Abstract
The blood–brain barrier (BBB) regulates passage of nutrients and signaling molecules from the circulation into the brain. Whether lipoproteins cross the BBB in vivo has been controversial, and no clear requirement for circulating lipoproteins in brain development has been shown. We address these issues in Drosophila, which has an functionally conserved BBB, and lipoproteins that resemble those of vertebrates. We show that the Drosophila lipoprotein lipophorin exists in two isoforms. Both isoforms cross the BBB, but accumulate on distinct subsets of cells within the brain. In addition to acting as a lipid carrier, lipophorin carries both sterol-linked and GPI-linked proteins into the circulation and transports them across the BBB. Finally, lipophorin promotes neuroblast proliferation by a mechanism that does not depend on delivery of dietary lipids. Transport of lipophorin and its cargo across the BBB represents a novel mechanism by which peripherally synthesized proteins might enter the brain and influence its development. Furthermore, lipid-linkage may be an efficient method to transport therapeutic molecules across the BBB.
Introduction
The BBB prevents unregulated passage of circulating molecules into the brain, maintaining a controlled and stable environment important for neuronal function (Banerjee and Bhat, 2007). To enter the brain, nutrients and signaling molecules must engage specific transport systems on BBB cells (Broadwell, 1989; Hervé et al., 2008). Lipoproteins mediate interorgan transport of nutritional and signaling lipids, and also bear a variety of lipid-linked signaling molecules (Panáková et al., 2005). Vertebrate BBB cells express lipoprotein receptors, and transcytose both LDL and HDL in tissue culture (Dehouck et al., 1997; Balazs et al., 2004). Furthermore, linking peptides derived from ApoE and ApoB to other molecules can promote their transport across the BBB (Spencer and Verma, 2007; Zensi et al., 2009). However, circulating lipoproteins have never been shown to enter to the brain in vivo. If lipoproteins do enter the brain, it is unclear what their function there would be since the brain does not use fatty acids for energy production (Yang et al., 1987). Furthermore, almost all cholesterol in the vertebrate brain is derived from endogenous synthesis rather than lipoprotein-mediated delivery (Dietschy and Turley, 2004; Dietschy, 2009).
The sterol auxotroph Drosophila melanogaster provides a powerful model system to study lipoproteins and the BBB. Their lipoproteins and lipoprotein receptors resemble those of vertebrates (van der Horst et al., 2002; Van der Horst et al., 2009; Khaliullina et al., 2010) and the functionally conserved BBB is formed by septate junctions composed of molecules almost identical to those in vertebrate tight junctions (Awasaki et al., 2008; Stork et al., 2008; Mayer et al., 2009). Here, we investigate whether the Drosophila lipoprotein lipophorin (Lpp) crosses the larval BBB, and whether it influences larval brain development.
Materials and Methods
Cloning
Lipophorin:GAL4.
gal4 cDNA was inserted between the 5.5 kbp promoter and 3 kbp 3′ UTR sequence of lipophorin and cloned into a vector containing a multiple cloning site and mini-white gene flanked by FRT sites.
Tagged mCherry reporters.
An 840 bp fragment containing the predicted signal sequence of lipophorin was fused to a HA-epitope triplet and cloned 5′ prime to mcherry to generate secreted HAmCherrySec. For HAmCherryGPI, we fused HAcherrySec to LFA3-derived sequences specifying GPI addition. For HAmCherrySterol, we fused HAcherrySec to sequences encoding amino acids 243–472 of Hh. For HAmCherry, we fused HAcherrySec to the CD8 transmembrane domain sequence and inserted 2 myc tags 5′ to and a v5 tag 3′ to the transmembrane domain. HAmCherry reporter constructs were cloned into pUAST.
Tagged tevP.
To generate cytoplasmic TevP, the tevP cDNA was cloned into pUAST. To generate a TevP localized to the secretory pathway, we inserted the Lpp translational start site and signal sequence, and 3 HA-epitope tags 5′ of tevP.
Fly stocks
Flies were raised on conventional cornmeal agar under a 12 h light/12 h dark cycle at 25°C. UAS-lipophorinRNAi fly lines are from the Vienna Drosophila RNAi Center and UAS-Dicer2 flies are from Bloomington Stock Center. The HAmCherrySterol enhancer trap harbors a UAS HAmCherrySterol construct expressed independently of GAL4.
Collections
Embryos collected at 4 h intervals were kept at 27°C. Wild-type and LppGAL/+; UAS;dsLpp/+ larvae were transferred to applejuice agar plates containing yeast. Wild-type larvae were also transferred to lipid-depleted medium (Panáková et al., 2005). For phospho-histoneH3 staining, LppGAL/+; UAS;dsLpp/+ larvae and lipid-depleted wild-type larvae were collected after 5 d. As normal second instar controls, we used wild-type larvae fed yeast-containing food for 41 h.
Hemolymph
Larvae.
Larvae were washed with ice-cold PBS, transferred into PBS + protease inhibitors and pierced with a tungsten needle. The hemolymph/PBS was centrifuged 30 min, 550 × g at 4°C. The supernatant was recentrifuged 3 h, 112,000 × g at 4°C, and its protein concentration measured using the Bradford kit (Promega).
Lpp labeling
Fluorescent lipoproteins were prepared according to the method of Khaliullina et al. (2010).
Immunohistochemistry
Larval tissues dissected on ice in PBS were fixed with 4%PFA at room temperature and stained in 10% NGS, 0.1% Triton X-100 PBS solution. Antibodies were diluted as follows: anti-HA16B12 1:1500 (Santa Cruz Biotechnology), anti-Elav7E8A10 1:1500 [Developmental Studies Hybridoma Bank (DSHB), University of Iowa, Iowa City, IA], anti-HRP-Cy5 1:500 (Dianova), anti-Lpp (Eugster et al., 2007) 1:2000, anti-ApoLII 1:2000 and anti-ApoI 1:1500 (Panáková et al., 2005), anti-GFP 1:1000 (Invitrogen), anti-Ci1552A1 (1:30) (Wang and Holmgren, 1999), anti-Dpn (1:30) (Boone and Doe, 2008), anti-Myc 1:1000 (Gramsch), anti-Prospero-MR1A 1:750 (DSHB), anti-Repo8D12 1:500 (DSHB) and anti-phospho-histoneH36G3 1:1500 (Cell Signaling Technology).
Phalloidin-Cy5 1:200 (Invitrogen) and FilipinIII 1:500 (Sigma). For quantitation, tissues were treated in parallel and imaged under identical conditions on the same day using a Zeiss confocal microscope. Data were analyzed using Fiji.
Quantification of CNS staining patterns
To quantify accumulation of HAmCherrygpi, HAmCherrysterol, and HAmCherrysec within the CNS, each section of a confocal stack comprising the entire CNS was examined by a blind observer, who enumerated Elav and HA-double-positive cells. To assess whether TEV-mediated cleavage of HAApoL-tevMyc increased accumulation in the optic anlage, a blind observer evaluated HA distribution in brains from animals expressing either HAApoL-tevMyc alone or HAApoL-tevMyc + TEV protease in the fat body.
Results
To ask whether the major Drosophila lipoprotein, lipophorin (Lpp), accumulated in the larval brain, we stained brains with an antibody directed against Lpp particles. Lpp is detectable on the BBB and within the CNS, suggesting these particles cross the BBB (Fig. 1A,D–I).
Figure 1.
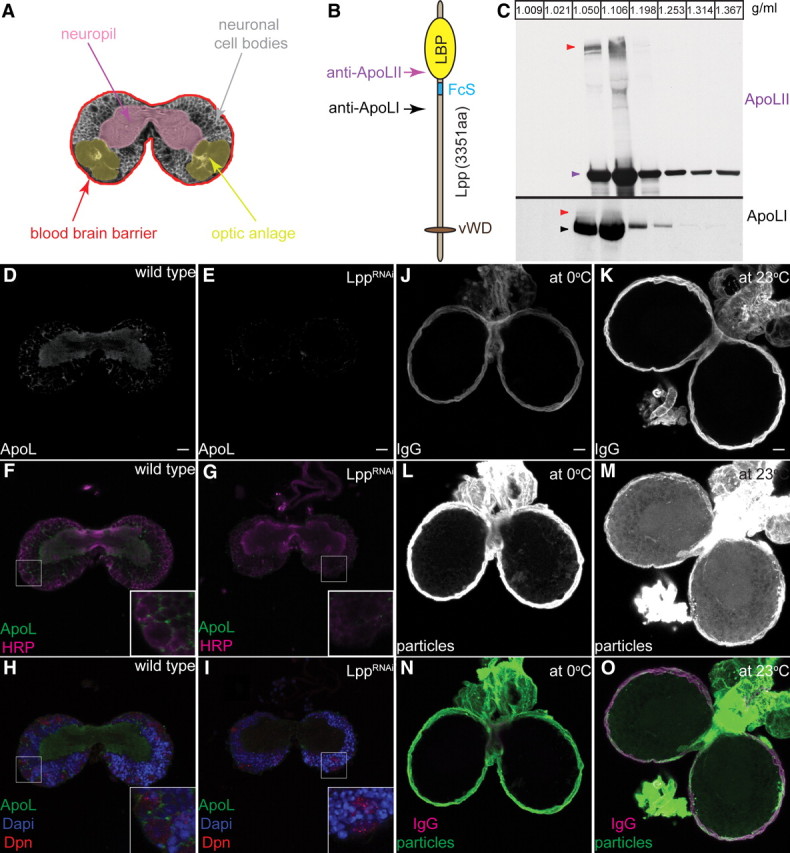
Lpp particles cross the larval BBB. A, Scheme depicts the larval CNS at the level of the commissure: neuropil (magenta), optic anlage (yellow), cell bodies (gray) and the BBB (red). B, Motifs within apolipophorin. Lipid-binding pocket (LBP) (yellow oval), Furin cleavage site (FcS) (blue line), von Willebrand factor D domain (vWD) homology region (brown oval). Arrows indicate the position of peptides used to generate anti-ApoLII (magenta) and anti-ApoLI (black). C, Western blots of larval hemolymph iodixanol density gradients probed with anti-ApoLII and anti-ApoLI. Arrowheads indicate ApoLII (magenta), ApoLI (black) and ApoFL (red). Fraction densities in g/ml. D–I, Confocal section at the level of the commissures from wild-type (D, F, H) and LppRNAi (E, G, I) CNS stained with anti-Lpp (D, E; F–I; green), anti-HRP (F, G; magenta), anti-Dpn (H, I; red) and DAPI (4′,6′-diamidino-2-phenylindole dihydrochloride) (H, I; blue). J–O, Wild-type larval brains incubated at 0°C (J, L, N) or 23°C (K, M, O) with labeled Lpp (L, M; N, O, in green) and labeled IgG (J, K; N, O; magenta). Ten micrometer projections at the level of the commissure.
To directly observe passage of Lpp across the BBB, we used fluorescently labeled Lpp particles. Alexa543-labeled lipophorin and Alexa488-labeled IgG were added to explanted second instar larval brains and incubated at 0°C. After 15 min at 0°C, both Alexa543-labeled Lpp and Alexa488-labeled IgG accumulate on the BBB but not within the CNS (Fig. 1J,L,N). When the incubation is performed at 23°C, Alexa488 IgG remains on the BBB and is still excluded from the CNS (Fig. 1K,O). In contrast, Alexa543-lipophorin enters the brain and accumulates on neuronal cell bodies, axon tracts and cells of the optic anlage (Fig. 1A,M,O). Thus, while IgG is efficiently excluded by the BBB, Lpp particles are actively transported across it.
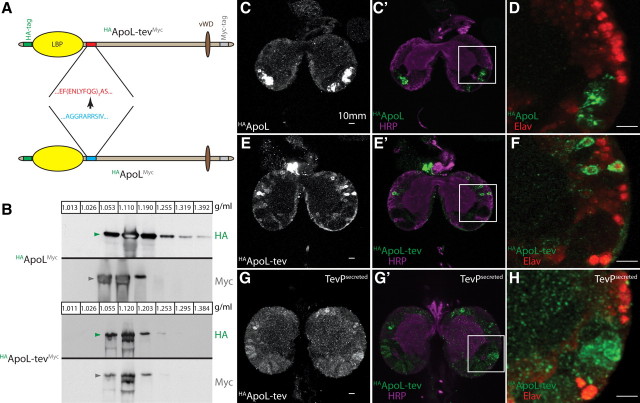
The protein moiety of Lpp, apolipophorin, undergoes furin-dependent cleavage, generating apolipophorin I (ApoLI) and apolipophorin II (ApoLII) (Panáková et al., 2005), both of which circulate on low-density lipophorin particles in the hemolymph (Fig. 1B,C). Western blotting suggests that a fraction of circulating lipophorin particles also contains uncleaved apolipophorin, which we denote ApoLFL (Fig. 1C, red arrowheads). To investigate whether apolipophorin cleavage might influence its passage across the BBB, we replaced the cleavage motif RXRR with a tobacco mosaic virus protease (TevP) cleavage site triplicate (ApoL-tev). We tagged both wild-type apolipophorin (HAApoLMyc) and ApoL-tev (HAApoL-tevMyc) with HA at the N terminus and Myc at the C terminus (Fig. 2A) and expressed these constructs in the fat body (the normal site of lipophorin production) under the control of LppGAL4.
Figure 2.
HAApoL-tevMyc and HAApoLII localize differently within the CNS. A, Replacement of the apolipophorin furin cleavage site (blue) with TEV-protease cleavage sequences (red). HA (green) and Myc (gray) tags are shown. Other motifs as in Figure 1B. B, Western blots of hemolymph iodixanol density gradients from larvae expressing either HAApoLMyc or HAApoL-tevMyc. Blots are probed with anti-HA (green arrowheads) and anti-Myc (gray arrowheads). Size heterogeneity of HAApoL-tevMyc detected with both N-terminal HA and C-terminal Myc tags suggests alternative splicing may generate different ApoLFL isoforms. Fraction densities in g/ml. C–H, Ten micrometer projections of second instar brains from larvae expressing either HAApoLMyc (C, C′, D), HAApoL-tevMyc (E, E′, F), or HAApoL-tevMyc + TevPsecreted (G, G′, H) in the fat body. Brains are stained with anti-HA (C, E, G; C′–H; green), anti-HRP (C′, E′, G′; magenta) and anti-Elav (D, F, H; red). Staining was observed only in the optic anlage for three of three brains expressing HAApoLMyc. Both neuronal and optic anlage staining was observed for five of six brains from animals expressing HAApoL-tevMyc + TevPsecreted. Optic anlage staining was never observed in five of five brains from animals expressing either HAApoL-tevMyc alone or + TevPcytoplasmic.
We first asked whether these tagged versions of apolipophorin were incorporated into lipophorin particles. Western blotting of larval hemolymph density gradients shows that HAApoLMyc is normally processed to HAApoLII and ApoLIMyc, which are incorporated into particles of the same density as wild-type Lpp (compare Fig. 2B, panels 1, 2 with Fig. 1C). As expected, HAApo-tevMyc is not detectably cleaved, and is incorporated in its full-length form into particles of the same density as wild-type ApoLFL (compare Fig. 2B, panels 3, 4 with Fig. 1C). Thus, both proteins produce Lpp particles of normal density—HAApoLMyc produces Lpp particles containing mostly the cleaved isoforms and HAApoL-tevMyc forms lipoproteins in which apolipophorin is uncleaved.
To ask whether these particles differ in their ability to cross the BBB, we expressed either HAApoLMyc or HAApoL-tevMyc under the control of LppGAL4. LppGAL4 is active only in the fat body and has no ectopic activity in the brain (supplemental Fig. S1A,B, available at www.jneurosci.org as supplemental material). Furthermore, neither HAApoLMyc nor HAApoL-tevMyc is detected in the brain when LppGAL4 is not present (supplemental Fig. S1C, available at www.jneurosci.org as supplemental material). In contrast, when these constructs are expressed in the fat body, both ApoL isoforms are found inside the CNS. Strikingly, they localize to different regions. Uncleavable HAApoL-tevMyc localizes to neurons, whereas lipoproteins containing HAApoLII are found almost exclusively in the optic anlage (Fig. 2C–F). To confirm that their different localization depends on apolipophorin cleavage, we induced cleavage of HAApoL-tevMyc by co-expressing the TevP in the fat body. When TevP is expressed in a form that can enter the secretory pathway, localization of HAApoL-tevMyc shifts toward the optic anlage (Fig. 2G,G′,H). These data show that different types of lipoproteins accumulate in specific regions of the brain after crossing the BBB.
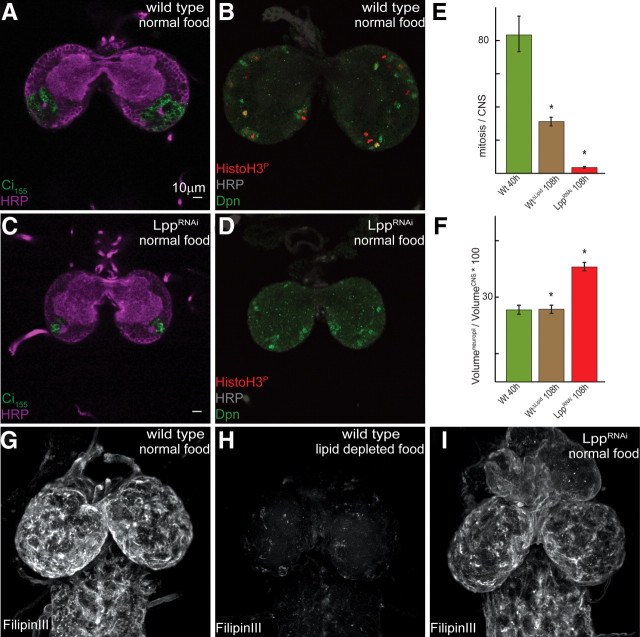
The first instar neuropil is composed of arborized neuronal projections formed by primary neurons that arise during embryogenesis. The neuropil increases in volume during early larval stages as primary neurons further extend neurites and increase their arborization. At the same time, continuous division of neuroblasts in the cortex generates what will become new secondary neurons (Larsen et al., 2009). To assess requirements for lipophorins in the brain at this stage, we used RNA interference to reduce lipophorin production in the fat body (supplemental Fig. S2A–C, available at www.jneurosci.org as supplemental material). LppRNAi larvae arrest in the second instar and live many days without developing further. Anti-HRP staining reveals that the second instar CNS of these larvae remains smaller than that of stage-matched control animals, and that this results from a reduction in the volume occupied by neuronal cell bodies and the optic anlage (Fig. 3A,C). Surprisingly, the size of the neuropil is even greater than that of wild-type second instar larvae (Fig. 3F; supplemental Fig. S3C,D, available at www.jneurosci.org as supplemental material). Staining glial cells with anti-Repo indicates that glial cell number is normal in these brains (supplemental Fig. S3G,H, available at www.jneurosci.org as supplemental material). Together, these data suggest that LppRNAi may block proliferation of neuroblasts without affecting neurite growth from primary neurons.
Figure 3.
Lpp loss of function blocks neuroblast proliferation. A–D, Confocal sections of second instar brains from wild-type (A, B) or LppRNAi (C, D) larvae, stained with anti-HRP (A, C; magenta), anti-Ci155 (A, C; green), anti-HRP (B, D; gray), anti-Dpn (B, D; green) or anti-HistoH3P (B, D; red). Ci-155 labels the optic anlage, Dpn marks neuroblasts, and phospho-histone H3 indicates mitotic cells. E, Quantification of phospho-histone H3-positive cells within second instar brains. Green bar, Wild-type larvae fed normal food (n = 5, 83.4 ± 21.5); brown bar, wild-type larvae fed lipid-depleted food (n = 5, 31.2 ± 5.2); red bar, LppRNAi larvae fed normal food (n = 6, 3.5 ± 1.0). Asterisks indicate significant differences from wild-type larvae fed normal food (p < 0.001). Error bars are SDs. F, Neuropil/CNS volume ratio of wild-type larvae raised on normal food (green bar) or lipid-depleted food (brown bar) and LppRNAi larvae raised on normal food (red bar). Asterisks indicate significant differences from wild-type larvae fed normally (p < 0.0001). Error bars are SDs. G–I, Projections of whole brains stained with FilipinIII from wild-type larvae raised on normal food (G) or lipid-depleted food (H) and LppRNAi larvae raised on normal food (I).
To investigate this further, we stained the CNS of LppRNAi and control animals with anti-phospho-histone H3, which detects cells in mitosis, and with anti-Deadpan (Dpn), which labels neuroblasts in the CNS (Boone and Doe, 2008). In the wild-type CNS, a significant fraction of Dpn-labeled neuroblasts also stain with anti-phospho-histone H3 (Fig. 3B). In contrast, proliferation in LppRNAi brains is dramatically reduced (Fig. 3E). Although Dpn-positive neuroblasts are still detected in the CNS, they do not stain with anti-phospho-histone H3 (Fig. 3D; not a single Dpn-positive neuroblast stained with anti-phospho-histone H3 in 10 LppRNAi brains examined). The few dividing cells observed in these brains were not Dpn-positive (supplemental Fig. S3A,B, available at www.jneurosci.org as supplemental material). Thus, LppRNAi blocks proliferation of neuroblasts, preventing generation of secondary neurons within the CNS.
To confirm that neurite extension from primary neurons was unaffected in LppRNAi animals, we labeled a single neuron that projects to the mushroom body and quantified the average length of its primary projection (before the first branch point). The average length does not differ in wild-type and LppRNAi animals (supplemental Fig. S4A,B, available at www.jneurosci.org as supplemental material). Furthermore, the subset of FasII-positive neuronal projections in LppRNAi larvae are larger than those of equally staged control animals (supplemental Fig. S4C,D, available at www.jneurosci.org as supplemental material), confirming that loss of Lpp does not reduce neurite growth from existing primary neurons. Because neurite growth demands the incorporation of large amounts of lipid into the plasma membrane (Pfenninger, 2009), these observations suggest that membrane lipids are not limiting in LppRNAi animals.
Although Drosophila requires sterols for development, they cannot synthesize them and depend on nutritional sources (Clayton, 1964). LppRNAi inhibits lipid mobilization from the gut (Panáková et al., 2005) and reduces sterol levels in the larval imaginal discs (Khaliullina et al., 2010). Removal of dietary sterol arrests larval growth and development in the first/second instar (M. Carvalho and S. Eaton, unpublished observations), similar to the arrest seen in LppRNAi larvae. Therefore, we wondered whether reduced proliferation in the brain might be an indirect consequence of the lack of sterols or other essential dietary lipids. To investigate this, we compared the effects of LppRNAi versus dietary lipid depletion. Staining larval brains with FilipinIII, to detect free sterols, reveals that dietary lipid depletion reduces brain sterol levels more than LppRNAi (Fig. 3G–I) However, while LppRNAi reduces mitosis dramatically, dietary lipid depletion has less severe effects (Fig. 3E,F; supplemental Figure S3C,D, available at www.jneurosci.org as supplemental material). Thus, reduced proliferation in the brain of LppRNAi animals is not a consequence of the lack of sterol or other dietary lipids.
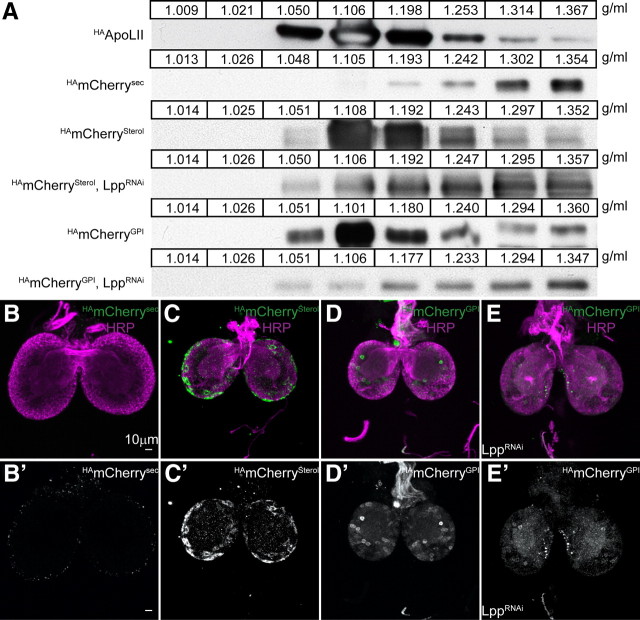
Since Lpp did not appear to promote proliferation by delivering dietary lipid, we wondered about other possible cargo. We previously reported that lipid-linked proteins (including GPI-linked, cholesterol-modified and palmitoylated proteins) associate with Lpp, permitting their local mobilization (Panáková et al., 2005; Eugster et al., 2007). We wondered whether Lpp association might also allow release of lipid-linked proteins into systemic circulation. To investigate this, we constructed HAmCherry fusion proteins that were either secreted (HAmCherrysec), or targeted to the membrane in different ways. Membrane association was conferred either by a transmembrane domain (HAmCherry), GPI linkage (HAmCherryGPI) or cholesterol modification (HAmCherrySterol). We expressed these constructs in the fat body, and analyzed larval hemolymph to determine whether they were released into systemic circulation. Both HAmCherrysec and the two lipid-linked forms circulate systemically. While soluble proteins such as HAmCherrysec are present only in high-density fractions, the majority of HAmCherrySterol and HAmCherryGPI cofractionates with Lpp particles (Fig. 4A). As expected, HAmCherry is not present in the hemolymph, although it is expressed at equivalent levels in the fat body (supplemental Fig. S1, available at www.jneurosci.org as supplemental material). Thus, GPI and sterol-linked proteins are released into systemic circulation on Lpp.
Figure 4.
HAmCherryGPI crosses the BBB on Lpp. A, Iodixanol density gradients of hemolymph from third instar wild-type (rows 1, 2, 3, 5) and LppRNAi larvae (rows 4, 6) expressing HAmCherrysec, HAmCherrysterol or HAmCherryGPI in the fat body. Western blots are probed with anti-ApoLII (first row) or anti-HA (rows 2–6). Fraction densities are shown in g/ml. B–D′, Forty micrometer projection at the level of the commissures from wild-type (B, C′) and LppRNAi (D, D′) second instar brains expressing HAmCherrysec (B, B′), HAmCherrysterol (C, C′), or HAmCherryGPI (D, E′) in the fat body stained with anti-HA (B′, C′, D′; B–D; green) and anti-HRP (B–D, magenta). HAmCherrysec is not found within the CNS (0 HAmCherry-positive neurons in 10 brains examined). HAmCherryGPI accumulates in the CNS (>50 HAmCherry-positive neurons in all 5 brains examined). HAmCherrysterol accumulates weakly in the CNS (5, 13, and 22 HAmCherry-positive neurons in 3 brains examined).
To ask whether Lpp was required for release of HAmCherrySterol and HAmCherryGPI into the hemolymph, we reduced Lpp levels in animals expressing either HAmCherrySterol or HAmCherryGPI in the fat body. Lpp knock-down dramatically reduces the amount of lipid-linked HAmCherry present in low-density fractions. (Fig. 4A, compare rows 3, 5 with rows 4, 6). This indicates that HAmCherrySterol and HAmCherryGPI are released on Lpp, when it is present, but that other release mechanisms operate in its absence.
To test whether Lpp particles might act as vehicles for the transport of lipid-linked proteins across the BBB, we asked whether expression of HAmCherry fusion proteins in the fat body resulted in their accumulation in the CNS. Although HAmCherrysec circulates systemically, it is not found within the CNS (Fig. 4B,B′). In contrast, when HAmCherryGPI is expressed in the fat body, it is detected on subsets of neurons within the brain at varying levels (Fig. 4D,D′). HAmCherryGPI accumulates only on neurons, and not in the optic anlage—similar to the localization of Lpp particles containing uncleaved HAApo-tevMyc. The presence of HAmCherryGPI in the brain does not result from GAL4-independent expression (supplemental Fig. S1D,E, available at www.jneurosci.org as supplemental material). Interestingly, although HAmCherrySterol, like HAmCherryGPI, circulates on Lpp particles it is distributed differently when the particles reach the CNS. HAmCherrySterol accumulates strongly on the BBB, while HAmCherryGPI does not. Furthermore, while HAmCherryGPI is detected on at least 50 neurons/brain, many fewer neurons are positive for HAmCherrySterol (Fig. 4C,D′). Thus, HAmCherryGPI crosses the BBB more efficiently than HAmCherrySterol, and the non-Lpp-associated HAmCherrysec does not cross the BBB at all.
HAmCherryGPI circulates in two forms, one Lpp-associated, and one of a higher density. To determine whether Lpp association was required for HAmCherryGPI to cross the BBB, we knocked down Lpp in animals expressing HAmCherryGPI in the fat body. Lpp knock-down reduces levels of HAmCherryGPI in the brain (compare Fig. 4D,D′,E,E′), suggesting that HAmCherryGPI can cross the BBB only when associated with Lpp. Together, our data indicate that lipid-linked proteins cross the BBB in a lipophorin-dependent manner.
Discussion
The possibility that lipoproteins cross the BBB has been suggested by the presence of lipoprotein receptors on BBB cells, and by the observation that peptides derived from ApoE and ApoB promote transport of linked proteins across the BBB (Balazs et al., 2004; Kreuter et al., 2007; Spencer and Verma, 2007; Candela et al., 2008; Zensi et al., 2009). Nevertheless, there has been no evidence to show that circulating lipoproteins cross the BBB in vivo, or what their function in the brain might be if they did.
We show that the Drosophila lipoprotein lipophorin crosses the BBB, and is essential for neuroblast proliferation. Our data suggest this requirement is independent of its function in lipid delivery. What other roles might Lpp play in brain development? The finding that lipophorin transports GPI-linked proteins into the CNS suggests a new range of mechanisms by which systemic signals might influence the nervous system: peripheral growth-promoting signals might be transported into the brain on Lpp. This may be of particular relevance to lipid-modified growth factors such as the EGF-related Spitz (Pan and Kastin, 1999; Miura et al., 2006). Furthermore, once inside the brain, Lpp may act as it does in imaginal discs (Panáková et al., 2005) to help mobilize signaling molecules (e.g., axon guidance cues), which act over large distances (Brankatschk and Dickson, 2006; Charron and Tessier-Lavigne, 2007).
Our observations suggest remarkable specificity in the trafficking of lipophorin and lipophorin-associated proteins in the nervous system. Two isoforms of Lpp differing only in the processing of apolipophorin both cross the BBB but accumulate in different regions of the CNS. Furthermore, although both sterol and GPI-linked proteins associate with Lpp, they are transported across the BBB with different efficiencies. This may reflect association with functionally different lipoprotein subpopulations, or differences in trafficking at the BBB. The cell biological basis of this specificity will be fascinating to unravel. Furthermore, these observations raise the possibility that linking therapeutic molecules to lipids could facilitate transport across the BBB by promoting association with lipoproteins.
Footnotes
This work was supported by a European Molecular Biology Organization postdoctoral fellowship to M.B., by a grant from the Deutsche Forschungsgemeinschaft to S.E., and by the Max Planck Gesellschaft. We are grateful to Volker Hartenstein, Elisabeth Knust, Kai Simons, Wilhelm Palm, Christina Eugster, Andreas Sagner, and Daiki Umetsu for helpful discussions and critical comments on the manuscript. We thank Sven Ssykor for help with transgenesis and stock maintenance.
References
- Awasaki T, Lai SL, Ito K, Lee T. Organization and postembryonic development of glial cells in the adult central brain of Drosophila. J Neurosci. 2008;28:13742–13753. doi: 10.1523/JNEUROSCI.4844-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balazs Z, Panzenboeck U, Hammer A, Sovic A, Quehenberger O, Malle E, Sattler W. Uptake and transport of high-density lipoprotein (HDL) and HDL-associated alpha-tocopherol by an in vitro blood-brain barrier model. J Neurochem. 2004;89:939–950. doi: 10.1111/j.1471-4159.2004.02373.x. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Bhat MA. Neuron-glial interactions in blood-brain barrier formation. Annu Rev Neurosci. 2007;30:235–258. doi: 10.1146/annurev.neuro.30.051606.094345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone JQ, Doe CQ. Identification of Drosophila type II neuroblast lineages containing transit amplifying ganglion mother cells. Dev Neurobiol. 2008;68:1185–1195. doi: 10.1002/dneu.20648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brankatschk M, Dickson BJ. Netrins guide Drosophila commissural axons at short range. Nat Neurosci. 2006;9:188–194. doi: 10.1038/nn1625. [DOI] [PubMed] [Google Scholar]
- Broadwell RD. Transcytosis of macromolecules through the blood-brain barrier: a cell biological perspective and critical appraisal. Acta Neuropathol. 1989;79:117–128. doi: 10.1007/BF00294368. [DOI] [PubMed] [Google Scholar]
- Candela P, Gosselet F, Miller F, Buee-Scherrer V, Torpier G, Cecchelli R, Fenart L. Physiological pathway for low-density lipoproteins across the blood-brain barrier: transcytosis through brain capillary endothelial cells in vitro. Endothelium. 2008;15:254–264. doi: 10.1080/10623320802487759. [DOI] [PubMed] [Google Scholar]
- Charron F, Tessier-Lavigne M. The Hedgehog, TGF-beta/BMP and Wnt families of morphogens in axon guidance. Adv Exp Med Biol. 2007;621:116–133. doi: 10.1007/978-0-387-76715-4_9. [DOI] [PubMed] [Google Scholar]
- Clayton RB. The utilization of sterols by insects. J Lipid Res. 1964;15:3–19. [PubMed] [Google Scholar]
- Dehouck B, Fenart L, Dehouck MP, Pierce A, Torpier G, Cecchelli R. A new function for the LDL receptor: transcytosis of LDL across the blood-brain barrier. J Cell Biol. 1997;138:877–889. doi: 10.1083/jcb.138.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietschy JM. Central nervous system: cholesterol turnover, brain development and neurodegeneration. Biol Chem. 2009;390:287–293. doi: 10.1515/BC.2009.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietschy JM, Turley SD. Thematic review series: brain lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J Lipid Res. 2004;45:1375–1397. doi: 10.1194/jlr.R400004-JLR200. [DOI] [PubMed] [Google Scholar]
- Eugster C, Panáková D, Mahmoud A, Eaton S. Lipoprotein-heparan sulfate interactions in the Hh pathway. Dev Cell. 2007;13:57–71. doi: 10.1016/j.devcel.2007.04.019. [DOI] [PubMed] [Google Scholar]
- Hervé F, Ghinea N, Scherrmann JM. CNS delivery via adsorptive transcytosis. Aaps J. 2008;10:455–472. doi: 10.1208/s12248-008-9055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaliullina H, Panáková D, Eugster E, Riedel F, Carvalho M, Eaton S. Patched regulates Smoothened trafficking using lipoprotein-derived lipids. Development. 2010 doi: 10.1242/dev.041392. in press. [DOI] [PubMed] [Google Scholar]
- Kreuter J, Hekmatara T, Dreis S, Vogel T, Gelperina S, Langer K. Covalent attachment of apolipoprotein A-I and apolipoprotein B-100 to albumin nanoparticles enables drug transport into the brain. J Control Release. 2007;118:54–58. doi: 10.1016/j.jconrel.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Larsen C, Shy D, Spindler SR, Fung S, Pereanu W, Younossi-Hartenstein A, Hartenstein V. Patterns of growth, axonal extension and axonal arborization of neuronal lineages in the developing Drosophila brain. Dev Biol. 2009;335:289–304. doi: 10.1016/j.ydbio.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer F, Mayer N, Chinn L, Pinsonneault RL, Kroetz D, Bainton RJ. Evolutionary conservation of vertebrate blood–brain barrier chemoprotective mechanisms in Drosophila. J Neurosci. 2009;29:3538–3550. doi: 10.1523/JNEUROSCI.5564-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura GI, Buglino J, Alvarado D, Lemmon MA, Resh MD, Treisman JE. Palmitoylation of the EGFR ligand Spitz by Rasp increases Spitz activity by restricting its diffusion. Dev Cell. 2006;10:167–176. doi: 10.1016/j.devcel.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Pan W, Kastin AJ. Entry of EGF into brain is rapid and saturable. Peptides. 1999;20:1091–1098. doi: 10.1016/s0196-9781(99)00094-7. [DOI] [PubMed] [Google Scholar]
- Panáková D, Sprong H, Marois E, Thiele C, Eaton S. Lipoprotein particles are required for Hedgehog and Wingless signalling. Nature. 2005;435:58–65. doi: 10.1038/nature03504. [DOI] [PubMed] [Google Scholar]
- Pfenninger KH. Plasma membrane expansion: a neuron's Herculean task. Nat Rev Neurosci. 2009;10:251–261. doi: 10.1038/nrn2593. [DOI] [PubMed] [Google Scholar]
- Spencer BJ, Verma IM. Targeted delivery of proteins across the blood-brain barrier. Proc Natl Acad Sci U S A. 2007;104:7594–7599. doi: 10.1073/pnas.0702170104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stork T, Engelen D, Krudewig A, Silies M, Bainton RJ, Klämbt C. Organization and function of the blood-brain barrier in Drosophila. J Neurosci. 2008;28:587–597. doi: 10.1523/JNEUROSCI.4367-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Horst DJ, van Hoof D, van Marrewijk WJ, Rodenburg KW. Alternative lipid mobilization: the insect shuttle system. Mol Cell Biochem. 2002;239:113–119. [PubMed] [Google Scholar]
- Van der Horst DJ, Roosendaal SD, Rodenburg KW. Circulatory lipid transport: lipoprotein assembly and function from an evolutionary perspective. Mol Cell Biochem. 2009;326:105–119. doi: 10.1007/s11010-008-0011-3. [DOI] [PubMed] [Google Scholar]
- Wang QT, Holmgren RA. The subcellular localization and activity of Drosophila cubitus interruptus are regulated at multiple levels. Development. 1999;126:5097–5106. doi: 10.1242/dev.126.22.5097. [DOI] [PubMed] [Google Scholar]
- Yang SY, He XY, Schulz H. Fatty acid oxidation in rat brain is limited by the low activity of 3-ketoacyl-coenzyme A thiolase. J Biol Chem. 1987;262:13027–13032. [PubMed] [Google Scholar]
- Zensi A, Begley D, Pontikis C, Legros C, Mihoreanu L, Wagner S, Büchel C, von Briesen H, Kreuter J. Albumin nanoparticles targeted with Apo E enter the CNS by transcytosis and are delivered to neurones. J Control Release. 2009;137:78–86. doi: 10.1016/j.jconrel.2009.03.002. [DOI] [PubMed] [Google Scholar]