Abstract
The World Health Organization now recognizes primary mediastinal B-cell lymphoma (PMBCL) as a unique clinical and biologic entity. PMBCL is distinct from other B-cell non-Hodgkin lymphoma subtypes and has features that overlap with classical Hodgkin lymphoma, including a peak incidence in the adolescent and young adult population, mediastinal presentation of disease, and molecular alterations in JAK2 and programmed death ligands. Because PMBCL is rare, there are few prospective clinical trials to guide therapy, resulting in no single standard of care. Given the long life expectancy of survivors of PMBCL, treatment approaches must balance maximizing cure while minimizing long-term toxicity. In this article, I review my approach to the treatment of PMBCL, incorporating data from adult and pediatric studies, as well as recent advances in our understanding of the molecular basis of PMBCL.
Introduction
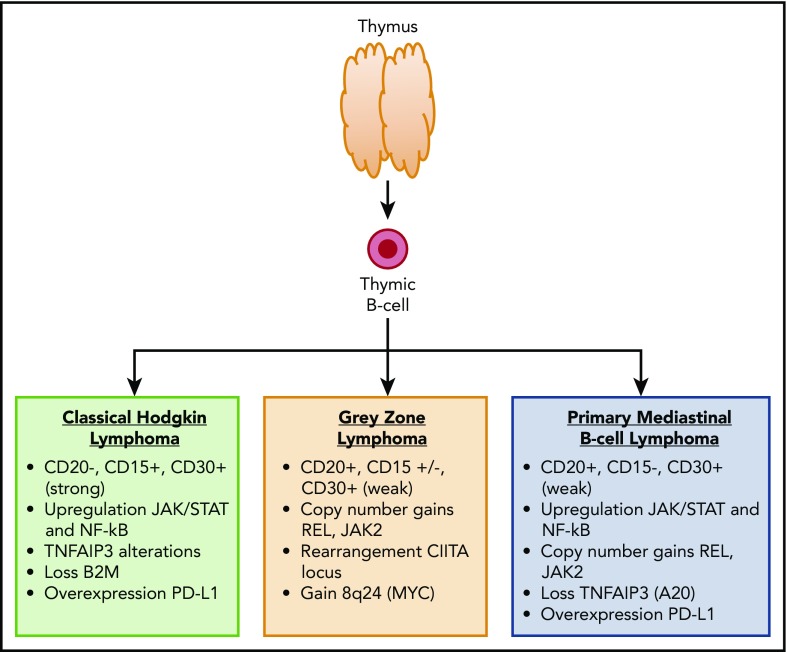
Primary mediastinal B-cell lymphoma (PMBCL) is a rare subtype of non-Hodgkin lymphoma that predominantly occurs in adolescents and young adults (AYAs). Although previously considered a subtype of diffuse large B-cell lymphoma (DLBCL), PMBCL is now recognized by the World Health Organization as a unique entity with distinct clinical and biologic features.1 The diagnosis of PMBCL can present a challenge because the histologic features overlap with nodular sclerosing Hodgkin lymphoma. The malignant cells express B-cell markers (CD19, CD20, CD22, CD79a) but not surface immunoglobulin. CD30 is expression is weak (vs strong in nodular sclerosing Hodgkin lymphoma), and CD15 is negative. B-cell transcription factors are often positive, including PAX5, OCT2, BCL6, and BOB1. Clinically, PMBCL typically presents as a bulky mediastinal mass. Local infiltration to the lung, chest wall, pleura, or pericardium is common. Unlike other non-Hodgkin lymphoma (NHL) subtypes, PMBCL has a female predominance.2 From a biologic standpoint, PMBCL shares many similarities with classical Hodgkin lymphoma, including constitutive activation of the JAK-STAT and NF-κB pathways3,4 (Figure 1). Similar to Hodgkin lymphoma, PMBCL exhibits immune evasion, likely as a result of downregulation of MHC class I and II and upregulation of programmed death ligands.5-7
Figure 1.
Immunophenotype and common molecular alterations among thymic B-cell–derived lymphomas.
The clinical management of PMBCL varies across centers, with no single standard of care. A variety of upfront chemotherapy approaches have been studied by adult and pediatric groups without consensus on the optimal regimen. In addition, the use of radiation therapy (RT) varies across centers. Although many approaches historically included consolidative radiation, there is an interest to reduce the exposure to RT in this young predominantly female population, given the risk for long-term toxicity. Some of the major unanswered clinical questions in PMBCL include: (1) what is the optimal upfront chemoimmunotherapy regimen?, (2) when should RT be used?, (3) can fluorodeoxyglucose (FDG)–positron emission tomography (PET) be used to stratify patients and guide therapy?, and (4) what is the role for novel agents? In this article, I present 2 case vignettes that highlight major clinical dilemmas in PMBCL, and I discuss my approach to their management, taking into consideration the emerging clinical literature, as well as our current understanding of disease biology.
Case 1
An 18-year-old woman presents with a 1-month history of back pain and a 2-week history of fever and progressive dyspnea. Bloodwork is remarkable for a lactate dehydrogenase level of 716 IU/L. A chest radiograph shows a markedly enlarged cardiomediastinal silhouette. A chest computed tomography (CT) scan reveals a 12.1 × 7.4-cm anterior mediastinal mass with mass effect on the aortic arch and main pulmonary artery and compression of the superior vena cava. There are several small pulmonary nodules up to 1 cm in size. There is a small pericardial effusion and moderate pleural effusions. A biopsy of the mediastinal mass is performed. Flow cytometry reveals an abnormal B-cell population that is CD19+, CD20+(bright), CD5−, CD10−, and surface immunoglobulin−. Microscopic examination reveals a diffuse proliferation of atypical medium- to large-sized lymphoid cells. The atypical cells are positive for CD20, BCL6, BCL2, MUM1, CD23 (partial), and CD30(weak). They are negative for CD3, CD10, and CD15. The Ki67 proliferative fraction is ∼90%. These findings, in conjunction with the clinical presentation, are consistent with PMBCL. A PET scan is performed. The mediastinal mass is FDG avid, with a standardized uptake value (SUV) max of 20.4. The pulmonary nodules are also FDG avid, with SUV ranging from 3.0 to 5.4. There are no abnormalities below the diaphragm. Bone marrow and cerebrospinal fluid are negative for lymphoma.
What is the optimal upfront therapy for PMBCL?
Because PMBCL is uncommon, there are few prospective trials to establish a standard therapeutic approach. In pediatric and adult patients, the majority of the data to support clinical management has been extrapolated from retrospective series or from subgroup analyses of prospective trials designed for DLBCL (adult trials) or Burkitt lymphoma (BL; pediatric trials).
Adult approach
Although there is no single approach to initial therapy, a rituximab and anthracycline–containing regimen is used at most centers (Table 1). In the United States, the CHOP regimen (cyclophosphamide, doxorubicin, vincristine, prednisone) and subsequent R-CHOP (rituximab-CHOP), which are well established in DLBCL, have historically been the standard treatment of PMBCL.8-10 Some European centers have used the more dose-dense V/MACOP-B (etoposide or methotrexate, doxorubicin, cyclophosphamide, vincristine, prednisone, bleomycin) regimens.11-13 R-CHOP and V/MACOP-B are typically administered in conjunction with consolidative radiation for the majority of patients.
Table 1.
Selected studies of adults with PMBCL treated in the rituximab era
| Reference | Study type | Treatment | Patients receiving RT, % | N | PFS, % |
|---|---|---|---|---|---|
| 19 | Retrospective | CHOP | Variable | 153 | 75 (5 y) |
| M/VACOP-B | |||||
| R-CHOP | |||||
| 22 | Retrospective | R-V/MACOP-B | 71 | 45 | 84 (5 y) |
| 20 | Prospective: subgroup analysis of MInT trial | R-CHOP-like (n=44) CHOP-like (n=43) |
70 | 87 | 78 (3-y EFS) 52 (3-y EFS) |
| 21 | Retrospective | R-CHOP CHOP |
76 | 76 48 | 81 (5 y) 47 (5 y) |
| 68 | Retrospective | R-CHOP | 77 | 63 | 68 (5 y) |
| 18 | Retrospective | R-VACOP-B (n = 30) R-CHOP (n = 13) VACAOP-B (n = 47) CHOP (n = 5) |
0 | 95 | 83 (5 y) 69 (5 y) 62 (5 y) 20 (5 y) |
| 42 | Retrospective | R-MACOP-B | 69 | 74 | 88 (10 y) |
| 69 | Prospective: subgroup analysis of UK NCRI RCHOP 14 vs 21 trial | R-CHOP 14 (n = 22) R-CHOP 21 (n = 28) |
58 | 50 | 84 (5 y) 80 (5 y) |
| 25, 26 | Prospective trial (n = 28) and subsequent patients treated according to the trial protocol (n = 26) | R-CHOP14-ICE | 0 | 54 | 78 (3 y) |
| 23 | Prospective | DA-EPOCH-R | 4 | 51 | 93 (3 y) |
| 29 | Retrospective | DA-EPOCH-R | 16 | 118 | 87 (3-y EFS) |
EFS, event-free survival; ICE, ifosfamide, carboplatin, etoposide; PFS, progression-free survival.
Retrospective studies in the pre-rituximab era suggest that outcomes with V/MACOP-B are superior to CHOP14-17; however, the addition of rituximab to CHOP may mitigate this effect.18,19 The largest comparison of CHOP and V/MACOP-B was a retrospective series that observed superior outcome among patients treated with a MACOP-B–like regimen over a CHOP-like regimen (10-year survival, 67% vs 35%; P = .0003).17 However, the addition of rituximab to CHOP has likely improved outcomes, making the distinction between R-CHOP and V/MACOP-B less clear. Although there are no randomized trials of R-CHOP vs CHOP specifically in PMBCL, data from prospective and retrospective series suggest an advantage to R-CHOP. A subgroup analysis of the Mabthera International Trial compared patients with PMBCL who were treated with CHOP-like chemotherapy with or without rituximab. Among 86 patients, the 3-year event-free survival (EFS) in the R-CHOP and CHOP groups was 78% and 52%, respectively (P = .012).20 Similar findings were observed in a large retrospective series (5-year PFS with R-CHOP and historic CHOP was 81% vs 47%, P < .0001).21 Rituximab has also been added to V/MACOP-B, but the benefit in this setting is less clear. Zinzani et al reported a 5-year PFS of 84% among 45 patients treated with rituximab + V/MACOP-B (R-V/MACOP-B), which was not statistically different from historic controls without rituximab.22 Comparisons among CHOP, R-CHOP, and R-V/MACOP-B have also been performed in retrospective studies. The British Columbia Cancer Agency (BCCA) reported their 5-year overall survival (OS) with V/MACOP-B, CHOP-like treatment, and R-CHOP to be 87%, 71%, and 82% respectively. In pairwise comparisons, the only 2 groups that were significantly different were V/MACOP-B and CHOP, suggesting that V/MACOP-B is superior to CHOP but not R-CHOP.19 In conclusion, R-CHOP and (R-)V/MACOP-B result in favorable outcomes for the majority of patients; however, they are usually given in combination with radiotherapy.
More recently, dose-intensive regimens without radiation have been investigated.23-26 Reducing the exposure to radiation is of particular interest in the AYA population given the risk for late effects, including breast cancer and cardiovascular disease.27,28 The National Cancer Institute (NCI) recently conducted a phase 2 trial of DA-EPOCH-R (dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, rituximab) without radiation in PMBCL. A 5-year EFS of 93% was observed among 51 patients.23 Similar results were observed in a single-center retrospective series of 16 patients, with no events reported.23 Our group recently conducted a multicenter retrospective study of DA-EPOCH-R in adults and children with PMBCL. Among 118 adults, the 3-year EFS was 87%.29 Of note, in our retrospective series, 15% of patients received consolidative radiotherapy. Based on encouraging results with DA-EPOCH-R, many centers in the United States consider this to be the standard of care for PMBCL; however, prospective multicenter studies are needed to validate the existing data.
Pediatric approach
Pediatric patients with BL, DLBCL, or PMBCL have historically been treated on the same protocols. These regimens consist of alternating cycles of dose-intensive multiagent chemotherapy, including doxorubicin, high-dose methotrexate, and intrathecal chemotherapy for central nervous system prophylaxis. Patients do not typically receive consolidative radiation. Outcomes among patients with PMBCL in pediatric trials have been inferior compared with the excellent outcomes in BL and DLBCL but are similar to what has been observed in adults (Table 2). The Berlin-Frankfurt-Munster Group reported pooled outcomes of children and adolescents with PMBCL treated in successive prospective trials from 1986 to 1999. Among 30 patients, the 5-year EFS was 70%.30 The international FAB/LMB 96 mature B-cell NHL (B-NHL) trial enrolled 42 patients with PMBCL. The 5-year EFS for PMBCL was 66%, which was inferior to DLBCL (5-year EFS 85%, P < .001).31 In recognition of inferior results in pediatric trials and the encouraging results from the NCI phase 2 trial, the most recent international pediatric mature B-NHL trial included a separate cohort of patients with PMBCL to evaluate the DA-EPOCH-R regimen. Among the 40 patients included in the primary analysis, the 2-year EFS is 69% (95% confidence interval, 52-82%).32 This is in contrast to the early encouraging results from the NHL-BFM group that reported a 2-year EFS of 92.8% with the DA-EPOCH-R regimen, although with a small number of patients (n = 15).33 Our retrospective review of DA-EPOCH-R found a 3-year EFS of 81% among 38 pediatric patients.29 This leaves the pediatric community with no single standard of care for frontline treatment of PMBCL. Many centers in the United States use DA-EPOCH-R, whereas many centers in Europe follow the FAB/LMB regimen, with or without rituximab.
Table 2.
Selected studies of children and adolescents with PMBCL
| Reference | Study type | Treatment | N | EFS, % |
|---|---|---|---|---|
| 30 | Prospective, subgroup analysis pooled data from 3 studies | NHL-BFM 86, 90, 95 | 28 | 70 (5 y) |
| 31 | Prospective, subgroup analysis | FAB/LMB 96 | 42 | 66 (5 y) |
| 32 | Prospective phase 2 | DA-EPOCH-R | 40 | 69 (2 y) |
| 29 | Retrospective | DA-EPOCH-R | 38 | 81 (3 y) |
EFS, event-free survival.
How I treat de novo PMBCL: a discussion of case 1
This patient has a typical presentation of PMBCL with a large mediastinal mass, pleural and pericardial effusions, and metastatic disease to the lungs. She has several risk factors that may be associated with inferior outcome, including lactate dehydrogenase > 2 times the upper limit of normal, mediastinal mass > 10 cm, and advanced-stage disease. She does not have disease in the bone marrow or central nervous system, which is also typical for PMBCL. Given the compression of major vessels from the mediastinal mass, as well as the pleural and pericardial effusions, she requires prompt initiation of therapy. This patient falls into the AYA range (defined by the NCI as age 15-39 years) and could reasonably be treated on an adult or pediatric regimen.
I use the DA-EPOCH-R regimen for the initial treatment of PMBCL. Although the only prospective data on this regimen in adults are from a single-center trial, retrospective multicenter studies by our group and other investigators also suggest good disease control without the use of RT for the majority of patients.23,29,34,35 The preliminary data from the pediatric prospective multicenter trial of DA-EPOCH-R was not as favorable (2-year EFS, 69%); however, with small numbers, the 95% confidence interval for EFS overlaps with outcomes reported in other series.32 Although RT is avoided for most patients treated with DA-EPOCH-R, there remains concern for long-term toxicity with this regimen due to high cumulative exposure to anthracycline (up to 395 mg/m2 of doxorubicin). One reasonable approach is to modify this regimen by capping the cumulative doxorubicin dose at 360 mg/m2 as was reported by the pediatric BFM group.33 Other toxicities to consider are the risk for secondary malignancy due to etoposide and gonadal toxicity from cyclophosphamide.
Case 1 continued
Once the diagnosis of PMBCL is confirmed, the patient starts treatment with DA-EPOCH-R. Her treatment course is remarkable for an upper extremity deep vein thrombosis during cycle 1, for which she is placed on prophylactic enoxaparin, and uncomplicated febrile neutropenia after cycle 2. She continues with DA-EPOCH-R for a total of 6 cycles. She is escalated to a maximum dose level of 4. Approximately 6 weeks after the completion of cycle 6, she undergoes a repeat PET/CT scan. The mediastinal mass now measures 6.3 × 1.7 cm and has an SUV uptake of 4.0 (liver uptake 3.5). The pulmonary modules have resolved. She is assigned a Deauville score of 4. Should she receive further therapy?
What is the role for consolidative radiation in PMBCL?
PMBCL is a radiotherapy-sensitive disease. Consolidative RT has been demonstrated to convert patients from a partial response (PR) to a complete response (CR) after chemotherapy.16,17,36 However, the role for RT in all patients, and particularly in patients with a good response to chemotherapy, is not known. A retrospective analysis of 465 patients with PMBCL, identified through the National Cancer Database, demonstrated an improvement in OS among patients who received RT compared with those treated with chemotherapy alone (5-year OS, 93% vs 83%; hazard ratio, 0.43; P = .002).37 In contrast, a retrospective review from the BCCA failed to show a benefit with regard to PFS or OS in the RT group among 153 patients treated with V/MACOP-B, CHOP, or R-CHOP.19 Similar results were found when comparing patients treated with R-CHOP or CHOP with or without RT.21 Recently, excellent outcomes have been reported using dose-intensive chemotherapy regimens without RT. The Memorial Sloan Kettering group reported a 3-year PFS of 88% among 54 patients with PMBCL treated with dose-dense R-CHOP, followed by ICE without RT.25,26 DA-EPOCH-R is also typically administered without RT, although a minority of patients in the NCI prospective trial (4%) and in our retrospective series (15%) did receive radiation.
Can FDG-PET determine which patients should receive RT?
FDG-PET is routinely performed at the completion of chemo-immunotherapy in PMBCL to assess remission status. A negative PET scan is defined by FDG uptake less than or equal to uptake in the liver (Deauville score 1-3).38 Patients with a negative end-of-therapy (EOT) PET scan have been found to have improved outcomes in prospective and retrospective series.29,35,39 In the prospective IELSG-26 study, the 5-year PFS for patients with a negative and positive EOT PET was 99% and 68%, respectively (P < .001).40 Of note, the majority of patients in that study received RT. In the DA-EPOCH-R prospective trial, EOT PET was also found to be predictive, although the effect was more modest (5-year EFS, 92% vs 80%; P = .043). In this trial, the EOT PET was found to have a negative predictive value of 100% but a positive predictive value of 17%.23 A similar trend was observed in our retrospective series, in which the negative and positive predictive values of EOT PET were 96% and 42%, respectively.29 This is likely due to posttreatment inflammatory tissue in the mediastinum, which results in a high rate of false positive results.
More recently, FDG-PET has been used to identify patients for whom RT can be safely omitted. The BCCA reported outcomes among 50 patients treated with R-CHOP and PET-directed RT. Patients with a positive EOT PET scan (38% of all patients) received consolidative RT, whereas those with a negative EOT PET scan were observed without further therapy. The 5-year time to progression (TTP) in the overall cohort was 83%. This was not statistically different than outcomes in a historic control when all patients received R-CHOP and RT.41 Zinzani et al have also reported a PET-guided approach to RT. Among 74 patients treated with R-MACOP-B, RT was administered only to patients with a positive EOT PET scan (69%). There was no statistical difference in PFS between the RT and no-RT groups (10-year PFS, 90.7% vs 90%; P = .85).42 Of note, neither study used Deauville criteria to define a positive scan. However, both studies suggest that FDG-PET may be used to guide RT. The IELSG is currently conducting a randomized phase 3 trial (IELSG-37) to evaluate the role of RT in PET-negative patients. After treatment with a rituximab and anthracycline–containing regimen, patients with an FDG-PET Deauville score of 1-3 will be randomly assigned to RT or observation. Patients with a Deauville score of 4-5 will be treated at the discretion of the physician.43 Future studies evaluating the predictive role of FDG-PET in PMCBL may also include parameters beyond the Deauville score. Total lesion glycolysis, metabolic tumor volume, and metabolic heterogeneity have also been evaluated as potential prognostic markers.44,45
How I treat PET-avid disease at the completion of chemoimmunotherapy: a discussion of case 1
The optimal approach to patients with a positive EOT PET scan is not clear and presents a particular challenge to clinicians as they balance maximizing cure with minimizing long-term toxicity. In case 1, the patient has a Deauville score of 4 and a max SUV of 4.0 after 6 cycles of DA-EPOCH-R. Approaches to patients with a Deauville score of 4 after treatment vary greatly. My personal approach would be to follow closely with a repeat FDG-PET in 6-8 weeks without further treatment. If the lesion is increasing in size or in FDG uptake on repeat imaging, I would then consider a biopsy to determine whether the patient has primary refractory disease. If the lesion is improved or unchanged on repeat imaging, I would continue to follow without intervention. This decision is based on updated data from the NCI phase 2 trial of DA-EPOCH-R, which reported outcomes stratified by EOT PET Deauville score. With no further therapy after DA-EPOCH-R, recurrences occurred in 1 of 57 (2%) patients with a Deauville score of 1-3, 1 of 18 (6%) patients with Deauville score of 4, and 4 of 8 (50%) patients with a Deauville score of 5.46 Therefore, in the setting of DA-EPOCH-R, a Deauville 4 EOT PET scan may reflect an inflammatory state rather than refractory disease.
Case 1 follow-up
This patient was observed after DA-EPOCH-R without further therapy. A repeat PET/CT scan 8 weeks later demonstrated stable size of the mediastinal mass and a decrease in the SUV uptake from 4.0 to 3.2. I elected not to repeat additional PET/CT scans without a change in clinical status. She is now 20 months off therapy and remains in CR.
Case 2
A 20-year-old woman presents to the emergency department with a 3-week history of cough and a 1-week history of a “lump” protruding from her left (L) chest wall. PET/CT scan reveals an anterior mediastinal mass (12.6 × 10.0 cm, SUV 23.8) and L infraclavicular and L subpectoral lymph nodes (1.8-2.3 cm, SUV 4.2-17.3). A biopsy of the mediastinal mass is consistent with PMBCL. She is treated with 6 cycles of DA-EPOCH-R. Her PET/CT scan at the end of therapy shows a decrease in the size and FDG uptake of the mediastinal mass, which is now 7.8 × 5.0 cm (SUV 2.1 [Deauville 3]). The infraclavicular and subpectoral lymph nodes have resolved. She does not receive radiation. Four months later she develops fever and cough. PET/CT scan reveals an increase in the size and FDG uptake of the mediastinal mass, which is now 9.4 × 6.3 cm (SUV 12.7), multiple new bilateral pulmonary nodules up to 1.5 cm in size (SUV 3.0-6.2), and a PET-avid lesion at the head of the pancreas (SUV 5.1). A biopsy of the mediastinal mass confirms relapsed PMBCL.
What are the treatment options for relapsed/refractory PMCBL?
Relapsed or refractory PMBCL typically occurs early, with a median time to progression of 8 months from diagnosis; the majority of cases occur while on therapy or within 12 months of completion.47,48 At the time of relapse, the disease can metastasize beyond the mediastinum, including extranodal sites, such as the liver, pancreas, kidney, and the central nervous system. For patients who have not previously received RT and have disease that is restricted to the mediastinum, RT alone may be curative.23,29 For all other patients, the treatment of relapsed/refractory disease is typically high-dose chemotherapy, with or without RT, followed by autologous stem cell transplant (auto-SCT). Second-line treatment regimens are similar to those used in DLBCL and include R-ICE, R-DHAP (rituximab, dexamethasone, high-dose cytarabine, cisplatin), and others.49,50 Relapsed disease can be refractory to chemotherapy, and outcomes in such cases are poor.48,50,51 For patients with chemotherapy-sensitive disease who undergo auto-SCT, outcomes are more favorable and comparable to relapsed DLBCL. A retrospective series of 44 patients with relapsed/refractory PMCBL treated with high-dose therapy and auto-SCT in Japan reported a 4-year PFS of 61%.47 Similar outcomes have been reported in other series.48,50,51 The strongest predictor of outcome across series has been chemotherapy-responsive disease prior to auto-SCT.48,50 Given the variable response to chemotherapy in the relapsed setting, novel agents are needed in PMCBL.
What is the role for targeted therapy in PMBCL?
PMBCL tumors harbor numerous molecular alternations that may be amenable to targeting with novel therapies. Potential targeted therapy for PMBCL includes agents directed at cellular surface markers, dysregulated cellular signaling, and programmed death ligands. Like Hodgkin lymphoma, PMBCL tumors express CD30, albeit with more variable expression. The anti-CD30 antibody drug conjugate brentuximab vedotin, which is approved in Hodgkin lymphoma, was evaluated in relapsed/refractory PMBCL in a phase 2 trial. Among the first 15 patients enrolled in the study, the overall response rate (ORR) was unexpectedly low at 13% (2/15), and the trial was stopped early because of drug inefficacy.52
Genomic alterations in the programmed T-cell death ligand (PDL) locus, 9p24.1, which lead to PDL1/2 amplification and overexpression, are common in PMBCL.53,54 Pembrolizumab is a humanized monoclonal antibody that binds PD-1, preventing the interaction between PD-1 and PD-1 ligands.55-57 In PMBCL, pembrolizumab has been evaluated in a phase 1b study of patients with relapsed/refractory disease.58 Among 17 patients, the ORR was 41%, including 2 patients with a CR and 5 patients with a PR. Eighty-one percent of patients (13/16) had a decrease in target lesions. Based on these encouraging results, a phase 2 trial of pembrolizumab in relapsed/refractory PMBCL is ongoing. An interim analysis of this trial reported that, among 49 patients, the ORR was 41%, including 4 CRs (14%) and 8 PRs (28%).59 Other checkpoint inhibitors are being studied in B-cell lymphoma, including PMBCL.60,61
Structural changes in 9p24 in PMBCL also lead to dysregulation of JAK/STAT signaling. PMBCL is likely dependent on JAK/STAT, as evidenced by growth inhibition of PMBCL preclinical models in response to selective JAK2 inhibition.62 The JAK inhibitor ruxolitinib and the JAK2/FLT3 inhibitor SB518 have been evaluated in Hodgkin lymphoma and PMBCL but with too few cases of PMBCL to draw definitive conclusions.63,64
Lastly, T cells genetically modified to express an anti-CD19 chimeric antigen receptor (CAR) have demonstrated activity in CD19+ B-cell lymphomas and may be an emerging therapy for PMBCL.65 Patients with PMBCL have been included in phase 1 and 2 trials of these agents with CRs reported in many cases, albeit with small numbers of patients.65-67 Final results in the multicenter setting are awaited to determine activity of CD19 CAR-T therapy in PMBCL.
How I treat relapsed PMBCL: case 2 discussion
The patient in case 2 developed relapsed PMBCL soon after the completion of therapy and presented with disseminated disease. This pattern is common among cases of relapsed/refractory PMBCL and requires aggressive therapy given the poor prognosis. My approach to this patient would be high-dose therapy to try to induce remission and auto-SCT if she demonstrates chemotherapy-sensitive disease. In this case, because the patient did not receive RT with initial therapy, RT before or after auto-SCT could be considered as well. I use R-ICE as a second-line induction therapy; however, a variety of other strategies are also reasonable. For patients who fail to respond to second-line therapy, I would consider enrolling in a clinical trial of a novel agent with priority for checkpoint inhibitors or CD19 CAR-T therapy given the encouraging preliminary results.
Case 2 follow-up
This patient was treated with R-ICE for 2 cycles but was noted after cycle 2 to have progressive disease in the mediastinum. She then went on to receive 2 cycles of rituximab + gemcitabine, vinorelbine, and doxorubicin. PET/CT scan after cycle 2 demonstrated a PR with a decrease in size and SUV of the mediastinal mass (now 8.3 × 5.2 cm, SUV 7.9) and resolution of the pancreatic lesion. She proceeded to auto-SCT after conditioning with carmustine, etoposide, cytarabine, mephalan (BEAM). After auto-SCT she received 40 Gy RT to the mediastinum. A PET/CT scan at the completion of treatment demonstrated a CR. She currently remains in CR 9 months after SCT.
Conclusions
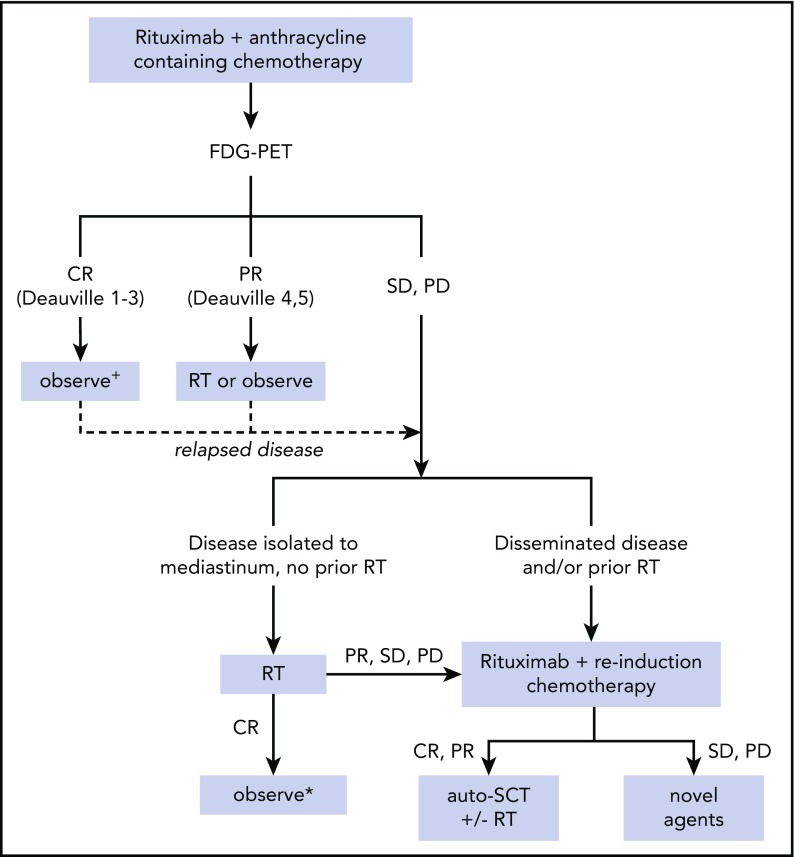
The unique clinical and biologic features of PMBCL warrant a treatment approach that is distinct from other B-NHL subtypes. Although different groups have used various chemo-immunotherapy regimens, some common themes have emerged (Figure 2). Initial treatment of PMBCL consists of rituximab and an anthracycline-containing chemotherapy regimen. FDG-PET at the completion of therapy is often used to determine response to treatment; however, this must be interpreted with caution because false positives are frequent. RT may help to consolidate remission, although it may not be required for all patients, especially those with a complete metabolic response after chemo-immunotherapy and those treated with intensive regimens, such as DA-EPOCH-R. When deciding on an upfront treatment approach, the potential toxicity of more intensive regimens, such as DA-EPOCH-R, should be weighed against the potential toxicity of RT. For patients with primary refractory or relapsed disease, outcomes are poor. High-dose therapy followed by auto-SCT is the standard of care; however, many patients have chemotherapy-refractory disease. RT may be sufficient for the subset of patients without prior RT and disease restricted to the mediastinum, although this has not been compared with combined-modality or chemotherapy-based strategies. Novel agents may benefit those with refractory disease and ultimately may be used in upfront therapy to improve initial response rates. Because PMBCL predominantly occurs in the AYA population, collaborations between pediatric and adult groups may help to advance outcomes in this rare NHL subtype.
Figure 2.
Proposed treatment algorithm for management of PMBCL. +Observation alone without RT after therapies other than DA-EPOCH-R has not been studied in prospective trials to date. The ongoing IELSG-37 trial seeks to determine whether RT can safely be omitted in these patients. *RT alone or combined modality with auto-SCT are both reasonable approaches in this scenario and have not been compared in clinical trials. PD, progressive disease; SD, stable disease.
Authorship
Contribution: L.G.-R. analyzed the literature and wrote the manuscript.
Conflict-of-interest disclosure: L.G.-R. declares no competing financial interests.
Correspondence: Lisa Giulino-Roth, Weill Cornell Medical College, 525 E 68th St, Payson 695, New York, NY 10065; e-mail: lgr2002@med.cornell.edu.
REFERENCES
- 1.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu PP, Wang KF, Xia Y, et al. Racial patterns of patients with primary mediastinal large B-cell lymphoma: SEER analysis. Medicine (Baltimore). 2016;95(27):e4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feuerhake F, Kutok JL, Monti S, et al. NFkappaB activity, function, and target-gene signatures in primary mediastinal large B-cell lymphoma and diffuse large B-cell lymphoma subtypes. Blood. 2005;106(4):1392-1399. [DOI] [PubMed] [Google Scholar]
- 4.Guiter C, Dusanter-Fourt I, Copie-Bergman C, et al. Constitutive STAT6 activation in primary mediastinal large B-cell lymphoma. Blood. 2004;104(2):543-549. [DOI] [PubMed] [Google Scholar]
- 5.Möller P, Lämmler B, Herrmann B, Otto HF, Moldenhauer G, Momburg F. The primary mediastinal clear cell lymphoma of B-cell type has variable defects in MHC antigen expression. Immunology. 1986;59(3):411-417. [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenwald A, Wright G, Leroy K, et al. Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. J Exp Med. 2003;198(6):851-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts RA, Wright G, Rosenwald AR, et al. Loss of major histocompatibility class II gene and protein expression in primary mediastinal large B-cell lymphoma is highly coordinated and related to poor patient survival. Blood. 2006;108(1):311-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abou-Elella AA, Weisenburger DD, Vose JM, et al. Primary mediastinal large B-cell lymphoma: a clinicopathologic study of 43 patients from the Nebraska Lymphoma Study Group. J Clin Oncol. 1999;17(3):784-790. [DOI] [PubMed] [Google Scholar]
- 9.Kirn D, Mauch P, Shaffer K, et al. Large-cell and immunoblastic lymphoma of the mediastinum: prognostic features and treatment outcome in 57 patients. J Clin Oncol. 1993;11(7):1336-1343. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez J, Pugh WC, Romaguera JE, et al. Primary mediastinal large cell lymphoma is characterized by an inverted pattern of large tumoral mass and low beta 2 microglobulin levels in serum and frequently elevated levels of serum lactate dehydrogenase. Ann Oncol. 1994;5(9):847-849. [DOI] [PubMed] [Google Scholar]
- 11.Bertini M, Orsucci L, Vitolo U, et al. Stage II large B-cell lymphoma with sclerosis treated with MACOP-B. Ann Oncol. 1991;2(10):733-737. [DOI] [PubMed] [Google Scholar]
- 12.Klimo P, Connors JM. MACOP-B chemotherapy for the treatment of diffuse large-cell lymphoma. Ann Intern Med. 1985;102(5):596-602. [DOI] [PubMed] [Google Scholar]
- 13.Zinzani PL, Bendandi M, Frezza G, et al. Primary mediastinal B-cell lymphoma with sclerosis: clinical and therapeutic evaluation of 22 patients. Leuk Lymphoma. 1996;21(3-4):311-316. [DOI] [PubMed] [Google Scholar]
- 14.Lazzarino M, Orlandi E, Paulli M, et al. Primary mediastinal B-cell lymphoma with sclerosis: an aggressive tumor with distinctive clinical and pathologic features. J Clin Oncol. 1993;11(12):2306-2313. [DOI] [PubMed] [Google Scholar]
- 15.Lazzarino M, Orlandi E, Paulli M, et al. Treatment outcome and prognostic factors for primary mediastinal (thymic) B-cell lymphoma: a multicenter study of 106 patients. J Clin Oncol. 1997;15(4):1646-1653. [DOI] [PubMed] [Google Scholar]
- 16.Todeschini G, Secchi S, Morra E, et al. Primary mediastinal large B-cell lymphoma (PMLBCL): long-term results from a retrospective multicentre Italian experience in 138 patients treated with CHOP or MACOP-B/VACOP-B. Br J Cancer. 2004;90(2):372-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zinzani PL, Martelli M, Bertini M, et al. ; International Extranodal Lymphoma Study Group (IELSG). Induction chemotherapy strategies for primary mediastinal large B-cell lymphoma with sclerosis: a retrospective multinational study on 426 previously untreated patients. Haematologica. 2002;87(12):1258-1264. [PubMed] [Google Scholar]
- 18.Avigdor A, Sirotkin T, Kedmi M, et al. The impact of R-VACOP-B and interim FDG-PET/CT on outcome in primary mediastinal large B cell lymphoma. Ann Hematol. 2014;93(8):1297-1304. [DOI] [PubMed] [Google Scholar]
- 19.Savage KJ, Al-Rajhi N, Voss N, et al. Favorable outcome of primary mediastinal large B-cell lymphoma in a single institution: the British Columbia experience. Ann Oncol. 2006;17(1):123-130. [DOI] [PubMed] [Google Scholar]
- 20.Rieger M, Osterborg A, Pettengell R, et al. ; MabThera International Trial (MInT) Group. Primary mediastinal B-cell lymphoma treated with CHOP-like chemotherapy with or without rituximab: results of the Mabthera International Trial Group study. Ann Oncol. 2011;22(3):664-670. [DOI] [PubMed] [Google Scholar]
- 21.Vassilakopoulos TP, Pangalis GA, Katsigiannis A, et al. Rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone with or without radiotherapy in primary mediastinal large B-cell lymphoma: the emerging standard of care. Oncologist. 2012;17(2):239-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zinzani PL, Stefoni V, Finolezzi E, et al. Rituximab combined with MACOP-B or VACOP-B and radiation therapy in primary mediastinal large B-cell lymphoma: a retrospective study. Clin Lymphoma Myeloma. 2009;9(5):381-385. [DOI] [PubMed] [Google Scholar]
- 23.Dunleavy K, Pittaluga S, Maeda LS, et al. Dose-adjusted EPOCH-rituximab therapy in primary mediastinal B-cell lymphoma. N Engl J Med. 2013;368(15):1408-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldschmidt N, Kleinstern G, Orevi M, et al. Favorable outcome of primary mediastinal large B-cell lymphoma patients treated with sequential RCHOP-RICE regimen without radiotherapy. Cancer Chemother Pharmacol. 2016;77(5):1053-1060. [DOI] [PubMed] [Google Scholar]
- 25.Moskowitz C, Hamlin PA, Maragulia J, Meikle J, Zelenetz AD. Sequential dose-dense RCHOP followed by ICE consolidation (MSKCC protocol 01–142) without radiotherapy for patients with primary mediastinal large B cell lymphoma. Blood. 2010;116(21):420. [Google Scholar]
- 26.Moskowitz CH, Schöder H, Teruya-Feldstein J, et al. Risk-adapted dose-dense immunochemotherapy determined by interim FDG-PET in advanced-stage diffuse large B-cell lymphoma. J Clin Oncol. 2010;28(11):1896-1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swerdlow AJ, Cooke R, Bates A, et al. Breast cancer risk after supradiaphragmatic radiotherapy for Hodgkin’s lymphoma in England and Wales: a National Cohort Study. J Clin Oncol. 2012;30(22):2745-2752. [DOI] [PubMed] [Google Scholar]
- 28.van Nimwegen FA, Schaapveld M, Cutter DJ, et al. Radiation dose-response relationship for risk of coronary heart disease in survivors of Hodgkin lymphoma. J Clin Oncol. 2016;34(3):235-243. [DOI] [PubMed] [Google Scholar]
- 29.Giulino-Roth L, O’Donohue T, Chen Z, et al. Outcomes of adults and children with primary mediastinal B-cell lymphoma treated with dose-adjusted EPOCH-R. Br J Haematol. 2017;179(5):739-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seidemann K, Tiemann M, Lauterbach I, et al. ; NHL Berlin-Frankfurt-Münster Group. Primary mediastinal large B-cell lymphoma with sclerosis in pediatric and adolescent patients: treatment and results from three therapeutic studies of the Berlin-Frankfurt-Münster Group. J Clin Oncol. 2003;21(9):1782-1789. [DOI] [PubMed] [Google Scholar]
- 31.Gerrard M, Waxman IM, Sposto R, et al. ; French-American-British/Lymphome Malins de Burkitt 96 (FAB/LMB 96) International Study Committee. Outcome and pathologic classification of children and adolescents with mediastinal large B-cell lymphoma treated with FAB/LMB96 mature B-NHL therapy. Blood. 2013;121(2):278-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burke GAA, Gross TG, Pillon M, et al. Results of Inter-B-NHL Ritux 2010 - phase II study of DA-EPOCH-R for children and adolescents with primary mediastinal large B-cell lymphoma (PMLBL) on behalf of European Intergroup for Childhood Non Hodgkin’s Lymphoma (EICNHL) and Children’s Oncology Group (COG) [abstract]. Blood. 2017;130(suppl 1). Abstract 4124. [Google Scholar]
- 33.Woessmann W, Lisfeld J, Burkhardt B; NHL-BFM Study Group. Therapy in primary mediastinal B-cell lymphoma. N Engl J Med. 2013;369(3):282-284. [DOI] [PubMed] [Google Scholar]
- 34.Binkley MS, Hiniker SM, Wu S, et al. A single-institution retrospective analysis of outcomes for stage I-II primary mediastinal large B-cell lymphoma treated with immunochemotherapy with or without radiotherapy. Leuk Lymphoma. 2016;57(3):604-608. [DOI] [PubMed] [Google Scholar]
- 35.Pinnix CC, Dabaja B, Ahmed MA, et al. Single-institution experience in the treatment of primary mediastinal B cell lymphoma treated with immunochemotherapy in the setting of response assessment by 18fluorodeoxyglucose positron emission tomography. Int J Radiat Oncol Biol Phys. 2015;92(1):113-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zinzani PL, Martelli M, Bendandi M, et al. Primary mediastinal large B-cell lymphoma with sclerosis: a clinical study of 89 patients treated with MACOP-B chemotherapy and radiation therapy. Haematologica. 2001;86(2):187-191. [PubMed] [Google Scholar]
- 37.Jackson MW, Rusthoven CG, Jones BL, Kamdar M, Rabinovitch R. Improved survival with combined modality therapy in the modern era for primary mediastinal B-cell lymphoma. Am J Hematol. 2016;91(5):476-480. [DOI] [PubMed] [Google Scholar]
- 38.Younes A, Hilden P, Coiffier B, et al. International Working Group consensus response evaluation criteria in lymphoma (RECIL 2017). Ann Oncol. 2017;28(7):1436-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Filippi AR, Piva C, Giunta F, et al. Radiation therapy in primary mediastinal B-cell lymphoma with positron emission tomography positivity after rituximab chemotherapy. Int J Radiat Oncol Biol Phys. 2013;87(2):311-316. [DOI] [PubMed] [Google Scholar]
- 40.Martelli M, Ceriani L, Zucca E, et al. [18F]fluorodeoxyglucose positron emission tomography predicts survival after chemoimmunotherapy for primary mediastinal large B-cell lymphoma: results of the International Extranodal Lymphoma Study Group IELSG-26 Study. J Clin Oncol. 2014;32(17):1769-1775. [DOI] [PubMed] [Google Scholar]
- 41.Savage KJ, Yenson PR, Shenkier T, et al. The outcome of primary mediastinal large B-cell lymphoma (PMBCL) in the R-CHOP treatment era. Blood. 2012;120(21):303.22596259 [Google Scholar]
- 42.Zinzani PL, Broccoli A, Casadei B, et al. The role of rituximab and positron emission tomography in the treatment of primary mediastinal large B-cell lymphoma: experience on 74 patients. Hematol Oncol. 2015;33(4):145-150. [DOI] [PubMed] [Google Scholar]
- 43.Cavalli F, Ceriani L, Zucca E. Functional imaging using 18-fluorodeoxyglucose PET in the management of primary mediastinal large B-cell lymphoma: the contributions of the International Extranodal Lymphoma Study Group. Am Soc Clin Oncol Educ Book. 2016;35:e368-e375. [DOI] [PubMed] [Google Scholar]
- 44.Ceriani L, Martelli M, Conconi A, et al. Prognostic models for primary mediastinal (thymic) B-cell lymphoma derived from 18-FDG PET/CT quantitative parameters in the International Extranodal Lymphoma Study Group (IELSG) 26 study. Br J Haematol. 2017;178(4):588-591. [DOI] [PubMed] [Google Scholar]
- 45.Ceriani L, Milan L, Martelli M, et al. Metabolic heterogeneity on baseline 18FDG-PET/CT scan is a predictor of outcome in primary mediastinal B-cell lymphoma. Blood. 2018;blood-2018-01-826958. [DOI] [PubMed] [Google Scholar]
- 46.Melani C, Advani RH, Roschewski M, et al. End-of-treatment CT and serial FDG-PET Imaging to assess residual disease in primary mediastinal B-cell lymphoma [abstract]. Blood. 2017;130(suppl 1). Abstract 2859. [Google Scholar]
- 47.Aoki T, Shimada K, Suzuki R, et al. High-dose chemotherapy followed by autologous stem cell transplantation for relapsed/refractory primary mediastinal large B-cell lymphoma. Blood Cancer J. 2015;5(12):e372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuruvilla J, Pintilie M, Tsang R, Nagy T, Keating A, Crump M. Salvage chemotherapy and autologous stem cell transplantation are inferior for relapsed or refractory primary mediastinal large B-cell lymphoma compared with diffuse large B-cell lymphoma. Leuk Lymphoma. 2008;49(7):1329-1336. [DOI] [PubMed] [Google Scholar]
- 49.Hamlin PA, Portlock CS, Straus DJ, et al. Primary mediastinal large B-cell lymphoma: optimal therapy and prognostic factor analysis in 141 consecutive patients treated at Memorial Sloan Kettering from 1980 to 1999. Br J Haematol. 2005;130(5):691-699. [DOI] [PubMed] [Google Scholar]
- 50.Sehn LH, Antin JH, Shulman LN, et al. Primary diffuse large B-cell lymphoma of the mediastinum: outcome following high-dose chemotherapy and autologous hematopoietic cell transplantation. Blood. 1998;91(2):717-723. [PubMed] [Google Scholar]
- 51.Hamlin PA, Dickson M, Kewalramani T, et al. Relapsed and refractory primary mediastinal diffuse large B-cell lymphoma: outcome with ICE-based treatment. Blood. 2006;108(11):3057. [Google Scholar]
- 52.Zinzani PL, Pellegrini C, Chiappella A, et al. Brentuximab vedotin in relapsed primary mediastinal large B-cell lymphoma: results from a phase 2 clinical trial. Blood. 2017;129(16):2328-2330. [DOI] [PubMed] [Google Scholar]
- 53.Joos S, Otaño-Joos MI, Ziegler S, et al. Primary mediastinal (thymic) B-cell lymphoma is characterized by gains of chromosomal material including 9p and amplification of the REL gene. Blood. 1996;87(4):1571-1578. [PubMed] [Google Scholar]
- 54.Twa DD, Chan FC, Ben-Neriah S, et al. Genomic rearrangements involving programmed death ligands are recurrent in primary mediastinal large B-cell lymphoma. Blood. 2014;123(13):2062-2065. [DOI] [PubMed] [Google Scholar]
- 55.Reck M, Rodríguez-Abreu D, Robinson AG, et al. ; KEYNOTE-024 Investigators. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823-1833. [DOI] [PubMed] [Google Scholar]
- 56.Robert C, Schachter J, Long GV, et al. ; KEYNOTE-006 investigators. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521-2532. [DOI] [PubMed] [Google Scholar]
- 57.Armand P, Shipp MA, Ribrag V, et al. Programmed death-1 blockade with pembrolizumab in patients with classical Hodgkin lymphoma after brentuximab vedotin failure. J Clin Oncol. 2016;34(31):3733-3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zinzani PL, Ribrag V, Moskowitz CH, et al. Safety and tolerability of pembrolizumab in patients with relapsed/refractory primary mediastinal large B-cell lymphoma. Blood. 2017;130(3):267-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zinzani PL, Thieblemont C, Melnichenko V, et al. Efficacy and safety of pembrolizumab in relapsed/refractory primary mediastinal large B-cell lymphoma (rrPMBCL): updated analysis of the Keynote-170 Phase 2 Trial [abstract]. Blood. 2017;130(suppl 1). Abstract 2833. [Google Scholar]
- 60.Armand P, Nagler A, Weller EA, et al. Disabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large B-cell lymphoma: results of an international phase II trial. J Clin Oncol. 2013;31(33):4199-4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lesokhin AM, Ansell SM, Armand P, et al. Nivolumab in patients with relapsed or refractory hematologic malignancy: preliminary results of a phase Ib study. J Clin Oncol. 2016;34(23):2698-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hao Y, Chapuy B, Monti S, Sun HH, Rodig SJ, Shipp MA. Selective JAK2 inhibition specifically decreases Hodgkin lymphoma and mediastinal large B-cell lymphoma growth in vitro and in vivo. Clin Cancer Res. 2014;20(10):2674-2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim SJ, Kang HJ, Dong-Yeop S, et al. The efficacy of JAK2 inhibitor in heavily pretreated classical Hodgkin lymphoma: a prospective pilot study of ruxolitinib in relapsed or refractory classical Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2016;128(22):1820. [Google Scholar]
- 64.Younes A, Romaguera J, Fanale M, et al. Phase I study of a novel oral Janus kinase 2 inhibitor, SB1518, in patients with relapsed lymphoma: evidence of clinical and biologic activity in multiple lymphoma subtypes. J Clin Oncol. 2012;30(33):4161-4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abramson JS, Palomba ML, Gordon LI, et al. CR rates in relapsed/refractory (R/R) aggressive B-NHL treated with the CD19-directed CAR T-cell product JCAR017 (TRANSCEND NHL 001) [abstract]. J Clin Oncol. 2017;35(15 suppl). Abstract 7513.
- 67.Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33(6):540-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Soumerai JD, Hellmann MD, Feng Y, et al. Treatment of primary mediastinal B-cell lymphoma with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone is associated with a high rate of primary refractory disease. Leuk Lymphoma. 2014;55(3):538-543. [DOI] [PubMed] [Google Scholar]
- 69.Gleeson M, Hawkes EA, Cunningham D, et al. Rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone (R-CHOP) in the management of primary mediastinal B-cell lymphoma: a subgroup analysis of the UK NCRI R-CHOP 14 versus 21 trial. Br J Haematol. 2016;175(4):668-672. [DOI] [PubMed] [Google Scholar]