Abstract
The posterior alpha rhythm (8–14 Hz), originating in occipito-parietal areas through thalamocortical generation, displays characteristics of visual activity in anticipation of visual events. Posterior alpha power is influenced by visual spatial attention via top-down control from higher order attention areas such as the frontal eye field. It covaries with visual cortex excitability, as tested through transcranial magnetic stimulation (TMS), and predicts the perceptual fate of a forthcoming visual stimulus. Yet, it is still unknown whether the nature of the relationship between this prestimulus alpha oscillation and upcoming perception is causal or only correlative. Here, we tested in the human brain whether the oscillation in the alpha band is causally shaping perception through directly stimulating visual areas via short trains of rhythmic TMS. We compared stimulation at alpha frequency (10 Hz) with two control frequencies in the theta (5 Hz) and beta bands (20 Hz), and assessed immediate perceptual outcomes. Target visibility was significantly modulated by alpha stimulation, relative to both control conditions. Alpha stimulation selectively impaired visual detection in the visual field opposite to the stimulated hemisphere, while enhancing detection ipsilaterally. These frequency-specific effects were observed both for stimulation over occipital and parietal areas of the left and right hemispheres and were short lived: they were observed by the end of the TMS train but were absent 3 s later. This shows that the posterior alpha rhythm is actively involved in shaping forthcoming perception and, hence, constitutes a substrate rather than a mere correlate of visual input regulation.
Introduction
Oscillatory activity is an intrinsic property of the neuronal elements that generate it (Buzsáki and Draguhn, 2004). Unsurprisingly, neuronal oscillations therefore display ongoing variability in power even at baseline (Linkenkaer-Hansen et al., 2001), in addition to showing sensory-driven changes (Bauer et al., 2006; Gross et al., 2007; van Dijk et al., 2008). More notably, trial-by-trial variability in oscillatory activity at baseline (e.g., before sensory input) also covaries with variability in forthcoming stimulus processing, as shown for specific oscillatory signatures over posterior recording sites (Womelsdorf et al., 2006; Hanslmayr et al., 2007; van Dijk et al., 2008). These prestimulus brain rhythms thus seem to be perceptually relevant. However, to what extent these rhythms shape the perceptual fate of upcoming visual input, as opposed to reflecting by-products of other, underlying mechanisms, is unknown.
The posterior alpha rhythm (8–14 Hz) is the most prominent oscillation during wakeful rest. It originates from occipito-parietal areas, where it is modulated by visual input (Berger, 1929; Adrian and Matthews, 1934; Hari et al., 1997). It is therefore thought to reflect the spontaneous rhythm of visual areas. Because the occipital alpha oscillation is synchronized to cyclic activity in visual thalamic relay neurons, it is likely involved in signal transmission at early input stages (Lorincz et al., 2009).
Occipito-parietal alpha oscillations also display characteristics of baseline hemodynamic signals in visual areas (Kastner et al., 1999) and visual thalamic nuclei (O'Connor et al., 2002). By analogy to this baseline activity, posterior alpha power is influenced by deployment of visual spatial attention (Sauseng et al., 2005; Thut et al., 2006), leading to retinotopically organized alpha changes in accordance with the focus of attention (Worden et al., 2000; Rihs et al., 2007) through top-down influences from the frontal eye field (Capotosto et al., 2009). Prestimulus alpha power also covaries with the excitability of the visual cortex, tested by single-pulse transcranial magnetic stimulation (TMS) over the occipital pole (Romei et al., 2008), and is predictive of forthcoming perception (Ergenoglu et al., 2004; Hanslmayr et al., 2007; van Dijk et al., 2008). Together, these findings point to a role of alpha oscillations in regulating the incoming information flow at early (retinotopic) processing stages.
The current study was designed to test the nature of the link between the alpha rhythm and perception (causal vs correlative). To this end, we applied rhythmic TMS over occipito-parietal areas at alpha frequency before stimulus onset, thereby mimicking the natural brain rhythms of attention deployment, and tested for frequency-specific perceptual consequences. Because prestimulus alpha power is inversely related to perception (Ergenoglu et al., 2004; Hanslmayr et al., 2007; van Dijk et al., 2008), in line with its suggested inhibitory role in brain operations (Klimesch et al., 2007), we expected mainly inhibitory effects of alpha stimulation. These should lead to retinotopically organized suppression of target visibility in the visual field opposite to the alpha-stimulated hemisphere, possibly in association with a secondary transcallosal disinhibitory (push–pull) effect in the visual field ipsilateral to TMS (Hilgetag et al., 2001).
Materials and Methods
Participants
Fourteen healthy volunteers with normal or corrected vision participated (8 women; mean age, 27.8 years; age range, 19–42 years). All participants took part in two to three sessions of 1–1.5 h each for the recording of a dataset of TMS over one hemisphere. Of the 14 volunteers, 10 participated in these initial two to three sessions only (5 left hemispheres and 5 right hemispheres), whereas 4 volunteers agreed to take part in a further two to three sessions of TMS over the opposite hemisphere. Therefore, 10 participants were tested over the left or right hemisphere (5 per hemisphere), whereas 4 participants were tested over both left and right hemispheres, resulting in a total of 2 × 9 participants tested per hemisphere. All participants gave written informed consent to participate in the study, which was approved by the ethics committee of the Faculty of Information and Mathematical Sciences, University of Glasgow.
Visual stimuli and task
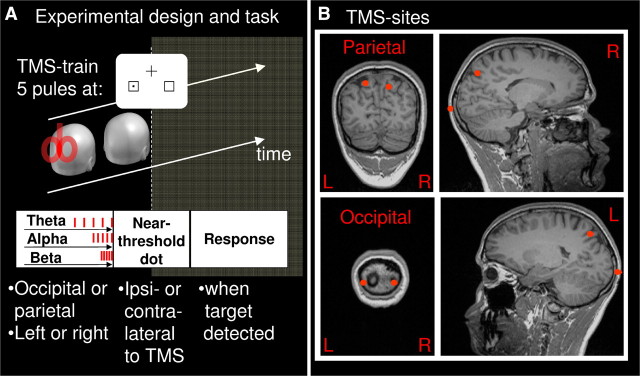
A fixation cross and two placeholders were continuously displayed on white background on a CRT monitor (85 Hz refresh rate). The fixation cross (0.7° of visual angle) was presented in the upper part of the screen, and the two placeholders (squares, 2.0°) were presented at 4.1°/3.7° eccentricity (horizontal/vertical) in the lower left visual field (LVF) and right visual field (RVF) [Fig. 1A (not drawn to proportion)]. Each target stimulus was preceded by a red fixation cross (500–700 ms duration), which served as a warning signal and temporarily replaced the black fixation cross. The target stimulus, which followed on this signal, consisted of a small black dot that was presented for 23.5 ms in the center of one of the placeholders. Target trials were randomly intermixed with catch trials (no target present, one-third of trials, i.e., one catch trial for each left or right target trial). The interstimulus interval (ISI) was 3 s.
Figure 1.
A, Experimental design and task. TMS was applied in trains of five pulses at alpha, theta, or beta frequency (10, 5, or 20 Hz; randomly intermixed) over one of four sites (occipital, parietal × left, right; block design). Real TMS blocks were intermingled with sham blocks (coil perpendicular to the scalp). To assess immediate effects of prestimulus TMS, a near-threshold stimulus was presented simultaneously with the last pulse (0 s) in one of two placeholders, either contralaterally or ipsilaterally to TMS (plus catch trials). To assess offline effects, target visibility was probed for six further time points post-TMS [3, 6, 9, 12, 15, and 18 s (not shown)]. Participants had to respond with the right index finger whenever they perceived a stimulus. The intertrain interval was 22 s. Fixation cross and placeholders stayed continuously on the screen. B, Stimulation sites for one representative participant.
Participants were asked to correctly report target presence whenever they perceived a dot by single button press with their right index (detection task). Response speed was not stressed at any time to favor the perceptual aspects of the task. Moreover, not stressing response speed served to minimize false alarms resulting from response anticipation (a likely confound of detection rate). Finally, targets were presented at threshold sizes (after individual titration before the experiment, same procedure as used in Hilgetag et al., 2001; Thut et al., 2006), avoiding floor or ceiling of perception and therefore ensuring that both TMS-induced suppression and enhancement of target visibility can be uncovered.
Experimental procedure
Target titration.
Targets were randomly presented in nine different sizes, ranging from 1 to 25 square-pixels (1 × 1, 2 × 1, 2 × 2, 3 × 2, 3 × 3, 4 × 3, 4 × 4, 5 × 4, 5 × 5, 1 pixel = 0.015°). The session consisted of two blocks of 270 trials each, lasting ∼10 min, for a total of 20 trials per target size and visual field. At the end of the session, a psychometric curve was drawn for each participant, and the target closest to threshold (one per visual field) was selected for the experiment.
Experiment.
Rhythmic TMS was then applied while participants were performing the lateralized visual detection task on threshold targets [mean target sizes were as follows: 3.7 × 3.2 pixels (0.056° × 0.048°) for RVF stimuli; 3.6 × 3.4 pixels (0.055° × 0.051°) for LVF stimuli].
Rhythmic TMS was administered in short trains of five pulses at one of three frequencies (5, 10, and 20 Hz, in random order) immediately before each seventh trial (target onset coincided with the fifth TMS pulse). This made it possible to test effects of rhythmic TMS on target visibility at seven time points after each TMS train (0, 3, 6, 9, 12, 15, and 18 s; ISI = 3 s) and led to an intertrain interval of 22 s. The experiment comprised nine blocks per hemisphere and site of stimulation (occipital left and/or right, parietal left and/or right, sham left and/or right) (∼4 min per block, counterbalanced within sessions). This resulted in a maximum of 2 × 810 real TMS pulses for a final nine trials per condition (ipsilateral, contralateral, and catch trials).
Occipital, parietal, and sham TMS
TMS was applied with a Magstim Rapid2 Transcranial Magnetic Stimulator (via a 70 mm figure-of-eight coil; Magstim) over the left or right occipital cortex (at the sites of maximum phosphene induction) or over the left or right parietal cortex [Talairach coordinates taken from a study on attention orienting by Corbetta et al., 1998, and neuronavigated via Brainsight (Rogue Research)] (see also Fig. 1B). The coordinates were as follows: average left occipital: −17.3 ± 2.5; −85.4 ± 1.5; −25.0 ±4.9; average right occipital: 28.2 ± 3.9; −83.7 ±2.0; −25.3 ±7.6; left parietal: −19, −63, 60; right parietal: 17, −65, 54. The parietal site was located in the intraparietal sulcus (Corbetta et al., 1998). During real TMS, the coil surface was in a tangential position to the scalp with its handle oriented parallel to the sagittal plane. Most of the current was therefore induced in the anterior–posterior (y-axis) and superior–inferior dimension (z-axis), with only minor contribution to the left–right dimension (x-axis).
Sham stimulation was performed to account for nonspecific TMS effects induced by the auditory clicks. Specifically, the TMS coil was positioned in between the occipital and parietal spots and oriented perpendicular to the scalp. Sham recordings were obtained for both hemispheres and all three frequencies of stimulation. Auditory clicks differed in our design in (1) lateralization (left, right) and (2) click-density over time (5, 10, 20/s), which could have biased visual target detection as a result of (1) lateralized cross-modal attention capture or (2) differential alerting potential (high click density in 20 Hz trains should have maximum alerting potential). Hence, the need to control for nonspecific influences on the dependent measure of interest via a sham condition.
TMS intensity
TMS intensity was chosen at phosphene threshold determined in blindfolded participants (or motor threshold when no consistent phosphenes could be obtained, n = 2 participants). Under blindfolding, occipital stimulation at experimental TMS intensity therefore evoked weak phosphenes in 50% of trials. In contrast, no participant reported seeing phosphenes while facing the white screen during the task on stimulation of the occipital target site. This is in accord with the phosphene threshold being increased with a concurrent visual detection task (Kammer et al., 2005). In addition, no participant experienced phosphenes with parietal TMS, neither when blindfolded nor when facing the white screen and performing the task. Hence, TMS was subthreshold (occipital TMS) or ineffective (parietal TMS) in evoking phosphenes at the experimental intensity, avoiding phosphenes that could distract from target detection. The average stimulation intensity (±SEM) was 61.2 ± 1.4% of maximum stimulator output.
Data analyses
The dependent measure of interest was hit rate as a result of the instructions emphasizing perceptual aspects of the task. Because no speeded response was required, reaction times were expected to be uninformative, which was confirmed by ANOVAs (same factorial designs as for hit rate) not revealing any significant effect.
Because hit rate was affected by nonspecific TMS effects (see below), we first sham normalized the data before examining TMS effects across conditions. Sham normalization consisted of subtracting the mean hit rate of each subject and sham TMS condition from the mean hit rate for that subject under the corresponding real condition (real TMS − sham TMS). To evaluate the immediate TMS effects on target visibility (0 s post-train delay), data were subjected to a mixed-design ANOVA with the between-subject factor hemisphere (left vs right) and the within-subject factors TMS frequency (5 Hz vs 10 Hz vs 20 Hz), TMS site (occipital vs parietal), and target position (contralateral vs ipsilateral to TMS). To examine target visibility over the entire intertrain interval (22 s), the within-subject factor of post-train delay (0, 3, 6, 9, 12, 15 and 18 s) was added. When appropriate, post hoc comparisons involved simple tests.
Results
We first checked for nonspecific effects attributable to the TMS-associated sensory events (the lateralized click trains) by examining performance in the sham control. We found a linear increase of detection rates at train offset (0 s delay) with increasing frequency of click trains [5 vs 10 vs 20 Hz (sham): 40.2 ± 4.5% vs 51.4 ± 3.9% vs 56.5 ± 3.1%; main effect: F(2,32) = 7.57, p = 0.002; linear polynomial contrast: F = 11.3, p = 0.0039], most likely the result of an enhanced alerting potential as click density increases from 5 to 20 Hz. Conversely, we found no effect on detection when targets were presented ipsilateral versus contralateral to the sham trains (49.1 ± 4.0% vs 49.7 ± 3.7% at 0 s delay; no main effect of target position: F(1,16) = 0.02, p = 0.90), speaking against cross-modal deployment of visual spatial attention by the lateralized click trains. To reveal the TMS-specific effects, we removed the nonspecific TMS confounds via sham normalization (detection rates in real TMS − sham control). In the following, we focus on this normalized measure (see the integral dataset, available at www.jneurosci.org as supplemental material).
TMS-specific effects on sham-normalized hit rate
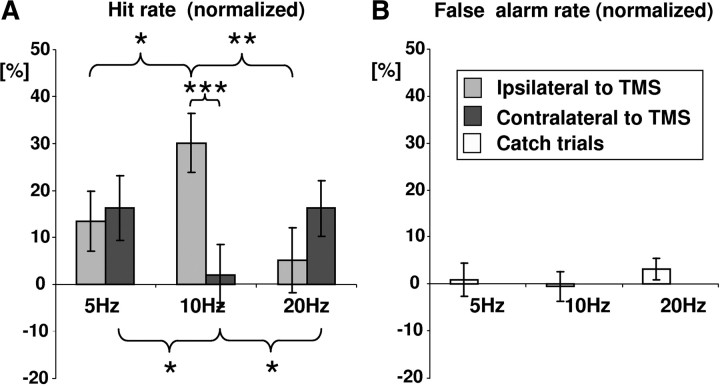
At the train offsets (0 s delay), we found a significant two-way interaction between TMS frequency (5 Hz vs 10 Hz vs 20 Hz) and target position (contralateral vs ipsilateral to TMS) (F(2,32) = 9.7; p = 0.0005), in line with rhythmic TMS affecting perception in a frequency- and space-specific manner. Target detection in the contralateral versus ipsilateral visual fields to TMS was differentially affected by 10 Hz TMS (simple effect of target position: F(1,16) = 13.5, p = 0.002) (Fig. 2A, middle bars) but not by 5 Hz (F(1,16) = 0.3, p = 0.63) or 20 Hz TMS (F(1,16) = 1.7, p = 0.21) (Fig. 2A, lateral bars). In the visual field contralateral to rhythmic stimulation, 10 Hz TMS significantly impaired target detection, relative to the 5 Hz (simple effect of TMS frequency: F(1,16) = 5.7, p = 0.029) and 20 Hz TMS (F(1,16) = 4.7, p = 0.045) (Fig. 2A, dark gray bars). Conversely, in the visual field ipsilateral to rhythmic stimulation, 10 Hz TMS significantly enhanced target detection, relative to the 5 Hz (F(1,16) = 6.0, p = 0.025) and 20 Hz conditions (F(1,16) = 12.4, p = 0.003) (Fig. 2A, light gray bars). There were no other significant main effects or interactions.
Figure 2.
Frequency- and space-specific biasing of visual detection through rhythmic TMS over occipito-parietal sites for performance at TMS train offset (immediate effects). A, Effects of rhythmic TMS (5, 10, and 20 Hz) on hit rate (sham normalized = real − sham) for target detection ipsilateral (light gray bars) and contralateral to TMS (dark gray bars). Data are collapsed over all four TMS positions (occipital, parietal × left, right), as frequency-specific effects did not interact with either TMS site or TMS side. B, False alarm rate to catch trials, collapsed over all stimulation sites. Asterisks point to significant differences: *p < 0.05; **p < 0.01; ***p < 0.001.
Notably, the two-way interaction between TMS frequency and target position depended neither on hemisphere (no three-way interaction with hemisphere: F(2,32) = 1.2, p = 0.32) nor TMS-Site (no three-way interaction with site: F(2,32) = 0.9, p = 0.42). This suggests that the 10 Hz-specific pattern of contralateral suppression with ipsilateral enhancement (Fig. 2A) generalizes across hemispheres (left, right) and sites (occipital, parietal) (supplemental Fig. 2, available at www.jneurosci.org as supplemental material).
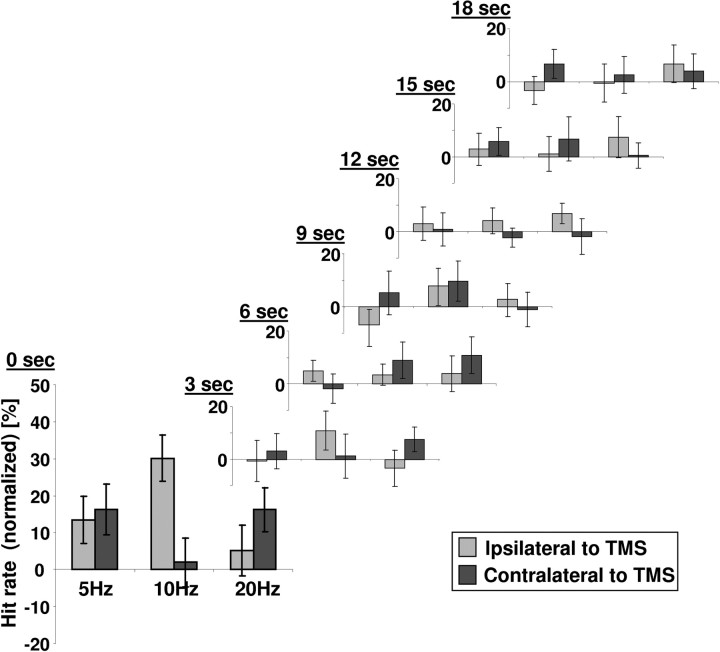
Finally, the 10 Hz-specific effects at 0 s delay (immediate effect) disappeared over the subsequent six time points of testing (3–18 s) within the intertrain interval (Fig. 3), as indicated by a significant three-way interaction of TMS frequency and target position with post-train delays (F(12,192) = 1.9, p = 0.035), and insignificant two-way interactions between TMS frequency and target position for all delays ≥3 s (F < 1.6, p > 0.22).
Figure 3.
Effect evolution over the intertrain interval (22 s). The frequency- and space-specific biasing of visual detection through rhythmic TMS is temporally restricted. It is present at train offset (0 s) but disappeared ≥3 s post-train.
Sham-normalized false alarm rates in catch trials
Finally, we calculated the false alarm rate for catch trials to establish that rhythmic TMS effects on hit rate reflect a perceptual bias. False alarm rates were small (Fig. 2B, illustrated for 0 s delay) and did not show any significant main effects or interactions across conditions.
Discussion
Our main finding is that stimulation of the cortical sites of alpha generation through rhythmic short-train TMS biases the visibility of lateralized stimuli in a spatially specific and frequency-specific manner. By the end of the five-pulse trains, occipital and parietal TMS at alpha frequency (10 Hz) impaired target visibility in the visual field contralateral to the stimulated hemisphere and enhanced it ipsilaterally relative to control stimulations in the theta (5 Hz) and beta (20 Hz) bands. Because alpha TMS suppressed contralateral perception, the alpha-specific effect seems primarily inhibitory in nature, in line with the alpha-inhibition hypothesis (Klimesch et al., 2007). Because perception of ipsilateral targets was also enhanced, however, local inhibitory effects by alpha TMS seem to release opposite homologous areas, contributing to a push–pull effect previously reported in the attention literature (Hilgetag et al., 2001). The finding that the spatially specific effects of prestimulus alpha TMS were symmetrically evoked not only over occipital and parietal cortex but also over the left and right hemisphere suggests that these effects originate in retinotopically organized areas, present in both the occipital and parietal lobes (for review, see Silver and Kastner, 2009). On the one hand, rhythmic alpha TMS over occipital and parietal areas might have selectively affected stimulus-related neurons of the respective retinotopic occipital and parietal maps. On the other hand, it is plausible that although the TMS coil was localized to either occipital or parietal cortex, there was a common effect site for both conditions (e.g., the occipital cortex) because of the two sites being naturally connected in a retinotopic, point-by-point manner to allow for transmission of top-down attention signals from parietal to early visual cortex (Silver and Kastner, 2009). Overall, our results provide evidence for a causal, mechanistic role of the alpha oscillation. The data indicate that alpha regulates the flow of incoming information by playing a role in anticipatory tuning of retinotopically organized areas.
The use of rhythmic transcranial stimulation at frequencies mimicking brain rhythms for the study of brain oscillations has recently gained renewed interest (Pogosyan et al., 2009; Sauseng et al., 2009; Thut and Miniussi, 2009). Regarding the alpha rhythm, parietal TMS at alpha frequency has been found to enhance performance in visual mental rotation of three-dimensional cubes (Klimesch et al., 2003) or visual working memory for unilateral targets when competing with contralateral distracters (Sauseng et al., 2009). In both cases, the focus was thus on internal visual representation, rather than perception of external stimuli. In both cases also, parietal alpha TMS can be interpreted as benefitting these internal representations by suppressing input from distracting visual information (Sauseng et al., 2009). Our data indicate that alpha oscillations play a key role in shaping the perceptual bias across the two hemi-fields beyond representational space. Interestingly, alpha TMS not only suppressed target visibility contralaterally but also enhanced it ipsilaterally, even in the absence of distracters (we used unilateral target displays). The beneficial effect of alpha stimulation on ipsilateral target visibility therefore cannot be explained by simply freeing capacity as a consequence of contralateral distracter suppression (as in Sauseng et al., 2009), but is likely to result from transcallosal network effects. This implicates posterior alpha oscillators in a push–pull mechanism shaping perceptual bias across the hemi-fields, in line with previous findings that a posterior alpha lateralization index, taking into account activity over both hemispheres, best indexes momentary attentional bias (Thut et al., 2006).
How could the effects of alpha TMS come about? Although speculative, the most likely explanation at this point is entrainment of the natural alpha rhythm (see also Sauseng et al., 2009). It has been shown that a single occipital TMS pulse delivered at a low, variable jitter rate triggers alpha oscillations in occipital regions (Rosanova et al., 2009). This can be explained as a result of resetting the natural rhythm of the stimulated corticothalamic circuit, much like the account for beta waves triggered by TMS over motor cortex (Van Der Werf and Paus, 2006). It is thus likely that alpha oscillations were evoked for all stimulation frequencies in the current study, at least by the initial pulse in the sequence. In contrast to Rosanova et al. (2009), we applied the subsequent TMS pulses at fixed rates. When occurring in phase with the TMS-initiated alpha oscillation (such as when applied at 10 Hz), these subsequent pulses would be expected to reset more and more neurons to oscillate in synchrony with the evolving alpha wave, resulting in progressive entrainment. Stimulation at 5 Hz might not evoke additive effects because TMS-induced alpha waves seem to last less than two cycles (Rosanova et al., 2009, their Fig. 1). Stimulation at 20 Hz might interfere with additive effects because each second pulse occurs in anti-phase with the alpha cycle (20 Hz). Note that this account would link TMS-induced alpha synchronization (power) with perception, the latter probed here for only one time point immediately post-TMS. It is conceivable that target visibility might further vary with the time point of testing within an alpha cycle, because visual perception not only fluctuates with alpha power but also with alpha phase (Busch et al., 2009; Mathewson et al., 2009). It is also conceivable that the strength of the effects reported here depends on the individual alpha frequency.
The reported result of contralateral suppression and/or ipsilateral release of visual detection is also in line with the hemispheric rivalry model (Kinsbourne, 1977), positing that spatial attention is governed by two homologous attention processors, located opposite each other in the left and right hemispheres. Because each processor controls attention toward the opposite hemi-field and because reciprocally interacting through inhibitory cross talk, the model posits that damage to one processor will suppress perception contralaterally and release it ipsilaterally, the latter effect being the result of disinhibition of the unaffected processor from the disrupted, contralateral counterpart. Corresponding results have been obtained previously with parietal TMS using so-called disruptive (“virtual lesion”) TMS protocols (Seyal et al., 1995; Hilgetag et al., 2001; Blankenburg et al., 2008), some of which include 10 Hz TMS over a short interval (online rTMS protocol). We show for the first time that the potential of behavioral suppression (and associated “paradoxical” enhancement) of online rTMS depends on the frequency applied (maximum for alpha TMS) in line with the inhibitory alpha hypothesis, at least for stimulation over alpha oscillating sites at short trains.
In summary, we find lateralized rhythmic TMS of the posterior brain at alpha frequency to bias perception away from contralateral and toward ipsilateral space. We conclude that visual receptive/attentive states in humans cannot only be inferred from prestimulus oscillatory EEG signatures (as shown previously for the posterior alpha rhythm by, for example, Thut et al., 2006; Hanslmayr et al., 2007; van Dijk et al., 2008), but can also be induced by rhythmic transcranial stimulation at these frequencies to bias upcoming perception in a specific direction.
Footnotes
We thank Simon Garrod for helpful comments.
References
- Adrian ED, Matthews BHC. The Berger rhythm: potential changes from the occipital lobes in man. Brain. 1934;57:355–385. doi: 10.1093/brain/awp324. [DOI] [PubMed] [Google Scholar]
- Bauer M, Oostenveld R, Peeters M, Fries P. Tactile spatial attention enhances gamma-band activity in somatosensory cortex and reduces low-frequency activity in parieto-occipital areas. J Neurosci. 2006;26:490–501. doi: 10.1523/JNEUROSCI.5228-04.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger H. Über das elektrenkephalogramm des menschen. Arch Psychiatr Nervenkr. 1929;87:527–570. [Google Scholar]
- Blankenburg F, Ruff CC, Bestmann S, Bjoertomt O, Eshel N, Josephs O, Weiskopf N, Driver J. Interhemispheric effect of parietal TMS on somatosensory response confirmed directly with concurrent TMS-fMRI. J Neurosci. 2008;28:13202–13208. doi: 10.1523/JNEUROSCI.3043-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch NA, Dubois J, VanRullen R. The phase of ongoing EEG oscillations predicts visual perception. J Neurosci. 2009;29:7869–7876. doi: 10.1523/JNEUROSCI.0113-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Capotosto P, Babiloni C, Romani GL, Corbetta M. Frontoparietal cortex controls spatial attention through modulation of anticipatory alpha rhythms. J Neurosci. 2009;29:5863–5872. doi: 10.1523/JNEUROSCI.0539-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, Linenweber MR, Petersen SE, Raichle ME, Van Essen DC, Shulman GL. A common network of functional areas for attention and eye movements. Neuron. 1998;21:761–773. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- Ergenoglu T, Demiralp T, Bayraktaroglu Z, Ergen M, Beydagi H, Uresin Y. Alpha rhythm of the EEG modulates visual detection performance in humans. Brain Res Cogn Brain Res. 2004;20:376–383. doi: 10.1016/j.cogbrainres.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Gross J, Schnitzler A, Timmermann L, Ploner M. Gamma oscillations in human primary somatosensory cortex reflect pain perception. PLoS Biol. 2007;5:e133. doi: 10.1371/journal.pbio.0050133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanslmayr S, Aslan A, Staudigl T, Klimesch W, Herrmann CS, Bäuml KH. Prestimulus oscillations predict visual perception performance between and within subjects. Neuroimage. 2007;37:1465–1473. doi: 10.1016/j.neuroimage.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Hari R, Salmelin R, Mäkelä JP, Salenius S, Helle M. Magnetoencephalographic cortical rhythms. Int J Psychophysiol. 1997;26:51–62. doi: 10.1016/s0167-8760(97)00755-1. [DOI] [PubMed] [Google Scholar]
- Hilgetag CC, Théoret H, Pascual-Leone A. Enhanced visual spatial attention ipsilateral to rTMS-induced “virtual lesions” of human parietal cortex. Nat Neurosci. 2001;4:953–957. doi: 10.1038/nn0901-953. [DOI] [PubMed] [Google Scholar]
- Kammer T, Puls K, Erb M, Grodd W. Transcranial magnetic stimulation in the visual system. II. Characterization of induced phosphenes and scotomas. Exp Brain Res. 2005;160:129–140. doi: 10.1007/s00221-004-1992-0. [DOI] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron. 1999;22:751–761. doi: 10.1016/s0896-6273(00)80734-5. [DOI] [PubMed] [Google Scholar]
- Kinsbourne M. Hemi-neglect and hemisphere rivalry. Adv Neurol. 1977;18:41–49. [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Gerloff C. Enhancing cognitive performance with repetitive transcranial magnetic stimulation at human individual alpha frequency. Eur J Neurosci. 2003;17:1129–1133. doi: 10.1046/j.1460-9568.2003.02517.x. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S. EEG alpha oscillations: the inhibition-timing hypothesis. Brain Res Rev. 2007;53:63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Linkenkaer-Hansen K, Nikouline VV, Palva JM, Ilmoniemi RJ. Long-range temporal correlations and scaling behavior in human brain oscillations. J Neurosci. 2001;21:1370–1377. doi: 10.1523/JNEUROSCI.21-04-01370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorincz ML, Kékesi KA, Juhász G, Crunelli V, Hughes SW. Temporal framing of thalamic relay-mode firing by phasic inhibition during the alpha rhythm. Neuron. 2009;63:683–696. doi: 10.1016/j.neuron.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathewson KE, Gratton G, Fabiani M, Beck DM, Ro T. To see or not to see: prestimulus alpha phase predicts visual awareness. J Neurosci. 2009;29:2725–2732. doi: 10.1523/JNEUROSCI.3963-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor DH, Fukui MM, Pinsk MA, Kastner S. Attention modulates responses in the human lateral geniculate nucleus. Nat Neurosci. 2002;5:1203–1209. doi: 10.1038/nn957. [DOI] [PubMed] [Google Scholar]
- Pogosyan A, Gaynor LD, Eusebio A, Brown P. Boosting cortical activity at beta-band frequencies slows movement in humans. Curr Biol. 2009;19:1637–1641. doi: 10.1016/j.cub.2009.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rihs TA, Michel CM, Thut G. Mechanisms of selective inhibition in visual spatial attention are indexed by alpha-band EEG synchronization. Eur J Neurosci. 2007;25:603–610. doi: 10.1111/j.1460-9568.2007.05278.x. [DOI] [PubMed] [Google Scholar]
- Romei V, Brodbeck V, Michel C, Amedi A, Pascual-Leone A, Thut G. Spontaneous fluctuations in posterior alpha-band EEG activity reflect variability in excitability of human visual areas. Cereb Cortex. 2008;18:2010–2018. doi: 10.1093/cercor/bhm229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosanova M, Casali A, Bellina V, Resta F, Mariotti M, Massimini M. Natural frequencies of human corticothalamic circuits. J Neurosci. 2009;29:7679–7685. doi: 10.1523/JNEUROSCI.0445-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Stadler W, Schabus M, Doppelmayr M, Hanslmayr S, Gruber WR, Birbaumer N. A shift of visual spatial attention is selectively associated with human EEG alpha activity. Eur J Neurosci. 2005;22:2917–2926. doi: 10.1111/j.1460-9568.2005.04482.x. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Heise KF, Gruber WR, Holz E, Karim AA, Glennon M, Gerloff C, Birbaumer N, Hummel FC. Brain oscillatory substrates of visual short-term memory capacity. Curr Biol. 2009;19:1846–1852. doi: 10.1016/j.cub.2009.08.062. [DOI] [PubMed] [Google Scholar]
- Seyal M, Ro T, Rafal R. Increased sensitivity to ipsilateral cutaneous stimuli following transcranial magnetic stimulation of the parietal lobe. Ann Neurol. 1995;38:264–267. doi: 10.1002/ana.410380221. [DOI] [PubMed] [Google Scholar]
- Silver MA, Kastner S. Topographic maps in human frontal and parietal cortex. Trends Cogn Sci. 2009;13:488–495. doi: 10.1016/j.tics.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut G, Miniussi C. New insights into rhythmic brain activity from TMS-EEG studies. Trends Cogn Sci. 2009;13:182–189. doi: 10.1016/j.tics.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Thut G, Nietzel A, Brandt SA, Pascual-Leone A. Alpha-band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. J Neurosci. 2006;26:9494–9502. doi: 10.1523/JNEUROSCI.0875-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Werf YD, Paus T. The neural response to transcranial magnetic stimulation of the human motor cortex. I. Intracortical and cortico-cortical contributions. Exp Brain Res. 2006;175:231–245. doi: 10.1007/s00221-006-0551-2. [DOI] [PubMed] [Google Scholar]
- van Dijk H, Schoffelen JM, Oostenveld R, Jensen O. Prestimulus oscillatory activity in the alpha band predicts visual discrimination ability. J Neurosci. 2008;28:1816–1823. doi: 10.1523/JNEUROSCI.1853-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womelsdorf T, Fries P, Mitra PP, Desimone R. Gamma-band synchronization in visual cortex predicts speed of change detection. Nature. 2006;439:733–736. doi: 10.1038/nature04258. [DOI] [PubMed] [Google Scholar]
- Worden MS, Foxe JJ, Wang N, Simpson GV. Anticipatory biasing of visuospatial attention indexed by retinotopically specific alpha-band electroencephalography increases over occipital cortex. J Neurosci. 2000;20(RC63) doi: 10.1523/JNEUROSCI.20-06-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]