Abstract
Misestimating risk could lead to disadvantaged choices such as initiation of drug use (or gambling) and transition to regular drug use (or gambling). Although the normative theory in decision-making under risks assumes that people typically take the probability-weighted expectation over possible utilities, experimental studies of choices among risks suggest that outcome probabilities are transformed nonlinearly into subjective decision weights by a nonlinear weighting function that overweights low probabilities and underweights high probabilities. Recent studies have revealed the neurocognitive mechanism of decision-making under risk. However, the role of modulatory neurotransmission in this process remains unclear. Using positron emission tomography, we directly investigated whether dopamine D1 and D2 receptors in the brain are associated with transformation of probabilities into decision weights in healthy volunteers. The binding of striatal D1 receptors is negatively correlated with the degree of nonlinearity of weighting function. Individuals with lower striatal D1 receptor density showed more pronounced overestimation of low probabilities and underestimation of high probabilities. This finding should contribute to a better understanding of the molecular mechanism of risky choice, and extreme or impaired decision-making observed in drug and gambling addiction.
Introduction
Life is filled with risks. Should I take an umbrella with me this morning? Should I buy car insurance? Which therapy or medicine will improve my health? To answer these questions, and choose, weighting the probability of the possible outcomes is crucial. In particular, misestimating risk could lead to disadvantaged choices such as initiation of drug use (or gambling) and transition to regular drug use (or gambling) (Kreek et al., 2005).
Normative theory in decision-making under risks assumes that people combine probabilities and valuation (utility) of possible outcomes in some way, most typically by taking the probability-weighted expectation over possible utilities. While this expected utility theory (von Neumann and Morgenstern, 1944) is the dominant model, a substantial body of evidence shows that decision makers systematically depart from it (Camerer and Loewenstein, 2004). One type of systematic departure is that subjective weights on probabilities appear to be nonlinear: people often overestimate low probabilities (e.g., playing lotteries) and underestimate high probabilities.
A leading alternative to the expected utility theory is the prospect theory (Tversky and Kahneman, 1992). In the prospect theory, objective probabilities, p, are transformed nonlinearly into decision weights w(p) by a weighting function. Experimental estimates suggest the weighting function is regressive, asymmetric, and inverse S-shaped, crossing the diagonal from above at an inflection point (about 1/3) where p = w(p). In an inverse S-shaped nonlinear weighting function, low probabilities are overweighted and moderate to high probabilities are underweighted. The function neatly explains the typically observed pattern of risk-seeking for low probability gain and risk aversion toward high probability gain.
Risky choice is one of the topics explored in a synthesis of economics and neuroscience called neuroeconomics. Neuroeconomics fMRI studies have demonstrated the neural basis for some other features of the prospect theory such as framing effects and loss aversion (De Martino et al., 2006; Tom et al., 2007). Recently, the neural basis for nonlinear weighting function has also been investigated by fMRI. Hsu et al. (2009) reported that the degree of nonlinearity in the neural response to anticipated reward in the striatum reflected the nonlinearity parameter as estimated behaviorally.
A deeper question is how modulatory neurotransmission is involved in the central process of decision-making (Trepel et al., 2005; Rangel et al., 2008; Fox and Poldrack, 2009). Investigation of the relationship between the dopamine (DA) system and prospect theory seems promising, considering the fact that DA is linked to risk-seeking behavior (Leyton et al., 2002) and is involved in disrupted decision-making observed in neuropsychiatric disorders such as drug/gambling addiction and Parkinson's disease (Zack and Poulos, 2004; Steeves et al., 2009). Trepel et al. (2005) speculated in a thoughtful review that DA transmission in the striatum might be involved in shaping probability weighting. Using positron emission tomography (PET), we tested this speculation directly by investigating how DA D1 and D2 receptors in the brain are associated with transformation of probabilities into decision weights. Phasic DA release occurs during reward and reward-predicting stimuli (Grace, 1991; Schultz, 2007). It is suggested that available striatal D1 receptors are preferentially stimulated by phasically released DA, whereas low-level baseline tonic DA release is enough for stimulating striatal D2 receptors (Frank et al., 2007; Schultz, 2007). Because estimating reward cue in our task is considered to induce phasic DA release, we hypothesized that the variability of available D1 receptors might be more associated with individual differences than that of available D2 receptors.
Materials and Methods
Subjects
Thirty-six healthy male volunteers (mean age ± SD, 25.2 ± 4.9 years) were studied. They did not meet the criteria for any psychiatric disorder based on unstructured psychiatric screening interviews. None of the controls were taking alcohol at the time, nor did they have a history of psychiatric disorder, significant physical illness, head injury, neurological disorder, or alcohol or drug dependence. Ten subjects were light to moderate cigarette smokers. All subjects were right-handed according to the Edinburgh Handedness Inventory. The vast majority of subjects were university students or graduate school students (three of the participants had finished university and were employed). All subjects underwent MRI to rule out cerebral anatomic abnormalities. After complete explanation of the study, written informed consent was obtained from all subjects, and the study was approved by the Ethics and Radiation Safety Committee of the National Institute of Radiological Sciences, Chiba, Japan.
Procedure
To estimate decision weight, certainty equivalents were determined outside the PET scanner. The behavioral experiment took place 1–2 h before the first PET scans. The procedure was based on the staircase procedure suggested by Tversky and Kahneman (1992), which is the most efficient method for estimating certainty equivalents (Paulus and Frank, 2006; Fox and Poldrack, 2009). A gamble's certainty equivalent is the amount of sure payoff at which a player is indifferent between the sure payoff and the gamble. Participants were presented with options between a gamble and a sure payoff on a computer monitor (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). Gambles were presented that had an objective probability p of paying a known outcome x (and paying zero otherwise). The different combinations of p and x are shown in supplemental Table 1, available at www.jneurosci.org as supplemental material. There were 22 gambles, and half of them were 10,000 yen (∼$100) gambles. Because 10,000 yen is the highest-value Japanese paper currency, 11 probabilities were used for 10,000 yen gambles to refine the estimation of weighting function. In each trial, the participants chose between a gamble and a sure payoff. The relative position (left and right) of the two options was randomized to counterbalance for order effects. The subjects were told to make hypothetical rather than actual gambles and were instructed as follows: “Two options for possible monetary gain will be presented to you. Option 1 is a sure payoff and option 2 is a gamble. For example, you will see the guaranteed 6,666 yen on one side of the monitor, and see a gamble in which you have a 50% chance of winning 10,000 yen on the other side. Make a choice between the two options according to your preference by pressing the right or left button. There is no correct answer and no time limit. Once you make a choice, the next options will be presented.”
Each time a choice was made between a gamble and a sure payoff in a trial, the amount of a sure payoff in the next trial was adjusted and eight trials per each gamble were iterated to successively narrow the range including the certainty equivalents. The adjustments in the amount of a sure payoff were made in the following manner. The initial range was set between 0 and x (the gamble outcome). The range was divided into thirds. The one-third and the two-thirds intersecting points of the initial range were used as sure payoff options in trials 1 and 2. If the participant accepted the sure option of the two-thirds and rejected that of the one-third in trials 1 and 2, the middle third portion of the initial range was used as a range for trials 3 and 4. If the participant accepted both sure options of the thirds, the lower third part was then used as a range. If the participant rejected both the sure options of the thirds, the upper third part was then used. The new range was again divided into thirds and the same procedure was iterated until the participant completed trial 8. The mean of the final range was used for a certainty equivalent (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). Once a certainty equivalent was estimated for a given gamble, the next gamble was chosen for estimation, and so on. The order of the gambles was randomized across the participants.
Behavioral data estimation
According to the prospect theory, the valuation V of a prospect that pays amount x with probability p is expressed as v(x, p) = w(p) v(x), where v is the subjective value of the amount x, and w is the decision weight of the objective probability p. The utility function is usually assumed to be a power function v(x) = xσ (results are typically similar to other functions). Although several estimations of the nonlinear probability weighing function have been used in previous experiments (Lattimore et al., 1992; Tversky and Kahneman, 1992; Wu and Gonzalez, 1996), we estimated probability weighting using the one-parameter function derived axiomatically by Prelec (1998), w(p) = exp{−(ln(1/p))α} with 0 < α < 1. This function typically fits as well as other functions with one or two parameters (Hsu et al., 2009), and because nonlinearity is fully captured by a single parameter, it is simple to correlate the degree of nonlinearity (α) across individuals with biological measures such as receptor density or fMRI signals (Hsu et al., 2009). This w(p) function has an inverted-S shape with a fixed inflection point at p = 1/e = 0.37 (at that point the probability 1/e also receives decision weight 1/e). The parameter α indicates the degree of nonlinearity. A smaller value of α (closer to 0) means a more nonlinear inflected weighting function and a higher value (closer to 1) means a more linear weighting function. At α = 1 the function is linear. The weighting function and utility function were estimated by least-squares method.
PET scanning
PET studies were performed on ECAT EXACT HR+ (CTI-Siemens). The system provides 63 planes and a 15.5 cm field of view. To minimize head movement, a head fixation device (Fixster) was used. A transmission scan for attenuation correction was performed using a germanium 68–gallium 68 source. Acquisitions were done in three-dimensional mode with the interplane septa retracted. The first group of 18 subjects (mean age ± SD, 24.7 ± 3.8 years) was studied for both D1 receptors and extrastriatal D2 receptors. These 18 subjects came to the PET center twice, once each for the studies of [11C]SCH23390 (R-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine) and [11C]FLB457 ((S)-N-((1-ethyl-2-pyrrolidinyl)methyl)-5-bromo-2,3-dimethoxybenzamide). For evaluation of D1 receptors, a bolus of 215.9 ± 9.8 MBq of [11C]SCH23390 with specific radioactivities (90.1 ± 38.5 GBq/μmol) was injected intravenously from the antecubital vein with a 20 ml saline flush. The fact that [11C]SCH23390 has high affinity for D1 receptors (Ekelund et al., 2007), and that D1 receptors are moderately expressed in the extrastriatal regions (approximately one-fifth of striatal D1 receptor density) (Ito et al., 2008) leads to good reproducibility of both striatal and extrastriatal [11C]SCH23390 bindings (Hirvonen et al., 2001). Although [11C]SCH23390 is a selective radioligand for D1 receptors, it has some affinity for 5HT2A receptors. However, 5HT2A receptor density in the striatum is negligible compared with D1 receptor density. 5HT2A receptor density is never negligible in the extrastriatal regions. Although previous reports in the literature have indicated that [11C]SCH23390 affinity for 5HT2A receptors relative to D1 receptors is negligible, a recent in vivo study reported that approximately one-fourth of the cortical signal of [11C]SCH23390 was due to binding to 5HT2A receptors, suggesting that cautious interpretation of the extrastriatal findings regarding this ligand is recommended (Ekelund et al., 2007). For evaluation of extrastriatal D2 receptors, a bolus of 218.3 ± 13.9 MBq of [11C]FLB457 with high specific radioactivities (238.0 ± 100.8 GBq/μmol) was injected in the same way. [11C]FLB457 has very high affinity for D2 receptors. It is a selective radioligand for D2 receptors and has good reproducibility of extrastriatal D2 bindings (Sudo et al., 2001). Dynamic scans were performed for 60 min for [11C]SCH23390 and 90 min for [11C]FLB457 immediately after the injection. Although [11C]FLB457 accumulates to a high degree in the striatum, striatal data were not evaluated since the duration of the [11C]FLB457 PET study was not sufficient to obtain equilibrium in the striatum (Olsson et al., 1999; Suhara et al., 1999). For radiation safety reason, striatal D2 receptors were evaluated in the second group of the other 18 subjects [mean age ± SD, 25.7 ± SD 5.9 years]. A bolus of 218.2 ± 10.1MBq of [11C]raclopride with a specific radioactivity of 451.1 ± 154.6 GBq/μmol was injected similarly. [11C]Raclopride is a selective radioligand for D2 receptors, and has good reproducibility of striatal D2 bindings (Volkow et al., 1993). Because the density of extrastriatal D2 receptors is less than one-tenth of striatal D2 receptors (Ito et al., 2008), [11C]raclopride is suitable for the evaluation of striatal D2 receptors, but not of extrastriatal D2 receptors, due to its moderate affinity for D2 receptors. Dynamic scans were performed for 60 min. All emission scans were reconstructed with a Hanning filter cutoff frequency of 0.4 (full width at half maximum, 7.5 mm). MRI was performed on Gyroscan NT (Philips Medical Systems) (1.5 T). T1-weighted images of the brain were obtained for all subjects. Scan parameters were 1-mm-thick, three-dimensional T1 images with a transverse plane (repetition time/echo time, 19/10 ms; flip angle, 30°; scan matrix, 256 × 256 pixels; field of view, 256 × 256 mm; number of excitations, 1).
Quantification of D1 and D2 receptors
Because one subject felt discomfort from the head fixation device during the [11C]FLB457 scan, the scan was discontinued and the data of this subject were excluded from the subsequent analysis. Quantitative analysis was performed using the three-parameter simplified reference tissue model (Lammertsma and Hume, 1996; Olsson et al., 1999). This method is well established for [11C]SCH23390, [11C]FLB457 and [11C]raclopride (Lammertsma and Hume, 1996; Olsson et al., 1999) and is widely used (Aalto et al., 2005; Takahashi et al., 2008; McNab et al., 2009; Takahashi et al., 2010), and it allows us to quantify DA receptors without arterial blood sampling, an invasive and time-consuming procedure. The cerebellum was used as reference region because it has been shown to be almost devoid of D1 and D2 receptors (Farde et al., 1987; Suhara et al., 1999). The model provides an estimation of the binding potential [BPND (nondisplaceable)] (Innis et al., 2007), which is defined by the following equation: BPND = k3/k4 = f2 Bmax/{Kd [1 + Σi Fi/Kdi]}, where k3 and k4 describe the bidirectional exchange of tracer between the free compartment and the compartment representing specific binding, f2 is the “free fraction” of nonspecifically bound radioligand in brain, Bmax is the receptor density, Kd is the equilibrium dissociation constant for the radioligand, and Fi and Kdi are the free concentration and the dissociation constant of competing ligands, respectively (Lammertsma and Hume, 1996). Based on this model, we created parametric images of BPND using the basis function method (Gunn et al., 1997) to conduct voxelwise statistical parametric mapping (SPM) analysis.
In addition to the SPM analysis, we conducted region-of-interest (ROI) analysis. The tissue concentrations of the radioactivities of [11C]SCH23390, [11C]FLB457 and [11C]raclopride were obtained from anatomically defined ROIs. The individual MRIs were coregistered on [11C]SCH23390, [11C]FLB457 and [11C]raclopride PET images of summated activity for 60, 90 and 60 min, respectively. The ROIs were defined on coregistered MRI with reference to the brain atlas. Given our hypothesis from the previous literature (Hsu et al., 2009), the ROIs were set on the striatum (caudate and putamen). Manual delineation of caudate and putamen ROIs was based on the dorsal caudate and dorsal putamen criteria, respectively, of Mawlawi et al. (2001). The average values of right and left ROIs were used to increase the signal-to-noise ratio for the calculations.
Statistical analysis
SPM analysis.
Parametric images of BPND of [11C]SCH23390, [11C] FLB457 and [11C]raclopride were analyzed using the SPM2 software package (Wellcome Department of Cognitive Neurology, London, UK) running with MATLAB (MathWorks). Parametric images of BPND were normalized into MNI (Montreal Neurological Institute) template space. Normalized BPND images were smoothed with a Gaussian filter to 8 mm full-width half-maximum. Using each of the individual behavioral parameters (α and σ) as covariate, regression analyses with the BPND images and the covariates were performed. A statistical threshold of p < 0.05 corrected for multiple comparisons across the whole brain was used, except for a priori hypothesized regions, which were thresholded at p < 0.001 uncorrected (r > 0.68) for examination of effect size (only clusters involving 10 or more contiguous voxels are reported). These a priori ROIs included the caudate and putamen.
ROI analysis.
Pearson's correlation coefficients between BPND of [11C]SCH23390 and [11C]raclopride in the ROIs and behavioral parameters (α and σ) were calculated using SPSS software. Because some subjects were smokers, we further calculated partial correlation coefficients between BPND of [11C]SCH23390 and [11C]raclopride and behavioral parameters to control for the potential influence of smoking (number of cigarettes per day).
Results
In the first group, with D1 receptors and extrastriatal D2 receptors investigated, the mean (SD) α of the weighting function and σ of the utility function were 0.58 (0.16) and 0.99 (0.33), respectively. The second group, in which striatal D2 receptors were investigated, the mean (SD) α and σ were 0.56 (0.19) and 0.98 (0.18), respectively, indicating that the two groups were comparable. Averaged weighting functions and value functions of the two groups are shown in Figure 1 and supplemental Figure 3 (available at www.jneurosci.org as supplemental material), respectively. Normalized parametric images of BPND of [11C]SCH23390, [11C]raclopride and [11C]FLB457 are shown in Figure 2A, B, and C, respectively. The mean BPND values of [11C]SCH23390 in the caudate and putamen were 1.86 ± 0.24 and 2.01 ± 0.22, and those of [11C]raclopride were 3.00 ± 0.32 and 3.61 ± 0.37, respectively. Voxel-by-voxel SPM analysis revealed significant positive correlation (r > 0.68, p < 0.001) between striatal D1 receptor binding and the nonlinearity parameter α of weighting function [right striatum, peak (30, −8, −4), 230 voxels; left striatum, peak (−20, −4, 8), 154 voxels] (Fig. 3A). Independent ROI analyses revealed that D1 receptor binding in the putamen showed a significant correlation with α (Fig. 3B; Table 1), and D1 receptor binding in the caudate showed a trend level correlation with α (Table 1). That is, people with lower striatal D1 receptor binding tend to be more risk-seeking for low probability gambles and more risk-averse for high probability gambles. SPM analysis showed that extrastriatal D1 binding was not correlated with α. SPM and ROI analyses revealed that neither striatal nor extrastriatal D2 receptor binding was correlated with α. None of [11C]SCH23390, [11C]FLB457 and [11C]raclopride binding was correlated with the power σ of the value function. Correlation analyses with controlling for the potential influence of smoking revealed identical results, indicating that the influence of smoking was minimal. The results of partial correlation analyses of ROIs between behavioral parameters (α and σ) and BPND values of [11C]SCH23390 and [11C]raclopride in the striatum after controlling for the potential influence of smoking are summarized in supplemental Table 2, available at www.jneurosci.org as supplemental material.
Figure 1.
The fitted probability weighting function with the Prelec model. The red line represents the first group (N = 18 subjects) with D1 receptors and extrastriatal D2 receptors investigated. The black line is the second group (N = 18 subjects) whose striatal D2 receptors were investigated.
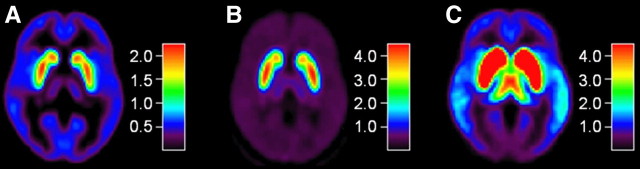
Figure 2.
Maps of DA D1 and D2 BP, averaged across participants (axial slices at the level of Z = 0 of MNI coordinates). A, D1 BP, measured with [11C]SCH23390 (N = 18 subjects). B, Striatal D2 BP, measured with [11C]raclopride (N = 18 subjects). C, Extrastriatal D2 BP, measured with [11C]FLB457 (N = 17 subjects). Although [11C]FLB457 accumulates to a high degree in the striatum, striatal data were not evaluated because the duration of the [11C]FLB457 PET study was not sufficient to obtain equilibrium in the striatum. The bar indicates the range of BP.
Figure 3.
Correlation between nonlinearity of probabilities weighting and D1 binding in the striatum (N = 18 subjects). A, Image showing regions of correlation between nonlinearity parameter of weighting function and D1 binding in the striatum. The bar shows the range of the correlation coefficient. B, Plots and regression line of correlation between α (nonlinearity parameter) and binding potential of the putamen (r = 0.66, p = 0.003).
Table 1.
Correlation between behavioral parameters (α and σ) and BPND values of [11C]SCH23390 (N = 18 subjects) and [11C]raclopride (N = 18 subjects) in the striatum
| α | σ | |
|---|---|---|
| D1 receptors | ||
| Caudate | 0.011 (r = 0.582) | 0.717 (r = 0.092) |
| Putamen | 0.003* (r = 0.658) | 0.260 (r = 0.280) |
| D2 receptors | ||
| Caudate | 0.305 (r = 0.256) | 0.218 (r = 0.305) |
| Putamen | 0.242 (r = 0.291) | 0.122 (r = 0.378) |
p values (correlation coefficients) are shown.
*p < 0.01.
Discussion
We provided the first evidence of a relation between striatal D1 receptor binding and nonlinear probability weighting during decision-making under risk. Based on circumstantial evidence (Kuhnen and Knutson, 2005; Wittmann et al., 2008) and a speculative review (Trepel et al., 2005), it has been suggested that curvature of the weighting function might be modulated by DA transmission. Utilizing a molecular imaging technique, we directly measured the relation between DA receptors and the nonlinearity of weighting function in vivo. Individuals with lower striatal D1 receptor binding showed more nonlinear probability weighting and more pronounced overestimation of low probabilities and underestimation of high probabilities. Low D1 receptor binding means that available receptors for phasically released DA are limited. In such case, phasic DA release in response to positive outcomes can stimulate limited D1 receptors in the striatum. In contrast, low-level baseline tonic DA release is enough for stimulating D2 receptors (Frank et al., 2007; Schultz, 2007). Therefore, the variability of D2 receptor binding might have less impact on current behavioral task during which phasic DA release occurs in response to reward cue.
This molecular imaging approach allows us to broaden our understanding of the neurobiological mechanism underlying nonlinear weighting beyond the current knowledge attained by neuroeconomics fMRI. An fMRI study using a value-titration paradigm has shown that differential anterior cingulate activation during estimation of high probabilities relative to low probabilities was positively correlated with Prelec's nonlinearity parameter α across subjects (Paulus and Frank, 2006). Another fMRI study with risks of electric shocks found similar nonlinear response in the caudate/subgenual anterior cingulate (Berns et al., 2008). More recently, Hsu et al. (2009), using a simpler exposure-choice paradigm, demonstrated that Prelec's nonlinearity parameter α was negatively correlated with striatal activity during reward anticipation under risk. That is, people with a greater degree of nonlinearity in striatal activation to anticipated reward tend to overestimate low probabilities (to be risk-seeking) and underestimate high probabilities (to be risk-averse).
Exploring novelty and risk-seeking behavior are, to some extent, desirable and advantageous for the survival and development of many species including human (Kelley et al., 2004). Being too risk-averse would lose opportunities to obtain possibly better outcomes. However, excessive risk-seeking may contribute to reckless choices such as initiation of drug use (or gambling) and transition to regular drug use (or gambling) (Kreek et al., 2005). Pathological gambling and drug addiction frequently co-occur, and it is suggested that the neurobiological mechanisms underlying the two conditions overlap (Tamminga and Nestler, 2006; Steeves et al., 2009). In fact, pharmacological therapy for drug addiction has been shown to also be effective when applied to pathological gambling (Tamminga and Nestler, 2006). Animal studies demonstrated that stimulation of D1 receptors by a selective agonist increased risky choice and blockade of D1 receptors decreased risky choice in rats. Although D2 agonist/antagonist showed similar actions, their effects were not as pronounced as those of D1 agonist/antagonist (St Onge and Floresco, 2009). A human genetic study reported that variants of the gene for D1 receptors were linked to risky and novelty-seeking behaviors (Comings et al., 1997), although the genes for other subtypes of DA receptors are also linked to those behaviors. More recently, a PET study suggested that reduced D1 receptor binding may be associated with an increased risk of relapse in drug addiction (Martinez et al., 2009).
The curvature of the weighting function is traditionally explained by the psychophysics of diminishing sensitivity, the idea that sensitivity to changes in probability decreases as probability moves away from the endpoints of 0 and 1 (Tversky and Kahneman, 1992). However, it has also been suggested that emotional responses to gambles influence weighting as well. In particular, the overweighting of low-probability gains may reflect hope of winning and the underweighting of high-probability gains may reflect fear of losing a “near sure thing” (Trepel et al., 2005). One study supportive of this hypothesis found more nonlinear weighting functions for gambles over emotional outcomes (kisses and shocks) than over money (Rottenstreich and Hsee, 2001). In this sense, individuals with lower striatal D1 binding might be interpreted as showing more “emotional” decision-making.
We used a simple behavioral task with only positive outcomes to estimate weighting function in this study. Any generalization of our findings needs to be approached with caution. We make more complex decisions in the real world where both positive and negative outcomes are possible, and have to pay attention to relative differences in the magnitude of gains and losses. A computational model has suggested that tonic D2 receptor stimulation in the striatum inhibits response to avoid negative outcomes (Frank et al., 2007), and other neurotransmitters such as serotonin and noradrenaline are thought to be involved in the complex decision-making process (Trepel et al., 2005; Frank et al., 2007; Cools et al., 2008; Doya, 2008). Using behavioral tasks with negative outcomes, future studies to investigate involvements of other neurotransmissions as well as other areas that are related to punishment or negative emotions such as the orbitofrontal cortex, insula and amygdala (Trepel et al., 2005; Pessiglione et al., 2006; Voon et al., 2010) are recommended. Furthermore, our subjects were relatively homogeneous in terms of economic status (the majority were students). Our findings might not be representative of various samples with different background and socioeconomic status. Notwithstanding this limitation, the present study illustrated that molecular imaging can provide a new research direction for neuroeconomics and decision-making studies by more directly investigating the association between striatal DA transmission and nonlinear probability weighting. This approach may shed light on neurotransmission effects on emotional and boundedly rational decision-making in our daily life. At the same time, understanding the molecular mechanism of extreme or impaired decision-making can contribute to the assessment and prevention of drug and gambling addiction and the development of novel pharmacological therapies for those addictions.
Footnotes
This study was supported by a consignment expense for Molecular Imaging Program on “Research Base for PET Diagnosis” from the Ministry of Education, Culture, Sports, Science and Technology (MEXT). We thank Katsuyuki Tanimoto and Takahiro Shiraishi for their assistance in performing the PET experiments at the National Institute of Radiological Sciences. We also thank Yoshiko Fukushima of the National Institute of Radiological Sciences for her help as clinical research coordinator.
References
- Aalto S, Brück A, Laine M, Någren K, Rinne J. Frontal and temporal dopamine release during working memory and attention tasks in healthy humans: a positron emission tomography study using the high-affinity dopamine D2 receptor ligand [11C] FLB 457. J Neurosci. 2005;25:2471–2477. doi: 10.1523/JNEUROSCI.2097-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns GS, Capra CM, Chappelow J, Moore S, Noussair C. Nonlinear neurobiological probability weighting functions for aversive outcomes. Neuroimage. 2008;39:2047–2057. doi: 10.1016/j.neuroimage.2007.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camerer C, Loewenstein G. Behavioral economics: past, present, future. In: Camerer C, Loewenstein G, Rabin M, editors. Advances in behavioral economics. Princeton: Princeton UP; 2004. pp. 3–51. [Google Scholar]
- Comings D, Gade R, Wu S, Chiu C, Dietz G, Muhleman D, Saucier G, Ferry L, Rosenthal RJ, Lesieur HR, Rugle LJ, MacMurray P. Studies of the potential role of the dopamine D1 receptor gene in addictive behaviors. Mol Psychiatry. 1997;2:44–56. doi: 10.1038/sj.mp.4000207. [DOI] [PubMed] [Google Scholar]
- Cools R, Roberts AC, Robbins TW. Serotoninergic regulation of emotional and behavioural control processes. Trends Cogn Sci. 2008;12:31–40. doi: 10.1016/j.tics.2007.10.011. [DOI] [PubMed] [Google Scholar]
- De Martino B, Kumaran D, Seymour B, Dolan RJ. Frames, biases, and rational decision-making in the human brain. Science. 2006;313:684–687. doi: 10.1126/science.1128356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doya K. Modulators of decision making. Nat Neurosci. 2008;11:410–416. doi: 10.1038/nn2077. [DOI] [PubMed] [Google Scholar]
- Ekelund J, Slifstein M, Narendran R, Guillin O, Belani H, Guo NN, Hwang Y, Hwang DR, Abi-Dargham A, Laruelle M. In vivo DA D1 receptor selectivity of NNC 112 and SCH 23390. Mol Imaging Biol. 2007;9:117–125. doi: 10.1007/s11307-007-0077-4. [DOI] [PubMed] [Google Scholar]
- Farde L, Halldin C, Stone-Elander S, Sedvall G. PET analysis of human dopamine receptor subtypes using 11C-SCH 23390 and 11C-raclopride. Psychopharmacology (Berl) 1987;92:278–284. doi: 10.1007/BF00210831. [DOI] [PubMed] [Google Scholar]
- Fox C, Poldrack R. Prospect theory and the brain. In: Glimcher PW, Camerer C, Fehr E, Poldrack R, editors. Neuroeconomics. London: Academic; 2009. pp. 145–174. [Google Scholar]
- Frank MJ, Scheres A, Sherman SJ. Understanding decision-making deficits in neurological conditions: insights from models of natural action selection. Philos Trans R Soc Lond B Biol Sci. 2007;362:1641–1654. doi: 10.1098/rstb.2007.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace A. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41:1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ. Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. Neuroimage. 1997;6:279–287. doi: 10.1006/nimg.1997.0303. [DOI] [PubMed] [Google Scholar]
- Hirvonen J, Någren K, Kajander J, Hietala J. Measurement of cortical dopamine d1 receptor binding with 11C [SCH23390]: a test-retest analysis. J Cereb Blood Flow Metab. 2001;21:1146–1150. doi: 10.1097/00004647-200110000-00002. [DOI] [PubMed] [Google Scholar]
- Hsu M, Krajbich I, Zhao C, Camerer CF. Neural response to reward anticipation under risk is nonlinear in probabilities. J Neurosci. 2009;29:2231–2237. doi: 10.1523/JNEUROSCI.5296-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Ito H, Takahashi H, Arakawa R, Takano H, Suhara T. Normal database of dopaminergic neurotransmission system in human brain measured by positron emission tomography. Neuroimage. 2008;39:555–565. doi: 10.1016/j.neuroimage.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Schochet T, Landry CF. Risk taking and novelty seeking in adolescence: introduction to part I. Ann N Y Acad Sci. 2004;1021:27–32. doi: 10.1196/annals.1308.003. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci. 2005;8:1450–1457. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- Kuhnen CM, Knutson B. The neural basis of financial risk taking. Neuron. 2005;47:763–770. doi: 10.1016/j.neuron.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4:153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- Lattimore P, Baker J, Witte A. The influence of probability on risky choice: a parametric examination. Behav Organ. 1992;17:377–400. [Google Scholar]
- Leyton M, Boileau I, Benkelfat C, Diksic M, Baker G, Dagher A. Amphetamine-induced increases in extracellular dopamine, drug wanting, and novelty seeking: a PET/[11C] raclopride study in healthy men. Neuropsychopharmacology. 2002;27:1027–1035. doi: 10.1016/S0893-133X(02)00366-4. [DOI] [PubMed] [Google Scholar]
- Martinez D, Slifstein M, Narendran R, Foltin RW, Broft A, Hwang DR, Perez A, Abi-Dargham A, Fischman MW, Kleber HD, Laruelle M. Dopamine D1 receptors in cocaine dependence measured with PET and the choice to self-administer cocaine. Neuropsychopharmacology. 2009;34:1774–1782. doi: 10.1038/npp.2008.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, Huang Y, Simpson N, Ngo K, Van Heertum R, Laruelle M. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. accuracy and precision of D2 receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab. 2001;21:1034–1057. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- McNab F, Varrone A, Farde L, Jucaite A, Bystritsky P, Forssberg H, Klingberg T. Changes in cortical dopamine D1 receptor binding associated with cognitive training. Science. 2009;323:800–802. doi: 10.1126/science.1166102. [DOI] [PubMed] [Google Scholar]
- Olsson H, Halldin C, Swahn CG, Farde L. Quantification of [11C]FLB 457 binding to extrastriatal dopamine receptors in the human brain. J Cereb Blood Flow Metab. 1999;19:1164–1173. doi: 10.1097/00004647-199910000-00013. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Frank LR. Anterior cingulate activity modulates nonlinear decision weight function of uncertain prospects. Neuroimage. 2006;30:668–677. doi: 10.1016/j.neuroimage.2005.09.061. [DOI] [PubMed] [Google Scholar]
- Pessiglione M, Seymour B, Flandin G, Dolan RJ, Frith CD. Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature. 2006;442:1042–1045. doi: 10.1038/nature05051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prelec D. The probability weighting function. Econometrica. 1998;66:497–527. [Google Scholar]
- Rangel A, Camerer C, Montague PR. A framework for studying the neurobiology of value-based decision making. Nat Rev Neurosci. 2008;9:545–556. doi: 10.1038/nrn2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenstreich Y, Hsee CK. Money, kisses, and electric shocks: on the affective psychology of risk. Psychol Sci. 2001;12:185–190. doi: 10.1111/1467-9280.00334. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral dopamine signals. Trends Neurosci. 2007;30:203–210. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Steeves TD, Miyasaki J, Zurowski M, Lang AE, Pellecchia G, Van Eimeren T, Rusjan P, Houle S, Strafella AP. Increased striatal dopamine release in Parkinsonian patients with pathological gambling: a [11C] raclopride PET study. Brain. 2009;132:1376–1385. doi: 10.1093/brain/awp054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Onge JR, Floresco SB. Dopaminergic modulation of risk-based decision making. Neuropsychopharmacology. 2009;34:681–697. doi: 10.1038/npp.2008.121. [DOI] [PubMed] [Google Scholar]
- Sudo Y, Suhara T, Inoue M, Ito H, Suzuki K, Saijo T, Halldin C, Farde L. Reproducibility of [11C]FLB 457 binding in extrastriatal regions. Nucl Med Commun. 2001;22:1215–1221. doi: 10.1097/00006231-200111000-00008. [DOI] [PubMed] [Google Scholar]
- Suhara T, Sudo Y, Okauchi T, Maeda J, Kawabe K, Suzuki K, Okubo Y, Nakashima Y, Ito H, Tanada S, Halldin C, Farde L. Extrastriatal dopamine D2 receptor density and affinity in the human brain measured by 3D PET. Int J Neuropsychopharmcol. 1999;2:73–82. doi: 10.1017/S1461145799001431. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Kato M, Takano H, Arakawa R, Okumura M, Otsuka T, Kodaka F, Hayashi M, Okubo Y, Ito H Suhara T. Differential contributions of prefrontal and hippocampal dopamine D1 and D2 receptors in human cognitive functions. J Neurosci. 2008;28:12032–12038. doi: 10.1523/JNEUROSCI.3446-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Takano H, Kodaka F, Arakawa R, Yamada M, Otsuka T, Hirano Y, Kikyo H, Okubo Y, Kato M, Obata T, Ito H, Suhara T. Contribution of dopamine D1 and D2 receptors to amygdala activity in human. J Neurosci. 2010;30:3043–3047. doi: 10.1523/JNEUROSCI.5689-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminga CA, Nestler EJ. Pathological gambling: focusing on the addiction, not the activity. Am J Psychiatry. 2006;163:180–181. doi: 10.1176/appi.ajp.163.2.180. [DOI] [PubMed] [Google Scholar]
- Tom SM, Fox CR, Trepel C, Poldrack RA. The neural basis of loss aversion in decision-making under risk. Science. 2007;315:515–518. doi: 10.1126/science.1134239. [DOI] [PubMed] [Google Scholar]
- Trepel C, Fox CR, Poldrack RA. Prospect theory on the brain? Toward a cognitive neuroscience of decision under risk. Brain Res Cogn Brain Res. 2005;23:34–50. doi: 10.1016/j.cogbrainres.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Tversky A, Kahneman D. Advances in prospect theory: cumulative representation of uncertainty. J Risk Uncertain. 1992;5:297–323. [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Dewey SL, Schlyer D, MacGregor R, Logan J, Alexoff D, Shea C, Hitzemann R, Angrist B, Wolf AP. Reproducibility of repeated measures of carbon-11-raclopride binding in the human brain. J Nucl Med. 1993;34:609–613. [PubMed] [Google Scholar]
- von Neumann J, Morgenstern O. Princeton: Princeton UP; 1944. Theory of games and economic behavior. [Google Scholar]
- Voon V, Pessiglione M, Brezing C, Gallea C, Fernandez HH, Dolan RJ, Hallett M. Mechanisms underlying dopamine-mediated reward bias in compulsive behaviors. Neuron. 2010;65:135–142. doi: 10.1016/j.neuron.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann BC, Daw ND, Seymour B, Dolan RJ. Striatal activity underlies novelty-based choice in humans. Neuron. 2008;58:967–973. doi: 10.1016/j.neuron.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Gonzalez R. Curvature of the probability weighting function. Manage Sci. 1996;42:1676–1690. [Google Scholar]
- Zack M, Poulos CX. Amphetamine primes motivation to gamble and gambling-related semantic networks in problem gamblers. Neuropsychopharmacology. 2004;29:195–207. doi: 10.1038/sj.npp.1300333. [DOI] [PubMed] [Google Scholar]