Abstract
In a random sequence of binary events where one alternative occurs more often than the other, humans tend to guess which of the two alternatives will occur next by trying to match the frequencies of previous occurrences. Based on split-brain and unilaterally damaged patients' performances, it has been proposed that the left hemisphere (LH) tends to match the frequencies, while the right hemisphere (RH) tends toward maximizing and always choosing the most frequent alternative. The current study used transcranial direct current stimulation (tDCS) to test this hemispheric asymmetry hypothesis by stimulating the dorsolateral prefrontal cortex of each hemisphere and simultaneously inhibiting the corresponding region in the homotopic hemisphere, while participants were engaged in a probabilistic guessing task. Results showed no difference in strategy between the three groups (RH anodal/LH cathodal, LH anodal/RH cathodal, no stimulation) as participants predominantly matched the frequencies of the two alternatives. However, when anodal tDCS was applied to the LH and cathodal tDCS applied to the RH, participants became quicker to select the most frequent alternative. This finding is in line with previous evidence on the involvement of the LH in probabilistic learning and reasoning and adds to a number of demonstrations of anodal tDCS leading to some behavioral enhancement or change in bias.
Introduction
In a random sequence of binary events with the probabilities P(A) > 0.5 and P(B) = 1 − P(A), the optimal strategy for predicting which event will occur next is to always choose the most frequent event. Consider a deck of cards composed of more red than black cards. Before each trial, all the cards are reshuffled, and one is given an incentive for correctly guessing which card is about to be picked out. The best strategy is to always predict “red.” Suppose the probabilities are 75% and 25% for red and black cards, respectively. A consistent prediction of red yields 75% correct and 25% incorrect guesses—[(0.75 × 1) + (0.25 × 0)]. This “maximizing” (MAX) strategy is adopted by many animals, while humans tend to match their predictions to the actual frequencies (Estes, 196l; Hinson and Staddon, 1983). In the example of the cards, humans, after noticing over several trials that there are more red than black cards, will tend to predict “red” on ∼75% of the trials and “black” on the other ∼25%. This frequency-matching (FM) strategy is less optimal since it yields <63% correct guesses—[(0.75 × 0.75) + (0.25 × 0.25)]. Why do humans perform worse than rats and pigeons on this task? It has been suggested that humans are prone to search for patterns and causality in sequences of events, even when told that the sequences are totally random (Estes, 196l; Yellott, 1969; Hinson and Staddon, 1983).
A study on split-brain and unilateral frontal brain-damaged patients found that when the two alternatives were presented in the right visual field [i.e., processed by the left hemisphere (LH)], patients used the FM strategy, but they moved steadily toward the MAX strategy for stimuli presented in the left visual field [i.e., to the right hemisphere (RH)] (Wolford et al., 2000). These findings inspired the “hemispheric asymmetry in reasoning” hypothesis in which the LH tends to search for a pattern and a causal relationship, whereas the RH that lacks these “interpreting” and “pattern-searching” qualities favors the maximizing strategy (Walsh, 2000; Wolford et al., 2000). However, subsequent studies with intact-brain participants have shown mixed results (Wolford et al., 2004; Miller et al., 2005). Furthermore, split-brain patients have displayed a FM strategy for faces presented to the RH (Miller and Valsangkar-Smyth, 2005).
The current study aimed to test this putative hemispheric asymmetry in guessing strategies by using transcranial direct current stimulation (tDCS). With tDCS, anodal stimulation causes membrane depolarization and increases neuronal firing rates, thus enhancing cerebral excitability, while cathodal stimulation diminishes it (Nitsche and Paulus, 2000, 2001; Nitsche et al., 2008; Galea et al., 2009; Jang et al., 2009; Stagg et al., 2009). There is of course no necessary correspondence between excitation/inhibition and behavioral improvements/impairments and cathodal stimulation can, in certain conditions, improve a behavioral function (e.g., Antal et al., 2004). tDCS enables one to stimulate an area in one hemisphere while simultaneously inhibiting the corresponding region in the other hemisphere. The stimulation sites in the current study included the right and left dorsolateral prefrontal cortices (DLPFCs). These sites were selected following previous reports on reasoning tasks (Wolford et al., 2000; Miller et al., 2005). Thus, we compared participants' guessing strategies in three conditions: RH anodal/LH cathodal, LH anodal/RH cathodal, and control group with no DC stimulation.
Materials and Methods
Participants.
Twenty-eight healthy college students participated in the study (14 males and 14 females; mean age 22.8 ± 3.5 years). Participants were all right handed as assessed by the Edinburgh Handedness Inventory (Oldfield, 1971), with normal or corrected-to-normal vision and without any known neurological or psychiatric conditions. All were naive to the nature of the experiment, and gave informed written consent before entering the study, which was approved by the UCL ethics committee. Participants were randomly assigned into one of three groups to receive either (1) active stimulation with the anodal electrode over the right dorsolateral prefrontal cortex and the cathodal electrode over the left DLPFC (referred to as “RH anodal/LH cathodal group”; n = 10; 5 males); (2) active stimulation with the anodal electrode over the left DLPFC and the cathodal electrode over the right DLPFC (referred to as “LH anodal/RH cathodal group”; n = 10; 5 males); or (3) no stimulation (referred to as “control group”; n = 8; 4 males).
Prediction task.
Participants were told that in each trial an asterisk (*) will appear on the screen either at the center of the upper half of the screen or at the center of the lower half of the screen, based upon the computer's random selection. The task was to guess where the asterisk was going to appear on the screen (top or bottom). The computer was programmed to present the asterisk in the upper half on 70% of the trials and in the lower half in the other 30% of trials in each block. The sequence of the trials within each block was randomly determined by the computer.
tDCS.
A direct current of 2 mA intensity was induced by two saline-soaked surface sponge electrodes (9 cm2) and delivered by a battery-driven, constant-current stimulator (Magstim). All participants indicated that they felt the stimulation. Previous studies have shown that this intensity of stimulation is safe in healthy volunteers (Iyer et al., 2005). For stimulation over the left DLPFC, the anodal electrode was placed over the left F3 (using the international EEG 10/20 system) and the cathodal electrode over the right F4 (LH anodal/RH cathodal group). For stimulation of the right DLPFC, the polarity was reversed: the anode was placed over F4 and the cathode over F3 (RH anodal/LH cathodal group).
Procedure.
Trials were delivered in five blocks of 100 trials each. tDCS started immediately before the prediction task began and was delivered during the whole course of the five-block experiment, which lasted ∼22 min. Each trial began with a question mark (?) presented at the center of the screen and the participants' task was to guess where the asterisk was going to appear on the screen (top or bottom) and press one of the designated buttons on the keyboard (M and B, respectively). One hundred milliseconds after the participant's guess, an asterisk was presented for 100 ms either in the top half of the screen or in the bottom half. The same procedure was repeated in the next trial, with a 1 s interval between trials. To motivate participants to perform optimally, they were told that if their correct predictions exceeded the average score by >10% they would receive an additional bonus of £5.
Results
The three dependent variables were as follows: (1) the participant's guess regarding the location of the asterisk; (2) accuracy—whether the prediction turned out to be correct or not; and (3) response time (RT)—the duration of time from the moment that each trial began, with the question mark on the screen, until the participant made a guess and pressed the corresponding button. Three participants (one in the LH anodal/RH cathodal group and two in the RH anodal/LH cathodal group) were excluded from further analysis as their performances were >2 SDs below the group's average.
Strategy choice
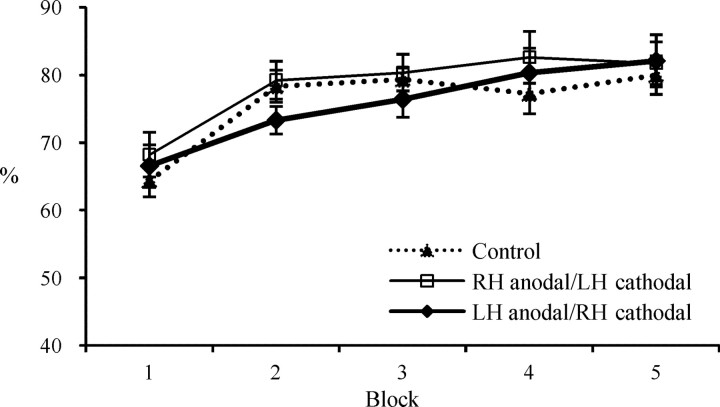
In all three groups, participants adopted the frequency-matching strategy and they predicted the screen location that was most frequent on average, in ∼80% of the trials in the final blocks (Fig. 1). A general mixed-design ANOVA of 5 blocks (repeated) by 3 stimulation groups as the between-subjects variable on the selection of the most frequent alternative showed no effect for stimulation group or block, and no interaction.
Figure 1.
Strategies were similar in all three groups. The most frequent alternative (top) was selected in ∼80% of the trials. Shown is mean ± SE in the control (broken line), RH anodal/LH cathodal (thin line), and LH anodal/RH cathodal (thick line) groups.
Accuracy
Since participants adopted the FM strategy, as is evident by the ∼80% selection of the most frequent alternative, their accuracy rates after the initial “information gathering” block ranged between 58 and 63% (Table 1). This is less optimal than if they had chosen the MAX strategy—always selecting the most frequent alternative—which would have yielded 70% accuracy. Nevertheless, despite the group's average, there were two individuals (from the LH anodal/RH cathodal group) who selected the “top” >94%, and therefore tended toward the maximizing strategy.
Table 1.
Adopting the frequency-matching strategy led to suboptimal accuracy rates
| Group | Block |
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Control | 53.9 ± 4.3 | 62.2 ± 6 | 61.1 ± 6 | 58 ± 6.2 | 61.7 ± 9.1 |
| LH anodal/RH cathodal | 59.2 ± 5.9 | 60.9 ± 4.4 | 60.2 ± 5.9 | 61.2 ± 7.6 | 61.9 ± 6 |
| RH anodal/LH cathodal | 56.2 ± 7.6 | 61 ± 4.9 | 62.1 ± 6.8 | 63 ± 8.5 | 62 ± 5.1 |
Shown is mean ± SD in the three groups along the five blocks.
A mixed-design ANOVA [5 blocks (repeated) by 3 groups] on the accuracy rates revealed no effect for group, a significant effect for block (F(4,88) = 3.69, p < 0.01), and no interaction. As can be seen in Table 1, accuracies were lower in the first block than in the subsequent blocks. Post hoc comparison with Bonferroni adjustments showed that the difference between the first and second blocks was significant only in the control condition (t(7) = 3.53, p < 0.005). The poorer accuracy in the first block reflects the fact that during the initial trials participants had no clue about the probabilities and therefore their accuracy rate was not very different from chance level (∼50%), but as they gradually discovered that there were more occurrences of the asterisk at the top, they developed a strategy for systematic guessing, which improved their overall accuracy rate (although it was not the optimal strategy).
Response times
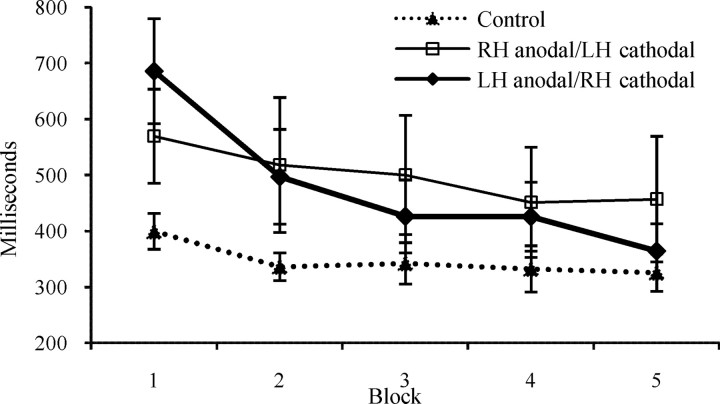
A mixed-design ANOVA [5 blocks (repeated) by 3 groups] revealed no effect for group, but a significant effect for block (F(4,88) = 20.3, p < 0.0001), as well as a significant interaction of block and group (F(8,88) = 4.52, p < 0.01). Post hoc comparisons (with Bonferroni adjustments) showed that in the first block, there was a significant difference in RTs between the control group and the LH anodal/RH cathodal group (t(7) = 2.98, p = 0.01). As can be seen in Figure 2, when the LH was stimulated anodally and the RH cathodally, participants' RTs consistently and significantly decreased, over the blocks of trials (block 1 > blocks 2, 3, 4 > block 5). This significant decrease in RTs along the blocks was unique to responses selecting the most frequent alternative in the LH anodal/RH cathodal group, while in the control (no stimulation) and the RH anodal/LH cathodal stimulation groups the decrease in RTs over the five blocks for both selecting the most or least frequent alternative was not significant. The development of strategy is reflected mainly in the later four blocks: the first block's RTs are qualitatively different from the subsequent four blocks, as participants initially are presumably gathering information and learning the probabilities. We therefore ran another ANOVA including only blocks 2–5. Again, there was no effect for stimulation group, but a significant effect for block (F(3,66) = 7.58, p < 0.001), as well as an interaction of block and group (F(6,66) = 2.17, p = 0.056).
Figure 2.
The largest reduction in response times (mean ± SE), along the blocks, occurred with LH anodal/RH cathodal stimulation. Broken line, Control; thin line, RH anodal/LH cathodal; thick line, LH anodal/RH cathodal.
To rule out the possibility that the reductions in RTs, along the blocks, in the LH anodal/RH cathodal group reflect merely a facilitation of the motor response, but not any cognitive facilitation of the decision process, we ran a control experiment. Eighteen participants were randomly assigned into one of three groups: control (no stimulation), LH anodal/RH cathodal, or RH anodal/LH cathodal. Each group included three males and three females. The stimulation protocol and the stimuli were the same as in the first experiment. The task was changed to a simple motor response, and participants were only asked to press a key when they saw the question mark appearing on the screen (a simple RT paradigm). The results are summarized in Table 2.
Table 2.
Differences in the motor component were small and nonsignificant
| Group | Block |
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Control | 273 ± 35 | 272 ± 52 | 278 ± 56 | 280 ± 52 | 282 ± 50 |
| LH anodal/RH cathodal | 250 ± 29 | 242 ± 32 | 248 ± 25 | 259 ± 43 | 266 ± 48 |
| RH anodal/LH cathodal | 255 ± 25 | 240 ± 26 | 237 ± 26 | 232 ± 24 | 245 ± 32 |
Shown is mean ± SD in the three groups along the five blocks.
A mixed-design ANOVA of 5 blocks (repeated) by 3 groups as the between-subjects variable on the RTs showed no significant effect for group or block, and no interaction. Furthermore, in this pure motor task, the differences in RTs between blocks or groups were in the range of <25 ms, far less that the effect we observed in the guessing task (a reduction of ∼300 ms from the initial block to the last block). Thus, the major portion of the RT gains in the LH anodal/RH cathodal group that we report here represents a cognitive facilitation of the decision process rather than a simple motor effect.
Discussion
Participants in all three groups adopted the FM strategy and chose the most frequent alternative on ∼80% of trials. Thus, in regard to the strategy chosen, there was no effect of the tDCS. Nevertheless, the tDCS did affect the speed of making a decision. Both stimulation conditions (LH anodal/RH cathodal and RH anodal/LH cathodal) initially slowed RT compared to the control group. A similar effect of tDCS slowing RT in a cognitive task was reported by Marshall et al. (2005). This may be explained by the fact that all subjects indicated clearly that they felt the DC stimulation, which distracted them to some degree, and interfered with their ability to fully concentrate on the task. Therefore, whereas in the control condition participants were able to focus their attention entirely on the task and responded, on average, at the 400–350 ms range, in both stimulation conditions their overall initial responses were relatively slower due to the partial distraction. However, as participants progressed along the blocks of trials, there was a clear and steady reduction in the amount of time they needed to select the most frequent alternative, in the LH anodal/RH cathodal group, but not when the least frequent option was chosen, nor in the control or RH anodal/LH cathodal groups.
The steadily decreasing RTs of the LH anodal/RH cathodal group, compared to the RH anodal/LH cathodal group, corresponds with previous evidence that the LH is specifically involved in probabilistic learning and reasoning. In a reasoning study, participants were presented with two statements followed by a conclusion and they had to make either a valid/invalid judgment using deductive reasoning, or a high/low likelihood judgment using probabilistic reasoning. An example of deductive reasoning is as follows: a) If John is an electrician, then he spent 2 years in high school. b) John is an electrician and owns a computer. c) John spent 2 years in high school. In this task the judgment involves the detection of logical necessity, and the information provided by the premises suffices for making the judgment. An example of the probabilistic reasoning is as follows: a) If John is a heart specialist, then he either bicycles to work or swims regularly. b) John is a heart specialist. c) John bicycles to work. In such a task the argument is (logically) invalid as the information in the premises is insufficient to constrain the conclusion; however, the task is to judge whether it is more likely to be true or false. This judgment is subjective in character and requires the integration of background knowledge (e.g., about jobs and recreation) not explicitly presented in the arguments. The results showed that probabilistic reasoning activated mostly LH regions, whereas deductive reasoning activated mostly RH areas (Parsons and Osherson, 2001). Similarly, in a probability classification learning task, participants learned gradually which of two outcomes would occur in each trial given the particular combination of associated cues. It was found that anodal, but not cathodal, tDCS to the left prefrontal cortex (PFC) improved performance and reduced errors (Kincses et al., 2004). Thus, it may be that the significant decrease in RTs that we observed selectively in the LH anodal/RH cathodal group reflects the LH specialization in processing probabilistic reasoning and learning, which the tDCS enhanced.
Our findings of a hemispheric asymmetry in tDCS effects on the pace of decision making, where only LH anodal/RH cathodal led to faster decisions, but the opposite electrode arrangement did not, can be further explained in terms of an interaction between the LH's propensity to overinterpret information (Gazzaniga, 2000; Walsh, 2000; Wolford et al., 2000) and the RH's capacity to “temper” this overinterpretation by supporting ambiguous or uncertain mental representations (Goel and Vartanian, 2005). In a study where participants judged the validity of certain and uncertain inferences (e.g., A > B, B > C, therefore A > C; A > B, A > C, therefore B > C, respectively), it was found that, relative to healthy controls, patients with RH prefrontal lesions made more errors when the information given to them was indeterminate and insufficient for the conclusion statement, whereas patients with LH lesions made more errors when they received the complete information (Goel et al., 2007). These findings were interpreted with reference to the notion that the left PFC is more adept at constructing determinate, precise, and unambiguous representations of the world. Thus, it automatically fills in any gaps in the available information, often prematurely or incorrectly. Conversely, the right PFC is more adept at constructing and maintaining fluid, indeterminate, vague, and ambiguous representations, enabling it to temper or “inhibit” premature interpretations by the left PFC. If, however, a right PFC lesion impairs this ability, then the LH's “interpreter” will impose a particular interpretation on the problem representation, rendering it determinate (Goel, 1995, 2002; Gazzaniga, 2000; Wolford et al., 2000; Goel et al., 2007). This view fits with the literature showing that inhibitory functions are controlled by the RH (Aron et al., 2003, 2004; Chikazoe et al., 2007, 2009; Forstmann et al., 2008; Xue et al., 2008; Berkman et al., 2009; Chamberlain et al., 2009; Goghari and MacDonald, 2009), and reports that the RH is associated with more careful strategies and less willingness to take risks, than the LH (e.g., Knoch et al., 2006; Fecteau et al., 2007; Gianotti et al., 2009). In our study, participants were motivated to guess optimally, and they were even promised a ”bonus“ for above-average performances. It seems that the RH anodal/LH cathodal stimulation promoted conscientious behavior, hesitation, and slower decisions, whereas the LH anodal/RH cathodal stimulation had an opposite effect—faster decisions, which may reflect less inhibition, premature conclusions, and some degree of increased willingness to take risks.
Three complementary lines of evidence lead us to suggest that our observation of reduced RTs in the LH anodal/RH cathodal group represents a cognitive facilitation in reasoning and decision-making processes. First, other studies support a general role of the LH in decision processes (Dee and Van Allen, 1973; Godefroy and Rousseaux, 1996; Heekeren et al., 2004, 2006; Rorie and Newsome, 2005). Second, there is sound evidence of the LH involvement in probabilistic reasoning (Parsons and Osherson, 2001; Kincses et al., 2004). Third, a study with a protocol similar to ours has shown that tDCS changes decision processes; specifically, RH stimulation decreases the willingness to take risks (Fecteau et al., 2007).
The absence of a tDCS effect on the strategy choice cannot be explained in terms of insufficient stimulation (e.g., low intensity, etc.), since the same stimulation did affect RTs. Also, another study using a similar stimulation protocol reported significant cognitive effects on decision making (Fecteau et al., 2007). Therefore, in regard to the guessing strategy participants adopt, it may be plausible to assume that the hemispheric asymmetry is exclusive to split-brain and unilaterally damaged patients, but may not occur in normal healthy subjects (Miller et al., 2005). Moreover, in a study with normal participants, individual differences were found and although ∼80% of participants tended toward FM, there were some ∼20% of maximizers (Miller et al., 2005). Similarly, in the current study, despite the group's average showing a FM strategy, there were individual participants who tended toward MAX, and the two extreme maximizers (selecting the “top” >94%) were from the LH anodal/RH cathodal group—contrary to the original prediction. Thus, it may be that even the hemispheric asymmetry found in patients was due to the small and imbalanced group—only two split-brain and five unilaterally damaged patients (of whom four had RH lesions and only one was with an LH lesion) (Wolford et al., 2000). Had the patient group been larger and more balanced, it may have been found that in some patients the RH can also adopt the FM strategy (Miller et al., 2005).
There might be another account for not observing a RH MAX strategy. In the only study (Wolford et al., 2004) that found some support for the original hemispheric asymmetry idea, for intact-brain participants, the tasks were not matched. Participants in that study were involved either in a secondary (verbal working memory) task that competed for LH resources and consequently let the RH dictate which strategy to adopt (MAX), or in a secondary (spatial memory) task that competed for RH resources, which preserved the FM strategy (Wolford et al., 2004). However, whereas the LH task involved constantly memorizing and updating the last three digits presented, the RH task was less demanding and involved memorizing only the last single polygon shape. In addition, the LH task demanded active memorizing of the three digits, while the RH task required participants only to passively maintain the shape in working memory and judge whether subsequent shapes were similar or different. Thus, the RH task required fewer resources than the LH task, and therefore enabled some strategic RH reasoning.
In conclusion, LH anodal/RH cathodal DC stimulation caused subjects to be significantly faster to select the most frequent alternative. This may reflect the LH's greater involvement in probabilistic learning and reasoning. Also, it may reflect the association of the LH with a greater willingness to take risks (Knoch et al., 2006; Fecteau et al., 2007) and its propensity for premature conclusions, which we may be guilty of here: further experiments will determine this.
Footnotes
We are members of the European Union Marie Curie Initial Training Network (ITN-LAN Grant PITN-G-2008-214570) funded under Framework 7.
References
- Antal A, Nitsche MA, Kruse W, Kincses TZ, Hoffmann KP, Paulus W. Direct current stimulation over V5 enhances visuomotor coordination by improving motion perception in humans. J Cogn Neurosci. 2004;16:521–527. doi: 10.1162/089892904323057263. [DOI] [PubMed] [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Berkman ET, Burklund L, Lieberman MD. Inhibitory spillover: intentional motor inhibition produces incidental limbic inhibition via right inferior frontal cortex. Neuroimage. 2009;47:705–712. doi: 10.1016/j.neuroimage.2009.04.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain SR, Hampshire A, Müller U, Rubia K, Del Campo N, Craig K, Regenthal R, Suckling J, Roiser JP, Grant JE, Bullmore ET, Robbins TW, Sahakian BJ. Atomoxetine modulates right inferior frontal activation during inhibitory control: a pharmacological functional magnetic resonance imaging study. Biol Psychiatry. 2009;65:550–555. doi: 10.1016/j.biopsych.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Chikazoe J, Konishi S, Asari T, Jimura K, Miyashita Y. Activation of right inferior frontal gyrus during response inhibition across response modalities. J Cogn Neurosci. 2007;19:69–80. doi: 10.1162/jocn.2007.19.1.69. [DOI] [PubMed] [Google Scholar]
- Chikazoe J, Jimura K, Asari T, Yamashita K, Morimoto H, Hirose S, Miyashita Y, Konishi S. Functional dissociation in right inferior frontal cortex during performance of go/no-go task. Cereb Cortex. 2009;19:146–152. doi: 10.1093/cercor/bhn065. [DOI] [PubMed] [Google Scholar]
- Dee HL, Van Allen MW. Speed of decision-making processes in patients with unilateral cerebral disease. Arch Neurol. 1973;28:163–166. doi: 10.1001/archneur.1973.00490210043004. [DOI] [PubMed] [Google Scholar]
- Estes WK. A descriptive approach to the dynamics of choice behavior. Behav Sci. 1961;6:177–184. doi: 10.1002/bs.3830060302. [DOI] [PubMed] [Google Scholar]
- Fecteau S, Knoch D, Fregni F, Sultani N, Boggio P, Pascual-Leone A. Diminishing risk-taking behavior by modulating activity in the prefrontal cortex: a direct current stimulation study. J Neurosci. 2007;27:12500–12505. doi: 10.1523/JNEUROSCI.3283-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstmann BU, Jahfari S, Scholte HS, Wolfensteller U, van den Wildenberg WP, Ridderinkhof KR. Function and structure of the right inferior frontal cortex predict individual differences in response inhibition: a model-based approach. J Neurosci. 2008;28:9790–9796. doi: 10.1523/JNEUROSCI.1465-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea JM, Jayaram G, Ajagbe L, Celnik P. Modulation of cerebellar excitability by polarity-specific noninvasive direct current stimulation. J Neurosci. 2009;29:9115–9122. doi: 10.1523/JNEUROSCI.2184-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaniga MS. Cerebral specialization and interhemispheric communication: does the corpus callosum enable the human condition? Brain. 2000;123:1293–1326. doi: 10.1093/brain/123.7.1293. [DOI] [PubMed] [Google Scholar]
- Gianotti LRR, Knoch D, Faber PL, Lehmann D, Pascual-Marqui RD, Diezi C, Schoch C, Eisenegger C, Fehr E. Tonic activity level in the right prefrontal cortex predicts individuals' risk taking. Psychol Sci. 2009;20:33–38. doi: 10.1111/j.1467-9280.2008.02260.x. [DOI] [PubMed] [Google Scholar]
- Godefroy O, Rousseaux M. Binary choice in patients with prefrontal or posterior brain damage. A relative judgement theory analysis. Neuropsychologia. 1996;34:1029–1038. doi: 10.1016/0028-3932(96)00012-7. [DOI] [PubMed] [Google Scholar]
- Goel V. Sketches of thought. Cambridge MA: MIT; 1995. [Google Scholar]
- Goel V. Planning: neural and psychological. In: Nadel L, editor. Encyclopedia of cognitive science. New York: Macmillan; 2002. pp. 697–703. [Google Scholar]
- Goel V, Vartanian O. Dissociating the roles of right ventral lateral and dorsal lateral prefrontal cortex in generation and maintenance of hypotheses in set-shift problems. Cereb Cortex. 2005;15:1170–1177. doi: 10.1093/cercor/bhh217. [DOI] [PubMed] [Google Scholar]
- Goel V, Tierney M, Sheesley L, Bartolo A, Vartanian O, Grafman J. Hemispheric specialization in human prefrontal cortex for resolving certain and uncertain inferences. Cereb Cortex. 2007;17:2245–2250. doi: 10.1093/cercor/bhl132. [DOI] [PubMed] [Google Scholar]
- Goghari VM, MacDonald AW., 3rd The neural basis of cognitive control: response selection and inhibition. Brain Cogn. 2009;71:72–83. doi: 10.1016/j.bandc.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heekeren HR, Marrett S, Bandettini PA, Ungerleider LG. A general mechanism for perceptual decision-making in the human brain. Nature. 2004;431:859–862. doi: 10.1038/nature02966. [DOI] [PubMed] [Google Scholar]
- Heekeren HR, Marrett S, Ruff DA, Bandettini PA, Ungerleider LG. Involvement of human left dorsolateral prefrontal cortex in perceptual decision making is independent of response modality. Proc Natl Acad Sci U S A. 2006;103:10023–10028. doi: 10.1073/pnas.0603949103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinson JM, Staddon JER. Matching, maximizing and hill-climbing. J Exp Anal Behav. 1983;40:321–331. doi: 10.1901/jeab.1983.40-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer MB, Mattu U, Grafman J, Lomarev M, Sato S, Wassermann EM. Safety and cognitive effect of frontal DC brain polarization in healthy individuals. Neurology. 2005;64:872–875. doi: 10.1212/01.WNL.0000152986.07469.E9. [DOI] [PubMed] [Google Scholar]
- Jang SH, Ahn SH, Byun WM, Kim CS, Lee MY, Kwon YH. The effect of transcranial direct current stimulation on the cortical activation by motor task in the human brain: an fMRI study. Neurosci Lett. 2009;460:117–120. doi: 10.1016/j.neulet.2009.05.037. [DOI] [PubMed] [Google Scholar]
- Kincses TZ, Antal A, Nitsche MA, Bártfai O, Paulus W. Facilitation of probabilistic classification learning by transcranial direct current stimulation of the prefrontal cortex in the human. Neuropsychologia. 2004;42:113–117. doi: 10.1016/s0028-3932(03)00124-6. [DOI] [PubMed] [Google Scholar]
- Knoch D, Gianotti LRR, Pascual-Leone A, Treyer V, Regard M, Hohmann M, Brugger P. Disruption of right prefrontal cortex by low-frequency repetitive transcranial magnetic stimulation induces risk-taking behavior. J Neurosci. 2006;26:6469–6472. doi: 10.1523/JNEUROSCI.0804-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L, Mölle M, Siebner HR, Born J. Bifrontal transcranial direct current stimulation slows reaction time in a working memory task. BMC Neurosci. 2005;6:23. doi: 10.1186/1471-2202-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MB, Valsangkar-Smyth M. Probability matching in the right hemisphere. Brain Cogn. 2005;57:165–167. doi: 10.1016/j.bandc.2004.08.038. [DOI] [PubMed] [Google Scholar]
- Miller MB, Valsangkar-Smyth M, Newman S, Dumont H, Wolford G. Brain activations associated with probability matching. Neuropsychologia. 2005;43:1598–1608. doi: 10.1016/j.neuropsychologia.2005.01.021. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527:633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57:1899–1901. doi: 10.1212/wnl.57.10.1899. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Antal A, Liebetanz D, Lang N, Tergau F, Paulus W. Neuroplasticty induced by transcranial direct current stimulation. In: Wassermann EM, Epstein CM, Ziemann U, Walsh V, Paus T, Lisanby SH, editors. Oxford handbook of transcranial stimulation. Oxford: Oxford UP; 2008. pp. 201–218. [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Parsons LM, Osherson D. New evidence for distinct right and left brain systems for deductive versus probabilistic reasoning. Cereb Cortex. 2001;11:954–965. doi: 10.1093/cercor/11.10.954. [DOI] [PubMed] [Google Scholar]
- Rorie AE, Newsome WT. A general mechanism for decision-making in the human brain? Trends Cogn Sci. 2005;9:41–43. doi: 10.1016/j.tics.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Best JG, Stephenson MC, O'Shea J, Wylezinska M, Kincses ZT, Morris PG, Matthews PM, Johansen-Berg H. Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. J Neurosci. 2009;29:5202–5206. doi: 10.1523/JNEUROSCI.4432-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh V. Hemispheric asymmetries: a brain in two minds. Curr Biol. 2000;10:R460–R462. doi: 10.1016/s0960-9822(00)00533-9. [DOI] [PubMed] [Google Scholar]
- Wolford G, Miller MB, Gazzaniga MS. The left hemisphere's role in hypothesis formation. J Neurosci. 2000;20(RC64):1–4. doi: 10.1523/JNEUROSCI.20-06-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolford G, Newman SE, Miller MB, Wig GS. Searching for patterns in random sequences. Can J Exp Psychol. 2004;58:221–228. doi: 10.1037/h0087446. [DOI] [PubMed] [Google Scholar]
- Xue G, Aron AR, Poldrack RA. Common neural substrates for inhibition of spoken and manual responses. Cereb Cortex. 2008;18:1923–1932. doi: 10.1093/cercor/bhm220. [DOI] [PubMed] [Google Scholar]
- Yellott JL. Probability learning with noncontingent success. J Math Psychol. 1969;6:541–575. [Google Scholar]