Abstract
Growth cones regulate the speed and direction of neuronal outgrowth during development and regeneration. How the growth cone spatially and temporally regulates signals from guidance cues is poorly understood. Through a proteomic analysis of purified growth cones we identified isoforms of the 14-3-3 family of adaptor proteins as major constituents of the growth cone. Disruption of 14-3-3 via the R18 antagonist or knockdown of individual 14-3-3 isoforms switches nerve growth factor- and myelin-associated glycoprotein-dependent repulsion to attraction in embryonic day 13 chick and postnatal day 5 rat DRG neurons. These effects are reminiscent of switching responses observed in response to elevated cAMP. Intriguingly, R18-dependent switching is blocked by inhibitors of protein kinase A (PKA), suggesting that 14-3-3 proteins regulate PKA. Consistently, 14-3-3 proteins interact with PKA and R18 activates PKA by dissociating its regulatory and catalytic subunits. Thus, 14-3-3 heterodimers regulate the PKA holoenzyme and this activity plays a critical role in modulating neuronal responses to repellent cues.
Introduction
Axons accurately project to specific targets during development by interpreting a complex environment containing attractive and repulsive cues (Huber et al., 2003). Binding of guidance cues to receptors on the surface of the growth cone stimulates intracellular signaling cascades that converge on cytoskeletal elements to control growth cone morphology (Huber et al., 2003). How the activation state of cytoskeletal regulatory proteins is spatially and temporally regulated is an important question which has only been partially elucidated. Local translation, insertion and removal of receptors at the plasma membrane as well as receptor silencing all affect growth cone responses (Stein and Tessier-Lavigne, 2001; Bouchard et al., 2004; Kim et al., 2005; Lin and Holt, 2007). Further, regulating intracellular concentrations of cyclic nucleotides affects whether neuronal processes are attracted or repelled by guidance cues in vitro and in vivo (Ming et al., 1997; Song et al., 1998; Song and Poo, 1999; Polleux et al., 2000; Shewan et al., 2002; Nishiyama et al., 2003). Antagonizing cAMP in embryonic neurons can convert growth cone attraction to repulsion while elevating cAMP in postnatal neurons can convert repulsion to attraction and can promote CNS regeneration following injury (Neumann et al., 2002; Qiu et al., 2002). Both type II cAMP-dependent protein kinase A (PKA) and the exchange protein activated by cAMP (Epac) have been implicated as cAMP effectors affecting neurite outgrowth and growth cone turning (Cai et al., 2001; Han et al., 2007; Murray and Shewan, 2008) but the mechanisms regulating these effectors are unclear.
In a proteomic analysis of a retinal ganglion cell (RGC) growth cone preparation we identified several members of the 14-3-3 protein family (Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation proteins); excellent candidates to regulate the spatial and temporal activity of cAMP and its effectors. The 14-3-3 proteins are adapter proteins that interact with specific phospho-serine and phospho-threonine motifs within a number of binding proteins and consequently regulate diverse cellular processes including cell survival, metabolism, proliferation and protein trafficking (van Heusden, 2005; Garbe and Bashaw, 2004; Clandinin and Zipursky, 2002; Jin et al., 2004). The 14-3-3 proteins function as homodimers and heterodimers to control the spatial and temporal activity of substrate proteins by regulating subcellular localization (Muslin and Xing, 2000; Nagata-Ohashi et al., 2004), binding partner proximity (Jones et al., 1995; Berruti, 2000; Van Der Hoeven et al., 2000) and by inducing conformational changes that can affect interactions between binding proteins and their substrates (Roy et al., 1998). In mammals, there are seven 14-3-3 isoforms, designated β or α (β,α), ε, η, γ, τ or θ (τ,θ), ζ or δ (ζ,δ), and σ/stratifin (σ) (Bridges and Moorhead, 2005). Multiple binding partners have been described for each 14-3-3 isoform; however, the functional relevance of these interactions has only been elucidated for a small subset of proteins.
The ability of 14-3-3 proteins to regulate the activity and localization of Ser/Thr phosphorylated substrates and our identification of 14-3-3 proteins as major constituents of the growth cone led us to investigate the role of 14-3-3 proteins in regulating neuronal growth cone responses to extracellular cues. We find that 14-3-3 proteins regulate PKA and loss of 14-3-3 function converts nerve growth factor (NGF)- and myelin-associated glycoprotein (MAG)-dependent repulsion to attraction in embryonic day 13 (E13) chick and postnatal day 5 (P5) rat DRG neurons. Thus, we have identified a novel molecular switch that may be harnessed to attenuate the inhibitory CNS environment following injury.
Materials and Methods
Reagents.
To construct expression vectors for 14-3-3 isoforms, cDNA was amplified by PCR from vectors provided by Dr. Tony Pawson (University of Toronto, Toronto, ON, Canada) (pcDNA3.1FLAG 14-3-3γ, pSport6 14-3-3ε, pOTB7 14-3-3ζ, pDNR-Lib 14-3-3β) or Open Biosource Systems (pOTB7 14-3-3τ/θ) and ligated into pcDNA3.1A 2xmyc. R18 and WLRL expression vectors were constructed by synthesizing oligos encoding the peptide sequence (R18: ccccactgtgtcccccgagatctttcgtggttagatttagaagcaaatatgtgtttaccc WLRL: ccccactgtgtcccccgagatctttcgtggttaaggttagaagcaaatatgtgtttaccc), ligating into pCS2+ EGFP and then subcloning R18 EGFP and WLRL EGFP into pHSVPrPUC. To construct expression vectors for PKA RIIα and PKA RIIβ, cDNA was amplified by PCR from pET-RIIα (mouse) and pET-RIIβ (rat) provided by Dr. Susan Taylor (University of California, San Diego, CA) and PCR products were ligated into pcDNA 3.1 V5 His.
HDAC2 antibody was purchased from Zymed Laboratories Inc. and used at a 1:2500 dilution for Western blotting. Neurofilament-200 antibody was purchased from Sigma-Aldrich and used at a dilution of 1:2000 for Western blotting. Anti-pan-β-, ζ-, ε-, γ-, τ- and η-14-3-3 primary antibodies were purchased from Santa Cruz Biotechnology and used at a 1:1000 (ζ, ε, γ, τ) or 1:3000 (β) dilution for Western blotting and 1:50 for immunofluorescence. Anti-PKA catalytic subunit and regulatory subunit II Beta primary antibodies were purchased from BD Biosciences and used at 1:1000 for Western blotting. Anti-phospho-PKA catalytic (Thr197) antibody was purchased from Cell Signaling Technology and used at 1:1000 for Western blotting. Anti-phospho I-1/DARPP-32 (Thr34) antibody was purchased from Novus Biologicals and used at 1:100 for immunofluorescence. Sp-cAMPS, Rp-cAMPS and KT-5720 were purchased from Sigma-Aldrich. Myristoylated PKI was purchased from Invitrogen.
Dorsal root ganglion cultures.
DRGs were dissected from E8 chicks, E13 chicks, or P5 rats in L-15 medium and plated on poly-l-lysine- and laminin-coated substrates as explants. For dissociated cultures, explants were treated with 0.25% trypsin-EDTA and triturated in medium to dissociate cells. DRGs were grown in F-12 medium or Neurobasal medium with B-27 (Invitrogen) for chick and rat DRGs, respectively. Medium was supplemented with 1% penicillin/streptomycin, and 10 ng/ml NGF (Calbiochem).
Proteomics.
E6 retinas were dissected from E6 chicks, cut into strips, placed on nitrocellulose membranes and grown on poly-l-lysine and laminin in a 1:1 DMEM/F-12 (Invitrogen) mixture with 10% FBS, 1% penicillin/streptomycin, 30 ng/ml BDNF and 1% N-2 nutritional supplement (Invitrogen). Axons with growth cones were separated from cell bodies using a sharp borosilicate glass capillary. Cell bodies were removed and remaining neurites and growth cones were incubated at 37°C for 30 min for recovery. Growth cones were collected in radioimmunoprecipitation assay buffer (20 mm HEPES, pH 7.5, 150 mm NaCl, 0.5% sodium deoxycholate, 0.1% SDS, 1% Triton X-100) and lysates separated by SDS-PAGE. Bands were processed by trypsin digestion and the resulting peptide mixtures were analyzed by nanoscale liquid chromatography quadrupole time-of-flight tandem mass spectrometry. Mass spectrometry profiles were analyzed using MASCOT software.
Immunofluorescence.
For analysis of 14-3-3 isoform expression, primary neuronal cultures were fixed with modified Davidson's Fix (30% ethanol, 10% acetic acid, 20% formaldehyde, 20% sucrose). Antigen blockade was performed with blocking peptide (Santa Cruz Biotechnology), by incubating the antibody with a 5:1 molar excess of the blocking peptide for 30 min at 37°C immediately before staining. To validate developmental differences in expression, E6 retinal explants and E8 DRG explants were cocultured in the same well of a 4-well chamber slide, fixed as described above and immunostained. Staining intensities were directly compared during image acquisition. For phosphorylated I-1 levels, P5 rat DRG explants were fixed with 4% PFA with 20% sucrose.
14-3-3 knockdown and virus preparation.
shRNA with a microRNA stem (shRNAmir) was designed against target sequences (see below) for each 14-3-3 isoform using Invitrogen's RNAi Designer. Oligonucleotides encoding the sequences were ligated into a pcDNA6.2-GW/EmGFP-miR vector and lentivirus was produced as described previously (Thomas et al., 2009). Sequences of oligos used for knockdown of each 14-3-3 isoform are as follows: 14-3-3β, TGCTGATAACGCTCAGCTTGCTCAGCGTTTTGGCCACTGACTGACGCTGAGCACTGAGCGTTAT; 14-3-3ζ, TGCTGATAAGCAACAGAGAGAAGGTTGTTTTGGCCACTGACTGACAACCTTCTCTGTTGCTTAT; 14-3-3γ, TGCTGATCAGAGTGGAGTCCTTGTAGGTTTTGGCCACTGACTGACCTACAAGGTCCACTCTGAT; 14-3-3τ, TGCTGTGAGGGTGCTGTCTTTGTAGGGTTTTGGCCACTGACTGACCCTACAAACAGCACCCTCA; 14-3-3ε, TGCTGAAACCTTGGACTCGCCAGTGTGTTTTGGCCACTGACTGACACACTGGCGTCCAAGGTTT; Control, TGCTGTTTGCAAAGAGCACTTTCTTTTCCACTACTGACGAAAGCCTTCTCTTGCAAA.
Dissociated P5 rat DRGs were transduced with recombinant lentivirus particles at the time of plating for 12 h and then cultured for 5 d in vitro. Cells were detached with 5 mm EDTA and replated for use in Dunn chamber turning assays or lysed for Western blotting. Knockdown of targeted isoforms and specificity was examined by Western blot. Densitometry was performed on three independent experiments. Band density of each isoform was normalized to GAPDH levels on the same blot and expressed as a percentage of expression in control lentivirus-transduced cells, or the mean expression of the isoform in lysates transduced for knockdown of the off-target isoforms.
Recombinant herpes simplex virus was produced by transfecting pHSVPrPUC plasmids into 2-2 Vero cells that were superinfected with 5dl 1.2 herpes simplex virus (HSV) helper virus 1 d later. Recombinant virus was amplified through three passages and stored at −80°C as described previously (Neve et al., 1997).
Dunn chamber turning assay.
Dissociated E8/E13 chick or P5 rat DRG neurons were cultured with 1 ng/ml (chick) or 5 ng/ml (rat) NGF overnight on #3D glass coverslips coated with poly-l-lysine and laminin (10 μg/ml). Coverslips were used for Dunn chamber assembly as described previously (Yam et al., 2009) to establish an NGF or MAG gradient (Outer well concentrations of 200 ng/ml MAG-Fc or 50 ng/ml NGF for chick DRGs and 100 ng/ml NGF for rat DRGs). Cell images were acquired every 3–4 min for 100–120 min on a temperature controlled stage. Neurites of at least 15 μm in length were tracked and the final position of the growth cone was used to determine the trajectory relative to the initial 15 μm. The angle turned over 2 h, relative to the gradient position, was recorded. Measurements are presented in rose histograms in bins of 10° with the length of each segment representing the frequency of measurements in percent. Only transduced neurons were quantified as assessed by GFP staining. Where indicated, DRGs were treated with Sp-cAMPS, Rp-cAMPS, KT-5720 or PKI for 1 h before imaging in the Dunn chamber.
Immunoprecipitation.
HEK293T cells were transfected overnight with Lipofectamine 2000 and harvested in NP-40 buffer (150 mm NaCl, 1% Nonidet P-40, 10 mm Tris-Cl, pH 8.0, 10% w/v glycerol) with Complete EDTA Free Protease Inhibitor Cocktail (Roche) and phosphatase inhibitors (5 mm NaF and 1 mm Na3VO4). Following sonication, lysates were precleared with protein A/G agarose beads (Santa Cruz Biotechnology) and then incubated with anti-V5- or anti-myc-conjugated agarose (Sigma). Precipitates were washed with NP-40 buffer and eluted in 2× SDS sample buffer.
For immunoprecipitations from DRGs, P5 rat DRG explants were collected and harvested in NP-40 buffer. Cleared lysates were incubated with anti-regulatory subunit β2 antibody (BD Bioscience) and protein A/G agarose beads. Precipitates were washed with NP-40 buffer and eluted in 2× SDS sample buffer. PC12 cells were transduced with recombinant HSV-WLRL-GFP or HSV-R18-GFP and plated overnight in RPMI medium (Invitrogen) with 10% horse serum, 5% fetal bovine serum, and 1% penicillin/streptomycin. Lysates were collected as above and incubated with anti-regulatory subunit β2 antibody (BD Bioscience) and protein A/G agarose beads. Precipitates were washed with NP-40 buffer and eluted in 2× SDS sample buffer.
Results
14-3-3 proteins are expressed in neuronal growth cones
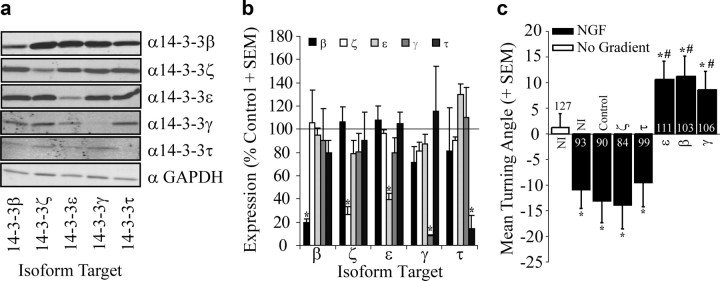
To identify constituents of neuronal growth cones, proteins from mechanically purified growth cone preparations from E6 chick RGCs were analyzed by tandem mass spectrometry (supplemental Fig. S1, available at www.jneurosci.org as supplemental material). Of the total number of tandem mass spectra assigned to peptides, 4.1% are assigned to members of the 14-3-3 family and 14-3-3 proteins represent 6.5% of total proteins suggesting that they are abundant components of growth cones (supplemental Fig. S2, available at www.jneurosci.org as supplemental material). To verify these results we identified antibodies specific for the β, ζ, ε, γ, and τ isoforms that recognize a protein of the correct molecular weight in brain lysates (supplemental Fig. S3, available at www.jneurosci.org as supplemental material). By immunofluorescence we find that 14-3-3 γ, ε, ζ and τ are expressed in E6 chick RGC growth cones confirming the results of our proteomic analysis, and we identified 14-3-3β as an additional isoform present in these growth cones (Fig. 1). To test whether 14-3-3 expression extends to other types of growth cones we analyzed 14-3-3 expression in chick DRG growth cones. Isoforms β, γ, ε and τ but not ζ are present in E8 chick DRG growth cones (Fig. 1). To determine whether 14-3-3 expression in growth cones is developmentally regulated we compared 14-3-3 expression in E8 and E13 chick DRGs. E12 represents a critical transitional time point in growth cone development, cAMP levels in growth cones are high during early development and these drop sharply at E12 in chick (equivalent to postnatal day 4 in rat). The change in cyclic nucleotide concentration mediates differential phenotypic responses to guidance cues such as netrins, neurotrophins and myelin-associated inhibitors (Song et al., 1997, 1998; Song and Poo, 1999; Shewan et al., 2002; Nishiyama et al., 2003). We find that expression of 14-3-3 β, γ, and τ are upregulated in E13 chick (Fig. 1). The altered expression of individual 14-3-3 isoforms suggests that they may developmentally regulate responses to individual guidance cues. By Western blotting we find that all 14-3-3 isoforms are upregulated in E13 chick DRG lysates (supplemental Fig. S4, available at www.jneurosci.org as supplemental material) suggesting that 14-3-3 expression can be locally regulated within the growth cone independent of the cell body.
Figure 1.
14-3-3 proteins are present in growth cones. Immunofluorescence staining of growth cones with isoform-specific 14-3-3 antibodies. For each antibody the immunofluorescence signal is lost by preadsorbing the antibody with isoform-specific blocking peptide (BP). RGC, retinal ganglion cell. Scale bar, 5 μm.
A 14-3-3 antagonist converts NGF-dependent repulsion to attraction
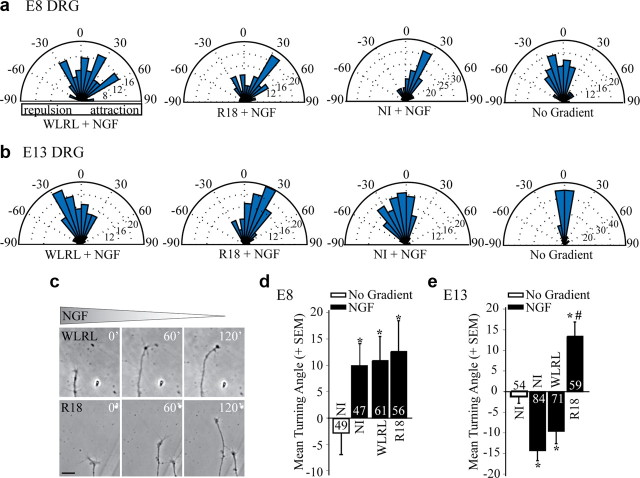
To determine whether 14-3-3 proteins regulate ligand-dependent growth cone responses we investigated the impact of a 14-3-3 antagonist on DRG growth cone turning in response to NGF using a Dunn chamber turning assay (Yam et al., 2009). R18 (PHCVPRDLSWLDLEANMCLP) is a potent 14-3-3 peptide antagonist, which disrupts binding of Ser/Thr phosphorylated proteins to 14-3-3 (Wang et al., 1999). We generated a recombinant R18-green fluorescent protein expressing herpes simplex virus (HSV-R18-GFP) and a control HSV-WLRL-GFP virus that does not bind to 14-3-3 because of a substitution in the core-binding motif (WLDL to WLRL) (Wang et al., 1999). R18-GFP binds to 14-3-3 and antagonizes a previously characterized 14-3-3ζ-Slingshot1 interaction (supplemental Fig. S5, available at www.jneurosci.org as supplemental material) (Nagata-Ohashi et al., 2004). We confirmed that application of an NGF gradient results in an attractive mean turning angle when applied to E8 chick DRG growth cones (Gundersen and Barrett, 1980; Gehler et al., 2004) and we find that this response is unaffected by HSV-R18-GFP (12.6 ± 5.8°; Fig. 2a,d). However, E13 chick DRG neurons are repelled by NGF (−14.3 ± 2.5°) and intriguingly this response is converted to an attractive response in HSV-R18-GFP but not HSV-WLRL-GFP-transduced growth cones (R18, 13.4 ± 3.4°; WLRL, −9.6 ± 3.1°; Fig. 2b,c,e) demonstrating that 14-3-3 proteins are important for conferring repulsive responses to NGF in postnatal neurons.
Figure 2.
The 14-3-3 antagonist R18 converts NGF-dependent repulsion to attraction in E13 chick DRG neurons. a, b, Rose histograms illustrating turning responses of E8 (a) or E13 (b) chick DRG neurons in response to an NGF gradient in a Dunn chamber turning assay (50 ng/ml NGF in outer chamber). Responses of individual neurons are clustered in 10° bins and the percentage of total neurons per bin is represented by the radius of each segment. Nontransduced neurons (NI) or neurons transduced with HSV-WLRL-GFP or HSV-R18-GFP for 12–16 h before the turning assay were analyzed. c, Phase images of representative E13 chick DRG growth cones transduced with WLRL or R18 and exposed to an NGF gradient in the Dunn chamber turning assay. Scale bar, 20 μm. d, e, Mean turning angles of E8 (d) or E13 (e) chick DRG neurons in the absence of a cue (No Gradient) or in response to 50 ng/ml NGF. Numbers within the bars indicate the number of growth cones measured over at least three independent experiments. Statistics were performed by one-way ANOVA with Tukey's post hoc test. *p < 0.05 compared with no gradient control; #p < 0.05 compared with the NGF-dependent repellent response in WLRL-transduced neurons.
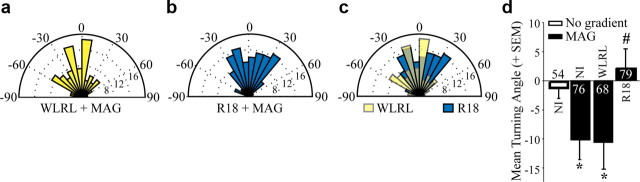
A 14-3-3 antagonist blocks MAG-dependent repulsion
To test whether 14-3-3 proteins regulate responses to other inhibitory cues we assessed the impact of HSV-R18-GFP on repulsion in response to the myelin-associated inhibitor MAG. Noninfected or control (HSV-WLRL-GFP)-transduced E13 chick DRGs are repelled by MAG (−11.3 ± 4.2° and −12.1 ± 4.5°, respectively; Fig. 3a,d). HSV-R18-GFP-transduced neurons are not repelled by MAG (4.0 ± 2.9°) but are not significantly attracted to MAG suggesting that R18 attenuates rather than switches the growth cone response to MAG (Fig. 3b,d). However, we noted that in the control WLRL-transduced DRG neurons a notable number of DRG neurons (43%) are not repelled by MAG (Fig. 3a) raising the possibility that the E13 chick DRG population may be heterogeneous in its responsiveness to MAG. When overlaying the turning data from WLRL- and R18-transduced neurons, a proportion of DRG neurons do become attracted to MAG when transduced with R18 (Fig. 3c). When neurons are grouped by turning angle into positive or negative bins (greater than or less than 0.5 SD of no gradient controls, respectively), 56.9% of WLRL-transduced neurons show negative turning, compared with 39% of R18-transduced neurons. Positive turning angles are observed for 49% of R18-transduced neurons, compared with 26.4% of WLRL neurons. We conclude that 14-3-3 proteins are important for conferring repulsive responses to both NGF and MAG in E13 chick DRG neurons and that loss of 14-3-3 can convert a repellent response to an attractive response.
Figure 3.
The 14-3-3 antagonist R18 blocks MAG-dependent repulsion of E13 chick DRG neurons. a, b, Rose histograms illustrate turning responses of E13 chick DRG neurons in response to a MAG-Fc gradient (200 ng/ml in outer chamber) in a Dunn chamber turning assay. Neurons were transduced with HSV-WLRL (a) or HSV-R18 (b) for 12–16 h before the turning assay. c, An overlay of the rose histograms presented in a and b illustrating a large population of R18-positive neurons with an attractive turning response to a MAG gradient. d, Mean turning angles of noninfected, WLRL- or R18-transduced E13 chick DRG neurons in the absence of a gradient or in response to MAG. Numbers within the bars indicate the number of growth cones measured over at least three independent experiments. Statistics were performed by one-way ANOVA with Tukey's post hoc test. *p < 0.05 compared with no gradient control; #p < 0.05 compared with the MAG-dependent repellent response in WLRL-transduced neurons.
Knockdown of specific 14-3-3 proteins converts NGF-dependent repulsion to attraction
R18 is a powerful reagent that simultaneously inhibits binding of all 14-3-3 isoforms to Ser/Thr phosphorylated targets allowing one to assess the global role of 14-3-3 proteins in a given phenotype. To verify that 14-3-3 proteins play a role in growth cone turning, we transduced neurons with lentiviruses expressing shRNA target sequences with a microRNA stem (shRNAmir) for knockdown of individual 14-3-3 isoforms. These experiments were performed in P4–P7 rat DRGs, a stage equivalent to ∼E13 in chick when cAMP levels have been developmentally downregulated. P5 rat DRG growth cones express all 14-3-3 isoforms examined (β, ζ, ε, γ, and τ; Fig. 1). Similar to E13 chick, the repellant response to NGF in P5 rat DRG neurons is switched to an attractive response with transduction of HSV-R18-GFP, but not HSV-WLRL-GFP (R18, 9.45 ± 4.75°; WLRL, −15.86 ± 5.05°). Transduction of P4 rat DRG neurons with lentivirus for 14-3-3 isoform-specific knockdown effectively and specifically reduces expression levels of the targeted isoform by 60–90% (Fig. 4a,b). In the Dunn chamber turning assay we find that knockdown of 14-3-3ε, β or γ but not ζ or τ converts NGF-dependent repulsion to attraction (Fig. 4c; ε, 10.6 ± 3.5°; β, 11.2 ± 3.9°; γ, 9.9 ± 3.3°; ζ, −13.9 ± 4.7°; τ. −9.5 ± 4.8°). These data provide an independent confirmation that 14-3-3 proteins are important for NGF-dependent repulsion and demonstrates that 14-3-3ε, β and γ mediate this response.
Figure 4.
Loss of 14-3-3ε, β or γ converts NGF-dependent repulsion to attraction in P5 rat DRG neurons. a, Lysates from P5 rat DRG neurons transduced with lentiviruses for knockdown of individual 14-3-3 isoforms and analyzed by Western blot with anti-GAPDH or anti-14-3-3 isoform-specific antibodies. The GAPDH blot shown is from the 14-3-3β gel and is representative of the equal loading achieved in these lysates. b, Expression of individual isoforms quantified by densitometry from Western blots of P5 rat DRG lysates transduced with lentiviruses for knockdown of individual isoforms. Densities were normalized to GAPDH levels for each blot. The mean expression of each isoform is expressed as a percentage of control from 3 independent experiments (+SEM). *p < 0.05 compared with expression in control miRNA-transduced neurons. c, Mean turning angles of P5 rat DRG neurons in the absence of a cue (No Gradient) or in response to 100 ng/ml NGF. Neurons were nontransduced (NI) or were transduced with lentiviruses for expression of a nontargeting control sequence or sequences for knockdown of individual 14-3-3 isoforms. Numbers within the bars indicate the number of growth cones measured over at least three independent experiments. Statistics were performed by one-way ANOVA with Tukey's post hoc test. *p < 0.05 compared with no gradient control; #p < 0.05 compared with the NGF-dependent repellent response in control shRNAmir-transduced neurons.
14-3-3 proteins switch NGF-dependent growth cone turning responses through PKA
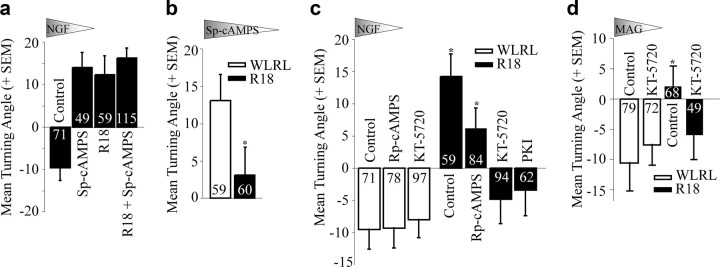
The switch in growth cone turning response is reminiscent of the switching responses observed in response to regulating levels of cyclic nucleotides (Song et al., 1997, 1998). Neurotrophin-dependent attraction can be converted to repulsion in the presence of the cAMP antagonist Rp-cAMPS and MAG-dependent repulsion can be converted to attraction with the cAMP agonist Sp-cAMPS (Song et al., 1997, 1998). We find that Sp-cAMPS converts NGF-dependent repulsion to attraction in E13 chick DRG neurons (12.5 ± 4.4°) similar to the effect of R18 (Fig. 5). Combinatorial treatment with Sp-cAMPS and R18 does not have an additive effect (16.5 ± 2.2°) suggesting that cAMP and 14-3-3 proteins function in a convergent pathway (Fig. 5a). Further, attractive turning in response to an Sp-cAMPS gradient (13.1 ± 3.5°) is blocked by the introduction of R18 (3.1 ± 3.7°) suggesting that 14-3-3 proteins function downstream of cAMP to affect growth cone turning (Fig. 5b). To further investigate the relationship between 14-3-3 proteins and cAMP-dependent signaling we asked whether R18-dependent switching in an NGF gradient would be affected by applying the cAMP antagonist Rp-cAMPs or the protein kinase A antagonists KT-5720 or PKI (Kase et al., 1987; Dalton and Dewey, 2006). Both the small molecule inhibitor KT-5720 and the myristoylated peptide PKI act by targeting the catalytic subunit of the PKA holoenzyme I and together these inhibitors selectively address the function of PKA (Murray, 2008). As expected, inhibition of cAMP or PKA did not impact NGF-dependent repulsion in WLRL-transduced E13 chick DRGs; a stage with low levels of cAMP (Fig. 5c,d; RpCAMPS, −9.3 ± 3.1°; KT-5720, −8.0 ± 2.9°). Intriguingly, we find that while Rp-cAMPS has no significant effect on NGF-dependent attraction of R18-transduced growth cones (6.2 ± 3.1°), R18-transduced neurons are no longer attracted to NGF when treated with KT-5720 or PKI (KT-5720, −4.8 ± 3.8°; PKI, −3.4 ± 4.0°; Fig. 5c). Similarly, repellant responses to MAG are restored in R18-transduced DRGs treated with KT-5720 (−6.0 ± 4.1; Fig. 5d). Together these findings suggest that antagonizing 14-3-3 proteins leads to activation of PKA consequently converting NGF- and MAG-dependent repulsion to attraction.
Figure 5.
14-3-3 proteins regulate growth cone turning responses through PKA. a–d, Mean turning angles of E13 chick DRG neurons in response to gradients of NGF (50 ng/ml), Sp-cAMPS (20 μm), or MAG-Fc (200 ng/ml). Neurons were transduced with HSV-WLRL-GFP or HSV-R18-GFP. Where indicated, neurons were treated with a bath application of 20 μm Sp-cAMPS, 20 μm Rp-cAMPS, 200 nm KT-5720, or 20 μm myristoylated PKI for 60 min before the turning analysis. Numbers within the bars indicate the number of growth cones measured over at least three independent experiments. Statistics for a, c, and d were performed by two-way ANOVA with Bonferroni post hoc tests. Statistics for b and PKI treatment were performed with unpaired Student's t test. *p < 0.05 compared with the WLRL-transduced control for each condition.
14-3-3 proteins bind and regulate PKA
The PKA holoenzyme is as a tetramer, with two catalytic subunits bound by two regulatory subunits that prevent the catalytic subunits from becoming active. cAMP binding to the regulatory subunits causes the release of the catalytic subunits and subsequent activation of PKA. We asked whether 14-3-3 isoforms could interact with PKA regulatory subunits α or β (RII α and RII β) that are known to be present in growth cones (Han et al., 2007). First, we performed coimmunoprecipitation experiments in HEK 293T cotransfected with myc-tagged 14-3-3 isoforms and V5-tagged RIIα or RIIβ. Immunoprecipitation of RIIα or RIIβ robustly coimmunoprecipitated 14-3-3γ and the strength of the coimmunoprecipitation was diminished by introducing R18 but not WLRL (Fig. 6a). RIIα weakly coimmunoprecipitated 14-3-3ε and both regulatory subunits failed to coimmunoprecipitate 14-3-3β, τ, or ζ. PKA binding to 14-3-3-γ and ε isoforms complements our turning data as knockdown of these isoforms resulted in switching from NGF-dependent repulsion to attraction. Immunoprecipitation of 14-3-3γ also coimmunoprecipitated RIIα and RIIβ (Fig. 6b). In this direction, the strength of the coimmunoprecipitation is weaker indicating that only a small percentage of 14-3-3 may interact with PKA. We also detect an interaction between endogenous 14-3-3γ and RIIβ in P5 rat DRG lysates (Fig. 6c) supporting the physiological relevance of the interaction.
Figure 6.
14-3-3 proteins bind PKA. a, b, 293T cells were cotransfected with V5-tagged RIIα or RIIβ and myc-tagged 14-3-3 constructs and GFP-WLRL or GFP-R18 and then subjected to immunoprecipitation with anti-V5 (a) or anti-myc (b) antibody. Cell lysates and immunoprecipitates were separated by SDS-PAGE and analyzed by Western blotting with anti-V5 and anti-myc antibodies. c, Cell lysates from P5 rat DRG neurons were subjected to immunoprecipitation with control IgG or anti-RIIβ antibody and cell lysates and immunoprecipitates were analyzed by Western blot with anti-14-3-3γ and anti-RIIβ antibodies.
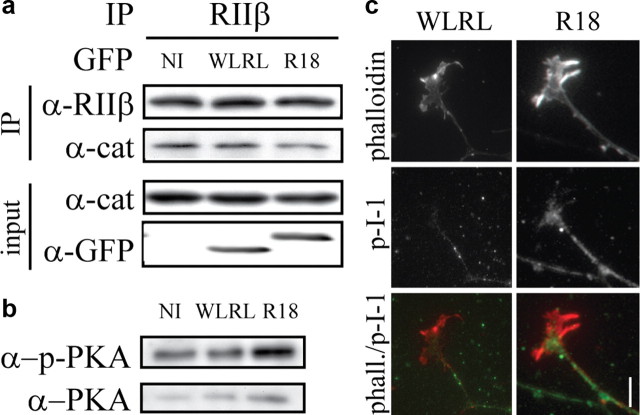
To test whether 14-3-3 binding could regulate PKA, we tested the impact of R18 on the interaction between the regulatory and catalytic subunits of PKA. PC12 cells were transduced with HSV-R18-EGFP or HSV-WLRL-EGFP. Endogenous PKA regulatory subunit (RIIβ) was immunoprecipitated from cell lysates and the amount of coimmunoprecipitating catalytic subunit (PKAcat) was assessed by Western blot. Introduction of R18 resulted in a 33% decrease in the amount of PKAcat that coimmunoprecipitated with RIIβ when compared with WLRL control lysates (Fig. 7a; p < 0.05 by paired Student's t test) indicating that 14-3-3 proteins stabilize binding between the regulatory and catalytic subunits of PKA. To determine whether the dissociation between regulatory and catalytic subunits correlated with an increase in PKA activity we monitored active PKA using an antibody that recognizes an activated form of the catalytic subunit of PKA (Parra and Zou, 2010). Lysates from DRGs expressing R18 showed a 37% increase in the levels of phospho-PKA compared with those expressing WLRL (Fig. 7b; p < 0.05 by paired Student's t test, n = 3). We further examined HSV-R18-EGFP- or HSV-WLRL-EGFP-transduced growth cones for indications of PKA activity. PKA regulates growth cone turning in part through targeting the PKA substrate PP-1 inhibitory protein I-1(Han et al., 2007). We find that an antibody that recognizes the phosphorylated form of I-1 robustly stains a subset of neurites in P5 rat DRG explants. We observe that phospho-I-1 staining extends beyond the neurite into the central domain of the growth cone in R18-transduced explants while the staining is largely restricted to the neurite in WLRL-transduced explants (Fig. 7c). The growth cone to neurite ratio of phosphorylated I-1 shows a 1.73-fold increase in R18-transduced explants compared with the WLRL control (p < 0.05 by Student's t test, n = 29 and 31 for WLRL- and R18-transduced growth cones, respectively) demonstrating that 14-3-3s regulate PKA activity in neuronal growth cones.
Figure 7.
14-3-3 proteins regulate the stability and activity of PKA holoenzyme. a, PC12 cells were transduced with HSV-WLRL or HSV-R18. Following transduction, cell lysates were subjected to immunoprecipitation with anti-RIIβ antibody. Cell lysates and immunoprecipitates were analyzed by Western blot with anti-RIIβ, anti-PKA catalytic subunit (cat) and anti-GFP antibodies. b, P5 rat DRGs were transduced with HSV-WLRL or HSV-R18. Cell lysates were analyzed by Western blot for levels of phosphorylated PKA catalytic subunit and total levels of PKA catalytic subunit. c, Immunofluorescence of P5 rat DRG growth cones transduced with HSV-WLRL or HSV-R18 and stained with an anti-phospho-I-1 antibody and rhodamine-phalloidin. Scale bar, 10 μm.
Discussion
Intracellular signaling within the growth cone is critical for interpreting extracellular cues and for regulating how growth cones navigate during development and following injury. In this study we have demonstrated that 14-3-3 proteins are major constituent proteins of growth cones. Our findings suggest that 14-3-3 proteins stabilize the PKA holoenzyme. Antagonizing 14-3-3 proteins activates PKA and converts NGF- and MAG-dependent repulsion to attraction through the regulation of PKA. These findings provide novel insights into the intracellular mechanisms that control axon repulsion and suggest a novel molecular mechanism to convert repellent responses to growth promoting responses, a process which could positively impact neuronal repair following CNS injury.
14-3-3 expression in growth cones
Here, we identify a number of growth cone constituent proteins from a mechanically isolated growth cone preparation. Of the proteins that we identified as growth cone constituents by tandem mass spectrometry, 85% overlap with those identified in a recent study of biochemically isolated growth cone particle fraction from rat forebrain (Nozumi et al., 2009). Both approaches identified 14-3-3ε, γ, ζ and τ as growth cone constituents while Nozumi and colleagues additionally identified 14-3-3β and η. As expected, the biochemical isolation is a valuable high yield approach, while the mechanical purification may more accurately predict growth cone constituent proteins.
14-3-3β, γ and τ are upregulated between E8 and E13 in chick DRG growth cones while 14-3-3ε and ζ are not. By Western blotting, a general increase in expression of each 14-3-3 isoform can be detected in E13 DRG cell lysates (supplemental Fig. S4, available at www.jneurosci.org as supplemental material) suggesting that 14-3-3 expression is regulated locally through translation of 14-3-3 mRNA as has been seen for other proteins (Lin and Holt, 2007). The weak 14-3-3 expression in E8 chick DRG growth cones may explain why responses to NGF are unaltered by antagonizing 14-3-3 function with R18 at this stage (Fig. 2). Alternatively, cAMP levels and associated PKA activity are elevated at E8 and thus R18-dependent enhancement of PKA activity would not necessarily be expected to affect attractive turning responses. For example, bath application of Sp-cAMPS to Xenopus spinal neurons does not significantly impact netrin-dependent attraction (Ming et al., 1997). The 14-3-3 proteins are, however, strongly expressed in E6 chick RGCs (Fig. 1). At this stage RGCs are undergoing a number of well characterized guidance decisions raising the possibility that 14-3-3 proteins may also play additional roles in regulating axon guidance during development through PKA-dependent or -independent mechanisms.
14-3-3 proteins regulate PKA
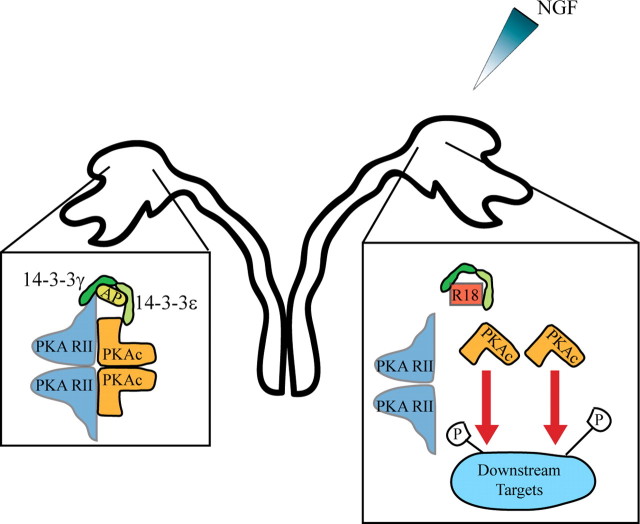
Our functional and biochemical data suggest a model whereby 14-3-3 proteins regulate PKA activity to modify neuronal responses to NGF and MAG (Fig. 8). We postulate that 14-3-3 proteins regulate PKA because R18 converts NGF- and MAG-dependent repulsion to attraction in the presence of the cAMP antagonist Rp-cAMPS but not the PKA antagonists KT5720 or PKI. Introduction of R18 dissociates the PKA catalytic and regulatory subunits suggesting that 14-3-3 proteins stabilize the PKA holoenzyme. However, it is also well established that the spatial localization and regulation of PKA activity is essential for proper growth cone turning in Xenopus spinal neurons (Han et al., 2007) and it is conceivable that 14-3-3 proteins may have a parallel function cooperating with A-Kinase anchoring proteins (AKAPs) which anchor PKA to phosphorylation targets consequently regulating local PKA activity. Upon local stimulation of DRG growth cones with an NGF gradient, we do not find any evidence of asymmetric localization of 14-3-3γ by immunofluorescence (data not shown). However, this does not exclude the possibility that localized changes in the phosphorylation state of 14-3-3 proteins or binding targets enable spatial regulation of PKA activity.
Figure 8.
Model for 14-3-3 regulation of PKA in growth cone turning response. In P5 rat DRG growth cones, 14-3-3γ/ε heterodimers (shown in green) bind to PKA directly or through an adaptor protein (AP) and downregulate the activity of the PKA holoenzyme, resulting in a repellant response to a gradient of NGF (left). In the presence of the R18 peptide (red square), 14-3-3 binding is disrupted, leading to a dissociation of the active catalytic subunits of PKA (orange) from the regulatory subunits (blue) and an increase in PKA phosphorylation of downstream targets. This switches the response of the growth cone from repulsion to attraction (right).
Knockdown of 14-3-3γ, β or ε expression mimics the ability of R18 to convert NGF-dependent repulsion to attraction. Biochemically we observe a robust interaction between PKA and 14-3-3γ but weak or no interaction with 14-3-3β or ε, respectively. One possible explanation for our findings is that 14-3-3γ/β and 14-3-3γ/ε heterodimers bind to PKA and that both complexes are necessary for regulating PKA, perhaps through favoring specific regulatory or catalytic PKA subunits. Our biochemical data suggest a model whereby 14-3-3γ may mediate binding to PKA and that 14-3-3β and 14-3-3ε may complex with PKA through binding to 14-3-3γ in an unstable heterodimer that is difficult to retain in our coimmunoprecipitation analysis. An alternative possibility is that a 14-3-3γ/ε heterodimer may stabilize PKA while 14-3-3β may regulate growth cone responses through a PKA-independent pathway. Treatment with the PKA inhibitors, KT-5720 and PKI did not completely restore repellent responses to MAG and NGF in R18-infected neurons (Fig. 5). While this could be ascribed to incomplete inactivation of PKA it does allow for the possibility that 14-3-3 proteins may affect growth cone responses through additional mechanisms. In addition to directly regulating PKA activity it is possible that 14-3-3s may also act as a tether to affect the localization of PKA. Further, 14-3-3 proteins may regulate growth cone responses by affecting the activity of cytoskeletal modulators including ADF/cofilin (Soosairajah et al., 2005), members of the CaMK pathway (Davare et al., 2004), myosin light chain phosphatase (Koga and Ikebe, 2008), and Rho GEFs (Meiri et al., 2009). It is also important to acknowledge that viral-mediated transduction of R18-GFP and shRNAmir affects 14-3-3 function throughout the cell body as well as the growth cone. The 14-3-3 proteins are expressed in a number of cellular compartments and given the wide range of binding partners previously reported (Jin et al., 2004), it is possible that 14-3-3s also alter growth cone responses through additional mechanisms such as transcriptional regulation and vesicle trafficking.
PKA and growth cone turning
How 14-3-3-dependent PKA activation may convert NGF- and MAG-dependent repulsion to attraction remains an open question. PKA can effect the insertion of guidance cue receptors into the plasma membrane (Bouchard et al., 2004). Both NGF and MAG can engage p75NTR as part of their receptor complex in certain contexts raising the possibility that receptor dynamics may be altered by PKA activation at the site of ligand binding. PKA also targets IP-1, an inhibitor of PP1. PP1 is an important downstream effector of Ca2+ signaling, another known modulator of growth cone response (Hong et al., 2000) and these findings suggest a high degree of cross talk between cAMP and Ca2+ signaling. This idea is further supported by studies suggesting cAMP levels can alter Ca2+ signals by modifying voltage-dependent Ca2+ channels (Henley et al., 2004) and through activation of ryanodine receptors, modify Ca2+-dependent release of intracellular Ca2+ (Ooashi et al., 2005). The 14-3-3 proteins may play a critical role in spatially and temporally regulating the interaction between PKA and such target substrates to regulate growth cone turning responses.
Footnotes
This study was supported by a grant to A.E.F. from the Canadian Institutes of Health Research (CIHR) and by the McGill Neuroengineering program. G.B.F. is supported by Fonds de la recherche en santé du Québec (FRSQ), and T.S. by the McGill Neuroengineering program and the Uehara Memorial Foundation. P.S.M. is a James McGill Professor. F.C. is a CIHR New Investigator and a FRSQ Chercheur-Boursier. A.E.F. is a Tier 2 Canada Research Chair and a Killam Scholar. We thank Alex Bell for valuable assistance with our proteomics analysis and Wayne Sossin for helpful comments and advice.
References
- Berruti G. A novel rap1/B-Raf/14-3-3 theta protein complex is formed in vivo during the morphogenetic differentiation of postmeiotic male germ cells. Exp Cell Res. 2000;257:172–179. doi: 10.1006/excr.2000.4877. [DOI] [PubMed] [Google Scholar]
- Bouchard JF, Moore SW, Tritsch NX, Roux PP, Shekarabi M, Barker PA, Kennedy TE. Protein kinase A activation promotes plasma membrane insertion of DCC from an intracellular pool: a novel mechanism regulating commissural axon extension. J Neurosci. 2004;24:3040–3050. doi: 10.1523/JNEUROSCI.4934-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges D, Moorhead GB. 14-3-3 proteins: a number of functions for a numbered protein. Sci STKE. 2005;2005:re10. doi: 10.1126/stke.2962005re10. [DOI] [PubMed] [Google Scholar]
- Cai D, Qiu J, Cao Z, McAtee M, Bregman BS, Filbin MT. Neuronal cyclic AMP controls the developmental loss in ability of axons to regenerate. J Neurosci. 2001;21:4731–4739. doi: 10.1523/JNEUROSCI.21-13-04731.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clandinin TR, Zipursky SL. Making connections in the fly visual system. Neuron. 2002;35:827–841. doi: 10.1016/s0896-6273(02)00876-0. [DOI] [PubMed] [Google Scholar]
- Dalton GD, Dewey WL. Protein kinase inhibitor peptide (PKI): a family of endogenous neuropeptides that modulate neuronal cAMP-dependent protein kinase function. Neuropeptides. 2006;40:23–34. doi: 10.1016/j.npep.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Davare MA, Saneyoshi T, Guire ES, Nygaard SC, Soderling TR. Inhibition of calcium/calmodulin-dependent protein kinase kinase by protein 14-3-3. J Biol Chem. 2004;279:52191–52199. doi: 10.1074/jbc.M409873200. [DOI] [PubMed] [Google Scholar]
- Garbe DS, Bashaw GJ. Axon guidance at the midline: From mutants to mechanisms. Crit Rev Biochem Mol Biol. 2004;39:319–341. doi: 10.1080/10409230490906797. [DOI] [PubMed] [Google Scholar]
- Gehler S, Gallo G, Veien E, Letourneau PC. p75 neurotrophin receptor signaling regulates growth cone filopodial dynamics through modulating RhoA activity. J Neurosci. 2004;24:4363–4372. doi: 10.1523/JNEUROSCI.0404-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen RW, Barrett JN. Characterization of the turning response of dorsal root neurites toward nerve growth factor. J Cell Biol. 1980;87:546–554. doi: 10.1083/jcb.87.3.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Han L, Tiwari P, Wen Z, Zheng JQ. Spatial targeting of type II protein kinase A to filopodia mediates the regulation of growth cone guidance by cAMP. J Cell Biol. 2007;176:101–111. doi: 10.1083/jcb.200607128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley JR, Huang KH, Wang D, Poo MM. Calcium mediates bidirectional growth cone turning induced by myelin-associated glycoprotein. Neuron. 2004;44:909–916. doi: 10.1016/j.neuron.2004.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong K, Nishiyama M, Henley J, Tessier-Lavigne M, Poo M. Calcium signalling in the guidance of nerve growth by netrin-1. Nature. 2000;403:93–98. doi: 10.1038/47507. [DOI] [PubMed] [Google Scholar]
- Huber AB, Kolodkin AL, Ginty DD, Cloutier JF. Signaling at the growth cone: ligand-receptor complexes and the control of axon growth and guidance. Annu Rev Neurosci. 2003;26:509–563. doi: 10.1146/annurev.neuro.26.010302.081139. [DOI] [PubMed] [Google Scholar]
- Jin J, Smith FD, Stark C, Wells CD, Fawcett JP, Kulkarni S, Metalnikov P, O'Donnell P, Taylor P, Taylor L, Zougman A, Woodgett JR, Langeberg LK, Scott JD, Pawson T. Proteomic, functional, and domain-based analysis of in vivo 14-3-3 binding proteins involved in cytoskeletal regulation and cellular organization. Curr Biol. 2004;14:1436–1450. doi: 10.1016/j.cub.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Jones DH, Ley S, Aitken A. Isoforms of 14-3-3 protein can form homo- and heterodimers in vivo and in vitro: implications for function as adapter proteins. FEBS Lett. 1995;368:55–58. doi: 10.1016/0014-5793(95)00598-4. [DOI] [PubMed] [Google Scholar]
- Kase H, Iwahashi K, Nakanishi S, Matsuda Y, Yamada K, Takahashi M, Murakata C, Sato A, Kaneko M. K-252 compounds, novel and potent inhibitors of protein kinase C and cyclic nucleotide-dependent protein kinases. Biochem Biophys Res Commun. 1987;142:436–440. doi: 10.1016/0006-291x(87)90293-2. [DOI] [PubMed] [Google Scholar]
- Kim TH, Lee HK, Seo IA, Bae HR, Suh DJ, Wu J, Rao Y, Hwang KG, Park HT. Netrin induces down-regulation of its receptor, Deleted in Colorectal Cancer, through the ubiquitin-proteasome pathway in the embryonic cortical neuron. J Neurochem. 2005;95:1–8. doi: 10.1111/j.1471-4159.2005.03314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga Y, Ikebe M. A novel regulatory mechanism of myosin light chain phosphorylation via binding of 14-3-3 to myosin phosphatase. Mol Biol Cell. 2008;19:1062–1071. doi: 10.1091/mbc.E07-07-0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AC, Holt CE. Local translation and directional steering in axons. EMBO J. 2007;26:3729–3736. doi: 10.1038/sj.emboj.7601808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiri D, Greeve MA, Brunet A, Finan D, Wells CD, LaRose J, Rottapel R. Modulation of Rho guanine exchange factor Lfc activity by protein kinase A-mediated phosphorylation. Mol Cell Biol. 2009;29:5963–5973. doi: 10.1128/MCB.01268-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming GL, Song HJ, Berninger B, Holt CE, Tessier-Lavigne M, Poo MM. cAMP-dependent growth cone guidance by netrin-1. Neuron. 1997;19:1225–1235. doi: 10.1016/s0896-6273(00)80414-6. [DOI] [PubMed] [Google Scholar]
- Murray AJ. Pharmacological PKA inhibition: all may not be what it seems. Sci Signal. 2008;1:re4. doi: 10.1126/scisignal.122re4. [DOI] [PubMed] [Google Scholar]
- Murray AJ, Shewan DA. Epac mediates cyclic AMP-dependent axon growth, guidance and regeneration. Mol Cell Neurosci. 2008;38:578–588. doi: 10.1016/j.mcn.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Muslin AJ, Xing H. 14-3-3 proteins: regulation of subcellular localization by molecular interference. Cell Signal. 2000;12:703–709. doi: 10.1016/s0898-6568(00)00131-5. [DOI] [PubMed] [Google Scholar]
- Nagata-Ohashi K, Ohta Y, Goto K, Chiba S, Mori R, Nishita M, Ohashi K, Kousaka K, Iwamatsu A, Niwa R, Uemura T, Mizuno K. A pathway of neuregulin-induced activation of cofilin-phosphatase Slingshot and cofilin in lamellipodia. J Cell Biol. 2004;165:465–471. doi: 10.1083/jcb.200401136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann S, Bradke F, Tessier-Lavigne M, Basbaum AI. Regeneration of sensory axons within the injured spinal cord induced by intraganglionic cAMP elevation. Neuron. 2002;34:885–893. doi: 10.1016/s0896-6273(02)00702-x. [DOI] [PubMed] [Google Scholar]
- Neve RL, Howe JR, Hong S, Kalb RG. Introduction of the glutamate receptor subunit 1 into motor neurons in vitro and in vivo using a recombinant herpes simplex virus. Neuroscience. 1997;79:435–447. doi: 10.1016/s0306-4522(96)00645-8. [DOI] [PubMed] [Google Scholar]
- Nishiyama M, Hoshino A, Tsai L, Henley JR, Goshima Y, Tessier-Lavigne M, Poo MM, Hong K. Cyclic AMP/GMP-dependent modulation of Ca2+ channels sets the polarity of nerve growth-cone turning. Nature. 2003;423:990–995. doi: 10.1038/nature01751. [DOI] [PubMed] [Google Scholar]
- Nozumi M, Togano T, Takahashi-Niki K, Lu J, Honda A, Taoka M, Shinkawa T, Koga H, Takeuchi K, Isobe T, Igarashi M. Identification of functional marker proteins in the mammalian growth cone. Proc Natl Acad Sci U S A. 2009;106:17211–17216. doi: 10.1073/pnas.0904092106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooashi N, Futatsugi A, Yoshihara F, Mikoshiba K, Kamiguchi H. Cell adhesion molecules regulate Ca2+-mediated steering of growth cones via cyclic AMP and ryanodine receptor type 3. J Cell Biol. 2005;170:1159–1167. doi: 10.1083/jcb.200503157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra LM, Zou Y. Sonic hedgehog induces response of commissural axons to Semaphorin repulsion during midline crossing. Nat Neurosci. 2010;13:29–35. doi: 10.1038/nn.2457. [DOI] [PubMed] [Google Scholar]
- Polleux F, Morrow T, Ghosh A. Semaphorin 3A is a chemoattractant for cortical apical dendrites. Nature. 2000;404:567–573. doi: 10.1038/35007001. [DOI] [PubMed] [Google Scholar]
- Qiu J, Cai D, Dai H, McAtee M, Hoffman PN, Bregman BS, Filbin MT. Spinal axon regeneration induced by elevation of cyclic AMP. Neuron. 2002;34:895–903. doi: 10.1016/s0896-6273(02)00730-4. [DOI] [PubMed] [Google Scholar]
- Roy S, McPherson RA, Apolloni A, Yan J, Lane A, Clyde-Smith J, Hancock JF. 14-3-3 facilitates Ras-dependent Raf-1 activation in vitro and in vivo. Mol Cell Biol. 1998;18:3947–3955. doi: 10.1128/mcb.18.7.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewan D, Dwivedy A, Anderson R, Holt CE. Age-related changes underlie switch in netrin-1 responsiveness as growth cones advance along visual pathway. Nat Neurosci. 2002;5:955–962. doi: 10.1038/nn919. [DOI] [PubMed] [Google Scholar]
- Song HJ, Poo MM. Signal transduction underlying growth cone guidance by diffusible factors. Curr Opin Neurobiol. 1999;9:355–363. doi: 10.1016/s0959-4388(99)80052-x. [DOI] [PubMed] [Google Scholar]
- Song HJ, Ming GL, Poo MM. cAMP-induced switching in turning direction of nerve growth cones. Nature. 1997;388:275–279. doi: 10.1038/40864. [DOI] [PubMed] [Google Scholar]
- Song H, Ming G, He Z, Lehmann M, McKerracher L, Tessier-Lavigne M, Poo M. Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science. 1998;281:1515–1518. doi: 10.1126/science.281.5382.1515. [DOI] [PubMed] [Google Scholar]
- Soosairajah J, Maiti S, Wiggan O, Sarmiere P, Moussi N, Sarcevic B, Sampath R, Bamburg JR, Bernard O. Interplay between components of a novel LIM kinase-slingshot phosphatase complex regulates cofilin. EMBO J. 2005;24:473–486. doi: 10.1038/sj.emboj.7600543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein E, Tessier-Lavigne M. Hierarchical organization of guidance receptors: silencing of netrin attraction by slit through a Robo/DCC receptor complex. Science. 2001;291:1928–1938. doi: 10.1126/science.1058445. [DOI] [PubMed] [Google Scholar]
- Thomas S, Ritter B, Verbich D, Sanson C, Bourbonnière L, McKinney RA, McPherson PS. Intersectin regulates dendritic spine development and somatodendritic endocytosis but not synaptic vesicle recycling in hippocampal neurons. J Biol Chem. 2009;284:12410–12419. doi: 10.1074/jbc.M809746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Hoeven PC, Van Der Wal JC, Ruurs P, Van Dijk MC, Van Blitterswijk J. 14-3-3 isotypes facilitate coupling of protein kinase C-zeta to Raf-1: negative regulation by 14-3-3 phosphorylation. Biochem J. 2000;345:297–306. doi: 10.1042/0264-6021:3450297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heusden GP. 14-3-3 proteins: regulators of numerous eukaryotic proteins. IUBMB Life. 2005;57:623–629. doi: 10.1080/15216540500252666. [DOI] [PubMed] [Google Scholar]
- Wang B, Yang H, Liu YC, Jelinek T, Zhang L, Ruoslahti E, Fu H. Isolation of high-affinity peptide antagonists of 14-3-3 proteins by phage display. Biochemistry. 1999;38:12499–12504. doi: 10.1021/bi991353h. [DOI] [PubMed] [Google Scholar]
- Yam PT, Langlois SD, Morin S, Charron F. Sonic hedgehog guides axons through a noncanonical, Src-family-kinase-dependent signaling pathway. Neuron. 2009;62:349–362. doi: 10.1016/j.neuron.2009.03.022. [DOI] [PubMed] [Google Scholar]