Abstract
The role of GABAB receptors in sleep is still poorly understood. GHB (γ-hydroxybutyric acid) targets these receptors and is the only drug approved to treat the sleep disorder narcolepsy. GABAB receptors are obligate dimers comprised of the GABAB2 subunit and either one of the two GABAB1 subunit isoforms, GABAB1a and GABAB1b. To better understand the role of GABAB receptors in sleep regulation, we performed electroencephalogram (EEG) recordings in mice devoid of functional GABAB receptors (1−/− and 2−/−) or lacking one of the subunit 1 isoforms (1a−/− and 1b−/−). The distribution of sleep over the day was profoundly altered in 1−/− and 2−/− mice, suggesting a role for GABAB receptors in the circadian organization of sleep. Several other sleep and EEG phenotypes pointed to a more prominent role for GABAB1a compared with the GABAB1b isoform. Moreover, we found that GABAB1a protects against the spontaneous seizure activity observed in 1−/− and 2−/− mice. We also evaluated the effects of the GHB-prodrug GBL (γ-butyrolactone) and of baclofen (BAC), a high-affinity GABAB receptor agonist. Both drugs induced a state distinct from physiological sleep that was not observed in 1−/− and 2−/− mice. Subsequent sleep was not affected by GBL whereas BAC was followed by a delayed hypersomnia even in 1−/− and 2−/− mice. The differential effects of GBL and BAC might be attributed to differences in GABAB-receptor affinity. These results also indicate that all GBL effects are mediated through GABAB receptors, although these receptors do not seem to be involved in mediating the BAC-induced hypersomnia.
Introduction
GABAB receptors are involved in epilepsy (Schuler et al., 2001; Gambardella et al., 2003), anxiety and depression (Mombereau et al., 2004; Jacobson et al., 2007a), nociception (Zarrindast et al., 2000), memory (Levin et al., 2004; Jacobson et al., 2007b), addiction (Cruz et al., 2004; Roberts, 2005; Filip and Frankowska, 2008), and potentially sleep (Juhász et al., 1994; Ulloor et al., 2004). Although a prominent role of GABAA receptors in sleep is firmly established and is central in the pharmacological management of disturbed sleep (Winsky-Sommerer, 2009), little is known about the importance of GABAB receptors in regulating sleep and the electroencephalogram (EEG). Although the effects of specific GABAB agonists, like baclofen (BAC), on rapid eye movement sleep (REMS) remain unclear (Finnimore et al., 1995; Ulloor et al., 2004), available data indicate that BAC increases non-REMS (NREMS) and promotes EEG slow (delta) waves (0.75–4.5 Hz) during NREMS (Finnimore et al., 1995; Darbari et al., 2005; Huang and Guilleminault, 2009).
γ-Hydroxybutyrate (GHB) is a GABA metabolite found in low concentrations throughout the mammalian brain (Bessman and Fishbein, 1963; Cash, 1994; Maitre, 1997). Since its synthesis in the 1960s (Laborit, 1964), GHB has been used as an anesthetic, sedative, and hypnotic agent (Laborit et al., 1960; Vickers, 1969). Because of its abuse potential, GHB is banned in many countries. GHB is approved as a treatment for narcolepsy with cataplexy (Fuller and Hornfeldt, 2003; U.S. Xyrem Multicenter Study Group, 2003). Although the mechanism of action is still unclear, GHB decreases excessive daytime sleepiness and attacks of cataplexy in narcolepsy patients (Xyrem International Study Group, 2005; Black and Houghton, 2006). Despite conflicting results suggesting that GHB acts via specific GHB receptors (Doherty et al., 1978; Castelli et al., 2004), compelling evidence suggests that most of the physiological and pharmacological effects of exogenous GHB are mediated through GABAB receptors (Waldmeier, 1991; Jensen and Mody, 2001; Kaupmann et al., 2003; Quéva et al., 2003; Carai et al., 2008).
Both in patients and in healthy subjects, GHB decreases sleep latency and promotes deep NREMS, evidenced by the marked increase in the prevalence and amplitude of EEG delta waves (Mamelak et al., 1977; Lapierre et al., 1990; Van Cauter et al., 1997). Animal studies also suggest that GHB promotes NREMS (Godschalk et al., 1977; Stock et al., 1978; Monti et al., 1979). However, it was also reported that GHB and its prodrug γ-butyrolactone (GBL) can induce paradoxical EEG slow/delta waves in awake humans (Mamelak et al., 1977; Van Cauter et al., 1997) and animals (Godschalk et al., 1977; Meerlo et al., 2004). This finding challenges the claimed physiological sleep-promoting effects of GHB. The first aim of the present study was to investigate the role of each of the known GABAB receptor subunits in sleep–wake regulation and in mediating the effects of GHB. The second aim was to perform a detailed sleep and EEG analysis to investigate whether the delta waves induced by GHB contribute to normal physiological sleep.
Materials and Methods
Animals and housing conditions.
All experiments were performed in accordance with the protocols approved by the Ethical Committee of the State of Vaud Veterinary Office, Switzerland.
GABAB1−/− (1−/−), GABAB2−/− (2−/−), GABAB1a−/− (1a−/−), and GABAB1b−/− (1b−/−) mice were generated on a BALB/c background as described previously (Schuler et al., 2001; Gassmann et al., 2004; Vigot et al., 2006). Adult male mice of the four genotypes along with their wild-type (WT) controls were used in baseline conditions, 6 h sleep deprivation, and experiments with GBL and saline injections (n = 8–9 mice per genotype; age, 10–15 weeks; weight, 24–31 g). For the BAC experiments, BALB/cJ (WT) mice were purchased from Jackson Laboratory. All mice were kept individually in polycarbonate cages (31 × 18 × 18 cm) under a 12 h light/dark cycle (lights on at 9:00 A.M.) at an ambient temperature of 24.5–25.5°C. Food and water were available ad libitum.
Surgery and sleep recordings.
EEG and electromyogram (EMG) electrodes were implanted while the mice were under deep anesthesia as previously described (Hasan et al., 2009). Four to six days of recovery from surgery were allowed before connecting animals to the recording leads. A minimum of 6 adaptation days (or 10 including recovery from surgery) were scheduled before data collection. The analog signals were digitized at 2 kHz and subsequently stored at 200 Hz on a hard disc. The EEG was subjected to a discrete Fourier transformation yielding power spectra (range, 0.75–90 Hz; frequency resolution, 0.25 Hz; time resolution, consecutive 4 s epochs; window function, hamming). Hardware (EMBLA) and software (Somnologica-3) were purchased from Medcare Flaga. Activity in the 50 Hz band was discarded from further analysis because of power line artifacts in the EEG of some of the animals.
Based on the EEG and EMG signals, the animals' behaviors were classified as REMS, NREMS, or wakefulness (Franken et al., 1998). In addition to these three behavioral states, seizures and drug (i.e., GBL or BAC)-induced states were also assessed (for description see GBL and BAC administration, below). All states were scored by visual inspection of the EEG and EMG signals displayed on a PC monitor. Four second epochs containing EEG artifacts were marked and excluded from EEG spectral analyses.
Five to 12 animals were recorded together in one experimental session (1−/− mice, n = 8; 2−/− mice, n = 8; 1a−/− mice, n = 8; 1b−/− mice, n = 9; WT mice, n = 8). At least two genotypes were included per session in an attempt to equally distribute the environmental variation over genotypes. Overall, eight sessions were necessary to complete the study.
Baseline and sleep-deprivation experiments.
EEG and EMG signals were recorded continuously for at least 48 h with the first 24 h serving as baseline followed by 6 h sleep deprivation (SD) starting at light onset and 18 h of recovery sleep. SD was achieved by gentle handling, consisting of introducing novel objects into the cage, approaching a pipette next to the mouse, or gentle cage tapping as soon as a sleeping behavior was observed. Due to health deterioration, particularly when disturbed, 1−/− and 2−/− mice were not included in the sleep-deprivation protocol. One subset of mice was used for the baseline conditions protocol (n = 8 per genotype) and one other subset of mice was included in the drug protocol (see below).
Mean EEG spectra were calculated over 4 s epochs scored as artifact-free NREMS, REMS, or wakefulness to construct behavioral state-specific spectral EEG profiles for baseline. EEG delta power (a measure of homeostatic sleep need) was calculated by averaging EEG power density in the 1–4 Hz range for 4 s epochs scored as NREMS. Time-course analysis of EEG delta power during baseline and after SD was described in detail previously (Franken et al., 2001). In short, the recording was divided into sections to which an equal number of 4 s epochs scored as NREMS contributed (i.e., percentiles). The first 6 h of the baseline light period was divided into six such sections; the second 6 h into four. The second 6 h of the recovery light period was divided into six sections, the dark periods of both the baseline and recovery dark periods into eight sections. The choice of the number of sections per recording period depended on NREMS prevalence. Delta power values were normalized by expressing them as a percentage of the individual mean value reached over the last 4 h of the main rest period when delta power is minimal during baseline.
The main rest period was calculated as described previously (Franken et al., 1999) with modifications. Mean sleep duration was calculated over a 2 h moving average at 15 min increments within individual mice. Fifteen-minute intervals in which mice slept more than their individual 24 h baseline mean were termed as rest. Fourteen or more 15 min rest intervals interrupted by <6 nonrest intervals constituted a rest period. Applying this algorithm to mice, generally one main rest period was obtained associated with the light period.
Sleep quality was assessed by analyzing its consolidation by counting the number of brief awakenings and the number of short and long NREMS episodes, as previously described (Franken et al., 1999).
GBL and BAC administration.
Five days after the sleep deprivation experiment, EEG and EMG signals were recorded continuously for six consecutive 24 h periods, starting at lights-on. Twenty-four hour baseline was followed by a saline day and 4 d with administration of four different doses of GBL (50, 100, 150, and 300 mg/kg) or 3 d with administration of three different doses of BAC (5, 7.5, and 10 mg/kg). WT mice taking part in the BAC experiment and 1−/− and 2−/− mice were not previously used in the sleep deprivation experiment. Out of concern of health conditions in 1−/− and 2−/− mice, the drug protocol was slightly simplified, i.e., only saline and the highest drug dose were tested (n = 3 per genotype per drug). To exclude any carry-over or tachyphylaxis due to our increasing dosing protocol, 18 wild-type BALB/cJ mice (n = 9 per drug) were studied in a randomized crossover experiment with GBL and BAC at the lowest and highest doses and saline. Mice were included in one of three conditions: (1) administration of the highest dose followed 24 h later by the lowest dose and 24 h later by saline; (2) administration of the highest dose followed by 48 h washout, then the lowest dose and then 24 h later saline; and (3) administration of saline followed 24 h later by the lowest dose and then 24 h later by the highest dose. The results indicated that the order of dose or duration of washout did not significantly affect the results for three main and tested sleep phenotypes: amount of drug-induced state, time course of delta power in NREMS following the drug-induced state, and amount of NREMS after drug administration (data not shown).
Drug doses were chosen according to the literature to cover a large range of sedative/hypnotic effects that could be compared between drugs (Schuler et al., 2001; de Fiebre et al., 2004; Meerlo et al., 2004; Koek et al., 2007). Saline, GBL, and BAC were intraperitoneally administrated 6 h after lights onset at Zeitgeber time (ZT) 6 (light onset being ZT0). At least 18 h were recorded after the last injection. BAC- and GBL-induced states were characterized by an increase of hypersynchronous slow waves and/or spiky EEG pattern following drug injection. The drug-induced state can be readily distinguished from the three classical behavioral states and were therefore analyzed separately. For both compounds, the drug-induced state was determined as follows: at the onset of the drugs effects, animals were awake while large-amplitude, short-lasting (2–4 s) burst of hypersynchronous slow waves appeared and progressively dominated the EEG until normal EEG activity could no longer be discerned. The first waking 4 s epoch in which abnormal EEG activity was observed was taken as drug-induced state onset. Toward the end of the drug-induced state, this alternation between normal waking and drug-induced state reappeared. The last 4 s epoch with abnormal EEG was taken as the end of the drug-induced state. The amount of drug-induced state was the sum of 4 s epochs scored as drug-induced state.
Four second epochs of NREMS and BAC- and GBL-induced states were subjected to spectral analysis to calculate the EEG power density in the delta frequency range (1–4 Hz). Time-course analysis of the delta power on the saline day and the 3 or 4 d with injections of BAC or GBL, respectively, were performed similarly to the baseline condition. Delta power during the BAC- and GBL-induced state is presented as a single time point averaging over the duration of the state, which was too short to reliably estimate a time course.
Spectral content of the EEG during NREMS, BAC-, and GBL-induced state was quantified as described above. EEG spectra were normalized to be directly comparable as follows: EEG power in each frequency bin for each mean NREMS or drug-induced state spectrum was expressed as a percentage of the mean NREMS EEG power determined over all artifact-free 4 s epochs during 4 h of the rest period in baseline within individual mice.
Analysis tools.
TMT Pascal Multi-Target5 software (TMT Development) was used to manage the data, SigmaPlot 10.0 (Systat Software) for graphics, and SAS Institute software, version 9.1, for statistical analyses.
Drugs.
Placebo was a saline solution (NaCl 0.9%, B. Braun Medical). GBL and racemic BAC (Sigma-Aldrich Chemie) were freshly diluted in saline solution to obtain different solutions of GBL (50, 100, 150, and 300 mg/kg) and BAC (5, 7.5, and 10 mg/kg) with an injection volume of 5 ml/kg of body weight.
GBL has a greater lipid solubility than GHB, allowing uniform and rapid absorption (Snead, 1991). The in vivo pharmacological properties of GBL are secondary to its final conversion into GHB (Rubin and Giarman, 1947). GBL is biologically inactive (Roth et al., 1966) and all its physiologic and behavioral effects are due to its rapid conversion (<1 min) to GHB by peripheral lactonases or by nonenzymatic hydrolysis (Roth et al., 1967; Carter et al., 2006).
Results
Spontaneous epileptiform activity in 1−/−, 2−/−, and 1a−/− mice
Both 1−/− and 2−/− mice lack functional GABAB receptors whereas 1a−/− mice still have functional GABAB1b,2 receptors and 1b−/− mice have GABAB1a,2 receptors. As previously observed (Schuler et al., 2001; Gassmann et al., 2004), all 1−/− and 2−/− mice displayed spontaneous seizures. Over the 24 h baseline recording period, five of eight mice of both 1−/− and 2−/− genotypes showed at least one seizure. Health status of 1−/− and 2−/− mice gradually deteriorated, manifested as weight loss, ruffled fur, and hunched posture accompanying the increasing number of seizures (up to 20 seizures per day). Only data from healthy animals were included in the analyses.
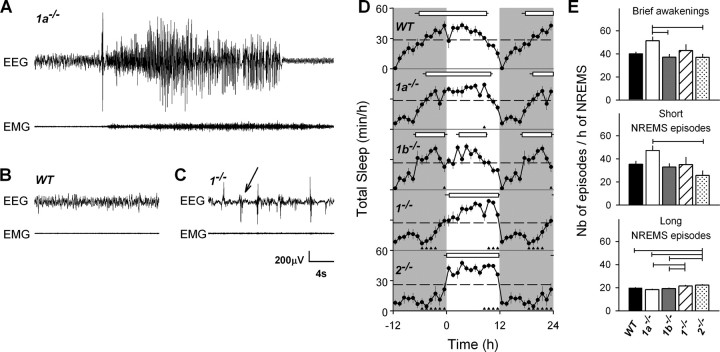
Interestingly, four of eight 1a−/− mice also exhibited similar spontaneous epileptiform activity (Fig. 1A), a phenotype never described before for this genotype. This epileptiform trait was, however, less severe, with the number of seizure never exceeding four per day and without affecting their overt health status. Almost all seizures observed in the three genotypes were of the clonic type lasting between 12 s and 1.5 min. On occasion, tonic-clonic seizures were observed after audiogenic stimuli, handling, or cage change. In addition, epileptic mice displayed high-voltage EEG spikes generally in the hours prior and/or following a seizure (Fig. 1B,C). Seizures occurred in all behavioral states and during both light and dark periods. No epileptiform activity was observed in any of the 1b−/− or WT mice.
Figure 1.
Sleep and EEG phenotypes for 1a−/−, 1b−/−, 1−/−, 2−/−, and WT mice. A, EEG and EMG signals illustrating a spontaneous clonic seizure in a 1a−/− mouse during undisturbed baseline conditions. This seizure occurred during NREMS (2 s before seizure onset). Both EEG amplitude and frequency were increased as well as muscle tone (EMG). Animal showed rearing and bilateral clonus of the forelimbs during the seizure. B, Twenty seconds of typical NREMS in a WT mouse characterized by a high amplitude of low-frequency EEG oscillations (delta waves) and reduced muscle tone. C, Example of abnormal EEG during well identified NREMS in a 1−/− mouse. Arrow points to an abrupt EEG sharp wave. This epileptiform activity during NREMS was seen only in 1−/− and 2−/− mice, and was present during >20% of their NREMS. Waking and REMS were also affected to a lesser extent. These abnormal EEG events were excluded from the spectral analysis depicted in Figure 3. D, Time course of hourly mean values of total sleep amount (NREMS + REMS; ± SEM, n = 8–9) during baseline. Values of the dark period (gray areas) were depicted twice to illustrate the changes at the dark-to-light transition. Horizontal dashed lines mark the mean baseline (0–24 h) value for total sleep. Genotype did not affect sleep amount but its distribution changed (two-way ANOVA with factor genotype, p = 0.11; hour, p < 0.0001; genotype × hour, p < 0.0001). Triangles below each curve indicate hourly intervals for which values differed from WT mice (Dunnett's two-tailed t test; p < 0.05). For each genotype, the main rest period is indicated by a horizontal bar connecting rest onset and end (mean ± SEM, n = 8–9). Rest periods were determined individually by selecting intervals in which NREMS and REMS were above the individual baseline mean (see Materials and Methods). In all 1b−/− mice, the main rest period was interrupted by a 3 h gap. E, Sleep fragmentation was quantified by counting the number of brief awakenings (<16 s; 1, 2, 3, or 4 s epochs of waking; top) interrupting sleep and the number of short (<1 min; <15 consecutive 4 s epoch of NREMS; center) and long (>1 min; bottom) NREMS episodes according to previously published criteria (Franken et al., 1999). Variables were expressed per hour of NREMS to correct for differences in total NREMS amount. Calculated over the 24 h of baseline, 1a−/− mice had more short NREMS episodes than 2−/− mice and more brief awakenings compared with 1b−/− and 2−/− mice (one-way ANOVA factor genotype, p = 0.017 and p = 0.0089, respectively). The number of long NREMS episodes was generally higher in 1−/− and 2−/− mice compared with 1a−/−, 1b−/−, and WT mice (one-way ANOVA factor genotype, p < 0.0001). Horizontal lines connect genotypes for which significant differences were observed (Tukey's test, p < 0.05).
Loss of GABAB receptors delays the rest period, reduces delta and theta activity in the NREMS EEG, and increases theta activity in the waking EEG
Although genotype did not affect behavioral state duration under baseline conditions (Table 1), a lack of functional GABAB receptors greatly altered the sleep–wake distribution (Fig. 1D). Although the main rest period in 1a−/−, 1b−/−, and WT mice was initiated several hours before light onset (−4.6 ± 0.7, −6.9 ± 0.4, and −6.2 ± 0.8 h, respectively; mean ± SEM), which is typical for male BALB/c mice (Franken et al., 1999; Shimomura et al., 2001), in 1−/− and 2−/− mice, the onset of the rest period was delayed by 6 h compared with WT and became closely associated with light onset. The end of the rest period was similarly delayed in 1−/− and 2−/− mice compared with 1a−/−, WT, and 1b−/− mice (11.7 ± 0.2, 11.8 ± 0.1, 10.0 ± 0.3, 9.0 ± 0.4, and 8.9 ± 0.3 h after light onset, respectively; 1-way ANOVA for rest onset and rest end, p < 0.0001; paired t test, p < 0.05). 1a−/− mice displayed an onset and end of their rest period intermediate between 1−/− and 2−/− mice on the one hand, and WT and 1b−/− mice on the other. The delay of the end of the rest period was also reflected in the increased time spent asleep in the second half of the light period with highest values reached in 1−/− and 2−/− mice, intermediate in 1a−/− mice, and lowest in WT and 1b−/− mice (Figs. 1D, 2A,C).
Table 1.
Behavioral states in baseline; 12 and 24 h values
| Waking (min) | NREMS (min) | REMS (min) | TS (min) | |
|---|---|---|---|---|
| 24 h period | ||||
| WT | 753.9 ± 5.6 | 608.6 ± 8.9 | 77.5 ± 5.2 | 686.1 ± 8.9 |
| 1a−/− | 752.1 ± 18.4 | 607.9 ± 19.3 | 79.9 ± 4.6 | 687.8 ± 19.3 |
| 1b−/− | 796.7 ± 14.5 | 561.6 ± 16.7 | 81.7 ± 3.5 | 643.3 ± 16.7 |
| 1−/− | 781.5 ± 29.6 | 570.6 ± 32.5 | 81.3 ± 6.0 | 651.8 ± 32.6 |
| 2−/− | 812.4 ± 27.1 | 543.0 ± 30.1 | 82.7 ± 4.7 | 625.7 ± 29.6 |
| p | 0.28 | 0.12 | 0.95 | 0.25 |
| 12 h light period | ||||
| WT | 328.5 ± 13.3b,c | 352.0 ± 15.9a,b | 39.5 ± 3.7a | 391.5 ± 15.9a,b |
| 1a−/− | 283.3 ± 7.8a,b | 386.8 ± 8.6b,c | 49.7 ± 4.5a,b | 436.5 ± 8.6b,c |
| 1b−/− | 350.9 ± 10.6c | 325.1 ± 12.1a | 44.0 ± 2.7a | 369.1 ± 12.1a |
| 1−/− | 244.5 ± 18.9a | 413.4 ± 20.5c | 58.8 ± 4.3b | 472.2 ± 19.9c |
| 2−/− | 244.7 ± 16.6a | 429.2 ± 16.4c | 64.4 ± 2.9b | 493.6 ± 16.5c |
| p | <0.0001 | <0.0001 | 0.0001 | <0.0001 |
| 12 h dark period | ||||
| WT | 425.4 ± 14.4a | 256.5 ± 17.5b | 38.0 ± 4.0b | 294.6 ± 17.5b |
| 1a−/− | 468.8 ± 16.9a,b | 221.0 ± 19.4b,c | 30.2 ± 2.7a,b | 251.2 ± 19.4b,c |
| 1b−/− | 445.8 ± 11.5a,b | 236.5 ± 14.9b | 37.7 ± 4.1b | 274.2 ± 14.9b |
| 1−/− | 537.0 ± 24.7b,c | 157.2 ± 29.7a,c | 22.4 ± 5.1a,b | 179.7 ± 29.5a,c |
| 2−/− | 587.7 ± 28.3c | 113.8 ± 18.2a | 18.2 ± 4.7a | 132.1 ± 33.0a |
| p | <0.0001 | <0.0001 | 0.0048 | <0.0001 |
Mean (±SEM; n = 8–9) artifact free recording time in 24 h baseline, 12 h light, and 12 h dark period for the time spent in waking, NREMS, REMS, and total sleep time (TS; NREMS + REMS). Behavioral states varied among genotypes (p values of one-way ANOVA indicated).
a–c, Tukey's test, p < 0.05; genotypes for which mean values significantly differed do not share the same character.
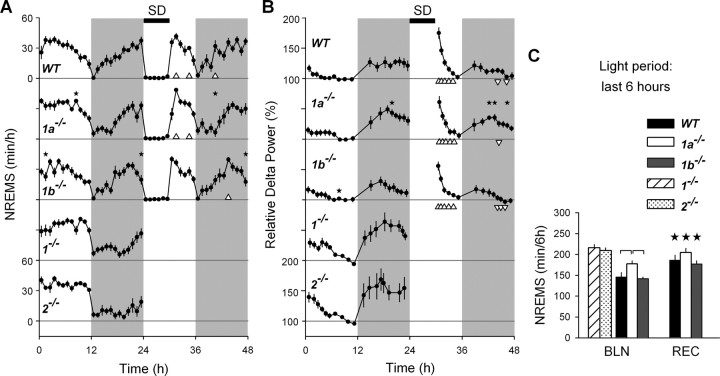
Figure 2.
Time course of the amount of NREMS and EEG delta power (1–4 Hz) in NREMS. A, B, Mean 1 h values for NREMS (A) and mean values for delta power (±SEM; B) during 24 h baseline (BLN; 0–24 h), 6 h sleep deprivation (SD; 24–30 h) and 18 h recovery (REC; 30–48 h) in WT, 1a−/−, and 1b−/− mice (n = 8, n = 8, and n = 9, respectively). For comparison, baseline results were also shown for 1−/− and 2−/− mice (n = 8 per genotype; these mice were not sleep deprived; see Materials and Methods). Delta power was expressed as a percentage of individual mean NREMS delta power over the last 4 h of the rest period. Gray areas mark the dark periods, white areas the light periods, and the black bar on the top indicates the 6 h SD. Stars above the curves of 1a−/− and 1b−/− mice indicate significant differences from WT (one-way ANOVA; Dunnett's two-tailed t test, p < 0.05). In recovery, triangles below the curves indicate hours at which values differed from baseline (t tests, p < 0.05, ▵ > baseline, ▿ < baseline). C, Mean values for NREMS amount during the last 6 h of the light periods of baseline and recovery in 1b−/− (n = 9), 1a−/− (n = 8), and WT (n = 8) mice. These two values were compared among the three genotypes. Black stars indicate significant recovery–baseline differences (one-way ANOVA; Tukey's test, p < 0.05). Horizontal connecting lines indicate significant differences among genotypes (one-way ANOVA; Tukey's test, p < 0.05). Amount of NREMS during the last 6 h of the baseline light period was shown also for 1−/− and 2−/− mice (n = 8 per genotype), but values were not included in the statistics.
Genotype difference in the duration of the rest period, calculated as the time span between the onset and end of the rest period, also distinguished 1−/− and 2−/− mice from 1a−/−, WT, and 1b−/− mice, the former two genotypes showing a significantly shorter rest period (1-way ANOVA for total rest duration, p < 0.0001; 1−/− = 2−/− < 1a−/− = WT = 1b−/−; Tukey's test, p < 0.05) (Fig. 1D). Also, for this phenotype, 1a−/− mice appeared intermediate between mice completely lacking GABAB receptors and WT and 1b−/− mice. Whereas all other genotypes displayed only one rest period per 24 h, 1b−/− mice showed a consistent 3.0 ± 0.2 h gap interrupting the rest period (Fig. 1D).
Apart from this marked redistribution of sleep and waking over the day, we also noticed genotype differences in sleep architecture at the level of individual sleep episodes. Judging by the increased number of brief awakenings (<16 s), sleep in 1a−/− mice was generally more fragmented compared with all other genotypes (Fig. 1E), consistent with the fragmented sleep recently reported in mice lacking functional GABAB receptors in orexin neurons specifically (Matsuki et al., 2009). In contrast, we found that an overall lack of functional GABAB receptors (i.e., in 1−/− and 2−/− mice) lead to a greater number of longer periods of sleep (>1 min) compared with the other genotypes (Fig. 1E). Thus, brain site-specific effects of GABAB receptors and subcellular localization of GABAB receptors subunits (Vigot et al., 2006; Ulrich and Bettler, 2007) can have a profound impact on the consolidation of sleep.
GABAB receptor genotype also affected EEG activity and the main spectral changes were found in frequencies <20 Hz. During NREMS, 1b−/− mice exhibited a reduced EEG activity in theta frequency range compared with WT (3.75–7.5 Hz) (Fig. 3). This decrease became more pronounced both in terms of amplitude and frequency range in 1−/− and 2−/− mice, which showed a strong decrease over a broad frequency range (1.75–10 Hz), including both delta and theta frequencies, compared with WT mice. This decrease is reminiscent of the reduction in EEG synchronization observed after the thalamic administration of a GABAB receptor antagonist (Juhász et al., 1994), underscoring the crucial role of GABAB receptors in thalamocortical oscillations characteristic of NREMS (Huguenard and Prince, 1994; Kim et al., 1997).
Figure 3.
Average EEG power spectra (±SEM) for NREMS, REMS, and waking during baseline. For clarity, only the frequency range for which major genotype differences were observed is shown (0.75–20 Hz at 0.25 Hz bins). Genotype affected the EEG spectra of the three behavioral states (two-way ANOVA for each state; factors genotype, bin, and their interaction, p < 0.0001). Colored triangles above each set of spectra indicate frequency bins for which power density differed from WT mice (Dunnett's two-tailed t test, p < 0.05) (black, NREMS; blue, REMS; red, waking; color coding of lines and triangles matches).
The waking EEG spectra of the latter two genotypes also markedly differed from 1a−/−, 1b−/−, and WT mice in that theta activity, especially ∼7 Hz, was more pronounced (Fig. 3 and data not shown for 1a−/− and 1b−/− mice). The increase in theta power during wakefulness might suggest an increase in active and exploratory behavior, which is associated with hippocampal theta oscillations (van Lier et al., 2003). Alternatively, GABAB receptors seem to be directly involved in theta rhythm generation (Vertes, 2005), although the REMS spectral signature, with its characteristic theta peak ∼7 Hz, remained unaffected by genotype.
The homeostatic regulation of sleep is not affected in mice lacking GABAB receptor subunits
A 6 h SD was performed to assess whether GABAB receptor subunits contribute to sleep homeostasis. Due to the health deterioration of 1−/− and 2−/− mice during SD, these two genotypes were excluded from this experiment. Recovery of sleep loss in the three remaining genotypes was evident by increases in both NREMS duration and in EEG delta power in the first 6 h after SD (i.e., recovery light period). This response did not differ among 1a−/−, 1b−/−, and WT mice (Fig. 2A–C). EEG delta power steeply declined over the course of recovery and fell below baseline in the subsequent recovery dark period. During this period, levels of delta power in 1a−/− mice were higher than those observed in 1b−/− and WT mice. This genotype difference was also observed in the dark period of baseline after a spontaneous period of wakefulness (Fig. 2B). Although the effect of SD could not be evaluated in 1−/− and 2−/− mice (see Materials and Methods), EEG delta power during baseline also decreased during the rest period and increased over the course of the active period. Like for 1a−/− mice, delta power levels reached in the baseline dark period in 1−/− and 2−/− mice seemed higher than WT and 1b−/− mice (Fig. 2B). These results, together with the effects observed after SD in the other three genotypes, suggest that GABAB receptors do not play a major role in sleep homeostasis as indexed by EEG delta power.
GBL, through GABAB receptors, induces an anesthetic-like state distinct from physiological sleep
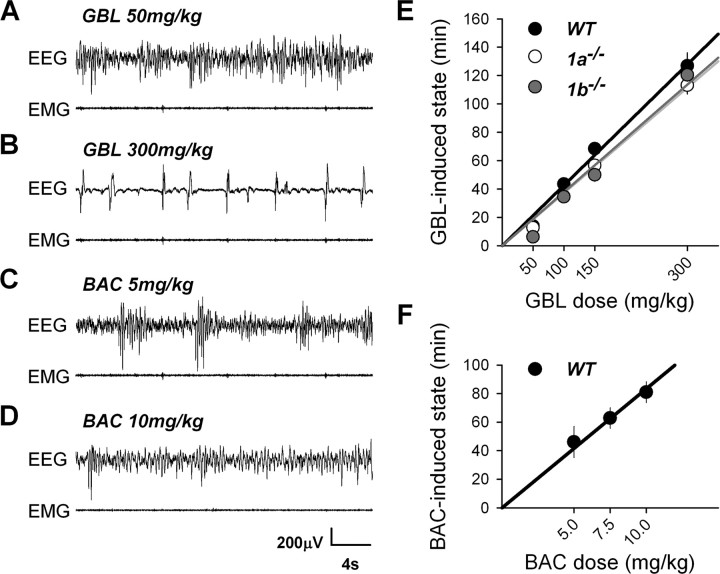
We tested the effects of GBL and BAC on sleep and the EEG at various doses. Order of dose did not affect the main drug effects presented here (see Materials and Methods). Administration of GBL or BAC did not affect behavior or the EEG in 1−/− or 2−/− mice. In contrast, GBL and BAC dose-dependently affected the EEG and behavior in WT, 1a−/−, and 1b−/− mice without noticeable behavioral or EEG differences among these three genotypes. At low doses of GBL (50 and 100 mg/kg), EEG slow waves appeared (Fig. 4A) and locomotor activity decreased, while animals remained behaviorally awake with eyes open and responded normally when stimulated. At higher GBL doses (150 and 300 mg/kg), mice became immobile with an unnatural flat body posture with hind limbs stretched sideways while eyes remained open. Their EEG displayed hypersynchronous slow waves and a spike-like pattern, which was more abundant after the highest dose (Fig. 4B). Importantly, at the highest GBL dose, animals became completely unresponsive to stimulation, resembling being in a state of deep anesthesia. BAC, administered in WT mice, also induced hypersynchronous slow waves and decreased locomotor activity. However, even at the highest dose (10 mg/kg), BAC did not induce the spike-like EEG pattern observed after 300 mg/kg of GBL (Fig. 4C,D). Moreover, although at 10 mg/kg animals were also immobile with abnormal flat posture and open eyes, they still responded to tactile stimuli. The EEG patterns combined with behavioral observations indicated that the state induced by the drugs could not be interpreted as either normal sleep or wakefulness. We therefore scored periods with abnormal EEG following drug administration as drug-induced state (see Materials and Methods). The GBL-induced state appeared 4–9 min after injection in 1a−/−, 1b−/−, and WT mice, and the BAC-induced state appeared significantly later (13–17 min; 1-way ANOVA, p < 0.001) in WT mice. The length of both drug-induced states varied according to dose and both 1a−/− and 1b−/− mice displayed an overall shorter GBL-induced state amount than WT mice did (Fig. 4E,F).
Figure 4.
A–D, Representative traces illustrating the effects of GBL and BAC on the EEG and EMG in WT animals after 50 (A) and 300 (B) mg/kg of GBL and after 5 (C) and 10 (D) mg/kg of BAC. Similar EEG and EMG patterns after GBL were found in 1a−/− and 1b−/− mice. GBL did not affect behavior or EEG in 1−/− or 2−/− mice (data not shown). E, Length of GBL-induced state in 1a−/−, 1b−/−, and WT mice. The length of GBL-induced state increased linearly and dose-dependently (linear regression; WT, n = 8, R2 = 0.99; 1a−/−, n = 8, R2 = 0.99; 1b−/−, n = 9, R2 = 0.96). The length of GBL-induced state varied with dose and genotype and was, in general, longer in WT mice (two-way ANOVA, factor genotype, p = 0.0011; factor dose, p < 0.0001; genotype × dose, p = 0.42; genotype, 1a−/− = 1b−/− < WT; Tukey's test, p < 0.05; dose:,50 < 100 < 150 < 300 mg/kg; Tukey's test, p < 0.05). F, Length of BAC-induced state after each dose of BAC in WT mice. The length of BAC-induced state increased linearly and dose-dependently within this dosage range (linear regression, WT, n = 8, R2 = 0.96; one-way ANOVA, factor dose, p = 0.033; 5 = 7.5 < 7.5 = 10 mg/kg; Tukey's test, p < 0.05).
Because of the induction of slow waves, especially at lower drug doses, reminiscent of those present during NREMS, we contrasted delta power during drug-induced state to the levels usually obtained during NREMS. Delta power during GBL-induced state increased from 50 to 100 mg/kg but did not further increase at higher doses (Fig. 5A). In 1a−/− and 1b−/− mice, delta power reached at the highest three doses was significantly higher compared with that reached after 50 mg/kg of GBL (Fig. 5A). Furthermore, in 1a−/− mice, levels reached at the three highest doses were approximately twofold higher compared with 1b−/− and WT mice and threefold higher than the baseline reference reached in NREMS. During the BAC-induced state, delta power levels remained within the baseline range determined for NREMS and did not differ among doses (Fig. 5B).
Figure 5.
EEG delta power (1.0–4.0 Hz) during the GBL- and BAC-induced state and its time course during subsequent NREMS (mean ± SEM). A, Delta power during GBL-induced state (triangles) increased from 50 to 100 mg/kg, where it reached a plateau (50 mg/kg, <3 highest doses in 1a−/− and 1b−/− mice; one-way ANOVA, Tukey's test, p < 0.05). Plateau levels reached were around twofold higher in 1a−/− mice than in 1b−/− and WT mice (one-way ANOVAs, p < 0.0001; Tukey's tests, p < 0.05, stars). For NREMS delta power (circles; mean ± SEM), a comparison among genotype (1a−/−, 1b−/−, WT), day (1–5), and time (18 intervals per d) was performed (three-way ANOVA, factor genotype, time, p < 0.0001; factor day, p = 0.0003; interaction genotype × day, p = 0.0020; genotype × time, p = 0.040; time × day, p = 0.10; genotype × day × time, p = 0.10). Although the time course of NREMS delta power did not differ among the three genotypes, the overall dynamic range was smaller in 1b−/− and larger in 1a−/− mice compared with WT mice (Tukey's tests, p < 0.05). For NREMS delta power, differences among drug days were observed, but not in a dose-dependent manner (Tukey's test, 150 = saline = 50 = 100 > 50 = 100 = 300 mg/kg). B, Delta power during BAC-induced state (black triangles) did not increase with dose in WT mice (one-way ANOVA, p = 0.64). BAC affected the time course of delta power in NREMS [circles; two-way ANOVA factor day (1–4), p = 0.079; factor time (18 intervals per d), p < 0.0001; interaction, p < 0.0001]. A large increase in NREMS delta power occurred after the BAC-induced state, followed by a decrease below saline levels during the subsequent dark period (white triangles mark significant differences from saline; Dunnett's two-tailed t test, p < 0.05). Note the dose-dependent decrease in delta power during the dark period (one-way ANOVA, factor day, p < 0.0001; saline > 5 = 7.5 > 7.5 = 10 mg/kg; Tukey's test, p < 0.05).
Similar to the analysis of delta power, we contrasted the full EEG spectra during the drug-induced state to the EEG spectra obtained during NREMS over the last 4 h of the baseline rest period. In addition, because, like GBL and BAC, SD also increased delta power (Fig. 2B), we compared the drug-induced state EEG spectra to the EEG spectra obtained during NREMS after 6 h SD. Spectral analyses revealed that the abnormal EEG activity following the injection of the highest GBL dose (300 mg/kg) (Fig. 4B) was due to a large increase of EEG activity in the low delta frequencies (0.75–1.5 Hz) reaching three- to fourfold higher levels than those reached after BAC and saline injections and ∼1.5-fold higher compared with the effects of SD (Fig. 6A). An equally large suppression of EEG activity was observed at frequencies >3 Hz with the largest reduction reached at ∼13 Hz (Fig. 6A). The GBL effects on the EEG spectra were dose-dependent (two-way ANOVA in WT mice: factor dose, p < 0.0001; factor bin, p < 0.0001; interaction, p < 0.0001) with a progressive increase with dose in the low delta frequencies (0.75–1.75 Hz) and a decrease with dose for frequencies >3 Hz (analyses not shown).
Figure 6.
EEG spectra during and after the drug-induced state for the highest doses of BAC (10 mg/kg) and GBL (300 mg/kg). All spectra (0.75–90 Hz; at 0.25 Hz bins) were expressed as a percentage of the NREMS EEG spectrum averaged over the last 4 h of the baseline rest period, thereby allowing direct comparison among genotypes, drugs, and conditions. A, BAC- and GBL-induced state EEG spectra in WT mice (blue and red lines, respectively). For comparison, EEG spectra during the first 20 min of NREMS after 6 h sleep deprivation (SD; black) and after saline administration (gray line) were included. Spectra significantly differed among conditions (two-way ANOVA for factors condition, bin, and interaction, p < 0.0001). Horizontal colored lines indicate frequency bins in which EEG power significantly differed (GBL vs SD, red; BAC vs SD, blue; GBL vs BAC, black; Tukey's test, p < 0.05). The GBL-induced state EEG spectrum differed strongly from that of the BAC-induced state, especially in the low delta (0.75–1.75 Hz) frequencies and for frequencies >3 Hz. B, GBL-induced state spectra in 1a−/−, 1b−/−, and WT mice (light gray, dark gray, and black lines, respectively; WT same as in A). Spectra significantly differed among genotypes (two-way ANOVA for factors genotype, bin, and interaction, p < 0.0001). Horizontal colored lines mark frequency bins in which genotypes differed (1a−/− vs 1b−/−, black; 1b−/− vs WT, dark gray; 1a−/− vs WT, light gray; Tukey's test, p < 0.05). EEG changes in 1b−/− mice closely resembled those of WT. C, EEG spectra during the first 20 min of NREMS after GBL- (red) and BAC- (blue) induced state and after SD (black) and saline (gray line) in WT mice. SD and saline spectra same as in A. Spectra were affected by condition (two-way ANOVA for factors condition, bin, and interaction, p < 0.0001; Tukey's test for genotype, p < 0.05), largely due to the low spectral values reached after GBL in frequencies <7 Hz. Statistics and color coding as in A. D, EEG spectra during the first 20 min of NREMS after GBL-induced state in 1a−/−, 1b−/−, and WT mice (color coding as in B). Spectra significantly differed among genotypes (two-way ANOVA for factors genotype, bin, and interaction, p < 0.0001; statistics and color coding as in B).
EEG spectra during the BAC-induced state revealed that only fast delta activity (4–5.25 Hz) contributed to the slow waves induced by this drug (Fig. 6A). Although the increase in this frequency range was similar to the increase observed in the NREMS EEG after SD, the effect of SD also included slower delta oscillations (Fig. 6A). EEG activity in higher frequencies (10.75–37.5 Hz), encompassing the sigma and beta ranges, was clearly reduced by BAC compared with the NREMS spectrum after saline injection (Fig. 6A and data not shown).
Genotype affected the drug-induced changes in EEG spectra. The most salient of these genotype differences are illustrated for the highest dose of GBL (300 mg/kg) in Figure 6B. In 1a−/− mice, the increase in low frequencies during the GBL-induced state was more pronounced compared with WT and 1b−/− mice. Moreover, the decrease in EEG power density for frequencies >3 Hz, equally observed in 1b−/− and WT mice, was less pronounced in 1a−/− mice. In general, 1b−/− mice displayed a GBL EEG signature very similar to that observed in WT mice (Fig. 6B). The same held true for the EEG spectra during subsequent NREMS (Fig. 6D).
BAC induces hypersomnia similar to that observed after sleep deprivation
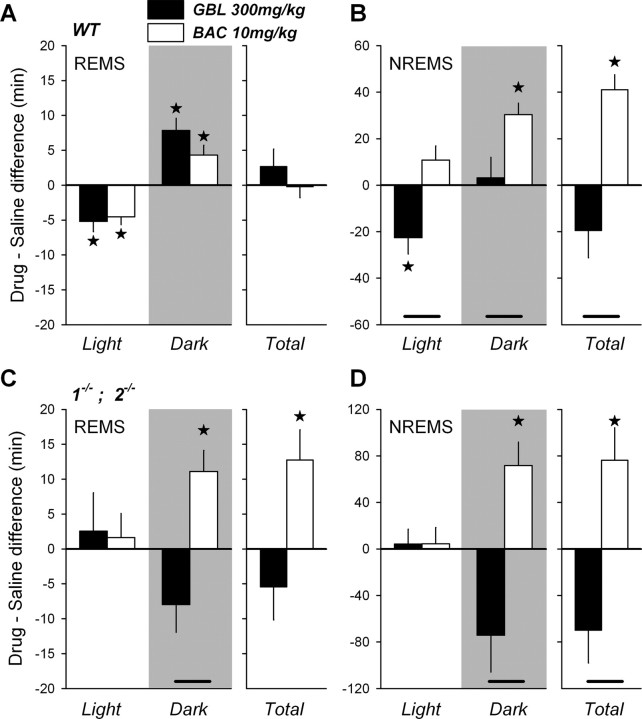
After the acute effects of the drugs on behavior and the EEG waned, normal behavioral states could again be assigned. We quantified the longer-term effects of both drugs on sleep and the EEG in WT as well as in 1−/− and 2−/− mice. Compared with individually matched recording periods after saline injections, both BAC and GBL initially suppressed REMS. This loss in REMS time was fully compensated over the course of the final 12 h of the recording period in WT mice (Fig. 7A). The effect on NREMS amount importantly differed between the two drugs, illustrated for the highest doses of BAC and GBL in Fig. 7B. Over the entire recovery period after BAC injection, mice spent 40 min more in NREMS than calculated over the same period after saline injection (Fig. 7B). Especially during the dark period, extra NREMS was accumulated. In stark contrast, GBL was followed by an immediate decrease of NREMS in the recovery light period (Fig. 7B) (Meerlo et al., 2004). During the subsequent dark period, no differences in NREMS time were observed. As a result of these opposing drug effects, at the end of the recording period, WT mice treated with BAC gained 1.0 h of NREMS compared with WT mice treated with GBL, indicating that BAC induced a long-term hypersomnia (one-way ANOVA, p = 0.0005) (Fig. 7A,B). Interestingly, hypersomnia was also observed after BAC administration in 1−/− and 2−/− mice now concerning both NREMS and REMS (Fig. 7C,D). This indicates that, in contrast to the acute effects of BAC, BAC-induced hypersomnia might not be mediated through GABAB receptors. In addition to sleep amounts, we also quantified the distribution and consolidation of sleep but did not observe significant changes in sleep fragmentation after any dose of GBL or BAC compared with saline conditions (data not shown).
Figure 7.
A–D, Drug–saline differences in NREMS and REMS length (mean ± SEM), counted from the end of drug-induced state in WT mice (A, B) or from the time of injection in 1−/− and 2−/− mice (C, D), to the end of the following dark period. Drug effects are shown only for the highest dose of GBL (300 mg/kg) and BAC (10 mg/kg). A, Both drugs decreased REMS during the remainder of the light period (Light) in WT mice (n = 8), a decrease that was compensated for during the subsequent dark period (Dark; gray area), resulting in no overall difference (Total). B, During the light period, NREMS amount significantly decreased only after GBL. In the subsequent dark period NREMS, BAC increased NREMS compared with saline, resulting in a large overall increase (hypersomnia). C, D, Although neither drug affected REMS (C) or NREMS (D) during the light period in 1−/− and 2−/− mice, in the subsequent dark period, BAC surprisingly increased both sleep states whereas GBL tended to decrease sleep. Over the entire 18 h following drug injection (Total), BAC strongly increased REMS and NREMS. Note that results from 1−/− and 2−/− mice were pooled (n = 3 per genotype per group), as no genotype differences were observed. Stars mark statistical differences from saline (one-way ANOVA, p < 0.05; paired t test, p < 0.05). Significant differences between drugs are shown by connecting lines (one-way ANOVA, p < 0.05; Tukey's test, p < 0.05).
Recovery from drug effects was also assessed at the level of delta power in NREMS. Despite the pronounced increase in EEG delta power during the GBL-induced state (Figs. 5A, 6A), the time course of delta activity during subsequent recovery sleep remained unaffected in the genotypes tested (i.e., 1a−/−, 1b−/−, and WT) (Fig. 5A). As expected, GBL also failed to alter the time course of EEG delta power during NREMS in 1−/− and 2−/− mice (data not shown). In stark contrast to the lack of an effect of GBL, the BAC-induced state was followed by an immediate increase in NREMS delta power, independent of dose (Fig. 5B). Delta power quickly decreased in the presence of NREMS and, in the dark period, values below those obtained during the same period after saline injection were reached. This decrease became more pronounced with increasing dose (one-way ANOVA factor dose, p < 0.0001; Tukey's test, saline > 5 = 7.5 > 7.5 = 10 mg/kg), consistent with the dose-dependent BAC-induced increase in NREMS time during the dark period (data not shown). In contrast to the BAC-induced hypersomnia (see above), the BAC-induced increase in delta power during NREMS was not observed in 1−/− and 2−/− mice (data not shown), suggesting that only the latter effect involves the GABAB receptor. The effects of BAC in WT mice on NREMS time and especially on the dynamics of delta power are very similar to the effects of SD. The similarity between the EEG effects of BAC and SD were not restricted to the delta frequencies. EEG spectra calculated over the first 20 min of NREMS following the BAC-induced state and SD were similar over a broad frequency range and differed only in the low delta frequencies (1–2 Hz) (Fig. 6C).
Discussion
We studied the role of GABAB receptors in sleep in mice lacking functional GABAB receptors or one of the two GABAB1 receptor isoforms. We identified a number of sleep and EEG phenotypes under baseline conditions and after the administration of GABAB-receptor agonists that not only separated 1−/− and 2−/− mice from 1a−/−, 1b−/−, and WT mice but also 1a−/− from 1b−/− and WT mice. Among the most salient phenotypes we observed in 1−/− and 2−/− mice were the presence of clonic seizures, the marked delay in the distribution of sleep over the 24 h day, the altered spectral composition of the NREMS and waking EEG, and the complete lack of the acute response to GBL and BAC. 1a−/− mice differed from 1b−/− and WT mice in that they showed seizures, their sleep was more fragmented and more prevalent in the second half of the light period, and they responded with a larger increase in EEG delta power after GBL administration. For several sleep and EEG phenotypes, 1a−/− thus seemed intermediate between 1b−/− and WT mice on one hand, and 1−/− and 2−/− mice on the other, suggesting functional differences between the two GABAB1 receptor isoforms. These differences are likely to be due to differential subcellular localizations of the two isoforms because binding pharmacology showed similar properties (Pérez-Garci et al., 2006; Vigot et al., 2006).
The GABAB1a receptor subunit protect against seizures
Spontaneous epileptiform activity has been reported in mice lacking functional GABAB receptors (Schuler et al., 2001; Gassmann et al., 2004). We discovered that mice lacking subunit GABAB1a also displayed spontaneous seizures, indicating a specific role for GABAB1a subunit in preventing seizures. GABAB1a and GABAB1b subunits localize to distinct synaptic sites, thereby conveying separate functions. Of relevance for the epileptiform trait is the fact that at hippocampal synapses, GABAB1a,2 receptors inhibit glutamate release, whereas GABAB1b,2 receptors predominantly mediate postsynaptic inhibition (Vigot et al., 2006). The lack of presynaptic inhibition of glutaminergic neurons in 1a−/− mice might have contributed to the presence of seizures. Functional differences between these two subunits might also have contributed to the sleep and EEG genotype differences we report here.
GABAB receptors determine the diurnal organization of sleep
The distribution of sleep and wakefulness over the 24 h day markedly differed among genotypes. BALB/c and BALB/cByJ mice initiated their main rest period in the middle of the dark period (Fig. 1) (Franken et al., 1999; Shimomura et al., 2001), whereas the rest period in 1−/− and 2−/− mice coincided largely with the light period, which is common for most other inbred strains. We and others attributed the earlier rest onset and resulting compression of the active period to the shorter endogenous circadian period length observed in BALB/c mice (Schwartz and Zimmerman, 1990; Franken et al., 1999; Shimomura et al., 2001). Several studies implicate GABAB receptors in circadian timing. Activation of GABAB receptors in the suprachiasmatic nucleus (SCN), the master circadian clock, phase-shifts circadian rhythms both in vitro and in vivo (Biggs and Prosser, 1998; Novak et al., 2004), and the effects of light on circadian phase are blocked by BAC (Ralph and Menaker, 1989; Crosio et al., 2000). It remains to be established whether the large delay in the timing of the rest period we report here is due to a role of GABAB receptors at the level of the (light) input to the SCN or at the level of rhythm generation itself.
GABAB receptor agonists do not promote physiological sleep
The lack of any behavioral and EEG effects of GBL in 1−/− and 2−/− mice clearly indicates that exogenous GHB acts via GABAB receptors only. A similar lack of effect in 1−/− mice has been reported for other variables, such as the GHB-induced decrease in locomotor activity and hypothermia (Kaupmann et al., 2003; Quéva et al., 2003). Our behavioral and EEG observations show that GBL does not induce physiological sleep, but a subanesthetic state with EEG hypersynchrony consistent with reports by others (Godschalk et al., 1977; Meerlo et al., 2004). Also, BAC did not initially induce physiological sleep and its acute effects in WT mice had some similarities with the acute effects of GBL. However, BAC even at the highest dose, failed to induce the spiky EEG pattern characteristic of the GBL-induced state, whereas the amount of the drug-induced state was comparable between the two drugs. First evidence of spiky EEG patterns appeared at an extremely high BAC dose (50 mg/kg), but at this dose the drug-induced state lasted ∼5 h (data not shown), demonstrating that the drug dynamics for EEG and behavioral aspects greatly differ.
Delta power during NREMS is in a quantitative and predictive relationship with prior wakefulness and is therefore thought to reflect a need or pressure for NREMS and its underlying homeostatically regulated recovery process (Franken et al., 2001). Delta power during NREMS is also considered a measure of the efficiency with which sleep need decreases during NREMS (Dijk and Beersma, 1989; Borbély and Achermann, 1999). The profound increase in EEG delta activity during the GBL-induced state did not affect the dynamics of delta power in subsequent NREMS, indicating that functionally, GBL-induced delta oscillations differ from those expressed during physiological NREMS.
The changes evoked by BAC on subsequent NREMS were even more remarkable than the lack of response observed after GBL; delta power increased and the subsequent recovery dynamics were highly similar to those observed after SD. This similarity was true for the entire NREMS EEG spectrum, supporting the puzzling conclusion that the BAC-induced state is functionally similar to intense wakefulness. Nevertheless, we cannot rule out that the increase in delta power is a residual direct effect of BAC on EEG synchronization rather than reflecting increased homeostatic drive. Also, the pattern of NREMS recovery, with its largest increase in the dark period, is reminiscent of the effect of SD (Franken et al., 1999). This delayed hypersomnia was also observed in 1−/− and 2−/− mice, suggesting that this aspect of the sleep response is most probably not mediated through GABAB receptors. Studies in human subjects reported a BAC-induced increase in NREMS (Guilleminault and Flagg, 1984; Finnimore et al., 1995) and somnolence as a side effect (Hulme et al., 1985; Huang and Guilleminault, 2009). In contrast and similar to our findings in mice, GHB given at night did not increase total sleep time in healthy men or narcolepsy patients (Van Cauter et al., 1997; Mamelak et al., 2004) and did not induce daytime somnolence and, importantly, reduces excessive daytime sleepiness in narcolepsy patients (Black and Houghton, 2006).
Although GABAB receptors mediate the acute effects of both GBL/GHB and BAC and the two drugs have several effects in common (e.g., hypothermia, catalepsy, sedation) (van Nieuwenhuijzen et al., 2009), the underlying mechanisms may not be identical (Koek et al., 2005, 2007). For instance, in mice, NMDA receptor antagonists enhanced the cataleptic effects of GHB but not those of BAC (Koek and France, 2008), suggesting a differential role of glutamate in GABAB receptor-mediated effects of GHB and BAC. Moreover, BAC inhibited both dopaminergic and GABAergic neurons in the ventral tegmental area, whereas GHB inhibited only GABAergic (Cruz et al., 2004). This discrepancy may be explained by the fact that GHB is a full, low-affinity agonist and BAC a full, high-affinity agonist of GABAB receptors (Lingenhoehl et al., 1999). Thus, low-affinity compounds can have very different or even opposite effects compared with high-affinity agonists. These differences in drug kinetics could be further modulated by potassium channel tetramerization domain-containing proteins that function as auxiliary subunits of GABAB receptors (Schwenk et al., 2010).
Conclusions
It is believed that GHB, by consolidating sleep and promoting EEG delta oscillations, reduces excessive daytime sleepiness and cataplexy associated with narcolepsy. Although it has been reported that GHB consolidates sleep in narcolepsy patients (Black et al., 2009) and that BAC promotes sleep efficiency in healthy subjects (Finnimore et al., 1995), we found no evidence for increased sleep consolidation after GBL or BAC in mice. Given the contradictory effects of both drugs on EEG and sleep among the various studies, species differences and potentially the dose used might play a role. Our in-depth quantitative EEG analyses show that, at least in the mouse, GBL and BAC do not promote physiological sleep at the doses used and that delta oscillations during the drug-induced state functionally differ from those during NREMS. We further identified several functional differences between the two GABAB1 isoforms, the most salient of which concerns the role of the GABAB1a subunit epileptogenesis and sleep consolidation. Finally, BAC, but not GHB, seems to mobilize a sleep homeostatic mechanism comprised of hypersomnia and increased EEG delta power. Identifying the cellular mechanism contributing to this differential response might give insight into the elusive sleep homeostatic process.
Footnotes
This work was supported by Swiss National Science Foundation Grants 3100A0-108478 (to M.T.) and 3100A0-117816 (B.B), and by the state of Vaud, Switzerland. We thank Martin Gassmann for his excellent help with the GABAB knock-out mice, Pich Moly Sun for her invaluable contribution to the baclofen experiment, and Yann Emmenegger for his generous technical support.
References
- Bessman SP, Fishbein WN. Gamma-hydroxybutyrate, a normal brain metabolite. Nature. 1963;200:1207–1208. doi: 10.1038/2001207a0. [DOI] [PubMed] [Google Scholar]
- Biggs KR, Prosser RA. GABAB receptor stimulation phase-shifts the mammalian circadian clock in vitro. Brain Res. 1998;807:250–254. doi: 10.1016/s0006-8993(98)00820-8. [DOI] [PubMed] [Google Scholar]
- Black J, Houghton WC. Sodium oxybate improves excessive daytime sleepiness in narcolepsy. Sleep. 2006;29:939–946. doi: 10.1093/sleep/29.7.939. [DOI] [PubMed] [Google Scholar]
- Black J, Pardi D, Hornfeldt CS, Inhaber N. The nightly administration of sodium oxybate results in significant reduction in the nocturnal sleep disruption of patients with narcolepsy. Sleep Med. 2009;10:829–835. doi: 10.1016/j.sleep.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Borbély AA, Achermann P. Sleep homeostasis and models of sleep regulation. J Biol Rhythms. 1999;14:557–568. doi: 10.1177/074873099129000894. [DOI] [PubMed] [Google Scholar]
- Carai MA, Lobina C, Maccioni P, Cabras C, Colombo G, Gessa GL. Gamma-aminobutyric acidB (GABAB)-receptor mediation of different in vivo effects of gamma-butyrolactone. J Pharmacol Sci. 2008;106:199–207. doi: 10.1254/jphs.fp0071487. [DOI] [PubMed] [Google Scholar]
- Carter LP, Koek W, France CP. Lack of effects of GHB precursors GBL and 1,4-BD following i.c.v. administration in rats. Eur J Neurosci. 2006;24:2595–2600. doi: 10.1111/j.1460-9568.2006.05146.x. [DOI] [PubMed] [Google Scholar]
- Cash CD. Gamma-hydroxybutyrate: an overview of the pros and cons for it being a neurotransmitter and/or a useful therapeutic agent. Neurosci Biobehav Rev. 1994;18:291–304. doi: 10.1016/0149-7634(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Castelli MP, Pibiri F, Carboni G, Piras AP. A review of pharmacology of NCS-382, a putative antagonist of gamma-hydroxybutyric acid (GHB) receptor. CNS Drug Rev. 2004;10:243–260. doi: 10.1111/j.1527-3458.2004.tb00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosio C, Cermakian N, Allis CD, Sassone-Corsi P. Light induces chromatin modification in cells of the mammalian circadian clock. Nat Neurosci. 2000;3:1241–1247. doi: 10.1038/81767. [DOI] [PubMed] [Google Scholar]
- Cruz HG, Ivanova T, Lunn ML, Stoffel M, Slesinger PA, Lüscher C. Bi-directional effects of GABA(B) receptor agonists on the mesolimbic dopamine system. Nat Neurosci. 2004;7:153–159. doi: 10.1038/nn1181. [DOI] [PubMed] [Google Scholar]
- Darbari FP, Melvin JJ, Piatt JH, Jr, Adirim TA, Kothare SV. Intrathecal baclofen overdose followed by withdrawal: clinical and EEG features. Pediatr Neurol. 2005;33:373–377. doi: 10.1016/j.pediatrneurol.2005.05.017. [DOI] [PubMed] [Google Scholar]
- de Fiebre CM, de Fiebre NE, Coleman SL, Forster MJ. Comparison of the actions of gamma-butyrolactone and 1,4-butanediol in Swiss-Webster mice. Pharmacol Biochem Behav. 2004;77:705–710. doi: 10.1016/j.pbb.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Beersma DG. Effects of SWS deprivation on subsequent EEG power density and spontaneous sleep duration. Electroencephalogr Clin Neurophysiol. 1989;72:312–320. doi: 10.1016/0013-4694(89)90067-9. [DOI] [PubMed] [Google Scholar]
- Doherty JD, Hattox SE, Snead OC, Roth RH. Identification of endogenous gamma-hydroxybutyrate in human and bovine brain and its regional distribution in human, guinea pig and rhesus monkey brain. J Pharmacol Exp Ther. 1978;207:130–139. [PubMed] [Google Scholar]
- Filip M, Frankowska M. GABA(B) receptors in drug addiction. Pharmacol Rep. 2008;60:755–770. [PubMed] [Google Scholar]
- Finnimore AJ, Roebuck M, Sajkov D, McEvoy RD. The effects of the GABA agonist, baclofen, on sleep and breathing. Eur Respir J. 1995;8:230–234. doi: 10.1183/09031936.95.08020230. [DOI] [PubMed] [Google Scholar]
- Franken P, Malafosse A, Tafti M. Genetic variation in EEG activity during sleep in inbred mice. Am J Physiol. 1998;275:R1127–R1137. doi: 10.1152/ajpregu.1998.275.4.R1127. [DOI] [PubMed] [Google Scholar]
- Franken P, Malafosse A, Tafti M. Genetic determinants of sleep regulation in inbred mice. Sleep. 1999;22:155–169. [PubMed] [Google Scholar]
- Franken P, Chollet D, Tafti M. The homeostatic regulation of sleep need is under genetic control. J Neurosci. 2001;21:2610–2621. doi: 10.1523/JNEUROSCI.21-08-02610.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DE, Hornfeldt CS. From club drug to orphan drug: sodium oxybate (Xyrem) for the treatment of cataplexy. Pharmacotherapy. 2003;23:1205–1209. doi: 10.1592/phco.23.10.1205.32756. [DOI] [PubMed] [Google Scholar]
- Gambardella A, Manna I, Labate A, Chifari R, La Russa A, Serra P, Cittadella R, Bonavita S, Andreoli V, LePiane E, Sasanelli F, Di Costanzo A, Zappia M, Tedeschi G, Aguglia U, Quattrone A. GABA(B) receptor 1 polymorphism (G1465A) is associated with temporal lobe epilepsy. Neurology. 2003;60:560–563. doi: 10.1212/01.wnl.0000046520.79877.d8. [DOI] [PubMed] [Google Scholar]
- Gassmann M, Shaban H, Vigot R, Sansig G, Haller C, Barbieri S, Humeau Y, Schuler V, Müller M, Kinzel B, Klebs K, Schmutz M, Froestl W, Heid J, Kelly PH, Gentry C, Jaton AL, Van der Putten H, Mombereau C, Lecourtier L, et al. Redistribution of GABAB(1) protein and atypical GABAB responses in GABAB(2)-deficient mice. J Neurosci. 2004;24:6086–6097. doi: 10.1523/JNEUROSCI.5635-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godschalk M, Dzoljic MR, Bonta IL. Slow wave sleep and a state resembling absence epilepsy induced in the rat by gamma-hydroxybutyrate. Eur J Pharmacol. 1977;44:105–111. doi: 10.1016/0014-2999(77)90096-6. [DOI] [PubMed] [Google Scholar]
- Guilleminault C, Flagg W. Effect of baclofen on sleep-related periodic leg movements. Ann Neurol. 1984;15:234–239. doi: 10.1002/ana.410150304. [DOI] [PubMed] [Google Scholar]
- Hasan S, Pradervand S, Ahnaou A, Drinkenburg W, Tafti M, Franken P. How to keep the brain awake? The complex molecular pharmacogenetics of wake promotion. Neuropsychopharmacology. 2009;34:1625–2640. doi: 10.1038/npp.2009.3. [DOI] [PubMed] [Google Scholar]
- Huang YS, Guilleminault C. Narcolepsy: action of two gamma-aminobutyric acid type B agonists, baclofen and sodium oxybate. Pediatr Neurol. 2009;41:9–16. doi: 10.1016/j.pediatrneurol.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Huguenard JR, Prince DA. Intrathalamic rhythmicity studied in vitro: nominal T-current modulation causes robust antioscillatory effects. J Neurosci. 1994;14:5485–5502. doi: 10.1523/JNEUROSCI.14-09-05485.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulme A, MacLennan WJ, Ritchie RT, John VA, Shotton PA. Baclofen in the elderly stroke patient its side-effects and pharmacokinetics. Eur J Clin Pharmacol. 1985;29:467–469. doi: 10.1007/BF00613463. [DOI] [PubMed] [Google Scholar]
- Jacobson LH, Bettler B, Kaupmann K, Cryan JF. Behavioral evaluation of mice deficient in GABA(B(1)) receptor isoforms in tests of unconditioned anxiety. Psychopharmacology (Berl) 2007a;190:541–553. doi: 10.1007/s00213-006-0631-9. [DOI] [PubMed] [Google Scholar]
- Jacobson LH, Kelly PH, Bettler B, Kaupmann K, Cryan JF. Specific roles of GABA(B(1)) receptor isoforms in cognition. Behav Brain Res. 2007b;181:158–162. doi: 10.1016/j.bbr.2007.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen K, Mody I. GHB depresses fast excitatory and inhibitory synaptic transmission via GABA(B) receptors in mouse neocortical neurons. Cereb Cortex. 2001;11:424–429. doi: 10.1093/cercor/11.5.424. [DOI] [PubMed] [Google Scholar]
- Juhász G, Emri Z, Kékesi KA, Salfay O, Crunelli V. Blockade of thalamic GABAB receptors decreases EEG synchronization. Neurosci Lett. 1994;172:155–158. doi: 10.1016/0304-3940(94)90685-8. [DOI] [PubMed] [Google Scholar]
- Kaupmann K, Cryan JF, Wellendorph P, Mombereau C, Sansig G, Klebs K, Schmutz M, Froestl W, van der Putten H, Mosbacher J, Bräuner-Osborne H, Waldmeier P, Bettler B. Specific gamma-hydroxybutyrate-binding sites but loss of pharmacological effects of gamma-hydroxybutyrate in GABA(B)(1)-deficient mice. Eur J Neurosci. 2003;18:2722–2730. doi: 10.1111/j.1460-9568.2003.03013.x. [DOI] [PubMed] [Google Scholar]
- Kim U, Sanchez-Vives MV, McCormick DA. Functional dynamics of GABAergic inhibition in the thalamus. Science. 1997;278:130–134. doi: 10.1126/science.278.5335.130. [DOI] [PubMed] [Google Scholar]
- Koek W, France CP. Cataleptic effects of gamma-hydroxybutyrate (GHB) and baclofen in mice: mediation by GABA(B) receptors, but differential enhancement by N-methyl-d-aspartate (NMDA) receptor antagonists. Psychopharmacology (Berl) 2008;199:191–198. doi: 10.1007/s00213-008-1160-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koek W, Carter LP, Lamb RJ, Chen W, Wu H, Coop A, France CP. Discriminative stimulus effects of gamma-hydroxybutyrate (GHB) in rats discriminating GHB from baclofen and diazepam. J Pharmacol Exp Ther. 2005;314:170–179. doi: 10.1124/jpet.105.083394. [DOI] [PubMed] [Google Scholar]
- Koek W, Mercer SL, Coop A. Cataleptic effects of gamma-hydroxybutyrate (GHB), its precursor gamma-butyrolactone (GBL), and GABAB receptor agonists in mice: differential antagonism by the GABAB receptor antagonist CGP35348. Psychopharmacology (Berl) 2007;192:407–414. doi: 10.1007/s00213-007-0718-y. [DOI] [PubMed] [Google Scholar]
- Laborit H. Sodium 4-hydroxybutyrate. Int J Neuropharmacol. 1964;3:433–451. doi: 10.1016/0028-3908(64)90074-7. [DOI] [PubMed] [Google Scholar]
- Laborit H, Jouany JM, Gerard J, Fabiani F. Summary of an experimental and clinical study on a metabolic substrate with inhibitory central action: sodium 4-hydroxybutyrate. Presse Med. 1960;68:1867–1869. [PubMed] [Google Scholar]
- Lapierre O, Montplaisir J, Lamarre M, Bedard MA. The effect of gamma-hydroxybutyrate on nocturnal and diurnal sleep of normal subjects: further considerations on REM sleep-triggering mechanisms. Sleep. 1990;13:24–30. doi: 10.1093/sleep/13.1.24. [DOI] [PubMed] [Google Scholar]
- Levin ED, Weber E, Icenogle L. Baclofen interactions with nicotine in rats: effects on memory. Pharmacol Biochem Behav. 2004;79:343–348. doi: 10.1016/j.pbb.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Lingenhoehl K, Brom R, Heid J, Beck P, Froestl W, Kaupmann K, Bettler B, Mosbacher J. Gamma-hydroxybutyrate is a weak agonist at recombinant GABA(B) receptors. Neuropharmacology. 1999;38:1667–1673. doi: 10.1016/s0028-3908(99)00131-8. [DOI] [PubMed] [Google Scholar]
- Maitre M. The gamma-hydroxybutyrate signalling system in brain: organization and functional implications. Prog Neurobiol. 1997;51:337–361. doi: 10.1016/s0301-0082(96)00064-0. [DOI] [PubMed] [Google Scholar]
- Mamelak M, Escriu JM, Stokan O. The effects of gamma-hydroxybutyrate on sleep. Biol Psychiatry. 1977;12:273–288. [PubMed] [Google Scholar]
- Mamelak M, Black J, Montplaisir J, Ristanovic R. A pilot study on the effects of sodium oxybate on sleep architecture and daytime alertness in narcolepsy. Sleep. 2004;27:1327–1334. doi: 10.1093/sleep/27.7.1327. [DOI] [PubMed] [Google Scholar]
- Matsuki T, Nomiyama M, Takahira H, Hirashima N, Kunita S, Takahashi S, Yagami K, Kilduff TS, Bettler B, Yanagisawa M, Sakurai T. Selective loss of GABA(B) receptors in orexin-producing neurons results in disrupted sleep/wakefulness architecture. Proc Natl Acad Sci U S A. 2009;106:4459–4464. doi: 10.1073/pnas.0811126106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerlo P, Westerveld P, Turek FW, Koehl M. Effects of gamma-hydroxybutyrate (GHB) on vigilance states and EEG in mice. Sleep. 2004;27:899–904. doi: 10.1093/sleep/27.5.899. [DOI] [PubMed] [Google Scholar]
- Mombereau C, Kaupmann K, Froestl W, Sansig G, van der Putten H, Cryan JF. Genetic and pharmacological evidence of a role for GABA(B) receptors in the modulation of anxiety- and antidepressant-like behavior. Neuropsychopharmacology. 2004;29:1050–1062. doi: 10.1038/sj.npp.1300413. [DOI] [PubMed] [Google Scholar]
- Monti JM, Altier H, D'Angelo L. The effects of the combined administration of gamma-hydroxybutyrate and diazepam on sleep parameters in the rat. J Neural Transm. 1979;45:177–183. doi: 10.1007/BF01250092. [DOI] [PubMed] [Google Scholar]
- Novak CM, Ehlen JC, Huhman KL, Albers HE. GABA(B) receptor activation in the suprachiasmatic nucleus of diurnal and nocturnal rodents. Brain Res Bull. 2004;63:531–535. doi: 10.1016/j.brainresbull.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Pérez-Garci E, Gassmann M, Bettler B, Larkum ME. The GABAB1b isoform mediates long-lasting inhibition of dendritic Ca2+ spikes in layer 5 somatosensory pyramidal neurons. Neuron. 2006;50:603–616. doi: 10.1016/j.neuron.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Quéva C, Bremner-Danielsen M, Edlund A, Ekstrand AJ, Elg S, Erickson S, Johansson T, Lehmann A, Mattsson JP. Effects of GABA agonists on body temperature regulation in GABA(B(1))−/− mice. Br J Pharmacol. 2003;140:315–322. doi: 10.1038/sj.bjp.0705447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph MR, Menaker M. GABA regulation of circadian responses to light. I. Involvement of GABAA-benzodiazepine and GABAB receptors. J Neurosci. 1989;9:2858–2865. doi: 10.1523/JNEUROSCI.09-08-02858.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DC. Preclinical evidence for GABAB agonists as a pharmacotherapy for cocaine addiction. Physiol Behav. 2005;86:18–20. doi: 10.1016/j.physbeh.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Roth RH, Delgado JM, Giarman NJ. Gamma-butyrolactone and gamma-hydroxybutyric acid. II. The pharmacologically active form. Int J Neuropharmacol. 1966;5:421–428. doi: 10.1016/0028-3908(66)90007-4. [DOI] [PubMed] [Google Scholar]
- Roth RH, Levy R, Giarman NJ. Dependence of rat serum lactonase upon calcium. Biochem Pharmacol. 1967;16:596–598. doi: 10.1016/0006-2952(67)90110-4. [DOI] [PubMed] [Google Scholar]
- Rubin BA, Giarman NJ. The therapy of experimental influenza in mice with antibiotic lactones and related compounds. Yale J Biol Med. 1947;19:1017–1022. [PMC free article] [PubMed] [Google Scholar]
- Schuler V, Lüscher C, Blanchet C, Klix N, Sansig G, Klebs K, Schmutz M, Heid J, Gentry C, Urban L, Fox A, Spooren W, Jaton AL, Vigouret J, Pozza M, Kelly PH, Mosbacher J, Froestl W, Käslin E, Korn R, et al. Epilepsy, hyperalgesia, impaired memory, and loss of pre- and postsynaptic GABA(B) responses in mice lacking GABA(B(1)) Neuron. 2001;31:47–58. doi: 10.1016/s0896-6273(01)00345-2. [DOI] [PubMed] [Google Scholar]
- Schwartz WJ, Zimmerman P. Circadian timekeeping in BALB/c and C57BL/6 inbred mouse strains. J Neurosci. 1990;10:3685–3694. doi: 10.1523/JNEUROSCI.10-11-03685.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenk J, Metz M, Zolles G, Turecek R, Fritzius T, Bildl W, Tarusawa E, Kulik A, Unger A, Ivankova K, Seddik R, Tiao JY, Rajalu M, Trojanova J, Rohde V, Gassmann M, Schulte U, Fakler B, Bettler B. Native GABA(B) receptors are heteromultimers with a family of auxiliary subunits. Nature. 2010;465:231–235. doi: 10.1038/nature08964. [DOI] [PubMed] [Google Scholar]
- Shimomura K, Low-Zeddies SS, King DP, Steeves TD, Whiteley A, Kushla J, Zemenides PD, Lin A, Vitaterna MH, Churchill GA, Takahashi JS. Genome-wide epistatic interaction analysis reveals complex genetic determinants of circadian behavior in mice. Genome Res. 2001;11:959–980. doi: 10.1101/gr.171601. [DOI] [PubMed] [Google Scholar]
- Snead OC., 3rd The gamma-hydroxybutyrate model of absence seizures: correlation of regional brain levels of gamma-hydroxybutyric acid and gamma-butyrolactone with spike wave discharges. Neuropharmacology. 1991;30:161–167. doi: 10.1016/0028-3908(91)90199-l. [DOI] [PubMed] [Google Scholar]
- Stock G, Heidt H, Buss J, Schlör KH. Sleep patterns in cat induced by gammahydroxybutyric acid. Electroencephalogr Clin Neurophysiol. 1978;44:523–527. doi: 10.1016/0013-4694(78)90037-8. [DOI] [PubMed] [Google Scholar]
- Ulloor J, Mavanji V, Saha S, Siwek DF, Datta S. Spontaneous REM sleep is modulated by the activation of the pedunculopontine tegmental GABAB receptors in the freely moving rat. J Neurophysiol. 2004;91:1822–1831. doi: 10.1152/jn.01104.2003. [DOI] [PubMed] [Google Scholar]
- Ulrich D, Bettler B. GABA(B) receptors: synaptic functions and mechanisms of diversity. Curr Opin Neurobiol. 2007;17:298–303. doi: 10.1016/j.conb.2007.04.001. [DOI] [PubMed] [Google Scholar]
- U.S. Xyrem Multicenter Study Group. A 12-month, open-label, multicenter extension trial of orally administered sodium oxybate for the treatment of narcolepsy. Sleep. 2003;26:31–35. [PubMed] [Google Scholar]
- Van Cauter E, Plat L, Scharf MB, Leproult R, Cespedes S, L'Hermite-Balériaux M, Copinschi G. Simultaneous stimulation of slow-wave sleep and growth hormone secretion by gamma-hydroxybutyrate in normal young men. J Clin Invest. 1997;100:745–753. doi: 10.1172/JCI119587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lier H, Coenen AM, Drinkenburg WH. Behavioral transitions modulate hippocampal electroencephalogram correlates of open field behavior in the rat: support for a sensorimotor function of hippocampal rhythmical synchronous activity. J Neurosci. 2003;23:2459–2465. doi: 10.1523/JNEUROSCI.23-06-02459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nieuwenhuijzen PS, McGregor IS, Hunt GE. The distribution of gamma-hydroxybutyrate-induced Fos expression in rat brain: comparison with baclofen. Neuroscience. 2009;158:441–455. doi: 10.1016/j.neuroscience.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Hippocampal theta rhythm: a tag for short-term memory. Hippocampus. 2005;15:923–935. doi: 10.1002/hipo.20118. [DOI] [PubMed] [Google Scholar]
- Vickers MD. Gammahydroxybutyric acid. Int Anesthesiol Clin. 1969;7:75–89. doi: 10.1097/00004311-196900710-00007. [DOI] [PubMed] [Google Scholar]
- Vigot R, Barbieri S, Bräuner-Osborne H, Turecek R, Shigemoto R, Zhang YP, Luján R, Jacobson LH, Biermann B, Fritschy JM, Vacher CM, Müller M, Sansig G, Guetg N, Cryan JF, Kaupmann K, Gassmann M, Oertner TG, Bettler B. Differential compartmentalization and distinct functions of GABAB receptor variants. Neuron. 2006;50:589–601. doi: 10.1016/j.neuron.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmeier PC. The GABAB antagonist, CGP 35348, antagonizes the effects of baclofen, gamma-butyrolactone and HA 966 on rat striatal dopamine synthesis. Naunyn Schmiedebergs Arch Pharmacol. 1991;343:173–178. doi: 10.1007/BF00168606. [DOI] [PubMed] [Google Scholar]
- Winsky-Sommerer R. Role of GABAA receptors in the physiology and pharmacology of sleep. Eur J Neurosci. 2009;29:1779–1794. doi: 10.1111/j.1460-9568.2009.06716.x. [DOI] [PubMed] [Google Scholar]
- Xyrem International Study Group. Further evidence supporting the use of sodium oxybate for the treatment of cataplexy: a double-blind, placebo-controlled study in 228 patients. Sleep Med. 2005;6:415–421. doi: 10.1016/j.sleep.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Zarrindast M, Valizadeh S, Sahebgharani M. GABA(B) receptor mechanism and imipramine-induced antinociception in ligated and non-ligated mice. Eur J Pharmacol. 2000;407:65–72. doi: 10.1016/s0014-2999(00)00648-8. [DOI] [PubMed] [Google Scholar]