Abstract
Although synaptophysin is one of the most abundant integral proteins of synaptic vesicle membranes, its contribution to neurotransmitter release remains unclear. One possibility is that through its association with dynamin it controls the fine tuning of transmitter release. To test this hypothesis, we took advantage of amperometric measurements of quantal catecholamine release from chromaffin cells. First, we showed that synaptophysin and dynamin interact in chromaffin granule-rich fractions and that this interaction relies on the C terminal of synaptophysin. Experimental maneuvers that are predicted to disrupt the association between these two proteins, such as injection of antibodies against dynamin or synaptophysin, or peptides homologous to the C terminal of synaptophysin, increased the quantal size and duration of amperometric spikes. In contrast, the amperometric current that precedes the spike remained unchanged, indicating that synaptophysin/dynamin association does not regulate the initial fusion pore, but it appears to target a later step of exocytosis to control the amount of catecholamines released during a single vesicle fusion event.
Introduction
As for neurotransmitter release at synapses, catecholamine release from adrenal chromaffin cells requires the concerted action of protein complexes that mobilize the secretory vesicle to the release site, fuse the vesicle membrane with the plasma membrane, empty its content, and retrieve the vesicle membrane for recycling. Being one of the most abundant integral proteins of synaptic vesicle membranes (Jahn et al., 1985), synaptophysin has been proposed to participate at several stages of the vesicle life cycle (Valtorta et al., 2004). This protein is also present in secretory granules of adrenal chromaffin cells (Obendorf et al., 1988; Schilling and Gratzl, 1988; Fournier et al., 1989) but at a lower density compared with synaptic vesicle membranes (Schilling and Gratzl, 1988).
Synaptophysin is composed of four transmembrane domains and cytosolic C and N termini (Südhof et al., 1987). The functional role of this protein has been controversial. Early studies supported the idea that synaptophysin was a component of the fusion pore formed during exocytosis (Thomas et al., 1988) and that it played a central role in neurotransmitter release (Alder et al., 1992a, 1992b; Yin et al., 2002). However, this idea was inconsistent with the lack of a neurotransmitter release phenotype of synaptophysin knock-out mice (McMahon et al., 1996). One possible explanation is the inherent redundancy of the system, which expresses related proteins such as synaptoporin and synaptogyrin (Janz et al., 1999; Spiwoks-Becker et al., 2001). More recent findings implicate synaptophysin in the rapid retrieval of synaptic vesicles through its association with dynamin (Daly et al., 2000; Daly and Ziff, 2002). The hypothesis set forth by these authors is that synaptophysin recruits the GTPase dynamin to the release site in a calcium-dependent manner to promote the rapid retrieval of synaptic vesicles (Daly et al., 2000). In chromaffin cells, disruption of dynamin GTPase activity increases the amount of catecholamines released per individual exocytotic event (Graham et al., 2002). These findings indicate that dynamin plays a pivotal role in controlling the amount of transmitters released during a single exocytotic event. However, the mechanism by which dynamin controls the quantal size (Q) remains unknown. To get additional insight into this mechanism, we characterized the interaction between synaptophysin and dynamin in chromaffin cells, and, taking advantage of the carbon-fiber amperometry technique, we studied how the disruption of the association between these proteins affects individual exocytotic events. Our results show that synaptophysin–dynamin association controls the quantal size and the duration of the exocytotic events. However, the amperometric current that precedes the amperometric spike remains unchanged, indicating that the synaptophysin–dynamin association does not regulate the initial fusion pore, but would become involved in a later step of exocytosis. This yet-to-be-identified step would control the amount of catecholamines released during a single vesicle exocytotic event.
Materials and Methods
Cloning, expression, and purification of C terminal of synaptophysin.
The plasmid Bluescript II SK containing the complete cDNA of synaptophysin was a gift from Dr. Rudolf E. Leube (University of Mainz, Mainz, Germany). The region encoding the C-terminal domain of synaptophysin (from amino acid 219 to amino acid 307) was amplified by PCR using the forward primer (5′-AAGGAGACAGGCTGGGCAGCC-3′) and reverse primer (5′-TTACATCTGATTGGAGAAGGAGGTGGG-3′) complementary to nucleotides 664-684 and 907-933 of the rat coding sequence, respectively (Leube et al., 1987). The resulting PCR product was digested with BamHI and EcoRI and subcloned into the expression plasmid pGEX-4T2 (GE Healthcare) and verified by DNA sequencing. The resulting clone was expressed in Escherichia coli BL21 cells according to standard methods, and the glutathione S-transferase (GST) fusion protein (GST-Cterm) was purified by batch binding from cell lysates to glutathione agarose beads (Invitrogen). GST protein was a gift from Dr. Patricia Hidalgo (Hannover Medizinishe Hocheshule, Hannover, Germany).
Synthesis of a synthetic peptide homologous to synaptophysin C terminal.
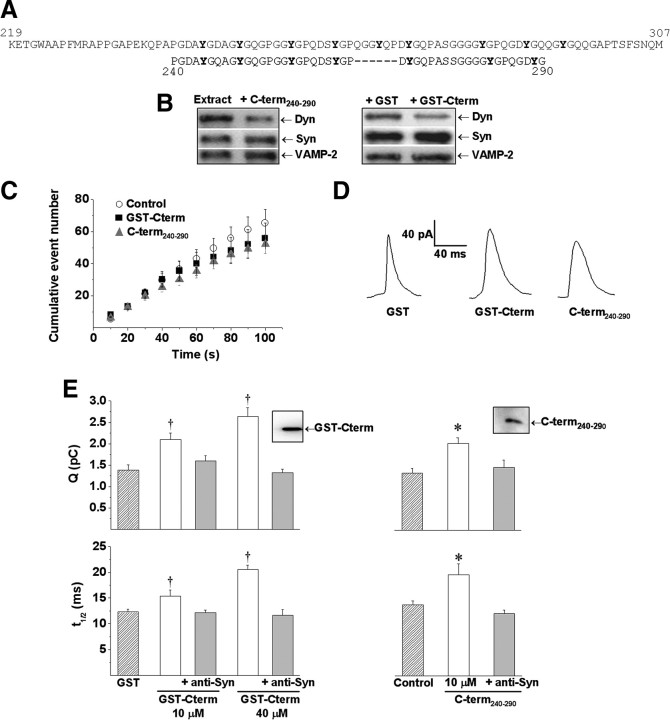
We designed a synthetic peptide homologous to a region of the C terminal of bovine synaptophysin (amino acids 240-290) that contains the segment necessary to interact with dynamin (Daly and Ziff, 2002) and with the monoclonal antibody against synaptophysin clone SY-38 (Knaus and Betz, 1990). For synthesis requirement, this peptide contains a deletion of six amino acids (265-270), but for simplicity we called it C-term240-290 (for sequence, see Fig. 4A). This peptide was synthesized by Invitrogen. The immunoreactivity of C-term240-290 to the anti-synaptophysin antibody (clone SY-38) was confirmed by Western blot after being subjected to electrophoresis on 12% SDS-polyacrylamide Tris-tricine gels (see Fig. 4D, inset).
Figure 4.
Microinjections of synaptophysin C-terminal derivatives increase duration and quantal size of depolarization-induced exocytotic events. Chromaffin cells were injected with GST (40 μm), GST-Cterm (10 and 40 μm), GST-Cterm plus anti-Syn antibody (10 and 40 μm), C-term240-290 (10 μm), or C-term240-290 plus anti-Syn antibody (10 μm). Thirty to forty-five minutes after injections, exocytosis responses evoked by high K+ were measured. A, Amino acid sequence of the C-terminal domain of rat synaptophysin (top, gray) and the synthetic peptide C-term240-290 (bottom, black). Tyrosine residues are shown in bold. Underlined amino acids indicate the epitope recognized by anti-Syn. B, Protein extracts from chromaffin cells were immunoprecipitated with a polyclonal anti-synaptophysin antibody, in the presence of 10 μm C-term240-290, GST, or GST-Cterm. Bound proteins were analyzed by immunoblotting with antibodies against Syn, Dyn, and VAMP-2. C, Cumulative histograms of the number of amperometric events from control cells (white circles), cells injected with 40 μm GST-Cterm (black square) or 10 μm C-term240-290 (gray triangles). D, Representative amperometric traces from cells injected with GST, GST-Cterm or C-term240-290. E, Averaged values for Q and t1/2. Data are means ± SEM of averages per cell (12–24 cells from 3 to 5 different cultures). *p < 0.05 compared with control cells. Insets: Immunoblotting showing that GST-Cterm and C-term240-290 are recognized by the anti-Syn antibody.
Cell cultures and microinjections.
Bovine adrenal chromaffin cells were isolated as previously described (Montiel et al., 2003). Briefly, cells were suspended in 1:1 mixture of DMEM and Ham′s F-12 (DMEM-F12) supplemented with 10% fetal calf serum, 50 IU/ml penicillin, and 100 μg/ml gentamicin. Cells were plated on 25-mm-diameter glass coverslips at a density of 2.5 × 104 cells/ml. Cells were kept in a water-saturated incubator at 37°C, in a 5% CO2/95% air atmosphere, and used in the experiments after 2–4 d of culture.
Chromaffin cells were injected using an InyectMan NI2 system (Eppendorf) and glass micropipettes with an internal tip diameter of 0.5 μm (Femtotips, Eppendorf). The injection time was 0.2 s at a pressure of 120 hPa. During microinjection, chromaffin cells were kept in DMEM-F12 without HCO3 to avoid changes in pH.
The monoclonal antibodies against dynamin (anti-Dyn, BD Bioscience), synaptophysin, clone SY-38 (anti-Syn, Millipore Bioscience Research Reagents), poly-His (anti-His, Covance), and C-term240-290 were injected at an approximate concentration of 10 μm. The GST-Cterm was injected at 10 and 40 μm.
Amperometric detection of exocytosis.
The exocytotic release of catecholamines was detected by amperometry, as previously described (Ardiles et al., 2006). Carbon fibers of 5 μm radius (Thornel P-55, Amoco) were used to manufacture the microelectrodes (Kawagoe et al., 1993). Electrochemical recordings were performed using an Axopatch 1C patch-clamp amplifier modified for this purpose according to the manufacturer instructions (Molecular Devices). Cells were placed in a perfusion chamber positioned on the stage of an inverted microscope and washed in Krebs-HEPES buffer solution (in mm: 140 NaCl, 5 KCl, 2 MgCl2, 2.5 CaCl2, 10 HEPES-NaOH, and 10 glucose, pH 7.4). Catecholamine release was stimulated by 10 s pressure ejection of 100 mm K+ solution from a micropipette positioned 10–15 μm from the cell. Amperometric records were low pass filtered at 1 kHz and digitized at 5 Hz with a PCI-6030E analog-to-digital converter (National Instruments) controlled by WinEDR software (University of Strathclyde). Data analysis was carried out using locally written macros for IGOR (Wavemetrics). These macros allowed the automatic digital filtering, secretory spike identification, and data analysis (Segura et al., 2000). All of the above macros and their user instructions can be downloaded from the following Web address: http://webpages.ull.es/users/rborges/. Spikes with amplitude <15 pA or rise time >15 ms as well as overlapping spikes were excluded from the analysis. Foot analysis was restricted to events with current and duration >2 pA and >2 ms, respectively.
Subcellular fractionation of chromaffin cells.
Chromaffin cells were centrifuged at 1000 × g for 10 min, and the pellet was resuspended in a buffer solution containing: 0.32 m sucrose, protease inhibitor mixture (Roche Applied Science), 10 mm Tris-HCl, pH 7.4. Then the supernatant was centrifuged at 20,000 × g for 20 min, and the pellet was layered over a continuous sucrose density gradient (1–2.2 m). Gradients were centrifuged at 4°C for 1 h at 100,000 × g in a Beckman Optima XL-100 K ultracentrifuge. Fractions of 300 μl were then collected from the bottom to top; aliquots were taken for immunoblotting analysis.
Chromaffin granules were purified from the 20,000 × g pellet using 1.6 m sucrose gradients (Smith and Winkler, 1967). The chromaffin granule-enriched fraction was solubilized in cold hypotonic buffer (1 mm HEPES/Na, pH 7.0, and protease inhibitor mixture) and centrifuged again at 100,000 × g for 1 h. The pellet was then resuspended in cold hypotonic buffer for immunoblotting analysis.
Immunoprecipitation and pull-down assays.
Proteins from chromaffin cells were extracted at 4°C in a lysis buffer (150 mm NaCl, 20 mm HEPES, 5 mm EDTA, pH 7.4, 1% Triton X-100) containing a protease inhibitor mixture (Roche Applied Science). In some experiments, EDTA was replaced by 2 mm EGTA or 2 mm CaCl2 and 2 mm MgCl2 in the lysis buffer. For immunoprecipitation assays, 1 μg of anti-Dyn, anti-Syn, or a polyclonal antibody against amino acids 41-62 of human synaptophysin (Abcam) was incubated for 2 h with 30 μl of A-Sepharose 4B beads (Invitrogen). After binding, the beads were washed three times in lysis buffer. Then, the antibody bound to the Sepharose beads were incubated for 1 h at 4°C with 800 μg of protein extracts in the presence of 10% bovine serum albumin. Bound proteins were eluted in SDS sample buffer and analyzed by immunoblotting for synaptophysin, dynamin, or vesicle-associated membrane protein 2 (VAMP-2). VAMP-2 was identified with a polyclonal antibody (Novus Biologicals).
For pull-down assays, 100 μl of glutathione-agarose beads (Invitrogen) were incubated with 120 μg of GST or GST-Cterm for 5 h at 4°C. Then, the GST fusion proteins immobilized on glutathione-agarose beads were incubated overnight at 4°C with 800 μg of protein extracts. After binding, the beads were washed three times in lysis buffer. Bound proteins were eluted in SDS sample buffer and analyzed by immunoblotting.
Statistics.
Results were expressed as means ± SEM. Amperometric spike data were grouped by individual cells; thus, the data presented correspond to means ± SEM of cell averages, and “n” refers to the number of cells. Statistical comparisons were performed using the nonparametric Mann–Whitney U test.
Results
Synaptophysin is a partner of dynamin in chromaffin granules
To characterize the interaction between synaptophysin and dynamin in chromaffin cells, we performed immunoprecipitation and pull-down assays, and analyzed the presence of both proteins in chromaffin granule membranes. As shown in Figure 1A, immunoprecipitation with the anti-Dyn antibody precipitated a comparable amount of synaptophysin from chromaffin cell extracts in the presence of Ca2+/Mg2+ or EGTA. Densitometric analysis of immunoblots obtained from six independent experiments shows nonsignificant differences for synaptophysin precipitated in both conditions. Thus, contrary to that observed in brain extracts (Daly and Ziff, 2002), in chromaffin cells free Ca2+ does not appear to influence synaptophysin–dynamin interaction, at least in vitro. This does not rule out though that cytosolic Ca2+ may regulate this interaction in vivo. Therefore, we immunoprecipitated cell extracts obtained immediately after depolarizing the cells with 100 mm KCl for 5 min (Fig. 1B). Densitometric analysis of immunoblots from nine experiments shows a 62 ± 15% increase in the amount of immunoprecipitated synaptophysin from extracts from depolarized cells. To test whether Ca2+ entry was mediating this increase, we depolarized the cells in the presence of EGTA or Co2+, a nonspecific calcium channel blocker. Surprisingly, we found that the EGTA or Co2+ treatment did not inhibit the depolarization-induced increase of synaptophysin–dynamin interaction (Fig. 1B). The analysis of five experiments shows nonsignificant differences.
Figure 1.
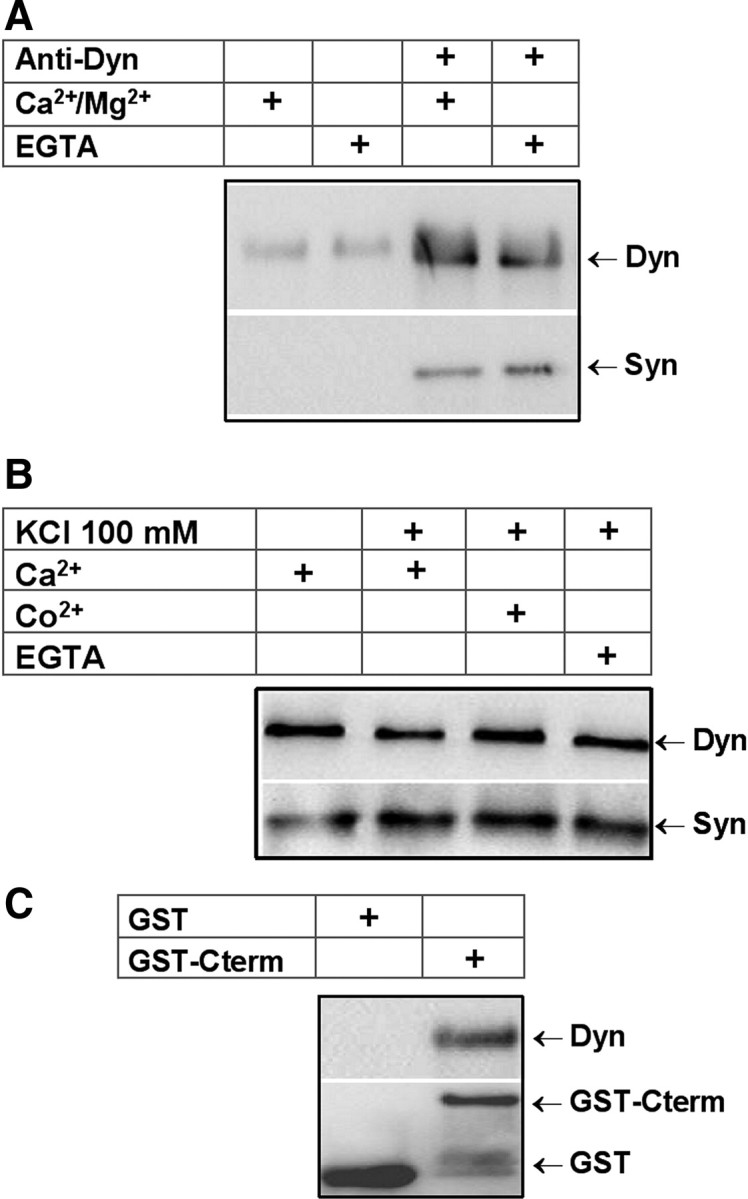
Synaptophysin binds to dynamin in bovine chromaffin cells. A, Protein extracts from cultured bovine chromaffin cells were incubated in the presence of Ca2+/Mg2+ or EGTA for 30 min, and then subjected to immunoprecipitation with a monoclonal anti-dynamin antibody. Bound proteins were analyzed by immunoblotting with anti-Dyn and anti-Syn antibodies. B, Protein extracts from non-depolarized chromaffin cells, or cells depolarized with 100 mm KCl (5 min) in the presence of 2.5 mm Ca2+, 5 mm EGTA, or 10 mm Co2+ were immunoprecipitated with a monoclonal anti-dynamin antibody. EGTA and Co2+ were present 15 min before and during the depolarizing pulse. Bound proteins were analyzed by immunoblotting with antibodies against Syn and Dyn. C, GST alone or GST-Cterm was immobilized on glutathione-agarose beads and incubated with protein extracts from chromaffin cells. The bound material was subjected to immunobloting with anti-Dyn and anti-GST antibodies.
In brain extract, synaptophysin was shown to interact with dynamin through its C-terminal domain (Daly and Ziff, 2002). Here, to confirm that in chromaffin cells the C terminal of synaptophysin was also mediating this interaction, we used a GST fusion protein comprising the C terminal of synaptophysin (GST-Cterm) to pull down dynamin. As shown in Figure 1C, GST-Cterm binds dynamin from chromaffin cell extracts.
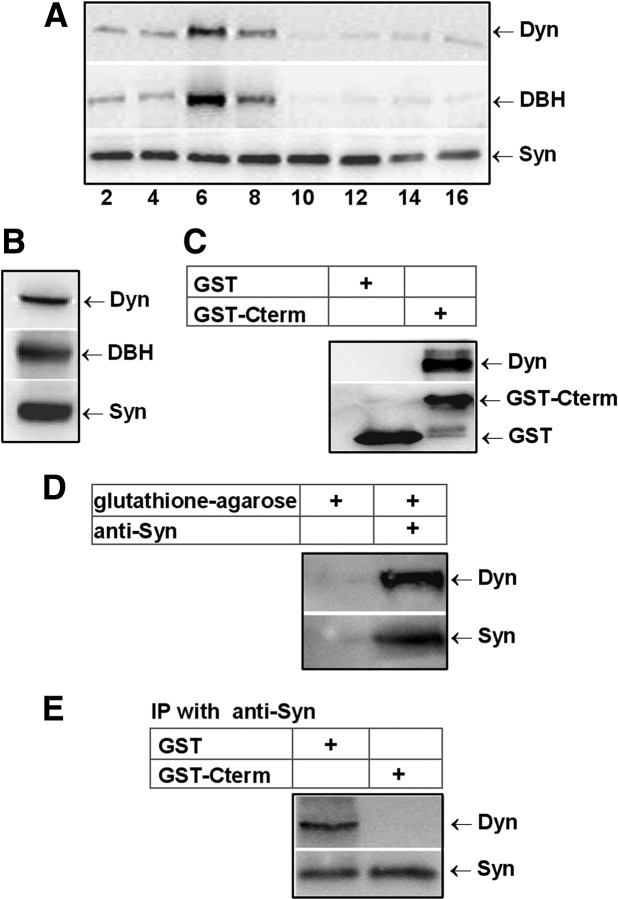
To investigate in which cellular compartment the dynamin–synaptophysin interaction occurs we analyzed the subcellular distribution of these proteins. To this end, cellular extracts were layered on a continuous sucrose density gradient (1.0–2.2 m sucrose), ultracentrifuged at 100,000 × g for 1 h and collected in 16 fractions that were assayed by immunoblot for dynamin, synaptophysin, and dopamine β-hydroxylase (DBH), a standard chromaffin granule marker. As shown in Figure 2A, the distribution of dynamin correlated quite well with the distributions of DBH (fractions 6–8), confirming its association with chromaffin granules. A similar distribution of dynamin in bovine chromaffin cells was previously observed by Galas et al. (2000). In contrast, synaptophysin is broadly distributed across the sucrose density gradients. This result is consistent with synaptophysin being present not only in chromaffin granules (Obendorf et al., 1988; Schilling and Gratzl, 1988; Fournier et al., 1989) but also in other organelles such as synaptic-like microvesicles (Annaert et al., 1993), trans-Golgi networks (Régnier-Vigouroux et al., 1991), endosomes (Tixier-Vidal et al., 1988), and recycling vesicular organelles (Cameron et al., 1991; Linstedt and Kelly, 1991).
Figure 2.
Immunodetection of dynamin and synaptophysin in chromaffin granules. A, Fractions 2–16 collected from a continuous sucrose density gradient (1.0–2.2 m sucrose) layered with protein extracts from chromaffin cells were subjected to gel electrophoresis and immunodetected with anti-Dyn, anti-DBH, and anti-Syn. B, Purified chromaffin granule membranes were subjected to immunoblotting with anti-Dyn, anti-DBH, and anti-Syn. C, GST alone or GST-Cterm were immobilized on glutathione-agarose beads and incubated with protein extracts from chromaffin granules. The bound material was subjected to immunobloting with anti-Dyn and anti-GST antibodies. D, Chromaffin granule extracts were subjected to immunoprecipitation with anti-Syn. Bound proteins were analyzed by immunoblotting with anti-Dyn and anti-Syn. E, Supernatants from pull-down assays were subjected to immunoprecipitation with anti-Syn, and then bound proteins were analyzed by immunoblotting with anti-Dyn and anti-Syn.
We also analyzed the presence of dynamin and synaptophysin in membranes isolated from purified chromaffin granules and investigate whether these proteins interact in these organelles. As shown in Figure 2B, dynamin and synaptophysin are present in chromaffin granule membranes, and both GST-Cterm and anti-Syn antibody efficiently bind dynamin from granule extracts (Fig. 2C,D). Interestingly, when the supernatants obtained from the pull-down assays were subjected to an immunoprecipitation using the anti-Syn antibody, we observed that no dynamin was immunoprecipitated from the supernatant obtained from the GST-Cterm pull-down assay (Fig. 2E). This result suggests that in chromaffin granules most dynamin is found as a dynamin–synaptophysin complex.
Microinjections of antibodies against synaptophysin or dynamin increase duration and quantal size of the exocytotic events
To investigate the role of synaptophysin–dynamin association in exocytosis, we injected chromaffin cells with proteins that interfere with either the function or association of these proteins. Compared with DNA transfection or protein dialysis via a patch pipette, microinjection of purified proteins is particularly well suited to study acute effects without affecting the cytosol composition. Exocytosis was monitored by amperometry, a technique that allows monitoring individual exocytotic events in real time as amperometric spikes. The time course and area of these spikes yield information about the kinetics and the mode of exocytosis (Fisher et al., 2001; Ardiles et al., 2007).
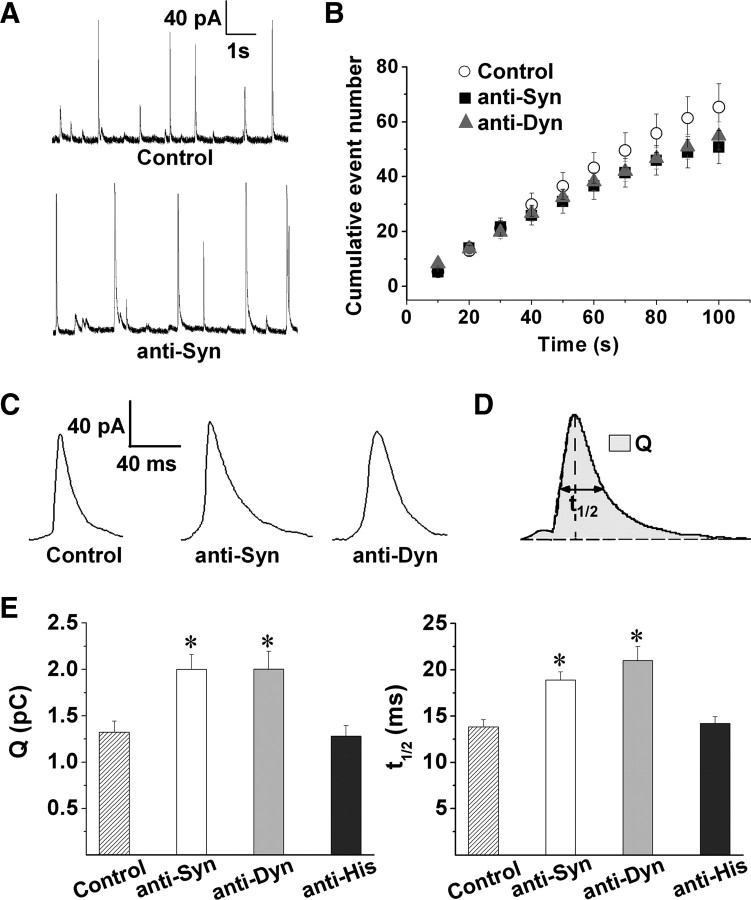
Figure 3A shows representative amperometric traces from experiments performed in chromaffin cells noninjected (control) or injected with anti-Syn. Figure 3B compares the cumulative histograms of the number of amperometric events from both conditions. A 10 s pulse of high K+ produced 65 ± 8 (n = 24) amperometric spikes over a period of 100 s in control cells. A similar number of exocytotic events evoked by high K+ was observed in cells injected with anti-Syn (51 ± 6 exocytotic events in 100 s). However, as shown in Figure 3C, the shape and size of the individual amperometric spikes appear modified by anti-Syn intracellular injection. Figure 3D summarizes the amperometric parameters analyzed: (1) the Q, which is proportional to the amount of catecholamines released during each exocytotic event; and (2) the half-width (t1/2) that reflects the duration of the individual exocytotic events. As shown in Figure 3E, Q was significantly larger in anti-Syn injected cells (2.0 ± 0.16 pC) than control cells (1.3 ± 0.1 pC). The injection of anti-Syn also slowed t1/2 significantly. Mean values for t1/2 were 14 ± 0.8 ms for control, and 19 ± 0.9 ms for anti-Syn-injected cells. We also analyzed other amperometric parameters, such as the peak amplitude (Imax), corresponding to the maximum concentration reaching the carbon electrode; the rise time, influenced by the size and expansion rate of the fusion pore (Neco et al., 2008); and the decay time, which reflects depletion of catecholamines. The latter parameter seems to be accelerated by the premature closure of the fusion pore (Elhamdani et al., 2001; Graham et al., 2002; Barclay et al., 2003). As shown in Table 1, injection of the anti-Syn significantly slowed the decay time, while the rise time and Imax remain unchanged.
Figure 3.
Microinjections of antibodies against synaptophysin or dynamin increase duration and quantal size of depolarization-induced exocytotic events. Chromaffin cells were injected with anti-Syn, anti-Dyn or anti-His. The exocytosis response was evoked by high K+ and measured 30–45 min after injections. A, Representative amperometric traces from noninjected cells (Control) or cells injected with anti-Syn. B, Cumulative histograms of the number of amperometric events from control (white circles), anti-Syn (black squares) and anti-Dyn injected cells (gray triangles). C, Representative amperometric spikes from noninjected cells (Control), anti-Syn and anti-Dyn injected cells. D, Representative amperometric spike with its parameters: Q and t1/2. E, Averaged values for Q and t1/2. Data are means ± SEM of averages per cell (14–24 cells from 3 to 5 different cultures). *p < 0.05 compared with control cells.
Table 1.
Amperometric spike parameters from chromaffin cells in different experimental conditions
| Imax (pA) | Rise time (ms) | Decay time (ms) | |
|---|---|---|---|
| Control | 83 ± 4 | 5.2 ± 0.3 | 7.7 ± 0.5 |
| Anti-Syn (10 μm) | 81 ± 7 | 5.9 ± 0.5 | 10.6 ± 0.8* |
| Anti-Dyn (10 μm) | 78 ± 6 | 5.4 ± 0.4 | 10.3 ± 0.7* |
| Anti-His (10 μm) | 77 ± 4 | 5.5 ± 0.4 | 8.1 ± 0.4 |
| GST (40 μm) | 88 ± 8 | 4.8 ± 0.3 | 7.3 ± 0.5 |
| GST-Cterm (10 μm) | 95 ± 4* | 4.9 ± 0.4 | 8.6 ± 0.6 |
| GST-Cterm + anti-Syn (10 μm) | 89 ± 5 | 4.7 ± 0.2 | 7.2 ± 0.4 |
| GST-Cterm (40 μm) | 89 ± 4 | 4.8 ± 0.4 | 11.9 ± 0.6* |
| GST-Cterm + anti-Syn (40 μm) | 90 ± 9 | 4.5 ± 0.5 | 7.1 ± 0.6 |
| C-term240-290 (10 μm) | 92 ± 4 | 5.7 ± 0.5 | 10.6 ± 1.0* |
| C-term240-290 + anti-Syn (10 μm) | 85 ± 9 | 5.0 ± 0.3 | 7.1 ± 0.3 |
Control cells correspond to noninjected cells. Cells were injected with monoclonal anti-Syn, anti-Dyn, or anti-His antibodies. Cells were also injected with the following peptides: GST, GST-Cterm, and C-term240-290. Peptides encompassing the C terminal of synaptophysin were also coinjected with anti-Syn (GST-Cterm + anti-Syn; C-term240-290 + anti-Syn). Rise time, Time needed to reach Imax; Decay time, time constant of a single exponential fit to the decay phase. Data are means ± SEM of cell median for each spike parameters (12–24 cells from 3–5 different cultures).
*p < 0.05 compared with control cells.
We also evaluated the effect of intracellular injection of anti-Dyn. Injection of this antibody did not affect the number of exocytotic events significantly (55 ± 5) (Fig. 3B). However, compared with noninjected control cells (Fig. 3E), anti-Dyn injection significantly increased Q and t1/2 (2.0 ± 0.19 pC and 21 ± 1.5 ms, respectively). As observed for anti-Syn, anti-Dyn also slowed the decay time, but neither Imax nor the rise time were affected (Table 1). These results agree with previous findings in chromaffin cells showing that disruption of dynamin function increases both catecholamine quantal size and duration of exocytotic events (Graham et al., 2002).
As a control for specificity of anti-Syn and anti-Dyn effects, we injected cells with an antibody against an antigen not present in chromaffin cells such as a standard polyhistidine tag (anti-His). Anti-His injected at the same concentration used for the other antibodies (10 μm) did not modify any of the different amperometric parameters (Fig. 3E, Table 1).
Disruption of synaptophysin–dynamin association with the C terminal of synaptophysin increases duration and quantal size of depolarization-induced exocytotic events
To disrupt the association between synaptophysin and dynamin, we injected chromaffin cells with GST-Cterm or C-term240-290, a synthetic peptide homologous to the C-terminal region of bovine synaptophysin encompassing both the minimal region necessary to interact with dynamin (Daly and Ziff, 2002) and the anti-Syn epitope (Knaus and Betz, 1990). Figure 4A shows the amino acid sequences of the C-terminal domain of rat synaptophysin used to construct GST-Cterm fusion protein, and the synthetic peptide C-term240-290. To demonstrate that these peptides indeed disrupt the synaptophysin–dynamin association, we immunoprecipitated with a polyclonal antibody against amino acids 41-62 of human synaptophysin protein extracts incubated with 10 μm GST-Cterm or C-term240-290. As shown in Figure 4B, coprecipitation of dynamin was considerably diminished in the presence of both peptides. Coprecipitation of dynamin decreased by 62 ± 10% (n = 4) and 78% (n = 2) in the presence of GST-Cterm or C-term240-290, respectively. As a control, we evaluated the effect of these peptides on the coprecipitation of VAMP-2, a known partner of synaptophysin in synaptic vesicles. The analysis shows that VAMP-2 coprecipitation was not affected in the presence of GST-Cterm or C-term240-290. VAMP-2/synaptophysin ratios were 1.02 ± 0.07 (n = 4) and 1.12 (n = 2).
Cumulative histograms of the number of events show that the intracellular injection of either GST-Cterm or C-term240-290 did not modify significantly the number of the exocytotic events induced by high K+ (Fig. 4C). Over a period of 100 s, GST-Cterm-injected cells (40 μm) and C-term240-290-injected cells (10 μm) produced 56 ± 9 (n = 19) and 52 ± 6 (n = 18) amperometric spikes, respectively. Figure 4D shows representative amperometric spikes of cells injected with either GST alone, GST-Cterm, or C-term240-290. The analysis of the amperometric parameters revealed that injection of GST-Cterm modified Q in a concentration-dependent manner (Fig. 4E). Q values for 10 and 40 μm GST-Cterm were 2.1 ± 0.2 and 2.6 ± 0.2 pC, respectively. These Q values were significantly higher than those from cells injected with GST alone (p < 0.05). Q value for GST alone was 1.4 ± 0.13 pC. As shown in Figure 4E, GST-Cterm, at both concentrations, also slowed t1/2 significantly, compared with cells injected with GST alone (p < 0.05). For 10 and 40 μm of GST-Cterm, t1/2 values were 15 ± 1.2 and 20 ± 0.9 ms, respectively. Only at 40 μm, GST-Cterm slowed the decay time significantly (p < 0.05 compared with GST alone and control cells; Table 1). The rise time was not affected by both concentrations of GST-Cterm, but compared with noninjected cells at 10 μm this recombinant protein increased Imax significantly (Table 1). As shown in Figure 4E, C-term240-290 (10 μm) increased Q (2.0 ± 0.1 pC) and t1/2 (20 ± 2 ms) (p < 0.05 compared with controls). Compared with control cells, this peptide also slowed the decay time significantly, but it did not modify Imax or the rise time (Table 1).
The epitope recognized by anti-Syn is located in the C-terminal tail of synaptophysin, which was confirmed by the Western blots shown in Figure 4E as insets. Therefore, the specificity of the effects of both antibody and peptides was tested by injecting anti-Syn preincubated with either GST-Cterm or C-term240-290. As shown in Figure 4E, coinjection of anti-Syn with either GST-Cterm or C-term240-290 did not modify the amperometric parameters.
The acute disruption of synaptophysin–dynamin association does not affect the fusion pore dynamics
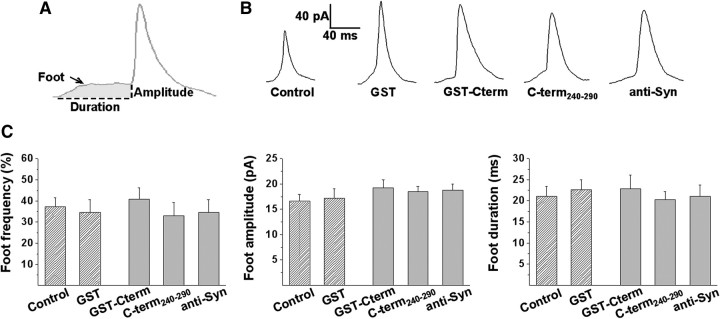
Preceding the amperometric spike of a standard exocytotic event, it is often possible to detect a small stepwise current usually referred to as “foot” that reflects the slow release of catecholamines through a narrow fusion pore (Chow et al., 1992). The amplitude of the foot currents is proportional to the fusion pore conductance (Albillos et al., 1997), whereas the duration of the foot signal is thought to reflect the stability of the fusion pore (Lindau and Alvarez de Toledo, 2003). Here, we examined the effects of GST-Cterm, C-term240-290, and anti-Syn on the frequency, duration, and amplitude of foot signals. Figure 5B shows examples of amperometric spikes from controls and cells injected with GST, GST-Cterm, C-term240-290, or anti-Syn. As shown in Figure 5C, 37 ± 4% of the amperometric spikes from control cells exhibited feet with an average duration and amplitude of 21 ± 2 ms and 17 ± 1 pA, respectively. The injection of GST, GST-Cterm, C-term240-290, or anti-Syn did not affect the frequency, amplitude, and duration of foot signals (Fig. 5C).
Figure 5.
Microinjections of either derivatives of the C terminal of synaptophysin or anti-Syn do not affect the fusion pore dynamics. A, Example traces with a long-lasting foot event (shaded area) showing foot amplitude and foot duration. B, Examples of spikes with foot signals from different experimental conditions. C, Averaged values for frequency (percentage of spikes with foot per cell), foot amplitude, and foot duration of amperometric spikes of cells noninjected (Control) or injected with GST (40 μm), GST-Cterm (40 μm), C-term240-290 (10 μm), or anti-Syn (10 μm). Data are means ± SEM average per cell (14–22 cells from 3 to 5 different cultures).
Discussion
In the present article, we studied the interaction of synaptophysin with dynamin in chromaffin cells and showed that synaptophysin is a partner of dynamin in chromaffin granules, and that disruption of the association between these proteins increases the quantal size and duration of individual exocytotic events.
Synaptophysin and dynamin are constitutively associated in chromaffin cells
In contrast to a previous study in brain synaptosomes (Daly and Ziff, 2002) using the same protocol, we observed that in chromaffin cells synaptophysin–dynamin interaction occurs in vitro independently of the presence of Ca2+ (Fig. 1A). We also show that also in vivo this interaction does not depend on cytosolic Ca2+ (Fig. 1B). The lack of Ca+2 dependence may stem from differences in the type of dynamin involved. Neurons express mostly dynamin-1, whereas chromaffin cells mainly express dynamin-2 (Galas et al., 2000). These two isoforms differ in their capability to interact with other proteins. Dynamin-2, for example, binds more efficiently to the postsynaptic protein Shank than dynamin-1 (Okamoto et al., 2001). In addition, each dynamin isoform has at least four alternatively spliced variants (Cao et al., 1998), and these splicings may also display differences in their affinities for partner proteins (McNiven et al., 2000).
A curious feature of the dynamin–synaptophysin association in chromaffin cells is that the interaction in vivo between these proteins is favored by cell depolarization, but this effect is independent of Ca2+ entry. We still have not been able to identify a Ca2+-independent mechanism for the depolarization-induced increase in dynamin–synaptophysin association. One possibility is suggested by the observation that membrane depolarization provoked by high K+ produces intracellular acidification in rat hippocampal slices (Zhan et al., 1998). This effect, only partially dependent on extracellular Ca2+, was proposed to stem from a metabolic accumulation of lactate secondary to an increased Na+-K+ ATPase activity. It is plausible that membrane depolarization of chromaffin cells also changes the cytosolic pH by a similar mechanism and that this acidification impacts dynamin–synaptophysin association.
Our results also indicate that synaptophysin and dynamin are constitutively associated in chromaffin cells and that this interaction seems important to keep dynamin bound to the chromaffin granules. Indeed, our results show that synaptophysin and dynamin are present in chromaffin granules (Fig. 2A,B), and that both anti-Syn and GST-Cterm efficiently bind dynamin from granule extracts (Fig. 2C,D). As shown by the distribution of these proteins in a continuous sucrose density gradient, synaptophysin is also present in other organelles, whereas dynamin cosediments with synaptophysin only in the chromaffin granule fraction (Fig. 2A). This preferential colocalization of dynamin with synaptophysin in chromaffin granule could be a consequence of: (1) other proteins present in the granules, such as syntaxin, contributing to the association of dynamin to chromaffin granules (Galas et al., 2000); and/or (2) the lipid composition of the granule membranes favoring the associations between synaptophysin and dynamin. The latter idea is supported by the observation that cholesterol content is higher in granules than in synaptic-like microvesicles such as those found in PC12 cells (Wang et al., 2006) and that cholesterol content regulates the association of synaptophysin with other proteins, such as synaptobrevin (Mitter et al., 2003).
How does the synaptophysin–dynamin association influence the quantal release of catecholamines?
The association of synaptophysin with dynamin has been implicated in two different processes. Daly et al. (2000) found that synaptophysin–dynamin complex is necessary for the rapid retrieval of synaptic vesicles, while Liang et al. (2007) proposed that it mediates opioid receptor endocytosis. Here, we propose that synaptophysin–dynamin association has a more subtle effect on the vesicle retrieval mechanism and that its role is to fine-tune the release of catecholamines.
Injections of derivatives of the C-terminal domain of synaptophysin (GST-Cterm and C-term240-290) increase duration and quantal size of the exocytotic events and slow down the decay of the amperometric spike (Fig. 4). Similar effects were observed when the cells were injected with antibodies against synaptophysin or dynamin (Fig. 3). These kinds of changes in the amperometric parameters could reflect: (1) a shift in the mode of exocytosis/endocytosis (Elhamdani et al., 2001; Graham et al., 2002; Barclay et al., 2003; Chen et al., 2005); or (2) an increment in the amount of catecholamines stored in the granules (Colliver et al., 2000). Different modes of exocytosis/endocytosis have been described in neuroendocrine cells, among them the kiss-and-run mechanism. In this mechanism, the fusion pore remains open for a few hundred milliseconds and then abruptly recloses, releasing a small amount of catecholamines, usually observed as “stand-alone-foot” events in amperometric recordings (Zhou et al., 1996; Albillos et al., 1997; Alés et al., 1999). A dynamin-dependent kiss-and-run mechanism, whereby dynamin facilitates the closure of the fusion pore, has also been proposed (Elhamdani et al., 2001; Graham et al., 2002). In this scheme, disruption of dynamin function would increase catecholamine quantal size in chromaffin cells, as observed in the present article (Fig. 3E) and by Graham et al. (2002). However, the molecular mechanisms by which dynamin regulates the quantal size is unknown. One possibility is that amphiphysin, one of the most important dynamin-binding partners at the synapse, recruits dynamin to the exocytotic sites. However, seemingly contradictory results have been reported for the effect of disrupting of the dynamin–amphiphysin association. Graham et al. (2002) and Chen et al. (2005) have shown an increase in the quantal size of the exocytotic events, while Fulop et al. (2008) have claimed a decrease. Elhamdani et al. (2006) found no effect. Here, we propose instead that synaptophysin is the partner that recruits dynamin to the chromaffin granules and that higher concentrations of dynamin at release sites will hasten the closure of the fusion pore.
An alternative explanation for the increase in catecholamine quantal size is an increment in the amount of catecholamines stored in the granules. To discard this possibility, we measured chromaffin granule sizes in cells injected with C-term240-290. As long as intragranular concentrations of catecholamine remain constant, an increase in its total content should be reflected in a change in the granule size (Colliver et al., 2000; Gong et al., 2003). As shown in supplemental Figure 1 (available at www.jneurosci.org as supplemental material), the intracellular injection of C-term240-290 did not significantly affect the size of either the granule or its dense core. Thus, as long as the concentration remains constant, disruption of the synaptophysin–dynamin association does not increase the intragranular catecholamine content. However, additional analyses are required to completely discard this possibility.
Disruption of synaptophysin–dynamin association does not regulate the initial fusion pore
It has been observed that synaptophysin forms homomultimers that upon reconstitution into lipid bilayers give rise to voltage-sensitive channels (Thomas et al., 1988; Gincel and Shoshan-Barmatz, 2002). Similar channel activity was observed when membranes from bovine neurohypophysial secretory granules were incorporated into lipid bilayers (Yin et al., 2002). These observations led to the idea that synaptophysin-formed channels contribute to the fusion pore (Thomas et al., 1988). However, their existence in native cell membranes has not yet been demonstrated. Thomas et al. (1988) and Yin et al. (2002) showed that channel activity decreases when exposed to the same antibody that we used in this current work. Thus, in a scenario that the fusion pore corresponds to synaptophysin-formed channels, their blockade by the antibody should inhibit the exocytotic release of transmitters and/or alter the fusion pore properties. Anti-Syn was shown to block peptide release from permeabilized neurohypophysial terminals (Yin et al., 2002) but not to interfere with the depolarization-induced exocytosis in PC-12 cells (Elferink et al., 1993). Here, we observed no effects of the anti-Syn on the spike frequency (Fig. 3B) or fusion pore properties as monitored by the foot current preceding the amperometric spike (Fig. 5C). On the other hand, disruption of synaptophysin–dynamin association with GST-Cterm or C-term240-290 also failed to alter either the fusion pore conductance and stability or the frequency of spikes and foot signals (Figs. 4B, 5C). Thus, synaptophysin does not appear to regulate the initial fusion pore but, as mentioned previously, after association with dynamin it would become involved in a later step of the exocytosis. This mechanism may play a key role in the tight regulation of hormone release under physiological conditions.
Footnotes
This work was supported by Fondecyt 1085266, DIPUV 08/2006, Anillo de Ciencia y Tecnología (ACT 46), and Proyecto PBCT Redes 24. We thank F. Vargas for his assistance in the electron microscopy, A. Ardiles for helping in the immunodetection of C-term240-290, Dr. P. Hidalgo (Hannover Medizinishe Hocheshule, Hannover, Germany) for providing the GST protein, Dr. R. E. Leube (University of Mainz, Mainz, Germany) for providing the synapotophysin cDNA, and Frigorifico Don Pedro for providing bovine adrenal glands.
References
- Albillos A, Dernick G, Horstmann H, Almers W, Alvarez de Toledo G, Lindau M. The exocytotic event in chromaffin cells revealed by patch amperometry. Nature. 1997;389:509–512. doi: 10.1038/39081. [DOI] [PubMed] [Google Scholar]
- Alder J, Lu B, Valtorta F, Greengard P, Poo MM. Calcium-dependent transmitter secretion reconstituted in Xenopus oocytes: requirement for synaptophysin. Science. 1992a;257:657–661. doi: 10.1126/science.1353905. [DOI] [PubMed] [Google Scholar]
- Alder J, Xie ZP, Valtorta F, Greengard P, Poo M. Antibodies to synaptophysin interfere with transmitter secretion at neuromuscular synapses. Neuron. 1992b;9:759–768. doi: 10.1016/0896-6273(92)90038-f. [DOI] [PubMed] [Google Scholar]
- Alés E, Tabares L, Poyato JM, Valero V, Lindau M, Alvarez de Toledo G. High calcium concentrations shift the mode of exocytosis to the kiss-and-run mechanism. Nat Cell Biol. 1999;1:40–44. doi: 10.1038/9012. [DOI] [PubMed] [Google Scholar]
- Annaert WG, Llona I, Backer AC, Jacob WA, De Potter WP. Catecholamines are present in a synaptic-like microvesicle-enriched fraction from bovine adrenal medulla. J Neurochem. 1993;60:1746–1754. doi: 10.1111/j.1471-4159.1993.tb13399.x. [DOI] [PubMed] [Google Scholar]
- Ardiles AO, Maripillán J, Lagos VL, Toro R, Mora IG, Villarroel L, Alés E, Borges R, Cárdenas AM. A rapid exocytosis mode in chromaffin cells with a neuronal phenotype. J Neurochem. 2006;99:29–41. doi: 10.1111/j.1471-4159.2006.04080.x. [DOI] [PubMed] [Google Scholar]
- Ardiles AO, González-Jamett AM, Maripillán J, Naranjo D, Caviedes P, Cárdenas AM. Calcium channel subtypes differentially regulate fusion pore stability and expansion. J Neurochem. 2007;103:1574–1581. doi: 10.1111/j.1471-4159.2007.04871.x. [DOI] [PubMed] [Google Scholar]
- Barclay JW, Craig TJ, Fisher RJ, Ciufo LF, Evans GJ, Morgan A, Burgoyne RD. Phosphorylation of Munc18 by protein kinase C regulates the kinetics of exocytosis. J Biol Chem. 2003;278:10538–10545. doi: 10.1074/jbc.M211114200. [DOI] [PubMed] [Google Scholar]
- Cameron PL, Südhof TC, Jahn R, De Camilli P. Colocalization of synaptophysin with transferrin receptors: implications for synaptic vesicle biogenesis. J Cell Biol. 1991;115:151–164. doi: 10.1083/jcb.115.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Garcia F, McNiven MA. Differential distribution of dynamin isoforms in mammalian cells. Mol Biol Cell. 1998;9:2595–2609. doi: 10.1091/mbc.9.9.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XK, Wang LC, Zhou Y, Cai Q, Prakriya M, Duan KL, Sheng ZH, Lingle C, Zhou Z. Activation of GPCRs modulates quantal size in chromaffin cells through G(betagamma) and PKC. Nat Neurosci. 2005;8:1160–1168. doi: 10.1038/nn1529. [DOI] [PubMed] [Google Scholar]
- Chow RH, von Rüden L, Neher E. Delay in vesicle fusion revealed by electrochemical monitoring of single secretory events in adrenal chromaffin cells. Nature. 1992;356:60–63. doi: 10.1038/356060a0. [DOI] [PubMed] [Google Scholar]
- Colliver TL, Pyott SJ, Achalabun M, Ewing AG. VMAT-Mediated changes in quantal size and vesicular volume. J Neurosci. 2000;20:5276–5282. doi: 10.1523/JNEUROSCI.20-14-05276.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly C, Ziff EB. Ca2+-dependent formation of a dynamin-synaptophysin complex: potential role in synaptic vesicle endocytosis. J Biol Chem. 2002;277:9010–9015. doi: 10.1074/jbc.M110815200. [DOI] [PubMed] [Google Scholar]
- Daly C, Sugimori M, Moreira JE, Ziff EB, Llinás R. Synaptophysin regulates clathrin-independent endocytosis of synaptic vesicles. Proc Natl Acad Sci U S A. 2000;97:6120–6125. doi: 10.1073/pnas.97.11.6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elferink LA, Peterson MR, Scheller RH. A role for synaptotagmin (p65) in regulated exocytosis. Cell. 1993;72:153–159. doi: 10.1016/0092-8674(93)90059-y. [DOI] [PubMed] [Google Scholar]
- Elhamdani A, Palfrey HC, Artalejo CR. Quantal size is dependent on stimulation frequency and calcium entry in calf chromaffin cells. Neuron. 2001;31:819–830. doi: 10.1016/s0896-6273(01)00418-4. [DOI] [PubMed] [Google Scholar]
- Elhamdani A, Azizi F, Solomaha E, Palfrey HC, Artalejo CR. Two mechanistically distinct forms of endocytosis in adrenal chromaffin cells: differential effects of SH3 domains and amphiphysin antagonism. FEBS Lett. 2006;580:3263–3269. doi: 10.1016/j.febslet.2006.04.083. [DOI] [PubMed] [Google Scholar]
- Fisher RJ, Pevsner J, Burgoyne RD. Control of fusion pore dynamics during exocytosis by Munc18. Science. 2001;291:875–878. doi: 10.1126/science.291.5505.875. [DOI] [PubMed] [Google Scholar]
- Fournier S, Novas ML, Trifaró JM. Subcellular distribution of 65,000 calmodulin-binding protein (p65) and synaptophysin (p38) in adrenal medulla. J Neurochem. 1989;53:1043–1049. doi: 10.1111/j.1471-4159.1989.tb07393.x. [DOI] [PubMed] [Google Scholar]
- Fulop T, Doreian B, Smith C. Dynamin I plays dual roles in the activity-dependent shift in exocytic mode in mouse adrenal chromaffin cells. Arch Biochem Biophys. 2008;477:146–154. doi: 10.1016/j.abb.2008.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galas MC, Chasserot-Golaz S, Dirrig-Grosch S, Bader MF. Presence of dynamin-syntaxin complexes associated with secretory granules in adrenal chromaffin cells. J Neurochem. 2000;75:1511–1519. doi: 10.1046/j.1471-4159.2000.0751511.x. [DOI] [PubMed] [Google Scholar]
- Gincel D, Shoshan-Barmatz V. The synaptic vesicle protein synaptophysin: purification and characterization of its channel activity. Biophys J. 2002;83:3223–3229. doi: 10.1016/S0006-3495(02)75324-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong LW, Hafez I, Alvarez de Toledo G, Lindau M. Secretory vesicles membrane area is regulated in tandem with quantal size in chromaffin cells. J Neurosci. 2003;23:7917–7921. doi: 10.1523/JNEUROSCI.23-21-07917.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham ME, O'Callaghan DW, McMahon HT, Burgoyne RD. Dynamin-dependent and dynamin-independent processes contribute to the regulation of single vesicle release kinetics and quantal size. Proc Natl Acad Sci U S A. 2002;99:7124–7129. doi: 10.1073/pnas.102645099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R, Schiebler W, Ouimet C, Greengard P. A 38,000-dalton membrane protein (p38) present in synaptic vesicles. Proc Natl Acad Sci U S A. 1985;82:4137–4141. doi: 10.1073/pnas.82.12.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janz R, Südhof TC, Hammer RE, Unni V, Siegelbaum SA, Bolshakov VY. Essential roles in synaptic plasticity for synaptogyrin I and synaptophysin I. Neuron. 1999;24:687–700. doi: 10.1016/s0896-6273(00)81122-8. [DOI] [PubMed] [Google Scholar]
- Kawagoe KT, Zimmerman JB, Wightman RM. Principles of voltammetry and microelectrode surface states. J Neurosci Methods. 1993;48:225–240. doi: 10.1016/0165-0270(93)90094-8. [DOI] [PubMed] [Google Scholar]
- Knaus P, Betz H. Mapping of a dominant immunogenic region of synaptophysin, a major membrane protein of synaptic vesicles. FEBS Lett. 1990;261:358–360. doi: 10.1016/0014-5793(90)80591-6. [DOI] [PubMed] [Google Scholar]
- Leube RE, Kaiser P, Seiter A, Zimbelmann R, Franke WW, Rehm H, Knaus P, Prior P, Betz H, Reinke H, Beyreuther K, Wiedenmann B. Synaptophysin: molecular organization and mRNA expression as determined from cloned cDNA. EMBO J. 1987;6:3261–3268. doi: 10.1002/j.1460-2075.1987.tb02644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang YJ, Wu DF, Yang LQ, Höllt V, Koch T. Interaction of the mu-opioid receptor with synaptophysin influences receptor trafficking and signaling. Mol Pharmacol. 2007;71:123–131. doi: 10.1124/mol.106.026062. [DOI] [PubMed] [Google Scholar]
- Lindau M, Alvarez de Toledo G. The fusion pore. Biochim Biophys Acta. 2003;1641:167–173. doi: 10.1016/s0167-4889(03)00085-5. [DOI] [PubMed] [Google Scholar]
- Linstedt AD, Kelly RB. Synaptophysin is sorted from endocytotic markers in neuroendocrine PC12 cells but not transfected fibroblasts. Neuron. 1991;7:309–317. doi: 10.1016/0896-6273(91)90269-6. [DOI] [PubMed] [Google Scholar]
- McMahon HT, Bolshakov VY, Janz R, Hammer RE, Siegelbaum SA, Südhof TC. Synaptophysin, a major synaptic vesicle protein, is not essential for neurotransmitter release. Proc Natl Acad Sci U S A. 1996;93:4760–4764. doi: 10.1073/pnas.93.10.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNiven MA, Kim L, Krueger EW, Orth JD, Cao H, Wong TW. Regulated interactions between dynamin and the actin-binding protein cortactin modulate cell shape. J Cell Biol. 2000;151:187–198. doi: 10.1083/jcb.151.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitter D, Reisinger C, Hinz B, Hollmann S, Yelamanchili SV, Treiber-Held S, Ohm TG, Herrmann A, Ahnert-Hilger G. The synaptophysin/synaptobrevin interaction critically depends on the cholesterol content. J Neurochem. 2003;84:35–42. doi: 10.1046/j.1471-4159.2003.01258.x. [DOI] [PubMed] [Google Scholar]
- Montiel C, Mendoza I, García CJ, Awad Y, García-Olivares J, Solís-Garrido LM, Lara H, García AG, Cárdenas AM. Distinct protein kinases regulate SNAP-25 expression in chromaffin cells. J Neurosci Res. 2003;71:353–364. doi: 10.1002/jnr.10499. [DOI] [PubMed] [Google Scholar]
- Neco P, Fernández-Peruchena C, Navas S, Gutiérrez LM, de Toledo GA, Alés E. Myosin II contributes to fusion pore expansion during exocytosis. J Biol Chem. 2008;283:10949–10957. doi: 10.1074/jbc.M709058200. [DOI] [PubMed] [Google Scholar]
- Obendorf D, Schwarzenbrunner U, Fischer-Colbrie R, Laslop A, Winkler H. In adrenal medulla synaptophysin (protein p38) is present in chromaffin vesicles and in a special vesicle population. J Neurochem. 1988;51:1573–1580. doi: 10.1111/j.1471-4159.1988.tb01127.x. [DOI] [PubMed] [Google Scholar]
- Okamoto PM, Gamby C, Wells D, Fallon J, Vallee RB. Dynamin isoform-specific interaction with the shank/ProSAP scaffolding proteins of the postsynaptic density and actin cytoskeleton. J Biol Chem. 2001;276:48458–48465. doi: 10.1074/jbc.M104927200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Régnier-Vigouroux A, Tooze SA, Huttner WB. Newly synthesized synaptophysin is transported to synaptic-like microvesicles via constitutive secretory vesicles and the plasma membrane. EMBO J. 1991;10:3589–35601. doi: 10.1002/j.1460-2075.1991.tb04925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling K, Gratzl M. Quantification of p38/synaptophysin in highly purified adrenal medullary chromaffin vesicles. FEBS Lett. 1988;233:22–24. doi: 10.1016/0014-5793(88)81348-6. [DOI] [PubMed] [Google Scholar]
- Segura F, Brioso MA, Gómez JF, Machado JD, Borges R. Automatic analysis for amperometrical recordings of exocytosis. J Neurosci Methods. 2000;103:151–156. doi: 10.1016/s0165-0270(00)00309-5. [DOI] [PubMed] [Google Scholar]
- Smith AD, Winkler H. A simple method for the isolation of adrenal chromaffin granules on a large scale. Biochem J. 1967;103:480–482. doi: 10.1042/bj1030480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiwoks-Becker I, Vollrath L, Seeliger MW, Jaissle G, Eshkind LG, Leube RE. Synaptic vesicle alterations in rod photoreceptors of synaptophysin-deficient mice. Neuroscience. 2001;107:127–142. doi: 10.1016/s0306-4522(01)00345-1. [DOI] [PubMed] [Google Scholar]
- Südhof TC, Lottspeich F, Greengard P, Mehl E, Jahn R. A synaptic vesicle protein with a novel cytoplasmic domain and four transmembrane regions. Science. 1987;238:1142–1144. doi: 10.1126/science.3120313. [DOI] [PubMed] [Google Scholar]
- Thomas L, Hartung K, Langosch D, Rehm H, Bamberg E, Franke WW, Betz H. Identification of synaptophysin as a hexameric channel protein of the synaptic vesicle membrane. Science. 1988;242:1050–1053. doi: 10.1126/science.2461586. [DOI] [PubMed] [Google Scholar]
- Tixier-Vidal A, Faivre-Bauman A, Picart R, Wiedenmann B. Immunoelectron microscopic localization of synaptophysin in a Golgi subcompartment of developing hypothalamic neurons. Neuroscience. 1988;26:847–861. doi: 10.1016/0306-4522(88)90104-2. [DOI] [PubMed] [Google Scholar]
- Valtorta F, Pennuto M, Bonanomi D, Benfenati F. Synaptophysin: leading actor or walk-on role in synaptic vesicle exocytosis? Bioessays. 2004;26:445–453. doi: 10.1002/bies.20012. [DOI] [PubMed] [Google Scholar]
- Wang R, Hosaka M, Han L, Yokota-Hashimoto H, Suda M, Mitsushima D, Torii S, Takeuchi T. Molecular probes for sensing the cholesterol composition of subcellular organelle membranes. Biochim Biophys Acta. 2006;1761:1169–1181. doi: 10.1016/j.bbalip.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Yin Y, Dayanithi G, Lemos JR. Ca(2+)-regulated, neurosecretory granule channel involved in release from neurohypophysial terminals. J Physiol. 2002;539:409–418. doi: 10.1113/jphysiol.2001.012943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan RZ, Fujiwara N, Tanaka E, Shimoji K. Intracellular acidification induced by membrane depolarization in rat hippocampal slices: roles of intracellular Ca2+ and glycolysis. Brain Res. 1998;780:86–94. doi: 10.1016/s0006-8993(97)01149-9. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Misler S, Chow RH. Rapid fluctuations in transmitter release from single vesicles in bovine adrenal chromaffin cells. Biophys J. 1996;70:1543–1552. doi: 10.1016/S0006-3495(96)79718-7. [DOI] [PMC free article] [PubMed] [Google Scholar]