed using a 2 × 2 × 12 (prenatal treatment × drug treatment × bins) repeated-measures ANOVA. Behavioral scores in the APO sensitivity test were analyzed by 2 × 2 (prenatal treatment × drug) ANOVAs. Locomotor and stereotypic measures obtained in the APO sensitivity test were separately analyzed for peripubertal (PND35) and adult (PND70) offspring. In the test of PPI, percentage PPI (%PPI) was analyzed using a 2 × 3 × 3 (prenatal treatment × prepulse level × pulse level) ANOVA, and startle reactivity to pulse-alone trials and prepulse-alone trials was analyzed using 2 × 3 (prenatal treatment × pulse level) and 2 × 3 (prenatal treatment × prepulse level) ANOVAs, respectively. The data obtained in the individual PPI tests were separately analyzed for peripubertal (PND35) and adult (PND70) offspring. The analysis of the effects of dopamine receptor antagonists (SCH23390, raclopride, or vehicle) on percentage PPI in adult (PND70) control or Poly-I:C offspring was conducted using a 2 × 3 × 3 × 3 (prenatal treatment × drug treatment × prepulse level × pulse level) ANOVA, and startle reactivity to pulse-alone trials and prepulse-alone trials was analyzed using 2 × 3 × 3 (prenatal treatment × drug treatment × pulse level) and 2 × 3 × 3 (prenatal treatment × drug treatment × prepulse level) ANOVAs, respectively. The analysis of the effects of dopamine receptor agonists (SKF38393, quinpirole, or vehicle) on percentage PPI in adult (PND70) C57BL/6 mice without any prenatal manipulation was conducted using 4 × 3 × 3 (drug treatment × prepulse level × pulse level) ANOVAs, and startle reactivity to pulse-alone trials and prepulse-alone trials was analyzed using 3 × 3 (drug treatment × pulse level) and 3 × 3 (drug treatment × prepulse level) ANOVAs, respectively. The data obtained in the SKF38393 and quinpirole experiments were separately analyzed.
Results
Prenatal immune activation induces age-dependent alterations in the number of midbrain dopamine cells
Disruption of structural and functional dopaminergic development may be at the core of the developmental neuropathology associated with prenatal exposure to infection (Meyer et al., 2008a; Meyer and Feldon, 2009). First, we tested this hypothesis by exploring whether maternal immune activation may affect the development of midbrain dopamine cells in the offspring. In the adult mammalian brain, the vast majority of midbrain dopaminergic cell bodies are located in discrete structures, namely, the SN and the VTA (Smidt and Burbach, 2007; Van den Heuvel and Pasterkamp, 2008). During development, dopamine cells are formed rostral to the mid-hindbrain border in the ventral aspect of the fetal mesencephalic flexure (Hynes and Rosenthal, 1999; Smidt and Burbach, 2007). The emergence of the dopamine phenotype in the fetal ventral midbrain in mice is characterized by the expression of TH starting at around GD11 (Bayer et al., 1995). TH is the rate-limiting enzyme of dopamine (and noradrenalin) synthesis in vivo and can therefore be used as a dopaminergic marker for the detection of mesencephalic dopamine cells (Bacopoulos and Bhatnagar, 1977). To evaluate whether prenatal immune activation may induce alterations in the number of mesencephalic dopamine cells, pregnant mice were subjected to a single exposure to the viral mimic Poly-I:C (5 mg/kg, i.v.) or corresponding vehicle (saline, i.v.) treatment in early/middle pregnancy (GD9). The brains of the resulting offspring were then collected when they reached the late fetal (GD19), peripubertal (PND 35) or adult (PND70) stage of development; and brain specimen were stained for TH by standard immunohistochemical procedures to visualize midbrain dopamine cells.
TH-positive dopamine neurons were clearly identifiable by the presence of darkly stained cells in the SN and VTA regions of fetal, peripubertal, and adult offspring (Fig. 1). Unbiased stereological estimation of TH-positive cells revealed a general age-dependent decrease in the number of dopamine cells in both midbrain regions (Fig. 1). Most importantly, Poly-I:C-induced prenatal immune challenge caused age-dependent alterations in the number of dopamine cells in both SN and VTA: the immunological manipulation led to a significant increase in TH-positive dopamine cells in the SN of fetal (GD19) and adult (PND70) offspring relative to control offspring (Fig. 1A), whereas it did not significantly alter the number of SN dopamine cells in peripubertal offspring (Fig. 1A). Similar to its effects on SN dopamine cells, prenatal immune activation also led to a significant increase in TH-positive dopamine cells in the VTA of adult (PND70) offspring compared with age-matched controls (Fig. 1B). However, prenatal immunological manipulation did not significantly influence the number of TH-positive cells in the VTA of fetal (GD19) or peripubertal (PND35) offspring relative to control treatment (Fig. 1B). These results demonstrate that maternal exposure to a viral-like acute phase response in early/middle pregnancy (GD9) in mice is sufficient to disrupt the normal development of dopaminergic structures in the offspring's midbrain.
Prenatal immune activation induces age-dependent changes in midbrain Nurr1 expression
The age-dependent nature of the changes in midbrain dopamine cell numbers observed in prenatally immune challenged offspring relative to age-matched controls may be related to infection-induced alterations in cellular and/or molecular factors that regulate the dopaminergic cell phenotype in midbrain regions. The orphan nuclear transcription factor Nurr1 is expressed in at least 95% of all TH-positive midbrain neurons from GD10-11 onwards and has been shown to critically control the expression of dopamine phenotypic markers such as TH (Smidt and Burbach, 2007). Hence, the age-dependent alterations of TH-positive cell numbers in the midbrain regions of prenatally immune challenged offspring may be mediated by infection-induced changes in Nurr1 expression. To seek evidence for this possibility, we analyzed the expression of Nurr1 protein in the VTA and SN of offspring derived from Poly-I:C- or vehicle-treated mothers at the fetal (GD19), peripubertal (PND35), and adult (PND70) stage of development.
Nurr1-positive cells were clearly visible in the selected midbrain regions (i.e., SN and VTA) at all three developmental stages examined (Fig. 2). In support of our hypothesis, the quantitative analysis of Nurr1-immunoreactive cells showed that prenatal immune activation led to age-dependent changes of Nurr1 protein expression in both midbrain areas (Fig. 2). Importantly, the prenatal Poly-I:C-induced changes in SN Nurr1 protein expression were highly consistent with the effects of the immunological manipulation on midbrain TH immunoreactivity: maternal Poly I:C exposure led to a significant increase in Nurr1-positive cells in the SN of fetal (GD19) and adult (PND70) offspring relative to control offspring (Fig. 2A), whereas it did not significantly alter the number of SN dopamine cells in peripubertal offspring (Fig. 2A). In addition, similar to its age-dependent effects on the number of TH-positive cells in the VTA (Fig. 1B), prenatal immune challenge led to a significant increase in Nurr1-positive cells in the VTA of adult (PND70) offspring compared with age-matched controls (Fig. 2B), while it did not significantly influence the number of Nurr1-immuoreactive cells in the VTA of fetal (GD19) or peripubertal (PND35) offspring relative to control treatment (Fig. 2B). Together, these data demonstrate that the age- and region-specific alterations in midbrain Nurr1 protein expression following prenatal Poly-I:C-induced immune activation stringently follow the age- and region-dependent changes in the number of TH-positive dopaminergic cells in the midbrain (Fig. 1).
Prenatal immune activation induces age-dependent changes in presynaptic dopaminergic markers in dorsal and ventral striatal regions
Alterations in the number of midbrain dopamine neurons may be closely linked to parallel disturbances in efferent dopaminergic midbrain pathways. Midbrain dopamine cells send prominent projections to dorsal and ventral parts of the striatum. In mice, dopaminergic cell bodies located in the SN project primarily to dorsal parts of the striatum (caudate–putamen, CPu), forming the nigrostriatal dopaminergic pathway (Van den Heuvel and Pasterkamp, 2008). On the other hand, ventral parts of the striatum (nucleus accumbens core and shell regions) receive prominent projections from dopaminergic cell bodies of the VTA, forming parts of the mesolimbic dopamine system (Van den Heuvel and Pasterkamp, 2008). Considering the notable effects of prenatal immune activation on maldevelopment of dopaminergic midbrain structures (Figs. 1, 2), we were interested to assess possible effects of the prenatal immunological insult on dopaminergic abnormalities in striatal regions.
First, we assessed the expression of the dopaminergic marker TH, which exhibits a presynaptic cellular localization in striatal regions. Pregnant mice were subjected to a single exposure to the viral mimic Poly-I:C (5 mg/kg, i.v.) or corresponding vehicle (saline, i.v.) treatment in early/middle pregnancy (GD9) as described before, and striatal TH immunoreactivity was analyzed in the brains of the resulting offspring at fetal (GD19), peripubertal (PND35) or adult (PND70) stage of development using optical densitometry. Striatal TH immunoreactivity was detected in brain specimen of all three developmental stages (Fig. 3) and was measured in the CPu and NAc core and shell subregions. In contrast to peripubertal and adult animals, NAc core and shell subregions were not readily distinguishable in fetal brain specimen. Therefore, the analyses of TH expression in the ventral striatum of fetuses were conducted by taking into account TH immunoreactivity in the complete NAc region. As can be seen in Figure 3A, maternal exposure to the viral mimic Poly-I:C significantly led to a small but significant increase in TH immunoreactivity in the fetal CPu compared with control fetal brains. Interestingly, however, the level of TH immunoreactivity was significantly reduced in the CPu of prenatally immune challenged offspring at peripubertal age relative to age-matched controls (Fig. 3A), and no significant difference in CPu TH immunoreactivity was present between adult offspring born to Poly-I:C- and vehicle-treated mothers (Fig. 3A). Prenatal immune activation also induced age-dependent changes in TH expression in ventral parts of the striatum: whereas TH immunoreactivity in both NAc core and shell was significantly reduced in peripubertal offspring born to immune-challenged mothers relative to age-matched controls (Fig. 3B), prenatal immune activation led to a significant increase in TH immunoreactivity in the two subregions of the ventral striatum at adult age in comparison with prenatal control treatment (Fig. 3B). The latter effects on adult TH immunoreactivity in the NAc are in full agreement with our own previous studies, which were conducted on perfused adult mouse brain tissue (Meyer et al., 2008b) and are also highly consistent with the immunohistochemical data obtained in other models of prenatal immune activation (Borrell et al., 2002; Romero et al., 2008). In contrast to its effects on peripubertal and adult TH density in the NAc, maternal immune activation did not significantly alter the expression of TH in the fetal NAc (Fig. 3B).
The prenatal infection-induced presynaptic dopaminergic abnormalities in striatal regions (as indexed by the age-dependent alterations of striatal TH expression) (Fig. 3) may be associated with accompanying changes in neuronal markers involved in the regulation of synaptic dopaminergic activity. Therefore, we also investigated the impact of prenatal immune challenge on the striatal expression of DAT, which is known to provide the primary mechanism through which dopamine is cleared from synapses in striatal regions (Masson et al., 1999). To this end, fetal (GD19), peripubertal (PND35), and adult (PND70) brain samples of offspring derived from Poly-I:C-exposed and control mothers were stained for DAT using immunohistochemistry. Again, DAT immunoreactivity was analyzed in dorsal and ventral regions of the striatum using optical densitometry. Striatal DAT immunoreactivity was detected in brain specimen of all three developmental stages (Fig. 4). However, DAT expression was low in fetal compared with postnatal (peripubertal and adult) brain samples (Fig. 4). Prenatal immune activation significantly affected striatal DAT immunoreactivity in a region- and age-dependent manner: Poly-I:C-exposed offspring displayed a small but significant decrease specifically in the ventral (i.e., NAc) but not dorsal (i.e., CPu) striatum at the fetal stage of development relative to control fetuses (Fig. 4). In addition, prenatal immune challenge led to a global reduction in striatal DAT expression when the offspring reached the peripubertal period of life in comparison with age-matched controls (Fig. 4). No significant differences were evident between striatal DAT immunoreactivity in adult offspring derived from immune-challenged and control mothers (Fig. 4).
Together, the analyses of striatal TH and DAT immunoreactivities highlight that in addition to its impact on the development of midbrain dopamine cells (Figs. 1, 2), maternal exposure to a viral-like acute phase response in early/middle pregnancy can lead to significant presynaptic dopaminergic abnormalities in the striatum, which are both region-specific and dependent on the precise stage of the offspring's development. Notably, similar to the ontogeny of dopaminergic maldevelopment in midbrain structures (Fig. 1), maternal immune challenge during pregnancy can induce dopamine-related striatal abnormalities starting already early in fetal life.
Prenatal immune activation induces a postpubertal onset of altered dopamine D1 and D2 receptor expression in dorsal and ventral striatal regions
In addition to the characterization of presynaptic dopaminergic markers in striatal structures (Figs. 3, 4), we were interested to explore whether prenatal immune challenge may also affect the developmental expression of striatal dopamine receptors. Here, we focused on two dopamine receptor subtypes known to be strongly expressed in striatal structures, namely the D1 and D2 dopamine receptor subtypes (D1R and D2R, respectively). Abnormal expression of these receptors has already been associated with the long-term neuropathological consequences of prenatal immune challenge (Meyer and Feldon, 2009). However, the developmental nature of dopamine receptor abnormalities following prenatal immune activation remains unknown. Therefore, we treated pregnant mice with Poly-I:C (5 mg/kg, i.v.) or corresponding vehicle (saline, i.v.) in early/middle pregnancy (GD9) as described before, and we analyzed striatal D1R and D2R expression in the brains of the resulting offspring at fetal (GD19), peripubertal (PND35) or adult (PND70) age using standard immunohistochemical procedures and optical densitometry.
D1R and D2R immunoreactivities were relatively low in fetal striatal areas compared with peripubertal and adult brain samples (Figs. 5, 6), and no significant group differences in D1R (Fig. 5) and D2R (Fig. 6) immunoreactivities were manifest at this early stage of development. Likewise, prenatal immune activation did not significantly affect striatal D1R (Fig. 5) or D2R expression (Fig. 6) in peripubertal offspring compared with prenatal control treatment. However, relative to control treatment, prenatal Poly-I:C-induced immune activation selectively increased striatal D1R and D2R immunoreactivities when the offspring reached the adult stage of development. More specifically, adult offspring born to immune-challenged mothers displayed a significant increase in D1R immunoreactivity in the CPu (Fig. 5A) and NAc shell (Fig. 5B) and a significant increase in D2R immunoreactivity specifically in the NAc shell (Fig. 6B) region of the striatum. Together, these findings clearly demonstrate that in contrast to the induction of presynaptic dopaminergic abnormalities in fetal and/or peripubertal life (Figs. 3, 4), prenatal immune activation induces a postpubertal onset of altered D1R and D2R expression in dorsal and ventral striatal regions. However, the direction of prenatal infection-induced changes in striatal D1R and D2R density appears to differ from the earlier findings by Ozawa and colleagues (2006) showing that subchronic administration of Poly-I:C from middle to late gestation (i.e., from GD12 to GD17) leads to decreased (rather than increased) D2R receptor binding in striatal regions. As extensively discussed previously (see Meyer et al., 2006, 2007, 2009a,b), these discrepant outcomes of prenatal Poly-I:C-induced immune activation on striatal dopamine receptor expression are likely to be accounted for by differences in the precise prenatal timing of the immunological insult.
Confirmation of the adult dopaminergic phenotype in perfused brain specimen
Since our original longitudinal investigation of prenatal immune activation effects on dopaminergic development from fetal to the adult stage of life was performed on nonperfused brain tissue, we were interested to confirm the prenatal Poly-I:C-induced neuroanatomical alterations using perfused brain samples. For this purpose, we focused on the dopaminergic phenotypes observed in adult (PND70) Poly-I:C relative to control offspring because this developmental stage corresponds to our endpoint phenotype. Pregnant mice were subjected to a single exposure to the viral mimic Poly-I:C (5 mg/kg, i.v.) or corresponding vehicle (saline, i.v.) treatment in early/middle pregnancy (GD9) as described before, and immunoreactivities of TH, DAT, D1R, and D2R were assessed in the adult offspring according to standard procedures using perfused brain samples.
As expected, the background levels of the dopaminergic immunostainings were generally lower in perfused brain sections (Fig. 7) compared with nonperfused brain sections (Figs. 1–6) of adult PolyI:C and control mice; and consequently, the optical densitometric measures of TH, DAT, D1R, and D2R in striatal regions were generally higher in perfused (Fig. 7) relative to nonperfused (Figs. 1–6) adult brains. Most importantly, however, the additional investigation of prenatal immune activation effects on dopaminergic markers in perfused adult brain samples confirmed the outcomes previously revealed in nonperfused fresh brain samples. Specifically, a significant increase in TH and D2R densities in the ventral (i.e., NAc core and shell) but not dorsal (i.e., CPu) striatum was noticeable in adult Poly-I:C offspring relative to age-matched controls (Fig. 7B,D). Furthermore, our analysis of striatal D1R immunoreactivity in perfused adult subjects also confirmed the significant effects of prenatal PolyI:C exposure on enhancement of D1R densities in the CPu and NAc shell regions (Fig. 7C). Consistent with the results obtained in the analysis of nonperfused adult brain samples, prenatal immune challenge did not significantly affect DAT density in dorsal and ventral striatal regions (data not shown). Finally, unbiased stereological estimation of midbrain dopamine cells in perfused brains samples also confirmed the significant increase in TH-positive cells in the SN and VTA regions of adult Poly-I:C offspring compared with adult control offspring (Fig. 7A). It is also important to point out that the magnitude of the various dopaminergic changes revealed in perfused brains of adult Poly-I:C offspring (10–20% difference to control levels) is highly consistent with the magnitude of prenatal Poly-I:C-induced changes observed in nonperfused brain samples. Together, the successful replication of the adult dopaminergic phenotype in perfused brain specimen (Fig. 7) readily excludes the possibility that the methods of tissue processing chosen for our original longitudinal investigation (Figs. 1–6) might have undermined a sensitive assessment of prenatal immune activation effects on fetal to adult dopaminergic development.
Differential ontogeny of altered sensitivity to direct and indirect dopamine receptor agonist treatment following prenatal immune activation
The differential ontogeny of prenatal infection-induced alterations in presynaptic and receptor-associated dopaminergic markers may critically influence the postnatal onset of dopamine-related functional impairments. To test this hypothesis, we conducted pharmacological and behavioral analyses in peripubertal (PND35) and adult (PND70) offspring born to mothers subjected to Poly-I:C or control treatment during pregnancy.
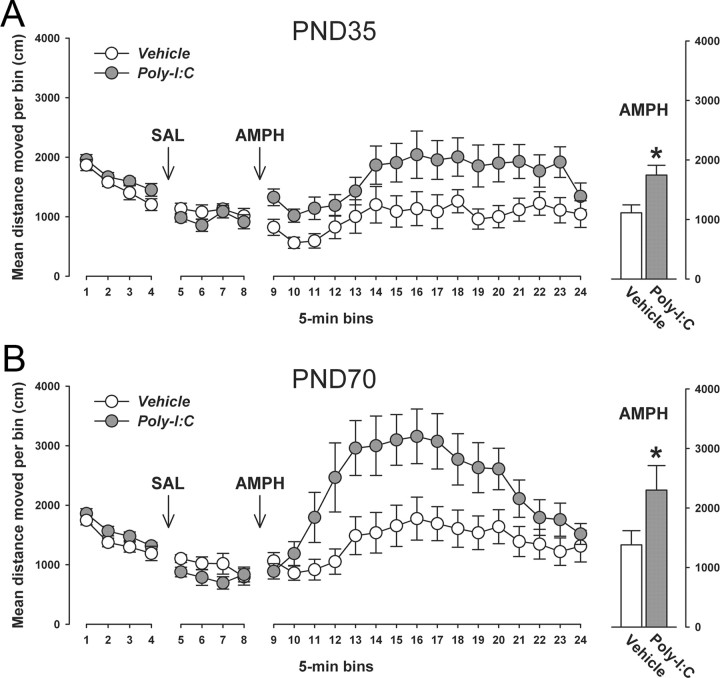
First, we explored the behavioral sensitivity of prenatally immune-challenged and control offspring to acute treatment with a low dose of the indirect dopamine receptor agonist AMPH. The neurochemical and behavioral effects of AMPH are primarily accounted for by stimulation of endogenous presynaptic dopamine release upon synaptic uptake mediated by DAT (Giros et al., 1996; Salahpour et al., 2008). The behavioral sensitivity to acute treatment with a low dose of AMPH (2.5 mg/kg, i.p.) was assessed in terms of measuring the drug's effect on enhancing locomotor activity in the open field. In this procedure, the animals were first habituated to the open field arena and then treated with saline solution only to measure basal locomotor activity. For both postnatal ages investigated (i.e., peripubertal and adult age), prenatal Poly-I:C-induced immune activation did neither significantly alter locomotor activity in the initial acclimatization period nor following saline treatment (Fig. 8). Subsequent to the saline phase, all animals were briefly removed from the open field, injected with AMPH, and immediately placed back to the same apparatus to monitor the locomotor response to the acute drug challenge. Figure 8 shows that prenatal immune activation led to a significant potentiation of the locomotor-enhancing effects of acute AMPH treatment. Importantly, this effect was clearly independent of the postnatal developmental stage of the animals (Fig. 8). Hence, both peripubertal (Fig. 8A) and adult (Fig. 8B) offspring born to Poly-I:C-treated mothers displayed a significant enhancement of the locomotor-stimulating effects of AMPH compared with age-matched control offspring.
Figure 8.
Prenatal immune activation induces enhancement of the behavioral sensitivity to acute amphetamine treatment regardless of the offspring's postnatal age. The behavioral sensitivity of prenatally Poly-I:C- or vehicle-treated offspring to a low dose of AMPH (2.5 mg/kg, i.p.) was assessed in an open field arena when they reached the peripubertal (PND35) or adult (PND70) stage of development. The line plots show locomotor activity as indexed by the total distance traveled in the entire open field across bins of 5 min during the initial acclimatization period as well as following saline (SAL) and AMPH administration; the bar plots represent the mean distance moved across the entire 80 min period following AMPH administration. A, No significant differences in basal locomotor activity (as measured during the initial acclimatization period and following SAL administration) were noticeable between Poly-I:C and control offspring at peripubertal age. Acute treatment with the low dose of AMPH led to a modest increase in locomotor activity in peripubertal offspring born to control mothers. The locomotor-enhancing effects of AMPH were significantly increased in peripubertal offspring born to Poly-I:C-treated mothers. *p < 0.05 represents the significant main effect of prenatal treatment in the 2 × 16 (prenatal treatment × bins) repeated-measures ANOVA of distance moved following AMPH treatment (F(1,25) = 4.27). The numbers of offspring included were N(vehicle) = 14, N(Poly-I:C) = 13. B, There were also no significant differences in basal locomotor activity between Poly-I:C and control offspring at the adult stage of life. Acute treatment with the low dose of AMPH led to marked increase in locomotor activity in peripubertal offspring born to control mothers. Again, the locomotor-stimulating effects of AMPH were significantly enhanced in adult offspring born to Poly-I:C-treated mothers. *p < 0.05 represents the significant main effect of prenatal treatment in the 2 × 16 (prenatal treatment × bins) repeated-measures ANOVA of distance moved following AMPH treatment (F(1,20) = 5.36). The numbers of offspring included were N(vehicle) = 10, N(Poly-I:C) = 12. All values in A and B are means ± SEM.
In the next step, we explored the effects of prenatal Poly-I:C-induced immune challenge on the sensitivity to direct pharmacological stimulation of dopamine receptors. In view of the age-dependent effects of the prenatal immunological manipulation on striatal D1R and D2R expression (Figs. 5–7), we hypothesized that offspring born to Poly-I:C-exposed mothers would display age-dependent alterations in the behavioral sensitivity to the mixed D1R/D2R agonist APO. Therefore, we treated peripubertal (PND35) or adult (PND70) PolyI:C and control offspring with a single dose of APO (0.75 mg/kg) or corresponding vehicle solution (VitC) and analyzed several behavioral measures following the acute pharmacological D1R/D2R stimulation, including horizontal locomotor activity and presence of stereotypic behaviors such as climbing, rearing, licking/grooming and sniffing/gnawing. Acute APO treatment similarly depressed horizontal locomotor activity in peripubertal (PND35) Poly-I:C and control offspring relative to corresponding vehicle (VitC) treatment (Fig. 9A). These effects of acute APO treatment are in full agreement with the known locomotor-depressing effects of low doses of APO in mice, which have been attributed to stimulation of presynaptic D2R-like auto-receptors exerting a negative feedback control over dopamine release (Singer et al., 2009 and references therein). Acute APO administration also markedly depressed horizontal locomotor activity in adult (PND70) offspring born to control mothers (Fig. 9B). Most interestingly, however, the horizontal locomotor activity pattern in APO-treated adult Poly-I:C offspring significantly differed from the pattern observed in APO-treated adult control offspring (Fig. 9B). In contrast to the persistent decrease in locomotor activity revealed in adult control offspring following acute APO treatment, horizontal locomotor activity in APO-treated adult Poly-I:C offspring was characterized by a transient decrease (evident only during the early post-treatment interval) and a subsequent increase (Fig. 9B). The latter effect (i.e., secondary increase in locomotor activity) was most pronounced between 15 and 30 min following APO treatment and is likely to represent enhanced signaling at postsynaptic dopamine receptors (Singer et al., 2009 and references therein).
Figure 9.
Postpubertal emergence of altered behavioral sensitivity to acute apomorphine treatment following prenatal immune activation. Locomotor activity was assessed in peripubertal (PND35) and adult (PND70) Poly-I:C and control offspring following acute treatment with a low dose of APO (0.75 mg/kg, s.c.) or vehicle (vitamin C solution, VitC) in a sawdust embedded wire-mesh cylinder. The line plots show horizontal locomotor activity as indexed by the total distance traveled in the test apparatus across bins of 5 min; the bar plots represent the mean distance moved across the entire 60 min period. A, APO treatment similarly depressed horizontal locomotor activity in peripubertal (PND35) Poly-I:C and control offspring relative to corresponding vehicle (VitC) treatment. **p < 0.01 represents the significant main effect of drug treatment in the 2 × 2 × 12 (prenatal treatment × drug treatment × bins) repeated-measures ANOVA of horizontal distance moved (F(1,29) = 8.82). The numbers of offspring included were N(VitC-treated vehicle offspring) = 7, N(APO-treated vehicle offspring) = 10, N(VitC-treated Poly-I:C offspring) = 7, N(APO-treated Poly-I:C offspring) = 9. B, In contrast to the persistent decrease in locomotor activity revealed in adult control (vehicle) offspring following acute APO treatment, horizontal locomotor activity in APO-treated adult Poly-I:C offspring was characterized by an initial decrease and a subsequent increase. The secondary increase in locomotor activity observed in APO-treated adult Poly-I:C offspring was most pronounced between 15 and 30 min following APO treatment and led to a significant attenuation of the APO-induced depression of locomotor activity typically seen in APO-treated adult control offspring. **p < 0.01, based on Fisher's LSD post hoc group comparisons following the presence of a significant (F(1,37) = 6.25, p < 0.05) prenatal treatment × drug treatment interaction in the initial 2 × 2 × 12 (prenatal treatment × drug treatment × bins) repeated-measures ANOVA of horizontal distance moved. The numbers of offspring included were N(VitC-treated vehicle offspring) = 9, N(APO-treated vehicle offspring) = 12, N(VitC-treated Poly-I:C offspring) = 8, N(APO-treated Poly-I:C offspring) = 12. All values in A and B are means ± SEM.
The quantitative analysis of stereotypic behaviors following acute APO treatment revealed no significant differences between peripubertal (PND35) or adult (PND70) PolyI:C and control offspring, suggesting that the prenatal immunological manipulation did not significantly influence the stereotypic responses to systemic APO treatment (data not shown). In both peripubertal and adult subjects, the drug treatment significantly increased and decreased licking/grooming and sniffing/gnawing behavior, respectively, regardless of the prenatal treatment histories. At PND35, the overall mean ± SEM time (seconds) of licking/grooming was 72.34 ± 6.88 and 41.20 ± 5.52 in APO- and vehicle-treated subjects, respectively (main effect of drug treatment: F(1,29) = 6.23, p < 0.05); and the overall mean ± SEM time (seconds) of sniffing/gnawing was 109.16 ± 8.71 and 133.56 ± 6.12 in peripubertal APO- and vehicle-treated subjects, respectively (main effect of drug treatment: F(1,29) = 4.69, p < 0.05). At PND70, the overall mean ± SEM time (seconds) of licking/grooming was 92.86 ± 9.93 and 53.77 ± 8.09 in APO- and vehicle-treated subjects, respectively (main effect of drug treatment: F(1,37) = 8.12, p < 0.01); and the overall mean ± SEM time (seconds) of sniffing/gnawing was 147.25 ± 9.59 and 176.94 ± 10.05 in peripubertal APO- and vehicle-treated subjects, respectively (main effect of drug treatment: F(1,37) = 4.17, p < 0.05).
Together, the AMPH and APO sensitivity tests conducted in peripubertal and adult offspring born to immune-challenged or control mothers demonstrates a differential ontogeny of altered sensitivity to direct and indirect dopamine receptor agonist treatment following prenatal immune activation. While prenatal Poly-I:C exposure leads to AMPH hypersensitivity both in per-puberty and adulthood, the effects of prenatal immune activation on altered sensitivity to APO treatment show a developmental delay and thus only emerge in adulthood. In support of our hypothesis, the latter effect thus shows a remarkable developmental correspondence with the postpubertal onset of enhanced striatal D1R and D2R density following prenatal immune challenge.
Postpubertal onset of sensorimotor gating deficiency following prenatal immune activation
We extended the functional investigations of dopamine-dependent behaviors to a test of sensorimotor gating. Sensorimotor gating was assessed using the paradigm of PPI of the ASR. PPI of the ASR refers to the reduction of startle reactivity to a loud startle-eliciting auditory stimulus (pulse) when this is shortly preceded by a weak acoustic stimulus (prepulse). PPI is known to be sensitive to experimental manipulations affecting (striatal) D1R and D2R activities (Ralph et al., 2001; Ralph-Williams et al., 2003). Therefore, one expectation from the results obtained in our longitudinal immunohistochemical analyses is that the postpubertal onset of altered striatal D1R and D2R expression following prenatal immune activation would be associated with the postpubertal onset of PPI abnormalities. The assessment of PPI in peripubertal and adult offspring born to Poly-I:C- or vehicle-treated mothers confirmed this prediction: prenatal immune challenge led to a significant attenuation of PPI specifically in adult offspring relative to prenatal control treatment (Fig. 10B), while it did not influence PPI at the periadolescent stage of development (Fig. 10A). Prenatal Poly-I:C exposure did also not significantly influence the startle reactivity as such (i.e., the reactivity to pulse-alone stimuli) or prepulse-elicited reactivity (i.e., the reactivity to prepulse-alone stimuli). The overall mean ± SEM startle reactivity (in arbitrary units) to pulse-alone stimuli was 13.03 ± 1.15 (100 dBA), 48.10 ± 3.34 (110 dBA) and 84.25 ± 5.36 (120 dBA) at the peripubertal stage of life, and 18.78 ± 2.90 (100 dBA), 52.48 ± 8.08 (110 dBA) and 90.36 ± 13.94 (120 dBA) at the adult stage of life. The overall mean ± SEM startle reactivity (in arbitrary units) to the prepulse-alone presentations was 5.33 ± 1.25 (71 dBA), 6.11 ± 0.93 (77 dBA), and 7.20 ± 1.55 (83 dBA) in preadolescence, and 6.76 ± 0.50 (71 dBA), 7.10 ± 0.58 (77 dBA), and 8.06 ± 0.61 (83 dBA) in adulthood.
Figure 10.
Postpubertal emergence of sensorimotor gating deficits following prenatal immune activation. The effects of prenatal Poly-I:C-induced immune challenge, relative to prenatal vehicle treatment, on sensorimotor gating in peripuberty (PND35) and adulthood (PND70) were investigated using the paradigm of PPI of the acoustic startle reflex. The line plots show %PPI as a function of the three intensities of prepulse (71, 77, and 83 dBA, which corresponded to 6, 12, and 18 dBA above background white noise, respectively) and of the three intensities of pulse (P-100, P-110, and P-120, which corresponded to pulse intensities of 100, 110, and 120 dBA, respectively). The bar plots depict mean %PPI across all prepulse and pulse levels used. A, No significant differences in PPI were apparent between peripubertal offspring born to Poly-I:C- and vehicle-treated mothers. The numbers of offspring included were N(vehicle) = 14, N(Poly-I:C) = 14. B, However, a significant overall reduction in %PPI emerged in prenatally Poly-I:C-treated offspring at adult age relative to adult control offspring. The PPI-disrupting effects of prenatal Poly-I:C exposure seemed most pronounced in conditions, in which the highest pulse stimulus (P-120) was used. **p < 0.01 represents the significant main effect of prenatal treatment in the 2 × 3 × 3 (prenatal treatment × prepulse level × pulse level) ANOVA of %PPI (F(1,26) = 11.01). The numbers of offspring included were N(vehicle) = 15, N(Poly-I:C) = 13. All values in A and B are means ± SEM.
Normalization of prenatal infection-induced PPI deficits by acute treatment with selective D1R and D2R antagonists
The developmental correspondence between the postpubertal onset of enhanced D1R and D2R expression in the striatum and disruption of PPI in prenatally immune-challenged offspring may be suggestive of a causal relationship, in which enhanced signaling at D1R and/or D2R may disrupt PPI in adult offspring born to immune-challenged mothers. We were interested to test the feasibility of this potential causal relationship. First, we confirmed the efficacy of enhanced stimulation of D1R or D2R to disrupt sensorimotor gating as assessed in our 3 × 3 (prepulse × pulse) parametric design of PPI. For this purpose, we adopted a dose–response study in which we subjected adult (PND70) C57BL/6 mice without any prenatal manipulation to acute treatment with the selective D1R agonist SKF38393 at a dose of 0 (=vehicle), 0.3, 1, or 3 mg/kg, or with the D2R-like agonist quinpirole at a dose of 0 (=vehicle), 1, 5, or 10 mg/kg. Acute treatment with the selective D1R agonist SKF38393 led to a dose-dependent attenuation of PPI: the highest dose of SKF38393 (3 mg/kg) significantly reduced %PPI relative to corresponding vehicle treatment, and this effect was noticeable across all pulse levels investigated (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). SKF38393 treatment did not significantly affect prepulse-elicited or pulse-elicited startle reactivity (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). Administration of the D2R-like agonist quinpirole also significantly attenuated %PPI. However, the efficacy of quinpirole to disrupt PPI clearly depended on the intensity of the pulse stimulus used: while only the highest dose (10 mg/kg) of quinpirole significantly reduced %PPI in trials, in which the lowest pulse (100 dBA) served as the pulse stimulus, quinpirole at all three doses (1, 5, or 10 mg/kg) were efficient in reducing %PPI in conditions in which the middle pulse intensity (110 dBA) was used (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). On the other hand, none of the three selected doses were capable of significantly affecting the expression of %PPI in conditions in which the highest pulse (120 dBA) served as the pulse stimulus (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). Interestingly, quinpirole treatment also markedly increased prepulse-elicited startle reactivity while sparing pulse-elicited startle reactivity (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). The effects of quinpirole on prepulse-elicited startle reactivity are highly similar to those observed following systemic APO treatment in C57BL/6 mice (Yee et al., 2004) and highlight that manipulations especially targeting D2Rs exert effects on sensorimotor gating beyond those captured in the expression of %PPI.
Hence, our dose–response studies of the effects of selective D1R and D2R-like agonists confirmed the sensitivity of our parametric PPI design for the assessment of the PPI-disrupting effects of enhanced D1R and D2R stimulation. Against this background, we went on to explore the hypothesis of causality in the association between enhanced striatal D1R and D2R expression and emergence of PPI deficiency in adult offspring born to immune-challenged mothers. This was achieved by investigating whether acute treatment with the selective D1R antagonist SCH23390 (1 mg/kg, s.c.) or with the selective D2R receptor antagonist raclopride (3 mg/kg, i.p.) before the testing would be effective in normalizing the prenatal infection-induced PPI deficits. SCH23390 and raclopride at the selected doses have been shown previously to be able to antagonize the PPI-disrupting effects of acute APO treatment in C57BL/6 mice (Ralph-Williams et al., 2003) and to normalize PPI deficits in DAT knock-out mice (Ralph et al., 2001), respectively. In support of a causal relationship, both pretreatment with SCH23390 and raclopride significantly enhanced PPI scores in adult prenatally Poly-I:C-treated offspring to levels comparable to those measured in vehicle-treated control offspring (Fig. 11A). Neither SCH23390 nor raclopride treatment significantly influenced PPI in adult offspring born to control mothers. Hence, the beneficial effects of the two dopamine receptor antagonists on PPI dysfunctions in prenatally immune-challenged offspring were not accounted for by a general enhancement of PPI, but rather to selective effects in subjects predisposed to adult brain pathology by the prenatal immunological insult.
Figure 11.
Effects of pharmacological dopamine D1 or D2 receptor blockade on prepulse inhibition and prepulse-induced startle reactivity in adult offspring born to immune-challenged or control mothers. The line plots show %PPI as a function of the three intensities of prepulse (71, 77, and 83 dBA, which corresponded to 6, 12, and 18 dBA above background white noise, respectively) and of the three intensities of pulse (P-100, P-110, and P-120, which corresponded to pulse intensities of 100, 110, and 120 dBA, respectively). The bar plots present mean %PPI across all prepulse and pulse levels used. A, The prenatal Poly-I:C-induced impairment in PPI was normalized by acute administration of the preferential dopamine D1 receptor antagonist SCH23390 (1 mg/kg, s.c.) or by the preferential dopamine D2 receptor antagonist raclopride (3 mg/kg, i.p.). *p < 0.05, based on Fisher's LSD post hoc group comparisons following the presence of a significant (F(2,55) = 4.55, p < 0.05) prenatal treatment × drug treatment interaction in the initial 2 × 3 × 3 × 3 (prenatal treatment × drug treatment × prepulse level × pulse level) ANOVA. B, Vehicle-treated offspring born to Poly-I:C-exposed mothers displayed a significant enhancement in prepulse-induced startle reactivity [in arbitrary units (AU)] compared with vehicle-treated offspring born to control mothers. This effect of the prenatal Poly-I:C challenge was normalized by acute administration of SCH23390 (1 mg/kg, s.c.) or raclopride (3 mg/kg, i.p.). On the other hand, acute raclopride treatment significantly increased prepulse-induced startle reactivity in offspring born to control mothers. *p < 0.05 and **p < 0.01, based on Fisher's LSD post hoc group comparisons following the presence of a significant (F(2,177) = 19.73, p < 0.001) prenatal treatment × drug treatment interaction in the initial 2 × 3 × 3 (prenatal treatment × drug treatment × prepulse level) ANOVA. The numbers of offspring included were N(control offspring/vehicle) = 10, N(control offspring/SCH23390) = 10, N(control offspring/raclopride) = 10, N(Poly-I:C offspring/vehicle) = 11, N(Poly-I:C offspring/SCH23390) = 10, N(Poly-I:C offspring/raclopride) = 10. All values in A and B are means ± SEM.
In agreement with our previous results, prenatal Poly-I:C exposure did not significantly influence the startle reactivity as such, that is, there were no significant differences between vehicle-treated offspring born to Poly-I:C-exposed and control mothers (data not shown). Furthermore, pretreatment with SCH23390 or raclopride did not significantly affect startle reactivity (data not shown). However, a significant increase in prepulse-elicited startle reactivity was noticed in vehicle-treated offspring born to Poly-I:C-challenged mothers relative to vehicle-treated offspring born to control mothers (Fig. 11B). Given the equivocal effects of prenatal Poly-I:C exposure on prepulse-elicited startle reactivity, it appears that the prenatal immunological manipulation may not induce robust effects on this measure. Rather, differences in prepulse-elicited startle reactivity following prenatal immune activation may only be unmasked by additional exposure to (mild) stress such as injections. However, it should be noted that the effects of prenatal Poly-I:C exposure on prepulse-elicited startle reactivity (as revealed in the pharmacological profiling of PPI in adult Poly-I:C and control subjects) are strikingly similar to the efficacy of pharmacological stimulation of D1R/D2R signaling by acute APO treatment to enhance prepulse-elicited startle reactivity (Yee et al., 2004). In view of the significant increases of striatal D1R and D2R densities in adult Poly-I:C offspring (Figs. 5–7), one implication would be that the enhancement of prepulse-elicited startle reactivity following prenatal immune activation may be also related to increased signaling at striatal D1R and D2Rs. In support of this hypothesis, acute administration of SCH23390 or raclopride significantly reduced the prenatal Poly-I:C-induced enhancement of prepulse-elicited startle reactivity (Fig. 11B). At the same time, however, pretreatment with raclopride in control offspring significantly increased prepulse-elicited startle reactivity compared with the levels of prepulse-elicited startle reactivity measured in vehicle-treated offspring born to control mothers. This indicated that raclopride exerted opposing effects on prepulse-elicited startle reactivity depending on whether basal prepulse-elicited startle reactivity is low (i.e., in vehicle-treated offspring born to control mothers) or high (i.e., in vehicle-treated offspring born to Poly-I:C-exposed mothers).
Discussion
The present study provides a systematic ontogenetic assessment of the structural and functional dopaminergic changes across early fetal to adult stages of development following prenatal immune activation. Our findings demonstrate that exposure to a viral-like acute phase response during early/middle gestation in mice leads to a complex pattern of age-dependent structural abnormalities in the mesoaccumbal and nigrostriatal dopamine systems. Importantly, we show that maternal immune activation during pregnancy compromises the integrity of the developing fetal dopamine system, suggesting that prenatal exposure to immunological insults induces primary defects in dopaminergic development.
Our study also emphasizes the critical influence of the offspring's precise developmental stage in the causal relationship between prenatal immune activation and emergence of dopaminergic neuropathology. Indeed, the magnitude and/or direction of the prenatal infection-induced neuropathological changes significantly differ between distinct prenatal and postnatal developmental stages. This suggests that prenatal immune activation does not induce static effects on dopaminergic development. Rather, the prenatal immunological insult induces early (fetal) abnormalities in specific dopaminergic systems, which then may interact with maturational processes to precipitate long-term dopaminergic abnormalities that are dependent on the actual stage of postnatal development. In agreement with this hypothesis, some of the effects of prenatal immune activation on dopaminergic structures appear to be progressive in nature and only emerge at the adult stage of development. One particular example of such a pathological progression is the increase in striatal D1R and D2R density following prenatal immune activation. On the other hand, some of the structural dopaminergic effects (e.g., altered striatal DAT expression) appear to be transient, with abnormalities existing only at specific stages of early (fetal and/or early postnatal) development. Finally, prenatal immune activation can also induce a biphasic pattern of dopaminergic abnormalities. The most compelling evidence for such an effect was obtained in the analyses of striatal TH density in fetal, peripubertal, and adult brain samples. For example, prenatal immune activation led to an initial decrease and final increase in accumbal TH density at peripubertal and adult stages of life, respectively, relative to age-matched control offspring. This biphasic effect is in full agreement with a recent neurochemical investigation of the effects of prenatal immune challenge in rats: using a model of prenatal immune activation by the bacterial endotoxin LPS, Romero et al. (2008) have recently shown that striatal dopamine levels are significantly decreased in LPS-exposed offspring at prepubertal age relative to age-matched control offspring, while dopamine levels are significantly enhanced in the striatum of LPS-treated offspring at adult age.
The development of the midbrain dopamine system is controlled by multiple cellular and molecular mechanisms (Smidt and Burbach, 2007). The transcription factor Nurr1 is known to be crucial for the induction and expression of the dopaminergic cell phenotype particularly in midbrain regions (Smidt and Burbach, 2007). For example, genetically induced Nurr1 deficiency leads to reduced TH expression in midbrain dopamine cells (Zetterström et al., 1997), suggesting that the expression of TH is critically controlled by Nurr1. Here, we found that for each specific developmental stage and midbrain region, the prenatal infection-induced increases in TH-positive cells were accompanied by a parallel enhancement of Nurr1 protein expression. This highlights that infection-induced changes in Nurr1 expression may have significantly contributed to altered dopaminergic development in general, and to enhanced expression of TH in midbrain regions in particular. However, the age-dependent nature of altered Nurr1 and TH expression suggests that additional factors acting upstream of Nurr1 may be involved in this association. Since prenatal immune challenge led to significant alterations in Nurr1 and TH expression specifically at the fetal and adult, but not at the peripubertal stage of development, such additional factors may involve cellular and/or molecular mechanisms controlling peripubertal maturation. Recent experimental evidence indicates that the protein Kisspeptin (Kiss1) may be involved in the peripubertal control of abnormal brain functions following prenatal immune challenge (Cardon et al., 2009). Future studies will thus be required to evaluate whether peripubertal alterations in Kiss1 may provide a satisfactory explanation for the age-dependent changes in Nurr1 and TH expression described here.
Even though some of the identified effects of prenatal Poly-I:C-induced immune challenge on dopaminergic markers in fetal, peripubertal, and adult offspring appear to be relatively small, they are likely to have a direct impact on dopamine-related brain functions. Indeed, the emergence of age-dependent alterations in dopamine-related brain structures seems to critically influence the ontogeny of specific dopamine-related behavioral and pharmacological abnormalities. Consistent with this suggestion, we found a striking developmental correspondence between the onset of enhanced striatal D1R/D2R expression and abnormalities in the behavioral sensitivity to the mixed D1R/D2R agonist APO as well as disruption of PPI in prenatally immune-challenged offspring, with both the neuroanatomical and behavioral effects emerging only in adult but not peripubertal subjects. The feasibility of a causal relationship between the postpubertal onset of enhanced D1R and D2R expression and PPI disruption was further supported by our findings that the prenatal Poly-I:C-induced PPI deficits can be normalized by acute treatment with the preferential D1R antagonist SCH23390 or the preferential D2R receptor antagonist raclopride. These beneficial effects of SCH23390 and raclopride corroborate previous studies in rats and mice showing that PPI deficiency following prenatal exposure to influenza virus (Shi et al., 2003) or the bacterial endotoxin LPS (Borrell et al., 2002) can be normalized by acute treatment with both typical and atypical antipsychotic drugs. However, since the pharmacological profiles of typical and atypical antipsychotic drugs include antagonism of multiple dopaminergic, serotonergic and noradrenergic receptors (Nestler et al., 2001), the precise contribution of dopaminergic mechanisms to the beneficial effects of anti-psychotic drugs on prenatal infection-induced PPI deficits remained elusive this far. The present results thus provide an important extension to these earlier findings by highlighting that selective D1R or D2R antagonism is sufficient for the normalization of PPI deficits following prenatal immune activation. A limitation of the present study is that we did not assess the potentials of selective dopamine receptor antagonists to correct the prenatal infection-induced alterations in APO sensitivity. This should be addressed in future studies to further consolidate the potential causal relationship between postpubertal onset of enhanced striatal D1R/D2R expression and abnormal behavioral sensitivity to the mixed D1R/D2R agonist APO.
In contrast to the postpubertal emergence of PPI impairment and altered locomotor response to systemic APO treatment, enhanced sensitivity to the indirect dopamine receptor agonist AMPH was manifest in prenatally immune-challenged offspring already at the peripubertal stage of development and persisted into adulthood. This effect is in line with previous reports in rats and mice showing that prenatal immune challenge by Poly-I:C in early/middle gestation (Meyer et al., 2008b), but not middle to late gestational periods (Zuckerman et al., 2003; Ozawa et al., 2006), can lead to a peripubertal AMPH hypersensitivity that precedes the later emergence of other behavioral, cognitive and pharmacological abnormalities in adult life. Together, these findings demonstrate that prenatal immune challenge during early fetal development can lead to the emergence of specific dopamine-related functional abnormalities during periadolescent brain maturation long before the onset of the full spectrum of functional abnormalities in adulthood.
When focusing on the long-term dopaminergic effects emerging in adulthood, the prenatal Poly-I:C-induced changes resemble some of the critical dopamine-related abnormalities implicated in schizophrenia, including enhanced striatal TH (Toru et al., 1988; Roberts et al., 2009) and D2R (Laruelle, 1998) densities, potentiated sensitivity to acute treatment with AMPH (Laruelle et al., 1999), and impairments in PPI (Braff et al., 2001). Interestingly, the magnitude of the observed dopaminergic changes in our model system (10–20% difference to control levels) is consistent with the magnitude of changes of striatal dopaminergic markers in patients with schizophrenia (Laruelle, 1998). The fact that only relatively modest (but significant) changes are observed both in our model system and the clinical condition in humans is not surprising because the relevant neuropathological alterations most likely stem from neurodevelopmental but not ongoing neurodegenerative processes, the latter of which may induce more prominent and progressive neuropathology.
As reviewed previously (Meyer et al., 2007, 2009a,b; Patterson, 2009), additional long-term effects of prenatal infection and/or immune activation have been described in numerous independent experimental investigations in both rats and mice, providing compelling evidence that prenatal immune activation models can successfully capture a variety of brain and behavioral abnormalities relevant to schizophrenia. It has been suggested that many of the environmental risk factors of schizophrenia, including maternal infection during pregnancy, may act by facilitating dopamine dysregulations (Di Forti et al., 2007; Murray et al., 2008). Our experimental data support this hypothesis by demonstrating that prenatal viral-like immune activation induces schizophrenia-related structural and functional abnormalities linked to dopaminergic changes in the mesoaccumbal and nigrostriatal pathways. Future studies will be needed to dissect the relative contribution of abnormal dopaminergic development to specific symptoms or symptom clusters involved in schizophrenia, and to explore the modulatory influences of environmental factors such as prenatal infection on these associations. This may open new avenues for the establishment of novel therapeutic interventions that may attenuate or even prevent the emergence of this disabling mental illness following early neurodevelopmental disruption of dopamine functions.
Footnotes
The research presented in this study was partially supported by Swiss National Science Foundation Grant 310000-118284/1, by Swiss Federal Institute of Technology Zurich Grant 11 07/03, and by a 2009 National Alliance for Research on Schizophrenia and Depression Distinguished Investigator Award to J.F. We are extremely grateful to the Animal Services Department Schwerzenbach, Swiss Federal Institute of Technology, for their excellent animal husbandry and care. We also remain indebted to Juliet Richetto and Celia Hampson-Reid for their assistance in parts of the behavioral and immunohistochemical analyses.
The authors declare no competing financial interests.
References
- Bacopoulos NG, Bhatnagar RK. Correlation between tyrosine hydroxylase activity and catecholamine concentration or turnover in brain regions. J Neurochem. 1977;29:639–643. doi: 10.1111/j.1471-4159.1977.tb07780.x. [DOI] [PubMed] [Google Scholar]
- Bayer SA, Wills KV, Triarhou LC, Ghetti B. Time of neuron origin and gradients of neurogenesis in midbrain dopaminergic neurons in the mouse. Exp Brain Res. 1995;105:191–199. doi: 10.1007/BF00240955. [DOI] [PubMed] [Google Scholar]
- Borrell J, Vela JM, Arévalo-Martin A, Molina-Holgado E, Guaza C. Prenatal immune challenge disrupts sensorimotor gating in adult rats: implications for the etiopathogenesis of schizophrenia. Neuropsychopharmacology. 2002;6:204–221. doi: 10.1016/S0893-133X(01)00360-8. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology. 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Brown AS. Prenatal infection as a risk factor for schizophrenia. Schizophr Bull. 2006;32:200–202. doi: 10.1093/schbul/sbj052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardon M, Ron-Harel N, Cohen H, Lewitus GM, Schwartz M. Dysregulation of kisspeptin and neurogenesis at adolescence link inborn immune deficits to the late onset of abnormal sensorimotor gating in congenital psychological disorders. Mol Psychiatry. 2009 doi: 10.1038/mp.2009.66. in press. [DOI] [PubMed] [Google Scholar]
- Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience. 2001;105:7–17. doi: 10.1016/s0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- Di Forti M, Lappin JM, Murray RM. Risk factors for schizophrenia–all roads lead to dopamine. Eur Neuropsychopharmacol. 2007;17:S101–107. doi: 10.1016/j.euroneuro.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Fatemi SH. London: Martin Dunitz-Taylor and Francis Group; 2005. Neuropsychiatric disorders and infection. [Google Scholar]
- Fortier ME, Joober R, Luheshi GN, Boksa P. Maternal exposure to bacterial endotoxin during pregnancy enhances amphetamine-induced locomotion and startle responses in adult rat offspring. J Psychiatr Res. 2004;38:335–345. doi: 10.1016/j.jpsychires.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. Amsterdam: Elsevier Academic; 2008. The mouse brain in stereotaxic coordinates. [Google Scholar]
- Gilmore JH, Jarskog LF. Exposure to infection and brain development: cytokines in the pathogenesis of schizophrenia. Schizophr Res. 1997;24:365–367. doi: 10.1016/s0920-9964(96)00123-5. [DOI] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Guillin O, Abi-Dargham A, Laruelle M. Neurobiology of dopamine in schizophrenia. Int Rev Neurobiol. 2007;78:1–39. doi: 10.1016/S0074-7742(06)78001-1. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, Møller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sørensen FB, Vesterby A, West MJ. The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS. 1988;96:857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- Howard CV, Reed MG. New York: Garland Science/BIOS Scientific; 2005. Unbiased stereology. [Google Scholar]
- Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III–the final common pathway. Schizophr Bull. 2009;35:549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Montgomery AJ, Asselin MC, Murray RM, Grasby PM, McGuire PK. Molecular imaging studies of the striatal dopaminergic system in psychosis and predictions for the prodromal phase of psychosis. Br J Psychiatry. 2007;51:s13–18. doi: 10.1192/bjp.191.51.s13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes M, Rosenthal A. Specification of dopaminergic and serotonergic neurons in the vertebrate CNS. Curr Opin Neurobiol. 1999;9:26–36. doi: 10.1016/s0959-4388(99)80004-x. [DOI] [PubMed] [Google Scholar]
- Koshibu K, Levitt P, Ahrens ET. Sex-specific, postpuberty changes in mouse brain structures revealed by three-dimensional magnetic resonance microscopy. Neuroimage. 2004;22:1636–1645. doi: 10.1016/j.neuroimage.2004.03.051. [DOI] [PubMed] [Google Scholar]
- Laruelle M. Imaging dopamine transmission in schizophrenia. A review and meta-analysis. Q J Nucl Med. 1998;42:211–221. [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A, Gil R, Kegeles L, Innis R. Increased dopamine transmission in schizophrenia: relationship to illness phases. Biol Psychiatry. 1999;46:56–72. doi: 10.1016/s0006-3223(99)00067-0. [DOI] [PubMed] [Google Scholar]
- Masson J, Sagné C, Hamon M, El Mestikawy S. Neurotransmitter transporters in the central nervous system. Pharmacol Rev. 1999;51:439–464. [PubMed] [Google Scholar]
- Meyer U, Feldon J. Prenatal exposure to infection: a primary mechanism for abnormal dopaminergic development in schizophrenia. Psychopharmacology. 2009;206:587–602. doi: 10.1007/s00213-009-1504-9. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Schedlowski M, Yee BK. Towards an immuno-precipitated neurodevelopmental animal model of schizophrenia. Neurosci Biobehav Rev. 2005;29:913–947. doi: 10.1016/j.neubiorev.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, Yee BK, Feldon J. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci. 2006;26:4752–4762. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Yee BK, Feldon J. The neurodevelopmental impact of prenatal infections at different times of pregnancy: the earlier the worse? Neuroscientist. 2007;13:241–256. doi: 10.1177/1073858406296401. [DOI] [PubMed] [Google Scholar]
- Meyer U, Engler A, Weber L, Schedlowski M, Feldon J. Preliminary evidence for a modulation of fetal dopaminergic development by maternal immune activation during pregnancy. Neuroscience. 2008a;154:701–709. doi: 10.1016/j.neuroscience.2008.04.031. [DOI] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Schwendener S, Knuesel I, Yee BK, Feldon J. Relative prenatal and postnatal maternal contributions to schizophrenia-related neurochemical dysfunction after in utero immune challenge. Neuropsychopharmacology. 2008b;33:441–456. doi: 10.1038/sj.npp.1301413. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Fatemi SH. In-vivo rodent models for the experimental investigation of prenatal immune activation effects in neurodevelopmental brain disorders. Neurosci Biobehav Rev. 2009a;33:1061–1079. doi: 10.1016/j.neubiorev.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Yee BK. A review of the fetal brain cytokine imbalance hypothesis of schizophrenia. Schizophr Bull. 2009b;35:959–972. doi: 10.1093/schbul/sbn022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RM, Lappin J, Di Forti M. Schizophrenia: from developmental deviance to dopamine dysregulation. Eur Neuropsychopharmacol. 2008;18:S129–134. doi: 10.1016/j.euroneuro.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE, Malenka RC. New York: McGraw-Hill; 2001. Molecular neuropharmacology: a foundation for clinical neuroscience. [Google Scholar]
- Ozawa K, Hashimoto K, Kishimoto T, Shimizu E, Ishikura H, Iyo M. Immune activation during pregnancy in mice leads to dopaminergic hyperfunction and cognitive impairment in the offspring: a neurodevelopmental animal model of schizophrenia. Biol Psychiatry. 2006;59:546–554. doi: 10.1016/j.biopsych.2005.07.031. [DOI] [PubMed] [Google Scholar]
- Patterson PH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res. 2009;204:313–321. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Halliday G, Watson C, Koutcherov Y, Wang H. Amsterdam: Elsevier Academic; 2007. Atlas of the developing mouse brain. [Google Scholar]
- Ralph RJ, Paulus MP, Fumagalli F, Caron MG, Geyer MA. Prepulse inhibition deficits and perseverative motor patterns in dopamine transporter knock-out mice: differential effects of D1 and D2 receptor antagonists. J Neurosci. 2001;21:305–313. doi: 10.1523/JNEUROSCI.21-01-00305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph-Williams RJ, Lehmann-Masten V, Geyer MA. Dopamine D1 rather than D2 receptor agonists disrupt prepulse inhibition of startle in mice. Neuropsychopharmacology. 2003;28:108–118. doi: 10.1038/sj.npp.1300017. [DOI] [PubMed] [Google Scholar]
- Roberts RC, Roche JK, Conley RR, Lahti AC. Dopaminergic synapses in the caudate of subjects with schizophrenia: relationship to treatment response. Synapse. 2009;63:520–530. doi: 10.1002/syn.20623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero E, Guaza C, Castellano B, Borrell J. Ontogeny of sensorimotor gating and immune impairment induced by prenatal immune challenge in rats: implications for the etiopathology of schizophrenia. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.44. in press. [DOI] [PubMed] [Google Scholar]
- Salahpour A, Ramsey AJ, Medvedev IO, Kile B, Sotnikova TD, Holmstrand E, Ghisi V, Nicholls PJ, Wong L, Murphy K, Sesack SR, Wightman RM, Gainetdinov RR, Caron MG. Increased amphetamine-induced hyperactivity and reward in mice overexpressing the dopamine transporter. Proc Natl Acad Sci U S A. 2008;105:4405–4410. doi: 10.1073/pnas.0707646105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci. 2003;23:297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer P, Feldon J, Yee BK. Interactions between the glycine transporter 1(GlyT1) inhibitor SSR504734 and psychoactive drugs in mouse motor behaviour. Eur Neuropsychopharmacol. 2009;19:571–580. doi: 10.1016/j.euroneuro.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Smidt MP, Burbach JP. How to make a mesodiencephalic dopaminergic neuron. Nat Rev Neurosci. 2007;8:21–32. doi: 10.1038/nrn2039. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Toru M, Watanabe S, Shibuya H, Nishikawa T, Noda K, Mitsushio H, Ichikawa H, Kurumaji A, Takashima M, Mataga N. Neurotransmitters, receptors and neuropeptides in postmortem brains of chronic schizophrenic patients. Acta Psychiatr Scand. 1988;78:121–137. doi: 10.1111/j.1600-0447.1988.tb06312.x. [DOI] [PubMed] [Google Scholar]
- Van den Heuvel DM, Pasterkamp RJ. Getting connected in the dopamine system. Prog Neurobiol. 2008;85:75–93. doi: 10.1016/j.pneurobio.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Yee BK, Russig H, Feldon J. Apomorphine-induced prepulse inhibition disruption is associated with a paradoxical enhancement of prepulse stimulus reactivity. Neuropsychopharmacology. 2004;29:240–248. doi: 10.1038/sj.npp.1300323. [DOI] [PubMed] [Google Scholar]
- Zetterström RH, Solomin L, Jansson L, Hoffer BJ, Olson L, Perlmann T. Dopamine neuron agenesis in Nurr1-deficient mice. Science. 1997;276:248–250. doi: 10.1126/science.276.5310.248. [DOI] [PubMed] [Google Scholar]
- Zuckerman L, Rehavi M, Nachman R, Weiner I. Immune activation during pregnancy in rats leads to a postpubertal emergence of disrupted latent inhibition, dopaminergic hyperfunction, and altered limbic morphology in the offspring: a novel neurodevelopmental model of schizophrenia. Neuropsychopharmacology. 2003;28:1778–1789. doi: 10.1038/sj.npp.1300248. [DOI] [PubMed] [Google Scholar]