Abstract
BACKGROUND & AIMS:
Many patients with gastroparesis are prescribed opioids for pain control, but indications for opioid prescriptions and the relationship of opioid use to gastroparesis manifestations are undefined. We characterized associations of use of potent vs weaker opioids and presentations of diabetic and idiopathic gastroparesis.
METHODS:
We collected data on symptoms, gastric emptying, quality of life, and health care resource use from 583 patients with gastroparesis (>10% 4-h scintigraphic retention) from the National Institute of Diabetes and Digestive and Kidney Diseases Gastroparesis Consortium, from January 2007 through November 2016. Patients completed medical questionnaires that included questions about opioid use. The opioid(s) were categorized for potency relative to oral morphine. Symptom severities were quantified by Patient Assessment of Upper Gastrointestinal Disorders Symptoms questionnaires. Subgroup analyses compared patients on potent vs weaker opioids and opioid effects in diabetic vs idiopathic etiologies.
RESULTS:
Forty-one percent of patients were taking opioids; 82% of these took potent agents (morphine, hydrocodone, oxycodone, methadone, hydromorphone, buprenorphine, or fentanyl). Abdominal pain was the reason for prescription for 61% of patients taking opioids. Mean scores for gastroparesis, nausea/vomiting, bloating/distention, abdominal pain, and constipation scores were higher in opioid users (P ≤ .05). Opioid use was associated with greater levels of gastric retention, worse quality of life, increased hospitalization, and increased use of antiemetic and pain modulator medications compared with nonusers (P ≤ .03). Use of potent opioids was associated with worse gastroparesis, nausea/vomiting, upper abdominal pain, and quality-of-life scores, and more hospitalizations compared with weaker opioids (tapentadol, tramadol, codeine, or propoxyphene) (P ≤ .05). Opioid use was associated with larger increases in gastric retention in patients with idiopathic vs diabetic gastroparesis (P = .008).
CONCLUSIONS:
Opioid use is prevalent among patients with diabetic or idiopathic gastroparesis, and is associated with worse symptoms, delays in gastric emptying, and lower quality of life, as well as greater use of resources. Potent opioids are associated with larger effects than weaker agents. These findings form a basis for studies to characterize adverse outcomes of opioid use in patients with gastroparesis and to help identify those who might benefit from interventions to prevent opioid overuse.
Keywords: Stomach, Nausea and Vomiting, Abdominal Pain, Diabetes Mellitus
Thirty-one percent to 50% of gastroparesis patients are prescribed opioids.1,2 Upper abdominal pain of at least moderate severity is reported by two thirds of patients and is more prevalent in idiopathic vs diabetic gastroparesis.2 Ratings of abdominal pain as the predominant symptom are associated with greater opioid use.2 Opioids inhibit gastric emptying in patients without gastroparesis.3–5 Features of opioid use in gastroparesis and indications for prescription are poorly characterized.
Consequences of opioids in gastroparesis are incompletely investigated. In opioid-induced nausea and vomiting (OINV), the distinct complication of nausea has been reported by 20% to 32%, and vomiting has been reported by 9% to 15% of patients prescribed opioids for noncancer pain.6,7 By comparison, opioid-induced constipation has 41% prevalence.6 Nausea and vomiting represent distressing complications of opioids and commonly result in stopping opioids for chronic pain.8
Opioids of different potency have variable propensities to elicit gastrointestinal (GI) complications. Potent agents such as oxycodone induce more severe side effects than weaker opioids such as tapentadol.9,10 Fewer patients on tapentadol discontinue therapy because of nausea.11 The effects of opioids of differing potency on manifestations of gastroparesis have not been compared.
We accessed data from Registries of the National Institute of Diabetes and Digestive and Kidney Diseases Gastroparesis Consortium to relate opioid use to clinical manifestations, quality of life, and resource utilization in a large multicenter gastroparesis cohort. We hypothesized the following: (1) opioids are prescribed frequently in gastroparesis; (2) opioid use is associated with worse gastric emptying delays, greater symptoms, lower quality of life, and increased resource use; (3) potent opioids are associated with greater impact than weaker agents; and (4) presentations relating to opioids are similar in diabetic vs idiopathic gastroparesis. These investigations provided insight into opioid associations and formed a foundation for future studies targeting treatment of opioid side effects in gastroparesis.
Methods
Patient Population
A total of 583 patients with diabetic or idiopathic gastroparesis (age, >18 y) were recruited at 8 centers of the National Institute of Diabetes and Digestive and Kidney Diseases Gastroparesis Clinical Research Consortium into Gastroparesis Registries 1 and 2 from January 2007 through November 2016 (ClinicalTrials.gov: NCT00398801 and NCT01696747). All reported gastroparesis symptoms of 12 weeks’ duration or longer (not necessarily contiguous) showed delayed gastric emptying (>10% 4-hour scintigraphic retention) for 6 months or less before enrollment.12 Scintigraphy was performed off opioids and other agents affecting gut transit (including prokinetics and anticholinergics) for 72 hours or more before testing. Esophagogastroduodenoscopy excluded organic diseases causing symptoms 12 months or less before enrollment. Patients were excluded for other conditions causing similar symptoms (obstruction, inflammatory bowel disease, eosinophilic gastroenteritis, neurologic, liver, or renal disease, metabolic causes, or prior gastric surgery [fundoplication, gastric resection, or pyloroplasty]). Institutional Review Board approval was obtained at Clinical Centers and the Data Coordinating Center. Patients provided written informed consent.
Opioid Intake
Questions on Baseline Medical History forms queried opioid use and the reasons for their use. For Gastroparesis Registry 1, selections included 1 or more of the following: (1) abdominal pain, (2) headache pain, (3) leg pain, and/or (4) other pain. For Gastroparesis Registry 2, patients chose 1 or more of the following: (1) pain related to gastroparesis symptoms, (2) headache pain, (3) leg pain, (4) back pain, and/or (5) other pain. The abdominal pain selection in Registry 1 and pain related to gastroparesis symptoms selection in Registry 2 were combined for this investigation.
On the Baseline Medical History, specific opioid(s) were stratified by potency in relation to oral morphine, which was assigned a relative potency of 1.0.13 Potent opioids showing potencies of 1.0 or greater included morphine (potency 1), hydrocodone (1), oxycodone (1.5), methadone (2.5), hydromorphone (5), buprenorphine (40), and fentanyl (50). Weaker agents included tapentadol (0.3), tramadol (0.1), codeine (0.1), and propoxyphene (0.05).
Data Acquisition
Demographic information was collected on Registration and Baseline Medical History forms (Supplementary Methods section). Gastric emptying was calculated from solid (99mTc-sulfur colloid) and liquid (111In-water) scintigraphy performed before enrollment (Supplementary Methods).12,14,15
Symptom severities were quantified by Patient Assessment of Upper Gastrointestinal Disorders Symptoms (PAGI-SYM) questionnaires enumerating 22 symptoms from 0 (no symptoms) to 5 (most severe), with 2-week recall.16 Overall gastroparesis severity was determined by Gastroparesis Cardinal Symptom Index (GCSI) scores, which included 9 PAGI-SYM questions.17 Subscale and individual PAGI-SYM scores quantified gastroparesis and lower GI symptom severity (Supplementary Methods section).
Quality of life was quantified by Patient Assessment of Upper Gastrointestinal Disorders Quality of Life (PAGI-QOL) surveys, which scored 30 factors from 0 (none of the time) to 5 (all of the time) in 5 domains (daily activities, clothing, diet, relationships, and psychological) over 2 weeks.18 PAGI-QOL scores were calculated from the means of all factors after reversing individual scores; 0 represented poor quality of life and 5 reflected excellent quality of life.
Health utilization parameters were determined from Baseline Medical History forms including gastroparesis hospitalizations in the past year and nonopioid medication intake (Supplementary Methods section).
Data Comparisons
Opioid use was related to demographic measures (age, sex, race), gastric emptying (2- and 4-hour solid; 1-hour liquid retention; percentage with mild, moderate, and severe retention), gastroparesis symptoms (overall GCSI, GCSI nausea/vomiting, fullness/early satiety, and bloating/distention subscales, individual PAGI-SYM upper abdominal pain scores), lower GI symptoms (individual PAGI-SYM lower abdominal pain, constipation, diarrhea scores), quality of life (PAGI-QOL scores), and resource use (numbers of hospitalizations, prokinetic, antiemetic, nonopiate analgesic, neuropathic pain modulator, laxative intake). Comparisons related potent vs weaker opioid use and opioid associations in diabetic vs idiopathic gastroparesis.
Statistical Analyses
Factor differences were compared for patients on vs not on opioids and potent vs weaker agents. Numbers and percentages or means ± SD were reported for categoric or continuous characteristics. P values were determined from Pearson chi-square analysis or the Fisher exact tests for categoric characteristics and 2-sample t tests for continuous measures. Logistic regression models comparing diabetic vs idiopathic gastroparetics included patient characteristics, etiology (diabetic vs idiopathic), and patient characteristic by etiology interaction terms. Multiple logistic regression assessed independent relationships of characteristics associated with opioids, selecting the model with the lowest Akaike Information Criteria from age, sex, race, ethnicity, overall GCSI scores, GCSI subscores (nausea/vomiting, early satiety/fullness, bloating/distention), individual PAGI-SYM scores (upper abdominal pain, lower abdominal pain, constipation, diarrhea), PAGI-QOL scores, yearly hospitalizations, and medication use (prokinetics, antiemetics, nonopiate analgesics, pain modulators); etiology and 4-hour retention were included but were not in the candidate set.19 Akaike Information Criteria measures trade-offs between goodness of fit vs model complexity, and is not based on P values. The Hosmer–Lemeshow goodness-of-fit test indicated adequate fit (P = .41).
Given the study’s exploratory nature, P values were 2-sided and nominal with significance at P = .05, a priori. Because these exploratory analyses aimed to generate new hypotheses to be tested in future confirmatory studies, multiple comparison corrections were not performed. Such adjustments reduce power to define differences, are unnecessary if exploratory questions are unrelated, and are only required for studies aiming to prove predefined hypotheses to endorse decision-making protocols.20 Stata Software (release v14; StataCorp LP, College Station, TX) and SAS (version 9.3; SAS Institute, Inc, Cary, NC) were used.
Results
Baseline Features
Opioid use was reported by 240 of 583 (41%) gastroparesis patients; most (198 of 240, 82%) were on potent agents, with 42 of 240 (18%) on weaker opioids. More than 60% took opioids for abdominal pain as the sole cause or with other pain syndromes; fewer than 40% took opioids for extragastric conditions (Table 1). Reasons for opioid use of potent vs weaker agents (P = .10) and diabetic vs idiopathic patients were similar (P = .55).
Table 1.
Self-Reported Reasons for Opioid Use in Gastroparesis
| Reason | All opioid users | On potent opioids | On weaker opioids | P value, potent vs weaker opioids | Diabetic patients | Idiopathic patients | P value, diabetic vs idiopathic etiology |
|---|---|---|---|---|---|---|---|
| Abdominal pain only | 96/227 (42%) | 82/186 (44%) | 14/41 (34%) | .10 | 34/84 (40%) | 62/143 (43%) | .55 |
| Abdominal pain plus other | 42/227 (19%) | 30/186 (16%) | 12/41 (29%) | 15/84 (18%) | 27/143 (19%) | ||
| Headache only | 8/227 (4%) | 7/186 (4%) | 1/41 (2%) | 1/84 (1%) | 7/143 (5%) | ||
| Back pain only | 9/227 (4%) | 5/186 (3%) | 4/41 (10%) | 3/84 (4%) | 6/143 (4%) | ||
| Leg pain only | 3/227 (1%) | 3/186 (2%) | 0/41 (0%) | 2/84 (2%) | 1/143 (1%) | ||
| Other | 69/227 (30%) | 59/186 (32%) | 10/41 (24%) | 29/84 (35%) | 40/143 (28%) |
Relation of Opioids to Clinical Variables
Demographic factors and gastric emptying.
Demographic characteristics were similar in opioid users vs patients not on opioids and in patients on potent vs weaker opioids (Table 2). Opioid use was associated with modestly worse gastric emptying (Table 2). Four-hour solid retention was greater on vs not on opioids (P = .008). Severe impairments (>35% 4-hour retention) were more prevalent on opioids (P = .02). Two-hour solid and 1-hour liquid retention were not different in relation to opioid use. Emptying was similar on potent vs weaker opioids.
Table 2.
Relationship of Opioid Use to Demographic Factors, Gastric Emptying, and Resource Utilization
| Category | Variable | On opioids | Not on opioids | P value, on vs not on opioids | On potent opioids | On weaker opioids | P value, on potent vs weaker opioids |
|---|---|---|---|---|---|---|---|
| Demographic factors | Age, y, means ± SD | 44 ± 13 | 42 ± 14 | .11 | 44 ± 13 | 44 ± 13 | .95 |
| Female sex | 202/240 (84%) | 286/343 (83%) | .80 | 165/198 (83%) | 37/42 (88%) | .64 | |
| Race | |||||||
| White | 84% | 89% | .18 | 84% | 86% | .87 | |
| Black | 12% | 8% | 12% | 10% | |||
| Other | 4% | 3% | 4% | 5% | |||
| Etiology | |||||||
| Diabetic | 38% | 32% | .13 | 39% | 31% | .39 | |
| Idiopathic | 62% | 68% | 61% | 69% | |||
| Pain severity | PAGI-SYM upper abdominal pain score ≥ 3 | 180/240 (75%) | 205/343 (60%) | .0001 | 155/198 (78%) | 25/42 (60%) | .02 |
| PAGI-SYM upper abdominal pain score <3 | 60/240 (25%) | 138/343 (40%) | 43/198 (22%) | 17/42 (40%) | |||
| Gastric emptying measures | 4-hour solid retention, means ± SD | 36% ± 22% | 32% ± 20% | .008 | 37% ± 23% | 33% ± 18% | .28 |
| 4-hour solid retention severity | |||||||
| Mild, 10%–20% retained | 29% | 38% | .02 | 29% | 29% | .17 | |
| Moderate, 20%–35% retained | 29% | 31% | 27% | 40% | |||
| Severe, >35% retained | 42% | 31% | 44% | 31% | |||
| 2-hour solid retention, means ± SD | 65% ± 19% | 64% ± 18% | .53 | 65% ± 19% | 65% ± 18% | .89 | |
| 1-hour liquid retention, means ± SD | 49% ± 17% | 50% ± 17% | .73 | 48% ± 19% | 50% ± 12% | .75 | |
| Health resource utilization | Hospitalized for gastroparesis in past year | 131/240 (55%) | 123/343 (36%) | <.0001 | 114/198 (58%) | 17/42 (40%) | .06 |
| Hospitalizations per year, means ± SD | 3.3 ± 5.5 | 1.5 ± 4.2 | <.0001 | 3.6 ± 5.7 | 1.8 ± 3.8 | .01 | |
| Medication use | |||||||
| Prokinetics | 129/240 (54%) | 182/343 (53%) | .87 | 106/198 (54%) | 23/42 (55%) | 1.00 | |
| Antiemetics | 173/240 (72%) | 172/343 (50%) | <.0001 | 146/198 (74%) | 27/42 (64%) | .26 | |
| Opiate analgesics | 145/240 (60%) | 204/343 (59%) | .82 | 114/198 (58%) | 31/42 (71%) | .06 | |
| Neuropathic pain modulators | 101/240 (42%) | 77/343 (22%) | <.0001 | 82/198 (41%) | 19/42 (45%) | .73 | |
| Laxatives | 36/75 (48%) | 51/141 (36%) | .09 | 22/48 (46%) | 14/27 (52%) | .64 |
PAGI-SYM, Patient Assessment of Upper Gastrointestinal Disorders Symptoms.
Gastroparesis and lower gastrointestinal symptoms.
Opioid intake was associated with higher symptom scores. Opioid use (P = .0001) and potent opioid use (P = .02) were higher among patients with PAGI-SYM upper abdominal pain scores of 3 or greater vs less than 3 (Table 2). Overall GCSI scores (P = .0001), and nausea/vomiting (P < .0001) and bloating/visible distention (P = .003) subscores, and upper abdominal pain scores (P < .0001) were greater on vs not on opioids (Figure 1A). Lower abdominal pain (P < .0001) and constipation scores (P = .05) were higher on opioids.
Figure 1.
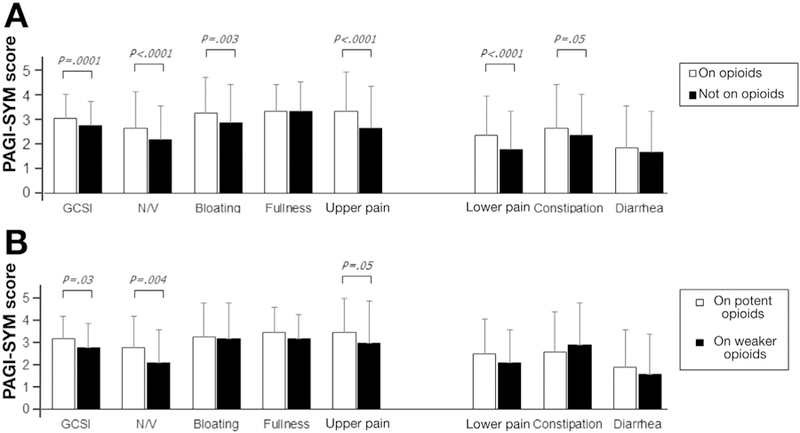
Symptom severities (Patient Assessment of Upper Gastrointestinal Disorders Symptoms [PAGI-SYM]) are related to opioid use. (A) Patients on opioids reported greater Gastroparesis Cardinal Symptom Index (GCSI) scores (P = .0001), higher subscores for nausea/vomiting (N/V) (P < .0001) and bloating/visible distention (P = .003), and individual scores for upper and lower pain (P < .0001) and constipation (P = .05) vs not taking opioids. (B) Patients on potent opioids reported higher GCSI scores (P = .03), N/V subscores (P = .004), and individual scores for upper pain (P = .05) vs taking weaker opioids.
Overall GCSI (P = .03), nausea/vomiting (P = .004), and upper abdominal pain scores (P = .05) were greater on potent vs weaker opioids (Figure 1B). Other symptoms were similar relating to opioid potency.
Quality of life and resource utilization.
Opioid use was associated with worse quality of life and increased resource use. PAGI-QOL scores were lower on vs not on opioids (P < .0001) and for potent vs weaker opioids (P = .03) (Figure 2). Greater percentages of patients on opioids were hospitalized in the past year (P < .0001), and yearly hospitalization numbers were higher (P < .0001) vs in patients not taking opioids (Table 2). Percentages of patients hospitalized for gastroparesis trended higher (P = .06), and numbers of hospitalizations were greater (P = .01) on potent vs weaker opioids. Greater percentages of opioid users reported antiemetic (P < .0001) and pain modulator (P < .0001) use. Trends showed more opioid users took laxatives (P = .09) vs patients not on opioids. There were trends to fewer potent opioid users on nonopiate analgesics vs weaker opioids (P = .06).
Figure 2.
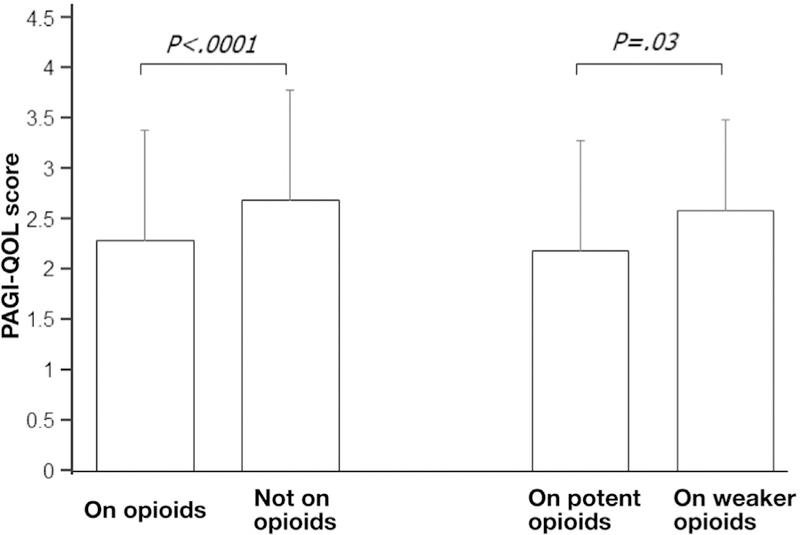
Patient Assessment of Upper Gastrointestinal Disorders Quality of Life (PAGI-QOL) scores are related to opioid use. Opioid users showed worse PAGI-QOL scores vs patients not on opioids (P < .0001). Patients taking potent opioids showed worse PAGI-QOL scores vs patients on weaker opioids (P = .03).
Subgroup Comparisons by Etiology
Subgroup analyses defined opioid associations by etiology (Table 3). Opioid use was similar in diabetic (91 of 200; 46%) and idiopathic (149 of 383; 39%) patients (P = .15). Opioid intake in diabetic gastroparesis was associated with higher overall GCSI (P = .02), nausea/vomiting (P = .01), and upper abdominal pain (P = .002) scores, lower PAGI-QOL scores (P = .0003), greater percentages of patients hospitalized (P = .03) and yearly hospitalizations (P = .01), and greater antiemetic (P < .0001), pain modulator (P = .0003), and laxative (P = .02) use. Opioid use in idiopathic gastroparesis was associated with greater overall GCSI (P = .002), nausea/vomiting (P = .001), bloating/distention (P = .01), and upper and lower (P < .0001) abdominal pain scores, worse PAGI-QOL scores (P = .006), greater percentages of patients hospitalized (P = .0002) and yearly hospitalizations (P = .0005), and greater antiemetic (P = .0004) and pain modulator (P = .0008) intake. Idiopathic patients showed greater increases in 4-hour retention (P = .008) and greater increases in lower abdominal pain scores with opioid use (P = .02) vs diabetic patients.
Table 3.
Differences in Opioid Associations in Diabetic vs Idiopathic Gastroparesis
| Variable | Diabetic gastroparesis |
Idiopathic gastroparesis |
P value, diabetic vs idiopathic | ||||
|---|---|---|---|---|---|---|---|
| On opioids | Not on opioids | P value | On opioids | Not on opioids | P value | ||
| 4-hour scintigraphic gastric retention, means ± SD | 40% ± 25% | 41% ± 25% | .81 | 34% ± 20% | 27% ± 16% | .91 | .008 |
| Symptoms | |||||||
| Overall GCSI score | 3.1 ± 1.0 | 2.8 ± 1.1 | .02 | 3.2 ± 1.1 | 2.8 ± 1.0 | .002 | .91 |
| Nausea/vomiting subscore | 3.0 ± 1.4 | 2.5 ± 1.5 | .01 | 2.6 ± 1.5 | 2.1 ± 1.3 | .001 | .96 |
| Bloating/distention subscore | 3.1 ± 1.5 | 2.7 ± 1.7 | .07 | 3.3 ± 1.4 | 3.0 ± 1.6 | .01 | .95 |
| Upper abdominal pain score | 3.3 ± 1.7 | 2.6 ± 1.7 | .002 | 3.4 ± 1.5 | 2.8 ± 1.7 | <.0001 | .93 |
| Lower abdominal pain score | 2.2 ± 1.6 | 2.0 ± 1.7 | .41 | 2.6 ± 1.6 | 1.8 ± 1.5 | <.0001 | .02 |
| Constipation score | 2.7 ± 1.7 | 2.3 ± 1.7 | .12 | 2.7 ± 1.9 | 2.4 ± 1.7 | .19 | .63 |
| PAGI-QOL, means ± SD | 2.2 ± 1.2 | 2.9 ± 1.2 | .0003 | 2.3 ± 1.0 | 2.6 ± 1.1 | .006 | .31 |
| Hospitalized for gastroparesis in past year | 59/91 (65%) | 54/109 (50%) | .03 | 72/149 (48%) | 69/234 (29%) | .0002 | .65 |
| Hospitalizations per year, means ± SD | 5.3 ± 7.3 | 2.8 ± 6.3 | .01 | 2.1 ± 3.6 | 0.9 ± 2.5 | .0005 | .09 |
| Medication use | |||||||
| Antiemetics | 70/91 (77%) | 53/109 (49%) | <.0001 | 103/149 (69%) | 119/234 (51%) | .0004 | .18 |
| Nonopiate analgesics | 48/91 (53%) | 62/109 (57%) | .56 | 97/149 (65%) | 142/234 (61%) | .38 | .29 |
| Pain modulators | 48/91 (53%) | 30/109 (30%) | .0003 | 53/149 (36%) | 47/234 (20%) | .0008 | .44 |
| Laxatives | 13/27 (48%) | 12/40 (30%) | .02 | 23/48 (48%) | 39/100 (39%) | .28 | .55 |
GCSI, Gastroparesis Cardinal Symptom Index; PAGI-QOL, Patient Assessment of Upper Gastrointestinal Disorders Quality of Life.
Multiple Logistic Regression
On regression analyses, factors positively associated with opioids included increasing age (P = .001), upper (P = .004) and lower (P = .02) abdominal pain, numbers of hospitalizations (P = .02), and antiemetic (P = .002) and pain modulator (P < .0001) use (Table 4).
Table 4.
Multiple Regression Analyses to Define Factors Relating to Opioid Use in Gastroparesis
| Variable | Odds ratio opioid use vs no opioid use | 95% CI | P value |
|---|---|---|---|
| Age, y, per 1-year increase | 1.02 | 1.01–1.04 | .001 |
| 4-hour gastric retention, per 1% increase in retention | 1.01 | 1.00–1.01 | .19 |
| PAGI-SYM upper abdominal pain score, per 1-point increase in severity score | 1.20 | 1.06–1.36 | .004 |
| PAGI-SYM lower abdominal pain score, per 1-point increase in severity score | 1.15 | 1.02–1.30 | .02 |
| Number of hospitalizations, per 1 inpatient stay increase | 1.05 | 1.01–1.10 | .02 |
| Antiemetic medications, yes vs no | 1.86 | 1.26–2.74 | .002 |
| Neuropathic pain medications, yes vs no | 2.10 | 1.42–3.09 | <.0001 |
| Diabetic etiology, vs idiopathic | 0.90 | 0.60–1.35 | .62 |
PAGI-SYM, Patient Assessment of Upper Gastrointestinal Disorders Symptoms.
Discussion
This investigation was a large analysis of factors associated with opioid use in gastroparesis. Its strengths included its multicenter structure with recruitment of well-characterized patients, prospective data collection, consistent validated survey administration in a standardized fashion, separate diabetic and idiopathic analyses, and relation of opioid potency to gastroparesis associations.
More than 40% of patients took opioids. We acknowledge that many patients were referred to tertiary centers for refractory symptoms including pain, which may have contributed to opioid use in this population. Our findings may not replicate those in community gastroparesis settings. These observations complement previous Gastroparesis Consortium reports correlating opioid intake with abdominal pain and parallel older observations of 31% to 50% opioid use in gastroparesis.1,2 These rates are unsurprising because 3% of adults in the United States use opioids for chronic pain.21 The high prevalence of opioid use in gastroparesis warrants consideration that this association is a component of the prescription opioid epidemic, which can have far-ranging consequences, including fatal outcomes.21,22
Opioid-induced gastric emptying delays have been established. μ-opioid agonists slow emptying, blunt motility, and promote endoscopic food retention.4,5,23 The stomach has high μ-receptor densities.24 We observed modestly worse emptying impairments in opioid users. Opioid use was somewhat more prevalent with severe delays (>35% 4-hour retention) and relatively lower with mild delays (<10% 4-hour retention). The cause of these incremental delays among opioid users is uncertain. Enrolled patients were required to discontinue opioids for at least 72 hours before scintigraphy. It is conceivable that stopping opioids for 72 hours was insufficient to reverse inhibitory opioid effects on gastric function. The possibility has not been investigated, but this cut-off value has been widely adopted because this is more than 5-fold greater than circulating half-lives of most opioids. Alternatively, it is possible that patients were noncompliant with requests to withhold opioids. Because emptying differences between opioid users and nonusers were modest, it is likely that surreptitious opioid intake during scintigraphy had limited impact in this cohort. Furthermore, although this was not a longitudinal outcomes study, 55 patients had available gastric emptying data on enrollment and at 48-week study visits showing similar 22% ± 14% improvements in 4-hour retention in 4 patients who stopped opioids for an uncertain length of time and 18% ± 28% in 47 patients remaining on opioids on follow-up evaluation (P = NS). Regardless, our protocols were unable to exclude opioid intake within 72 hours of emptying testing, as mentioned later.
Symptom severity in gastroparesis was associated strongly with opioid use. Prior articles reported nausea in 20% to 32% and vomiting in 9% to 15% of patients receiving opioids.6,7 In addition to inhibiting gastric motility, opioids elicited nausea and vomiting via actions on the vestibular apparatus and area postrema.8 We observed increased upper and lower GI symptoms on opioids on univariate and regression analyses. We emphasize that these analyses defined associations and did not prove causation, thus we cannot state that greater nausea and vomiting were direct consequences of opioids or if patients had worse symptoms at baseline requiring prescription analgesics. The strong correlation of symptom severity with opioid use coupled with less robust emptying impairments in opioid users emphasize that other mechanisms such as luminal hypersensitivity or blunted fundic accommodation mediate abdominal pain in gastroparesis as previously proposed.2
Opioid use in gastroparesis related to worse quality of life on PAGI-QOL surveys and greater resource use quantified by hospitalizations. Differences in nonopioid medication intake in patients on opioids included higher antiemetic and pain modulator use on univariate and regression analyses. These measures were not quantified previously in gastroparesis or OINV, although nausea and vomiting were rated the most distressing side effects and most frequent reason for stopping opioids.8 Our findings raise questions that warrant further study. Were opioids preferentially prescribed during inpatient stays to expedite hospital discharge without giving neuro-modulators a chance to work? Why were older patients more likely to receive opioids when associations of age with pain in gastroparesis were not observed?2 Should clinicians exercise care in prescribing medications for pain in older gastroparetic patients?
Factors associated with opioid use were correlated to gastroparesis etiology. We previously noted more prevalent severe upper abdominal pain in idiopathic vs diabetic gastroparesis.2 In this study, opioid use was associated with increased abdominal pain with both etiologies and little difference was seen in opioid associations in diabetic vs idiopathic gastroparesis on univariate and regression analyses. The only factors that diverged by etiology relating to opioids were worse emptying delays in idiopathic gastroparetic patients and greater lower abdominal pain in diabetic patients.
We compared factors associated with opioids equipotent or stronger than morphine vs weaker agents. Other investigators have related opioid potency to OINV severity. Less-potent medications (tramadol, tapentadol) elicit less nausea and vomiting than morphine or oxycodone.9,10,25 Patients are less likely to discontinue tapentadol because of nausea than oxycodone.11 Despite these lesser effects, weaker opioids still inhibit gut transit.3,9,10 We observed higher levels of some gastroparesis symptoms, worse quality of life, and more hospitalizations with potent vs weaker agents. Such findings support prescribing less-potent opioids for chronic pain syndromes in gastroparesis whenever possible. Similar to OINV, emptying delays were not different in gastroparesis patients on potent vs weaker opioids.3,9
This investigation had limitations. As stated earlier, we relied on patient self-report to characterize opioid use. It was not feasible to measure opioid levels in this large cohort and pharmacy records were inaccessible. Our surveys did not relate opioid dosing timing with reports of pain, thus we were unable to assess temporal relations of opioid use with other factors. However, recall times of surveys quantifying symptoms and quality of life were 2 weeks.16–18 Thus, we believe our findings likely incorporated any transient adverse effects of opioids on these measures. Unfortunately because the number and breadth of surveys completed by our patients on enrollment were extensive, no dosing of any medications was recorded. Thus, stratifications by opioid potency were based on the drugs themselves and not on opioid doses, frequency of intake, or duration of use. Consequently, dose-response relationships of opioid intake and clinical manifestations were not generated. We believe it is unlikely this limitation led to overstating the impact of opioids on gastroparesis manifestations. Scenarios can be envisioned in which some opioid users were taking exceedingly low or infrequent opioid doses, or some patients were taking large numbers of weaker opioids whereas others were on infrequent potent opioid agents. However, both of these possibilities likely would diminish rather than strengthen the observed associations of opioids in gastroparesis using our analyses. This investigation also did not address if patients with worse gastric emptying impairments experienced more severe pain and other symptoms that could lead to more frequent opioid prescription. However, prior Gastroparesis Consortium research observed no relation of abdominal pain, nausea and vomiting, or bloating subscores with gastric retention rates.2,26 Only early satiety and postprandial fullness scores were higher with worse emptying delays in prior studies.27 A recalculation of pain severity in the current gastroparesis cohort was performed and showed no difference in 4-hour gastric retention among those with pain scores of 3 or higher (moderate or greater severity) vs less than 3 (34.2% ± 21.3% vs 32.3% ± 20.9%, respectively; P = .32), similar to older findings.
These findings are important because they provide the foundation for future research on opioid-dependent gastroparesis. Therapy of gastroparesis pain is unsatisfactory and includes prokinetics for nausea and vomiting as well as antidepressants and pain modulators.28,29 No medications are Food and Drug Administration approved for OINV; a systematic review suggested variable efficacy of antiemetics to reverse OINV.30 Metoclopramide and ondansetron were ineffective, but small studies observed benefits with olanzapine and risperidone.31–33 Peripheral opiate antagonists (methylnaltrexone, naloxegol) are approved for opioid-induced constipation but have not been tested in OINV. However, methylnaltrexone prevents morphine-induced vomiting in dogs, gastric emptying delays in human beings, and decreased gastric residuals and improved enteral nutrition tolerance in a hospitalized patient on opioids.34–36 These observations raise hope of multifaceted approaches to treating opioid-requiring painful gastroparesis with antiemetics to improve symptoms, peripheral opiate antagonists to block gut effects of opioids, and neuromodulators to limit opioid dependence.
In conclusion, these comprehensive, novel data show that opioid use is prevalent and associates with more severe gastric emptying delays, greater symptoms, worse quality of life, and higher resource use in gastroparesis. Potent opioids may be associated with greater increases in some factors vs weaker agents. These findings provide insight into this common problem and form a basis to study therapy of adverse consequences of opioid use in gastroparesis. This investigation also highlights factors that may help identify patients who can be targeted for future interventions to prevent overuse of opioid agents in gastroparesis.
Supplementary Material
What You Need to Know.
Background
Indications for opioid prescription and the relationship between opioid use and manifestations of gastroparesis are undefined. We characterized associations between opioid use and presentations of gastroparesis in relation to opioid potency.
Findings
Opioids are taken frequently by patients with gastroparesis, often for abdominal pain. Opioid use was associated with worse symptoms and quality of life, greater resource utilization, and gastric emptying delays in patients with gastroparesis.
Implications for patient care
We found an association between gastroparesis and opioid use and characterized the clinical effects. Our findings might be used in studies of the consequences of opioid use by patients with gastroparesis and to identify patients who might benefit from interventions to prevent opioid overuse.
Acknowledgments
The members of the Gastroparesis Clinical Research Consortium are as follows.
Clinical centers: Johns Hopkins University, Baltimore, MD: Pankaj Jay Pasricha, MD (Principal Investigator), John Clarke, MD, and Yale Kim, MHS, MS; Stanford University, Stanford, CA: Linda Nguyen, MD (Principal Investigator), and Nighat Ullah, MD; California Pacific Medical Center, San Francisco, CA: William Snape, MD (Principal Investigator), Nata DeVole, RN, Mary Greene (2009–2011), Candice Lee, Courtney Ponsetto (2009–2010), and Katerina Shetler, MD; Temple University, Philadelphia, PA: Henry P. Parkman, MD (Principal Investigator), Steven Kantor, Vanessa Lytes, MSN, CRNP, Amiya Palit, MD, and Kellie Simmons, NP; Texas Tech University Health Sciences Center, El Paso, TX: Richard W. McCallum, MD (Principal Investigator), Reza Hejazi, MD (2009–2011), Kathy Roeser (2008–2009), Irene Sarosiek, MD, Denise Vasquez, RN, and Natalia Vega, RN; University of Louisville, Louisville, KY: Thomas Abell, MD (Principal Investigator), Karen Beatty, RN, Lisa Hatter, RN, Ronna Howard, and Lindsay Nowotny, PA-C; University of Mississippi Medical Center, Jackson, MS: Shou Tang, MD (Principal Investigator), Om S. Amin, MD (2010–2011), Olivia Henry, MS, RD, Archana Kedar, MD, Valerie McNair (2008–2012), Susanne Pruett, RN (2007–2008), Margaret Smith, RN, and Danielle Spree, RN (2007–2010); University of Michigan, Ann Arbor, MI: William Hasler, MD (Principal Investigator), Michelle Castle (2008–2011), Radoslav Coleski, MD (2007–2009), and Sophanara Wootten; Wake Forest University, Winston-Salem, NC: Kenneth Koch, MD (Principal Investigator), Lynn Baxter, Anya Brown, Samantha Culler (2009–2012), Judy Hooker, and Paula Stuart, PA.
Resource centers: Mayo Clinic College of Medicine (Pathology Analyses Center), Rochester, MN: Gianrico Farrugia, MD (Principal Investigator), Madhusudan Grover, MD, and Cheryl Bernard; MetroHealth Medical Center, Cleveland, OH: Jorge Calles-Escandon, MD; National Institute of Diabetes, Digestive and Kidney Diseases, Bethesda, MD: Jose Serrano, MD, PhD (Program Official), Frank Hamilton, MD, MPH (Project Scientist), Steven James, MD, Rebecca Torrance, RN, MSN, and Rebekah Van Raaphorst, MPH; Johns Hopkins University, Bloomberg School of Public Health (Data Coordinating Center), Baltimore, MD: James Tonascia, PhD (Principal Investigator), Patricia Belt, Erin Corless Hallinan, MHS, Ryan Colvin, MPH (2007–2010), Michele Donithan, MHS, Mika Green, MA (2009–2012), Milana Isaacson, Wana Kim (2009–2011), Linda Lee, MD, Patrick May, MS, Laura Miriel, Alice Sternberg, ScM, Mark Van Natta, MHS, Ivana Vaughn, MPH, Laura Wilson, ScM, and Katherine Yates, ScM.
Funding
This project received support from the National Institute of Diabetes and Digestive and Kidney Diseases (grants U01DK073983, U01DK073975, U01DK073985, U01DK074007, U01DK073974, and U01DK074008) as part of its funding of the Gastroparesis Clinical Research Consortium.
Abbreviations used in this paper:
- GCSI
Gastroparesis Cardinal Symptom Index
- GI
gastrointestinal
- OINV
opioid-induced nausea and vomiting
- PAGI-QOL
Patient Assessment of Upper Gastrointestinal Quality of Life
- PAGI-SYM
Patient Assessment of Upper Gastrointestinal Symptoms
Footnotes
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at https://doi.org/10.1016/j.cgh.2018.10.013.
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Cherian D, Sachdeva P, Fisher RS, et al. Abdominal pain is a frequent symptom of gastroparesis. Clin Gastroenterol Hepatol 2010;8:676–681. [DOI] [PubMed] [Google Scholar]
- 2.Hasler WL, Wilson LA, Parkman HP, et al. Factors related to abdominal pain in gastroparesis: contrast to patients with predominant nausea and vomiting. Neurogastroenterol Motil 2013; 25:427–e301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jeong ID, Camilleri M, Shin A, et al. A randomized, placebo-controlled trial comparing the effects of tapentadol and oxycodone on gastrointestinal and colonic transit in healthy humans. Aliment Pharmacol Ther 2012;35:1088–1096. [DOI] [PubMed] [Google Scholar]
- 4.Gonenne J, Camilleri M, Ferber I, et al. Effect of alvimopan and codeine on gastrointestinal transit: a randomized controlled study. Clin Gastroenterol Hepatol 2005;3:784–791. [DOI] [PubMed] [Google Scholar]
- 5.Yuan CS, Foss JF, O’Connor M, et al. Effects of low-dose morphine on gastric emptying in healthy volunteers. J Clin Pharmacol 1998;38:1017–1020. [DOI] [PubMed] [Google Scholar]
- 6.Kalso E, Edwards JE, Moore RA, et al. Opioids in chronic non-cancer pain: systematic review of efficacy and safety. Pain 2004;112:372–380. [DOI] [PubMed] [Google Scholar]
- 7.Tuteja AK, Biskupiak J, Stoddard GJ, et al. Opioid-induced bowel disorders and narcotic bowel syndrome in patients with chronic non-cancer pain. Neurogastroenterol Motil 2010; 22:424–430. [DOI] [PubMed] [Google Scholar]
- 8.Porreca F, Ossipov MH. Nausea and vomiting side effects with opioid analgesics during treatment of chronic pain: mechanisms, implications, and management options. Pain Med 2009; 10:654–662. [DOI] [PubMed] [Google Scholar]
- 9.Vorsanger G, Xiang J, Biondi D, et al. Post hoc analyses of data from a 90-day clinical trial evaluating the tolerability and efficacy of tapentadol immediate release and oxycodone immediate release for the relief of moderate to severe pain in elderly and nonelderly patients. Pain Res Manag 2011; 16:245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buynak R, Shapiro DY, Okamoto A, et al. Efficacy and safety of tapentadol extended release for the management of chronic low back pain: results of a prospective, randomized, double-blind, placebo- and active-controlled phase III study. Expert Opin Pharmacother 2010;11:1787–1804. [DOI] [PubMed] [Google Scholar]
- 11.Vorsanger G, Xiang J, Okamoto A, et al. Evaluation of study discontinuations with tapentadol immediate release and oxycodone immediate release in patients with low back or osteoarthritis pain. J Opioid Manag 2010;6:169–179. [DOI] [PubMed] [Google Scholar]
- 12.Tougas G, Eaker EY, Abell TL, et al. Assessment of gastric emptying using a low fat meal: establishment of international control values. Am J Gastroenterol 2000;95:1456–1462. [DOI] [PubMed] [Google Scholar]
- 13.Anderson R, Saiers JH, Abram S, et al. Accuracy in equianalgesic dosing. Conversion dilemmas. J Pain Symptom Manage 2001;21:397–406. [DOI] [PubMed] [Google Scholar]
- 14.Sachdeva P, Malhotra N, Pathikonda M, et al. Gastric emptying of solids and liquids for evaluation for gastroparesis. Dig Dis Sci 2011;56:1138–1146. [DOI] [PubMed] [Google Scholar]
- 15.Abell TL, Camilleri M, Donahoe K, et al. Consensus recommendations for gastric emptying scintigraphy: a joint report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. Am J Gastroenterol 2008;103:753–763. [DOI] [PubMed] [Google Scholar]
- 16.Rentz AM, Kahrilas P, Stanghellini V, et al. Development and psychometric evaluation of the patient assessment of upper gastrointestinal symptom severity index (PAGI-SYM) in patients with upper gastrointestinal disorders. Qual Life Res 2004; 13:1737–1749. [DOI] [PubMed] [Google Scholar]
- 17.Revicki DA, Rentz AM, Dubois D, et al. Development and validation of a patient-assessed gastroparesis symptom severity measure: the Gastroparesis Cardinal Symptom Index. Aliment Pharmacol Ther 2003;18:141–150. [DOI] [PubMed] [Google Scholar]
- 18.De la Loge C, Trudeau E, Marquis P, et al. Cross-cultural development and validation of a patient self-administered questionnaire to assess quality of life in upper gastrointestinal disorders: the PAGI-QOL. Qual Life Res 2004;13:1751–1762. [DOI] [PubMed] [Google Scholar]
- 19.Akaike H A new look at the statistical model identification. IEEE Trans Autom Control 1974;19:716–723. [Google Scholar]
- 20.Bender R, Lange S. Adjusting for multiple testing: when and how? J Clin Epidemiol 2001;54:343–349. [DOI] [PubMed] [Google Scholar]
- 21.Florence CS, Zhou C, Luo F, et al. The economic burden of prescription opioid overdose, abuse, and dependence in the United States, 2013. Med Care 2016;54:901–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilkerson RG, Kim HK, Windsor TA, et al. The opioid epidemic in the United States. Emerg Med Clin North Am 2016;34:e1–e23. [DOI] [PubMed] [Google Scholar]
- 23.Coleski R, Baker JR, Hasler WL. Endoscopic gastric food retention in relation to scintigraphic gastric emptying delays and clinical factors. Dig Dis Sci 2016;61:2593–2601. [DOI] [PubMed] [Google Scholar]
- 24.Fickel J, Bagnol D, Watson SJ, et al. Opioid receptor expression in the rat gastrointestinal tract: a quantitative study with comparison to the brain. Brain Res Mol Brain Res 1997;46:1–8. [DOI] [PubMed] [Google Scholar]
- 25.Tzschentke TM, Christoph T, Kogel B, et al. (1R,2R)-3-(3-dimethylamino-1-ethyl-2-methyl-propyl)-phenol hydrochloride (tapentadol HCl): a novel μ-opioid receptor agonist/norepinephrine reuptake inhibitor with broad-spectrum analgesic properties. J Pharmacol Exp Ther 2007;323:265–276. [DOI] [PubMed] [Google Scholar]
- 26.Parkman HP, Hallinan EK, Hasler WL, et al. Nausea and vomiting in gastroparesis: similarities and differences in idiopathic and diabetic gastroparesis. Neurogastroenterol Motil 2016; 28:1902–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parkman HP, Hallinan EK, Hasler WL, et al. Early satiety and postprandial fullness in gastroparesis correlate with gastroparesis severity, gastric emptying, and water load testing. Neurogastroparesis Motil 2017;29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bielefeldt K, Raza N, Zickmund SL. Different faces of gastroparesis. World J Gastroenterol 2009;15:6052–6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anaparthy R, Pehlivanov N, Grady J, et al. Gastroparesis and gastroparesis-like syndrome: response to therapy and its predictors. Dig Dis Sci 2009;54:1003–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davis MP, Hallerberg G. Palliative Medicine Study Group of the Multinational Association of Supportive Care in Cancer. A systematic review of the treatment of nausea and/or vomiting in cancer unrelated to chemotherapy or radiation. J Pain Symptom Manag 2010;39:756–767. [DOI] [PubMed] [Google Scholar]
- 31.Hardy J, Daly S, McQuade B, et al. A double-blind, randomized, parallel group, multinational, multicenter study comparing a single dose of ondansetron 24 mg p.o. with placebo and metoclopramide 10 mg t.d.s. p.o. in the treatment of opioid-induced nausea and emesis in cancer patients. Support Care Cancer 2002;10:231–236. [DOI] [PubMed] [Google Scholar]
- 32.Torigoe K, Nakahara K, Rahmadi M, et al. Usefulness of olanzapine as an adjunct to opioid treatment and for the treatment of neuropathic pain. Anesthesiology 2012;116:159–169. [DOI] [PubMed] [Google Scholar]
- 33.Okamoto Y, Tsuneto S, Matsuda Y, et al. A retrospective chart review of the antiemetic effectiveness of risperidone in refractory opioid-induced nausea and vomiting in advanced cancer patients. J Pain Symptom Manag 2007;34:217–222. [DOI] [PubMed] [Google Scholar]
- 34.Foss JF, Bass AF, Goldberg LI. Dose-related antagonism of the emetic effect of morphine by methylnaltrexone in dogs. J Clin Pharmacol 1993;33:747–751. [DOI] [PubMed] [Google Scholar]
- 35.Murphy DB, Sutton JA, Prescott LF, et al. Opioid-induced delay in gastric emptying: a peripheral mechanism in humans. Anesthesiology 1997;87:765–770. [DOI] [PubMed] [Google Scholar]
- 36.Woo M, O’Connor M, Yuan CS, et al. Reversal of opioid-induced gastric dysfunction in a critically ill burn patient after methyl-naltrexone. Anesth Analg 2008;107:1965–1967. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


