Abstract
Serotonergic neurotransmission is involved in the regulation of physiological functions such as mood, sleep, memory, and appetite. Within the serotonin transmitter system, both the postsynaptically located serotonin 2A (5-HT2A) receptor and the presynaptic serotonin transporter (SERT) are sensitive to chronic changes in cerebral 5-HT levels. Additionally, experimental studies suggest that alterations in either the 5-HT2A receptor or SERT level can affect the protein level of the counterpart. The aim of this study was to explore the covariation between cerebral 5-HT2A receptor and SERT in vivo in the same healthy human subjects. Fifty-six healthy human subjects with a mean age of 36 ± 19 years were investigated. The SERT binding was imaged with [11C]3-amino-4-(2-dimethylaminomethyl-phenylsulfanyl)-benzonitrile (DASB) and 5-HT2A receptor binding with [18F]altanserin using positron emission tomography. Within each individual, a regional intercorrelation for the various brain regions was seen with both markers, most notably for 5-HT2A receptor binding. An inverted U-shaped relationship between the 5-HT2A receptor and the SERT binding was identified. The observed regional intercorrelation for both the 5-HT2A receptor and the SERT cerebral binding suggests that, within the single individual, each marker has a set point adjusted through a common regulator. A quadratic relationship between the two markers is consistent with data from experimental studies of the effect on SERT and 5-HT2A receptor binding of chronic changes in 5-HT levels. That is, the observed association between the 5-HT2A receptor and SERT binding could be driven by the projection output from the raphe nuclei, but other explanations are also at hand.
Introduction
Central serotonin [5-hydroxytryptamine (5-HT)] function has a profound role in the normal brain function, in which it modulates mood, sex, appetite, sleep, memory, emotion, and endocrine responses. Serotonin is synthesized and released by neurons that have their cell bodies in the raphe nuclei in the brainstem, and the effect of its release is mediated through at least 15 presynaptic and postsynaptic receptors.
The presynaptically located serotonin transporter (SERT) is crucial for the regulation of 5-HT transmission because it controls the 5-HT availability at the site of the postsynaptic receptors by high-affinity reuptake of released 5-HT (Blakely et al., 1994). The SERT protein is expressed in high density in dorsal and median raphe nuclei, caudate, putamen, and thalamus but in relatively low densities in cortex (Varnas et al., 2004). More specifically, SERTs are localized on cell bodies in the raphe nucleus as well as on serotonergic axons and nerve terminals (Zhou et al., 1998, 2000).
The 5-HT neurotransmission is mediated through different postsynaptic receptors such as the 5-HT2A. This receptor is heterogeneously distributed with high receptor concentrations in several cortical areas (Pazos et al., 1987; Adams et al., 2004; Varnas et al., 2004) in which they are located primarily postsynaptically (and perisynaptically) (Miner et al., 2003). The cerebral 5-HT2A receptors are mainly expressed on glutamatergic pyramidal neurons, cholinergic neurons, and GABAergic interneurons (Morilak et al., 1994; Santana et al., 2004), and, in most areas of the forebrain, regional variations in their density correspond closely to the 5-HT axon innervation (Blue et al., 1988).
Cerebral 5-HT2A receptors and SERT protein levels both respond to chronic changes in 5-HT levels. In vitro autoradiography and homogenate binding experiments in rats suggest that a decrease in SERT binding levels occur after pharmacologically induced chronic extracellular 5-HT depletion in two (Rattray et al., 1996; Rothman et al., 2003) of three studies (no change seen in the study by Dewar et al., 1992). Chronically elevated extracellular 5-HT levels have usually been achieved by chronic treatment with the specific selective serotonin reuptake inhibitor (SSRI) compounds sertraline, citalopram, and paroxetine, and, in 10 (Kovachich et al., 1992; Pineyro et al., 1994; Benmansour et al., 1999; Horschitz et al., 2001; Benmansour et al., 2002; Gould et al., 2003, 2006; Rossi et al., 2008) of 15 experimental settings (no change reported in the studies by Graham et al., 1987; Kovachich et al., 1992; Cheetham et al., 1993; Gobbi et al., 1997; Gould et al., 2006), decreased SERT levels have been found. It can be argued, however, that chronic blockade of the SERT may lead to regulation of its expression and that the primary cause for the SERT downregulation is unrelated to 5-HT levels. However, support for a direct 5-HT effect comes from monoamine oxidase A knock-out mice. These mice have increased extracellular 5-HT levels and reduced SERT levels (Evrard et al., 2002). For the 5-HT2A receptors, moderate 5-HT depletion results in an inverse correlation between 5-HT and 5-HT2A receptor levels (Heal et al., 1985; Cahir et al., 2007), and chronically increased 5-HT levels after SSRI treatment results in a reduction in 5-HT2A receptor binding (Nelson et al., 1989; Cowen, 1990; Maj et al., 1996; Gunther et al., 2008; Licht et al., 2009). The latter observation has also been made in humans; chronic (Spigset and Mjorndal, 1997; Meyer et al., 2001) but not acute (Pinborg et al., 2004) SSRI treatment decreases cortical 5-HT2A receptor binding. Likewise, acute tryptophan depletion in humans, resulting in a transient reduction in cerebral 5-HT levels, does not alter cerebral SERT binding (Praschak-Rieder et al., 2004, 2005; Talbot et al., 2005). In the absence of competition effects, it is unlikely that acutely induced alterations in cerebral 5-HT levels alone would lead to immediate changes in SERT binding as assessed with in vivo imaging. Protein expression and trafficking are considered to be more long-term processes, and, accordingly, >5 d of 5-HT depletion is required to see a downregulation in SERT binding (Rattray et al., 1996).
The aim of this study was, for the first time, to investigate the in vivo relationship between SERT and 5-HT2A receptor binding in a large group of healthy human subjects using the selective positron emission tomography (PET) radioligands [11C]3-amino-4-(2-dimethylaminomethyl-phenylsulfanyl)-benzonitrile (DASB) and [18F]altanserin. Provided that in normal healthy individuals there is some interindividual variation in the dorsal raphe output and because both 5-HT2A receptors and SERT protein levels are sensitive to a common regulating factor in terms of chronic changes in cerebral 5-HT levels, it was the expectation that 5-HT2A receptors and SERT protein levels each show a within-subject interregional correlation and that the two markers are correlated. More specifically, we hypothesized, first, that a high degree of covariation between brain regions was present for both SERT and 5-HT2A receptors and, second, that the relationship between [18F]altanserin and [11C]DASB binding could be described as an inverted U-shape.
Materials and Methods
Participants and interviews.
Fifty-six adult human subjects were included in the study. The mean age was 36 ± 19 years (range, 20–82 years); 22 were females, and 34 were males. Written informed consent was obtained according to the Declaration of Helsinki II, and the study had been approved by the local ethics committee (KF 02-058/99, KF 12-122/99, KF 12-113/00, KF 12-152/01, KF 01-001/02, KF 11-061/03 and KF 12-142/03, KF 01-124/04, KF 01-156/04, KF 01 2006-20). All subjects were screened for medical history, had a normal neurological examination, and were lifetime naive for antipsychotics and antidepressants. None of the subjects had stimulant abuse or history of neurological or psychiatric disorders.
PET imaging.
All subjects were PET scanned with both [11C]DASB and [18F]altanserin on an 18-ring GE-Advance scanner (GE Healthcare) operating in three-dimensional acquisition mode producing 35 image slices with an interslice distance of 4.25 mm. The total axial field of view was 15.2 cm with an approximate in-plane resolution of down to 5 mm. Reconstruction, attenuation, and scatter correction procedures were conducted according to DeGrado et al. (1994). All subjects were scanned in a resting state.
For [18F]altanserin, subjects underwent a 40 min scan under tracer steady-state conditions as described by Pinborg et al. (2003). [18F]altanserin was produced with a mean specific activity of 58 ± 36 GBq/μmol (range, 10–182 GBq/μmol), and subjects received 297 ± 52 MBq (range, 204–437 MBq), corresponding to 7.4 ± 5.4 nmol [18F]altanserin. For [11C]DASB, a dynamic 90-min-long emission recording was initiated during intravenous injection during 12 s of 485 ± 86 MBq (range, 279–601 MBq) [11C]DASB, with specific activity of 29 ± 16 GBq/μmol (range, 9–82 GBq/μmol). The two PET scans obtained for each individual were acquired with an average gap between scans of 104 ± 200 d (range, 0–793 d).
The outcome parameter for [18F]altanserin binding was the binding potential of specific tracer binding (BPP). Cerebellum was used as a reference region because it represents nonspecific binding only (Pinborg et al., 2003). In steady state, BPP is defined as follows:
where CVOI and CND are steady-state mean count density in the volume of interest (VOI) and in the reference region, respectively, CP is the steady-state activity of nonmetabolized tracer in plasma, fP is the free fraction of radiotracer, Bmax is the density of receptor sites available for tracer binding, and Kd is the affinity constant of the radiotracer to the receptor. The outcome parameter of the [11C]DASB binding is the nondisplaceable binding potential, designated BPND. We used a modified reference tissue model designed specifically for quantification of [11C]DASB (MRTM/MRTM2) as described and evaluated by Ichise et al. (2003) using the software PMOD version 2.9 (PMOD Technologies). For further details about [18F]altanserin and [11C]DASB imaging and quantification, please see (Erritzoe et al., 2009) and (Frokjaer et al., 2009), respectively.
Magnetic resonance imaging.
Magnetic resonance imaging (MRI) of the brain was acquired on a Siemens Magnetom Trio 3 T MR scanner with an eight-channel head coil (In Vivo Clinical Research). High-resolution three-dimensional T1-weighted, sagittal, magnetization prepared rapid gradient echo scans of the head and T2-weighted scans of the whole brain were acquired. Both T1 and T2 images were corrected for spatial distortions because of nonlinearity in the gradient system if the scanner (Jovicich et al., 2006) using the Gradient Non-Linearity Distortion Correction software distributed by the Biomedical Informatics Research Network (http://www.nbirn.net). Subsequently, non-uniformity correction of the T1 images was performed with two iterations of the N3 program (Sled et al., 1998). The resulting T1 images were intensity normalized to a mean value of 1000. To enable extraction of the PET VOI signal from gray matter voxels only, MR images were segmented into gray matter, white matter, and CSF tissue classes using SPM2 (Welcome Department of Cognitive Neurology, University College London, UK) and the hidden Markov random field model as implemented in the SPM2 VBM toolbox (http://dbm.neuro.uni-jena.de/vbm/). This was done for the subcortical high-binding region and for neocortex but not for midbrain because the segmentation within this region is not considered reliable. Therefore, all midbrain voxels were included in the analysis. A brain mask based on the gradient nonlinearity corrected T2 image was used to exclude extracerebral tissue.
Volumes of interest.
VOIs were automatically delineated on each individual's transaxial MRI slices in a strictly user-independent manner (Svarer et al., 2005). With this approach, a template set of 10 MRIs is automatically coregistered to a new subject's MR image. The identified transformation parameters are used to define VOIs in the new subject MRI space, and, through the coregistering, these VOIs are transferred onto the PET images.
For both the SERT and 5-HT2A receptor, we computed an average binding potential for neocortex for each subject, and this served as the primary outcome. This region consisted of a volume-weighted average of eight cortical regions (orbitofrontal cortex, medial inferior frontal cortex, superior frontal cortex, superior temporal cortex, medial inferior temporal cortex, sensory motor cortex, parietal cortex, and occipital cortex). In addition, for SERT binding, the midbrain and a subcortical high-binding region consisting of a volume-weighted average of caudate, putamen, and thalamus were defined. The cerebellum, except the vermis, was defined and used for nonspecific binding measurements for both markers because this region has only negligible amounts of 5-HT2A receptors and SERT (Pazos et al., 1987; Cortés et al., 1988; Kish et al., 2005).
Statistics.
For both measurements, the correlation structure between regions was assessed, and graphical representations of the correlation matrices (Murdoch and Chow, 1996) were presented. Furthermore, a principal component analysis was performed as an explorative analysis of the inter-regional variation for both markers.
To explore the association between 5-HT2A receptor binding (BPP) in neocortex and SERT binding (BPND) in midbrain, caudate–putamen–thalamus (subcortical high-binding region), and neocortex, respectively, a nonparametric model using penalized regression splines with the smoothness automatically selected by a generalized cross-validation criterion (Wood, 2008) was first applied.
We aimed for a parametric model allowing for an easier interpretation. Based on a nonparametric regression analysis, the association between the two markers was subsequently modeled using up to an order of three polynomials of the 5-HT2A BPP. The subsequent model selection was based on Akaike's information criterion (AIC), for which all three regional relationships was in favor of a quadratic expression.
To address potential confounding effects of (1) a long time period between measurements of the two markers and (2) the relatively few included subjects with very high 5-HT2A receptor binding, the quadratic effect was estimated post hoc in two different subsamples: one subsample of subjects with a maximum 2 week interval between the two measurements, and one subsample in which subjects with high 5-HT2A BPP were omitted (the five largest 5-HT2A BPP values corresponding to a cutoff point at the 90% quantile).
Results
For the 56 healthy individuals, the average neocortical [11C]DASB BPND was 0.23 ± 0.05 (range, 0.13–0.37), midbrain BPND was 1.81 ± 0.22 (range, 1.34–2.27), and the subcortical BPND was 1.63 ± 0.19 (range, 1.27–2.07). The average neocortical [18F]altanserin BPP was 1.63 ± 0.58 (range, 0.69–3.56).
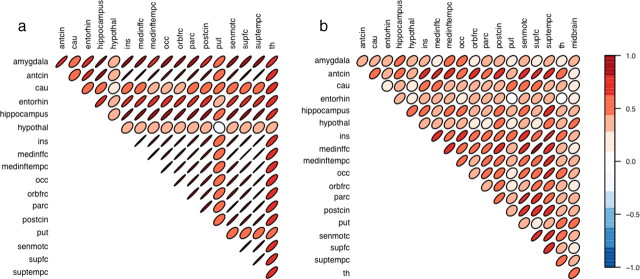
Graphical representations of the inter-regional correlation matrices (Murdoch and Chow, 1996) are shown in Figure 1. They reveal high pairwise correlations between regions. The inter-regional correlation was most pronounced in the 5-HT2A marker (Fig. 1a) in which the principal component analysis revealed a first principal component, with the distinctive features being global changes in the 5-HT2A level (i.e., a random intercept) and accounting for 80% of the total variation in the dataset.
Figure 1.
Region-wise correlation matrices for [18F]altanserin BPP (a) and [11C]DASB BPND (b) with each entry in the matrix being represented by a level curve from a bivariate normal distribution with the relevant correlation structure. The shape, direction, and the color of the ellipses designates the magnitude and the direction of the correlation.
The estimated shape of the curve with pointwise approximate 95% confidence limits from the nonparametric analysis are presented in Figure 2a–c. Based on this explorative analysis and in agreement with the previously mentioned experimental results, an inverted U-shaped relationship between SERT and 5-HT2A receptor binding seems reasonable. The estimated quadratic association between cortical 5-HT2A receptor binding and cortical and subcortical SERT binding are presented in Figure 2d–f together with the estimated curves in the subsample of individuals with <2 weeks separating the two measurements. The p values for the second-order term in the model were 0.0045, 0.0003, and 0.0836 for the association between the 5-HT2A receptor binding in neocortex and the SERT binding in the subcortical high-binding region, neocortex, and in midbrain, respectively. Although the relationship in the midbrain was only borderline significant, the AIC was still in favor of the quadratic model in this region. Including an interaction term in the main model with the grouping defined by more or less than 2 weeks between measurements, we did not find any significant differences in the shape of the curves between the two groups. The quadratic relation between the two markers was also seen for two of three of the investigated relations in the subset of individuals excluding the top 10% 5-HT2A BPP values, but the relationship was no longer statistically significant in midbrain SERT versus cortical 5-HT2A.
Figure 2.
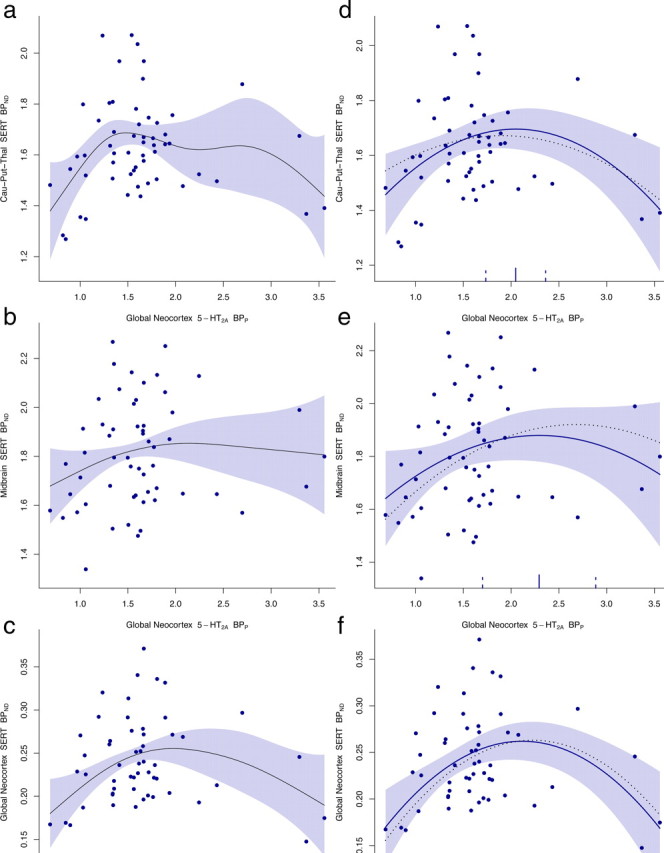
Left, The estimated regression functions from nonparametric analyses with approximate 95% pointwise confidence limits, in caudate–putamen–thalamus (a), midbrain (b), and neocortex (c). Right, Estimated quadratic relationship between neocortical 5-HT2A receptor BPP (x-axis) and SERT BPND (y-axis) with pointwise 95% confidence limits in caudate–putamen–thalamus (d), midbrain (e), and neocortex (f), respectively. A subsample of individuals with a maximum 2 week interval between the two measurements is represented with a dotted line. The estimated top points of the quadratic curves (d–f) with approximate 95% confidence limits are shown on the x-axis.
Discussion
This is the first study to directly compare the cerebral in vivo binding of the 5-HT2A receptor and the SERT in the same healthy individuals. In our sample of 56 healthy people, we found a high degree of covariation in the regional brain density of both [11C]DASB and [18F]altanserin, most notably for [18F]altanserin. We also confirmed our hypothesis of an inverted U-shaped association between cortical 5-HT2A receptor and both cortical and subcortical SERT binding.
A high degree of covariation in [18F]altanserin binding was found, accounting for 80% of the total variation in the dataset and was particularly pronounced across the cortical brain regions. We are unaware of other studies having addressed statistically the issue of covariation in receptor/transporter binding between regions within the same individual. When examining global effects, our finding supports the use of a single outcome parameter, in this case the volume-weighted average cortical 5-HT2A receptor BPP. We noted that, for the subcortical brain structures caudate nucleus, putamen, hypothalamus, and thalamus, the intercorrelation in BPP was somewhat smaller, although a tendency for an intercorrelation was almost invariably seen. In the subcortical structures, the density of 5-HT2A receptors is low. Therefore, it should be considered whether the lower covariation in these regions is caused by increased noise levels. There is for [11C]DASB, however, less covariation in the subcortical regions than in cortex, despite a low cortical SERT binding. One possible explanation is that receptors and transporters in brain regions receiving projections from the different subgroups of serotonergic neurons in the midbrain vary in their response to serotonergic activity. It is known that neurons in the dorsomedial and the ventral, paramedian part of the dorsal raphe nucleus account for the vast majority of mesostriatal 5-HT projections (Lowry, 2002). It might be that the regulation of receptors/transporters of these neurons and those in their projection areas is independent from the regulation of markers on other projections.
Although often implicitly inferred, 5-HT2A binding levels are occasionally interpreted as reflecting cerebral 5-HT levels, with high 5-HT levels resulting in lower 5-HT2A receptor binding and vice versa (Adams et al., 2005; Haugbøl et al., 2007; Meyer, 2007). This interpretation is in accordance with our observation of a high interregional correlation of 5-HT2A receptor binding, which is suggestive of a common regulator, presumably the raphe serotonergic output. Given that the 5-HT2A receptor binding is accepted as a surrogate marker of cerebral 5-HT levels and that SERT binding adjusts to 5-HT levels in a manner suggested from some, although not all, experimental studies (Graham et al., 1987; Kovachich et al., 1992; Cheetham et al., 1993; Pineyro et al., 1994; Rattray et al., 1996; Gobbi et al., 1997; Benmansour et al., 1999; Horschitz et al., 2001; Benmansour et al., 2002; Evrard et al., 2002; Gould et al., 2003, 2006; Rothman et al., 2003), our observation of an inverted U-shaped relation between 5-HT2A receptor and SERT binding may be the result of inter-individual differences in cerebral baseline 5-HT levels.
So far, only one study has examined the relationship between postsynaptic 5-HT receptor and SERT binding in the human brain previously. In 12 healthy individuals, Lundberg et al. (2007) investigated both the 5-HT1A receptor binding and SERT binding with PET. They found a positive correlation between the two markers in the raphe nuclei and in the hippocampus but not in the frontal cortex. The positive correlation in raphe is not unexpected because both markers exhibit proportionality to the number of serotonergic neurons. Based on animal studies (Le Poul et al., 1995; Cahir et al., 2007; Riad et al., 2008), however, the anticipation would be an inverted U-shaped relationship between 5-HT1A receptor and SERT binding in hippocampus and frontal cortex, because 5-HT1A receptor levels seem to be relatively resistant to changes in cerebral 5-HT levels. The authors did not attempt to model an inverted U-formed relationship, and the relatively low sample size may also have been prohibitive for a more extensive analysis.
A potential confound with our study is that, in some individuals, there was an interval of more than 2 years between the two PET acquisitions. We have, however, in a longitudinal study shown that 5-HT2A receptor binding remains relatively stable over 2 years (Marner et al., 2009). We also ensured that the outcome was the same in the subsample of the cohort that was scanned within 2 weeks and in the full sample. Because we had relatively few individuals with very high 5-HT2A receptor binding (Fig. 1), which could have driven the association with SERT, we conducted a post hoc analysis in which we excluded the subjects with the 10% largest BPP values. The quadratic relationship with 5-HT2A BPP was confirmed for the caudate–putamen–thalamus as well as for the cortical SERT region, whereas the relation was not significant between midbrain SERT BPND and cortical 5-HT2A BPP in this subsample. The degree of noise in the latter region might have accounted for this difference.
Apart from a compensatory regulation of the serotonergic markers secondary to differences in cerebral 5-HT levels, alternative interpretations should also be considered. Instead of cerebral 5-HT level being the primary regulator of the two markers, SERT or 5-HT2A receptors may also both independently regulate the levels of the other marker.
Thus, primary differences in the cerebral SERT levels, caused by either genetic or environmental factors, do influence cerebral 5-HT levels, and this could then in turn regulate 5-HT2A receptor protein levels. By its selective reuptake of 5-HT, SERT is the key regulator of the synaptic levels of this transmitter. For example, the short allele of the SCL6A4 serotonin transporter-linked polymorphic region (5-HTTLPR) polymorphism confers decreased SERT expression (Lesch et al., 1996; Little et al., 1998; Heinz et al., 2000) and binding, especially when taking the triallelic variation in the 5-HTTLPR into account (Praschak-Rieder et al., 2007; Reimold et al., 2007), although conflicting data exist (Greenberg et al., 1999; Mann et al., 2000; Preuss et al., 2000; van Dyck et al., 2004; Parsey et al., 2006). Currently, there are no published studies available to address whether the SCL6A4 5-HTTLPR polymorphism or other functional SERT polymorphisms have any impact on cerebral 5-HT2A receptor binding in humans. A number of studies do, however, investigate 5-HT2A receptor levels in transgenic mice that either underexpress or overexpress SERT. SERT knock-out mice have a sixfold increase in extracellular 5-HT (Bengel et al., 1998), and, in these mice, a 42% decrease is seen in the cortical but not in the striatal 5-HT2A receptor density (Rioux et al., 1999). Consistent with this observation, pharmacological stimulation with a 5-HT2A receptor agonist was associated with a marked attenuation of the head-twitch response and in phospholipase A2 signaling in SERT knock-out compared with SERT overexpressing mice (Qu et al., 2005). Similarly, in SERT overexpressing mice with decreased extracellular 5-HT levels, increased functional sensitivity of the 5-HT2A receptor was found; 5-HT2A receptor agonist stimulation induced a greater increase in both c-Fos and Arc mRNA expression in cortical brain regions and more head twitches compared with wild-type mice (Jennings et al., 2008), although the authors were unable to demonstrate significantly increased 5-HT2A receptor binding. Based on these studies, it is not possible to assess the independent role of cerebral SERT levels and function versus that of 5-HT levels on cortical 5-HT2A receptor levels, but we consider it more likely that the alterations in 5-HT2A receptor levels and function are generated through changes in 5-HT levels.
Last, the possibility of the 5-HT2A receptor as the primary site of regulation should also be considered: stimulation of this receptor causes a decrease in the firing of raphe nuclei 5-HT neurons (Boothman et al., 2003) through a negative feedback possibly mediated by glutamatergic projections from cortex to raphe GABA interneurons (Sharp et al., 2007). A high level of 5-HT2A receptor activity could therefore potentially decrease 5-HT neuron firing from raphe nuclei and thus, through decreased 5-HT release, and lower subcortical and cortical SERT levels, but it does not fully explain the observed inverted U-formed association.
In conclusion, the observed regional intercorrelation for both 5-HT2A receptor and SERT cerebral binding suggests that, within the single individual, each marker has a set point adjusted through a common regulator. We suggest that this regulator could be extracellular 5-HT levels and, accordingly, that 5-HT levels vary between individuals, depending on genetic and/or environmental influence.
Footnotes
The study was sponsored by The Danish Medical Research Council, H:S (Copenhagen Hospital Cooperation) Research Council, Copenhagen University Hospital, and The Lundbeck Foundation.
The authors declare no competing financial interests.
References
- Adams KH, Pinborg LH, Svarer C, Hasselbalch SG, Holm S, Haugbøl S, Madsen K, Frøkjaer V, Martiny L, Paulson OB, Knudsen GM. A database of [18F]-altanserin binding to 5-HT2A receptors in normal volunteers: normative data and relationship to physiological and demographic variables. Neuroimage. 2004;21:1105–1113. doi: 10.1016/j.neuroimage.2003.10.046. [DOI] [PubMed] [Google Scholar]
- Adams KH, Hansen ES, Pinborg LH, Hasselbalch SG, Svarer C, Holm S, Bolwig TG, Knudsen GM. Patients with obsessive-compulsive disorder have increased 5-HT2A receptor binding in the caudate nuclei. Int J Neuropsychopharmacol. 2005;8:391–401. doi: 10.1017/S1461145705005055. [DOI] [PubMed] [Google Scholar]
- Bengel D, Murphy DL, Andrews AM, Wichems CH, Feltner D, Heils A, Mössner R, Westphal H, Lesch KP. Altered brain serotonin homeostasis and locomotor insensitivity to 3, 4-methylenedioxymethamphetamine (“Ecstasy”) in serotonin transporter-deficient mice. Mol Pharmacol. 1998;53:649–655. doi: 10.1124/mol.53.4.649. [DOI] [PubMed] [Google Scholar]
- Benmansour S, Cecchi M, Morilak DA, Gerhardt GA, Javors MA, Gould GG, Frazer A. Effects of chronic antidepressant treatments on serotonin transporter function, density, and mRNA level. J Neurosci. 1999;19:10494–10501. doi: 10.1523/JNEUROSCI.19-23-10494.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benmansour S, Owens WA, Cecchi M, Morilak DA, Frazer A. Serotonin clearance in vivo is altered to a greater extent by antidepressant-induced downregulation of the serotonin transporter than by acute blockade of this transporter. J Neurosci. 2002;22:6766–6772. doi: 10.1523/JNEUROSCI.22-15-06766.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakely RD, De Felice LJ, Hartzell HC. Molecular physiology of norepinephrine and serotonin transporters. J Exp Biol. 1994;196:263–281. doi: 10.1242/jeb.196.1.263. [DOI] [PubMed] [Google Scholar]
- Blue ME, Yagaloff KA, Mamounas LA, Hartig PR, Molliver ME. Correspondence between 5-HT2 receptors and serotonergic axons in rat neocortex. Brain Res. 1988;453:315–328. doi: 10.1016/0006-8993(88)90172-2. [DOI] [PubMed] [Google Scholar]
- Boothman LJ, Allers KA, Rasmussen K, Sharp T. Evidence that central 5-HT2A and 5-HT2B/C receptors regulate 5-HT cell firing in the dorsal raphe nucleus of the anaesthetised rat. Br J Pharmacol. 2003;139:998–1004. doi: 10.1038/sj.bjp.0705328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahir M, Ardis T, Reynolds GP, Cooper SJ. Acute and chronic tryptophan depletion differentially regulate central 5-HT1A and 5-HT 2A receptor binding in the rat. Psychopharmacology (Berl) 2007;190:497–506. doi: 10.1007/s00213-006-0635-5. [DOI] [PubMed] [Google Scholar]
- Cheetham SC, Viggers JA, Slater NA, Heal DJ, Buckett WR. [3H]paroxetine binding in rat frontal cortex strongly correlates with [3H]5-HT uptake: effect of administration of various antidepressant treatments. Neuropharmacology. 1993;32:737–743. doi: 10.1016/0028-3908(93)90181-2. [DOI] [PubMed] [Google Scholar]
- Cortés R, Soriano E, Pazos A, Probst A, Palacios JM. Autoradiography of antidepressant binding sites in the human brain: localization using [3H]imipramine and [3H]paroxetine. Neuroscience. 1988;27:473–496. doi: 10.1016/0306-4522(88)90282-5. [DOI] [PubMed] [Google Scholar]
- Cowen PJ. A role for 5-HT in the action of antidepressant drugs. Pharmacol Ther. 1990;46:43–51. doi: 10.1016/0163-7258(90)90033-x. [DOI] [PubMed] [Google Scholar]
- DeGrado TR, Turkington TG, Williams JJ, Stearns CW, Hoffman JM, Coleman RE. Performance characteristics of a whole-body PET scanner. J Nucl Med. 1994;35:1398–1406. [PubMed] [Google Scholar]
- Dewar KM, Grondin L, Carli M, Lima L, Reader TA. [3H]paroxetine binding and serotonin content of rat cortical areas, hippocampus, neostriatum, ventral mesencephalic tegmentum, and midbrain raphe nuclei region following p-chlorophenylalanine and p-chloroamphetamine treatment. J Neurochem. 1992;58:250–257. doi: 10.1111/j.1471-4159.1992.tb09303.x. [DOI] [PubMed] [Google Scholar]
- Erritzoe D, Frokjaer VG, Haugbol S, Marner L, Svarer C, Holst K, Baaré WF, Rasmussen PM, Madsen J, Paulson OB, Knudsen GM. Brain serotonin 2A receptor binding: relations to body mass index, tobacco and alcohol use. Neuroimage. 2009;46:23–30. doi: 10.1016/j.neuroimage.2009.01.050. [DOI] [PubMed] [Google Scholar]
- Evrard A, Malagié I, Laporte AM, Boni C, Hanoun N, Trillat AC, Seif I, De Maeyer E, Gardier A, Hamon M, Adrien J. Altered regulation of the 5-HT system in the brain of MAO-A knock-out mice. Eur J Neurosci. 2002;15:841–851. doi: 10.1046/j.1460-9568.2002.01917.x. [DOI] [PubMed] [Google Scholar]
- Frokjaer VG, Vinberg M, Erritzoe D, Svarer C, Baaré W, Budtz-Joergensen E, Madsen K, Madsen J, Kessing LV, Knudsen GM. High familial risk for mood disorder is associated with low dorsolateral prefrontal cortex serotonin transporter binding. Neuroimage. 2009;46:360–366. doi: 10.1016/j.neuroimage.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Gobbi M, Crespi D, Foddi MC, Fracasso C, Mancini L, Parotti L, Mennini T. Effects of chronic treatment with fluoxetine and citalopram on 5-HT uptake, 5-HT1B autoreceptors, 5-HT3 and 5-HT4 receptors in rats. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:22–28. doi: 10.1007/pl00005024. [DOI] [PubMed] [Google Scholar]
- Gould GG, Pardon MC, Morilak DA, Frazer A. Regulatory effects of reboxetine treatment alone, or following paroxetine treatment, on brain noradrenergic and serotonergic systems. Neuropsychopharmacology. 2003;28:1633–1641. doi: 10.1038/sj.npp.1300236. [DOI] [PubMed] [Google Scholar]
- Gould GG, Altamirano AV, Javors MA, Frazer A. A comparison of the chronic treatment effects of venlafaxine and other antidepressants on serotonin and norepinephrine transporters. Biol Psychiatry. 2006;59:408–414. doi: 10.1016/j.biopsych.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Graham D, Tahraoui L, Langer SZ. Effect of chronic treatment with selective monoamine oxidase inhibitors and specific 5-hydroxytryptamine uptake inhibitors on [3H]paroxetine binding to cerebral cortical membranes of the rat. Neuropharmacology. 1987;26:1087–1092. doi: 10.1016/0028-3908(87)90252-8. [DOI] [PubMed] [Google Scholar]
- Greenberg BD, Tolliver TJ, Huang SJ, Li Q, Bengel D, Murphy DL. Genetic variation in the serotonin transporter promoter region affects serotonin uptake in human blood platelets. Am J Med Genet. 1999;88:83–87. [PubMed] [Google Scholar]
- Günther L, Liebscher S, Jähkel M, Oehler J. Effects of chronic citalopram treatment on 5-HT1A and 5-HT2A receptors in group- and isolation-housed mice. Eur J Pharmacol. 2008;593:49–61. doi: 10.1016/j.ejphar.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Haugbøl S, Pinborg LH, Regeur L, Hansen ES, Bolwig TG, Nielsen FA, Svarer C, Skovgaard LT, Knudsen GM. Cerebral 5-HT2A receptor binding is increased in patients with Tourette's syndrome. Int J Neuropsychopharmacol. 2007;10:245–252. doi: 10.1017/S1461145706006559. [DOI] [PubMed] [Google Scholar]
- Heal DJ, Philpot J, Molyneux SG, Metz A. Intracerebroventricular administration of 5,7-dihydroxytryptamine to mice increases both head-twitch response and the number of cortical 5-HT2 receptors. Neuropharmacology. 1985;24:1201–1205. doi: 10.1016/0028-3908(85)90155-8. [DOI] [PubMed] [Google Scholar]
- Heinz A, Jones DW, Mazzanti C, Goldman D, Ragan P, Hommer D, Linnoila M, Weinberger DR. A relationship between serotonin transporter genotype and in vivo protein expression and alcohol neurotoxicity. Biol Psychiatry. 2000;47:643–649. doi: 10.1016/s0006-3223(99)00171-7. [DOI] [PubMed] [Google Scholar]
- Horschitz S, Hummerich R, Schloss P. Down-regulation of the rat serotonin transporter upon exposure to a selective serotonin reuptake inhibitor. Neuroreport. 2001;12:2181–2184. doi: 10.1097/00001756-200107200-00027. [DOI] [PubMed] [Google Scholar]
- Ichise M, Liow JS, Lu JQ, Takano A, Model K, Toyama H, Suhara T, Suzuki K, Innis RB, Carson RE. Linearized reference tissue parametric imaging methods: application to [11C]DASB positron emission tomography studies of the serotonin transporter in human brain. J Cereb Blood Flow Metab. 2003;23:1096–1112. doi: 10.1097/01.WCB.0000085441.37552.CA. [DOI] [PubMed] [Google Scholar]
- Jennings KA, Sheward WJ, Harmar AJ, Sharp T. Evidence that genetic variation in 5-HT transporter expression is linked to changes in 5-HT2A receptor function. Neuropharmacology. 2008;54:776–783. doi: 10.1016/j.neuropharm.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Greve D, Haley E, van der Kouwe A, Gollub R, Kennedy D, Schmitt F, Brown G, Macfall J, Fischl B, Dale A. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage. 2006;30:436–443. doi: 10.1016/j.neuroimage.2005.09.046. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Furukawa Y, Chang LJ, Tong J, Ginovart N, Wilson A, Houle S, Meyer JH. Regional distribution of serotonin transporter protein in postmortem human brain: is the cerebellum a SERT-free brain region? Nucl Med Biol. 2005;32:123–128. doi: 10.1016/j.nucmedbio.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Kovachich GB, Aronson CE, Brunswick DJ. Effect of repeated administration of antidepressants on serotonin uptake sites in limbic and neocortical structures of rat brain determined by quantitative autoradiography. Neuropsychopharmacology. 1992;7:317–324. [PubMed] [Google Scholar]
- Le Poul E, Laaris N, Doucet E, Laporte AM, Hamon M, Lanfumey L. Early desensitization of somato-dendritic 5-HT1A autoreceptors in rats treated with fluoxetine or paroxetine. Naunyn Schmiedebergs Arch Pharmacol. 1995;352:141–148. doi: 10.1007/BF00176767. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Müller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Licht CL, Marcussen AB, Wegener G, Overstreet DH, Aznar S, Knudsen GM. The brain 5-HT4 receptor binding is down-regulated in the Flinders Sensitive Line depression model and in response to paroxetine administration. J Neurochem. 2009;109:1363–1374. doi: 10.1111/j.1471-4159.2009.06050.x. [DOI] [PubMed] [Google Scholar]
- Little KY, McLaughlin DP, Zhang L, Livermore CS, Dalack GW, McFinton PR, DelProposto ZS, Hill E, Cassin BJ, Watson SJ, Cook EH. Cocaine, ethanol, and genotype effects on human midbrain serotonin transporter binding sites and mRNA levels. Am J Psychiatry. 1998;155:207–213. doi: 10.1176/ajp.155.2.207. [DOI] [PubMed] [Google Scholar]
- Lowry CA. Functional subsets of serotonergic neurones: implications for control of the hypothalamic-pituitary-adrenal axis. J Neuroendocrinol. 2002;14:911–923. doi: 10.1046/j.1365-2826.2002.00861.x. [DOI] [PubMed] [Google Scholar]
- Lundberg J, Borg J, Halldin C, Farde L. A PET study on regional coexpression of 5-HT1A receptors and 5-HTT in the human brain. Psychopharmacology (Berl) 2007;195:425–433. doi: 10.1007/s00213-007-0928-3. [DOI] [PubMed] [Google Scholar]
- Maj J, Bijak M, Dziedzicka-Wasylewska M, Rogoz R, Rogoz Z, Skuza G, Tokarski T. The effects of paroxetine given repeatedly on the 5-HT receptor subpopulations in the rat brain. Psychopharmacology (Berl) 1996;127:73–82. doi: 10.1007/BF02805977. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Huang YY, Underwood MD, Kassir SA, Oppenheim S, Kelly TM, Dwork AJ, Arango V. A serotonin transporter gene promoter polymorphism (5-HTTLPR) and prefrontal cortical binding in major depression and suicide. Arch Gen Psychiatry. 2000;57:729–738. doi: 10.1001/archpsyc.57.8.729. [DOI] [PubMed] [Google Scholar]
- Marner L, Knudsen GM, Haugbøl S, Holm S, Baaré W, Hasselbalch SG. Longitudinal assessment of cerebral 5-HT2A receptors in healthy elderly volunteers: an [18F]-altanserin PET study. Eur J Nucl Med Mol Imaging. 2009;36:287–293. doi: 10.1007/s00259-008-0945-4. [DOI] [PubMed] [Google Scholar]
- Meyer JH. Imaging the serotonin transporter during major depressive disorder and antidepressant treatment. J Psychiatry Neurosci. 2007;32:86–102. [PMC free article] [PubMed] [Google Scholar]
- Meyer JH, Kapur S, Eisfeld B, Brown GM, Houle S, DaSilva J, Wilson AA, Rafi-Tari S, Mayberg HS, Kennedy SH. The effect of paroxetine on 5-HT2A receptors in depression: an [18F]setoperone PET imaging study. Am J Psychiatry. 2001;158:78–85. doi: 10.1176/appi.ajp.158.1.78. [DOI] [PubMed] [Google Scholar]
- Miner LA, Backstrom JR, Sanders-Bush E, Sesack SR. Ultrastructural localization of serotonin2A receptors in the middle layers of the rat prelimbic prefrontal cortex. Neuroscience. 2003;116:107–117. doi: 10.1016/s0306-4522(02)00580-8. [DOI] [PubMed] [Google Scholar]
- Morilak DA, Somogyi P, Lujan-Miras R, Ciaranello RD. Neurons expressing 5-HT2 receptors in the rat brain: neurochemical identification of cell types by immunocytochemistry. Neuropsychopharmacology. 1994;11:157–166. doi: 10.1038/sj.npp.1380102. [DOI] [PubMed] [Google Scholar]
- Murdoch DJ, Chow ED. A graphical display of large correlation matrices. Am Stat. 1996;50:178–180. [Google Scholar]
- Nelson DR, Thomas DR, Johnson AM. Pharmacological effects of paroxetine after repeated administration to animals. Acta Psychiatr Scand Suppl. 1989;350:21–23. doi: 10.1111/j.1600-0447.1989.tb07162.x. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Hastings RS, Oquendo MA, Hu X, Goldman D, Huang YY, Simpson N, Arcement J, Huang Y, Ogden RT, Van Heertum RL, Arango V, Mann JJ. Effect of a triallelic functional polymorphism of the serotonin-transporter-linked promoter region on expression of serotonin transporter in the human brain. Am J Psychiatry. 2006;163:48–51. doi: 10.1176/appi.ajp.163.1.48. [DOI] [PubMed] [Google Scholar]
- Pazos A, Probst A, Palacios JM. Serotonin receptors in the human brain-IV. Autoradiography mapping of serotonin-2 receptors. Neuroscience. 1987;21:123–139. doi: 10.1016/0306-4522(87)90327-7. [DOI] [PubMed] [Google Scholar]
- Pinborg LH, Adams KH, Svarer C, Holm S, Hasselbalch SG, Haugbøl S, Madsen J, Knudsen GM. Quantification of 5-HT2A receptors in the human brain using [18F]altanserin-PET and the bolus/infusion approach. J Cereb Blood Flow Metab. 2003;23:985–996. doi: 10.1097/01.WCB.0000074092.59115.23. [DOI] [PubMed] [Google Scholar]
- Pinborg LH, Adams KH, Yndgaard S, Hasselbalch SG, Holm S, Kristiansen H, Paulson OB, Knudsen GM. [18F]altanserin binding to human 5HT2A receptors is unaltered after citalopram and pindolol challenge. J Cereb Blood Flow Metab. 2004;24:1037–1045. doi: 10.1097/01.WCB.0000126233.08565.E7. [DOI] [PubMed] [Google Scholar]
- Piñeyro G, Blier P, Dennis T, de Montigny C. Desensitization of the neuronal 5-HT carrier following its long-term blockade. J Neurosci. 1994;14:3036–3047. doi: 10.1523/JNEUROSCI.14-05-03036.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praschak-Rieder N, Hussey D, Wilson AA, Carella A, Lee M, Dunn E, Willeit M, Bagby RM, Houle S, Meyer JH. Tryptophan depletion and serotonin loss in selective serotonin reuptake inhibitor-treated depression: an [18F] MPPF positron emission tomography study. Biol Psychiatry. 2004;56:587–591. doi: 10.1016/j.biopsych.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Praschak-Rieder N, Wilson AA, Hussey D, Carella A, Wei C, Ginovart N, Schwarz MJ, Zach J, Houle S, Meyer JH. Effects of tryptophan depletion on the serotonin transporter in healthy humans. Biol Psychiatry. 2005;58:825–830. doi: 10.1016/j.biopsych.2005.04.038. [DOI] [PubMed] [Google Scholar]
- Praschak-Rieder N, Kennedy J, Wilson AA, Hussey D, Boovariwala A, Willeit M, Ginovart N, Tharmalingam S, Masellis M, Houle S, Meyer JH. Novel 5-HTTLPR allele associates with higher serotonin transporter binding in putamen: a [11C] DASB positron emission tomography study. Biol Psychiatry. 2007;62:327–331. doi: 10.1016/j.biopsych.2006.09.022. [DOI] [PubMed] [Google Scholar]
- Preuss UW, Soyka M, Bahlmann M, Wenzel K, Behrens S, de Jonge S, Krüger M, Bondy B. Serotonin transporter gene regulatory region polymorphism (5-HTTLPR), [3H]paroxetine binding in healthy control subjects and alcohol-dependent patients and their relationships to impulsivity. Psychiatry Res. 2000;96:51–61. doi: 10.1016/s0165-1781(00)00190-6. [DOI] [PubMed] [Google Scholar]
- Qu Y, Villacreses N, Murphy DL, Rapoport SI. 5-HT2A/2C receptor signaling via phospholipase A2 and arachidonic acid is attenuated in mice lacking the serotonin reuptake transporter. Psychopharmacology (Berl) 2005;180:12–20. doi: 10.1007/s00213-005-2231-5. [DOI] [PubMed] [Google Scholar]
- Rattray M, Baldessari S, Gobbi M, Mennini T, Samanin R, Bendotti C. p-Chlorphenylalanine changes serotonin transporter mRNA levels and expression of the gene product. J Neurochem. 1996;67:463–472. doi: 10.1046/j.1471-4159.1996.67020463.x. [DOI] [PubMed] [Google Scholar]
- Reimold M, Smolka MN, Schumann G, Zimmer A, Wrase J, Mann K, Hu XZ, Goldman D, Reischl G, Solbach C, Machulla HJ, Bares R, Heinz A. Midbrain serotonin transporter binding potential measured with [11C]DASB is affected by serotonin transporter genotype. J Neural Transm. 2007;114:635–639. doi: 10.1007/s00702-006-0609-0. [DOI] [PubMed] [Google Scholar]
- Riad M, Rbah L, Verdurand M, Aznavour N, Zimmer L, Descarries L. Unchanged density of 5-HT(1A) autoreceptors on the plasma membrane of nucleus raphe dorsalis neurons in rats chronically treated with fluoxetine. Neuroscience. 2008;151:692–700. doi: 10.1016/j.neuroscience.2007.11.024. [DOI] [PubMed] [Google Scholar]
- Rioux A, Fabre V, Lesch KP, Moessner R, Murphy DL, Lanfumey L, Hamon M, Martres MP. Adaptive changes of serotonin 5-HT2A receptors in mice lacking the serotonin transporter. Neurosci Lett. 1999;262:113–116. doi: 10.1016/s0304-3940(99)00049-x. [DOI] [PubMed] [Google Scholar]
- Rossi DV, Burke TF, McCasland M, Hensler JG. Serotonin-1A receptor function in the dorsal raphe nucleus following chronic administration of the selective serotonin reuptake inhibitor sertraline. J Neurochem. 2008;105:1091–1099. doi: 10.1111/j.1471-4159.2007.05201.x. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Jayanthi S, Wang X, Dersch CM, Cadet JL, Prisinzano T, Rice KC, Baumann MH. High-dose fenfluramine administration decreases serotonin transporter binding, but not serotonin transporter protein levels, in rat forebrain. Synapse. 2003;50:233–239. doi: 10.1002/syn.10266. [DOI] [PubMed] [Google Scholar]
- Santana N, Bortolozzi A, Serrats J, Mengod G, Artigas F. Expression of serotonin1A and serotonin2A receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cereb Cortex. 2004;14:1100–1109. doi: 10.1093/cercor/bhh070. [DOI] [PubMed] [Google Scholar]
- Sharp T, Boothman L, Raley J, Quérée P. Important messages in the “post”: recent discoveries in 5-HT neurone feedback control. Trends Pharmacol Sci. 2007;28:629–636. doi: 10.1016/j.tips.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Spigset O, Mjorndal T. Effect of fluvoxamine on platelet 5-HT2A receptors as studied by [3H]lysergic acid diethylamide ([3H]LSD) binding in healthy volunteers. Psychopharmacology (Berl) 1997;133:39–42. doi: 10.1007/s002130050368. [DOI] [PubMed] [Google Scholar]
- Svarer C, Madsen K, Hasselbalch SG, Pinborg LH, Haugbøl S, Frøkjaer VG, Holm S, Paulson OB, Knudsen GM. MR-based automatic delineation of volumes of interest in human brain PET images using probability maps. Neuroimage. 2005;24:969–979. doi: 10.1016/j.neuroimage.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Talbot PS, Frankle WG, Hwang DR, Huang Y, Suckow RF, Slifstein M, Abi-Dargham A, Laruelle M. Effects of reduced endogenous 5-HT on the in vivo binding of the serotonin transporter radioligand 11C-DASB in healthy humans. Synapse. 2005;55:164–175. doi: 10.1002/syn.20105. [DOI] [PubMed] [Google Scholar]
- van Dyck CH, Malison RT, Staley JK, Jacobsen LK, Seibyl JP, Laruelle M, Baldwin RM, Innis RB, Gelernter J. Central serotonin transporter availability measured with [123I]beta-CIT SPECT in relation to serotonin transporter genotype. Am J Psychiatry. 2004;161:525–531. doi: 10.1176/appi.ajp.161.3.525. [DOI] [PubMed] [Google Scholar]
- Varnäs K, Halldin C, Hall H. Autoradiographic distribution of serotonin transporters and receptor subtypes in human brain. Hum Brain Mapp. 2004;22:246–260. doi: 10.1002/hbm.20035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SN. Fast stable direct fitting and smoothness selection for generalized additive models. J R Stat Soc B. 2008;70:495–518. [Google Scholar]
- Zhou FC, Tao-Cheng JH, Segu L, Patel T, Wang Y. Serotonin transporters are located on the axons beyond the synaptic junctions: anatomical and functional evidence. Brain Res. 1998;805:241–254. doi: 10.1016/s0006-8993(98)00691-x. [DOI] [PubMed] [Google Scholar]
- Zhou FC, Sari Y, Zhang JK. Expression of serotonin transporter protein in developing rat brain. Brain Res Dev Brain Res. 2000;119:33–45. doi: 10.1016/s0165-3806(99)00152-2. [DOI] [PubMed] [Google Scholar]