Abstract
Activation of Notch signaling induces the expression of transcriptional repressor genes such as Hes1, leading to repression of proneural gene expression and maintenance of neural stem/progenitor cells. However, a requirement for Notch signaling in the telencephalon was not clear, because in Hes1;Hes3;Hes5 triple-mutant mice, neural stem/progenitor cells are depleted in most regions of the developing CNS, but not in the telencephalon. Here, we investigated a role for Notch signaling in the telencephalon by generating tamoxifen-inducible conditional knock-out mice that lack Rbpj, an intracellular signal mediator of all Notch receptors. When Rbpj was deleted in the embryonic brain, almost all telencephalic neural stem/progenitor cells prematurely differentiated into neurons and were depleted. When Rbpj was deleted in the adult brain, all neural stem cells differentiated into transit-amplifying cells and neurons. As a result, neurogenesis increased transiently, but 3 months later all neural stem cells were depleted and neurogenesis was totally lost. These results indicated an absolute requirement of Notch signaling for the maintenance of neural stem cells and a proper control of neurogenesis in both embryonic and adult brains.
Introduction
Neurogenesis occurs in both developing and adult brains (Gage, 2000; Temple, 2001; Ming and Song, 2005; Imayoshi et al., 2008b, 2009; Kriegstein and Alvarez-Buylla, 2009). In the embryonic brain, radial glial cells sequentially give rise to distinct types of neurons or basal progenitors (Rakic, 1995; Haubensak et al., 2004; Miyata et al., 2004; Noctor et al., 2004), and finally differentiate into astrocytes. Radial glial cells are thus considered embryonic neural stem/progenitor cells (Fishell and Kriegstein, 2003; Götz and Huttner, 2005; Miller and Gauthier, 2007). In the adult brain, neural stem cells exist in two regions, the subventricular zone (SVZ) of the lateral ventricle and the subgranular zone of the hippocampal dentate gyrus, and neurogenesis occurs continuously in these two regions (Alvarez-Buylla et al., 2001; Doetsch, 2003). It was shown that subsets of GFAP+ cells (type B cells) function as neural stem cells in the adult SVZ (Doetsch et al., 1999; Imura et al., 2003). Type B cells divide slowly and give rise to rapidly proliferating cells called “transit-amplifying cells” (type C cells), which then differentiate into neuroblasts (type A cells) (Zhao et al., 2008; Kriegstein and Alvarez-Buylla, 2009).
It has been shown that Notch signaling plays an important role in neurogenesis in both embryonic and adult brains (Chambers et al., 2001; Hitoshi et al., 2002; Mason et al., 2005; Androutsellis-Theotokis et al., 2006; Mizutani et al., 2007; Yoon et al., 2008). Upon activation of Notch by its ligands (e.g., Delta1), the Notch intracellular domain (NICD) is released from the membrane and translocates to the nucleus, where NICD forms a complex with Rbpj. The NICD-Rbpj complex induces the expression of transcriptional repressor genes such as Hes1 and Hes5, which repress proneural gene expression and thereby inhibit neuronal differentiation. Thus, activation of Notch signaling leads to maintenance of the neural stem cell population, whereas inactivation of Notch signaling induces neuronal differentiation and depletes the neural stem cell population (Bertrand et al., 2002; Ross et al., 2003; Kageyama et al., 2008; Kopan and Ilagan, 2009). We previously generated mouse mutants that lacked the Notch effector genes Hes1, Hes3, and Hes5, and found that virtually all neural stem cells prematurely differentiated into neurons and were depleted in most regions of the CNS (Hatakeyama et al., 2004). However, in the telencephalon, many radial glial cells were maintained and proliferated almost normally even in Hes1;Hes3;Hes5 triple-mutant mice, suggesting that the mechanism for maintenance of neural stem cells is different between the telencephalon and other regions (Imayoshi et al., 2008a). Thus, whether there is a requirement for Notch signaling for maintenance of neural stem cells in embryonic and adult forebrains remains to be determined.
Here, we generated Rbpj-conditional knock-out mice by crossing Rbpj-floxed mice and tamoxifen-inducible Nestin-CreERT2 mice, because conventional Rbpj-null mice die very early. We found that by inactivation of Rbpj, neural stem cells are completely lost in both the developing and adult telencephalon, revealing an absolute requirement for Notch signaling for maintenance of the telencephalic neural stem cells.
Materials and Methods
Breeding and tamoxifen treatment of mice.
Rbpj-conditional mutant mice were obtained by crossing homozygous Rbpj-floxed mice (Han et al., 2002) with Rbpj (+/−) (Oka et al., 1995) and Nes-CreERT2 mice (Imayoshi et al., 2006). Line5-1 and line4 of Nes-CreERT2 mice were used for the analysis of Rbpj-conditional mutant embryos and postnatal mice, respectively. These mice were maintained on C57BL/6;ICR mixed background. Plug date was defined as embryonic day 0.5 (E0.5). Stock solution of tamoxifen (20 mg/ml; Sigma-Aldrich) was prepared in corn oil (Sigma-Aldrich). For activation of CreERT2, 6 mg of tamoxifen per 35 g of body weight was administered by oral gavage to the pregnant mice at E9.5 or E14.5. CreERT2 activation in pups was triggered by intraperitoneal injection of 30 μl of tamoxifen solution once each at postnatal day 1 (P1) and P7. For activation of CreERT2 in adult mice, 10 mg of tamoxifen was orally administered to 2-month-old mice once a day for 4 consecutive days. For a higher degree of Cre-mediated recombination, other rounds of injections were given after the initial tamoxifen treatment. For estimating Cre recombination efficiency, Rosa26-loxP-stop-loxP-CFP mice (Srinivas et al., 2001) were crossed with Nes-CreERT2 mice and recombined cells were identified by CFP expression.
Generation of Hes5-nlsLacZ mice.
For the Hes5-nlsLacZ targeting vector, IRES (internal ribosome entry site)-nls (nuclear localization signal)-attached LacZ and PGK (phosphoglycerate kinase)-puromycin resistance gene cassette were inserted into the SacI sites of Hes5 gene. This engineered genomic fragment was inserted into the pBluescript II SK+ vector (Stratagene) with a diphtheria toxin A-negative selection cassette. The targeting vector was linearized and electroporated into TT2 embryonic stem cells, and puromycin-resistant clones were selected. Genomic DNA from drug-resistant cells was digested with EcoRI and analyzed by Southern blot using a 0.45 kb EcoRI-SpeI fragment as a 5′ external probe for Hes5. Transmission of the target allele into the germline was confirmed by Southern blot. Genotypes were determined by PCR using the following primers: Hes5-WT-Fw, 5′-TGCCCGCGCCTATATAGGGCTGGCGTGCTG-3′; Hes5-WT-Rv, 5′-TGCACTTACTCGGTTTTTCTCCTTGGGACT-3′; and Hes5-KO-Rv, 5′-CCTTCACGACATTCAACAGACCTTGCATTC-3′. The sizes of the PCR products for mutant allele and wild-type allele are 400 bp and 200 bp, respectively.
Bromodeoxyuridine administration.
Bromodeoxyuridine (BrdU) (Sigma-Aldrich) was dissolved in 0.1 m PBS, pH7.4. BrdU (50 mg per g of body weight) was given to pregnant mice at E15.5 for 30 min by a single intraperitoneal injection at a concentration of 10 mg/ml. BrdU (200 mg per g of body weight) was given to adult mice for 2 h by a single intraperitoneal injection at a concentration of 10 mg/ml. For long-term BrdU administration, drinking water containing 1 mg/ml BrdU was given to mice for 14 consecutive days, and they were killed immediately or 12 d later.
Cytosine-β-d-arabinofuranoside infusion.
Tamoxifen was administered to 2-month-old adult mice, and 10 d later these mice were anesthetized with ketamine (37.5 mg/kg, i.p.) and implanted with an osmotic pump (Alzet model 1002). For cytosine-β-d-arabinofuranoside (AraC) infusions, the cannula was implanted stereotaxically in the left lateral ventricle (anterior, −0.2 mm; lateral, 0.8 mm; depth, 2 mm relative to bregma and the surface of the brain). AraC (2%; Sigma-Aldrich) was dissolved in Ringer's solution with 1 mg/ml mouse serum albumin (Sigma-Aldrich). Seven days after infusion, the pump was removed, and mice were killed immediately or 10 d later. BrdU was given for 4 h by a single intraperitoneal injection just before death. The SVZ of the right lateral ventricle (the opposite side of cannula-injected hemisphere) was analyzed.
X-gal staining.
For whole-mount X-gal staining, dissected embryos were fixed in 2% formaldehyde and 0.2% glutaraldehyde in PBS for 60 min at 4°C, rinsed twice in 0.1 m phosphate buffer, pH 7.4, 2 mm MgCl2, 0.01% sodium deoxycholate, 0.02% NP-40, and then stained at 37°C for 8 h in X-gal stain buffer (2 mg of X-gal in 2 mm MgCl2, 0.01% sodium deoxycholate, 0.02% NP-40, 5 mm K3Fe(CN)6, 5 mm K4Fe(CN)6 in 0.1 m phosphate buffer). Stained embryos were washed twice in PBS and postfixed with 4% paraformaldehyde (PFA) in PBS for 2 h at room temperature.
For X-gal staining of tissue sections, embryos and brains were fixed with 4% PFA in PBS at 4°C for 60 min, washed in PBS, equilibrated in 20% sucrose/PBS before mounting OCT compound, and frozen at −80°C. Frozen sections were made by cryostat at 16 μm thickness, and then X-gal staining was performed as described for whole-mount staining.
Tissue preparation and immunocytochemistry.
Embryos were fixed in 4% PFA/PBS overnight at 4°C, washed in ice-cold PBS three times, equilibrated in 20% sucrose/PBS at 4°C, embedded in OCT compound (Sakura Finetek), and frozen at −80°C. Pups and adult mice were deeply anesthetized and transcardially perfused with 30 ml of PBS and 30 ml of 4% PFA/PBS. Brains were postfixed in the perfusion solution overnight at 4°C and then cryoprotected for 24 h in 20% sucrose in PBS. Brains were embedded in OCT compound and frozen at −80°C. Sections were made by cryostat at 16 μm thickness and incubated in 5% normal goat serum (NGS) and 0.1% Triton X-100/PBS at room temperature for 1 h. Then, these sections were incubated with primary antibodies diluted in 0.1% Triton X-100/PBS containing 1% NGS overnight at 4°C, washed with PBS, and incubated with regular secondary antibodies conjugated with Alexa488 or Alexa594 (1:200; Invitrogen) for 1 h at room temperature. For BrdU staining, sections were pretreated with 1N HCl for 20 min at 37°C to denature cellular DNA. Sections were mounted with Fluormount-G (Southern Biotech) and photographed with LSM510 confocal microscopy (Zeiss). The following primary antibodies (final dilution and source) were used: mouse anti-βIII-tubulin (1:500; Babco), rat anti-BrdU (1:50; Oxford Biotech), goat anti-doublecortin (DCX; 1:200; Santa Cruz Biotechnology), rabbit anti-LacZ (1:5000; Cortex Biochem), mouse anti-GFAP (1:200; Sigma-Aldrich), mouse anti-mammalian achaete-schute homolog 1 (Mash1; 1:20; Pharmingen), mouse anti-S100β (1:500; Sigma-Aldrich), rabbit anti-S100β (1:2000; DAKO), mouse anti-Cre (1:200; Millipore Bioscience Research Reagents), mouse anti-NeuN (1:200; Millipore Bioscience Research Reagents) and mouse anti-Ki67 (1:50; BD Biosciences).
Quantification of labeled cells and statistical analysis.
The lateral SVZ of the lateral ventricle of more than three adult mice per age group was analyzed in each experiment. A minimum of 10 coronal sections, throughout the anterior–posterior extent of the SVZ of the lateral ventricle, was assessed for each animal. Cells were scored on the basis of staining for BrdU or Mash1 in their nuclei. Stained cells within the SVZ were counted and expressed as the number of cells per 5 mm along the ventricle for each image. Student's t test (one-tailed) was performed to calculate p values and to determine whether the results were significantly different.
Results
Notch signaling is active in the developing and adult forebrain
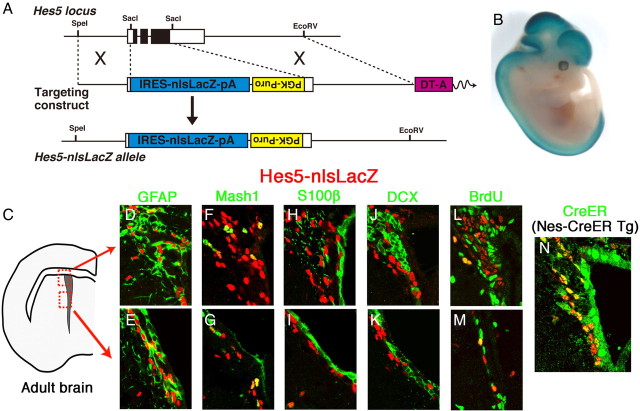
To understand the significance of Notch signaling in the telencephalon, we first determined to what extent Notch signaling is active in this region. In our previous analysis using Hes1 reporter mice, we found that the Notch effector Hes1 is highly expressed by neural stem/progenitor cells in both developing and adult telencephalon (Ohtsuka et al., 2006), suggesting that Notch signaling is active in this region. Because Hes1 expression is also regulated by signaling pathways other than Notch signaling, we next examined the expression of Hes5, a more faithful Notch effector. To this end, we visualized the expression of Hes5 by knocking in the nlsLacZ cDNA at the Hes5 locus (Hes5-nlsLacZ mice) (Fig. 1A). In these mice, LacZ was expressed from the endogenous Hes5 promoter, and LacZ was found to be highly expressed in the ventricular zone of the developing telencephalon (Fig. 1B and supplemental Fig. S1, available at www.jneurosci.org as supplemental material). LacZ was also expressed in the adult brain; hence, we decided to determine the LacZ-expressing cell types in more detail.
Figure 1.
Notch signaling activity in the SVZ of the adult brain. A, Generation of Hes5-nlsLacZ knock-in mice. In the Hes5-nlsLacZ allele, the entire coding region of the Hes5 gene was replaced by the IRES-nlsLacZ expression cassette. B, X-gal staining of E12.5 Hes5-nlsLacZ embryo. C–N, Immunostaining of coronal sections of the SVZ of the lateral ventricle in Hes5-nlsLacZ mice. Most nlsLacZ+ cells expressed GFAP (D, E). A small number of nlsLacZ+ cells expressed Mash1 (F, G). However, nlsLacZ-expressing cells were negative for S100β (H, I) and DCX (J, K). Some nlsLacZ+ cells incorporated BrdU (L, M). Almost all nlsLacZ+ cells expressed CreERT2 in Hes5-nlsLacZ and Nes-CreERT2 double-transgenic mice (N).
In the SVZ and its surrounding regions of the adult brain of Hes5-nlsLacZ mice (Fig. 1C), almost all LacZ-positive cells expressed GFAP, a type B cell and astrocyte marker (96.5 ± 0.4%) (Fig. 1D,E and supplemental Table S1, available at www.jneurosci.org as supplemental material). The majority of Mash1+ type C cells were negative for LacZ expression, whereas a small number of coexpressing cells were observed (6.8 ± 0.8%) (Fig. 1F,G and supplemental Table S1, available at www.jneurosci.org as supplemental material). In addition, after prolonged exposure to BrdU (14 d), only a few of LacZ-positive cells were labeled with BrdU (4.7 ± 1.3%) (Fig. 1L,M and supplemental Table S1, available at www.jneurosci.org as supplemental material), suggesting that the majority of Hes5-expressing cells were not actively dividing. Furthermore, in Nes-CreERT2;Hes5-nlsLacZ mice, which express CreERT2 in type B cells (Imayoshi et al., 2008b), many cells coexpressed LacZ and CreERT2 (Fig. 1N). However, none of the ependymal cells (S100β+) and neuroblasts (DCX+) expressed LacZ in Hes5-nlsLacZ mice (Fig. 1H–K). Thus, LacZ expression occurred in almost all type B cells as well as in astrocytes and a small number of type C cells but not in others. Because the LacZ protein is stable, it is likely that the expression could remain transiently in type C cells even after the Hes5 promoter was repressed, suggesting that Notch signaling is active mostly in type B cells. All these data suggest that Notch signaling is active in neural stem/progenitor cells of both the developing and the adult forebrain.
Notch signaling is required for the maintenance of neural stem cells in the embryonic telencephalon
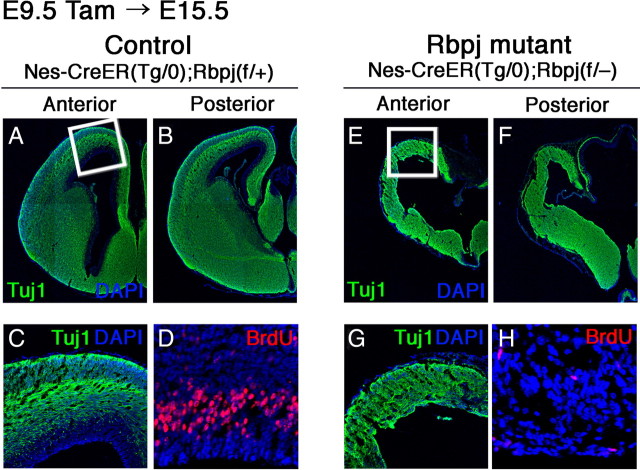
To determine the role of Notch signaling in telencephalic neural stem cells, we conditionally knocked out Rbpj by using Rbpj-floxed mice and Nes-CreERT2 mice. The latter mice specifically induce Cre recombinase activity in neural stem cells after tamoxifen treatment. We first assessed the Cre recombination efficiency at various stages (embryonic, perinatal, and adult stages) by using Rosa26 reporter mice, and found that >80% of neural stem/progenitor cells (Ki67+) displayed Cre-mediated recombination after tamoxifen administration (supplemental Fig. S2, available at www.jneurosci.org as supplemental material) (Imayoshi et al., 2006, 2008b). We next examined the role of Notch signaling in the early development of the telencephalon. Tamoxifen was given to pregnant mothers carrying Nes-CreERT2;Rbpjfloxed/− embryos so that the Cre recombinase was activated in the nervous system of Rbpj-floxed embryos at E9.5 and the brains were examined at E15.5. Tamoxifen-treated Nes-CreERT2;Rbpjfloxed/+ mice, which retained one wild-type Rbpj allele, were normal and used as the control in subsequent experiments. In the control brain, postmitotic neurons (Tuj1+) existed in the outer layers, and radial glial cells actively proliferated in the ventricular zone (VZ) at E15.5 (BrdU+) (Fig. 2A–D). In contrast, in Rbpj-conditional knock-out mice, brain size was significantly smaller than controls (Fig. 2, compare A,B with E,F). In addition, almost all cells became postmitotic neurons (Tuj1+) (Fig. 2G), and BrdU-incorporating dividing radial glial cells were greatly reduced in number (Fig. 2H). Expression of radial glial cell markers such as Nestin and GLAST was also severely downregulated (data not shown). As a result, the VZ was totally missing in Rbpj-mutant brains (Fig. 2G). These results indicate that in the absence of Rbpj, the telencephalic radial glial cells are not maintained; they prematurely differentiate into neurons. Acceleration of neurogenesis and reduction of radial glial cells were already observed at E12.5 (data not shown). Thus, Notch signaling is required for the maintenance of embryonic neural stem cells in the early developing telencephalon.
Figure 2.
Premature loss of neural stem cells in the telencephalon of Rbpj-conditional knock-out embryos. A–H, Histological analysis was done with the control (A–D) and Rbpj-mutant (E–H) mice at E15.5. Tamoxifen was orally administered to pregnant mothers at E9.5. In control embryos, neurons (Tuj1+) reside in the outer layers and BrdU-incorporated dividing stem/progenitor cells are located in the ventricular zone (A–D). In Rbpj-mutant embryos, neurons are located throughout the neuroepithelium, and the number of BrdU-incorporated cells was dramatically reduced (E–H). Boxed regions in A and E are enlarged in C and G, respectively. D and H are enlarged view of the dorsal telencephalon.
We next investigated the role of Rbpj in the brain development at a later stage. Tamoxifen was given to Nes-CreERT2;Rbpjfloxed/− embryos at E14.5, and the brains were examined at E18.5. In the control brain, there were numerous BrdU-incorporating neural stem/progenitor cells in the VZ and the SVZ of the lateral ventricle (Fig. 3A–D). DCX+ postmitotic neurons were present in the cortex but were rarely observed in the VZ (Fig. 3E–G). In contrast, the number of BrdU-incorporating neural stem/progenitor cells was greatly reduced in many regions of the Rbpj-mutant brain (Fig. 3H). This reduction was evident in the dorsal and lateral walls of the lateral ventricle (Fig. 3I,J). However, in the ventral region, the number of dividing cells increased (Fig. 3H,K), suggesting that other mitogenic signaling pathways are dominant in the ventral region and that Notch signaling might negatively regulate cell proliferation in this region. In the Rbpj-mutant brain, DCX+ neurons were located in the inner region as well as in the cortex, and as a result the VZ was not clear (Fig. 3L–N). These results indicate that in the absence of Rbpj, neural stem/progenitor cells are not maintained; they prematurely differentiate into neurons in many brain regions, except for the ventral region at a later stage. Thus, Notch signaling is required for maintenance of proliferating radial glial cells by inhibiting premature neuronal differentiation in the developing telencephalon. However, in the ventral region at a later developmental stage, Notch signaling inhibited cell proliferation, and the proliferative activity of neural stem/progenitor cells changed.
Figure 3.
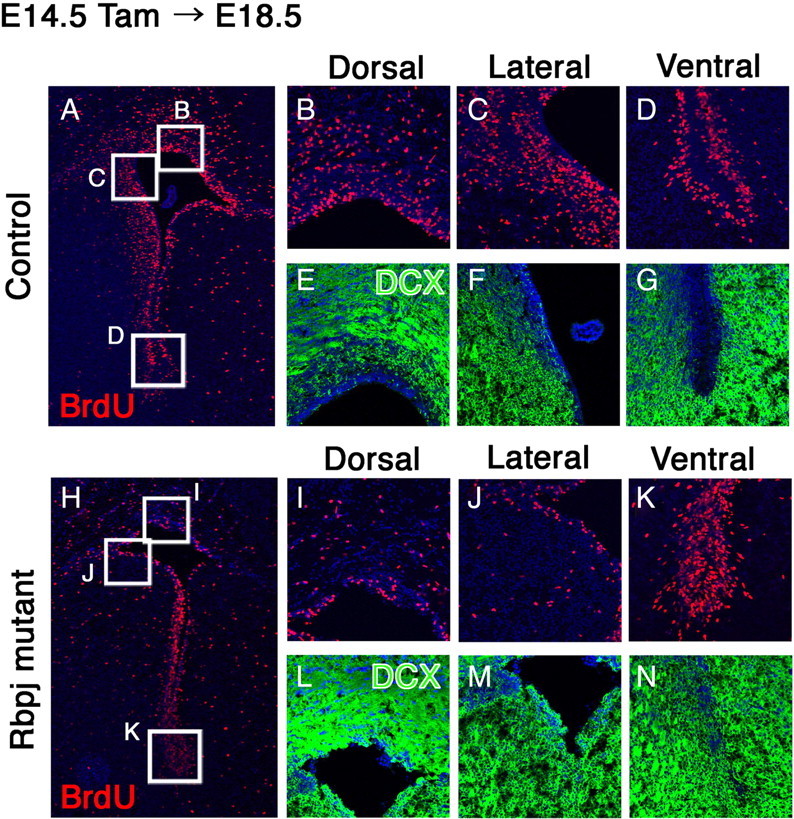
Rbpj is required for maintenance of neural stem/progenitor cells in the late embryonic telencephalon. A–N, Histological analysis was done with control (A–G) and Rbpj-conditional knock-out (H–N) mice at E18.5. Tamoxifen was administered to pregnant mothers at E14.5. In Rbpj-mutant embryos, the number of BrdU-incorporated dividing progenitors was significantly reduced in the dorsal and lateral regions of the SVZ of the lateral ventricle (H–J) compared with controls (A–C). However, in the ventral region, the number of BrdU-incorporated cells increased in Rbpj-mutant embryos (H, K), compared with controls (A, D). Neurons were located outside of the ventricular zone in control mice (E–G), whereas numerous DCX-positive neurons were observed in the ventricular zone of Rbpj-mutant mice (L–N), indicating premature neurogenesis.
Notch signaling regulates neurogenesis in the postnatal period
It has been shown that radial glial cells persist for several days after birth in the mouse brain, but mostly disappear by P15 (Tramontin et al., 2003), whereas GFAP+ astrocytes are mostly absent at P0 but are formed in the SVZ later (Gates et al., 1995; Tramontin et al., 2003), suggesting that radial glial cells present at birth become GFAP+ astrocytes during this postnatal period and that some of the GFAP+ cells function as adult-type neural stem cells (Merkle et al., 2004). Thus, the features of neural stem cells gradually change during this postnatal period: from the embryonic radial glial cells to adult astrocyte-like neural stem cells (Kriegstein and Alvarez-Buylla, 2009).
We next determined the role of Notch signaling in this transition period. Tamoxifen was given to Nes-CreERT2;Rbpjfloxed/− neonatal mice, and the brains were examined at P7 and P14. In the absence of Rbpj, dividing cells (Ki67+) were more abundant at P7 (supplemental Fig. S3E–H, available at www.jneurosci.org as supplemental material), compared with controls (supplemental Fig. S3A–D, available at www.jneurosci.org as supplemental material). This abundance was in sharp contrast to the embryonic brain, in which the number of dividing neural stem/progenitor cells was greatly reduced in the absence of Rbpj, but the situation was similar to the ventral region of the later-stage embryonic brain, in which the number of dividing cells increased in the absence of Rbpj (Fig. 3K). These results indicate that at this postnatal period, Notch signaling inhibits cell proliferation, suggesting that the cell proliferation depends on a different mitogenic factor. At P14, neurogenesis (DCX+) was significantly increased in the absence of Rbpj (supplemental Fig. S3J, available at www.jneurosci.org as supplemental material), compared with controls (supplemental Fig. S3I, available at www.jneurosci.org as supplemental material). However, this increase was transient, and neurogenesis no longer occurred in the mutant 6 weeks later (supplemental Fig. S3L, available at www.jneurosci.org as supplemental material), although neurogenesis continued in the control SVZ (supplemental Fig. S3K, available at www.jneurosci.org as supplemental material). Thus, Notch signaling regulates cell proliferation and long-term neurogenesis in the postnatal brain. The phenotypes observed postnatally in Rbpj-mutant mice were similar to the ones observed in the adult brain (see below), suggesting that the transition from embryonic to adult neural stem cells accounts for different features of Rbpj-dependent cell proliferation between embryonic and postnatal periods.
Transient increase in cell proliferation and neurogenesis in the absence of Rbpj
To determine the role of Rbpj in type B neural stem cells in the adult brain, tamoxifen was given to Nes-CreERT2;Rbpjfloxed/− adult mice. Ten days after tamoxifen administration, BrdU pulse-labeling experiments, which preferentially labels transit-amplifying type C cells (Doetsch et al., 2002), were performed. In the absence of Rbpj, cells that incorporated BrdU significantly increased in number (360.9 ± 37.7 cells per 5 mm along the ventricle) (supplemental Fig. S4H–K,O, available at www.jneurosci.org as supplemental material) compared with the controls (189.9 ± 29.5) (supplemental Fig. S4A–D,O, available at www.jneurosci.org as supplemental material). Furthermore, the number of cells expressing Mash1, a type C cell marker (Parras et al., 2004; Kim et al., 2007), also significantly increased (487.1 ± 44.4) (supplemental Fig. S4L,M,O, available at www.jneurosci.org as supplemental material) compared with the controls (211.8 ± 54.1) (supplemental Fig. S4E,F,O), but the number of DCX+ neuroblasts was not changed (supplemental Fig. S4G,N, available at www.jneurosci.org as supplemental material). Thus, 10 d after inactivation of Rbpj, there was a specific increase in the number of transit-amplifying type C cells. Analysis of Hes5-nlsLacZ mice indicated that more cells coexpressed LacZ and Mash1 as early as 5 d after Rbpj inactivation (17.7 ± 2.1%) than in the control mice (7.5 ± 0.7%) (supplemental Fig. S5, available at www.jneurosci.org as supplemental material), suggesting that neuronal differentiation of Hes5-expressing type B cells is enhanced in the absence of Rbpj.
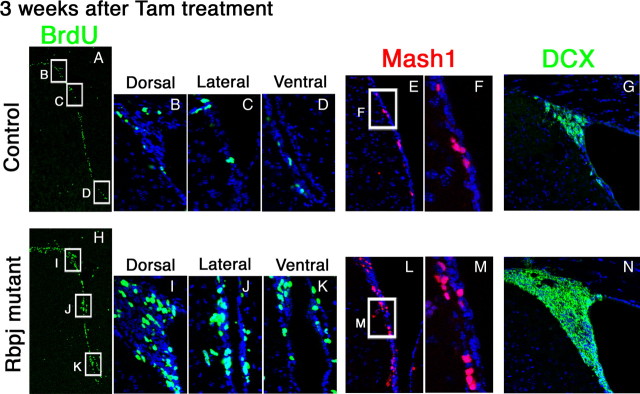
Three weeks after tamoxifen administration, cells that incorporated BrdU in BrdU pulse-labeling experiments and Mash1+ cells significantly increased in number (BrdU+, 698.1 ± 44.1; Mash1+, 699.6 ± 48.3) (Fig. 4H–M), compared with controls (BrdU+, 201.8 ± 34.4; Mash1+, 233.2 ± 69.2) (Fig. 4A–F). This increase in the number of type C cells also occurred in dorsal, lateral, and ventral regions (Fig. 4H–K), similar to the postnatal stage of the embryonic telencephalon (supplemental Fig. S3E–H, available at www.jneurosci.org as supplemental material). At this stage, the number of DCX+ neuroblasts also significantly increased (Fig. 4N), compared with controls (Fig. 4G). We also quantified newly born neurons by labeling dividing cells with BrdU for 7 d and chasing their progeny in the olfactory bulb 21 d later (supplemental Fig. S6A, available at www.jneurosci.org as supplemental material). In this experiment, the number of newly born neurons (NeuN+) in the olfactory bulb that had incorporated BrdU significantly increased in Rbpj-mutant mice compared with controls (supplemental Fig. S6B–D, available at www.jneurosci.org as supplemental material). Thus, inactivation of Rbpj leads to an increase in the number of transit-amplifying type C cells, leading to increased neurogenesis. These results suggest that Notch signaling inhibits transition from slowly dividing type B cells to transit-amplifying type C cells.
Figure 4.
Increase in the number of transit-amplifying cells and neuroblasts in the Rbpj-mutant adult brain. A–N, Coronal sections of the SVZ of the lateral ventricles of control (A–G) and Rbpj-conditional knock-out (H–N) mice that were treated with tamoxifen 3 weeks before. BrdU was administered for 2 h before mice were killed. The number of BrdU-incorporated cells in the SVZ significantly increased in Rbpj-mutant mice (H–K) compared with controls (A–D). The number of Mash1+ transit-amplifying cells also increased in Rbpj-mutant mice (L, M) compared with controls (E, F). Boxed regions in panels E and L are enlarged in F and M, respectively. Neurogenesis (DCX+) was enhanced in Rbpj-mutants (N) compared with controls (G).
Notch signaling is required forlong-term maintenance of adultneural stem cells
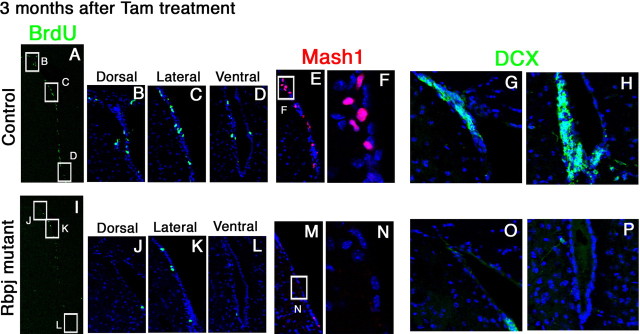
We next examined the long-term outcome of increased neurogenesis from type B cells in the adult brain. Three months after tamoxifen administration into Nes-CreERT2;Rbpjfloxed/− adult mice, cells that incorporated BrdU in BrdU pulse-labeling experiments and Mash1+ cells were significantly reduced in number (BrdU+, 17.3 ± 5.7; Mash1+, 12.6 ± 6.6) (Fig. 5I–N), compared with the controls (BrdU+, 169.9 ± 39.2; Mash1+, 218.0 ± 39.9) (Fig. 5A–F). This severe reduction in the number of type C cells occurred similarly in dorsal, lateral, and ventral regions (Fig. 5I–L). Furthermore, in the absence of Rbpj, DCX+ neuroblasts were mostly missing at this stage (Fig. 5O,P), whereas there were many in the controls (Fig. 5G,H). These results indicate that increased neurogenesis by inactivation of Rbpj is only transient (Fig. 4N), and that Notch signaling is required for long-term neurogenesis in the adult brain.
Figure 5.
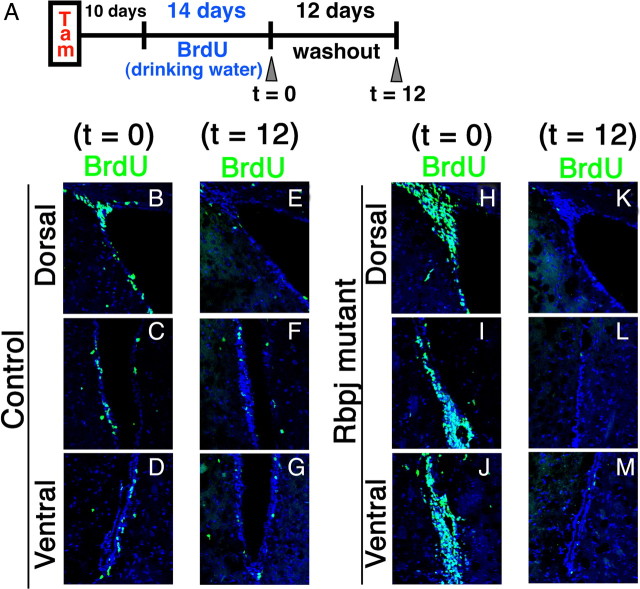
Depletion of dividing progenitor cells and neurogenesis in the Rbpj-mutant adult brain. A–P, Coronal sections of the SVZ of the lateral ventricles from control (A–H) and Rbpj-conditional knock-out (I–P) mice that had been treated with tamoxifen 3 months before. BrdU was administered for 2 h before death. The number of cells that incorporated BrdU in the SVZ was markedly reduced in Rbpj-mutant mice (I–L) compared with controls (A–D). The number of Mash1+ transit-amplifying cells was also reduced in Rbpj-mutant mice (M, N) compared with controls (E, F). Boxed regions in panels E and M are enlarged in F and N, respectively. Neurogenesis (DCX+) was greatly attenuated in Rbpj-mutant mice (O, P) compared with controls (G, H).
The above results suggest the possibility that by inactivation of Notch signaling all slowly dividing neural stem cells become transit-amplifying cells and neurons, leading to a transient increase in neurogenesis, but that the depletion of neural stem cells eventually causes loss of neurogenesis. To investigate this possibility, we quantified the label-retaining cells, a feature observed for slowly dividing neural stem cells, in Rbpj-conditional knock-out mice that had been injected with tamoxifen 10 d before. BrdU was given to both Rbpj-conditional knock-out mice and control mice for 14 consecutive days to label not only transit-amplifying type C cells but also slowly dividing type B cells (Fig. 6A). At day 0 after BrdU administration (t = 0), all dividing cells, including slowly dividing neural stem cells, were labeled by prolonged exposure to BrdU, whereas slowly dividing neural stem cells selectively retained BrdU 12 d after BrdU administration (t = 12), because fast-cycling transit-amplifying cells diluted out the BrdU (Capela and Temple, 2002). In agreement with the above results (Fig. 4 and supplemental Fig. S4, available at www.jneurosci.org as supplemental material), dividing cells significantly increased in number in Rbpj-mutant mice (1533.0 ± 24.5) (Fig. 6H–J), compared with the controls (491.1 ± 58.5) (Fig. 6B–D) at t = 0. At t = 12, there were numerous label-retaining cells in the control mice (77.3 ± 13.8) (Fig. 6E–G), whereas the number of BrdU+ cells was severely reduced in Rbpj-mutant mice (24.5 ± 5.3) (Fig. 6K–M). These results indicate that slowly dividing type B cells are severely reduced in number as early as 10 d after inactivation of Rbpj. At this stage, transit-amplifying type C cells proliferate extensively (supplemental Fig. S4, available at www.jneurosci.org as supplemental material). These results suggest that by inactivation of Notch signaling, slowly dividing type B cells are not properly maintained and mostly become transit-amplifying type C cells.
Figure 6.
Depletion of slowly dividing neural stem cells in the Rbpj-mutant adult brain. A, Experimental design. B–M, Dividing progenitors (t = 0; B–D, H–J) and slowly dividing cells, ones that retained labeling even after 12 d (t = 12; E–G, K–M), were labeled with BrdU in control (B–G) and Rbpj-mutant (H–M) mice.
It was recently shown that in the absence of Rbpj, ependymal cells enter the cell cycle and differentiate into neurons, leading to collapse of the lateral ventricles (Carlén et al., 2009). Although our Nes-CreERT2 mice efficiently induce recombination in ependymal cells after tamoxifen treatment (Imayoshi et al., 2008b), almost all ependymal cells (S100β+) were negative for Ki67, a marker for proliferating cells, in Rbpj-conditional knock-out mice, indicating that very few ependymal cells entered the cell cycle (supplemental Fig. S7, available at www.jneurosci.org as supplemental material). Furthermore, even 3 months after tamoxifen treatment, ependymal cells and the lateral ventricles were properly maintained in Rbpj-conditional knock-out mice (supplemental Fig. S7J,J', available at www.jneurosci.org as supplemental material). These results suggest that Notch signaling is not important for maintenance of ependymal cells. In agreement with this notion, LacZ expression was not observed in ependymal cells (S100β+) of Hes5-nlsLacZ mice, suggesting that Notch signaling is not active in these cells (Fig. 1H,I).
Notch signaling is required for neuronal regeneration in AraC-treated brains
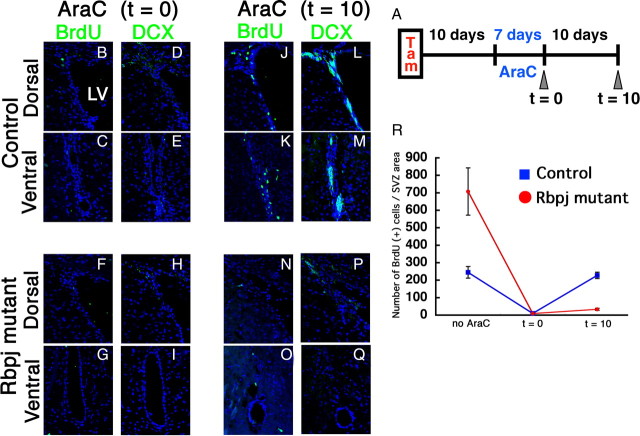
It has been shown that treatment with the antimitotic drug AraC kills transit-amplifying cells but does not affect slowly dividing cells (Morshead et al., 1994; Doetsch et al., 1999). Thus, although neurogenesis is transiently impaired by AraC treatment, the damaged brains are later reconstituted by slowly dividing type B cells (Doetsch et al., 1999). We next examined this regeneration process of control mice and Rbpj-conditional knock-out mice that had been injected with tamoxifen 10 d before (Fig. 7A). At day 0 after AraC treatment (t = 0), cells that incorporated BrdU in BrdU pulse-labeling experiments (transit-amplifying cells) and DCX+ neuroblasts were mostly missing in both Rbpj-conditional knock-out mice (Fig. 7F–I) and control mice (Fig. 7B–E). At day 10 after AraC treatment (t = 10), however, the BrdU+ transit-amplifying cell population returned to normal numbers (Fig. 7J,K,R) and resumed neurogenesis in the control brain (Fig. 7L,M). In contrast, in the Rbpj-mutant brain, BrdU+ transit-amplifying cells did not reappear (Fig. 7N,O,R), and thus neurogenesis did not resume by day 10 after AraC treatment (Fig. 7P,Q). The lack of reconstitution was probably because without Rbpj, slowly dividing type B cells were mostly missing at this stage. These results indicate that Notch signaling regulates neuronal regeneration in AraC-treated brains by maintaining slowly dividing neural stem cells.
Figure 7.
Impairment of regeneration after AraC treatment in Rbpj-mutant adult brain. A, Experimental design. B–Q, Coronal sections of the SVZ showing BrdU-incorporating cells soon after the removal of the osmotic pump (t = 0; B–I) and 10 d after the pump removal (t = 10; J–Q) in AraC-treated control (B–E, J–M) and AraC-treated Rbpj-mutant (F–I, N–Q) mice. R, Quantification of the number of BrdU-incorporating cells in the SVZ. BrdU was administered for 4 h before death.
Discussion
Requirement of Notch signaling for maintenance of neural stem cells in the embryonic and adult brain
The role of Notch signaling in neural development has been intensively analyzed by conditional inactivation of Rbpj, and it has been shown that Notch signaling is required for both maintenance of neural stem/progenitor cells and specification of glial cells (Taylor et al., 2007; Gao et al., 2009). However, the functions of Notch signaling in the embryonic and adult forebrain remains to be determined. We previously generated Hes1;Hes3;Hes5 triple knock-out mice and found that virtually all neural stem/progenitor cells prematurely differentiated into neurons and were depleted in most regions of the developing CNS, indicating an absolute requirement for Notch signaling for maintenance of embryonic neural stem/progenitor cells (Hatakeyama et al., 2004; Kageyama et al., 2007). However, in the developing telencephalon, many radial glial cells were maintained and proliferated almost normally even in the absence of Hes1, Hes3, and Hes5 (Imayoshi et al., 2008a), suggesting that the mechanism for maintenance of neural stem cells is different between the telencephalon and other regions. Here, to examine the role of Notch signaling in the telencephalic development, we generated Rbpj-conditional knock-out mice, and found that in the absence of Notch signaling, neural stem cells are completely lost in the embryonic telencephalon. These results indicate an absolute requirement of Notch signaling for the maintenance of neural stem cells of the developing telencephalon. In this region, it is likely that another Hes1-related gene could compensate for the lack of Hes1, Hes3, and Hes5. In agreement with this idea, we previously found that the Hes1-related repressor gene Hey1 is highly expressed in the developing telencephalon, and that this expression is upregulated in the absence of Hes1, Hes3, and Hes5 (Imayoshi et al., 2008a). Thus, Hes1, Hes3, Hes5, and Hey1 could compensate for each other to regulate telencephalic development. Our preliminary analysis of Hes1;Hes3;Hes5;Hey1-mutant mice suggest that this is indeed the case (our unpublished data).
In the present study, we also examined the role of Notch signaling in the adult brain. It was previously shown that inactivation of Notch1 promotes neuronal differentiation in the hippocampal dentate gyrus of the adult brain (Breunig et al., 2007). However, it was not clear whether only subsets or all neural stem cells are regulated by Notch signaling. It was also reported that when Rbpj is deleted in type C cells by using CaMKII-Cre driver, some oligodendrocyte precursors are formed at the expense of neurons, suggesting that Notch signaling is required for maturation of neurons (Fujimoto et al., 2009). However, the role for Notch signaling in adult neural stem cells remained to be analyzed. Our data suggest that without Notch signaling, virtually all neural stem cells differentiated into transit-amplifying cells and then into neurons in the SVZ of the lateral ventricles of the adult brain. As a result, although neurogenesis was increased transiently, neural stem cells were depleted, and eventually neurogenesis was completely lost. We also found similar results in the subgranular zone of the hippocampal dentate gyrus, although loss of neural stem cells was not complete in this region probably because of lower Cre recombinase activity (our unpublished data). These results suggest an absolute requirement for Notch signaling for maintenance of neural stem cells in both embryonic and adult brains. Because, based on the Hes5 reporter expression, Notch signaling is inactive in the majority of transit-amplifying cells, it seems that inactivation of Notch signaling is required for a transition from neural stem cells to transit-amplifying cells. It remains to be determined how such activation and inactivation of Notch signaling are controlled.
It was recently reported that in the absence of Rbpj or in response to stroke, ependymal cells the enter cell cycle and differentiate into neurons, leading to collapse of the lateral ventricles (Carlén et al., 2009). However, in our Rbpj-conditional knock-out mice, very few ependymal cells entered the cell cycle, and these cells and the lateral ventricles were properly maintained even 3 months after inactivation of Rbpj. Thus, Rbpj-dependent Notch signaling is not important for maintenance of ependymal cells, and contribution of these cells to neurogenesis, if any, seems to be minimal.
Similarity and difference in phenotypes of Notch signaling inactivation between embryonic and adult brains
In both Rbpj-mutant embryonic and adult brains, neuronal differentiation is accelerated, resulting in depletion of neural stem cells. Thus, the final outcome of Rbpj-conditional knock-out mice is the same between embryonic and adult brains. However, the immediate outcome is different. In the embryonic brain, inactivation of Rbpj leads to a decrease in cell proliferation because actively dividing neural stem cells prematurely differentiate into postmitotic neurons. In contrast, in the adult brain, inactivation of Rbpj leads to an increase in cell proliferation because all slowly dividing neural stem cells become transit-amplifying cells. The difference in the immediate outcome is thus due to the different features of embryonic and adult neurogenesis. An increase in cell proliferation after inactivation of Rbpj was initially observed in the ventral region of the telencephalon of late-stage embryos, but this phenotype expands to the lateral and dorsal regions during the postnatal period. Thus, it is likely that the transition from embryonic to adult neural stem cells occurs from the ventral to the lateral and dorsal regions during the perinatal period; but the mechanism showing how this transition is controlled remains to be determined.
This transition could be partly attributable to changes in Shh dependency. Shh signaling has been shown to be important for the proliferation and maintenance of adult neural stem cells (Lai et al., 2003; Machold et al., 2003; Palma et al., 2005). Slowly diving adult neural stem cells and transit-amplifying cells respond to Shh, but these Shh-sensitive cells seem to appear after late embryonic periods (Ahn and Joyner, 2005). In the absence of Shh signaling, SVZ neural stem cells are not affected before birth. However, at early postnatal periods, neural stem cells are depleted after the transient expansion of neuroblasts in the absence of Smoothened, an essential effector of Shh, although expansion of Mash1+ type C cells was not observed, suggesting that Shh signaling regulates both maintenance of type B cells and proliferation of type C cells (Machold et al., 2003; Balordi and Fishell, 2007a,b). Thus, a requirement for Shh signaling for maintenance of neural stem cells might be a fundamental event in the transition from embryonic to adult neural stem cells when the active proliferation loses the dependency on Rbpj.
In the adult brain, all neural stem cells differentiated into transit-amplifying cells in the absence of Rbpj, which increased cell proliferation. However, transit-amplifying cells proliferated only transiently, and soon differentiated into postmitotic neurons. It remains to be determined why these cells soon lost mitotic activity.
Regulation of proliferation of neural stem cells
Adult neural stem cells are often said to be “slowly dividing” or “quiescent,” although it is not clear whether they are mostly dormant and only transiently enter the cell cycle or whether they continuously divide but have a long G1 phase. Our study indicates that Notch signaling is required not only for actively dividing embryonic neural stem cells but also for slowly dividing or quiescent adult neural stem cells. The precise mechanism for regulation of such different cell division modes is not known. One possibility is that the expression mode of Notch effectors such as Hes1 could be different between embryonic and adult neural stem cells. Hes1 expression is oscillating in actively dividing embryonic neural stem cells (Shimojo et al., 2008). Although Hes1 is required for maintenance and proliferation of embryonic neural stem cells, cells with sustained Hes1 expression divide very slowly or do not divide, indicating that sustained Hes1 expression inhibits cell cycle progression (Baek et al., 2006). In agreement with this notion, sustained Hes1 expression downregulates G1 cyclin expression (Ström et al., 2000; Baek et al., 2006; Shimojo et al., 2008). Thus, one interesting possibility is that Hes1 expression is sustained in adult neural stem cells. Further studies will be required to clarify whether Hes1 expression mode is different between embryonic and adult neural stem cells, and if this is the case, how different expression mode of Hes1 is controlled.
Footnotes
This work was supported by grants-in-aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan. I.I. was supported by Research Fellowships of the Japan Society for the Promotion of Science for Young Scientists. We thank F. Costantini and T. Honjo for Rosa26-loxP-stop-loxP-CFP mice and Rbpj mutant mice, respectively. We also thank T. Inoue for generating Hes5-nlsLacZ mice and A. Eisch for critical reading of this manuscript.
References
- Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding sonic hedgehog. Nature. 2005;437:894–897. doi: 10.1038/nature03994. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, García-Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci. 2001;2:287–293. doi: 10.1038/35067582. [DOI] [PubMed] [Google Scholar]
- Androutsellis-Theotokis A, Leker RR, Soldner F, Hoeppner DJ, Ravin R, Poser SW, Rueger MA, Bae SK, Kittappa R, McKay RD. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature. 2006;442:823–826. doi: 10.1038/nature04940. [DOI] [PubMed] [Google Scholar]
- Baek JH, Hatakeyama J, Sakamoto S, Ohtsuka T, Kageyama R. Persistent and high levels of Hes1 expression regulate boundary formation in the developing central nervous system. Development. 2006;133:2467–2476. doi: 10.1242/dev.02403. [DOI] [PubMed] [Google Scholar]
- Balordi F, Fishell G. Hedgehog signaling in the subventricular zone is required for both the maintenance of stem cells and the migration of newborn neurons. J Neurosci. 2007a;27:5936–5947. doi: 10.1523/JNEUROSCI.1040-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balordi F, Fishell G. Mosaic removal of hedgehog signaling in the adult SVZ reveals that the residual wild-type stem cells have a limited capacity for self-renewal. J Neurosci. 2007b;27:14248–14259. doi: 10.1523/JNEUROSCI.4531-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand N, Castro DS, Guillemot F. Proneural genes and the specification of neural cell types. Nat Rev Neurosci. 2002;3:517–530. doi: 10.1038/nrn874. [DOI] [PubMed] [Google Scholar]
- Breunig JJ, Silbereis J, Vaccarino FM, Sestan N, Rakic P. Notch regulates cell fate and dendrite morphology of newborn neurons in the postnatal dentate gyrus. Proc Natl Acad Sci U S A. 2007;104:20558–20563. doi: 10.1073/pnas.0710156104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capela A, Temple S. LeX/ssea-1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron. 2002;35:865–875. doi: 10.1016/s0896-6273(02)00835-8. [DOI] [PubMed] [Google Scholar]
- Carlén M, Meletis K, Göritz C, Darsalia V, Evergren E, Tanigaki K, Amendola M, Barnabé-Heider F, Yeung MSY, Naldini L, Honjo T, Kokaia Z, Shupliakov O, Cassidy RM, Lindvall O, Frisén J. Forebrain ependymal cells are Notch-dependent and generate neuroblasts and astrocytes after stroke. Nat Neurosci. 2009;12:259–267. doi: 10.1038/nn.2268. [DOI] [PubMed] [Google Scholar]
- Chambers CB, Peng Y, Nguyen H, Gaiano N, Fishell G, Nye JS. Spatiotemporal selectivity of response to Notch1 signals in mammalian forebrain precursors. Development. 2001;128:689–702. doi: 10.1242/dev.128.5.689. [DOI] [PubMed] [Google Scholar]
- Doetsch F. The glial identity of neural stem cells. Nat Neurosci. 2003;6:1127–1134. doi: 10.1038/nn1144. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Caillé I, Lim DA, García-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Petreanu L, Caille I, Garcia-Verdugo JM, Alvarez-Buylla A. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 2002;36:1021–1034. doi: 10.1016/s0896-6273(02)01133-9. [DOI] [PubMed] [Google Scholar]
- Fishell G, Kriegstein AR. Neurons from radial glia: the consequences of asymmetric inheritance. Curr Opin Neurobiol. 2003;13:34–41. doi: 10.1016/s0959-4388(03)00013-8. [DOI] [PubMed] [Google Scholar]
- Fujimoto M, Takagi Y, Muraki K, Nozaki K, Yamamoto N, Tsuji M, Hashimoto N, Honjo T, Tanigaki K. RBP-J promotes neuronal differentiation and inhibits oligodendroglial development in adult neurogenesis. Dev Biol. 2009;332:339–350. doi: 10.1016/j.ydbio.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Gao F, Zhang Q, Zheng MH, Liu HL, Hu YY, Zhang P, Zhang ZP, Qin HY, Feng L, Wang L, Han H, Ju G. Transcriptional factor RBP-J-mediated signaling represses the differentiation of neural stem cells into intermediate neural progenitors. Mol Cell Neurosci. 2009;40:442–450. doi: 10.1016/j.mcn.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Gates MA, Thomas LB, Howard EM, Laywell ED, Sajin B, Faissner A, Götz B, Silver J, Steindler DA. Cell and molecular analysis of the developing and adult mouse subventricular zone of the cerebral hemispheres. J Comp Neurol. 1995;361:249–266. doi: 10.1002/cne.903610205. [DOI] [PubMed] [Google Scholar]
- Götz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell Biol. 2005;6:777–788. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- Han H, Tanigaki K, Yamamoto N, Kuroda K, Yoshimoto M, Nakahata T, Ikuta K, Honjo T. Inducible gene knockout of transcription factor recombination signal binding protein-J reveals its essential role in T versus B lineage decision. Int Immunol. 2002;14:637–645. doi: 10.1093/intimm/dxf030. [DOI] [PubMed] [Google Scholar]
- Hatakeyama J, Bessho Y, Katoh K, Ookawara S, Fujioka M, Guillemot F, Kageyama R. Hes genes regulate size, shape and histogenesis of the nervous system by control of the timing of neural stem cell differentiation. Development. 2004;131:5539–5550. doi: 10.1242/dev.01436. [DOI] [PubMed] [Google Scholar]
- Haubensak W, Attardo A, Denk W, Huttner WB. Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: a major site of neurogenesis. Proc Natl Acad Sci U S A. 2004;101:3196–3201. doi: 10.1073/pnas.0308600100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitoshi S, Alexson T, Tropepe V, Donoviel D, Elia AJ, Nye JS, Conlon RA, Mak TW, Bernstein A, van der Kooy D. Notch pathway molecules are essential for the maintenance, but not the generation, of mammalian neural stem cells. Genes Dev. 2002;16:846–858. doi: 10.1101/gad.975202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imayoshi I, Ohtsuka T, Metzger D, Chambon P, Kageyama R. Temporal regulation of Cre recombinase activity in neural stem cells. Genesis. 2006;44:233–238. doi: 10.1002/dvg.20212. [DOI] [PubMed] [Google Scholar]
- Imayoshi I, Shimogori T, Ohtsuka T, Kageyama R. Hes genes and neurogenin regulate non-neural versus neural fate specification in the dorsal telencephalic midline. Development. 2008a;135:2531–2541. doi: 10.1242/dev.021535. [DOI] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, Mori K, Ikeda T, Itohara S, Kageyama R. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008b;11:1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Ohtsuka T, Kageyama R. Continuous neurogenesis in the adult brain. Dev Growth Differ. 2009;51:379–386. doi: 10.1111/j.1440-169X.2009.01094.x. [DOI] [PubMed] [Google Scholar]
- Imura T, Kornblum HI, Sofroniew MV. The predominant neural stem cell isolated from postnatal and adult forebrain but not early embryonic forebrain expresses GFAP. J Neurosci. 2003;23:2824–2832. doi: 10.1523/JNEUROSCI.23-07-02824.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama R, Ohtsuka T, Kobayashi T. The Hes gene family: repressors and oscillators that orchestrate embryogenesis. Development. 2007;134:1243–1251. doi: 10.1242/dev.000786. [DOI] [PubMed] [Google Scholar]
- Kageyama R, Ohtsuka T, Shimojo H, Imayoshi I. Dynamic Notch signaling in neural progenitor cells and a revised view of lateral inhibition. Nat Neurosci. 2008;11:1247–1251. doi: 10.1038/nn.2208. [DOI] [PubMed] [Google Scholar]
- Kim EJ, Leung CT, Reed RR, Johnson JE. In vivo analysis of Ascl1 defined progenitors reveals distinct developmental dynamics during adult neurogenesis and gliogenesis. J Neurosci. 2007;27:12764–12774. doi: 10.1523/JNEUROSCI.3178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopan R, Ilagan MXG. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai K, Kaspar BK, Gage FH, Schaffer DV. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat Neurosci. 2003;6:21–27. doi: 10.1038/nn983. [DOI] [PubMed] [Google Scholar]
- Machold R, Hayashi S, Rutlin M, Muzumdar MD, Nery S, Corbin JG, Gritli-Linde A, Dellovade T, Porter JA, Rubin LL, Dudek H, McMahon AP, Fishell G. Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron. 2003;39:937–950. doi: 10.1016/s0896-6273(03)00561-0. [DOI] [PubMed] [Google Scholar]
- Mason HA, Rakowiecki SM, Raftopoulou M, Nery S, Huang Y, Gridley T, Fishell G. Notch signaling coordinates the patterning of striatal compartments. Development. 2005;132:4247–4258. doi: 10.1242/dev.02008. [DOI] [PubMed] [Google Scholar]
- Merkle FT, Tramontin AD, García-Verdugo JM, Alvarez-Buylla A. Radial glia give rise to adult neural stem cells in the subventricular zone. Proc Natl Acad Sci U S A. 2004;101:17528–17532. doi: 10.1073/pnas.0407893101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller FD, Gauthier AS. Timing is everything: making neurons versus glia in the developing cortex. Neuron. 2007;54:357–369. doi: 10.1016/j.neuron.2007.04.019. [DOI] [PubMed] [Google Scholar]
- Ming G, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Miyata T, Kawaguchi A, Saito K, Kawano M, Muto T, Ogawa M. Asymmetric production of surface-dividing and non-surface-dividing cortical progenitor cells. Development. 2004;131:3133–3145. doi: 10.1242/dev.01173. [DOI] [PubMed] [Google Scholar]
- Mizutani K, Yoon K, Dang L, Tokunaga A, Gaiano N. Differential Notch signalling distinguishes neural stem cells from intermediate progenitors. Nature. 2007;449:351–355. doi: 10.1038/nature06090. [DOI] [PubMed] [Google Scholar]
- Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, Weiss S, van der Kooy D. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071–1082. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Martínez-Cerdeño V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004;7:136–144. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- Ohtsuka T, Imayoshi I, Shimojo H, Nishi E, Kageyama R, McConnell SK. Visualization of embryonic neural stem cells using Hes promoters in transgenic mice. Mol Cell Neurosci. 2006;31:109–122. doi: 10.1016/j.mcn.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Oka C, Nakano T, Wakeham A, de la Pompa JL, Mori C, Sakai T, Okazaki S, Kawaichi M, Shiota K, Mak TW, Honjo T. Disruption of the mouse RBP-Jk gene results in early embryonic death. Development. 1995;121:3291–3301. doi: 10.1242/dev.121.10.3291. [DOI] [PubMed] [Google Scholar]
- Palma V, Lim DA, Dahmane N, Sánchez P, Brionne TC, Herzberg CD, Gitton Y, Carleton A, Alvarez-Buylla A, Ruiz i Altaba A. Sonic hedgehog controls stem cell behavior in the postnatal and adult brain. Development. 2005;132:335–344. doi: 10.1242/dev.01567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parras CM, Galli R, Britz O, Soares S, Galichet C, Battiste J, Johnson JE, Nakafuku M, Vescovi A, Guillemot F. Mash1 specifies neurons and oligodendrocytes in the postnatal brain. EMBO J. 2004;23:4495–4505. doi: 10.1038/sj.emboj.7600447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. A small step for the cell, a giant leap for mankind: a hypothesis of neocortical expansion during evolution. Trends Neurosci. 1995;18:383–388. doi: 10.1016/0166-2236(95)93934-p. [DOI] [PubMed] [Google Scholar]
- Ross SE, Greenberg ME, Stiles CD. Basic helix-loop-helix factors in cortical development. Neuron. 2003;39:13–25. doi: 10.1016/s0896-6273(03)00365-9. [DOI] [PubMed] [Google Scholar]
- Shimojo H, Ohtsuka T, Kageyama R. Oscillations in Notch signaling regulate maintenance of neural progenitors. Neuron. 2008;58:52–64. doi: 10.1016/j.neuron.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ström A, Arai N, Leers J, Gustafsson JA. The Hairy and Enhancer of Split homologue-1 (HES-1) mediates the proliferative effect of 17beta-estradiol on breast cancer cell lines. Oncogene. 2000;19:5951–5953. doi: 10.1038/sj.onc.1203990. [DOI] [PubMed] [Google Scholar]
- Taylor MK, Yeager K, Morrison SJ. Physiological Notch signaling promotes gliogenesis in the developing peripheral and central nervous system. Development. 2007;134:2435–2447. doi: 10.1242/dev.005520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple S. The development of neural stem cells. Nature. 2001;414:112–117. doi: 10.1038/35102174. [DOI] [PubMed] [Google Scholar]
- Tramontin AD, García-Verdugo JM, Lim DA, Alvarez-Buylla A. Postnatal development of radial glia and ventricular zone (VZ): a continuum of the neural stem cell compartment. Cereb Cortex. 2003;13:580–587. doi: 10.1093/cercor/13.6.580. [DOI] [PubMed] [Google Scholar]
- Yoon KJ, Koo BK, Im SK, Jeong HW, Ghim J, Kwon MC, Moon JS, Miyata T, Kong YY. Mind bomb 1-expressing intermediate progenitors generate Notch signaling to maintain radial glial cells. Neuron. 2008;58:519–531. doi: 10.1016/j.neuron.2008.03.018. [DOI] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]