Abstract
Acute intoxication with organophosphate cholinesterase inhibitors (OPs) is a significant human health threat, and current medical countermeasures for OP poisoning are of limited therapeutic efficacy. The rat model of acute intoxication with diisopropylfluorophosphate (DFP) is increasingly being used to test candidate compounds for efficacy in protecting against the immediate and long-term consequences of acute OP toxicity. In this model, rats are typically pretreated with pyridostigmine bromide (PB), a reversible cholinesterase inhibitor, to enhance survival. However, PB pretreatment is not likely in most scenarios of civilian exposure to acutely neurotoxic levels of OPs. Therefore, the goal of this study was to determine whether PB pretreatment significantly increases survival in DFP-intoxicated rats. Adult male Sprague Dawley rats were injected with DFP (4 mg/kg, s.c.) or vehicle (VEH) followed 1 min later by combined i.m. injection of atropine sulfate (2 mg/kg) and 2-pralidoxime (25 mg/kg). Animals were pretreated 30 min prior to these injections with PB (0.1 mg/kg, i.m.) or an equal volume of saline. DFP triggered rapid and sustained seizure behavior irrespective of PB pretreatment, and there was no significant difference in average seizure behavior score during the first 4 h following injection between DFP animals pretreated with PB or not. PB pretreatment also had no significant effect on survival or brain AChE activity at 24 h post-DFP exposure. In summary, PB pretreatment is not necessary to ensure survival of rats acutely intoxicated with DFP, and eliminating PB pretreatment in the rat model of acute DFP intoxication would increase its relevance to acute OP intoxication in civilians.
Keywords: Carbamate, cholinesterase, neurotoxicity, organophosphates, preclinical model, prophylaxis
Introduction
Cholinesterase inhibiting organophosphorus compounds (OPs) figure prominently in the civilian chemical threat spectrum (Jett and Spriggs, 2018). Credible scenarios of civilian exposure to acutely neurotoxic levels of OPs include intentional release in a terrorist attack, accidental release in a natural disaster or industrial accident (Jett and Spriggs, 2018), or self-poisoning with OPs, with the last accounting for about one-third of the world’s suicide cases (Mew et al., 2017, Pereira et al., 2014). Current medical countermeasures for OP poisoning are of limited therapeutic efficacy (Jett, 2016, Rosenbaum and Bird, 2010), and there is a real need for improved treatments to terminate OP-induced seizures and mitigate long-term neurological sequelae in survivors (Jett and Spriggs, 2018).
Preclinical models are critical tools in drug discovery, and the rat model of acute intoxication with diisopropylfluorophosphate (DFP) is increasingly being used to test candidate therapeutics for efficacy in mitigating the immediate and long-term consequences of acute OP intoxication (Deshpande et al., 2010, Pessah et al., 2016, Pouliot et al., 2016). While less potent than the OP warfare agents, DFP has nearly identical neurotoxic effects (Pouliot et al., 2016, Siso et al., 2017, Sogorb et al., 2015), and is itself considered a credible threat agent (Jett and Spriggs, 2018). The rat model of acute DFP intoxication typically includes pretreatment with the carbamate pyridostigmine bromide (PB) (Deshpande et al., 2010, Li et al., 2011, Pouliot et al., 2016) to enhance survival of intoxicated animals (Kim et al., 1999). PB is FDA-approved for military use as a prophylactic to increase survival during combat situations when exposure to the OP warfare agent soman is anticipated (Lorke and Petroianu, 2019). PB is a reversible cholinesterase inhibitor, and its protective action is presumed to be mediated by reversible carbamylation of the active site of AChE to prevent soman from binding to and irreversibly inhibiting AChE (Lorke and Petroianu, 2019). However, prophylactic use of PB is not an option in most scenarios of civilian exposure to acutely neurotoxic levels of OPs. Thus, questions have been raised as to whether inclusion of PB pretreatment in the rat model represents a realistic model of civilian mass casualties or suicides involving OPs. Therefore, the goal of this study was to determine whether PB pretreatment is needed to ensure survival of DFP-intoxicated rats.
Materials and Methods
Materials
DFP was purchased from Sigma-Aldrich (St. Louis, MO, USA), and confirmed to be ~90 ± 7% pure using previously described NMR methodology (Gao et al., 2016). On the day of experimentation, DFP was prepared in sterile, ice-cold phosphate buffered saline (PBS, 3.6 mM Na2HPO4, 1.4 mM NaH2PO4, 150 mM NaCl, pH 7.2) within 5 min of injection into animals. Pyridostigmine bromide (>98% pure) was purchased from TCI America, Portland, OR, USA; atropine sulfate (>97% pure) and 2-pralidoxime (2-PAM, >97% pure) were purchased from Sigma-Aldrich. All four compounds were aliquoted upon receipt from the manufacturer and stored at −80°C. Under these conditions, DFP is stable for at least 400 days (Heiss et al., 2016).
Animal exposures
All animal experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publication No. 8023, revised 1978) following protocols approved by the University of California-Davis Institutional Animal Care and Use Committee. Adult male Sprague Dawley rats (50–60 days old, 225 −250 g, Charles River Laboratories, Hollister, CA) were individually housed in standard plastic shoebox cages in facilities fully accredited by AAALAC International under controlled environmental conditions (22 ± 2°C, 40–50% humidity, 12 h light-dark cycle). Food and water were provided ad libitum. Experiments were repeated in two cohorts of animals.
Animals were randomly divided into groups using a random number generator. For seizure and survival studies, two groups were set up: (1) DFP pretreated with saline; and (2) DFP pretreated with PB. For AChE studies, two additional groups were set up: (3) vehicle for DFP (VEH) pretreated with saline; and (4) VEH pretreated with PB. In this study, PB was tested at 0.1 mg/kg, which is a commonly used dose in this model (Ferchmin et al., 2014, Kim et al., 1999, Li et al., 2011, Rojas et al., 2015, Siso et al., 2017) that is relevant to the human dose equivalent of PB prophylaxis for soman intoxication (Lorke and Petroianu, 2019). Animals were injected i.m. with PB (0.1 mg/kg in sterile isotonic saline) or an equivalent volume (100 μl) of sterile isotonic saline 30 min prior to injection of either DFP (4 mg/kg, s.c.) or an equivalent volume (300 μl) of vehicle (sterile PBS, s.c.). One min later, all animals were administered a combined i.m. injection of atropine sulfate (2 mg/kg) and 2-pralidoxime (2-PAM, 25 mg/kg) in sterile saline. At 4–5 h post-exposure, all DFP animals were administered 10 ml of 5% dextrose in sterile saline (s.c., Baxter Healthcare Co., Deerfield, IL, USA) to replace lost fluids and prevent hypoglycemia, and then returned to their home cages and provided soft chow (Pessah et al., 2016).
Outcomes assessment
Seizure behavior was continuously monitored for 4 h after DFP or VEH injection and scored using a 5-point scoring metric (Figure 1) by individuals blinded to experimental group. Seizure scores were collected at 5 min intervals from 0 to 120 min post- DFP and at 20 min intervals from 120 to 240 min post DFP. The average seizure score was calculated as a time-weighted average of the animal’s individual seizure scores across the 240 min of observation.
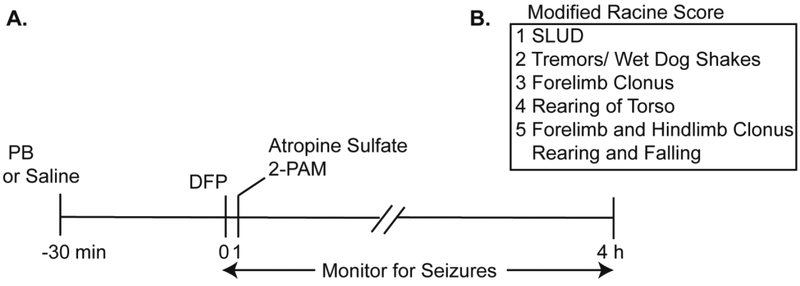
Figure 1. Schematic illustrating the DFP exposure paradigm.
A. Adult Sprague Dawley rats were administered either pyridostigmine bromide (0.1 mg/kg) or an equivalent volume (100 μl) of vehicle (isotonic saline) via i.m. injection. Thirty min later, animals received a s.c. injection of DFP (4 mg/kg) or vehicle (PBS) followed 1 min later by a combined i.m. injection of atropine sulfate (2 mg/kg) and 2-PAM (25 mg/kg). B. Seizure behavior was scored during the first 4 h post-injection using a modified Racine scale. SLUD indicates any of the following symptoms of cholinergic crisis: salivation, lacrimation, urination or defecation.
At 24 h post-exposure, a subset of animals was deeply anesthetized with 5% isoflurane to collect brain tissue for acetylcholinesterase (AChE) analyses. Deeply anesthetized animals were transcardially perfused with 100 ml PBS, brains were quickly removed and the cerebellum dissected out, flash frozen on dry ice and stored at −80°C. On the day of analysis, samples were thawed on ice, and then homogenized in lysis buffer (0.1 M phosphate, pH 8.0 containing 0.1% Triton) using a Dounce homogenizer, centrifuged at 13,400 × g, and the supernatant collected for analysis. AChE activity was determined using the standard Ellman assay (Ellman et al., 1961) with 5,5′-dithio-bis(2-nitrobenzoic acid) (DTNB) and acetylthiocholine iodide (ASChI) as substrate; 100 μM tetraisopropyl pyrophosphoramide was included in the assay to inhibit pseudocholinesterase. AChE activity was normalized using protein concentration as determined using the BCA assay as described by the manufacturer (Pierce, Rockford, IL).
Statistical methods
Time of death post-exposure (in h) was captured for animals that died during the first 24 h following DFP exposure. All animals still alive at 24 h were censored at that time. Kaplan-Meier curves used to illustrate the survival patterns of DFP animals with (n=49) or without PB pretreatment (n=112) were compared using the Wilcoxon test, which is more appropriate when the survival curves cross. Average seizure scores were collected for most but not all DFP animals and these data were compared between DFP animals pretreated with PB (n=44) versus saline (n=109) using the Wilcoxon rank sum test, a non-parametric test, since the underlying assumption of normality was violated in the data. AChE activity data were analyzed using oneway ANOVA with a post hoc Sidak’s multiple comparisons test.
Results
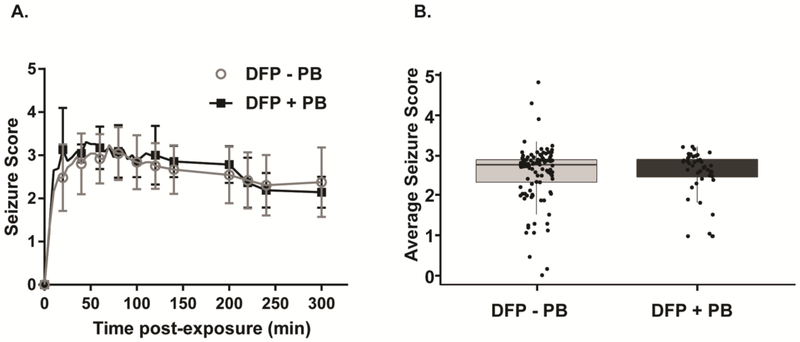
DFP triggered seizure behavior within minutes of exposure that was sustained for hours in both animals pretreated with PB and those that were not (Figure 2A). To determine whether PB treatment significantly altered seizure behavior, we compared the average seizure score between the animals that were pretreated with PB versus the animals that did not receive PB pretreatment (Figure 2B). We found that there was no significant difference in the average seizure scores between these groups (p=0.7). In DFP animals pretreated with PB (n= 44), the mean seizure score was 2.57 (0.61 SD) and the median score (25 – 75 percentile) was 2.71 (2.45–2.92). In DFP animals not pretreated with PB (n=109), the mean seizure score was 2.56 (0.71 SD) and the median was 2.77 (2.33–2.90).
Figure 2. Pyridostigmine bromide (PB) pretreatment has no significant effect on DFP-induced seizure behavior.
A. Seizure scores were obtained at 5 min intervals during the first 120 min following administration of DFP, and at 20 min intervals between 120 and 240 min post-exposure. Data points correspond to the mean seizure score (± S.D.) at each observation point (n=109 DFP - PB and 44 + DFP). B. Average seizure score over the 4 h post-exposure in DFP animals pretreated with 0.1 mg/kg PB or not. Each dot represents the average seizure score of an individual animal; the box, the 25%-ile to 75%-ile; the horizontal bar, the mean; and the bar, the S.D. (n = 109 DFP - PB; 42 DFP + PB).
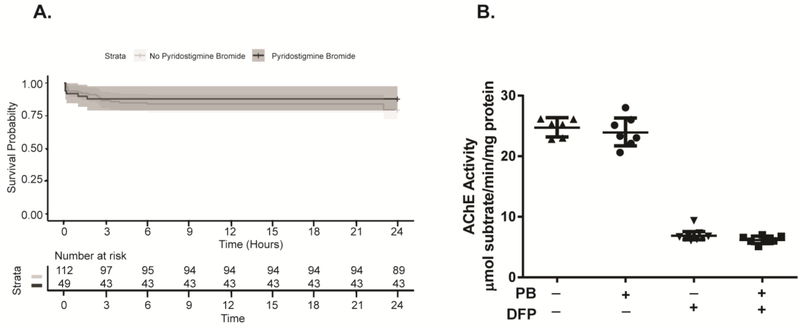
At 24 h post-exposure, 89 of 112 (79.5%) DFP animals not pretreated with PB were still alive, while 43 of 49 (87.8%) DFP animals pretreated with PB were still alive. Figure 3A illustrates the survival curves for the two groups, which were not significantly different (p=0.3). AChE activity at 24 h post-exposure was measured in the cerebellum of a subset of animals (n=6–9 per group) (Figure 3B). In VEH animals, the AChE activity in this brain region was approximately 24–25 μmol substrate/min/mg protein; however, acute DFP intoxication reduced AChE levels to approximately 6–7 μmol substrate/min/mg protein. There were significant differences between the VEH and DFP animals pretreated with PB and between the VEH and DFP animals not pretreated with PB [(17.83. (15.48 to 20.17) (adjusted p value = <0.001) and 17.84 (15.53 to 20.16) (adjusted p value = <0.001), respectively]. In contrast, there were no significant differences between VEH animals that received PB pretreatment versus those that did not, nor were there significant differences between DFP animals that received PB pretreatment versus those that did not. The mean difference in the VEH animals in the Sideks test was −7.386 with a 95% confidence intervals of difference that was −3.182 to 1.705 (adjusted p value =0.9515). Similarly, in the DFP animals, the mean difference was −7.231 (−2.934 – 1.492) (adjusted p value = 0.9320).
Figure 3. Pyridostigmine bromide (PB) pretreatment has no significant effect on 24 h survival or brain AChE activity in DFP-intoxicated rats.
A. Kaplan-Meier curves illustrating the 24 h survival patterns of DFP animals pretreated with PB (n=49) or not (n=112). No significant differences between these groups were identified using the Wilcoxon rank sum test. B. AChE activity in the cerebellum at 24 h post-exposure. Each dot represents a single animal; the horizontal line, the mean; and the error bars, the S.D. (n = 6–9 animals per group).
Discussion
Numerous laboratories have demonstrated that the rat model of acute DFP intoxication recapitulates the neurotoxic effects observed in preclinical models of acute intoxication with OP warfare agents (de Araujo Furtado et al., 2012, Pereira et al., 2014), including rapid and profound inhibition of AChE, seizures that rapidly progress to status epilepticus, and widespread neurodegeneration, neuroinflammation, and oxidative stress that persists in multiple brain regions for days to weeks post-exposure (Deshpande et al., 2010, Hobson et al., 2017, Kim et al., 1999, Li et al., 2011, Liang et al., 2018, Pouliot et al., 2016, Rojas et al., 2015, Siso et al., 2017, Wu et al., 2018). The rat model of acute DFP intoxication typically includes pretreatment with PB and post-exposure treatment with atropine sulfate and 2-PAM (Deshpande et al., 2010, Li et al., 2011, Pouliot et al., 2016), which are thought to be necessary for animals to survive the peripheral cholinergic symptoms of acute OP intoxication (Kim et al., 1999). We have previously reported that post-exposure treatment with atropine sulfate and 2-PAM is required for DFP-intoxicated animals to survive (Pessah et al., 2016). In contrast, the data shown here indicate that pretreatment with PB is not required nor does it enhance survival of adult male rats acutely intoxicated with DFP.
At the dose used in these studies (0.1 mg/kg, i.m.), PB pretreatment did not significantly alter DFP inhibition of AChE activity in the cerebellum at 24 h post-exposure. This is consistent with reports that PB does not penetrate the CNS (Lorke and Petroianu, 2019). OPs are thought to trigger seizures via inhibition of AChE in the brain (Chen, 2012), thus, the lack of effect of PB pretreatment on DFP inhibition of brain cholinesterase activity is consistent with the observation that PB pretreatment also had no significant effect on seizure behavior in DFP-intoxicated animals during the first 4 h post-exposure. One caveat is that we did not assess seizures by electroencephalography (EEG); however, it has been demonstrated that in rats acutely intoxicated with DFP, seizure behavior is highly correlated with abnormal EEG activity indicative of seizure activity (Pouliot et al., 2016).
Our findings indicate that PB pretreatment does not significantly enhance survival at 24 h. However, a key question is whether PB pretreatment significantly improves survival at later time points. While we did not collect data to address this question as part of this study, our laboratory’s experience with hundreds of DFP animals indicates that >90% of DFP animals that survive the first 24 h post-exposure will survive for months post-exposure irrespective of PB pre-treatment. Collectively, these data support the elimination of PB pretreatment from the rat model of acute DFP intoxication to increase its relevance to civilian scenarios of acute OP intoxication. Moreover, because PB interacts with AChE in the periphery, thereby potentially modifying effects of DFP on peripheral targets that influence CNS pathology (notably cardiac and immune function), inclusion of PB pretreatment in the rat DFP model has the potential to confound translation of animal model data to the human situation.
Acknowledgements:
This work was supported by the National Institutes of Health [CounterACT Program grant number NS079202]. MG was supported by a predoctoral fellowship from training grant GM099608 and by a predoctoral fellowship from the David and Dana Loury Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chen Y Organophosphate-induced brain damage: mechanisms, neuropsychiatric and neurological consequences, and potential therapeutic strategies. Neurotoxicology. 2012;33:391–400. [DOI] [PubMed] [Google Scholar]
- de Araujo Furtado M, Rossetti F, Chanda S, Yourick D. Exposure to nerve agents: from status epilepticus to neuroinflammation, brain damage, neurogenesis and epilepsy. Neurotoxicology. 2012;33:1476–90. [DOI] [PubMed] [Google Scholar]
- Deshpande LS, Carter DS, Blair RE, DeLorenzo RJ. Development of a prolonged calcium plateau in hippocampal neurons in rats surviving status epilepticus induced by the organophosphate diisopropylfluorophosphate. Toxicol Sci. 2010;116:623–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres V Jr., Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochemical Pharmacology. 1961;7:88–95. [DOI] [PubMed] [Google Scholar]
- Ferchmin PA, Andino M, Reyes Salaman R, Alves J, Velez-Roman J, Cuadrado B, et al. 4R-cembranoid protects against diisopropylfluorophosphate-mediated neurodegeneration. Neurotoxicology. 2014;44:80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Naughton SX, Wulff H, Singh V, Beck WD, Magrane J, et al. Diisopropylfluorophosphate impairs the transport of membrane-bound organelles in rat cortical axons. J Pharmacol Exp Ther. 2016;356:645–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss DR, Zehnder DW 2nd, Jett DA, Platoff GE Jr., Yeung DT, Brewer BN. Synthesis and storage stability of diisopropylfluorophosphate. J Chem. 2016;2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson BA, Siso S, Rowland DJ, Harvey DJ, Bruun DA, Garbow JR, et al. From the cover: Magnetic resonance imaging reveals progressive brain injury in rats acutely intoxicated with diisopropylfluorophosphate. Toxicol Sci. 2017;157:342–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jett DA. The NIH Countermeasures Against Chemical Threats Program: overview and special challenges. Annals of the New York Academy of Sciences. 2016;1374:5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jett DA, Spriggs SM. Translational research on chemical nerve agents. Neurobiol Dis. 2018. [DOI] [PubMed] [Google Scholar]
- Kim YB, Hur GH, Shin S, Sok DE, Kang JK, Lee YS. Organophosphate-induced brain injuries: delayed apoptosis mediated by nitric oxide. Environ Toxicol Pharmacol. 1999;7:147–52. [DOI] [PubMed] [Google Scholar]
- Li Y, Lein PJ, Liu C, Bruun DA, Tewolde T, Ford G, et al. Spatiotemporal pattern of neuronal injury induced by DFP in rats: a model for delayed neuronal cell death following acute OP intoxication. Toxicol Appl Pharmacol. 2011;253:261–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang LP, Pearson-Smith JN, Huang J, McElroy P, Day BJ, Patel M. Neuroprotective effects of AEOL10150 in a rat organophosphate model. Toxicol Sci. 2018;162:611–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorke DE, Petroianu GA. Reversible cholinesterase inhibitors as pretreatment for exposure to organophosphates. A review. J Appl Toxicol. 2019;39:101–16. [DOI] [PubMed] [Google Scholar]
- Mew EJ, Padmanathan P, Konradsen F, Eddleston M, Chang SS, Phillips MR, et al. The global burden of fatal self-poisoning with pesticides 2006–15: Systematic review. J Affect Disord. 2017;219:93–104. [DOI] [PubMed] [Google Scholar]
- Pereira EF, Aracava Y, DeTolla LJ Jr., Beecham EJ, Basinger GW Jr., Wakayama EJ, et al. Animal models that best reproduce the clinical manifestations of human intoxication with organophosphorus compounds. J Pharmacol Exp Ther. 2014;350:313–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessah IN, Rogawski MA, Tancredi DJ, Wulff H, Zolkowska D, Bruun DA, et al. Models to identify treatments for the acute and persistent effects of seizure-inducing chemical threat agents. Ann N Y Acad Sci. 2016;1378:124–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouliot W, Bealer SL, Roach B, Dudek FE. A rodent model of human organophosphate exposure producing status epilepticus and neuropathology. Neurotoxicology. 2016;56:196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas A, Ganesh T, Lelutiu N, Gueorguieva P, Dingledine R. Inhibition of the prostaglandin EP2 receptor is neuroprotective and accelerates functional recovery in a rat model of organophosphorus induced status epilepticus. Neuropharmacology. 2015;93:15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum C, Bird SB. Non-muscarinic therapeutic targets for acute organophosphorus poisoning. J Med Toxicol. 2010;6:408–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siso S, Hobson BA, Harvey DJ, Bruun DA, Rowland DJ, Garbow JR, et al. Editor’s Highlight: Spatiotemporal progression and remission of lesions in the rat brain following acute intoxication with diisopropylfluorophosphate. Toxicol Sci. 2017;157:330–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogorb M, Estevez J, Vilanova E. Toxicokinetics and toxicodynamics of DFP In: Gupta RC, editor. Handbook of Toxicology of Chemical Warfare Agents. 2 ed. USA: Elsevier; 2015. [Google Scholar]
- Wu X, Kuruba R, Reddy DS. Midazolam-resistant seizures and brain injury after acute intoxication of diisopropylfluorophosphate, an organophosphate pesticide and surrogate for nerve agents. J Pharmacol Exp Ther. 2018;367:302–21. [DOI] [PMC free article] [PubMed] [Google Scholar]