Abstract
The generation of a precise number of neural cells and the determination of their laminar fate are tightly controlled processes during development of the cerebral cortex. Using genetic tracing in mice, we have identified a population of glutamatergic neurons generated by Dbx1-expressing progenitors at the pallial–subpallial boundary predominantly at embryonic day 12.5 (E12.5) and subsequent to Cajal–Retzius cells. We show that these neurons migrate tangentially to populate the cortical plate (CP) at all rostrocaudal and mediolateral levels by E14.5. At birth, they homogeneously populate cortical areas and represent <5% of cortical cells. However, they are distributed into neocortical layers according to their birthdates and express the corresponding markers of glutamatergic differentiation (Tbr1, ER81, Cux2, Ctip2). Notably, this population dies massively by apoptosis at the completion of corticogenesis and represents 50% of dying neurons in the postnatal day 0 cortex. Specific genetic ablation of these transient Dbx1-derived CP neurons leads to a 20% decrease in neocortical cell numbers in perinatal animals. Our results show that a previously unidentified transient population of glutamatergic neurons migrates from extraneocortical regions over long distance from their generation site and participates in neocortical radial growth in a non-cell-autonomous manner.
Introduction
The generation of a precise number of neurons during development is a crucial step in the formation of functional neural circuits (Kriegstein et al., 2006). In the developing cerebral cortex growth factors, regulators of the cell cycle and transcription factors intrinsic to the neocortical primordium control neurogenesis (Dehay and Kennedy, 2007; O'Leary et al., 2007). However, secretion of growth factors from signaling centers at the borders of the pallium during early stages of development and from ingrowing thalamic afferents during midcorticogenesis has been shown to provide an extrinsic control of neocortical growth (Dehay et al., 2001). In addition, an activity-dependent induction of programmed cell death (PCD) contributes to a specific refinement of the number of cortical neurons at early postnatal stages (Haydar et al., 1999; Verney et al., 2000). Nevertheless, the contribution of intrinsic versus extrinsic cues in the final output of corticogenesis remains an open question.
In the mammalian neocortex, neurons organize in a laminated structure according to an “inside-out” sequence (Rakic, 1974). During development, the first generated neurons constitute the preplate (PP), which is later split into an upper marginal zone (MZ) and a lower subplate layer (SP) by successive waves of glutamatergic neurons that form the cortical plate (CP) (Berry et al., 1964). In contrast to GABAergic interneurons, which invade the developing cortex by tangential migration, glutamatergic neurons are thought to be born locally from pallial progenitors and to mostly reach their final laminar destination via radial glia-mediated migration (Rakic, 1972; Marín-Padilla, 1998; Kriegstein and Noctor, 2004).
Glutamatergic Cajal–Retzius (CR) and subplate/pioneer cells are among the earliest generated neuronal classes populating the preplate and have been shown to play crucial roles in cortical development by controlling lamination and thalamic axons pathfinding (Meyer et al., 1998; Supèr et al., 1998; Zecevic and Rakic, 2001). CR subtypes arise from focal progenitor domains at the edges of the developing pallium and invade the preplate by tangential migration (Takiguchi-Hayashi et al., 2004; Bielle et al., 2005; Yoshida et al., 2006). Notably, both CR and SP cells mostly disappear at the end of development (Meyer et al., 1998; Supèr et al., 1998). Nevertheless, a much higher number of transient cells and structures appear to exist as suggested by the observation of transient markers staining (Mitrovic and Schachner, 1996; Huh et al., 1997) and transient axonal projections (Innocenti and Price, 2005). Furthermore, up to 30% of cortical cells have been described to undergo apoptosis in the CP during the first 2 postnatal weeks in rodents (Ferrer et al., 1990; Haydar et al., 1999; Verney et al., 2000). Despite these observations, the existence, disappearance, and function of cortical transient cells have so far remained elusive because of the inability to label them throughout their life.
In this paper, we characterize a previously unidentified transient population of glutamatergic neurons in the developing cortical plate that migrate tangentially from the pallial–subpallial boundary (PSB) and play a crucial role in the control of cell numbers in the neocortex.
Materials and Methods
Animals.
All animals were kept in C57BL/6 background and use of mice in this study was approved by the Veterinary Services of Paris. Transient labeling of Dbx1-expressing progenitors and Dbx1-derived early postmitotic cells was realized using the Dbx1nlslacZ mouse line (Pierani et al., 2001). Permanent tracing of Dbx1-derived cells, from embryonic stages to adulthood, was performed using Dbx1iresCRE animals (Bielle et al., 2005) in which the coding sequence of the CRE recombinase has been inserted by homologous recombination in the 3′-untranslated region of the Dbx1 gene and therefore expressing the CRE under the control of the Dbx1 promoter. These animals were crossed with either the TauloxP-stop-loxP-MARCKSeGFP-IRES-nlslacZ or the ROSA26loxP-stop-loxP-YFP reporter mouse lines (Srinivas et al., 2001; Hippenmeyer et al., 2005), which label exclusively postmitotic neurons and progenitors/neurons/glial lineages, respectively. A higher total number of labeled Dbx1-derived neurons was observed in Dbx1CRE;TauGFP-IRES-nlslacZ with respect to Dbx1CRE;ROSA26YFP embryos, likely because of a higher sensitivity of CRE-mediated recombination and/or higher expression levels at the Tau locus. The conditional ablation of Dbx1-derived cells was performed by crossing the Dbx1loxP-stop-loxP-DTA mouse line (Bielle et al., 2005) with the E1-Ngn2/CRE(iresGFP) strain (Berger et al., 2004) expressing the CRE recombinase and the green fluorescent protein (GFP) under the control of the E1 enhancer element of the Ngn2 gene. Embryos and postnatal animals were genotyped by PCR using primers specific for the different alleles. For staging of animals, midday of vaginal plug was considered as embryonic day 0.5 (E0.5).
Tissue preparation, immunohistochemistry, XGal staining, and in situ hybridization.
Embryos were fixed by immersion in 4% paraformaldehyde (PFA) in 0.1 m phosphate buffer (PB), pH 7.2, for 2 h at 4°C, and postnatal animals were anesthetized and perfused with 4% PFA in 0.1 m PB for 10 min. Dissected brains were then rinsed in PBS for 2 h, cryoprotected overnight in 30% sucrose in 0.1 m PB, and embedded in OCT compound (Sakura). Embedded tissues were sectioned on a cryostat with a 12–14 μm for embryonic and 35 μm step for postnatal stages. Fluorescent immunohistochemistry, Nissl staining, in situ hybridization, and XGal staining were performed as previously described (Bielle et al., 2005; Dubreuil et al., 2008). For 3,3′-diaminobenzidine tetrahydrochloride (DAB) staining, anti-GFP and anti-βGal antibodies were detected with a biotinylated secondary antibody using the Elite Vectastain ABC kit (Vector Laboratories) (see Figs. 4L, 6A–D; supplemental Figs. 2, 3, available at www.jneurosci.org as supplemental material). For DAB immunostaining subsequent to vesicular glutamate transporter 2 (VGlut2) mRNA in situ detection, the hybridization was processed in the absence of proteinase K treatment. The double immunohistochemistry using rabbit anti-GABA and rabbit anti-glutamate in supplemental Figure 1 (available at www.jneurosci.org as supplemental material) was performed using Zenon Alexa Fluor 647 rabbit IgG according to the manufacturer's protocol (Invitrogen). Primary antibodies produced in mice were as follows: anti-Reelin (Calbiochem; G10; 1:500), anti-Ngn2 (gift from D. J. Anderson), anti-Mash1 (BD Biosciences Pharmingen; 1:10), anti-NeuN (Euromedex; 1:400); primary antibodies produced in rabbit were as follows: anti-Calbindin (Swant; CB8a; 1:4000), anti-activated Caspase3 (Cell Signaling; 5A1; 1:800), anti-Cux2 (Santa Cruz; CDP M-222; 1:300), anti-GABA (Sigma-Aldrich; 1:4000), anti-glutamate (Sigma-Aldrich; 1:5000), anti-Dbx1 (gift from S. Morton and T. M. Jessell, Howard Hughes Medical Institute, Columbia University, New York, NY; 1:10,000), anti-Olig2 (Millipore; AB9610; 1:500), anti-ER81 (gift from S. Arber, Biozentrum, Basel, Switzerland, and T. M. Jessell), anti-Tbr1 (Millipore Bioscience Research Reagents; 1:4000), anti-Tbr2 (Millipore Bioscience Research Reagents; 1:8000), anti-GFP (Invitrogen; 1:2000). We also used rat anti-Ctip2 (Abcam; 1:300), chick anti-GFP (AvesLab; 1:2000), chick anti-βGal (Abcam; 1:4000), and guinea pig anti-VGlut2 (gift from T. Kaneko, Kyoto University, Kyoto, Japan; 1:1000). Birthdating experiments were performed on animals obtained from pregnant females injected intraperitoneally with a single dose of 5-bromodeoxyuridine (BrdU) (Sigma-Aldrich; 50 mg/kg). BrdU staining was performed using rat anti-BrdU (Accurate Chemical; 1:400) after 5 min of 4 m HCl treatment. 4′,6-Diamidino-2-phenylindole (DAPI) staining was performed using Vectashield Mounting with DAPI from Biovalley. All fluorescent secondary antibodies were purchased from Jackson ImmunoResearch. Terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick end labeling (TUNEL) staining was performed according to the manufacturer's protocol (Roche).
Figure 4.
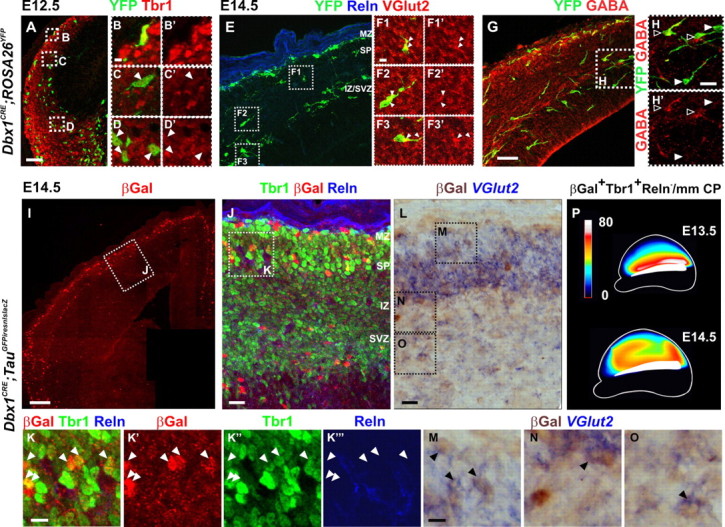
Dbx1-derived glutamatergic neurons present the morphology of tangentially migrating cells and progressively populate the entire pallium by E14.5. A–D′, Immunostaining for YFP and Tbr1 on coronal sections of E12.5 Dbx1CRE;ROSA26YFP animals shows a deep stream of YFP+Tbr1+ cells emerging from the PSB (D, D′) and showing a typical morphology of migrating cells up to the lateral and dorsolateral pallium (C, C′). B and B′ correspond to a Dbx1-derived CR neuron in the MZ (Reln staining not shown). E–H′, Immunostaining for YFP (E–H) and VGlut2 (E–F3′) or GABA (G–H′) on serial coronal sections of E14.5 Dbx1CRE;ROSA26YFP animals labeling either Dbx1-derived glutamatergic neurons (F1–F3′,H,H′, white arrowheads) or inhibitory interneurons (H, H′, black arrowheads). Both populations migrate through the IZ. Some YFP+VGlut2+ neurons are observed invading the CP by radial migration (F1, F1′). Images in F1–F3′ represent a 1-μm-thick confocal plane. I–K‴, Numerous βGal+Tbr1+Reln− cells are detected at rostrodorsal levels in the CP of Dbx1CRE;TauGFPiresnlslacZ animals at E14.5. βGal+Tbr1+Reln+ CR cells position specifically in the MZ. K–K‴, High magnifications of boxed region in J. K′–K‴ show single-channel images. βGal+Tbr1+Reln− cells are distributed in the dorsal CP by E14.5 (white arrowheads). L–O, Immunostaining for βGal after in situ hybridization with a VGlut2 mRNA probe shows double-labeled cells in the IZ, SP, and CP of E14.5 Dbx1CRE;TauGFPiresnlslacZ rostrodorsal cortices. M–O, High magnifications of boxed regions in L. P, Schematic representation of the distribution of βGal+Tbr1+Reln− neurons in the CP of E13.5 and E14.5 Dbx1CRE;TauGFPiresnlslacZ cortices (lateral view) (for values, see supplemental Table 1, available at www.jneurosci.org as supplemental material). Scale bars: I, 100 μm; A, E, G, 50 μm; J, L, 20 μm; F1, K, M, 10 μm.
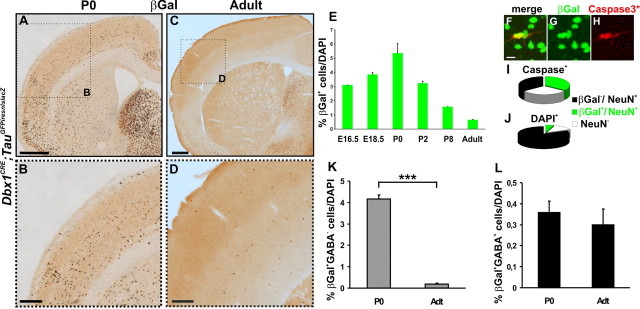
Figure 6.
Glutamatergic Dbx1-derived cells undergo programmed cell death at birth. A–D, βGal immunohistochemistry on coronal sections of Dbx1CRE;TauGFPiresnlslacZ brains shows a strong decrease in the number of βGal+ neurons in the adult neocortex (C, D), compared with neonatal (A, B) animals. B, D, High magnifications of boxed regions in A and C, respectively. E, Graph represents the percentage of βGal+ cells relative to DAPI+ nuclei in the CP/layers II–VI from E16.5 to adulthood (n = 3095 at E16.5, n = 16,785 at E18.5, n = 34,712 at P0, n = 19,600 at P2, n = 9865 at P8, and n = 34,712 in adults). F–H, Activated-Caspase3 immunohistochemistry shows colocalization with βGal+ cells in P0 Dbx1CRE;TauGFPiresnlslacZ cerebral cortices. I, Graph shows the proportion of βGal+ cells relative to Caspase3*+ cells. βGal+ neurons represent 34.9 ± 0.39% of total Caspase3*+ cells (n = 111 of 316 cells) and 50.26 ± 1.51% of NeuN+Caspase3*+ neurons (n = 97 of 192 cells); 29.69 ± 2.42% of total cortical Caspase3*+ cells are NeuN− and, thus, are likely to represent glial cells (n = 83/275). J, NeuN staining on coronal sections of P0 cortices labels 87.13 ± 2.33% of total DAPI+ nuclei and βGal+NeuN+ cells represent 4.02 ± 0.09% of the total NeuN+ nuclei. K, L, Graphs represent the percentage of βGal+GABA−/DAPI+ (K) and βGal+GABA+/DAPI+ (L) neurons at P0 and in adults. The percentage of βGal+GABA− neurons is decreased by 95.6% between P0 and adult stages (from 4.16 ± 0.18 to 0.18 ± 0.00063%; n = 15,037 at P0 and n = 1000 in adults; p = 0.00042), whereas that of βGal+GABA+ cells does not change (from 0.36 ± 0.05 to 0.29 ± 0.027%; n = 1343 at P0 and n = 1554 in adults; p = 0.54794). Results are expressed as mean ± SEM. ***p < 0.001, t test. Scale bars: A, C, 500 μm; B, D, 200 μm; F, 10 μm.
Slice culture, transplantation experiments, and CMTMR injections.
Three hundred micrometer slices of E12.5 Dbx1CRE;ROSA26YFP, Dbx1CRE;TauGFPiresnlslacZ or control embryos were prepared from fresh brain tissue using a vibratome and cultured for 2 d as previously described (Bielle et al., 2005). For grafting experiments, ventricular zone (VZ) explants were dissected from the PSB of Dbx1CRE;TauGFPiresnlslacZ embryos and used to replace the VZ at the PSB at the same rostrocaudal (RC) level of control slices collected from littermate wild-type embryos. For the PSB removal experiments, the PSB was dissected out from Dbx1CRE;TauGFPiresnlslacZ embryos before culturing. For (5-(6)-(((4-chloromethyl)benzoyl)amino)tetramethylrhodamine (CMTMR) injections, a pellet of 0.7 μm tungsten beads (Bio-Rad; M-10) coated with CellTracker Orange (CMTMR) in 10 mm DMSO (Invitrogen) was inserted into the VZ of the PSB. After 2 d in vitro (DIV), slices were fixed for 45 min with 4% PFA in 0.1 m PB and rinsed for 1 h in PBS before immunostaining with primary antibodies in PBS, 0.2% Triton, and 1% horse serum overnight. After three washes in PBS, 0.2% Triton, and 1% horse serum, the slices were incubated overnight with secondary fluorescent antibodies in the same buffer. For immunostaining of CMTMR experiments, 100 μm serial sections were prepared from each slice and incubated with different primary antibodies.
Data collection.
Countings of cells labeled by immunofluorescence were performed manually on 12–14 μm sections for E11.5 to E14.5 embryonic stages and on 35 μm sections for E16.5 to postnatal stages [with the exception of (1)] using the ImageJ software. Countings of cells labeled by DAB immunostaining and DAPI were performed semiautomatically on 35 μm sections for E16.5 to adult stages.
(1) For cell density estimation of NeuN+, Olig2+, and glutamate+ cells of the total number of DAPI+, and of βGal+ cells of NeuN+, glutamate+, and DAPI+ cells (Table 1) at postnatal day 0 (P0), cells were manually counted in >20 boxes of 100 × 100 μm in the dorsolateral cortex at three rostrocaudal levels on one single 1-μm-thick confocal section.
(2) For analysis of the distribution of βGal+GABA− neurons (see Fig. 1B), the whole cortical surface on coronal sections of P0 brains was divided in four mediolateral (ML) regions, labeled cells were counted in each region and normalized by square millimeter of cortical surface. Counting was performed at three RC levels.
(3) For analysis of the distribution of βGal+GABA+, βGal+GABA−, βGal+BrdU+GABA−, and βGal+ neurons in the CP, the thickness of the dorsolateral CP at three RC levels was divided into 10 bins of equal size, and cells were counted in each delineated area (see Figs. 1C, 2D; supplemental Fig. 1, available at www.jneurosci.org as supplemental material).
(4) For quantifications of cell density changes during aging, DAPI+ cells were counted in 12–18 boxes of 100 × 100 μm for each age, arbitrarily distributed in the CP (supplemental Fig. 3, available at www.jneurosci.org as supplemental material).
(5) The kinetics of disappearance of Dbx1-derived cells was obtained by counting the total number of labeled cells by βGal or yellow fluorescent protein (YFP) staining in the whole cortex on five sections of 35 μm distributed along the RC axis. Regions of interest corresponding to the cortical plate were designed using free hand lines selection. As the size of counted regions varied, the number of cells were normalized by square millimeter of cortical regions. Semiautomatic image processing was realized using the ImageJ software (ImageJ; W. S. Rasband, National Institutes of Health, Bethesda, MD; http://rsb.info.nih.gov/ij/; 1997–2007). Cells segmentation was done by applying gray-level thresholding on wavelets transformed images (third wavelet coefficient image). A B-spline wavelet “à trous” was used for transformation. ImageJ watershed was used to separate joined spots. Spots containing >8 pixels (magnification, 5×; pixel size, 1.29 μm) were counted as cells. Manual countings were also performed on each animal to control the validity of this technique. Evaluation of Dbx1-derived cells over the total number of DAPI+ cells was then obtained by dividing the βGal+ per square millimeter by DAPI per square millimeter at corresponding stages (see Fig. 6E; supplemental Fig. 3, available at www.jneurosci.org as supplemental material).
(6) The cell density per square millimeter of glutamatergic and GABAergic populations in P0 and adults animals (see Fig. 6K,L) was performed by counting the βGal+GABA− per square millimeter and βGal+GABA+ per square millimeter neurons at both ages in three coronal sections on three different animals. The evaluation of glutamatergic versus GABAergic cell density on DAPI was then normalized for DAPI per square millimeter at corresponding stages.
(7) To represent the distribution of βGal+Tbr1+Reln− neurons in a lateral view of whole-mount brains at E13.5 and E14.5 (see Fig. 4P), labeled cells were counted in 16 boxes spanning the PP/CP along the RC and ML axis and normalized for 1 mm of CP length (supplemental Table 1, available at www.jneurosci.org as supplemental material). Each region was assigned a gray value corresponding to the βGal+Tbr1+Reln− neurons density using the gradient mesh tool in Adobe Illustrator CS3. Gray values were converted in colors following the inverted royal lookup table in ImageJ software.
(8) For quantification of CP thickness in control and mutant animals, Nissl staining was used to delineate the cortical plate, based on the differential cell density (see Fig. 7K–R).
(9) For quantifications in Dbx1DTA;Dbx1nlsLacZ control and E1-Ngn2/CRE;Dbx1DTA;Dbx1nlsLacZ triple mutant animals, the number of XGal+ cells in the CP was counted in bands that were placed over the rostrodorsal and caudolateral regions of the pallium in region-matched control and mutant sections. All measurements realized in these boxes were normalized for the number of XGal+ cells per millimeter of CP (supplemental Fig. 4, available at www.jneurosci.org as supplemental material).
Table 1.
Dbx1-derived neurons in the P0 cerebral cortex
| Percentage (%) | |
|---|---|
| βGal+/DAPI+ | 4.54 ± 0.90 |
| NeuN+/DAPI+ | 87.13 ± 2.33 |
| βGal+NeuN+/NeuN+ | 4.02 ± 0.09 |
| βGal+Glu+/Glu+ | 4.49 ± 1.16 |
| βGal+Glu+/DAPI+ | 3.99 ± 1.04 |
| βGal+GABA+/DAPI+ | 0.42 ± 0.14 |
| βGal+NeuN+/βGal+ | 96.97 ± 0.05 |
| βGal+Glu+/βGal+ | 91.38 ± 3.55 |
| βGal+GABA+/βGal+ | 7.85 ± 0.75 |
Results are expressed as mean ± SEM.
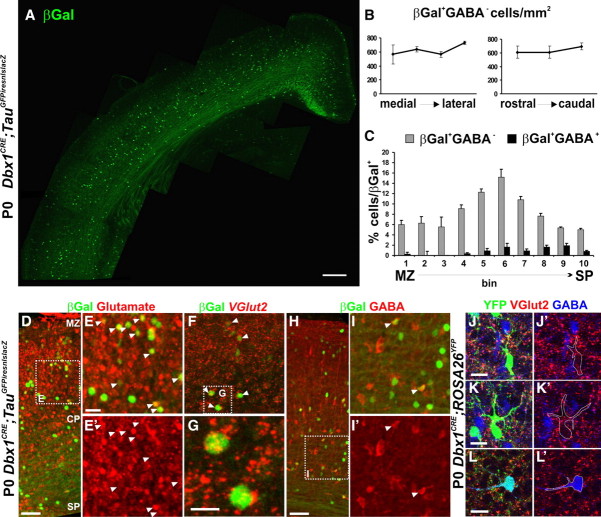
Figure 1.
Distribution and neurochemical properties of Dbx1-derived neurons in the cortical plate. A, Immunohistochemistry for βGal on coronal sections of P0 Dbx1CRE;TauGFPiresnlslacZ animals shows the distribution of Dbx1-derived neurons in the neocortex. B, Graphs show the homogeneous distribution of βGal+GABA− neurons per square millimeter along the mediolateral and rostrocaudal axis (see Materials and Methods). C, Histogram shows the distribution of βGal+GABA− and βGal+GABA+ neurons relative to total number of βGal+ neurons (in percentage) in the dorsolateral neocortex divided in 10 bins in its radial dimension. Results are expressed as mean ± SEM. D–I′, Immunohistochemistry for βGal and glutamate (D–E′) or GABA (H–I′) on coronal sections of P0 Dbx1CRE;TauGFPiresnlslacZ animals. F, G, Fluorescent in situ hybridization for VGlut2 followed by βGal immunostaining on P0 Dbx1CRE;TauGFPiresnlslacZ brains. E, G, I, High magnifications of dashed boxes in D, F, and H, respectively. The vast majority of Dbx1-derived neurons are Glu+ (91.38 ± 3.55%; n = 743 of 813) or VGlut2+ (83.6 ± 1.06%; n = 98 of 116), whereas a small percentage are GABA+ (7.85 ± 0.75%; n = 108 of 1380) or VGlut2− (6.3 ± 0.53%; n = 7 of 116). The white arrowheads show colabeled cells. J–L, Immunohistochemistry for VGlut2, GABA, and YFP on Dbx1CRE;ROSA26YFP animal at P0 shows the neurochemical properties of Dbx1-derived cells in the CP. Many of the YFP+ cells express VGlut2+ but not GABA (J–K′). Conversely, the YFP+GABA+ cells are not labeled with VGlut2 (L, L′). Images in J–L′ represent a 1-μm-thick confocal plane. Scale bars: A, 200 μm; D, E, 50 μm; F, J, K, L, 20 μm; G, 10 μm.
Figure 2.
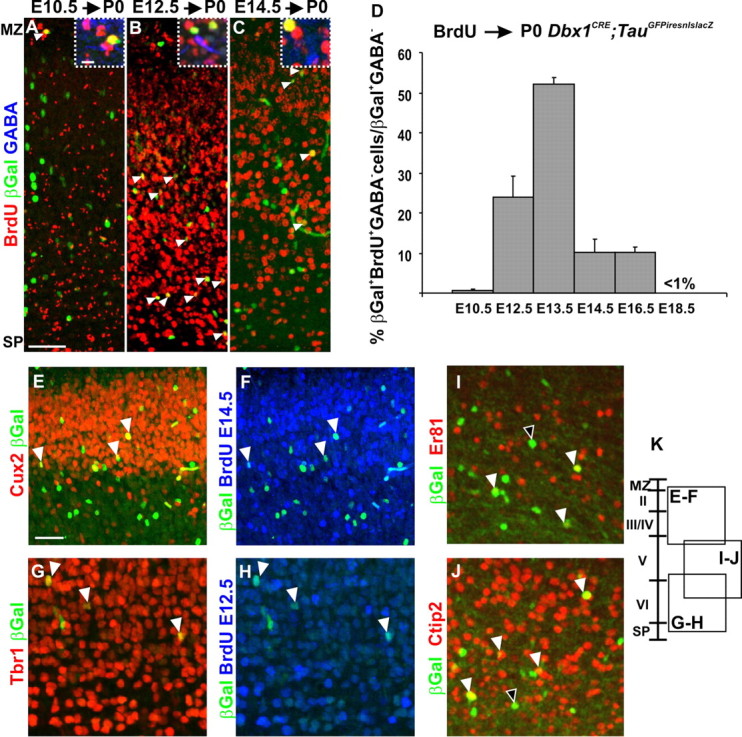
Birthdating of the glutamatergic Dbx1-derived cell population. A–C, BrdU staining at P0 on Dbx1CRE;TauGFPiresnlslacZ animals after a pulse shows that βGal+ neurons born at E10.5 are positioned in the MZ (A); those born at E12.5, in deep layers (B); and those born at E14.5, in more superficial layers (C) (white arrowheads). The dashed boxes in A–C are high magnifications of βGal+BrdU+GABA− neurons used for quantification in D. D, Graph represents the percentage of βGal+BrdU+GABA−/total βGal+GABA− neurons after a pulse of BrdU at different embryonic stages and analyzed at P0. Glutamatergic Dbx1-derived neurons in the CP are mainly born between E11.5 (24.09 ± 5.17%; n = 19 of 78) and E14.5 (10.24 ± 3.07%; n = 54 of 527), with a peak at E12.5 (51.99 ± 1.89%; n = 79 of 152). Results are expressed as mean ± SEM. E–H, βGal+ neurons are predominantly Tbr1+ (G) and Cux2+ (E) when born at E12.5 (G, H) and E14.5 (E, F), respectively (BrdU in blue). I, J, Some of βGal+ neurons positioned in layer V express ER81 (I) or Ctip2 (J). The black and white arrowheads show single- and double-labeled cells, respectively. K, Diagram represents the positions where the respective images have been taken in the dorsolateral cortex of P0 Dbx1CRE;TauGFPiresnlslacZ animals. Scale bars: A, E, 50 μm; A, boxed region, 10 μm.
Figure 7.
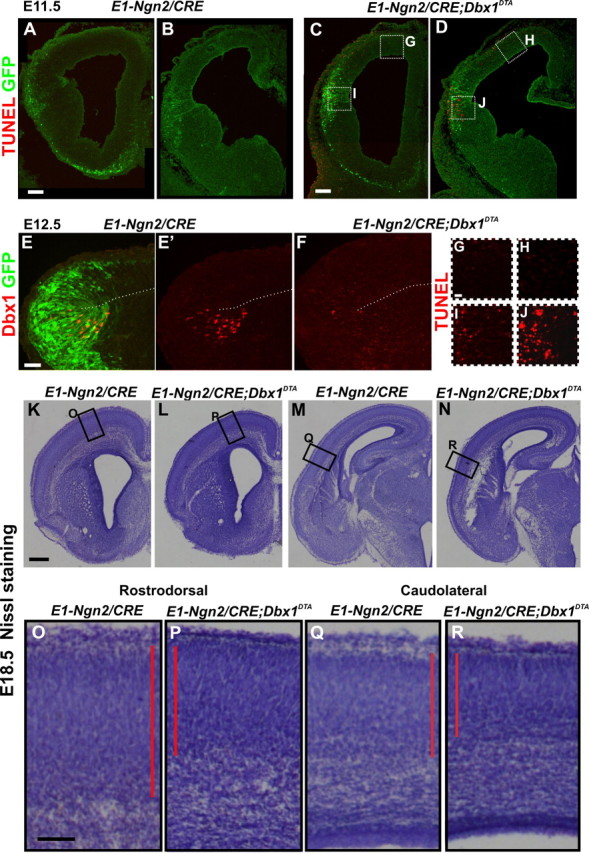
Decrease of cortical plate thickness in E1-Ngn2/CRE;Dbx1DTA animals upon ablation of CP transient cells. A–D, G–J, TUNEL and GFP staining of E11.5 E1-Ngn2/CRE(iresGFP) control (A, B) and E1-Ngn2/CRE;Dbx1DTA (C, D) telencephalons. G–J, High magnifications of boxed regions in C and D. More TUNEL+ cells are detected at the PSB caudally (J) than rostrally (I) in mutants, whereas no cell death is observed in the dorsolateral pallium (G, H) and control telencephalons. E, F, Immunohistochemistry for GFP and Dbx1 on E12.5 embryos showing that the E1-Ngn2/CRE(iresGFP) enhancer is expressed in all Dbx1+ progenitors at the PSB in control animals (E, E′). The number of Dbx1+ progenitors is dramatically reduced in E1-Ngn2/CRE;Dbx1DTA animals at the PSB (compare E′, F). K–R, Nissl staining on coronal sections of E18.5 control (K, M, O, Q) and E1-Ngn2/CRE;Dbx1DTA mutant (L, N, P, R) cortices at rostrodorsal (K, L, O, P) and caudolateral (M, N, Q, R) levels showing a decrease in CP thickness in mutant compared with control animals. Scale bars: K, 250 μm; A, C, 100 μm; E, O, 50 μm; G, 20 μm.
Statistical analysis.
For all experiments, results have been obtained from at least three animals or three pairs of control and mutant littermates, excepted for the kinetics of Dbx1-derived cells loss in Dbx1CRE;ROSA26YFP (n = 1–2 animals) (supplemental Fig. 3, available at www.jneurosci.org as supplemental material). For all experiments, the number of counted cells is indicated in Results or figure legends. For all quantifications, normal distribution was confirmed and unpaired, two-tailed t test on group means were performed for statistical analysis, using Microsoft Excel software (*p < 0.05, **p < 0.01, ***p < 0.001).
Image acquisition.
Bright-field images of brain sections were acquired using a color camera (Zeiss Axiocam HRc) coupled to a Zeiss Axiovert 200 microscope and immunofluorescence images using an inverted confocal microscope (Leica TCS SP5 AOBS tandem resonant scanner).
Quantitative real-time PCR.
Twenty nanograms of RNA extracted from the entire pallium (including the PSB) (PSB+) or from pallial territories dissected dorsally to the PSB (PSB−) at E12.5 were used for cDNA synthesized with the SuperScript VILO cDNA Synthesis kit (Invitrogen), following the manufacturer's instructions. Real-time PCR was performed on a Roche LightCycler according to the manufacturer's instructions for the SYBR Green detection kit. Primers were designed using PrimerBank (Spandidos et al., 2010) and Primer3 (Rozen and Skaletsky, 2000). The primers were verified for specificity with Primer-Blast from NCBI. Expression of each gene was calculated relative to that of the mRNA for the ribosomal protein rpS17 (Sansom et al., 2005) in the same PSB+ and PSB− samples and in three independent experiments. Relative quantifications of gene expression were calculated as described by Livak and Schmittgen (2001). The PCR efficiency for each primer pair was estimated with the LightCycler software using a calibration dilution curve for each primer set. Primers used were as follows: Pax6, forward, GCAGATGCAAAAGTCCAGGTG; Pax6, reverse, CAGGTTGCGAAGAACTCTGTTT; Dbx1, forward, TGAAGGACTCGCAGGTGA; Dbx1, reverse, GGTCTGGATGGGGGTTTAGTTTT).
Results
Dbx1-derived neurons populate the neonatal cortical plate
Using permanent genetic tracing, we have previously shown that Dbx1-expressing progenitors generate Reln+ Cajal–Retzius neurons residing in the MZ/layer I of the early postnatal cerebral cortex. However, Reln− Dbx1-derived neurons were also detected in the CP (Bielle et al., 2005). To further characterize Dbx1-derived neurons in the CP, we used permanent tracing in P0 Dbx1CRE;TauGFPiresnlslacZ brains (Fig. 1A). This reporter line has been shown to label exclusively neuronal populations (Hippenmeyer et al., 2005). We confirmed that most Dbx1-derived βGal+ cells expressed the neuronal marker NeuN (Table 1) at P0 and none coexpressed glial markers such as Olig2 and GFAP (supplemental Fig. 1, available at www.jneurosci.org as supplemental material) (data not shown). Dbx1-derived neurons (βGal+NeuN+) represented 4.02 ± 0.09% of the total NeuN+ population and 4.54 ± 0.90% of the total cells (DAPI+) in the P0 cerebral cortex (Table 1). We then analyzed their neurotransmitter phenotype. Using immunostainings for βGal and glutamate, we found that 91.38 ± 3.55% of βGal+ neurons were Glu+ (Fig. 1A,D–E′, Table 1). Corroborating this, we found by fluorescent in situ hybridization for the VGlut2 mRNA followed by βGal+ immunostaining that the large majority of βGal+ in Dbx1CRE; TauGFPiresnlslacZ P0 brains unambiguously coexpressed VGlut2 (83.6 ± 1.06%) (Fig. 1F,G). At P0, Dbx1-derived Glu+ neurons represented 4.49 ± 1.16% of the total Glu+ neurons and 3.99 ± 1.04% of the total DAPI+ cells (Table 1). Coimmunostaining for GABA revealed that only 7.85 ± 0.75% of βGal+ were GABA+ (Fig. 1H–I′), suggesting that βGal+Glu+ and βGal+GABA+ cells accounted for virtually all βGal+ neurons labeled in the TauloxP-stop-loxPGFPiresnlslacZ reporter line. Additional support to the view that Glu+ and GABA+ cells constitute complementary subsets of Dbx1-derived neurons came from an absence of colabeling of glutamate with GABA in cortical neurons (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). In addition, coimmunostaining of VGlut2, GABA, and YFP on P0 Dbx1CRE;ROSA26YFP brains, a line that allows labeling of cell bodies, confirmed the colabeling of VGlut2 in Dbx1-derived YFP+ cells and the mutually exclusive labeling of VGlut2 or GABA in cortical neurons (Fig. 1J–L′). Thus, we parsimoniously mapped the distribution of glutamatergic Dbx1-derived neurons considering them as βGal+GABA− neurons. Quantification of the number of βGal+GABA− neurons at P0 at three RC levels and along the entire ML axis showed that they distributed homogeneously throughout the cortex (Fig. 1B). We also specifically quantified βGal+GABA− neurons in motor, somatosensory, and visual cortex and confirmed that their number does not differ significantly between primary cortical areas (data not shown). Nevertheless, quantifications revealed that both βGal+GABA+ and βGal+GABA− populations preferentially positioned in deep cortical layers (Fig. 1C), suggesting an early birthdate for Dbx1-derived neurons in the CP.
We conclude that, in addition to CR cells in layer I, Dbx1-derived neurons in the neonatal CP include a major population of glutamatergic neurons and a minor population of GABAergic neurons. The glutamatergic population homogeneously distributes in cortical territories and represents ∼4% of the total number of cortical cells at P0.
Dbx1-derived neurons are born during early stages of neurogenesis and distribute in the CP according to their birthdates
To study the precise timing of generation and its relationship to the laminar distribution of Dbx1-derived CP neurons, we performed BrdU injections at different embryonic stages and analyzed P0 Dbx1CRE;TauGFPiresnlslacZ brains by coimmunostaining for βGal, GABA, and BrdU (Fig. 2A–D). Quantifications revealed that βGal+GABA− neurons were born between E11.5 and E16.5 with 51.99 ± 1.89% of the cells being generated at E12.5 (Fig. 2D). βGal+GABA− neurons were found along the radial dimension of the CP but preferentially (∼40%) in layer V (Fig. 1C; supplemental Fig. 1, available at www.jneurosci.org as supplemental material). The precise distribution of βGal+BrdU+ GABA− cells in the CP after a pulse at each age is plotted in supplemental Figure 1 (available at www.jneurosci.org as supplemental material). When BrdU injection was performed at E10.5, βGal+BrdU+GABA− cells were only detected in the MZ, corresponding to CR neurons (Fig. 2A). After injection at E12.5, βGal+BrdU+GABA− cells were mainly distributed in deep layers (Fig. 2B), whereas those generated at E14.5 were positioned in superficial layers (Fig. 2C). To assess whether their position in the CP was associated with a laminar fate, we performed immunostaining with Tbr1, Cux2 and ER81 or Ctip2 (markers of deep layers, superficial layers, and of subpopulations of layer V glutamatergic neurons, respectively) (Molyneaux et al., 2007). Double labeling with βGal+ was observed for all four markers (Fig. 2E,G,I,J), therefore suggesting that Dbx1-expressing progenitors generate glutamatergic neurons fated to all layers. Moreover, triple immunostaining of βGal with Tbr1 or Cux2 on BrdU injection at E12.5 and E14.5, respectively, revealed that Dbx1-derived neurons are fated to deep and superficial layers according to their birthdates (Fig. 2E–H). Thus, the glutamatergic Dbx1-derived population is generated with a strong peak at E12.5 and distributes predominantly in deep cortical layers according to its birthdate.
Glutamatergic Dbx1-derived CP neurons are generated by progenitors at the PSB
The Dbx1 gene was shown to be specifically expressed in the ventricular zone of the ventral pallium (VP) at the PSB with a caudalhighrostrallow gradient, in the septum and in the preoptic area (POA)/anterior entopenduncular area (AEP) (Yun et al., 2001; Medina et al., 2004; Bielle et al., 2005; Hirata et al., 2009). We previously reported that Dbx1-expressing progenitors at the PSB generate Reln+ Cajal–Retzius neurons between E10.5 and E11.5 (Bielle et al., 2005). We also observed that the caudal PSB appeared to give rise to numerous Reln− cells invading the dorsal pallium tangentially at E12.5 both in the preplate and intermediate zone (IZ)/subventricular zone (SVZ) (Bielle et al., 2005). To characterize the site of origin of CP Dbx1-derived neurons, we used immunohistochemistry for Ngn2, a marker of pallial progenitors fated to the glutamatergic lineage, and Mash1, a marker of subpallial progenitors at E12.5 (Guillemot, 2005). We confirmed that Dbx1 is expressed in pallial Ngn2+Mash1− progenitors at the PSB in both E11.5 and E12.5 telencephalons, whereas Dbx1+ progenitors in the subpallium coexpress Mash1 (Fig. 3A,B) (data not shown). Quantitative PCR (qPCR) analysis using RNA extracted from the whole pallium (PSB+) or the pallium dorsal to the PSB (PSB−) of E12.5 embryos confirmed the absence of Dbx1 expression in other pallial regions than the PSB/VP (Fig. 3C).
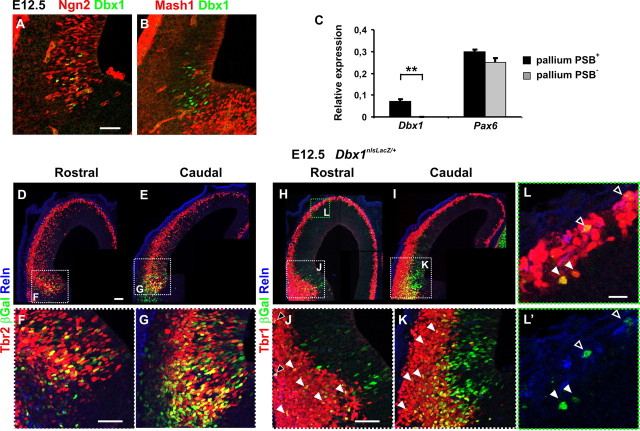
Figure 3.
Dbx1-expressing progenitors at the PSB generate glutamatergic neurons at early stages of development. A, B, Immunostaining for Dbx1 on E12.5 coronal sections show that Dbx1-expressing progenitors at the PSB are pallial since they all express Ngn2 (A) but not Mash1 (B). C, Graphs represent the expression value ratios of the Dbx1 and Pax6 genes relative to that of the ribosomal protein rpS17 (see Materials and Methods) in RNA samples extracted from the entire pallium including the PSB (pallium PSB+) and from pallial territories dissected dorsally to the PSB (pallium PSB−). The values represent the mean of a triplicate (mean ± SD; **p = 0.0021 for Dbx1). The results show the absence of Dbx1 mRNA expression in the pallium PSB− sample, whereas Pax6 expression is slightly reduced because of its higher expression at the PSB. D–L′, Immunostaining for βGal (D–L′), Tbr2 (D–G), Tbr1 (H–L), and Reln (D–L′) on coronal sections of E12.5 Dbx1nlsLacZ/+ embryos. F, G, J, and K are high magnifications of the dashed white boxes in D, E, H, and I, respectively. L, L′, High magnification of green dashed box in H. More βGal+ cells are detected at the caudal than at the rostral PSB (compare F with G and J with K). The vast majority of βGal+ neurons express Tbr2 at the basal VZ/SVZ (D–G) and all express Tbr1 in the mantle zone (H–L, white and black arrowheads in J, K). βGal+Tbr1+Reln+ cells are detected in the lateral and dorsolateral rostral cortex (black arrowheads in J, and L, L′, respectively) but not in the lateral caudal cortex (K). However, the vast majority of βGal+ cells in the lateral cortex are Tbr1+Reln− (J–L′, white arrowheads) and few Tbr1+ Reln− cells are observed in the dorsal pallium (L, L′, white arrowheads) at this stage. **p < 0.01, t test. Scale bars: A, D, F, J, 50 μm; L, 20 μm.
To label progenitors and early postmitotic Dbx1-derived neurons, we traced them using Dbx1nlsLacz/+ embryos (Pierani et al., 2001; Bielle et al., 2005) and performed coimmunostaining of βGal with Tbr2 and Tbr1, two genes sequentially expressed during the differentiation of glutamatergic neurons (Englund et al., 2005). In E12.5 embryos, more βGal+ cells were detected at the caudal PSB than at the rostral PSB correlating with the number of Dbx1-expressing progenitors (Fig. 3D–K). Many βGal+ cells in the VZ and most of those located at the basal VZ/SVZ at both rostral and caudal PSB coexpressed Tbr2 (Fig. 3D–G). In addition, all βGal+ cells in the postmitotic compartment expressed Tbr1 (Fig. 3H–L). Several βGal+Tbr1+Reln+ CR neurons were observed in the lateral MZ at rostral levels but very few at caudal levels (Fig. 3J,K, black arrowheads). At this stage, βGal+ Tbr1+Reln− neurons in the lateral PP were oriented parallel to the pial surface, suggesting their tangential migration through the PP layer (Fig. 3J,K) (see also Fig. 4A). In the dorsal pallium, however, very few Tbr1+Reln− Dbx1-derived neurons were observed at this stage (Fig. 3L,L′). No βGal+Calbindin+ interneurons, shown to be the phenotype of POA Dbx1-derived interneurons migrating into the amygdala (Hirata et al., 2009), were detected in the pallium of Dbx1nlsLacz/+ animals (data not shown). Together with the absence of generation of GABAergic neurons in PSB explant cultures (Bielle et al., 2005), our results show that Dbx1-expressing progenitors at the PSB generate exclusively glutamatergic cells at E12.5 in a caudalhighrostrallow gradient and suggest that the caudal PSB generate mostly Reln− neurons. These results also confirm the focal expression of the Dbx1 gene at the PSB and exclude low levels of expression in the dorsal pallium.
Glutamatergic Dbx1-derived neurons migrate tangentially to invade the cortical plate
Since βGal labeling is transient in Dbx1nlsLacz/+ animals, we used permanent labeling in Dbx1CRE;ROSA26YFP and Dbx1CRE; TauGFPiresnlslacZ animals to follow Dbx1-derived neurons at later developmental stages. In E12.5 Dbx1CRE;ROSA26YFP embryos, a deep stream of YFP+Tbr1+ cells emerging from the PSB and showing a typical morphology of migrating cells was observed in the lateral and dorsolateral pallium (Fig. 4A–D′). By E14.5, tangentially oriented YFP+ cells were present in the PP/CP and IZ/SVZ and reached the dorsal pallium (Fig. 4E–H). At this stage, several YFP+ cells in the IZ expressed VGlut2 (Fig. 4E–F3′) or GABA (Fig. 4G–H′). Moreover, YFP+Reln+ cells, corresponding to CR cells, and YFP+GABA+ neurons appeared to account for all Dbx1-derived cells migrating in the MZ at this stage (Fig. 4E,G) (data not shown). Glutamatergic YFP+VGlut2+ and YFP+Tbr1+ cells were also found radially oriented (Fig. 4E,F1,F1′; supplemental Fig. 2, available at www.jneurosci.org as supplemental material) likely in the process of entering the lower CP. These results suggest that glutamatergic (Reln−) Dbx1-derived neurons are tangentially migrating through the nascent CP and the IZ/SVZ but not the MZ. Similar migratory routes were observed using Dbx1CRE;TauGFPiresnlslacZ embryos at E13.5 and E14.5 (supplemental Fig. 2, available at www.jneurosci.org as supplemental material; Fig. 4I–L). We confirmed the glutamatergic phenotype of Dbx1-derived neurons in the IZ and CP using in situ hybridization for the vesicular glutamate transporter VGlut2 mRNA followed by anti-βGal immunostaining (Fig. 4L–O). Quantification of the number of Dbx1-derived βGal+Tbr1+Reln− neurons in Dbx1CRE;TauGFPiresnlslacZ embryos between E13.5 and E14.5 (Fig. 4P) confirmed a progressive increase in the dorsal CP at both rostral and caudal levels (Fig. 4I–K,P) and that many of the Dbx1-derived neurons in the CP are glutamatergic at this stage. The schematic representation of the distribution of βGal+ Tbr1+Reln− neurons in the CP also showed that the number of Dbx1-derived glutamatergic neurons appeared to increase at the rostral PSB at E14.5, suggesting a caudal-to-rostral (along the PSB), in addition to a lateral-to-dorsal, trajectory of migration of these cells (Fig. 4P; supplemental Table 1, available at www.jneurosci.org as supplemental material, for values).
To confirm the origin and tangential migration of Dbx1-derived Tbr1+Reln− neurons, we grafted PSB explants of E12.5 Dbx1CRE;TauGFPiresnlslacZ embryos onto the PSB of WT slices. After 2 DIV culture, βGal+Tbr1+Reln− neurons were detected in the lateral and dorsal pallium (Fig. 5A–D″). In contrast, when the PSB was removed from E12.5 Dbx1CRE;TauGFPiresnlslacZ slices and cultured for 2 d, almost exclusively βGal+Tbr1+Reln+ CR cells were detected in the dorsal pallium correlating with their earlier birthdate and migration into the dorsal pallium from the PSB before its removal or from the remaining septum (Fig. 5E–H″). Finally, we inserted a bead coated with CellTracker Orange (CMTMR) at the PSB on E12.5 coronal and sagittal sections of Dbx1CRE;ROSA26YFP embryos (Fig. 5I–K‴) (data not shown). After 2 DIV, we observed colabeling of CMTMR/YFP with Tbr1 but not with GABA in cells migrating toward the dorsal pallium (Fig. 5I,J″, K–K‴, respectively).
Figure 5.
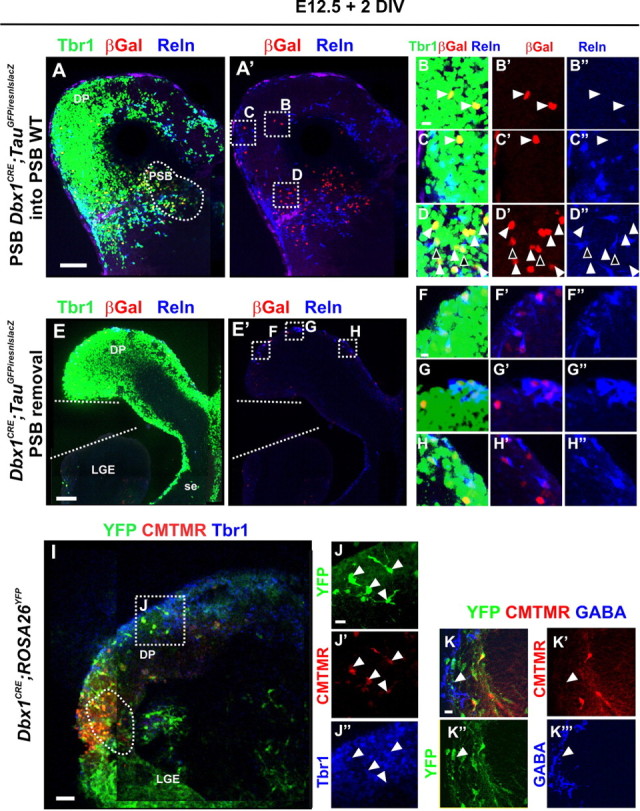
Dbx1-derived glutamatergic cells emerge from the PSB at E12.5 and invade the dorsal pallium by tangential migration. A–H″, Immunostaining for βgal, Tbr1, and Reln. A–D″, Transplant experiments of PSB explants (A, dashed region) from E12.5 Dbx1CRE;TauGFPiresnlslacZ animals into the PSB of E12.5 WT coronal brain slices and cultured for 2 DIV. βGal+Tbr1+Reln− neurons migrate tangentially from the grafted explant into the dorsal pallium (B–C″) in addition to the lateral pallium (D–D″) (white arrowheads). Migration of βGal+Tbr1+Reln+ neurons is also detected in the lateral pallium (D–D″) (black arrowheads). E–H″, Removal of the PSB from E12.5 Dbx1CRE;TauGFPiresnlslacZ coronal slices shows that no βGal+Tbr1+Reln− neurons are generated in the dorsal pallium or the septum. F–H″, Only βGal+Tbr1+Reln+ neurons are observed in the dorsal (F–G″) or medial (H–H″) pallium after 2 DIV, corresponding to the Cajal–Retzius cells generated by Dbx1-expressing cells at the septum and at the PSB earlier than E12.5. I–K‴, A bead coated with CellTracker Orange (CMTMR) (dashed region at the PSB in I) was placed at the VZ of the PSB on 300 μm coronal slices of E12.5 Dbx1CRE;ROSA26YFP embryos and cultured for 2 DIV. I–J″, Immunostaining for YFP and Tbr1 on 100 μm sections shows glutamatergic Dbx1-derived cells in the dorsal pallium (J–J″, white arrowheads) that have incorporated the CMTMR and thus originated from the PSB. K–K‴, Immunostaining for YFP and GABA on serial sections of the same experiment shows that none of the Dbx1-derived GABAergic cells in the pallium have incorporated the CMTMR confirming that the Dbx1-derived neurons migrating from the PSB are exclusively glutamatergic. DP, Dorsal pallium; LGE, lateral ganglionic eminence; se, septum. Scale bars: A, E, I, 100 μm; B, F, K, 10 μm.
Together, our data show that Dbx1-expressing progenitors at the PSB generate, in addition to Tbr1+Reln+ CR cells between E10.5 and E11.5, a population of glutamatergic Tbr1+Reln− neurons at E12.5, which migrate tangentially from caudal-to-rostral along the PSB and from lateral-to-dorsal to populate the entire developing cortex by E14.5.
Massive apoptotic cell death of CP Dbx1-derived glutamatergic neurons in the postnatal cortex
We next examined the distribution of the CP Dbx1-derived neurons in adult Dbx1CRE;TauGFPiresnlslacZ brains. Surprisingly, the number of βGal+ cells was dramatically decreased compared with P0 (Fig. 6A–D). We quantified the total number of Dbx1-derived neurons at different embryonic and postnatal stages taking into account the decrease in cell density (βGal+/DAPI+ nuclei) (for methods, see supplemental Fig. 3, available at www.jneurosci.org as supplemental material). We observed an increase in the percentage of Dbx1-derived neurons between E16.5 and P0 in the CP, whereas a strong decrease was detected between P0 and P8 (Fig. 6E). Similar results were obtained using two additional reporter mouse lines, namely Dbx1CRE;βactin:lacZ and Dbx1CRE;ROSA26YFP, which labels Dbx1-derived neurons and both neuronal and glial lineages, respectively (supplemental Fig. 3, available at www.jneurosci.org as supplemental material) (data not shown). Notably, when we quantified the number of glutamatergic (βGal+GABA−/DAPI+) and GABAergic (βGal+GABA+/DAPI+) neurons in Dbx1CRE;TauGFPiresnlslacZ animals, we found that 95.57% of the glutamatergic population disappeared in adults, whereas the percentage of Dbx1-derived GABAergic cells did not significantly change (Fig. 6K,L).
We then tested whether the loss of Dbx1-derived glutamatergic cells was attributable to apoptotic cell death by coimmunostaining with activated-Caspase3 (Caspase3*), a specific marker of apoptosis. We observed that in P0 Dbx1CRE;TauGFPiresnlslacZ cerebral cortices, 34.9% of the total cells undergoing apoptotic cell death were Dbx1-derived (βgal+ Caspase3*+) (Fig. 6F–I). Moreover, we evaluated the proportion of neurons relative to the total dying cells in wild-type cortices using NeuN staining; 70.3% of the total apoptotic cells at P0 were NeuN+ (NeuN+Caspase3*+), and therefore, 29.7% likely correspond to glial cells. Among the NeuN+ population undergoing apoptosis, 50.3% were Dbx1-derived neurons (βgal+ NeuN+Caspase3*+) (Fig. 6I,J).
We conclude that Dbx1-derived glutamatergic, but not GABAergic neurons, in the CP represent a transient population undergoing programmed cell death starting at P0 and account for 50% of the total apoptotic neurons in the CP at this stage.
Reduction of cortical plate thickness upon ablation of glutamatergic neurons derived from Dbx1+ progenitors at the PSB
To study the function of PSB-derived CP glutamatergic transient neurons, we performed genetic ablation using the Dbx1LoxP-stop-LoxP-DTA mouse line (Bielle et al., 2005) crossed with the E1-Ngn2/CRE(iresGFP) transgenic line (Berger et al., 2004). In the telencephalon, CRE expression is restricted to subsets of ventral and lateral pallium progenitors at early stages and is not observed in the subpallium (Berger et al., 2004). TUNEL staining analysis revealed that dying cells are first detected at E11.5 mainly at the caudal PSB in the basal VZ/SVZ (Fig. 7A–D). A peak of cell death was observed at the PSB at E12.5 and was almost undetectable by E14.5 in mutant animals (Fig. 7I,J; supplemental Fig. 4, available at www.jneurosci.org as supplemental material), as expected from the dynamic of Dbx1 expression (Yun et al., 2001; Medina et al., 2004; Bielle et al., 2005). No cell death was observed in the cortex dorsally to the PSB at any embryonic stages tested (E11.5, E12.5, E14.5, E16.5, and E18.5) (Fig. 7G,H; supplemental Fig. 4, available at www.jneurosci.org as supplemental material) (data not shown). The effective ablation at the PSB was confirmed by the almost complete loss of Dbx1 protein at E12.5 (Fig. 7E,F). In addition, in E1-Ngn2/CRE;Dbx1DTA;Dbx1nlsLacZ triple mutant compared with Dbx1LoxP-stop-LoxP-DTA;Dbx1nlsLacZ control embryos, we observed a 30–50% decrease in the number of XGal+ cells in the PP/CP starting caudolaterally at E12.5 and progressing toward the rostrodorsal cortex at E14.5 (supplemental Fig. 4, available at www.jneurosci.org as supplemental material). To ensure that the ablation did not affect CR cells, we performed in situ hybridization for Reln and p73 mRNAs, which label CR cells (Cabrera-Socorro et al., 2007), between E11.5 and E14.5 in E1-Ngn2/CRE;Dbx1DTA telencephalons and detected no difference in the number of CR neurons upon ablation (supplemental Fig. 5, available at www.jneurosci.org as supplemental material) (data not shown). We also observed no changes in interneuron number and/or distribution at E12.5 using Calbindin staining (supplemental Fig. 5, available at www.jneurosci.org as supplemental material). Last, the ablation did not lead to defects in the expression of signaling molecules at the PSB, namely Tgfα, Sfrp2, and Wnt7b, or in the establishment of the PSB as shown by the similar domains of Ngn2 and Dlx1 expression (supplemental Fig. 5, available at www.jneurosci.org as supplemental material). We conclude that E1-Ngn2/CRE;Dbx1DTA embryos show a specific ablation of Dbx1-expressing progenitors at the PSB and of 30–50% of CP glutamatergic transient neurons without affecting CR cells or subpallially derived interneuron generation and migration.
We then analyzed the mutant phenotype at perinatal stages before the onset of death of the transient Dbx1-derived CP neurons. Mutant brains appeared slightly smaller, and we observed an overall 18–22% reduction in thickness of the cortical plate at all RC and ML levels of the neocortex using Nissl staining at E18.5 to P2 (Fig. 7K–R) (data not shown). The thickness of the CP seemed to be affected in all cortical areas in a similar manner upon Dbx1-expressing progenitor ablation. Cell density was similar between control and mutant brains (14,911 ± 4380 cells/mm2 in controls and 13,000 ± 3668 cells/mm2 in mutants; p = 0.33) and no changes in density of GABA staining was observed at P2 (data not shown).
Together, these results show that genetic ablation of Dbx1-derived CP transient neurons generated at the PSB leads to a decrease in cell numbers throughout the neocortex. Since this neuronal population represents <5% of cortical cells, together our data strongly suggest that they play a crucial role in corticogenesis at a distance from their generation site in a non-cell-autonomous manner.
Discussion
We have identified a novel transient population of glutamatergic neurons generated from Dbx1-expressing progenitors at the PSB mostly at E12.5. This population invades the PP/CP by tangential migration with a caudolateral-to-rostromedial progression starting at E12.5 and redistributes homogeneously in the cortical plate along the RC and ML axis afterward. Beginning at birth, CP Dbx1-derived neurons undergo PCD and massively disappear during the first postnatal week. Specific ablation of this cell population leads to a reduction in CP thickness at neonatal stages, suggesting that they play a crucial role in cortical growth at long distance from their site of origin in a non-cell-autonomous manner.
Focal origins of glutamatergic cortical neurons and tangential migration
We described by genetic tracing the progressive tangential dispersion of a subpopulation of glutamatergic neurons arising at early stages of PP/CP formation from a focal pallial progenitor domain at the PSB. Although previously thought to be a feature of GABAergic interneurons (Marín and Rubenstein, 2003), tangential migration of glutamatergic neurons has now been reported for several populations during development (Walsh and Cepko, 1992; O'Rourke et al., 1995; Rakic, 1995), including Satb2+ and Cajal–Retzius neurons (Takiguchi-Hayashi et al., 2004; Bielle et al., 2005; Britanova et al., 2006; Yoshida et al., 2006). At least two of the glutamatergic transient neurons forming the preplate/early CP, CR and Dbx1-derived CP neurons, have now been shown by genetic tracing to be generated at the borders of the developing pallium. Subplate and pioneer cells are also thought to derive in humans and rodents, respectively, from extraneocortical territories (Meyer et al., 1998; Morante-Oria et al., 2003; Bystron et al., 2006). Our results support the notion that tangential dispersion of glutamatergic postmitotic neurons is more represented than previously thought and is used by many of the earliest-born neurons in the developing pallium. Moreover, our results show that Dbx1-expressing progenitors at the PSB sequentially generate neurons expressing the appropriate sequence of layer specific markers described for successive offspring of cortical progenitors (Molyneaux et al., 2007). Thus, despite their focal origin and long-range migration, Dbx1-derived CP neurons position into the CP according to their birthdates, supporting the hypothesis of intrinsic cues governing laminar fate (McConnell and Kaznowski, 1991; Desai and McConnell, 2000).
We have shown that CP transient neurons reach the cortex by migrating along the SP and IZ/SVZ but appear to avoid the MZ. In the IZ, they intermingle with GABAergic neurons and the onset of invasion of pallial territories also strongly parallels that of inhibitory interneurons. This suggests that similar molecular mechanisms might govern tangential migration in the IZ for both GABAergic and CP transient neurons. Indeed, in the MZ, similar cues have been shown to control the migration of GABAergic and CR cells, namely the CXCL12/CXCR4 pathway (Stumm et al., 2003; Borrell and Marín, 2006; Paredes et al., 2006). However, in contrast to GABAergic and CR neurons, CP transient neurons do not appear to be prevented to enter the CP. Moreover, in contrast to CR subtypes, which distribute in specific proportions in pallial regions and have been suggested to respond to contact-inhibitory interactions (Bielle et al., 2005; Borrell and Marín, 2006), CP transient neurons distribute homogeneously in all developing cortical regions. These results suggest that they may respond differently to cues that govern the invasion of cortical territories by other tangentially migrating neurons.
Early preplate neurons, pioneer functions, and programmed cell death in cortical development
Specific subpopulations of neurons have been suggested to undergo PCD in the cerebral cortex such as CR and SP cells and hippocampal interneurons (Supèr et al., 1998). PCD is a well known developmental event and cortical cell death has been shown to involve up to 30% of the cells and to mainly occur around P4–P6 in superficial layers (Verney et al., 2000). Indeed, the peak of PCD is commonly associated with increased sensory activity corresponding to the period of synaptogenesis and axonal outgrowth/targeting (Buss et al., 2006; Heck et al., 2008; Morishita and Hensch, 2008). However, only recent genetic tracing has allowed the life span of specific cell populations, namely CR neurons (Bielle et al., 2005) and oligodendrocytes (Kessaris et al., 2006), to be assessed. We describe here that glutamatergic, but not GABAergic, Dbx1-derived neurons populating the CP constitute an early-born population that undergoes almost complete PCD at birth and represents 50% of the total dying neurons at this stage. Our results describe for the first time precise kinetics of massive neuronal death in a specific, and previously unidentified, glutamatergic population in the CP and attribute a function to it in regulating cortical growth. Notably, Dbx1-derived CP neurons death begins before the peak of activity-dependent PCD and coincides with the end of the proliferation of progenitors during development (Caviness et al., 1995). Together with the role of CR cells in controlling radial migration and that of SP cells in axonal pathfinding (Supèr et al., 1998), our results strongly suggest that the early PP/CP are populated by many transient neurons, which orchestrate crucial steps in the construction of the cerebral cortex architecture. Notably, processes of proliferation, migration, and axonal pathfinding are strongly reduced or extinguished in the mature cortex. Thus, the disappearance of transient cell/structures might be crucial for the accomplishment of development, and, indeed, their perdurance has been described in pathological conditions (Blümcke et al., 2002; Kostović and Judas, 2007). Interestingly, at least two of these transient populations, CR and CP transient neurons, are sequentially born at early stages (E10–E12) from Dbx1-expressing progenitors at the PSB. Hence, these early generated neurons might be intrinsically determined from their early birthdate and their site of origin to a short transient life span during which they perform specific developmental functions.
Our results strongly suggest that Dbx1-derived CP transient glutamatergic neurons function in a non-cell-autonomous manner since they only represent <5% of cortical cells and DTA-mediated ablation is strictly cell autonomous. Our data open the question as to whether CP transient neurons might affect corticogenesis while invading the pallium or at later stages of development when positioned in the CP before undergoing PCD. The distribution of CP transient neurons in all cortical territories by E14.5 together with the homogeneous defects of cortical thickness along the RC axis and the absence of an increase in cell death in E1-Ngn2/CRE;Dbx1DTA mutants suggest that CP transient neurons might affect progenitors' proliferation/differentiation but not survival at midcorticogenesis. Indeed, a feedback control from the postmitotic compartment on the VZ has been suggested to affect the generation of later-born neurons (Polleux et al., 1998; Viti et al., 2003; Dehay and Kennedy, 2007) as well as the switch from neurogenesis to gliogenesis (Seuntjens et al., 2009). Nevertheless, the final positioning of CP transient neurons into the CP is also consistent with a function for CP transient neurons at late stages of development, such as in the establishment of transient connections.
Acquisition of Dbx1-derived transient neurons at the PSB and the evolution of the neocortex
The PSB has been the center of debate in studies on cortical evolution and the acquisition of a new progenitor domain in the VP at the PSB or of migration trajectories from this domain have been proposed to play a crucial role in the “complexification” of the claustro-amygdaloid complex and of the lateral pallium (LP) in mammals (Molnár and Butler, 2002; Medina et al., 2004). However, its role in neocortical evolution is still not yet established. Based on analogy of connectivity, Karten (1997) suggested that the redistribution by tangential migration of cells generated by the DVR (dorsal ventricular ridge) domain, a derivative of the VP/LP in birds and reptiles, contributed to form the neocortex. However, until recently, tangential migration of glutamatergic cells from the VP/PSB toward the dorsal pallium was not demonstrated in mammals and was further excluded to occur in sauropsids (Métin et al., 2007).
We now have shown that tangential migration of glutamatergic neurons from the borders of the developing pallium (VP/PSB and pallial septum) occurs in mice for CR subtypes, populating the MZ between E10.5 and E11.5 (Bielle et al., 2005), and CP transient neurons invading the PP/CP between E12.5 and E14.5. CR neurons act as mediators of cortical patterning and arealization (Griveau et al., 2010), and thus are involved in tangential growth of cortical areas, and the amplification of the number of Reln+ cells has been shown in mammals (Bar et al., 2000; Cabrera-Socorro et al., 2007). Our results suggest that CP transient neurons are required for radial growth of the neocortex. Since Dbx1 is not expressed at the PSB in chicks (Bielle et al., 2005), we suggest that together our results might reconcile theories of cortical evolution by providing support for the acquisition of new progenitor domain(s) at the PSB, or antihem, and of tangentially migrating glutamatergic “signaling” cells in mammals. These transient neurons, by participating in controlling growth from extraneocortical origins through cell migration, could be one of the evolutionary steps to pattern large structure such as the cerebral cortex and increase vertebrate brain complexity (Supèr and Uylings, 2001).
Footnotes
This work was supported by Agence Nationale de la Recherche Grant ANR-07-NEURO-046-01, Fondation pour le Recherche Médicale Grant INE20060306503, Association pour la Recherche sur le Cancer Grant 4940, and Ville de Paris Grant 2006 ASES 102 (A.P.). A.T. was the recipient of a Fellowship from the French Ministry of Education. A.G. was the recipient of fellowships from the French Ministry of Education and the Association pour la Recherche sur le Cancer. U.B. was the recipient of fellowships from Neuropôle de Recherche Francilien and Fondation pour la Recherche Médicale. A.P. is a Centre National de la Recherche Scientifique Investigator. We apologize to the authors of original work not cited. Because of space limitations, we cited review articles where possible. We thank F. Guillemot and A. Stoykova for providing us the E1-Ngn2/CRE mouse line, D. J. Anderson for the Ngn2 antibodies, S. Arber and T. M. Jessell for the ER81 antibodies, and T. Kaneko for anti-VGlut2 antibodies. We are grateful to the Imaging Facility for precious help with confocal microscopy. We thank V. Dubreuil for advice on fluorescent in situ hybridization and S. Karaz and A. Djemat for technical assistance. We are indebted to F. Causeret for modeling CP transient neurons distribution. We thank F. Causeret, S. Garel, M. Barber, and M. Wassef for critical reading of this manuscript. A.T., A.G., and A.P. conceived the study and wrote this manuscript. A.T. performed most of the experiments. A.G. initiated and performed part of the analysis of the mutant animals. L.V. performed some of the DAB immunohistochemistry experiments. T.P. designed the semiautomated counting method. U.B. performed the qPCR experiments. A.P. supervised the project.
References
- Bar I, Lambert de Rouvroit C, Goffinet AM. The evolution of cortical development. An hypothesis based on the role of the Reelin signaling pathway. Trends Neurosci. 2000;23:633–638. doi: 10.1016/s0166-2236(00)01675-1. [DOI] [PubMed] [Google Scholar]
- Berger J, Eckert S, Scardigli R, Guillemot F, Gruss P, Stoykova A. E1-Ngn2/Cre is a new line for regional activation of Cre recombinase in the developing CNS. Genesis. 2004;40:195–199. doi: 10.1002/gene.20081. [DOI] [PubMed] [Google Scholar]
- Berry M, Rogers AW, Eayrs JT. Pattern of cell migration during cortical histogenesis. Nature. 1964;203:591–593. doi: 10.1038/203591b0. [DOI] [PubMed] [Google Scholar]
- Bielle F, Griveau A, Narboux-Nême N, Vigneau S, Sigrist M, Arber S, Wassef M, Pierani A. Multiple origins of Cajal-Retzius cells at the borders of the developing pallium. Nat Neurosci. 2005;8:1002–1012. doi: 10.1038/nn1511. [DOI] [PubMed] [Google Scholar]
- Blümcke I, Thom M, Wiestler OD. Ammon's horn sclerosis: a maldevelopmental disorder associated with temporal lobe epilepsy. Brain Pathol. 2002;12:199–211. doi: 10.1111/j.1750-3639.2002.tb00436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrell V, Marín O. Meninges control tangential migration of hem-derived Cajal-Retzius cells via CXCL12/CXCR4 signaling. Nat Neurosci. 2006;9:1284–1293. doi: 10.1038/nn1764. [DOI] [PubMed] [Google Scholar]
- Britanova O, Alifragis P, Junek S, Jones K, Gruss P, Tarabykin V. A novel mode of tangential migration of cortical projection neurons. Dev Biol. 2006;298:299–311. doi: 10.1016/j.ydbio.2006.06.040. [DOI] [PubMed] [Google Scholar]
- Buss RR, Sun W, Oppenheim RW. Adaptive roles of programmed cell death during nervous system development. Annu Rev Neurosci. 2006;29:1–35. doi: 10.1146/annurev.neuro.29.051605.112800. [DOI] [PubMed] [Google Scholar]
- Bystron I, Rakic P, Molnár Z, Blakemore C. The first neurons of the human cerebral cortex. Nat Neurosci. 2006;9:880–886. doi: 10.1038/nn1726. [DOI] [PubMed] [Google Scholar]
- Cabrera-Socorro A, Hernandez-Acosta NC, Gonzalez-Gomez M, Meyer G. Comparative aspects of p73 and Reelin expression in Cajal-Retzius cells and the cortical hem in lizard, mouse and human. Brain Res. 2007;1132:59–70. doi: 10.1016/j.brainres.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Caviness VS, Jr, Takahashi T, Nowakowski RS. Numbers, time and neocortical neuronogenesis: a general developmental and evolutionary model. Trends Neurosci. 1995;18:379–383. doi: 10.1016/0166-2236(95)93933-o. [DOI] [PubMed] [Google Scholar]
- Dehay C, Kennedy H. Cell-cycle control and cortical development. Nat Rev Neurosci. 2007;8:438–450. doi: 10.1038/nrn2097. [DOI] [PubMed] [Google Scholar]
- Dehay C, Savatier P, Cortay V, Kennedy H. Cell-cycle kinetics of neocortical precursors are influenced by embryonic thalamic axons. J Neurosci. 2001;21:201–214. doi: 10.1523/JNEUROSCI.21-01-00201.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai AR, McConnell SK. Progressive restriction in fate potential by neural progenitors during cerebral cortical development. Development. 2000;127:2863–2872. doi: 10.1242/dev.127.13.2863. [DOI] [PubMed] [Google Scholar]
- Dubreuil V, Ramanantsoa N, Trochet D, Vaubourg V, Amiel J, Gallego J, Brunet JF, Goridis C. A human mutation in Phox2b causes lack of CO2 chemosensitivity, fatal central apnea, and specific loss of parafacial neurons. Proc Natl Acad Sci U S A. 2008;105:1067–1072. doi: 10.1073/pnas.0709115105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund C, Fink A, Lau C, Pham D, Daza RA, Bulfone A, Kowalczyk T, Hevner RF. Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J Neurosci. 2005;25:247–251. doi: 10.1523/JNEUROSCI.2899-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I, Bernet E, Soriano E, del Rio T, Fonseca M. Naturally occurring cell death in the cerebral cortex of the rat and removal of dead cells by transitory phagocytes. Neuroscience. 1990;39:451–458. doi: 10.1016/0306-4522(90)90281-8. [DOI] [PubMed] [Google Scholar]
- Griveau A, Borello U, Causeret F, Tissir F, Boggetto N, Karaz S, Pierani A. A novel role for Dbx1-derived Cajal-Retzius cells in early regionalization of the cerebral cortical neuroepithelium. PloS Biol. 2010;8:e1000440. doi: 10.1371/journal.pbio.1000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemot F. Cellular and molecular control of neurogenesis in the mammalian telencephalon. Curr Opin Cell Biol. 2005;17:639–647. doi: 10.1016/j.ceb.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Haydar TF, Kuan CY, Flavell RA, Rakic P. The role of cell death in regulating the size and shape of the mammalian forebrain. Cereb Cortex. 1999;9:621–626. doi: 10.1093/cercor/9.6.621. [DOI] [PubMed] [Google Scholar]
- Heck N, Golbs A, Riedemann T, Sun JJ, Lessmann V, Luhmann HJ. Activity-dependent regulation of neuronal apoptosis in neonatal mouse cerebral cortex. Cereb Cortex. 2008;18:1335–1349. doi: 10.1093/cercor/bhm165. [DOI] [PubMed] [Google Scholar]
- Hippenmeyer S, Vrieseling E, Sigrist M, Portmann T, Laengle C, Ladle DR, Arber S. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol. 2005;3:e159. doi: 10.1371/journal.pbio.0030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata T, Li P, Lanuza GM, Cocas LA, Huntsman MM, Corbin JG. Identification of distinct telencephalic progenitor pools for neuronal diversity in the amygdala. Nat Neurosci. 2009;12:141–149. doi: 10.1038/nn.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh Y, Kim C, Lee W, Kim J, Ahn H. Age-related change in the neuropeptide Y and NADPH-diaphorase-positive neurons in the cerebral cortex and striatum of aged rats. Neurosci Lett. 1997;223:157–160. doi: 10.1016/s0304-3940(97)13430-9. [DOI] [PubMed] [Google Scholar]
- Innocenti GM, Price DJ. Exuberance in the development of cortical networks. Nat Rev Neurosci. 2005;6:955–965. doi: 10.1038/nrn1790. [DOI] [PubMed] [Google Scholar]
- Karten HJ. Evolutionary developmental biology meets the brain: the origins of mammalian cortex. Proc Natl Acad Sci U S A. 1997;94:2800–2804. doi: 10.1073/pnas.94.7.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessaris N, Fogarty M, Iannarelli P, Grist M, Wegner M, Richardson WD. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat Neurosci. 2006;9:173–179. doi: 10.1038/nn1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostović I, Judas M. Transient patterns of cortical lamination during prenatal life: do they have implications for treatment? Neurosci Biobehav Rev. 2007;31:1157–1168. doi: 10.1016/j.neubiorev.2007.04.018. [DOI] [PubMed] [Google Scholar]
- Kriegstein AR, Noctor SC. Patterns of neuronal migration in the embryonic cortex. Trends Neurosci. 2004;27:392–399. doi: 10.1016/j.tins.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Kriegstein A, Noctor S, Martínez-Cerdeño V. Patterns of neural stem and progenitor cell division may underlie evolutionary cortical expansion. Nat Rev Neurosci. 2006;7:883–890. doi: 10.1038/nrn2008. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔC(T) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Marín O, Rubenstein JL. Cell migration in the forebrain. Annu Rev Neurosci. 2003;26:441–483. doi: 10.1146/annurev.neuro.26.041002.131058. [DOI] [PubMed] [Google Scholar]
- Marín-Padilla M. Cajal-Retzius cells and the development of the neocortex. Trends Neurosci. 1998;21:64–71. doi: 10.1016/s0166-2236(97)01164-8. [DOI] [PubMed] [Google Scholar]
- McConnell SK, Kaznowski CE. Cell cycle dependence of laminar determination in developing neocortex. Science. 1991;254:282–285. doi: 10.1126/science.254.5029.282. [DOI] [PubMed] [Google Scholar]
- Medina L, Legaz I, González G, De Castro F, Rubenstein JL, Puelles L. Expression of Dbx1, Neurogenin 2, Semaphorin 5A, Cadherin 8, and Emx1 distinguish ventral and lateral pallial histogenetic divisions in the developing mouse claustroamygdaloid complex. J Comp Neurol. 2004;474:504–523. doi: 10.1002/cne.20141. [DOI] [PubMed] [Google Scholar]
- Métin C, Alvarez C, Moudoux D, Vitalis T, Pieau C, Molnár Z. Conserved pattern of tangential neuronal migration during forebrain development. Development. 2007;134:2815–2827. doi: 10.1242/dev.02869. [DOI] [PubMed] [Google Scholar]
- Meyer G, Soria JM, Martínez-Galán JR, Martín-Clemente B, Fairén A. Different origins and developmental histories of transient neurons in the marginal zone of the fetal and neonatal rat cortex. J Comp Neurol. 1998;397:493–518. [PubMed] [Google Scholar]
- Mitrovic N, Schachner M. Transient expression of NADPH diaphorase activity in the mouse whisker to barrel field pathway. J Neurocytol. 1996;25:429–437. doi: 10.1007/BF02284813. [DOI] [PubMed] [Google Scholar]
- Molnár Z, Butler AB. The corticostriatal junction: a crucial region for forebrain development and evolution. Bioessays. 2002;24:530–541. doi: 10.1002/bies.10100. [DOI] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Menezes JR, Macklis JD. Neuronal subtype specification in the cerebral cortex. Nat Rev Neurosci. 2007;8:427–437. doi: 10.1038/nrn2151. [DOI] [PubMed] [Google Scholar]
- Morante-Oria J, Carleton A, Ortino B, Kremer EJ, Fairén A, Lledo PM. Subpallial origin of a population of projecting pioneer neurons during corticogenesis. Proc Natl Acad Sci U S A. 2003;100:12468–12473. doi: 10.1073/pnas.1633692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita H, Hensch TK. Critical period revisited: impact on vision. Curr Opin Neurobiol. 2008;18:101–107. doi: 10.1016/j.conb.2008.05.009. [DOI] [PubMed] [Google Scholar]
- O'Leary DD, Chou SJ, Sahara S. Area patterning of the mammalian cortex. Neuron. 2007;56:252–269. doi: 10.1016/j.neuron.2007.10.010. [DOI] [PubMed] [Google Scholar]
- O'Rourke NA, Sullivan DP, Kaznowski CE, Jacobs AA, McConnell SK. Tangential migration of neurons in the developing cerebral cortex. Development. 1995;121:2165–2176. doi: 10.1242/dev.121.7.2165. [DOI] [PubMed] [Google Scholar]
- Paredes MF, Li G, Berger O, Baraban SC, Pleasure SJ. Stromal-derived factor-1 (CXCL12) regulates laminar position of Cajal–Retzius cells in normal and dysplastic brains. J Neurosci. 2006;26:9404–9412. doi: 10.1523/JNEUROSCI.2575-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierani A, Moran-Rivard L, Sunshine MJ, Littman DR, Goulding M, Jessell TM. Control of interneuron fate in the developing spinal cord by the progenitor homeodomain protein Dbx1. Neuron. 2001;29:367–384. doi: 10.1016/s0896-6273(01)00212-4. [DOI] [PubMed] [Google Scholar]
- Polleux F, Dehay C, Kennedy H. Neurogenesis and commitment of corticospinal neurons in reeler. J Neurosci. 1998;18:9910–9923. doi: 10.1523/JNEUROSCI.18-23-09910.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Mode of cell migration to the superficial layers of fetal monkey neocortex. J Comp Neurol. 1972;145:61–83. doi: 10.1002/cne.901450105. [DOI] [PubMed] [Google Scholar]
- Rakic P. Neurons in rhesus monkey visual cortex: systematic relation between time of origin and eventual disposition. Science. 1974;183:425–427. doi: 10.1126/science.183.4123.425. [DOI] [PubMed] [Google Scholar]
- Rakic P. Radial versus tangential migration of neuronal clones in the developing cerebral cortex. Proc Natl Acad Sci U S A. 1995;92:11323–11327. doi: 10.1073/pnas.92.25.11323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- Sansom SN, Hébert JM, Thammongkol U, Smith J, Nisbet G, Surani MA, McConnell SK, Livesey FJ. Genomic characterisation of a Fgf-regulated gradient-based neocortical protomap. Development. 2005;132:3947–3961. doi: 10.1242/dev.01968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seuntjens E, Nityanandam A, Miquelajauregui A, Debruyn J, Stryjewska A, Goebbels S, Nave KA, Huylebroeck D, Tarabykin V. Sip1 regulates sequential fate decisions by feedback signaling from postmitotic neurons to progenitors. Nat Neurosci. 2009;12:1373–1380. doi: 10.1038/nn.2409. [DOI] [PubMed] [Google Scholar]
- Spandidos A, Wang X, Wang H, Seed B. PrimerBank: a resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucleic Acids Res. 2010;38:D792–D799. doi: 10.1093/nar/gkp1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumm RK, Zhou C, Ara T, Lazarini F, Dubois-Dalcq M, Nagasawa T, Höllt V, Schulz S. CXCR4 regulates interneuron migration in the developing neocortex. J Neurosci. 2003;23:5123–5130. doi: 10.1523/JNEUROSCI.23-12-05123.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supèr H, Uylings HB. The early differentiation of the neocortex: a hypothesis on neocortical evolution. Cereb Cortex. 2001;11:1101–1109. doi: 10.1093/cercor/11.12.1101. [DOI] [PubMed] [Google Scholar]
- Supèr H, Soriano E, Uylings HB. The functions of the preplate in development and evolution of the neocortex and hippocampus. Brain Res Brain Res Rev. 1998;27:40–64. doi: 10.1016/s0165-0173(98)00005-8. [DOI] [PubMed] [Google Scholar]
- Takiguchi-Hayashi K, Sekiguchi M, Ashigaki S, Takamatsu M, Hasegawa H, Suzuki-Migishima R, Yokoyama M, Nakanishi S, Tanabe Y. Generation of reelin-positive marginal zone cells from the caudomedial wall of telencephalic vesicles. J Neurosci. 2004;24:2286–2295. doi: 10.1523/JNEUROSCI.4671-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verney C, Takahashi T, Bhide PG, Nowakowski RS, Caviness VS., Jr Independent controls for neocortical neuron production and histogenetic cell death. Dev Neurosci. 2000;22:125–138. doi: 10.1159/000017434. [DOI] [PubMed] [Google Scholar]
- Viti J, Gulacsi A, Lillien L. Wnt regulation of progenitor maturation in the cortex depends on Shh or fibroblast growth factor 2. J Neurosci. 2003;23:5919–5927. doi: 10.1523/JNEUROSCI.23-13-05919.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh C, Cepko CL. Widespread dispersion of neuronal clones across functional regions of the cerebral cortex. Science. 1992;255:434–440. doi: 10.1126/science.1734520. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Assimacopoulos S, Jones KR, Grove EA. Massive loss of Cajal-Retzius cells does not disrupt neocortical layer order. Development. 2006;133:537–545. doi: 10.1242/dev.02209. [DOI] [PubMed] [Google Scholar]
- Yun K, Potter S, Rubenstein JL. Gsh2 and Pax6 play complementary roles in dorsoventral patterning of the mammalian telencephalon. Development. 2001;128:193–205. doi: 10.1242/dev.128.2.193. [DOI] [PubMed] [Google Scholar]
- Zecevic N, Rakic P. Development of layer I neurons in the primate cerebral cortex. J Neurosci. 2001;21:5607–5619. doi: 10.1523/JNEUROSCI.21-15-05607.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]