Introduction
Charcot–Marie–Tooth disease (CMT) encompasses a group of mostly non-syndromic inherited peripheral motor and sensory neuropathies, which are genetically heterogeneous and have a prevalence of ∼17–40:100,000 worldwide—one of the most common neurogenetic disorders (Martyn and Hughes, 1997). The X-linked form of CMT (CMT1X) is the second most common form among all CMT patients with a frequency of 7–18% (average 12% internationally) (Nicolaou et al., 2010; Braathen et al., 2011). CMT1X is caused by >400 different mutations in the GJB1 gene that encodes the gap junction (GJ) protein connexin32 (Cx32). Cx32 forms both intercellular (between adjacent cells), as well as intracellular, “reflexive” (between layers of the same cell) GJs in both peripheral and central myelinating cells that play an important role in the homeostasis of myelinated axons. In vitro and in vivo models of the disease have demonstrated impaired formation of GJs by mutant Cx32 and that loss of Cx32 function accounts for the peripheral neuropathy. An effective therapy for CMT1X remains to be developed.
Clinical and pathological features of CMT1X
The clinical phenotype of CMT1X is characterized by slowly progressive weakness and atrophy starting in distal leg muscles. Difficulty running and frequently sprained ankles may be reported as initial symptoms, typically beginning by 10 years of age or earlier in most affected males (Birouk et al., 1998; Shy et al., 2007; Yiu et al., 2011). Hands may be affected later on, particularly the thenar muscles. Sensory loss in distal limbs, sometimes combined with painful sensory paresthesias may also develop. Compared with affected males, heterozygous females may be asymptomatic, or may develop milder clinical manifestations at an older age, or at least have electrophysiological evidence or subtle clinical findings of peripheral neuropathy. However, exceptionally severe and early neuropathy phenotypes have been reported also in female CMT1X patients (Wicklein et al., 1997; Dubourg et al., 2001; Kuntzer et al., 2003; Karadima et al., 2004; Liang et al., 2005). Thus, CMT1X is best described as an X-linked dominant disease (Kleopa and Scherer, 2006). The variable phenotype in women may be explained by the random X-chromosome inactivation in each myelinating cell (Siskind et al., 2011), so that different fractions of myelinating Schwann cells express the mutant GJB1 allele in each case (Scherer et al., 1998).
Electrophysiological studies typically show intermediate or only mild slowing of motor nerve conduction velocities in the majority of CMT1X patients as well as distal axonal loss which progresses with age (Rozear et al., 1987; Hahn et al., 1990, 1999; Nicholson and Nash, 1993; Rouger et al., 1997; Birouk et al., 1998; Senderek et al., 1999), but can be found even in young children (Yiu et al., 2011). Thus, CMT1X is distinct from most other forms of demyelinating CMT1 that are caused by mutations in myelin-related genes expressed by Schwann cells.
Pathologically, sural nerve biopsy shows early axonal alterations and less prominent demyelination in CMT1X compared with other CMT1 types (Senderek et al., 1999; Hahn et al., 2001; Hattori et al., 2003). Typical features include age-related loss of myelinated fibers and, in parallel, an increasing number of regenerated axon clusters (Rozear et al., 1987; Hahn et al., 1990, 1999; Nicholson and Nash, 1993; Birouk et al., 1998; Sander et al., 1998; Senderek et al., 1998, 1999; Tabaraud et al., 1999; Gutierrez et al., 2000; Vital et al., 2001; Kleopa et al., 2006). Many myelin sheaths are inappropriately thin for the axonal diameter, suggesting chronic segmental demyelination and remyelination or remyelination after axonal regeneration (Sander et al., 1998; Hahn et al., 2001; Vital et al., 2001; Hattori et al., 2003; Kleopa et al., 2006). Ultrastructural studies have shown enlargement and widening of the adaxonal Schwann cell cytoplasm, myelin discompaction, and vesicle formation between degenerating innermost myelin layers (Senderek et al., 1999; Hahn et al., 2001; Kuntzer et al., 2003; Kleopa et al., 2006), as well as increased packing density of axonal neurofilaments (Hahn et al., 2001). Thus, CMT1X shows more prominent axonal pathology and not the obvious changes of demyelination and remyelination that are typical for other demyelinating neuropathies, indicating that GJs formed by Cx32 are crucial for the axon itself as much as for its myelin sheath.
In addition to peripheral neuropathy, CMT1X patients often have asymptomatic evidence of CNS involvement, such as abnormal brainstem auditory evoked potentials (Nicholson and Corbett, 1996; Nicholson et al., 1998). Moreover, CMT1X mutations have been increasingly associated with clinical CNS phenotypes. Signs of chronic corticospinal tract dysfunction such as spasticity, extensor plantar responses and hyperactive reflexes have been reported in patients with the A39V (Marques et al., 1999), T55I (Panas et al., 1998), M93V (Bell et al., 1996), R164Q (Panas et al., 1998), R183H (Bort et al., 1997), T191 frameshift (Lee et al., 2002), and L143P (Kleopa et al., 2006) mutations. Duplication of amino acids 55–61 is associated with progressive cerebellar ataxia, dysarthria, and delayed central somatosensory responses (Kawakami et al., 2002).
Acute transient encephalopathy syndromes associated with brain MRI changes have been described in CMT1X patients with the T55I, R75W, E102del, R142W, R164W and C168Y mutations (Panas et al., 2001; Paulson et al., 2002; Schelhaas et al., 2002; Hanemann et al., 2003; Taylor et al., 2003). In most patients encephalopathy developed under conditions of metabolic stress caused by travel to high altitudes (Paulson et al., 2002), febrile illness (Schelhaas et al., 2002; Hanemann et al., 2003), hyperventilation (Srinivasan et al., 2008), or concussion (Halbrich et al., 2008). CNS dysfunction caused by GJB1 mutations has even been reported in childhood as early as 5 years of age (Siskind et al., 2009) and overall does not appear to correlate with the stage and severity of the peripheral neuropathy: in some cases it was the first manifestation of CMT1X, while in others with exceptionally severe neuropathy, no clinical CNS phenotypes were present.
Although many cell types express Cx32, peripheral neuropathy is usually the sole clinical manifestation of GJB1 mutations except for the occasional CNS phenotypes described above. The coexpression of other connexins in other tissues appears to provide at least partial functional redundancy that protects against the loss of Cx32. Oligodendrocytes, for example, coexpress Cx47, and at least in rodents, the loss of both Cx32 and Cx47 is far more deleterious than the loss of either one alone (Menichella et al., 2003; Odermatt et al., 2003). However, loss-of-function mutations in GJC2 encoding Cx47 in humans cause Pelizeaus-Merzbacher-like disease (Uhlenberg et al., 2004; Tress et al., 2011). Thus, in humans and not in mice the loss of Cx47 alone produces a severe leukodystrophy. Although myelinating Schwann cells in rodents also express Cx29 (the human orthologue is Cx31.3), Cx29/Cx31.3 does not prevent the development of demyelinating neuropathy, likely because it does not form functional GJs (Ahn et al., 2008; Sargiannidou et al., 2008).
Genetic and neurobiological basis of CMT1X
GJs are cell membrane channels found in most tissues, connecting adjacent cells or, in the case of the myelin sheath, different layers of the same cell (Bruzzone et al., 1996; White and Paul, 1999). Channels are composed of two apposed connexons (or hemichannels) and each connexon is composed of a hexamer of connexin molecules arranged around a central pore. The GJ channel of ∼1.2 nm diameter allows the transfer of molecules smaller that 1000 Da, including ions and second messengers, enabling metabolic cooperation, spatial buffering, and electrical coupling (Bruzzone et al., 1996). Connexins belong to a family of >20 highly conserved integral membrane proteins that are usually named according to their predicted molecular mass (Willecke et al., 2002). They are highly homologous with the same overall topology: a cytoplasmic N terminus, four transmembrane domains with α helix structure, one intracellular and two extracellular loops, and a cytoplasmic C terminus (Bruzzone et al., 1996; Unger et al., 1999; White and Paul, 1999). The first transmembrane domain forms the central pore (Maeda et al., 2009). The intracellular loop and C-terminal domain are the most divergent parts of the connexins and determine the differences in their molecular mass (Willecke et al., 2002). The extracellular loops regulate the connexon–connexon interactions via disulfide bonds formed by conserved cysteine residues.
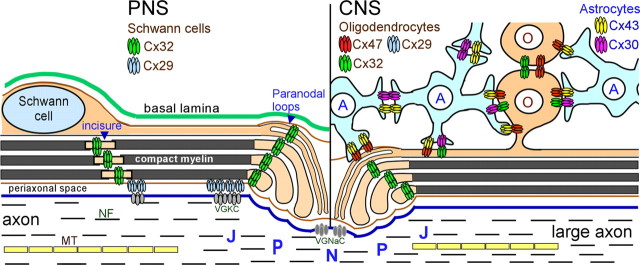
Cx32 is a myelin-related protein localized to non-compact myelin of incisures and paranodes. GJ-like structures were first observed by freeze-fracture electron microscopy at these areas (Schnapp and Mugnaini, 1978; Sandri et al., 1982; Tetzlaff, 1982). The localization of Cx32 in the same areas by immunohistochemistry (Bergoffen et al., 1993; Scherer et al., 1995) suggested that Cx32 forms these GJs between the layers of the Schwann cell myelin sheath (Fig. 1). This localization has been confirmed by freeze-fracture replica immunogold labeling (Meier et al., 2004). Diffusion of low molecular mass fluorescent dyes across the myelin sheath has been documented by fluorescence microscopy following injection in the perinuclear region of living myelinating Schwann cells (Balice-Gordon et al., 1998). This radial pathway formed by GJs at these locations would be up to a 1000-fold shorter than the circumferential pathway following the Schwann cell cytoplasm (Scherer et al., 1995). Whether this shortcut is disrupted in CMT1X and how this disturbs the homeostasis of myelinated axons has not been fully elucidated.
Figure 1.
Expression of Cx32 in PNS and CNS myelinated fibers. Schematic diagram showing the localization of Cx32 and other GJ proteins in myelinating Schwann cells in the PNS (left) and in the CNS (right) oligodendrocytes (O) as well as astrocytes (A). In Schwann cells Cx32 forms GJs through the non-compact myelin areas including the paranodes and Schmidt-Lantermann incisures. Coexpressed Cx29 likely forms hemichannels in the innermost aspect of these areas adjacent to the axonal membrane and apposing voltage-gated potassium channels (VGKC). In the CNS, all oligodendrocytes express Cx47 in cell bodies and proximal processes, and Cx47 forms most O:A GJs, while both Cx47 and Cx32 form O:O GJs. In large myelinated fibers they coexpress Cx32, and in small myelinated fibers (data not shown), Cx29. Astrocytes connect with oligodendrocytes and other astrocytes mainly with Cx43 and Cx30. NF, Neurofilaments; MT, microtubule; N, node; P, paranode; J, juxtaparanode; VGNaC, voltage-gated sodium channels.
Following the first report by Bergoffen et al. (1993), >400 mutations in GJB1 have been described, predicted to affect all regions of the Cx32 protein (listed in http://www.molgen.ua.ac.be/cmtmutations/). Only one of the reported amino acid changes is a polymorphism (Brozková et al., 2010), indicating that most affected residues are highly conserved and indeed required for the normal function of Cx32. Many of the mutations have been reported more than once, either representing founder effects or mutational “hot spots” in GJB1. In a few CMT1X kindreds the entire coding region of GJB1 is deleted (Gonzaga-Jauregui et al., 2010). In addition, several mutations in the noncoding 5′-untranslated region of the GJB1 gene likely abolish the expression of Cx32 by affecting the promoter or the translation of Cx32 mRNA (Murphy et al., 2011).
Clinical studies of a large number of CMT1X patients with different GJB1 mutations have shown that disability increases with age, and that the degree of disability was comparable to that observed in patients with a documented GJB1 deletion (Shy et al., 2007). Thus, different GJB1 mutations, including deletions and frame shift mutations, appear to cause a similar degree of neuropathy (Shy et al., 2007; Lin et al., 2010), indicating that most GJB1 mutations cause loss of function. The results of clinical studies are in agreement with pathological studies showing that the severity of changes in CMT1X nerve biopsies are not associated with particular GJB1 mutations (Hahn et al., 2000; Nakagawa et al., 2001; Hattori et al., 2003). Moreover, a GJB1 frameshift mutation caused a CNS phenotype similar to those caused by missense mutations (Sakaguchi et al., 2011), suggesting that CNS phenotypes in CMT1X also lack any consistent genotype-phenotype correlation.
Models of CMT1X and the molecular mechanisms of Cx32 mutants
When expressed in Xenopus oocytes, many Cx32 mutants that cause CMT1X fail to form functional channels and some of them also exert dominant-negative effects on the coexpressed WT Cx32 (Bruzzone et al., 1994). Other mutants form functional channels with altered biophysical characteristics, such as reduced pore diameter that may prevent the diffusion of second messengers like IP3, cAMP, and Ca2+ (Oh et al., 1997). The position of the Cx32 mutation alone does not necessarily predict the molecular and functional consequences, as the R15Q and H94Q mutants form normal functional channels, whereas R15W and H94Y do not (Abrams et al., 2001). When expressed in mammalian cells with more stringent protein trafficking requirements, Cx32 mutants are often retained intracellularly, even if they form rare GJ-like plaques (Omori et al., 1996; Yoshimura et al., 1998; Yum et al., 2002). They are localized predominately in the ER or Golgi (Deschênes et al., 1997; Oh et al., 1997; Martin et al., 2000; Matsuyama et al., 2001; Kleopa et al., 2002, 2006; Yum et al., 2002) and are degraded via endosomal and proteasomal pathways (VanSlyke et al., 2000; Kleopa et al., 2002). Several mutants, the majority of which occur in the C-terminal domain, were mainly localized on the cell membrane and show no significant difference from WT protein (Kleopa et al., 2002; Yum et al., 2002), but they may be less stable or have abnormal biophysical properties (Rabadan-Diehl et al., 1994; Castro et al., 1999; Abrams et al., 2000). In one case they form leaky hemichannels with severe phenotype (Liang et al., 2005).
The cellular effects of Cx32 mutants in vitro highlight some structure–function correlations for Cx32 (Abrams et al., 2000). N-terminal mutations result in altered biophysical properties and may cause reversal of gating polarity by negative charge substitutions. This is in keeping with the role of this domain in the insertion of the nascent polypeptide chain into the ER, and along with the first transmembrane domain in the regulation of voltage gating (Maeda et al., 2009). Shifted voltage gating and abnormally increased opening have been shown for several mutants affecting the first and second transmembrane domain, which cause conformational changes (Abrams et al., 2002). Mutations affecting the cysteine residues in the two extracellular loops, which mediate the interactions between apposed connexons, lead to a loss of functional channels. Mutations of the intracellular loop and C-terminal domain may affect pH gating (Castro et al., 1999). Two mutations that affect a consensus prenylation motif of Cx32 (C280G and S281X) abolish prenylation, a lipid modification (Huang et al., 2005).
Several animal models have provided further insights into CMT1X pathogenesis. Mice with targeted deletion of the Gjb1/cx32 gene develop a progressive, predominantly motor demyelinating peripheral neuropathy beginning at ∼3 months of age (Anzini et al., 1997; Scherer et al., 1998). Heterozygous females have fewer demyelinated and remyelinated axons than age-matched Gjb1/cx32-null females or males (Scherer et al., 1998), in keeping with the clinical phenotype of affected women who are obligate carriers of CMT1X. Expression of wild-type human Cx32 protein driven by the rat Mpz promoter prevents demyelination in Gjb1/cx32-null mice (Scherer et al., 2005), confirming that the loss of Schwann cell-autonomous expression of Cx32 is sufficient to account for CMT1X pathology.
In addition, Cx32 KO mice show subtle CNS myelin defects, including diminished myelinated fiber and myelin volume density, particularly in white matter tracts with prominent Cx32 expression, such as the ventral and dorsal funiculus of the spinal cord (Sargiannidou et al., 2009), but also in the neocortex (Sutor et al., 2000). Optic nerves, which do not significantly express Cx32 (Kleopa et al., 2004), are free of pathology (Scherer et al., 1998; Sargiannidou et al., 2009).
Transgenic mice expressing the 175fs, T55I, R75W, R142W, C280G, and S281X Cx32 mutations have also been generated. No Cx32 protein could be detected in 175fs transgenic mice despite expression of the transgenic mRNA (Abel et al., 1999). In contrast, R142W transgenic mice showed retention of the mutant protein in the perinuclear region and developed a mild demyelinating neuropathy (Scherer et al., 1999). Moreover, the presence of the mutant Cx32 reduced the level of the endogenous mouse Cx32, indicating that R142W may have dominant-negative interactions with the WT protein. However, this is not clinically relevant in patients with CMT1X as only one GJB1 allele is expressed in each cell. The R142W mutant did not affect the coexpressed Cx29 in Schwann cells (Jeng et al., 2006). The C280G and S281X mutants were properly localized to incisures and paranodes of myelinating Schwann cells and even prevented demyelination in Gjb1/cx32-null mice, indicating that they may form functional channels in the myelin sheath (Huang et al., 2005). How they cause neuropathy in humans remains unclear.
Transgenic mice that express the T55I and R75W mutants in both Schwann cells and oligodendrocytes were generated to clarify whether Cx32 mutants associated with CNS phenotypes (Kleopa et al., 2002) could have gain-of-function effects in oligodendrocytes. In myelinating cells, as in cultured cells, these Cx32 mutants are retained intracellularly and fail to reach the membrane and to form GJ-like plaques. The T55I mutant is localized in the ER and the R75W mostly in the Golgi. On a Gjb1-null background they cause a progressive demyelinating neuropathy as well as mild CNS myelination defects. However, they do not appear to affect the expression and localization of coexpressed Cx29 in Schwann cells or Cx29 and Cx47 in oligodendrocytes. Similar to R142W, and without clinical relevance, the Golgi-retained R75W mutant impairs the expression of endogenous Cx32 on a WT background. Thus, the loss of Cx32 function appears to be the main effect of the T55I and R75W mutants, in both the PNS and the CNS (Sargiannidou et al., 2009).
Together, the effects of Cx32 mutants expressed in vivo correlate with their localization in myelinating cells and in transfected cell lines (Deschênes et al., 1997; Kleopa et al., 2002; Yum et al., 2002), and indicate that altered synthesis or trafficking and loss of GJ function is the fundamental mechanism in most CMT1X mutants both in PNS and in CNS myelinating cells.
Axonal involvement in CMT1X
Axonal pathology in CMT patients is an important determinant of disability, and correlates with clinical progression not only in axonal forms but also in primarily demyelinating CMT types (Krajewski et al., 2000). In the typical demyelinating CMT1 forms, axonal pathology is thought to be secondary to demyelination (Giese et al., 1992; Martini et al., 1995; Sancho et al., 1999). In contrast, the mixed axonal and demyelinating clinicopathological features of CMT1X (Hattori et al., 2003; Yiu et al., 2011) suggest that axonal alterations develop early, and even in the absence of demyelination. Experimentally, mutant Schwann cells from CMT1X patients transplanted into sciatic nerves of nude mice induced increased density of axonal neurofilaments, depletion of microtubules, and increased density of vesicles and mitochondria without demyelination (Sahenk and Chen, 1998).
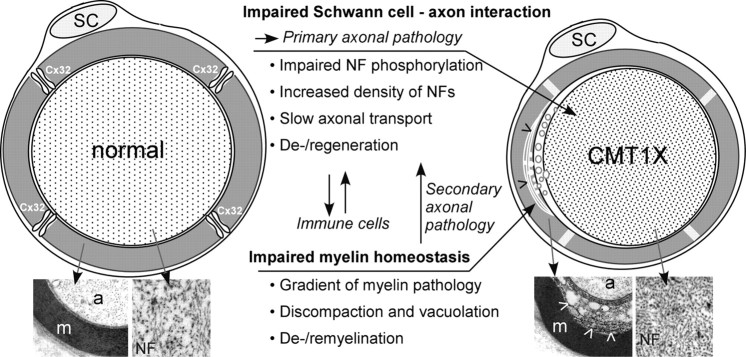
Although the initial characterization of Gjb1-null mice showed that demyelination preceded axonal loss (Anzini et al., 1997; Scherer et al., 1998; Sargiannidou et al., 2009), we have recently reinvestigated this issue in Gjb1-null mice at the ages of 2–4 months, before the onset of demyelination (Vavlitou et al., 2010). At the age of 4 months, only ∼5% of fibers are abnormally myelinated and only ∼1% are completely demyelinated, whereas at 2 months, demyelination has not yet started (Anzini et al., 1997; Sargiannidou et al., 2009). This study showed that the diameter of the myelinated axons was progressively reduced in Gjb1-null mice while neurofilaments were increasingly dephosphorylated and more densely packed. These cytoskeletal alterations were associated with slowing of axonal transport (Vavlitou et al., 2010). Thus, impaired cytoskeletal organization and axonal transport defects appear to precede demyelination in this mouse model, providing clues to the mechanisms of early axonopathy in CMT1X. Disturbed axon-glial signaling and glial support of axon function (Nave and Trapp, 2008) is likely to account for this axonal pathology independent of myelination (Fig. 2).
Figure 2.
Pathomechanisms in CMT1X. This diagram illustrates the main molecular-pathological mechanisms occurring in myelinated fibers of CMT1X patients, which have also been reproduced in animal models. Loss of GJs formed by Cx32 in non-compact myelin areas of myelinated fibers leads to impaired Schwann cell–axon interaction and primary axonal pathology. Major aspects of this include impaired phosphorylation and increased packing density of neurofilaments (NF), slowing of axonal transport, and axonal de- and regeneration. In parallel, the myelin sheath shows characteristic alterations including a gradient of pathology with discompaction of the innermost layers and vacuole formation (arrowheads), leading eventually to de- and remyelination. Electron microscopy images of control and CMT1X nerve fibers illustrate these changes (m, myelin; a, axon). Immune cells may be recruited to the nerves following these pathological changes, and their action may further exacerbate CMT1X pathological changes.
Future challenges and perspectives in CMT1X
CMT1X, like other CMT forms, remains an incurable disease. Both clinical studies of large series of CMT1X patients as well as established disease models (above) suggest that most GJB1 mutations appear to cause CMT1X through loss of normal Cx32 function. Since CMT1X results from cell-autonomous loss-of-function Cx32 mutations (Scherer et al., 2005), therapeutic gene delivery specifically to Schwann cells is likely to succeed in preventing the neuropathy and should be a priority in future research efforts. There have been previous attempts to deliver therapeutic genes to intact Schwann cells with adenovirus and adeno-associated virus, which did not provide significant results (Sørensen et al., 1998), mainly due to inefficient transduction in the cells of interest and host immune response. Alternative methods of gene delivery have also failed, as transient expression limits the nonviral gene delivery approach using naked DNA, and the stem cell approach is hampered by the difficulties of differentiation into Schwann cells (Shy, 2006).
Lentiviral vectors may be a possible tool for gene delivery to Schwann cells, as they have reached the clinical trial stage (Levine et al., 2006) and clinical translation of such vectors aimed at treating neuromuscular disorders may ultimately be feasible. They combine the advantages of long-term transgene expression (Kordower et al., 2000; Azzouz et al., 2004), minimal immunogenicity, ability to accommodate larger transgenes, and the capacity to form pseudotypes with a wide variety of different glycoproteins (Federici et al., 2009). Specific expression in Schwann cells can be achieved under the control of a myelin-related promoter such as the myelin protein zero (MPZ) or even the “glial promoter” of GJB1 itself.
One concern is the dominant effect on endogenous WT Cx32 of Golgi-retained mutants such as R75W (Sargiannidou et al., 2009) or R142W (Jeng et al., 2006), which have been expressed in vivo. While this dominant-negative effect is not clinically relevant for patients, since only one GJB1 allele is expressed in each cell, it may raise concerns for future gene replacement therapies. In this scenario, the endogenous mutant protein could potentially attenuate the expression and function of exogenously delivered WT Cx32. Whether this possibility will affect the outcome of such treatment may also depend on the expression levels achieved by the gene delivery methods, and will need to be addressed and clarified in future trials using the available CMT1X mouse models.
Further questions on CMT1X pathogenesis remain. We still have no exact knowledge of the molecules transported through Cx32 GJs in PNS and CNS and how their impaired transport affects the integrity of myelin and axon. The early abnormalities in axonal cytoskeleton dynamics in Gjb1-null mice (Vavlitou et al., 2010) suggest that signaling pathways that regulate axonal cytoskeleton, for example through phosphorylation, are impaired. Perhaps the loss of a signal that depends on these GJs initiates the axonal alterations. The IP3-receptor-3 colocalizes with Cx32 in the paranodal areas of Schwann cells (Toews et al., 2007). During neural activity, intracellular Ca2+ rises through the IP3 signaling cascade and undergoes rhyanodin-dependent released from ER specifically at areas of non-compact myelin (Lev-Ram and Ellisman, 1995). Loss of Cx32 GJs may impair the radial spread of Ca2+ signals and second messengers initiated during action potential propagation (Toews et al., 2007). In vitro studies have shown that GJs allow the diffusion of Ca2+-mobilizing second messengers across coupled cells (Anselmi et al., 2008) and intracellular calcium concentration regulates Cx32 hemichannel opening (De Vuyst et al., 2006). It remains to be determined whether Cx32 GJs in non-compact myelin areas of myelinated fibers serve as conduits for the rapid radial spread of these Ca2+ and IP3 signals required for axonal integrity.
Impaired energy supply is another possibility, since Schwann cell GJs play a role in delivering glucose to the axons (Véga et al., 2003). Finally, a number of studies support the role of secondary immune mechanisms in the pathological changes observed in CMT1X mouse models (Kobsar et al., 2003; Groh et al., 2010). This appears to be a more general phenomenon shared by other inherited demyelinating neuropathies (Ip et al., 2006). Whether immunomodulation could offer additional therapeutic opportunities for CMT patients and CMT1X in particular requires further study.
Footnotes
Editor's Note: Disease Focus articles provide brief overviews of a neural disease or syndrome, emphasizing potential links to basic neural mechanisms. They are presented in the hope of helping researchers identify clinical implications of their research. For more information, see http://www.jneurosci.org/misc/ifa_minireviews.dtl.
The contributions of many collaborators to the work summarized here, especially Dr. Steven S. Scherer, are greatly acknowledged. Work in the author's laboratory has been funded by the Cyprus Research Promotion Foundation (Grant HEALTH/BIOS/0609/BIE/09), the USA National Multiple Sclerosis Society (Grant RG3457A2/1), and the Cyprus Telethon (2006–2011).
References
- Abel A, Bone LJ, Messing A, Scherer SS, Fischbeck KH. Studies in transgenic mice indicate a loss of connexin32 function in X-linked Charcot-Marie-Tooth disease. J Neuropathol Exp Neurol. 1999;58:702–710. doi: 10.1097/00005072-199907000-00004. [DOI] [PubMed] [Google Scholar]
- Abrams CK, Oh S, Ri Y, Bargiello TA. Mutations in connexin 32: the molecular and biophysical bases for the X-linked form of Charcot-Marie-Tooth disease. Brain Res Rev. 2000;32:203–214. doi: 10.1016/s0165-0173(99)00082-x. [DOI] [PubMed] [Google Scholar]
- Abrams CK, Freidin MM, Verselis VK, Bennett MV, Bargiello TA. Functional alterations in gap junction channels formed by mutant forms of connexin 32: evidence for loss of function as a pathogenic mechanism in the X-linked form of Charcot-Marie-Tooth disease. Brain Res. 2001;900:9–25. doi: 10.1016/s0006-8993(00)03327-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrams CK, Bennett MV, Verselis VK, Bargiello TA. Voltage opens unopposed gap junction hemichannels formed by a connexin 32 mutant associated with X-linked Charcot-Marie-Tooth disease. Proc Natl Acad Sci U S A. 2002;99:3980–3984. doi: 10.1073/pnas.261713499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn M, Lee J, Gustafsson A, Enriquez A, Lancaster E, Sul JY, Haydon PG, Paul DL, Huang Y, Abrams CK, Scherer SS. Cx29 and Cx32, two connexins expressed by myelinating glia, do not interact and are functionally distinct. J Neurosci Res. 2008;86:992–1006. doi: 10.1002/jnr.21561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anselmi F, Hernandez VH, Crispino G, Seydel A, Ortolano S, Roper SD, Kessaris N, Richardson W, Rickheit G, Filippov MA, Monyer H, Mammano F. ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear. Proc Natl Acad Sci U S A. 2008;105:18770–18775. doi: 10.1073/pnas.0800793105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzini P, Neuberg DH, Schachner M, Nelles E, Willecke K, Zielasek J, Toyka KV, Suter U, Martini R. Structural abnormalities and deficient maintenance of peripheral nerve myelin in mice lacking the gap junction protein connexin32. J Neurosci. 1997;17:4545–4551. doi: 10.1523/JNEUROSCI.17-12-04545.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzouz M, Ralph GS, Storkebaum E, Walmsley LE, Mitrophanous KA, Kingsman SM, Carmeliet P, Mazarakis ND. VEGF delivery with retrogradely transported lentivector prolongs survival in a mouse ALS model. Nature. 2004;429:413–417. doi: 10.1038/nature02544. [DOI] [PubMed] [Google Scholar]
- Balice-Gordon RJ, Bone LJ, Scherer SS. Functional gap junctions in the Schwann cell myelin sheath. J Cell Biol. 1998;142:1095–1104. doi: 10.1083/jcb.142.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell C, Willison H, Clark C, Haites N. CNS abnormalities in a family with a connexin32 mutation and peripheral neuropathy. Eur J Hum Genet. 1996;4:S136. [Google Scholar]
- Bergoffen J, Scherer SS, Wang S, Oronzi-Scott M, Bone LJ, Paul DL, Chen K, Lensch MW, Chance PF, Fischbeck KH. Connexin mutations in X-linked Charcot-Marie-Tooth disease. Science. 1993;262:2039–2042. doi: 10.1126/science.8266101. [DOI] [PubMed] [Google Scholar]
- Birouk N, LeGuern E, Maisonobe T, Rouger H, Gouider R, Gugenheim M, Tardieu S, Gugenheim M, Routon MC, Léger JM, Agid Y, Brice A, Bouche P. X-linked Charcot-Marie-Tooth disease with connexin 32 mutations—clinical and electrophysiological study. Neurology. 1998;50:1074–1082. doi: 10.1212/wnl.50.4.1074. [DOI] [PubMed] [Google Scholar]
- Bort S, Nelis E, Timmerman V, Sevilla T, Cruz-Martínez A, Martínez F, Millán JM, Arpa J, Vílchez JJ, Prieto F, Van Broeckhoven C, Palau F. Mutational analysis of the MPZ, PMP22 and Cx32 genes in patients of Spanish ancestry with Charcot-Marie-Tooth disease and hereditary neuropathy with liability to pressure palsies. Hum Genet. 1997;99:746–754. doi: 10.1007/s004390050442. [DOI] [PubMed] [Google Scholar]
- Braathen GJ, Sand JC, Lobato A, Høyer H, Russell MB. Genetic epidemiology of Charcot-Marie-Tooth in the general population. Eur J Neurol. 2011;18:39–48. doi: 10.1111/j.1468-1331.2010.03037.x. [DOI] [PubMed] [Google Scholar]
- Brozková D, Mazanec R, Haberlová J, Sakmaryová I, Subrt I, Seeman P. Six new gap junction beta 1 gene mutations and their phenotypic expression in Czech patients with Charcot-Marie-Tooth disease. Genet Test Mol Biomarkers. 2010;14:3–7. doi: 10.1089/gtmb.2009.0093. [DOI] [PubMed] [Google Scholar]
- Bruzzone R, White TW, Scherer SS, Fischbeck KH, Paul DL. Null mutations of connexin32 in patients with X-linked Charcot-Marie-Tooth disease. Neuron. 1994;13:1253–1260. doi: 10.1016/0896-6273(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Bruzzone R, White TW, Paul DL. Connections with connexins: the molecular basis of direct intercellular signaling. Eur J Biochem. 1996;238:1–27. doi: 10.1111/j.1432-1033.1996.0001q.x. [DOI] [PubMed] [Google Scholar]
- Castro C, Gómez-Hernandez JM, Silander K, Barrio LC. Altered formation of hemichannels and gap junction channels caused by C-terminal connexin-32 mutations. J Neurosci. 1999;19:3752–3760. doi: 10.1523/JNEUROSCI.19-10-03752.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschênes SM, Walcott JL, Wexler TL, Scherer SS, Fischbeck KH. Altered trafficking of mutant connexin32. J Neurosci. 1997;17:9077–9084. doi: 10.1523/JNEUROSCI.17-23-09077.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vuyst E, Decrock E, Cabooter L, Dubyak GR, Naus CC, Evans WH, Leybaert L. Intracellular calcium changes trigger connexin 32 hemichannel opening. EMBO J. 2006;25:34–44. doi: 10.1038/sj.emboj.7600908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubourg O, Tardieu S, Birouk N, Gouider R, Léger JM, Maisonobe T, Brice A, Bouche P, LeGuern E. Clinical, electrophysiological and molecular genetic characteristics of 93 patients with X-linked Charcot–Marie–Tooth disease. Brain. 2001;124:1958–1967. doi: 10.1093/brain/124.10.1958. [DOI] [PubMed] [Google Scholar]
- Federici T, Kutner R, Zhang XY, Kuroda H, Tordo N, Boulis NM, Reiser J. Comparative analysis of HIV-1-based lentiviral vectors bearing lyssavirus glycoproteins for neuronal gene transfer. Genet Vaccines Ther. 2009;7:1. doi: 10.1186/1479-0556-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese KP, Martini R, Lemke G, Soriano P, Schachner M. Mouse P0 gene disruption leads to hypomyelination, abnormal expression of recognition molecules, and degeneration of myelin and axons. Cell. 1992;71:565–576. doi: 10.1016/0092-8674(92)90591-y. [DOI] [PubMed] [Google Scholar]
- Gonzaga-Jauregui C, Zhang F, Towne CF, Batish SD, Lupski JR. GJB1/Connexin 32 whole gene deletions in patients with X-linked Charcot-Marie-Tooth disease. Neurogenetics. 2010;11:465–470. doi: 10.1007/s10048-010-0247-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groh J, Heinl K, Kohl B, Wessig C, Greeske J, Fischer S, Martini R. Attenuation of MCP-1/CCL2 expression ameliorates neuropathy in a mouse model for Charcot-Marie-Tooth 1X. Hum Mol Genet. 2010;19:3530–3543. doi: 10.1093/hmg/ddq269. [DOI] [PubMed] [Google Scholar]
- Gutierrez A, England JD, Sumner AJ, Ferer S, Warner LE, Lupski JR, Garcia CA. Unusual electrophysiological findings in X-linked dominant Charcot-Marie-Tooth disease. Muscle Nerve. 2000;23:182–188. doi: 10.1002/(sici)1097-4598(200002)23:2<182::aid-mus6>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Hahn AF, Brown WF, Koopman WJ, Feasby TE. X-linked dominant hereditary motor and sensory neuropathy. Brain. 1990;113:1511–1525. doi: 10.1093/brain/113.5.1511. [DOI] [PubMed] [Google Scholar]
- Hahn AF, Bolton CF, White CM, Brown WF, Tuuha SE, Tan CC, Ainsworth PJ. Genotype/phenotype correlations in X-linked Charcot-Marie-Tooth disease. Ann N Y Acad Sci. 1999;883:366–382. [PubMed] [Google Scholar]
- Hahn AF, Ainsworth PJ, Naus CC, Mao J, Bolton CF. Clinical and pathological observations in men lacking the gap junction protein connexin 32. Muscle Nerve Suppl. 2000;9:S39–S48. [PubMed] [Google Scholar]
- Hahn AF, Ainsworth PJ, Bolton CF, Bilbao JM, Vallat JM. Pathological findings in the X-linked form of Charcot-Marie-Tooth disease: a morphometric and ultrastructural analysis. Acta Neuropathol. 2001;101:129–139. doi: 10.1007/s004010000275. [DOI] [PubMed] [Google Scholar]
- Halbrich M, Barnes J, Bunge M, Joshi C. A V139M mutation also causes the reversible CNS phenotype in CMTX. Can J Neurol Sci. 2008;35:372–374. doi: 10.1017/s0317167100008994. [DOI] [PubMed] [Google Scholar]
- Hanemann CO, Bergmann C, Senderek J, Zerres K, Sperfeld AD. Transient, recurrent, white matter lesions in X-linked Charcot-Marie-Tooth disease with novel connexin 32 mutation. Arch Neurol. 2003;60:605–609. doi: 10.1001/archneur.60.4.605. [DOI] [PubMed] [Google Scholar]
- Hattori N, Yamamoto M, Yoshihara T, Koike H, Nakagawa M, Yoshikawa H, Ohnishi A, Hayasaka K, Onodera O, Baba M, Yasuda H, Saito T, Nakashima K, Kira J, Kaji R, Oka N, Sobue G. Demyelinating and axonal features of Charcot-Marie-Tooth disease with mutations of myelin-related proteins (PMP22, MPZ and Cx32): a clinicopathological study of 205 Japanese patients. Brain. 2003;126:134–151. doi: 10.1093/brain/awg012. [DOI] [PubMed] [Google Scholar]
- Huang Y, Sirkowski EE, Stickney JT, Scherer SS. Prenylation-defective human connexin32 mutants are normally localized and function equivalently to wild-type connexin32 in myelinating Schwann cells. J Neurosci. 2005;25:7111–7120. doi: 10.1523/JNEUROSCI.1319-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip C, Kroner A, Fischer S, Berghoff M, Kobsar I, Mäurer M, Martini R. Role of immune cells in animal models for inherited peripheral neuropathies. Neuromolecular Med. 2006;8:175–190. doi: 10.1385/nmm:8:1-2:175. [DOI] [PubMed] [Google Scholar]
- Jeng LJ, Balice-Gordon RJ, Messing A, Fischbeck KH, Scherer SS. The effects of a dominant connexin32 mutant in myelinating Schwann cells. Mol Cell Neurosci. 2006;32:283–298. doi: 10.1016/j.mcn.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Karadima G, Panas M, Floroskufi P, Kalfakis N, Vassilopoulos D. A V38A mutation in X-linked Charcot-Marie-Tooth neuropathy with unusual clinical features. J Neurol. 2004;251:222–223. doi: 10.1007/s00415-004-0284-8. [DOI] [PubMed] [Google Scholar]
- Kawakami H, Inoue K, Sakakihara I, Nakamura S. Novel mutation in X-linked Charcot-Marie-Tooth disease associated with CNS impairment. Neurology. 2002;59:923–926. doi: 10.1212/wnl.59.6.923. [DOI] [PubMed] [Google Scholar]
- Kleopa KA, Scherer SS. Molecular genetics of X-linked Charcot-Marie-Tooth disease. Neuromolecular Med. 2006;8:107–122. doi: 10.1385/nmm:8:1-2:107. [DOI] [PubMed] [Google Scholar]
- Kleopa KA, Yum SW, Scherer SS. Cellular mechanisms of connexin32 mutations associated with CNS manifestations. J Neurosci Res. 2002;68:522–534. doi: 10.1002/jnr.10255. [DOI] [PubMed] [Google Scholar]
- Kleopa KA, Orthmann JL, Enriquez A, Paul DL, Scherer SS. Unique distribution of gap junction proteins connexin29, connexin32, and connexin47 in oligodendrocytes. Glia. 2004;47:346–357. doi: 10.1002/glia.20043. [DOI] [PubMed] [Google Scholar]
- Kleopa KA, Zamba-Papanicolaou E, Alevra X, Nicolaou P, Georgiou DM, Hadjisavvas A, Kyriakides T, Christodoulou K. Phenotypic and cellular expression of two novel connexin32 mutations causing CMT1X. Neurology. 2006;66:396–402. doi: 10.1212/01.wnl.0000196479.93722.59. [DOI] [PubMed] [Google Scholar]
- Kobsar I, Berghoff M, Samsam M, Wessig C, Mäurer M, Toyka KV, Martini R. Preserved myelin integrity and reduced axonopathy in connexin32-deficient mice lacking the recombination activating gene-1. Brain. 2003;126:804–813. doi: 10.1093/brain/awg072. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L, McBride J, Chen EY, Palfi S, Roitberg BZ, Brown WD, Holden JE, Pyzalski R, Taylor MD, Carvey P, Ling Z, Trono D, Hantraye P, Déglon N, Aebischer P. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson's disease. Science. 2000;290:767–773. doi: 10.1126/science.290.5492.767. [DOI] [PubMed] [Google Scholar]
- Krajewski KM, Lewis RA, Fuerst DR, Turansky C, Hinderer SR, Garbern J, Kamholz J, Shy ME. Neurological dysfunction and axonal degeneration in Charcot-Marie-Tooth disease. Brain. 2000;123:1516–1527. doi: 10.1093/brain/123.7.1516. [DOI] [PubMed] [Google Scholar]
- Kuntzer T, Dunand M, Schorderet DF, Vallat JM, Hahn AF, Bogousslavsky J. Phenotypic expression of a Pro 87 to Leu mutation in the connexin 32 gene in a large Swiss family with Charcot-Marie-Tooth neuropathy. J Neurol Sci. 2003;207:77–86. doi: 10.1016/s0022-510x(02)00394-5. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Nelson I, Houlden H, Sweeney MG, Hilton-Jones D, Blake J, Wood NW, Reilly MM. Six novel connexin32 (GJB1) mutations in X-linked Charcot-Marie-Tooth disease. J Neurol Neurosurg Psychiatry. 2002;73:304–306. doi: 10.1136/jnnp.73.3.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine BL, Humeau LM, Boyer J, MacGregor RR, Rebello T, Lu X, Binder GK, Slepushkin V, Lemiale F, Mascola JR, Bushman FD, Dropulic B, June CH. Gene transfer in humans using a conditionally replicating lentiviral vector. Proc Natl Acad Sci U S A. 2006;103:17372–17377. doi: 10.1073/pnas.0608138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev-Ram V, Ellisman MH. Axonal activation-induced calcium transients in myelinating Schwann cells, sources, and mechanisms. J Neurosci. 1995;15:2628–2637. doi: 10.1523/JNEUROSCI.15-04-02628.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang GS, de Miguel M, Gómez-Hernández JM, Glass JD, Scherer SS, Mintz M, Barrio LC, Fischbeck KH. Severe neuropathy with leaky connexin32 hemichannels. Ann Neurol. 2005;57:749–754. doi: 10.1002/ana.20459. [DOI] [PubMed] [Google Scholar]
- Lin P, Mao F, Liu Q, Yang W, Shao C, Yan C, Gong Y. A novel deletion mutation in GJB1 causes X-linked Charcot-Marie-Tooth disease in a Han Chinese family. Muscle Nerve. 2010;42:922–926. doi: 10.1002/mus.21790. [DOI] [PubMed] [Google Scholar]
- Maeda S, Nakagawa S, Suga M, Yamashita E, Oshima A, Fujiyoshi Y, Tsukihara T. Structure of the connexin 26 gap junction channel at 3.5 A resolution. Nature. 2009;458:597–602. doi: 10.1038/nature07869. [DOI] [PubMed] [Google Scholar]
- Marques W, Jr, Sweeney JG, Wood NW, Wroe SJ. Central nervous system involvement in a novel connexin 32 mutation affecting identical twins. J Neurol Neurosurg Psychiatry. 1999;66:803–804. doi: 10.1136/jnnp.66.6.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PE, Mambetisaeva ET, Archer DA, George CH, Evans WH. Analysis of gap junctions assembly using mutated connexins detected in Charcot-Marie-Tooth X-linked disease. J Neurochem. 2000;74:711–720. doi: 10.1046/j.1471-4159.2000.740711.x. [DOI] [PubMed] [Google Scholar]
- Martini R, Zielasek J, Toyka KV, Giese KP, Schachner M. Protein zero (P0)-deficient mice show myelin degeneration in peripheral nerves characteristic of inherited human neuropathies. Nat Genet. 1995;11:281–286. doi: 10.1038/ng1195-281. [DOI] [PubMed] [Google Scholar]
- Martyn CN, Hughes RA. Epidemiology of peripheral neuropathy. J Neurol Neurosurg Psychiatry. 1997;62:310–318. doi: 10.1136/jnnp.62.4.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama W, Nakagawa M, Moritoyo T, Takashima H, Umehara F, Hirata K, Suehara M, Osame M. Phenotypes of X-linked Charcot-Marie-Tooth disease and altered trafficking of mutant Connexin 32 (GJB1) J Hum Genet. 2001;46:307–313. doi: 10.1007/s100380170064. [DOI] [PubMed] [Google Scholar]
- Meier C, Dermietzel R, Davidson KG, Yasumura T, Rash JE. Connexin32-containing gap junctions in Schwann cells at the internodal zone of partial myelin compaction and in Schmidt-Lanterman incisures. J Neurosci. 2004;24:3186–3198. doi: 10.1523/JNEUROSCI.5146-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menichella DM, Goodenough DA, Sirkowski E, Scherer SS, Paul DL. Connexins are critical for normal myelination in the CNS. J Neurosci. 2003;23:5963–5973. doi: 10.1523/JNEUROSCI.23-13-05963.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SM, Polke J, Manji H, Blake J, Reiniger L, Sweeney M, Houlden H, Brandner S, Reilly MM. A novel mutation in the nerve-specific 5′UTR of the GJB1 gene causes X-linked Charcot-Marie-Tooth disease. J Peripher Nerv Syst. 2011;16:65–70. doi: 10.1111/j.1529-8027.2011.00321.x. [DOI] [PubMed] [Google Scholar]
- Nakagawa M, Takashima H, Umehara F, Arimura K, Miyashita F, Takenouchi N, Matsuyama W, Osame M. Clinical phenotype in X-linked Charcot-Marie-Tooth disease with an entire deletion of the connexin 32 coding sequence. J Neurol Sci. 2001;185:31–37. doi: 10.1016/s0022-510x(01)00454-3. [DOI] [PubMed] [Google Scholar]
- Nave KA, Trapp BD. Axon-glial signaling and the glial support of axon function. Annu Rev Neurosci. 2008;31:535–561. doi: 10.1146/annurev.neuro.30.051606.094309. [DOI] [PubMed] [Google Scholar]
- Nicholson G, Corbett A. Slowing of central conduction in X-linked Charcot-Marie-Tooth neuropathy shown by brain auditory evoked responses. J Neurol Neurosurg Psychiatry. 1996;61:43–46. doi: 10.1136/jnnp.61.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson G, Nash J. Intermediate nerve conduction velocities define X-linked Charcot-Marie-Tooth neuropathy families. Neurology. 1993;43:2558–2564. doi: 10.1212/wnl.43.12.2558. [DOI] [PubMed] [Google Scholar]
- Nicholson GA, Yeung L, Corbett A. Efficient neurophysiological selection of X-linked Charcot-Marie-Tooth families. Neurology. 1998;51:1412–1416. doi: 10.1212/wnl.51.5.1412. [DOI] [PubMed] [Google Scholar]
- Nicolaou P, Zamba-Papanicolaou E, Koutsou P, Kleopa KA, Georghiou A, Hadjigeorgiou G, Papadimitriou A, Kyriakides T, Christodoulou K. Charcot-Marie-Tooth disease in Cyprus: epidemiological, clinical and genetic characteristics. Neuroepidemiology. 2010;35:171–177. doi: 10.1159/000314351. [DOI] [PubMed] [Google Scholar]
- Odermatt B, Wellershaus K, Wallraff A, Seifert G, Degen J, Euwens C, Fuss B, Büssow H, Schilling K, Steinhäuser C, Willecke K. Connexin 47 (Cx47)-deficient mice with enhanced green fluorescent protein reporter gene reveal predominant oligodendrocytic expression of Cx47 and display vacuolized myelin in the CNS. J Neurosci. 2003;23:4549–4559. doi: 10.1523/JNEUROSCI.23-11-04549.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S, Ri Y, Bennett MV, Trexler EB, Verselis VK, Bargiello TA. Changes in permeability caused by connexin 32 mutations underlie X-linked Charcot-Marie-Tooth disease. Neuron. 1997;19:927–938. doi: 10.1016/s0896-6273(00)80973-3. [DOI] [PubMed] [Google Scholar]
- Omori Y, Mesnil M, Yamasaki H. Connexin 32 mutations from X-linked Charcot-Marie-Tooth disease patients: functional defects and dominant negative effects. Mol Biol Cell. 1996;7:907–916. doi: 10.1091/mbc.7.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panas M, Karadimas C, Avramopoulos D, Vassilopoulos D. Central nervous system involvement in four patients with Charcot-Marie-Tooth disease with connexin 32 extracellular mutations. J Neurol Neurosurg Psychiatry. 1998;65:947–948. doi: 10.1136/jnnp.65.6.947a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panas M, Kalfakis N, Karadimas C, Vassilopoulos D. Episodes of generalized weakness in two sibs with the C164T mutation of the connexin 32 gene. Neurology. 2001;57:1906–1908. doi: 10.1212/wnl.57.10.1906. [DOI] [PubMed] [Google Scholar]
- Paulson HL, Garbern JY, Hoban TF, Krajewski KM, Lewis RA, Fischbeck KH, Grossman RI, Lenkinski R, Kamholz JA, Shy ME. Transient central nervous system white matter abnormality in X-linked Charcot-Marie-Tooth disease. Ann Neurol. 2002;52:429–434. doi: 10.1002/ana.10305. [DOI] [PubMed] [Google Scholar]
- Rabadan-Diehl C, Dahl G, Werner R. A connexin-32 mutation associated with Charcot-Marie-Tooth disease does not affect channel formation in oocytes. FEBS Lett. 1994;351:90–94. doi: 10.1016/0014-5793(94)00819-1. [DOI] [PubMed] [Google Scholar]
- Rouger H, LeGuern E, Birouk N, Gouider R, Tardieu S, Plassart E, Gugenheim M, Vallat JM, Louboutin JP, Bouche P, Agid Y, Brice A. Charcot-Marie-Tooth disease with intermediate motor nerve conduction velocities: characterization of 14 Cx32 mutations in 35 families. Hum Mutat. 1997;10:443–452. doi: 10.1002/(SICI)1098-1004(1997)10:6<443::AID-HUMU5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Rozear MP, Pericak-Vance MA, Fischbeck K, Stajich JM, Gaskell PC, Jr, Krendel DA, Graham DG, Dawson DV, Roses AD. Hereditary motor and sensory neuropathy, X-linked: a half century follow-up. Neurology. 1987;37:1460–1465. doi: 10.1212/wnl.37.9.1460. [DOI] [PubMed] [Google Scholar]
- Sahenk Z, Chen L. Abnormalities in the axonal cytoskeleton induced by a connexin32 mutation in nerve xenografts. J Neurosci Res. 1998;51:174–184. doi: 10.1002/(SICI)1097-4547(19980115)51:2<174::AID-JNR6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Sakaguchi H, Yamashita S, Miura A, Hirahara T, Kimura E, Maeda Y, Terasaki T, Hirano T, Uchino M. A novel GJB1 frameshift mutation produces a transient CNS symptom of X-linked Charcot-Marie-Tooth disease. J Neurol. 2011;258:284–290. doi: 10.1007/s00415-010-5752-8. [DOI] [PubMed] [Google Scholar]
- Sancho S, Magyar JP, Aguzzi A, Suter U. Distal axonopathy in peripheral nerves of PMP22 mutant mice. Brain. 1999;122:1563–1577. doi: 10.1093/brain/122.8.1563. [DOI] [PubMed] [Google Scholar]
- Sander S, Nicholson GA, Ouvrier RA, McLeod JG, Pollard JD. Charcot-Marie-Tooth disease: histopathological features of the peripheral myelin protein (PMP22) duplication (CMT1A) and connexin32 mutations (CMTX1) Muscle Nerve. 1998;21:217–225. doi: 10.1002/(sici)1097-4598(199802)21:2<217::aid-mus9>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Sandri C, Van Buren JM, Akert K. Membrane morphology of the vertebrate nervous system. Prog Brain Res. 1982;46:201–265. [PubMed] [Google Scholar]
- Sargiannidou I, Ahn M, Enriquez AD, Peinado A, Reynolds R, Abrams C, Scherer SS, Kleopa KA. Human oligodendrocytes express Cx31.3: function and interactions with Cx32 mutants. Neurobiol Dis. 2008;30:221–233. doi: 10.1016/j.nbd.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargiannidou I, Vavlitou N, Aristodemou S, Hadjisavvas A, Kyriacou K, Scherer SS, Kleopa KA. Connexin32 mutations cause loss of function in Schwann cells and oligodendrocytes leading to PNS and CNS myelination defects. J Neurosci. 2009;29:4736–4749. doi: 10.1523/JNEUROSCI.0325-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schelhaas HJ, Van Engelen BG, Gabreëls-Festen AA, Hageman G, Vliegen JH, Van Der Knaap MS, Zwarts MJ. Transient cerebral white matter lesions in a patient with connexin 32 missense mutation. Neurology. 2002;59:2007–2008. doi: 10.1212/01.wnl.0000038390.29853.46. [DOI] [PubMed] [Google Scholar]
- Scherer SS, Deschênes SM, Xu YT, Grinspan JB, Fischbeck KH, Paul DL. Connexin32 is a myelin-related protein in the PNS and CNS. J Neurosci. 1995;15:8281–8294. doi: 10.1523/JNEUROSCI.15-12-08281.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer SS, Xu YT, Nelles E, Fischbeck K, Willecke K, Bone LJ. Connexin32-null mice develop a demyelinating peripheral neuropathy. Glia. 1998;24:8–20. doi: 10.1002/(sici)1098-1136(199809)24:1<8::aid-glia2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Scherer SS, Bone LJ, Deschênes SM, Abel A, Balice-Gordon R, Fischbeck K. The role of the gap junction protein connexin32 in the pathogenesis of X-linked Charcot-Marie-Tooth disease. In: Cardew G, editor. Gap junction-mediated intercellular signalling in health and disease. New York: Wiley; 1999. pp. 175–185. [DOI] [PubMed] [Google Scholar]
- Scherer SS, Xu YT, Messing A, Willecke K, Fischbeck KH, Jeng LJ. Transgenic expression of human connexin32 in myelinating Schwann cells prevents demyelination in connexin32-null mice. J Neurosci. 2005;25:1550–1559. doi: 10.1523/JNEUROSCI.3082-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnapp BJ, Mugnaini E. Membrane architecture of myelinated fibers as seen by freeze-fracture. In: Waxman SG, editor. Physiology and pathobiology of axons. New York: Raven; 1978. pp. 83–123. [Google Scholar]
- Senderek J, Bergmann C, Quasthoff S, Ramaekers VT, Schröder JM. X-linked dominant Charcot-Marie-Tooth disease: nerve biopsies allow morphological evaluation and detection of connexin32 mutations (Arg15Trp, Arg22Gln) Acta Neuropathol. 1998;95:443–449. doi: 10.1007/s004010050823. [DOI] [PubMed] [Google Scholar]
- Senderek J, Hermanns B, Bergmann C, Boroojerdi B, Bajbouj M, Hungs M, Ramaekers VT, Quasthoff S, Karch D, Schröder JM. X-linked dominant Charcot-Marie-Tooth neuropathy: clinical, electrophysiological, and morphological phenotype in four families with different connexin32 mutations. J Neurol Sci. 1999;167:90–101. doi: 10.1016/s0022-510x(99)00146-x. [DOI] [PubMed] [Google Scholar]
- Shy ME. Therapeutic strategies for the inherited neuropathies. Neuromolecular Med. 2006;8:255–278. doi: 10.1385/nmm:8:1-2:255. [DOI] [PubMed] [Google Scholar]
- Shy ME, Siskind C, Swan ER, Krajewski KM, Doherty T, Fuerst DR, Ainsworth PJ, Lewis RA, Scherer SS, Hahn AF. CMT1X phenotypes represent loss of GJB1 gene function. Neurology. 2007;68:849–855. doi: 10.1212/01.wnl.0000256709.08271.4d. [DOI] [PubMed] [Google Scholar]
- Siskind CE, Murphy SM, Ovens R, Polke J, Reilly MM, Shy ME. Phenotype expression in women with CMT1X. J Peripher Nerv Syst. 2011;16:102–107. doi: 10.1111/j.1529-8027.2011.00332.x. [DOI] [PubMed] [Google Scholar]
- Siskind C, Feely SM, Bernes S, Shy ME, Garbern JY. Persistent CNS dysfunction in a boy with CMT1X. J Neurol Sci. 2009;279:109–113. doi: 10.1016/j.jns.2008.12.031. [DOI] [PubMed] [Google Scholar]
- Sørensen J, Haase G, Krarup C, Gilgenkrantz H, Kahn A, Schmalbruch H. Gene transfer to Schwann cells after peripheral nerve injury: a delivery system for therapeutic agents. Ann Neurol. 1998;43:205–211. doi: 10.1002/ana.410430210. [DOI] [PubMed] [Google Scholar]
- Srinivasan J, Leventer RJ, Kornberg AJ, Dahl HH, Ryan MM. Central nervous system signs in X-linked Charcot-Marie-Tooth disease after hyperventilation. Pediatr Neurol. 2008;38:293–295. doi: 10.1016/j.pediatrneurol.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Sutor B, Schmolke C, Teubner B, Schirmer C, Willecke K. Myelination defects and neuronal hyperexcitability in the neocortex of connexin 32-deficient mice. Cereb Cortex. 2000;10:684–697. doi: 10.1093/cercor/10.7.684. [DOI] [PubMed] [Google Scholar]
- Tabaraud F, Lagrange E, Sindou P, Vandenberghe A, Levy N, Vallat JM. Demyelinating X-linked Charcot-Marie-Tooth disease: unusual electrophysiological findings. Muscle Nerve. 1999;22:1442–1447. doi: 10.1002/(sici)1097-4598(199910)22:10<1442::aid-mus16>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Taylor RA, Simon EM, Marks HG, Scherer SS. The CNS phenotype of X-linked Charcot-Marie-Tooth disease: more than a peripheral problem. Neurology. 2003;61:1475–1478. doi: 10.1212/01.wnl.0000095960.48964.25. [DOI] [PubMed] [Google Scholar]
- Tetzlaff W. Tight junction contact events and temporary gap junctions in the sciatic nerve fibres of the chicken during Wallerian degeneration and subsequent regeneration. J Neurocytol. 1982;11:839–858. doi: 10.1007/BF01153522. [DOI] [PubMed] [Google Scholar]
- Toews JC, Schram V, Weerth SH, Mignery GA, Russell JT. Signaling proteins in the axoglial apparatus of sciatic nerve nodes of Ranvier. Glia. 2007;55:202–213. doi: 10.1002/glia.20448. [DOI] [PubMed] [Google Scholar]
- Tress O, Maglione M, Zlomuzica A, May D, Dicke N, Degen J, Dere E, Kettenmann H, Hartmann D, Willecke K. Pathologic and phenotypic alterations in a mouse expressing a connexin47 missense mutation that causes Pelizaeus-Merzbacher-like disease in humans. PLoS Genet. 2011;7:e1002146. doi: 10.1371/journal.pgen.1002146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlenberg B, Schuelke M, Rüschendorf F, Ruf N, Kaindl AM, Henneke M, Thiele H, Stoltenburg-Didinger G, Aksu F, Topaloğlu H, Nürnberg P, Hübner C, Weschke B, Gärtner J. Mutations in the gene encoding gap junction protein alpha 12 (Connexin 46.6) cause Pelizaeus-Merzbacher-like disease. Am J Hum Genet. 2004;75:251–260. doi: 10.1086/422763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger VM, Kumar NM, Gilula NB, Yeager M. Three-dimensional structure of a recombinant gap junction membrane channel. Science. 1999;283:1176–1180. doi: 10.1126/science.283.5405.1176. [DOI] [PubMed] [Google Scholar]
- VanSlyke JK, Deschênes SM, Musil LS. Intracellular transport, assembly, and degradation of wild-type and disease-linked mutant gap junction proteins. Mol Biol Cell. 2000;11:1933–1946. doi: 10.1091/mbc.11.6.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavlitou N, Sargiannidou I, Markoullis K, Kyriacou K, Scherer SS, Kleopa KA. Axonal pathology precedes demyelination in a mouse model of X-linked demyelinating/type I Charcot-Marie Tooth neuropathy. J Neuropathol Exp Neurol. 2010;69:945–958. doi: 10.1097/NEN.0b013e3181efa658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Véga C, Martiel JL, Drouhault D, Burckhart MF, Coles JA. Uptake of locally applied deoxyglucose, glucose and lactate by axons and Schwann cells of rat vagus nerve. J Physiol. 2003;546:551–564. doi: 10.1113/jphysiol.2002.029751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vital A, Ferrer X, Lagueny A, Vandenberghe A, Latour P, Goizet C, Canron MH, Louiset P, Petry KG, Vital C. Histopathological features of X-linked Charcot-Marie-Tooth disease in 8 patients from 6 families with different connexin32 mutations. J Peripher Nerv Syst. 2001;6:79–84. doi: 10.1046/j.1529-8027.2001.01011.x. [DOI] [PubMed] [Google Scholar]
- White TW, Paul DL. Genetic diseases and gene knockouts reveal diverse connexin functions. Annu Rev Physiol. 1999;61:283–310. doi: 10.1146/annurev.physiol.61.1.283. [DOI] [PubMed] [Google Scholar]
- Wicklein EM, Orth U, Gal A, Kunze K. Missense mutation (R15W) of the connexin32 gene in a family with X chromosomal Charcot-Marie-Tooth neuropathy with only female family members affected. J Neurol Neurosurg Psychiatry. 1997;63:379–381. doi: 10.1136/jnnp.63.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willecke K, Eiberger J, Degen J, Eckardt D, Romualdi A, Güldenagel M, Deutsch U, Söhl G. Structural and functional diversity of connexin genes in the mouse and human genome. Biol Chem. 2002;383:725–737. doi: 10.1515/BC.2002.076. [DOI] [PubMed] [Google Scholar]
- Yiu EM, Geevasinga N, Nicholson GA, Fagan ER, Ryan MM, Ouvrier RA. A retrospective review of X-linked Charcot-Marie-Tooth disease in childhood. Neurology. 2011;76:461–466. doi: 10.1212/WNL.0b013e31820a0ceb. [DOI] [PubMed] [Google Scholar]
- Yoshimura T, Satake M, Ohnishi A, Tsutsumi Y, Fujikura Y. Mutations of connexin32 in Charcot-Marie-Tooth disease type X interfere with cell-to-cell communication but not cell proliferation and myelin-specific gene expression. J Neurosci Res. 1998;51:154–161. doi: 10.1002/(SICI)1097-4547(19980115)51:2<154::AID-JNR4>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Yum SW, Kleopa KA, Shumas S, Scherer SS. Diverse trafficking abnormalities of Connexin32 mutants causing CMTX. Neurobiol Dis. 2002;11:43–52. doi: 10.1006/nbdi.2002.0545. [DOI] [PubMed] [Google Scholar]