Abstract
Head and neck squamous cell carcinomas (HNSCC) includes multiple subsites that exhibit differential treatment outcome, which is in turn reflective of tumor stage/histopathology and molecular profile. This study hypothesized that the molecular profile is an accurate prognostic adjunct in patients triaged based on clinico-pathological characteristics. Towards this effect, publically available micro-array datasets (n = 8), were downloaded, classified based on HPV association (n = 83) and site (tongue n = 88; laryngopharynx n = 53; oropharynx n = 51) and re-analyzed (Genespring; v13.1). The significant genes were validated in respective cohorts in The Cancer Genome Atlas (TCGA) for correlation with clinico-pathological parameters/survival. The gene entities (n = 3258) identified from HPV based analysis, when validated in TCGA identified the subset specifically altered in HPV+ HNSCC (n = 63), with three genes showing survival impact (RPP25, NUDCD2, NOVA1). Site-specific meta-analysis identified respective differentials (tongue: 3508, laryngopharynx: 4893, oropharynx: 2386); validation in TCGA revealed markers with high incidence (altered in >10% of patients) in tongue (n = 331), laryngopharynx (n = 701) and oropharynx (n = 404). Assessment of these genes in clinical sub-cohorts of TCGA indicated that early stage tongue (MTFR1, C8ORF33, OTUD6B) and laryngeal cancers (TWISTNB, KLHL13 and UBE2Q1) were defined by distinct prognosticators. Similarly, correlation with perineural/angiolymophatic invasion, identified discrete marker panels with survival impact (tongue: NUDCD1, PRKC1; laryngopharynx: SLC4A1AP, PIK3CA, AP2M1). Alterations in ANO1, NUDCD1, PIK3CA defined survival in tongue cancer patients with nodal metastasis (node+ECS-), while EPS8 is a significant differential in node+ECS- laryngopharyngeal cancers. In oropharynx, wherein HPV is a major etiological factor, distinct prognosticators were identified in HPV+ (ECHDC2, HERC5, GGT6) and HPV- (GRB10, EMILIN1, FNDC1). Meta-analysis in combination with TCGA validation carried out in this study emphasized on the molecular heterogeneity inherent within HNSCC; the feasibility of leveraging this information for improving prognostic efficacy is also established. Subject to large scale clinical validation, the marker panel identified in this study can prove to be valuable prognostic adjuncts.
1. Introduction
Accurate molecular prognosticators predictive of survival in patients diagnosed with cancer can be an invaluable adjunct to the existing clinical and pathological parameters. In head and neck squamous cell carcinoma (HNSCC), advanced stage tumors, nodal metastasis, and presence of aggressive pathological features (perineural (PNI)/lymphovascular invasion (LVI)) are poor prognosticators indicating an increased risk of metastatic disease and reduced disease free/overall survival [1–3]. Notwithstanding the adoption of multi-modal treatment strategies based on these parameters, survival has not improved [4], indicating the need for additional and more accurate factors to improve disease management. Currently, TNM staging is a primary parameter for triaging the patients based on prognosis, however stage-independent prognostic impact attributed to the presence of pathological parameters such as PNI, angiolymphatic invasion (ALI) and LVI [5,6] indicates the need for a multi-parameter assessment. Whether additional consideration of underlying molecular parameters can further improve prognostication needs to be investigated.
Prognostic impact of the clinico-pathological parameters is site-dependent; recent studies have identified differential impact of the pathological parameters in the prognosis of tongue, buccal and laryngopharyngeal cancers possibly owing to the underlying molecular pathways involved in the carcinogenesis and progression of the disease [7–9]. Additionally, Human papilloma virus (HPV) -associated etiology is an increasingly evident prognostic factor; oropharynx being the most commonly associated site [10] [11] [12]. Among pathological parameters, nodal metastasis is a significant prognosticator in HNSCC; the primary tumor site, presence of PNI, extra-capsular spread (ECS), being additional contributing factors [2–4,13,14]. PNI is a factor that can determine lymph node involvement, recurrence, disease-free and overall survival necessitating appropriate treatment management if susceptibility is detected. Elective neck dissection is prescribed for T1/T2N0 PNI+ patients [15,16]. Additionally, PNI was identified as a prognostic factor in early stage tongue cancer, while LVI was a significant parameter in late stage buccal mucosal cancers [3]. These studies point out to etiological/biology-driven differences in the survival impact of the existing clinical and pathological parameters; identifying the underlying biological parameters may be significant in improving their prognostic impact.
Advancement in the technologies and high throughput global profiling have led to the identification of a repertoire of candidate markers that can qualify as molecular prognosticators [17–20]. These markers can be predictive (to evaluate the likelihood of benefit from a specific clinical intervention) or prognostic (to evaluate the patient’s overall outcome). A cataloguing of the candidate markers that correlated with the aetiology and pathological parameters in a site-dependent manner, might provide insights into the molecules associated with treatment response/survival in each site and will therefore be more specific. This study proposed that molecular markers can serve as accurate adjuncts to existing prognostic parameters in head and neck cancers. Towards this effect, this study planned to mine the existing high throughput data to identify a panel of potential markers associated with etiological/clinical/pathological features in the three major sites in HNSCC; oral cavity, laryngopharynx and oropharynx.
2. Materials and methods
2.1 Data mining and meta-analysis
The electronic search strategy included in the study is described as below (S1 Fig and Fig 1). Publicly available raw microarray data derived in head and neck cancer and normal sub sites of head and neck were mined from Gene Expression Omnibus (GEO) (NCBI; http://www.ncbi.nlm.nih.gov/geo/) and Array Express (EBI) [http://www.ebi.ac.uk/arrayexpress] [21]. A comprehensive search was carried out in the public databases for a period of January 2007 to December 2016. The datasets were searched using the keywords, “Head and Neck Cancer”, tongue squamous cell carcinoma, “oral tongue cancer”, “larynx”, laryngeal, “laryngeal squamous cell carcinoma”, “pharynx”, “pharyngeal”, “pharyngeal squamous carcinoma”, “oral cancer”“oropharyngeal” and oropharynx, while the search were filtered based on organism (“Homo sapiens” or “human”) and the assay systems (“RNA assay”, “array assay” or “expression profiling by array”). As a next step the identified series or datasets were screened for microarray performed by using Affymetrix Human Genome U133 plus 2.0 [Affymetrix Inc., California, USA], which is the most advanced microarray platform, with maximum number of probes. Eligible studies were finally selected based on the following inclusion and exclusion criteria for the identification of series. The inclusion criteria were i) experimental studies carried out by Affymetrix U133 plus 2.0 with available raw data ii) treatment naïve HNSCC patient’s iii) normal site of tongue, larynx pharynx and oropharynx. The exclusion criteria were i) studies carried out on HNSCC cell lines ii) studies with xenograft samples and iii) patient’s with treatment or recurrence or non-tissue sample (blood). The series selected were carried forward for meta-analysis.
Fig 1. Over all work flow of site specific meta-analysis.
Raw data (Affymetrix U133 Plus 2.0 microarray) of head and neck cancer series from publically available databases were downloaded and re-analyzed in Genespring statistical software. During analysis, post data upload onto the software, normalization (RMA) was carried out and samples grouped either into HPV positive/negative or based on sites (tongue, laryngopharyngeal and oropharyngeal cancer). In each site, further sample grouping was carried out into tumor and normal. Gene level experiment was performed in the software and PCA/clustering analysis was carried out to remove the discordant samples. Significant gene entities were identified (fold change >2.0, p-value <0.05) from each analysis/experiment. The annotation of the significant genes was carried out by TOPPFUN gene enrichment analysis (GO analysis/pathways). For validation, The Cancer Genome Atlas (TCGA) Head and Neck squamous cell carcinoma (HNSCC) cohort was classified based on HPV (HPV+/-) and site (tongue, laryngopharyngeal and oropharyngeal cancer). Patient cohorts in each site were further classified based on stage/pathological parameters and/or HPV. The significant gene entities identified from meta-analysis were validated in their respective cohorts; differential mRNA expression (z-score) and association with survival (OS/DFS) were the main endpoints.
Meta-analysis was carried out based on the stepwise protocol described previously [21] and as per the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines. Briefly, raw datasets (.CEL files) were downloaded from the database and meta-analysis carried out using the GeneSpring software (http://genespring-support.com) [v13.1, Agilent, California, USA] to avoid analytical bias from individual studies. The samples were grouped based on two parameters initially i) HPV association ii) site. For HPV based analysis, the tumor samples pertaining HPV status (positive/negative) were classified and re-analyzed as single experiment. In order to analyze individual sites (tongue, laryngopharyngeal and oropharynx), the samples were grouped into ‘tumor’ and ‘normal’ for re-analysis independently. For both the analytical pipelines, the samples were baseline transformed and normalized by Robust Multi-array Analysis (RMA). Gene level experiment was carried out by combining the arithmetic mean of all probes mapping to the same probe ID. Principal Component Analysis (PCA) was carried out for removal of outliers as a quality control check in the software (stratification of tumor and normal samples separately). Unpaired t-test (unequal variance) was carried out on the samples to obtain the significant gene entities on which fold change analysis was executed. Asymptotic p-value computation and Benjamini Hochberg FDR multiple testing correction was carried out to obtain gene entities with p-value <0.05 and fold change (FC) of > 2.0. The significant gene entities were extracted and exported to excel files for further downstream analysis.
2.2 Patient based validation in The Cancer Genome Atlas (TCGA)
Independent validation was carried out using TCGA database (http://www.cbioportal.org) [22,23]. The HNSCC patients (Provisional; n = 528 samples) in TCGA was categorized into different sites and further into sub cohorts based on the clinical information provided (HPV status, site, stage, and pathological parameters). The validation was carried out using two pipelines i) HPV associated meta-analysis gene list was validated in the HPV+ and HPV- cohort of TCGA ii) meta-analysis derived significant gene list obtained from each site was validated in the site-specific cohort in the TCGA HNSCC database. In both cases, the validation was carried out with regard to incidence of gene alteration (percentage alteration), differential profiling (mRNA expression status using z-score) and prognostic efficacy (correlation with survival) (Fig 1).
The mRNA expression z-scores (RNA Seq V2 RSEM) of significant gene entities were downloaded from TCGA HNSCC. The patient samples were categorized into HPV+/HPV- and within the sites further classified based on clinical and pathological condition. As a first tier analysis, the significance of the z-score distribution between the different groups; HPV, clinical stages (stage I-II vs stage III-IV) and pathological conditions (ALI, PNI, Node, ECS) was evaluated using the t-test unpaired unequal variance (p<0.05). The second tier analysis was carried out with the significant gene set to assess the heterogeneity of expression within each sub cohort of patient. This was assessed by evaluating the percentage of patients showing alteration in the gene (z-score threshold: ±2) in each cohort and difference in level of expression in the altered set (t-test unpaired unequal variance) (Fig 1).
Prognostic efficacy was assessed with markers identified at two levels i) mRNA z-score based differentials ii) markers with higher incidence of alterations in patients (>20%) (Fig 1). The markers were assessed for their survival impact in patients within the TCGA cohort categorized based on HPV and clinical stage (early and late stage), nodal metastasis (with and without extracapsular spread) and pathological (perineural invasion, angiolymphatic invasion, positive lymph node hematoxylin and eosin staining microscopy count, extracapsular spread pathologic) parameters within each site. For each analysis, TCGA samples without definite information for these parameters were excluded from the study. The contribution of these markers to the prognostic efficacy of these parameters (disease-free (DFS), overall survival (OS)) was assessed. The significant marker panel (p-value <0.04) were also evaluated for the prognostic efficacy in the different sub cohorts of patients in each site (Kaplan Meier plots; log rank test, p<0.05) (Fig 1).
2.3 Functional annotation of significant gene entities
Functional annotation of the significant gene entities was carried out by Gene Ontology [(GO; Biological Process (BP), Cellular Component (CC), and Molecular Function (MF)] and pathway analysis [Kyoto Encyclopedia of Genes and Genomes (KEGG), Reactome pathway] (p value: <0.05) using TOPPFUN (Transcriptome, ontology, phenotype, proteome, and pharmacome annotations based gene list functional enrichment analysis; https://toppgene.cchmc.org/enrichment.jsp) (Fig 1).
3. Results
3.1 Data mining and meta-analysis
A total of 8 datasets, wherein analysis was carried out using the Affymetrix platform (U133 plus 2.0) were included in this study (S1 File). The samples were categorized based on HPV status and then site. Among the series downloaded, two series had information on HPV status; a total of 97 tumor samples (HPV positive n = 12; Negative n = 85) were selected, 83 included in the final analysis after PCA/clustering analysis and set of 3258 gene entities were identified.
The tongue, laryngopharyngeal and oropharyngeal cancer samples (both tumor and adjacent normal) were separated out from the 8 series for analysis; a total of 192 samples (tumors (T): 154, normal (N): 38) were collected from the series, which included tongue (T = 62; N = 26), laryngopharyngeal (T = 46, N = 7) and oropharyngeal (T = 46; N = 5) cancers. The site-specific samples were grouped and analyzed separately in the GeneSpring for the identification of significant gene entities. PCA analysis removed the outliers and the samples that qualified were taken for further analysis in each site (tongue = 73, laryngopharyngeal = 48, oropharyngeal cancer = 43). Site-specific meta-analysis lead to the identification of the statistically significant gene entities (fold change (FC)> 2.0, p-value<0.05) specific to each site; 3508 in tongue samples, 4893 from laryngopharyngeal and 2386 from oropharyngeal cancers.
A comparison across the different sites indicated that 698 genes were common across all the 3 sites (S2 File). The significant gene entities from each site, when cross compared with the previously published database of 181 gene entities (up regulated: 168; down regulated: 13) [21] indicated that 66.3% (n = 119/181) of the genes in the database were identified in tongue cancer, while 109/181 genes (60.22%) were common with laryngopharyngeal subset and 95/181 genes (52.48%) were common with oropharyngeal cancer.
3.2 Molecular profile of HPV associated HNSCC
The 3258 gene entities (up = 2140; down = 1118) (S3A File) identified from analysis of HPV associated samples were annotated by GO analysis; the genes belonged to the extracellular matrix (GO: 0005201; 38.5%; n = 30) and structural molecule activity (GO: 0005198, 18.6%; n = 142) categories in molecular functions (MF). The major cellular component (CC) was complex of collagen trimers/collagen trimers (GO: 0098644/GO: 0005581; 30–47.8%), while among the biological processes (BP), positive regulation of reactive oxygen species (GO: 1903428, 32.2%; n = 19) and collagen catabolic process (GO: 0030574, 29.85%; n = 20) showed maximum representation (S3B File). Pathway analysis for the same gene entities (n = 3258) in TOPPFUN identified collagen assembly (38%; n = 23), PI3K-Akt signaling pathway (19.29%; n = 66), Focal adhesion (21.1%; n = 42) (KEGG) and GPCR ligand binding (19.56%; n = 89), Extracellular matrix organization (24.83%; n = 74) and Class A/1 (Rhodopsin-like receptors) (19.87%; n = 64) (Reactome) (S3C File) being the top pathways.
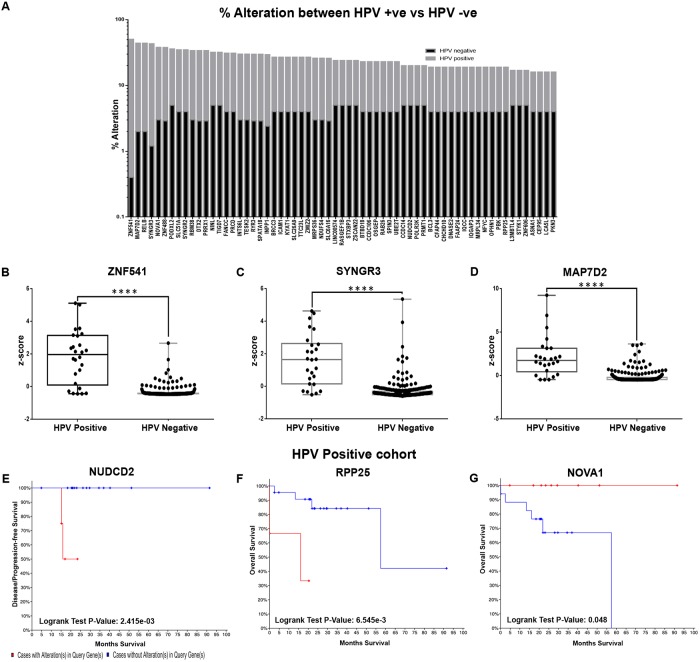
The genes were validated in the TCGA HNSCC cohort with HPV information (n = 271; HPV+: 26, HPV-: 245) for their percentage alteration and the mRNA expression status; a total of 833 genes were altered in more than 5% and 267 genes in >10% of the cohort (S4A, S4B and S4C File). In order to document differences in alteration status between the HPV+/HPV- cohorts, the alteration status and expression levels of this subset (n = 267) were assessed separately in each cohort. Analysis to identify the genes exclusively altered in the HPV+ cohort, indicated that alterations in 63/267 genes were specific to the HPV+ (altered in >10% of cohort) with minimal (altered in <5% of the cohort) or no alterations in the patients negative for HPV (Fig 2A; S4D File). Among these, ZNF541 (Zinc Finger Protein 541) showed the highest alteration in 50% of HPV+ cases as compared to 0.5% in HPV- cases. The other top genes altered in HPV positive cohort includes SYNGR3 (Synaptogyrin 3, 42% vs 1.2%), MAP7D2 (MAP7 Domain Containing 2, 42% vs 2%), RELB (RELB Proto-Oncogene, NF-KB Subunit, 42% vs 2%), and ZNF488 (Zinc Finger Protein 488, 35% vs 3%) (Fig 2A). Assessment of the mRNA expression levels (z-score) indicated that this cohort of 63 genes showed significant differential expression between the two cohorts (p<0.04) (Fig 2B–2D; S4D File). The survival impact of these genes in patients positive for HPV (n = 26) was assessed; RPP25 showed association with overall survival (p = 6.545e-3), NUDCD2 (NudC Domain Containing 2) was associated with DFS (p = 2.415e-03), while NOVA1 (Neuro-Oncological Ventral Antigen 1) was a good prognosticator for overall survival in patients positive for HPV (p = 0.048) (Fig 2E–2G; S4E File).
Fig 2. Marker profile in HPV+ HNSCC in terms of alterations, differential expression and prognostic efficacy.
Comparison of the significant gene entities across HPV positive and negative cohort for their percentage alteration identified a total of 63 genes as highly altered in HPV positive cohort as compared to HPV negative cohort (A). Assessment of expression levels (mRNA z-score) indicated that all these genes were significant differentials (p<0.05) between the two cohorts; the profile of top 3 genes (both alterations and z-score); ZNF541 (50%), SYNGR3 (42%), MAP7D2 (42%) in HPV positive TCGA cohort is represented as boxplots. (B-D). The selected markers (n = 63) were analysed for their significant association with disease free and overall survival in patients with HPV positive cancer in TCGA. KM analysis indicated that NUDCD2 (E) was associated with DFS (p = 2.415e-03), RPP25 (F; p = 6.545e-3) and NOVA1 (G; good prognosticator, p = 0.048) were associated with OS. (P-value; **** p<0.00005).
3.3 Molecular prognosticators of tongue cancer
The significant genes identified from the meta-analysis of the tongue cancer cohort (n = 3508; up = 1753; down = 1755) (S5A File) were annotated based on the gene ontology classes. GO analysis (n = 3508) indicated that in molecular functions (MF), CXCR chemokine receptor binding (GO: 0045236; 64.7%; n = 11) and structural constituent of muscle (GO: 0008307; 63.4%; n = 26) category showed maximum representation. Among the cellular components (CC), TAP complex (GO: 0042825, 100%; n = 4), epidermal lamellar body (GO: 0097209, 100%; n = 4) and central spindling complex (GO: 0097149, 100%; n = 3) were highly represented, while in the biological process (BP), regulation of endodermal cell differentiation (GO: 1903224, 100%; n = 7), regulation of endodermal cell fate specification (GO: 0042663, 100%; n = 5) and regulation of protein kinase C activity (GO: 1900019, 100%; n = 4) showed maximum representation (S5B File). Pathway analysis carried out (n = 331) in TOPPFUN identified N-glycosylation by oligosaccharyl transferase (71.42%; n = 5) and proteasome (48.8%; n = 22) as the top pathways in KEGG with most number of overlapping genes. While in Reactome, the top pathways were Phosphorylation of Emi1 (83.3%; n = 5), Unwinding of DNA (66.67%; n = 8) and Platelet Adhesion to exposed collagen (53.8%; n = 7) (S5C File). The subset of significant genes (n = 3508) were assessed for their association with clinical/pathological parameters and survival.
3.3.1 Markers associated with early stage tongue cancer
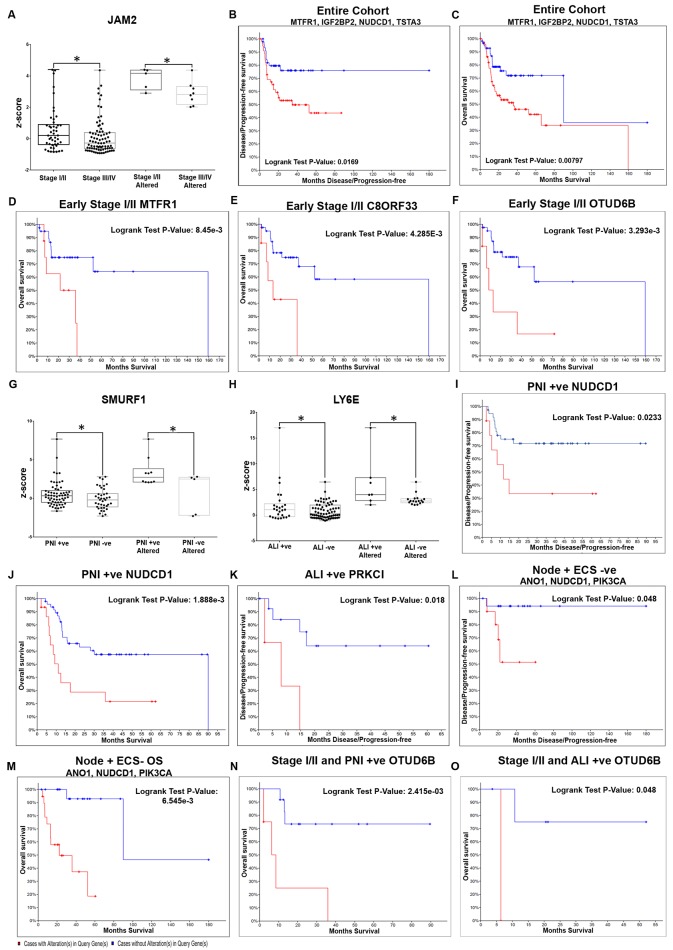
In order to validate the gene entities in an independent cohort of patients, the TCGA patient cohort was used. The alteration status was evaluated in tongue cancer-specific patient cohort of TCGA (n = 132) to identify the highly prevalent genes; 331 genes were altered in at least 10% of the cohort, while 56 genes were altered in more than 20% (S5D File). To delineate the expression of these markers in different clinical stages, the TCGA tongue cancer patients were further classified into early/late stages and the expression profiling compared. mRNA z-score based differential profiling of the genes (n = 331) between the early and late stage cohorts indicated 14 genes as statistically significant differentials (p = >0.05) (S6A File); JAM2 (Junctional Adhesion Molecule) showing a significant alteration (FC>2 fold; p = 0.05) in the early stage patients (Table 1, Fig 3A). Evaluation of prognostic efficacy in early stage patients indicated that none of them were associated with survival.
Table 1. Differential mRNA z-score expression profiling between the altered cases in association with clinico-pathological parameters in tongue cancer.
| Percentage altered in cohort | |||||
|---|---|---|---|---|---|
| S.No | Parameter | Gene | Early stage | Late stage | p-value |
| 1 | Clinical stage | JAM2 | 10.63829787 | 9.756097561 | 0.049438 |
| Positive | Negative | ||||
| 2 | PNI | SMURF1 | 16.12903226 | 12.82051282 | 0.040467 |
| 3 | ALI | LY6E | 26.92307692 | 23.94366197 | 0.019605 |
| SUPT16H | 15.38461538 | 7.042253521 | 0.009972 | ||
| MFN1 | 11.53846154 | 22.53521127 | 0.023416 | ||
Fig 3. Molecular markers with significant differential expression and prognostic efficacy in tongue cancer.
Significant differentials from tongue cancer meta-analysis were validated in terms of mRNA expression levels (z-score) across stage/pathological parameters in the TCGA tongue cancer cohort. In early stage tongue cancers, JAM2 showed a significantly upregulation (±2 fold) as compared to late stage (A), with no effect on survival. Assessment of prognostic efficacy (genes >20% alterations) indicated that in the entire cohort (B and C), a combination of MTFR1, IGF2BP2, NUDCD1 and TSTA3 clearly showed a poor DFS (p = 0.0169) and OS (p = 0.00794). In early stage cancer (D-F), MTRF1 (p = 8.450e-3), C8ORF33 (p = 4.285e-3) and OTUD6B (p = 3.293e-3) were individually associated with overall survival in early stage tongue cancer. Comparison of the differentials based on mRNA expression levels (z-score) between patients positive for pathological parameters (PNI, ALI, nodal metastasis) in tongue cancer TCGA cohort indicated that in the PNI+ cases, SMURF1 was upregulated (G), while the gene LY6E was altered maximum in ALI+ cases (H). KM plot analysis in PNI+ patients (I and J) indicated that NUDCD1 showed an association with poor DFS (p = 0.023) and OS (p = 1.888e-3), while in ALI+ cases (K) PRKCI was associated with poor OS (p = 0.018). Analysis in the node +/ECS- cohort (L and M) of TCGA revealed that a combination of ANO1, NUDCD1 and PIK3CA was a poor prognosticator (DFS p = 0.0236 and OS p = 1.419e-4). Analysis in a further defined cohort of early stage tongue cancers positive for PNI or ALI, identified OTUD6B as a poor prognosticator (N and O). (P-value; * p<0.05).
As an additional effort to identify significant prognosticators, prognostic impact (overall/disease-free survival) was also investigated using the gene set altered in higher percentage of patients (>20%) in i) the overall tongue cancer cohort ii) early stage cohort iii) late stage cohort. In the overall tongue cohort, 4 genes showed an association for DFS and/or OS (p<0.05) individually and in combination. A combination of NUDCD1 (NudC domain containing 1) (Table 2), TSTA3 (Tissue specific transplantation antigen P35B), MTFR1 (Mitochondrial fission regulator 1) and IGF2BP2 (Insulin-like growth factor 2 mRNA binding protein 2) (S6B File) clearly demarcated the patients with poor survival in tongue cancer; patients with over-expression showed low median survival (35.91 vs 90.05 months; p = 0.007) and low DFS (34.76 vs NA months; p = 0.01) when compared with the cohort without alterations (Figs 3B and 4C).
Table 2. Prognosticators of survival (DFS and OS) in patients with tongue cancer in various clinic-pathological parameters.
| S.No | Clinico-pathological parameter | Genes | Overall Survival Logrank Test P-Value |
Disease Free Survival Logrank Test P-Value# |
|---|---|---|---|---|
| 1 | Entire cohort (differential expression) | NUDCD1, TSTA3, MTFR1 and IGF2BP2 | 0.007 | 0.01 |
| 2 | Entire cohort | NUDCD1 | 0.0331 | 0.00958 |
| 3 | Stage I-II | C8ORF33 | 4.28e-03 | |
| OTUD6B | 0.0032 | |||
| MTFR1 | 0.0084 | |||
| 4 | Stage III-IV | NUDCD1 | 0.0253 | |
| CLPTM1L | 0.0285 | |||
| MAPRE1 | 0.034 | |||
| 5 | PNI + | NUDCD1 | 0.00187 | 0.0228 |
| TFG* | 0.019 | 0.0331 | ||
| BRIX1* | 0.0475 | 0.0437 | ||
| 6 | ALI + | PRKCI | 0.01 | |
| WDR70* | 0.0338 | |||
| BRIX1* | 0.0474 | |||
| 7 | Node+ ECS- | ANO1 | 0.00843 | 0.0133 |
| NUDCD1 | 0.00246 | 0.015 | ||
| ANO1, NUDCD1 and PIK3CA (Combination) | 6.545e-3 | 0.048 | ||
| 8 | Node+ ECS+ | GMPS | 0.0254 | |
| TSTA3 | 0.0373 | |||
| 9 | Stage I-II and PNI + | OTUD6B | 0.00166 | 0.0287 |
| 10 | Stage I-II and ALI + | OTUD6B | 0.0455 |
*Genes which are good prognosticators in their respective clinic-pathological parameter,
#genes were sorted based on DFS.
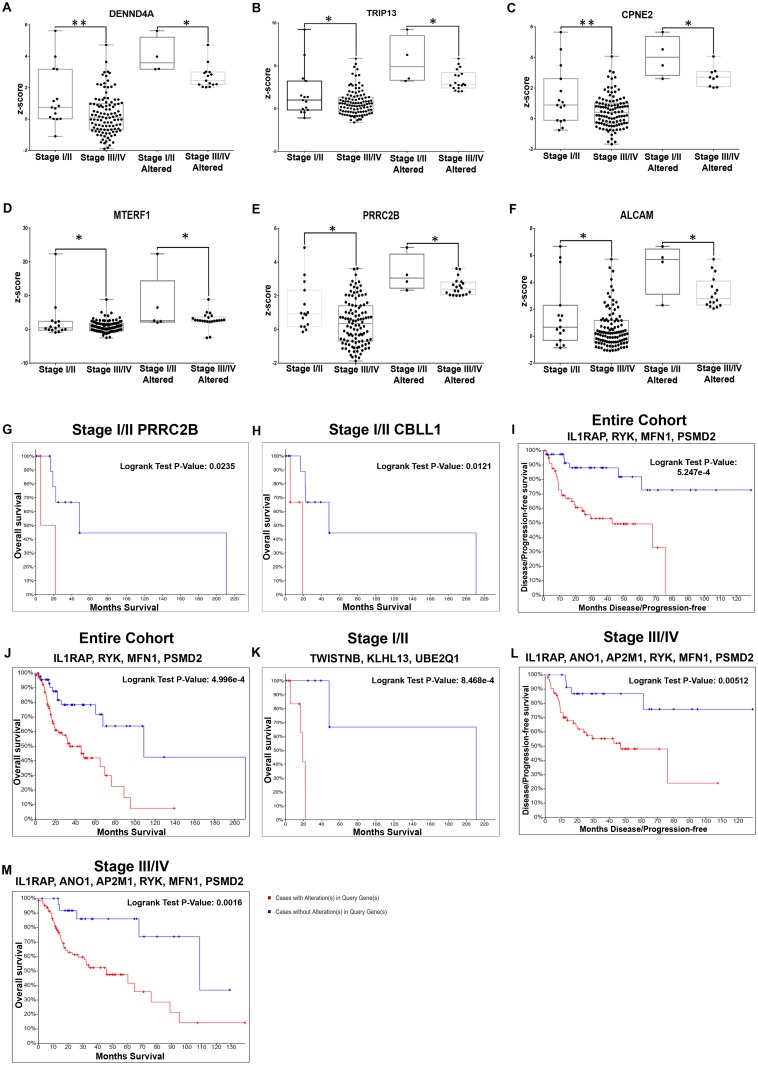
Fig 4. Molecular markers with significant differential expression and prognostic efficacy in laryngopharyngeal cancers.
Meta-analysis based differentials in laryngopharyngeal cancers were validated in the TCGA cohort. mRNA expression levels (z-score) of the genes when compared stage-wise in the TCGA laryngopharyngeal cancer cohort identified 19 differentials in early stage cancers, the top six markers (DENND4A, TRIP13, CPNE2, MTERF1, PRRC2B and ALCAM) are represented here (A-F). KM plot analysis of these genes in patients with early stage laryngopharyngeal cancers indicated PRRC2B (G; p = 0.023) and CBLL1 (H; p = 0.0121) showed an association with overall survival. Evaluation of the prognostic efficacy of the gene subset (altered in >20% of the patients) in the entire cohort of laryngopharyngeal cancers in TCGA identified the combination of IL1RAP, RYK, MFN1 and PSMD2 with low median survival in cases with alteration (DFS p = 0.0004; OS p = 0.0005) (I and J). Similar analysis in early stage patients (K) showed TWISTNB, KLHL13 and UBE2Q1 to be associated with overall survival (p = 8.468e-4), while in late stage patients, the combination of IL1RAP, ANO1, RYK, AP2M1, MFN1 and PSMD2 were associated with poor prognosis (L and M). (P-value; * p<0.05; ** p<0.005).
Subsequent analysis to identify the markers that could distinguish patients of poor prognosis in early stage tongue cancer cohort of TCGA (stage I-II; n = 47) identified a three gene panel that independently demarcated patients with poor overall survival; MTFR1 (identified in the overall tongue cancer cohort) (p = 8.450e-3), C8ORF33 (Chromosome 8 open reading frame 33, p = 4.285e-3) and OTUD6B (OTU domain containing 6B, p = 3.293e-3) (Table 2; Fig 3D–3F; S6B File), although the marker combination was not significant. Interestingly, this analysis also revealed that NUDCD1 that was significantly correlated with all tongue cancer patients, was relevant (p<0.05) in advanced cancers (stage III-IV; n = 81) (S6B File), not in patients with early cancers.
3.3.2 Markers with prognostic efficacy in patients with pathologically distinct tongue cancer
To identify the genes associated with severe pathological parameters in tongue cancer, the cohort of 331 genes was validated in the TCGA tongue cancer patients with perineural invasion (PNI, n = 65) or angiolymphatic invasion (ALI, n = 26). A panel of 17 genes were differentials in PNI+ or ALI+ as compared to the negative patients (p<0.05) (S6A File); SMURF1 (SMAD Specific E3 Ubiquitin Protein Ligase 1) (Fig 3G) was significantly altered in PNI+ patients and a panel of 3 genes were significantly altered in ALI+ patients (FC>±2; p<0.05) (Table 1; Fig 3H). None of these genes showed a survival impact. Prognostic efficacy assessed with genes having alterations in a larger cohort of patients (>20%) in the multiple pathological sub-cohorts (PNI+, ALI+, node+, node+/ECS+ and node+/ECS-) identified 4 genes in PNI+ patients (n = 62); NUDCD1 (Fig 3I and 3J), TFG (TRK-Fused Gen), TMEM267 (Transmembrane Protein 267) and BRIX1 (BRX1, Biogenesis of Ribosomes) as significant in survival prediction (OS and/or DFS; p<0.05), while PRKCI (Protein kinase C, iota) (Fig 3K) WDR70 (WD repeat domain 70) and BRIX1 were predictors in patients with ALI+ (n = 26) (Table 2; S6B File) although the marker combination was not significant in both the parameters.
A similar investigation into markers associated extracapsular spread (ECS) in tongue cancer indicated a panel of 17 differentials in node+ ECS+ patients (p<0.05) (S6A File). However, none of these genes were significantly altered or showed any significant survival impact. In next level analysis of the prognostic efficacy of genes with alterations in >20% of patients of node positive patients (n = 68), NUDCD1, PUF60 (poly-U binding splicing factor 60KDa), ANO1 (Anoctamin 1) and RHOA (Ras Homolog Family Member A) showed an association with OS/DFS (S6B File). These genes showed no correlation with survival in negative patient cohort. Analysis of the sub cohorts with/without ECS (ECS+: 24; ECS-: 39) indicated that NUDCD1 and ANO1 were significant prognosticators (Table 2; S6B File) in the ECS- cohort. In this sub-cohort, patient with overexpression of the marker combination, ANO1, NUDCD1 and PIK3CA (Phosphatidylinositol-4, 5-Bisphosphate 3-Kinase Catalytic Subunit Alpha) showed low median survival (22.34 vs 90.05 months; p = 0.00014) (Fig 3L and 3M) indicating their relevance in cohort which is otherwise considered to have a good prognosis. Further in the ECS+ patients (n = 24), wherein presence of ECS itself indicated poor prognosis, GMPS (Guanine monophosphate synthetase) (DFS; p = 0.025), TSTA3 (OS; p = 0.0373) were additional prognosticators (Table 2; S6B File).
Multiple level classification including stage and pathological parameters indicated that in the early stage patients with poor prognosis (PNI+ and/or ALI+), OTUD6B was an additional prognosticator (p<0.05) (Table 2; Fig 3N and 3O).
3.4 Molecular prognosticators of laryngopharyngeal cancers
A total of 4893 genes (up = 2017; down = 2876) from laryngopharyngeal samples with FC >2 and a p-value of <0.05 were identified (S7A File). GO analysis of the significant (n = 4893) genes indicated that among molecular functions (MF), DNA replication origin binding (GO: 0003688) showed maximum representation (88.88%; n = 8) followed by CXCR chemokine receptor binding (GO: 0045236) (64.70%; n = 11). Condensed nuclear chromosome outer kinetochore (GO: 0000942, 100%; n = 4) was the major cellular component, while in the biological process (BP), regulation of endodermal cell differentiation (GO: 1903224, 100%; n = 7) and regulation of endodermal cell fate specification (GO: 0042663, 100%; n = 5) showed maximum representation (S7B File). Pathway analysis identified DNA replication (52.77%; n = 19), Cell cycle (45.16%; n = 56), condensation of Prometaphase Chromosomes (88.18%; n = 9), Zinc influx into cells by the SLC39 gene family (80%; n = 8) and Unwinding of DNA (75%; n = 9) (S7C File) as the top relevant pathways.
3.4.1 Markers associated with early stage laryngopharyngeal cancers
These significant gene entities were validated in the TCGA HNSC laryngopharyngeal specific samples (n = 127) in terms of prevalence, differential expression and prognostic efficacy. Assessment of alteration status identified that 701 genes were altered in mRNA expression in >10% of patients and 137 genes were altered in at least 20%. The expression of the 701 genes was looked into in the TCGA laryngopharyngeal subset (n = 127) classified based on stage; 82 genes were differentially regulated (p<0.05) between the early/late stage laryngopharyngeal cancers, out of which 19 genes showed a significant alteration (FC >±2) in the expression levels (Table 3; Fig 4A–4F; S8A File). These differential genes were assessed for their impact on overall and disease free survival; PRRC2B (Proline Rich Coiled-Coil 2B, p = 0.0235) and CBLL1 (Cbl proto-oncogene, E3 ubiquitin protein ligase-like 1, p = 0.0121) impacted overall survival of early stage laryngopharyngeal patients (Fig 4G and 4H; S8B File).
Table 3. Differential mRNA z-score expression profiling between the altered cases in association with clinico-pathological parameters in laryngopharyngeal cancer.
| Percentage altered in cohort | |||||
|---|---|---|---|---|---|
| S.No | Parameter | Gene | Early stage | Late stage | p-value |
| 1 | Clinical stage | MTERF1 | 33.33333333 | 20.56074766 | 0.039969451 |
| DENND4A | 26.66666667 | 14.01869159 | 0.011957913 | ||
| TRIP13 | 26.66666667 | 16.82242991 | 0.016278625 | ||
| ALCAM | 26.66666667 | 14.95327103 | 0.022132352 | ||
| CPNE2 | 26.66666667 | 8.411214953 | 0.027141198 | ||
| PRRC2B | 26.66666667 | 18.69158879 | 0.027273281 | ||
| P3H2 | 20 | 17.75700935 | 0.000163948 | ||
| ORC5 | 20 | 22.42990654 | 0.003092435 | ||
| CBLL1 | 20 | 20.56074766 | 0.005484005 | ||
| ANKIB1 | 20 | 17.75700935 | 0.011002909 | ||
| SMIM13 | 20 | 12.14953271 | 0.011095341 | ||
| NUP155 | 20 | 16.82242991 | 0.037923343 | ||
| RBM48 | 13.33333333 | 17.75700935 | 6.57699e-05 | ||
| SKP2 | 13.33333333 | 14.01869159 | 0.00127573 | ||
| STK38L | 13.33333333 | 10.28037383 | 0.001602153 | ||
| PON2 | 13.33333333 | 13.08411215 | 0.00565697 | ||
| CYP3A5 | 13.33333333 | 12.14953271 | 0.007557119 | ||
| CDK6 | 13.33333333 | 9.345794393 | 0.015929102 | ||
| WAPL | 13.33333333 | 8.411214953 | 0.020646728 | ||
| Gene | Positive | Negative | p-value | ||
| 2 | PNI | RSRC1 | 51.72413793 | 46.15384615 | 0.006797288 |
| PIK3CA | 48.27586207 | 38.46153846 | 0.034488768 | ||
| DCUN1D1 | 44.82758621 | 28.84615385 | 0.047521591 | ||
| FYTTD1 | 37.93103448 | 21.15384615 | 0.03346135 | ||
| SLC4A1AP | 20.68965517 | 5.769230769 | 0.017492707 | ||
| CWC22 | 17.24137931 | 19.23076923 | 0.015213151 | ||
| COMMD2 | 10.34482759 | 5.769230769 | 0.011896098 | ||
| 3 | ALI | PAK2 | 45.94594595 | 35.55555556 | 0.011671511 |
| FXR1 | 43.24324324 | 20 | 0.043115543 | ||
| MFN1 | 37.83783784 | 31.11111111 | 0.005977193 | ||
| SLC4A1AP | 16.21621622 | 6.666666667 | 0.012880852 | ||
| 4 | Node positive ECS | EPS8 | 19.23076923 | 38.0952381 | 0.032931489 |
Prognostic efficacy was assessed for genes (>20%; n = 137) in i) entire cohort (n = 127) of laryngopharyngeal patients ii) early stage patients. Assessment in entire cohort identified 27 significant predictors of survival (OS and/or DFS) (Table 4; S9A File); patients with overexpression of IL1RAP (Interleukin 1 Receptor Accessory Protein), RYK (Receptor-like tyrosine kinase), PSMD2 (Proteasome (prosome, macropain) 26S subunit, non-ATPase, 2) and MFN1 (Mitofusin 1) showed low median survival (34.07 vs 108.81 months; p = 0.0005) and low DFS (42.77 vs NA months; p = 0.0004) when compared with the cohort without alterations (Fig 4I and 4J; S9B File). Assessment of the prognostic efficacy in the early stage laryngopharyngeal samples (I-II; N = 15) revealed a total of 12 gene predictor panel completely unique to this cohort (Table 4; S9A File). Among these genes, the combination of TWISTNB (TWIST Neighbor), KLHL13 (Kelch-like 13) and UBE2Q1 (Ubiquitin-conjugating enzyme E2Q family member 1) and WBP11 (WW Domain Binding Protein 11) proved to be accurate survival predictors (low median survival (18.96 vs 210.81 months; p = 0.0008) (Fig 4K; S9B File). As observed in the case of tongue, genes associated with overall cohort, IL1RAP, ANO1, RYK, AP2M1, MFN1 and PSMD2 were significant prognosticators only in the late stage not in early cancers. (Table 4; Fig 4L and 4M; S9A and S9B File).
Table 4. List of significant genes with both disease free and overall survival of different clinic-pathological parameters in TCGA laryngopharyngeal cohort.
| S.No | Clinico-pathological parameter | Genes | Overall Survival Logrank Test P-Value | Disease Free Survival Logrank Test P-Value# |
|---|---|---|---|---|
| 1 | Entire cohort | IL1RAP | 0.015 | 3.47e-06 |
| RYK | 0.00979 | 7.25e-04 | ||
| AP2M1 | 0.0288 | 0.00106 | ||
| ANO1 | 0.0465 | 0.00153 | ||
| MFN1 | 0.0094 | 0.0081 | ||
| PSMD2 | 8.94e-04 | 0.00994 | ||
| PIK3CA | 0.0449 | 0.0145 | ||
| TRMT12 | 0.0422 | 0.0346 | ||
| IGF2BP2 | 0.0277 | 0.04 | ||
| ZNF12 | 0.0426 | 0.044 | ||
| IL1RAP, RYK, MFN1 and PSMD2 (Combination) | 5.25e-04 | 5.00e-04 | ||
| 2 | Stage I-II* | TWISTNB | 0.00166 | |
| KLHL13 | 0.00173 | |||
| UBE2Q1 | 0.0037 | |||
| WBP11 | 0.0108 | |||
| ATP1B1 | 0.0235 | |||
| ZNF687 | 0.0253 | |||
| FXR1 | 0.0453 | |||
| PFN2 | 0.0453 | |||
| EPS8 | 0.0453 | |||
| WDR53 | 0.0453 | |||
| SDE2 | 0.0465 | |||
| TERF1 | 0.0453 | |||
| TWISTNB, KLHL13, UBE2Q1 and WBP11(combination) | 8.47e-04 | |||
| 3 | Stage III-IV | IL1RAP | 0.00396 | 1.32e-07 |
| ANO1 | 0.0186 | 5.25e-04 | ||
| RYK | 0.0106 | 0.00175 | ||
| AP2M1 | 0.00474 | 0.00356 | ||
| MFN1 | 0.00881 | 0.0148 | ||
| PSMD2 | 0.0012 | 0.0153 | ||
| TRMT12 | 0.0353 | 0.0275 | ||
| IGF2BP2 | 0.0207 | 0.0429 | ||
| IL1RAP, ANO1, RYK, AP2M1, MFN1 and PSMD2 (combination) | 0.0016 | 0.00512 | ||
| 4 | PNI + | AP2M1 | 0.0133 | 0.0259 |
| 5 | ALI + | RYK | 0.00245 | 0.00717 |
| IL1RAP | 0.0333 | 0.0125 | ||
| AP2M1 | 0.0359 | 0.035 | ||
| RYK and IL1RAP (combination) | 6.79e-04 | 0.0209 | ||
| 6 | Node+ ECS+ | STK3 | 0.00163 | 0.00106 |
| TMEM267 | 0.00298 | 0.00121 | ||
| CLPTM1L | 0.0489 | 0.0118 | ||
| PSMD2 | 0.00504 | 0.0175 | ||
| STK3, TMEM267 and PSMD2 (combination) | 6.11e-04 | 0.00401 | ||
| 7 | Node+ ECS- | VKORC1L1 | 0.0323 | 0.00545 |
*In stage I-II all the genes were associated with only overall survival,
#genes were sorted based on DFS.
3.4.2 Markers correlating with pathological parameters
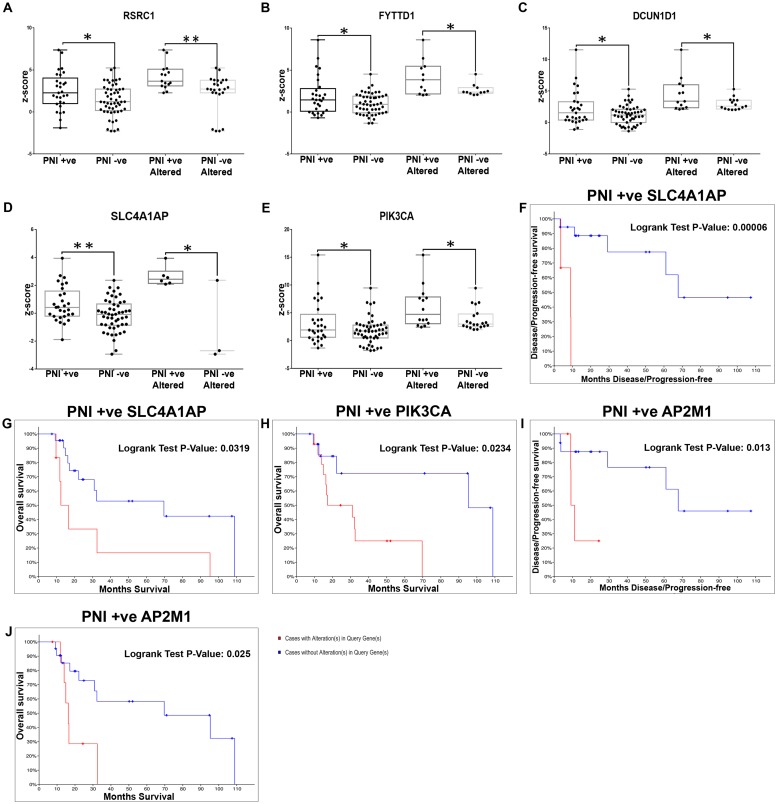
The genes (n = 701) were assessed for differential expression in the laryngopharyngeal patients triaged based on pathological categories. In the PNI+ subset (n = 29), 7 genes showed statistically significant alteration as compared to PNI- patients (p<0.05) (Fig 5A–5E; S8A File); SLC4A1AP (Solute Carrier Family 4 Member 1 Adaptor Protein, OS; p = 0.03 and DFS; p = 6.57e-05) and PIK3CA (OS; p = 0.0234) added to the survival impact of perineural invasion in PNI+ cases. (Fig 5F–5H; S8B File). Additional analysis to identify independent prognosticators in the PNI+ laryngopharyngeal cancer patients, identified AP2M1 (Adaptor Related Protein Complex 2 Subunit Mu 1) (Table 4; Fig 5I and 5J) as the most significant predictor (DFS and OS) in addition to a panel of 12 genes which could predict disease free/overall survival (S9A File).
Fig 5. Molecular markers with significant differential expression and prognostic efficacy in laryngopharyngeal cancers with perineural invasion.
Validation of the differentials identified in the laryngopharyngeal cancer within the TCGA cohort of patients with perineural invasion identified 7 gene panel based on differences in mRNA expression levels (z-score), the top 5 based on percentage alteration being RSRC1, FYTTD1, DCUN1D1, SLC4A1AP and PIK3CA was upregulated in altered cases of PNI positive patients (A-E). From among this cohort, SLC4A1AP showed an association in both DFS (F; p = 0.00006569) and OS (G; p = 0.0319), whereas PIK3CA was associated with overall survival (H; p = 0.0234). Assessment of the prognostic efficacy of the genes with alterations >20% identified AP2M1 as the sole marker with survival impact on both OS (I; p = 0.013) and DFS (J; p = 0.025). (P-value; * p<0.05; ** p<0.005).
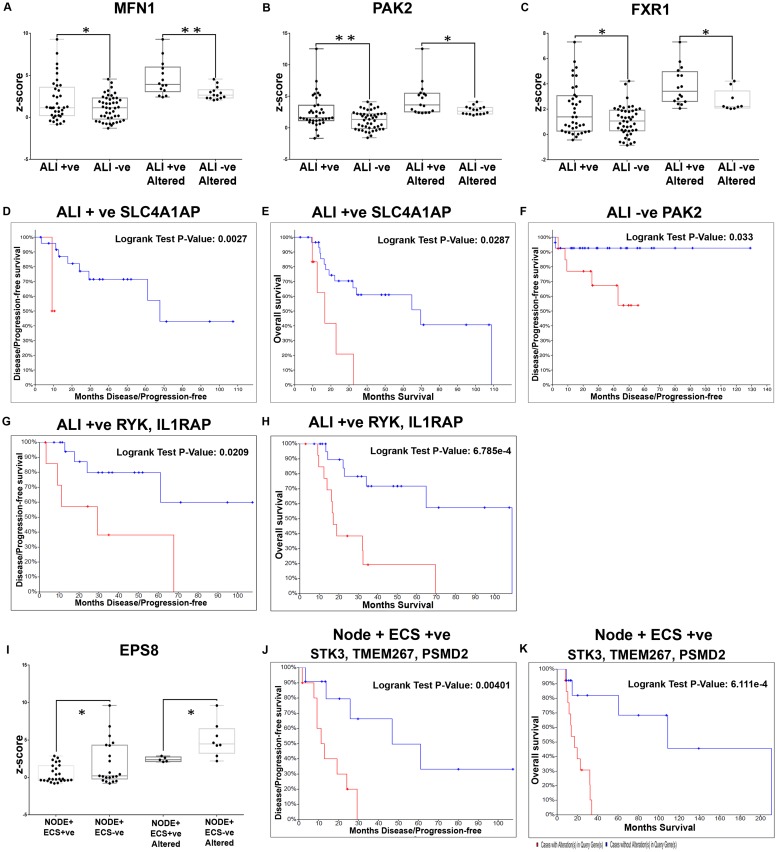
In the ALI+ set (n = 37), 4 genes [MFN1, PAK2 (P21 (RAC1) Activated Kinase 2), SLC4A1AP and FXR1 (Fragile X mental retardation syndrome-related protein 1)] were significant differentials (Table 3; Fig 6A–6C; S8A File), with SLC4A1AP being a determinant of both OS (p = 0.0027) and DFS (p = 0.0287) (Fig 6D and 6E). PAK2 was associated with disease free survival (p = 0.033; Fig 6F) in ALI- cases (S8B File). Additional analysis (genes with alterations in >20% patients) in ALI+ patients revealed that RYK, AP2M1 and IL1RAP that relevant in the overall cohort were significantly predictors of OS and DFS (Table 4; S9A File). Among these markers, the overexpression of the combination of RYK and IL1RAP in ALI+ patients, led to low median survival (17.12 vs 108.87 months; p = 0.0006) and low DFS (29.2 vs NA months; p = 0.02) when compared with the cohort without alterations (Fig 6G and 6H; S9B File). The assessment of differential prognosticators of early stage laryngopharyngeal patients with PNI/ALI+ was not carried out due to less number of patients in this cohort.
Fig 6. Molecular markers with significant differential expression and prognostic efficacy in laryngopharyngeal cancers with angiolymphatic invasion and nodal metastasis.
Validation of the differentials identified in the laryngopharyngeal cancer within the TCGA cohort of patients with angiolymphatic invasion identified 4 genes with significant difference in mRNA expression levels (z-score) in TCGA cohort laryngopharyngeal cancer with ALI; the top three genes being MFN1, PAK2 and FXR1 (A-C). Among this cohort, SLC4A1AP showed an association in both OS (D; p = 0.002785) and DFS (E; p = 0.0287) of ALI+ patients, while PAK2 showed association with ALI- in DFS alone (F; p = 0.033). Prognostic assessment of the subset of meta-analysis based differentials altered in >20% of the patients identified the combination of RYK and IL1RAP to be associated with poor survival (G and H). EPS8 was the only differential in node in node+ECS- patients with no survival impact (I) validation of the differentials associated with node positive with/without ECS indicated that EPS8 was the only differential (downregulated) in node+/ECS+ cohort with no association with survival. Prognostic efficacy (subset altered in >20% patients) indicated that STK3, TMEM267 and PSMD2 were the prognosticators of poor survival (J and K). (P-value; * p<0.05; ** p<0.005).
EPS8 (Epidermal growth factor receptor kinase substrate 8) was the sole marker that showed significant upregulation (>2 fold) in the patients with node+ ECS+ as compared to node+ ECS- patients (Fig 6I), but without any impact on the survival of the patients. In node positive laryngopharyngeal patients (n = 54) prognostic efficacy was also assessed with genes having increased alterations (>20%), 17 markers were poor predictors of survival (S9A File); among which IL1RAP and PSMD2 were predictors in the entire cohort. Further, assessment in node+ ECS+ patients (n = 26), identified 21 genes associated with DFS and/or OS; PSMD2 being significant in this sub cohort also (S9A File). A combination panel of STK3 (Serine/Threonine Kinase 3), TMEM267 and PSMD2 clearly demarcated the patients [node+ ECS+] with poor median survival (17.12 vs 108.87 months; p = 0.0006) and low DFS (11.2 vs 46.81 months; p = 0.004) when compared with the cohort without alterations (Fig 6J and 6K, S9B File). In [node+ ECS-] patients (n = 21), who are known to have a better prognosis than the [node+ ECS+] patients, alterations in VKORC1L1 (Vitamin K epoxide reductase complex, subunit 1-like 1), KLHL12 (Kelch-like 12), PAIP1 (poly (A) binding protein interacting protein 1), CBLL1 and PUS7 (Pseudouridine Synthase 7) could predict OS/DFS (Table 4; S9A File).
3.5 Molecular prognosticators of oropharyngeal cancers
Meta-analysis identified 2386 genes (Up = 1767; Down = 619) in oropharynx samples (S10A File) (n = 51). GO analysis carried out to evaluate the functional classes indicated that in molecular functions (MF), extracellular matrix constituent conferring elasticity (GO: 0030023) category showed maximum representation (100%; n = 6) followed by 2'-5'-oligoadenylate synthetase activity (GO: 0001730, 100%; n = 4). Among the cellular components (CC), laminin-1 complex (GO: 0005606 100%; n = 3) and collagen type VI trimer (GO: 0005589, 100%; n = 3) classes were highly represented, while in the biological process (BP), adenine biosynthetic process (GO: 0046084, 100%; n = 4) and adenine metabolic process (GO: 0046083, 100%; n = 4) showed maximum representation (S10B File). Analysis also identified N-glycosylation by oligosaccharyl transferase (71.42%; n = 5), Guanine ribonucleotide biosynthesis IMP = > GDP,GTP (46.15%; n = 6), Glycolysis (Embden-Meyerhof pathway), glucose = > pyruvate (44%; n = 25) and ECM-receptor interaction (36.58%; n = 30) were significant pathways in KEGG, while TFAP2 (AP-2) family regulates transcription of cell cycle factors (100%; n = 5), Antagonism of Activin by Follistatin (100%; n = 4) and Phosphorylation of proteins involved in the G2/M transition by Cyclin A:Cdc2 complexes 100%; n = 3) were major pathways in Reactome (S10C File).
Validation in the TCGA oropharyngeal cancer cohort (n = 32) indicated that 404 genes were altered at the mRNA expression level in at least 10% of the cohort, while a sub-set of 73 genes showed a high prevalence and were altered in >20% of the patients (S11A File). Analysis of these genes in terms of z-score analysis and prognosis in correlation with stage/pathological parameters and survival analysis was not carried out due to the low number of samples in the sub-categories.
3.5.1 Markers correlating to HPV-associated oropharyngeal cancers
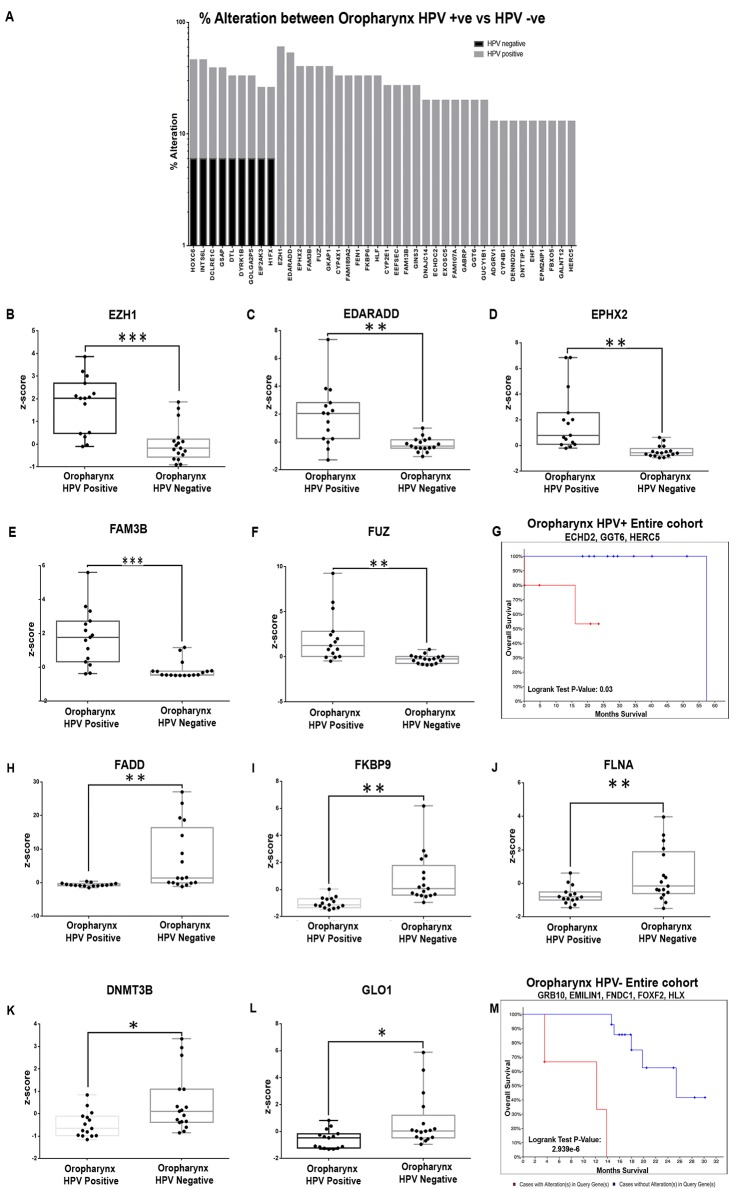
The markers were assessed for their relevance in HPV associated oropharyngeal cancers; the gene set was validated for alterations/survival impact in the TCGA oropharyngeal cohort segregated according to HPV status. Among the 404 gene set, 128 genes were altered in positive cohort (n = 15), while 131 were altered in the negative cohort (n = 17) (S11B and S11C File). Comparison of the alteration status across the two cohorts indicated that 51 genes in HPV+ and 55 in HPV- were either altered only in the respective cohorts or showed a high percentage alteration (S11D File). Assessment of the mRNA expression level (z-score) differences in these genes indicated that 31 genes in HPV+ cohort (Fig 7A) and 27 genes in HPV- cohort showed significant difference in the expression levels between the two cohorts (p<0.04).
Fig 7. Marker profile of HPV+/HPV- oropharyngeal cancers in terms of percentage alterations, differential expression and survival.
The significant gene entities identified from the meta-analysis of oropharyngeal cancers were compared with the oropharyngeal HPV positive and negative cohort from TCGA. A total of 31 genes showed to be highly altered in HPV positive cohort with minimal/ no alteration in negative cohort. The top 5 genes altered exclusively in HPV+ cohort included EZH1 (60%), EDARADD (42%), EPHX2 (42%), FAM3B (42%) and FUZ (35%) (A). Differential profiling of these genes based on mRNA expression levels (z-score) indicated that these genes showed a significantly upregulation in oropharyngeal HPV+ cases (B-F). The selected markers (n = 31) were analysed for their significant association with disease free and overall survival in oropharyngeal HPV+ cohort in TCGA; KM plot analysis identified that a combination of ECHD2, GGT6 and HERC5 (all genes uniquely altered in the HPV+ cohort) showed a significant association with overall survival (G; p = 0.0321). Similar validation of the genes in the HPV- cohort identified 27 genes altered; mRNA expression levels (z-score) of the top 5 genes [FADD (41%), FKBP9 (24%), FLNA (24%) DNMT3B (18%) and GLO1 (18%)] (H-L) indicated that they were significantly upregulated in the HPV+ cohort. Assessment of prognostic efficacy in HPV- cohort showed that a combination of GRB10, EMILIN1, FNDC1, FOXF2 and HLX showed a significant association with overall survival (M; p = 2.939e-6). (P-value; * p<0.05; ** p<0.005; *** p<0.0005).
The significantly altered genes in the HPV+ cohort include EZH1 (Enhancer Of Zeste 1 Polycomb Repressive Complex 2 Subunit, 60% vs 0%), EDARADD (Ectodysplasin-A Receptor-Associated Adapter Protein Associated Death Domain, 42% vs 1.2%), EPHX2 (Epoxide Hydrolase 2, 42% vs 2%), FAM3B (Family With Sequence Similarity 3 Member B, 42% vs 2%), and FUZ (Fuzzy Planar Cell Polarity Protein, 35% vs 3%) (Fig 7B–7F). Assessment of prognostic efficacy in HPV+ cohort indicated that although these genes were differentials, they did not have a survival impact. Among the genes altered in >20% of patients, ECHDC2 (Enoyl-CoA Hydratase Domain Containing 2, p = 2.22e-03), GGT6 (Gamma-Glutamyltransferase 6, p = 2.22e-03), HERC5 (HECT And RLD Domain Containing E3 Ubiquitin Protein Ligase 5, p = 0.018), EEFSEC (Eukaryotic Elongation Factor, Selenocysteine-TRNA Specific, p = 0.013), FEN1 (Flap Structure-Specific Endonuclease 1, p = 0.02), ADGRV1 (Adhesion G Protein-Coupled Receptor V1) and GINS3 (GINS Complex Subunit 3) were associated with overall survival/DFS (p = 0.03); a combination of the genes ECHD2, GGT6 and HERC5 showed a significant association with overall survival in entire cohort (p = 0.0321) (Fig 7G; S12A File).
A similar differential analysis in the HPV- cohort indicated a panel completely distinct from the HPV+ oropharyngeal cancers; FADD (Fas Associated via Death Domain, 41%), FKBP9 (FKBP Prolyl Isomerase 9, 24%), FLNA (Filamin A, 24%) and DNMT3B (DNA Methyl transferase 3 Beta, 18%) with high prevalence (Fig 7H–7L). Assessment of prognostic efficacy of the differentials (n = 55) indicated that in HPV- cohort, FARSA (Phenylalanyl-TRNA Synthetase Subunit Alpha) showed an association with both overall and disease free survival (p<0.03), while GRB10 (Growth Factor Receptor Bound Protein 10), EMILIN1 (Elastin Microfibril Interfacer 1), FNDC1 Fibronectin Type III Domain Containing 1), FOXF2 (Forkhead Box F2), HLX (H2.0 Like Homeobox), GTF2F1 (General Transcription Factor IIF Subunit 1) were associated either with overall or disease free survival (p< 0.01). A combination of the genes GRB10, EMILIN1, FNDC1, FOXF2 and HLX showed a significant association with overall survival (p = 2.939e-6) (Fig 7M; S12B File).
Discussion
Heterogeneity in treatment outcome is a major aspect of concern in solid tumors. In head and neck cancers (HNSCC), diverse behavioral and molecular etiologies drive tumors in anatomically different sites that further vary in the underlying biological basis, histology and treatment response. Consequently, with treatment currently being administered based on clinical/histological parameters, accurate prognostication is still a rising challenge. Given the availability of multi-modality treatments as first line therapy, designing treatment strategies based on accurate understanding of treatment outcome is imperative; identification of molecular markers that can improve accuracy of prognosis is hence of extreme significance. The cataloging of biomarkers that correlate to tumor biology, its clinicopathological characteristics and evaluation of their prognostic ability is an essential step towards identifying candidate markers of clinical utility [8,24]. Significant advances in global profiling technologies has improved the understanding of the mechanisms underlying the disease and thereby generating a repertoire of biomarkers that can be assessed/validated for their clinical relevance. This study attempted to identify clinically relevant, molecular prognosticators pertaining to etiology, site, stage and pathological severity, by the meta-analysis approach.
Etiology based categorization was carried out in terms of HPV associated disease, HPV being the major cause of viral associated disease in HNSCC. Although oropharyngeal cancers are the major site with HPV-associated etiology, recent studies have attributed a role in oral cancers too [11] [25] [26]. In our study, although 63 genes, including RELB, the upregulation of which leads to TRAF regulated over expression of HPV E6 protein [27], only three showed a survival impact. RPPP25, NUDCD2 were poor prognosticators, while, interestingly, NOVA1 was a good prognosticator in patients with HPV. NOVA1 is known to be involved in the downregulation of E6 and E7 proteins in HPV associated cancers [28] and is hence indicated as a good prognosticator in HPV+ patients. These genes can be possible prognosticators in HPV+ cancers, subject to large scale clinical validation.
Site-specific meta-analysis of publicly available data identified a subset of genes that were common across the three major sites of HNSCC (tongue, laryngopharynx, oropharynx); the pathways that were primarily enriched included focal adhesion and proteoglycans in cancer, and cell cycle, mitotic pathway, which are well known in various cancers [29–37]. Differential expression profiling of the tongue cancer cohort triaged based on the stage, pathology identified multiple candidate markers; JAM2, SMURF1, LY6E, MFN1 and SUPT16H. JAM2 is reported to be instrumental for metastatic progression in breast and colon cancer [38,39], while others are known regulators of migratory/invasive properties in many cancers [31,40–44]. Analysis in laryngopharyngeal cohort also identified markers of early stage disease with PNI/ALI which included MFN1, PAK2, PIK3CA, FXR1 (differentials), PRRC2B and CBLL1 (with prognostic efficacy), all of which were previously identified to play a major role in head and neck oncogenesis as well as in other cancers [45–52] EPS8, identified in ECS- laryngopharyngeal patients in this study, is known to be involved in the proliferation apoptosis, adhesion and migration in other cancers including HNSCC [53–56]. The correlation of these markers with the clinically/pathologically classified sub-cohorts (ALI+/PNI+) indicated their possible relevance as predictive panel, with a subset of genes providing survival impact. A comparison of the prognosticators identified for tongue and laryngopharynx indicated that AP2M1 and IGF2BP2 are common across sites, with deregulation of AP2M1, a protein trafficking molecule, signifying contradictory effects in tongue and laryngeal cancer. NUDCD1 also known as CML66, located on the chromosome 8q23, was one of the significant unique poor prognosticator in advanced tongue cancers along with MTFR1, IGF2BP2, TSTA3; genes that associated with survival in tongue cancer, many of these genes have been identified as involved in tumorigenesis, metastases, immune suppression in solid tumors [57–60]. The prognosis of laryngeal cancers could be improved by addition of markers such as IL1RAP, LANCL2, RYK, and SLC33A1. IL1RAP, interleukin involved in synthesis of pro inflammatory proteins [61], RYK a member receptor protein tyrosine kinases have been reported in drug resistance, cell motility, anchorage independent cell growth and other tumorigenic properties [62–64].
In tongue and laryngopharyngeal cancers, advanced stage of the disease signifies poor prognosis. Nevertheless, studies have also shown a subset of early stage patients that have an extremely poor prognosis [65,66]. Biomarkers that can further classify these patients based on outcome can be immensely valuable in this context. Early stage tongue cancer patients were significantly classified into patients with poor/good survival based on alterations in AP2M1, CTBP1, and MTFR1 all of whom were associated with prognosis in other studies [60,67,68]. Additionally OTUD6B alterations, which was prognostic in advanced stage (III-IV) patients was also significant predictor of survival in early stage patients with perineural invasion/angiolymphatic invasion (Stage I-II/PNI+/ALI+), indicating that this marker is a clear indicator of advanced disease. Expectedly, the prognosticators of early stage laryngopharyngeal cohort were completely unique and included TWISTNB and UBE2Q1, previously designated poor prognosticators in breast and hepatocellular cancers [69,70].
Pathological parameters that include perineural invasion, angiolymphatic invasion, and extracapsular spread signify poor prognosis; assessment of markers associated with these parameters, identified the adjunct predictors. Interestingly, there were no common significant predictor of ALI/PNI across the two sites (MFN1 was a differential but did not show relevance as a prognosticator). In PNI/ALI+ tongue cancer, the panel of TFG (tumor suppressor gene) [71], TMEM267, BRIX1 (good prognosticators), NUDCD1 and PRKCI (poor prognosticators) together could categorize the patients based on survival; PRKC1 expression is known to be associated with metastasis and poor prognosis in esophageal/ovarian cancer [72,73]. The predictor panel in patients with PNI/ALI in laryngopharyngeal cancer consisted of AP2M1, LANCL2, PFN2, RPN1, MAP3K13, WWTR1 and IL1RAP; WWTR1 being a transcriptional coactivator regulating cell proliferation, differentiation, survival and apoptosis in oral cancer cells [74,75], while LANCL2 is associated with the EGFR pathway in many cancer [76,77]. The association of these markers with PNI and ALI indicated their possible role in the processes apart from their significance as adjunct prognosticators. Assessment of ECS, an additional prognosticator in advanced stage disease, indicated a distinct set of markers in tongue cancer (EXT1, GMPS, TSTA3), while in laryngopharyngeal cancers a majority of the markers were common those associated with late stage disease. Notably, in tongue cancer patients without ECS, the markers of advanced disease were poor prognosticators indicating their relevance as adjunct prognosticators in the absence of clinical/pathological parameters.
The oropharyngeal cohort was assessed separately, given the high prevalence of HPV-associated etiology. Low sample numbers precluded extensive validation in TCGA patient cohort; nevertheless a distinct alteration pattern of candidate genes was observed between HPV+ and HPV–cohorts of oropharyngeal cancers. A distinct set of prognosticators were identified for HPV+ and HPV- oropharyngeal cancers; ECHDC2, GGT6, HERC5 were showed to be associated with HPV+; HERC5 has a known anti-viral activity [78] [79] and its possible role in HNSCC HPV associated cancer is yet to be studied. On the other hand alterations in GRB10, EMILIN1, FNDC1, FOXF2 and HLX, determined poor prognosis in the HPV- cohort; this subset is involved in multiple carcinogenic processes in different solid tumors [80] [81] [82] [83] [84] [85].
The clinical relevance of molecular prognosticators is contentious, given the vast repertoire of data, and the lack of adequate validation to accurately pin-point the beneficial patient cohort. In view of the extremely clinical, pathological, cellular and molecular heterogeneity inherent in all cancers including head and neck cancers, the utility of the biomarkers need to be customized to the sub-categories of patients with definite clinico-pathological parameters prior to validation. This study was an attempt to leverage the existing high throughput studies to specify the predictive/prognostic marker pattern that correlate to the various clinic-pathological sub-types in tongue/ laryngopharyngeal/oropharyngeal cancers, the most common sub-sites of head and neck cancer. Although a common thread of pathways/biomarkers was observed, the distinct marker subset that represented each of the sub-cohorts emphasizes the point of discussion. The primary limitation was that this study was confined to three sub sites; it can be expanded to other sites in order to enable accurate triaging of patients based on risk and thereby provide appropriate treatment. Marker panels that classified the sub-cohorts with historically good prognosis, such as the patients with early stage disease, patients without extra-capsular spread, can prove to be invaluable candidates to improve on the current prognostic indicators (S2 Fig). Distinct and large scale clinical validation is mandatory prior to the adoption of these markers into a clinical setting; nevertheless this study points to the need of customized marker mapping in patients and provides a database of annotated candidates for subsequent validation.
Supporting information
The selection pipeline for the series is indicated including the comprehensive search criteria, screening process and details of the eligible studies.
(TIF)
A pictorial representation of all markers that were significantly associated with prognosticators of HPV associated cancer and different subsites of HNSCC (tongue, laryngopharyngeal and Oropharyngeal cancer) with various clinic-pathological parameters.
(TIF)
(XLSX)
(XLSX)
List of differentially expressed genes obtained from HPV based analysis (A). List of Gene Ontology categories from TOPPFUN enrichment analysis for HPV associated cancer (B). List of KEGG and Reactome pathways obtained from TOPPFUN enrichment analysis for HPV associated cancer (C).
(XLSX)
List of genes altered (gene expression) in >10% of the TCGA HPV specific cohort (A). List of genes altered (gene expression) in >10% of the TCGA HPV positive specific cohort (B). List of genes altered (gene expression) in >10% of the TCGA HPV negative specific cohort (C). List of significant genes altered (gene expression) only in HPV positive specific cohort (D). Kaplan-Meier analysis of individual genes for their survival in HPV positive cohort (E).
(XLSX)
List of differentially expressed genes obtained from Tongue cancer (A). List of Gene Ontology categories from TOPPFUN enrichment analysis for tongue cancer gene entities (B). List of KEGG and Reactome pathways obtained from TOPPFUN enrichment analysis for tongue cancer gene entities (C). List of genes altered (gene expression) in >10% of the TCGA tongue specific cohort (D).
(XLSX)
List of significant markers which are differentially expressed in various sub cohorts of patients with tongue cancer in TCGA (A). Kaplan-Meier curve analysis of significant gene entities in various sub cohorts of patient with tongue cancer in TCGA (B).
(XLSX)
List of differentially expressed genes obtained from laryngopharyngeal specific cohort (A). Gene Ontology categories from TOPPFUN enrichment analysis for laryngopharyngeal cancer gene entities (B). KEGG and Reactome pathways obtained from TOPPFUN enrichment analysis for laryngopharyngeal cancer gene entities (C). List of genes altered (mRNA expression) in >10% of the TCGA laryngopharyngeal specific cohort (D).
(XLSX)
List of significant gene entities which are differentially expressed in various sub cohorts of patients with laryngopharyngeal cancer in TCGA (A). Kaplan-Meier analysis of differential genes for their survival in various sub cohorts of patients with laryngopharyngeal cancer in TCGA (B).
(XLSX)
Kaplan-Meier analysis of individual genes for their survival in TCGA laryngopharyngeal cancer cohort (A). Kaplan-Meier analysis of combination markers for their survival in TCGA laryngopharyngeal cancer cohort (B).
(XLSX)
List of differentially expressed genes obtained from Oropharyngeal cohort (A). List of Gene Ontology categories from TOPPFUN enrichment analysis for Oropharyngeal cohort (B). List of KEGG and Reactome pathways obtained from TOPPFUN enrichment analysis for Oropharyngeal cohort (C).
(XLSX)
List of genes altered (gene expression) in >10% of the TCGA Oropharynx HPV specific cohort (A). List of genes altered (gene expression) in >10% of the TCGA Oropharynx HPV positive specific cohort (B). List of genes altered (gene expression) in >10% of the TCGA HPV negative specific cohort (C). List of genes altered (gene expression) only in HPV positive specific cohort (D).
(XLSX)
Kaplan-Meier analysis of individual genes for their survival in Oropharyngeal HPV positive TCGA patients (A). Kaplan-Meier analysis of individual genes for their survival in Oropharyngeal HPV negative TCGA patients (B).
(XLSX)
(DOC)
Acknowledgments
The project was funded by Indian Council of Medical Research, India (No: 5/8/10-18 (Oto)/CFP/11-NCD-1). We acknowledge Dr. Moni Abraham Kuriakose, Chief, Surgical oncology and Head, Head and Neck Oncology department and Dr. Vijay Pillai, Consultant, Head and Neck Oncology Department, Mazumdar Shaw Medical Center, Bangalore for their guidance in inferring the data. We thankfully acknowledged the financial assistance from Indian Council of Medical Research (ICMR), in the form of Senior Research Fellowship (SRF), New Delhi, India to the first author Mr. Ram Bhupal Reddy (No.3/2/2/277/2014/NCD-III).
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study received financial support from the Indian Council of Medical Research (ICMR) (https://www.icmr.nic.in) through grant 5/8/10-18 (Oto)/CFP/11-NCD-1 (to AS) and in the form of Senior Research Fellowship (SRF) 3/2/2/277/2014/NCD-III (to RBR). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Vasan K, Low TH, Gupta R, Ashford B, Asher R, et al. (2018) Lymph node ratio as a prognostic factor in metastatic cutaneous head and neck squamous cell carcinoma. Head Neck 40: 993–999. 10.1002/hed.25066 [DOI] [PubMed] [Google Scholar]
- 2.Wreesmann VB, Katabi N, Palmer FL, Montero PH, Migliacci JC, et al. (2016) Influence of extracapsular nodal spread extent on prognosis of oral squamous cell carcinoma. Head Neck 38 Suppl 1: E1192–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu SA, Wang CC, Jiang RS, Lee FY, Lin WJ, et al. (2017) Pathological features and their prognostic impacts on oral cavity cancer patients among different subsites—A singe institute’s experience in Taiwan. Sci Rep 7: 7451 10.1038/s41598-017-08022-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jerjes W, Upile T, Petrie A, Riskalla A, Hamdoon Z, et al. (2010) Clinicopathological parameters, recurrence, locoregional and distant metastasis in 115 T1-T2 oral squamous cell carcinoma patients. Head Neck Oncol 2: 9 10.1186/1758-3284-2-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bottcher A, Dommerich S, Sander S, Olze H, Stromberger C, et al. (2016) Nodal yield of neck dissections and influence on outcome in laryngectomized patients. Eur Arch Otorhinolaryngol 273: 3321–3329. 10.1007/s00405-016-3928-2 [DOI] [PubMed] [Google Scholar]
- 6.Wurdemann N, Wagner S, Sharma SJ, Prigge ES, Reuschenbach M, et al. (2017) Prognostic Impact of AJCC/UICC 8th Edition New Staging Rules in Oropharyngeal Squamous Cell Carcinoma. Front Oncol 7: 129 10.3389/fonc.2017.00129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jadhav KB, Gupta N (2013) Clinicopathological prognostic implicators of oral squamous cell carcinoma: need to understand and revise. N Am J Med Sci 5: 671–679. 10.4103/1947-2714.123239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas GR, Nadiminti H, Regalado J (2005) Molecular predictors of clinical outcome in patients with head and neck squamous cell carcinoma. Int J Exp Pathol 86: 347–363. 10.1111/j.0959-9673.2005.00447.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta S, Kushwaha VS, Verma S, Khan H, Bhatt ML, et al. (2016) Understanding molecular markers in recurrent oral squamous cell carcinoma treated with chemoradiation. Heliyon 2: e00206 10.1016/j.heliyon.2016.e00206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Culie D, Garrel R, Viotti J, Schiappa R, Chamorey E, et al. (2018) Impact of HPV-associated p16-expression and other clinical factors on therapeutic decision-making in patients with oropharyngeal cancer: A GETTEC multicentric study. Eur J Surg Oncol 44: 1908–1913. 10.1016/j.ejso.2018.05.022 [DOI] [PubMed] [Google Scholar]
- 11.Valls-Ontanon A, Hernandez-Losa J, Somoza Lopez de Haro R, Bellosillo-Paricio B, Ramon YCS, et al. (2019) Impact of human papilloma virus in patients with oral and oropharyngeal squamous cell carcinomas. Med Clin (Barc) 152: 174–180. [DOI] [PubMed] [Google Scholar]
- 12.Maghami E, Koyfman SA, Weiss J (2018) Personalizing Postoperative Treatment of Head and Neck Cancers. Am Soc Clin Oncol Educ Book 38: 515–522. 10.1200/EDBK_201087 [DOI] [PubMed] [Google Scholar]
- 13.Carlton JA, Maxwell AW, Bauer LB, McElroy SM, Layfield LJ, et al. (2017) Computed tomography detection of extracapsular spread of squamous cell carcinoma of the head and neck in metastatic cervical lymph nodes. Neuroradiol J 30: 222–229. 10.1177/1971400917694048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewis JS Jr., Carpenter DH, Thorstad WL, Zhang Q, Haughey BH (2011) Extracapsular extension is a poor predictor of disease recurrence in surgically treated oropharyngeal squamous cell carcinoma. Mod Pathol 24: 1413–1420. 10.1038/modpathol.2011.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang X, Tian X, Wu K, Liu W, Li S, et al. (2018) Prognostic impact of perineural invasion in early stage oral tongue squamous cell carcinoma: Results from a prospective randomized trial. Surg Oncol 27: 123–128. 10.1016/j.suronc.2018.02.005 [DOI] [PubMed] [Google Scholar]
- 16.Huang TY, Hsu LP, Wen YH, Huang TT, Chou YF, et al. (2010) Predictors of locoregional recurrence in early stage oral cavity cancer with free surgical margins. Oral Oncol 46: 49–55. 10.1016/j.oraloncology.2009.10.011 [DOI] [PubMed] [Google Scholar]
- 17.Roesch-Ely M, Nees M, Karsai S, Ruess A, Bogumil R, et al. (2007) Proteomic analysis reveals successive aberrations in protein expression from healthy mucosa to invasive head and neck cancer. Oncogene 26: 54–64. 10.1038/sj.onc.1209770 [DOI] [PubMed] [Google Scholar]
- 18.Suresh A, Vannan M, Kumaran D, Gumus ZH, Sivadas P, et al. (2012) Resistance/response molecular signature for oral tongue squamous cell carcinoma. Dis Markers 32: 51–64. 10.3233/DMA-2012-0860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ziober AF, D’Alessandro L, Ziober BL (2010) Is gene expression profiling of head and neck cancers ready for the clinic? Biomark Med 4: 571–580. 10.2217/bmm.10.71 [DOI] [PubMed] [Google Scholar]
- 20.Goonesekere NC, Wang X, Ludwig L, Guda C (2014) A meta analysis of pancreatic microarray datasets yields new targets as cancer genes and biomarkers. PLoS One 9: e93046 10.1371/journal.pone.0093046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reddy RB, Bhat AR, James BL, Govindan SV, Mathew R, et al. (2016) Meta-Analyses of Microarray Datasets Identifies ANO1 and FADD as Prognostic Markers of Head and Neck Cancer. PLoS One 11: e0147409 10.1371/journal.pone.0147409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, et al. (2013) Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 6: pl1 10.1126/scisignal.2004088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, et al. (2012) The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2: 401–404. 10.1158/2159-8290.CD-12-0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silva P, Homer JJ, Slevin NJ, Musgrove BT, Sloan P, et al. (2007) Clinical and biological factors affecting response to radiotherapy in patients with head and neck cancer: a review. Clin Otolaryngol 32: 337–345. 10.1111/j.1749-4486.2007.01544.x [DOI] [PubMed] [Google Scholar]
- 25.Prawdzic Senkowska A, Kiczmer P, Strzelczyk JK, Kowalski D, Krakowczyk L, et al. (2019) Impact of HPV infection on gene expression and methylation in oral cancer patients. J Med Microbiol 68: 440–445. 10.1099/jmm.0.000898 [DOI] [PubMed] [Google Scholar]
- 26.Cohen N, Fedewa S, Chen AY (2018) Epidemiology and Demographics of the Head and Neck Cancer Population. Oral Maxillofac Surg Clin North Am 30: 381–395. 10.1016/j.coms.2018.06.001 [DOI] [PubMed] [Google Scholar]
- 27.Zhang J, Chen T, Yang X, Cheng H, Spath SS, et al. (2018) Attenuated TRAF3 Fosters Activation of Alternative NF-kappaB and Reduced Expression of Antiviral Interferon, TP53, and RB to Promote HPV-Positive Head and Neck Cancers. Cancer Res 78: 4613–4626. 10.1158/0008-5472.CAN-17-0642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu J, Liu H, Yang Y, Wang X, Liu P, et al. (2019) Genome-Wide Profiling of Cervical RNA-Binding Proteins Identifies Human Papillomavirus Regulation of RNASEH2A Expression by Viral E7 and E2F1. MBio 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ansari SS, Sharma AK, Zepp M, Ivanova E, Bergmann F, et al. (2018) Upregulation of cell cycle genes in head and neck cancer patients may be antagonized by erufosine’s down regulation of cell cycle processes in OSCC cells. Oncotarget 9: 5797–5810. 10.18632/oncotarget.23537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo D, Fu J (2018) Identifying characteristic miRNAs-genes and risk pathways of multiple sclerosis based on bioinformatics analysis. Oncotarget 9: 5287–5300. 10.18632/oncotarget.23866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tao Y, Sun C, Zhang T, Song Y (2017) SMURF1 promotes the proliferation, migration and invasion of gastric cancer cells. Oncol Rep 38: 1806–1814. 10.3892/or.2017.5825 [DOI] [PubMed] [Google Scholar]
- 32.Sun J, Yan J, Yuan X, Yang R, Dan T, et al. (2016) A computationally constructed ceRNA interaction network based on a comparison of the SHEE and SHEEC cell lines. Cell Mol Biol Lett 21: 21 10.1186/s11658-016-0022-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang M, Gao CE, Li WH, Yang Y, Chang L, et al. (2017) Microarray based analysis of gene regulation by mesenchymal stem cells in breast cancer. Oncol Lett 13: 2770–2776. 10.3892/ol.2017.5776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osathanon T, Nowwarote N, Pavasant P (2016) Expression and influence of Notch signaling in oral squamous cell carcinoma. J Oral Sci 58: 283–294. 10.2334/josnusd.15-0535 [DOI] [PubMed] [Google Scholar]
- 35.Zhou X, Zheng R, Zhang H, He T (2015) Pathway crosstalk analysis of microarray gene expression profile in human hepatocellular carcinoma. Pathol Oncol Res 21: 563–569. 10.1007/s12253-014-9855-x [DOI] [PubMed] [Google Scholar]
- 36.Huang WC, Chan SH, Jang TH, Chang JW, Ko YC, et al. (2014) miRNA-491-5p and GIT1 serve as modulators and biomarkers for oral squamous cell carcinoma invasion and metastasis. Cancer Res 74: 751–764. 10.1158/0008-5472.CAN-13-1297 [DOI] [PubMed] [Google Scholar]
- 37.Oh JE, Kim JO, Shin JY, Zhang XH, Won HS, et al. (2013) Molecular genetic characterization of p53 mutated oropharyngeal squamous cell carcinoma cells transformed with human papillomavirus E6 and E7 oncogenes. Int J Oncol 43: 383–393. 10.3892/ijo.2013.1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lian S, Meng L, Xing X, Yang Y, Qu L, et al. (2016) PRL-3 promotes cell adhesion by interacting with JAM2 in colon cancer. Oncol Lett 12: 1661–1666. 10.3892/ol.2016.4836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Culhane AC, Quackenbush J (2009) Confounding effects in "A six-gene signature predicting breast cancer lung metastasis". Cancer Res 69: 7480–7485. 10.1158/0008-5472.CAN-08-3350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lv Y, Song Y, Ni C, Wang S, Chen Z, et al. (2018) Overexpression of Lymphocyte Antigen 6 Complex, Locus E in Gastric Cancer Promotes Cancer Cell Growth and Metastasis. Cell Physiol Biochem 45: 1219–1229. 10.1159/000487453 [DOI] [PubMed] [Google Scholar]
- 41.Yeom CJ, Zeng L, Goto Y, Morinibu A, Zhu Y, et al. (2016) LY6E: a conductor of malignant tumor growth through modulation of the PTEN/PI3K/Akt/HIF-1 axis. Oncotarget 7: 65837–65848. 10.18632/oncotarget.11670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kwon A, Lee HL, Woo KM, Ryoo HM, Baek JH (2013) SMURF1 plays a role in EGF-induced breast cancer cell migration and invasion. Mol Cells 36: 548–555. 10.1007/s10059-013-0233-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao J, Zhang J, Yu M, Xie Y, Huang Y, et al. (2013) Mitochondrial dynamics regulates migration and invasion of breast cancer cells. Oncogene 32: 4814–4824. 10.1038/onc.2012.494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kwei KA, Shain AH, Bair R, Montgomery K, Karikari CA, et al. (2011) SMURF1 amplification promotes invasiveness in pancreatic cancer. PLoS One 6: e23924 10.1371/journal.pone.0023924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gupta A, Ajith A, Singh S, Panday RK, Samaiya A, et al. (2018) PAK2-c-Myc-PKM2 axis plays an essential role in head and neck oncogenesis via regulating Warburg effect. Cell Death Dis 9: 825 10.1038/s41419-018-0887-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garcia-Escudero R, Segrelles C, Duenas M, Pombo M, Ballestin C, et al. (2018) Overexpression of PIK3CA in head and neck squamous cell carcinoma is associated with poor outcome and activation of the YAP pathway. Oral Oncol 79: 55–63. 10.1016/j.oraloncology.2018.02.014 [DOI] [PubMed] [Google Scholar]
- 47.Qie S, Majumder M, Mackiewicz K, Howley BV, Peterson YK, et al. (2017) Fbxo4-mediated degradation of Fxr1 suppresses tumorigenesis in head and neck squamous cell carcinoma. Nat Commun 8: 1534 10.1038/s41467-017-01199-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fan Y, Yue J, Xiao M, Han-Zhang H, Wang YV, et al. (2017) FXR1 regulates transcription and is required for growth of human cancer cells with TP53/FXR2 homozygous deletion. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pattle SB, Utjesanovic N, Togo A, Wells L, Conn B, et al. (2017) Copy number gain of 11q13.3 genes associates with pathological stage in hypopharyngeal squamous cell carcinoma. Genes Chromosomes Cancer 56: 185–198. 10.1002/gcc.22425 [DOI] [PubMed] [Google Scholar]
- 50.Majumder M, House R, Palanisamy N, Qie S, Day TA, et al. (2016) RNA-Binding Protein FXR1 Regulates p21 and TERC RNA to Bypass p53-Mediated Cellular Senescence in OSCC. PLoS Genet 12: e1006306 10.1371/journal.pgen.1006306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Du L, Chen X, Cao Y, Lu L, Zhang F, et al. (2016) Overexpression of PIK3CA in murine head and neck epithelium drives tumor invasion and metastasis through PDK1 and enhanced TGFbeta signaling. Oncogene 35: 4641–4652. 10.1038/onc.2016.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park J, Kim JM, Park JK, Huang S, Kwak SY, et al. (2015) Association of p21-activated kinase-1 activity with aggressive tumor behavior and poor prognosis of head and neck cancer. Head Neck 37: 953–963. 10.1002/hed.23695 [DOI] [PubMed] [Google Scholar]
- 53.Huang R, Liu H, Chen Y, He Y, Kang Q, et al. (2018) EPS8 regulates proliferation, apoptosis and chemosensitivity in BCR-ABL positive cells via the BCR-ABL/PI3K/AKT/mTOR pathway. Oncol Rep 39: 119–128. 10.3892/or.2017.6102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nasri E, Wiesen LB, Knapik JA, Fredenburg KM (2018) Eps8 expression is significantly lower in p16(+) head and neck squamous cell carcinomas (HNSCCs) compared with p16(-) HNSCCs. Hum Pathol 72: 45–51. 10.1016/j.humpath.2017.10.021 [DOI] [PubMed] [Google Scholar]
- 55.Shin WS, Shim HJ, Lee YH, Pyo M, Park JS, et al. (2017) PTK6 Localized at the Plasma Membrane Promotes Cell Proliferation and MigratiOn Through Phosphorylation of Eps8. J Cell Biochem 118: 2887–2895. 10.1002/jcb.25939 [DOI] [PubMed] [Google Scholar]
- 56.Chen C, Liang Z, Huang W, Li X, Zhou F, et al. (2015) Eps8 regulates cellular proliferation and migration of breast cancer. Int J Oncol 46: 205–214. 10.3892/ijo.2014.2710 [DOI] [PubMed] [Google Scholar]
- 57.Sarkar S, Bristow CA, Dey P, Rai K, Perets R, et al. (2017) PRKCI promotes immune suppression in ovarian cancer. Genes Dev 31: 1109–1121. 10.1101/gad.296640.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barghash A, Golob-Schwarzl N, Helms V, Haybaeck J, Kessler SM (2016) Elevated expression of the IGF2 mRNA binding protein 2 (IGF2BP2/IMP2) is linked to short survival and metastasis in esophageal adenocarcinoma. Oncotarget 7: 49743–49750. 10.18632/oncotarget.10439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun Y, Liu X, Zhang Q, Mao X, Feng L, et al. (2016) Oncogenic potential of TSTA3 in breast cancer and its regulation by the tumor suppressors miR-125a-5p and miR-125b. Tumour Biol 37: 4963–4972. 10.1007/s13277-015-4178-4 [DOI] [PubMed] [Google Scholar]
- 60.Wang W, Lim WK, Leong HS, Chong FT, Lim TK, et al. (2015) An eleven gene molecular signature for extra-capsular spread in oral squamous cell carcinoma serves as a prognosticator of outcome in patients without nodal metastases. Oral Oncol 51: 355–362. 10.1016/j.oraloncology.2014.12.012 [DOI] [PubMed] [Google Scholar]
- 61.Wesche H, Korherr C, Kracht M, Falk W, Resch K, et al. (1997) The interleukin-1 receptor accessory protein (IL-1RAcP) is essential for IL-1-induced activation of interleukin-1 receptor-associated kinase (IRAK) and stress-activated protein kinases (SAP kinases). J Biol Chem 272: 7727–7731. 10.1074/jbc.272.12.7727 [DOI] [PubMed] [Google Scholar]
- 62.Adamo A, Fiore D, De Martino F, Roscigno G, Affinito A, et al. (2017) RYK promotes the stemness of glioblastoma cells via the WNT/ beta-catenin pathway. Oncotarget 8: 13476–13487. 10.18632/oncotarget.14564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stanam A, Gibson-Corley KN, Love-Homan L, Ihejirika N, Simons AL (2016) Interleukin-1 blockade overcomes erlotinib resistance in head and neck squamous cell carcinoma. Oncotarget 7: 76087–76100. 10.18632/oncotarget.12590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hamilton G, Rath B, Klameth L, Hochmair M (2015) Receptor tyrosine kinase expression of circulating tumor cells in small cell lung cancer. Oncoscience 2: 629–634. 10.18632/oncoscience.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ord RA, Isaiah A, Dyalram D, Lubek JE (2018) Is Long-Term Follow-Up Mandatory for Stage I Oral Tongue Cancer? J Oral Maxillofac Surg. [DOI] [PubMed] [Google Scholar]
- 66.Deneuve S, Bodard AG, Delquignies E, Janot F, Zrounba P, et al. (2017) Can Early Dissection of Cervical Lymph Nodes Improve Prognosis in Squamous Cell Carcinomas of the Hard Palate. ORL J Otorhinolaryngol Relat Spec 79: 314–322. 10.1159/000480351 [DOI] [PubMed] [Google Scholar]
- 67.Le Duff M, Gouju J, Jonchere B, Guillon J, Toutain B, et al. (2018) Regulation of senescence escape by the cdk4-EZH2-AP2M1 pathway in response to chemotherapy. Cell Death Dis 9: 199 10.1038/s41419-017-0209-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Deng Y, Liu J, Han G, Lu SL, Wang SY, et al. (2010) Redox-dependent Brca1 transcriptional regulation by an NADH-sensor CtBP1. Oncogene 29: 6603–6608. 10.1038/onc.2010.406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang B, Deng C, Wang L, Zhou F, Zhang S, et al. (2018) Upregulation of UBE2Q1 via gene copy number gain in hepatocellular carcinoma promotes cancer progression through beta-catenin-EGFR-PI3K-Akt-mTOR signaling pathway. Mol Carcinog 57: 201–215. 10.1002/mc.22747 [DOI] [PubMed] [Google Scholar]
- 70.Tanabe Y, Tsuda H, Yoshida M, Yunokawa M, Yonemori K, et al. (2017) Pathological features of triple-negative breast cancers that showed progressive disease during neoadjuvant chemotherapy. Cancer Sci 108: 1520–1529. 10.1111/cas.13274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mencinger M, Panagopoulos I, Andreasson P, Lassen C, Mitelman F, et al. (1997) Characterization and chromosomal mapping of the human TFG gene involved in thyroid carcinoma. Genomics 41: 327–331. [DOI] [PubMed] [Google Scholar]
- 72.Tsang TY, Wei W, Itamochi H, Tambouret R, Birrer MJ (2017) Integrated genomic analysis of clear cell ovarian cancers identified PRKCI as a potential therapeutic target. Oncotarget 8: 96482–96495. 10.18632/oncotarget.19946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang YL, Chu JY, Luo ML, Wu YP, Zhang Y, et al. (2008) Amplification of PRKCI, located in 3q26, is associated with lymph node metastasis in esophageal squamous cell carcinoma. Genes Chromosomes Cancer 47: 127–136. 10.1002/gcc.20514 [DOI] [PubMed] [Google Scholar]
- 74.Hiemer SE, Zhang L, Kartha VK, Packer TS, Almershed M, et al. (2015) A YAP/TAZ-Regulated Molecular Signature Is Associated with Oral Squamous Cell Carcinoma. Mol Cancer Res 13: 957–968. 10.1158/1541-7786.MCR-14-0580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li Z, Wang Y, Zhu Y, Yuan C, Wang D, et al. (2015) The Hippo transducer TAZ promotes epithelial to mesenchymal transition and cancer stem cell maintenance in oral cancer. Mol Oncol 9: 1091–1105. 10.1016/j.molonc.2015.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reis-Filho JS, Pinheiro C, Lambros MB, Milanezi F, Carvalho S, et al. (2006) EGFR amplification and lack of activating mutations in metaplastic breast carcinomas. J Pathol 209: 445–453. 10.1002/path.2004 [DOI] [PubMed] [Google Scholar]
- 77.Katoh M, Katoh M (2003) Identification and characterization of human ZPBP-like gene in silico. Int J Mol Med 12: 399–404. [PubMed] [Google Scholar]
- 78.Paparisto E, Woods MW, Coleman MD, Moghadasi SA, Kochar DS, et al. (2018) Evolution-Guided Structural and Functional Analyses of the HERC Family Reveal an Ancient Marine Origin and Determinants of Antiviral Activity. J Virol 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhao C, Hsiang TY, Kuo RL, Krug RM (2010) ISG15 conjugation system targets the viral NS1 protein in influenza A virus-infected cells. Proc Natl Acad Sci U S A 107: 2253–2258. 10.1073/pnas.0909144107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kang YH, Lee KA, Kim JH, Park SG, Yoon DY (2010) Mitomycin C modulates DNA-double strand break repair genes in cervical carcinoma cells. Amino Acids 39: 1291–1298. 10.1007/s00726-010-0568-5 [DOI] [PubMed] [Google Scholar]
- 81.Bunbanjerdsuk S, Vorasan N, Saethang T, Pongrujikorn T, Pangpunyakulchai D, et al. (2019) Oncoproteomic and gene expression analyses identify prognostic biomarkers for second primary malignancy in patients with head and neck squamous cell carcinoma. Mod Pathol. [DOI] [PubMed] [Google Scholar]
- 82.Modica TME, Maiorani O, Sartori G, Pivetta E, Doliana R, et al. (2017) The extracellular matrix protein EMILIN1 silences the RAS-ERK pathway via alpha4beta1 integrin and decreases tumor cell growth. Oncotarget 8: 27034–27046. 10.18632/oncotarget.15067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ren J, Niu G, Wang X, Song T, Hu Z, et al. (2018) Overexpression of FNDC1 in Gastric Cancer and its Prognostic Significance. J Cancer 9: 4586–4595. 10.7150/jca.27672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lo PK (2018) FOXF2 differentially regulates expression of metabolic genes in non-cancerous and cancerous breast epithelial cells. Trends Diabetes Metab 1. 10.15761/TDM.1000103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Piragyte I, Clapes T, Polyzou A, Klein Geltink RI, Lefkopoulos S, et al. (2018) A metabolic interplay coordinated by HLX regulates myeloid differentiation and AML through partly overlapping pathways. Nat Commun 9: 3090 10.1038/s41467-018-05311-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The selection pipeline for the series is indicated including the comprehensive search criteria, screening process and details of the eligible studies.
(TIF)
A pictorial representation of all markers that were significantly associated with prognosticators of HPV associated cancer and different subsites of HNSCC (tongue, laryngopharyngeal and Oropharyngeal cancer) with various clinic-pathological parameters.
(TIF)
(XLSX)
(XLSX)
List of differentially expressed genes obtained from HPV based analysis (A). List of Gene Ontology categories from TOPPFUN enrichment analysis for HPV associated cancer (B). List of KEGG and Reactome pathways obtained from TOPPFUN enrichment analysis for HPV associated cancer (C).
(XLSX)
List of genes altered (gene expression) in >10% of the TCGA HPV specific cohort (A). List of genes altered (gene expression) in >10% of the TCGA HPV positive specific cohort (B). List of genes altered (gene expression) in >10% of the TCGA HPV negative specific cohort (C). List of significant genes altered (gene expression) only in HPV positive specific cohort (D). Kaplan-Meier analysis of individual genes for their survival in HPV positive cohort (E).
(XLSX)
List of differentially expressed genes obtained from Tongue cancer (A). List of Gene Ontology categories from TOPPFUN enrichment analysis for tongue cancer gene entities (B). List of KEGG and Reactome pathways obtained from TOPPFUN enrichment analysis for tongue cancer gene entities (C). List of genes altered (gene expression) in >10% of the TCGA tongue specific cohort (D).
(XLSX)
List of significant markers which are differentially expressed in various sub cohorts of patients with tongue cancer in TCGA (A). Kaplan-Meier curve analysis of significant gene entities in various sub cohorts of patient with tongue cancer in TCGA (B).
(XLSX)
List of differentially expressed genes obtained from laryngopharyngeal specific cohort (A). Gene Ontology categories from TOPPFUN enrichment analysis for laryngopharyngeal cancer gene entities (B). KEGG and Reactome pathways obtained from TOPPFUN enrichment analysis for laryngopharyngeal cancer gene entities (C). List of genes altered (mRNA expression) in >10% of the TCGA laryngopharyngeal specific cohort (D).
(XLSX)
List of significant gene entities which are differentially expressed in various sub cohorts of patients with laryngopharyngeal cancer in TCGA (A). Kaplan-Meier analysis of differential genes for their survival in various sub cohorts of patients with laryngopharyngeal cancer in TCGA (B).
(XLSX)
Kaplan-Meier analysis of individual genes for their survival in TCGA laryngopharyngeal cancer cohort (A). Kaplan-Meier analysis of combination markers for their survival in TCGA laryngopharyngeal cancer cohort (B).
(XLSX)
List of differentially expressed genes obtained from Oropharyngeal cohort (A). List of Gene Ontology categories from TOPPFUN enrichment analysis for Oropharyngeal cohort (B). List of KEGG and Reactome pathways obtained from TOPPFUN enrichment analysis for Oropharyngeal cohort (C).
(XLSX)
List of genes altered (gene expression) in >10% of the TCGA Oropharynx HPV specific cohort (A). List of genes altered (gene expression) in >10% of the TCGA Oropharynx HPV positive specific cohort (B). List of genes altered (gene expression) in >10% of the TCGA HPV negative specific cohort (C). List of genes altered (gene expression) only in HPV positive specific cohort (D).
(XLSX)
Kaplan-Meier analysis of individual genes for their survival in Oropharyngeal HPV positive TCGA patients (A). Kaplan-Meier analysis of individual genes for their survival in Oropharyngeal HPV negative TCGA patients (B).
(XLSX)
(DOC)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.