Abstract
Severe stress induces changes in neuronal function that are implicated in stress-related disorders such as depression. The molecular mechanisms underlying the response of the brain to stress remain primarily unknown, however. Signal regulatory protein α (SIRPα) is an Ig-superfamily protein that undergoes tyrosine phosphorylation and binds the protein tyrosine phosphatase Shp2. Here we show that mice expressing a form of SIRPα that lacks most of the cytoplasmic region manifest prolonged immobility (depression-like behavior) in the forced swim (FS) test. FS stress induced marked tyrosine phosphorylation of SIRPα in the brain of wild-type mice through activation of Src family kinases. The SIRPα ligand CD47 was important for such SIRPα phosphorylation, and CD47-deficient mice also manifested prolonged immobility in the FS test. Moreover, FS stress-induced tyrosine phosphorylation of both the NR2B subunit of the NMDA subtype of glutamate receptor and the K+-channel subunit Kvβ2 was regulated by SIRPα. Thus, tyrosine phosphorylation of SIRPα is important for regulation of depression-like behavior in the response of the brain to stress.
Introduction
Stress is a biologically important factor with the potential to markedly perturb the physiological or psychological homeostasis of an organism. The brain is one of the organs most affected by stress in mammals (Kim and Diamond, 2002; Nestler et al., 2002), but it is able to respond effectively and to adapt to many types of stress. Chronic or acute exposure to severe stress, however, often results in long-term neuronal changes that are implicated in stress-related disorders such as depression. For instance, stress is thought to downregulate the expression in the hippocampus of brain-derived neurotrophic factor (BDNF), which promotes the growth and development of immature neurons and enhances the survival of adult neurons. The expression of BDNF in the hippocampus is indeed reduced in suicidal depressed individuals but increased in patients receiving antidepressant medication, suggesting that a defect in BDNF signaling may contribute to the pathogenesis of depression (Nestler et al., 2002; Duman and Monteggia, 2006; Martinowich et al., 2007). However, the molecular mechanisms underlying the response of the brain to stress as well as the pathogenesis of stress-related disorders remain unclear.
Signal regulatory protein α (SIRPα; also known as SHPS-1, p84, and BIT) is a transmembrane protein that contains three Ig-like domains in its extracellular region as well as putative tyrosine phosphorylation sites in its cytoplasmic region (Barclay, 2009; Matozaki et al., 2009). SIRPα is expressed throughout the brain but is particularly abundant in synapse-rich regions (Jiang et al., 1999; Mi et al., 2000; Ohnishi et al., 2005). Various receptor-type tyrosine kinases, including the BDNF receptor TrkB, as well as Src family kinases (SFKs) mediate the tyrosine phosphorylation of SIRPα (Matozaki et al., 2009), which then binds and activates the protein tyrosine phosphatases Shp1 and Shp2 (Neel et al., 2003; Matozaki et al., 2009). Shp1 is expressed predominantly in hematopoietic cells, whereas Shp2 is expressed in most cell types including neurons. Shp2 is thus likely an important mediator of SIRPα signaling in neurons, but the functional relevance of the SIRPα–Shp2 complex in the brain has remained unknown. CD47 is a member of the Ig superfamily of proteins that possesses five transmembrane domains (Brown and Frazier, 2001) and functions as a ligand for the extracellular region of SIRPα (Jiang et al., 1999; Barclay, 2009). Similar to SIRPα, CD47 is expressed throughout the brain, with the regions in which it is particularly abundant overlapping extensively with those enriched in SIRPα (Mi et al., 2000; Ohnishi et al., 2005). SIRPα and CD47 thus constitute a cell–cell communication system that likely plays an important role in the brain.
Here we have found that SIRPα mutant mice manifest depression-like behavior in the Porsolt forced swim (FS) test (Porsolt et al., 1977). Furthermore, FS stress induced rapid tyrosine phosphorylation of SIRPα by SFKs in the brain. Both norepinephrine (NE) and CD47 were found to be important for the FS stress-induced tyrosine phosphorylation of SIRPα. SIRPα was also found to regulate the FS stress-induced tyrosine phosphorylation of other neuronal proteins.
Materials and Methods
Animals.
Mice that express a mutant form of SIRPα (Inagaki et al., 2000) and CD47-deficient mice (Oldenborg et al., 2000) were backcrossed to the C57BL/6N or C57BL/6J background, respectively, for >10 generations. Fyn-deficient mice were as described previously (Yagi et al., 1993). For behavioral analysis, heterozygous mutant mice were crossed, and the resulting homozygous mutant animals and their wild-type (WT) littermates were studied. Mice obtained by mating of homozygotes were used for other experiments. TrkBlox/lox and CaMKII-Cre mice were generated as described previously (Minichiello et al., 1999). Homozygous floxed mice harboring the CaMKII-Cre transgene were crossed with the corresponding homozygous floxed mice, and the resulting conditional TrkB-deficient mice and their littermates that did not harbor the Cre transgene were studied. Male mice were used in all experiments. Mice were bred and maintained at the Institute of Experimental Animal Research of Gunma University under specific pathogen-free conditions. They were housed in an air-conditioned room with a 12 h light/dark cycle. All mice were handled in accordance with the animal care guidelines of Gunma University.
Primary antibodies and reagents.
Hybridoma cells producing a rat a monoclonal antibody (mAb) to mouse SIRPα (P84) were kindly provided by C. F. Lagenaur (University of Pittsburgh, Pittsburg, PA). Rabbit polyclonal antibodies (pAbs) specific for tyrosine-phosphorylated SIRPα (anti-pSIRPα) were generated in response to a synthetic phosphopeptide corresponding to amino acids 496-506 (PSFSEpYASVQV) of mouse SIRPα and were purified from serum by affinity chromatography with the synthetic peptide covalently coupled to epoxy-activated Sepharose 6B (GE Healthcare). Rabbit pAbs to NR2B and to the Tyr1472-phosphorylated form of NR2B (pY1472) (Nakazawa et al., 2006) were kindly provided by T. Yamamoto (University of Tokyo, Tokyo, Japan); a rat mAb to Fyn (γC3) (Yasunaga et al., 1996) was provided by T. Yagi (Osaka University, Osaka, Japan); and a mouse mAb to TrkB (Iwasaki et al., 1997) was provided by S. Koizumi (Novartis Pharma K.K., Ibaraki, Japan). Rabbit pAbs to CD47 were generated in response to a glutathione S-transferase fusion protein containing 34 amino acids corresponding to the COOH terminus of mouse CD47 form 4 (Murata et al., 2006). Rabbit pAbs to SIRPα and mouse mAbs to phosphotyrosine (4G10) and to PSD-95 were obtained from Millipore. A rat mAb to mouse CD47 (miap 301) was from BD PharMingen. Rabbit pAbs to Shp2 and to pan-Trk were from Santa Cruz Biotechnology. A mouse mAb to phosphotyrosine (PY100) and rabbit pAbs to the Tyr416-phosphorylated form of c-Src, to Akt, and to phospho-Akt was from Cell Signaling Technology. Mouse mAbs to Kvβ2 (K17/70) and to Kv1.4 (K13/31) were obtained from NeuroMab. Rabbit pAbs to mitogen-activated protein kinase (MAPK) and to phospho-MAPK were from Promega. A mouse mAb to Src as well as to PP2 and PP3 was obtained from Calbiochem. Rabbit pAbs to MAP2 and to GluR1 as well as a mouse mAb to Tau-1 were from Millipore. Rabbit pAbs to calbindin (Nakagawa et al., 1998) were provided by M. Watanabe (Hokkaido University, Sapporo, Japan). A mouse mAb to synaptophysin (171B5) (Obata et al., 1987) was provided by S. C. Fujita (Mitsubishi Kagaku Institute of Life Sciences, Tokyo, Japan). Imipramine hydrochloride, fluoxetine hydrochloride, reboxetine, desipramine hydrochloride, prazosin hydrochloride, propranolol hydrochloride, yohimbine hydrochloride, as well as recombinant human BDNF and insulin-like growth factor 1 (IGF-1) were obtained from Sigma.
Pharmacological studies.
Imipramine (25 mg per kilogram of body mass), fluoxetine (25 mg/kg), reboxetine (20 mg/kg), desipramine (10 mg/kg), or the same volume of saline was injected intraperitoneally into WT mice. Five minutes after the injection, hippocampal tissue was dissected for immunoblot analysis.
Mouse behavioral tests.
A battery of mouse behavioral tests was performed as described previously (Miyakawa et al., 2001, 2003; Morishima et al., 2005; Shibata et al., 2007). The tests included the wire hang test, grip strength test, light–dark transition test, open-field test, elevated plus-maze test, rotarod test, hot plate test, social interaction test, startle response–prepulse inhibition test, Porsolt FS test, eight-arm radial maze test, cued and contextual fear-conditioning test, tail suspension test, and home cage monitoring. In all tests, digital image data were recorded with a charge-coupled device (CCD) camera and were analyzed with NIH Image software (developed by W. Rasband at the National Institute of Mental Health, Bethesda, MD) modified appropriately for each test (Miyakawa et al., 2001) (available through O'Hara & Co.). For behavioral studies with SIRPα mutant mice, WT and SIRPα mutant males were subjected sequentially to the behavioral tests described above. For the FS test, the apparatus consisted of one or four Plexiglas cylinders (height, 20 cm; diameter, 10 cm) that were filled with water up to a height of 7.5 cm and maintained at room temperature overnight. Mice were placed individually into the cylinders, and their behavior was recorded with a CCD camera over a 10 min test period. Images were captured at a rate of one frame per second. For each pair of successive frames, the area (pixels) within which the mouse moved was measured. When the area was below a certain threshold (20 pixels), the mouse was judged “immobile”; when it equaled or exceeded the threshold, the mouse was considered to be “moving.” The optimal threshold (number of pixels) for the definition of immobility was determined by adjustment based on human observation. For the tail suspension test, mice were suspended 30 cm above the floor in a visually isolated area by adhesive tape placed ∼1 cm from the tip of the tail, and their behavior was recorded over a 10 min test period. Assessment of forelimb grip strength was performed with a grip strength meter (O'Hara & Co.). Mice were lifted and held by their tail so that their forepaws could grasp a wire grid. They were then gently pulled backward by the tail with their posture parallel to the surface of the table until they released the grid. The peak force applied by the mouse forelimbs was recorded in newtons. Each mouse was tested three times, and the greatest value measured was used for statistical analysis. For the open-field test, we placed each mouse in the center of an open-field apparatus (40 × 40 × 30 cm; AccuScan Instruments). The total distance traveled (centimeters) and vertical activity (rearing measured by counting the number of photobeam interruptions) were recorded.
Learned helplessness behavioral tests were performed with the CompACT shuttle avoidance system (Muromachi Kikai Co.). This apparatus was divided into two equal compartments with a removable stainless plate. Mice were subjected to 360 inescapable electric foot shocks (0.16 mA, 2 s duration with an interval of 9 s) in one of the two closed compartments on 2 consecutive days. Control animals were treated similarly without foot shocks. Twenty-four hours after the second shock procedure, a two-way conditioned avoidance test was performed with the apparatus that were separated with a stainless plate with small gate for the test session. Each trial started with a light stimulus of 5 s, announcing a subsequent foot shock of maximum 10 s duration. The intertrial interval was 30 s. The following behavioral reactions were defined: escape latency, time needed for shuttling to the other section in reaction to the electric shock; and failure, when no attempt to escape was made. Total time of testing for helplessness was about 30 min, the exact time period depending on the animal's ability to learn the paradigm.
Immunoblot analysis.
For immunoblot analysis of brain homogenates, mice were killed by cervical dislocation, and the cerebral cortex or hippocampus was dissected into ice-cold PBS and homogenized at 4°C in lysis buffer (20 mm Tris-HCl, pH 7.6, 140 mm NaCl, 1 mm EDTA, 1 mm EGTA, 10 mm sodium fluoride, 1% NP-40, 1 mm phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 1 mm sodium vanadate). The homogenates were then subjected directly to immunoblot analysis or were centrifuged at 21,000 × g for 15 min at 4°C, with the resulting supernatants being subjected to immunoprecipitation. For immunoblot analysis of cultured hippocampal neurons, the cells were lysed on ice in lysis buffer, the lysates were centrifuged at 21,000 × g for 15 min at 4°C, and the resulting supernatants were subjected to the analysis.
Histological analysis.
For immunofluorescence analysis, mice were anesthetized with ether and perfused transcardially with 4% paraformaldehyde and 7% saturated picric acid in 0.1 m phosphate buffer, pH 7.4, supplemented with heparin (50 U/ml). Brain tissue was dissected and fixed again in the same solution overnight at 4°C with gentle shaking. Tissue was then transferred sequentially to a series of sucrose solutions [7, 20, and 30% (w/v) in PBS] for cryoprotection, embedded in OCT compound (Sakura Fine Technical), and rapidly frozen in liquid nitrogen. Frozen sections with a thickness of 10 μm were prepared with a cryostat, mounted on glass slides, and air dried. For staining with anti-pSIRPα, sections were autoclaved in Retrievagen A (pH 6.0; BD PharMingen) at 110°C for 15 min for antigen retrieval. All sections were then incubated for 1 h at room temperature in buffer G (PBS supplemented with 5% goat serum and 0.1% Triton X-100) and stained for 2 h at room temperature with primary antibodies diluted in the same buffer. They were then washed with PBS and exposed to secondary antibodies conjugated with the fluorescent dye Cy3 (Jackson ImmunoResearch Laboratories). Sections were also stained with 4′,6-diamidino-2-phenylindole (Nacalai Tesque) as indicated. Fluorescence images were acquired with a fluorescence microscope (DM RXA; Leica) equipped with a cooled CCD camera (Cool SNAP HQ; Roper Scientific) and IPLab Image analysis software (Scanalytic).
For hematoxylin–eosin staining, mice were perfused with 4% paraformaldehyde in 0.1 m sodium phosphate buffer, after which the brain was removed, reexposed to the same fixative, embedded in paraffin, and sectioned at a thickness of 10 μm. Sections were stained with hematoxylin–eosin to reveal neuronal structures. For Golgi staining, the brain was dissected, sectioned at a thickness of ∼2 mm, and fixed for 5–6 d in the dark with a solution containing 5% glutaraldehyde and 2% potassium dichromate. The tissue was then incubated with 1% silver nitrate for an additional 5–6 d, washed with distilled water, sectioned at a thickness of 200 μm with a vibratome, dehydrated in a series of ethanol solutions (50, 70, 95, and 100%, sequentially), exposed to xylene, and mounted with Eukitt (O. Kindler).
Cell culture and transfection.
Hippocampal neurons were prepared and cultured as described previously (Murata et al., 2006). For examination of the effects of interaction between CD47 and SIRPα, HEK293T cells were transfected with an expression vector for mouse CD47 (Murata et al., 2006) or for green fluorescent protein with the use of the LipofectAMINE2000 reagent (Invitrogen). Twenty-four hours after transfection, the cells were detached from the culture dish and cultured for 3 h with hippocampal neurons that had been prepared from CD47-deficient mice 14 d before the experiment. The cocultured cells were then fixed and stained both with a mAb to CD47 and with anti-pSIRPα, as described previously (Murata et al., 2006).
In vivo microdialysis. In vivo microdialysis was performed with male mice at 10–14 weeks of age essentially as described previously (Ago et al., 2006). In brief, mice were anesthetized by an intraperitoneal injection of sodium pentobarbital (40 mg/kg), and a guide cannula (one site per animal) for a dialysis probe (Eicom) was implanted stereotaxically in the hippocampus (anterior, −3.0 mm; lateral, −2.8 mm; ventral, −4.2 mm relative to the bregma and skull) (Franklin and Paxinos, 1997). The cannula was cemented in place with dental acrylic, and the animals were maintained warm and allowed to recover from anesthesia. The active probe membrane was 2 mm in length. On the day after surgery, the probe was perfused with Ringer's solution (147.2 mm NaCl, 4.0 mm KCl, and 2.2 mm CaCl2; Fuso Pharmaceutical Industries) at a constant flow rate of 2 μl/min. Experiments were initiated after a stabilization period of 3 h. Microdialysis samples (40 μl) were collected every 20 min and assayed for NE by HPLC with electrochemical detection.
Calcium imaging.
Mouse hippocampal neurons cultured for 14 d on glass-bottom plates coated with poly-d-lysine (25 μg/ml) were incubated for 30 min at 37°C in the dark with perfusion buffer (15 mm HEPES-NaOH, pH 7.4, 140 mm NaCl, 4.7 mm KCl, 11 mm glucose, 1.2 mm KH2PO4, 2.5 mm CaCl2, 1.2 mm MgSO4) supplemented with 10 μm fura-2 AM (Nacalai Tesque). The neurons were washed with perfusion buffer (without fura-2 AM), and the culture plate was placed on the stage of an inverted microscope (ECLIPSE TE300; Nikon). The neurons were then perfused at a rate of 1.0 ml/min with perfusion buffer at room temperature. The cytosolic Ca2+ concentration of individual neurons was determined at the soma by dual-wavelength fluorometry on the basis of the fluorescence intensity ratio measured with excitation at 340 and 380 nm and emission at 510 nm. Images were captured every 10 s with a cooled CCD camera (Orca; Hamamatsu Photonics) and were analyzed with AquaCosmos software (Hamamatsu Photonics). A stock solution of NE (100 mm in water) was added to the perfusion buffer to yield the desired final concentration of NE for the indicated time.
Measurement of tissue content of monoamines and their metabolites.
Mice were killed by decapitation, and the hippocampus was removed rapidly, weighed, and homogenized in 0.1 m perchloric acid containing 0.1 mm EDTA to yield a 20% (w/v) homogenate. After the addition of 10 μl of isoproterenol (10 ng/μl) as an internal control, the homogenate was maintained on ice for 30 min and centrifuged at 20,000 × g for 15 min at 4°C. The resultant supernatant was passed through an Ultrafree-MC filter (Millipore), and the pH of the filtrate was adjusted to 3.0 by the addition of 1 m sodium acetate. The amounts of monoamines and their metabolites were then assayed with the use of an HPLC system equipped with an Eicompak SC-5ODS column (Eicom) and an electrochemical detector (ECD-300; Eicom); the graphite electrode was set to 750 mV relative to an Ag/AgCl reference electrode. The mobile phase consisted of 83 mm acetate–citrate buffer, pH 3.5, 190 mg/L sodium-1-decanesulfonic acid (Wako), 5 mg/L EDTA (Dojindo), and 17% methanol.
Affinity purification of tyrosine-phosphorylated proteins and mass spectrometric analysis.
Mice were killed by cervical dislocation after exposure to FS stress for 10 min, and the hippocampus was dissected into ice-cold PBS and homogenized in ice-cold homogenization buffer (20 mm Tris-HCl, pH 7.6, 140 mm NaCl, 1 mm EDTA, 1 mm EGTA, 10 mm sodium fluoride) containing 1 mm phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, and 1 mm sodium vanadate. The homogenate was centrifuged at 21,000 × g for 20 min at 4°C, and the resulting pellet was washed once with homogenization buffer, lysed with lysis buffer for 1 h on ice, and subjected to ultrasonic treatment. The suspension was then centrifuged at 21,000 × g for 20 min at 4°C, and the resulting supernatant was applied to a column of agarose conjugated to the 4G10 mAb to phosphotyrosine (Millipore). The bead-bound proteins were washed with lysis buffer (20 mm Tris-HCl, pH 7.6, 140 mm NaCl, 1 mm EDTA, 1 mm EGTA, 10 mm sodium fluoride, 1% NP-40, 1 mm phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 1 mm sodium vanadate), eluted with 0.1 m phenyl phosphate in lysis buffer, and concentrated by ultrafiltration with a Centricon device (Millipore). Proteins were separated by SDS-PAGE and visualized by silver staining, and a band corresponding to a 38 kDa protein was excised from the gel and subjected to matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry as described previously (Ohe et al., 2003) with an Ultraflex TOF/TOF instrument (Bruker Daltonics). The protein was identified by comparison of the molecular masses of peptides determined by MALDI-TOF mass spectrometry and theoretical peptide masses for proteins registered in the NCBInr database.
Construction of expression vectors for Kvβ2 and its mutants.
cDNA fragments encoding the mouse Kvβ2 and Kv1.4 were amplified by reverse transcription-PCR using total RNA prepared from hippocampus of mouse C57BL/6 as a template and subcloned in the pCAGGS vector, which was kindly provided by J. Miyazaki (Osaka University, Osaka, Japan). Mutant forms of Kvβ2 (Y2F, Y25F, Y29F) were constructed by a PCR-based method. Primers used were as follows: Kcnab2f, 5′-TTTTGGCAAAGAATTCTGGATAAGTGTGGCC-3′; Kcnab2r, 5′- CCTGAGGAGTGAATTCTGGGGAGTAGACTTA-3′; Y2Ff, 5′-CTGGATAAGTGTGGCCGGCCCCATGTTCCCGGAATCAACCACG-3′; Y25Ff, 5′-ATCTTCAGTACTCGTTATGGG-3′; Y25Fr, 5′-CCCATAACGAGTACTGAAGATCATCCCGGGGGA-3′; Y29Ff, 5′-CGTTTTGGGAGTCCCAAAAGA-3′; and Y29Fr, 5′- TCTTTTGGGACTCCCAAAACGAGTACTGTAGAT-3′. These primers were designed to introduce an amino acid substitution from Tyr to Phe at the position of 2, 25, or 29 of Kvβ2 by overlap extension mutagenesis (Heckman and Pease, 2007). For the construction of Y2F, the first PCR product was amplified with a primer pair, Y25Ff and Kcnab2r, using cDNA of WT Kvβ2 as a template, and it was then used as a template in the second PCR using a primer pair, Kcnab2f and Kcnab2r. For the construction of Y25F and Y29F, two PCR products were amplified with primer pair 1 (Kcnab2f and Y25Fr for Y25F; Kcnab2f and Y29Fr for Y29F) and primer pair 2 (Kcnab2r and Y25Ff for Y25F; Kcnab2r and Y29Ff for Y29F) using cDNA of WT Kvβ2 as a template, and they were then mixed and used as a template in the second PCR. The two PCR products were hybridized at the overlapping region, and a full-length cDNA with a point mutation were amplified. Resulting PCR products were subcloned in the pCAGGS. DNA sequences of all constructs were confirmed by DNA sequence analyses.
Other methods.
HEK293T cells were cultured under a humidified atmosphere of 5% CO2 in air at 37°C and in DMEM (Sigma) supplemented with 10% fetal bovine serum (Sigma). Chinese hamster ovary (CHO) cell lines that stably express WT mouse SIRPα (CHO-SIRPα) or a mutant form of SIRPα, in which all four tyrosine residues in the cytoplasmic region are replaced by phenylalanine (CHO-SIRPα-4F), were described previously (Sato et al., 2003). Plasma corticosterone levels were assayed with an enzyme immunoassay kit (Assay Designs). The intracellular content of cAMP was extracted from cultured hippocampal neurons (14 d in vitro) with 0.1 m HCl and assayed with an enzyme immunoassay kit (Cayman Chemical).
Statistical analysis.
Data are presented as means ± SEM. and were analyzed by Student's t test or repeated-measures ANOVA with the use of StatView 5.0 software (SAS Institute). A p value of <0.05 was considered statistically significant.
Results
SIRPα mutant mice manifest an increased immobility time in the FS test
We previously generated SIRPα mutant mice (Inagaki et al., 2000) that express a form of SIRPα lacking most of the cytoplasmic region instead of the WT protein (supplemental Fig. 1A–C, available at www.jneurosci.org as supplemental material). The mutant SIRPα protein thus does not undergo tyrosine phosphorylation or form a complex with Shp2. The SIRPα mutant animals were backcrossed to the C57BL/6N background for >10 generations for the present study and manifested no gross abnormality in general behavior or activity. We also did not detect any differences between WT and SIRPα mutant mice in brain morphology as revealed by hematoxylin–eosin staining, in morphology of dendrites and density of spines as revealed by Golgi staining, or in the expression of various neuronal proteins (supplemental Fig. 1D–G, available at www.jneurosci.org as supplemental material), suggesting that CD47-SIRPα signaling might play a regulatory role in the adult brain. To investigate the function of SIRPα in the brain, we performed a battery of behavioral tests (Miyakawa et al., 2001) with the SIRPα mutant mice. We found that the mutant animals manifested an increased immobility time, compared with that of WT mice, in the Porsolt FS test (Fig. 1A). FS stress induces long-term behavioral changes (Porsolt et al., 1977; Nestler et al., 2002; Urani et al., 2005). During their first exposure to FS stress, mice exhibit both active (such as swimming) and passive (such as floating) behaviors. During their second exposure (24 h later), active behavior is suppressed, and passive behavior (immobility) is enhanced. This increase in immobility time is thought to represent a state of despair or depression because it is markedly reduced by treatment with various classes of antidepressant (Nestler et al., 2002; Urani et al., 2005). The increased immobility time of SIRPα mutant mice relative to that of WT mice was most pronounced in the second exposure to FS stress (Fig. 1A). We did not detect any difference in behavior between SIRPα mutant and WT mice in the tail suspension test (Fig. 1B), in which an increase in immobility time also represents a state of despair (Cryan et al., 2005; Urani et al., 2005). In addition, we also did not find any difference in behavior between SIRPα mutant and WT mice in the learned helplessness test, another animal model of depression (Seligman and Maier, 1967) (data not shown). Furthermore, no differences were apparent between the two types of mice in the grip strength test (Fig. 1C) or the open-field test (Fig. 1D), suggesting that neuromuscular function and locomotor activity are normal in the mutant mice.
Figure 1.
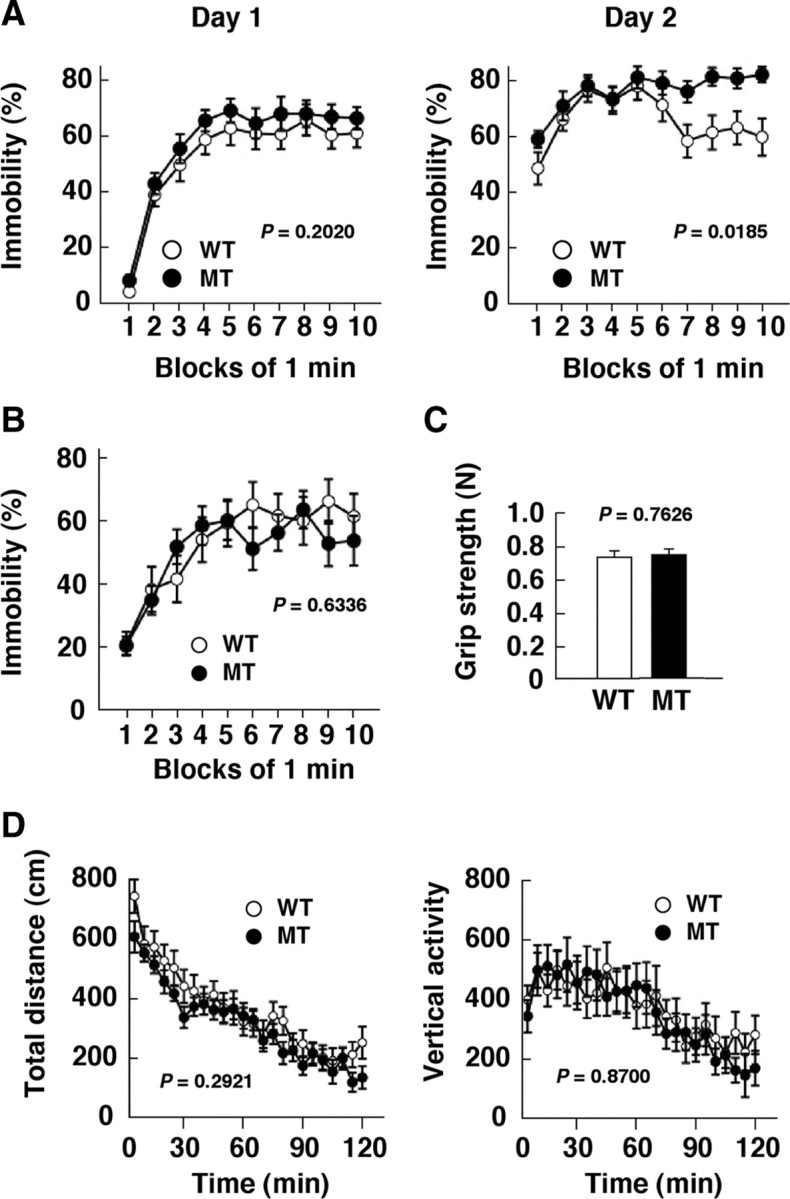
Increased immobility time of SIRPα mutant mice in the FS test. A, WT and SIRPα mutant mice at 18–20 weeks of age (n = 20 for each genotype) were subjected to the FS test, and their immobility time was measured every 1 min for a 10 min period on the first (left) and second (right) days of the test. B, WT and SIRPα mutant mice were subjected to the tail suspension test, and their immobility time was measured every 1 min for a 10 min period. C, Forelimb grip strength of WT and SIRPα mutant mice as measured with a grip strength meter. D, Total distance traveled (left) and vertical activity (right) in the open-field test were measured for SIRPα mutant and WT mice every 5 min for a 120 min period. All data are means ± SEM, and p values for comparison between WT and SIRPα mutant mice were determined by repeated-measures ANOVA (A, B, D) or Student's t test (C). MT, SIRPα mutant.
SIRPα undergoes tyrosine phosphorylation and binds Shp2 in the brain in response to FS stress
To investigate the molecular mechanism by which SIRPα regulates depression-like behavior in the FS test, we examined the effect of FS stress on the tyrosine phosphorylation of SIRPα in the brain. We prepared pAbs specific for SIRPα phosphorylated on a tyrosine residue (Tyr501) in the cytoplasmic region of the protein (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). Immunoblot analysis with these antibodies (anti-pSIRPα) revealed that exposure of WT mice to FS stress for 10 min indeed induced a pronounced increase in the tyrosine phosphorylation of SIRPα in both the hippocampus and cerebral cortex (Fig. 2A). In addition, FS stress markedly increased the association of Shp2 with tyrosine-phosphorylated SIRPα (Fig. 2B). The effect of FS stress on tyrosine phosphorylation of SIRPα was evident at 2 min, reached a maximum between 5 and 20 min, and was barely detectable at 40 min after initiation of the stress (Fig. 2C). This pattern of tyrosine phosphorylation of SIRPα was apparent in the second as well as in the first exposure of WT mice to FS stress (data not shown). Immunofluorescence analysis of the brain with anti-pSIRPα revealed that the increase in the level of tyrosine phosphorylation of SIRPα induced by FS stress was most prominent in the hippocampus (particularly in the stratum lucidum of the CA3 region), amygdala (particularly in the amygdalostriatal transition area), and thalamus but was also apparent in other parts of the brain including the cortex (Fig. 2D). Immunoblot analysis of hippocampal homogenates with a mAb to phosphotyrosine also revealed that an ∼90 kDa protein underwent marked tyrosine phosphorylation in response to FS stress in WT mice but not in SIRPα mutant mice (Fig. 2E), suggesting that this protein is SIRPα and that SIRPα is a major tyrosine-phosphorylated protein in the brain of animals exposed to FS stress.
Figure 2.
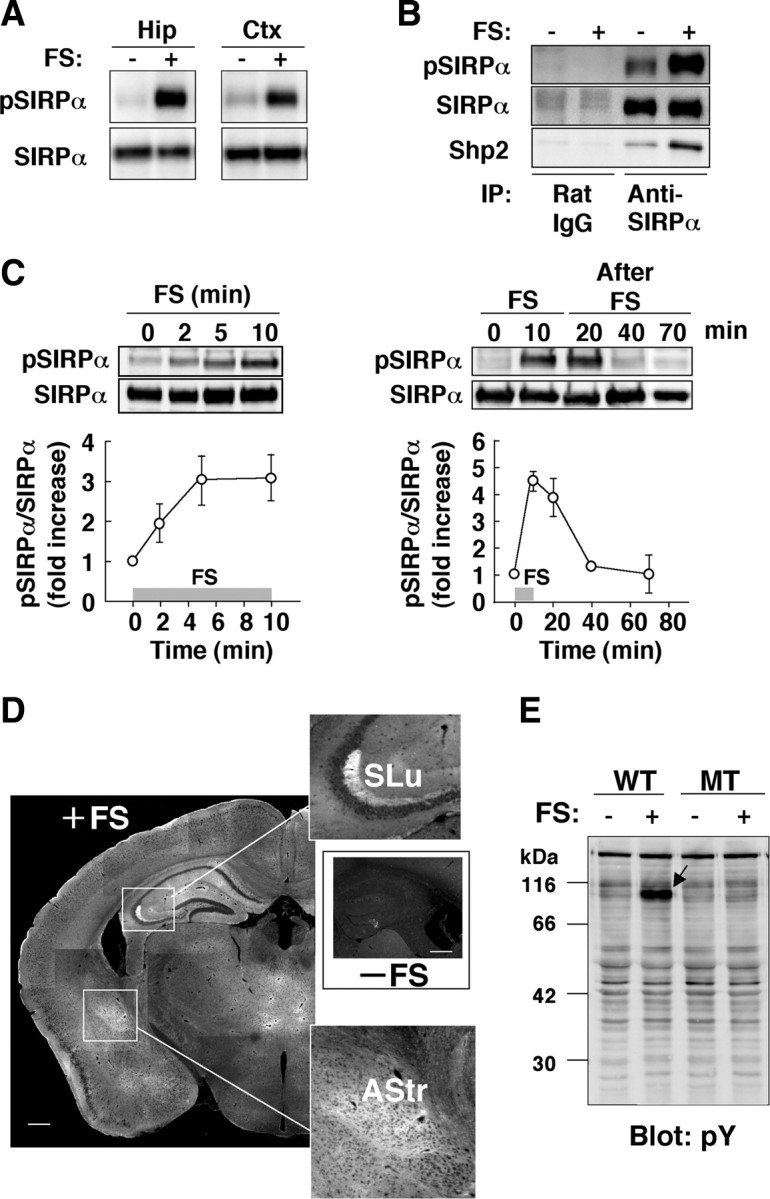
SIRPα is a major tyrosine-phosphorylated protein and binds Shp2 in response to FS stress in the brain. A, WT mice were subjected to FS stress (FS+) or allowed to stand in water (FS−) for 10 min, after which homogenates of the hippocampus (Hip) or cerebral cortex (Ctx) were immediately prepared and subjected to immunoblot analysis with anti-pSIRPα or pAbs to SIRPα. B, Hippocampal homogenates prepared from WT mice treated as in A were subjected to immunoprecipitation (IP) with a mAb to SIRPα or control rat IgG, and the resulting precipitates were subjected to immunoblot analysis with anti-pSIRPα, pAbs to SIRPα, or pAbs to Shp2. C, WT mice were subjected to FS stress for the indicated times (gray bar, 0–10 min; left) or were subjected to FS stress for 10 min and then returned to their cages (right). Hippocampal homogenates were prepared at the indicated times and subjected to immunoblot analysis as in A. The ratio of the intensity of the pSIRPα band to that of the SIRPα band was determined and expressed as fold increase relative to the basal value; data are means ± SEM. for a total of four mice in two independent experiments, with the exception of the 40 min time point in the right panel (two mice in one experiment). D, The brain of WT mice was fixed immediately after FS stress (+FS; left) or control treatment (−FS; middle right) for 10 min, and frozen sections were prepared and subjected to immunofluorescence staining with anti-pSIRPα. The boxed regions in the left panel are shown at higher magnification in the top and bottom right panels. SLu, Stratum lucidum; AStr, amygdalostriatal transition area. Scale bars, 500 μm. E, Hippocampal homogenates prepared from WT or SIRPα mutant (MT) mice treated as in A were subjected to immunoblot analysis with a mAb (PY100) to phosphotyrosine (pY). The arrow indicates an ∼90 kDa protein that underwent pronounced tyrosine phosphorylation in response to FS stress. Data in A, B, D, and E are representative of three separate experiments.
FS stress induces tyrosine phosphorylation of SIRPα through activation of SFKs
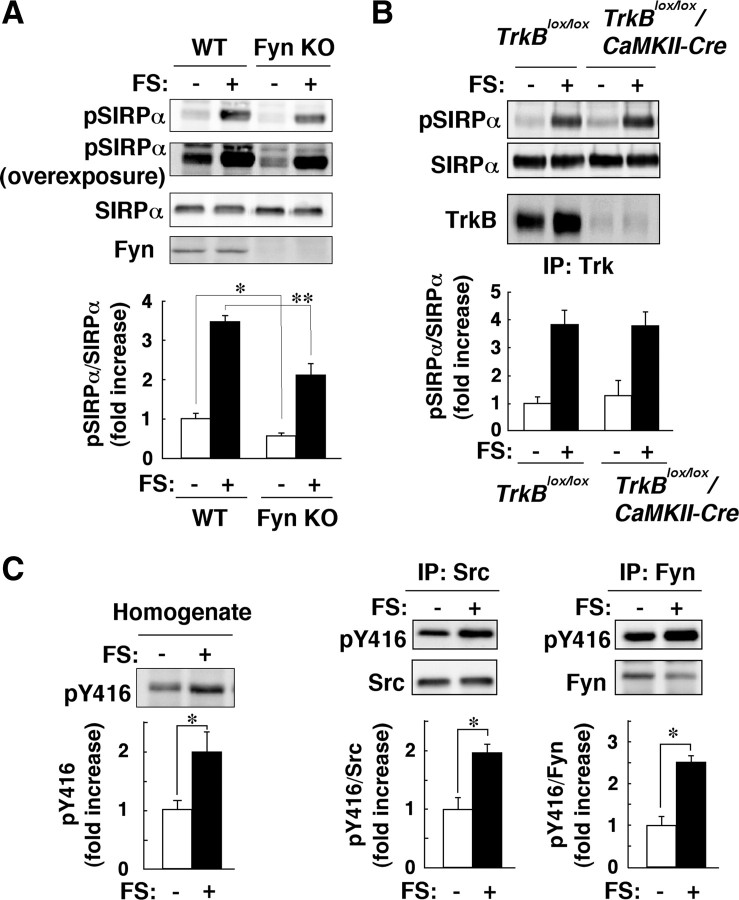
We next investigated the molecular mechanism underlying the FS stress-induced tyrosine phosphorylation of SIRPα in the hippocampus. Given that both SFKs and TrkB have been shown to phosphorylate SIRPα (Matozaki et al., 2009), we first examined the effect of FS stress on the tyrosine phosphorylation of SIRPα in mice deficient in the SFK Fyn (Yagi et al., 1993). The extents of both basal and FS stress-induced tyrosine phosphorylation of SIRPα in the hippocampus were markedly reduced in Fyn-deficient mice compared with those apparent in WT mice (Fig. 3A). In contrast, the FS stress-induced tyrosine phosphorylation of SIRPα in the hippocampus was not impaired in mice deficient in TrkB specifically in the forebrain (Fig. 3B), which were generated by crossing mice harboring a “floxed” TrkB allele with transgenic mice expressing Cre recombinase under the control of the promoter for the calmodulin-dependent kinase II (CaMKII) gene (Minichiello et al., 1999).
Figure 3.
FS stress induces tyrosine phosphorylation of SIRPα through activation of SFKs. A, WT or Fyn-deficient (Fyn KO) mice were subjected to FS stress (FS+) or control treatment (FS−) for 10 min, after which homogenates of the hippocampus were immediately prepared and subjected to immunoblot analysis with anti-pSIRPα, pAbs to SIRPα, or a mAb to Fyn. The ratio of the intensity of the pSIRPα band to that of the SIRPα band is presented as fold increase relative to the WT control (FS−) value; data are means ± SEM. for a total of eight WT and nine Fyn-deficient mice for each condition in four independent experiments. *p < 0.05; **p < 0.01, Student's t test. B, Hippocampal homogenates of mice with forebrain-specific deficiency of TrkB (TrkBlox/lox/CaMKII-Cre) or control (TrkBlox/lox) animals treated as in A were subjected to immunoblot analysis with anti-pSIRPα or pAbs to SIRPα. The homogenates were also subjected to immunoprecipitation with pAbs to pan-Trk, and the resulting precipitates were subjected to immunoblot analysis with a mAb to TrkB. The ratio of the intensity of the pSIRPα band to that of the SIRPα band is presented as fold increase relative to the TrkBlox/lox control (FS−) value; data are means ± SEM. for a total of three TrkBlox/lox and four TrkBlox/lox/CaMKII-Cre mice for each condition in three independent experiments. C, Hippocampal homogenates prepared from WT mice treated as in A were subjected to immunoblot analysis with pAbs to the Tyr416-phosphorylated form of c-Src (pY416; left). The homogenates were also subjected to immunoprecipitation with mAbs to Src (middle) or to Fyn (right), and the resulting precipitates were subjected to immunoblot analysis with pAbs to Tyr416-phosphorylated Src or with mAbs to Src or to Fyn. The intensity of the pY416 band (left) and the ratios of the intensity of this band to that of the Src or Fyn bands (middle and right) are presented as fold increase relative to the control (FS−) value; data are means ± SEM. from a total of four mice for each condition in two independent experiments. *p < 0.05, Student's t test. IP, Immunoprecipitation.
We thus next examined whether FS stress activates SFKs in the hippocampus of WT mice. An increase in the level of phosphorylation at the autophosphorylation site of SFKs (Tyr416 for avian c-Src) reflects an increase in the tyrosine kinase activity of these enzymes (Xu et al., 1999). Immunoblot analysis with pAbs to the Tyr416-phosphorylated form of c-Src revealed that the extent of phosphorylation at the autophosphorylation site of SFKs in hippocampal homogenates was markedly increased in response to exposure of WT mice to FS stress (Fig. 3C). In addition, the extent of autophosphorylation of either Src or Fyn immunoprecipitated from the hippocampus with corresponding specific antibodies was also increased by FS stress (Fig. 3C).
Both antidepressants and NE induce tyrosine phosphorylation of SIRPα
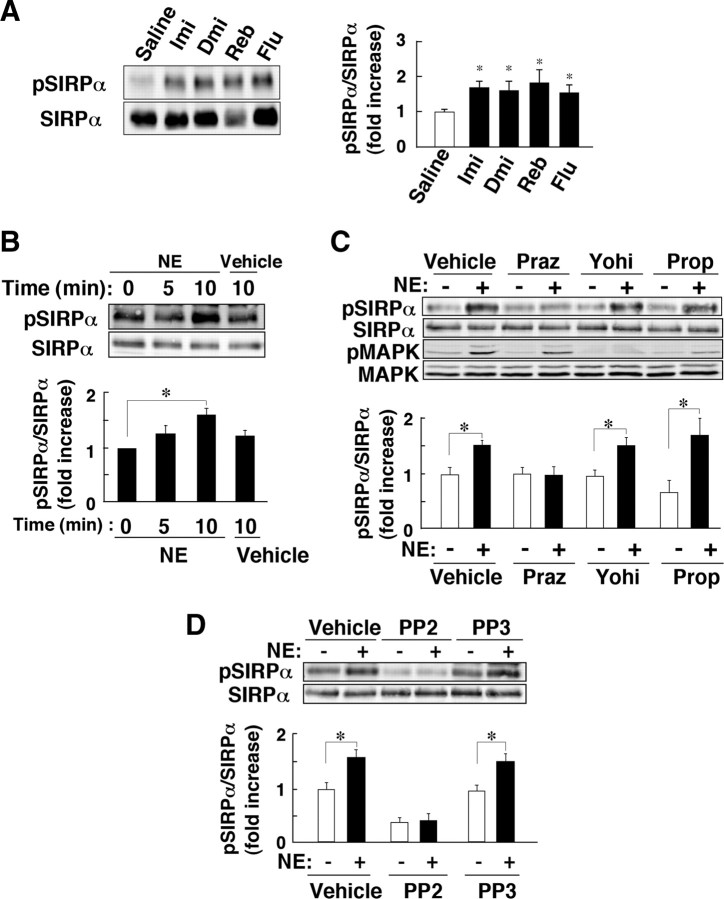
We next sought to identify the factor that is responsible for SFK activation and the induction of tyrosine phosphorylation of SIRPα in the hippocampus by FS stress. FS stress induces a marked increase in the extracellular concentrations of NE and serotonin in the brain of rats (Page et al., 2003) or mice (Fujino et al., 2002) (see supplemental Fig. 3, available at www.jneurosci.org as supplemental material). We thus investigated the possible roles of NE and serotonin in the FS stress-induced tyrosine phosphorylation of SIRPα in the hippocampus. We first examined the effects of antidepressants on SIRPα phosphorylation in the hippocampus of WT animals. The tricyclic antidepressants imipramine and desipramine each increase the extracellular concentrations of monoamines in the brain by inhibiting their uptake by monoamine transporters (Wong et al., 2000). Administration of imipramine or desipramine markedly increased the tyrosine phosphorylation of SIRPα in the hippocampus of WT mice (Fig. 4A). In addition, both reboxetine, a selective NE reuptake inhibitor (SNRI), and fluoxetine, a selective serotonin reuptake inhibitor (SSRI) (Wong et al., 2000), also induced tyrosine phosphorylation of SIRPα in the hippocampus (Fig. 4A), suggesting that NE or serotonin may contribute to the effect of FS stress on SIRPα phosphorylation.
Figure 4.
Antidepressants and NE induce tyrosine phosphorylation of SIRPα. A, WT mice were treated with imipramine (Imi), desipramine (Dmi), reboxetine (Reb), fluoxetine (Flu), or saline. Hippocampal homogenates were prepared 5 min after drug administration and subjected to immunoblot analysis with anti-pSIRPα or pAbs to SIRPα. The ratio of the intensity of the pSIRPα band to that of the SIRPα band is presented as fold increase relative to the control (Saline) value; data are means ± SEM. for a total of six mice for each condition in three independent experiments. *p < 0.05 versus saline value (Student's t test). B, Cultured mouse hippocampal neurons (18 d in vitro) were exposed for the indicated times to 10 μm NE or vehicle (distilled water), after which cell lysates were prepared and subjected to immunoblot analysis as in A. C, Mouse hippocampal neurons were incubated first for 30 min with 10 μm prazosin (Praz), 10 μm yohimbine (Yohi), 10 μm propranolol (Prop), or vehicle (distilled water) and then for 10 min in the additional absence or presence of 10 μm NE. Cell lysates were then subjected to immunoblot analysis with anti-pSIRPα, pAbs to SIRPα, or pAbs to phosphorylated (pMAPK) or total (MAPK) forms of MAPK. D, Mouse hippocampal neurons were incubated first for 30 min with 20 μm PP2 or PP3 or with vehicle (dimethyl sulfoxide) and then for 10 min in the additional absence or presence of 10 μm NE. Cell lysates were then subjected to immunoblot analysis as in A. In B–D, the ratio of the intensity of the pSIRPα band to that of the SIRPα band is expressed as fold increase relative to the corresponding control value; data are means ± SEM. for three independent experiments. *p < 0.05, Student's t test.
We next examined whether NE is able to induce tyrosine phosphorylation of SIRPα in cultured mouse hippocampal neurons. Exposure of the neurons to 10 μm NE for 5–10 min indeed triggered tyrosine phosphorylation of SIRPα (Fig. 4B). Prazosin, an antagonist of α1-adrenergic receptors, abolished the NE-induced tyrosine phosphorylation of SIRPα in hippocampal neurons (Fig. 4C). Neither yohimbine, an antagonist of α2-adrenergic receptors, nor propranolol, an antagonist of β-adrenergic receptors, had such an effect, but these agents did block the NE-induced activation of MAPK (Fig. 4C). These results suggested that α1-adrenergic receptors, but not α2- or β-adrenergic receptors, mediate the NE-induced tyrosine phosphorylation of SIRPα in hippocampal neurons. Src participates in α1-adrenergic receptor-mediated effects of NE (Lindquist et al., 2000). Indeed, PP2, an inhibitor of SFKs, but not its inactive analog PP3, abolished the NE-induced tyrosine phosphorylation of SIRPα in hippocampal neurons (Fig. 4D), suggesting that SFKs are also important for this effect of NE.
Importance of CD47 for the FS stress-induced tyrosine phosphorylation of SIRPα as well as for regulation of immobility in the FS test
The brain regions in which CD47 is particularly abundant overlap extensively with those enriched in SIRPα (Mi et al., 2000; Ohnishi et al., 2005). In addition, interaction of CD47 with SIRPα induces tyrosine phosphorylation of SIRPα in cultured endothelial cells and macrophages (Matozaki et al., 2009). We thus next examined whether CD47 is required for the FS stress-induced tyrosine phosphorylation of SIRPα in the hippocampus with the use of CD47-deficient mice (Oldenborg et al., 2000). Immunoblot analysis with anti-pSIRPα revealed that both the basal and FS stress-induced levels of tyrosine phosphorylation of SIRPα in the hippocampus were markedly reduced in CD47-deficient mice compared with those in WT mice (Fig. 5A), implicating CD47 in this effect of FS stress. To determine whether the trans-interaction of CD47 with SIRPα indeed promotes tyrosine phosphorylation of the latter protein in hippocampal neurons, we cultured neurons that lack CD47 but express endogenous SIRPα with HEK293T cells overexpressing CD47. Contact of the CD47-deficient neurons with neighboring CD47-expressing HEK293T cells resulted in a pronounced increase in the tyrosine phosphorylation of SIRPα at the contact site (Fig. 5B). These results suggested that trans-interaction of SIRPα on neurons with CD47 expressed by neighboring neurons or glial cells might promote tyrosine phosphorylation of SIRPα. In contrast, forced expression of CD47 in CD47-deficient neurons failed to induce tyrosine phosphorylation of endogenous SIRPα in the neurons (data not shown), suggesting that cis-interaction of SIRPα with CD47 is not important for SIRPα phosphorylation.
Figure 5.
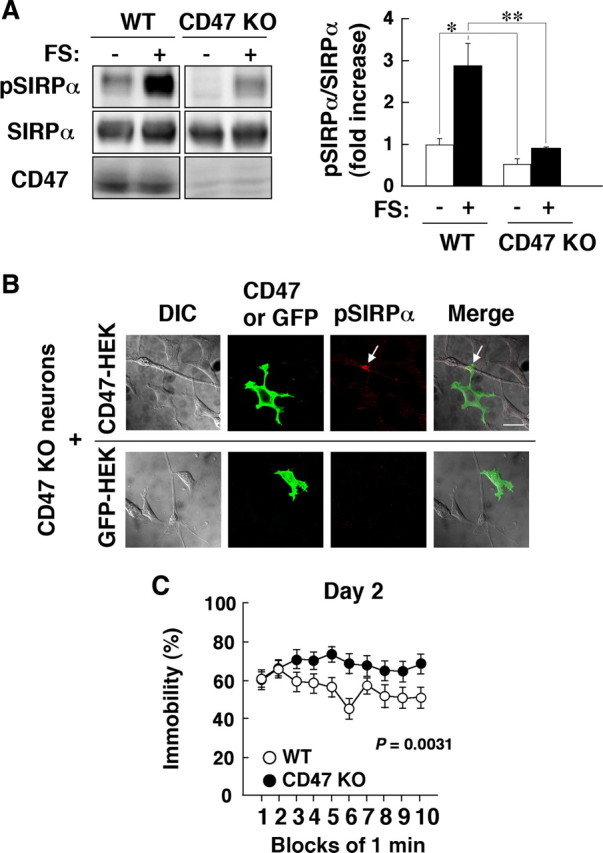
Importance of CD47 both for the FS stress-induced tyrosine phosphorylation of SIRPα in the hippocampus and for regulation of immobility in the FS test. A, WT or CD47-deficient (CD47 KO) mice were subjected to FS stress (FS+) or control treatment (FS−) for 10 min, after which hippocampal homogenates were immediately prepared and subjected to immunoblot analysis with anti-pSIRPα or with pAbs to SIRPα or to CD47. The ratio of the intensity of the pSIRPα band to that of the SIRPα band is expressed as fold increase relative to the WT control (FS−) value; data are means ± SEM. for a total of four (FS−) or six (FS+) mice in two independent experiments. *p < 0.05; **p < 0.01, Student's t test. B, Hippocampal neurons from CD47-deficient mice (14 d in vitro) were cultured for 3 h with HEK293T cells that had been transfected with an expression vector either for CD47 (top) or for green fluorescent protein (GFP; bottom). The cells were then fixed and subjected to immunofluorescence staining with anti-pSIRPα (red). The cells were also stained with a mAb to CD47 (green; top) or examined for GFP fluorescence (green; bottom). Differential interference contrast (DIC) and merged images are also shown. Arrows indicate pSIRPα immunoreactivity at a site of contact between a neuron and a CD47-expressing HEK293T cell. Scale bar, 20 μm. C, WT or CD47-deficient mice at 12–18 weeks of age (n = 20 for each genotype) were subjected to the FS test and analyzed as in Figure 1A. The results obtained on the second day of the test are shown. Data are means ± SEM, and the p value for comparison between WT and CD47-deficient mice was determined by repeated-measures ANOVA.
We next examined whether CD47 ablation might affect immobility time in the FS test. CD47-deficient mice manifested a significant increase in immobility time on the second day of the test compared with that apparent for WT mice (Fig. 5C). Given that CD47 is important for the FS stress-induced tyrosine phosphorylation of SIRPα, the increased immobility time observed in both SIRPα mutant and CD47-deficent mice suggests that the interaction of these two proteins plays a key role in regulation of depression-like behavior in the FS test.
FS stress-induced tyrosine phosphorylation of NR2B and Kvβ2 in the hippocampus and its dysregulation in SIRPα mutant mice
We also investigated the molecular mechanism underlying the prolonged immobility of SIRPα mutant mice in the FS test. Among various factors implicated in regulation of immobility in the FS test, monoamines, hormones related to the hypothalamic–pituitary–adrenal axis, and neurotrophic factors such as BDNF and IGF-1, are thought to be particularly important (Nestler et al., 2002; Urani et al., 2005; Duman and Monteggia, 2006). However, we found that neither the increase in the extracellular concentration of NE in the hippocampus induced by FS stress, the NE content of the hippocampus, nor the NE-stimulated generation of second messengers in hippocampal neurons differed between WT and SIRPα mutant mice (supplemental Fig. 3A–D, available at www.jneurosci.org as supplemental material). Administration of imipramine markedly shorten the immobility time in both WT and SIRPα mutant mice (supplemental Fig. 3E, available at www.jneurosci.org as supplemental material), suggesting that SIRPα is dispensable for the effect of imipramine on the immobility in FS test. In addition, no marked differences in the circadian pattern of the plasma corticosterone concentration or in the FS stress-induced increase in the plasma corticosterone level were observed between WT and SIRPα mutant mice (supplemental Fig. 4A,B, available at www.jneurosci.org as supplemental material). Moreover, signaling by either BDNF or IGF-1 did not appear to be impaired in hippocampal neurons derived from SIRPα mutant mice (supplemental Fig. 4C,D, available at www.jneurosci.org as supplemental material). We thus next investigated whether FS stress induces tyrosine phosphorylation of other molecules and whether such tyrosine phosphorylation might be dysregulated in SIRPα mutant mice. Fyn is implicated in tyrosine phosphorylation of the NR2B subunit of the NMDA-sensitive glutamate receptor (Nakazawa et al., 2006). We found that FS stress increased the extent of NR2B phosphorylation on Tyr1472 in the hippocampus of WT mice and that this effect of FS stress was mostly abolished in Fyn-deficient mice (Fig. 6A). Moreover, the FS stress-induced tyrosine phosphorylation of NR2B was greatly diminished in the hippocampus of SIRPα mutant mice compared with that apparent in WT animals (Fig. 6B).
Figure 6.
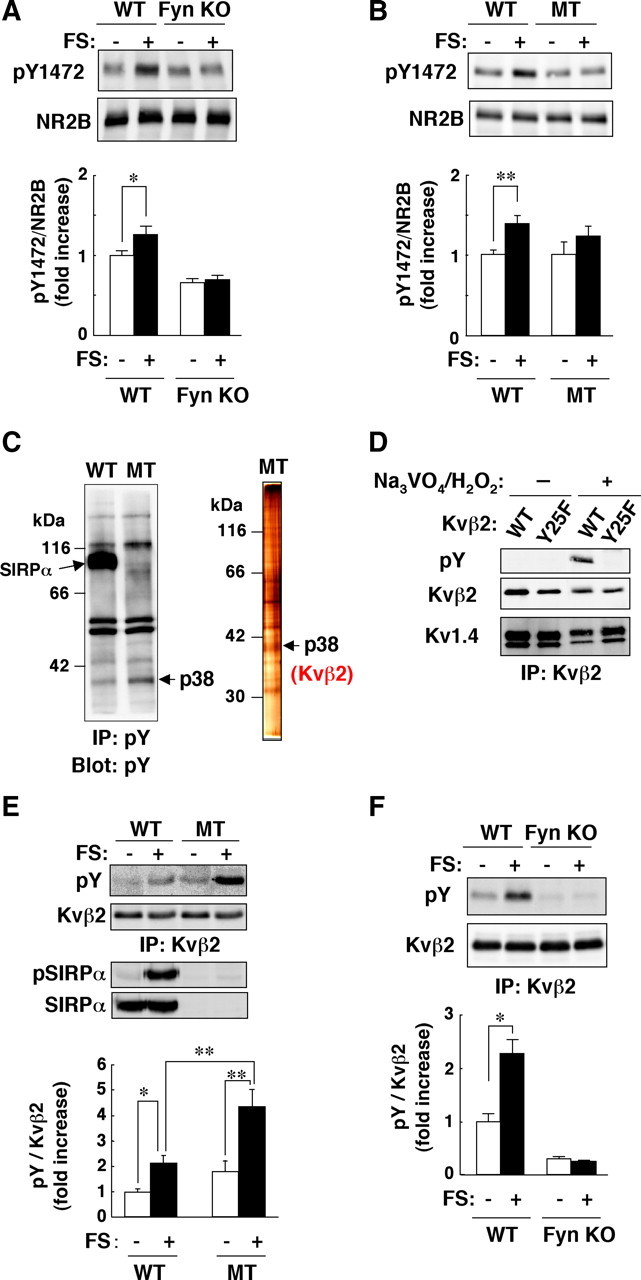
FS stress-induced tyrosine phosphorylation of NR2B and Kvβ2 in the hippocampus and its dysregulation in SIRPα mutant mice. A, B, WT and Fyn-deficient (Fyn KO; A) or SIRPα mutant (MT; B) mice were subjected to FS stress (FS+) or control treatment (FS−) for 10 min, after which hippocampal homogenates were immediately prepared and subjected to immunoblot analysis with pAbs to the Tyr1472-phosphorylated (pY1472) or total forms of the NR2B subunit of the NMDA receptor. The ratio of the intensity of the pY1472 band to that of the NR2B band is expressed as fold increase relative to the WT control (FS−) value; data are means ± SEM. for a total of seven WT and eight Fyn-deficient mice (A) or 11 or 12 WT and SIRPα mutant mice (B) for each condition in at least three independent experiments. *p < 0.05; **p < 0.01, Student's t test. C, Hippocampal membranes prepared from WT or SIRPα mutant mice exposed to FS stress as in A were subjected to immunoprecipitation with a mAb (4G10) to phosphotyrosine (pY), and the resulting precipitates were subjected to immunoblot analysis with the same antibody (left). Tyrosine-phosphorylated proteins purified from the membrane fraction of the SIRPα mutant mice with the use of 4G10-conjugated agarose were separated by SDS-PAGE and visualized by silver staining (right). Arrows indicate the positions of SIRPα and a 38 kDa protein (Kvβ2). D, HEK293T cells were transfected with an expression vector for Kv1.4 and that either for WT or Y25F mutant of Kvβ2. Forty-eight hours after transfection, the cells were treated with pervanadate (250 μm Na3VO4 and 250 μm H2O2) for 10 min. Cell lysates were then prepared and subjected to immunoprecipitation with a mAb to Kvβ2, and the resulting precipitates were subjected to immunoblot analysis with mAbs to phosphotyrosine (4G10), to Kvβ2, or to Kv1.4. Data are representative of three separate experiments. E, Hippocampal homogenates from WT or SIRPα mutant mice exposed to FS stress as in A were subjected to immunoprecipitation with a mAb to Kvβ2, and the resulting precipitates were subjected to immunoblot analysis with mAbs to phosphotyrosine (4G10) or to Kvβ2. The homogenates were also subjected to immunoblot analysis with anti-pSIRPα or pAbs to SIRPα. The ratio of the intensity of the pY band to that of the Kvβ2 band is expressed as fold increase relative to the WT control (FS−) value; data are means ± SEM. for a total of six mice of each genotype and for each condition in three independent experiments. *p < 0.05; **p < 0.01, Student's t test. F, Hippocampal homogenates from WT or Fyn-deficient mice exposed to FS stress as in A were subjected to immunoprecipitation with a mAb to Kvβ2, and the resulting precipitates were subjected to immunoblot analysis with mAbs to phosphotyrosine (4G10) or to Kvβ2. The ratio of the intensity of the pY band to that of the Kvβ2 band is expressed as fold increase relative to the WT control (FS−) value; data are means ± SEM. for a total of five (FS−) or six (FS+) mice of each genotype for each condition in two independent experiments. *p < 0.05, Student's t test. IP, Immunoprecipitation.
We also found that tyrosine phosphorylation of a 38 kDa protein was markedly increased in a membrane fraction prepared from the hippocampus of SIRPα mutant mice subjected to FS stress compared with that apparent for WT mice (Fig. 6C). The 38 kDa protein was affinity purified with the use of an agarose-conjugated mAb to phosphotyrosine, separated by SDS-PAGE, and visualized by silver staining (Fig. 6C). Analysis of the purified protein by MALDI-TOF mass spectrometry revealed it to be Kvβ2, a subunit of voltage-dependent K+ channels (National Center for Biotechnology Information accession number NM_010598), which interact with the pore-forming α subunits of the Shaker (Kv1) subfamily of K+ channels (Lai and Jan, 2006). Large-scale phosphoproteomic analysis suggested that Tyr25 of mouse Kvβ2 are potentially phosphorylated in the brain (Ballif et al., 2008). To identify the tyrosine phosphorylation site of Kvβ2, WT and mutant forms of Kvβ2 (Y2F, Y25F, or Y29F), in which Tyr2, Tyr25, or Tyr29 was substituted with Phe, were transiently coexpressed with Kv1.4, an α-subunit of Kv1 subfamily channels, in HEK293T cells, and the cells were treated with pervanadate, an inhibitor of protein tyrosine phosphatase, to enhance protein tyrosine phosphorylation in the cells. Treatment of transfected cells with pervanadate caused tyrosine phosphorylation of WT (Fig. 6D) as well as Y2F and Y29F mutants of Kvβ2 (data not shown), but not the Y25F mutant of Kvβ2 (Fig. 6D), suggesting that Tyr25 is indeed a major site for tyrosine phosphorylation of Kvβ2. Tyrosine phosphorylation of Kvβ2 was also confirmed in vivo. Immunoprecipitation performed with hippocampal homogenates and a mAb to Kvβ2 showed that FS stress induced the tyrosine phosphorylation of this protein in both WT and SIRPα mutant mice and that this effect was more pronounced in the mutant animals (Fig. 6E). We also found that the tyrosine phosphorylation of Kvβ2 was markedly reduced in the hippocampus of Fyn-deficient mice compared with that apparent with WT animals (Fig. 6F).
Discussion
The FS test has provided a useful model for characterization of the neurobiological and genetic basis of stress-induced responses that underlie despair or depression. It has also been used to predict the antidepressant efficacy of new medications in human subjects (Cryan et al., 2002; Urani et al., 2005). However, the intracellular signaling in neurons that contributes to FS stress-induced biological responses has remained unknown. We have now shown that FS stress activates SFKs and thereby induces tyrosine phosphorylation of SIRPα and formation of the SIRPα–Shp2 complex in the brain. Such SIRPα phosphorylation was prominent in the hippocampus, cortex, and amygdala, brain regions that are thought to be closely related to the pathogenesis of depression (Nestler et al., 2002). Both the hippocampus and cortex are implicated in the cognitive aspects of depression, such as memory impairment and a feeling of worthlessness. The amygdala and striatum are important for emotional memory and are implicated in the anxiety and reduced motivation associated with depression. We have now found that mice expressing a mutant form of SIRPα that lacks tyrosine phosphorylation sites and is therefore not able to form a complex with Shp2 manifest prolonged immobility in the FS test. The SFK-mediated tyrosine phosphorylation of SIRPα thus represents a novel molecular basis for the brain response to stress and the regulation of depression-like behavior in the FS test.
The SIRPα mutant mice did not exhibit an increased immobility time in the tail suspension test. Consistent with this finding, tail suspension stress had no effect on the tyrosine phosphorylation of SIRPα in the brain (data not shown). The FS test and tail suspension test are not necessarily interchangeable. In particular, the SSRI class of antidepressants induces a reproducible response in the tail suspension test but not in the FS test (Cryan et al., 2002, 2005). Other acute stressors that we tested were restraint stress and cold stress. Restraint stress also failed to induce a marked increase in the tyrosine phosphorylation of SIRPα in the brain (data not shown). Cold exposure slightly increased the tyrosine phosphorylation of SIRPα, although to a lesser extent than those induced by FS stress (data not shown).
The mechanism by which FS stress activates SFKs in the brain remains unclear but may be dependent, at least in part, on NE or serotonin. Indeed, FS stress increases the extracellular concentrations of monoamines such as NE (see supplemental Fig. 3, available at www.jneurosci.org as supplemental material) and serotonin (Fujino et al., 2002) in the mouse brain. We also showed that antidepressants, including tricyclic antidepressant, an SNRI, and an SSRI, induced the tyrosine phosphorylation of SIRPα in the hippocampus. Moreover, we found that NE directly induced the tyrosine phosphorylation of SIRPα in cultured hippocampal neurons in a manner dependent on α1-adrenergic receptors and SFKs. We also found that activation of protein kinase C (PKC) participates in the NE-induced tyrosine phosphorylation of SIRPα in cultured hippocampal neurons; this effect of NE was thus mimicked by an activator of PKC and was blocked by a PKC inhibitor (data not shown). α1-Adrenergic receptors are coupled to the heterotrimeric GTP-binding protein Gq and stimulate polyphosphoinositide hydrolysis, resulting in the activation of PKC. In addition, PKC promotes activation of SFKs in hippocampal neurons (Salter and Kalia, 2004). NE-induced activation of PKC might thus contribute, at least in part, to the activation of SFKs by FS stress.
Various homophilic or heterophilic interactions between transmembrane proteins, such as between cadherins, ephrin and Eph, semaphorins and plexins, Notch ligands and Notch, or many Ig-superfamily proteins, play important roles in axonal guidance, synaptogenesis, and synaptic plasticity underlying memory formation (Grunwald and Klein, 2002; Yamagata et al., 2003; Maness and Schachner, 2007). The importance of such proteins in the regulation of stress responses has remained primarily unknown, however, although chronic stress has been shown to downregulate expression of neural cell adhesion molecule, a neuronal Ig-superfamily protein, in the hippocampus and might thereby impair synaptic plasticity and cognitive function (Sandi, 2004). We have now shown that CD47 is required for the FS stress-induced tyrosine phosphorylation of SIRPα in the hippocampus. Moreover, CD47-deficient mice manifested prolonged immobility in the FS test, suggesting that SIRPα in neurons regulates depression-like behavior through its trans-interaction with CD47 in neighboring neurons or glia. Although CD47 was previously shown to promote activation of SFKs (Murata et al., 2006), the FS stress-induced activation of SFKs in the hippocampus was not impaired in CD47-deficient mice (data not shown). The interaction of SIRPα with CD47 might therefore be important for proper localization or stabilization of SIRPα at the plasma membrane that allows for its efficient tyrosine phosphorylation by SFKs. Ten alternative sequence patterns have been identified in the ligand-binding, NH2-terminal IgV domain of human SIRPα (Takenaka et al., 2007). Such polymorphism of SIRPα was proposed to serve as a strong genetic determinant of the engraftment of human hematopoietic stem cells, given that the interaction of CD47 (on these cells) with SIRPα (on macrophages) is important for such engraftment (Takenaka et al., 2007). Polymorphism of SIRPα might thus also be a genetic determinant of neurological or psychiatric disorders such as depression.
The precise molecular mechanism underlying the prolonged immobility of SIRPα mutant mice in the FS test remains unclear. However, we found that FS stress increased the level of phosphorylation of the NR2B subunit of the NMDA receptor at Tyr1472 in the hippocampus in a manner dependent on both Fyn and SIRPα. Tyrosine phosphorylation of NMDA receptors, which are essential for regulation of synaptic plasticity underlying learning and memory, is thought to increase receptor activity, although the precise mechanism responsible for this effect remains unknown (Salter and Kalia, 2004). Tyr1472 of NR2B, which is a major tyrosine phosphorylation site of NMDA receptors, has been shown to be required for fear-related learning and synaptic plasticity in the amygdala (Nakazawa et al., 2006). Immobility time during the second exposure of mice to FS stress is greater than that during the first exposure, indicating that the FS test provides a measure of learning and memory acquisition or consolidation as well as of despair and depression (De Pablo et al., 1989). NMDA receptors are thought to play a role in the increased immobility time observed in the second exposure of rat to FS stress (Padovan and Guimaraes, 2004). Tyrosine phosphorylation of the NR2B subunit of these receptors might thus contribute to regulation of depression-like behavior in the FS test. Furthermore, the dysregulation of FS stress-induced tyrosine phosphorylation of NR2B apparent in SIRPα mutant mice suggests that SIRPα might regulate depression-like behavior in the FS test by modulating NR2B function.
We also found that Kvβ2 in the hippocampus undergoes tyrosine phosphorylation in response to FS stress. Such tyrosine phosphorylation of Kvβ2 was markedly diminished in Fyn-deficient mice, suggesting that Fyn mediates this process. In contrast, tyrosine phosphorylation of Kvβ2 was markedly enhanced in SIRPα mutant mice. The mechanism responsible for hyperphosphorylation of Kvβ2 in the mutant animals is unclear. Kvβ2 is a member of the Kvβ family of proteins (Kvβ1, Kvβ2, Kvβ3), which interact with the cytoplasmic T1 region of the pore-forming α subunits of the Shaker subfamily of K+ channels (Lai and Jan, 2006). Although Kvβ2, unlike Kvβ1 and Kvβ3, lacks a ball-and-chain domain and does not confer the property of rapid inactivation on noninactivating Kv1 α subunits, it does increase the rate of inactivation of endogenously inactivating Kv1.4 currents (McCormack et al., 1995). The functional relevance of tyrosine phosphorylation of Kvβ2 is unknown, although we identified Tyr25 as a major site of tyrosine phosphorylation of exogenously expressed Kvβ2 in HEK293T cells. Intraventricular injection of K+-channel blockers in mice induces antidepressant effects in the FS test (Galeotti et al., 1999). In addition, ablation of TREK-1, a member of the family of two-pore domain K+ channels, also exerts an antidepressant effect in this test (Heurteaux et al., 2006), suggesting that K+ channels play important roles in regulation of depression-like behavior in the FS test. FS stress-induced tyrosine phosphorylation of Kvβ2 by SFK and its regulation by SIRPα may contribute to regulation of neuronal excitability through modulation of K+ currents.
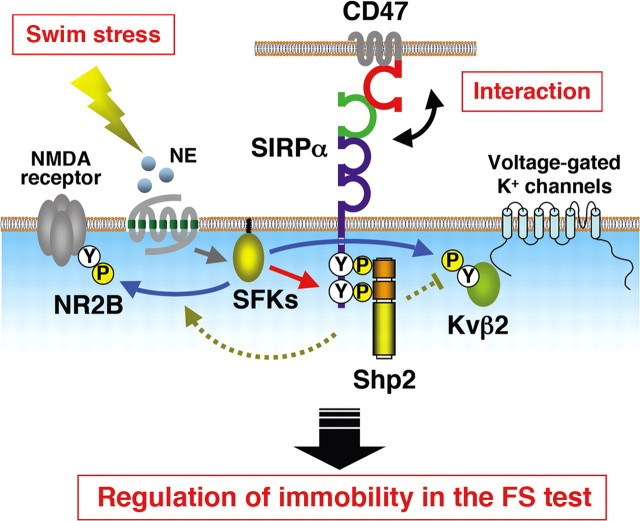
In conclusion, we propose a model for the regulation of behavioral immobility in the FS test by tyrosine phosphorylation of SIRPα (Fig. 7). FS stress induces activation of SFKs and the consequent tyrosine phosphorylation of a variety of neuronal proteins. These tyrosine-phosphorylated proteins, in particular SIRPα, are potential targets for the development of new drugs for mood disorders such as depression.
Figure 7.
Model for the regulation of behavioral immobility in the FS test by tyrosine phosphorylation of SIRPα. Severe stress such as that associated with forced swimming induces activation of SFKs and the consequent tyrosine phosphorylation of SIRPα and its formation of a complex with Shp2 (red arrow). The stress-induced increase in the extracellular concentration of NE contributes, at least in part, to SFK activation (gray arrow). Trans-interaction of SIRPα with CD47 is indispensable for the swim stress-induced tyrosine phosphorylation of SIRPα. Thus, the NE-induced tyrosine phosphorylation of SIRPα likely requires CD47. Swim stress also induces tyrosine phosphorylation both of the NR2B subunit of the NMDA receptor and of the Kvβ2 subunit of voltage-gated K+ channels (blue arrows). The phosphorylation of these molecules is regulated by SIRPα (green dotted lines), and such regulation likely underlies the modulation by SIRPα of behavioral immobility in the FS test.
Footnotes
This work was supported by the following: a Grant-in-Aid for Scientific Research on Priority Areas Cancer; a Grant-in-Aid for Scientific Research on Priority Areas Molecular Brain Science; a Grant-in-Aid for Scientific Research on Priority Areas Integrative Brain Research; a Grant-in-Aid for Scientific Research (B) and (C); a grant of the Global COE Program from the Ministry of Education, Culture, Sports, Science, and Technology of Japan; a grant from the Institute for Bioinformatics Research and Development, Japan Science and Technology Agency; grants from the Swedish Research Council (31X-14286), Takeda Science Foundation, Life Science Foundation of Japan, and the Faculty of Medicine, Umeå University; and a Young Researcher Award from Umeå University, Sweden. We thank T. Yagi, S. Yuasa, and K. Hattori for Fyn-deficient mice and a mAb to Fyn; E. Kramer and R. Klein for TrkB floxed mice and CaMKII-Cre mice; S. Koizumi for a mAb to TrkB; T. Yamamoto and T. Nakazawa for pAbs to Tyr1472-phosphorylated NR2B and to NR2B; C. F. Lagenaur for a mAb to SIRPα; H. Hirai, N. Hosoi, and A. Saiki for technical support; T. Kato for comments and discussion; S. C. Fujita, M. Watanabe, and J. Miyazaki for reagents; and H. Kobayashi, K. Tomizawa, E. Urano, and Y. Kusakari for technical assistance.
References
- Ago Y, Nakamura S, Uda M, Kajii Y, Abe M, Baba A, Matsuda T. Attenuation by the 5-HT1A receptor agonist osemozotan of the behavioral effects of single and repeated methamphetamine in mice. Neuropharmacology. 2006;51:914–922. doi: 10.1016/j.neuropharm.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Ballif BA, Carey GR, Sunyaev SR, Gygi SP. Large-scale identification and evolution indexing of tyrosine phosphorylation sites from murine brain. J Proteome Res. 2008;7:311–318. doi: 10.1021/pr0701254. [DOI] [PubMed] [Google Scholar]
- Barclay AN. Signal regulatory protein α (SIRPα)/CD47 interaction and function. Curr Opin Immunol. 2009;21:47–52. doi: 10.1016/j.coi.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EJ, Frazier WA. Integrin-associated protein (CD47) and its ligands. Trends Cell Biol. 2001;11:130–135. doi: 10.1016/s0962-8924(00)01906-1. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci. 2002;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- De Pablo JM, Parra A, Segovia S, Guillamon A. Learned immobility explains the behavior of rats in the forced swimming test. Physiol Behav. 1989;46:229–237. doi: 10.1016/0031-9384(89)90261-8. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. San Diego: Academic; 1997. The mouse brain in stereotaxic coordinates. [Google Scholar]
- Fujino K, Yoshitake T, Inoue O, Ibii N, Kehr J, Ishida J, Nohta H, Yamaguchi M. Increased serotonin release in mice frontal cortex and hippocampus induced by acute physiological stressors. Neurosci Lett. 2002;320:91–95. doi: 10.1016/s0304-3940(02)00029-0. [DOI] [PubMed] [Google Scholar]
- Galeotti N, Ghelardini C, Caldari B, Bartolini A. Effect of potassium channel modulators in mouse forced swimming test. Br J Pharmacol. 1999;126:1653–1659. doi: 10.1038/sj.bjp.0702467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald IC, Klein R. Axon guidance: receptor complexes and signaling mechanisms. Curr Opin Neurobiol. 2002;12:250–259. doi: 10.1016/s0959-4388(02)00323-9. [DOI] [PubMed] [Google Scholar]
- Heckman KL, Pease LR. Gene splicing and mutagenesis by PCR-driven overlap extension. Nat Protoc. 2007;2:924–932. doi: 10.1038/nprot.2007.132. [DOI] [PubMed] [Google Scholar]
- Heurteaux C, Lucas G, Guy N, El Yacoubi M, Thummler S, Peng XD, Noble F, Blondeau N, Widmann C, Borsotto M, Gobbi G, Vaugeois JM, Debonnel G, Lazdunski M. Deletion of the background potassium channel TREK-1 results in a depression-resistant phenotype. Nat Neurosci. 2006;9:1134–1141. doi: 10.1038/nn1749. [DOI] [PubMed] [Google Scholar]
- Inagaki K, Yamao T, Noguchi T, Matozaki T, Fukunaga K, Takada T, Hosooka T, Akira S, Kasuga M. SHPS-1 regulates integrin-mediated cytoskeletal reorganization and cell motility. EMBO J. 2000;19:6721–6731. doi: 10.1093/emboj/19.24.6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki Y, Ishikawa M, Okada N, Koizumi S. Induction of a distinct morphology and signal transduction in TrkB/PC12 cells by nerve growth factor and brain-derived neurotrophic factor. J Neurochem. 1997;68:927–934. doi: 10.1046/j.1471-4159.1997.68030927.x. [DOI] [PubMed] [Google Scholar]
- Jiang P, Lagenaur CF, Narayanan V. Integrin-associated protein is a ligand for the P84 neural adhesion molecule. J Biol Chem. 1999;274:559–562. doi: 10.1074/jbc.274.2.559. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Lai HC, Jan LY. The distribution and targeting of neuronal voltage-gated ion channels. Nat Rev Neurosci. 2006;7:548–562. doi: 10.1038/nrn1938. [DOI] [PubMed] [Google Scholar]
- Lindquist JM, Fredriksson JM, Rehnmark S, Cannon B, Nedergaard J. β3- and α1-Adrenergic Erk1/2 activation is Src- but not Gi-mediated in Brown adipocytes. J Biol Chem. 2000;275:22670–22677. doi: 10.1074/jbc.M909093199. [DOI] [PubMed] [Google Scholar]
- Maness PF, Schachner M. Neural recognition molecules of the immunoglobulin superfamily: signaling transducers of axon guidance and neuronal migration. Nat Neurosci. 2007;10:19–26. doi: 10.1038/nn1827. [DOI] [PubMed] [Google Scholar]
- Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nat Neurosci. 2007;10:1089–1093. doi: 10.1038/nn1971. [DOI] [PubMed] [Google Scholar]
- Matozaki T, Murata Y, Okazawa H, Ohnishi H. Functions and molecular mechanisms of the CD47-SIRPα signalling pathway. Trends Cell Biol. 2009;19:72–80. doi: 10.1016/j.tcb.2008.12.001. [DOI] [PubMed] [Google Scholar]
- McCormack K, McCormack T, Tanouye M, Rudy B, Stuhmer W. Alternative splicing of the human Shaker K+ channel β1 gene and functional expression of the β2 gene product. FEBS Lett. 1995;370:32–36. doi: 10.1016/0014-5793(95)00785-8. [DOI] [PubMed] [Google Scholar]
- Mi ZP, Jiang P, Weng WL, Lindberg FP, Narayanan V, Lagenaur CF. Expression of a synapse-associated membrane protein, P84/SHPS-1, and its ligand, IAP/CD47, in mouse retina. J Comp Neurol. 2000;416:335–344. [PubMed] [Google Scholar]
- Minichiello L, Korte M, Wolfer D, Kuhn R, Unsicker K, Cestari V, Rossi-Arnaud C, Lipp HP, Bonhoeffer T, Klein R. Essential role for TrkB receptors in hippocampus-mediated learning. Neuron. 1999;24:401–414. doi: 10.1016/s0896-6273(00)80853-3. [DOI] [PubMed] [Google Scholar]
- Miyakawa T, Yamada M, Duttaroy A, Wess J. Hyperactivity and intact hippocampus-dependent learning in mice lacking the M1 muscarinic acetylcholine receptor. J Neurosci. 2001;21:5239–5250. doi: 10.1523/JNEUROSCI.21-14-05239.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa T, Leiter LM, Gerber DJ, Gainetdinov RR, Sotnikova TD, Zeng H, Caron MG, Tonegawa S. Conditional calcineurin knockout mice exhibit multiple abnormal behaviors related to schizophrenia. Proc Natl Acad Sci U S A. 2003;100:8987–8992. doi: 10.1073/pnas.1432926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishima Y, Miyakawa T, Furuyashiki T, Tanaka Y, Mizuma H, Nakanishi S. Enhanced cocaine responsiveness and impaired motor coordination in metabotropic glutamate receptor subtype 2 knockout mice. Proc Natl Acad Sci U S A. 2005;102:4170–4175. doi: 10.1073/pnas.0500914102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata T, Ohnishi H, Okazawa H, Murata Y, Kusakari S, Hayashi Y, Miyashita M, Itoh H, Oldenborg PA, Furuya N, Matozaki T. CD47 promotes neuronal development through Src- and FRG/Vav2-mediated activation of Rac and Cdc42. J Neurosci. 2006;26:12397–12407. doi: 10.1523/JNEUROSCI.3981-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Watanabe M, Isobe T, Kondo H, Inoue Y. Cytological compartmentalization in the staggerer cerebellum, as revealed by calbindin immunohistochemistry for Purkinje cells. J Comp Neurol. 1998;395:112–120. doi: 10.1002/(sici)1096-9861(19980525)395:1<112::aid-cne8>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Nakazawa T, Komai S, Watabe AM, Kiyama Y, Fukaya M, Arima-Yoshida F, Horai R, Sudo K, Ebine K, Delawary M, Goto J, Umemori H, Tezuka T, Iwakura Y, Watanabe M, Yamamoto T, Manabe T. NR2B tyrosine phosphorylation modulates fear learning as well as amygdaloid synaptic plasticity. EMBO J. 2006;25:2867–2877. doi: 10.1038/sj.emboj.7601156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neel BG, Gu H, Pao L. The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci. 2003;28:284–293. doi: 10.1016/S0968-0004(03)00091-4. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- Obata K, Kojima N, Nishiye H, Inoue H, Shirao T, Fujita SC, Uchizono K. Four synaptic vesicle-specific proteins: identification by monoclonal antibodies and distribution in the nervous tissue and the adrenal medulla. Brain Res. 1987;404:169–179. doi: 10.1016/0006-8993(87)91368-0. [DOI] [PubMed] [Google Scholar]
- Ohe Y, Ohnishi H, Okazawa H, Tomizawa K, Kobayashi H, Okawa K, Matozaki T. Characterization of nucleotide pyrophosphatase-5 as an oligomannosidic glycoprotein in rat brain. Biochem Biophys Res Commun. 2003;308:719–725. doi: 10.1016/s0006-291x(03)01454-2. [DOI] [PubMed] [Google Scholar]
- Ohnishi H, Kaneko Y, Okazawa H, Miyashita M, Sato R, Hayashi A, Tada K, Nagata S, Takahashi M, Matozaki T. Differential localization of Src homology 2 domain-containing protein tyrosine phosphatase substrate-1 and CD47 and its molecular mechanisms in cultured hippocampal neurons. J Neurosci. 2005;25:2702–2711. doi: 10.1523/JNEUROSCI.5173-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science. 2000;288:2051–2054. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- Padovan CM, Guimaraes FS. Antidepressant-like effects of NMDA-receptor antagonist injected into the dorsal hippocampus of rats. Pharmacol Biochem Behav. 2004;77:15–19. doi: 10.1016/j.pbb.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Page ME, Brown K, Lucki I. Simultaneous analyses of the neurochemical and behavioral effects of the norepinephrine reuptake inhibitor reboxetine in a rat model of antidepressant action. Psychopharmacology (Berl) 2003;165:194–201. doi: 10.1007/s00213-002-1269-x. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Salter MW, Kalia LV. Src kinases: a hub for NMDA receptor regulation. Nat Rev Neurosci. 2004;5:317–328. doi: 10.1038/nrn1368. [DOI] [PubMed] [Google Scholar]
- Sandi C. Stress, cognitive impairment and cell adhesion molecules. Nat Rev Neurosci. 2004;5:917–930. doi: 10.1038/nrn1555. [DOI] [PubMed] [Google Scholar]
- Sato R, Ohnishi H, Kobayashi H, Kiuchi D, Hayashi A, Kaneko Y, Honma N, Okazawa H, Hirata Y, Matozaki T. Regulation of multiple functions of SHPS-1, a transmembrane glycoprotein, by its cytoplasmic region. Biochem Biophys Res Commun. 2003;309:584–590. doi: 10.1016/j.bbrc.2003.08.031. [DOI] [PubMed] [Google Scholar]
- Seligman ME, Maier SF. Failure to escape traumatic shock. J Exp Psychol. 1967;71:1–9. doi: 10.1037/h0024514. [DOI] [PubMed] [Google Scholar]
- Shibata M, Yamasaki N, Miyakawa T, Kalaria RN, Fujita Y, Ohtani R, Ihara M, Takahashi R, Tomimoto H. Selective impairment of working memory in a mouse model of chronic cerebral hypoperfusion. Stroke. 2007;38:2826–2832. doi: 10.1161/STROKEAHA.107.490151. [DOI] [PubMed] [Google Scholar]
- Takenaka K, Prasolava TK, Wang JC, Mortin-Toth SM, Khalouei S, Gan OI, Dick JE, Danska JS. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nat Immunol. 2007;8:1313–1323. doi: 10.1038/ni1527. [DOI] [PubMed] [Google Scholar]
- Urani A, Chourbaji S, Gass P. Mutant mouse models of depression: candidate genes and current mouse lines. Neurosci Biobehav Rev. 2005;29:805–828. doi: 10.1016/j.neubiorev.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Wong EH, Sonders MS, Amara SG, Tinholt PM, Piercey MF, Hoffmann WP, Hyslop DK, Franklin S, Porsolt RD, Bonsignori A, Carfagna N, McArthur RA. Reboxetine: a pharmacologically potent, selective, and specific norepinephrine reuptake inhibitor. Biol Psychiatry. 2000;47:818–829. doi: 10.1016/s0006-3223(99)00291-7. [DOI] [PubMed] [Google Scholar]
- Xu W, Doshi A, Lei M, Eck MJ, Harrison SC. Crystal structures of c-Src reveal features of its autoinhibitory mechanism. Mol Cell. 1999;3:629–638. doi: 10.1016/s1097-2765(00)80356-1. [DOI] [PubMed] [Google Scholar]
- Yagi T, Aizawa S, Tokunaga T, Shigetani Y, Takeda N, Ikawa Y. A role for Fyn tyrosine kinase in the suckling behaviour of neonatal mice. Nature. 1993;366:742–745. doi: 10.1038/366742a0. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Sanes JR, Weiner JA. Synaptic adhesion molecules. Curr Opin Cell Biol. 2003;15:621–632. doi: 10.1016/s0955-0674(03)00107-8. [DOI] [PubMed] [Google Scholar]
- Yasunaga M, Yagi T, Hanzawa N, Yasuda M, Yamanashi Y, Yamamoto T, Aizawa S, Miyauchi Y, Nishikawa S. Involvement of Fyn tyrosine kinase in progression of cytokinesis of B lymphocyte progenitor. J Cell Biol. 1996;132:91–99. doi: 10.1083/jcb.132.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]