Abstract
Several animal studies have demonstrated functional roles of dopamine (DA) D1 and D2 receptors in amygdala activity. However, the contribution of DA D1 and D2 receptors to amygdala response induced by affective stimuli in human is unknown. To investigate the contribution of DA receptor subtypes to amygdala reactivity in human, we conducted a multimodal in vivo neuroimaging study in which DA D1 and D2 receptor bindings in the amygdala were measured with positron emission tomography (PET), and amygdala response induced by fearful faces was assessed by functional magnetic resonance imaging (fMRI) in healthy volunteers. We used multimodality voxelwise correlation analysis between fMRI signal and DA receptor binding measured by PET. DA D1 binding in the amygdala was positively correlated with amygdala signal change in response to fearful faces, but DA D2 binding in the amygdala was not related to amygdala signal change. DA D1 receptors might play a major role in enhancing amygdala response when sensory inputs are affective.
Introduction
The amygdala plays a central role in processing affective stimuli, and in particular, threatening stimuli in the brain (LeDoux, 2000). The amygdala receives a moderate innervation of dopaminergic fibers (Asan, 1998), and both dopamine (DA) D1 and D2 receptors are expressed in this region (Ito et al., 2008), although the latter exhibit lower expression (Scibilia et al., 1992). DA release in the amygdala is increased in response to stress (Inglis and Moghaddam, 1999). It has been shown in animal studies that DA potentiates the response of the amygdala by augmenting excitatory sensory input and attenuating inhibitory prefrontal input to the amygdala (Rosenkranz and Grace, 2002). Systemic and local applications into the amygdala of D1 agonist and antagonist are known to potentiate and decrease fear response in animals, respectively. Although some studies reported that applications of D2 agonist and antagonist induced similar effects, the results were less consistent compared with D1-mediated effects (for review, see Pezze and Feldon, 2004; de la Mora et al., 2009).
A human functional magnetic resonance imaging (fMRI) study reported that doparminergic drug therapy such as levodopa or DA agonists partially restored amygdala response due to emotional task in Parkinson's disease patients who showed no significant amygdala response during drug-off states (Tessitore et al., 2002). In addition, another fMRI study of healthy volunteers has demonstrated that amphetamine potentiated the response of the amygdala during an emotional task (Hariri et al., 2002). More recently, Kienast et al. (2008) reported that dopamine storage capacity in human amygdala, measured with 6-[18F]fluoro-l-DOPA positron emission tomography (PET), was positively correlated with functional magnetic resonance imaging (fMRI) signal changes in amygdala. However, the contribution of DA D1 and D2 receptors to amygdala response induced by affective stimuli is unknown in human. To investigate the relation between amygdala reactivity and dopamine receptor subtype, we conducted a multimodal in vivo neuroimaging study in which DA D1 and D2 receptor bindings in the amygdala were measured with PET, and amygdala response by novel faces with either neutral or fearful expression was assessed with fMRI. Based on animal pharmacological studies, we hypothesized that D1, but not D2 receptors, would predict amygdala response.
Materials and Methods
Subjects
Twenty-one male volunteers [mean age 23.1 ± (SD) 3.6 years] were studied. They did not meet the criteria for any psychiatric disorder based on unstructured psychiatric screening interviews. None of the controls were taking alcohol at the time, nor did they have a history of psychiatric disorder, significant physical illness, head injury, neurological disorder, or alcohol or drug dependence. All subjects were right-handed according to the Edinburgh Handedness Inventory. All subjects underwent MRI to rule out cerebral anatomic abnormalities. After complete explanation of the study, written informed consent was obtained from all subjects, and the study was approved by the Ethics and Radiation Safety Committee of the National Institute of Radiological Sciences, Chiba, Japan.
fMRI procedure
Stimulus materials were taken from the Karolinska Directed Emotional Faces (KDEF) (Lundqvist et al., 1998). Thirty neutral and 30 fear faces were used, with half of them being male faces. The pictures were projected via a computer and a telephoto lens onto a screen mounted on a head-coil. The experimental design consisted of 5 blocks for each of the 2 conditions (neutral, fear) interleaved with 21 s rest periods. The order of presentation for the 2 conditions (neutral and fear) was randomized. During the baseline condition, subjects viewed a crosshair pattern projected to the center of the screen. In each 21 s block, 6 different faces of the same emotional class were presented for 3.5 s each. During the scans, the subjects were instructed to judge the gender of each face using selection buttons.
fMRI scanning
The images were acquired with a 3.0 Tesla Excite system (General Electric). Functional images of 126 volumes were acquired with T2*-weighted gradient echo planar imaging sequences sensitive to the blood oxygenation level-dependent (BOLD) contrast. Each volume consisted of 40 transaxial contiguous slices with a slice thickness of 3 mm to cover almost the whole brain (flip angle, 90°; echo time, 50 ms; repetition time, 3500 ms; matrix, 64 × 64; field of view, 24 × 24 cm).
Analysis of fMRI data
Data analysis was performed with the statistical parametric mapping software package (SPM2) (Wellcome Department of Cognitive Neurology, London, UK) running with MATLAB (MathWorks). All volumes were realigned to the first volume of each session to correct for subject motion and were spatially normalized to the standard space defined by the Montreal Neurological Institute (MNI) template. After normalization, all scans had a resolution of 2 × 2 × 2 mm3. Functional images were spatially smoothed with a three-dimensional isotropic Gaussian kernel (full-width at half-maximum of 8 mm). Low-frequency noise was removed by applying a high-pass filter (cutoff period = 128 s) to the fMRI time series at each voxel. A temporal smoothing function was applied to the fMRI time series to enhance the temporal signal-to-noise ratio. Significant hemodynamic changes for each condition were examined using the general linear model with boxcar functions convolved with a hemodynamic response function. Statistical parametric maps for each contrast of t-statistic were calculated on a voxel-by-voxel basis.
We assessed the contrasts of fear and neutral minus baseline (F&N-B). A random effects model, which estimates the error variance for each condition across the subjects, was implemented for group analysis. The contrast images were obtained from single-subject analysis and entered into the group analysis. A one-sample t test was applied to determine group response for each effect. Significant amygdala activations were identified if they reached the extent threshold of p < 0.05 corrected for multiple comparisons, with a height threshold of p < 0.001, uncorrected.
PET scanning
After the fMRI session, each participant underwent PET scanning. The interval between fMRI session and PET scan was 3–5 h. PET studies were performed on ECAT EXACT HR+ (CTI-Siemens). The system provides 63 planes and a 15.5 cm field of view. To minimize head movement, a head fixation device (Fixster) was used. A transmission scan for attenuation correction was performed using a germanium 68–gallium 68 source. Acquisitions were done in three-dimensional mode with the interplane septa retracted. For evaluation of D1 receptors, a bolus of 219.7 ± 6.9 MBq of [11C]SCH23390 with specific radioactivities (95.7 ± 35.5 GBq/μmol) was injected intravenously from the antecubital vein with a 20 ml saline flush. For evaluation of extrastriatal DA D2 receptors, a bolus of 218.1 ± 14.7 MBq of [11C]FLB457 with high specific radioactivities (221.6 ± 94.9 GBq/μmol) was injected in the same way. Dynamic scans were performed for 60 min for [11C]SCH23390 and 90 min for [11C]FLB457 immediately after the injection. All emission scans were reconstructed with a Hanning filter cutoff frequency of 0.4 (full-width at half-maximum, 7.5 mm). MRI was performed on Gyroscan NT (Philips Medical Systems) (1.5 T). T1-weighted images of the brain were obtained for all subjects. Scan parameters were 1-mm-thick, three-dimensional T1 images with a transverse plane (repetition time/echo time, 19/10 ms; flip angle, 30°; scan matrix, 256 × 256 pixels; field of view, 256 × 256 mm; and number of excitations, 1).
Quantification of DA D1 and D2 receptors
Quantitative analysis was performed using the three-parameter simplified reference tissue model (Lammertsma and Hume, 1996; Olsson et al., 1999). The cerebellum was used as a reference region because it has been shown to be almost devoid of DA D1 and D2 receptors (Farde et al., 1987; Olsson et al., 1999; Suhara et al., 1999). The model provides an estimation of the binding potential (BPND (nondisplaceable)) (Innis et al., 2007), which is defined by the following equation: BPND = k3/k4 = f2 Bmax/{Kd [1 + Σi Fi/Kdi]}, where k3 and k4 describe the bidirectional exchange of tracer between the free compartment and the compartment representing specific binding, f2 is the “free fraction” of nonspecifically bound radioligand in brain, Bmax is the receptor density, Kd is the equilibrium dissociation constant for the radioligand, and Fi and Kdi are the free concentration and dissociation constant of competing ligands, respectively (Lammertsma and Hume, 1996). Tissue concentrations of the radioactivities of [11C]SCH23390 and [11C]FLB457 were obtained from regions of interest (ROIs) defined on PET images of summated activity for 60 min and 90 min, respectively, with reference to the individual MRIs coregistered on summated PET images and the brain atlas. Given our hypothesis of amygdala activation during viewing novel neutral and fearful faces, ROIs were set on the bilateral amygdala. The method for defining the boundaries of the amygdala was adapted from previously described methods (Kates et al., 1997; Convit et al., 1999). In short, the amygdala ROIs consisted of three axial slices. The anterior and posterior boundaries were identified at the level of the optic chiasm and the temporal horn of the lateral ventricle, respectively. The superior and inferior-lateral boundaries were identified at the level of the mammalian body and the temporal lobe white matter and extension of the temporal horn, respectively. We also created parametric images of BPND using the basis function method (Gunn et al., 1997) to conduct voxelwise SPM analysis in addition to ROI analysis.
Statistical analysis
ROI correlation analysis.
Estimates of percentage signal change of fear vs baseline condition were extracted from the amygdala for each participant using the MarsBaR toolbox (Brett et al., 2002). The bilateral amygdala ROIs were defined from the WFU-Pickatlas SPM tool (Maldjian et al., 2003) with the aal atlas (Tzourio-Mazoyer et al., 2002). Correlation between BPND of [11C]SCH23390 and [11C]FLB457 in the bilateral amygdala and bilateral amygdala fMRI signal change were calculated using SPSS.
Confirmatory SPM correlation analysis.
Parametric images of BPND of [11C]SCH23390 and [11C]FLB457 were analyzed using SPM2. Exactly the same image preprocessings of normalization and smoothing that were used in fMRI data analysis were applied to parametric images of BPND. To conduct multimodality voxelwise correlation analysis between the BOLD signal and DA receptor binding, we used the biological parametric mapping toolbox for SPM (Casanova et al., 2007). Significant clusters were identified if they reached the extent threshold of p < 0.05 corrected for multiple comparisons, with a height threshold of R > 0.6 (p < 0.003 uncorrected).
Results
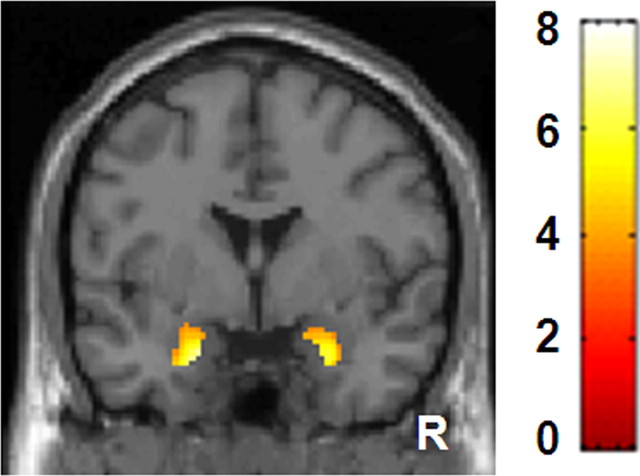
Since the face pictures consisted of Caucasian faces (racial outgroup), even novel neutral faces produced amygdala response in several participants (Hart et al., 2000; Schwartz et al., 2003), leading to a blunted contrast of fear minus neutral. Therefore, we combined neutral and fear conditions and used F&N-B contrast for analyses. Group analysis of F&N-B contrast revealed significant bilateral amygdala responses [right amygdala (26, 0, −26), t = 4.43, 93 voxels, left amygdala (−20, −2, −26), Z = 4.96, 101 voxels] (Fig. 1). The mean BPND of [11C]SCH23390 in the right and left amygdala were 0.38 ± 0.08 and 0.39 ± 0.11, respectively. The mean BPND of [11C]FLB457 in the right and left amygdala were 2.49 ± 0.50 and 2.50 ± 0.44, respectively.
Figure 1.
Images showing brain response induced by fear and neutral minus baseline condition. Bilateral amygdala responses are shown. The bar shows the range of the t-value. R indicates right.
Correlation analysis of biological parametric mapping revealed that the BPND value of [11C]SCH23390 in the right amygdala was positively correlated with the BOLD signals in the right amygdala of F&N-B contrast [peak (28, 2, −28), 24 voxels] (Fig. 2A). ROIs analysis also revealed a similar significant correlation (r = 0.59, p = 0.005) in the right amygdala (Fig. 2B), but not in the left amygdala (r = 0.18, p = 0.43). According to biological parametric mapping analysis, the BPND value of [11C]FLB457 in the amygdala was not correlated with BOLD signals in the amygdala of F&N-B contrast. ROIs analysis showed that right and left amygdala D2 binding was not correlated with the BOLD signals in the right (r = 0.26, p = 0.27) and left amygdala (r = 0.28, p = 0.23), respectively. Both biological parametric mapping analysis and ROIs analysis showed that D1 binding in the right and left amygdala was not correlated with D2 binding in the right (r = 0.24, p = 0.30) and left amygdala (r = 0.16, p = 0.49), respectively. We used anatomically defined ROIs of the amygdala rather than functional ROIs defined by fMRI in the ROI correlation analysis because it is difficult to place functionally defined ROIs on individual PET data. Anatomically defined ROIs of the amygdala were larger than functionally defined amygdala ROIs. This fact was advantageous in increasing the signal-to-noise ratio in the PET analysis, but led to blunted BOLD signal changes in the amygdala. However, BOLD signal changes derived from both ROI methods were highly correlated with each other. For example, very high correlation (r = 0.80, p < 0.001) was observed in the right amygdala. Thus, regardless of ROI definition method, we obtained similar results from ROI correlation analyses between BOLD signal changes and DA receptor binding in the amygdala.
Figure 2.
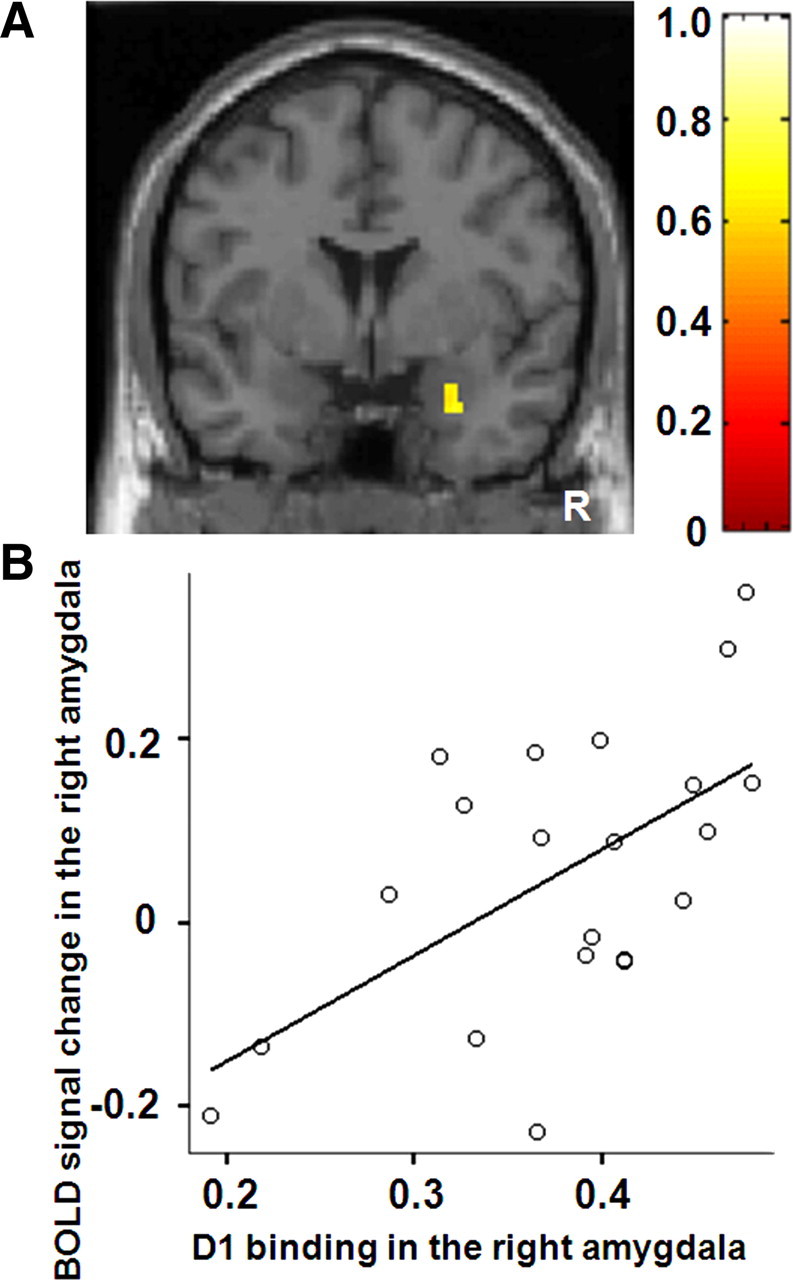
A, SPM correlation analysis revealed significant positive linear correlations between D1 binding in the right amygdala and right amygdala signal change. The bar shows the range of the correlation coefficient. B, ROI correlation analysis also revealed similar correlations. R indicates right.
Discussion
Using a multimodality in vivo neuroimaging approach, we first directly compared amygdala DA D1 and D2 receptor bindings, indices of receptor availability, with amygdala response evoked by novel or fearful stimuli in human. We found that DA D1 receptors, but not D2 receptors, predicted amygdala response induced by novel facial stimuli with either neutral or fearful expression. Our findings broaden our knowledge about dopaminergic transmission in amygdala response beyond the recent study (Kienast et al., 2008) that elucidated the relation between presynaptic dopamine synthesis and amygdala reactivity.
Human neuroimaging studies reported that DA potentiated amygdala response evoked by affective stimuli (Hariri et al., 2002; Tessitore et al., 2002). In rat studies, Rosenkranz and Grace (2002) demonstrated that DA enhances the response of the amygdala by augmenting excitatory sensory input via DA D2 receptor stimulation and attenuating inhibitory prefrontal input to the amygdala through DA D1 receptor stimulation. More recently, it was demonstrated that both D1 and D2 receptor stimulations directly enhanced the excitability of amygdala projection neurons via postsynaptic mechanism (Rosenkranz and Grace, 2002; Kröner et al., 2005; Yamamoto et al., 2007). Amygdala projection neurons are under inhibitory control by GABAergic interneurons (Royer et al., 1999). Both projection neurons and interneurons in the amygdala express DA D1 and D2 receptors (Rosenkranz and Grace, 1999). It has been shown that DA and D1 receptor stimulation augments interneuron excitability and increases the frequency of IPSC in amygdala projection neurons (Kröner et al., 2005). This is a counterintuitive result, considering the fact that DA disinhibits amygdala response in vivo. However, Marowsky et al. (2005) found that a subpopulation of amygdala interneurons (paracapsular intercalated cells), located between the major input and output stations of amygdala, is suppressed by DA through D1 receptor stimulation. DA D2 receptors also play a role in disinhibiting amygdala response by decreasing inhibition onto projection neurons and increasing inhibition onto interneurons (Bissière et al., 2003).
Although detailed examination of subnuclei of the amygdala is difficult in this imaging method, the dorsal portion of the amygdala roughly corresponds to the central nuclei of amygdala (CeA) and the ventral portion of the amygdala corresponds to the basolateral nuclei of amygdala (BLA) and intercalated cell masses (ICM) (Whalen et al., 2009). The amygdala clusters identified both in fMRI task effect analysis and in correlation analysis between D1 binding and amygdala reactivity were located in the ventral portion of the amygdala. Thus, our findings seem to mainly reflect BLA and ICM properties. It is worth mentioning that the highest density of D1 receptors within the amygdala was found in the ICM, followed by BLA, and the expression of D1 receptors is low in CeA (de la Mora et al., 2009; Muly et al., 2009). In contrast, D2 receptors are mainly distributed in CeA (de la Mora et al., 2009). Both D1 and D2 receptors are expressed both postsynaptically in dendrites and presynaptically in axon terminals (Pinto and Sesack, 2008; Muller et al., 2009; Muly et al., 2009), but D1 receptors in BLA are mainly expressed in the dendrites, indicating that DA directly modulates the excitability of BLA projection neurons and interneurons. At the same time, DA also acts on presynaptic D1 receptors to increase the probability of neurotransmitter release from glutamatergic terminals (Muly et al., 2009). Thus, the net DA effect on D1 receptors in the amygdala is a complex mixture of post- and presynaptic actions at several sites.
Although both DA D1 and D2 receptors contribute to potentiating amygdala response via various mechanisms as described above, our finding suggested that DA D1 receptors play a major role in the overall potentiation of amygdala response. At a behavioral level, previous animal studies repeatedly reported that D1 agonist and antagonist applications into the amygdala potentiated and decreased fear response, respectively. However, the effects of D2 agonist/antagonist on fear response have not been well established (Pezze and Feldon, 2004; de la Mora et al., 2009). Thus, the current finding could be regarded as being consistent with previous behavioral pharmacological studies. The combination of PET molecular imaging and fMRI seems to represent a powerful approach for understanding molecular functions in system neuroscience. However, this study has several limitations. First, current PET techniques for human do not have enough spatial resolution to distinguish subnuclei of the amygdala. Although analysis of parametric images of BPND has become well established (Gunn et al., 1997) and is used in many [11C]SCH23390 and [11C]FLB457 studies (Cervenka et al., 2006; Takahashi et al., 2008; Karlsson et al., 2009; McNab et al., 2009), a very small region or a single voxel is susceptible to partial volume effect. Thus, it is recommended that parametric image analysis should be used in combination with ROI analysis. At the same time, current results merit further investigation with a higher resolution PET scanner. Second, PET imaging cannot tell us the exact location of DA receptors expressed in projection neurons and interneurons. Future animal studies or in vitro studies would complement our findings to determine which D1 receptor-mediated mechanism is most responsible for the overall amygdala response. Third, differences in DA receptor occupancies by endogenous DA might affect BP, leading to different excitabilities of neurons. It is known that BP of [11C]SCH23390 is not sensitive to competitive endogenous dopamine even if massive dopamine is released by amphetamine (Abi-Dargham et al., 1999; Chou et al., 1999). However, it is possible that differences in receptor affinity might contribute to differences in DA receptor occupancies, although Farde et al. (1995) reported that variability in D2 receptor affinity is smaller than that in D2 receptor density. Finally, gender and race effects might also be possible. Any generalization should be approached with caution. Notwithstanding these limitations, we expect our finding to contribute to a broadening of the knowledge of the molecular mechanism of functional abnormalities of the amygdala implicated in neuropsychiatric disorders such as schizophrenia (Takahashi et al., 2004), depression (Drevets, 2000) and Parkinson's disease (Tessitore et al., 2002).
Footnotes
This study was supported by a consignment expense for Molecular Imaging Program on “Research Base for PET Diagnosis” from the Ministry of Education, Culture, Sports, Science and Technology. Takanori Kochiyama and Yoko Ikoma are greatly acknowledged for her comments. We thank K. Tanimoto and T. Shiraishi for their assistance in performing the PET experiments at the National Institute of Radiological Sciences. We also thank Y. Fukushima, K. Suzuki, and I. Izumida of the National Institute of Radiological Sciences for their help as clinical research coordinators.
References
- Abi-Dargham A, Simpson N, Kegeles L, Parsey R, Hwang DR, Anjilvel S, Zea-Ponce Y, Lombardo I, Van Heertum R, Mann JJ, Foged C, Halldin C, Laruelle M. PET studies of binding competition between endogenous dopamine and the D1 radiotracer [11C] NNC 756. Synapse. 1999;32:93–109. doi: 10.1002/(SICI)1098-2396(199905)32:2<93::AID-SYN3>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Asan E. The catecholaminergic innervation of the rat amygdala. Adv Anat Embryol Cell Biol. 1998;142:1–118. doi: 10.1007/978-3-642-72085-7. [DOI] [PubMed] [Google Scholar]
- Bissière S, Humeau Y, Lüthi A. Dopamine gates LTP induction in lateral amygdala by suppressing feedforward inhibition. Nat Neurosci. 2003;6:587–592. doi: 10.1038/nn1058. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J, Valabregue R, Poline J. Region of interest analysis using the MarsBar toolbox [abstract]. Paper presented at 8th International Conference on Functional Mapping of the Human Brain; Sendai, Japan. 2002. [Google Scholar]
- Casanova R, Srikanth R, Baer A, Laurienti PJ, Burdette JH, Hayasaka S, Flowers L, Wood F, Maldjian JA. Biological parametric mapping: a statistical toolbox for multimodality brain image analysis. Neuroimage. 2007;34:137–143. doi: 10.1016/j.neuroimage.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervenka S, Pålhagen SE, Comley RA, Panagiotidis G, Cselényi Z, Matthews JC, Lai RY, Halldin C, Farde L. Support for dopaminergic hypoactivity in restless legs syndrome: a PET study on D2-receptor binding. Brain. 2006;129:2017–2028. doi: 10.1093/brain/awl163. [DOI] [PubMed] [Google Scholar]
- Chou YH, Karlsson P, Halldin C, Olsson H, Farde L. A PET study of D1-like dopamine receptor ligand binding. Psychopharmacology. 1999;146:220–227. doi: 10.1007/s002130051110. [DOI] [PubMed] [Google Scholar]
- Convit A, McHugh P, Wolf OT, de Leon MJ, Bobinski M, De Santi S, Roche A, Tsui W. MRI volume of the amygdala: a reliable method allowing separation from the hippocampal formation. Psychiatry Res. 1999;90:113–123. doi: 10.1016/s0925-4927(99)00007-4. [DOI] [PubMed] [Google Scholar]
- de la Mora MP, Gallegos-Cari A, Arizmendi-Garcia Y, Marcellino D, Fuxe K. Role of dopamine receptor mechanisms in the amygdaloid modulation of fear and anxiety: structural and functional analysis. Prog Neurobiol. 2009 doi: 10.1016/j.pneurobio.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Prog Brain Res. 2000;126:413–431. doi: 10.1016/S0079-6123(00)26027-5. [DOI] [PubMed] [Google Scholar]
- Farde L, Halldin C, Stone-Elander S, Sedvall G. PET analysis of human dopamine receptor subtypes using 11C-SCH 23390 and 11C-raclopride. Psychopharmacology (Berl) 1987;92:278–284. doi: 10.1007/BF00210831. [DOI] [PubMed] [Google Scholar]
- Farde L, Hall H, Pauli S, Halldin C. Variability in D2-dopamine receptor density and affinity: a PET study with [11C] raclopride in man. Synapse. 1995;20:200–208. doi: 10.1002/syn.890200303. [DOI] [PubMed] [Google Scholar]
- Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ. Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. Neuroimage. 1997;6:279–287. doi: 10.1006/nimg.1997.0303. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Smith WG, Weinberger DR. Dextroamphetamine modulates the response of the human amygdala. Neuropsychopharmacology. 2002;27:1036–1040. doi: 10.1016/S0893-133X(02)00373-1. [DOI] [PubMed] [Google Scholar]
- Hart AJ, Whalen PJ, Shin LM, McInerney SC, Fischer H, Rauch SL. Differential response in the human amygdala to racial outgroup vs ingroup face stimuli. Neuroreport. 2000;11:2351–2355. doi: 10.1097/00001756-200008030-00004. [DOI] [PubMed] [Google Scholar]
- Inglis FM, Moghaddam B. Dopaminergic innervation of the amygdala is highly responsive to stress. J Neurochem. 1999;72:1088–1094. doi: 10.1046/j.1471-4159.1999.0721088.x. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Ito H, Takahashi H, Arakawa R, Takano H, Suhara T. Normal database of dopaminergic neurotransmission system in human brain measured by positron emission tomography. Neuroimage. 2008;39:555–565. doi: 10.1016/j.neuroimage.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Karlsson S, Nyberg L, Karlsson P, Fischer H, Thilers P, Macdonald S, Brehmer Y, Rieckmann A, Halldin C, Farde L, Bäckman L. Modulation of striatal dopamine D1 binding by cognitive processing. Neuroimage. 2009;48:398–404. doi: 10.1016/j.neuroimage.2009.06.030. [DOI] [PubMed] [Google Scholar]
- Kates W, Abrams M, Kaufmann W, Breiter S, Reiss A. Reliability and validity of MRI measurement of the amygdala and hippocampus in children with fragile X syndrome. Psychiatry Res. 1997;75:31–48. doi: 10.1016/s0925-4927(97)00019-x. [DOI] [PubMed] [Google Scholar]
- Kienast T, Hariri AR, Schlagenhauf F, Wrase J, Sterzer P, Buchholz HG, Smolka MN, Gründer G, Cumming P, Kumakura Y, Bartenstein P, Dolan RJ, Heinz A. Dopamine in amygdala gates limbic processing of aversive stimuli in humans. Nat Neurosci. 2008;11:1381–1382. doi: 10.1038/nn.2222. [DOI] [PubMed] [Google Scholar]
- Kröner S, Rosenkranz JA, Grace AA, Barrionuevo G. Dopamine modulates excitability of basolateral amygdala neurons in vitro. J Neurophysiol. 2005;93:1598–1610. doi: 10.1152/jn.00843.2004. [DOI] [PubMed] [Google Scholar]
- Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4:153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lundqvist D, Flykt A, Ohman A. Psychology section. Stockholm, Sweden: Department of Clinical Neuroscience, Karolinska Institute; 1998. The Karolinska Directed Emotional Faces. [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Marowsky A, Yanagawa Y, Obata K, Vogt KE. A specialized subclass of interneurons mediates dopaminergic facilitation of amygdala function. Neuron. 2005;48:1025–1037. doi: 10.1016/j.neuron.2005.10.029. [DOI] [PubMed] [Google Scholar]
- McNab F, Varrone A, Farde L, Jucaite A, Bystritsky P, Forssberg H, Klingberg T. Changes in cortical dopamine D1 receptor binding associated with cognitive training. Science. 2009;323:800–802. doi: 10.1126/science.1166102. [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Dopaminergic innervation of pyramidal cells in the rat basolateral amygdala. Brain Struct Funct. 2009;213:275–288. doi: 10.1007/s00429-008-0196-y. [DOI] [PubMed] [Google Scholar]
- Muly EC, Senyuz M, Khan ZU, Guo JD, Hazra R, Rainnie DG. Distribution of D1 and D5 dopamine receptors in the primate and rat basolateral amygdala. Brain Struct Funct. 2009;213:375–393. doi: 10.1007/s00429-009-0214-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson H, Halldin C, Swahn CG, Farde L. Quantification of [11C]FLB 457 binding to extrastriatal dopamine receptors in the human brain. J Cereb Blood Flow Metab. 1999;19:1164–1173. doi: 10.1097/00004647-199910000-00013. [DOI] [PubMed] [Google Scholar]
- Pezze MA, Feldon J. Mesolimbic dopaminergic pathways in fear conditioning. Prog Neurobiol. 2004;74:301–320. doi: 10.1016/j.pneurobio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Pinto A, Sesack SR. Ultrastructural analysis of prefrontal cortical inputs to the rat amygdala: spatial relationships to presumed dopamine axons and D1 and D2 receptors. Brain Struct Funct. 2008;213:159–175. doi: 10.1007/s00429-008-0180-6. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA. Modulation of basolateral amygdala neuronal firing and afferent drive by dopamine receptor activation in vivo. J Neurosci. 1999;19:11027–11039. doi: 10.1523/JNEUROSCI.19-24-11027.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA. Cellular mechanisms of infralimbic and prelimbic prefrontal cortical inhibition and dopaminergic modulation of basolateral amygdala neurons in vivo. J Neurosci. 2002;22:324–337. doi: 10.1523/JNEUROSCI.22-01-00324.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer S, Martina M, Paré D. An inhibitory interface gates impulse traffic between the input and output stations of the amygdala. J Neurosci. 1999;19:10575–10583. doi: 10.1523/JNEUROSCI.19-23-10575.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz CE, Wright CI, Shin LM, Kagan J, Whalen PJ, McMullin KG, Rauch SL. Differential amygdalar response to novel versus newly familiar neutral faces: a functional MRI probe developed for studying inhibited temperament. Biol Psychiatry. 2003;53:854–862. doi: 10.1016/s0006-3223(02)01906-6. [DOI] [PubMed] [Google Scholar]
- Scibilia RJ, Lachowicz JE, Kilts CD. Topographic nonoverlapping distribution of D1 and D2 dopamine receptors in the amygdaloid nuclear complex of the rat brain. Synapse. 1992;11:146–154. doi: 10.1002/syn.890110208. [DOI] [PubMed] [Google Scholar]
- Suhara T, Sudo Y, Okauchi T, Maeda J, Kawabe K, Suzuki K, Okubo Y, Nakashima Y, Ito H, Tanada S, Halldin C, Farde L. Extrastriatal dopamine D2 receptor density and affinity in the human brain measured by 3D PET. Int J Neuropsychopharmcol. 1999;2:73–82. doi: 10.1017/S1461145799001431. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Koeda M, Oda K, Matsuda T, Matsushima E, Matsuura M, Asai K, Okubo Y. An fMRI study of differential neural response to affective pictures in schizophrenia. Neuroimage. 2004;22:1247–1254. doi: 10.1016/j.neuroimage.2004.03.028. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Kato M, Takano H, Arakawa R, Okumura M, Otsuka T, Kodaka F, Hayashi M, Okubo Y, Ito H, Suhara T. Differential contributions of prefrontal and hippocampal dopamine D1 and D2 receptors in human cognitive functions. J Neurosci. 2008;28:12032–12038. doi: 10.1523/JNEUROSCI.3446-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessitore A, Hariri AR, Fera F, Smith WG, Chase TN, Hyde TM, Weinberger DR, Mattay VS. Dopamine modulates the response of the human amygdala: a study in Parkinson's disease. J Neurosci. 2002;22:9099–9103. doi: 10.1523/JNEUROSCI.22-20-09099.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Davis C, Oler JA, Kim H, Kim J, Neta M. Human amygdala responses to facial expressions of emotions. In: Whalen PJ, Phelps EA, editors. The human amygdala. New York: Guilford; 2009. pp. 265–288. [Google Scholar]
- Yamamoto R, Ueta Y, Kato N. Dopamine induces a slow afterdepolarization in lateral amygdala neurons. J Neurophysiol. 2007;98:984–992. doi: 10.1152/jn.00204.2007. [DOI] [PubMed] [Google Scholar]