Abstract
The study involves analyzing the performance of bivoltine Bombyx mori larvae reared on different host plants varieties. The consumption rate (CR) of different strains of B. mori was high when fed with Jorhat and TR10 mulberry plant varieties. Jorhat and TR10 mulberry plant varieties were found to contain significant amount of calcium, potassium, magnesium and phosphorus. Local (Hmute) mulberry plant variety had high amount of protein, carbohydrate and reducing sugar. Majority of the B. mori strains reared on Jorhat and TR10 mulberry plant varieties had high level of fibroin protein which resulted in increased silk productivity than those larvae reared with other mulberry varieties. The filament length was higher when reared on Jorhat and TR10 mulberry plant varieties. CSR4 × CSR2, FC1 × FC2, and FC2 × FC1 strains reared on Jorhat and TR10 mulberry plant varieties performed well in terms of economic parameters. Proteins and other nutrients in combination with high levels of micronutrients are very much essential for better silk quality. The present study attempted to identify the most suitable host plants for silkworm rearing under mountainous agro-ecological conditions which can lead to sustainable production of silk in relation to physiological and economic parameters.
Keywords: bivoltine, Bombyx mori, food consumption, micronutrients, economics, mulberry
Introduction
Sericulture is a sustainable, eco-friendly and agro-forestry oriented trade comprising cultivation of mulberry plant varieties, rearing of silkworms, and production of silk. It is one of the most labor-intensive sectors and has played a critical role in rural development and economic growth. Most of the marketable silk around the world is being produced from the mulberry silkworm, Bombyx mori L. (Yogananda-Murthy et al., 2013). B. mori is an essentially monophagous and host plant-specific insect that feeds solely on mulberry leaves (Morus alba, Family: Moracea) (Savithri et al., 2013). Two kinds of silk proteins have been distinguished as major components of silk cocoons, the first being fibroin – a fibrous protein secreted in the lumen of the posterior silk gland of B. mori and the second being sericin, a natural macromolecular protein that serves as an adhesive to unite fibroin for making silk cocoons of silkworm, B. mori (Sabina et al., 2012).
India has rich resources of mulberry varieties that are traditionally cultivated, and a few exotic varieties have been introduced from time to time. Besides the influence of environmental factors, the silk productivity is related to the quantity and quality of mulberry leaves (Nagaraju, 2002). Nutritional physiology has a vital role in influencing the performances of different stages of silkworm. To better understand the chemical ecology of the insect-plant relationship, studying the quantitative aspect of nutrition in the insect is important (Waldbauer, 1968). Development of silkworm is greatly influenced by the nutrient composition of the mulberry host leaves, which is also the determining factor of the quality of silk (Jyothi et al., 2014). The performance of silkworm is evident by the digestion and assimilation of the nutritional materials present in mulberry leaves (Lalfelpuii et al., 2014a,b). The life cycle routine of the silkworm from identical genetic stock varies significantly based on nutritional quality of mulberry leaves (Rahmathulla, 2011). As such, the amount of food consumed and the quantity digested by the silkworms have a direct effect on its physiological performance and silk production. Pioneering research has proved that deficiency of certain nutrients or imbalance of nutrient on the diet affects the digestibility and metabolic activity of larvae (Waldbauer, 1964).
Several scientists have established variations in biochemical components of mulberry leaves. Bose et al. (1991) and Neog et al. (2011) revealed that mulberry varieties differ significantly in the composition of nutrients. The protein content (soluble and crude) of mulberry leaves is the paramount nutritional factor in determining the life cycle performance of silkworm (Pillai and Jolly, 1985). Machii and Katagiri (1991) indicated that the nutritive value of mulberry leaves depends on nitrogen and amino acid contents. Carbohydrates are very important for maintaining healthy growth of young silkworm larvae. Fats or lipids are particularly the main forms of energy reserves and are important for the proper development of wild silkmoth, Antheraea assama (Kataky and Hazarika, 1997). These studies have mainly focused on the growth and reproduction of the silkworm. Different mulberry varieties react to climatic conditions, particularly temperature, which affects their quality (Lalfelpuii et al., 2014b). To select a silkworm strain for its commercial exploitation, attributes like geographical environment, viability to rear, silk traits, etc., are to be taken into consideration. Therefore, it is required that strains are selected for particular geographical environments by utilizing the races acclimatized to that location (Lalfelpuii et al., 2014a). However, not much research has been done to understand the role of nutrients on the economic parameters, especially in a high-altitude hilly mountainous agro-climatic zone.
The amount of food consumed and the quantity digested by the silkworms will have direct effects on its performance, mating success and reproduction. Deficiency of certain nutrients or a nutritionally imbalanced diet affects the digestibility and metabolic activity of larvae (Waldbauer, 1964). Therefore, we would expect to find that different silkworm strains performed best on its most preferred host plants (Slansky and Scriber, 1985). The rearing performance of silkworm races are described in terms of larval weight and improved economic traits like cocoon weight, shell weight, and silk percentage (Gangawar, 2010; Seidavi, 2011). Cocoon weight and shell weight are the most important characteristics evaluated for productivity (Gaviria et al., 2006).
In the present study, an attempt has been made to assess the performance of B. mori on four mulberry varieties (Jorhat, TR10, BC2-59, and the local Hmute) to identify the most suitable host plant with regard to the economic traits of different bivoltine silkworm strains in the Eastern Himalayan region of Northeast India.
Materials and Methods
Mulberry Cultivation and Study Site
Cuttings of mulberry varieties viz. Jorhat, TR10, and BC2-59 were procured from Research Extension Centre, Central Silk Board, Shillong, India. The local (Hmute) mulberry variety was collected from Aizawl, Northeast India. Humte is found extensively in the hills and jungles of Mizoram in wild conditions, and it possesses comparatively high adaptability to Mizoram agro-climatic conditions (Supplementary Table 1). The selected mulberry varieties were grown in the experimental field of the Department of Biotechnology, Mizoram University, Aizawl (altitude of 950 MSL; Longitude 92°38′ to 92°42′E; Latitude 23°42′ to 23°46′N). Aizawl has mild climate temperatures ranging from 20 to 30°C in summer and 3 to 20°C in winter. It rains heavily from May to September, with little rain in the dry (cold) season, and the climate pattern is moist tropical to moist sub-tropical with average state rainfall of 215 cm and precipitation of 85.09 cm per annum. Cultivation of mulberry plant varieties was done as per the agronomic practices followed for rain-fed mulberry accessions (Kant and Bhat, 2010).
Mass Culture of Silkworm
Disease-free layings of six bivoltine silkworm strains – SK6 × SK7, SK7 × SK6, CSR2 × CSR4, CSR4 × CSR2, FC1 × FC2, and FC2 × FC1 – were procured from germplasm of National Silkworm Seed Organization, Bangalore, India and one pure Japanese strain J112 (S7) was procured from germplasm of the Department of Sericulture, Aizawl, India.
Silkworm rearing was done as per standard rearing package (Krishnaswami, 1978). All seven bivoltine races of B. mori were reared with locally available mulberry leaves (Hmute) up to second instar to initiate and uniformly stabilize the cultures before starting the experiments on different host plant leaves. From the third instar active feeding stage onward, the larvae were individually fed with four different mulberry varieties (temperature 25°C and relative humidity 72%) until the end of the larval instars. There were 84 experimental sets, and each experimental set comprised of a rearing tray with 20 larvae (7 silkworm races × 4 host plant varieties × 3 replicates = 84 sets × 20 larvae = 1680 larvae). The larvae were fed ad libitum four times daily (4 h interval) with chopped mature leaves obtained from the newly formed twigs on the top of the mulberry plants (Supplementary Figure 1). The initial and final weights (leftover) of the leaves were measured. The leaves were collected every day at 06:00 h and stored in moist gunny bags. The first feed was given at 07:00 h while the last feeding was at 19:00 h, and this routine was continued until cocoon formation. Nets with meshes were laid on the top of the rearing tray, and fresh leaves were spread above them. The tray was cleaned daily and the excreta and leftover food was discarded. The larvae were dusted with bleaching powder (3%) before the first feed of every instar (Krishnaswami, 1978).
Macro- and Micronutrient Analysis of Mulberry Plants
A total of 100 mg of fresh mature mulberry leaves of different host plants were subjected to biochemical analysis. Quantitative estimation of total proteins (Lowry et al., 1951), total carbohydrates (Yemm and Willis, 1954), total lipids (Bligh and Dyer’s, 1959), total amino acids (Moore and Stein, 1948) and total reducing sugar by the Dinitrosalicylic method (Miller, 1972) were measured. Moisture content of mulberry leaves was measured by computing the difference between initial and final dried weight (incubated at 37°C) and represented in percentage (Shobana et al., 2010).
For micronutrient analysis, mature leaves of the four mulberry varieties were washed with distilled water, shade dried at room temperature and ground to powder using a mixer grinder. Wet digestion was performed to estimate the level of nutrients. An accurately weighed leaf powder (0.3 g) was transferred to closed flask. A total of 5 mL of 30% Hydrogen peroxide and 10 mL of concentrated sulphuric acid solutions was added and the sample was digested until the solution became clear. After digestion, the sample was diluted to 100 mL with distilled water in a volumetric flask. The samples were used for analysis of minerals such as magnesium, calcium, potassium, and phosphorus (Jackson, 1973).
Food Utilization Indices
A food utilization experiment was performed using 84 experimental sets, and each experimental set comprised of a rearing tray with 20 larvae (7 silkworm races × 4 host plant varieties × 3 replicates = 84 sets × 20 larvae = 1680 larvae). Larvae were maintained under controlled conditions (temperature: 25 ± 2°C; moisture content 70–80%; light: dark 12:12 h), and weight of the larva, food consumed and feces produced were measured daily. The host plant feed was changed daily and the fresh as well as leftover leaves were weighed. The experiment was continued for 6 days, and observations were recorded every 24 h. Food utilization indices (all based on dry weight) were calculated (Waldbauer, 1968; Slansky and Scriber, 1985):
| Growth rate (GR) | = P/T |
| Consumption rate (CR) | = E/T |
| Consumption index (CI) | = E/TA |
| Approximate digestibility (AD) (%) | = 100 (E - F)/E |
| Efficiency of conversion of ingested food (ECI) (%) | = 100P/E |
| Efficiency of conversion of digested food (ECD) (%) | = 100P/(E - F) |
Where, A: mean dry weight of animal during T; E: dry weight of food eaten; F: dry weight of feces produced; P: dry weight gain of insects; T: duration of the experimental period.
Silkworm Larva Performance on Different Diets
The weights of larva, cocoon, shell, and filament were measured using electronic balance (±0.01 g; Mettler, United States). Shell percentage and denier were calculated using the formula: Shell (%) = Cocoon shell weight/cocoon weight × 100; Denier = [(Filament weight (g)/filament length (meter)] × 9000. The length of the silk thread (bave) from the cocoon was reeled using a reeling apparatus (eprouvette) and was measured in meters. The silk filament length represents the percentage of silk by measuring the length of the bave contained in the shell (FAO, 1999).
Biochemical Estimation of B. mori
The silkworm larva that fed on different host plants were used for estimation of biochemical parameters. Larval hemolymph and silk gland proteins were estimated using Lowry et al. (1951). Fibroin and sericin percentage were analyzed from cocoon (Thirumalaisamy et al., 2009). Enzyme assay from midgut tissue extracts were performed by dissecting the midgut. A total of 25 mg of the midgut was homogenized (200 μL phosphate buffered saline) and centrifuged at 3000 rpm for 10 min. Enzyme analysis was performed using this supernatant. Quantitative amylase activity of different midgut samples was analyzed using standard protocol (Nagaraju and Abraham, 1995). Mid gut (50 μL) samples were mixed with citrate buffer (50 μL) and allowed to incubate at 37°C for 3 min and the reaction was inhibited by adding 3, 5-Dinitrosalicylic acid (200 μL). The reaction mixture was kept in boiling temperature in a water bath, and the color developed was read at 540 nm. Protease activity of different midgut samples was analyzed using standard protocol (Eguchi and Iwamoto, 1976). 30 μL of 1% casein was taken in centrifuge tubes followed by 30 μL of 0.1 M borate buffer (pH 11.0) and 60 μL of 10% Trichloroacetic acid. To this, 50 μL of the samples was added and centrifuged at 3000 rpm for 10 min. The sample mixture (200 μL) was mixed with sodium hydroxide (20 μL, 0.5 N) and folin-ciocalteu reagent (50 μL) and incubated for 30 min. The absorbance was measured at 660 nm.
Statistical Analysis
All data on food consumption and biochemical analysis were affirmed as mean ± SE by using statistical software OriginPro 8 v8.0724, Northampton, MA, United States. The normality distribution of the variables was tested using one sample Kolmogorov–Smirnov test. Differences in measured parameters among the groups were analyzed by a one-way analysis of variance (ANOVA) test due to normal distribution. The results were analyzed by ANOVA followed by a Duncan test for post hoc comparisons, and class predictions were computed by R statistical package (at P < 0.05) (Duncan, 1955).
Results
Biochemical Parameters of Mulberry Host Plants
There were differences in the levels of biochemical components among the tested mulberry cultivars (Table 1). Total protein content varied significantly between the different mulberry varieties (Hmute > TR10 > BC2-59 > Jorhat). Lipid content was highest in the Jorhat mulberry variety and lowest in BC2-59. Carbohydrate content was high in the Hmute variety and low in the BC2-59 variety. Total amino acid, water content, potassium, and calcium were highest in TR10 and lowest in the BC2-59 mulberry plant variety. Magnesium and phosphorus were high in the Jorhat mulberry plant variety.
Table 1.
Biochemical parameters of mulberry plant varieties.
| Host plant | Protein (μg/mL) | Amino acid (μg/mL) | Lipid (μg/mL) | Carbohydrate (μg/mL) | Reducing sugar (μg/mL) | Water content | Magnesium (mM/L) | Calcium (mM/L) | Potassium (mM/L) | Phosphorous (mM/L) |
|---|---|---|---|---|---|---|---|---|---|---|
| Hmute | 1766.2 ± 4.72a | 34.99 ± 1.28b | 930 ± 2.96b | 3842.83 ± 4.97a | 193.23 ± 5.49a | 1.268 ± 0.73a | 0.562 ± 0.082a | 1.362 ± 0.074b | 1.752 ± 0.086b | 0.352 ± 0.049b |
| Jorhat | 449.4 ± 3.91d | 34.07 ± 1.03b | 970 ± 3.61a | 3839.06 ± 7.36b | 131.05 ± 9.31c | 1.244 ± 0.39a | 0.678 ± 0.079a | 1.582 ± 0.115a | 1.793 ± 0.095b | 1.672 ± 0.064a |
| BC2-59 | 473.7 ± 3.82c | 31.47 ± 1.95b | 880 ± 3.72c | 3746.09 ± 7.48c | 164.91 ± 4.47b | 1.215 ± 0.41a | 0.529 ± 0.161a | 1.322 ± 0.108b | 1.872 ± 0.061ab | 0.363 ± 0.029b |
| TR10 | 909.8 ± 5.49b | 40.58 ± 1.68a | 930 ± 3.29b | 3841.56 ± 5.72a | 168.53 ± 6.28b | 1.272 ± 0.86a | 0.645 ± 0.038a | 1.583 ± 0.093a | 1.952 ± 0.072a | 0.371 ± 0.037b |
| F-value 3,11 | 18391 | 6.314 | 116.96 | 53.995 | 14.834 | 0.001732 | 0.4809 | 2.004 | 1.237 | 197.12 |
| p-value | <0.0001 | <0.0167 | <0.0001 | <0.0001 | <0.0012 | <0.9999 | <0.7046 | <0.1921 | <0.3583 | <0.0001 |
The data are mean ± standard error (n = 3 observations). p-values were determined by one-way ANOVA with subsequent Tukey’s post hoc test. Values of treatment groups with different alphabetical letters show statistically significant difference at p < 0.05 and values with similar letters show no significance at p > 0.05. The F-value is the ratio of the mean regression sum of squares divided by the mean error sum of squares (variance within samples/variance between samples).
Food Utilization Indices
The food consumption and ingestion indices of fifth instar B. mori larvae reared on Hmute, Jorhat, BC2-59, and TR10 mulberry plant varieties are shown in Table 2. Consumption Rate (CR), Consumption Index (CI), and Approximate Digestibility (AD) were high in TR10-reared larvae for all the B. mori strains tested. The growth rate (GR) was highest in larvae fed Hmute for both the SK6 × SK7 and CSR2 × CSR4 strains. In the FC2 × FC1 strain, the GR and CI were highest in larvae fed with the Jorhat plant variety. Significant variations were observed in the ECI and ECD parameters between the silkworm strains and mulberry varieties. High ECI (22.49%) and ECD (24.55%) were recorded in the SK6 × SK7 strain reared on Hmute mulberry varieties.
Table 2.
Food utilization efficiency measures of fifth instar larvae of B. mori strains reared on different mulberry varieties.
| Strain | Host plant | GR (g/larvae/day) | CR (g/larvae/day) | CI (g/larvae/day) | AD (%) | ECI (%) | ECD (%) |
|---|---|---|---|---|---|---|---|
| SK6 × SK7 | Hmute | 0.076 ± 0.016a | 0.345 ± 0.086a | 0.196 ± 0.025a | 91.58 ± 1.90a | 22.49 ± 0.42a | 24.55 ± 0.68a |
| Jorhat | 0.067 ± 0.017b | 0.331 ± 0.125a | 0.172 ± 0.045a | 92.03 ± 2.01a | 20.24 ± 0.66b | 21.99 ± 0.78b | |
| BC2-59 | 0.049 ± 0.015b | 0.390 ± 0.149a | 0.164 ± 0.024a | 91.88 ± 1.59a | 12.55 ± 0.54d | 13.66 ± 0.91c | |
| TR10 | 0.060 ± 0.017b | 0.521 ± 0.135a | 0.251 ± 0.040a | 93.76 ± 1.85a | 15.43 ± 0.77c | 12.26 ± 0.64c | |
| p-value | <0.6982 | <0.7099 | <0.3460 | <0.8356 | <0.0001 | <0.0001 | |
| F-Value 3,11 | 0.4910 | 0.4725 | 1.278 | 0.2842 | 54.411 | 63.853 | |
| SK7 × SK6 | Hmute | 0.058 ± 0.015a | 0.348 ± 0.129a | 0.162 ± 0.028a | 90.79 ± 1.84a | 16.88 ± 0.54a | 18.59 ± 0.70a |
| Jorhat | 0.067 ± 0.019b | 0.466 ± 0.137a | 0.246 ± 0.030a | 93.20 ± 1.89a | 14.49 ± 0.78b | 15.55 ± 0.87b | |
| BC2-59 | 0.061 ± 0.019b | 0.388 ± 0.125a | 0.194 ± 0.060a | 92.07 ± 1.69a | 15.77 ± 0.49ab | 17.13 ± 0.85ab | |
| TR10 | 0.064 ± 0.016b | 0.520 ± 0.133a | 0.263 ± 0.026a | 94.07 ± 1.34a | 16.18 ± 0.89ab | 13.15 ± 0.72c | |
| p-value | <0.9842 | <0.7925 | <0.2976 | <0.5801 | <0.1804 | <0.0067 | |
| F-Value 3,11 | 0.04988 | 0.3470 | 1.456 | 0.6960 | 2.087 | 8.718 | |
| CSR2 × CSR4 | Hmute | 0.096 ± 0.018a | 0.335 ± 0.147a | 0.219 ± 0.036ab | 92.83 ± 1.76a | 28.65 ± 0.63a | 30.87 ± 0.82a |
| Jorhat | 0.046 ± 0.006ab | 0.458 ± 0.168a | 0.164 ± 0.062b | 94.65 ± 1.76a | 10.17 ± 0.59d | 10.74 ± 0.65c | |
| BC2-59 | 0.059 ± 0.008ab | 0.391 ± 0.128a | 0.184 ± 0.025b | 91.83 ± 1.64a | 15.28 ± 0.65c | 16.64 ± 0.68b | |
| TR10 | 0.083 ± 0.019b | 0.525 ± 0.136a | 0.325 ± 0.036a | 93.57 ± 1.22a | 19.63 ± 0.69b | 16.88 ± 0.96b | |
| p-value | <0.1234 | <0.8110 | <0.1008 | <0.6643 | <0.0001 | <0.0001 | |
| F-Value 3,11 | 2.612 | 0.3199 | 2.912 | 0.5465 | 149.30 | 117.79 | |
| CSR4 × CSR2 | Hmute | 0.068 ± 0.016b | 0.348 ± 0.125a | 0.192 ± 0.024a | 90.58 ± 1.67a | 19.72 ± 0.87ab | 21.77 ± 0.67a |
| Jorhat | 0.241 ± 0.018a | 0.452 ± 0.126a | 0.171 ± 0.029a | 94.82 ± 1.97a | 5.33 ± 0.88c | 5.62 ± 0.95c | |
| BC2-59 | 0.078 ± 0.015b | 0.384 ± 0.127a | 0.224 ± 0.046a | 92.17 ± 1.77a | 20.48 ± 0.49a | 22.22 ± 0.73a | |
| TR10 | 0.068 ± 0.015b | 0.514 ± 0.129a | 0.285 ± 0.052a | 94.47 ± 1.67a | 17.93 ± 0.58b | 14.15 ± 0.63b | |
| p-value | <0.0001 | <0.7991 | <0.2677 | <0.3476 | <0.0001 | <0.0001 | |
| F-Value 3,11 | 28.035 | 0.3373 | 1.584 | 1.273 | 95.777 | 107.04 | |
| Strain | Host plant | GR (g/insect/day) | CR (g/insect/day) | CI (g/insect/day) | AD (%) | ECI (%) | ECD (%) |
| FC1 × FC2 | Hmute | 0.063 ± 0.017a | 0.355 ± 0.149a | 0.176 ± 0.043b | 89.90 ± 2.01a | 17.91 ± 0.84b | 19.93 ± 0.91b |
| Jorhat | 0.049 ± 0.007a | 0.471 ± 0.137a | 0.279 ± 0.026ab | 92.68 ± 1.56a | 10.45 ± 0.76c | 11.28 ± 0.73c | |
| BC2-59 | 0.078 ± 0.016a | 0.395 ± 0.126a | 0.268 ± 0.038ab | 91.86 ± 1.95a | 19.75 ± 0.57ab | 21.50 ± 0.96b | |
| TR10 | 0.081 ± 0.016a | 0.523 ± 0.157a | 0.334 ± 0.039a | 93.92 ± 1.94a | 20.29 ± 0.85a | 39.37 ± 0.89a | |
| P-value | <0.4306 | <0.8394 | <0.0875 | <0.5223 | <0.0001 | <0.0001 | |
| F-Value 3,11 | 1.027 | 0.2787 | 3.129 | 0.8116 | 35.514 | 180.83 | |
| FC2 × FC1 | Hmute | 0.071 ± 0.016a | 0.357 ± 0.126a | 0.195 ± 0.061a | 89.92 ± 1.98b | 20.00 ± 0.85a | 22.25 ± 0.69a |
| Jorhat | 0.078 ± 0.013a | 0.471 ± 0.129a | 0.282 ± 0.050a | 92.90 ± 1.94ab | 16.72 ± 0.67b | 18.00 ± 0.89b | |
| BC2-59 | 0.076 ± 0.017a | 0.395 ± 0.129a | 0.240 ± 0.025a | 91.27 ± 1.89b | 19.34 ± 0.78a | 21.19 ± 0.80a | |
| TR10 | 0.062 ± 0.015a | 0.525 ± 0.128a | 0.279 ± 0.027a | 93.69 ± 1.44a | 16.83 ± 0.69b | 12.58 ± 0.90c | |
| p-value | <0.8820 | <0.7924 | <0.4921 | <0.5038 | <0.0293 | <0.0001 | |
| F-Value 3,11 | 0.2169 | 0.3471 | 0.8776 | 0.8516 | 5.086 | 27.761 | |
| J112 | Hmute | 0.067 ± 0.018c | 0.406 ± 0.128a | 0.215 ± 0.028a | 61.47 ± 1.23c | 16.58 ± 0.86c | 26.98 ± 0.98d |
| Jorhat | 0.131 ± 0.015a | 0.492 ± 0.126a | 0.447 ± 0.139a | 66.38 ± 1.88b | 26.62 ± 0.85a | 40.10 ± 0.67a | |
| BC2-59 | 0.077 ± 0.019bc | 0.400 ± 0.087a | 0.236 ± 0.034a | 59.08 ± 1.45c | 19.42 ± 0.69b | 32.88 ± 0.79b | |
| TR10 | 0.118 ± 0.019ab | 0.552 ± 0.066a | 0.461 ± 0.135a | 70.69 ± 1.01a | 25.23 ± 0.75a | 30.39 ± 0.95c | |
| p-value | <0.0931 | <0.0931 | <0.2298 | <0.0019 | <0.0019 | <0.0001 | |
| F-Value 3,11 | 3.033 | 0.7038 | 1.774 | 13.117 | 36.152 | 42.143 | |
GR (Growth rate); CR (Consumption rate); CI (Consumption index); AD (Approximate digestibility); ECI (Efficiency of conversion of ingested food); ECD (Efficiency of conversion of digested food). The data are mean ± standard error (n = 3 observations). p-values were determined by one-way ANOVA with subsequent Tukey’s post hoc test. Values of treatment groups with different alphabetical letters show statistically significant difference at p < 0.05, and values with similar letters show no significance at p > 0.05. The F-value is the ratio of the mean regression sum of squares divided by the mean error sum of squares (variance within samples/variance between samples).
Economic Performance of B. mori
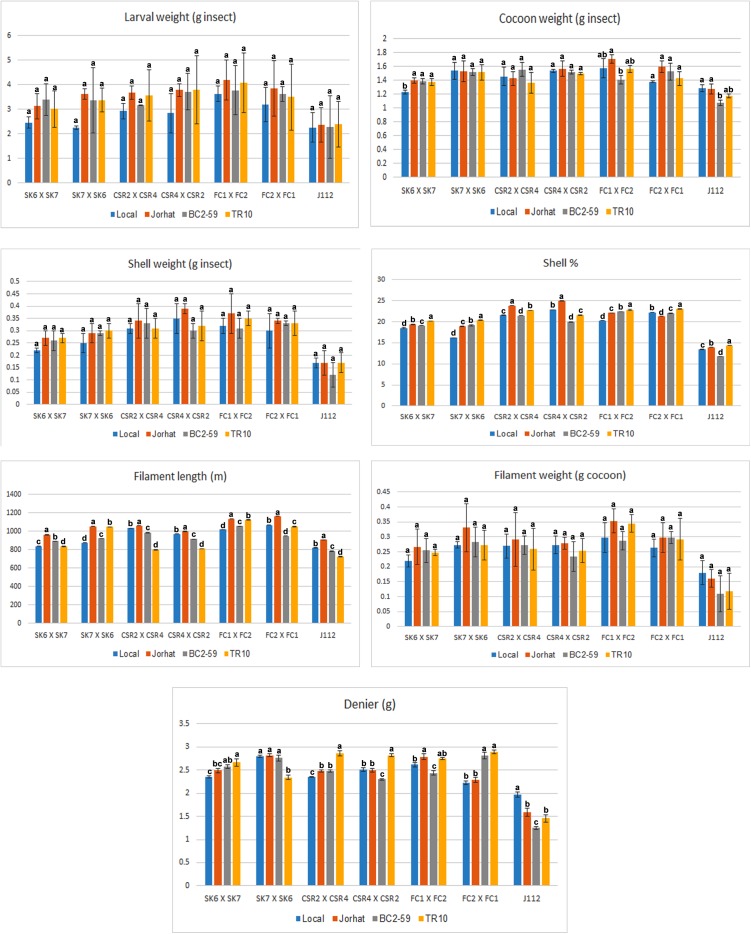
The silkworm economic parameters such as cocoon weight, shell weight, and filament length were high on larvae fed with the Jorhat mulberry plant variety in all the B. mori strains tested. A higher value of Shell percentage was evident on all the B. mori strains reared with the TR10 mulberry plant variety except CSR2 × CSR4 and CSR4 × CSR2. Filament length was higher when the larvae were reared with the Jorhat mulberry plant variety (Figure 1).
FIGURE 1.
Economic parameters of B. mori strains reared on different mulberry varieties. Six bivoltine silkworm strains were used – SK6 × SK7, SK7 × SK6, CSR2 × CSR4, CSR4 × CSR2, FC1 ×FC2, FC2 × FC1 – as well as one multivoltine strain, J112. Host plants used – Hmute, Jorhat, BC2-59, and TR10. Data are presented as mean ± standard error mean (n = 1680 larvae/treatment). There were 84 experimental sets and each experimental set comprised of a rearing tray with 20 larvae (7 silkworm races × 4 host plant varieties × 3 replicates = 84 sets × 20 larvae = 1680). Statistical comparison was performed using one-way ANOVA followed by Tukey’s post hoc tests for all pair-wise multiple comparisons. Bars with different letters (a, b, c, d) indicate that treatment groups are significantly different at P < 0.05 and with same letters indicate that treatment groups are not statistically significant at P > 0.05.
The highest shell weight and shell percentage were found in the SK7 × SK6 strain reared on TR10. Filament length and weight were higher when the larvae were reared with the Jorhat plant variety. In the CSR2 × CSR4 strain, the shell weight as well as filament length were high on the Jorhat plant variety-reared larvae. Denier was higher on the TR10 mulberry plant variety. The shell weight and filament length were higher in the CSR4 × CSR2 strain reared with the Jorhat mulberry plant variety. The larval weight of the FC1 × FC2 strain was highest on Jorhat followed by the TR10 mulberry plant variety. The FC2 × FC1 strain had high larval, cocoon, shell, and filament weight in larvae reared on the Jorhat mulberry plant variety. The larval strains reared on the TR10 mulberry plant variety exhibited high Denier in all the larval strains except SK7 × SK6 and J112 (Figure 1).
Biochemical and Enzymatic Activities of B. mori Strains
Silk is mainly composed of two proteins, fibroin and sericin, accounting for about 70 and 30% of total silk cocoon weight, respectively. The SK6 × SK7 cocoon had high fibroin (74.0%) and sericin (26.0%) contents when reared on the Jorhat mulberry variety. The CSR2 × CSR4 strain cocoon had 71.01 and 28.99% of fibroin and sericin, respectively, when reared on Jorhat mulberry plant variety. The silkworm strains FC1 × FC2 and SK7 × SK6 had a high fibroin to sericin ratio when reared on the TR10 host plant (Table 3). TR10 mulberry plant variety-reared larvae had highest hemolymph protein among all the host plants tested in the present study. The highest silk gland protein content was observed in larvae reared on the BC2-59 mulberry plant variety. Maximum amylase activity was found in those larval midgut samples reared on the Jorhat mulberry variety (Table 3).
Table 3.
Biochemical and Enzymatic profiles of B. mori strains reared on different host plants.
| Strain | Host plant | Fibroin % (Cocoon) | Sericin % (Cocoon) | Hemolymph protein (μg/mL) | Silk gland protein (μg/mL) | Amylase (μg/mL) | Protease (μg/mL) |
|---|---|---|---|---|---|---|---|
| SK6 × SK7 | Hmute | 70.30 ± 0.05c | 29.70 ± 0.04b | 416.44 ± 0.88b | 365 ± 1.20b | 1.01 ± 0.08b | 0.20 ± 0.08a |
| Jorhat | 74.00 ± 0.12a | 26.00 ± 0.07d | 472.61 ± 0.90a | 525 ± 2.23a | 1.38 ± 0.06a | 0.20 ± 0.04a | |
| BC2-59 | 72.32 ± 0.09b | 27.68 ± 0.06c | 416.20 ± 1.30b | 332 ± 1.85c | 0.60 ± 0.04c | 0.06 ± 0.07a | |
| TR10 | 66.37 ± 0.05d | 33.63 ± 0.07a | 414.91 ± 0.96b | 282 ± 1.78d | 0.61 ± 0.03c | 0.20 ± 0.05a | |
| P-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.3475 | |
| F-Value 3,11 | 1571.6 | 2881.3 | 768.23 | 3393.3 | 44.437 | 1.273 | |
| SK7 × SK6 | Hmute | 74.47 ± 0.08c | 25.53 ± 0.06c | 407.37 ± 1.20b | 354 ± 1.30b | 0.93 ± 0.03a | 0.19 ± 0.01b |
| Jorhat | 65.62 ± 0.07d | 34.38 ± 0.04a | 363.17 ± 0.81c | 425 ± 1.85a | 1.02 ± 0.06a | 0.28 ± 0.02a | |
| BC2-59 | 74.29 ± 0.06b | 25.71 ± 0.03b | 336.83 ± 1.57d | 348 ± 1.42c | 0.62 ± 0.08b | 0.05 ± 0.03c | |
| TR10 | 76.04 ± 0.03a | 23.96 ± 0.04d | 410.61 ± 0.75a | 256 ± 1.56d | 1.05 ± 0.02a | 0.26 ± 0.02a | |
| P-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0016 | <0.0016 | |
| F-Value 3,11 | 5646.2 | 11586 | 997.23 | 2009.1 | 13.699 | 24.074 | |
| CSR2 × CSR4 | Hmute | 62.74 ± 0.10d | 37.26 ± 0.01a | 329.57 ± 0.89d | 380 ± 3.01b | 0.72 ± 0.06b | 0.19 ± 0.04a |
| Jorhat | 71.01 ± 0.04a | 28.99 ± 0.14d | 376.17 ± 0.78c | 345 ± 1.03c | 0.95 ± 0.05a | 0.19 ± 0.05a | |
| BC2-59 | 68.60 ± 0.03b | 31.40 ± 0.07c | 395.54 ± 0.85b | 511 ± 1.29a | 0.66 ± 0.09b | 0.07 ± 0.07a | |
| TR10 | 67.08 ± 0.10c | 32.92 ± 0.08b | 484.79 ± 0.63a | 272 ± 2.05d | 0.62 ± 0.09b | 0.09 ± 0.06a | |
| P-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0548 | <0.3390 | |
| F-Value 3,11 | 2150.1 | 1560.6 | 6714.0 | 2503.1 | 3.903 | 1.302 | |
| CSR4 × CSR2 | Hmute | 68.83 ± 0.09c | 31.17 ± 0.07b | 425.98 ± 1.76c | 425 ± 1.05b | 0.74 ± 0.03a | 0.20 ± 0.03a |
| Jorhat | 94.97 ± 0.08a | 5.03 ± 0.06d | 287.49 ± 0.84d | 348 ± 1.68c | 0.67 ± 0.05ab | 0.08 ± 0.02b | |
| BC2-59 | 67.47 ± 0.13d | 32.53 ± 0.16a | 438.57 ± 0.64b | 611 ± 2.54a | 0.61 ± 0.03b | 0.09 ± 0.04b | |
| TR10 | 69.35 ± 0.05b | 30.65 ± 0.10c | 488.10 ± 0.59a | 427 ± 1.89b | 0.71 ± 0.04ab | 0.06 ± 0.02b | |
| P-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.1732 | <0.0338 | |
| F-Value 3,11 | 20665 | 15885 | 6484.1 | 3579.7 | 2.141 | 4.798 | |
| FFC1 × FC2 | Hmute | 68.83 ± 0.09b | 31.17 ± 0.07b | 424.98 ± 1.76b | 425 ± 1.05b | 0.71 ± 0.01a | 0.20 ± 0.03a |
| Jorhat | 79.25 ± 0.07d | 20.75 ± 0.05c | 482.41 ± 0.90a | 422 ± 1.09b | 0.73 ± 0.02a | 0.08 ± 0.05b | |
| BC2-59 | 68.16 ± 0.04c | 31.84 ± 0.05a | 393.81 ± 0.95c | 624 ± 1.65a | 0.70 ± 0.03a | 0.17 ± 0.04ab | |
| TR10 | 83.91 ± 0.07a | 16.09 ± 0.04d | 425.27 ± 0.79b | 282 ± 2.06c | 0.74 ± 0.04a | 0.07 ± 0.09b | |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.7278 | <0.3446 | |
| F-Value 3,11 | 12465 | 21136 | 1004.4 | 8549.7 | 0.4444 | 1.282 | |
| FC2 × FC1 | Hmute | 71.20 ± 0.04c | 28.80 ± 0.10b | 385.21 ± 2.64b | 480 ± 2.20b | 0.61 ± 0.13a | 0.08 ± 0.02b |
| Jorhat | 71.92 ± 0.02b | 28.08 ± 0.03c | 389.47 ± 0.73b | 478 ± 1.07b | 1.27 ± 0.48a | 0.27 ± 0.05a | |
| BC2-59 | 74.00 ± 0.05a | 26.00 ± 0.02d | 263.04 ± 2.00c | 619 ± 3.09a | 0.61 ± 0.18a | 0.09 ± 0.08b | |
| TR10 | 66.15 ± 0.06d | 33.85 ± 0.03a | 434.90 ± 1.57a | 478 ± 1.61b | 1.26 ± 0.14a | 0.22 ± 0.02ab | |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.2062 | <0.0618 | |
| F-Value 3,11 | 5477.5 | 3636.7 | 1551.2 | 1086.7 | 1.911 | 3.698 | |
| J112 | Hmute | 73.76 ± 0.06b | 26.24 ± 0.03c | 325.98 ± 1.76c | 128 ± 1.09c | 0.85 ± 0.04a | 0.07 ± 0.02a |
| Jorhat | 71.30 ± 0.16c | 28.70 ± 0.02b | 225.49 ± 0.84d | 311 ± 2.06b | 0.67 ± 0.02b | 0.05 ± 0.03a | |
| BC2-59 | 59.40 ± 0.02d | 40.60 ± 0.13a | 429.10 ± 0.64b | 123 ± 2.12c | 0.71 ± 0.02b | 0.06 ± 0.02a | |
| TR10 | 84.17 ± 0.04a | 15.83 ± 0.06d | 476.57 ± 0.59a | 325 ± 3.00a | 0.86 ± 0.04c | 0.07 ± 0.01a | |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0054 | <0.8910 | |
| F-Value 3,11 | 13263 | 18982 | 10975 | 2618.4 | 9.358 | 0.2037 | |
The data are mean ± standard error (n = 3 observations). p-values were determined by one-way ANOVA with subsequent Tukey’s post hoc test. Values of treatment groups with different alphabetical letters show statistically significant difference at p < 0.05, and values with similar letters show no significance at p > 0.05. The F-value is the ratio of the mean regression sum of squares divided by the mean error sum of squares (variance within samples/variance between samples).
Discussion
Bombyx mori is a monophagous insect that feeds exclusively on mulberry leaf for its growth as well as accumulation of energy during non-feeding stages (larval, molting, spinning, pupal, and adult). The larvae need food that not only meets its nutritional requirements but must also be capable of being assimilated and converted into energy required for growth and development. The present work was performed to study the comparative analysis of different mulberry plant varieties to evaluate differences in their biochemical nutritive values and their physiological effect on different strains of B. mori. It is one of the most promising income sources and will involve less investment through better utilization of the resources with the knowledge of the plant nutrient profile and ecological suitability of the silkworm strains.
In the present study, analysis of biochemical composition of four different varieties of mulberry leaves showed that the levels of protein, carbohydrate, and reducing sugar were greater in the Hmute mulberry plant variety. The TR10 and Jorhat mulberry varieties showed significant amounts of calcium, potassium, magnesium and phosphorus, and in turn also performed well in terms of insect fibroin content, filament length and percentage of shell. Shinde et al. (2014) stated that silk productivity and quality are based on mulberry plant variety, its nutrients and climatic conditions. Quality of feed plays a remarkable role and is an important parameter used for evaluation for choosing suitable varieties for silkworm rearing as the nutrient quality of food plants, which affects its conversion into insect biomass, and this in turn affects the economic traits of cocoons (Das et al., 2001; Kumar and Vadamalai, 2010).
Nutritional efficiency study of all the bivoltine silkworm strains used in our study revealed variation in their nutritional requirements when reared with different host plants. Significant differences in growth rate (GR) were observed in most of the strains reared on four different host plants. High GR was observed in insects fed with Hmute, Jorhat, and TR10 mulberry plant variety leaves. CR was significantly higher in TR10 and Jorhat mulberry plant varieties for all the silkworm strains tested. Approximate digestibility (AD) is the indicator showing the amount of food consumed by the larva versus retention of food in the midgut, reflecting the effectiveness of digestive enzymes and ingestion of nutrients from the food. High AD means nutrients in the diet are ingested appropriately and converted into energy. Based on the value of the AD, it was observed that the TR10 mulberry plant variety is the best plant for digestibility in all the Bombyx strains tested. Silkworm nutrition is an important component in silk production, and this constituent determines the silk quality and silk trade all over the world. Low-quality mulberry plant feed significantly influences the performance of the life cycle process (growth of larva, developmental period, fecundity, silk production, and quality of silk) in terms of quality of leaf (Ramesha et al., 2010) and metabolic conversion of food into silk substance (Parra and Kogan, 1981; Paul et al., 1992; Levesque et al., 2002).
Significant improvement in larval growth characters were observed in larvae reared on high amino acid-containing mulberry plants (Radjabi, 2010). In the present study, similar results were obtained from our experiment – Hmute and TR10 mulberry plant variety-reared insects showed high growth rate due to the presence of high amino acid contents. There was a strong positive correlation between leaf moisture content and approximate digestibility (Radha, 2013), and in our study as well, most of the strains performed best on its preferred host plant TR10 mulberry plant variety. High food index values were observed in the chosen B. mori strains because of the high nutrient quality present in the TR10 mulberry plant variety. This observation corroborates the findings of Applebaum (1985) and Slansky and Scriber (1985), establishing the influence of insect digestion on the nutritional composition of a host. Rahmathulla et al. (2005) emphasized the association between mulberry varieties and food indices, especially ECD and ECI with insect biomass.
Assal et al. (1994) studied the nutritional behavior of B. mori with Fenoxycarb and reported the importance of food indices in terms of silk production. Kumara and Roy (2011) used food indices as a vital tool to identify profitable silkworm breeds. In our study, B. mori reared on TR10 and Jorhat mulberry plant varieties had higher mineral contents and consequently, consumption indices as well as approximate digestibility were found to be higher when compared to other food sources. Larvae fed with diet containing more minerals divert minimum energy for maintenance, by which the larvae can channel maximum energy for silk production. Yamamoto and Fujimaki (1982) and Sabhat et al. (2011) reported the alterations in food indices among the different breeds and same breed of B. mori when fed on the leaves of different nutritional quality.
Improvement in the leaf silk conversion ability of a given mulberry genotype or silkworm race will add to its economics (Trivedi and Nair, 1998). A large amount of data are available on the evaluation of mulberry varieties against silkworm rearing and economic parameters (Iwanari and Ohno, 1969; Thangamani and Vivekanandan, 1984; Das and Vijayaraghavan, 1990; Gangwar, 2011). In our study, significant differences were observed in larval parameters and commercial cocoon characters for all the silkworm strain reared with different mulberry varieties.
According to Fonseca et al. (1990) and Seidavi (2011), rearing success of silkworm races varied significantly even under the same conditions, with some of them being better performers, whereas some races showed poor performance. The present study also confirms the same where different host plants were evaluated for different silkworm strains. Gangawar (2010) found that among eight mulberry varieties screened for nutritional suitability, silkworm larvae fed on BR2 variety leaves showed increased larval weight and better economic traits in comparison to other varieties. In the present study, silk filament length of cocoons recovered from silkworms reared on different mulberry varieties falls within the range between 600 and 1500 m (FAO, 1999), and cocoons recovered from silkworms reared on Jorhat mulberry variety leaves produced the longest filament length. Cocoon and shell weights are the important parameters evaluated for productivity, and shell weight percentage is the amount of raw silk that is reeled from the cocoons and varies according to age and strain of silkworm (Gaviria et al., 2006).
Feeding with high content of potassium, magnesium and calcium in mulberry leaves significantly increased the shell percentage (Mane et al., 1998), which has been found in the larvae reared on TR10 and Jorhat plant varieties, which may in turn be due to the high amount of amino acids, potassium, magnesium, calcium present in the leaves (Verma and Atwal, 1963). Rearing performance of all the silkworm strains used in our study proved to be better when fed with Jorhat mulberry variety leaves followed by TR10. Among the different silkworm strains used, the CSR4 × CSR2 strain fed with the Jorhat mulberry variety exhibited the best performance. The FC2 × FC1 strain fed with the Jorhat mulberry variety proved promising. Besides the Jorhat mulberry variety, the FC1 × FC2 strain fed with the TR10 mulberry plant variety also showed good performance. These plant varieties supported superior growth and development of silkworm larvae, which is reflected in quality cocoon characteristics. From the present results, B. mori strains CSR4 × CSR2, FC1 × FC2, and FC2 × FC1 fed with Jorhat and TR10 mulberry plant varieties turn out to be superior among other B. mori strains as well as host plants used in this experiment.
Different strains of B. mori showed variations in fibroin percentage, hemolymph protein, silk gland protein, amylase and protease for different mulberry plant varieties. The conversion of host plant nutrients into silk protein mainly takes place during the larval stages, and protein metabolism is a major biochemical process that helps in characterizing different stages of development (Chen and Pan, 1996). Nutrition is linked to the physiology of digestion, and the ability of silkworms to secrete digestive enzymes is influenced by the nutrient profile of the diet (Kellner et al., 1887). The differences in enzyme activity, in our study, indicated that some silkworm strains fed with different host plants are more efficient in biomass deconstruction, while others are not. Activity of the enzyme amylase was high in those larvae reared with the Jorhat mulberry variety and the TR10 mulberry variety, which might be due to the sufficient amount of raw substrate resulting from high food intake. The proteolytic enzyme, proteases, are found in abundance in the late larval stages of silkworms, which aids the digestion of leaf fibrous proteins found in their coarse leaf diet (Ito, 1978). The proteolytic activity of the gut in relation to protein diet has been studied in many insects (Hamano and Mukaiyama, 1970). In the present study, protease activity was high in larvae fed with the Jorhat mulberry plant variety, and it is presumed that the diet may facilitate the enzyme to act on their substrate.
Hemolymph serves as a reservoir for nutrients and metabolites during metamorphosis. The cellular structures of the silk gland also differentiate and repair themselves for synthesis of silk proteins by utilizing the free amino acids present in the hemolymph (Mathur et al., 1989; Mahmoud et al., 2013). The silk is secreted by the silk glands, which are a reservoir for two silk proteins, fibroin and sericin (Zhang et al., 2006). In the present study, the silk gland contained more protein content when compared to hemolymph in the majority of silkworm strains used. B. mori produces twin threads of silk fibroin coated by a protective layer of sericin, and the silk protein is an essential constituent of cocoon filament (Komatsu, 1975). In our study, we have observed that fibroin percentage is significantly higher for most of the B. mori strains fed with the TR10 and Jorhat mulberry plant varieties, which may be due to the presence of high amino acid and carbohydrate levels. Additionally, hemolymph protein was significantly higher for TR10 mulberry plant variety-reared larva, followed by Jorhat mulberry plant variety-reared larva, which significantly increases the fibroin percentage in silk gland as the amino acids resulting from digestion are transported directly to the silk gland via hemolymph (Bricteux et al., 1965). Approximate digestibility was found high for the TR10 mulberry plant variety- followed by Jorhat mulberry plant variety-reared larva, maybe due to the presence of high amylase in the larva reared on TR10 and the Jorhat plant variety.
Silkworm requires specific nutrient components such as essential sugars, amino acids, proteins, and vitamins for its optimal growth and development (Sengupta et al., 1972; Khedr et al., 2013). Poor nutrition diets will directly affect the primary biochemical and physiological metabolism in insects, and in turn alter the detoxification system leading to increased susceptibility to diseases (Lindroth et al., 1991).
Sericulture industry can develop the rural economy of any state as it is a part of the tradition and culture of the local populace and hence is an eco-friendly production process with skilled households. It is one of the most promising income resources without spending much for its cultivation and better utilization with the knowledge of the plant nutrient sources and ecological suitability. The optimal levels of macronutrients in the host plants are sufficient for silkworm larval growth, whereas high levels of micronutrients are very much essential for better silk quality. The findings of the present study can form a platform for further research on silkworm physiology, especially under the hilly and high altitude agro-climatic conditions. From the results, we have observed that B. mori strains CSR4 × CSR2, FC1 × FC2, and FC2 × FC1 and mulberry varieties Jorhat and TR10 performed better in silkworm rearing tests under hilly tropical agro-climatic conditions. Such mulberry varieties and silkworm strains can be recommended for more field trials by farmers and can be used for sustainable growth and development of the sericulture industry.
Author Contributions
LR, GG, BC, and NSK participated in the research design. LR and SG conducted the experiments. LR, SG, BC, TB, and NSK performed the data analysis. LR, SG, GG, TB, and NSK wrote or contributed to the writing of the manuscript. SS involved in major revision of the manuscript in both data analysis and rewriting the manuscript. All authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank the Mizoram University, which provided all the essential facilities to carry out the work, the Department of Biotechnology, New Delhi, India for support in the form of the Bioinformatics Infrastructural Facility [BT/BI/12/060/2012 (NERBIF-MUA)], the Advanced level State Biotech Hub (BT/04/NE/2009 Dated: August 29, 2014), as well as the DBT-eLibrary Consortium (DeLCON).
Footnotes
Funding. The authors thank the Program for Excellent Young Teachers at the Hangzhou Normal University (Grant No. JTAS 2011-01-031), which supported the present work.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2019.00878/full#supplementary-material
References
- Applebaum S. V. (1985). “Biochemistry of digestion,” in Comprehensive Insect Physiology, Biochemistry and Pharmacology, eds Kerku G. A., Gilbert L. T. (Oxford: Pergamon Press; ), 279–312. [Google Scholar]
- Assal O. M., Benedetti R., Cappellozza L., Cappellozza S. (1994). The nutritional behaviour of three different strains of mulberry silkworm (Bombyx mori) in relation to insegar (fenopxycarb). Sericologia 34 233–244. [Google Scholar]
- Bligh E. G., Dyer W. J. (1959). A rapid method of total lipid extraction and purification. Can. J. Biochem. Phys. 37 911–917. [DOI] [PubMed] [Google Scholar]
- Bose P. C., Mazumder S. K., Sengupta K. (1991). A comparative biochemical study of six mulberry (M. alba L.) varieties. Indian J. Seric. 30 83–87. [Google Scholar]
- Bricteux G. S., Jeuniaux C. H., Florkin M. (1965). Contributions à la biochimie du ver à soie—XXX. Biosynthèse de tréhalose et de glycogène à partir de glucose-1-phosphate. Comp. Biochem. Physiol. 16 333–340. 10.1016/0010-406x(65)90300-2 [DOI] [PubMed] [Google Scholar]
- Chen C., Pan S. (1996). Assay of superoxide dismutase activity by combining electrophoresis and densitometry. Bot. Bull. Acad. Sin. 37 107–111. [Google Scholar]
- Das B. C., Sahu P. K., Sengupta T., Misra A. K., Saratchandra B., Sen S. K. (2001). Genetic variability in some physiological traits in mulberry. Indian J. Plant. Physiol. 6 162–165. 10.3390/plants8050118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das P. K., Vijayaraghavan K. (1990). Studies on the effect of different mulberry varieties and seasons on the larval development and cocoon characters of silkworm Bombyx mori L. Indian J. Seric. 29 44–53. [Google Scholar]
- Duncan D. B. (1955). Multiple range and multiple F-tests. Biometrics 11 1–42. [Google Scholar]
- Eguchi M., Iwamoto A. (1976). Alkaline proteases in the midgut tissue and digestive fluid of the silkworm Bombyx mori L. Insect Biochem. 6 491–496. 10.1016/0020-1790(76)90073-1 [DOI] [Google Scholar]
- FAO (1999). “Silk reeling and testing manual,” Agricultural Services, Bulletin No. 136 Rome, 65–75. [Google Scholar]
- Fonseca T. C., Almeida J. E., Fonseca A. S. (1990). Effect of mulberry selection on silkworm feeding. Sericologia 30 475–477. [Google Scholar]
- Gangawar S. K. (2010). Impact of varietal feeding of eight Mulberry varieties on Bombyx mori L. Agric. Biol. J. North Americ. 1 350–354. 10.5251/abjna.2010.1.3.350.354 [DOI] [Google Scholar]
- Gangwar S. K. (2011). Screening of region and season specific bivoltine silkworm hybrid breeds of west bengal in spring and summer season of uttar pradesh climatic condition. Int. J. Plant Ann. Environ. Sci. 1 74–87. [Google Scholar]
- Gaviria D. A., Aguilar E., Serrano H. J., Alegria A. H. (2006). DNA fingerprinting using AFLP markers to search for makers associated with yield attributes in the silkworm Bombyx mori. J. Insect Sci. 6 1–10. 10.1673/2006_06_15.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamano K., Mukaiyama F. (1970). Some properties of digestive fluid proteases in the silkworm, Bombyx mori, with reference to the relation between dissociation degree and nutritive value of some proteins. J. Seric. Sci. Japan 39 371–376. [Google Scholar]
- Ito T. (1978). “Silkworm nutrition,” in The Silkworm, an Important Laboratory Tool, ed. Tazima Y. (Mishima: Kodansha Ltd.). [Google Scholar]
- Iwanari Y., Ohno Y. (1969). Rearing experiments of silkworms on the artificial diets composed mainly of the mulberry leaf powder harvested on the late autumnal and there after season. J. Seric. Sci. 38 307–315. [Google Scholar]
- Jackson M. L. (1973). Soil Chemical Analysis. New Jersey, NJ: Prentice Hall of Englewood cliffs. [Google Scholar]
- Jyothi M., Pratap M., Thimma N. S. (2014). Studies on biochemical constituents of different genotypes of Morus alba L. Int. J. Pharm Bio. Sci. 5 835–840. [Google Scholar]
- Kant R., Bhat M. M. (2010). Mulberry foliar fungal diseases and insect pests calendar in Uttarakand. Indian Silk 45 10–13. [Google Scholar]
- Kataky A., Hazarika L. K. (1997). Fats and fatty acids in the development stages of Antheraea assama and its hosts plants. Sericologia 37 511–515. [Google Scholar]
- Kellner O., Kakizaki S., Matsuoka M., Yoshu T. (1887). On the physiology of the silk worm. By Alexander pringle jameson and william ringrose gelston atkins. Landw. Versuchs-stationen. 33:381. [Google Scholar]
- Khedr M. M. A., El-Shafiey S. N., Mead H. M. I. (2013). Influence of fortification of mulberry leaves with natural and synthetic multivitamins on growth and development of Bombyx mori L. Egypt. J. Plant Prot. Path. 4 111–123. [Google Scholar]
- Komatsu K. (1975). Studies on dissolution behaviors and structural characteristic of silk Sericin. Bull. Sericult. Exp. Sta. 26 135–256. [Google Scholar]
- Krishnaswami S. (1978). Mulberry Cultivation in South India. Bulletin No.1. Mysore: Central Sericulture research and Training, Institute. [Google Scholar]
- Kumar R., Vadamalai E. (2010). Rearing Performance of Eri Silkworm Philosamia ricini in Monsoon Season of Uttar Pradesh. Asian J. Exp. Biol. Sci. 1 303–310. [Google Scholar]
- Kumara N., Roy S. P. (2011). Some aspects of the identification of nutritionally efficient silkworms, their metabolic rate and sustainable development as energy resources. Bioscan 6 475–481. [Google Scholar]
- Lalfelpuii R., Choudhury B. N., Gurusubramanian G., Senthil Kumar N. (2014a). Effect of different mulberry plant varieties on growth and economic parameters of the silkworm Bombyx mori in Mizoram. Sci. Vis. 14 34–38. [Google Scholar]
- Lalfelpuii R., Choudhury B. N., Gurusubramanian G., Senthil Kumar N. (2014b). Influence of medicinal plant extracts on the growth and economic parameters of mulberry silkworm, Bombyx mori L. Sericologia 54 275–282. [Google Scholar]
- Levesque K. R., Levesque K. R., Fortin M., Mauffette Y. (2002). Temperature and food quality effects on growth, consumption and post ingestive utilization efficiencies of the forest tent caterpillar Malacosoma disstria. Bull. Entomol. 92 127–136. 10.1079/ber2002153 [DOI] [PubMed] [Google Scholar]
- Lindroth R. L., Barman M. A., Weisbra A. V. (1991). Nutritient deficiencies and the gypsy moth, L. dispar: effects on larval performance and detoxification enzyme activities. J. Insect Physiol. 37 45–52. 10.1016/0022-1910(91)90018-u [DOI] [Google Scholar]
- Lowry O. H., Rosebrough N. F., Farr A. L., Randall R. J. (1951). Protein measurement with Folin phenol reagent. J. Biol. Chem. 193 267–275. [PubMed] [Google Scholar]
- Machii H., Katagiri K. (1991). Varietal differences in nutritive value of mulberry leaves for rearing of silkworms. JARQ 25 202–208. [Google Scholar]
- Mahmoud S. M., Akila M. E., Moustafa A. A., El-Banna A. A., Moustafa M. N. (2013). Enzymatic activity of the silkworm, Bombyx mori L. hemolymph reared on different mulberry varieties. Egypt. J. Agric. Res. 91 1407–1413. [Google Scholar]
- Mane J., Patil G. M., Vage M. (1998). Fortification of castor leaves with inorganic minerals to increase economic traits of eri silkworm, Samia Cynthia ricini. Sci. Cult. 64:34. [Google Scholar]
- Mathur S. K., Roy A. K., Sen S. K., Subba-Rao G. (1989). Studies on the growth of silkworm, Bombyx mori L. (Lepidoptera:Bombycidae) under tropical conditions. Indian J. Seric. 28 71–79. [Google Scholar]
- Miller G. L. (1972). Use of dinitrosalicyclic reagent fordeternination of reducing sugars. Anal. Chem. 31 426–428. [Google Scholar]
- Moore R. A., Stein W. H. (1948). Photometric ninhydrin method for use in the chromatography of amino acids. J. Biol. Chem. 176 367–388. [PubMed] [Google Scholar]
- Nagaraju J. (2002). Application of genetic principles in improving silk production. Curr. Sci. 83 409–415. [Google Scholar]
- Nagaraju J., Abraham E. G. (1995). Purification and characterization of digestive amylase from the tasar silkworm, Antheraea mylitta (Lepidoptera:Saturniidae). Comp. Biochem. Physiol. 110 201–209. 10.1016/0305-0491(94)00121-a [DOI] [Google Scholar]
- Neog K., Unni B., Ahmed G. (2011). Studies on the influence of host plants and effect of chemical stimulants on the feeding behavior in the muga silkworm, Antheraea assamensis. J. Insect Sci. 11:133. 10.1673/031.011.13301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra J. R. P., Kogan M. (1981). Comparative analysis of methods for measurements of food intake and utilization using the soybean looper, Pseudoplusia includens and artificial media. Entomol. Exp. Appl. 30 45–57. 10.1111/j.1570-7458.1981.tb03583.x [DOI] [Google Scholar]
- Paul D. C., Subba-Rao G., Deb D. C. (1992). Impact of dietary moisture on nutritional indices and growth of Bombyx mori and concomitant larval duration. J. Insect. Physiol. 38 229–235. 10.1016/0022-1910(92)90071-k [DOI] [Google Scholar]
- Pillai S. V., Jolly M. S. (1985). An evaluation on the quality of mulberry varieties raised under hill conditions and the crop results of Bombyx mori L. Indian L. Seric. 24 48–52. [Google Scholar]
- Radha R. (2013). Studies on the feeding and nutritional influence on the growth and reproduction of monarch butterfly, Danaus Chryssipus (Insecta:Lepidoptera). Int. Res. J. Env. Sci. 2 7–13. [Google Scholar]
- Radjabi R. (2010). Effect of mulberry leaves enrichment with Amino Acid Supplementary nutrients on silkworm Bombyx mori L. at North of Iran. Acad. J. Entomol. 3 45–51. [Google Scholar]
- Rahmathulla V. K. (2011). Management of climatic fractors for successful silkworm (Bombyxmori Bombyxmori L.) crop and higher silk production: a review. Psyche 2012 1–12. 10.1155/2012/121234 [DOI] [Google Scholar]
- Rahmathulla V. K., Haque R. S. Z., Himanthraj M. T., Vindhya G. S., Rajan R. K. (2005). Food ingestion, assimilation and conversion efficiency of mulberry silkworm, Bombyx mori L. Int. J. Indust. Entomol. 11 1–12. 10.1673/031.012.8201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesha C., Anuradha C. M., Lakshmi H., Kumari S. S., Seshagiri S. V., Goel A. K., et al. (2010). Nutrigenetic traits analysis for the identification of nutritionally efficient silkworm germplasm breeds. Biotechnology 9 131–140. 10.3923/biotech.2010.131.140 [DOI] [Google Scholar]
- Sabhat A., Malik M. A., Malik F. A., Sofi A. M., Mir M. R. (2011). Nutritional efficiency of selected silkworm breeds of Bombyx mori L. reared on different varieties of mulberry under temperate climate of Kashmir. Afr. J. Agric. Res. 6 120–126. [Google Scholar]
- Sabina A., Taseem A., Malik M. F., Trag A. R., Raies A. (2012). Comparative silk protein expression of different hybrid varieties of Bombyx mori. Trends in Life Sci. 1 12–16. [Google Scholar]
- Savithri G., Sujathamma P., Asha K. V. (2013). Silkworm Bombyx mori an economic insect. Int. J. Sci. Res. 2 535–537. 10.15373/22778179/july2013/187 [DOI] [Google Scholar]
- Seidavi A. (2011). Evaluation of the genetic potential of six native strains of silkworm Bombyx mori L. Afr. J. Agric. 6 4816–4823. [Google Scholar]
- Sengupta K., Singh B. D., Mustafi J. C. (1972). Nutrition of silkworm, Bombyx mori L. studies on the enrichment of mulberry leaf with various sugars, proteins, amino acids and vitamins for vigorous growth of the worm and increased cocoon crop protection. Indian J. Seric. 11 11–27. [Google Scholar]
- Shinde K. S., Avhad S. B., Hiware C. J. (2014). Impact of spacing’s and fertilizer’s on the production of M5 Mulberry variety. Int. J. Interdiscip. Multidiscip. Stud. 1 344–348. [Google Scholar]
- Shobana K., Murugan K., Naresh K. A. (2010). Influence of host plants on feeding, growth and reproduction of Papilio polytes (the common mormon). J. Insect Phys. 56 1065–1070. 10.1016/j.jinsphys.2010.02.018 [DOI] [PubMed] [Google Scholar]
- Slansky F., Scriber J. M. (1985). “Food consumption and utilization,” in Comprehensive Insect physiol. Biochem Pharmacol, eds Kerdut G. A., Gilbert L. I. (Oxford: Pergamon Press; ). [Google Scholar]
- Thangamani R., Vivekanandan M. (1984). Physiological studies and leaf nutrient analysis in the evaluation of best mulberry varieties. Sericologia 24 317–324. [Google Scholar]
- Thirumalaisamy R., Gowrishankar J., Suganthapriya S., Prakash B., Kumar A. L., Arunachalam G. (2009). Genetic variability in Morus alba L. by biochemical and bioassay methods for increased silk productivity. J. Biomed. Sci. Res. 1 11–18. [Google Scholar]
- Trivedi K., Nair K. S. (1998). Dietary efficiency and silk productivity. Indian Silk 36 5–7. [Google Scholar]
- Venkatash C. M., Rayer S. G. (2003). Evaluation of bivoltine x bivoltine hybrids of silkworm, Bombyx mori L. on V1 and M5 mulberry varieties under dharward conditions. Indian J. Sericult. 42 183–185. [Google Scholar]
- Verma A. N., Atwal A. S. (1963). Effect of chloromycetin, glycine and molasses on the growth and production of silk by Bombyx mori L. Indian J. Seric. 2 1–3. [Google Scholar]
- Waldbauer G. P. (1964). The consumption, digestion and utilization of solanaceous and non-solanaceous plant by larvae of the tobacco hornworm Ptotoparce sexta (Johan) (Lepidoptera:Sphingidae). Entomol. Exp. Appl. 21 254–260. [Google Scholar]
- Waldbauer G. P. (1968). The consumption and utilization of food by insects. Adv. Insect Physiol. 5 229–288. 10.1016/s0065-2806(08)60230-1 [DOI] [Google Scholar]
- Yamamoto T., Fujimaki T. (1982). Interstrain differences in food efficiency of the silkworm Bombyx mori reared on artificial diet. J. Seric. Sci. 51 312–315. [Google Scholar]
- Yemm E. W., Willis A. J. (1954). The estimation of carbohydrates in plants extracts by anthrone. Biochem. J. 57 508–514. 10.1042/bj0570508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogananda-Murthy V. N., Ramesh H. L., Munirajappa (2013). Impact of feed selected mulberry germplasm varieties on silkworm (Bombyx mori L.) through bioassay techniques for commercial exploitation. Asian J. Nat. App. Sci. 2 56–64. [Google Scholar]
- Zhang P. B., Aso Y. K., Yamamoto B. Y., Wang Y. Q., Tsuchida K. Y., Kawaguchi H., et al. (2006). Proteome analysis of silk gland proteins from the silkworm, Bombyx mori. Proteomics 6 2586–2599. 10.1002/pmic.200500348 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.