Abstract
Aims/Hypothesis:
The purpose of this prospective case-control study is to test the hypothesis that while long-term improvements in insulin sensitivity (SI) accompanying weight loss after Roux-en-Y gastric bypass (RYGB) would be similar in obese participants with (T2DM) and without (Controls) type 2 diabetes mellitus, stimulated islet cell insulin responses would differ, increasing (recovering) in the T2DM group but decreasing in Controls. These changes would occur in conjunction with favourable alterations in meal-related gut hormone secretion, insulin processing, and lipid metabolism.
Methods:
Forty participants with T2DM and 22 Controls from the Longitudinal Assessment of Bariatric Surgery (LABS-2) study were enrolled in a separate, longitudinal cohort (LABS-3 Diabetes) to examine the mechanisms of post-surgical diabetes improvement. Study procedures included measures of SI, islet secretory response, and gastrointestinal hormone secretion to both intravenous glucose (FSIVGTT) and a mixed meal (MM), prior to and up to 24 months after RYGB.
Results:
Post-operatively, weight loss and SI-FISIVGTT improvement was similar in both groups, whereas the acute insulin response to glucose (AIRglu) decreased in the Controls and increased in T2DM. The resulting disposition indices (DIFSIVGTT) increased by 3- to 9-fold in both groups. In contrast, during the MM total insulin responsiveness did not significantly change in either group despite durable increases of up to 8-fold in postprandial GLP-1 levels, and SI MM and DIMM increased only in the T2DM group. Peak postprandial glucagon levels increased in both groups.
Conclusions/Interpretation:
For up to two years following RYGB, obese participants without T2DM show improvements in disposition indices that approach population norms. Those with T2DM recover islet cell insulin secretion response yet continue to manifest abnormal insulin processing with disposition indices that remain well below population norms. These data suggest that rather than waiting for lifestyle or medical failure, RYGB is ideally considered before, or as soon as possible after, onset of T2DM. ClinicalTrials Number: NCT00433810.
Keywords: obesity, gastric bypass, insulin sensitivity, insulin secretion, disposition index, meal test, frequently-sampled intravenous glucose tolerance test, proinsulin, lipids, remission, GLP-1, GIP, glucagon
Introduction
Bariatric/metabolic surgery has been shown to improve glycaemic control and often induce remission of type 2 diabetes (T2DM) in individuals with obesity [1–3]. Previous studies have consistently demonstrated improvements in insulin sensitivity proportional to the amount of weight loss following bariatric surgery [4–6]. Given the importance of declining islet-cell function in the pathogenesis of T2DM [7] and the prominent effects of Roux-en-Y gastric bypass (RYGB) on secretion of gut hormones involved in islet-cell secretory responses, we designed a study to better understand the effect of RYGB on islet-cell function in response to both oral (mixed meal) and intravenous nutrient stimulation. Study times after surgery included an early point during which weight loss was still ongoing and a later visit when weight was at or near its nadir [8].
We hypothesized that as a result of altered nutrient flow through the gastrointestinal (GI) tract after RYGB, changes in key gut hormones known to influence islet-cell insulin secretion (such as GLP-1 and possibly GIP) would occur early and be maintained long-term. In addition, we compared responses between participants with T2DM and those without diabetes (Controls), hypothesizing that in both groups insulin sensitivity would improve with post-surgical weight loss, and that the proinsulin-to-insulin ratio would decrease, reflecting improved islet-cell insulin processing efficiency. On the other hand, we hypothesized that the post-surgical stimulated-islet-cell secretory responses would differ. In Controls in whom hyperinsulinemia compensates for insulin resistance allowing maintenance of normal glucose levels at baseline, insulin secretion would be reduced after surgery, reflecting decreased demand accompanying improved insulin sensitivity. In contrast, patients with T2DM, in whom defective islet function manifests in baseline hyperglycaemia, post-RYGB stimulated insulin secretory capacity would recover (increase). If the latter is shown to be true, these data would not only provide important insights into mechanisms of diabetes remission and prevention after RYGB, but would also place this procedure among a handful of interventions shown to reverse the decline in beta cell function that accompanies onset and progression of T2DM [9].
Methods
The Longitudinal Assessment of Bariatric Surgery (LABS) study is a prospective, longitudinal cohort study with three phases: LABS-1, LABS-2, and LABS-3 [10]. LABS-1 is complete and reported 30-day post-surgical adverse outcomes in nearly 5,000 participants [11]. LABS-2 is a cohort of 2,458 participants and has a primary goal of evaluating long-term efficacy of bariatric surgery [8]. The LABS-3 Diabetes sub-study comprised a sub-cohort from the LABS-2 study recruited specifically to better understand the physiological mechanisms of glucose metabolism improvement following RYGB.
Participants and Study Visits
Candidates were approached for inclusion if they were scheduled for RYGB at a participating LABS-2 site at the University of Pittsburgh, University of Washington, or Oregon Health & Science University (ESM Figure 1). Subjects with diabetes were included if they were documented to have a fasting plasma glucose ≥7.0 and ≤10 mmol/L or haemoglobin A1c (HbA1c) ≥6.5% (48 mmol/mol) and ≤8.5% (69 mmol/mol), and they were treated with lifestyle alone or either metformin, sulfonylurea, or both. Exclusion criteria included type 1 diabetes, creatinine >150 μmol/l, treatment with insulin or other non-metformin/non- sulfonylurea diabetes medications, treatment with weight-loss medications, and individuals unlikely or unwilling to comply with follow-up visits. Participants on metformin without a pre-treatment glucose or HbA1c meeting criteria for diabetes were considered to be on this therapy for diabetes prevention, weight loss, or polycystic ovarian syndrome, and were excluded. Control participants (those without diabetes) were included if their fasting plasma glucose was <7.0 mmol/l and/or their HbA1c was <6.5% (48 mml/mol), and they were not taking any diabetes medications. They were matched to subjects with diabetes based on age, sex, and pre-surgical body mass index (BMI). All studies were approved by the investigational review boards at each site, and consent was obtained from each participant before enrolment.
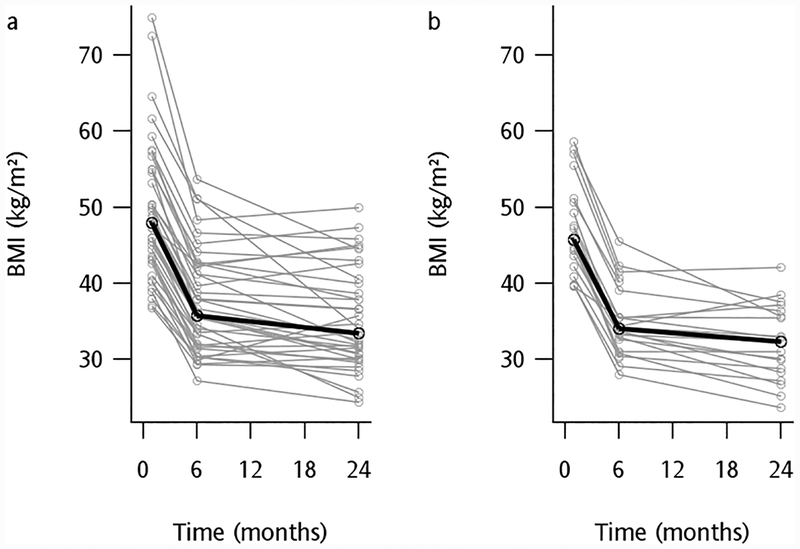
Figure 1.
Mean (bold black line) and individual (grey lines) body mass index (BMI) change from baseline to 6 and 24 months after RYGB in 40 participants with T2DM (a) and 22 Control participants without diabetes (b). Median total weight loss after 6 months was 24% in participants with T2DM and 26% in Controls (p=0.27), and after 24 months was 29% in participants with T2DM and 32% in Controls (p=0.57).
Within one week prior to each research study visit, participants met with a registered dietitian to receive instruction regarding a standardized diet consisting of ~30% total calories from fat, ~10–15% from protein, and ~55–60% from carbohydrates. During this time, participants with diabetes also stopped their diabetes medications and self-monitored their capillary blood glucoses at home. All other usual medications for co-morbid conditions (i.e., hypertension, gastroesophageal reflux, and hyperlipidaemia) were continued, as was treatment for obstructive sleep apnoea.
At the end of this pre-study week, participants presented to the clinical research centres at their respective institutions following an overnight fast on each of two days, scheduled not more than a week apart. On one day, fasting blood samples were drawn for lipids and proinsulin levels followed by a mixed-meal (MM) (BOOST Plus®: 360 Kcal, 45 gm carbohydrate, 14 gm fat, 14 gm protein), with blood samples obtained for glucose, insulin, C-peptide, glucagon, GLP-1, and GIP at time −15, 0, 10, 20, 30, 60, 90, 120, 150, 180, and 240 minutes after meal consumption. On the other day, they underwent an insulin-modified frequently-sampled intravenous glucose tolerance test (FSIVGTT) [12].
Following these baseline studies, participants underwent identical procedures as described above at 6 and 24 months after RYGB.
Biochemical Analysis
Determination of glucose was performed by the hexokinase method using Roche reagents. Levels of insulin and C-peptide were measured by a two-site enzymometric assay using Tosoh reagents on a Tosoh 2000 autoanalyzer (Tosoh Corp., Tokyo, Japan). Proinsulin levels were determined by radioimmunoassay using a Millipore kit. All lipid analyses were performed at the Northwest Lipid Metabolism and Diabetes Research laboratory, University of Washington, Seattle, WA.
Total GLP-1, total GIP, total adiponectin, and glucagon were measured by the Oregon Health & Science University Oregon Clinical and Translational Research Laboratory, Portland, Oregon. To determination total GLP-1 and total GIP, EDTA vacutainers prepared with 500 KIU aprotinin and 10 ul dipeptidyl-peptidase-4 inhibitor /ml of whole blood were collected on ice. To determinate adiponectin, EDTA vacutainers prepared with 500 KIU aprotinin/ml whole blood were collected on ice. These three analytes were measured using single-plex immunoassays from Meso Scale Discovery (MSD, Gaithersburg, MD, USA) and counted with a Sector Imager. Glucagon was measured from heparin vacutainers on ice prepared with 500 KIU aprotinin/ml whole blood using an RIA from EMD Millipore (Billerica, MA, USA 01821). Specificity of the glucagon assay was established by testing cross-reactivity with several gut hormones: oxyntomodulin (0.02%), 7–37 GLP-1 (none), 7–36 GLP-1 (<0.01%), and 1–37 GLP-1 (none).
Definition of Diabetes and Diabetes Remission
Diabetes remission included both partial and complete remission per the American Diabetes Association Consensus Group recommendation [13] as a HbA1c <6.5% (or fasting glucose ≤125 mg/dl if a HbA1c was not available) and the absence of concurrent pharmacologic therapy for diabetes.
Modelling for insulin secretory response and insulin sensitivity
Insulin sensitivity (SI), beta cell responsivity to glucose (Φ total, Φ basal, Φ dynamic, and Φ static), and hepatic insulin extraction (HE basal and HE) were determined during the MM as previously described [14]. Disposition index (DI) during the MM was derived from the product of SI and Φ total. Parameters of insulin sensitivity and secretion response to intravenous glucose during the FSIVGTT, including insulin sensitivity (SI), glucose effectiveness (SG), and acute insulin response (AIRglu), were modelled as previously described [15]. The disposition index (DI) during the FSIVGTT was derived from the product of SI and AIRglu.
Statistical Analysis
Forty subjects with diabetes and twenty without diabetes (sixty subjects total) were estimated to provide an effective sample size to detect modest-to-large effects in insulin sensitivity and stimulated islet cell secretion for within-person analyses and large-to-very-large effect sizes for between-group analyses based on prior studies [16, 17]. For continuous measures, participants in the T2DM and Control groups were compared based on a Wilcoxon Rank Sum test to assess statistical significance of differences, unless normally distributed, in which case paired t-tests were performed. For categorical measures, the frequency and percent of each category was reported, and a Pearson Chi square test was used for statistical significance. For tables with small cell frequencies, Fisher’s exact test was used. Measures of insulin secretion and sensitivity from the FSIVGTT and MM over time were modelled using generalized linear models that included a heterogeneous auto-regressive working correlation matrix to account for the correlation between successive measures. The model assumed a gamma distribution for the measures to account for highly skewed nature of the data. The mean measures at 6 months and 24 months were compared to baseline using a Wald t-test. Missing values were treated as random occurrences. The generalized linear models used here utilized likelihood-based inference under which estimates are unbiased when data are missing completely at random.
Results
Sixty-two participants (40 with T2DM and 22 Controls) were enrolled and were not different by group with regard to percentages of women and race, or for baseline age, BMI, percent body fat, or presence of obstructive sleep apnoea (Table 1). In participants with T2DM (n=24) in whom the information was obtained, the median duration of diabetes was 3.0 years. Baseline HbA1c and fasting levels of glucose, proinsulin, and proinsulin:insulin ratios were all higher in the participants with T2DM compared to Controls, but fasting insulin, C-peptide, and total adiponectin levels were not different (Table 2).
Table 1.
Baseline characteristics of participants with and without (Controls) type 2 diabetes
| Variable | n | Diabetes | n | Controls | p-value |
|---|---|---|---|---|---|
| Female (%) | 40 | 75 | 22 | 77 | 0.84 |
| Race (%) | 40 | 22 | 0.94 | ||
| White | 95 | 95 | |||
| Other | 5 | 5 | |||
| Age (years) | 40 | 52 (47, 57) | 22 | 52 (45, 54) | 0.88 |
| Body Mass Index (kg/m2) | 40 | 47.9 (43.1, 54.8) | 22 | 45.7 (43.6, 50.7) | 0.57 |
| Duration of Diabetes (years) | 24 | 3.0 (1.75, 5.5) | -- | ||
| Diabetes Treatment | 40 | 29 (72.5%) | -- | ||
| No Medications (diet alone) (%) | 40 | 11 (27.5%) | -- | ||
| Metformin (%) | 40 | 21 (52.5%) | -- | ||
| Sulphonylurea (%) | 40 | 10 (25%) | -- | ||
| Obstructive Sleep Apnoea (%) | 40 | 31 (77.5%) | 22 | 20 (90.9%) | 0.30 |
Results are median (25th percentile, 75th percentile).
Table 2.
Fasting levels of metabolic parameters prior to and after Roux-en-Y gastric bypass in participants with type 2 diabetes (T2DM) and participants without diabetes
| Variable | Group | n | Baseline | n | 6-Month | p-value (vs baseline) | n | 24-Month | p-value (vs baseline) |
|---|---|---|---|---|---|---|---|---|---|
| Fasting Glucose (mmol/l) | T2DM | 39 | 7.30 (6.73–7.87) | 38 | 5.21 (4.93–5.49) | <0.001 | 35 | 5.24 (5.03–5.45) | <0.001 |
| Control | 21 | 5.26 (5.09–5.43) | 22 | 4.74 (4.58–4.9) | <0.001 | 17 | 5.00 (4.84–5.15) | 0.011 | |
| p-value (Diabetes vs. Control) | <0.001 | 0.004 | 0.07 | ||||||
| Fasting Insulin (pmol/l) | T2DM | 39 | 129.7 (102.8–156.5) | 37 | 47.7 (36.9–58.6) | <0.001 | 35 | 41.5 (32.4–50.6) | <0.001 |
| Control | 21 | 130.5 (91.4–169.5) | 22 | 37.2 (30.4–44) | <0.001 | 17 | 36.9 (29.1–44.7) | <0.001 | |
| p-value (Diabetes vs. Control) | 0.97 | 0.11 | 0.45 | ||||||
| Haemoglobin A1c (%) | T2DM | 36 | 6.4 (6.1–6.6) | -- | -- | 35 | 5.2 (5.1–5.4) | <0.001 | |
| (mmol/mmol) | 46 (43–49) | 33 (32–36) | |||||||
| Control | 22 | 5.3 (5.2–5.4) | -- | -- | 15 | 5.0 (4.9–5.1) | <0.001 | ||
| (mmol/mmol) | 34 (33–36) | 31 (30–32) | |||||||
| p-value (T2DM vs. Con) | <0.001 | -- | -- | 0.017 | |||||
| C-peptide (nmol/l) | T2DM | 39 | 1.3 (1.1–1.5) | 38 | 0.70 (0.60–0.83) | <0.001 | 35 | 0.70 (0.57–0.80) | <0.001 |
| Control | 21 | 1.1 (0.90–1.4) | 22 | 0.57 (0.50–0.67) | <0.001 | 17 | 0.67 (0.57–0.77) | <0.001 | |
| p-value (T2DM vs. Con) | 0.25 | 0.06 | 0.86 | ||||||
| Proinsulin (pmol/l) | T2DM | 39 | 40 (26–54) | 36 | 12 (8.8–15) | <0.001 | 35 | 8.9 (6.0–12) | <0.001 |
| Control | 21 | 18 (14–21) | 22 | 6.5 (5.6–7.4) | <0.001 | 17 | 5.2 (4.7–5.8) | <0.001 | |
| p-value (T2DM vs. Con) | 0.002 | 0.001 | 0.017 | ||||||
| Proinsulin:Insulin Ratio | T2DM | 39 | 2.2 (1.8–2.7) | 36 | 1.8 (1.6–2.1) | 0.05 | 35 | 1.8 (1.2–2.3) | 0.13 |
| Control | 21 | 1.1 (0.9–1.4) | 22 | 1.3 (1.1–1.5) | 0.17 | 17 | 1.1 (0.91–1.4) | 0.89 | |
| p-value (T2DM vs. Con) | <0.001 | 0.002 | 0.036 | ||||||
| Adiponectin (ug/ml) | T2DM | 40 | 7.35 (6.17–8.53) | 37 | 11.6 (9.9–13.3) | <0.001 | 35 | 14.7 (12.0–17.5) | <0.001 |
| Control | 21 | 8.38 (7.08–9.67) | 22 | 11.7 (9.06–14.3) | 0.001 | 17 | 14.6 (11.6–17.6) | <0.001 | |
| p-value (T2DM vs. Con) | 0.25 | 0.97 | 0.95 |
Results are mean (95% CI). Estimates are based on Wald t-test from generalized linear model.
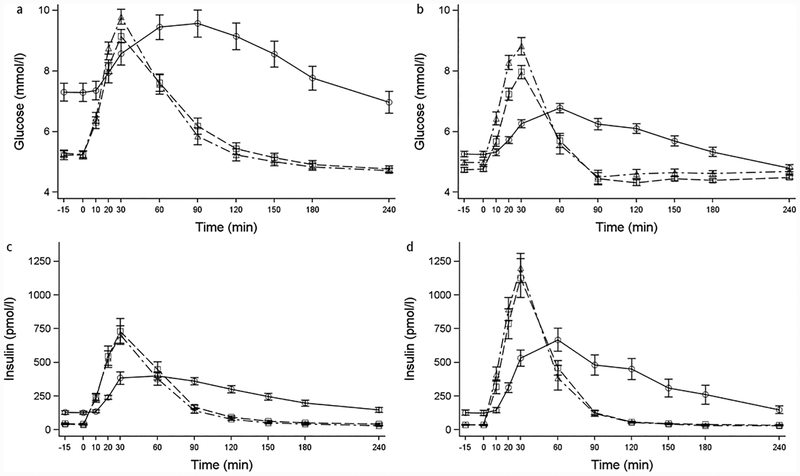
Weight loss was similar in both groups at 6 (24% in T2DM and 26% in Controls, p=0.27) and 24 months (29% in T2DM and 32% in Controls, p=0.57) (Figure 1). Accompanying this weight loss, fasting glucose and insulin levels were lower than baseline at each follow-up visit in both groups (Figure 2, Table 2). Compared with baseline, two years after surgery HbA1c levels significantly decreased to 5.0% (31 mmol/mol) in Controls and 5.2% (33 mmol/mol) in the T2DM group (Table 2), remaining marginally higher in the T2DM group compared to Controls (p=0.017). Compared with baseline, the time to peak glucose and insulin levels during the MM shifted to an earlier time point (30 vs. 60–90 minutes) in each group at both follow-up visits (P<0.001, Figure 2, Table 3). On the other hand, the peak postprandial glucose level increased compared with baseline in the Control group (P<0.001) and was not different from baseline in the T2DM group (Figure 2, Table 3). Similarly, peak postprandial insulin concentrations at each follow-up time point were higher compared with baseline in both groups (Figure 2, Table 3). Despite these high early postprandial levels for both glucose and insulin following a meal, area-under-the curve (AUC) levels decreased by 6 months and remained lower than baseline at the 24-month visit (Table 3).
Figure 2.
Mean and standard error bars for glucose ((a) and (b)) and insulin ((c) and (d)) levels during the mixed meal at baseline after RYGB for participants with T2DM ((a) and (c)) and Control participants ((b) and (d)) without diabetes. –○– Baseline. –□– 6 months. –Δ– 24 months.
Table 3.
Baseline and post-surgical levels of glucose, insulin, and gut hormones secreted following a mixed meal prior to and after Roux-en-Y gastric bypass in participants with type 2 diabetes (T2DM) and participants without diabetes (Control).
| Variable | Group | n | Baseline | n | 6-Month | p-value (vs baseline) | n | 24-Month | p-value (vs baseline) |
|---|---|---|---|---|---|---|---|---|---|
| Peak Glucose (mmol/l) | T2DM | 39 | 9.96 (9.14–10.78) | 38 | 9.32 (8.89–9.75) | 0.09 | 35 | 9.79 (9.32–10.25) | 0.69 |
| Control | 21 | 6.88 (6.57–7.18) | 22 | 7.98 (7.56–8.4) | <0.001 | 17 | 9.05 (8.62–9.48) | <0.001 | |
| p-value (Diabetes vs. Control) | <0.001 | <0.001 | 0.022 | ||||||
| Glucose AUC (mmol-4 hr/l) | T2DM | 39 | 2130 (1942–2319) | 38 | 1523 (1445–1602) | <0.001 | 35 | 1472 (1385–1559) | <0.001 |
| Control | 21 | 1448 (1382–1514) | 22 | 1278 (1234–1321) | <0.001 | 17 | 1347 (1298–1395) | 0.022 | |
| p-value (Diabetes vs. Control) | <0.001 | <0.001 | 0.014 | ||||||
| Peak Insulin (pmol/l) | T2DM | 39 | 491.9 (405.1–578.7) | 38 | 807.5 (617–997.9) | <0.001 | 35 | 771.4 (641.5–901.4) | <0.001 |
| Control | 21 | 778.3 (596.8–959.7) | 22 | 1137.4 (863.7–1411.2) | 0.003 | 17 | 1221 (1022.1–1419.8) | <0.001 | |
| p-value (Diabetes vs. Control) | 0.006 | 0.05 | <0.001 | ||||||
| Insulin AUC (pmol-4hr/l) | T2DM | 39 | 66376 (57212–75539) | 38 | 51305 (41779–60832) | <0.001 | 35 | 44929 (38306–51552) | <0.001 |
| Control | 21 | 89867(66916–112818) | 22 | 57628(46770–68487) | 0.001 | 17 | 57365(46776–67954) | 0.006 | |
| p-value (Diabetes vs. Control) | 0.06 | 0.39 | 0.05 | ||||||
| Fasting GIP (pmol/l) | T2DM | 40 | 16.5 (13.3–19.7) | 38 | 13.5 (12–15.1) | 0.029 | 35 | 14.6 (12.9–16.3) | 0.17 |
| Control | 21 | 15.1 (12.3–18) | 22 | 12.5 (10.8–14.2) | 0.1 | 17 | 14.7 (12.9–16.5) | 0.78 | |
| p-value (T2DM vs Ctrl) | 0.52 | 0.38 | 0.94 | ||||||
| Peak GIP (pmol/l) | T2DM | 40 | 140.4 (124–156.9) | 38 | 150.9 (131.1–170.7) | 0.29 | 35 | 159.4 (133.7–185.1) | 0.14 |
| Control | 21 | 129.2 103.8–154.6) | 22 | 156.6 (130.3–182.8) | 0.036 | 17 | 135.1 (112.4–157.9) | 0.62 | |
| p-value (Diabetes vs. Control) | 0.46 | 0.73 | 0.16 | ||||||
| GIP AUC (pmol-4hr/l) | T2DM | 40 | 16167 (14522–17813) | 38 | 11727 (10355–13099) | <0.001 | 35 | 12435 (10544–14325) | <0.001 |
| Control | 21 | 15879 (13280–18478) | 22 | 13126 (11146–15107) | 0.004 | 17 | 12048 (10042–14055) | 0.001 | |
| p-value (Diabetes vs. Control) | 0.85 | 0.25 | 0.78 | ||||||
| Fasting GLP-1 (pmol/l) | T2DM | 40 | 4.3 (3.5–5.2) | 37 | 3.5 (2.9–4) | 0.04 | 35 | 3.3 (2.8–3.9) | 0.011 |
| Control | 20 | 4.4 (3.3–5.4) | 21 | 2.7 (2.1–3.3) | <0.001 | 17 | 3.5 (2.7–4.3) | 0.05 | |
| p-value (Diabetes vs. Control) | 0.94 | 0.08 | 0.75 | ||||||
| Peak GLP-1 (pmol/l) | T2DM | 40 | 9.7 (7.4–11.9) | 38 | 68.5 (59.2–77.7) | <0.001 | 35 | 66.9 (58.3–75.5) | <0.001 |
| Control | 21 | 12.2 (3.1–21.3) | 22 | 66.2 (49.7–82.6) | <0.001 | 17 | 69.2 (56.5–82) | <0.001 | |
| p-value (Diabetes vs. Control) | 0.59 | 0.81 | 0.76 | ||||||
| GLP-1 AUC (pmol/l) | T2DM | 40 | 1445 (1243–1647) | 38 | 4441 (3971–4912) | <0.001 | 35 | 4067 (3629–4505) | <0.001 |
| Control | 21 | 1399 (1068–1730) | 22 | 4194 (3435–4953) | <0.001 | 17 | 3910 (3282–4538) | <0.001 | |
| p-value (Diabetes vs. Control) | 0.82 | 0.58 | 0.69 | ||||||
| Fasting Glucagon (ng/l) | T2DM | 40 | 78.5 (71.6–85.4) | 38 | 66.1 (59.9–72.4) | <0.001 | 35 | 66.8 (61.5–72.1) | <0.001 |
| Control | 21 | 85 (72–97.9) | 22 | 70 (60.8–79.2) | <0.001 | 17 | 68.6 (59–78.2) | <0.001 | |
| p-value (Diabetes vs. Control) | 0.38 | 0.49 | 0.75 | ||||||
| Peak Glucagon (ng/l) | T2DM | 40 | 102.2 (91.9–112.5) | 38 | 113.3 (106.5–120) | 0.045 | 35 | 112.3 (105.4–119.2) | 0.05 |
| Control | 21 | 99.2 (84.7–113.8) | 22 | 105.9 (94.9–116.8) | 0.17 | 17 | 109.9 (97.5–122.3) | 0.05 | |
| p-value (T2DM vs Ctrl) | 0.74 | 0.25 | 0.74 | ||||||
| Glucagon AUC (ng-4 hr/l) | T2DM | 40 | 20490 (18782–22198) | 38 | 21880 (20834–22925) | 0.06 | 35 | 21526 (20124–22928) | 0.19 |
| Control | 21 | 21356 (18312–24401) | 22 | 22023 (19867–24179) | 0.44 | 17 | 23083 (20385–25780) | 0.14 | |
| p-value (Diabetes vs. Control) | 0.62 | 0.91 | 0.31 |
Mean (95% CI). Estimates are based on Wald t-test from generalized linear model.
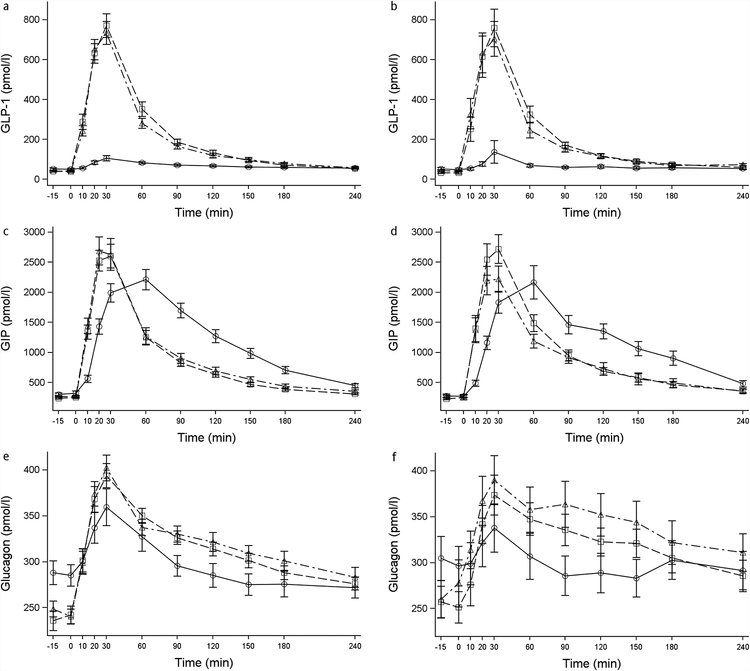
Although fasting levels of GIP were not different and fasting GLP-1 levels trended lower after surgery compared to baseline in both groups (Table 3), the postprandial increases of these hormones following a mixed meal mirrored those of glucose and insulin in both groups (Figure 3). Peak GIP shifted 30 minutes earlier and AUC GIP amounts were lower compared with baseline (Figure 3, Table 3). Peak GLP-1 levels increased 7- to 8-fold, and AUC levels were significantly increased at 6 and 24 months after surgery compared to baseline (Figure 3, Table 3). Also in both groups, fasting glucagon levels decreased after surgery but peak levels increased and AUC levels trended higher following the MM at both the 6 and 24-month time points (Figure 3, Table 3).
Figure 3.
Mean and standard error bars for glucagon-like peptide 1 (GLP-1) ((a) and (b)), glucose-dependent insulinotropic peptide (GIP) ((c) and (d)), and glucagon ((e) and (f)) during the mixed meal at baseline, 6, and 24 months after RYGB for participants with T2DM ((a), (c), and (e)) and Control participants ((b), (d), and (f)) without diabetes. –○– Baseline. –□– 6 months. –Δ– 24 months.
Glucose metabolism parameters following a mixed meal
Using a standardized MM to derive meal-related parameters of insulin secretion and sensitivity [14], both groups exhibited reduced basal (fasting) beta cell responsivity (Φb) and slight increases in basal hepatic insulin extraction (Table 4) (P<0.001 for all comparisons) at 6 and 24 months after RYGB. During the 24-month follow-up, despite an overall 32% reduction in the amount of secreted insulin per unit increase of glucose during the dynamic phase of the meal, or dynamic beta cell responsivity (Φd), Φd remained significantly higher in the Control group than the T2DM group, in which Φd did not significantly change after surgery (Table 4). Static beta cell responsivity (Φs), the amount of insulin secreted in response to glucose during the static phase compared to basal state, as well as total Φ, the overall beta cell responsivity derived from Φd and Φs, were higher in the Control group compared to the T2DM group at each study visit, but did not significantly change in either group as a result of the surgery and accompanying weight loss (Table 4). Conversely, hepatic insulin extraction (HE index) during the meal was greater in the T2DM group than the Control group at baseline and during follow-up, but did not significantly change in either group following surgery. Insulin sensitivity derived during the meal test (SI-MM) improved 6 months after surgery in both groups, and continued to increase in the T2DM group thereafter, but was no longer significantly different from baseline in the Control group after 24 months, despite continued weight loss (Table 4). Despite this increase in SI-MM, insulin sensitivity remained lower in the T2DM than Control group at all three study visits. The meal-derived disposition index (DIMM,) representing insulin secretion response to a given level of insulin sensitivity (calculated as the product of total Φ and SI-MM), was lower in the T2DM group than Control group at each visit. In the T2DM group, DIMM was higher 6 and 24 months after surgery compared with baseline, but not in the Control group where it remained high and unchanged from baseline throughout (Table 4, Figure 4).
Table 4.
Parameters of insulin sensitivity and secretion derived from the mixed-meal test prior to and after Roux-en-Y gastric bypass in participants with type 2 diabetes (T2DM) and participants without diabetes (Control).
| Variable | Group | n | Baseline | n | 6-Month | p-value (vs baseline) | n | 24-Month | p-value (vs baseline) |
|---|---|---|---|---|---|---|---|---|---|
| Basal β-cell responsivity, Φb (10−9) | Diabetes | 39 | 34 (30–39) | 38 | 25 (21–29) | <0.001 | 34 | 23 (20–27) | <0.001 |
| Control | 18 | 41 (33–49) | 22 | 23 (20–26) | <0.001 | 17 | 24 (19–28) | <0.001 | |
| p-value (Diabetes vs. Control) | 0.16 | 0.31 | 0.96 | ||||||
| Dynamic β-cell responsivity, Φd (10−9) | Diabetes | 39 | 2428 (1997–2859) | 38 | 2181 (1793–2569) | 0.22 | 34 | 1967 (1496–2439) | 0.10 |
| Control | 18 | 4566 (3414–5719) | 22 | 2623 (2114–3131) | <0.001 | 17 | 3120 (2442–3798) | 0.017 | |
| p-value (Diabetes vs. Control) | 0.001 | 0.17 | 0.007 | ||||||
| Static β-cell responsivity, Φs (10−9 / min) | Diabetes | 39 | 97 (77–117) | 38 | 125 (101–150) | 0.004 | 34 | 106 (88–125) | 0.41 |
| Control | 18 | 233 (183–283) | 22 | 212 (156–267) | 0.55 | 17 | 208 (175–241) | 0.36 | |
| p-value (Diabetes vs. Control) | <0.001 | 0.006 | <0.001 | ||||||
| Total Φ (10−9 / min) | Diabetes | 39 | 100 (80–120) | 38 | 131 (105–156) | 0.01 | 34 | 111 (92–130) | 0.38 |
| Control | 18 | 238 (188–288) | 22 | 218 (162–274) | 0.58 | 17 | 217 (183–251) | 0.45 | |
| p-value (Diabetes vs. Control) | <0.001 | 0.006 | <0.001 | ||||||
| SI-MM (10−5 l· kg−1 min−1/pmol l-1) | Diabetes | 33 | 5.6 (4.0–7.1) | 38 | 7.7 (6.0–9.5) | 0.023 | 34 | 9.4(7.6–11.2) | <0.001 |
| Control | 20 | 7.3 (3.0–11.5) | 22 | 10.7 (5.3–16.0) | 0.009 | 17 | 9.5(6.3–12.6) | 0.16 | |
| p-value (Diabetes vs. Control) | 0.46 | 0.3 | 0.97 | ||||||
| DIMM (SI-MM × Total Φ) | Diabetes | 33 | 1022 (596–1448) | 38 | 1639 (1170–2108) | 0.037 | 34 | 1682 (1240–2124) | 0.025 |
| Control | 18 | 2673 (1313–4034) | 22 | 3224 (2103–4345) | 0.47 | 17 | 2866 (2070–3663) | 0.81 | |
| p-value (Diabetes vs. Control) | 0.024 | 0.011 | 0.011 | ||||||
| HE basal | Diabetes | 39 | 0.78 (0.75–0.8) | 38 | 0.86 (0.85–0.88) | <0.001 | 34 | 0.88 (0.87–0.89) | <0.001 |
| Control | 20 | 0.75 (0.72–0.79) | 22 | 0.86(0.84–0.87) | <0.001 | 17 | 0.88 (0.86–0.9) | <0.001 | |
| p-value (Diabetes vs. Control) | 0.28 | 0.63 | 0.93 | ||||||
| HE index | Diabetes | 39 | 0.70 (0.68–0.73) | 38 | 0.71 (0.68–0.74) | 0.43 | 34 | 0.72 (0.7–0.75) | 0.21 |
| Control | 20 | 0.62 (0.55–0.69) | 22 | 0.63 (0.58–0.68) | 0.7 | 17 | 0.66 (0.61–0.72) | 0.16 | |
| p-value (Diabetes vs. Control) | 0.022 | 0.004 | 0.05 |
Results are mean (95% CI). Estimates are based on Wald t-test from generalized linear model. SI-insulin sensitivity. Phi (Φ): basal-basal insulin secretion. Phi dynamic: dynamic insulin secretion. Phi static: static insulin secretion. Phi Total: total insulin secretion. DI: disposition index. HEb: Hepatic extraction of insulin, basal. HEindex: Hepatic extraction of insulin, index. MM: mixed meal.
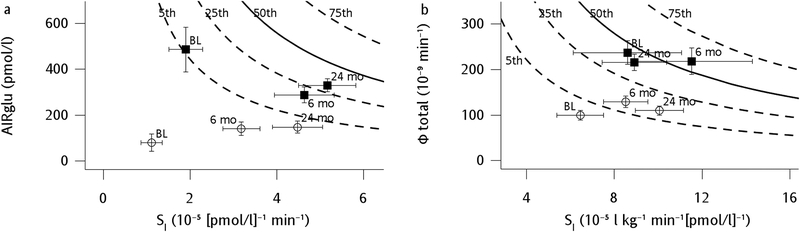
Figure 4.
Changes in disposition index’s after RYGB for participants with T2DM (–○–) and Control participants without diabetes (–⬛–). Mean and standard error bars for acute insulin response to glucose (AIRglu) vs. insulin sensitivity (SI) derived from a frequently-sampled intravenous glucose tolerance test (a) and insulin secretion (Phi Total) vs. SI derived from a mixed-meal test (b). Background solid and dotted lines represent percentiles derived normal populations as previously described [15, 47].
Glucose metabolism parameters derived from FSIVGTTs
Derived measures of insulin secretion and sensitivity during the FSIVGTT (Table 5) showed that the acute insulin response to IV glucose (AIRglu) significantly increased in the T2DM participants and but decreased in Controls. Insulin sensitivity (SI-FSIVGTT) was greater at baseline in the Control group compared to the T2DM group, and both groups experienced significant improvements in insulin sensitivity such that by 24 months they were no longer significantly different from one another. Despite divergent changes in AIRglu, the FSIVGTT-derived disposition index (DIFSIVGTT) increased roughly 9-fold in the Diabetes group and 2.5-fold in Controls by 24 months after surgery compared to baseline (Table 5, Figure 4). Glucose effectiveness (Sg) was not different between the groups or affected by surgery and weight loss.
Table 5.
Parameters of insulin sensitivity and secretion derived from the insulin-modified frequently sampled intravenous glucose tolerance test prior to and after Roux-en-Y gastric bypass in participants with type 2 diabetes (T2DM) and participants without diabetes (Control).
| Variable | Group | n | Baseline | n | 6-Month | p-value (vs baseline) | n | 24-Month | p-value (vs baseline) |
|---|---|---|---|---|---|---|---|---|---|
| AIRglu (pmol/l) | Diabetes | 39 | 80 (8–153) | 37 | 147 (91–203) | 0.017 | 35 | 150 (101–199) | 0.028 |
| Control | 22 | 486 (297–674) | 22 | 288 (221–355) | 0.008 | 17 | 312 (260–363) | 0.048 | |
| p-value (Diabetes vs. Control) | <0.001 | 0.002 | <0.001 | ||||||
| SI-FSIVGTT (10−5 pmol/l−1 min−1) | Diabetes | 37 | 0.90 (0.60–1.3) | 37 | 2.8 (2.2–3.4) | <0.001 | 34 | 4.3 (3.3–5.3) | <0.001 |
| Control | 22 | 1.6 (1.1–2.2) | 22 | 4.2 (3.1–5.2) | <0.001 | 17 | 5.0 (4.1–5.9) | <0.001 | |
| p-value (Diabetes vs. Control) | 0.041 | 0.025 | 0.27 | ||||||
| SG (/min) | Diabetes | 27 | 14 (13–16) | 29 | 14 (13–16) | 0.94 | 26 | 15 (13–16) | 0.64 |
| Control | 19 | 15 (12–17) | 17 | 14 (12–16) | 0.88 | 15 | 16 (13–19) | 0.23 | |
| p-value (Diabetes vs. Control) | 0.8 | 0.88 | 0.36 | ||||||
| DIFSIVGTT (SI-FSIVGTT × AIRglu) (/min) | Diabetes | 39 | 73 (33–114) | 37 | 326 (225–427) | <0.001 | 35 | 654 (307–1000) | 0.001 |
| Control | 22 | 597 (407–788) | 22 | 957 (809–1106) | 0.002 | 17 | 1493 (1197–1788) | <0.001 | |
| p-value (Diabetes vs. Control) | <0.001 | <0.001 | <0.001 |
Mean (95% CI). Estimates are based on Wald t-test from generalized linear model. SI: insulin sensitivity. SG: glucose effectiveness. AIRglu: acute insulin response to glucose. DI: disposition index. FSIVGTT: frequently-sampled intravenous glucose tolerance test.
Diabetes remission and characteristics of participants with diabetes that persisted or recurred after surgery
None of the Control group participants developed diabetes during the 24-month follow-up. Of the 40 participants with diabetes enrolled at baseline, 34 had data to determine diabetes status at 24 months. Of these, three were classified as having diabetes at 24 months (91% remission rate). Two of these three participants were taking diabetes medication at 6 months, whereas the third was not taking any diabetes medications at either of those time points.
Discussion
The current study was undertaken to compare two-year changes in insulin sensitivity and secretory responses, and levels of gastrointestinal hormones, measured during oral (mixed-meal) and intravenous (FSIVGTT) challenges in obese participants with and without diabetes following RYGB.
Although previously reported [18–21], in the largest studied groups to date after RYGB we found that fasting glucose and insulin levels declined, haemoglobin A1c levels improved in both participants with and without diabetes to become nearly identical and not clinically meaningfully different, and a fundamental change in post-meal glucose appearance and disappearance was readily apparent. Through creation of a gastrojejunostomy that bypasses the pyloric valve, gastric emptying is accelerated [21–23] and peak post-prandial glucose levels are shifted to an earlier time point after meal ingestion (30-minutes) in both groups. Also notable after surgery were that peak postprandial glucose levels remained equal to baseline levels in the participants with T2DM and were actually higher than baseline in Controls, as well as rapid declines in postprandial glucose levels following these peaks in both groups, resulting in lower AUC glucose and HbA1c levels during follow-up (i.e., improved glucose tolerance and glycaemic control, respectively). Such marked glucose fluctuations that include early postprandial hyperglycaemia, referred to as glycaemic variability or “dysglycemia,” have been suggested to play a role in diabetes complications independently of HbA1c levels [24]. However, several cohort studies that have included large numbers of post-RYGB participants have reported reduced microvascular and macrovascular diabetes-related complications and mortality compared to non-surgical controls [25, 26], suggesting that despite this postprandial hyperglycaemia, the overall reductions in fasting and postprandial glucose exposure following RYGB are beneficial.
Plasma insulin responses, as well as those of GIP and GLP-1, were also augmented in a pattern that mirrored the accelerated postprandial glucose appearance and disappearance following surgery. The 7- to 8-fold increases in peak and AUC GLP-1 levels after surgery seen in both groups are thought to contribute to improved insulin secretion [27–30] and remained robust through 24-months. Counter-intuitively, we found that despite large post-surgical weight loss and increases in peak insulin, glucose, and GLP-1 levels [31, 32], post-meal AUC glucagon levels did not change, and peak concentrations actually increased. This paradoxical meal-related glucagon response has previously been reported after RYGB in smaller studies [33–36], and in this report we confirm that this is not due to cross-reactivity of our assay with either GLP-1 or oxyntomodulin. Interestingly, our data would be consistent with a recently demonstrated dual role for increased glucagon and GLP-1 levels to facilitate long-term weight-loss maintenance after RYGB through appetite regulation [37].
Insulin sensitivity measured during the FSIVGTT increased post-operatively in both groups. In agreement with our hypothesis and extending findings of a previous smaller study [38], the acute insulin response to IV glucose decreased in the Control group but increased in the T2DM group up to two years after RYGB. Despite opposing stimulated insulin responses, RYGB restored diminished pancreatic insulin secretory capacity (as evidenced by increases in DIFSIVGTT) in both groups, with the Controls improving from the 5th to the 25th percentile of normative population values. A larger relative increase in DIFSIVGTT occurred in the T2DM group, but the pre-surgery secretory defect was so profound that even with an approximately 9-fold increase, the DIFSIVGTT still remained below the 5th percentile of the normal values 24 months after surgery. That is, despite equal weight loss, similarly impressive gains in insulin sensitivity by 24 months, and less “glucotoxicity” [39], the insulin secretory capacity of T2DM participants did not recover fully enough after two years to even match presurgical values of Controls. Likewise, proinsulin levels and insulin processing efficiency improved in both groups after surgery but still showed persistent defects (higher levels of proinsulin and proinsulin:insulin ratio) in the T2DM group compared with Controls at each visit.
Of note, two years after surgery, fasting plasma glucose or haemoglobin A1c levels reverted to “normal” in over 90% of the cohort with diabetes at baseline, becoming virtually indistinguishable from the control group without diabetes. As discussed above, however, this does not mean the groups became metabolically equivalent. Indeed, recent reports have shown that a persistent impairment in insulin secretion is a major factor contributing to the failure to achieve or sustain diabetes remission after surgically-induced weight loss [40, 41]. These reports and our data indicate that simple clinical indicators used to determine normal glucose tolerance or “nondiabetic” status in patients who have undergone weight loss surgeries fail to adequately describe the metabolic heterogeneity within this group or adequately assign risk for subsequent long-term deterioration in glucose control.
Previous studies have shown improvements of insulin secretion in response to both IV and oral (glucose and mixed-meal) challenges within 3 months of RYGB and biliopancreatic diversion [42, 43]. Longer-term, however, we find that the changes of insulin sensitivity and insulin secretion derived from the responses to a mixed-meal differed from the responses to a FSIVGTT. Basal insulin responsiveness (Φb) derived from the mixed meal improved in both groups. However, despite a robust increase in postprandial GLP-1 levels that persisted for two years after RYGB, which should correspond with improved beta cell glucose sensitivity [6, 44–46], it is somewhat surprising that the corresponding measures of meal-related dynamic insulin responsiveness (Φd) did not change in the T2DM group and decreased in the Controls, and that total insulin responsiveness (Total Φ) did not change in either group. One potential explanation for this discrepancy between oral and IV stimulated insulin secretory responses is that Φ values are normalized to glucose levels during modelling of the MM data, whereas calculations of AIRglu from the FSIVGTT are not. Another is that the Φ and SI values derived during the mixed meal were already close to population norms (see Figure 4) in the Control group, so did not have much room for improvement. It is also possible that improvements of SIMM in the T2DM group after surgery resulted in a reduced demand on islet cells, in which case maintenance of insulin responsiveness represents an improvement in the relative islet insulin secretory capacity, as reflected in a significant increase in DIMM in this group.
In summary, we demonstrate that for up to two years after RYGB, obese adults experience improvements in SI as well as improved insulin secretory responses to IV glucose as measured by DI with weight loss, despite opposite directionality of AIRglu responses in those with T2DM (increased) vs. without T2DM (decreased). The gastric bypass procedure is therefore among a handful of interventions shown to reverse the decline in beta cell function that accompanies onset and progression of T2DM. For participants with T2DM, however, the baseline beta cell secretory defect was so profound that even with a marked post-surgical improvement, mean DI remained below the 5th percentile of a normative population two years after surgery. Combining our findings with recent reports that islet-cell recovery is an important determinant of diabetes remission status following bariatric surgery [40, 41, 46], consideration should be given to recommending RYGB as early as possible after the diagnosis of diabetes (the median diabetes duration in our study was only 3 years) or, more optimally, in obese patients at risk for diabetes where we show that restoration of beta cell secretory capacity to parameters within population norms is more likely. Our data also highlight a paradoxical increase in peak post-prandial glucagon levels that, rather than negatively impacting postprandial glucose metabolism, supports an alternative, beneficial role for glucagon (along with markedly higher meal-stimulated GLP-1 levels) in appetite regulation and weight-loss maintenance after RYGB. Finally, studying the durability of beta cell recovery during longer-term follow-up will be vital for a deeper understanding of the mechanisms involved in T2DM improvement, remission, and recurrence following RYGB.
Supplementary Material
Research in context.
What is already known about this subject?
Gastric bypass surgery improves weight and glucose metabolism in obese patients.
Mechanisms for both of these effects are thought to include enhanced secretion of key gut hormones.
What is the key question?
Over a two year period, how does gastric bypass change glucose and gut hormone levels and what are the corresponding changes in insulin sensitivity, insulin secretory capacity, and disposition index in obese patients with and without diabetes?
What are the new findings?
Increases in postprandial GLP-1 secretion (nearly eight-fold compared to baseline) and peak glucagon levels in both groups after gastric bypass are durable to two years.
Gastric bypass improves disposition index in those without diabetes to nearly match population norms after two years even though insulin secretion to IV glucose declines.
In patients with diabetes, disposition indices and insulin secretion to IV glucose both improve after gastric bypass, but after two years still remain well below population norms.
How might this impact on clinical practice in the foreseeable future?
In order to maximize islet cell recovery, gastric bypass should be considered prior to onset of diabetes.
Acknowledgments:
We would like to thank Jason Ng, MD and Jolene Lowry, MD at the University of Pittsburgh for their contributions to the conduct of the study, as well as the LABS personnel, including: Oregon Health & Science University: Chelsea Cassady, BS, Emily Coburn, MPH, Emily Moher, MPH, Clifford Deveney, MD, Katherine Elder, PhD, Stefanie Greene, Jonathan Purnell, MD, Robert O’Rourke, MD, Chad Sorenson, Bruce M. Wolfe, MD, Legacy Good Samaritan Hospital, Portland, OR: Emma Patterson, MD, William Raum, MD, Lisa VanDerWerff, PAC, Jason Kwiatkowski, PAC, University of Pittsburgh Medical Center, Pittsburgh, PA: Anita P. Courcoulas, MD, MPH, FACS, William Gourash, MSN, CRNP, Carol A. McCloskey, MD, Ramesh Ramanathan, MD, Melissa Kalarchian PhD, Marsha Marcus PhD, Eleanor Shirley, MA, Angela Turo, BS, University of Washington, Seattle, WA: David R. Flum, MD, MPH, E. Patchen Dellinger, MD, Saurabh Khandelwal, MD, Skye D. Stewart, MS, CCRC, Morgan M. Cooley, Rebecca Blissell, Megan J. Miller, MEd, David E. Cummings, MD, Karen E. Foster-Schubert, MD Virginia Mason Medical Center, Seattle, WA: Richard Thirlby, MD Lily Chang, MD, Jeffrey Hunter, MD, Ravi Moonka, MD, Debbie Ng, MPH, MA Data Coordinating Center, Graduate School of Public Health at the University of Pittsburgh, Pittsburgh, PA: Steven H. Belle, PhD, MScHyg, Wendy C. King, PhD, Debbie Martin, BA, Rocco Mercurio, MBA, Abdus Wahed, PhD, Frani Averbach, MPH, RDN National Institute of Diabetes and Digestive and Kidney Diseases: Mary Horlick, MD, Carolyn W. Miles, PhD, Myrlene A. Staten, MD, Susan Z. Yanovski, MD
Funding
This clinical study was a cooperative agreement funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Grant numbers: R01 - DK103842; DCC -U01 DK066557; Columbia - U01-DK66667 (in collaboration with Cornell University Medical Center CTRC, Grant UL1-RR024996); University of Washington - U01-DK66568 (in collaboration with CTRC, Grant M01RR-00037); Neuropsychiatric Research Institute - U01-DK66471; East Carolina University – U01-DK66526; University of Pittsburgh Medical Center – U01-DK66585 (in collaboration with CTRC, Grant UL1-RR024153); Oregon Health & Science University – U01-DK66555. PJH’s laboratory receives funding from NIH grants: DK-09596, DK-092993, HL-107256, HL-121324 and a Multi-campus Award from the University of California, Office of the President.
Duality of Interest:
JQP receives consultant income for services on an advisory board for Novo Nordisk and receives grant funding from the National Institutes of Health (NIH). CC holds 10 patents and patent applications related to glucose sensors and artificial pancreas, received non-financial support from Roche and Dexcom,Inc., research support from Dexcom, Inc., Sanofi Aventis, Adocia, and consultant income for services on an advisory board for Novo Nordisk. From 207–2017, DEK was a full-time employee of Merck & Co. Inc, receiving salary and stock, but is now retired. DEC receives research support from Johnson & Johnson, the NIH, and Medtronic. DRF recieves consulting fees from Pacira Pharm, and Patient Centered outcomes with Surgical Consulting LLC. APC receives grant support from the NIH and Covidien/Ethicon J&J. GSJ, ASW, CDM, FP, RLP, BHG, MS, KSF, PJH, and BMW have no conflicts to declare.
Abbreviations:
- AIRglu
Acute insulin response to glucose
- Φ
Beta cell responsivity to glucose
- HE
Hepatic insulin extraction
- DI
Disposition index
- GLP-1
Glucagon-like peptide 1
- GIP
Gastric inhibitory polypeptide
- SG
Glucose effectiveness
- SI
Insulin sensitivity
- LABS Study
Longitudinal Assessment of Bariatric Surgery Study
- MM
Mixed meal
- RYGB
Roux-en-Y gastric bypass
Footnotes
Data Availability:
The datasets generated during and/or analysed during the current study are available by request from the NIH/NIDDK (https://www.niddkrepository.org/home/).
References
- [1].Pories WJ, MacDonald KG Jr., Morgan EJ, et al. (1992) Surgical treatment of obesity and its effect on diabetes: 10-y follow-up. Am J Clin Nutr 55: 582S–585S [DOI] [PubMed] [Google Scholar]
- [2].Purnell JQ, Selzer F, Wahed AS, et al. (2016) Type 2 Diabetes Remission Rates After Laparoscopic Gastric Bypass and Gastric Banding: Results of the Longitudinal Assessment of Bariatric Surgery Study. Diabetes Care 39: 1101–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Schauer PR, Bhatt DL, Kirwan JP, et al. (2017) Bariatric Surgery versus Intensive Medical Therapy for Diabetes - 5-Year Outcomes. N Engl J Med 376: 641–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Campos GM, Rabl C, Peeva S, et al. (2010) Improvement in peripheral glucose uptake after gastric bypass surgery is observed only after substantial weight loss has occurred and correlates with the magnitude of weight lost. J Gastrointest Surg 14: 15–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bradley D, Conte C, Mittendorfer B, et al. (2012) Gastric bypass and banding equally improve insulin sensitivity and beta cell function. J Clin Invest 122: 4667–4674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Camastra S, Muscelli E, Gastaldelli A, et al. (2013) Long-term effects of bariatric surgery on meal disposal and beta-cell function in diabetic and nondiabetic patients. Diabetes 62: 3709–3717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kahn SE (2001) Clinical review 135: The importance of beta-cell failure in the development and progression of type 2 diabetes. The Journal of clinical endocrinology and metabolism 86: 4047–4058 [DOI] [PubMed] [Google Scholar]
- [8].Courcoulas AP, Christian NJ, Belle SH, et al. (2013) Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA 310: 2416–2425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Halban PA, Polonsky KS, Bowden DW, et al. (2014) beta-cell failure in type 2 diabetes: postulated mechanisms and prospects for prevention and treatment. Diabetes Care 37: 1751–1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Belle SH, Berk PD, Courcoulas AP, et al. (2007) Safety and efficacy of bariatric surgery: Longitudinal Assessment of Bariatric Surgery. Surg Obes Relat Dis 3: 116–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Longitudinal Assessment of Bariatric Surgery C, Flum DR, Belle SH, et al. (2009) Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med 361: 445–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Prigeon RL, Roder ME, Porte D Jr., Kahn SE (1996) The effect of insulin dose on the measurement of insulin sensitivity by the minimal model technique. Evidence for saturable insulin transport in humans. J Clin Invest 97: 501–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Buse JB, Caprio S, Cefalu WT, et al. (2009) How do we define cure of diabetes? Diabetes care 32: 2133–2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cobelli C, Dalla Man C, Toffolo G, Basu R, Vella A, Rizza R (2014) The oral minimal model method. Diabetes 63: 1203–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kahn SE, Prigeon RL, McCulloch DK, et al. (1993) Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes 42: 1663–1672 [DOI] [PubMed] [Google Scholar]
- [16].Guldstrand M, Ahren B, Adamson U (2003) Improved beta-cell function after standardized weight reduction in severely obese subjects. Am J Physiol Endocrinol Metab 284: E557–565 [DOI] [PubMed] [Google Scholar]
- [17].Guidone C, Manco M, Valera-Mora E, et al. (2006) Mechanisms of recovery from type 2 diabetes after malabsorptive bariatric surgery. Diabetes 55: 2025–2031 [DOI] [PubMed] [Google Scholar]
- [18].Halverson JD, Kramer J, Cave A, Permutt A, Santiago J (1982) Altered glucose tolerance, insulin response, and insulin sensitivity after massive weight reduction subsequent to gastric bypass. Surgery 92: 235–240 [PubMed] [Google Scholar]
- [19].le Roux CW, Aylwin SJ, Batterham RL, et al. (2006) Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg 243: 108–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Johansson HE, Ohrvall M, Haenni A, et al. (2007) Gastric bypass alters the dynamics and metabolic effects of insulin and proinsulin secretion. Diabetic medicine : a journal of the British Diabetic Association 24: 1213–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rodieux F, Giusti V, D’Alessio DA, Suter M, Tappy L (2008) Effects of gastric bypass and gastric banding on glucose kinetics and gut hormone release. Obesity (Silver Spring) 16: 298–305 [DOI] [PubMed] [Google Scholar]
- [22].Chaikomin R, Doran S, Jones KL, et al. (2005) Initially more rapid small intestinal glucose delivery increases plasma insulin, GIP, and GLP-1 but does not improve overall glycemia in healthy subjects. Am J Physiol Endocrinol Metab 289: E504–507 [DOI] [PubMed] [Google Scholar]
- [23].Wang G, Agenor K, Pizot J, et al. (2012) Accelerated gastric emptying but no carbohydrate malabsorption 1 year after gastric bypass surgery (GBP). Obes Surg 22: 1263–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hirsch IB, Brownlee M (2010) Beyond hemoglobin A1c--need for additional markers of risk for diabetic microvascular complications. JAMA 303: 2291–2292 [DOI] [PubMed] [Google Scholar]
- [25].Adams TD, Gress RE, Smith SC, et al. (2007) Long-term mortality after gastric bypass surgery. N Engl J Med 357: 753–761 [DOI] [PubMed] [Google Scholar]
- [26].Sjostrom L, Peltonen M, Jacobson P, et al. (2014) Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 311: 2297–2304 [DOI] [PubMed] [Google Scholar]
- [27].Laferrere B, Heshka S, Wang K, et al. (2007) Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care 30: 1709–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Korner J, Inabnet W, Febres G, et al. (2009) Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes (Lond) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Salehi M, Prigeon RL, D’Alessio DA (2011) Gastric bypass surgery enhances glucagon-like peptide 1-stimulated postprandial insulin secretion in humans. Diabetes 60: 2308–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Van der Schueren BJ, Homel P, Alam M, et al. (2012) Magnitude and variability of the glucagon-like peptide-1 response in patients with type 2 diabetes up to 2 years following gastric bypass surgery. Diabetes Care 35: 42–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W (1993) Preserved incretin activity of glucagon-like peptide 1 [7–36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest 91: 301–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hare KJ, Vilsboll T, Asmar M, Deacon CF, Knop FK, Holst JJ (2010) The glucagonostatic and insulinotropic effects of glucagon-like peptide 1 contribute equally to its glucose-lowering action. Diabetes 59: 1765–1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Goldfine AB, Mun EC, Devine E, et al. (2007) Patients with neuroglycopenia after gastric bypass surgery have exaggerated incretin and insulin secretory responses to a mixed meal. J Clin Endocrinol Metab 92: 4678–4685 [DOI] [PubMed] [Google Scholar]
- [34].Nannipieri M, Baldi S, Mari A, et al. (2013) Roux-en-Y gastric bypass and sleeve gastrectomy: mechanisms of diabetes remission and role of gut hormones. J Clin Endocrinol Metab 98: 4391–4399 [DOI] [PubMed] [Google Scholar]
- [35].Salehi M, Gastaldelli A, D’Alessio DA (2014) Altered islet function and insulin clearance cause hyperinsulinemia in gastric bypass patients with symptoms of postprandial hypoglycemia. J Clin Endocrinol Metab 99: 2008–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Campos GM, Rabl C, Havel PJ, et al. (2014) Changes in post-prandial glucose and pancreatic hormones, and steady-state insulin and free fatty acids after gastric bypass surgery. Surg Obes Relat Dis 10: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Cegla J, Troke RC, Jones B, et al. (2014) Coinfusion of low-dose GLP-1 and glucagon in man results in a reduction in food intake. Diabetes 63: 3711–3720 [DOI] [PubMed] [Google Scholar]
- [38].Polyzogopoulou EV, Kalfarentzos F, Vagenakis AG, Alexandrides TK (2003) Restoration of euglycemia and normal acute insulin response to glucose in obese subjects with type 2 diabetes following bariatric surgery. Diabetes 52: 1098–1103 [DOI] [PubMed] [Google Scholar]
- [39].Turner RC, McCarthy ST, Holman RR, Harris E (1976) Beta-cell function improved by supplementing basal insulin secretion in mild diabetes. Br Med J 1: 1252–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Nannipieri M, Mari A, Anselmino M, et al. (2011) The role of beta-cell function and insulin sensitivity in the remission of type 2 diabetes after gastric bypass surgery. J Clin Endocrinol Metab 96: E1372–1379 [DOI] [PubMed] [Google Scholar]
- [41].Lund MT, Hansen M, Skaaby S, et al. (2015) Preoperative beta-cell function in patients with type 2 diabetes is important for the outcome of Roux-en-Y gastric bypass surgery. J Physiol 593: 3123–3133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Salinari S, Bertuzzi A, Asnaghi S, Guidone C, Manco M, Mingrone G (2009) First-phase insulin secretion restoration and differential response to glucose load depending on the route of administration in type 2 diabetic subjects after bariatric surgery. Diabetes Care 32: 375–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Martinussen C, Bojsen-Moller KN, Dirksen C, et al. (2015) Immediate enhancement of first-phase insulin secretion and unchanged glucose effectiveness in patients with type 2 diabetes after Roux-en-Y gastric bypass. Am J Physiol Endocrinol Metab 308: E535–544 [DOI] [PubMed] [Google Scholar]
- [44].Jorgensen NB, Jacobsen SH, Dirksen C, et al. (2012) Acute and long-term effects of Roux-en-Y gastric bypass on glucose metabolism in subjects with Type 2 diabetes and normal glucose tolerance. Am J Physiol Endocrinol Metab 303: E122–131 [DOI] [PubMed] [Google Scholar]
- [45].Camastra S, Gastaldelli A, Mari A, et al. (2011) Early and longer term effects of gastric bypass surgery on tissue-specific insulin sensitivity and beta cell function in morbidly obese patients with and without type 2 diabetes. Diabetologia 54: 2093–2102 [DOI] [PubMed] [Google Scholar]
- [46].Dutia R, Brakoniecki K, Bunker P, et al. (2014) Limited recovery of beta-cell function after gastric bypass despite clinical diabetes remission. Diabetes 63: 1214–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Dalla Man C, Campioni M, Polonsky KS, et al. (2005) Two-hour seven-sample oral glucose tolerance test and meal protocol: minimal model assessment of beta-cell responsivity and insulin sensitivity in nondiabetic individuals. Diabetes 54: 3265–3273 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.