Abstract
Gonadotropin-releasing hormone (GnRH) neurons in the basal forebrain are the final common pathway through which the brain regulates reproduction. GnRH secretion occurs in a pulsatile manner, and indirect evidence suggests the kisspeptin neurons in the arcuate nucleus (ARC) serve as the central pacemaker that drives pulsatile GnRH secretion. The purpose of this study was to investigate the possible coexpression of kisspeptin, neurokinin B (NKB), and dynorphin A (Dyn) in neurons of the ARC of the goat and evaluate their potential roles in generating GnRH pulses. Using double and triple labeling, we confirmed that all three neuropeptides are coexpressed in the same population of neurons. Using electrophysiological techniques to record multiple-unit activity (MUA) in the medial basal hypothalamus, we found that bursts of MUA occurred at regular intervals in ovariectomized animals and that these repetitive bursts (volleys) were invariably associated with discrete pulses of luteinizing hormone (LH) (and by inference GnRH). Moreover, the frequency of MUA volleys was reduced by gonadal steroids, suggesting that the volleys reflect the rhythmic discharge of steroid-sensitive neurons that regulate GnRH secretion. Finally, we observed that central administration of Dyn-inhibit MUA volleys and pulsatile LH secretion, whereas NKB induced MUA volleys. These observations are consistent with the hypothesis that kisspeptin neurons in the ARC drive pulsatile GnRH and LH secretion, and suggest that NKB and Dyn expressed in those neurons are involved in the process of generating the rhythmic discharge of kisspeptin.
Introduction
The pulsatile release of gonadotropin-releasing hormone (GnRH) is a prerequisite for sustaining normal gonadotropin secretion in mammals (Knobil, 1980; Karsch, 1984); however, the cellular and molecular mechanisms that generate the rhythmic discharge of GnRH are unknown. Kisspeptin neurons in the hypothalamus play a key role in the regulation of GnRH neurons (Oakley et al., 2009), but the precise nature of the interaction between kisspeptin and GnRH neurons is just emerging. Several recent studies provide tantalizing—albeit indirect—evidence that the rhythmic discharge of kisspeptin neurons actually drives pulsatile GnRH secretion. For example, Keen et al. (2008) have shown that pulses of kisspeptin in the median eminence (ME) of the monkey occur in temporal association with GnRH pulses. Moreover, Roseweir et al. (2009) have demonstrated in several species that a kisspeptin antagonist blocks pulsatile GnRH/luteinizing hormone (LH) secretion. Thus, it is conceivable that kisspeptin neurons represent the proximate source of the GnRH pulse generator.
Kisspeptin neurons in the arcuate nucleus (ARC) coexpress neurokinin B (NKB) and dynorphin A (Dyn), at least in some species (Goodman et al., 2007; Navarro et al., 2009), and fibers containing both NKB and Dyn surround and appose Dyn/NKB-containing somata in the ARC (Burke et al., 2006). Central administration of either an NKB receptor (NK3) agonist or a Dyn receptor [the κ-opiate receptor (KOR)] antagonist profoundly influences GnRH/LH secretion (Goodman et al., 2004; Sandoval-Guzmán and Rance, 2004); moreover, mutations in either Trc3 or Tacr3 (which encode NKB and NK3, respectively) cause severe gonadotropin deficiency (Topaloglu et al., 2009). In the mouse, kisspeptin neurons express NK3 and the KOR (Navarro et al., 2009), indicating that kisspeptin/NKB/Dyn neurons form a network, coupled through autosynaptic processes. Finally, kisspeptin-containing fibers densely innervate GnRH fibers in the ME (Ramaswamy et al., 2008). These observations suggest that an interaction between kisspeptin/NKB/Dyn neurons and GnRH neurons produce the pacemaker events that generate pulsatile GnRH secretion—yet evidence for this concept remains circumstantial.
We postulated that kisspeptin, NKB, and Dyn act together to generate the rhythmic activity of kisspeptin/NKB/Dyn neurons, which in turn generates pulsatile secretion of GnRH. First, we sought to determine whether kisspeptin, NKB, and Dyn are coexpressed in neurons in the ARC of the goat, as has been reported in some other species (Goodman et al., 2007; Navarro et al., 2009). Second, we recorded multiple-unit electrical activity (MUA) in close proximity to kisspeptin neurons in the ARC and examined the association between MUA volleys and ultradian bursts of LH secretion, as previously reported (Ohkura et al., 2009). Finally, we tested the hypothesis that NKB and Dyn play crucial roles in driving GnRH pulse generator activity by analyzing the effects of centrally administered NKB, Dyn, and a KOR antagonist on pulsatile MUA and LH secretion. We present evidence that kisspeptin, NKB, and Dyn act as comodulators to produce the rhythmic discharge of kisspeptin neurons in the ARC, whose network serves as the pacemaker for the GnRH pulse generator.
Materials and Methods
Animals
Adult (3- to 8-year-old) ovariectomized (OVX) Shiba goats (Capra hircus), weighing 20–35 kg, were used. The goats were loosely held in an individual stanchion in a condition-controlled room (12 h light/dark cycle, 23°C, and 50% relative humidity). They were fed daily with a standard pelleted diet and hay. Water was always available. All experimental procedures were approved by the National Institute of Agrobiological Sciences Committee for the Care and Use of Experimental Animals.
PCR and gene cloning
We amplified goat NKB and Preprodynorphin (PDYN) gene fragments by PCR using goat cDNA derived from the hypothalamus as templates. The PCR primers for the amplification of NKB and PDYN were based on bovine sequences on GenBank. We used the following primers: NKB sense (S), ATGCGGAGCACCCTGCTGTT; antisense (AS), CATTCCACACTTGGAGGGTA; PDYN S, TGTGCTGTGAAGACCCAGGA; AS, ACCGAGTGACCACCTTGAACTG. Each fragment was inserted into the pTA2 vector (Toyobo). GenBank accession numbers are AB499062 (goat NKB) and AB499063 (goat PDYN).
Histochemistry
Tissue preparation.
Three goats were killed with an overdose of sodium pentobarbital (25 mg/kg body weight). The heads were perfused bilaterally through the carotid arteries with 4 L of 10 mm PBS containing 3000 U heparin/L and 0.7% sodium nitrite, followed by 4 L of fixative consisting of 4% paraformaldehyde in 0.1 m phosphate buffer, pH 7.4. A block of brain containing the mediobasal hypothalamus was removed from the brain and immersed in the same fixative overnight at 4°C, then in 20% sucrose in 0.1 m phosphate buffer at 4°C until it sank. Coronal sections of two goats were cut at 50 μm on a freezing microtome. They were collected in the cryoprotectant solution (Watson et al., 1986) and kept at −20°C until used for double immunohistochemistry. The brain blocks of one goat were cut at 12 μm on a cryostat, and sections were subjected to in situ hybridization.
Double-label immunohistochemistry for kisspeptin and NKB/Dyn.
Free-floating sections were rinsed with PBS containing 0.3% of Triton X-100 (PBST) and incubated with PBST containing 1% BSA (PBST-BSA) and 20% fetal bovine serum for 1 h. Sections were then incubated with a solution containing a monoclonal antibody to kisspeptin (1:4000; Takeda; no. 254) (Ohkura et al., 2009; Takase et al., 2009) and either a polyclonal antibody to NKB (1:4000; Peninsula Laboratories) or Dyn (1:4000; Phoenix Pharmaceuticals) in PBST-BSA containing 2% fetal bovine serum for 72 h at 4°C. After three 15 min washes with PBST, sections were incubated with a solution containing biotinylated horse anti-mouse IgG (1:200; Vector Laboratories) and Alexa 555-conjugated anti-rabbit IgG (1:200; Invitrogen) in PBST-BSA containing 2% fetal bovine serum for 3 h, and for 1 h in streptavidin-Alexa 488 (1:200; Invitrogen) in PBST at room temperature. Sections were mounted on gelatin-coated slides and coverslipped with water-soluble mounting medium (Vector Laboratories). Sections were observed under a fluorescent microscopy (ECLIPSE E800M; Nikon) equipped with a CCD camera (AxioCam HRc; Zeiss), and the two fluorescent images were merged with the aid of computer software (AxioVision; Zeiss).
To confirm specificity, anti-kisspeptin, -NKB, and -Dyn antibodies were preabsorbed with 10 nmol of goat kisspeptin partial peptide (Ohkura et al., 2009), NKB (Sigma-Aldrich), or Dyn (Phoenix), respectively, and they were used for immunohistochemistry as mentioned above. Preincubation of the antibodies with corresponding peptides completely eliminated positive signals (data not shown), confirming specificity of each antibody.
Numbers of kisspeptin-immunopositive neurons and double-labeled neurons for kisspeptin and NKB or kisspeptin and Dyn were counted under the microscope in four sections that contained the caudal portion of the ARC in each animal. Densely packed clusters of kisspeptin neurons in which individual cells could not be distinguished (see Fig. 1A) were excluded from the count.
Figure 1.
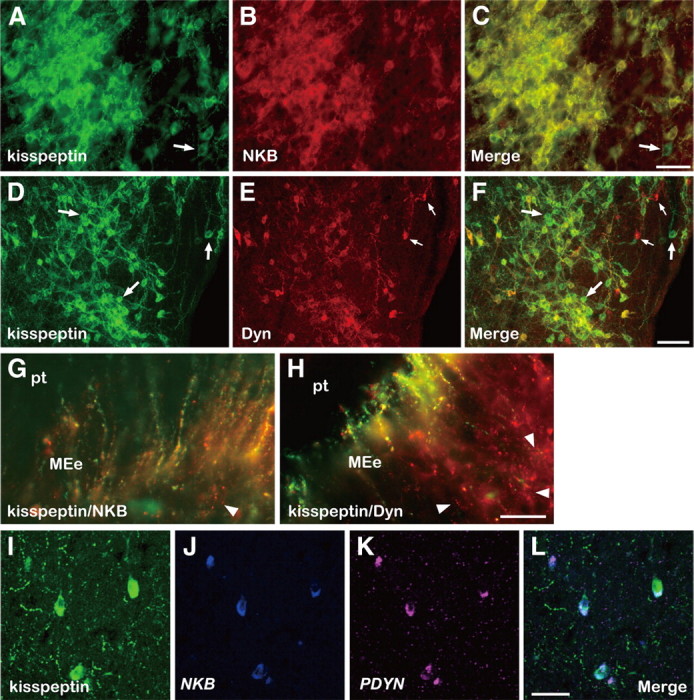
Colocalization of kisspeptin, NKB, and Dyn in the caudal ARC and ME of the goat. Photomicrographs of sections of the ARC stained by immunocytochemistry for kisspeptin (A) and NKB (B), or kisspeptin (D) and Dyn (E), are shown. C and F are computer-aid merged images of A and B, or D and E, respectively. An arrow in A or C indicates a cell body containing immunoreactivity (ir) for kisspeptin but not NKB-ir. The large arrows in D or F, or small arrows in E or F, show some cell bodies containing exclusively kisspeptin-ir or Dyn-ir, respectively. Note that numerous kisspeptin/NKB- or kisspeptin/Dyn-positive fibers surround immunopositive cell bodies. G and H are merged images of sections of the ME double stained for kisspeptin/NKB or kisspeptin/Dyn, respectively. The arrowhead in G indicates a fiber with only NKB-ir. The arrowheads in H show fibers with exclusively Dyn-ir. I–K show kisspeptin-ir and positive signals for NKB and PDYN in triple-label histochemistry, respectively. L is a merged image of I–K. MEe, External layer of median eminence; pt, pars tuberalis. Scale bars: A–C, I–L, 50 μm; D–F, 100 μm; G, H, 25 μm.
Triple labeling of kisspeptin, NKB, and PDYN.
For in situ hybridization, riboprobes were labeled with digoxigenin (DIG) and fluorescein isothiocyanate (FITC), respectively, for NKB and PDYN probes. They were synthesized by using T3 and T7 RNA polymerases (Stratagene) and a DIG- or FITC-labeling mixture (Roche). Double labeling of mRNA and proteins on the same section was performed as previously described (Wakabayashi et al., 2007; Ohkura et al., 2009). Briefly, to detect DIG-labeled NKB probe after hybridization, peroxidase-conjugated anti-DIG Fab fragment (1:250; Roche), tyramide–biotin amplification system (PerkinElmer Life Sciences), and Alexa 647-conjugated streptavidin (1:400; Invitrogen) were used. To detect FITC-labeled PDYN probe, alkaline phosphatase-conjugated anti-FITC Fab fragment (1:250; Roche) and Fast Red substrate (Dako) were used. For immunohistochemistry of kisspeptin and visualization of signal, the anti-kisspeptin monoclonal antibody (1:5000) and Alexa 488-conjugated anti-mouse IgG antibody (1:400; Invitrogen) were used, respectively. Triple-labeled sections were observed by confocal microscopy (TCS-SP5; Leica) at a thickness of 1 μm.
Surgery and steroid treatments
Each animal was anesthetized under halothane anesthesia and stereotaxically implanted with an array of bilateral recording electrodes. The electrode array was aimed at the cluster of kisspeptin neurons that are concentrated in the posterior part of the ARC, as previously described (Ohkura et al., 2009). The electrodes consisted of six Teflon-insulated platinum–iridium wires (75 μm in diameter). For intracerebroventricular administration, an 18-gauge stainless-steel guide cannula was also inserted so that the tip was positioned 5 mm above the lateral ventricle (LV). After confirming the position by radioventriculography, the electrodes and guide cannula were fixed to the skull.
SILASTIC (silicone) tubing (inner diameter, 3 mm; outer diameter, 5 mm; length, 20 mm; Dow Corning) was filled with crystalline estradiol (E2) (Sigma-Aldrich) and implanted subcutaneously in six OVX goats (OVX plus E2) to produce E2 levels simulating the luteal phase of the estrous cycle (4–8 pg/ml). Those low levels of E2 restrain GnRH/LH release (Ichimaru et al., 2001) but do not induce an LH surge. Experiments in the OVX-plus-E2 animals were conducted between 1 and 3 weeks after treatment. Furthermore, a silicon sheet (5 mm thickness; 5 × 8 cm) filled with 1 g of crystalline progesterone (P) (Sigma-Aldrich) was subcutaneously implanted in four OVX-plus-E2-treated animals (OVX plus E2 plus P) to mimic levels of P normally found in the luteal phase (1–3 ng/ml). The animals were subjected to experiments between 1 and 3 weeks after the treatment.
MUA recording and blood sampling
MUA was recorded as previously described (Mori et al., 1991; Ichimaru et al., 2001; Ohkura et al., 2009) with a slight modification. Briefly, signals were passed through a buffer amplifier integrated circuit directly plugged into an electrode assembly. After additional amplification and amplitude discrimination, MUA signals were stored as counts per 20 s on a personal computer. A characteristic increase in the MUA (MUA volley) was considered to be the electrophysiological manifestation of the neural element that generates pulsatile GnRH secretion. The MUA was recorded throughout the experimental period.
Blood samples were collected through a jugular catheter every 3–6 min for 6 h in the OVX and OVX-plus-E2-treated animals, or every 3–12 min for 6–8 h in the OVX-plus-E2-plus-P-treated animals. Blood was centrifuged and plasma was separated and stored at −30°C until assayed for LH.
Central administration of NKB, Dyn, or KOR antagonist (nor-binaltorphimine)
NKB was initially dissolved in 0.1N NaOH and further diluted with saline to make working concentrations of 0.2 or 2 nmol/400 μl. The final concentrations of 0.1N NaOH in the working solution were 5 × 10−5 and 5 × 10−4 N, respectively. We had established that these trace amounts of NaOH had no discernable effect on the MUA and plasma LH concentrations; thus, saline (at this concentration) was used as a vehicle control. On the day of the experiment, the mean intervolley interval was calculated during a control period in each goat. NKB solution (400 μl) or vehicle was injected into the LV for 60 s starting at the midpoint between MUA volleys that was calculated during the control period. The injection cannula was maintained in place for another 60 s after the injection. The OVX animals (n = 5) received both 0.2 and 2 nmol of NKB, whereas only 2 nmol of NKB was administered to the OVX-plus-E2- and OVX-plus-E2-plus-P-treated animals (n = 4 per group). Dyn was dissolved in saline, and 400 μl of either 0.2 or 2 nmol Dyn solution was injected into the LV of four OVX animals, respectively, following the same protocol for dilution and delivery as was used for NKB. A vehicle injection was performed in each of the three treatment groups: OVX (n = 7), OVX-plus-E2-treated (n = 4), and OVX-plus-E2-plus-P-treated (n = 4), and these data served as the reference control for both the NKB and Dyn experiments. The κ-opioid receptor antagonist nor-binaltorphimine (nor-BNI) (Sigma-Aldrich) was dissolved in saline and continuously infused into the LV at a rate of 60 nmol · 600 μl−1 · h−1 for 2 h in four OVX goats using a microinfusion pump (Chemyx) after a 2 h control period. The dose of each substance used in each formal experiment was determined in several pilot studies, in which only the MUA was monitored. All solutions given to the animals were sterilized by passing them through a 0.22 μm filter immediately before administration. Some goats were repeatedly used in several experiments. Vehicle and doses of NKB, Dyn, and nor-BNI were administered in random order within individuals. Each administration was separated from the previous one by at least 2 d.
Electrode placement
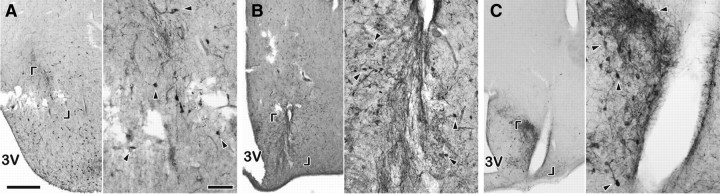
Two animals (nos. Y69 and 709) died suddenly by accident during the experiment. The brains from these animals were removed postmortem and soaked in 4% paraformaldehyde for 6–8 weeks. Another animal (no. 713), in which the MUA was successfully recorded, was killed, and its head was perfused as described above. All brains were processed for immunohistochemistry through the use of the anti-kisspeptin antibody, and immunopositive signals were visualized by 3,3′-diaminobenzidine reaction.
Radioimmunoassay for LH
LH concentrations in single aliquots of 50 μl plasma sample were measured by a double-antibody radioimmunoassay (Mori and Kano, 1984) with rabbit anti-ovine LH serum (Ohkura et al., 2004) and expressed in terms of ovine LH standard (NIDDK-oLH-I-4). Goat anti-rabbit IgG serum (PGKR001; Shibayagi) was used as second antibody. The least detectable LH concentration was 0.19 ng/ml for 50 μl plasma samples, and the intraassay and interassay coefficients of variation were 5.1% at 3.82 ng/ml and 6.5% at 3.55 ng/ml, respectively.
Data analysis
For analysis of the MUA data, the duration and amplitude of MUA volleys and the intervolley interval were determined for each individual before and after each injection. The mean value and SD of all MUA data (spikes per 20 s) on the experimental day were calculated. When the count at a time point exceeded twice the SD of that mean value, it was designated the start of a “volley.” The end of the volley was determined by the same criterion. Since this criterion could not be adapted to multiple volleys induced by the NKB injection (see Fig. 3C), the start and end of the volley were determined manually from the appearance of the MUA profile in those occasions. The duration of the volley was designated as the interval between the start and end of a given volley, and the intervolley interval was the time interval between the start of two successive volleys. The amplitude of the volley was obtained by subtracting the baseline level (the mean value of all MUA data) from the highest count in a given volley. For the purpose of comparison, the mean amplitude of MUA volleys observed before the injection on the experimental day was calculated, and the amplitude of the volley was expressed as a percentage of the mean value. LH pulses were identified by the PULSAR computer program (Merriam and Wachter, 1982).
Figure 3.
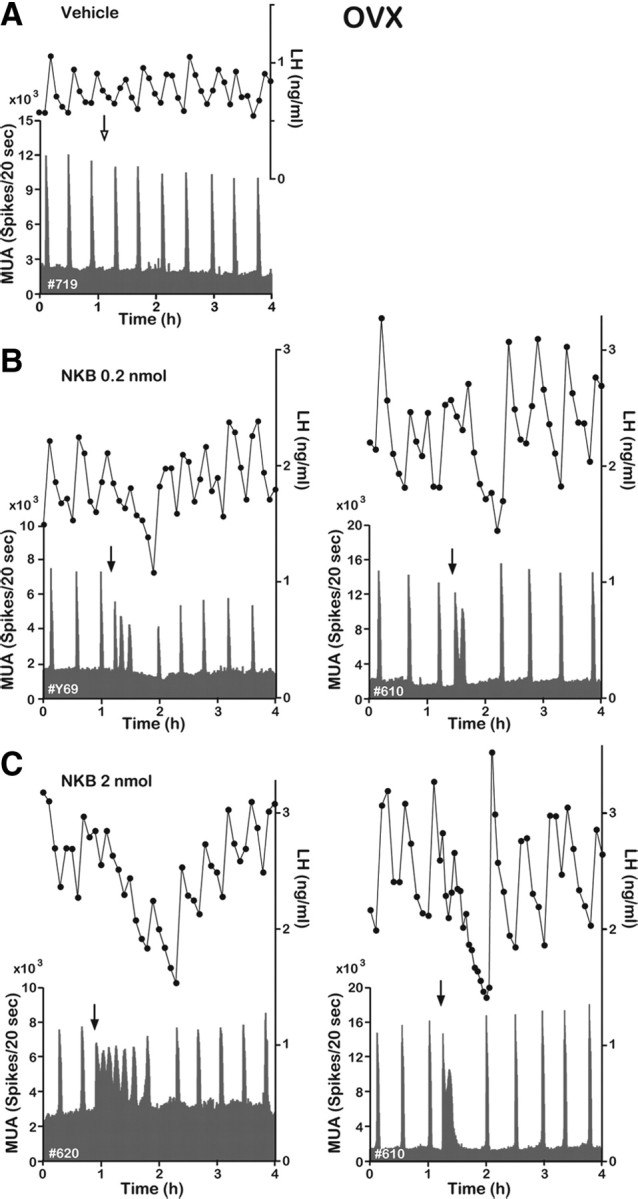
Effect of NKB on the MUA and plasma LH in the OVX goat. Representative profiles of the MUA and plasma LH concentrations in OVX animals that received an intracerebroventricular injection of vehicle (A) or 0.2 (B) or 2 nmol (C) of NKB are shown. The arrow indicates timing of injection.
The effects of NKB on the MUA and plasma LH concentrations were analyzed by comparing the number of MUA volleys, the mean duration and amplitude of those volleys, the number of LH pulses, and LH secretion during the 1 h period after the injection. For the purpose of comparison of LH secretion, the area under the curve of the LH profile during the 1 h period after the injection was expressed as the percentage of that occurring during the 1 h period before the injection. In the OVX animals, statistical differences between vehicle control and NKB treatments were analyzed by two-way ANOVA, followed by the Dunnett post hoc test for multiple comparisons. In the OVX-plus-E2-treated animals, differences between control and 2 nmol NKB treatments were analyzed by a paired t test. The effect of Dyn injection on the MUA volley interval was analyzed by two-way ANOVA, followed by the Dunnett post hoc test for multiple comparisons. The MUA volley interval and duration during the 2 h nor-BNI infusion period were compared with those in the 2 h preinfusion period using a paired t test.
Results
Colocalization of NKB and Dyn with kisspeptin in the ARC
First, we observed a cluster of cell bodies with kisspeptin immunoreactivity in the caudal region of the ARC, which were surrounded by kisspeptin-containing fibers with distinct varicosities (Fig. 1A). Second, we found NKB-containing cell bodies in the caudal ARC (Fig. 1B), with immunoreactivity for NKB completely overlapping that for kisspeptin, not only in somata but also in fibers, as can be seen in a merged image (Fig. 1C). Occasional cell bodies showing only kisspeptin immunoreactivity were seen (Fig. 1C, arrow); however, 2037 (99.5%) of 2047 kisspeptin-positive neurons contained NKB. Third, we found that the majority of kisspeptin-positive neurons in the ARC also contained Dyn. Although a few neurons showing only kisspeptin (Fig. 1F, large arrows) or Dyn (Fig. 1F, small arrows) were found, 1470 (78.0%) of 1885 kisspeptin-containing cells coexpressed Dyn (Fig. 1F). In the ME, we found many kisspeptin-containing fibers and varicosities that also contained either NKB (Fig. 1G) or Dyn (Fig. 1H), which projected to the external layer adjacent to the pars tuberalis. Fibers containing exclusively NKB were rare (Fig. 1G, arrowhead), whereas those containing exclusively Dyn were relatively abundant (Fig. 1H, arrowheads). Some fibers containing only kisspeptin were also observed in this region (data not shown). Triple labeling of kisspeptin by immunohistochemistry (Fig. 1I) and NKB (Fig. 1J) and PDYN (Fig. 1K) by in situ hybridization revealed that all three substances were concomitantly expressed in the ARC (Fig. 1L).
Effects of central administration of NKB on MUA and LH
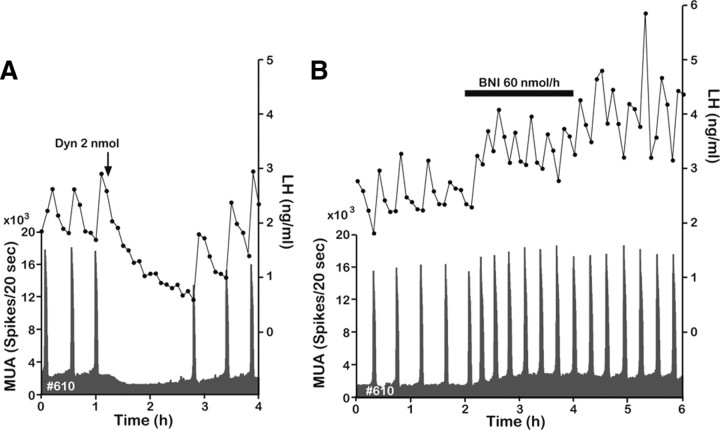
MUA was recorded through an electrode aimed at the caudal portion of the ARC, where kisspeptin neurons were densely clustered (Fig. 1A). The electrode trace and terminal location of the bundle were confirmed in three animals in which MUA was successfully recorded (Fig. 2). In the untreated OVX animals, spontaneous MUA volleys occurred with a relatively constant interval of ∼20–30 min. Each MUA volley was invariably followed by an LH pulse (Fig. 3A), confirming that the MUA volley is an electrophysiological manifestation of the GnRH pulse generator. Although intracerebroventricular injection of the vehicle alone had no effect on the occurrence of the next MUA volley and LH pulse (Fig. 3A), injection of 0.2 nmol of NKB immediately induced several MUA volleys that had a much shorter intervolley interval (Fig. 3B). Latencies from the start of injection to the appearance of the MUA volley were ∼40–120 s. Administration of 2 nmol of NKB induced an even more profound change in the MUA (Fig. 3C). Although profiles of the MUA after the NKB injection varied among animals, the general pattern of change was similar among the five animals examined (i.e., a rapid occurrence of initial MUA volley after the injection followed by one or multiple volleys with a longer duration and a shorter intervolley interval). This abrupt action of NKB persisted for as long as 50 min, and after a short pause, the normal spontaneous MUA volleys were reestablished (Fig. 3C). However, although LH concentrations appeared to increase slightly (albeit not significantly) immediately after the NKB injection, LH levels decreased thereafter, and MUA volleys evoked by NKB were not obviously accompanied by corresponding LH pulses (Fig. 3B,C). Table 1 summarizes changes in several parameters of the MUA and LH concentrations during the 1 h period after the vehicle or NKB injection. In the OVX animals, the frequency and mean duration of the MUA volleys were significantly increased, whereas the mean amplitude of the MUA volley was decreased by the 2 nmol NKB treatment. LH secretion was significantly reduced (compared with pretreatment values) after the 2 nmol NKB treatment, but the number of LH pulses was not different between the treatments. There was no significant effect of the smaller NKB dose (0.2 nmol) on either the LH or MUA parameters.
Figure 2.
Photomicrographs showing the placement of MUA recording electrodes in three goats [no. Y69 (A), no. 709 (B), and no. 713 (C)] from which MUA was successfully recorded. Sections were immunostained for kisspeptin. A pair of brackets indicates the area where a trace of a bundle of electrodes is observed, and a magnification of the indicated area is shown on the right side of each panel. Some kisspeptin-immunopositive cell bodies are shown by arrowheads. Note that because it was not possible to perfuse goats Y69 and 709, immunostaining of their sections was poor. 3V, Third ventricle. Scale bars: left panels, 500 μm; right panels, 100 μm.
Table 1.
MUA and LH profiles during the 1 h period after vehicle or NKB injection
| Treatment | No. of animals | MUA |
LH concentrations |
|||
|---|---|---|---|---|---|---|
| No. of volleys | Mean duration (s) | Mean amplitude (%)a | No. of pulses | AUC (%)b | ||
| OVX | ||||||
| Vehicle | 5 | 2.4 ± 0.24 | 127.3 ± 9.8 | 101.9 ± 3.5 | 0.8 ± 0.49 | 104.0 ± 9.5 |
| 0.2 nmol | 4 | 3.5 ± 0.29 | 193.3 ± 32.4 | 84.8 ± 8.5 | 1.5 ± 0.29 | 91.6 ± 8.0 |
| 2 nmol | 5c | 5.0 ± 0.82** | 234.8 ± 37.2* | 79.2 ± 4.3* | 0.6 ± 0.24 | 84.3 ± 5.0** |
| OVX + E2 | ||||||
| Vehicle | 4 | 1.3 ± 0.25 | 42.5 ± 10.3 | 104.2 ± 6.7 | 1.3 ± 0.25 | 89.1 ± 4.6 |
| 2 nmol | 4 | 1.8 ± 0.75 | 128.3 ± 34.7 | 111.2 ± 9.8 | 1.3 ± 0.25 | 96.0 ± 5.2 |
aValues are expressed as a percentage of the mean amplitude of MUA volleys observed before the injection on the experimental day.
bThe area under the curve (AUC) of LH concentrations. Values are expressed as percentage of the AUC during the 1 h period before the injection.
cMUA data were analyzed in only four goats because electrical noise prevented accurate analysis in one animal.
*p < 0.05 and
**p < 0.01 versus vehicle control.
The frequency of MUA volleys was reduced under the E2 treatment (becoming stabilized within 5 d). The intervolley interval varied among E2-treated animals but ranged from 35 to 90 min (Fig. 4A). Central injection of 2 nmol of NKB induced from 1 to 3 MUA volleys (Fig. 4B,C). Although the mean duration of the MUA volley appeared to increase after NKB, difference between the vehicle and NKB treatments was not statistically significant (Table 1). Neither the number of LH pulses nor the LH secretion was affected by the NKB treatment in the OVX-plus-E2-treated animals. In the E2-plus-P-treated animals, the intervolley interval was prolonged, ranging from 2.5 to 9 h (Fig. 4D), and varied within an individual, even within a single day. Therefore, injection of NKB (or vehicle alone) was performed 2 h after a spontaneously occurring MUA volley in the E2-plus-P-treated animals. Despite the profound inhibition of MUA produced by the E2-plus-P treatment, the injection of 2 nmol of NKB induced an MUA volley within 150 s from the start of injection, which was followed by an unequivocal LH pulse (Fig. 4E,F).
Figure 4.
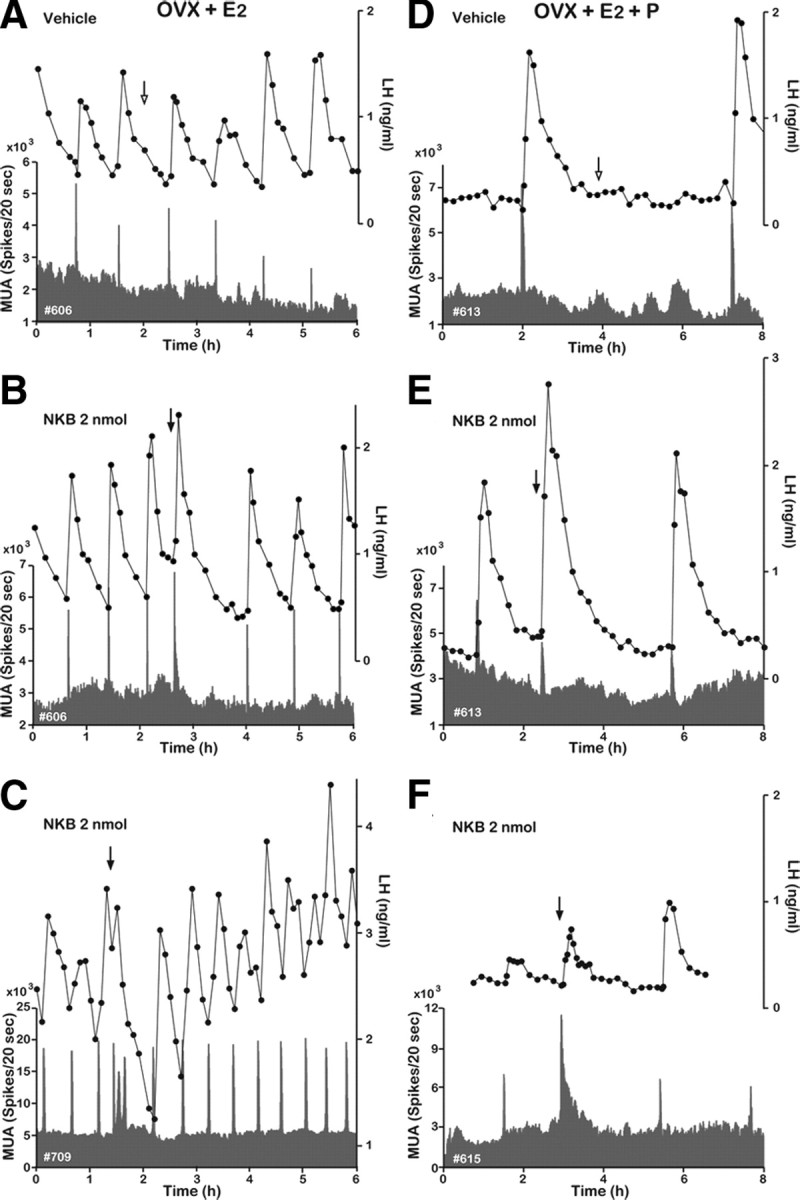
Effect of NKB on MUA and plasma LH in OVX-plus-E2- and OVX-plus-E2-plus-P-treated goats. Representative profiles of MUA and plasma LH concentrations in OVX-plus-E2 (A–C) and OVX-plus-E2-plus-P (D–F) goats that received an intracerebroventricular injection of vehicle (A, D) or 2 nmol (B, C, E, F) of NKB are shown. The arrow indicates timing of injection.
Effects of central administration of Dyn and KOR antagonist (nor-BNI) on MUA and LH
A representative profile of the MUA and plasma LH concentrations in each treatment is shown in Figure 5. After injection of 0.2 nmol of Dyn, the intervolley interval was slightly increased (34.5 ± 3.40 min; n = 4), but this was not statistically significant. After administration of 2 nmol of Dyn, the frequency of the MUA volleys was dramatically suppressed (Fig. 5A), which resulted in a significant increase in the intervolley interval (62.6 ± 14.50 min; n = 4; p < 0.01) compared with that in the control (26.6 ± 0.95 min; n = 6). In some animals, a small decline in baseline activity of the MUA was observed after the Dyn injection (Fig. 5A). Plasma LH concentrations were gradually decreased until the next MUA volley occurred. Administration of nor-BNI accelerated the occurrence of MUA volleys (Fig. 5B). During the 2 h infusion period, the intervolley interval was significant decreased (19.5 ± 2.23 min; n = 4; p < 0.01), whereas the volley duration was significant increased (226.7 ± 23.70 s; p < 0.05) compared with those in the 2 h preinfusion period (25.5 ± 1.65 min and 185.0 ± 14.71 s, respectively).
Figure 5.
Effects of Dyn and nor-BNI on the MUA and plasma LH in the OVX goat. A, Representative profiles of MUA and plasma LH concentrations in one animal that received an intracerebroventricular injection of 2 nmol of Dyn are shown. The arrow indicates timing of injection. B, Representative profiles of MUA and plasma LH concentrations in one animal that received an intracerebroventricular infusion of nor-BNI at a rate of 60 nmol · 600 μl−1 · h−1 for 2 h are shown. The bar indicates the period of infusion.
Discussion
We have demonstrated that NKB and Dyn are colocalized in most of the kisspeptin neurons in the ARC of the goat. This is in agreement with previous reports that NKB neurons in the ARC of the rat and sheep coexpress Dyn (Burke et al., 2006; Foradori et al., 2006) and that kisspeptin neurons in the ARC of the sheep and mouse coexpress both NKB and Dyn (Goodman et al., 2007; Navarro et al., 2009). In the ME, we observed rich projections of kisspeptin/NKB/Dyn fibers to the external layer, where kisspeptin axons are intimately associated with GnRH axons in the monkey (Ramaswamy et al., 2008). Since kisspeptin neurons in the preoptic area contain neither NKB nor Dyn (Goodman et al., 2007), it is likely that the kisspeptin/NKB/Dyn fibers in the ME originate from kisspeptin/NKB/Dyn neurons in the ARC. We also observed a dense network of varicose fibers containing either kisspeptin/NKB or kisspeptin/Dyn that surround kisspeptin/NKB/Dyn cell bodies in the ARC. A similar dense plexus of NKB/Dyn fibers has been reported in the ARC of the rat and sheep (Burke et al., 2006; Foradori et al., 2006), and an electron microscopic study in the sheep revealed that Dyn neurons in the ARC receive synaptic contact with Dyn fibers (Foradori et al., 2002). Active neurotransmitters at those synapses likely include NKB and Dyn, because Dyn neurons in the ARC of the rat contain NK3 (Burke et al., 2006) and Kiss1 neurons in the ARC of the mouse express NK3 and KOR (Navarro et al., 2009). These anatomical observations suggest that kisspeptin/NKB/Dyn neurons in the ARC comprise an oscillatory feedback loop interconnected through collaterals that synchronize their activity, as previously proposed (Burke et al., 2006; Foradori et al., 2006; Rance, 2009; Navarro et al., 2009). However, despite the solid anatomical evidence among diverse mammalian species for such a network, evaluating its physiological significance and possible relationship to pulsatile GnRH secretion calls for ancillary experimental approaches.
We recorded MUA in close proximity to kisspeptin/NKB/Dyn neurons in the ARC and found clear evidence for periodic bursts that are invariably associated with LH pulses as previously reported (Ohkura et al., 2009). Furthermore, we demonstrated that the frequency of MUA bursting (and LH pulses) is profoundly influenced by the prevailing sex steroid milieu. Finally, we showed that central administration of NKB evokes MUA bursting, whereas Dyn suppresses the occurrence of spontaneous MUA volleys and nor-BNI increases their frequency. These observations are consistent with the hypothesis that NKB and Dyn in kisspeptin neurons of the ARC are involved in pulsatile kisspeptin secretion, which drives ultradian GnRH/LH release. Although we cannot prove that the MUA bursting recorded in the ARC reflects activity of ARC kisspeptin neurons, several lines of evidence support this proposition. First, kisspeptin secreted into the ME of the monkey is episodic and temporally associated with pulsatile GnRH secretion (Keen et al., 2008). Second, administration of a kisspeptin antagonist suppresses pulsatile GnRH/LH secretion (Roseweir et al., 2009). Third, in the control group, LH pulses accompanied the volleys of the MUA that we observed in the vicinity of kisspeptin/NKB/Dyn neurons. Finally, kisspeptin/NKB/Dyn neurons in the ARC have been proposed to be a primary site of negative feedback action of E2 and P (Smith et al., 2005a,b, 2007; Burke et al., 2006; Foradori et al., 2006; Goodman et al., 2007), and we demonstrated here that E2 and P suppress the occurrence of MUA volleys.
The present results indicate several important aspects of the action of NKB. First, administration of NKB induced an immediate activation of MUA volleys in the OVX goat, perhaps reflecting synchronized activation of kisspeptin/NKB/Dyn neurons in the ARC. Second, the activation of kisspeptin/NKB/Dyn neurons by NKB resulted in the occurrence of multiple MUA volleys with short intervolley intervals, rather than a single sustained rise in the MUA, suggesting that the stimulatory effect of NKB on kisspeptin/NKB/Dyn neurons is intermittently extinguished by some endogenous inhibitory drive. It appears that the inhibitory drive becomes strengthened by the NKB treatment, since there was a slight pause before the resumption of normal spontaneous MUA volleys. Third, the administration of NKB to the OVX goat increased MUA but decreased LH secretion, which is consistent with previous observations that an NK3 agonist inhibits LH release in rats and mice (Sandoval-Guzmán and Rance, 2004; Navarro et al., 2009). There are several possible explanations for this apparent dissociation between MUA and LH secretion after NKB administration. One possibility is that the prolonged activation of kisspeptin neurons causes a desensitization of the kisspeptin–GnRH–LH cascade. Indeed, it has been reported that continuous administration of either kisspeptin (Seminara et al., 2006) or GnRH (Belchetz et al., 1978) results in reduced LH secretion. Another possibility is that, in addition to activating kisspeptin neurons, NKB also acts on NKB receptors on GnRH nerve terminals in the ME (Krajewski et al., 2005) to inhibit GnRH secretion, perhaps through a Gi-coupled mechanism (Laniyonu et al., 1988). In fact, this may explain why bursts of endogenous kisspeptin release result in only brief bouts of GnRH/LH release, despite results from in vitro electrophysiological studies indicating that a brief application of kisspeptin induces a prolonged activation of GnRH neurons in the mouse (Han et al., 2005; Pielecka-Fortuna et al., 2008). Since NKB is likely released along with kisspeptin into the ME, it is possible that kisspeptin activates GnRH axon terminals, whereas NKB acting through a slower Gi-coupled NK3 pathway would act to turn off the response to kisspeptin. This speculative inhibitory signal could be accomplished by some other pathway involving interneurons and either NKB or Dyn (or even some other not-yet-identified cotransmitters).
In contrast to NKB, Dyn produced a dose-dependent reduction of MUA volleys, perhaps reflecting the effect of Dyn on the kisspeptin/NKB/Dyn network. Furthermore, nor-BNI significantly reduced the intervolley interval and increased the volley duration, suggesting that Dyn/KOR signaling participates in sharpening of the MUA volley. We envision that NKB/NK3 signaling evokes synchronized bursts of firing among kisspeptin/NKB/Dyn neurons, whereas Dyn/KOR signaling acts with a phase lag to extinguish this activity, leading to the generation of periodic bursts of kisspeptin neuronal activity—and hence GnRH secretion. Indeed, there is precedence for this argument. For example, activation of NK3 increases firing in noradrenergic and dopaminergic neurons (Jung et al., 1996; Nalivaiko et al., 1997), whereas NK3 antagonists attenuate pharmacologically induced firing of dopaminergic neurons (Gueudet et al., 1999). Furthermore, it has been suggested that Dyn/KOR expressed in vasopressin neurons modulates the phasic firing of those neurons and the release of vasopressin by an autosynaptic loop (Brown et al., 1998; Iremonger and Bains, 2009).
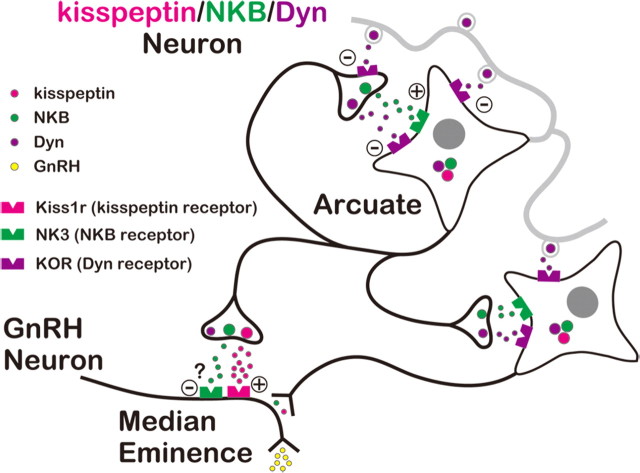
Our results are consistent with a model for the generation of rhythmic oscillation of activity in kisspeptin/NKB/Dyn neurons in the ARC and the pulsatile release of GnRH proposed by Navarro et al. (2009) and summarized in Figure 6. Briefly, kisspeptin/NKB/Dyn neurons in the ARC send axons to GnRH terminals in the ME, whereas their collaterals and dendrites form a neural circuit connecting the ensemble. Within this circuit, the stimulatory drive of NKB gradually increases, and when it overcomes the inhibition provided by Dyn/KOR signaling, these cells become spontaneously active. This initial activation is amplified by a regenerative feedback mechanism through NKB/NK3 signaling, which propagates among cells in the circuit to evoke the synchronized bursting of kisspeptin/NKB/Dyn neurons. This is followed by a delayed inhibition of kisspeptin/NKB/Dyn neurons, mediated by Dyn acting through KOR on the cell bodies, as well as on the presynaptic structure of axonal collaterals, which extinguishes the NKB-induced bursting, thus generating pulsatile kisspeptin and GnRH secretion.
Figure 6.
Schematic representation of the role of ARC kisspeptin/NKB/Dyn neurons in the generation of the pulsatile GnRH release. According to this model, kisspeptin/NKB/Dyn neurons in the ARC form a neural circuit by their collaterals and dendrites. Within the neural circuit, NKB/NK3 signaling plays the role of accelerator, whereas Dyn/KOR signaling serves as a brake on activation of kisspeptin/NKB/Dyn neurons. Through the reciprocal actions of NKB/NK3 and Dyn/KOR signaling, rhythmic oscillation of neural activity is generated in kisspeptin/NKB/Dyn neurons, which in turn induces pulsatile kisspeptin release at the ME and hence pulsatile GnRH release into the portal circulation. Thus, ARC kisspeptin/NKB/Dyn neurons would act as the GnRH pulse generator through the coordinated interaction between three peptides. See text for details.
According to this model, any dysfunction of the NKB/Dyn system would compromise pulsatile kisspeptin and GnRH/LH release. Indeed, Topaloglu et al. (2009) have recently shown that mutations of either Trc3 or Tacr3, which encode NKB and NK3, respectively, produce gonadotropin deficiency and pubertal failure in humans. Furthermore, mice bearing deletions of either Dyn or KOR show reduced LH secretion, probably because of a desensitization of the kisspeptin–GnRH–LH cascade by continuous activation of kisspeptin/NKB/Dyn neurons (Navarro et al., 2009). The negative-feedback actions of E2 on GnRH/LH secretion can also be explained by this model. Rising levels of E2 would reduce the stimulatory drive of NKB/NK3 signaling in ARC kisspeptin/NKB/Dyn neurons by suppressing the expression of NKB (Rance and Bruce, 1994; Dellovade and Merchenthaler, 2004; Navarro et al., 2009) as well as NK3 (Navarro et al., 2009), which would reduce kisspeptin/NKB/Dyn (MUA) burst frequency. In concert, we observed that the intervolley interval in the OVX-plus-E2-treated animal was nearly twice that observed in the OVX state. The weak response of MUA and LH secretion to exogenous NKB in the OVX-plus-E2 animals may be attributable to the low expression of NK3 as well as the low level of endogenous NKB that is necessary for the regenerative self-amplification. Since Dyn inhibits MUA volleys and central administration of nor-BNI reverses the inhibitory effect of P on pulsatile LH secretion (Gallo, 1990; Goodman et al., 2004), Dyn/KOR signaling could also mediate the negative-feedback action of P.
Footnotes
This work was supported in part by the Program for Promotion of Basic Research Activities for Innovative Bioscience of Japan and Grant-in-Aid for Young Scientists Start-up 2088042, the Eunice Kennedy Shriver National Institute of Child Health and Human Development–National Institutes of Health (NIH) through Cooperative Agreement U54 HD12629 and NIH Grant R01 HD049651, and the Marie Curie International Outgoing Fellowship within the 7th European Community Framework Programme. We are grateful to Drs. T. Ohtaki and H. Matsumoto (Takeda Pharmaceutical) for providing anti-rat kisspeptin monoclonal antibody (Takeda; no. 254), and Dr. A. F. Parlow and the National Hormone and Peptide Program for reagents used in the LH RIA. We also thank Dr. K. Moriya-Ito for her confocal microscopic observations and Y. Sakairi for her technical assistance.
References
- Belchetz PE, Plant TM, Nakai Y, Keogh EJ, Knobil E. Hypophysial responses to continuous and intermittent delivery of hypopthalamic gonadotropin-releasing hormone. Science. 1978;202:631–633. doi: 10.1126/science.100883. [DOI] [PubMed] [Google Scholar]
- Brown CH, Ludwig M, Leng G. κ-Opioid regulation of neuronal activity in the rat supraoptic nucleus in vivo. J Neurosci. 1998;18:9480–9488. doi: 10.1523/JNEUROSCI.18-22-09480.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke MC, Letts PA, Krajewski SJ, Rance NE. Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: morphologic evidence of interrelated function within the arcuate nucleus. J Comp Neurol. 2006;498:712–726. doi: 10.1002/cne.21086. [DOI] [PubMed] [Google Scholar]
- Dellovade TL, Merchenthaler I. Estrogen regulation of neurokinin B gene expression in the mouse arcuate nucleus is mediated by estrogen receptor alpha. Endocrinology. 2004;145:736–742. doi: 10.1210/en.2003-0894. [DOI] [PubMed] [Google Scholar]
- Foradori CD, Coolen LM, Fitzgerald ME, Skinner DC, Goodman RL, Lehman MN. Colocalization of progesterone receptors in parvicellular dynorphin neurons of the ovine preoptic area and hypothalamus. Endocrinology. 2002;143:4366–4374. doi: 10.1210/en.2002-220586. [DOI] [PubMed] [Google Scholar]
- Foradori CD, Amstalden M, Goodman RL, Lehman MN. Colocalisation of dynorphin A and neurokinin B immunoreactivity in the arcuate nucleus and median eminence of the sheep. J Neuroendocrinol. 2006;18:534–541. doi: 10.1111/j.1365-2826.2006.01445.x. [DOI] [PubMed] [Google Scholar]
- Gallo RV. Kappa-opioid receptor involvement in the regulation of pulsatile luteinizing hormone release during early pregnancy in the rat. J Neuroendocrinol. 1990;2:685–691. doi: 10.1111/j.1365-2826.1990.tb00465.x. [DOI] [PubMed] [Google Scholar]
- Goodman RL, Coolen LM, Anderson GM, Hardy SL, Valent M, Connors JM, Fitzgerald ME, Lehman MN. Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin-releasing hormone neurons in sheep. Endocrinology. 2004;145:2959–2967. doi: 10.1210/en.2003-1305. [DOI] [PubMed] [Google Scholar]
- Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CV, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148:5752–5760. doi: 10.1210/en.2007-0961. [DOI] [PubMed] [Google Scholar]
- Gueudet C, Santucci V, Soubrié P, Le Fur G. Blockade of neurokinin3 receptors antagonizes drug-induced population response and depolarization block of midbrain dopamine neurons in guinea pigs. Synapse. 1999;33:71–79. doi: 10.1002/(SICI)1098-2396(199907)33:1<71::AID-SYN7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steine RA, Herbison AE. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimaru T, Mori Y, Okamura H. A possible role of neuropeptide Y as a mediator of undernutrition to the hypothalamic gonadotropin-releasing hormone pulse generator in goats. Endocrinology. 2001;142:2489–2498. doi: 10.1210/endo.142.6.8002. [DOI] [PubMed] [Google Scholar]
- Iremonger KJ, Bains JS. Retrograde opioid signaling regulates glutamatergic transmission in the hypothalamus. J Neurosci. 2009;29:7349–7358. doi: 10.1523/JNEUROSCI.0381-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung M, Michaud JC, Steinberg R, Barnouin MC, Hayar A, Mons G, Souilhac J, Emonds-Alt X, Soubrié P, Le Fur G. Electrophysiological, behavioural and biochemical evidence for activation of brain noradrenergic systems following neurokinin NK3 receptor stimulation. Neuroscience. 1996;74:403–414. doi: 10.1016/0306-4522(96)00150-9. [DOI] [PubMed] [Google Scholar]
- Karsch FJ. Hormonal control of reproduction. The hypothalamus and anterior pituitary gland. In: Austin CR, Short RV, editors. Reproduction in mammals. Ed 2. Vol 3. Cambridge, UK: Cambridge UP; 1984. pp. 1–20. [Google Scholar]
- Keen KL, Wegner FH, Bloom SR, Ghatei MA, Terasawa E. An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology. 2008;149:4151–4157. doi: 10.1210/en.2008-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobil E. The neuroendocrine control of the menstrual cycle. Recent Prog Horm Res. 1980;36:53–88. doi: 10.1016/b978-0-12-571136-4.50008-5. [DOI] [PubMed] [Google Scholar]
- Krajewski SJ, Anderson MJ, Iles-Shih L, Chen KJ, Urbanski HF, Rance NE. Morphologic evidence that neurokinin B modulates gonadotropin-releasing hormone secretion via neurokinin 3 receptors in the rat median eminence. J Comp Neurol. 2005;489:372–386. doi: 10.1002/cne.20626. [DOI] [PubMed] [Google Scholar]
- Laniyonu A, Sliwinski-Lis E, Fleming N. Different tachykinin receptor subtypes are coupled to the phosphoinositide or cyclic AMP signal transduction pathways in rat submandibular cells. FEBS Lett. 1988;240:186–190. doi: 10.1016/0014-5793(88)80365-x. [DOI] [PubMed] [Google Scholar]
- Merriam GR, Wachter KW. Algorithms for the study of episodic hormone secretion. Am J Physiol. 1982;243:E310–E318. doi: 10.1152/ajpendo.1982.243.4.E310. [DOI] [PubMed] [Google Scholar]
- Mori Y, Kano Y. Changes in plasma concentrations of LH, progesterone and oestradiol in relation to the occurrence of luteolysis, oestrus and time of ovulation in the Shiba goat (Capra hircus) J Reprod Fertil. 1984;72:223–230. doi: 10.1530/jrf.0.0720223. [DOI] [PubMed] [Google Scholar]
- Mori Y, Nishihara M, Tanaka T, Shimizu T, Yamaguchi M, Takeuchi Y, Hoshino K. Chronic recording of electrophysiological manifestation of the hypothalamic gonadotropin-releasing hormone pulse generator activity in the goat. Neuroendocrinology. 1991;53:392–395. doi: 10.1159/000125746. [DOI] [PubMed] [Google Scholar]
- Nalivaiko E, Michaud JC, Soubrié P, Le Fur G, Feltz P. Tachykinin neurokinin-1 and neurokinin-3 receptor-mediated responses in guinea-pig substantia nigra: an in vitro electrophysiological study. Neuroscience. 1997;78:745–757. doi: 10.1016/s0306-4522(96)00625-2. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of GnRH secretion by Kiss1/Dynorphin/Neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29:11859–11866. doi: 10.1523/JNEUROSCI.1569-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev. 2009;30:713–743. doi: 10.1210/er.2009-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkura S, Ichimaru T, Itoh F, Matsuyama S, Okamura H. Further evidence for the role of glucose as a metabolic regulator of hypothalamic gonadotropin-releasing hormone pulse generator activity in goats. Endocrinology. 2004;145:3239–3246. doi: 10.1210/en.2003-1516. [DOI] [PubMed] [Google Scholar]
- Ohkura S, Takase K, Matsuyama S, Mogi K, Ichimaru T, Wakabayashi Y, Uenoyama Y, Mori Y, Steiner RA, Tsukamura H, Maeda KI, Okamura H. Gonadotropin-releasing hormone pulse generator activity in close proximity to the arcuate kisspeptin neurons in the goat. J Neuroendocrinol. 2009;21:813–821. doi: 10.1111/j.1365-2826.2009.01909.x. [DOI] [PubMed] [Google Scholar]
- Pielecka-Fortuna J, Chu Z, Moenter SM. Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology. 2008;149:1979–1986. doi: 10.1210/en.2007-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswamy S, Guerriero KA, Gibbs RB, Plant TM. Structural interactions between kisspeptin and GnRH neurons in the mediobasal hypothalamus of the male rhesus monkey (Macaca mulatta) as revealed by double immunofluorescence and confocal microscopy. Endocrinology. 2008;149:4387–4395. doi: 10.1210/en.2008-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rance NE. Menopause and the human hypothalamus: evidence for the role of kisspeptin/neurokinin B neurons in the regulation of estrogen negative feedback. Peptides. 2009;30:111–122. doi: 10.1016/j.peptides.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rance NE, Bruce TR. Neurokinin B gene expression is increased in the arcuate nucleus of ovariectomized rats. Neuroendocrinology. 1994;60:337–345. doi: 10.1159/000126768. [DOI] [PubMed] [Google Scholar]
- Roseweir AK, Kauffman AS, Smith JT, Guerriero KA, Morgan K, Pielecka-Fortuna J, Pineda R, Gottsch ML, Tena-Sempere M, Moenter SM, Terasawa E, Clarke IJ, Steiner RA, Millar RP. Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J Neurosci. 2009;29:3920–3929. doi: 10.1523/JNEUROSCI.5740-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval-Guzmán T, Rance NE. Central injection of senktide, an NK3 receptor agonist, or neuropeptide Y inhibits LH secretion and induces different patterns of Fos expression in the rat hypothalamus. Brain Res. 2004;1026:307–312. doi: 10.1016/j.brainres.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Seminara SB, Dipietro MJ, Ramaswamy S, Crowley WF, Jr, Plant TM. Continuous human metastin 45–54 infusion desensitizes G protein-coupled receptor 54-induced gonadotropin-releasing hormone release monitored indirectly in the juvenile male rhesus monkey (Macaca mulatta): a finding with therapeutic implications. Endocrinology. 2006;147:2122–2126. doi: 10.1210/en.2005-1550. [DOI] [PubMed] [Google Scholar]
- Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA. Differential regulation of Kisspeptin mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005a;146:2976–2984. doi: 10.1210/en.2005-0323. [DOI] [PubMed] [Google Scholar]
- Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005b;146:3686–3692. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- Smith JT, Clay CM, Caraty A, Clarke IJ. Kisspeptin messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology. 2007;148:1150–1157. doi: 10.1210/en.2006-1435. [DOI] [PubMed] [Google Scholar]
- Takase K, Uenoyama Y, Inoue N, Matsui H, Yamada S, Shimizu M, Homma T, Tomikawa J, Kanda S, Matsumoto H, Oka Y, Tsukamura H, Maeda K-I. Possible role of oestrogen in pubertal increase of kiss1/kisspeptin expression in discrete hypothalamic areas of female rats. J Neuroendocrinol. 2009;21:527–537. doi: 10.1111/j.1365-2826.2009.01868.x. [DOI] [PubMed] [Google Scholar]
- Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Ozbek MN, Imamoglu S, Akalin NS, Yuksel B, O'Rahilly S, Semple RK. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet. 2009;41:354–358. doi: 10.1038/ng.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi Y, Ohkura S, Okamura H, Mori Y, Ichikawa M. Expression of a vomeronasal receptor gene (V1r) and G protein alpha subunits in goats, Capra hircus, olfactory receptor neurons. J Comp Neurol. 2007;503:371–380. doi: 10.1002/cne.21394. [DOI] [PubMed] [Google Scholar]
- Watson RE, Jr, Wiegand SJ, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides. 1986;7:155–159. doi: 10.1016/0196-9781(86)90076-8. [DOI] [PubMed] [Google Scholar]