Abstract
Dronedarone is recommended for the treatment of atrial fibrillation. However, we do not know its effect on vascular remodeling. This study was designed to assess whether dronedarone has the potential to improve the intramyocardial artery remodeling induced by chronic hypertension. Ten-month-old male spontaneously hypertensive rats (SHR) were randomly assigned to receive dronedarone (100 mg/kg) or vehicle. Age-matched male Wistar-Kyoto rats served as controls. After 14 days of treatment, we studied the structure (geometry and fibrosis) of the intramyocardial artery using histological analysis. Nitric oxide (NO) in plasma was analyzed. In the untreated SHR, we observed a significant increase in external diameter, lumen diameter, wall width, cross-sectional area, and collagen volume density, as was expected in the experimental model. Dronedarone induced a significant decrease in wall width, cross-sectional area, and collagen volume density in SHR-D in comparison with untreated SHR. The values obtained in SHR-D were similar in the WKY control group. We found significantly higher NO levels in plasma in SHR-D than in untreated SHR. Dronedarone improves the intramyocardial artery remodeling induced by chronic hypertension in SHR through increased nitric oxide bioavailability.
1. Introduction
Hypertension leads to adverse remodeling (structural alterations) in the coronary artery wall and therefore increases the incidence of cardiovascular events [1, 2]. Antihypertensive therapy reverses vascular structural alterations and reduces cardiovascular morbidity [3, 4]. Therefore, the benefits by improving structural alterations in the coronary artery in patients with hypertension are an important factor in the selection of antihypertensive therapy [5].
Dronedarone is a novel antiarrhythmic drug for atrial fibrillation, acting as a multichannel blocker [6]. Dronedarone reduced the incidence of hospitalization due to cardiovascular events or death in patients with atrial fibrillation [7, 8]; however it is avoided in patients with unstable chronic heart failure [9]. In a preclinical study, we previously analyzed early regression of left ventricular hypertrophy following short-term use of antiarrhythmic agents and we found that dronedarone produces regression of left ventricular hypertrophy after two weeks of treatment [10]. However, its effect on intramyocardial artery remodeling has not been analyzed to date. Left ventricular hypertrophy, which is the usual complication of hypertension, is associated with structural changes, namely, coronary remodeling [11]; therefore we tested the hypothesis that short-term administration (two weeks) of dronedarone could improve the intramyocardial artery remodeling.
This study was designed to evaluate the efficacy of dronedarone in attenuating the intramyocardial branch of the obtuse marginal artery remodeling in spontaneously hypertensive rats (SHR), the most widely used animal models for human essential hypertension, left ventricular hypertrophy, and coronary damage.
2. Methods
2.1. Ethics
The study was performed in accordance with European Union Guidelines to use experimental animals (Directive 2010/63/EU and Spanish Law RD 53/2013) and was approved by the Ethics Committee of Hospital Gregorio Marañón, Madrid, Spain.
2.2. Animals and Experimental Protocols
Ten-month-old male SHR were randomly assigned to receive oral dronedarone (100 mg/kg once daily) (SHR-D, n=11) for a period of 14 days or to receive vehicle (SHR, n=11). A third group of normotensive control rats (WKY, n=11) was also added. Once the treatment was finished, the rats were killed by decapitation after sedation with diazepam 4 mg/kg and ketamine 10 mg/kg (intraperitoneal injection).
2.3. In Vivo Measurements (Arterial Pressure and Heart Rate)
As described in detail in previous studies [12] systolic arterial pressure (SAP) and heart rate (HR) were measured using the plethysmographic method on the tail artery of conscious animals (Niprem 546, Cibertec, Madrid, Spain). Several determinations were made before and after treatment, and the findings were considered valid if 10 consecutive measurements were within 10 mmHg of each other.
2.4. Intramyocardial Artery Structure
We analyzed 6 animals for each group as described in detail previously [12–14]. Hearts were excised and fixed in 4% paraformaldehyde. An equatorial cross section of the left ventricle was embedded in paraffin. Sections (3 sections/rat) were cut (thickness, 5 μm) and stained with orcein [12]. The intramyocardial branch of the obtuse marginal artery (branch of the circumflex coronary artery) of the left ventricle was located, and the external diameter (ED) (lumen diameter + tunica intima + tunica media + tunica adventitia) and lumen diameter (LD) of the intramyocardial artery were measured as described [12]. Wall width (WW) was calculated as (ED−LD)/2, the wall-to-lumen ratio (W/L) as (WW/LD)×100, and the media cross-sectional area (CSA) (tunica intima + tunica media + tunica adventitia) as (π/4)×(ED2−LD2) [13]. For collagen staining, 5 μm sections (3 sections/rat) of paraffin blocks were stained with picrosirius red, and the collagen volume density (CD) was determined using a stereological method [14].
2.5. Measurement of Plasma Nitrite Level
We analyzed 5 animals from each group. The same protocols described in previous studies [15] were followed in the present one. Plasma nitrite level was assessed using a Griess reaction-based protocol adapted from other authors [16, 17]. To eliminate thiols that interfere with the Griess reaction, 100 μL of plasma or nitrite standard was mixed with 10 μL of N-ethylenediamine. The protein from samples was precipitated by addition of 110 μL of trichloroacetic acid. 50 μL of supernatant (obtained by centrifugation) was mixed with 50 μL of saturated vanadium chloride, 25 μL of sulfanilamide, and 25 μL of N-naphthyl-ethylenediamine. The mixture was incubated at 37°C for 1 hour, and absorbance was read at 540 nm.
2.6. Statistical Analysis
The results were expressed as median and 25th and 75th percentiles and analyzed with the Mann-Whitney U test (arterial structure parameters and nitrites). The results were expressed as mean ± SEM and analyzed using repeated-measures analysis of variance (physiological parameters). Statistical significance was set at P ≤ 0.05. The analysis was performed using IBM SPSS Statistics for Windows, version 20.0.
3. Results
3.1. Administration of Dronedarone Lowers Blood Pressure and Heart Rate
Rat weight was significantly higher in WKY than in SHR and SHR-D (460.40±1.12 vs. 401.22±3.02 g, P<0.01, and 460.40±1.12 vs. 380.51±6.21 g, P<0.001, respectively); however, the difference was not statistically different between SHR and SHR-D.
Dronedarone significantly reduced SAP and HR in SHR-D with respect to control SHR. SAP remained unchanged in SHR-D and WKY (Table 1).
Table 1.
Systolic blood pressure and heart rate from WKY, SHR, and SHR-D.
| Groups | Time treatment (days) | SBP (mmHg) | HR (bpm) |
|---|---|---|---|
| WKY (n=6) | 0 | 130 ± 21 | 414 ± 20 |
| 14 | 137 ± 23 | 401 ± 24 | |
| SHR (n=6) | 0 | 174 ± 15∗∗ | 395 ± 21 |
| 14 | 180 ± 11∗∗ | 410 ± 18 | |
| SHR-D (n=6) | 0 | 176 ± 21∗∗ | 398 ± 12 |
| 14 | 141 ± 12## | 301 ± 17∗∗∗, ### |
SBP, systolic blood pressure; HR, heart rate. Values are shown as mean ± SEM. Statistically significant differences are shown (∗∗P < 0.01 versus WKY; ∗∗∗P < 0.001 versus WKY; ##P < 0.01 versus SHR; ###P < 0.001 versus SHR).
3.2. Administration of Dronedarone Improves Structural Intramyocardial Artery Remodeling
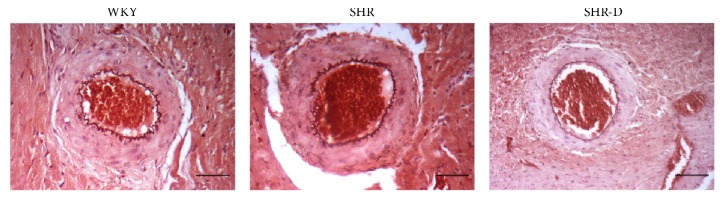
The effect of dronedarone on artery morphology is shown in Table 2 and Figure 1. SHR presented hypertrophic outward remodeling associated with a significant increase in CSA and LD in the intramyocardial branch of the obtuse marginal artery when compared with WKY. CSA and WW were significantly lower after 2 weeks of treatment with dronedarone in SHR-D than in SHR, and no differences were observed with respect to WKY. LD in SHR-D did not differ from that in SHR. ED was significantly increased in SHR when compared with WKY; however, administration of dronedarone significantly decreased this parameter in SHR-D when compared with SHR.
Table 2.
Geometry and collagen in the intramyocardial artery from WKY, SHR, and SHR-D.
| Structural parameters | WKY | SHR | SHR-D |
|---|---|---|---|
| ED (μm) | 151 (150-219) | 349 (312-377)∗∗ | 250 (248-303)∗∗,# |
| LD (μm) | 68 (60-129) | 201 (110-212)∗ | 176 (144-226)∗ |
| WW (μm) | 43 (36-47) | 82 (71-107)∗∗ | 49 (38-52)## |
| W/L (%) | 60 (37-74) | 46 (33-80) | 27 (18-36)∗,# |
| CSA (μm2) | 15058 (14381-21195) | 70227 (54016-82310)∗∗ | 31885 (25215-38623)## |
| CD (%) | 13.83 (11.81-21.40) | 41.66 (40.68-52.78)∗∗ | 15.38 (12.57-17.25)## |
| NO (μM) | 1.76 (1.68-1.84) | 1.35 (1.32-1.40)∗ | 1.66 (1.46-1.80) # |
ED, external diameter; LD, lumen diameter; WW, wall width; W/L, wall/lumen ratio; CSA, cross-sectional area; CD, collagen volume density; NO, nitric oxide. Data are expressed as median (25th-75th percentiles). Statistically significant differences are shown ( ∗P<0.05 versus WKY; ∗∗P<0.01 versus WKY; #P<0.05 versus SHR; ##P<0.01 versus SHR). Geometry and collagen (n=6) and NO (n=5).
Figure 1.
Histological sections of the intramyocardial branch of the obtuse marginal artery from WKY, SHR, and SHR-D (orcein, 100x, 100 μm).
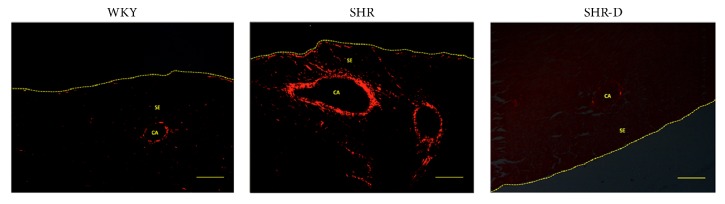
The effect of dronedarone on intramyocardial artery fibrosis is shown in Table 2 and Figure 2. Vascular collagen was expressed as CD. Compared with WKY rats, the CD of the intramyocardial artery was significantly increased in SHR. Dronedarone significantly reduced the collagen fiber content, and the CD value was comparable to that of WKY.
Figure 2.
Images of collagen staining (picrosirius red, 40x, 200 μm) of the intramyocardial branch of the obtuse marginal artery from WKY, SHR, and SHR-D. CA, coronary artery; SE, subepicardium.
3.3. Administration of Dronedarone Increases the Bioavailability of Nitric Oxide (NO)
Compared with WKY rats, the NO of the intramyocardial artery was significantly decreased in SHR. Dronedarone significantly increased the NO, and this parameter was comparable to that of WKY (Table 2).
4. Discussion
Our results show that two weeks of treatment with dronedarone improves the intramyocardial artery remodeling in adult SHR through increased nitric oxide bioavailability.
In the literature, we find that antihypertensive and antiarrhythmic drugs produce regression coronary artery remodeling after chronic treatment in SHR: administration of losartan for 5 weeks reduced wall thickness and CSA, amlodipine and enalapril led to a reduction in media thickness and CSA after 12 weeks, perindopril and indapamide led to a reduction in CSA and vessel diameter after 8 weeks, and lisinopril reduced the thickness of the coronary artery media after 12 weeks [18–21]. Furthermore, short-term administration of esmolol (48 hours) reduced wall thickness, CSA, and vessel diameter [15, 22]. However, the effect of dronedarone on vascular remodeling has not been investigated to date.
Functional and structural alterations of the coronary circulation have been well documented in left ventricular hypertrophy [1]. In a preclinical study, we show that short-term treatment (two weeks) with dronedarone has the potential to reverse the left ventricular hypertrophy induced by arterial hypertension in the SHR model of compensated ventricular hypertrophy [10]. In this context, we found it of interest to assess the impact of dronedarone in attenuating intramyocardial artery remodeling. And it is plausible that dronedarone induces changes in intramyocardial remodeling.
Dronedarone is a new drug (class III antiarrhythmic) used for the maintenance of sinus rhythm and control of ventricular rate [23]. Dronedarone produces a multichannel blockade of the sodium, potassium, and calcium channels and exhibits antiadrenergic properties [6]. Dronedarone reduces heart rate and blood pressure, and it has a direct cardioprotective effect (reduction in infarct size in an animal model of ischemia/reperfusion). Therefore, dronedarone reduces the risk of acute coronary syndrome [24]. This cardioprotective effect could be related to the improvement of structural alterations in intramyocardial arteries observed in the present study.
In the present study, the mechanism underlying dronedarone improving the intramyocardial artery remodeling could be related in part to an increase in the bioavailability of NO. Coronary artery remodeling in the presence of hypertension implies structural changes [25], but also endothelial dysfunction [26, 27]. Dronedarone acts on the coronary artery producing vasodilatation by activating the nitric oxide synthase pathway [28]. NO inhibits smooth muscle cell (SMC) proliferation of the arterial wall [29], decreasing wall thickness. Therefore, NO is a key regulator of cardiovascular remodeling thanks to its antihypertrophic and direct antiproliferative effects [30]. Our group has demonstrated that another antihypertensive drug (esmolol) reverses structural alterations in the coronary arteries (decreased media thickness and SMC count) by increasing the bioavailability of NO in SHR [15, 22]. With the data available from the literature, the mechanisms discussed above remain speculative and future studies are required to gain insight into the mechanism underlying dronedarone-induced protective effects on intramyocardial arteries remodeling.
Our results provide preclinical data on the beneficial effects of dronedarone on intramyocardial artery remodeling, which is associated with an increased incidence of cardiovascular events [1, 2]. During the last decade, dronedarone has been studied extensively in a wide range of experimental and clinical settings. These studies have revelated that dronedarone is an interesting antiarrhythmic agent for the treatment of atrial fibrillation [31]. Dronedarone reduces cardiovascular morbidity and mortality in clinically stable patients with other risk factors for recurrent atrial fibrillation [23]. Atrial fibrillation is the most common arrhythmia in clinical practice with significant morbidity and mortality [6]. In fact, atrial fibrillation induces ischemic microcirculatory flow abnormalities in the ventricle contributing to the risk of acute coronary syndromes [32]. Therefore, dronedarone is now widely used to treat patients with atrial fibrillation. However, regression of structural alterations in the intramyocardial arteries in patients with atrial fibrillation could play a key role in the selection of antiarrhythmic therapy. Further studies are needed to clarify the underlying mechanisms of positive effect of dronedarone on vascular remodeling.
The present study is subject to limitations. This study was designed to evaluate the efficacy of dronedarone in attenuating the intramyocardial branch of the obtuse marginal artery remodeling in SHR. Our results show that dronedarone improves structural intramyocardial artery remodeling; however, vascular remodeling in the presence of hypertension implies not only alterations in the structure of vessels, but also endothelial dysfunction [26, 27]. Thus, a major goal of research could be to analyze the effect of dronedarone on coronary artery vasodilator function. On the other hand, the complicated mechanisms underlying vascular remodeling involve not only decreased NO bioavailability. In fact, activation of the pathways of vascular smooth muscle contraction, vascular oxidative stress, and inflammation are implicated [27]. Therefore, we need to explore new mechanisms related to the positive effect of dronedarone on vascular remodeling.
In conclusion, this study suggests that dronedarone improves the intramyocardial artery remodeling induced by chronic hypertension in SHR through increased nitric oxide bioavailability.
Acknowledgments
This study was supported by Fondo de Investigaciones Sanitarias (PI13/01261, PI16/02069) and Fondos Feder.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Disclosure
Preliminary results of this study were presented in the 45th Physiological Society of Thailand Annual Meeting 2017: “Translational Physiology: Implications in Health and Disease.”
Conflicts of Interest
The authors declare that they have no conflicts of interest regarding the publication of this paper.
References
- 1.Camici P. G., Olivotto I., Rimoldi O. E. The coronary circulation and blood flow in left ventricular hypertrophy. Journal of Molecular and Cellular Cardiology. 2012;52(4):857–864. doi: 10.1016/j.yjmcc.2011.08.028. [DOI] [PubMed] [Google Scholar]
- 2.Intengan H. D., Schiffrin E. L. Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension. 2001;38(3, part 2):581–587. doi: 10.1161/hy09t1.096249. [DOI] [PubMed] [Google Scholar]
- 3.Rehman A., Schiffrin E. L. Vascular effects of antihypertensive drug therapy. Current Hypertension Reports. 2010;12(4):226–232. doi: 10.1007/s11906-010-0117-3. [DOI] [PubMed] [Google Scholar]
- 4.Schiffrin E. L. Vascular remodeling and endothelial function in hypertensive patients: effects of antihypertensive therapy. Scandinavian Cardiovascular Journal, Supplement. 1998;32(47):15–21. doi: 10.1080/140174398428009. [DOI] [PubMed] [Google Scholar]
- 5.Agabiti-Rosei E. Structural and functional changes of the microcirculation in hypertension: influence of pharmacological therapy. Drugs. 2003;63(1):19–29. doi: 10.2165/00003495-200363991-00003. [DOI] [PubMed] [Google Scholar]
- 6.Tadros R., Nattel S., Andrade J. G. Dronedarone: basic pharmacology and clinical use. Cardiac Electrophysiology Clinics. 2016;8(2):453–465. doi: 10.1016/j.ccep.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Cohen M., Boiangiu C. The management of patients with atrial fibrillation and dronedarone's place in therapy. Advances in Therapy. 2011;28(12):1059–1077. doi: 10.1007/s12325-011-0086-1. [DOI] [PubMed] [Google Scholar]
- 8.Hohnloser S. H., Crijns H. J. G. M., Eickels M. V., et al. Effect of dronedarone on cardiovascular events in atrial fibrillation. The New England Journal of Medicine. 2009;360(7):668–678. doi: 10.1056/nejmoa0803778. [DOI] [PubMed] [Google Scholar]
- 9.Lee E. J., Kim J. Evaluation of dronedarone as a therapeutic option for patients with atrial fibrillation. Journal of Clinical Pharmacy and Therapeutics. 2014;39(2):112–117. doi: 10.1111/jcpt.12128. [DOI] [PubMed] [Google Scholar]
- 10.Quintana-Villamandos B., Gómes De Diego J. J., Delgado-Martos M. J., et al. Dronedarone produces early regression of myocardial remodelling in structural heart disease. PLoS ONE. 2017;12(11) doi: 10.1371/journal.pone.0188442.e0188442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Escobar E. Hypertension and coronary heart disease. Journal of Human Hypertension. 2002;16(S1):S61–S63. doi: 10.1038/sj.jhh.1001345. [DOI] [PubMed] [Google Scholar]
- 12.Quintana-Villamandos B., Delgado-Martos M. J., Delgado-Baeza E. Adverse remodeling of the obtuse marginal artery in compensatory hypertrophied myocardium from spontaneously hypertensive rats. Cardiovascular Pathology. 2017;26:51–54. doi: 10.1016/j.carpath.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Yokoyama H., Averill D. B., Brosnihan K. B., Smith R. D., Schiffrin E. L., Ferrario C. M. Role of blood pressure reduction in prevention of cardiac and vascular hypertrophy. American Journal of Hypertension. 2005;18(7):922–929. doi: 10.1016/j.amjhyper.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 14.Gundersen H. J. G., Bendtsen T. F., Korbo L., et al. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS-Acta Pathologica, Microbiologica et Immunologica Scandinavica. 1988;96(5):379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- 15.Arnalich-Montiel A., González M. C., Delgado-Baeza E., et al. Short-term esmolol improves coronary artery remodeling in spontaneously hypertensive rats through increased nitric oxide bioavailability and superoxide dismutase activity. BioMed Research International. 2014;2014:9. doi: 10.1155/2014/531087.531087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giustarini D., Rossi R., Milzani A., Dalle-Donne I. Nitrite and nitrate measurement by griess reagent in human plasma: evaluation of interferences and standardization. Methods in Enzymology. 2008;440:361–380. doi: 10.1016/s0076-6879(07)00823-3. [DOI] [PubMed] [Google Scholar]
- 17.Miranda K. M., Espey M. G., Wink D. A. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide: Biology and Chemistry. 2001;5(1):62–71. doi: 10.1006/niox.2000.0319. [DOI] [PubMed] [Google Scholar]
- 18.Koprdova R., Cebova M., Kristek F. Long-term effect of losartan administration on blood pressure, heart and structure of coronary artery of young spontaneously hypertensive rats. Physiological Research. 2009;58(3):327–335. doi: 10.33549/physiolres.931528. [DOI] [PubMed] [Google Scholar]
- 19.Sharifi A. M., Li J. S., Endemann D., Schiffrin E. L. Effects of enalapril and amlodipine on small-artery structure and composition, and on endothelial dysfunction in spontaneously hypertensive rats. Journal of Hypertension. 1998;16(4):457–466. doi: 10.1097/00004872-199816040-00007. [DOI] [PubMed] [Google Scholar]
- 20.Neglia D., Fommei E., Varela-Carver A., et al. Perindopril and indapamide reverse coronary microvascular remodelling and improve flow in arterial hypertension. Journal of Hypertension. 2011;29(2):364–372. doi: 10.1097/HJH.0b013e328340a08e. [DOI] [PubMed] [Google Scholar]
- 21.Brilla C. G., Janicki J. S., Weber K. T. Impaired diastolic function and coronary reserve in genetic hypertension: role of interstitial fibrosis and medial thickening of intramyocardial coronary arteries. Circulation Research. 1991;69(1):107–115. doi: 10.1161/01.res.69.1.107. [DOI] [PubMed] [Google Scholar]
- 22.Quintana-Villamandos B., Arnalich-Montiel A., Arribas S., et al. Early regression of coronary artery remodeling with esmolol and DDAH/ADMA pathway in hypertensive rats. Hypertension Research. 2016;39(10):692–700. doi: 10.1038/hr.2016.57. [DOI] [PubMed] [Google Scholar]
- 23.Baroletti S., Catella J., Ehle M., Cheng J. W. Dronedarone: a review of characteristics and clinical data. Critical Pathways in Cardiology: A Journal of Evidence-Based Medicine. 2010;9(2):94–101. doi: 10.1097/HPC.0b013e3181dda1e6. [DOI] [PubMed] [Google Scholar]
- 24.Heijman J., Heusch G., Dobrev D. Pleiotropic effects of antiarrhythmic agents: dronedarone in the treatment of atrial fibrillation. Clinical Medicine Insights: Cardiology. 2013;7:127–140. doi: 10.4137/CMC.S8445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Renna N. F., Heras N. D. L., Miatello R. M. Pathophysiology of vascular remodeling in hypertension. International Journal of Hypertension. 2013;2013:7. doi: 10.1155/2013/808353.808353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Favero G., Paganelli C., Buffoli B., Rodella L. F., Rezzani R. Endothelium and its alterations in cardiovascular diseases: life style intervention. BioMed Research International. 2014;2014:28. doi: 10.1155/2014/801896.801896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feihl F., Liaudet L., Levy B. I., Waeber B. Hypertension and microvascular remodelling. Cardiovascular Research. 2008;78(2):274–285. doi: 10.1093/cvr/cvn022. [DOI] [PubMed] [Google Scholar]
- 28.Guiraudou P., Pucheu S. C., Gayraud R., et al. Involvement of nitric oxide in amiodarone- and dronedarone-induced coronary vasodilation in guinea pig heart. European Journal of Pharmacology. 2004;496(1-3):119–127. doi: 10.1016/j.ejphar.2004.05.046. [DOI] [PubMed] [Google Scholar]
- 29.Böger R. H. Asymmetric dimethylarginine (ADMA) modulates endothelial function - therapeutic implications. Vascular Medicine. 2003;8(3):149–151. doi: 10.1191/1358863x03vm501ed. [DOI] [PubMed] [Google Scholar]
- 30.Simko F., Simko J. The potential role of nitric oxide in the hypertrophic growth of the left ventricle. Physiological Research. 2000;49(1):37–46. [PubMed] [Google Scholar]
- 31.Rosa G. M., Bianco D., Parodi A., et al. Pharmacokinetic and pharmacodynamic profile of dronedarone, a new antiarrhythmic agent for the treatment of atrial fibrillation. Expert Opinion on Drug Metabolism & Toxicology. 2014;10(12):1751–1764. doi: 10.1517/17425255.2014.974551. [DOI] [PubMed] [Google Scholar]
- 32.Bukowska A., Hammwöhner M., Sixdorf A., et al. Dronedarone prevents microcirculatory abnormalities in the left ventricle during atrial tachypacing in pigs. British Journal of Pharmacology. 2012;166(3):964–980. doi: 10.1111/j.1476-5381.2011.01784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.