Abstract
One approach to quantifying biological diversity consists of characterizing the statistical distribution of specific properties of a taxonomic group or habitat. Microorganisms living in fluid environments, and for whom motility is key, exploit propulsion resulting from a rich variety of shapes, forms, and swimming strategies. Here, we explore the variability of swimming speed for unicellular eukaryotes based on published data. The data naturally partitions into that from flagellates (with a small number of flagella) and from ciliates (with tens or more). Despite the morphological and size differences between these groups, each of the two probability distributions of swimming speed are accurately represented by log-normal distributions, with good agreement holding even to fourth moments. Scaling of the distributions by a characteristic speed for each data set leads to a collapse onto an apparently universal distribution. These results suggest a universal way for ecological niches to be populated by abundant microorganisms.
Research organism: Other
Introduction
Unicellular eukaryotes comprise a vast, diverse group of organisms that covers virtually all environments and habitats, displaying a menagerie of shapes and forms. Hundreds of species of the ciliate genus Paramecium (Wichterman, 1986) or flagellated Euglena (Buetow, 2011) are found in marine, brackish, and freshwater reservoirs; the green algae Chlamydomonas is distributed in soil and fresh water world-wide (Harris et al., 2009); parasites from the genus Giardia colonize intestines of several vertebrates (Adam, 2001). One of the shared features of these organisms is their motility, crucial for nutrient acquisition and avoidance of danger (Bray, 2001). In the process of evolution, single-celled organisms have developed in a variety of directions, and thus their rich morphology results in a large spectrum of swimming modes (Cappuccinelli, 1980).
Many swimming eukaryotes actuate tail-like appendages called flagella or cilia in order to generate the required thrust (Sleigh, 1975). This is achieved by actively generating deformations along the flagellum, giving rise to a complex waveform. The flagellar axoneme itself is a bundle of nine pairs of microtubule doublets surrounding two central microtubules, termed the '9 + 2' structure (Nicastro et al., 2005), and cross-linking dynein motors, powered by ATP hydrolysis, perform mechanical work by promoting the relative sliding of filaments, resulting in bending deformations.
Although eukaryotic flagella exhibit a diversity of forms and functions (Moran et al., 2014), two large families, ‘flagellates’ and ‘ciliates’, can be distinguished by the shape and beating pattern of their flagella. Flagellates typically have a small number of long flagella distributed along the bodies, and they actuate them to generate thrust. The set of observed movement sequences includes planar undulatory waves and traveling helical waves, either from the base to the tip, or in the opposite direction (Jahn and Votta, 1972; Brennen and Winet, 1977). Flagella attached to the same body might follow different beating patterns, leading to a complex locomotion strategy that often relies also on the resistance the cell body poses to the fluid. In contrast, propulsion of ciliates derives from the motion of a layer of densely-packed and collectively-moving cilia, which are short hair-like flagella covering their bodies. The seminal review paper of Brennen and Winet (1977) lists a few examples from both groups, highlighting their shape, beat form, geometric characteristics and swimming properties. Cilia may also be used for transport of the surrounding fluid, and their cooperativity can lead to directed flow generation. In higher organisms this can be crucial for internal transport processes, as in cytoplasmic streaming within plant cells (Allen and Allen, 1978), or the transport of ova from the ovary to the uterus in female mammals (Lyons et al., 2006).
Here, we turn our attention to these two morphologically different groups of swimmers to explore the variability of their propulsion dynamics within broad taxonomic groups. To this end, we have collected swimming speed data from literature for flagellated eukaryotes and ciliates and analyze them separately (we do not include spermatozoa since they lack (ironically) the capability to reproduce and are thus not living organisms; their swimming characteristics have been studied by Tam and Hosoi, 2011). A careful examination of the statistical properties of the speed distributions for flagellates and ciliates shows that they are not only both captured by log-normal distributions but that, upon rescaling the data by a characteristic swimming speed for each data set, the speed distributions in both types of organisms are essentially identical.
Results and discussion
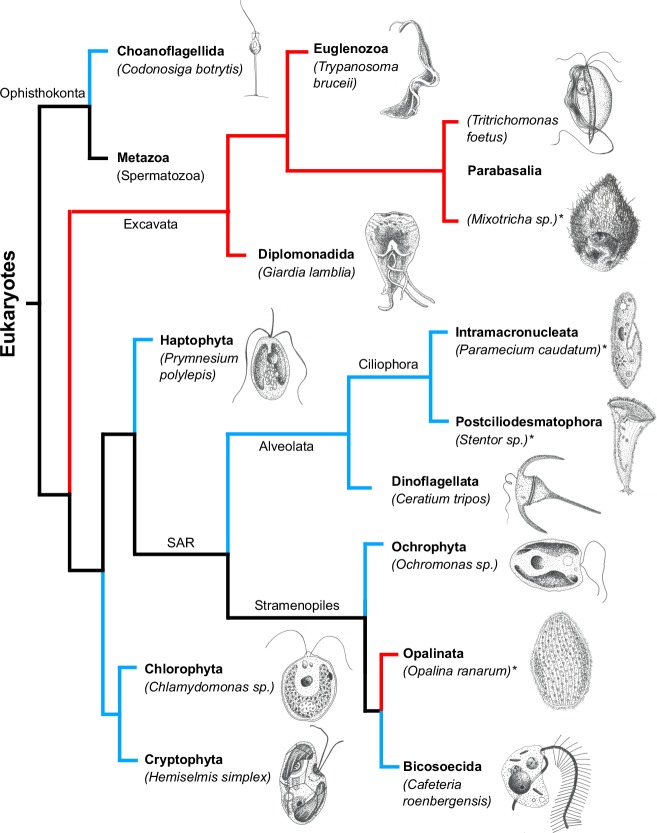
We have collected swimming data on 189 unicellular eukaryotic microorganisms ( flagellates and ciliates) (see Appendix 1 and Source data 1). Figure 1 shows a tree encompassing the phyla of organisms studied and sketches of a representative organism from each phylum. A large morphological variation is clearly visible. In addition, we delineate the branches involving aquatic organisms and parasitic species living within hosts. Both groups include ciliates and flagellates.
Figure 1. The tree of life (cladogram) for unicellular eukaryotes encompassing the phyla of organisms analyzed in the present study.
Aquatic organisms (living in marine, brackish, or freshwater environments) have their branches drawn in blue while parasitic organisms have their branches drawn in red. Ciliates are indicated by an asterisk after their names. For each phylum marked in bold font, a representative organism has been sketched next to its name. Phylogenetic data from Hinchliff et al. (2015).
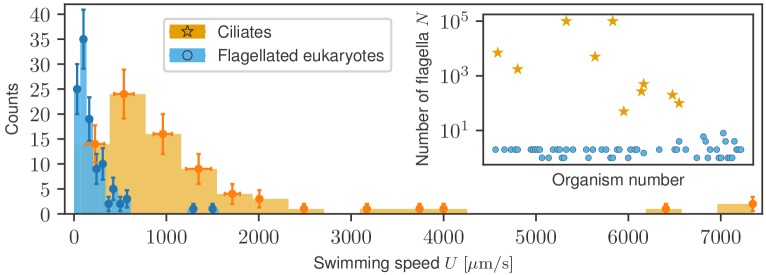
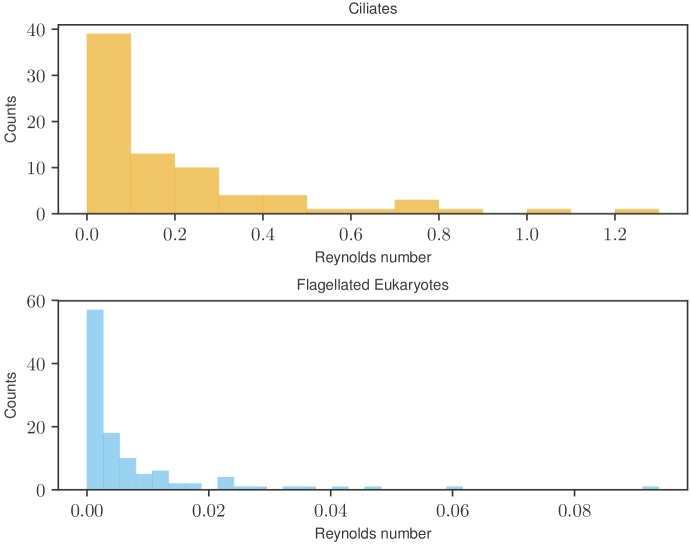
Due to the morphological and size differences between ciliates and flagellates, we investigate separately the statistical properties of each. Figure 2 shows the two swimming speed histograms superimposed, based on the raw distributions shown in Figure 2—figure supplement 1, where bin widths have been adjusted to their respective samples using the Freedman-Diaconis rule (see Materials and methods). Ciliates span a much larger range of speeds, up to 7 mm/s, whereas generally smaller flagellates remain in the sub-mm/s range. The inset shows that the number of flagella in both groups leads to a clear division. To compare the two groups further, we have also collected information on the characteristic sizes of swimmers from the available literature, which we list in Appendix 1. The average cell size differs fourfold between the populations (31 µm for flagellates and 132 µm for ciliates) and the distributions, plotted in Figure 2—figure supplement 2, are biased towards the low-size end but they are quantitatively different. In order to explore the physical conditions, we used the data on sizes and speeds to compute the Reynolds number for each organism, where is the kinematic viscosity of water, with the viscosity and the density. Since almost no data was available for the viscosity of the fluid in swimming speed measurements, we assumed the standard value for water for all organisms. The distribution of Reynolds numbers (Figure 2—figure supplement 3), shows that ciliates and flagellates operate in different ranges of , although for both groups , imposing on them the same limitations of inertia-less Stokes flow (Purcell, 1977; Lauga and Powers, 2009).
Figure 2. Histograms of swimming speed for ciliates and flagellates demonstrate a similar character but different scales of velocities.
Data points represent the mean and standard deviation of the data in each bin; horizontal error bars represent variability within each bin, vertical error bars show the standard deviation of the count. Inset: number of flagella displayed, where available, for each organism exhibits a clear morphological division between ciliates and flagellates.
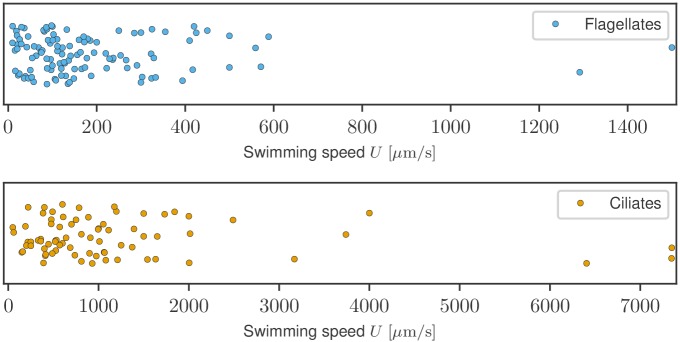
Figure 2—figure supplement 1. Linear distribution of swimming speed data.
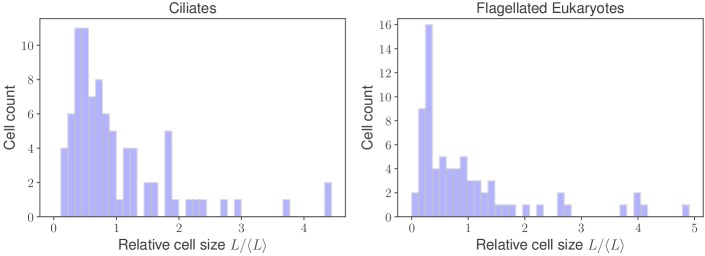
Figure 2—figure supplement 2. Distribution of organism sizes in analyzed groups.
Figure 2—figure supplement 3. Distribution of Reynolds numbers for organisms in analyzed groups.
Furthermore, studies of green algae (Short et al., 2006; Goldstein, 2015) show that an important distinction between the smaller, flagellated species and the largest multicellular ones involves the relative importance of advection and diffusion, as captured by the Péclet number , where is a typical organism size and is the diffusion constant of a relevant molecular species. Using the average size of the cell body in each group of the present study (, ) and the median swimming speeds (, ), and taking , we find and , which further justifies analyzing the groups separately; they live in different physical regimes.
Examination of the mean, variance, kurtosis, and higher moments of the data sets suggest that the probabilities of the swimming speed are well-described by log-normal distributions,
| (1) |
normalized as , where and are the mean and the standard deviation of . The median of the distribution is , with units of speed. Log-normal distributions are widely observed across nature in areas such as ecology, physiology, geology and climate science, serving as an empirical model for complex processes shaping a system with many potentially interacting elements (Limpert et al., 2001), particularly when the underlying processes involve proportionate fluctuations or multiplicative noise (Koch, 1966).
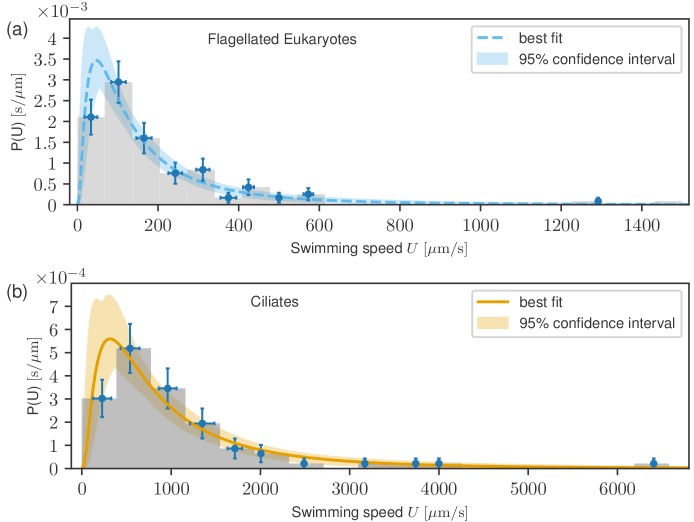
The results of fitting (see Materials and methods) are plotted in Figure 3, where the best fits are presented as solid curves, with the shaded areas representing 95% confidence intervals. For flagellates, we find the and while for ciliates, we obtain and . Log-normal distributions are known to emerge from an (imperfect) analogy to the Gaussian central limit theorem (see Materials and methods). Since the data are accurately described by this distribution, we conclude that the published literature includes a sufficiently large amount of unbiased data to be able to see the whole distribution.
Figure 3. Probability distribution functions of swimming speeds for flagellates (a) and ciliates (b) with the fitted log-normal distributions.
Data points represent uncertainties as in Figure 2. Despite the markedly different scales of the distributions, they have similar shapes.
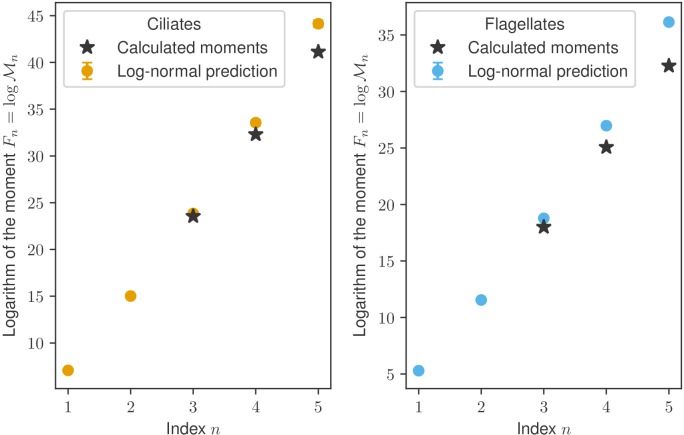
Figure 3—figure supplement 1. Higher moments of the swimming speed distributions obtained from the data compared with those calculated from the fitted log-normal distribution.
We next compare the statistical variability within groups by examining rescaled distributions (Goldstein, 2018). As each has a characteristic speed , we align the peaks by plotting the distributions versus the variable for each group. Since has units of 1/speed, we are thus led to the form for some function . For the log-normal distribution, with the median, we find
| (2) |
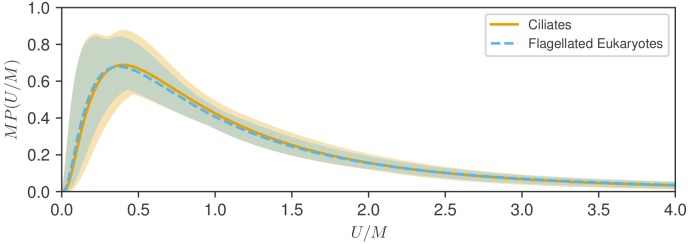
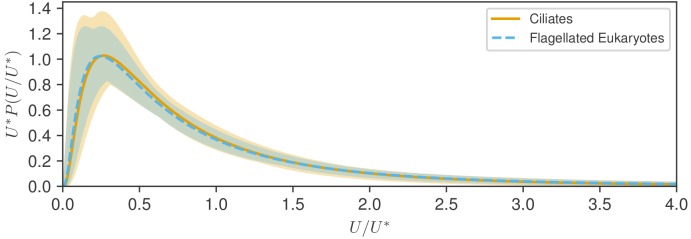
which now depends on the single parameter and has a median of unity by construction. To study the similarity of the two distributions we plot the functions for each. As seen in Figure 4, the rescaled distributions are essentially indistinguishable, and this can be traced back to the near identical values of the variances , which are within 5% of each other. The fitting uncertainties shown shaded in Figure 4 suggest a very similar range of variability of the fitted distributions. Furthermore, both the integrated absolute difference between the distributions (0.028) and the Kullback-Leibler divergence (0.0016) are very small (see Materials and methods), demonstrating the close similarity of the two distributions. This similarity is robust to the choice of characteristic speed, as shown in Figure 4—figure supplement 1, where the arithmetic mean is used in place of the median.
Figure 4. Test of rescaling hypothesis.
Shown are the two fitted log-normal curves for flagellates and ciliates, each multiplied by the distribution median , plotted versus speed normalized by . The distributions for show remarkable similarity and uncertainty of estimation.
Figure 4—figure supplement 1. Data collapse as in the main figure, but using the mean speeds instead of the median .
In living cells, the sources for intrinsic variability within organisms are well characterized on the molecular and cellular level (Kirkwood et al., 2005) but less is known about variability within taxonomic groups. By dividing unicellular eukaryotes into two major groups on the basis of their difference in morphology, size and swimming strategy, we were able to capture in this paper the log-normal variability within each subset. Using a statistical analysis of the distributions as functions of the median swimming speed for each population we further found an almost identical distribution of swimming speeds for both types of organisms. Our results suggest that the observed log-normal randomness captures a universal way for ecological niches to be populated by abundant microorganisms with similar propulsion characteristics. We note, however, that the distributions of swimming speeds among species do not necessarily reflect the distributions of swimming speeds among individuals, for which we have no available data.
Materials and methods
Data collection
Data for ciliates were sourced from 26 research articles, while that for flagellates were extracted from 48 papers (see Appendix 1). Notably, swimming speeds reported in the various studies have been measured under different physiological and environmental conditions, including temperature, viscosity, salinity, oxygenation, pH and light. Therefore we consider the data not as representative of a uniform environment, but instead as arising from a random sampling of a wide range of environmental conditions. In cases where no explicit figure was given for in a paper, estimates were made using other available data where possible. Size of swimmers has also been included as a characteristic length for each organism. This, however, does not reflect the spread and diversity of sizes within populations of individual but is rather an indication of a typical size, as in the considered studies these data were not available. Information on anisotropy (different width/length) is also not included.
No explicit criteria were imposed for the inclusion in the analyses, apart from the biological classification (i.e. whether the organisms were unicellular eukaryotic ciliates/flagellates). We have used all the data found in literature for these organisms over the course of an extensive search. Since no selection was made, we believe that the observed statistical properties are representative for these groups.
Data processing and fitting the log-normal distribution
Bin widths in histograms in Figure 2 and Figure 3 have been chosen separately for ciliates and flagellated eukaryotes according to the Freedman-Diaconis rule (Freedman and Diaconis, 1981) taking into account the respective sample sizes and the spread of distributions. The bin width is then given by the number of observations and the interquartile range of the data as
| (3) |
Within each bin in Figure 3, we calculate the mean and the standard deviation for the binned data, which constitute the horizontal error bars. The vertical error bars reflect the uncertainty in the number of counts in bin . This is estimated to be Poissonian, and thus the absolute error amounts to . Notably, the relative error decays with the number of counts as .
In fitting the data, we employ the log-normal distribution Equation (1). In general, from from data comprising measurements, labelled (), the -th arithmetic moment is the expectation , or
| (4) |
Medians of the data were found by sorting the list of values and picking the middlemost value. For a log-normal distribution, the arithmetic moments are given solely by and of the associated normal distribution as
| (5) |
where we have defined and , and note that is the median of the distribution. Thus, the mean is and the variance is . From the first and second moments, we estimate
| (6) |
Having estimated and , we can compute the higher order moments from Equation (5) and compare to those calculated directly from the data, as shown in Figure 3—figure supplement 1.
To fit the data, we have used both the MATLAB fitting routines and the Python scipy.stats module. From these fits we estimated the shape and scale parameters and the 95% confidence intervals in Figure 3 and Figure 4. We emphasize that the fitting procedures use the raw data via the maximum likelihood estimation method, and not the processed histograms, hence the estimated parameters are insensitive to the binning procedure.
For rescaled distributions, the average velocity for each group of organisms was calculated as , with . Then, data in each subset have been rescaled by the area under the fitted curve to ensure that the resulting probability density functions are normalized as
| (7) |
In characterizations of biological or ecological diversity, it is often assumed that the examined variables are Gaussian, and thus the distribution of many uncorrelated variables attains the normal distribution by virtue of the Central Limit Theorem (CLT). In the case when random variables in question are positive and have a log-normal distribution, no analogous explicit analytic result is available. Despite that, there is general agreement that a sum of independent log-normal random variables can be well approximated by another log-normal random variable. It has been proven by Szyszkowicz and Yanikome (2009) that the sum of identically distributed equally and positively correlated joint log-normal distributions converges to a log-normal distribution of known characteristics but for uncorrelated variables only estimations are available (Beaulieu et al., 1995). We use these results to conclude that our distributions contain enough data to be unbiased and seen in full.
Comparisons of distributions
In order to quantify the differences between the fitted distributions, we define the integrated absolute difference between two probability distributions and () as
| (8) |
As the probability distributions are normalized, this is a measure of their relative ’distance’. As a second measure, we use the Kullback-Leibler divergence (Kullback and Leibler, 1951),
| (9) |
Note that and therefore is not a distance metric in the space of probability distributions.
Acknowledgements
This project has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program (grant agreement 682754 to EL), and from Established Career Fellowship EP/M017982/1 from the Engineering and Physical Sciences Research Council and Grant 7523 from the Gordon and Betty Moore Foundation (REG).
Appendix 1
The Appendix contains the data which form the basis of our study. The tables contain data on the sizes and swimming speed of ciliates organisms and flagellated eukaryotes from the existing literature. Data for ciliates were sourced from 26 research articles, while data for the flagellates were extracted from 48 papers. In the cases where two or more sources reported contrasting figures for the swimming speed, the average value is reported in our tables. The data itself is available in Source data 1.
Data for swimming flagellates
Abbreviations: dflg. – dinoflagellata; dph – dinophyceae; chlph. – chlorophyta; ochph. (het.) –ochrophyta (heterokont); srcm. – sarcomastigophora, pyr. – pyramimonadophyceae; prym. – prymnesiophyceae; dict. – dictyochophyceae; crypt. – cryptophyceae; chrys. – chrysophyceae
| Species | Phylum | Class | [] | [] | References |
|---|---|---|---|---|---|
| Alexandrium minutum | dflg. | dph. | 21.7 | 222.5 | (Lewis et al., 2006) |
| Alexandrium ostenfeldii | dflg. | dph. | 41.1 | 110.5 | (Lewis et al., 2006) |
| Alexandrium tamarense | dflg. | dph. | 26.7 | 200 | (Lewis et al., 2006) |
| Amphidinium britannicum | dflg. | dph. | 51.2 | 68.7 | (Bauerfeind et al., 1986) |
| Amphidinium carterae | dflg. | dph. | 16 | 81.55 | (Gittleson et al., 1974; Bauerfeind et al., 1986) |
| Amphidinium klebsi | dflg. | dph. | 35 | 73.9 | (Gittleson et al., 1974) |
| Apedinella spinifera | ochph. (het.) | dict. | 8.25 | 132.5 | (Throndsen, 1973) |
| Bodo designis | euglenozoa | kinetoplastea | 5.5 | 39 | (Visser and Kiørboe, 2006) |
| Brachiomonas submarina | chlph. | chlorophyceae | 27.5 | 96 | (Bauerfeind et al., 1986) |
| Cachonina (Heterocapsa) niei | dflg. | dph. | 21.4 | 302.8 | (Levandowsky and Kaneta, 1987; Kamykowski and Zentara, 1977) |
| Cafeteria roenbergensis | bygira (heterokont) | bicosoecida | 2 | 94.9 | (Fenchel and Blackburn, 1999) |
| Ceratium cornutum | dflg. | dph. | 122.3 | 177.75 | (Levandowsky and Kaneta, 1987; Metzner, 1929) |
| Ceratium furca | dflg. | dph. | 122.5 | 194 | (Peters, 1929) |
| Ceratium fusus | dflg. | dph. | 307.5 | 156.25 | (Peters, 1929) |
| Ceratium hirundinella | dflg. | dph. | 397.5 | 236.1 | (Levandowsky and Kaneta, 1987) |
| Ceratium horridum | dflg. | dph. | 225 | 20.8 | (Peters, 1929) |
| Ceratium lineatus | dflg. | dph. | 82.1 | 36 | (Fenchel, 2001) |
| Ceratium longipes | dflg. | dph. | 210 | 166 | (Peters, 1929) |
| Ceratium macroceros | dflg. | dph. | 50 | 15.4 | (Peters, 1929) |
| Ceratium tripos | dflg. | dph. | 152.3 | 121.7 | (Peters, 1929; Bauerfeind et al., 1986) |
| Chilomonas paramecium | cryptophyta | crypt. | 30 | 111.25 | (Lee, 1954; Jahn and Bovee, 1967; Gittleson et al., 1974) |
| Chlamydomonas reinhardtii | chlph. | chlorophyceae | 10 | 130 | (Gittleson et al., 1974; Roberts, 1981; Guasto et al., 2010) |
| Chlamydomonas moewusii | chlph. | chlorophyceae | 12.5 | 128 | (Gittleson et al., 1974) |
| Chlamydomonas sp. | chlph. | chlorophyceae | 13 | 63.2 | (Lowndes, 1944; Lowndes, 1941; Bauerfeind et al., 1986) |
| Crithidia deanei | euglenozoa | kinetoplastea | 7.4 | 45.6 | (Gadelha et al., 2007) |
| Crithidia fasciculata | euglenozoa | kinetoplastea | 11.1 | 54.3 | (Gadelha et al., 2007) |
| Crithidia (Strigomonas) oncopelti | euglenozoa | kinetoplastea | 8 .1 | 18.5 | (Roberts, 1981; Gittleson et al., 1974) |
| Crypthecodinium cohnii | dflg. | dph. | n/a | 122.8 | (Fenchel, 2001) |
| Dinophysis acuta | dflg. | dph. | 65 | 500 | (Peters, 1929) |
| Dinophysis ovum | dflg. | dph. | 45 | 160 | (Buskey et al., 1993) |
| Dunaliella sp. | chlph. | chlorophyceae | 10.8 | 173.5 | (Gittleson et al., 1974; Bauerfeind et al., 1986) |
| Euglena gracilis | euglenozoa | euglenida (eugl.) | 47.5 | 111.25 | (Lee, 1954; Jahn and Bovee, 1967; Gittleson et al., 1974) |
| Euglena viridis | euglenozoa | euglenida (eugl.) | 58 | 80 | (Holwill, 1975; Roberts, 1981; Lowndes, 1941) |
| Eutreptiella gymnastica | euglenozoa | euglenida (aphagea) | 23.5 | 237.5 | (Throndsen, 1973) |
| Eutreptiella sp. R | euglenozoa | euglenida | 50 | 135 | (Throndsen, 1973) |
| Exuviaella baltica (Prorocentrum balticum) | dflg. | dph. | 15.5 | 138.9 | (Wheeler, 1966) |
| Giardia lamblia | srcm. | zoomastigophora | 11.25 | 26 | (Lenaghan et al., 2011; Campanati et al., 2002; Chen et al., 2012) |
| Gonyaulax polyedra | dflg. | dph. | 39.2 | 254.05 | (Hand et al., 1965; Gittleson et al., 1974; Kamykowski et al., 1992) |
| Gonyaulax polygramma | dflg. | dph. | 46.2 | 500 | (Levandowsky and Kaneta, 1987) |
| Gymnodinium aureolum | dflg. | dph. | n/a | 394 | (Meunier et al., 2013) |
| Gymnodinium sanguineum (splendens) | dflg. | dph. | 47.6 | 220.5 | (Kamykowski et al., 1992; Levandowsky and Kaneta, 1987) |
| Gymnodinium simplex | dflg. | dph. | 10.6 | 559 | (Jakobsen et al., 2006) |
| Gyrodinium aureolum | dflg. | dph. | 30.5 | 139 | (Bauerfeind et al., 1986; Throndsen, 1973) |
| Gyrodinium dorsum (bi-flagellated) | dflg. | dph. | 37.5 | 324 | (Hand et al., 1965; Gittleson et al., 1974; Kamykowski et al., 1992; Levandowsky and Kaneta, 1987; Brennen and Winet, 1977) |
| Gyrodinium dorsum (uni-flagellated) | dflg. | dph. | 34.5 | 148.35 | (Hand and Schmidt, 1975) |
| Hemidinium nasutum | dflg. | dph. | 27.2 | 105.6 | (Levandowsky and Kaneta, 1987; Metzner, 1929) |
| Hemiselmis simplex | cryptophyta | crypt. | 5.25 | 325 | (Throndsen, 1973) |
| Heterocapsa pygmea | dflg. | dph. | 13.5 | 102.35 | (Bauerfeind et al., 1986) |
| Heterocapsa rotundata | dflg. | dph. | 12.5 | 323 | (Jakobsen et al., 2006) |
| Heterocapsa triquetra | dflg. | dph. | 17 | 97 | (Visser and Kiørboe, 2006) |
| Heteromastix pyriformis | chlph. | nephrophyseae | 6 | 87.5 | (Throndsen, 1973) |
| Hymenomonas carterae | haptophyta | prym. | 12.5 | 87 | (Bauerfeind et al., 1986) |
| Katodinium rotundatum (Heterocapsa rotundata) | dflg. | dph. | 10.8 | 425 | (Levandowsky and Kaneta, 1987; Throndsen, 1973) |
| Leishmania major | euglenozoa | kinetoplastea | 12.5 | 36.4 | (Gadelha et al., 2007) |
| Menoidium cultellus | euglenozoa | euglenida (eugl.) | 45 | 136.75 | (Holwill, 1975; Votta et al., 1971) |
| Menoidium incurvum | euglenozoa | euglenida (eugl.) | 25 | 50 | (Lowndes, 1941; Gittleson et al., 1974) |
| Micromonas pusilla | chlph. | mamiellophyceae | 2 | 58.5 | (Bauerfeind et al., 1986; Throndsen, 1973) |
| Monas stigmata | ochph. (het.) | chrys. | 6 | 269 | (Gittleson et al., 1974) |
| Monostroma angicava | chlph. | ulvophyceae | 6.7 | 170.55 | (Togashi et al., 1997) |
| Nephroselmis pyriformis | chlph. | nephrophyseae | 4.8 | 163.5 | (Bauerfeind et al., 1986) |
| Oblea rotunda | dflg. | dph. | 20 | 420 | (Buskey et al., 1993) |
| Ochromonas danica | ochph. (het.) | chrys. | 8.7 | 77 | (Holwill and Peters, 1974) |
| Ochromonas malhamensis | ochph. (het.) | chrys. | 3 | 57.5 | (Holwill, 1974) |
| Ochromonas minima | ochph. (het.) | chrys. | 5 | 75 | (Throndsen, 1973) |
| Olisthodiscus luteus | ochph. (het.) | raphidophyceae | 22.5 | 90 | (Bauerfeind et al., 1986; Throndsen, 1973) |
| Oxyrrhis marina | dflg. | oxyrrhea | 39.5 | 300 | (Boakes et al., 2011; Fenchel, 2001) |
| Paragymnodinium shiwhaense | dflg. | dph. | 10.9 | 571 | (Meunier et al., 2013) |
| Paraphysomonas vestita | ochph. (het.) | chrys. | 14.7 | 116.85 | (Christensen-Dalsgaard and Fenchel, 2004) |
| Pavlova lutheri | haptophyta | pavlovophyceae | 6.5 | 126 | (Bauerfeind et al., 1986) |
| Peranema trichophorum | euglenozoa | euglenida (heteronematales) | 45 | 20 | (Lowndes, 1941; Gittleson et al., 1974; Brennen and Winet, 1977) |
| Peridinium bipes | dflg. | dph. | 42.9 | 291 | (Fenchel, 2001) |
| Peridinium cf. quinquecorne | dflg. | dph. | 19 | 1500 | (Bauerfeind et al., 1986; Levandowsky and Kaneta, 1987; Horstmann, 1980) |
| Peridinium cinctum | dflg. | dph. | 47.5 | 120 | (Bauerfeind et al., 1986; Levandowsky and Kaneta, 1987; Metzner, 1929) |
| Peridinium (Protoperidinium) claudicans | dflg. | dph. | 77.5 | 229 | (Peters, 1929) |
| Peridinium (Protoperidinium) crassipes | dflg. | dph. | 102 | 100 | (Peters, 1929) |
| Peridinium foliaceum | dflg. | dph. | 30.6 | 185.2 | (Kamykowski et al., 1992) |
| Peridinium (Bysmatrum) gregarium | dflg. | dph. | 32.5 | 1291.7 | (Levandowsky and Kaneta, 1987) |
| Peridinium (Protoperidinium) ovatum | dflg. | dph. | 61 | 187.5 | (Peters, 1929) |
| Peridinium (Peridiniopsis) penardii | dflg. | dph. | 28.8 | 417 | (Sibley et al., 1974) |
| Peridinium (Protoperidinium) pentagonum | dflg. | dph. | 92.5 | 266.5 | (Peters, 1929) |
| Peridinium (Protoperidinium) subinerme | dflg. | dph. | 50 | 285 | (Peters, 1929) |
| Peridinium trochoideum | dflg. | dph. | 25 | 53 | (Levandowsky and Kaneta, 1987) |
| Peridinium umbonatum | dflg. | dph. | 30 | 250 | (Levandowsky and Kaneta, 1987; Metzner, 1929) |
| Phaeocystis pouchetii | haptophyta | prym. | 6.3 | 88 | (Bauerfeind et al., 1986) |
| Polytoma uvella | chlph. | chlorophyceae | 22.5 | 100.9 | (Lowndes, 1944; Gittleson et al., 1974; Lowndes, 1941) |
| Polytomella agilis | chlph. | chlorophyceae | 12.4 | 150 | (Gittleson and Jahn, 1968; Gittleson and Noble, 1973; Gittleson et al., 1974; Roberts, 1981) |
| Prorocentrum mariae-lebouriae | dflg. | dph. | 14.8 | 141.05 | (Kamykowski et al., 1992; Bauerfeind et al., 1986; Miyasaka et al., 1998) |
| Prorocentrum micans | dflg. | dph. | 45 | 329.1 | (Bauerfeind et al., 1986; Levandowsky and Kaneta, 1987) |
| Prorocentrum minimum | dflg. | dph. | 15.1 | 107.7 | (Bauerfeind et al., 1986; Miyasaka et al., 1998) |
| Prorocentrum redfieldii Bursa (P.triestinum) | dflg. | dph. | 33.2 | 333.3 | (Sournia, 1982) |
| Protoperidinium depressum | dflg. | dph. | 132 | 450 | (Buskey et al., 1993) |
| Protoperidinium granii (Ostf.) Balech | dflg. | dph. | 57.5 | 86.1 | (Sournia, 1982) |
| Protoperidinium pacificum | dflg. | dph. | 54 | 410 | (Buskey et al., 1993) |
| Prymnesium polylepis | haptophyta | prym. | 9.1 | 45 | (Dölger et al., 2017) |
| Prymnesium parvum | haptophyta | prym. | 7.2 | 30 | (Dölger et al., 2017) |
| Pseudopedinella pyriformis | ochph. (het.) | dict. | 6.5 | 100 | (Throndsen, 1973) |
| Pseudoscourfieldia marina | chlph. | pyr. | 4.1 | 42 | (Bauerfeind et al., 1986) |
| Pteridomonas danica | ochph. (het.) | dict. | 5.5 | 179.45 | (Christensen-Dalsgaard and Fenchel, 2004) |
| Pyramimonas amylifera | chlph. | pyr. | 24.5 | 22.5 | (Bauerfeind et al., 1986) |
| Pyramimonas cf. disomata | chlph. | pyr. | 9 | 355 | (Throndsen, 1973) |
| Rhabdomonas spiralis | euglenozoa | euglenida (aphagea) | 27 | 120 | (Holwill, 1975) |
| Rhodomonas salina | cryptophyta | crypt. | 14.5 | 588.5 | (Jakobsen et al., 2006; Meunier et al., 2013) |
| Scrippsiella trochoidea | dflg. | dph. | 25.3 | 87.6 | (Kamykowski et al., 1992; Bauerfeind et al., 1986; Sournia, 1982) |
| Spumella sp. | ochph. (het.) | chrys. | 10 | 25 | (Visser and Kiørboe, 2006) |
| Teleaulax sp. | cryptophyta | crypt. | 13.5 | 98 | (Meunier et al., 2013) |
| Trypanosoma brucei | euglenozoa | kinetoplastea | 18.8 | 20.5 | (Rodríguez et al., 2009) |
| Trypanosoma cruzi | euglenozoa | kinetoplastea | 20 | 172 | (Jahn and Fonseca, 1963; Brennen and Winet, 1977) |
| Trypanosoma vivax | euglenozoa | kinetoplastea | 23.5 | 29.5 | (Bargul et al., 2016) |
| Trypanosoma evansi | euglenozoa | kinetoplastea | 21.5 | 16.1 | (Bargul et al., 2016) |
| Trypanosoma congolense | euglenozoa | kinetoplastea | 18 | 9.7 | (Bargul et al., 2016) |
| Tetraflagellochloris mauritanica | chlph. | chlorophyceae | 4 | 300 | (Barsanti et al., 2016) |
Appendix 2
Data for swimming ciliates
Abbreviations: imnc. = intramacronucleata; pcdph. = postciliodesmatophora; olig. – oligohymenophorea; spir. – spirotrichea; hettr. – heterotrichea; lit. – litostomatea; eugl. – euglenophyceae
| Species | Phylum | Class | [] | [] | References |
|---|---|---|---|---|---|
| Amphileptus gigas | imnc. | lit. | 808 | 608 | (Bullington, 1925) |
| Amphorides quadrilineata | imnc. | spir. | 138 | 490 | (Buskey et al., 1993) |
| Balanion comatum | imnc. | prostomatea | 16 | 220 | (Visser and Kiørboe, 2006) |
| Blepharisma | pcdph. | hettr. | 350 | 600 | (Sleigh and Blake, 1977; Roberts, 1981) |
| Coleps hirtus | imnc. | prostomatea | 94.5 | 686 | (Bullington, 1925) |
| Coleps sp. | imnc. | prostomatea | 78 | 523 | (Bullington, 1925) |
| Colpidium striatum | imnc. | olig. | 77 | 570 | (Beveridge et al., 2010) |
| Condylostoma patens | pcdph. | hettr. | 371 | 1061 | (Bullington, 1925; Machemer, 1974) |
| Didinium nasutum | imnc. | lit. | 140 | 1732 | (Bullington, 1925; Machemer, 1974; Roberts, 1981; Sleigh and Blake, 1977) |
| Euplotes charon | imnc. | spir. | 66 | 1053 | (Bullington, 1925) |
| Euplotes patella | imnc. | spir. | 202 | 1250 | (Bullington, 1925) |
| Euplotes vannus | imnc. | spir. | 82 | 446 | (Wang et al., 2008; Ricci et al., 1997) |
| Eutintinnus cf. pinguis | imnc. | spir. | 147 | 410 | (Buskey et al., 1993) |
| Fabrea salina | pcdph. | hettr. | 184.1 | 216 | (Marangoni et al., 1995) |
| Favella panamensis | imnc. | spir. | 238 | 600 | (Buskey et al., 1993) |
| Favella sp. | imnc. | spir. | 150 | 1080 | (Buskey et al., 1993) |
| Frontonia sp. | imnc. | olig. | 378.5 | 1632 | (Bullington, 1925) |
| Halteria grandinella | imnc. | spir. | 50 | 533 | (Bullington, 1925; Gilbert, 1994) |
| Kerona polyporum | imnc. | spir. | 107 | 476.5 | (Bullington, 1925) |
| Laboea strobila | imnc. | spir. | 100 | 810 | (Buskey et al., 1993) |
| Lacrymaria lagenula | imnc. | lit. | 45 | 909 | (Bullington, 1925) |
| Lembadion bullinum | imnc. | olig. | 43 | 415 | (Bullington, 1925) |
| Lembus velifer | imnc. | olig. | 87 | 200 | (Bullington, 1925) |
| Mesodinium rubrum | imnc. | lit. | 38 | 7350 | (Jonsson and Tiselius, 1990; Riisgård and Larsen, 2009; Crawford and Lindholm, 1997) |
| Metopides contorta | imnc. | armophorea | 115 | 359 | (Bullington, 1925) |
| Nassula ambigua | imnc. | nassophorea | 143 | 2004 | (Bullington, 1925) |
| Nassula ornata | imnc. | nassophorea | 282 | 750 | (Bullington, 1925) |
| Opalina ranarum | placidozoa (heterokont) | opalinea | 350 | 50 | (Blake, 1975; Sleigh and Blake, 1977) |
| Ophryoglena sp. | imnc. | olig. | 325 | 4000 | (Machemer, 1974) |
| Opisthonecta henneg | imnc. | olig. | 126 | 1197 | (Machemer, 1974; Jahn and Hendrix, 1969) |
| Oxytricha bifara | imnc. | spir. | 282 | 1210 | (Bullington, 1925) |
| Oxytricha ferruginea | imnc. | spir. | 150 | 400 | (Bullington, 1925) |
| Oxytricha platystoma | imnc. | spir. | 130 | 520 | (Bullington, 1925) |
| Paramecium aurelia | imnc. | olig. | 244 | 1650 | (Bullington, 1925; Bullington, 1930) |
| Paramecium bursaria | imnc. | olig. | 130 | 1541.5 | (Bullington, 1925; Bullington, 1930) |
| Paramecium calkinsii | imnc. | olig. | 124 | 1392 | (Bullington, 1930; Bullington, 1925) |
| Paramecium caudatum | imnc. | olig. | 225.5 | 2489.35 | (Bullington, 1930; Jung et al., 2014) |
| Paramecium marinum | imnc. | olig. | 115 | 930 | (Bullington, 1925) |
| Paramecium multimicronucleatum | imnc. | olig. | 251 | 3169.5 | (Bullington, 1930) |
| Paramecium polycaryum | imnc. | olig. | 91 | 1500 | (Bullington, 1930) |
| Paramecium spp. | imnc. | olig. | 200 | 975 | (Jahn and Bovee, 1967; Sleigh and Blake, 1977; Roberts, 1981) |
| Paramecium tetraurelia | imnc. | olig. | 124 | 784 | (Funfak et al., 2015) |
| Paramecium woodruffi | imnc. | olig. | 160 | 2013.5 | (Bullington, 1930) |
| Porpostoma notatum | imnc. | olig. | 107.7 | 1842.2 | (Fenchel and Blackburn, 1999) |
| Prorodon teres | imnc. | prostomatea | 175 | 1066 | (Bullington, 1925) |
| Spathidium spathula | imnc. | lit. | 204.5 | 526 | (Bullington, 1925) |
| Spirostomum ambiguum | pcdph. | hettr. | 1045 | 810 | (Bullington, 1925) |
| Spirostomum sp. | pcdph. | hettr. | 1000 | 1000 | (Sleigh and Blake, 1977) |
| Spirostomum teres | pcdph. | hettr. | 450 | 640 | (Bullington, 1925) |
| Stenosemella steinii | imnc. | spir. | 83 | 190 | (Buskey et al., 1993) |
| Stentor caeruleus | pcdph. | hettr. | 528.5 | 1500 | (Bullington, 1925) |
| Stentor polymorphus | pcdph. | hettr. | 208 | 887 | (Bullington, 1925; Sleigh and Aiello, 1972; Sleigh, 1968) |
| Strobilidium spiralis | imnc. | spir. | 60 | 330 | (Buskey et al., 1993) |
| Strobilidium velox | imnc. | spir. | 43 | 150 | (Gilbert, 1994) |
| Strombidinopsis acuminatum | imnc. | spir. | 80 | 390 | (Buskey et al., 1993) |
| Strombidium claparedi | imnc. | spir. | 69.5 | 3740 | (Bullington, 1925) |
| Strombidium conicum | imnc. | spir. | 75 | 570 | (Buskey et al., 1993) |
| Strombidium sp. | imnc. | spir. | 33 | 360 | (Buskey et al., 1993) |
| Strombidium sulcatum | imnc. | spir. | 32.5 | 995 | (Fenchel and Jonsson, 1988; Fenchel and Blackburn, 1999
Fenchel and Blackburn, 1999) |
| Stylonichia sp. | imnc. | spir. | 167 | 737.5 | (Bullington, 1925; Machemer, 1974) |
| Tetrahymena pyriformis | imnc. | olig. | 72.8 | 475.6 | (Sleigh and Blake, 1977; Roberts, 1981; Brennen and Winet, 1977) |
| Tetrahymena thermophila | imnc. | olig. | 46.7 | 204.5 | (Wood et al., 2007) |
| Tillina magna | imnc. | colpodea | 162.5 | 2000 | (Bullington, 1925) |
| Tintinnopsis kofoidi | imnc. | spir. | 100 | 400 | (Buskey et al., 1993) |
| Tintinnopsis minuta | imnc. | spir. | 40 | 60 | (Buskey et al., 1993) |
| Tintinnopsis tubulosa | imnc. | spir. | 95 | 160 | (Buskey et al., 1993) |
| Tintinnopsis vasculum | imnc. | spir. | 82 | 250 | (Buskey et al., 1993) |
| Trachelocerca olor | pcdph. | karyorelictea | 267.5 | 900 | (Bullington, 1925) |
| Trachelocerca tenuicollis | pcdph. | karyorelictea | 432 | 1111 | (Bullington, 1925) |
| Uroleptus piscis | imnc. | spir. | 203 | 487 | (Bullington, 1925) |
| Uroleptus rattulus | imnc. | spir. | 400 | 385 | (Bullington, 1925) |
| Urocentrum turbo | imnc. | olig. | 90 | 700 | (Bullington, 1925) |
| Uronema filificum | imnc. | olig. | 25.7 | 1372.7 | (Fenchel and Blackburn, 1999) |
| Uronema marinum | imnc. | olig. | 56.9 | 1010 | (Fenchel and Blackburn, 1999) |
| Uronema sp. | imnc. | olig. | 25 | 1175 | (Sleigh and Blake, 1977; Roberts, 1981) |
| Uronychia transfuga | imnc. | spir. | 118 | 6406 | (Leonildi et al., 1998) |
| Uronychia setigera | imnc. | spir. | 64 | 7347 | (Leonildi et al., 1998) |
| Uronemella spp. | imnc. | olig. | 28 | 250 | (Petroff et al., 2015) |
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
Maciej Lisicki, Email: maciej.lisicki@fuw.edu.pl.
Eric Lauga, Email: e.lauga@damtp.cam.ac.uk.
Arup K Chakraborty, Massachusetts Institute of Technology, United States.
Arup K Chakraborty, Massachusetts Institute of Technology, United States.
Funding Information
This paper was supported by the following grants:
H2020 European Research Council 682754 to Eric Lauga.
Engineering and Physical Sciences Research Council EP/M017982/ to Raymond E Goldstein.
Gordon and Betty Moore Foundation 7523 to Raymond E Goldstein.
Additional information
Competing interests
Reviewing editor, eLife.
No competing interests declared.
Author contributions
Conceptualization, Data curation, Software, Formal analysis, Validation, Investigation, Methodology, Writing—original draft, Writing—review and editing.
Data curation, Software, Formal analysis, Investigation, Visualization, Methodology, Writing—original draft, Writing—review and editing.
Investigation, Methodology, Writing—review and editing.
Conceptualization, Formal analysis, Supervision, Funding acquisition, Validation, Investigation, Methodology, Writing—original draft, Project administration, Writing—review and editing.
Additional files
Data availability
All data generated or analysed during this study are included in the manuscript.
References
- Adam RD. Biology of giardia lamblia. Clinical Microbiology Reviews. 2001;14:447–475. doi: 10.1128/CMR.14.3.447-475.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen NS, Allen RD. Cytoplasmic streaming in green plants. Annual Review of Biophysics and Bioengineering. 1978;7:497–526. doi: 10.1146/annurev.bb.07.060178.002433. [DOI] [PubMed] [Google Scholar]
- Bargul JL, Jung J, McOdimba FA, Omogo CO, Adung'a VO, Krüger T, Masiga DK, Engstler M. Species-Specific adaptations of trypanosome morphology and motility to the mammalian host. PLOS Pathogens. 2016;12:e1005448. doi: 10.1371/journal.ppat.1005448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsanti L, Coltelli P, Evangelista V, Frassanito AM, Gualtieri P. Swimming patterns of the quadriflagellate Tetraflagellochloris mauritanica (Chlamydomonadales, chlorophyceae) Journal of Phycology. 2016;52:209–218. doi: 10.1111/jpy.12384. [DOI] [PubMed] [Google Scholar]
- Bauerfeind E, Elbrächter M, Steiner R, Throndsen J. Application of laser Doppler spectroscopy (LDS) in determining swimming velocities of motile phytoplankton. Marine Biology. 1986;93:323–327. doi: 10.1007/BF00401099. [DOI] [Google Scholar]
- Beaulieu NC, Abu-Dayya AA, McLane PJ. Estimating the distribution of a sum of independent lognormal random variables. IEEE Transactions on Communications. 1995;43:2869. doi: 10.1109/26.477480. [DOI] [Google Scholar]
- Beveridge OS, Petchey OL, Humphries S. Mechanisms of temperature-dependent swimming: the importance of physics, physiology and body size in determining protist swimming speed. Journal of Experimental Biology. 2010;213:4223–4231. doi: 10.1242/jeb.045435. [DOI] [PubMed] [Google Scholar]
- Blake JR. Hydromechanical aspects of ciliary propulsion. In: Wu T, Brokaw C. J, Brennen C, editors. Swimming and Flying in Nature. New York: Plenum; 1975. pp. 185–209. [Google Scholar]
- Boakes DE, Codling EA, Thorn GJ, Steinke M. Analysis and modelling of swimming behaviour in Oxyrrhis marina. Journal of Plankton Research. 2011;33:641–649. doi: 10.1093/plankt/fbq136. [DOI] [Google Scholar]
- Bray D. Cell Movements. New York: Garland Science; 2001. [Google Scholar]
- Brennen C, Winet H. Fluid mechanics of propulsion by cilia and flagella. Annual Review of Fluid Mechanics. 1977;9:339–398. doi: 10.1146/annurev.fl.09.010177.002011. [DOI] [Google Scholar]
- Buetow DE. Euglena. American Cancer Society; 2011. [DOI] [Google Scholar]
- Bullington WE. A study of spiral movement in the ciliate infusoria. Archiv für Protistenkunde. 1925;50:219–274. [Google Scholar]
- Bullington WE. A further study of spiraling in the ciliate Paramecium, with a note on morphology and taxonomy. Journal of Experimental Zoology. 1930;56:423–449. doi: 10.1002/jez.1400560404. [DOI] [Google Scholar]
- Buskey EJ, Coulter C, Strom S. Locomotory patterns of microzooplankton: potential effects on food selectivity of larval fish. Bulletin of Marine Science. 1993;53:29–43. [Google Scholar]
- Campanati L, Holloschi A, Troster H, Spring H, de Souza W, Monteiro-Leal LH. Video-microscopy observations of fast dynamic processes in the protozoon Giardia lamblia. Cell Motility and the Cytoskeleton. 2002;51:213–224. doi: 10.1002/cm.10026. [DOI] [PubMed] [Google Scholar]
- Cappuccinelli P. The Movement of Eukaryotic Cells. Netherlands, Dordrecht: Springer; 1980. [DOI] [Google Scholar]
- Chen J, Lenaghan SC, Zhang M. Analysis of dynamics and planar motion strategies of a swimming microorganism - Giardia lamblia. IEEE International Conference on Robotics and Automation; 2012. pp. 4204–4209. [DOI] [Google Scholar]
- Christensen-Dalsgaard KK, Fenchel T. Complex flagellar motions and swimming patterns of the flagellates Paraphysomonas vestita and Pteridomonas danica. Protist. 2004;155:79–87. doi: 10.1078/1434461000166. [DOI] [PubMed] [Google Scholar]
- Crawford DW, Lindholm T. Some observations on vertical distribution and migration of the phototrophic ciliate Mesodinium rubrum (= Myrionecta rubra) in a stratified brackish inlet. Aquatic Microbial Ecology. 1997;13:267–274. doi: 10.3354/ame013267. [DOI] [Google Scholar]
- Dölger J, Nielsen LT, Kiørboe T, Andersen A. Swimming and feeding of mixotrophic biflagellates. Scientific Reports. 2017;7:39892. doi: 10.1038/srep39892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenchel T. How dinoflagellates swim. Protist. 2001;152:329–338. doi: 10.1078/1434-4610-00071. [DOI] [PubMed] [Google Scholar]
- Fenchel T, Blackburn N. Motile chemosensory behaviour of phagotrophic protists: mechanisms for and efficiency in congregating at food patches. Protist. 1999;150:325–336. doi: 10.1016/S1434-4610(99)70033-7. [DOI] [PubMed] [Google Scholar]
- Fenchel T, Jonsson PR. The functional biology of Strombidium sulcatum, a marine oligotrich ciliate (Ciliophora, oligotrichina) Marine Ecology Progress Series. 1988;48:1–15. doi: 10.3354/meps048001. [DOI] [Google Scholar]
- Freedman D, Diaconis P. Zeitschrift Fü Wahrscheinlichkeitstheorie Und Verwandte Gebiete. Vol. 57. Springer; 1981. On the histogram as a density estimator: L2 theory; pp. 453–476. [Google Scholar]
- Funfak A, Fisch C, Abdel Motaal HT, Diener J, Combettes L, Baroud CN, Dupuis-Williams P. Paramecium swimming and ciliary beating patterns: a study on four RNA interference mutations. Integrative Biology. 2015;7:90–100. doi: 10.1039/c4ib00181h. [DOI] [PubMed] [Google Scholar]
- Gadelha C, Wickstead B, Gull K. Flagellar and ciliary beating in trypanosome motility. Cell Motility and the Cytoskeleton. 2007;64:629–643. doi: 10.1002/cm.20210. [DOI] [PubMed] [Google Scholar]
- Gilbert JJ. Jumping behavior in the oligotrich ciliates Strobilidium velox and Halteria grandinella, and its significance as a defense against rotifer predators. Microbial Ecology. 1994;27:189–200. doi: 10.1007/BF00165817. [DOI] [PubMed] [Google Scholar]
- Gittleson SM, Hotchkiss SK, Valencia FG. Locomotion in the marine dinoflagellate Amphidinium carterae (Hulburt) Transactions of the American Microscopical Society. 1974;93:101–105. doi: 10.2307/3225224. [DOI] [Google Scholar]
- Gittleson SM, Jahn TL. Flagellar activity of Polytomella agilis. Transactions of the American Microscopical Society. 1968;87:464–471. doi: 10.2307/3224220. [DOI] [Google Scholar]
- Gittleson SM, Noble RM. Locomotion in Polytomella agilis and Polytoma uvella. Transactions of the American Microscopical Society. 1973;93:101–105. doi: 10.2307/3225176. [DOI] [Google Scholar]
- Goldstein RE. Green algae as model organisms for biological fluid dynamics. Annual Review of Fluid Mechanics. 2015;47:343–375. doi: 10.1146/annurev-fluid-010313-141426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RE. Are theoretical results 'Results'? eLife. 2018;7:e40018. doi: 10.7554/eLife.40018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guasto JS, Johnson KA, Gollub JP. Oscillatory flows induced by microorganisms swimming in two dimensions. Physical Review Letters. 2010;105:168102. doi: 10.1103/PhysRevLett.105.168102. [DOI] [PubMed] [Google Scholar]
- Hand WG, Collard PA, Davenport D. The effects of temperature and salinity change on swimming rate in the dinoflagellates, Gonyaulax and Gyrodinium. The Biological Bulletin. 1965;128:90–101. doi: 10.2307/1539392. [DOI] [Google Scholar]
- Hand WG, Schmidt JA. Phototactic orientation by the marine dinoflagellate Gyrodinium dorsum kofoid. ii. flagellar activity and overall response mechanism. The Journal of Protozoology. 1975;22:494–498. doi: 10.1111/j.1550-7408.1975.tb05217.x. [DOI] [Google Scholar]
- Harris EH, Stern DB, Witman GB. The Chlamydomonas Sourcebook. 2nd edition. London: Academic Press; 2009. [Google Scholar]
- Hinchliff CE, Smith SA, Allman JF, Burleigh JG, Chaudhary R, Coghill LM, Crandall KA, Deng J, Drew BT, Gazis R, Gude K, Hibbett DS, Katz LA, Laughinghouse HD, McTavish EJ, Midford PE, Owen CL, Ree RH, Rees JA, Soltis DE, Williams T, Cranston KA. Synthesis of phylogeny and taxonomy into a comprehensive tree of life. PNAS. 2015;112:12764–12769. doi: 10.1073/pnas.1423041112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holwill MEJ. Hydrodynamic aspects of ciliary and flagellar movement. In: Sleigh M. A, editor. Cilia and Flagella. London: Academic Press; 1974. pp. 143–176. [Google Scholar]
- Holwill MEJ. The role of body oscillation in the propulsion of microorganisms. In: Wu T, Brokaw C. J, Brennen C, editors. Swimming and Flying in Nature. New York: Plenum; 1975. pp. 133–141. [Google Scholar]
- Holwill ME, Peters PD. Dynamics of the hispid flagellum of Ochromonas danica. The role of mastigonemes. The Journal of Cell Biology. 1974;62:322–328. doi: 10.1083/jcb.62.2.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horstmann U. Observations on the peculiar diurnal migration of a red tide dinophyceae in tropical shallow waters. Journal of Phycology. 1980;16:481–485. doi: 10.1111/j.1529-8817.1980.tb03064.x. [DOI] [Google Scholar]
- Jahn TL, Bovee EC. Motile behavior of protozoa. In: Chen T. T, editor. Research in Protozoology. New York: Pergamon; 1967. pp. 41–200. [DOI] [Google Scholar]
- Jahn TL, Fonseca JR. Mechanisms of locomotion of flagellates: v: Trypanosoma lewisi and T. cruzi. The Journal of Protozoology. 1963;11 doi: 10.1111/j.1550-7408.1963.tb01688.x. [DOI] [PubMed] [Google Scholar]
- Jahn TL, Hendrix EM. Locomotion of the telotrich ciliate Opisthonecta henneguyi. Revista De La Sociedad Mexicana De Historia Natural. 1969;30:103–131. [Google Scholar]
- Jahn TL, Votta JJ. Locomotion of protozoa. Annual Review of Fluid Mechanics. 1972;4:93–116. doi: 10.1146/annurev.fl.04.010172.000521. [DOI] [Google Scholar]
- Jakobsen HH, Everett LM, Strom SL. Hydromechanical signaling between the ciliate Mesodinium pulex and motile protist prey. Aquatic Microbial Ecology. 2006;44:197–206. doi: 10.3354/ame044197. [DOI] [Google Scholar]
- Jonsson PP, Tiselius P. Feeding behaviour, prey detection and capture efficiency of the copepod Acartia tonsa feeding on planktonic ciliates. Marine Ecology Progress Series. 1990;60:35–44. doi: 10.3354/meps060035. [DOI] [Google Scholar]
- Jung I, Powers TR, Valles JM. Evidence for two extremes of ciliary motor response in a single swimming microorganism. Biophysical Journal. 2014;106:106–113. doi: 10.1016/j.bpj.2013.11.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamykowski D, Reed RE, Kirkpatrick GJ. Comparison of sinking velocity, swimming velocity, rotation and path characteristics among six marine dinoflagellate species. Marine Biology. 1992;113:319–328. [Google Scholar]
- Kamykowski D, Zentara S-J. The diurnal vertical migration of motile phytoplankton through temperature gradients1. Limnology and Oceanography. 1977;22:148–151. doi: 10.4319/lo.1977.22.1.0148. [DOI] [Google Scholar]
- Kirkwood TB, Feder M, Finch CE, Franceschi C, Globerson A, Klingenberg CP, LaMarco K, Omholt S, Westendorp RG. What accounts for the wide variation in life span of genetically identical organisms reared in a constant environment? Mechanisms of Ageing and Development. 2005;126:439–443. doi: 10.1016/j.mad.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Koch AL. The logarithm in biology. 1. Mechanisms generating the log-normal distribution exactly. Journal of Theoretical Biology. 1966;12:276–290. doi: 10.1016/0022-5193(66)90119-6. [DOI] [PubMed] [Google Scholar]
- Kullback S, Leibler RA. On information and sufficiency. The Annals of Mathematical Statistics. 1951;22:79–86. doi: 10.1214/aoms/1177729694. [DOI] [Google Scholar]
- Lauga E, Powers TR. The hydrodynamics of swimming microorganisms. Reports on Progress in Physics. 2009;72:096601. doi: 10.1088/0034-4885/72/9/096601. [DOI] [Google Scholar]
- Lee JW. The effect of pH on forward swimming in Euglena and Chilomonas. Transactions of the American Microscopical Society. 1954;84:395–406. [Google Scholar]
- Lenaghan SC, Davis CA, Henson WR, Zhang Z, Zhang M. High-speed microscopic imaging of flagella motility and swimming in Giardia lamblia trophozoites. PNAS. 2011;108:E550–E558. doi: 10.1073/pnas.1106904108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonildi A, Erra F, Banchetti R, Ricci N. The ethograms of Uronychia transfuga and Uronychia setigera (ciliata, hypotrichida): a comparative approach for new insights into the behaviour of protozoa. European Journal of Protistology. 1998;34:426–435. doi: 10.1016/S0932-4739(98)80011-4. [DOI] [Google Scholar]
- Levandowsky M, Kaneta PJ. Behaviour in dinoflagellates. In: Taylor F. J. R, editor. The Biology of Dinoflagellates (Botanical Monographs) Vol. 21. Blackwell Scientific; 1987. pp. 360–397. [Google Scholar]
- Lewis NI, Xu W, Jericho SK, Kreuzer HJ, Jericho MH, Cembella AD. Swimming speed of three species of Alexandrium (Dinophyceae) as determined by digital in-line holography. Phycologia. 2006;45:61–70. doi: 10.2216/04-59.1. [DOI] [Google Scholar]
- Limpert E, Stahel WA, Abbt M. Log-normal distributions across the sciences: keys and clues. BioScience. 2001;51:341–352. doi: 10.1641/0006-3568(2001)051[0341:LNDATS]2.0.CO;2. [DOI] [Google Scholar]
- Lowndes AG. On flagellar movement in unicellular organisms. Proceedings of the Zoological Society of London. 1941;A111:111–134. doi: 10.1111/j.1469-7998.1941.tb08476.x. [DOI] [Google Scholar]
- Lowndes AG. The swimming of Monas stigmatica pringsheim and peranema Trichophorum (Ehrbg.) Stein. and Volvox sp. Additional experiments on the working of a flagellum. Proceedings of the Zoological Society of London. 1944;114:325–338. doi: 10.1111/j.1096-3642.1944.tb00228.x. [DOI] [Google Scholar]
- Lyons RA, Saridogan E, Djahanbakhch O. The reproductive significance of human fallopian tube cilia. Human Reproduction Update. 2006;12:363–372. doi: 10.1093/humupd/dml012. [DOI] [PubMed] [Google Scholar]
- Machemer H. Ciliary activity and metachronism in protozoa. In: Sleigh M. A, editor. Cilia and Flagella. London: Academic Press; 1974. pp. 199–287. [Google Scholar]
- Marangoni R, Batistini A, Puntoni S, Colombetti G. Temperature effects on motion parameters and the phototactic reaction of the marine ciliate Fabrea salina. Journal of Photochemistry and Photobiology B: Biology. 1995;30:123–127. doi: 10.1016/1011-1344(95)07160-4. [DOI] [Google Scholar]
- Metzner P. Bewegungsstudien an Peridineen. Z.Bot. 1929;22:225–265. [Google Scholar]
- Meunier CL, Schulz K, Boersma M, Malzahn AM. Impact of swimming behaviour and nutrient limitation on predator–prey interactions in pelagic microbial food webs. Journal of Experimental Marine Biology and Ecology. 2013;446:29–35. doi: 10.1016/j.jembe.2013.04.015. [DOI] [Google Scholar]
- Miyasaka I, Nanba K, Furuya K, Nimura Y. High-speed video observation of swimming behavior and flagellar motility of Prorocentrum minimum (Dinophyceae) Protoplasma. 1998;204:38–46. doi: 10.1007/BF01282292. [DOI] [Google Scholar]
- Moran J, McKean PG, Ginger ML. Eukaryotic flagella: variations in form, function, and composition during evolution. BioScience. 2014;64:1103–1114. doi: 10.1093/biosci/biu175. [DOI] [Google Scholar]
- Nicastro D, McIntosh JR, Baumeister W. 3D structure of eukaryotic flagella in a quiescent state revealed by cryo-electron tomography. PNAS. 2005;102:15889–15894. doi: 10.1073/pnas.0508274102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters N. Über Orts- und Geisselbewegung bei marinen Dinoflagellaten. Archiv für Protistenkunde. 1929;67:291–321. [Google Scholar]
- Petroff AP, Pasulka AL, Soplop N, Wu XL, Libchaber A. Biophysical basis for convergent evolution of two veil-forming microbes. Royal Society Open Science. 2015;2:150437. doi: 10.1098/rsos.150437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell EM. Life at low Reynolds number. American Journal of Physics. 1977;45:3–11. doi: 10.1119/1.10903. [DOI] [Google Scholar]
- Ricci N, Luverà G, Cacciatori M, Banchetti R, Lueken W. The effects of 2 μm Hg++ on the ethogram of Euplotes vannus (Ciliata, Hypotrichida) European Journal of Protistology. 1997;33:63–71. doi: 10.1016/S0932-4739(97)80021-1. [DOI] [Google Scholar]
- Riisgård HU, Larsen PS. Ciliary-propelling mechanism, effect of temperature and viscosity on swimming speed, and adaptive significance of ‘jumping’ in the ciliate Mesodinium rubrum. Marine Biology Research. 2009;5:585–595. doi: 10.1080/17451000902729704. [DOI] [Google Scholar]
- Roberts AM. Hydrodynamics of protozoan swimming. In: Levandowski M, Hunter S, editors. Biochemistry and Physiology of Protozoa. Academic Press; 1981. pp. 5–66. [Google Scholar]
- Rodríguez JA, Lopez MA, Thayer MC, Zhao Y, Oberholzer M, Chang DD, Kisalu NK, Penichet ML, Helguera G, Bruinsma R, Hill KL, Miao J. Propulsion of african trypanosomes is driven by bihelical waves with alternating chirality separated by kinks. PNAS. 2009;106:19322–19327. doi: 10.1073/pnas.0907001106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short MB, Solari CA, Ganguly S, Powers TR, Kessler JO, Goldstein RE. Flows driven by flagella of multicellular organisms enhance long-range molecular transport. PNAS. 2006;103:8315–8319. doi: 10.1073/pnas.0600566103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley TH, Herrgesell PL, Knight AW. Density dependent vertical migration in the freshwater dinoflagellate Peridinium penardii (lemm.) lemm. fo. Californicum javorn. Journal of Phycology. 1974;10:475–476. doi: 10.1111/j.1529-8817.1974.tb02743.x. [DOI] [Google Scholar]
- Sleigh MA. Patterns of ciliary beating. Symposia of the Society for Experimental Biology. 1968;22:131–150. [PubMed] [Google Scholar]
- Sleigh MA. Cilia and Flagella. London: Academic Press; 1975. [Google Scholar]
- Sleigh MA, Aiello E. The movement of water by cilia. Archiv für Protistenkunde. 1972;50:219–274. [Google Scholar]
- Sleigh MA, Blake JR. Methods of ciliary propulsion and their size limitations. In: Pedley T. J, editor. Scale Effects in Animal Locomotion. Academic Press; 1977. pp. 243–256. [Google Scholar]
- Sournia A. Form and function in marine phytoplankton. Biological Reviews. 1982;57:347–394. doi: 10.1111/j.1469-185X.1982.tb00702.x. [DOI] [Google Scholar]
- Szyszkowicz S, Yanikome H. Limit theorem on the sum of identically distributed equally and positively correlated joint lognormals. IEEE Transactions on Communications. 2009;57:3538–3542. doi: 10.1109/TCOMM.2009.12.070539. [DOI] [Google Scholar]
- Tam D, Hosoi AE. Optimal kinematics and morphologies for spermatozoa. Physical Review E. 2011;83 doi: 10.1103/PhysRevE.83.045303. [DOI] [PubMed] [Google Scholar]
- Throndsen J. Motility in some marine nanoplankton flagellates. Norwegian Journal of Zoology. 1973;21:193–200. [Google Scholar]
- Togashi T, Motomura T, Ichimura T. Production of anisogametes and gamete motility dimorphism in Monostroma angicava. Sexual Plant Reproduction. 1997;10:261–268. doi: 10.1007/s004970050096. [DOI] [Google Scholar]
- Visser AW, Kiørboe T. Plankton motility patterns and encounter rates. Oecologia. 2006;148:538–546. doi: 10.1007/s00442-006-0385-4. [DOI] [PubMed] [Google Scholar]
- Votta JJ, Jahn TL, Griffith DL, Fonseca JR. Nature of the flagellar beat in Trachelomonas volvocina, Rhabdomonas spiralis, Menoidium cultellus, and Chilomonas paramecium. Transactions of the American Microscopical Society. 1971;90:404–412. doi: 10.2307/3225455. [DOI] [PubMed] [Google Scholar]
- Wang W, Shor LM, LeBoeuf EJ, Wikswo JP, Taghon GL, Kosson DS. Protozoan migration in bent microfluidic channels. Applied and Environmental Microbiology. 2008;74:1945–1949. doi: 10.1128/AEM.01044-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler B. Phototactic vertical migration in Exuviaella baltica. Botanica Marina. 1966;9:15–17. doi: 10.1515/botm.1966.9.1-2.15. [DOI] [Google Scholar]
- Wichterman R. The Biology of Paramecium. Springer; 1986. [Google Scholar]
- Wood CR, Hard R, Hennessey TM. Targeted gene disruption of dynein heavy chain 7 of Tetrahymena thermophila results in altered ciliary waveform and reduced swim speed. Journal of Cell Science. 2007;120:3075–3085. doi: 10.1242/jcs.007369. [DOI] [PubMed] [Google Scholar]