Abstract
GABAA receptors (GABAA-Rs) play a significant role in mediating fast synaptic inhibition and it is the main inhibitory receptor in the CNS. The role of Wnt signaling in coordinating synapse structure and function in the mature CNS is poorly understood. In previous studies we found that Wnt ligands can modulate excitatory synapses through remodeling both presynaptic and postsynaptic regions. In this current study we provide evidence for the effect of Wnt-5a on postsynaptic GABAA-Rs. We observed that Wnt-5a induces surface expression and maintenance of this receptor in the neuronal membrane. The evoked IPSC recordings in rat hippocampal slice indicate that Wnt-5a can regulates postsynaptically the hippocampal inhibitory synapses. We found also that Wnt-5a: (a) induces the insertion and clustering of GABAA-Rs in the membrane; (b) increases the amplitude of GABA-currents due exclusively to postsynaptic mechanisms; (c) does not affect the endocytic process, but increases the receptor recycling. Finally, all these effects on the GABAA-Rs are mediated by the activation of calcium/calmodulin-dependent kinase II (CaMKII). Therefore, we postulate that Wnt-5a, by activation of CaMKII, induces the recycling of functional GABAA-Rs on the mature hippocampal neurons.
Introduction
GABAA-Rs mediate most of the fast inhibitory neurotransmission in the brain (Macdonald and Olsen, 1994; Jacob et al., 2008). They are heteropentameric ligand-gated ion channels composed mainly of α, β, and γ2 subunits (Rudolph and Möhler, 2006). Postsynaptic aggregation of receptors is thought to be essential for synaptic transmission (Nusser et al., 1998; Collingridge et al., 2004). However, the mechanisms of postsynaptic clustering of GABAA-Rs and of the dynamic modulation of synaptic GABAA-Rs are not well understood (Jacob et al., 2008). In particular, insulin leads to a rapid recruitment of GABAA-Rs to postsynaptic sites, increasing the amplitude of GABAA-Rs mediated miniature IPSCs (mIPSCs) (Wan et al., 1997; Wang et al., 2003). In contrast, activation of TrkB receptors by brain-derived neurotrophic factor (BDNF) results in rapid downregulation of GABAergic mIPSC in a major subset of cultured hippocampal neurons (Brünig et al., 2001).
Wnt signaling is essential for neuronal development and the maintenance of the nervous system (Logan and Nusse, 2004; Ciani and Salinas, 2005; Salinas and Zou, 2008; Inestrosa and Arenas, 2010). Wnt proteins signal through at least three different pathways: Canonical Wnt pathway characterized by an increase of cytoplasmic β-catenin levels, which enters the nucleus where it coactivates transcription of Wnt target genes (Logan and Nusse, 2004; Toledo et al., 2008). Two noncanonical Wnt signaling pathways: Wnt/PCP (Planar Cell Polarity), in which Wnt acts via monomeric GTPases and C-Jun N-terminal kinase (JNK), and Wnt/Ca2+, in this case Wnt ligands can activate CaMKII and protein kinase C (PKC) (Montcouquiol et al., 2006). There are 19 identified Wnt genes in the vertebrate genome. The expression of Wnt ligands and proteins of the Wnt signaling machinery in the mature nervous system suggests that Wnt signaling plays a key role in neuroprotection and synaptic plasticity (Ahmad-Annuar et al., 2006; Salinas and Zou, 2008; Inestrosa and Arenas, 2010). Previously, it has been demonstrated that Wnt ligands regulate neurogenesis of hippocampal stem cells in the adult rat and human (Lie et al., 2005; He and Shen, 2009) and modulate long-term potentiation (LTP) in mouse hippocampal slices (Chen et al., 2006). We demonstrated that Wnt-7a acts activating the Wnt canonical pathway (Cerpa et al., 2008) and that Wnt-5a acts activating the noncanonical pathways (Farías et al., 2009). Previously, we found that Wnt signaling induces a short-term modulation in the synaptic vesicle cycle, synaptic transmission and synaptic structure in mature hippocampal neurons (Cerpa et al., 2008; Farías et al., 2009, 2010). In addition, we described that Wnt regulates the synaptic localization, the number and size of α7-nicotinic acetylcholine receptor (α7-nAChR) clusters (Farías et al., 2007). Interestingly, α7-nAChR is a member of the ligand-gated ion-channel superfamily that also comprises GABAA-Rs.
Owing to the fact that the cellular mechanisms that neurons use to regulate GABAA-Rs cell surface stability and activity has been of considerable interest, we focused on studying the role of the Wnt pathway on the modulation of inhibitory synapses, particularly in at the clustering and surface expression of GABAA-Rs.
Materials and Methods
Materials
Culture media, 2-amino-5-phosphonovaleric acid (APV), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), tetrodotoxin (TTX), insulin, anti-GABAA (γ2 subunit receptor) and fumagillin from Sigma; anti α1 subunits of GABAA-Rs, anti-synapsin-1 and anti-Wnt-5a (Santa Cruz Biotechnology Inc), mouse anti-PSD-95 (UC Davis/NINDS/NIMH, CA); Foxy-5 was obtained from Genemed Synthesis; Lithium, BDNF, 3,3′,5,5′-tetramethylbenzidine (TMB), Immuno pure ABC Peroxidase Staining Kit (Pierce). The dynamin blocking P4 peptide was purchased from Tocris Bioscience. KN-93, TAT-TI-JIP153-163 and Gö6976 (12-(2-cyanoethyl)-6,7,12,13-tetrahydro-13-methyl-5-oxo-5H-indolo(2,3-a) pyrrolo(3,4-c)-carbazole) were obtained from Calbiochem.
Hippocampal neuronal cultures
Hippocampal neurons were obtained from 18-d-old Sprague Dawley rat embryos. Hippocampi were aseptically dissected and trypsinized for 20 min. After centrifugation for 1 min, cells were seeded in phenol-red-free DMEM plus 10% horse serum into 1% poly-l-lysine-coated plates. After 120 min, the medium was removed and Neurobasal medium was added containing 1% B27 supplement from Invitrogen. On day 3 of culture, hippocampal neurons were treated with 2 μm 1-β-d-arabinofuranosylcytosine (AraC) for 24 h. Fifteen- to 18-d-old neuron cultures were used for various experiments; the average number of neurons in each experiment was ∼95% of the cells present in the cultures (Alvarez et al., 2004; Farías et al., 2007, 2009).
Conditioned medium containing Wnt ligand
To generate secreted Wnt ligand, HEK-293 cells were stably transfected by Lipofectamine 2000 (Invitrogen) according to manufacturer's instructions with constant and equal amounts of empty vector pcDNA or pcDNA containing sequences encoding Wnt-ligand or soluble Frizzled receptor protein (sFRP-1) coupled to the sequence encoding a hemagglutinin (HA) tag. Wnt-7a was a gift from Dr. Patricia Salinas (University College London, UK), Wnt-5a was a gift from Randall T. Moon (University of Washington, Seattle, WA), Wnt-3a a gift from Dr. Roel Nusse (Stanford University, Palo Alto, CA) and sFRP was a gift from Dr. Jeremy Nathans (John Hopkins University School of Medicine, Baltimore, MD). For Wnt-conditioned, control media or media containing sFRP transfected HEK-293 cells were grown to 85% confluence and maintained in Neurobasal medium without supplements by 60 h. Wnt secretion was verified by Western blot using a HA-specific antibody (Millipore). For the electrophysiological studies the media containing Wnt ligands was dialyzed against ACSF for 16 h at 4°C.
Quantification of cell surface GABAA-Rs by a colorimetric ELISA assay (Wang et al., 2003)
Briefly, hippocampal neurons were washed with PBS and starved with Neurobasal without supplement. At the end of each experiment, the cells were fixed with 4% paraformaldehyde in PBS for 3 min at room temperature. The fixation was then neutralized by incubation with 1% glycine at 4°C for 10 min. To determine the total amount of GABAA-Rs proteins, the cells were permeabilized by incubation with 0.2% Triton X-100. The wells were blocked with 3% BSA at 4°C for at least 3 h. A primary antibody for GABAA-Rs α1 (Santa Cruz Biotechnology Inc.) or γ2 (Sigma) subunits was then added to the cultures at a dilution of 1:250 and maintained at 4°C for 12 h. The cells were extensively washed and the antibody was detected using the Ultra-Sensitive ABC Peroxidase Staining Kits (Pierce). The colorimetric reaction using TMB substrate was measured at 450 nm or stopped by addition of 0.25 ml of 3 N HCl for 10 min at room temperature.
Quantification of cell surface GABAA-Rs by biotinylation assay (Cuitino et al., 2005)
Briefly, hippocampal neurons were biotinylated with sulfo-NHS-LC-biotin (Pierce) to a final concentration of 0.5 mg/ml for 45 min at 4°C. After biotinylation steps, the free biotin was quenched by incubation with 50 mm NH4Cl for 10 min. Cells were lysed in ice-cold SA buffer (150 mm NaCl, 20 mm Tris pH 8.0, 5 mm EDTA, 1% Triton X-100, 0.2% BSA and protease inhibitors). Nuclear and cellular debris was removed by centrifugation at 14,000 × g for 5 min at 4°C and the biotinylated cell-surface proteins were then adsorbed to streptavidin agarose beads for 16 h at 4°C. Beads were washed and the bound proteins were analyzed by SDS-PAGE followed by immunoblotting. The values for biotinylated cargo proteins were normalized to total cargo proteins expressed in the cells.
Image analysis and quantification
Hippocampal neurons were cultivated at a density 30,000 cells/coverslip. For cell surface GABAA-Rs staining, cells were fixed with 4% paraformaldehyde/4% sucrose for 20 min at room temperature. Fixed cells were washed and incubated with an antibody to α1 or γ2 GABAA-Rs subunit (Santa Cruz Biotechnology Inc. and Sigma, respectively). For the staining of other proteins, cells were fixed as described above and permeabilized by incubation in PBS-0.2% Triton X-100, and stained with the following antibodies: synapsin I (Santa Cruz Biotechnology Inc.) and PSD-95 (UC Davis/NINDS/NIMH). Finally, cells were incubated with Alexa 543, Alexa 488, and/or Alexa 633 (Pierce) for 30 min at 37°C. To determine which Wnt signaling was involved in the increase of GABAA-Rs on the cell surface, the hippocampal neurons were preincubated with fumagillin (50 nm) for 16 h. Then, KN-93 (10 μm), Gö6976 (200 nm) and TAT-TI-JIP (1 μm) were coincubated with Foxy-5 for different times. To analyze receptor clustering, we quantified the number of clusters per neurite length with ImageJ program [National Institutes of Health (NIH), Bethesda, MD]. Neurons on coverslips were imaged using a confocal microscope LSM 5 Pascal with a 63×/1.4 numerical aperture oil-immersion objective. Images used for quantification were taken with identical microscope settings and analyzed using ImageJ software (NIH). GABAA-Rs images from 10 microscope fields for each condition, of three independent experiments, were registered. Each field containing processes for 1 neuron were studied, in which 3 neurites per neuron were selected using the phalloidin staining to label neuronal processes. To quantify GABAA-R clusters, images of individual neurites were isolated, background for neurite free fields were subtracted and adjusted to the threshold. GABAA-R cluster number and size were obtained with the Particle Analysis tool using a size particle limit of 0.05–1 μm2. Cluster number was normalized against neurite length to obtain cluster density.
Slice preparation and electrophysiological analysis
Procedures for animal care, surgery, and slice preparation were in accordance with the guidelines for the care and use of laboratory animals adopted by the Society for Neuroscience. The procedures will be described briefly because they have been extensively detailed previously (Fuenzalida et al., 2007).
Slice preparation.
Young Wistar rats (15–20 d of age) were decapitated, and the brain was removed and submerged in cold (∼4°C) artificial CSF (ACSF; in mm: 124.00 NaCl, 2.69 KCl, 1.25 KH2PO4, 2 Mg2SO4, 26 NaHCO3, 2.50 CaCl2 and 10.00 glucose). The pH was stabilized at 7.4 by bubbling the ACSF with carbogen (95% O2, 5% CO2). Transverse hippocampal slices (300–350 μm thick) were cut with a Vibroslice microtome (VSL, WPI, Sarasota, FL) and incubated in ACSF for >1 h at room temperature and incubated in the ACSF (∼1 h, at room temperature, 20–22°C). Slices were transferred to a 2 ml chamber fixed to binocular stereo microscope (MSZ-10, Nikon). Slices were superfused with carbogen-bubbled ACSF (2 ml/min) and maintained at room temperature. All recordings were made under CNQX (20 μm) and APV (50 μm) (Sigma) were added to ACSF perfusion media to suppress excitatory α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPA) and N-methyl d-aspartate receptor (NMDAR)-transmission.
Stimulation, recording, and analysis.
Single cell recordings were made in the whole-cell configuration with fire-polished pipettes (3–5 MΩ) filled with intracellular solution (see below), connected to an EPC-7 patch-clamp amplifier (Heka Instruments), filtered at 3.0 kHz, sampled at 4.0 kHz using an A/D converter (ITC-16, InstruTech), and stored with Pulse FIT software (Heka Instruments). Single-electrode voltage-clamp recordings were obtained from pyramidal neurons of CA1. In the voltage-clamp configuration, the series resistance was compensated to ∼70%, and neurons were accepted only when the seal resistance was ∼1GΩ and the series resistance (7–14 MΩ) did not change ∼10% during the experiment. The intracellular solution contained (in mm): 97.5 K-gluconate, 32.5 KCl, 10.0 HEPES, 1 MgCl2, 5 EGTA, and 4 sodium salt (Na-ATP), pH 7.2. Experiments started after a 5–10 min stabilization period following entry into the intracellular compartment with patch electrodes. The voltage-clamp recordings were rejected when the access resistance (7–15 MΩ) increased 20% during the experiment. The spontaneous or mini IPSPs, sIPSC and mIPSC respectively, were analyzed offline, using an analysis software (Minianalysis, Synaptosoft), which allowed visual detection of events. Considering the intra- and extracellular chloride concentration, the reversal potential of the IPSC was ∼60 mV. Then, to dissecting IPSC from EPSC, all the cells were voltage-clamped at 0 mV (holding potential). The dynamin blocking Peptide P4 (Tocris Bioscience) was dissolved at 50 μm in the internal solution described above, as described (Kittler et al., 2000). To determine whether Wnt-5a induces the surface expression of receptor by JNK, PKC, or CaMKII, we dissolved the inhibitors TAT-TI-JIP (1 μm), Gö6976 (200 nm) and KN-93 (10 μm) in the internal solution. The evoked IPSC was elicited using concentric electrodes (platinum/iridium, FHC Inc.), placed at stratum radiatum close to the pyramidal layer (∼10–20 μm). The GABAergic neurons were activated by bipolar cathodic stimulation through an isolation unit (Isoflex, A.M.P.I.). Voltage-clamp data were high-pass filtered at 3.0 kHz and sampled at rates between 6.0 and 10.0 kHz, through a Digidata 1322A (Molecular Devices).
Recycling assay of GABAA-Rs on hippocampal neurons
Hippocampal neurons DIV 18 were biotinylated with 0.5 mg/ml Sulfo-NHS-SS-Biotin (Pierce) in Neurobasal medium at 37°C for 60 min. Dishes were placed on ice and the remaining biotin on the cell surface was stripped with 2–5 ml of ice-cold cleaving buffer (in mm: 50 glutathione, 75 NaCl, 10% bovine serum albumin, 0.1 CaCl2, 1 MgCl2, and 0.075 N NaOH) at 4°C for 30 min and quenched with 5 mg/ml iodoacetamide at 4°C for 15 min. Afterward, neurons were washed and returned to the 37°C incubator for 15 min, with and without the Wnt-5a ligand, to allow recycling of endocytosed cargo proteins back to cell surface. Then newly appeared cell surface biotin was again stripped with cleaving buffer at 4°C for 30 min and quenched again with iodoacetamide. Finally neurons were washed and lysed in ice-cold SA buffer at 4°C. Nuclear and cellular debris was removed by centrifugation at 14,000 × g for 5 min at 4°C and the supernatants were precipitated with streptavidin agarose beads (Pierce) for 16 h at 4°C. The beads were washed and the samples were prepared for immunoblot analysis. The values for biotinylated cargo proteins protected from glutathione treatment were normalized to total cargo proteins expressed in the cells (Morimoto et al., 2005). In an alternative approach, the recycling of the receptor was evaluated by immunofluorescence. For these studies, neurons were incubated for 30 min at 37°C with an antibody against γ2 subunits (Sigma). Then, the cells were acid stripped for 5 min with 0.1 m glycine-0.1 m NaCl, pH 3.0 at 4°C, before the treatment with Wnt-5a. The neurons were treated with Wn-5a by the times indicated at 37°C, allowing that the prelabeled subunit recycled to the cell surface. The neurons were fixed with 4% paraformaldehyde/4% sucrose for 10 min at 4°C and 10 min at room temperature. The surface receptors were detected in the absence of detergent using Alexa-conjugated secondary antibody, to detect receptors that reappear in the plasma membrane from the intracellular pool (Vargas et al., 2008). Then, the neurons were permeabilized by incubation in PBS-0.2% Triton X-100 for staining gephyrin (BD Transduction Laboratories). The image analysis for determinate the number and size of the GABAA-R clusters was accomplished as described above.
Statistical analysis
Statistical analysis was performed using statistical software Prism 5 (GraphPad Software Inc.). Values are expressed as mean ± SEM. Statistical significance of differences was assessed with the nonpaired Student's t test or ANOVA, and non-normally distributed data were analyzed using the Mann–Whitney or Kruskal–Wallis test (p < 0.05 was considered significant).
Results
Wnt-5a ligand induces neuronal surface expression of GABAA-Rs
Previous studies suggest that an important factor in regulating the efficacy of GABAergic inhibition is the number and stability of postsynaptic GABAA-Rs. Thus, a direct relationship between the number of synaptic GABAA-Rs and the strength of the inhibitory synapses has been demonstrated (Otis et al., 1994; Collingridge et al., 2004). On account of that in the brain are expressed >19 subunits of the GABAA-Rs, combinatorial subunit composition leads to different subtypes of receptors with different pharmacological properties and subcellular localization (Rudolph and Möhler, 2006; Jacob et al., 2008). We analyzed the effect of Wnt-5a in receptors containing the α1 and γ2 subunits. Importantly, γ2 is critical for the expression, trafficking, clustering and synaptic localization of the major heteropentameric receptor expressed in brain (Essrich et al., 1998). We used a quantitative colorimetric assay to calculate the surface expression of GABAA-Rs (Wang et al., 2003). We evaluated the effect of Wnt-5a, a ligand that leads to the activation of the Wnt/PCP and Wnt/Ca2+ pathways in hippocampal neurons (Farías et al., 2009). We observed that Wnt-5a induced a rapid increase of ∼25% of surface expression of GABAA-Rs, reaching ∼40% after 15 min of treatment. This effect was maintained for up to 60 min, measured by the detection of GABAA-Rs α1 and γ2 subunits (α1-GABAA-Rs and γ2-GABAA-Rs) (Fig. 1A,B). To test the specificity of the Wnt-5a effect, the Wnt ligand was incubated with a soluble frizzled receptor protein antagonist (sFRP). sFPR binds to the Wnt ligand, thereby preventing the interaction with cellular membrane-bound Frizzled receptor (Rattner et al., 1997). The coincubation of the Wnt-5a with sFRP prevented the increase of the surface expression of the GABAA-Rs triggered by the ligand (Fig. 1A,B). To further establish that the observed effects were due to Wnt-5a and not to other proteins present in the conditioned medium, we used a formylated hexapeptide (Foxy-5) derived from the sequence of the Wnt-5a ligand (Genemed Synthesis). This peptide mimics the full molecule in cultures of hippocampal neuron (Farías et al., 2009; our unpublished data). Treatment with the Foxy-5 (50 μm) increases the surface expression of α1-GABAA-Rs by 40% at 15 min of treatment and continues for 60 min (Fig. 1C). In addition, we observed a similar effect in γ2 containing GABAA-Rs (Fig. 1D). We confirmed that treatment with Insulin (0.5 μm) produces a significant increase in cell surface GABAA-Rs after 5 min (supplemental Fig. S1A, available at www.jneurosci.org as supplemental material), which then decrease staying above control levels for the remaining 60 min (Wang et al., 2003). Previously, our laboratory had demonstrated that Wnt-7a, a canonical Wnt ligand, was able to regulate the presynaptic region (Farías et al., 2007; Cerpa et al., 2008). Therefore, we evaluated the potential contribution of this pathway in the surface expression of GABAA-Rs. We used Wnt-7a ligand and lithium, an activator of canonical Wnt pathway that inhibits glycogen synthase kinase-3β (GSK-3β) a key enzyme of this signaling pathway (Inestrosa and Arenas, 2010). However, neither of both substances changed the surface expression of GABAA-Rs (supplemental Fig. S1B,C, available at www.jneurosci.org as supplemental material). This result suggests that Wnt-5a, but not Wnt-7a, specifically regulates the surface expression of the GABAA-Rs in neurons.
Figure 1.
A–D, The insertion of the α1- and γ2-GABAA receptor in the neuronal surface is stimulated by Wnt-5a in a time-dependent manner. Hippocampal neurons 15 DIV were incubated at 37°C with Wnt-5a ligand (A, B) or Wnt-5a/sFRP (C, D) Foxy-5 (50 μm). Neurons were labeled with an antibody against GABAA-Rs (α1 or γ2 subunits, extracellular epitopes). The ABC system was used with TMB as substrate, the final reaction was measured at 450 nm. Error bars indicate SEM (n = 4). *p < 0.05.
To corroborate these observations we performed experiments of surface biotinylation in cultured hippocampal neurons. As it was expected, treatment with Wnt-5a for 15 min increased the surface expression of γ2 containing GABAA-Rs without affecting the total amount of this GABAA-Rs subunit (Fig. 2A,B). We did not observe any effect of Wnt-5a on transferrin receptor, TfR, another constitutively expressed receptor in hippocampal neurons (Parton et al., 1992). Treatment with sFRP abolished the effect of Wnt-5a, however, Wnt-3a, a canonical Wnt ligand (Alvarez et al., 2004), did not trigger any effects (Fig. 2A,B). Taken into account these results, they indicate that Wnt-5a, but not canonical ligands, such as Wnt-3a and Wnt-7a, increase the surface expression of GABAA-Rs composed of α1 and γ2 subunits in neurons.
Figure 2.
The clustering of the γ2-GABAA receptor on the neuronal surface is specifically induced by Wnt-5a. A, Neurons (18 DIV) were treated with control media, Wnt-3a or with Wnt-5a ligand for 15 min, or coincubating with Wnt-5a/sFRP. Then neurons were labeled with Sulfo-NHS-LC-Biotin and processed at 4°C. The GABAA-Rs present in neuronal surface and total protein was analyzed using SDS-PAGE and Western blot with the antibody against the γ2 subunit. Transferrin (TfR), another receptor, was also detected as control. B, Quantification of the blots (n = 3). C, GABAA-Rs were immunostained with antibody to γ2 subunits in nonpermeabilized cells showing clustering of the receptor. Representative images of control hippocampal neurons or those treated with Wnt-5a or Foxy-5, at the time indicated, are shown. D, The number of clusters in 20 μm of neurite (Da) and the size (μm2, normalized to the control) (Db) were analyzed (n = 5). Error bars indicate SEM. *p < 0.05.
To study the clustering of GABAA-Rs at the cell surface, hippocampal pyramidal neurons were analyzed by immunofluorescence using specific antibodies to GABAA-Rs subunits, in particular directed toward the external epitopes of the γ2 or α1 subunits (Fig. 2C; data not shown). The neurons were incubated with Wnt-5a or Foxy-5 at the time indicated. The number of GABAA-R clusters in dendritic networks increase during the first 5 min after Wnt-5a or Foxy-5 treatment and remain elevated for the rest of the experiment (Fig. 2Da). Interestingly the size of the clusters did not increase (Fig. 2Db). Together, these results indicate that Wnt-5a and Foxy-5 increases the number but not the size of the GABAA-R clusters.
Wnt-5a increases the efficacy of GABA synapses at the postsynaptic level
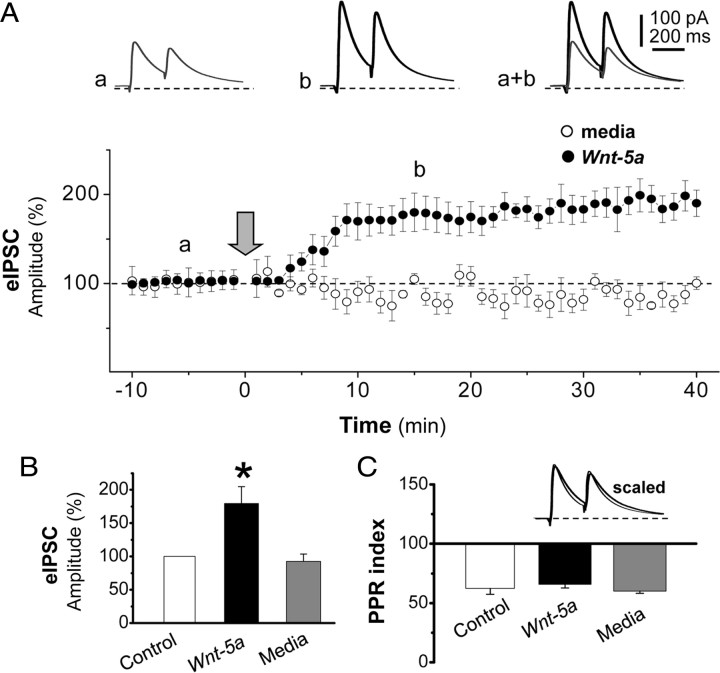
To determine whether the increase in GABAA-Rs surface expression induced by Wnt-5a leads to a concomitant increase in the number of functional receptors, we analyzed the effect of Wnt-5a over evoked GABAA receptor-mediated IPSC (eIPSC) in hippocampal CA1 pyramidal neurons. In whole-cell configuration at holding potential of 0 mV (close to the reversal potential of the glutamatergic current), the eIPSC evoked by the paired-pulse protocol were isolated in the presence of the AMPA and NMDA ionotropic glutamatergic receptor antagonists CNQX (20 μm) and APV (50 μm), respectively (Fig. 3A, top recordings). After stable baseline responses obtained for at least 10 min, the bath applications of Wnt-5a ligand induced a strong, fast and long-lasting increase of eIPSC peak amplitude (179.8 ± 24.9%; p < 0.05; n = 10) (Figs. 3A, filled circle, 3B, black bar). This effect is illustrated by a representative neuron in Figure 3A, top recordings. The superimposed recordings showed the changes in the amplitude of eIPSC before (gray trace) and during perfusion of Wnt-5a (black line). In contrast, the bath application of control media failed to induce any significant changes in the inhibitory synaptic efficacy (93.5 ± 10.6%; p > 0.05; n = 4; Fig. 3A, open circles). To establish the specificity of the Wnt-5a effect, we used an antibody against Wnt-5a. Treatment with this antibody abolished the effect of Wnt-5a ligand (data no shown).
Figure 3.
Wnt-5a ligand increases the amplitude of evoked IPSC, without affecting the paired-pulse index. Intracellular recording of a CA1 pyramidal cells after Wnt-5a ligand treatment at a holding membrane potential of 0 mV and in the presence of 50 μm APV and 20 μm CNQX. A, Averaged evoked IPSCs by paired-pulse protocols (eIPSCs), before (Aa) and after 15 min (Ab) of continued perfusion with Wnt-5a. Time course of the effect of Wnt-5a (black circles) on eIPSC amplitudes is shown. B, Quantification of the average amplitude of eIPSCs before (media) and after 15 min of treatment (control or Wnt-5a). C, Determination of the PPR index before (media) and after 15 min of treatment (control or Wnt-5a). “Media” corresponds to the values of eIPSCs when the cells have not been treated. “Control” corresponds to the values of eIPSCs when the cells were treated with vehicle. Error bars indicate SEM (n = 10). *p < 0.01.
It has been established that the induction and expression of long-lasting changes in the synaptic efficacy can be determined by pre and/or postsynaptic mechanism (Citri and Malenka, 2008). Thus, to establish whether the Wnt-5a-dependent eIPSC potentiation is due to presynaptic or postsynaptic mechanisms, we analyzed the paired-pulse index in presence of Wnt-5a. According to previous studies (Murthy et al., 1997) all of the tested eIPSCs showed paired-pulse depression (38.6 ± 4.0%; n = 10; Fig. 3C, white column) that did not change with the application of Wnt-5a (35.5 ± 3.0%; p < 0.05; n = 10; Fig. 3C, black column) or control media (40.1 ± 1.7%; p > 0.05; n = 4). The scaled superimposed recordings in Figure 3C shows that the Wnt-5a failed to induce changes in the time course and paired-pulse relationship of eIPSC. The above results indicate that the potentiation of eIPSC induced by Wnt-5a is exclusively mediated by postsynaptic mechanisms.
In addition, short- or long-term changes in the presynaptic excitability of inhibitory neurons that increase the spontaneous firing of inhibitory interneurons, may result in the increase of efficacy of GABA-mediated inhibitory current. To analyze whether Wnt-5a increases the amplitude and frequency of GABAA-mediated postsynaptic currents simultaneously, we analyzed the spontaneous and miniature postsynaptic inhibitory current (sIPSC and mIPSC, respectively). Similarly to experimental conditions to the evoked IPSC, sIPSC and mIPSC were recorded in whole-cell configuration at a holding potential of 0 mV, in the presence of CNQX (20 μm) and APV (50 μm). The GABAergic mIPSCs were recorded in the presence of TTX (0.5 μm). Wnt-5a application induces a strong increase of the amplitude without affecting the frequency of sIPSC and mIPSCs (Fig. 4). Figure 4 illustrates single recordings of representative neurons of sIPSC and mIPSCs before and during application of Wnt-5a (15 min). Wnt-5a increase the amplitude of both spontaneous and miniature synaptic current. In average, the sIPSC amplitude increased from 33.1 ± 2.1–47.1 ± 2.3 pA (n = 10) while mIPSC amplitude increased from 20.0 ± 1.5–28.0 ± 1.7 pA (n = 10). However, the frequency of sIPSC and mIPSC was similar before and during bath application of Wnt-5a. The frequency sIPSC in control conditions was 8.7 ± 0.8 Hz and 9.1 ± 0.4 Hz in the presence Wnt-5a (15 min of treatment). Similarly, in controls the mIPSC frequency was of 1.5 ± 0.1 Hz and 1.45 ± 0.1 Hz under Wnt-5a. In addition, rise time and decay time constant of mIPSC were unaffected by Wnt-5a (data not shown). Because an increase in the frequency but not in the amplitude of mIPSCs is generally thought to reflect a presynaptic increase in probability of transmitter release (Malenka and Nicoll, 1999), both the paired-pulse protocol and the frequency measurements of spontaneous events indicate that the presynaptic excitability and probability of GABA release was unaffected by exposure to Wnt-5a. Additionally, the evoked, spontaneous and mini IPSCs were GABAA-mediated because they were blocked by picrotoxin (data no shown). Together, these results suggest that the noncanonical Wnt ligand, Wnt-5a, induces a rapid increase in the response mediated by GABAA-Rs, due to an increase in the number of functional receptors present on the surface plasma-membrane of hippocampal neurons.
Figure 4.
Wnt-5a ligand increases the amplitude of spontaneous and miniature IPSCs. Events were recorded from voltage-clamped (0 mV) CA1 pyramidal cells in the presence of 50 μm APV and 20 μm CNQX. Recording of spontaneous and miniature IPSCs, sIPSC and mIPSC respectively, in cells treated with control or Wnt-5a.
Wnt-5a modulates the recycling of GABAA-Rs
Previous studies have revealed that neuronal GABAA-Rs undergo significant rates of constitutive endocytosis (Kittler et al., 2000; Collingridge et al., 2004; Jacob et al., 2008), a process that has been established to regulate synaptic inhibition (Kittler et al., 2004; Jacob et al., 2008). Internalized GABAA-Rs are then subjected to either rapid recycling or targeted for lysosomal degradation. To understand the mechanism by which Wnt-5a regulates the surface expression of GABAA-Rs, first, we investigated whether the endocytic process of receptor was modified. To test this, we performed whole-cell patch-clamp electrophysiological experiments using P4, a peptide that interferes with the function of the GTPase dynamin, thus blocking clathrin-dependent endocytosis of GABAA-Rs (Kittler et al., 2000). P4 produced an increase in the amplitude and frequency of mIPSCs and increased synaptic GABAA-Rs in cultured cortical neurons (Kittler et al., 2000). We reasoned that if the P4 peptide and Wnt-5a mediate their effects by targeting different components of the same endocytic pathway, their effects should not be additive on GABAA-Rs-mediated current. Interestingly, we observed an additive effect during cotreatment with P4 peptide and Wnt-5a (15 min of treatment). A single recording of a representative neuron of mIPSCs before and during application of Wnt-5a is illustrated (Fig. 5). Treatment with the P4 peptide induces a clear increase in the amplitude of GABAA-Rs current without affecting the frequency of mIPSCs (Fig. 5). We observed that the cotreatment with P4 peptide/Wnt-5a induces a further increase in the amplitude of mIPSCs (P4: 36.0 ± 3.2 pA, n = 6; P4 + Wnt-5a: 48.4 ± 2.8, n = 6) without affecting the frequency of mIPSCs (P4: 1.1 ± 0.3 Hz, n = 6; P4 + Wnt-5a: 1.0 ± 0.2 Hz, n = 6). These results suggest that the P4 peptide and Wnt-5a act in different pathways to increase the neural surface expression of GABAA-Rs.
Figure 5.
Wnt-5a ligand does not affect the endocytosis of GABAA-Rs. Events were recorded from voltage-clamped (0 mV) CA1 pyramidal cells in the presence of 50 μm APV, 20 μm CNQX and 0.5 μm TTX. The P4 peptide was injected by the patch pipette. Illustrates the single recordings of representative neurons of mIPSCs in the absence or in the presence of Wnt-5a (15 min).
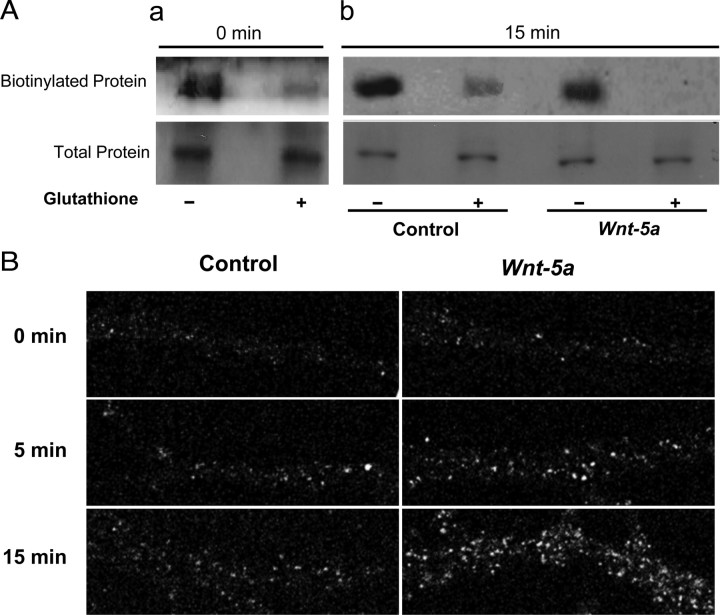
Afterward, we investigated whether the recycling process was modified by Wnt-5a treatment. To identify the recycling of this receptor, we used labeling with Sulfo-NHS-SS-Biotin (cleavable biotin), which allows the receptors to follow reinsertion from internal compartments to the cell surface (Morimoto et al., 2005). Neurons were labeled with cleavable biotin and incubated using different periods of times with Wnt-5a, allowing the recycling of endocytic cargo proteins back to the cell surface. The neurons were lysed, a part of the extract was prepared for Western blot analysis, and the other part was precipitated with streptavidin. We observed that a fraction of receptor is precipitated with streptavidin before and after the treatment with glutathione (Fig. 6Aa), indicating that there is an intracellular pool of the labeled receptor which is protected from the reduction with glutathione (0 min). Treatment with Wnt-5a induces a rapid recycling of the receptor toward the cell surface, as it is demonstrated by a minor protection from the reduction of the biotinylated receptor (Fig. 6Ab). The quantification of the receptor that returns to the surface (+glutathione) is expressed as a percentage of the total receptor labeled with cleavable biotin (−glutathione). In basal conditions, the basal recycling is ∼40%. That is, 60% of the receptor does not recycle to the surface and thus remains intracellular linked to biotin (Fig. 6Ab, line +glutathione in Biotinylated proteins). Wnt-5a treatment induces a strong increase of recycling reaching 90%. In other words, only 10% of the labeled GABAA-Rs was protected from the reduction, indicating that a greater number of biotinylated receptors are in the cell surface when the neurons were incubated with glutathione (Fig. 6Ab, line +glutathione in Biotinylated proteins). These results clearly indicate that the treatment with Wnt-5a induces an increase in the recycling of the GABAA-Rs.
Figure 6.
GABAA Receptors are recycled on the neuronal surface under the effect of Wnt-5a ligand. A, Neurons were incubated for 1 h at 37°C with Sulfo-NHS-SS-Biotin. Aa, Control cells at 0 min were reduced or not with glutathione. Ab, Neurons were stimulated for 15 min with Wnt-5a at 37°C. Surface Sulfo-NHS-SS-Biotin was reduced or not with glutathione, biotinylated and total γ2-GABAA-Rs were detected. B, Neurons were labeled with an antibody against γ2 subunits at 37°C and then incubated with Wnt-5a. The γ2 subunits were detected in nonpermeabilized neurons and correspond to the receptor recycling to the surface cell.
One alternative approach to evaluate the recycling is prelabeled neurons with γ2-specific antibody and then incubated neurons with Wnt-5a. Neurons were returned at 37°C for a different period of time in the presence of Wnt-5a, allowing recycling of marked receptor to the surface of the cell. The appearance of the receptor previously marked on the cell surface indicates the rescue of neuronal receptors from degradation. Image of representative neuron before and after the treatment with Wnt-5a is illustrated (Fig. 6B). Initially, GABAA-Rs clusters are few and small (cluster/20 μm: 2.65 ± 0.29; cluster area was considerate as 1). However, after 5 min of treatment with Wnt-5a, GABAA-Rs clusters increase significantly in number (control: 6.14 ± 0.64; Wnt-5a: 11.04 ± 0.49), but after 15 min of treatment the clusters numbers increase even more (control: 15.80 ± 0.49; Wnt-5a: 21.55 ± 0.69). In addition, the size of GABAA-Rs clusters increased significantly at 5 min (control: 1.22 ± 0.03; Wnt-5a: 1.40 ± 0.03) and at 15 min after treatment with Wnt-5a (control: 1.68 ± 0.1; Wnt-5a: 1.91 ± 0.02). The number of GABAA-R clusters increase nearly twice in control conditions and nearly four times in neurons treated with Wnt-5a (Fig. 6B). The size of the clusters (the clusters area) increase ∼68% in control conditions and 90% in cells treated with Wnt-5a. Both the size and the number remained high during 60 min of Wnt-5a treatment (data not show). The localization, the number and size of the clusters, and the total levels of synaptic protein gephyrin did not change in the presence of Wnt-5a (supplemental Fig. S2, available at www.jneurosci.org as supplemental material). These results indicate that the treatment with Wnt-5a increases the recycling of GABAA-Rs. It happens because, a significant proportion of internalized GABAA-Rs are rapidly recycled back to the plasma membrane and virtually no GABAA-R degradation could be detected within the first hour (Kittler et al., 2004).
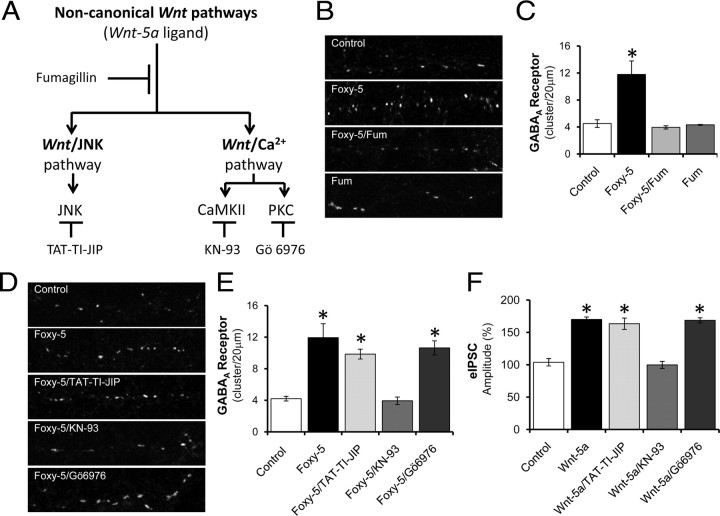
The noncanonical Wnt/Ca2+ pathway regulates the increase of the surface expression of GABAA-Rs induced by Wnt-5a
Previously, we demonstrated that Wnt-5a and Foxy-5 act as noncanonical ligands in mature hippocampal neurons, activating both Wnt signaling pathway, Wnt/JNK and Wnt/Ca2+ (Farías et al., 2009; our unpublished observations). To determine whether the noncanonical Wnt pathways are involved in the regulation of the surface expression of the GABAA-Rs induced by Wnt-5a or Foxy-5, we used fumagillin (Fum), a synthetic inhibitor of the noncanonical Wnt pathways. This inhibitor acts downstream of the Wnt receptors, but upstream of CaMKII and JNK without affecting the canonical Wnt/β-catenin pathway (Farías et al., 2009) (Fig. 7A). Previously, we described that Fum blocked the activation of JNK and CaMKII induced by Wnt-5a or Foxy-5 in mature hippocampal neurons (Farías et al., 2009; our unpublished observations). Consistent with our previous studies, the effect induced by Foxy-5 (50 μm) at 15 min on the surface clustering of GABAA-Rs is inhibited by Fum (Fig. 7B,C). To dissect whether Wnt-5a induces the surface expression of receptor by the Wnt/JNK or the Wnt/Ca2+ pathways, we used inhibitors for the three well known effectors of these pathways: TAT-TI-JIP to inhibit JNK activity of the Wnt/JNK pathway and KN-93 and Gö6976 to inhibit CaMKII and PKC activities of the Wnt/Ca2+ pathway (Fig. 7A). Hippocampal neurons were incubated with Foxy-5 for 15 min in the presence of the inhibitors mentioned and the clusters number per μm of neurite was measured. TAT-TI-JIP did not affect significantly the cluster number of GABAA-Rs induced by Foxy-5 (Fig. 7D,E). However, when we analyzed the contribution of the CaMKII and PKC, we found that only in the presence of the CaMKII inhibitor, the effect of Foxy-5 on the surface expression of the GABAA-Rs was completely blocked (Fig. 7D,E). The inhibitors alone do not affect the surface expression of GABAA-Rs (supplemental Fig. S3A,B, available at www.jneurosci.org as supplemental material). To confirm that the Wnt/Ca2+ pathway is implicated in the increase of functional GABAA-Rs, we analyzed the effect of TAT-TI-JIP, KN-93 and Gö6976 over evoked GABAA-R-mediated eIPSCs, in hippocampal CA1 pyramidal neurons. We observed that the effect of Wnt-5a (Fig. 7F) or Foxy-5 (supplemental Fig. S3C, available at www.jneurosci.org as supplemental material) is not blocked by TAT-TI-JIP or by Gö6976. Consistently with the results obtained by the immunofluorescence assay, the effect of Wnt-5a and Foxy-5 is completely inhibited by KN-93 (Fig. 7F; supplemental Fig. S3C, available at www.jneurosci.org as supplemental material). These results suggest that CaMKII is the main mediator of the effect of Wnt-5a over GABAA-Rs. The inhibitors alone do not affect the amplitude of the eIPSCs mediated by GABAA-Rs (supplemental Fig. S3D, available at www.jneurosci.org as supplemental material). Together, these results indicate that the noncanonical Wnt/Ca2+ pathway is required to modulate the recycling of functional GABAA-Rs on hippocampal neurons.
Figure 7.
Noncanonical Wnt/Ca2+ pathway signaling is involved in the surface expression of GABAA-Rs induced by Wnt-5a. A, The scheme indicates the two noncanonical Wnt pathways described for Wnt-5a, Wnt/Ca2+ and Wnt/JNK. The effectors for each pathway: CaMKII, PKC and JNK are shown as well as the drugs used to inhibit these kinases: TAT-TI-JIP, KN-93 and Gö6976. Fumagillin (Fum) is a general inhibitor of the noncanonical Wnt pathways. B, Representative neurite images of hippocampal neurons exposed to Foxy-5 for 15 min in the presence or absence of Fum. C, Quantification of γ2-GABAA-R clusters number/20 μm neurite shown in B). D, Representative neurite images of hippocampal neurons incubated with Foxy-5 or coincubated with Foxy-5 and inhibitors indicated, for 15 min. E, Quantification of γ2-GABAA-R clusters number/20 μm neurite of the treatments indicated in D). F, Quantification of average amplitude of eIPSCs in presence of Wnt-5a or coincubated with the inhibitors indicated for 15 min of treatment. The inhibitors were injected by the patch pipette. Error bars indicate SEM (n = 10). *p < 0.05.
Discussion
GABAA-Rs are critical mediators of synaptic inhibition in the brain (Macdonald and Olsen, 1994; Jacob et al., 2008). At synapses, GABAA-Rs constitutively undergo significant rates of constitutive endocytosis, via clathrin-coated pits in a dynamin-dependent process; the internalized GABAA-Rs are then subjected to either rapid recycling or targeted for lysosomal degradation (Kittler et al., 2004; Jacob et al., 2008). Therefore, changes in the rates of GABAA-R endocytosis and/or endocytic sorting represent potentially powerful mechanisms to regulate GABAA-R cell surface number and inhibitory synaptic transmission (Collingridge et al., 2004; Kittler et al., 2004). A direct relationship between the number of postsynaptic GABAA-Rs and the strength of the synapse has been demonstrated (Nusser et al., 1997, 1998). Therefore, to maintain a stable cell-surface receptor number, continual membrane insertion of newly synthesized or recycled receptors is required (Kennedy and Ehlers, 2006). However, how neurons facilitate the insertion of GABAA-Rs into synaptic membranes remains to be determined. This issue is not only of importance for inhibitory synaptic transmission, in fact, the major sites of excitatory synaptic transmission in the brain, the AMPA-type glutamate receptors also cycle between the plasma membrane and intracellular compartments playing a role in synaptic plasticity (Citri and Malenka, 2008). To address the mechanisms underlying GABAA-R membrane trafficking we have studied the role of the Wnt signaling pathway. Our results demonstrate that treatment with the noncanonical Wnt-5a ligand significantly increases the amount of the functional GABAA-Rs on the neuronal cell surface, increasing the number of clusters and the amplitude of the inhibitory currents.
Since the presynaptic and postsynaptic regions strongly interact, alterations in structuring the presynaptic terminal or the postsynaptic region are accompanied by a parallel change in the opposite synaptic site (Ahmad-Annuar et al., 2006; Citri and Malenka, 2008; Salinas and Zou, 2008). However, when we analyzed the scaffold protein gephyrin we did not observed any change in the presence of Wnt-5a treatment, during the same time frame where Wnt-5a increased the surface expression of GABAA-Rs. These results suggest that Wnt-5a does not affect the organization of the whole inhibitory postsynaptic region, at least on the same time scale. In fact, our studies were performed after short-term exposure to Wnt ligands, therefore we do not know whether a long-term exposure to Wnts will affect the presynaptic counterpart as a consequence of the postsynaptic differentiation. Previous studies in our laboratory demonstrated that Wnt-5a does not affect the clustering of different presynaptic proteins until 60 min (Cerpa et al., 2008, Farías et al., 2010; Inestrosa and Arenas, 2010). Therefore, although an increase in cluster number could indicate new, unsilenced synapses, it is more likely that this result from an increase in receptor levels above the immunocytochemical detection threshold at previously existing synapses. Furthermore, because we do not observed changes in paired-pulse relationship or mIPSC frequency in CA1 neurons in hippocampal slices after Wnt-5a or Foxy-5 application, it appears that the increase in surface receptors happens at preexisting synapses.
In addition, we demonstrate that the regulation of the expression of GABAA-Rs induced by Wnt-5a on the cell surface is due to the fact that this ligand increases the recycling of the receptor without affecting the endocytic process or the total protein level. These effects induced by the Wnt-5a are specific since they are blocked by sFRP, a soluble antagonist of Wnt signaling. Moreover, the increase induced by Wnt-5a in the surface expression of the GABAA-Rs was reproduced by a formylated hexapeptide that mimics Wnt-5a effect (Foxy-5). These results suggest that Wnt-5a facilitates the membrane insertion of GABAA-Rs. However, the precise mechanisms that mediate the stabilization of GABAA-Rs on the neuronal surface induced by Wnt-5a remains to be established. But, at least an increase in the recycling of GABAA-Rs is triggered by the Wnt-5a ligand.
During the last years, it has been observed that the phosphorylation of GABAA-Rs subunits is an important mechanism that dynamically modulates GABAA-Rs trafficking at synapses (Brandon et al., 2002) The GABAA-Rs is phosphorylated by diverse kinases including the cAMP-dependent protein kinase (PKA), PKC, CaMKII, Protein kinase B (Akt) and tyrosine kinases of the Src family. On this context, Brandon and coworkers described that GABAA receptor function, dependent upon the subtype analyzed, and it can be differentially modulated by phosphorylation of key residues within the intracellular loop of receptor β1–3 and γ2 subunits. Interestingly, PKC and CaMKII are involved in the noncanonical Wnt signaling pathway initiated by Wnt-5a (Farías et al., 2009, 2010; Inestrosa and Arenas, 2010). Previous studies have demonstrated that these kinases modulate differentially the receptor stability in the cell surface. Thus, the PKC activation promotes GABAA receptor endocytosis and decreases cell surface expression of the receptor. This phenomenon is accompanied by strong decreases in GABA-gated chloride currents (Herring et al., 2005). In addition, it has been demonstrated that CaMKII activation promotes the recruitment of postsynaptic GABAA-Rs, enhancing the amplitude of GABA whole-cell currents and IPSCs (Churn and DeLorenzo, 1998; Wei et al., 2004). In accordance with the studies mentioned above, we demonstrated that the noncanonical Wnt/Ca2+ pathway, particularly CaMKII, is required to modulate the effect of Wnt-5a on GABAA-Rs.
GABAA-Rs not only function as chloride channels that also regulate membrane voltage and conductance, but also play a crucial role in the establishment of functional synapses, as well as its maturation and stabilization (Ben-Ari, 2002; Ben-Ari et al., 2004). In addition, they are involved in the control of the excitability of the brain, circadian rhythms, cognition, sleeping-wakening cycle, learning and memory (Rudolph and Möhler, 2006). Functional adaptation of GABAergic synapses can generally be achieved by changes in either the neurotransmitter release properties of GABAergic neurons or changes in gene expression, cellular distribution, or function of postsynaptic GABAA-Rs. However, experimental evidences suggest that the synaptic efficacy of GABAergic synapses is tightly correlated with the number of postsynaptic GABAA-Rs (Kittler et al., 2000, Jacob et al., 2008). Therefore, changes in the trafficking of these receptors could regulate neuronal plasticity and contribute to the manifestation of a wide range of neurological and psychiatric disorders including epilepsy (Naylor et al., 2005), mood disorders such as anxiety and depression (Brambilla et al., 2003; Tunnicliff and Malatynska, 2003), and alcoholism (Kumar et al., 2003).
There are a wide variety of receptors that can be distinguished based on their pharmacologic profile, in their subcellular localization or simply by the combination of their subunits. The main GABAA-Rs expressed in the brain are composed by 2 α1 subunits, 2 β subunits and a γ2 subunit (Jacob et al., 2008). The γ2 subunit has been described as the responsible for the sensitivity to benzodiazepines, the synaptic localization, and the trafficking modulation of the receptors (Essrich et al., 1998; Connolly et al., 1999). But the current view is that gephyrin stabilizes receptor clusters in the postsynaptic membrane by preventing their lateral diffusion and/or internalization. In the present study we have described that Wnt-5a modulates the surface expression of the α1 as γ2 subunit in mature hippocampal neurons, increasing the number of the clusters and the colocalization with gephyrin, conferring to the Wnt signaling pathway a key role in the maintenance of the GABAergic synapses in the brain.
The Wnt signaling pathway has been involved in various cellular processes, including functions in the neuronal development and maintenance of the nervous system (Lie et al., 2005; Ahmad-Annuar et al., 2006; Chen et al., 2006; Salinas and Zou, 2008; Inestrosa and Arenas, 2010). Wnt proteins signal through at least three different pathways. In the canonical pathway, Wnt ligand increases cytoplasmic β-catenin levels, allowing β-catenin to enter the nucleus where it co-activates the transcription of Wnt target genes (Logan and Nusse, 2004; Toledo et al., 2008). Several “noncanonical” Wnt signaling pathways do not affect gene transcription through β-catenin, they mediate other cellular processes through different molecular intermediates instead, including the regulation of monomeric GTPases of the Rho/Rac family and changes in intracellular calcium levels (Montcouquiol et al., 2006; Salinas and Zou, 2008). Our laboratory has demonstrated that the Wnt signaling regulates the presynaptic localization of α7-nAChRs (Farías et al., 2007), it induces recycling and exocytosis of synaptic vesicles (Cerpa et al., 2008) and the clustering of PSD-95 at the postsynaptic region (Farías et al., 2009). However, little is known about its role in mature neurons, even though Wnt ligands and proteins that mediate their signaling are expressed in the mature nervous system (Inestrosa and Arenas, 2010). In the present work, we are proposing that the Wnt signaling pathway has a key role in the homeostasis of inhibitory neuronal synapses, suggesting a possible effect on the plasticity of the inhibitory synapses.
Receptor translocation has important implications for synaptic function and given that GABAA-Rs cycle between synaptic sites and intracellular endocytic structures (Kittler et al., 2000, 2004; Jacob et al., 2008). The capacity of neurons to modulate the removal and/or insertion of GABAA-Rs in synaptic membranes may have profound effects on the efficacy of synaptic transmission (Otis et al., 1994). Thus, the rapid increase of GABAA-Rs induced by Wnt-5a in the postsynaptic domain of inhibitory synapses provides an additional mechanism for the induction of synaptic plasticity in these synapses.
Footnotes
This work was supported by grants from Fondo de Financiamiento de Centros de Excelencia en Investigacíon N° 13980001, Millenium Institute for Fundamental and Applied Biology, and Basal Center for Excellence in Science and Technology, Centro de Envejecimiento y Regeneración (CARE) N° PFB12/2007 to N.C.I.; Fondo Nacional de Desarrollo Cientifico y Tecnológico (FONDECYT) N°1071001 and Iniciativa Científica Milenio N° ICM-P074-048-F to A.C.; FONDECYT N° 1061074 and Dirección de Investigación y Desarrollo de la Universidad de Valparaíso (DIPUV) N° 08/2005 to C.B.; FONDECYT N° 11090059 and DIPUV N°46/2007 to M.F.; and a predoctoral fellowship from FONDECYT to L.C. We thank Dr. Patricia C. Salinas, Jeremy Nathans, Randall Moon, and Roel Nusse for providing the Wnt-7a, Wnt-3a, Wnt-5a, and sFRP-1 HA-constructs; and Gloria Méndez and Francisco Romero for technical support.
References
- Ahmad-Annuar A, Ciani L, Simeonidis I, Herreros J, Fredj NB, Rosso SB, Hall A, Brickley S, Salinas PC. Signaling across the synapse: a role for Wnt and Dishevelled in presynaptic assembly and neurotransmitter release. J Cell Biol. 2006;174:127–139. doi: 10.1083/jcb.200511054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez AR, Godoy JA, Mullendorff K, Olivares GH, Bronfman M, Inestrosa NC. Wnt-3a overcomes β-amyloid toxicity in rat hippocampal neurons. Exp Cell Res. 2004;297:186–196. doi: 10.1016/j.yexcr.2004.02.028. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of GABA during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Khalilov I, Represa A, Gozlan H. Interneurons set the tune of developing networks. Trends Neurosci. 2004;27:422–427. doi: 10.1016/j.tins.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Perez J, Barale F, Schettini G, Soares JC. GABAergic dysfunction in mood disorders. Mol Psychiatry. 2003;8:721–737. doi: 10.1038/sj.mp.4001362. 715. [DOI] [PubMed] [Google Scholar]
- Brandon N, Jovanovic J, Moss S. Multiple roles of protein kinases in the modulation of γ-aminobutyric acid (A) receptor function and cell surface expression. Pharmacol Ther. 2002;94:113–122. doi: 10.1016/s0163-7258(02)00175-4. [DOI] [PubMed] [Google Scholar]
- Brünig I, Penschuck S, Berninger B, Benson J, Fritschy JM. BDNF reduces miniature inhibitory postsynaptic currents by rapid down regulation of GABAA receptor surface expression. Eur J Neurosci. 2001;13:1320–1328. doi: 10.1046/j.0953-816x.2001.01506.x. [DOI] [PubMed] [Google Scholar]
- Cerpa W, Godoy JA, Alfaro I, Farías GG, Metcalfe MJ, Fuentealba R, Bonansco C, Inestrosa NC. Wnt-7a modulates the synaptic vesicle cycle and synaptic transmission in hippocampal neurons. J Biol Chem. 2008;283:5918–5927. doi: 10.1074/jbc.M705943200. [DOI] [PubMed] [Google Scholar]
- Chen J, Park CS, Tang SJ. Activity-dependent synaptic Wnt release regulates hippocampal long term potentiation. J Biol Chem. 2006;281:11910–11916. doi: 10.1074/jbc.M511920200. [DOI] [PubMed] [Google Scholar]
- Churn SB, DeLorenzo RJ. Modulation of GABAergic receptor binding by activation of calcium and calmodulin-dependent kinase II membrane phosphorylation. Brain Res. 1998;809:68–76. doi: 10.1016/s0006-8993(98)00834-8. [DOI] [PubMed] [Google Scholar]
- Ciani L, Salinas PC. WNTs in the vertebrate nervous system: from patterning to neuronal connectivity. Nat Rev Neurosci. 2005;6:351–362. doi: 10.1038/nrn1665. [DOI] [PubMed] [Google Scholar]
- Citri A, Malenka RC. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology. 2008;33:18–41. doi: 10.1038/sj.npp.1301559. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Isaac JT, Wang YT. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci. 2004;5:952–962. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- Connolly CN, Uren JM, Thomas P, Gorrie GH, Gibson A, Smart TG, Moss SJ. Endocytosis of homomeric γ2 subunit splice variants of γ-aminobutyric acid type A subcellular localization and receptors. Mol Cell Neurosci. 1999;13:259–271. doi: 10.1006/mcne.1999.0746. [DOI] [PubMed] [Google Scholar]
- Cuitino L, Matute R, Retamal C, Bu G, Inestrosa NC, Marzolo MP. ApoER2 is endocytosed by a clathrin-mediated process involving the adaptor protein Dab2 independent of its Rafts' association. Traffic. 2005;6:820–838. doi: 10.1111/j.1600-0854.2005.00320.x. [DOI] [PubMed] [Google Scholar]
- Essrich C, Lorez M, Benson JA, Fritschy JM, Lüscher B. Postsynaptic clustering of major GABAA receptor subtypes requires the γ2 subunit and gephyrin. Nat Neurosci. 1998;1:563–571. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- Farías GG, Vallés AS, Colombres M, Godoy JA, Toledo EM, Lukas RJ, Barrantes FJ, Inestrosa NC. Wnt-7a induces presynaptic colocalization of α7-nicotinic acetylcholine receptors and adenomatous polyposis coli in hippocampal neurons. J Neurosci. 2007;27:5313–5325. doi: 10.1523/JNEUROSCI.3934-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farías GG, Alfaro IE, Cerpa W, Grabowski CP, Godoy JA, Bonansco C, Inestrosa NC. Wnt-5a/JNK signaling promotes the clustering of PSD-95 in hippocampal neurons. J Biol Chem. 2009;284:15857–15866. doi: 10.1074/jbc.M808986200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farías GG, Godoy JA, Cerpa W, Varela-Nallar L, Inestrosa NC. Wnt signaling modulates pre- and postsynaptic maturation: therapeutic considerations. Dev Dyn. 2010;239:94–101. doi: 10.1002/dvdy.22065. [DOI] [PubMed] [Google Scholar]
- Fuenzalida M, Fernandez de Sevilla D, Buño W. Changes of the EPSP waveform regulate the temporal window for spike-timing-dependent plasticity. J Neurosci. 2007;27:11940–11948. doi: 10.1523/JNEUROSCI.0900-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He P, Shen Y. Interruption of β-catenin signaling reduces neurogenesis in Alzheimer's disease. J Neurosci. 2009;29:6545–6557. doi: 10.1523/JNEUROSCI.0421-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring D, Huang R, Singh M, Dillon GH, Leidenheimer NJ. PKC modulation of GABAA receptor endocytosis and function is inhibited by mutation of a di-leucine motif within the receptor β2 subunit. Neuropharmacology. 2005;48:181–194. doi: 10.1016/j.neuropharm.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Inestrosa NC, Arenas E. Emerging roles of Wnts in the adult nervous system. Nat Rev Neurosci. 2010;11:77–86. doi: 10.1038/nrn2755. [DOI] [PubMed] [Google Scholar]
- Jacob TC, Moss SJ, Jurd R. GABAA receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci. 2008;9:331–343. doi: 10.1038/nrn2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MJ, Ehlers MD. Organelles and trafficking machinery for postsynaptic plasticity. Annu Rev Neurosci. 2006;29:325–362. doi: 10.1146/annurev.neuro.29.051605.112808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, Delmas P, Jovanovic JN, Brown DA, Smart TG, Moss SJ. Constitutive endocytosis of GABAA receptors by an association with the adaptin AP2 complex modulates inhibitory synaptic currents in hippocampal neurons. J Neurosci. 2000;20:7972–7977. doi: 10.1523/JNEUROSCI.20-21-07972.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, Thomas P, Tretter V, Bogdanov YD, Haucke V, Smart TG, Moss SJ. Huntingtin-associated protein 1 regulates inhibitory synaptic transmission by modulating γ-aminobutyric acid type A receptor membrane trafficking. Proc Natl Acad Sci U S A. 2004;101:12736–12741. doi: 10.1073/pnas.0401860101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Kralic JE, O'Buckley TK, Grobin AC, Morrow AL. Chronic ethanol consumption enhances internalization of α1 subunit-containing GABAA receptors in cerebral cortex. J Neurochem. 2003;86:700–708. doi: 10.1046/j.1471-4159.2003.01894.x. [DOI] [PubMed] [Google Scholar]
- Lie DC, Colamarino SA, Song HJ, Désiré L, Mira H, Consiglio A, Lein ES, Jessberger S, Lansford H, Dearie AR, Gage FH. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Olsen RW. GABAA receptor channels. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation—a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Montcouquiol M, Crenshaw EB, 3rd, Kelley MW. Non-canonical Wnt signaling and neural polarity. Annu Rev Neurosci. 2006;29:363–386. doi: 10.1146/annurev.neuro.29.051605.112933. [DOI] [PubMed] [Google Scholar]
- Morimoto S, Nishimura N, Terai T, Manabe S, Yamamoto Y, Shinahara W, Miyake H, Tashiro S, Shimada M, Sasaki T. Rab13 mediates the continuous endocytic recycling of occludin to the cell surface. J Biol Chem. 2005;280:2220–2228. doi: 10.1074/jbc.M406906200. [DOI] [PubMed] [Google Scholar]
- Murthy VN, Sejnowski TJ, Stevens CF. Heterogeneous release properties of visualized individual hippocampal synapses. Neuron. 1997;18:599–612. doi: 10.1016/s0896-6273(00)80301-3. [DOI] [PubMed] [Google Scholar]
- Naylor DE, Liu H, Wasterlain CG. Trafficking of GABAA receptors, loss of inhibition, and a mechanism for pharmacoresistance in status epilepticus. J Neurosci. 2005;25:7724–7733. doi: 10.1523/JNEUROSCI.4944-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Cull-Candy S, Farrant M. Differences in synaptic GABAA receptor number underlies variation in GABA mini amplitude. Neuron. 1997;19:697–709. doi: 10.1016/s0896-6273(00)80382-7. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Hájos N, Somogyi P, Mody I. Increased number of synaptic GABAA receptors underlies potentiation at hippocampal inhibitory synapses. Nature. 1998;395:172–177. doi: 10.1038/25999. [DOI] [PubMed] [Google Scholar]
- Otis TS, De Koninck Y, Mody I. Lasting potentiation of inhibition is associated with an increased number of gamma-aminobutyric acid type A receptors activated during miniature inhibitory postsynaptic currents. Proc Natl Acad Sci U S A. 1994;91:7698–7702. doi: 10.1073/pnas.91.16.7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton RG, Simons K, Dotti CG. Axonal and dendritic endocytic pathways in cultured neurons. J Cell Biol. 1992;119:123–137. doi: 10.1083/jcb.119.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattner A, Hsieh JC, Smallwood PM, Gilbert DJ, Copeland NG, Jenkins NA, Nathans J. A family of secreted proteins contains homology to the cysteine-rich ligand-binding domain of frizzled receptors. Proc Natl Acad Sci U S A. 1997;94:2859–2863. doi: 10.1073/pnas.94.7.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U, Möhler H. GABA-based therapeutic approaches: GABAA receptor subtype functions. Curr Opin Pharmacol. 2006;6:18–23. doi: 10.1016/j.coph.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Salinas PC, Zou Y. Wnt signaling in neural circuit assembly. Annu Rev Neurosci. 2008;31:339–358. doi: 10.1146/annurev.neuro.31.060407.125649. [DOI] [PubMed] [Google Scholar]
- Toledo EM, Colombres M, Inestrosa NC. Wnt signaling in neuroprotection and stem cell differentiation. Prog Neurobiol. 2008;86:281–296. doi: 10.1016/j.pneurobio.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Tunnicliff G, Malatynska E. Central GABAergic systems and depressive illness. Neurochem Res. 2003;28:965–976. doi: 10.1023/a:1023287729363. [DOI] [PubMed] [Google Scholar]
- Vargas KJ, Terunuma M, Tello JA, Pangalos MN, Moss SJ, Couve A. The availability of surface GABAB receptors is independent of γ-aminobutyric acid but controlled by glutamate in central neurons. J Biol Chem. 2008;283:24641–24648. doi: 10.1074/jbc.M802419200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Q, Xiong ZG, Man HY, Ackerley CA, Braunton J, Lu WY, Becker LE, MacDonald JF, Wang YT. Recruitment of functional GABAA receptors to postsynaptic domains by insulin. Nature. 1997;388:686–690. doi: 10.1038/41792. [DOI] [PubMed] [Google Scholar]
- Wang Q, Liu L, Pei L, Ju W, Ahmadian G, Lu J, Wang Y, Liu F, Wang YT. Control of synaptic strength, a novel function of Akt. Neuron. 2003;38:915–928. doi: 10.1016/s0896-6273(03)00356-8. [DOI] [PubMed] [Google Scholar]
- Wei J, Zhang M, Zhu Y, Wang JH. Ca2+-calmodulin signalling pathway up-regulates GABA synaptic transmission through cytoskeleton-mediated mechanisms. Neuroscience. 2004;127:637–647. doi: 10.1016/j.neuroscience.2004.05.056. [DOI] [PubMed] [Google Scholar]