Summary
Background
Rapid reversal of vitamin K antagonist (VKA)-induced anticoagulation is often necessary for patients needing urgent surgical or invasive procedures. The optimum means of VKA reversal has not been established in comparative clinical trials. We compared the efficacy and safety of four-factor prothrombin complex concentrate (4F-PCC) with that of plasma in VKA-treated patients needing urgent surgical or invasive procedures.
Methods
In a multicentre, open-label, phase 3b randomised trial we enrolled patients aged 18 years or older needing rapid VKA reversal before an urgent surgical or invasive procedure. We randomly assigned patients in a 1:1 ratio to receive vitamin K concomitant with a single dose of either 4F-PCC (Beriplex/Kcentra/Confidex; CSL Behring, Marburg, Germany) or plasma, with dosing based on international normalised ratio (INR) and weight. The primary endpoint was effective haemostasis, and the co-primary endpoint was rapid INR reduction (≤1·3 at 0·5 h after infusion end). The analyses were intended to evaluate, in a hierarchical fashion, first non-inferiority (lower limit 95% CI greater than −10% for group difference) for both endpoints, then superiority (lower limit 95% CI >0%) if non-inferiority was achieved. Adverse events and serious adverse events were reported to days 10 and 45, respectively. This trial is registered at ClinicalTrials.gov, number NCT00803101.
Findings
181 patients were randomised (4F-PCC n=90; plasma n=91). The intention-to-treat efficacy population comprised 168 patients (4F-PCC, n=87; plasma, n=81). Effective haemostasis was achieved in 78 (90%) patients in the 4F-PCC group compared with 61 (75%) patients in the plasma group, demonstrating both non-inferiority and superiority of 4F-PCC over plasma (difference 14·3%, 95% CI 2·8–25·8). Rapid INR reduction was achieved in 48 (55%) patients in the 4F-PCC group compared with eight (10%) patients in the plasma group, demonstrating both non-inferiority and superiority of 4F-PCC over plasma (difference 45·3%, 95% CI 31·9–56·4). The safety profile of 4F-PCC was generally similar to that of plasma; 49 (56%) patients receiving 4F-PCC had adverse events compared with 53 (60%) patients receiving plasma. Adverse events of interest were thromboembolic adverse events (six [7%] patients receiving 4F-PCC vs seven [8%] patients receiving plasma), fluid overload or similar cardiac events (three [3%] patients vs 11 [13%] patients), and late bleeding events (three [3%] patients vs four [5%] patients).
Interpretation
4F-PCC is non-inferior and superior to plasma for rapid INR reversal and effective haemostasis in patients needing VKA reversal for urgent surgical or invasive procedures.
Funding
CSL Behring.
Introduction
Patients receiving therapy with a vitamin K antagonist (VKA) have an increased risk of bleeding during surgical and procedural interventions.1 Therefore, guidelines recommend temporary interruption of VKA therapy 5 days before elective surgery to minimise perioperative bleeding.1 However, when patients need an urgent procedure, VKA reversal is often performed in the acute setting. Findings from a 2012 clinical trial underlined the risks involved, showing that the frequency of periprocedural bleeding in patients receiving VKA therapy was 3·3% for elective procedures, but 21·6% for emergency procedures.2 Although vitamin K alone can be effective, reversal can take several hours.3 Therefore, emergency reversal additionally necessitates the rapid replacement of vitamin K-dependent coagulation factors (ie, factors II, VII, IX, and X).
In some countries, including the USA, plasma is the most commonly used agent for rapid VKA reversal. Although plasma contains the vitamin K-dependent coagulation factors, it needs ABO typing and thawing before use, and is associated with long infusion times.4–6 More importantly, it can be associated with severe adverse outcomes including transfusion-related acute lung injury and transfusion-associated circulatory overload.7 Non-activated prothrombin complex concentrates contain vitamin K-dependent coagulation factors and are categorised as three-factor (3F-PCC) or four-factor (4F-PCC) prothrombin complex concentrates (depending on whether they contain clinically relevant amounts of factor VII).8 Prothrombin complex concentrates are stored at room temperature as a lyophilised powder, do not need ABO typing, can be prepared within minutes, and can be delivered in smaller volumes with shorter infusion times than can plasma.4
Adequately powered comparative trials investigating the optimum means of VKA reversal have not been done in patients needing urgent interventions, and the best method to promptly reverse VKAs remains unclear. The only plasma-controlled randomised clinical trial was a single-centre study of 40 patients (20 per group) undergoing semiurgent cardiac surgery, which was underpowered to detect significant differences in haemostatic efficacy.9 We therefore did a randomised clinical trial to compare 4F-PCC with plasma for urgent VKA reversal in patients needing urgent surgical or invasive procedures.
Methods
Study design and participants
In a randomised, open-label, active-controlled, non-inferiority, multicentre, phase 3b clinical trial, we enrolled patients in 33 hospitals (18 in the USA, two in Belarus, four in Bulgaria, two in Lebanon, one in Romania, and six in Russia).
Patients with an international normalised ratio (INR) of 2·0 or higher receiving VKA therapy and needing an urgent surgical or invasive procedure within 24 h were eligible for the study. The decision about the need for surgical treatment and rapid VKA reversal was made by the clinical care teams. Exclusion criteria included requirement for a procedure for which an accurate estimate of blood loss was not possible (eg, ruptured aneurysm or trauma) or coagulopathy that could be corrected solely through administration of vitamin K and withdrawal of VKA therapy. Full inclusion and exclusion criteria are provided in the appendix.
As part of ongoing review of the investigational new drug application, the United States Food and Drug Administration (FDA) reviewed the study protocol after the trial had been initiated. On July 20, 2011, after 157 patients had been enrolled, the FDA requested that enrolment of patients needing non-surgical invasive procedures be halted because of concern that no differences in haemostatic efficacy would be detected. No interim safety or efficacy analysis was done at this time. Sites were notified via letter on July 26, 2011, to immediately cease enrolment of this population, and a final protocol amendment was made on Sept 7, 2011. Patients needing urgent surgical procedures continued to be enrolled as planned.
The study was approved by the independent ethics committees and institutional review boards of the participating centres, in accordance with local legal requirements; written informed consent was obtained from all patients.
Randomisation and masking
Investigators called a 24 h randomisation centre and transmitted deidentified data for the randomisation procedure. We randomly assigned patients in a 1:1 ratio using a computerised system to receive either 4F-PCC (Beriplex/Kcentra/Confidex; CSL Behring, Marburg, Germany) or plasma. Treatment assignment was done by a centrally managed, biased-coin minimisation method,10 which is an adaptive randomisation scheme (appendix). This method also controlled for balance, both overall and within centres, between treatment groups within urgent surgical or invasive procedures with use of two levels of stratification: one based on the type of procedure, and one on the vitamin K dose given.
The first level of strata was: all cranial neurosurgical procedures; all cardiothoracic surgical procedures; all major orthopaedic surgical procedures (eg, open reduction internal fixation of hip); all other surgical procedures (such as general surgery, ear-nose-throat, noncranial neurological [eg, spine procedures], urological, gynaecological, cardio-vascular [eg, femoropopliteal bypass procedures], and minor orthopaedic interventions [eg, open reduction of ulna fracture]); and all invasive procedures (recruitment to this category was halted after protocol amendment). The second level of strata was oral vitamin K dose less than or equal to 2 mg; oral vitamin K dose more than 2 mg; and any intravenous vitamin K dose.
Surgery type was classified by the treating physician according to the first level of strata. The trial was open label; clinicians, study staff, and trial participants could not be blinded to treatment allocation because of the inherent characteristics of the study agents. The safety adjudication board (described below) was masked to treatment allocation.
Procedures
On day 1, patients received an intravenous infusion of study treatment based on baseline INR (assessed ≤3 h before start of infusion) and bodyweight, as described by Sarode and colleagues.11 Patients with baseline INR of 2 or higher but lower than 4 were given 4F-PCC at a dose of 25 IU factor IX per kg bodyweight or plasma 10 mL/kg bodyweight; those with baseline INR of 4 to 6 (inclusive) were given 4F-PCC at a dose of 35 IU factor IX per kg or plasma 12 mL/kg; and those with baseline INR higher than 6 were given 4F-PCC at a dose of 50 IU factor IX per kg or plasma 15 mL/kg. Patients weighing more than 100 kg were given doses based on a bodyweight of 100 kg.
4F-PCC was given at an infusion rate of 3 IU/kg per min or less; plasma was infused as rapidly as possible and at the discretion of the treating clinical team. Thus, the plasma infusion rate represented standard care and maximised patient safety (because of concern that some patients might not be able to tolerate rapid volume load). Additionally, vitamin K was to be given to all patients according to American College of Chest Physicians12 guidelines (≤5 mg orally, followed by 1–2 mg orally if required, in patients needing urgent surgery; 10 mg by slow intravenous infusion in patients with major bleeding) or local practice if different (ie, 2–10 mg). Vitamin K administration was not standardised in the protocol because of variations in local practice and guidelines.
We recorded the total volume and total infusion time of each study product. Additional blood products and haemostatic agents given were documented from randomisation to 24 h after start of study product infusion or end of the surgery, whichever came later. Blood samples were drawn for determination of INR and levels of vitamin K-dependent coagulation factors and proteins C and S before study product infusion and at 0·5 h, 1 h, 3 h, 6 h, and 24 h after start of infusion, in addition to INR at 0·5 h after end of infusion. We assessed baseline INR 3 h or less before start of infusion.
Adverse events and serious adverse events were recorded by the investigators and assessed by an independent data and safety monitoring board, unblinded to study treatment. After study launch, the data and safety monitoring board requested that a blinded safety adjudication board be established to review possible thromboembolic serious adverse events, late bleeding events, and deaths. Serious adverse events possibly consistent with thrombotic events or late bleeds, as well as death cases, were referred to the safety adjudication board. Adjudication results were provided to the data and safety monitoring board on an ongoing basis. Adverse events were assessed up to day 10 (visit window days 7–11) and serious adverse events up to day 45 (visit window days 43–51). Fluid overload events were identified according to the Medical Dictionary for Regulatory Activities version 12.0 terms: fluid overload, pulmonary oedema, cardiac failure congestive, cardiac failure chronic, and cardiac failure.
Outcomes
The primary endpoint was haemostasis during urgent surgical or invasive procedures in the intention-to-treat efficacy (ITT-E) population. We categorised haemostasis as a binary endpoint (effective or non-effective) and this endpoint was assessed from the start of infusion to the end of the procedure. We defined effective haemostasis as: intraoperative (or intra-procedural) blood loss not exceeding predicted blood loss by 30% or 50 mL; and normal or mildly abnormal haemostasis (surgeon assessed); and no administration of non-study coagulation products. Predicted blood loss was determined by the local surgeon before the start of surgery, using all clinical information available, based on the assumption of a similar non-coagulopathic patient undergoing the same intervention. We based actual blood loss (ABL) on the anaesthesiologist’s record of estimated blood loss during the procedure. If an anaesthesiologist was not present during the procedure, ABL was estimated by the surgeon or physician performing the procedure. Missing haemostatic efficacy assessments resulted in a rating of non-effective haemostasis.
The coprimary endpoint was rapid INR reduction (INR ≤1·3 at 0·5 h after the end of infusion) in the ITT-E population. A missing INR value at this timepoint, or administration of additional coagulation factor-containing products (non-study plasma, whole blood, or other non-study products containing coagulation factors, excluding packed red blood cells or platelets) from the start of treatment infusion to the start of the procedure resulted in a rating of no rapid decrease in INR.
There were four prespecified secondary endpoints: time to INR reduction (INR ≤1·3) from start of infusion; units of red blood cells (defined as packed red blood cells and whole blood) given from start of surgery to 24 h after start of surgery; proportion of patients receiving red blood cells from start of surgery to 24 h after start of surgery; and plasma levels of vitamin K-dependent coagulation factors and proteins C and S. Planned exploratory endpoints (intended for future analyses) are listed in the appendix.
Statistical analysis
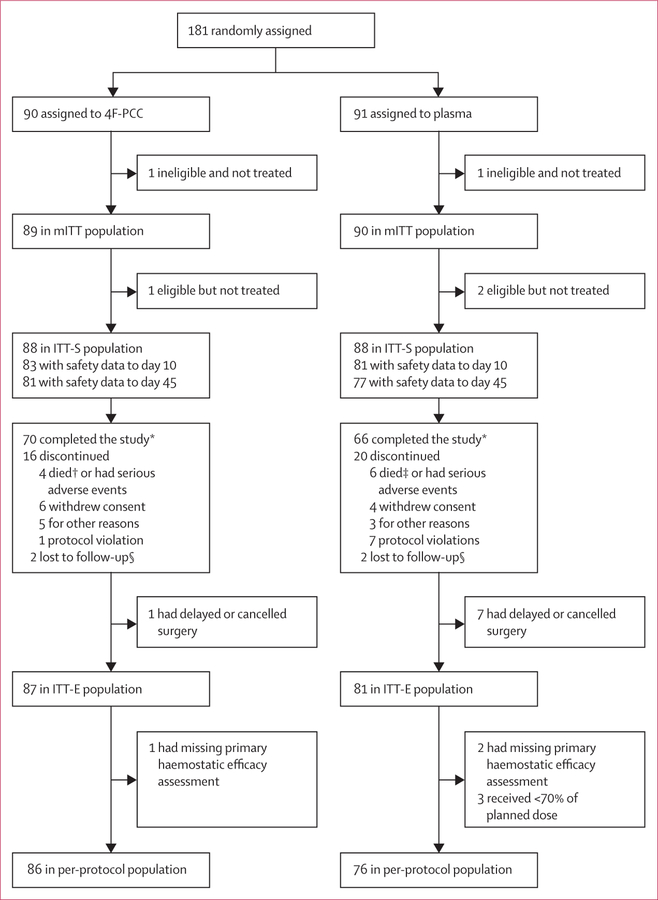
There were four analysis populations (figure 1). The modified intention-to-treat (mITT) population comprised all randomly assigned patients who were either eligible for the study but did not receive any portion of study product, or received any portion of study product. The intention-to-treat safety (ITT-S) population comprised all eligible patients from the mITT population who had received any portion of study product. The ITT-E population comprised all patients from the ITT-S population who had an INR higher than 1·3 before study product infusion and underwent the intended procedure; both the primary and coprimary endpoints were assessed in this population. Finally, the per-protocol population comprised all ITT-E patients who did not have any major protocol deviations.
Figure 1. Patient flow.
4F-PCC=four-factor prothrombin complex concentrate. mITT=modified intention-to-treat. ITT-E=intention-to-treat efficacy. ITT-S=intention-to-treat safety. *Study included viral follow-up to day 90. †One death occurred after study day 45 (day 48; worsening of cardiopulmonary disease). ‡Eight deaths in total in plasma group; one plasma death occurred in a completed patient and one plasma death occurred in a patient with a protocol violation. §Patient not able to be reached for follow-up.
The study was designed to test the hypothesis that 4F-PCC was non-inferior to plasma with regard to the primary and coprimary endpoints. We performed non-inferiority analyses in the ITT-E population via calculations (using the Newcombe-Wilson score method)13 of the two-sided 95% CI, equivalent to a one-sided type I error rate of 0·025, for the difference in the proportions of patients achieving effective haemostasis, and separately for rapid INR reduction. For both haemostasis and INR reduction, non-inferiority was demonstrated if the lower limit of the 95% CI for the between-group difference (4F-PCC minus plasma) was greater than −10%. Because there is little evidence available about the haemostatic efficacy of plasma versus placebo in patients on VKA therapy requiring urgent interventions,14 there was no independent way of determining a non-inferiority margin in terms of preserving some portion of the effect of plasma versus placebo. Therefore, the non-inferiority margin of −10% was chosen based on clinical judgment. 4F-PCC could be successfully claimed to be non-inferior to plasma if non-inferiority was shown for both the primary and co-primary endpoints. If non-inferiority was shown, 4F-PCC was also to be tested for superiority compared with plasma for each of these endpoints. Superiority for an endpoint could be declared if the lower limit of the 95% CI exceeded zero. Because testing for superiority after demonstration of non-inferiority15 does not increase type I error, a test for superiority could be done on the nominal one-sided α-level of 0·025 after demonstration of non-inferiority.
We did sample size calculations on the haemostatic efficacy endpoint and assumed that effective haemostasis would be achieved by 85% of patients in the plasma group and 90% of patients in the 4F-PCC group. Based on the Newcombe-Wilson score method for CI calculations,13 a non-inferiority margin of −10% and a dropout rate of 10%, the power to show non-inferiority would exceed 80% for two treatment groups of 88 patients (total target sample size of 176 patients). No sample size calculation was done on the INR endpoint because of the assumption of similar values for the percentages in the two study groups.
We adjusted four p values, one from each of the four secondary analyses, using the method of Holm.16 This adjustment controlled the overall type I error and preserved the 0·05 significance level. We described time to INR correction by Kaplan-Meier estimation, and assessed significance of treatment differences using the log-rank test. Between-group differences for number of units of red blood cells transfused were assessed by Wilcoxon-rank-sum test. We compared the proportions of patients receiving one or more transfusions of red blood cells using Newcombe-Wilson score test. Plasma levels of vitamin K-dependent coagulation factors and proteins C and S were summarised by descriptive statistics and, in post-hoc analyses, group differences were compared by two-sided Wilcoxon test.
We applied an ANCOVA model with predicted blood loss as a dependent variable, treatment, sex, surgical type, and study site as factors, and preinfusion haemoglobin as a covariate to establish whether predicted blood loss differed by treatment, which would suggest bias in the estimation of predicted blood loss.
We compared incidences of thromboembolic events, fluid overload events, and deaths between treatment groups using Newcombe-Wilson CIs with continuity correction; other safety outcomes were analysed descriptively. We computed p values using the χ2 test for homogeneity or Fisher’s exact test when any of the cell sizes were small (less than five).
As a result of the protocol amendment to halt enrolment of patients undergoing non-surgical invasive procedures, we also planned to do non-inferiority and superiority analyses of the haemostatic efficacy and rapid INR reduction endpoints with the exclusion of patients needing non-surgical invasive procedures.
We analysed data with SAS version 9.3. This trial is registered at ClinicalTrials.gov, number NCT00803101.
Role of the funding source
This research was funded by CSL Behring. A steering committee of academic medical experts and rep-resentatives of the funder oversaw the design and conduct of the study. The funder participated in the selection of the board members. The funder was responsible for data collection, management, and analysis of the data according to a predefined statistical analysis plan. Preparation and review of the Article and the decision to submit for publication was done by a publication steering committee that included academic medical experts and representatives of the funder. Medical writing assistance was paid for by the funder. JNG and RS had full access to all the data in the study and took responsibility for the integrity and accuracy of the data analysis.
Results
181 patients were enrolled in the trial between Feb 3, 2009, and Nov 28, 2012 (figure 1); the study completed on Feb 21, 2013. We randomly assigned 90 patients to receive 4F-PCC and 91 patients to receive plasma. The mITT population included 179 patients (89 in the 4F-PCC group and 90 in the plasma group) who were randomly assigned and were either eligible or received treatment (two patients who were randomly assigned but not eligible and not treated were excluded). The ITT-S population included 176 patients (88 in the 4F-PCC group and 88 in the plasma group); reasons for loss and exclusion are shown in figure 1. The ITT-E population included 168 patients (87 in the 4F-PCC group and 81 in the plasma group). 28 patients (13 receiving 4F-PCC and 15 receiving plasma) who needed non-surgical invasive procedures were enrolled in the study. Table 1 shows patients’ baseline data and characteristics. A detailed list of surgeries and procedures is shown in the appendix.
Table 1:
Demographic and baseline characteristics (ITT-E population)
| 4F-PCC (n=87) | Plasma (n=81) | |
|---|---|---|
| Sex | ||
| Female | 37 (43%) | 31 (38%) |
| Male | 50 (57%) | 50(62%) |
| Age, years | 69·4 (13·5) | 66·0 (13·2) |
| Region | ||
| USA | 44 (51%) | 37 (46%) |
| Europe/Lebanon | 43 (49%) | 44 (54%) |
| Body-mass index, kg/m2 | 27·9 (6·4) | 28·5 (7·9) |
| Baseline INR | 2·90 (2·0–17·0) | 2·90 (2·0–26·7) |
| Type of surgery or procedure | ||
| Cranial neurosurgical | 1 (1%) | 1 (1%) |
| Cardiothoracic surgical | 3 (3%) | 3 (4%) |
| Major orthopaedic surgical | 20 (23%) | 15 (19%) |
| Other surgical | 50 (57%) | 47 (58%) |
| Invasive | 13 (15%) | 15 (19%) |
| Vitamin K dose | ||
| Oral vitamin K dose ≤2 mg | 6 (7%) | 2 (2%) |
| Oral vitamin K dose >2 mg | 9 (10%) | 10 (12%) |
| Any IV vitamin K | 70 (80%) | 69 (85%) |
| No vitamin K | 1 (1%) | 0 |
| Not available | 1 (1%) | 0 |
| Reason for oral VKA therapy | ||
| Arrhythmia | 42 (48%) | 31 (38%) |
| Vascular disease | 17 (20%) | 18 (22%) |
| Artificial heart valve or joint | 13 (15%) | 14 (17%) |
| Thromboembolic event | 12 (14%) | 16 (20%) |
| Other | 3 (3%) | 2 (2%) |
Data are n (%), mean (SD), or median (IQR). 4F-PCC=four-factor prothrombin complex concentrate. INR=international normalised ratio. ITT-E=intention-to-treat efficacy. IV=intravenous. VKA=vitamin K antagonist.
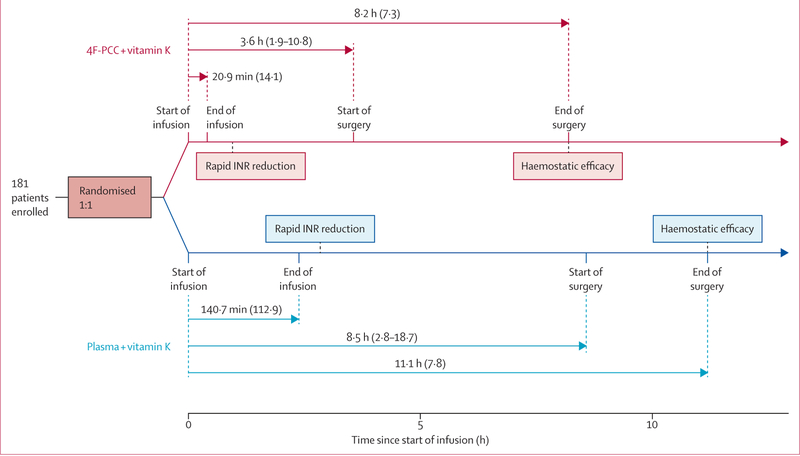
The timing of the study treatment and interventions is shown in figure 2. Delivery of plasma (mean volume 818·7 mL [SD 230·8]) was as fast as could be done by the clinical team within the confines of local practice (including type-specific matching, thawing, delivery, and infusion). 4F-PCC was reconstituted from a lyophilised powder and infused (mean volume 89·7 mL [SD 31·9]). Vitamin K was given a median of 13 min before 4F-PCC (IQR 40 min before, 26 min after), and a median of 15 min before plasma (IQR 55 min before, 55 min after; post-hoc analysis). Two patients in the 4F-PCC group and no patients in the plasma group received no vitamin K during the study. 15 patients in the 4F-PCC group and 12 in the plasma group received vitamin K by a non-intravenous route. The median time from start of infusion to start of urgent surgical procedure was longer in the plasma group (8·5 h [IQR 2·8–18·7]) than in the 4F-PCC group (3·6 h [1·9–10·8]; p=0·0098; post-hoc analysis).
Figure 2. Study overview (ITT-E population).
Data are mean (SD) or median (IQR). ITT-E=intention-to-treat efficacy. 4F-PCC=four-factor prothrombin complex concentrate. INR=international normalised ratio.
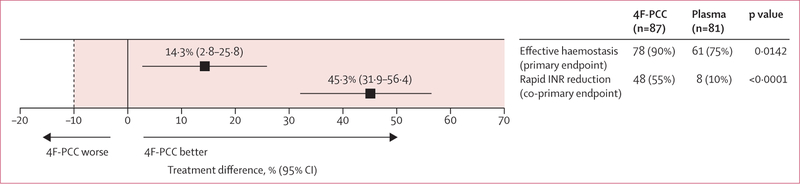
The primary endpoint, effective haemostasis in the ITT-E population, was achieved by 78 (90%) patients in the 4F-PCC group and 61 (75%) patients in the plasma group (figure 3). The treatment difference was 14·3% (95% CI 2·8 to 25·8, p=0·0142). Because the lower limit of the confidence interval for the treatment difference (2·8%) exceeded the non-inferiority margin of −10%, non-inferiority was shown for haemostatic efficacy. Analysis of superiority (lower limit of the 95% CI >0) showed that 4F-PCC was superior to plasma for this endpoint. Furthermore, we noted superiority for haemostatic efficacy for 4F-PCC compared with plasma when the per-protocol population was used (78 [91%] patients in the 4F-PCC group vs 58 [76%] patients in the plasma group; difference 14·4%, 95% CI 3·0 to 26·0, p=0·0128; appendix).
Figure 3. Primary and co-primary endpoints.
Figure shows effective haemostasis (haemostatic efficacy rating of excellent or good) and rapid INR reduction (INR ≤1·3 at 0·5 h after end of infusion) by non-inferiority analysis in the ITT-E population. Treatment difference refers to between-group difference of 4F-PCC minus plasma. Tinted area shows zone of non-inferiority, bounded by non-inferiority margin (dotted line) set at −10%. Superiority margin was set at 0% (solid line), meaning that 4F-PCC is superior to plasma if the lower limit of the 95% CI is to the right of the solid line. 4F-PCC=four-factor prothrombin complex concentrate. INR=international normalised ratio. ITT-E=intention-to-treat efficacy.
Intraoperative blood loss was used as part of the assessment of haemostatic efficacy. To address the possibility that enrolling investigators estimated predicted blood loss differently depending on treatment group in this open-label study, we assessed for such bias using an ANCOVA model. We found no statistically significant difference between treatment groups in terms of predicted blood loss (mean predicted blood loss 173·7 mL [SD 188·2] for 4F-PCC vs 173·3 mL [188·7] for plasma), supporting the finding of no bias on the part of the investigators, and the least-squares means of the predicted blood loss were similar for both treatments (175·6 [95% CI 86·1–265·1] for 4F-PCC vs 187·9 [104·1–271·6] for plasma; difference −12·3% [95% CI −64·6 to 40·1]; p=0·64).
For patients undergoing any surgical procedure (ie, excluding patients who underwent non-surgical invasive procedures), the treatment difference for effective haemostasis was 15·1% (95% CI 1·9 to 28·2, p=0·0237; 65 [88%] patients in the 4F-PCC group vs 48 [73%] patients in the plasma group), demonstrating superiority of 4F-PCC compared with plasma. Although the numbers in each group were small, limiting any conclusions, the treatment difference was 13·3% (95% CI −11·4 to 37·9, p=0·48; 13 [100%] patients in the 4F-PCC group vs 13 [87%] patients in the plasma group) for patients needing non-surgical invasive procedures. Table 2 shows the treatment differences for various prespecified subgroups.
Table 2:
Haemostatic efficacy and rapid INR reduction by type of procedure (ITT-E population)
| 4F-PCC (n=87) |
Plasma (n=81) |
Treatment difference (95% CI)* | p value* | |||
|---|---|---|---|---|---|---|
| Endpoint achieved | Endpoint not achieved | Endpoint achieved | Endpoint not achieved | |||
| Haemostatic efficacy endpoint | ||||||
| Cranial neurosurgical | 0 | 1 (100%) | 1 (100%) | 0 | NA† | ·· |
| Cardiothoracic surgical | 3 (100%) | 0 | 3 (100%) | 0 | 0% | 1·00 |
| Major orthopaedic surgical | 16 (80%) | 4 (20%) | 9 (60%) | 6 (40%) | 20·0% (−9·6 to 47·0) | 0·27 |
| Other surgical | 46 (92%) | 4 (8%) | 35 (74%) | 12 (26%) | 17·5% (2·6 to 32·3) | 0·0279 |
| Invasive procedure | 13 (100%) | 0 | 13 (87%) | 2 (13%) | 13·3% (−11·4 to 37·9) | 0·48 |
| Rapid INR reduction endpoint (INR ≤1·3 at 0·5 h after end of infusion) | ||||||
| Cranial neurosurgical | 0 | 1 (100%) | 0 | 1 (100%) | NA† | ·· |
| Cardiothoracic surgical | 2 (67%) | 1 (33%) | 0 | 3 (100%) | 66·7% (−5·9 to 93·9) | 0·4 |
| Major orthopaedic surgical | 11 (55%) | 9 (45%) | 2 (13%) | 13 (87%) | 41·7% (9·5 to 63·1) | 0·0158 |
| Other surgical | 27 (54%) | 23 (46%) | 2 (4%) | 45 (96%) | 49·7% (32·9 to 63·1) | <0·0001 |
| Invasive procedure | 8 (62%) | 5 (38%) | 4 (27%) | 11 (73%) | 34·9% (−1·4 to 60·9) | 0·12 |
Treatment difference refers to between-group difference of 4F-PCC minus plasma. Endpoint achieved refers to effective haemostasis or rapid reduction in INR; endpoint not achieved refers to non-effective haemostasis or no rapid reduction in INR. 4F-PCC=four-factor prothrombin complex concentrate. ITT-E=intention-to-treat efficacy. NA=not available.
95% CIs and p values generated post hoc using a basic χ2 test for homogeneity (Fisher’s exact test used for small cell sizes).
Analysis not done because fewer than five patients were in this subgroup.
The coprimary endpoint, rapid INR reduction in the ITT-E population, was achieved by 48 (55%) patients in the 4F-PCC group compared with eight (10%) patients in the plasma group. The treatment difference was 45·3% (95% CI 31·9 to 56·4, p<0·0001), demonstrating both non-inferiority and superiority for rapid INR reduction. Furthermore, superiority was shown for 4F-PCC compared with plasma for the per-protocol population (difference 45·3%, 95% CI 31·5 to 56·5, p<0·0001; 48 [56%] patients in the 4F-PCC group vs eight [11%] patients in the plasma group; appendix).
The treatment difference was 48·0% (95% CI 33·9 to 59·5, p<0·0001; 40 [54%] patients in the 4F-PCC group vs four [6%] patients in the plasma group) for patients undergoing any surgical procedure, demonstrating superiority of 4F-PCC over plasma. The treatment difference was 34·9% (95% CI −1·4 to 60·9, p=0·12; eight [62%] patients in the 4F-PCC group vs four [27%] patients in the plasma group) for patients undergoing a non-surgical invasive procedure. Additionally, in pre-specified subgroup analyses, we also noted treatment differences in favour of 4F-PCC for the major orthopaedic and other surgical enrolment strata (table 2).
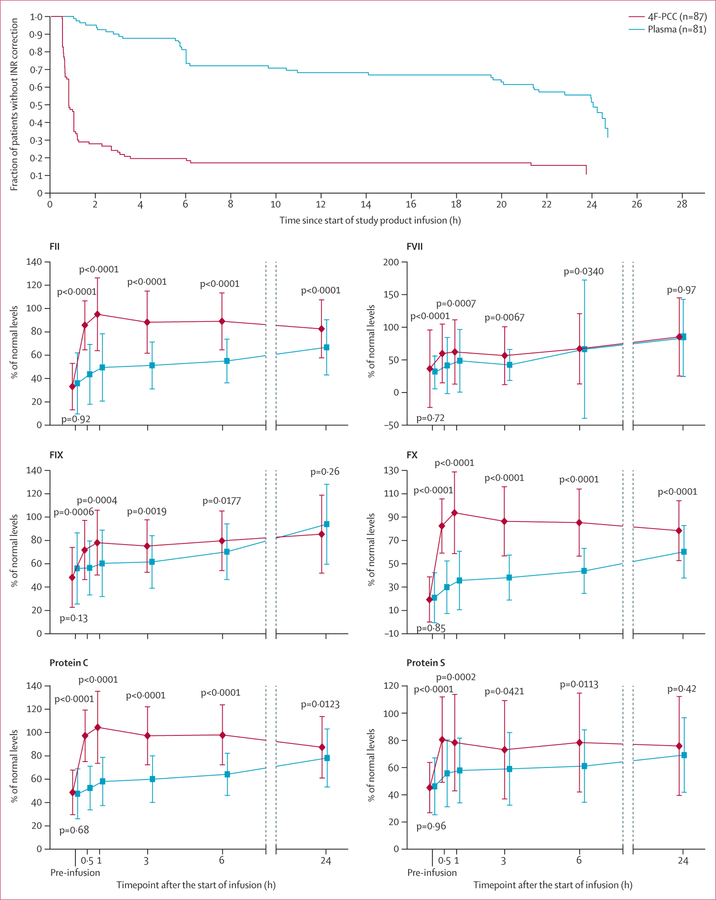
Patients in the 4F-PCC group achieved INR of 1·3 or lower more rapidly than did those in the plasma group (figure 4). 1 h after the start of infusion, 47 patients (54%) in the 4F-PCC group had an INR of 1·3 or lower compared with no patients in the plasma group (p<0·0001).
Figure 4. Secondary endpoints.
Figure shows INR correction and factor-level repletion in the ITT-E population. Data are proportion of patients or mean percentage of normal levels (SD). 4F-PCC=four-factor prothrombin complex concentrate. INR=international normalised ratio. ITT-E=intention-to-treat efficacy.
Plasma levels of vitamin K-dependent coagulation factors and proteins C and S were significantly higher in the 4F-PCC group than in the plasma group at 0·5 h, 1 h, 3 h, and 6 h after start of infusion (all p values <0·05; figure 4).
Few patients in either group received red blood cells (14 [16%] in the 4F-PCC group and 12 [15%] in the plasma group); we noted no significant difference between groups (p=0·83). Additionally, the mean number of red blood cell units transfused per patient was similar between groups (0·3 units [SD 0·9] for 4F-PCC vs 0·4 units [1·0] for plasma, p=0·91).
We assessed safety outcomes in the ITT-S population. 49 patients in the 4F-PCC group and 53 patients in the plasma group had at least one adverse event (p=0·54; table 3). The frequency of adverse events and serious adverse events, including those related to treatment, was generally similar between groups. In particular, the proportion of patients with adverse events related to study treatment was eight (9%) in the 4F-PCC group and 15 (17%) in the plasma group (difference 8·0%, 95% CI −18·9 to 3·0, p=0·18; post-hoc analysis). Adverse events that occurred in at least 5% of patients in any treatment group after study product infusion are reported in the appendix.
Table 3:
Summary of adverse events (ITT-S population)
| 4F-PCC (n=88) |
Plasma (n=88) |
|
|---|---|---|
| Any adverse event | 49 (56%) | 53 (60%) |
| Related adverse event* | 8 (9%) | 15 (17%) |
| Adverse event leading to treatment discontinuation | 0 | 0 |
| Serious adverse event | 22 (25%) | 23 (26%) |
| Related serious adverse event* | 3 (3%) | 3 (3%) |
| Adverse events of interest | ||
| Deaths to day 45† | 3 (3%) | 8 (9%) |
| Thromboembolic adverse event‡ | 6 (7%) | 7 (8%) |
| Fluid overload or similar cardiac event | 3 (3%) | 11 (13%) |
| Bleeding after primary outcome assessment | 3 (3%) | 4 (5%) |
Adverse events with missing treatment associations were classified as related to treatment.
Defined as events that were related to study treatment according to the investigator.
One additional death in the 4F-PCC group occurred after study day 45 (day 48; worsening of cardiopulmonary disease).
Thromboembolic adverse events included: six patients (seven events§) in the 4F-PCC group (deep-vein thrombosis [two events], thrombosis, ischaemic stroke [two events], vena cava filter insertion, and catheter-related complication) and seven patients (seven events) in the plasma group (acute myocardial infarction [two events], deep-vein thrombosis, ischaemic stroke [two events], pulmonary embolism, and transient ischaemic attack).
One deep-vein thrombosis and one stroke occurred in the same patient. 4F-PCC=four-factor prothrombin complex concentrate. ITT-S=intention-to-treat safety.
Thromboembolic adverse events were reported during the study for six (7%) patients in the 4F-PCC group and seven (8%) in the plasma group (difference −1·1%, 95% CI −10·3 to 8·0, p=0·77). Three (3%) patients in the 4F-PCC group developed fluid overload or similar cardiac events compared with 11 (13%) in the plasma group (difference −9·1%, 95% CI −18·6 to −0·1, p=0·0478). A total of seven patients (three [3%] for 4F-PCC and four [5%] for plasma) experienced possible late bleeding events that were reviewed by the safety adjudication board (appendix).
By the day 45 visit, there were three deaths in the 4F-PCC group and eight in the plasma group, of which one (acute myocardial infarction; plasma group) was considered by the safety adjudication board to be treatment related. The difference in rates was −5·7% (95% CI −14·6 to 2·7, p=0·21), and was not considered significant. Individual mortality data are detailed in the appendix.
Discussion
Because non-inferiority was achieved for both the primary and the coprimary endpoints, non-inferiority was achieved for 4F-PCC compared with plasma overall in this open-label phase 3b study. To our knowledge, this trial is the first adequately powered comparison of 4F-PCC and plasma for rapid VKA reversal in patients needing urgent surgical or invasive interventions. Not only was 4F-PCC non-inferior to plasma for haemostatic efficacy (the comparison we were primarily powered to test), but it was additionally superior for this endpoint (an effect we had less than 70% power to detect). 4F-PCC was also both non-inferior and superior to plasma for the coprimary endpoint of rapid INR reduction.
Only one other randomised trial has addressed this question, a cardiac surgery study with only 20 patients per group that assessed INR reduction but not haemostatic efficacy.9 Our results are consistent with findings from this trial and those from previous retrospective cohort studies (including for trauma and spontaneous haemorrhage), which have shown that 4F-PCCs used for VKA reversal can more rapidly replace vitamin K-dependent coagulation factors and lower INR than plasma.17–20
The time between start of infusion and start of surgery was significantly shorter in the 4F-PCC group than in the plasma group. The shorter administration time and rapid INR reduction due to higher levels of vitamin K-dependent coagulation factors in 4F-PCC probably contributed to the decreased time to start of surgery that we noted in the 4F-PCC group compared with the plasma group. We do not believe plasma may have been systematically infused more quickly; because it requires ABO typing and thawing, there is local variation in how quickly it can be obtained, and both patient-level and provider-level variation in how quickly the clinical team can infuse it. Because of ethical and logistic considerations, we could not mandate an infusion rate faster than local practice and clinicians could provide. Findings from observational studies have in fact shown substantially slower plasma infusion for VKA reversal in standard clinical practice than we noted in this trial, suggesting that patients in this trial truly did receive plasma as rapidly as was logistically feasible.21–24 Whether variations in plasma infusion rates affect haemostatic efficacy is not clear.
Additionally, vitamin K dose and administration route were not rigidly defined by the protocol. During trial planning, there were ethical concerns that local teams would need leeway in such dosing, depending on the clinical situation. This factor seems unlikely to be a substantial confounder because we noted no evidence that vitamin K dosing was different between study groups.
Although thromboembolic complications are often listed as a concern in VKA reversal, there was no evidence of an increased risk of such for 4F-PCC compared with plasma. However, the study was not powered to detect between-group differences in the incidence of these events. Thus far, no randomised trial has shown a difference in thromboembolic event rates between prothrombin complex concentrate and plasma, probably because they were not adequately powered to do so.9,11 A recent observational study examining the effect of introducing a 4F-PCC to the emergency department found similar results.17 Two recent comprehensive reviews, based on single-group studies of prothrombin complex concentrates, also concluded that there is a low risk of thromboembolic events in patients treated with prothrombin complex concentrates for VKA reversal, and that underlying disease and dosing may be important factors in increasing risk.25,26 Thromboembolism might occur with the same frequency in this patient population irrespective of the means used to reverse VKA.27,28
Fluid overload events, however, occurred more frequently in the plasma group than in the 4F-PCC group. Therefore, our data suggest that patients at higher risk of volume overload who need VKA reversal might specifically benefit from 4F-PCC rather than plasma.
We note that most patients in the ITT-E population completed the study to the primary endpoint (86 [99%] of 87 patients in the 4F-PCC group and 79 [98%] of 81 in the plasma group), and safety data to day 10 (including mortality) were available for 164 (93%) of 176 patients. Ten patients withdrew consent and eight withdrew from the study for other reasons; no safety data are available after withdrawal. 40 patients did not complete the study to the 90-day viral safety endpoint, which affected only the viral assessment and not our ability to analyse the primary, secondary, or other safety outcomes. Trial discontinuations did not seem to occur disproportionately in one study group compared with the other.
Eight patients had their procedure cancelled or delayed beyond 24 h, suggesting that some enrolled patients did not ultimately need an emergent or urgent procedure. This finding highlights the clinical reality of emergency care, in which decisions need to be made rapidly based on information available at the time. The clinical situation can then evolve, and for some patients their clinical status changed in a way that could not be predicted. To maximise generalisability, the criteria for entry included a judgment on the part of the participant’s clinical care team that an urgent or emergency procedure was indicated and that pre-procedural urgent VKA reversal was necessary.
Overall study enrolment was fairly slow. We noted that there were several exclusion criteria, and with enrolment occurring in the acute setting, many eligible patients were probably treated by the clinical care teams before the research teams had time to approach participants and go through the informed consent process. Additionally, the scientific literature regarding which patients might benefit from VKA reversal continues to evolve,1 and many potential participants could have been judged by the clinical teams to not need urgent reversal.
This study had several limitations. First, the study team members, clinicians, and participants could not be blinded to treatment allocation. Such blinding could not logistically or ethically be done because of the underlying differences in delivery (PCC is reconstituted and infused quickly in a small volume, whereas type-specific plasma must be prepared and dispensed from the local blood bank in multiple bags that must be thawed and infused separately). We attempted to control for this factor by assessing whether the prediction of expected blood loss was different between treatment groups, and found no effect. Second, there was variability in timing of plasma infusion. We did note that infusion times were more rapid and consistent than those documented in routine clinical practice,21–23 emphasising that plasma infusion was probably done as efficiently as could be achieved in view of the logistics of delivering it. Third, this study was powered to detect differences in efficacy but not differences between groups for safety outcomes; therefore, we cannot rule out differences in rare adverse events between treatment groups. Fourth, the haemostatic efficacy endpoint, although often used in haemostasis trials, includes a potentially subjective component, because clinicians must estimate predicted blood loss and might need to estimate ABL. We optimised this endpoint as much as possible; to our knowledge, it is the best currently available endpoint that can be applied across a range of surgical and invasive interventions.
This study focused on urgent reversal of VKAs rather than direct factor inhibitors. We note that direct factor IIa and Xa inhibitors are now available and are becoming more frequently used as an alternative to VKAs. Because we did not enrol patients taking these newer drugs, we cannot provide much-needed data on how best to reverse them.
In conclusion, these data show that 4F-PCC is an effective and superior alternative to plasma in terms of haemostatic efficacy and rapid INR reduction for the rapid reversal of VKA therapy before urgent procedures (panel).
Supplementary Material
Panel: Research in context.
Systematic review
We used previously published systematic reviews6,18,19,25,26 to assess the existing evidence for the use of plasma or 4F-PCCs for vitamin K antagonist (VKA) reversal. We also searched PubMed for comparative studies of VKA reversal with four-factor prothrombin complex concentrate (4F-PCC) or plasma reported since the publication of these systematic reviews (July, 2011, to March, 2014) with the terms (“prothrombin complex concentrates”[Supplementary Concept] OR “Factor IX”[MeSH]) AND “anticoagulants” [Pharmacological Action]. No language restrictions were used and the search was done on March 10, 2014. The results of these systematic reviews and the more recent references11,17,24 suggested that international normalised ratio (INR) reduction (in patients with spontaneous bleeding, trauma, or before a surgical procedure) is more effectively and rapidly achieved with 4F-PCC than with plasma or 3F-PCC. However, there was little comparative evidence about clinical outcomes or safety.
Interpretation
For the endpoint of rapid INR reduction, the results from our trial are consistent with previously published (mainly observational) data and demonstrate that 4F-PCC is non-inferior and superior to plasma for rapid INR reduction in patients on VKA therapy. Furthermore, we noted that 4F-PCC could be given more rapidly than plasma, which is in agreement with previously published (retrospectively collected) data.24 For the endpoint of clinical efficacy, we found no other adequately powered trial examining reversal of VKA therapy in patients needing urgent surgical procedures, and this trial therefore offers new insights into their treatment. We noted that 4F-PCC was superior to plasma for haemostatic efficacy. Although our study was not powered to assess safety, we did not detect any between-treatment differences for the occurrence of thromboembolic events or deaths, a finding in agreement with the existing scientific literature.11,17,25,26 Additionally, although these data guide clinicians on how best to achieve urgent VKA reversal, the scientific literature concerning which patients should be urgently reversed before surgical or invasive interventions continues to evolve; for example, findings from a recent trial showed the safety of pacemaker placement without interruption of anticoagulation.29
See Online for appendix
Acknowledgments
Medical writing assistance was provided by Rianne Stacey (Fishawack Communications, Abingdon, UK) and was funded by CSL Behring. Joerg Pawlitschko (Aptiv Solutions, Cologne, Germany) created all table shells and performed the data analyses reported in the Article. Martin L Lee (UCLA School of Public Health, Los Angeles, CA, USA) provided additional statistical assistance. Antoinette Mangione, Astrid Schneider, and Billie Durn (CSL Behring, King of Prussia, PA, USA) contributed to the data review and quality check of the Article. BAH is currently employed by GlaxoSmithKline.
Footnotes
Declaration of interests
JNG has received consulting fees and a research grant from CSL Behring. MAR is a member of a CSL Behring speakers bureau. TJM Jr has received consulting fees from CSL Behring and has been a member of a CSL Behring speakers bureau. RG-A is an employee of CSL Behring LLC. BAH was an employee of CSL Behring LLC at the time the study was conducted. RS has received consulting fees and honoraria from CSL Behring and has served as a member of advisory boards for Octapharma, Instrument Laboratories, Alexion, and Kedrion. BL declares no competing interests.
Contributor Information
Joshua N Goldstein, Massachusetts General Hospital, Boston, MA, USA.
Majed A Refaai, University of Rochester Medical Center, Rochester, NY, USA.
Truman J Milling, Jr, Seton/UT Southwestern Clinical Research Institute of Austin, Dell Children’s Medical Center, University Medical Center at Brackenridge, Austin, TX, USA.
Brandon Lewis, St Joseph Regional Health Center, Bryan, Texas A&M Health Science Center, College Station, TX, USA.
Robert Goldberg-Alberts, CSL Behring LLC, King of Prussia, PA, USA.
Bruce A Hug, CSL Behring LLC, King of Prussia, PA, USA.
Ravi Sarode, UT Southwestern Medical Center, Dallas, TX, USA.
References
- 1.Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th edn: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141: e326S–50S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Healey JS, Eikelboom J, Douketis J, et al. Periprocedural bleeding and thromboembolic events with dabigatran compared with warfarin: results from the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) randomized trial. Circulation 2012; 126: 343–48. [DOI] [PubMed] [Google Scholar]
- 3.Holbrook A, Schulman S, Witt DM, et al. Evidence-based management of anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th edn: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141: e152S–84S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G. Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th edn: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141: e44S–88S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bechtel BF, Nunez TC, Lyon JA, Cotton BA, Barrett TW. Treatments for reversing warfarin anticoagulation in patients with acute intracranial hemorrhage: a structured literature review. Int J Emerg Med 2011; 4: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang L, Stanworth S, Hopewell S, Doree C, Murphy M. Is fresh-frozen plasma clinically effective? An update of a systematic review of randomized controlled trials. Transfusion 2012; 52: 1673–86. [DOI] [PubMed] [Google Scholar]
- 7.Food and Drug Administration (FDA). 2012. Fatalities reported to FDA following blood collection and transfusion—annual summary for fiscal year 2012 http://www.fda.gov/downloads/BiologicsBloodVaccines/SafetyAvailability/ReportaProblem/TransfusionDonationFatalities/UCM346856.pdf (accessed Sept 1, 2013).
- 8.Holland L, Warkentin TE, Refaai M, Crowther MA, Johnston MA, Sarode R. Suboptimal effect of a three-factor prothrombin complex concentrate (Profilnine-SD) in correcting supratherapeutic international normalized ratio due to warfarin overdose. Transfusion 2009; 49: 1171–77. [DOI] [PubMed] [Google Scholar]
- 9.Demeyere R, Gillardin S, Arnout J, Strengers PF. Comparison of fresh frozen plasma and prothrombin complex concentrate for the reversal of oral anticoagulants in patients undergoing cardiopulmonary bypass surgery: a randomized study. Vox Sang 2010; 99: 251–60. [DOI] [PubMed] [Google Scholar]
- 10.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics 1975; 31: 103–15. [PubMed] [Google Scholar]
- 11.Sarode R, Milling TJ, Refaai MA, et al. Efficacy and safety of a four-factor prothrombin complex concentrate (4F-PCC) in patients on vitamin K antagonists presenting with major bleeding: a randomized, plasma-controlled, phase IIIb study. Circulation 2013; 128: 1234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition). Chest 2008; 133: 160S–98S. [DOI] [PubMed] [Google Scholar]
- 13.Newcombe RG. Interval estimation for the difference between independent proportions: comparison of eleven methods. Stat Med 1998; 17: 873–90. [DOI] [PubMed] [Google Scholar]
- 14.Murad MH, Stubbs JR, Gandhi MJ, et al. The effect of plasma transfusion on morbidity and mortality: a systematic review and meta-analysis. Transfusion 2010; 50: 1370–83. [DOI] [PubMed] [Google Scholar]
- 15.Committee for Proprietary Medicinal Products (CPMP). 2000. Points to consider on switching between superiority and non-inferiority http://www.emea.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003658.pdf (accessed July 5, 2013).
- 16.Holm S A simple sequentially rejective multiple test procedure. Scan J Statist 1979; 6: 65–70. [Google Scholar]
- 17.Hickey M, Gatien M, Taljaard M, Aujnarain A, Giulivi A, Perry JJ. Outcomes of urgent warfarin reversal with frozen plasma versus prothrombin complex concentrate in the emergency department. Circulation 2013; 128: 360–64. [DOI] [PubMed] [Google Scholar]
- 18.Lin DM, Murphy LS, Tran MH. Use of prothrombin complex concentrates and fibrinogen concentrates in the perioperative setting: a systematic review. Transfus Med Rev 2013; 27: 91–104. [DOI] [PubMed] [Google Scholar]
- 19.Voils SA, Baird B. Systematic review: 3-factor versus 4-factor prothrombin complex concentrate for warfarin reversal: does it matter? Thromb Res 2012; 130: 833–40. [DOI] [PubMed] [Google Scholar]
- 20.Quick JA, Bartels AN, Coughenour JP, Barnes SL. Experience with prothrombin complex for the emergent reversal of anticoagulation in rural geriatric trauma patients. Surgery 2012; 152: 722–26. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein JN, Thomas SH, Frontiero V, et al. Timing of fresh frozen plasma administration and rapid correction of coagulopathy in warfarin-related intracerebral hemorrhage. Stroke 2006; 37: 151–55. [DOI] [PubMed] [Google Scholar]
- 22.Jones CA, Petrozzino JJ, Hoesche J, Krol EM, Freeman K. Perceptions about time for normalization of international normalized ratio in patients requiring acute warfarin reversal when using fresh-frozen plasma. Am J Emerg Med 2013; 31: 878–79. [DOI] [PubMed] [Google Scholar]
- 23.Menzin J, White LA, Friedman M, et al. Factors associated with failure to correct the international normalised ratio following fresh frozen plasma administration among patients treated for warfarin-related major bleeding: an analysis of electronic health records. Thromb Haemost 2012; 107: 662–72. [DOI] [PubMed] [Google Scholar]
- 24.Majeed A, Meijer K, Larrazabal R, et al. Mortality in vitamin K antagonist-related intracerebral bleeding treated with plasma or 4-factor prothrombin complex concentrate. Thromb Haemost 2014; 111: 233–39. [DOI] [PubMed] [Google Scholar]
- 25.Dentali F, Marchesi C, Pierfranceschi MG, et al. Safety of prothrombin complex concentrates for rapid anticoagulation reversal of vitamin K antagonists a meta-analysis. Thromb Haemost 2011; 106: 429–38. [DOI] [PubMed] [Google Scholar]
- 26.Sorensen B, Spahn DR, Innerhofer P, Spannagl M, Rossaint R. Clinical review: prothrombin complex concentrates—evaluation of safety and thrombogenicity. Crit Care 2011; 15: 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prandoni P, Noventa F, Ghirarduzzi A, et al. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism: a prospective cohort study in 1,626 patients. Haematologica 2007; 92: 199–205. [DOI] [PubMed] [Google Scholar]
- 28.Witt DM, Delate T, Garcia DA, et al. Risk of thromboembolism, recurrent hemorrhage, and death after warfarin therapy interruption for gastrointestinal tract bleeding. Arch Intern Med 2012; 172: 1484–91. [DOI] [PubMed] [Google Scholar]
- 29.Birnie DH, Healey JS, Wells GA, et al. Pacemaker or defibrillator surgery without interruption of anticoagulation. N Engl J Med 2013; 368: 2084–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.