Abstract
Objective:
A randomized controlled trial of quitline-like phone counseling (QL) versus telemedicine integrated into primary care (ITM) compared the effectiveness of these modalities for smoking cessation. Study design and components were based on Self-Determination Theory (SDT). The purpose of this study was to test our SDT-based model in which perceived healthcare provider autonomy support, working alliance, autonomous motivation, and perceived competence were hypothesized to mediate the effects of ITM on smoking cessation.
Methods:
Rural smokers (560) were randomized to receive 4 sessions over a 3-month period of either QL or ITM. Follow-up assessments were conducted at months 3, 6, and 12. The primary outcome was biochemically verified 7-day point prevalence at 12-months. Structural equation modeling with latent change scores was used for the analysis.
Results:
Participants in the ITM condition reported greater increases in perceived healthcare provider autonomy support (PAS) at end of treatment, which in turn was associated with enhanced perceived competence to quit smoking (PC). Increased PC was associated with a higher likelihood of cessation at 12-months. Mediation analysis demonstrated significant indirect effects, including a path from ITM to increases in PAS to increases in PC to cessation at 12-months (Indirect effect = 0.0183, 95% CI [.003, .0434]).
Conclusions:
When integrated into primary care, ITM may influence smoking cessation by enhancing the extent to which smokers feel supported by their providers and thereby increase their perceived ability to quit. Findings suggest that locating tobacco treatment services in health care provider offices imparts a motivational benefit for cessation.
Trial Registration:
Clinical Trials Registration: NCT00843505
Keywords: Telemedicine, Internet, Rural, Smoking Cessation, RCT, Primary Care
Introduction
Tobacco use causes approximately 5.4 million deaths a year worldwide (World Health Organization, 2012). Although the prevalence of smoking has declined dramatically over the past 40 years in the U.S.(Centers for Disease Control (CDC), 2006), adult smoking rates in rural areas remains above 20% (Center for Behavioral Health Statistics and Quality, 2015).
This may be due, in part, to the fact that our health care system does a poor job of delivering evidence-based tobacco treatment to rural smokers. FDA-approved cessation medications double to triple the odds of quitting (Fiore et al., 2008) and supportive counseling doubles quit rates in the range of 4 sessions of treatment and is more effective at higher “doses” (Fiore et al., 2008). Primary health care providers, however, consistently fail to provide tobacco treatment. In 2015, only 57% of smokers reported that a health care provider advised them to quit (Centers for Disease Control (CDC), 2017). Among smokers who made quit attempts, 6.8% reported using counseling, 29.0% medication, and 4.7% used both (Centers for Disease Control (CDC), 2017).
Two significant approaches have emerged with potential to increase the provision of tobacco treatment by rural health care providers. One method is through tobacco quitlines which are used in conjunction with fax referral to improve the efficiency of providing longitudinal supportive counseling. Nearly all (49) state quitlines offer fax referral systems, in which health care providers identify tobacco users and then fax to quitlines the contact information for consenting patients who will receive their tobacco cessation behavioral care from the quitline (North American Quitline Consortium, 2008). The other method of care provision is through telemedicine. Telemedicine is the use of telecommunications for medical diagnosis and patient care across a distance and is often used to provide medical care to patients in remote locations (Scannell, Perednia, & Kissman, 1995). Broadly defined, telemedicine encompasses video, telephone, text message, and even website-focused approaches. A Cochrane review of interactive telemedicine-delivered care found that telemedicine was as effective as usual care in the treatment of heart failure and substance use disorders, and was more effective than usual care in achieving blood glucose control (Flodgren, Rachas, Farmer, Inzitari, & Shepperd, 2015). A Cochrane review of telemedicine versus face-to-face patient care for a numerous health issues found that telemedicine was as effective as face-to-face treatment and achieved high levels of satisfaction among patients and providers (Currell, Urquhart, Wainwright, & Lewis, 2000). At the time of the trial many rural Kansans lacked sufficient internet speed and computer equipment to handle video transmissions, but most primary care and safety net clinics had the ability to transmit video. This drove the decision to examine the effects of real-time, interactive video counseling, delivered via patients’ medical homes, on smoking cessation.
Given the potential of both phone and telemedicine to be used for tobacco treatment it is unclear whether there might be advantages of one over the other. Long-term quit rates for in-person face-to-face counseling (17%) are somewhat higher than those reported for telephone counseling (13%) (Fiore et al., 2008). In face to face interactions counselors may benefit from patient non-verbal cues that could help to improve the quality of communications. For example face to face interactions allow for observation of facial reactions and body language that can facilitate the judgement of affect or engagement (Foley & Gentile, 2010). With visual awareness of the patient’s reactions to the counseling process the counselor may better understand the patient’s perspective and provide more accurate reflective statements and information. Telemedicine software can also facilitate the sharing of documents such as action plans, worksheets, or educational materials targeted to the needs expressed by the patient in the counseling session. By fully integrating counselor/patient verbal and visual communication, telemedicine may therefore overcome numerous limitations of phone counseling. Patients may consequently experience an enhanced therapeutic relationship.
A recent randomized controlled clinical trial (RCT)—Connect2Quit—compared the effectiveness of telemedicine counseling that was integrated into rural smokers’ primary care clinics (Integrated Telemedicine—ITM) versus telephone counseling, similar to telephone quitline counseling, delivered to smokers in their homes (QL) (Mussulman et al., 2014). Based on principles of Self-Determination Theory (SDT) and psychotherapy research on the importance of the therapeutic alliance for counseling outcomes (Lambert & Barley, 2001; Orlinsky, Ronnestad, & Wilutzski, 2004; Williams, Gagne, Ryan, & Deci, 2002b) it was hypothesized that ITM would outperform telephone counseling. In the pre-specified primary outcome analysis of the trial we found no difference between ITM and QL conditions in rates of 12-month verified abstinence (9.8%, 27/280 vs 12%, 34/286; p =.406), average number of cigarettes per day (p = .721), or quit attempts (p = .347) (Richter et al., 2015).
Although the primary outcome analyses yielded findings contrary to our hypothesis we also pre-specified treatment targets or mediators hypothesized to influence smoking behavior. Incorporating and evaluating the role of hypothesized mediators in clinical trials is recommended for more precise interpretation of the results (Czajkowski et al., 2015). We primarily used Self-determination Theory (SDT) as a basis for our mediation model. SDT posits that when practitioners provide information and resources in a way that patient’s perceive supports their autonomy, patients internalize motivation for change, view themselves as competent to change, and are more likely to achieve lasting behavior change (Deci & Ryan, 1985). Important elements of autonomy-supportive counseling include listening carefully to patients perspectives, providing relevant information, inviting questions, offering treatment options, supporting patient attempts at behavior change, and minimizing control (Williams, Gagne, Ryan, & Deci, 2002a). This type of intervention leads to increased autonomous motivation, increased competence, and behavior change (Deci & Ryan, 1985). SDT has been effectively used in many clinical settings, for numerous health, mental health, and behavioral outcomes (Deci & Ryan, 1985, 2002; Williams et al., 2002c), including smoking cessation (Williams et al., 2006; Williams, Niemiec, Patrick, Ryan, & Deci, 2016).
In addition to the SDT framework we incorporated the construct of therapeutic alliance into our mediation model. Therapeutic alliance is an inherent aspect of counseling (i.e., independent of the counseling approach) and is well established as a key predictor of therapeutic outcome including smoking behavior (Ahn & Wampold, 2001; Klemperer, Hughes, Callas, & Solomon, 2017). Therapeutic alliance encompasses the bond between the counselor and patient as well as their agreement on methods and goals of treatment (Lambert, 2016). Previous research has observed a positive association between therapeutic alliance and autonomous motivation (McBride et al., 2010). Thus, it is expected that facets of ITM that are hypothesized to promote autonomous motivation may also promote participant-rated therapeutic alliance. In addition to a collaborative relational style, the theoretical conceptualization of therapeutic alliance has been expanded to reflect the idea that the quality of the working relationship depends upon the ease with which therapeutic ruptures and disagreements are resolved (Doran, 2016). Given that telemedicine may facilitate the judgement of affect through the presence of visual cues, it is possible that ITM may allow for more rapid identification of tension or disagreement. Relative to telephone counseling, this enhancement may allow for the more rapid resolution of difficult moments in counseling thereby promoting, or maintaining, alliance.
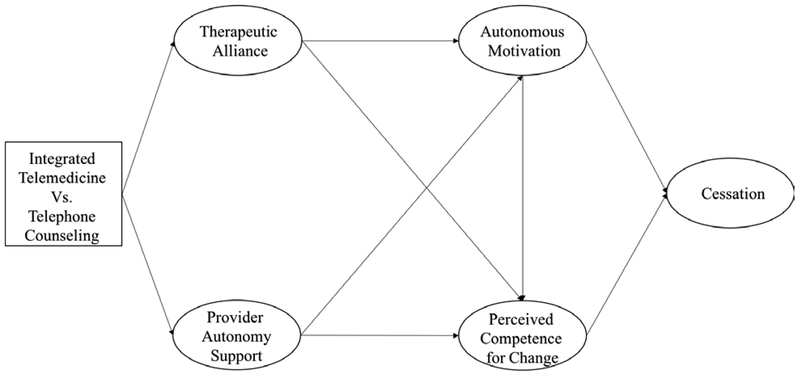
Figure 1 depicts the conceptual model of how we hypothesized SDT constructs and therapeutic alliance would facilitate smoking cessation in the context of ITM. We hypothesized that ITM would create a stronger therapeutic alliance and better facilitate the counselor’s ability to offer autonomy-supportive counseling. Although we trained counselors in both phone and ITM to use an autonomy supportive communication style, we hypothesized that ITM would facilitate more supportive counseling interactions than phone counseling because of the availability of visual cues and the facilitation of information exchange targeted to expressed patient needs. We also hypothesized that because ITM sessions were located in primary care offices, providers would be more likely to address smoking cessation informally or formally with the patients in that study condition. We hypothesized that the patients in ITM would therefore be more likely to perceive that their health care provider supported them making an attempt to quit than patients who received counseling over the phone at home. We further hypothesized that this might lead to greater perceived autonomy support from their provider even though the provider was not directly engaged in counseling. This was predicted to result in greater perceived competence and autonomous motivation for quitting and in turn an increased likelihood of quitting.
Figure 1.
Conceptual model of how the SDT Process Influences Cessation
The aim of this study was to conduct a secondary analysis to evaluate this SDT-based conceptual model to further our insight into the effects of telemedicine and quitline phone counseling for smoking cessation and better interpret the results of our clinical trial.
Methods
Study Design
The design of this study (Connect2Quit) has been published previously (Mussulman et al., 2014). The study enrolled 566 smokers who were recruited through 20 primary care clinics throughout the state of Kansas from June, 2009 through June, 2011. Six participants died during the course of the study and thus were excluded from the present analysis (n = 560). Participants were recruited through radio, community health fairs, religious organizations, and businesses. Participants were randomly assigned to the ITM and QL conditions, respectively. Four counseling sessions occurred at weeks 0, 1, 4, and 8. Follow-up assessments were conducted at months 3, 6, and 12. The reimbursement schedule for the study was $20 at baseline, $20 at 3 months, $20 at 6 months, and $50 at 12 months. Participating primary care clinics received $1,000 for incidental costs related to the study and a computer and PVX software used to implement the ITM intervention. The University of Kansas Medical Center approved all study procedures. Written informed consent was obtained from each study participant prior to enrollment in the trial.
Eligibility
Eligible participants were required to have a physician participating in the study, be 18 years or older, smoke 5 or more cigarettes per day for at least one year, smoke 25 or more days out of the past 30, speak English or Spanish, and have a telephone. Exclusion criteria included using non-cigarette tobacco products, currently taking smoking cessation products, currently breast feeding or planning to get pregnant, planning to move in the next year, not having a healthcare provider, or living with a current study participant (Mussulman et al., 2014).
Intervention
Study staff-delivered components.
In the week following enrollment, all participants received a mailed packet of educational materials on smoking cessation, a timeline of study activities, and individualized information on appropriate quit smoking medications given insurance and medical status. Participants were randomly assigned to either the ITM or QL treatment conditions.
Participants in the ITM condition received four sessions of smoking cessation counseling located in their primary care clinic. The QL condition consisted of four sessions that were conducted via telephone in participants’ homes or by mobile phone. The content of the QL condition was designed to mimic tobacco quitline counseling. The content of counseling in the ITM and QL conditions was based on the Combined Behavioral Intervention (CBI), which integrates Motivational Interviewing and Cognitive Behavioral Therapy (“Brief treatments for cannabis dependence: findings from a randomized multisite trial,” 2004; Miller, 2004; Stephens, Babor, Kadden, & Miller, 2002). The counseling in both treatment arms was adapted to adhere to principles of Self-Determination Theory (SDT). Self-Determination Theory posits that interventions that support participant autonomy facilitate the internalization of motivation to change and increase perceived competence to do so and thereby make behavior change more likely (Deci & Ryan, 1985). Components of counseling consistent with SDT included listening to participant perspectives, providing relevant information, inviting questions, supporting attempts at behavior change while minimizing counselor pressure and control so that patients could make their own choices related to quitting (Williams et al., 2002a). Quit Tips and pharmacotherapy guidance were provided to all patients during the first counseling session. A 5-Day Quit Plan was also completed during the first counseling session and updated as desired by the participant.
Physician office involvement.
Patients were recruited from primary-care clinics. Providers were informed about the study and agreed to facilitate recruitment by identifying and referring smokers as well as providing a room to place the equipment. Although participants’ physicians did not have a formal role in the intervention, participants in both ITM and QL arms were encouraged to collaborate with their physicians on quitting smoking by sharing with them their Quit Plans and requesting advice and scripts for smoking cessation medications.
Measures
Healthcare Provider Autonomy Support.
The Healthcare Climate Questionnaire, short-form (HCCQ) was used in the present study as a measure of participants’ perception of support provided by their healthcare providers and has been used previously within the context of smoking cessation (Williams et al., 2002a; Williams, Grow, Freedman, Ryan, & Deci, 1996). The short-form version of the HCCQ has 6 items (e.g., My health care providers listen to how I would like to do things regarding my smoking) and is scored on a 1 (strongly disagree) to 7 (strongly agree) scale. The HCCQ was administered at study baseline and the 3-month follow-up. The HCCQ at study baseline (ωTotal = .90, 95% CI [.88, .91]) and at 3-months (ωTotal = .93, 95% CI [.91, .94]) had satisfactory internal consistency. The HCCQ will be referred to subsequently by the construct it represents, provider autonomy support (PAS).
Working Alliance.
The Working Alliance Inventory, short-form (WAI) was administered to measure the therapeutic alliance perceived by participants with their smoking cessation counselor. The WAI is a 12-item measure (e.g., My Connect 2 Quit counselor and I agreed about the things I need to do help improve my situation) scored on a 7-point Likert-scale ranging from 1 (never) to 7 (always) (Tracey & Kokotovic, 1989). The WAI was administered at the 3-month follow-up only because it could not be used prior to the participants receiving counseling. The WAI had satisfactory internal consistency (ωTotal = .85, 95% CI [.78, .90]).
Perceived Competence for Smoking Cessation.
The Perceived Competence Scale for Smoking Cessation (PC) was administered to measure the degree to which participants felt able to quit smoking. The Perceived Competence scale is a 4-item measure (e.g., I feel confident in my ability to quit smoking) scored on a 7-point Likert-scale ranging from 1 (strongly disagree) to 7 (strongly agree) (Williams, Freedman, & Deci, 1998). The internal consistencies for PC at study baseline (ωTotal = .82, 95% CI [.79, .85]) and at 3-months (ωTotal = .94, 95% CI [.93, .95]) were satisfactory.
Autonomous Motivation.
The Treatment Self-Regulation Questionnaire (TSRQ) was administered to measure the degree to which participants had internalized their motivation to quit smoking. The version of the TSRQ used in the present study was adapted specifically for smoking cessation (Ryan & Connell, 1989; Williams et al., 2002a). The measure has 12 items (e.g., The reason I would quit smoking is because I feel that I want to take responsibility for my own health) scored on a 7-point Likert scale from 1 (strongly disagree) to 7 (strongly agree). The present analysis used only the six items composing the Autonomous Motivation (AM) subscale of the TSRQ. The internal consistency of the AM subscale was adequate at both study baseline (ωTotal = .84, 95% CI [.80, .88]) and 3-months (ωTotal = .89, 95% CI [.85, .92]).
Smoking cessation.
Two indicators of smoking cessation were used as outcome measures. The primary outcome of the trial and the primary focus of our analysis was verified quit at 12 months (M12QUIT), assessed using 7-day point prevalence that was verified using salivary cotinine, exhaled carbon monoxide (CO), or by proxy. A 15ng/ml cut-point was used to differentiate smokers and nonsmokers (SRNT Subcommittee on Biochemical Verification, 2002). Samples were returned to the University of Kansas Medical Center and stored in a freezer. Samples were sent to Dr. Neal Benowtiz at the University of California, San Francisco for analysis using a gas chromatography technique (Jacob, Wilson, & Benowitz, 1981; Spierto et al., 1988). Participants who reported quitting and were taking nicotine replacement therapy were asked to provide an exhaled CO sample. Samples ≤ 10 ppm were considered abstinent (Hughes et al., 2003). A secondary outcome of the trial was prolonged abstinence defined as self-reported continuous abstinence following a “grace period” of 1 month from beginning of treatment to allow treatment to take effect (Hughes et al., 2003). We supplemented our main analysis with an examination of the SDT-based model using prolonged abstinence as the outcome.
The self-reported number of days quit in the past 6-months and number of quit attempts made in the last 6-months were used in bivariate correlations with the SDT constructs. These self-reported smoking cessation outcomes were collected during the 12-month follow-up assessment.
Data Analysis
In order to test the conceptual model, structural equation modeling (SEM) using latent change scores (LCS) was used to model the inter-relationships among changes in the SDT constructs and their associations with smoking cessation. The analysis focused on 12-month abstinence, which was biochemically confirmed and the primary outcome of the trial, but we also repeated all analyses using prolonged abstinence. The use of LCS is preferable to that of observed change scores because measurement error can be modeled separately in the latent variable framework, whereas change scores are contaminated by measurement error when calculated directly from the observed data (McArdle & Hamagami, 2003). Latent change scores are explicitly modeled distinct latent variables and represent the change in a latent construct occurring between adjacent time-points (Selig & Preacher, 2009). In this analysis, all latent change scores represent change in SDT constructs occurring between the assessments at baseline and 3-months. Latent change scores are estimated by constraining the paths from latent variable (LV)Time 1 to LVTime2 and from ΔLVTime2,1 to LVTime2 to one (MacKinnon, 2008; McArdle & Hamagami, 2003). In order to account for the dependence among observations due to repeated measurements, clustered standard errors were estimated using the TYPE = COMPLEX function in Mplus, version 7.4 (Muthén & Muthén, 1998–2011).
Mediation models using LCS were adapted from LCS mediation models discussed in Selig and Preacher (2009) and MacKinnon (2008). The product-of-coefficients approach was used to estimate point estimates of the indirect effects. Assessing the statistical significance of indirect effects using p-values is liable to Type II error (Fritz & MacKinnon, 2007; Hayes & Scharkow, 2013). Confidence intervals for the indirect effects were estimated using Monte Carlo simulation (MC) (Preacher & Selig, 2012). For each indirect effect, the constituent unstandardized regression coefficients and asymptotic covariance matrix of these estimates were used as the parameters of a multivariate normal sampling distribution. A large sampling distribution (1 × 107) for each regression coefficient was obtained. The product of the sampling distributions for the regression coefficients formed the sampling distribution for the indirect effect, of which the 95% confidence interval was derived by obtaining the values at the 2.5th and 97.5th percentiles.
Six mediation paths were estimated. Indirect effect 1 was a path from study arm (ITM vs. QL) to verified quit at 12-months through ΔPAS and ΔAM. Indirect effect 2 was comprised by a path from study arm to cessation through WAI and ΔPC. Indirect effect 3 was a path from study arm to cessation through ΔPAS and ΔPC. Indirect Effect 4 was comprised by a path from study arm to cessation through WAI and ΔAM. Indirect effect 5 was comprised of a path from study arm to cessation through WAI, ΔAM and ΔPC. Lastly, indirect effect 6 was comprised of a path from study arm to cessation through, ΔPAS, ΔAM and ΔPC.
Before specifying the structural model, longitudinal invariance was tested for each measure with repeated assessments in order to establish the invariance of SDT-related constructs over time (Little, Preacher, Selig, & Card, 2007). Strong invariance (item loadings and intercepts constrained to equality across time) was achieved for HCCQ and PC. Weak invariance (item loadings constrained to equality) was achieved for PC. In addition, the measurement model for the WAI was also specified to ensure adequate fit and construct validity. Satisfactory model fit was achieved for a single-factor measurement model for the WAI (χ2 (52) = 129.90, RMSEA =.061, 90% CI [.048, .074], TLI =.879).
The structural model was adjusted for nicotine dependence, age, gender, and education. Rates of missing data were 17%, 14%, and 12% for months 3, 6, and 12, respectively. Missing data were handled using full information maximum likelihood (FIML). Fit statistics for the structural model could not be estimated due to the inclusion of the categorical outcome (M12QUIT) and the use of clustered standard errors. All confirmatory and structural models were estimated using robust maximum likelihood in Mplus, version 7.4 (Muthén & Muthén, 1998–2011). Monte Carlo simulation of the confidence intervals for the indirect effects was conducted in R, version 3.3.1 (R Core Team, 2016).
Results
Preliminary Analyses
Sample characteristics by treatment arm are presented in Table 1. No significant differences were observed with respect to treatment arm. As reported previously in the main outcomes paper (Richter et al., 2015), the rate of self-reported 7-day point prevalent abstinence at 3-months for the quitline and ITM groups were 19.4% and 23.2% at 3-months, respectively. The rates of self-reported 7-day point prevalent abstinence for the quitline and ITM groups at 6-months were 20.4% and 20.3%, respectively. The rates of biochemically verified abstinence at 12-months for the quitline and ITM groups were 12% and 9.8%, respectively. The rates of prolonged abstinence for the quitline and ITM groups was 8.1% and 7.6%, respectively. There were no significant group differences at any of the time points.
Table 1.
Baseline Characteristics Stratified by Arm
| Quitline (N = 284) |
Integrated Telemedicine (N = 276) |
||||
|---|---|---|---|---|---|
| Variable | N (%) or M (SD) | N (%) or M (SD) | p-Value | ||
| Age | 47.51 | (12.96) | 47.27 | (12.81) | 0.83 |
| Female | 190 | (66.90) | 173 | (62.68) | 0.33 |
| Education ≥ HS | 126 | (44.37) | 115 | (41.67) | 0.55 |
| Has health insurance | 178 | (62.68) | 174 | (63.04) | 0.93 |
| FTND, baseline | 4.85 | (2.35) | 4.91 | (2.2) | 0.74 |
| CPD, baseline | 19.22 | (9.84) | 20.27 | (10.69) | 0.23 |
| PC, baseline | 19.90 | (6.06) | 19.82 | (5.83) | 0.88 |
| AM, baseline | 38.71 | (5.30) | 39.08 | (4.76) | 0.38 |
| HCCQ, baseline | 30.63 | (10.48) | 31.17 | (9.82) | 0.52 |
| WAI, month 3 | 69.25 | (8) | 70.38 | (5.80) | 0.10 |
FTND = Fagerström Test of Nicotine Dependence, CPD = Cigarettes per day, PC = Perceived Competence to Quit Smoking, AM = Autonomous Motivation, HCCQ = Healthcare Climate Questionnaire, WAI = Working Alliance Inventory
The SDT-related constructs were moderately correlated concurrently and prospectively (Table 2). Self-Determination Theory constructs at baseline and 3-months were weakly-to-moderately associated with smoking outcomes at 12-months. The number of days quit in the last 6-months, reported at 12-months, was positively associated with autonomous motivation at baseline (r = .12, p = .01) and 3-months (r = .19, p < .01), perceived competence for quitting at 3-months (r = .31, p < .01), and healthcare provider support at 3-months (r = .14, p = .01). The number of quit attempts in the past 6-months at the end of study follow-up was positively associated with perceived competence at baseline (r = .12, p = .01).
Table 2.
Correlation Matrix of Self-Determination Theory Constructs and Smoking Cessation Outcomes
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Autonomous Motivation, Baseline | - | ||||||||
| 2 | Autonomous Motivation, Month 3 | 0.58** | - | |||||||
| 3 | Working Alliance Inventory, Month 3 | 0.27** | 0.42** | - | ||||||
| 4 | Perceived Competence to Quit Smoking, Baseline | 0.38** | 0.28** | 0.13* | - | |||||
| 5 | Perceived Competence to Quit Smoking, Month 3 | 0.26** | 0.4** | 0.22** | 0.41** | - | ||||
| 6 | Healthcare Climate Questionnaire, Baseline | 0.17** | 0.12* | 0.15** | 0.16** | 0.1* | - | |||
| 7 | Healthcare Climate Questionnaire, Month 3 | 0.08 | 0.2** | 0.23** | 0.12* | 0.25** | 0.35** | - | ||
| 8 | Number of days quit in last 6-months, Month 12 | 0.12* | 0.19** | 0.1 | 0.04 | 0.31** | 0.1 | 0.14* | - | |
| 9 | Number of quit attempts in past 6-months, Month 12 | 0.07 | 0.08 | 0.04 | 0.12* | 0.1 | 0.09 | 0.07 | 0.07 | - |
p <.05
p <.01
Structural Equation Model and Mediation Analysis
Because results of the model using 7-day point prevalence verified cessation as the outcome were very similar to the model for prolonged abstinence we present the findings for the primary outcome only. The results of the structural equation model are depicted in Figure 2. Relative to the Quitline condition (QL), Integrated Telemedicine (ITM) was associated with significantly greater gains in perceived healthcare provider autonomy support (ΔPAS) during the 3-month intervention period (b = .347, p < .001). Contrary to our hypothesis, being in the telemedicine arm did not lead to greater perceived working alliance (WAI) at 3-months. Intervention condition did not have significant associations with change in autonomous motivation (ΔAM) or perceived competence to quit (ΔPC) or verified smoking cessation at 12-months.
Figure 2. Structural Model Diagram and Parameter Estimates.
Note: PAS = Healthcare provider autonomy support, PC = Perceived Competence to Quit Smoking, AM = Autonomous Motivation Subscale, WAI = Working Alliance Inventory, ITM = Integrated Telemedicine, QL = Quitline, * = Fixed parameters. Ovals represent latent variables and rectangles represent observed variables. Regression coefficients are unstandardized for latent variables. Coefficients for predictors of smoking cessation are odds ratios. The structural model was adjusted for age, gender, education, and nicotine dependence.
The structural model showed different patterns of association between WAI and ΔPAS and the latent changes scores for AM and PC. Increases in healthcare provider support were positively associated with increases in ΔPC (b = .136, p < .001) and were significantly associated with change in ΔAM (b = .052, p < .01.). Working alliance at 3-months was not associated with increases in ΔPC (b = .360 p = n.s.), but was positively associated with ΔAM (b = .562, p < .01).
The latent change score for PC was the only SDT construct that predicted biochemically verified smoking cessation at 12-months (OR = 1.474, p < .01). Nicotine dependence, as measured by the FTND, was associated with a reduced likelihood of smoking cessation (OR = .838, p = .001).
Estimated indirect effects with their respective 95% confidence intervals are presented in Table 3. The path from arm to ΔPAS to ΔPC to M12Quit was statistically significant (indirect effect = .0183, 95% CI [.003, .044]). This indirect effect can be expressed and interpreted as an odds ratio (Huang, Sivaganesan, Succop, & Goodman, 2004; Preacher, 2015). The indirect effect coefficients in this analysis represent the product of the regression coefficient for each individual path comprising the indirect effect. In other words, they represent a composite effect of treatment arm and first and second-order mediators. Thus, the joint of effect of ITM and intra-individual increases in perceived autonomy support and perceived competence to quit smoking, is associated with a 1.8% increase in odds of smoking cessation compared to telephone counseling. The indirect effect from arm to ΔPAS to ΔAM to ΔPC to M12 Quit was also significant (indirect effect = .003, 95% CI [.002, .015]). For this path, the joint effect of ITM and intra-individual changes in provider autonomy support, autonomous motivation and perceived competence is associated with a 0.3% increase in odds of cessation.
Table 3.
Point Estimates and 95% Confidence Intervals for Indirect Effects
| Path | Point Estimate | 95% Confidence Interval | |
|---|---|---|---|
| Lower Limit | Upper Limit | ||
| Arm → ΔPAS → ΔAM → M12 Quit | −0.0028 | −0.0106 | 0.0044 |
| Arm → WAI → ΔPC → M12 Quit | 0.0067 | −0.016 | 0.0218 |
| Arm → ΔPAS → ΔPC → M12 Quit | 0.0183 | 0.003 | 0.0434 |
| Arm → WAI → ΔAM → M12 Quit | −0.0042 | −0.0156 | 0.0228 |
| Arm → WAI → ΔAM → ΔPC → M12 Quit | 0.0043 | −0.0162 | 0.0393 |
| Arm → ΔPAS → ΔAM → ΔPC → M12 Quit | 0.0029 | 0.0015 | 0.0151 |
Note. Estimates are on the logit scale.
ΔPAS = Healthcare Provider Autonomy Support Latent Change Score, ΔAM = Autonomous Motivation Latent Change Score, ΔPC = Perceived Competence to Quit Smoking Latent Change Score, WAI = Working Alliance Inventory at 3-Months
Discussion
Whereas the primary outcome analysis (Richter et al., 2015) showed no difference between treatments in their effects on cessation, the present analysis provides deeper insight into effective pathways and mechanisms of action. We found that integrated telemedicine predicted perceived autonomy support from health care providers at 3-months after randomization, which in turn was directly associated with perceived competence to quit at 3-months after randomization, which predicted smoking cessation after treatment. Additionally, a second indirect effect was observed that extended the first by incorporating change in autonomous motivation. These pathways were consistent with the SDT intervention model, and provide evidence that integrated telemedicine outperforms phone counseling in facilitating greater perceived provider support for cessation. These results are supported by our previously published finding that ITM participants were more likely to recommend the intervention to other smokers than phone counseling participants (Richter et al., 2015).
The effective pathways identified through SEM did not involve all of the mechanisms we originally hypothesized would contribute to the primary outcome—this perhaps accounts for the observation of no overall group differences in outcome. First, the effects of the interventions on targeted mediators were mixed. Integrated telemedicine positively influenced change in perceived provider autonomy support, but surprisingly, the visual cues in ITM did not appear to lead to enhanced working alliance relative to phone. We used the HCCQ to assess support from participants’ health care providers, and the WAI to evaluate participants’ interactions with their smoking cessation counselors. We may have not detected a difference in alliance because participants in ITM attended fewer sessions than those receiving phone counseling, such that any advantages to communicating via telemedicine were overwhelmed by the superior dose of phone contact. This dose imbalance would not affect perceived health care provider support, because only ITM participants had sessions at their providers’ offices. Alternatively, telemedicine for smoking cessation may simply have no alliance related advantages over phone counseling. Telemedicine appears to perform as well as face-to-face treatment for a number of conditions, and a number of trials have demonstrated that telephone performs as well as face-to-face counseling at delivering interventions for a number of health interventions, including genetic counseling and obesity treatment (Appel et al., 2011; Harrigan et al., 2016; Schwartz et al., 2014). The logical extension is that telephone may perform as well as telemedicine counseling.
Second, targeted mediators were positively correlated with second order SDT mediators and influenced them as expected. In two prior studies of SDT interventions on smoking cessation, changes in HCCQ enhanced autonomous motivation, which in turn increased rates of cessation (Williams et al., 2002b; Williams et al., 2006). In the present analysis, a similar effect was observed. Provider autonomy support was associated with greater change in autonomous motivation, which in turn was associated with increased PC and greater odds of cessation. Perceived autonomy support may have been bolstered by the proximity of providers during counseling sessions, and by additional information and treatment assistance they may have provided before, during, and/or after each session. Thus, this indirect effect is consistent with that observed in previous SDT-informed interventions for smoking cessation (Williams et al., 2006).
Third, second order mediators had mixed effects on the outcome. Consistent with Williams and colleagues (2006), the ITM intervention significantly increased perceived autonomy support, which was associated with greater changes in perceived competence for quitting smoking, which in turn was significantly associated with smoking cessation. Receiving counseling via telemedicine in the medical home enhanced perceived competence in a way that home-based phone counseling did not. Perhaps the perception that the health care team was prepared to expend office space, provider time, and technology on smoking cessation—in addition to the enhanced feelings of support from the providers—provided evidence that providers believed in the participants’ ability to quit, which in turn helped participants believe in their own ability to quit. The association between perceived competence and quitting smoking accords with current literature. Perceived competence and self-efficacy, which are distinct but similar concepts related to the ability to perform behaviors, are among the most reliable psychological predictors of health behavior change (Ng et al., 2012; Strecher, DeVellis, Becker, & Rosenstock, 1986).
This suggests that cessation counseling at a distance—that is in some way integrated into the medical office milieu—enhances efforts to quit smoking by boosting autonomy support directly and perceived competence indirectly. Integrating distance counseling in busy and often crowded clinical practices might be a challenge, but merits consideration. The parent study found that costs for delivering ITM versus phone counseling were equivalent, except for the cost of the clinic space for the ITM session (Richter et al., 2015). One solution might be to hold ITM sessions after clinic business hours, which would extend the utility of the office without taking examining room time away from the treatment of other conditions. Reimbursement for telemedicine-delivered care, which is available in some states, obviates this issue and facilitates individuals to choose their preferred approach to smoking cessation interventions.1
The findings provide support for the SDT model that was examined and replicates findings from 2 prior SDT based smoking cessation trials as well as a study of SDT in diabetes self-management (Williams et al., 2006; Williams, McGregor, Zeldman, Freedman, & Deci, 2004; Williams, Niemiec, Patrick, Ryan, & Deci, 2009; Williams et al., 2016). The present study extends the findings from those trials which used face to face and phone counseling by showing that ITM is also effective at fostering cessation through autonomy support, autonomous motivation, and perceived competence. Our results also indicated that the indirect effects of the intervention were ultimately mediated through perceived competence rather than autonomous motivation. This is consistent with both prior SDT based smoking cessation studies as well as a study of SDT in diabetes self-management (Williams et al., 2004).
Taken as a whole, results indicate that SDT is a useful model for understanding health behavior change and for identifying key behavioral mechanisms for facilitating behavior change. The two statistically significant indirect effects observed in this analysis were entirely consistent with SDT. Nevertheless, the magnitude of the indirect effect of telemedicine on cessation was small and we found relatively small effects between the first and second order mediators. In particular, the effect of autonomy support on autonomous motivation and competence was near zero (b = .05). Thus, the intervention was able to increase provider autonomy support, however the downstream effect of enhancing autonomy support on second order mediators was limited in this trial. This is consistent with the lack of an overall difference in cessation between ITM and phone that was observed in the primary outcome analysis (Richter et al., 2015). This result may also be related to the fact that we did not assess autonomous motivation for medication adherence. Williams et al. (2006) used a combined measure of autonomous motivation that included autonomous motivation for medication use and autonomous motivation for cessation. They found a significant path between autonomous motivation and cessation via medication use. Thus our findings may underestimate the effect of autonomous motivation on cessation. Moreover, Williams et al. (2006) operationalized abstinence by means of a latent variable that incorporated 7-day point prevalence abstinence, longest number of days not smoking, and number of days since last cigarette. Both these measurement differences may have contributed to the lack of a direct association between autonomous motivation and cessation found in the present study.
The study had a number of strengths and limitations. This study is an excellent test of the interventions and the effects of SDT, because we specified our conceptual model a priori, carefully measured each observed variable and putative SDT mechanism, and collected repeated measures longitudinally to assess the effects of the intervention on SDT mechanisms across time. Because SDT has been widely used for health behavior change, including smoking cessation, we were able to employ validated measures of theoretical components. One limitation is that we opted to measure autonomy support via only the Health Care Climate Questionnaire, and we asked participants to report solely on the support provided by health care providers. In addition, we did not posit a priori hypotheses regarding how treatment might engage controlled motivation in service of behavior change because the intervention implemented in this study was designed to be autonomy supportive. Although previous SDT research has also not observed an association between controlled motivation and smoking cessation (Williams et al., 2006), it may be useful for future SDT-informed research to include controlled motivation to confirm these findings and to provide a more complete picture of the interrelationships between SDT constructs.
With regard to the Working Alliance Questionnaire, we asked participants to report solely on the alliance they felt with their smoking cessation counselors. We could have asked for participants to report on the autonomy support and therapeutic alliance they experienced from both their health care providers and their smoking cessation counselors, but this would have created additional respondent burden and potentially confused patients through excess repetition of the same measures. There may also be other constructs and pathways that are not based on SDT or working alliance that influenced the outcomes of the trial. Study findings are not generalizable outside of the treatment of rural smokers. Last, although our mediators were measured longitudinally, it is important to not assume causal associations between these factors. This is particularly the case with regard to change in autonomous motivation and change in perceived competence, as these constructs were measured concurrently.
In spite of these limitations, the present findings suggest the study interventions generally influenced SDT constructs as expected. However, the indirect effect of telemedicine on cessation through SDT constructs was small and not sufficient to yield clinically meaningful differences in cessation. This is consistent with the null findings of the primary outcome analysis and highlights how mediation analysis can be used to gain better insight into the mechanisms of change underlying the results of intervention trials.
Acknowledgements:
The project was supported by Award Number R01HL087643 from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, And Blood Institute or the National Institutes of Health.
Funding Source: Award Number R01HL087643 from the National Heart, Lung, and Blood Institute
Footnotes
Related presentations:
Liebmann, E., Preacher, K.J., Richter, K.P., Cupertino, A.P., Catley, D. (April, 2018) Testing the Mechanisms of Action of Integrated Telemedicine for Smoking Cessation Using Self-Determination Theory. Society of Behavioral Medicine Conference: New Orleans, LA.
Reviewer 2 is credited with providing language that clearly articulates this point.
References
- Ahn H.-n., & Wampold BE (2001). Where oh where are the specific ingredients? A meta-analysis of component studies in counseling and psychotherapy. Journal of Counseling Psychology, 48(3), 251–257. doi: 10.1037/0022-0167.48.3.251 [DOI] [Google Scholar]
- Appel LJ, Clark JM, Yeh HC, Wang NY, Coughlin JW, Daumit G, … Brancati FL (2011). Comparative effectiveness of weight-loss interventions in clinical practice. N Engl J Med, 365(21), 1959–1968. doi: 10.1056/NEJMoa1108660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brief treatments for cannabis dependence: findings from a randomized multisite trial. (2004). J Consult Clin Psychol, 72(3), 455–466. doi: 10.1037/0022-006x.72.3.455 [DOI] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics and Quality. (2015). Behavioral health trends in the United States: Results from the 2014 National Survey on Drug Use and Health HHS Publication (No. SMA 15–4927, NSDUH Series H-50). http://www.samhsa.gov/data/.
- Centers for Disease Control (CDC). (2006). Tobacco use among adults--United States, 2005. MMWR. Morbidity and Mortality Weekly Report, 55(42), 1145–1148. [PubMed] [Google Scholar]
- Centers for Disease Control (CDC). (2017). Tobacco use among adults--United States, 2005–2015. MMWR. Morbidity and Mortality Weekly Report, 65(52), 1457–1464. [DOI] [PubMed] [Google Scholar]
- Currell R, Urquhart C, Wainwright P, & Lewis R (2000). Telemedicine versus face to face patient care: effects on professional practice and health care outcomes. Cochrane Database Syst Rev (2), Cd002098. doi: 10.1002/14651858.cd002098 [DOI] [PubMed] [Google Scholar]
- Czajkowski SM, Powell LH, Adler N, Naar-King S, Reynolds KD, Hunter CM, … Charlson ME (2015). From ideas to efficacy: The ORBIT model for developing behavioral treatments for chronic diseases. Health Psychol, 34(10), 971–982. doi: 10.1037/hea0000161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deci E, & Ryan R (1985). Intrinsic motivation and self-determination in human behavior. New York: Plenum. [Google Scholar]
- Deci E, & Ryan R (2002). Handbook of self-determination research. Rochester, NY: University of Rochester Press. [Google Scholar]
- Doran JM (2016). The working alliance: Where have we been, where are we going? Psychother Res, 26(2), 146–163. doi: 10.1080/10503307.2014.954153 [DOI] [PubMed] [Google Scholar]
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, & et al. (2008). Treating tobacco use and dependence 2008 update. Clinical practice guidelines. Retrieved from Rockville, MD: Accessed: [Google Scholar]
- Flodgren G, Rachas A, Farmer AJ, Inzitari M, & Shepperd S (2015). Interactive telemedicine: effects on professional practice and health care outcomes. Cochrane Database Syst Rev (9), Cd002098. doi: 10.1002/14651858.CD002098.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley GN, & Gentile JP (2010). Nonverbal communication in psychotherapy. Psychiatry (Edgmont), 7(6), 38–44. [PMC free article] [PubMed] [Google Scholar]
- Fritz MS, & MacKinnon DP (2007). Required Sample Size to Detect the Mediated Effect. Psychological science, 18(3), 233–239. doi: 10.1111/j.1467-9280.2007.01882.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrigan M, Cartmel B, Loftfield E, Sanft T, Chagpar AB, Zhou Y, … Irwin ML (2016). Randomized Trial Comparing Telephone Versus In-Person Weight Loss Counseling on Body Composition and Circulating Biomarkers in Women Treated for Breast Cancer: The Lifestyle, Exercise, and Nutrition (LEAN) Study. J Clin Oncol, 34(7), 669–676. doi: 10.1200/jco.2015.61.6375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF, & Scharkow M (2013). The relative trustworthiness of inferential tests of the indirect effect in statistical mediation analysis: does method really matter? Psychol Sci, 24(10), 1918–1927. doi: 10.1177/0956797613480187 [DOI] [PubMed] [Google Scholar]
- Huang B, Sivaganesan S, Succop P, & Goodman E (2004). Statistical assessment of mediational effects for logistic mediational models. Stat Med, 23(17), 2713–2728. doi: 10.1002/sim.1847 [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, & Swan GE (2003). Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res, 5(1), 13–25. [PubMed] [Google Scholar]
- Jacob P 3rd, Wilson M, & Benowitz NL (1981). Improved gas chromatographic method for the determination of nicotine and cotinine in biologic fluids. J Chromatogr, 222(1), 61–70. [DOI] [PubMed] [Google Scholar]
- Klemperer EM, Hughes JR, Callas PW, & Solomon LJ (2017). Working alliance and empathy as mediators of brief telephone counseling for cigarette smokers who are not ready to quit. Psychol Addict Behav, 31(1), 130–135. doi: 10.1037/adb0000243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert MJ (2016). The therapeutic alliance: An evidence-based guide to practice. Psychotherapy Research, 26(2), 59–61. [Google Scholar]
- Lambert MJ, & Barley DE (2001). Research summary on the therapeutic relationship and psychotherapy outcome. Psychotherapy: Theory, research, practice, training, 38(4), 357. [Google Scholar]
- Little TD, Preacher KJ, Selig JP, & Card NA (2007). New developments in latent variable panel analyses of longitudinal data. International Journal of Behavioral Development, 31(4), 357–365. [Google Scholar]
- MacKinnon D (2008). Introduction to Statistical Mediation Analysis. New York: Lawrence Erlbaum Associates. [Google Scholar]
- McArdle JJ, & Hamagami F (2003). Structural equation models for evaluating dynamic concepts within longitudinal twin analyses. Behav Genet, 33(2), 137–159. [DOI] [PubMed] [Google Scholar]
- McBride C, Zuroff DC, Ravitz P, Koestner R, Moskowitz DS, Quilty L, & Bagby RM (2010). Autonomous and controlled motivation and interpersonal therapy for depression: moderating role of recurrent depression. Br J Clin Psychol, 49(Pt 4), 529–545. doi: 10.1348/014466509x479186 [DOI] [PubMed] [Google Scholar]
- Miller W (2004). Combined behavioral intervention manual: a clinical research guid for therapists treating people with alcohol abuse and dependence (Vol. 1). Bethesda, MD: DHHS (NIH). [Google Scholar]
- Mussulman L, Ellerbeck EF, Cupertino AP, Preacher KJ, Spaulding R, Catley D, … Richter KP (2014). Design and participant characteristics of a randomized-controlled trial of telemedicine for smoking cessation among rural smokers. Contemp Clin Trials, 38(2), 173–181. doi: 10.1016/j.cct.2014.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (1998–2011). Mplus User’s Guide (Sixth ed.). Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- Ng JY, Ntoumanis N, Thogersen-Ntoumani C, Deci EL, Ryan RM, Duda JL, & Williams GC (2012). Self-Determination Theory Applied to Health Contexts: A Meta-Analysis. Perspect Psychol Sci, 7(4), 325–340. doi: 10.1177/1745691612447309 [DOI] [PubMed] [Google Scholar]
- North American Quitline Consortium. (2008). Draft results from the 2008 North American Quitline Consortium annual survey. Retrieved from http://www.naquitline.org/resource/resmgr/survey_2008/2008surveynotespres.pdf
- Orlinsky D, Ronnestad M, & Wilutzski U (2004). Fifty years of psychotherapy process-outcome research: Continuity and change In Lambert MJ (Ed.), Bergin and Garfield’s Handbook of Psychotheraphy and Behavior Change (5 ed.). New York: John Wiley & Sons. [Google Scholar]
- Preacher KJ (2015). Advances in mediation analysis: a survey and synthesis of new developments. Annu Rev Psychol, 66, 825–852. doi: 10.1146/annurev-psych-010814-015258 [DOI] [PubMed] [Google Scholar]
- Preacher KJ, & Selig JP (2012). Advantages of Monte Carlo confidence intervals for indirect effects. Communication Methods and Measures, 6(2), 77–98. [Google Scholar]
- R Core Team. (2016). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; Retrieved from https://www.r-project.org/ [Google Scholar]
- Richter KP, Shireman TI, Ellerbeck EF, Cupertino AP, Catley D, Cox LS, … Lambart L (2015). Comparative and cost effectiveness of telemedicine versus telephone counseling for smoking cessation. J Med Internet Res, 17(5), e113. doi: 10.2196/jmir.3975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan RM, & Connell JP (1989). Perceived locus of causality and internalization: examining reasons for acting in two domains. J Pers Soc Psychol, 57(5), 749–761. [DOI] [PubMed] [Google Scholar]
- Scannell K, Perednia D, & Kissman H (1995). Telemedicine: past, present, future. Retrieved from http://www.nlm.nih.gov/archive/20040830/pubs/cbm/telembib.html. Accessed: November 29, 2005.
- Schwartz MD, Valdimarsdottir HB, Peshkin BN, Mandelblatt J, Nusbaum R, Huang AT, … King L (2014). Randomized noninferiority trial of telephone versus in-person genetic counseling for hereditary breast and ovarian cancer. J Clin Oncol, 32(7), 618–626. doi: 10.1200/jco.2013.51.3226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selig JP, & Preacher KJ (2009). Mediation models for longitudinal data in developmental research. Research in Human Development, 6(2–3), 144–164. [Google Scholar]
- Spierto F, Hannon W, Kendrick J, Bernert J, Pirkle J, & Gargiullo P (1988). Urinary cotinine levels in women enrolled in a smoking cessation study during and after pregnancy. J Smoking-Related Dis, 5(2), 65–76. [Google Scholar]
- SRNT Subcommittee on Biochemical Verification. (2002). Biochemical verification of tobacco use and cessation. Nicotine Tob Res, 4(2), 149–159. doi: 10.1080/14622200210123581 [DOI] [PubMed] [Google Scholar]
- Stephens RS, Babor TF, Kadden R, & Miller M (2002). The Marijuana Treatment Project: rationale, design and participant characteristics. Addiction, 97 Suppl 1, 109–124. [DOI] [PubMed] [Google Scholar]
- Strecher VJ, DeVellis BM, Becker MH, & Rosenstock IM (1986). The role of self-efficacy in achieving health behavior change. Health Educ Q, 13(1), 73–92. doi: 10.1177/109019818601300108 [DOI] [PubMed] [Google Scholar]
- Tracey TJ, & Kokotovic AM (1989). Factor structure of the working alliance inventory. Psychological Assessment: A journal of consulting and clinical psychology, 1(3), 207. [Google Scholar]
- Williams GC, Freedman ZR, & Deci EL (1998). Supporting autonomy to motivate patients with diabetes for glucose control. Diabetes care, 21(10), 1644–1651. [DOI] [PubMed] [Google Scholar]
- Williams GC, Gagne M, Ryan RM, & Deci EL (2002a). Facilitating autonomous motivation for smoking cessation. Health Psychol, 21(1), 40–50. [PubMed] [Google Scholar]
- Williams GC, Gagne M, Ryan RM, & Deci EL (2002b). Facilitating autonomous motivation for smoking cessation Health Psychol (Vol. 21, pp. 40–50). [PubMed] [Google Scholar]
- Williams GC, Grow VM, Freedman ZR, Ryan RM, & Deci EL (1996). Motivational predictors of weight loss and weight-loss maintenance. Journal of personality and social psychology, 70(1), 115. [DOI] [PubMed] [Google Scholar]
- Williams GC, McGregor HA, Sharp D, Levesque C, Kouides RW, Ryan RM, & Deci EL (2006). Testing a self-determination theory intervention for motivating tobacco cessation: supporting autonomy and competence in a clinical trial. Health Psychol, 25(1), 91–101. doi: 10.1037/0278-6133.25.1.91 [DOI] [PubMed] [Google Scholar]
- Williams GC, McGregor HA, Zeldman A, Freedman ZR, & Deci EL (2004). Testing a self-determination theory process model for promoting glycemic control through diabetes self-management. Health Psychol, 23(1), 58–66. doi: 10.1037/0278-6133.23.1.58 [DOI] [PubMed] [Google Scholar]
- Williams GC, Minicucci DS, Kouides RW, Levesque CS, Chirkov VI, Ryan RM, & Deci EL (2002c). Self-determination, smoking, diet and health. Health education research, 17(5), 512–521. [DOI] [PubMed] [Google Scholar]
- Williams GC, Niemiec CP, Patrick H, Ryan RM, & Deci EL (2009). The importance of supporting autonomy and perceived competence in facilitating long-term tobacco abstinence. Ann Behav Med, 37(3), 315–324. doi: 10.1007/s12160-009-9090-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GC, Niemiec CP, Patrick H, Ryan RM, & Deci EL (2016). Outcomes of the Smoker’s Health Project: a pragmatic comparative effectiveness trial of tobacco-dependence interventions based on self-determination theory. Health Educ Res, 31(6), 749–759. doi: 10.1093/her/cyw046 [DOI] [PMC free article] [PubMed] [Google Scholar]