Abstract
Background:
Previous studies indicate that youth with posttraumatic stress disorder (PTSD) have abnormal activation in brain regions important for emotion processing. It is unknown whether symptom improvement is accompanied by normative changes in these regions. This study identified neural changes associated with symptom improvement with the long-term goal of identifying malleable targets for interventions.
Methods:
A total of 80 functional magnetic resonance imaging (fMRI) scans were collected, including 20 adolescents with PTSD (ages 9–17) and 20 age- and sex-matched healthy control subjects, each scanned before and after a 5-month period. Trauma-focused cognitive behavioral therapy was provided to the PTSD group to ensure improvement in symptoms. Whole brain voxel-wise activation and region of interest analyses of facial expression task data were conducted to identify abnormalities in the PTSD group versus HC at baseline (BL), and neural changes correlated with symptom improvement from BL to EOS of study (EOS).
Results:
At BL, the PTSD group had abnormally elevated activation in the cingulate cortex, hippocampus, amygdala, and medial frontal cortex compared to HC. From BL to EOS, a PTSD symptoms improved an average of 39%. Longitudinal improvement in symptoms of PTSD was associated with decreasing activation in posterior cingulate, mid-cingulate, and hippocampus, while improvement in dissociative symptoms was correlated with decreasing activation in the amygdala.
Conclusions:
Abnormalities in emotion-processing brain networks in youth with PTSD normalize when symptoms improve, demonstrating neural plasticity of these regions in young patients and the importance of early intervention.
INTRODUCTION
The long-lasting negative effects of childhood abuse are well-documented, including increased risk for physical illess (Monnat and Chandler 2015), cognitive dysfunction (De Bellis, Woolley, and Hooper 2013), and psychiatric disorders such as PTSD (Choi et al. 2017). For children and adolescents, even sub-syndromal symptoms of PTSD can cause significant impairments in school, family and social domains (Cohen and Mannarino 2010; Cohen, Deblinger, and Mannarino 2018; Carrion et al. 2002), which in turn could impair development of academic and social skills. It is clear that effective treatment is vitally important for the 5% of youth who suffer from PTSD (Merikangas et al. 2010).
Advances in treatment for PTSD may ultimately be facilitated by better understanding of how brain function changes when symptoms improve. The brain model of PTSD is based on human and animal studies of fear processing, with critical structures including the amygdala, hippocampus, medial prefrontal and anterior cingulate corticies (Mahan and Ressler 2012; Sheynin and Liberzon 2017). Neuroimaging studies of adults with PTSD support this model, often reporting aberrant structure and function of these brain regions (Patel et al. 2012). Compared to controls, PTSD is consistently associated with greater amygdala activation and lower frontal cortex activation, while hippocampal abnormalities are inconsistently reported (Henigsberg et al. 2019). Neuroimaging studies of pediatric PTSD are fewer than in adults but also have demonstrated abnormalities in functional activation of amygdala, anterior cingulate cortex and hippocampus (Carrion et al. 2010; Garrett et al. 2012; Aghajani et al. 2016; van den Bulk et al. 2016; Weems et al. 2015; Wolf and Herringa 2016; van Hoof et al. 2017) and abnormal connectivity between amygdala and frontal cortex (Keding and Herringa 2016; Wolf and Herringa 2016; Keding and Herringa 2015). Neurobiological evidence of a dissociative subtype of PTSD has been presented in several studies by Lanius and colleagues (Lanius et al. 2010; Nicholson et al. 2015; Nicholson et al. 2016; Daniels et al. 2016), suggesting that the dissociative subtype of PTSD involves unique biological abnormalities.
It is possible that abnormalities in these regions are normalized when symptoms improve, for example, following successful interventions. Identifying changes in brain function that accompany symptom improvement could lead to better understanding of the mechanisms of recovery and potentially serve as targets for novel interventions. Treatment studies with neuroimaging outcome measures provide a way to investigate the neural mechanisms of symptom improvement. A recent review of the emerging literature of neuroimaging treatment studies concluded that successful psychotherapy for adults with PTSD was consistently accompanied by decreased activation of the amygdala and insula and increased activation in the dorsal anterior cingulate and hippocampus (Malejko et al. 2017). A real-time fMRI neurofeedback study in adults training to decrease activation of amygdala during presentation of trauma words is accompanied by activation of dorsolateral prefrontal cortex (Nicholson et al. 2018). One of the few studies of psychotherapy for youth with PTSD reported that pre/post symptom improvement was correlated with post-treatment connectivity between right amygdala and insula, and between left amygdala and posterior cingulate (Cisler et al. 2016). A critical limitation of many neuroimaging treatment studies is the absence of a healthy comparison group, which restricts the ability to identify abnormal brain function at pre-treatment, to determine whether pre/post changes represent normalization of brain function versus development of compensatory networks, and take into consideration the effects of repeated scanning.
In the current study, we used a longitudinal design to investigate the neurofunctional correlates of symptom improvement in youth with PTSD. To ensure symptom improvement during the study, youth with PTSD received an evidence-based treatment that has been proven to reduce symptoms of PTSD in this age group: Trauma-Focused Cognitive-Behavioral Therapy (TF-CBT) (Cohen and Mannarino 2010; Deblinger et al. 2006; Cohen, Mannarino, and Knudsen 2005; Cohen et al. 2004; Leenarts et al. 2013). We included a healthy comparison group, scanned at a similar interval, for comparison with the PTSD group at BL, and to assess the effects of repeated scanning on measures of brain function. We hypothesized that the PTSD group would show abnormalities in emotion processing regions before treatment, that activation in these regions would significantly change when symptoms improved, and that brain changes would be correlated with improvement in PTSD.
METHODS
Participants
This study was approved by the Stanford University Institutional Review Board and funded by a career development award to the first author. Written informed consent or assent was obtained from all caregivers and children. Participants in the PTSD group were recruited primarily by referral from the Stanford Child and Adolescent Psychiatry Outpatient Clinic. Participants in the Healthy Control (HC) group were recruited from the surrounding communities using advertisements.
Inclusion and Exclusion criteria
Participants in both PTSD and HC groups were required to be ages 9–17 and to have self-reported a Tanner Stage of 2 or above, indicating post-onset of puberty. Exclusions included MRI contraindications, orthodontic braces, psychoactive medications, learning disability, neurological disorders, traumatic brain injury, major medical illness, IQ < 70, or current hospitalization.
Participants in the PTSD group were required to have a history of interpersonal trauma, such as physical abuse, sexual abuse, witnessing violence or other maltreatment-related trauma. In addition, participants were required to have current and significant symptoms of PTSD, defined as a rating of “3” or “4” on at least one item from each symptom cluster (reexperiencing, avoidance, hyperarousal) on the UCLA PTSD Reaction Index for DSM-4 (PTSD-RI) indicating significant impairment (Cohen and Mannarino 2010). Exclusionary comorbid diagnoses were psychotic disorder, Bipolar I, severe substance abuse or dependence, acute suicidal ideation requiring hospitalization, and autism spectrum disorder. Participants in the HC group were required to have no current or past psychiatric diagnosis and no history of trauma.
Behavioral Assessments
The PTSD-Reaction Index (PTSD-RI) (Steinberg et al. 2013) was completed at both timepoints as the primary PTSD symptom measure. The PTSD-RI is one of the most widely-used instruments to assess PTSD symptom severity, administered as a brief self-report rating scale with separate versions for parent and child. The average of the parent and child total score was used as the consensus rating, as a non-biased method of taking into account both child and parent report. The Kiddie Schedule for Affective Disorders and Schizophrenia interview (KSADS) (Kaufman et al. 1997) was administered to all participants by a trained graduate student to screen for Axis I diagnoses in the HC group and to assess all diagnoses in the PTSD group. The Wechsler Abbreviated Scale of Intelligence (WASI) (Wechsler 1997) was administered by a trained graduate student, to measure IQ. Additional assessments were given to assess the groups.
Trauma-Focused Cognitive Behavioral Therapy (TF-CBT)
TF-CBT is a flexible, protocolized treatment that offers education and coping skills to help children and caregivers actively process traumatic experiences in the context of individual and conjoint child-caregiver sessions (Cohen, Deblinger, and Mannarino 2018). Treatment was provided in weekly 90-minute sessions; 60 minutes with the youth and 30 minutes with the parent. TF-CBT can be completed in approximately 12 sessions, however, patients may require more sessions if they have multiple traumas or more severe symptoms, if they miss sessions, and for other reasons. To address the full spectrum of treatment needs, we allowed a total duration of 20 weeks +/− 2 weeks.
MRI Data Acquisition is described in the Supplementary Materials.
Facial Expression Task with scrambled images as contrasting condition
Facial expressions have been used extensively to study emotion processing in psychiatric patients of all ages, including those with pediatric PTSD (Sabatinelli et al. 2011). For this study, photographs of faces with happy, angry, and neutral expressions were used in order to probe responses to a range of emotions. While many studies use neutral facial expressions as the contrasting condition for affective faces, e.g. in order to remove activation associated with viewing faces, there is a growing body of evidence that youth with a history of interpersonal trauma (Garrett et al. 2012) and children and adults with depression and anxiety (Thomas et al. 2001; Filkowski and Haas 2017; Oliveira et al. 2013) have an emotional response (e.g. amygdala activation) in response to neutral faces, possibly because neutral faces are ambiguous and perceived as potentially threatening, particularly for victims of maltreatment. In this case, subtracting neutral faces from affective faces would substract away activation associated with an emotional response, which is our variable of interest. Accordingly, our study used scrambled images as the contrasting condition. Scrambled images were created by re-arranging the voxels of the affective face photographs into a complex pattern with the same colors as the face stimuli. As a contrasting condition, scrambled images subtract away activation associated with perception of a complex colorful visual stimulus, as well as the motor button press response.
During the task, participants responded to each face by pressing button ‘1’ for photographs of a female face, and button ‘2’ for a male face, and alternated buttons 1 and 2 during blocks of scrambled images. Faces included a variety of racial backgrounds and overall were half male/ half female, and no pictures were repeated. Each face was presented for 3 seconds, in blocks of 8 faces of the same expression. The cycle was repeated 4 times, with the order of the blocks pseudo-randomized to reduce order effects. Total task time was 8.5 minutes. The task was presented by Eprime software (http://www.pstnet.com/), which also collected behavioral responses via a custom-built response box. Task stimuli were projected from the head of the scanner bore onto a screen attached to the head coil, and viewed via a mirror above the participant. FMRI data collection was synchronized with task onset using a pulse trigger from Eprime to the scanner.
Whole Brain Voxel-wise fMRI Analyses
Individual subjects’ statistical analyses were performed at BL and EOS using fixed effects models. A boxcar design convolved with the hemodynamic response function was used to model the task, combining across the four blocks of each condition. Contrasts included (1) happy minus scrambled, (2) angry minus scrambled, and (3) neutral minus scrambled. Whole brain voxel-wise group analyses were conducted using the ‘Multivariate and Repeated Measures for Neuroimaging’ (MRM) toolbox, which allows advanced statistical modeling of repeated measures mixed effects designs using a multivariate form of the general linear model implemented in matlab (McFarquhar et al. 2016). Analyses in MRM included comparison of PTSD versus HC groups at BL (main effect of group and group x face interaction). We tested for longitudinal changes in activation from BL to EOS using a group (PTSD vs HC) x time (BL vs EOS) interaction. Correlations with symptom improvement were tested within the PTSD group. For all analyses, BL symptom severity was included as a covariate of no interest. Statistical thresholds were set using permutation-based inference, with a cluster-setting threshold of p=.001 and family wise error correction of p< .05 at the cluster level, following the recommendations of Eklund and colleagues (Eklund, Nichols, and Knutsson 2016).
Regions of Interest defined by Meta-analysis
In addition to the whole brain analyses, we conducted an independent analysis constrained to regions that have been consistently implicated in previous neuroimaging studies of symptom improvement following treatments for PTSD. A meta-analysis is a non-biased way to define regions of interest based on published studies, hence avoiding circularity issues. Although several meta-analyses on abnormalites in PTSD have been published, currently there are no published coordinate-based meta-analyses of neuroimaging studies of treatments for PTSD. Therefore we conducted our own coordinate-based meta-analysis to identify consistent findings across previous neuroimaging studies. Coordinate-based methods bypass the error inherent in combining results based on each authors’ assignment of brain region labels. BrainMap and GingerALE software (http://www.brainmap.org) were used to identify previously published studies and conduct the meta-analysis (details are provided in Supplementary materials). For each of the ROIs identified by meta-analysis, a 12 mm diameter sphere was created at the peak voxel location. Mean activation in each sphere was extracted using the REX toolbox (Duff, Cunnington, and Egan 2007) and exported to SAS software (SAS Institute, Cary, NC)
Group analyses of ROI activation in SAS included comparison of PTSD and HC groups at BL using analysis of variance. All regions were included as outcomes in a single model, with group as fixed factor (PTSD versus HC), and covarying for PTSD symptom severity at pre-treatment (Kraemer et al. 2001). To test for group differences in BL/EOS change, a mixed-effects regression was used, with group as fixed factor and time as repeated factor (BL vs EOS), adjusting for symptom severity at BL. To test for correlations between longitudinal changes in symptom severity and brain activation within the PTSD group, nonparametric Spearman’s correlations were conducted, with correction for multiple comparisons setting a threshold of p=.05/6 regions = .008. Finally, because previous studies have demonstrated significant variance in neuroimaging data attributable to dissociative symptoms (Nicholson et al. 2015), exploratory Spearman’s correlations were conducted with BL/EOS changes in ADES scores.
RESULTS
Thirty-two youth were enrolled in the PTSD group. Of these, six participants did not meet study criteria, three did not want to participate in psychotherapy, and two did not want to have an MRI scan. One participant withdrew from the study after the first session of TF-CBT, due to transportation issues. This left a final PTSD group of 20 youth who completed TF-CBT and BL and 2 scans. Twenty-three youth were enrolled in the HC group. Two participants were excluded for a history of trauma, and one was withdrawn by the parent for developing health concerns unrelated to the study. This left a final HC group of 20 youth who completed both scans.
Table 1 lists the demographic descriptors of the PTSD and HC groups. The groups were matched by age, sex and IQ and were 90% female. Both groups contained a mixture of races. None of the participants in either group was taking psychotropic medication. Supplementary Table S1 shows BL and EOS symptom scores for the PTSD and HC groups. As expected, the PTSD group had significantly higher PTSD, dissociation, depression, and internalizing/externalizing scores compared to controls. All participants in the PTSD group met DSM-IV criteria for PTSD at pre-treatment, and 50% met criteria for a current major depressive disorder. Trauma histories of the PTSD group are summarized in Supplementary Table S2. Eighty percent of participants reported sexual abuse trauma, and 90% reported multiple interpersonal traumas. Average duration of trauma was 5 years. These characteristics suggest that our sample is generalizable to the population of youth with moderate PTSD symptom severity.
Table 1.
Description of the PTSD and HC groups
| Descriptor | Posttraumatic Stress Disorder Group (N=20) | Healthy Control Group (N=20) | PTSD vs HC | ||
|---|---|---|---|---|---|
| p,effect size7 | |||||
| Female / Male | 18 F / 2 M | 90 / 10 | 18 F/2 M | 90/ 10 | equal |
| Race: White | 10 | 50 | 15 | 75 | Chi2 =2.67, p=.19 |
| Current Psychotropic Meds | None | None | None | None | N/A |
| Comorbid Diagnoses @ BL | |||||
| p,effect size7 | |||||
| Age at Pre-Treatment (years) | 15.3 (1.9) | 10.4–17.7 | 14.5 (2) | 11.2–17.5 | p=.18, e.s.=.05 |
| Estimate of IQ1 | 110 (9.6) | 93–126 | 113 (11) | 86–130 | p=.39, e.s.=.02 |
| PTSD3 at Baseline | 39.1 (10.6) | 24–58 | N/A | N/A | N/A |
| PTSD3 at End of Study | 22.9 (9.5) | 5–41 | N/A | N/A | N/A |
| PTSD3BL vs EOS | PTSD group: F (1,19)= 37; p=.0001, e.s.=.94 | ||||
| Dissociative4 at Baseline | 2.8 (1.7) | 0.1–5.2 | 0.6 (0.7) | 0–3.3 | P=.0001;e.s.=.71 |
| Dissociative4 at End of Study | 1.8 (1.3) | 0.3–5.6 | 0.5 (0.5) | 0–1.8 | P=.0001,e.s.=.64 |
| Dissociative4 BL vs EOS | PTSD group: F (1,19)= 18.3, p=.0001, e.s.=.49; HC group: F < 1, p=.53, e.s.=.022 | ||||
| Depression5 at Baseline | 23.6 (9.0) | 6–38 | 3.5 (3.6) | 0–13 | P=.0001; e.s.=.86 |
| Depression5 at End of Study | 13.7 (8.9) | 0–31 | 3.4 (3.0) | 0–12 | P=.0001, e.s.=.70 |
| Depression5 BL vs EOS | PTSD group: F (1,19)= 20.1, p=.0001, e.s.=.51; HC group: F < 1, p=.87, e.s.=.001 | ||||
| Externalizing6at Baseline | 54.8 (9.4) | 39–69 | 39.2 (7.4) | 32–54 | P=.0001, e.s.=.48 |
| Externalizing6 at End of Study | 50.9 (7.4) | 39–63 | 38.9 (6.7) | 32–52 | P=.0001, e.s.=.43 |
| Internalizing6 at Baseline | 64.4 (8.1) | 48–78 | 42.1 (9.3) | 31–61 | P=.0001, e.s.=.63 |
| Internalizing6 at End of Study | 55.4 (10.0) | 31–73 | 45.6 (7.6) | 31–59 | P=.001, e.s.=.24 |
IQ: Wechsler Abbreviated Scale of Intelligence (WASI);
Anorexia Nervosa, Oppositional Defiant Disorder;
UCLA PTSD Reaction Index for DSM-IV;
A-DES (Adolescent Dissociative Experiences Scale);
CDI (Children’s Depression Inventory instrument);
Child Behavior Checklist (CBCL);
partial eta squared
Symptom Improvement Following Therapy
From BL to EOS, symptoms of PTSD improved significantly, with 17 of the 20 patients (85%) showed an average improvement of 50% (SD= 14.7%; range of 20% to 79%). The remaining 3 patients showed no improvement or worsening symptom scores. Across all 20 patients, PTSD RI scores improved an average of 39%. This effect size is comparable to those reported in TF-CBT randomized clinical trials with children of similar ages and mixed trauma types (Cohen, Mannarino, and Deblinger 2017). In addition to significant improvement in PTSD symptoms (F(1,19)=37.03, p=.0001), dissociative symptoms (F(1,19)=17.56, p=.0001) and depression symptoms (F(1,19)=20.09, p=.001) also improved signficantly.
Task Performance in Scanner
A group x time ANOVA found no main effects or interactions in the analysis of task accuracy. Analysis of response times showed a main effect of group for happy (p<.04), angry (p< .04) and neutral faces (p<.02) such that the PTSD group responded more slowly than the HC group at both BL and EOS. Additional details are given in the Supplementary Materials.
Results of Meta-analysis to generate regions of interest
The meta-analysis identified 6 regions that have been consistently reported across previous studies of symptom improvement following treatments for PTSD: mid-cingulate (Brodmann area 24), dorsal cingulate (Brodmann area 32), left amygdala, right hippocampus, right medial frontal gyrus (Brodmann Area 9), and right lateral orbital gyrus (Brodmann area 47). Studies included in the meta-analysis are given in Table S2, and resulting brain regions in Table S3.
Results of PTSD vs HC group comparisons at Baseline
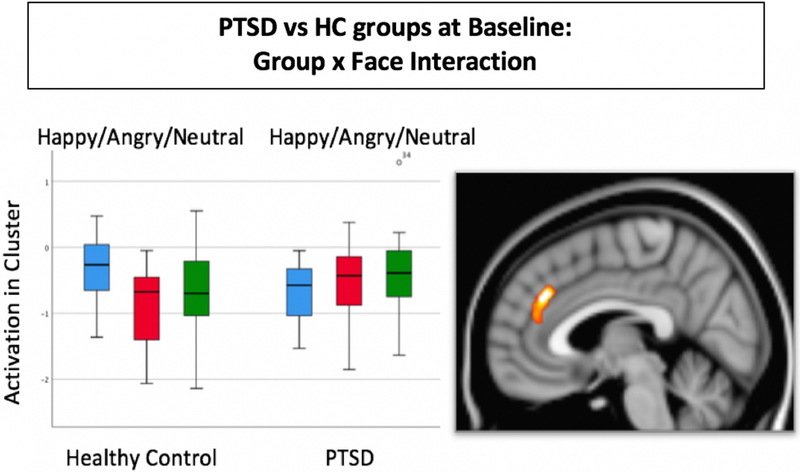
At BL, the whole brain voxel-wise comparison of the PTSD vs HC groups found no significant clusters for the main effect of group, but a significant group x face interaction was found in a single cluster located in the dorsal anterior cingulate cortex. As shown in Figure 1, the interaction was due to a different pattern of activation across the happy, angry, and neutral faces. Post-hoc whole brain comparisons conducted in order to interpret the interaction found no significant group differences any of the faces individually, when tested on the whole-brain level. In an independent analysis of group differences at BL constrained to the regions of interest defined by the meta-analysis, a significant group x face interaction was found for all regions combined (F=2.27, p=.039). Follow-up comparisons conducted to interpret the interaction showed that the PTSD had significantly greater activation than the HC group for neutral faces but not for happy or angry faces. Significant group differences for neutral faces were found in the mid-cingulate (F(2) = 13.6, p = .001), dorsal cingulate (F(2) = 4.8, p = .035), left amygdala (F(2) = 6.5, p = .015), medial frontal cortex (F(2) = 7.9, p = .008) and right hippocampus (F(2) = 5.7, p = .022). No group differences were found in the lateral orbital region of interest.
Figure 1.
Result of whole brain analysis comparing PTSD to HC groups at baseline. A significant interaction of group x face was found in a single cluster that included the dorsal anterior cingulate (Brodmann’s Area 32) and medial frontal gyrus (BA 9), p=.012, k=258 voxels, max voxel: x=4, y=36, z=30). Threshold was set at p=.05 family-wise error corrected for multiple comparisons at the cluster level.
Results of Group x Time interaction analysis
The whole brain analysis of the interaction of group (PTSD vs HC) x time (BL vs EOS) resulted in a significant cluster located in the posterior cingulate cortex and precuneus. This cluster is shown and details are given in Figure 2. Activation in the posterior cingulate decreased over time in the PTSD group, and stayed the same in the HC group. Post-hoc whole-brain comparisons conducted to interpet the interaction found that group differences were not significant at BL or EOS when analyzed separately.
Figure 2.
Top: Results of whole-brain analysis showing significant group x time interaction cluster in the posterior cingulate/precuneus (Brodmann Area 30/31/19; cluster size=241 voxels, peak voxel: x=6, y=−64, z=14; p=.023 corrected for multiple comparisons at cluster level. Extracted data are plotted to illustrate the nature of the interaction. Bottom: Results of whole-brain correlation with Bl to EOS symptom improvement within the PTSD group. The cluster is located in the posterior cingulate/precuneus/visual cortex (Brodmann Areas 30/31/18; cluster size=492 voxels; peak voxel: x=−6, y=−66, z=20; p=.013 cluster corrected). Extracted data are plotted for illustrative purposes only.
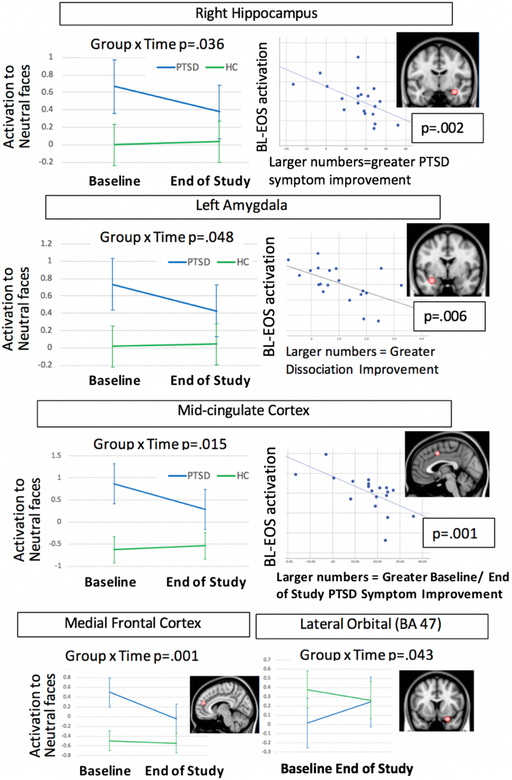
A group x time interaction analysis also was conducted using the ROIs defined by the meta-analysis. We constrained out analyses to the regions (mid-cingulate, dorsal cingulate, medial frontal, amygdala, hippocampus) and conditions (neutral faces) where we found abnormalities at BL. Results showed significant group x time interactions for the mid-cingulate (F(1,38) = 6.5, p = .015), medial frontal cortex (F(1,38) = 8.59, p=.006), right hippocampus (F(1,38) = 4.7, p = .036), and left amygdala (F(1,38) = 4.2, p = .048). In all cases, the PTSD group showed decreasing activation from BL to EOS, and the HC group showed no change over time. The group x time interations was not significant for the dorsal cingulate. Figure 3 illustrates the group x time interactions for each ROI.
Figure 3:
Summary of region of interest analysis results, including for each region the group x time interaction, correlation with symptom improvement, and brain image showing location of the spherical region of interest
An exploratory mixed-effects analysis was used to test for group x time interactions in the lateral orbital frontal cortex, as this was the only region where activation increased from BL to EOS in the PTSD group. The lateral orbital region also showed a significant group x time interaction for all facial expressions combined (F(1,38)= 4.39, p = .04).
Correlations with symptom improvement within PTSD group
The whole brain analysis within the PTSD group identified one cluster where changes in activation from BL to EOS were significantly correlated with improvement in PTSD symptoms. As shown in Figure 2, this cluster is located in the precuneus and posterior cingulate, and overlaps the cluster identified in the group x time interaction.
The region of interest analysis found significant Spearman’s correlations (after correction for multiple comparisons) between PTSD symptom improvement and brain changes in the mid-cingulate for neutral faces (rho = −.71, p = .001) and for happy faces (Spearmans rho = −.57, p = .008), and the right hippocampus for neutral faces (rho = −.65, p = .002). In all cases, symptom improvement was correlated with decreasing activation from BL to EOS. Significant correlations with improvement in symptoms of dissociation were found for the left amygdala (angry faces, rho = −.59, p = .006), dorsal cingulate (angry faces rho = −.58, p = .007), and right lateral orbital (happy faces, rho = −.60, p = .005). No significant correlations were found with depression symptoms. Inspection of the scatterplots shown in Figure 3 confirmed that these correlations could not be attributed to outliers. As a follow-up analysis to help explain our findings, we evaluated the correlation between pre/post improvement in symptoms of PTSD, dissociation, and depression. We found that none of these measures were significantly correlated (p’s > .05). However, by inspecting scatterplots of correlations between symptoms we found that the subject with pre/post worsening of PTSD symptoms was an outlier. Therefore, we recalculated without this subject and found a significant correlation between pre/post dissociation and pre/post PTSD symptoms after removing that subject, (rho= .58, p=.009). However, with the outlier subject removed the correlations with pre/post amygdala remained the same: there was a significant correlation with pre/post dissociation (rho= −.59, p=.007) but not pre/post PTSD symptoms (rho = −.39, p=.097).
Exploratory post-hoc analyses were conducted to examine correlations between improvement in each symptom subscale (re-experiencing, avoidance, hyperarousal) and pre/post changes in regions of interest. We used a threshold of p=.05/6 regions = .008. Results showed that pre/post changes in mid-cingulate activation to neutral faces were correlated with improvements in re-experiencing (rho= - .59, p=.007) but not with other subscales. Similarly, pre/post changes in hippocampus to neutral faces were correlated with improvements in re-experiencing only (rho= - .57, p=.009). Lastly, pre/post changes in medial frontal cortex (to neutral faces) were correlated with improvements in both re-experiencing (rho=.57, p=.008) and hyperarousal (rho=−.61, p=.004), but not avoidance. No other regions showed pre/post correlations with any sub-scale symptom improvement.
Table 2 summarizes all results and suggests interpretations.
Table 2:
Summary of Results and Interpretation
| Method | Region | Abnormal at BL | Group x time interaction | Correlation with PTSD symptom improvement | Correlation with dissociative symptom improvement | pre/post change in HC group | Interpretation |
|---|---|---|---|---|---|---|---|
| ROI | hippocam pus | For neutral faces | For neutral faces | Neutral faces | Decreasing activation is a potential mechanism of improvement in PTSD (re-experiencing) | ||
| ROI | mid-cingulate | For neutral faces | For neutral faces | For neutral faces | Decreasing activation is a potential mechanism of improvement in PTSD (re-experiencing) | ||
| ROI | amygdala | For neutral faces | For neutral faces | For angry faces | Decreasing activation is a potential mechanism of improvement in dissociation | ||
| ROI | medial frontal | For neutral faces | For neutral faces | Decreasing activation is general effect (re-experiencing and hyperarousal) | |||
| ROI | lateral orbital frontal | for all faces combined | for happy faces | Increasing activation is a potential compensatory mechanism | |||
| ROI | dorsal ACC | For neutral faces | x angry faces | ||||
| Whole brain | anterior cingulate /medial frontal cluster | Group x face interaction | |||||
| Whole brain | posterior cingulate / precuneus cluster | Across all faces | Across all faces | Decreasing activation is a potential compensatory mechanism |
DISCUSSION
This study investigated longitudinal changes in brain function associated with symptom improvement in youth with PTSD as compared to a matched healthy control group. Results showed that, at BL, the PTSD group had abnormal activation in the cingulate cortex, amygdala, hippocampus, and medial frontal cortex. When symptoms of PTSD improved significantly, activation in these regions decreased to the level of the control group. Furthermore, changes in the mid-cingulate and hippocampus were correlated with improvement in symptoms of PTSD, while changes in the amygdala was correlated with improvement in symptoms of dissociation. Longitudinal analyses of the control group confirmed that there were no significant changes in activation attributed to repeated scanning. These results add to the emerging literature showing neural plasticity with symptom improvement in youth with PTSD.
Of all brain regions investigated, the mid-cingulate and hippocampus show the most consistent evidence as potential mechanisms of PTSD symptom improvement. These regions were abnormal at BL, changed significantly at EOS, and were correlated with improvement in symptoms of PTSD, particularly with improvement in symptoms of re-experiencing. The mid-cingulate was significantly elevated at BL compared to the HC group in both the whole-brain and region of interest analyses. Abnormalities in the cingulate cortex have been frequently reported in the PTSD neuroimaging literature (Kaczkurkin et al. 2017; Rinne-Albers et al. 2017) and previous meta-analyses have shown this region to be consistently hyperactivated in adults with PTSD (Etkin and Wager 2007), consistent with our results. The mid-cingulate is believed to subserve an emotion evaluation/salience monitoring role (Etkin, Egner, and Kalisch 2011), so decreasing activation that is correlated with less re-experiencing could indicate that patients are less focused on trauma-related recall. Previous studies have reported structural abnormalities in the (dorsal) cingulate cortex in adolescents with PTSD (Rinne-Albers et al. 2017). In another study, greater cingulate activation at BL was found to predict worse treatment outcome and long-term symptom severity (Kennis Ph et al. 2017). A post-hoc analysis of our data produced a similar result: higher mid-cingulate activation at BL was significantly associated with less symptom improvement after treatment (rho= −.62, p=.004).
The hippocampus also shows evidence as a potential mechanism of symptom improvement. The hippocampus has long been a focus of investigation in the PTSD literature. A recent analysis of almost 800 patients reported significantly smaller hippocampi in adults with PTSD compared to trauma-exposed controls (Logue et al. 2018). However, hippocampal abnormalities are seen across a range of psychiatric disorders, so its specificity in PTSD is unknown, and meta-analyses of pediatric literature have found inconsistent results (Milani et al. 2017). Furthermore, a twin study suggested that hippocampal abnormalities are a risk factor for developing PTSD, rather than a result of PTSD (Gilbertson et al. 2002). Our study adds to this literature by showing that abnormal hippocampal activation normalizes as symptoms of PTSD improve.
Our study found abnormally increased amygdala activation at BL, which has been reported previously in studies of youth with PTSD, including fMRI studies using facial expression tasks (Garrett et al. 2012; Cisler et al. 2013; van Hoof et al. 2017). In the current study, we found a significant decrease in amygdala activation with symptom improvement, but this decrease was correlated with improvement in symptoms of dissociation, not PTSD (scatterplot shown in Figure 3). Dissociation is considered a maladaptive strategy for regulating emotion that may contribute to the maintenance of PTSD and is targeted by exposure to trauma reminders during psychotherapy. Although improvement in dissociation is correlated with improvement in PTSD, dissociation accounts for greater variability in amygdala activation in our study. This finding could indicate that amygdala abnormalities are linked to emotion regulation generally rather than to PTSD specifically. In addition, previous studies by Lanius and colleagues have shown that neural abnormalities in PTSD vary significantly depending on the dissociative subtype (Lanius et al. 2005; Nicholson et al. 2015). Our data support the importance of dissociative symptoms in the neural profile of PTSD.
A strength of our study is the inclusion of a healthy control group, scanned twice, allowing us to identify abnormalities at pre-treatment and to test whether observed brain changes are attributable to repeated scanning. We did not find longitudinal changes in the HC group, so our results are not likely to be related to repeated scanning of PTSD patients. Comparisons with the HC group at pre-treatment enabled us to determine that 5 out of 6 regions of interest were abnormally elevated in the PTSD group, and specifically for neutral facial expressions. This is interesting because many investigators use neutral faces as a contrast condition that is subtracted from affective faces. Our results suggest that subtractly neutral faces could subtract away amygdala activation and other emotion-related brain responses. Our finding is consistent with our previous analyses (Garrett et al. 2012) and others (Filkowski and Haas 2017; Thomas et al. 2001) that have recognized that neutral facial expressions can be viewed as ambiguous and potentially threatening, particularly by adolescents who are victims of interpersonal trauma who may be hypervigilant to potentially threatening facial expressions as a way to avoid harm.
Our whole brain analysis found a group x time interaction in the posterior cingulate cortex. The PCC plays a role in autobiographical memory (Maddock 1999), therefore elevated PCC activation in PTSD could be linked to intrusive trauma memories. Although it is not typically associated with the fear inhibition network, the PCC is frequently found to be abnormal in fMRI studies of youth with maltreatment-related PTSD (Sun et al. 2018; van Hoof et al. 2017). In adults with PTSD, a meta-analysis found that the PCC is consistently implicated across neuroimaging studies (Ramage et al. 2013). In addition, several connectivity studies in PTSD implicate the default mode network, of which the PCC is an important hub (Miller et al. 2017; Patriat et al. 2016; King et al. 2016). Further investigation of the role of the PCC in PTSD are warranted by this growing evidence.
A limitation of our study is our small sample size, although it is similar to the sample size of recent longitudinal studies that include neuroimaging and treatment (Cisler et al. 2016; Shou et al. 2017; Helpman et al. 2016; van Rooij et al. 2016). We improved power by acquiring a relatively homogeneous sample that is limited to interpersonal trauma, free from psychoactive medications, and matched to the HC group. Our sample was composed primarily of female participants and may not be generalizable to male patients, although PTSD is more common in female youth (Merikangas et al. 2010). Future studies should use an RCT design in order to attribute BL/EOS changes to specific treatments. Despite limitations, our study contributes to a growing literature investigating potential mechanistic targets for novel interventions.
Supplementary Material
Acknowledgement:
The authors acknowledge the helpful comments and suggestions provided by Dr. Peter Fox, M.D. regarding data analysis
REFERENCES
- Aghajani M, Veer IM, van Hoof MJ, Rombouts SA, van der Wee NJ, and Vermeiren RR. 2016. ‘Abnormal functional architecture of amygdala-centered networks in adolescent posttraumatic stress disorder’, Hum Brain Mapp, 37: 1120–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion VG, Haas BW, Garrett A, Song S, and Reiss AL. 2010. ‘Reduced hippocampal activity in youth with posttraumatic stress symptoms: an FMRI study’, J Pediatr Psychol, 35: 559–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion VG, Weems CF, Ray R, and Reiss AL. 2002. ‘Toward an empirical definition of pediatric PTSD: the phenomenology of PTSD symptoms in youth’, J Am Acad Child Adolesc Psychiatry, 41: 166–73. [DOI] [PubMed] [Google Scholar]
- Choi NG, DiNitto DM, Marti CN, and Segal SP. 2017. ‘Adverse childhood experiences and suicide attempts among those with mental and substance use disorders’, Child Abuse Negl, 69: 252–62. [DOI] [PubMed] [Google Scholar]
- Cisler JM, Scott Steele J, Smitherman S, Lenow JK, and Kilts CD. 2013. ‘Neural processing correlates of assaultive violence exposure and PTSD symptoms during implicit threat processing: a network-level analysis among adolescent girls’, Psychiatry Res, 214: 238–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM, Sigel BA, Steele JS, Smitherman S, Vanderzee K, Pemberton J, Kramer TL, and Kilts CD. 2016. ‘Changes in functional connectivity of the amygdala during cognitive reappraisal predict symptom reduction during trauma-focused cognitive-behavioral therapy among adolescent girls with post-traumatic stress disorder’, Psychol Med, 46: 3013–23. [DOI] [PubMed] [Google Scholar]
- Cohen JA, Deblinger E, and Mannarino AP. 2018. ‘Trauma-focused cognitive behavioral therapy for children and families’, Psychother Res, 28: 47–57. [DOI] [PubMed] [Google Scholar]
- Cohen JA, Deblinger E, Mannarino AP, and Steer RA. 2004. ‘A multisite, randomized controlled trial for children with sexual abuse-related PTSD symptoms’, J Am Acad Child Adolesc Psychiatry, 43: 393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JA, and Mannarino AP. 2010. ‘Psychotherapeutic options for traumatized children’, Curr Opin Pediatr, 22: 605–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JA, Mannarino AP, and Deblinger E. 2017. ‘Research on TF-CBT’ in, Treating Trauma and Traumatic Grief in Children and Adolescents (Guilford Press: New York: ). [Google Scholar]
- Cohen JA, Mannarino AP, and Knudsen K. 2005. ‘Treating sexually abused children: 1 year follow-up of a randomized controlled trial’, Child Abuse Negl, 29: 135–45. [DOI] [PubMed] [Google Scholar]
- Daniels JK, Frewen P, Theberge J, and Lanius RA. 2016. ‘Structural brain aberrations associated with the dissociative subtype of post-traumatic stress disorder’, Acta Psychiatr Scand, 133: 232–40. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Woolley DP, and Hooper SR. 2013. ‘Neuropsychological findings in pediatric maltreatment: relationship of PTSD, dissociative symptoms, and abuse/neglect indices to neurocognitive outcomes’, Child Maltreat, 18: 171–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deblinger E, Mannarino AP, Cohen JA, and Steer RA. 2006. ‘A follow-up study of a multisite, randomized, controlled trial for children with sexual abuse-related PTSD symptoms’, J Am Acad Child Adolesc Psychiatry, 45: 1474–84. [DOI] [PubMed] [Google Scholar]
- Duff EP, Cunnington R, and Egan GF. 2007. ‘REX: response exploration for neuroimaging datasets’, Neuroinformatics, 5: 223–34. [DOI] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, and Knutsson H. 2016. ‘Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates’, Proc Natl Acad Sci U S A, 113: 7900–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, and Kalisch R. 2011. ‘Emotional processing in anterior cingulate and medial prefrontal cortex’, Trends Cogn Sci, 15: 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, and Wager TD. 2007. ‘Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia’, Am J Psychiatry, 164: 1476–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filkowski MM, and Haas BW. 2017. ‘Rethinking the Use of Neutral Faces as a Baseline in fMRI Neuroimaging Studies of Axis-I Psychiatric Disorders’, J Neuroimaging, 27: 281–91. [DOI] [PubMed] [Google Scholar]
- Garrett AS, Carrion V, Kletter H, Karchemskiy A, Weems CF, and Reiss A. 2012. ‘Brain activation to facial expressions in youth with PTSD symptoms’, Depress Anxiety, 29: 449–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, and Pitman RK. 2002. ‘Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma’, Nat Neurosci, 5: 1242–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helpman L, Marin MF, Papini S, Zhu X, Sullivan GM, Schneier F, Neria M, Shvil E, Malaga Aragon MJ, Markowitz JC, Lindquist MA, Wager T, Milad M, and Neria Y. 2016. ‘Neural changes in extinction recall following prolonged exposure treatment for PTSD: A longitudinal fMRI study’, Neuroimage Clin, 12: 715–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henigsberg N, Kalember P, Petrovic ZK, and Secic A. 2019. ‘Neuroimaging research in posttraumatic stress disorder - Focus on amygdala, hippocampus and prefrontal cortex’, Prog Neuropsychopharmacol Biol Psychiatry, 90: 37–42. [DOI] [PubMed] [Google Scholar]
- Kaczkurkin AN, Burton PC, Chazin SM, Manbeck AB, Espensen-Sturges T, Cooper SE, Sponheim SR, and Lissek S. 2017. ‘Neural Substrates of Overgeneralized Conditioned Fear in PTSD’, Am J Psychiatry, 174: 125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, and Ryan N. 1997. ‘Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADSPL): initial reliability and validity data’, J Am Acad Child Adolesc Psychiatry, 36: 980–8. [DOI] [PubMed] [Google Scholar]
- Keding TJ, and Herringa RJ. 2015. ‘Abnormal structure of fear circuitry in pediatric post-traumatic stress disorder’, Neuropsychopharmacology, 40: 537–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keding TJ, and Herringa RJ. 2016. ‘Paradoxical Prefrontal-Amygdala Recruitment to Angry and Happy Expressions in Pediatric Posttraumatic Stress Disorder’, Neuropsychopharmacology, 41: 2903–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennis Ph DM, van Rooij Ph D. Sjh, Reijnen MSc A., and Geuze Ph DE. 2017. ‘The predictive value of dorsal cingulate activity and fractional anisotropy on long-term PTSD symptom severity’, Depress Anxiety, 34: 410–18. [DOI] [PubMed] [Google Scholar]
- King AP, Block SR, Sripada RK, Rauch S, Giardino N, Favorite T, Angstadt M, Kessler D, Welsh R, and Liberzon I. 2016. ‘Altered Default Mode Network (Dmn) Resting State Functional Connectivity Following a Mindfulness-Based Exposure Therapy for Posttraumatic Stress Disorder (Ptsd) in Combat Veterans of Afghanistan and Iraq’, Depress Anxiety, 33: 289–99. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Stice E, Kazdin A, Offord D, and Kupfer D. 2001. ‘How do risk factors work together? Mediators, moderators, and independent, overlapping, and proxy risk factors’, Am J Psychiatry, 158: 848–56. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Vermetten E, Loewenstein RJ, Brand B, Schmahl C, Bremner JD, and Spiegel D. 2010. ‘Emotion modulation in PTSD: Clinical and neurobiological evidence for a dissociative subtype’, Am J Psychiatry, 167: 640–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Bluhm RL, Densmore M, Boksman K, Neufeld RW, Gati JS, and Menon RS. 2005. ‘Functional connectivity of dissociative responses in posttraumatic stress disorder: a functional magnetic resonance imaging investigation’, Biol Psychiatry, 57: 873–84. [DOI] [PubMed] [Google Scholar]
- Leenarts LE, Diehle J, Doreleijers TA, Jansma EP, and Lindauer RJ. 2013. ‘Evidence-based treatments for children with trauma-related psychopathology as a result of childhood maltreatment: a systematic review’, Eur Child Adolesc Psychiatry, 22: 269–83. [DOI] [PubMed] [Google Scholar]
- Logue MW, van Rooij SJH, Dennis EL, Davis SL, Hayes JP, Stevens JS, Densmore M, Haswell CC, Ipser J, Koch SBJ, Korgaonkar M, Lebois LAM, Peverill M, Baker JT, Boedhoe PSW, Frijling JL, Gruber SA, Harpaz-Rotem I, Jahanshad N, Koopowitz S, Levy I, Nawijn L, O’Connor L, Olff M, Salat DH, Sheridan MA, Spielberg JM, van Zuiden M, Winternitz SR, Wolff JD, Wolf EJ, Wang X, Wrocklage K, Abdallah CG, Bryant RA, Geuze E, Jovanovic T, Kaufman ML, King AP, Krystal JH, Lagopoulos J, Bennett M, Lanius R, Liberzon I, McGlinchey RE, McLaughlin KA, Milberg WP, Miller MW, Ressler KJ, Veltman DJ, Stein DJ, Thomaes K, Thompson PM, and Morey RA. 2018. ‘Smaller Hippocampal Volume in Posttraumatic Stress Disorder: A Multisite ENIGMA-PGC Study: Subcortical Volumetry Results From Posttraumatic Stress Disorder Consortia’, Biol Psychiatry, 83: 244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddock RJ 1999. ‘The retrosplenial cortex and emotion: new insights from functional neuroimaging of the human brain’, Trends Neurosci, 22: 310–6. [DOI] [PubMed] [Google Scholar]
- Mahan AL, and Ressler KJ. 2012. ‘Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder’, Trends Neurosci, 35: 24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malejko K, Abler B, Plener PL, and Straub J. 2017. ‘Neural Correlates of Psychotherapeutic Treatment of Post-traumatic Stress Disorder: A Systematic Literature Review’, Front Psychiatry, 8: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarquhar M, McKie S, Emsley R, Suckling J, Elliott R, and Williams S. 2016. ‘Multivariate and repeated measures (MRM): A new toolbox for dependent and multimodal group-level neuroimaging data’, Neuroimage, 132: 373–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, Benjet C, Georgiades K, and Swendsen J. 2010. ‘Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication--Adolescent Supplement (NCS-A)’, J Am Acad Child Adolesc Psychiatry, 49: 980–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milani AC, Hoffmann EV, Fossaluza V, Jackowski AP, and Mello MF. 2017. ‘Does pediatric post-traumatic stress disorder alter the brain? Systematic review and meta-analysis of structural and functional magnetic resonance imaging studies’, Psychiatry Clin Neurosci, 71: 154–69. [DOI] [PubMed] [Google Scholar]
- Miller DR, Hayes SM, Hayes JP, Spielberg JM, Lafleche G, and Verfaellie M. 2017. ‘Default Mode Network Subsystems are Differentially Disrupted in Posttraumatic Stress Disorder’, Biol Psychiatry Cogn Neurosci Neuroimaging, 2: 363–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnat SM, and Chandler RF. 2015. ‘Long Term Physical Health Consequences of Adverse Childhood Experiences’, Sociol Q, 56: 723–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson AA, Densmore M, Frewen PA, Theberge J, Neufeld RW, McKinnon MC, and Lanius RA. 2015. ‘The Dissociative Subtype of Posttraumatic Stress Disorder: Unique Resting-State Functional Connectivity of Basolateral and Centromedial Amygdala Complexes’, Neuropsychopharmacology, 40: 2317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson AA, Rabellino D, Densmore M, Frewen PA, Paret C, Kluetsch R, Schmahl C, Theberge J, Ros T, Neufeld RWJ, McKinnon MC, Reiss JP, Jetly R, and Lanius RA. 2018. ‘Intrinsic connectivity network dynamics in PTSD during amygdala downregulation using real-time fMRI neurofeedback: A preliminary analysis’, Hum Brain Mapp, 39: 4258–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson AA, Sapru I, Densmore M, Frewen PA, Neufeld RW, Theberge J, McKinnon MC, and Lanius RA. 2016. ‘Unique insula subregion resting-state functional connectivity with amygdala complexes in posttraumatic stress disorder and its dissociative subtype’, Psychiatry Res Neuroimaging, 250: 61–72. [DOI] [PubMed] [Google Scholar]
- Oliveira L, Ladouceur CD, Phillips ML, Brammer M, and Mourao-Miranda J. 2013. ‘What does brain response to neutral faces tell us about major depression? evidence from machine learning and fMRI’, PLoS One, 8: e60121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R, Spreng RN, Shin LM, and Girard TA. 2012. ‘Neurocircuitry models of posttraumatic stress disorder and beyond: a meta-analysis of functional neuroimaging studies’, Neurosci Biobehav Rev, 36: 2130–42. [DOI] [PubMed] [Google Scholar]
- Patriat R, Birn RM, Keding TJ, and Herringa RJ. 2016. ‘Default-Mode Network Abnormalities in Pediatric Posttraumatic Stress Disorder’, J Am Acad Child Adolesc Psychiatry, 55: 319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramage AE, Laird AR, Eickhoff SB, Acheson A, Peterson AL, Williamson DE, Telch MJ, and Fox PT. 2013. ‘A coordinate-based meta-analytic model of trauma processing in posttraumatic stress disorder’, Hum Brain Mapp, 34: 3392–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinne-Albers MA, Pannekoek JN, van Hoof MJ, van Lang ND, Lamers-Winkelman F, Rombouts SA, van der Wee NJ, and Vermeiren RR. 2017. ‘Anterior cingulate cortex grey matter volume abnormalities in adolescents with PTSD after childhood sexual abuse’, Eur Neuropsychopharmacol, 27: 1163–71. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D, Fortune EE, Li Q, Siddiqui A, Krafft C, Oliver WT, Beck S, and Jeffries J. 2011. ‘Emotional perception: meta-analyses of face and natural scene processing’, Neuroimage, 54: 2524–33. [DOI] [PubMed] [Google Scholar]
- Sheynin J, and Liberzon I. 2017. ‘Circuit dysregulation and circuit-based treatments in posttraumatic stress disorder’, Neurosci Lett, 649: 133–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou H, Yang Z, Satterthwaite TD, Cook PA, Bruce SE, Shinohara RT, Rosenberg B, and Sheline YI. 2017. ‘Cognitive behavioral therapy increases amygdala connectivity with the cognitive control network in both MDD and PTSD’, Neuroimage Clin, 14: 464–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg AM, Brymer MJ, Kim S, Briggs EC, Ippen CG, Ostrowski SA, Gully KJ, and Pynoos RS. 2013. ‘Psychometric properties of the UCLA PTSD reaction index: part I’, J Trauma Stress, 26: 1–9. [DOI] [PubMed] [Google Scholar]
- Sun D, Haswell CC, Morey RA, and De Bellis MD. 2018. ‘Brain structural covariance network centrality in maltreated youth with PTSD and in maltreated youth resilient to PTSD’, Dev Psychopathol: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Dahl RE, Ryan ND, Birmaher B, Eccard CH, Axelson D, Whalen PJ, and Casey BJ. 2001. ‘Amygdala response to fearful faces in anxious and depressed children’, Arch Gen Psychiatry, 58: 1057–63. [DOI] [PubMed] [Google Scholar]
- van den Bulk BG, Somerville LH, van Hoof MJ, van Lang ND, van der Wee NJ, Crone EA, and Vermeiren RR. 2016. ‘Amygdala habituation to emotional faces in adolescents with internalizing disorders, adolescents with childhood sexual abuse related PTSD and healthy adolescents’, Dev Cogn Neurosci, 21: 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hoof MJ, van den Bulk BG, Rombouts Sarb, Rinne-Albers MAW, van der Wee NJA, van IJzendoorn M. H., and Vermeiren Rrjm. 2017. ‘Emotional face processing in adolescents with childhood sexual abuse-related posttraumatic stress disorder, internalizing disorders and healthy controls’, Psychiatry Res Neuroimaging, 264: 52–59. [DOI] [PubMed] [Google Scholar]
- van Rooij SJ, Kennis M, Vink M, and Geuze E. 2016. ‘Predicting Treatment Outcome in PTSD: A Longitudinal Functional MRI Study on Trauma-Unrelated Emotional Processing’, Neuropsychopharmacology, 41: 1156–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D 1997. WASI Wechsler Abbreviated Scale of Intelligence (3rd edition) (Psychological Corporationj; ). [Google Scholar]
- Weems CF, Klabunde M, Russell JD, Reiss AL, and Carrion VG. 2015. ‘Post-traumatic stress and age variation in amygdala volumes among youth exposed to trauma’, Soc Cogn Affect Neurosci, 10: 1661–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf RC, and Herringa RJ. 2016. ‘Prefrontal-Amygdala Dysregulation to Threat in Pediatric Posttraumatic Stress Disorder’, Neuropsychopharmacology, 41: 822–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.