Abstract
Organophosphate esters (OPEs) are commonly used as plasticizers and flame retardants in consumer products, and exposure is relatively ubiquitous in most populations studied. This may be of concern as some OPEs may be neurotoxic, endocrine-disrupting, and interfere with behavioral development; however, observational evidence is limited. We used data from the Pregnancy, Infection, and Nutrition Study, a prospective birth cohort study, to investigate associations between maternal OPE metabolite concentrations during pregnancy and behavioral development in offspring. Women provided a urine sample during pregnancy that was analyzed for concentrations of OPE metabolites, including diphenyl phosphate (DPHP), bis(1,3-dichloro-2-propyl phosphate) (BDCIPP), isopropyl-phenyl phenyl phosphate (ip-PPP), and 1-hydroxyl-2-propyl bis(1-chloro-2-propyl) phosphate (BCIPHIPP). Offspring’s behavioral development was assessed by the Behavioral Assessment System for Children (2nd Edition) (BASC-2) at approximately 36 months. Linear regression was used to estimate associations between tertiles in specific gravity-corrected OPE metabolite concentrations and children’s scores on the BASC-2, adjusted for maternal age, maternal BMI, maternal race, maternal education, familial income, maternal depression, quality of the home environment, and sex. Higher BDCIPP concentrations were associated with higher scores on the Behavioral Symptoms Index (1st vs. 3rd tertile: β = 3.03; 95% CI = 0.40, 5.67) and Externalizing Problems (1st vs. 3rd tertile: β = 2.49; 95% CI: −0.12, 5.10) composites. Among BASC-2 scales, BDCIPP was most strongly associated with Withdrawal, Attention Problems, Depression, Hyperactivity, and Aggression. DPHP concentrations were also associated with higher scores on the Externalizing Problems and Behavioral Symptoms Index composites, but not as strongly as BDCIPP. Conversely, higher concentrations of ip-PPP were associated with fewer adverse behavioral symptoms, including an inverse association with the Internalizing Problems composite (1st vs. 3rd tertile: β = −3.74; 95% CI = −6.75, −0.74) and constituent scales. BCIPHIPP was not strongly associated with any measured behavioral outcomes. Our results suggest that greater maternal exposure to tris(1,3-dichloro-2-propyl phosphate) (TDCIPP, parent compound of BDCIPP) and, to a lesser degree, triphenyl phosphate (TPHP, parent compound of DPHP) during pregnancy is associated with adverse behavioral development in children. Our study contributes to the growing body of evidence pertaining to adverse developmental effects of prenatal OPE exposure and highlights the need for further research to characterize risks associated with this ubiquitous family of chemicals.
Keywords: Behavior, OPE, OPFR, Organophosphate, Flame Retardant, Neurodevelopment
1. Introduction
Organophosphate esters (OPEs) are used in the production of a variety of consumer products. These compounds primarily function as flame retardants but are also used as plasticizers in other applications [1−3]. Utilization of OPEs has increased in recent years because they can be used to meet flammability standards for polyurethane foam in place of polybrominated diphenyl ethers (PBDEs), a phased-out class of flame retardant compounds [3−6]. In addition to polyurethane foam, other products also contain OPEs, including construction materials [2, 7], electronics [2, 8], children’s products [9, 10], nail polish [11], and recreational equipment [12−14]. OPEs are applied as additive compounds that are not chemically bound to products during production, thus they subsequently volatilize and leach into surrounding environments and media [1−3].
Because of their application to a wide variety of common consumer products and propensity to volatilize and leach, OPEs are present in many human environments including residential housing, office buildings, shopping centers, schools, child care facilities, and automobiles [3, 15−19]. Inhalation and ingestion of suspended particles in indoor environments are well-documented sources of exposure [3, 20−23], though dermal exposure [11, 24, 25] and other pathways (e.g., dietary intake, drinking water) may also contribute to exposure [3, 21, 26]. Inside the body, OPEs are quickly metabolized, primarily to their respective diesters and monoesters, which are excreted in urine [1, 21, 27, 28]. Although they have half-lives on the order of hours [29−32], urinary OPE metabolite concentrations appear to be relatively consistent over periods from weeks to months. For example, validation studies among pregnant women indicate that singular spot assessments can reflect exposure across periods of months with fair to good reliability [33, 34]. As such, urinary concentrations of OPE metabolites are useful OPE biomarkers for biomonitoring surveys [35] and epidemiologic studies [22, 36−40], where they are detected with high frequency. The 2013–2014 National Health and Nutrition Examination Survey (NHANES) detected urinary biomarkers of triphenyl phosphate (TPHP) and tris(1,3-dichloro-2-propyl) phosphate (TDCIPP), two of the most well-studied OPEs, in greater than 90% of participants and with median concentrations of approximately 0.8 ng/ml [35]. Exposure is also pervasive among pregnant women and women of reproductive age [22, 34, 36−38, 41, 42]. Further, investigators have detected OPEs at the maternal-fetal interface (e.g., placental tissue, chorionic villi and deciduae), indicating potential maternal-fetal transfer of exposure [43, 44].
Mounting evidence from both experimental and observational settings indicates that certain OPEs are biologically active and can affect behavioral development through both endocrine- and neurologically-mediated pathways, including thyroid-activity disruption [45−48], sex steroid-activity disruption [45, 49−51], and direct neurotoxic effects [52−55]. To date, only two observational studies of the behavioral effects of early life exposures to OPEs have been published [56, 57]. Both studies found that exposure to these compounds was associated with behavioral problems in children, particularly externalizing behaviors. Together, the available mechanistic and observational evidence suggests that exposure to at least some OPE compounds in early life may be associated with adverse behavioral outcomes in children, particularly externalizing-like behaviors, such as hyperactivity and attention problems. However, currently available data from humans is limited and further investigation of behavioral effects of early life exposure to OPEs is warranted.
We used data from a prospective birth cohort study of children born to women living in North Carolina between 2004 and 2006 to investigate relationships between biomarkers of OPE exposures during pregnancy and behavioral outcomes among offspring at approximately three years of age.
2. Materials and methods
2.1. Study sample
The PIN Kids study is ancillary to the Pregnancy Infection and Nutrition Study - phase 3 (PIN3) and the PIN Postpartum Study [58]. PIN3 enrolled pregnant women before 20 weeks gestation from the University of North Carolina Hospital in Chapel Hill, NC and followed them to delivery to investigate risk factors for preterm birth; enrollment spanned 2001–2005. Women were recruited from prenatal care clinics before 20 weeks gestation if they were English-speaking, older than 16 years of age, carrying a singleton pregnancy, and intended to deliver at University of North Carolina hospitals. In 2003, the PIN Postpartum study began to enroll PIN3 participants who delivered live infants without major birth defects to study maternal weight retention and mental health for the first year postpartum (n=689). Women were released from PIN Postpartum participation if they became pregnant again during follow-up or moved out of the area. Participants provided information about their health, nutrition, and lifestyle through interviews and questionnaires; they also provided second trimester urine and blood samples. In 2004, PIN Kids began to follow the growth and development of the children of PIN Postpartum participants age three years, by collecting data through maternal interview and child developmental assessment in the home (n=577). Eligibility for this analysis required stored maternal prenatal urine to assay OPE metabolites and completion of the BASC2 at 3 years.
PIN protocols had been approved by the Institutional Review Board of the University of North Carolina at Chapel Hill and all participating mothers had given written informed consent and parental permission for their child’s involvement.
2.2. Measurement of OPE metabolite concentrations
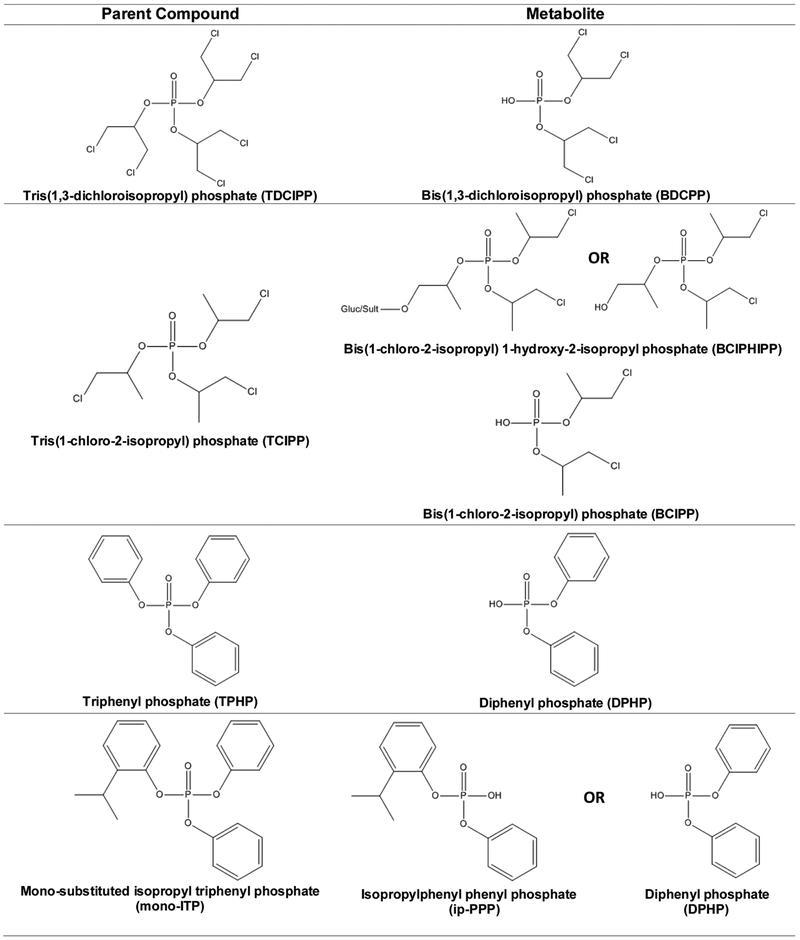
OPEs were measured in urine samples collected by UNC’s General Clinical Research Center at approximately 24 to 29 weeks gestation (Median: 27; IQR: 27−28) [36]. Time of urine collection was not standardized, though >95% of samples were collected between 0700 and 1200 hours. Samples were aliquoted into polyethylene storage tubes and frozen at −80°C until analysis (between 10 and 13 years after collection). Urine samples were analyzed for concentrations of six OPE metabolites, including diphenyl phosphate (DPHP), bis(1,3-dichloro-2-propyl) phosphate (BDCIPP), isopropyl-phenyl phenyl phosphate (ip-PPP), 1-hydroxyl-2-propyl bis(1-chloro-2-propyl) phosphate (BCIPHIPP), bis(1-chloro-2-propyl) phosphate (BCIPP), and tert-butyl-phenyl phenyl phosphate (tb-PPP) (Figure 1).
Figure 1.
OPE parent compounds and metabolites of interest to the Pregnancy, Infection, and Nutrition Study.
Analytic methods of OPE urinalysis are described in detail elsewhere [36, 38, 59]. Briefly, samples were extracted using previously described enzyme deconjugation and solid phase extraction techniques [59] adapted for 5 ml of urine [38]. Samples were analyzed using electrospray ionization liquid chromatography tandem mass spectrometry. Standard reference materials were included to assess assay performance, and average of batch-specific coefficients of variation ranged from 11% to 16%. Method detection limits (MDLs) were calculated to be three times the standard deviation of the laboratory blanks, normalized to the average urine volume (3 ml). Samples were analyzed in three batches and MDLs were calculated separately for each batch; MDLs ranged from 127 to 243 pg/ml for DPHP, 60 to 197 pg/ml for BDCIPP, 37 to 177 pg/ml for ip-PPP, 3 to 33 pg/ml for BCIPHIPP, 136 to 333 pg/ml for BCIPP, and 213–846 pg/ml for tb-PPP.
Specific gravity (SG) was measured in each urine sample using a digital handheld refractometer (Atago). OPE metabolite concentrations were standardized for SG using the method proposed by Boeniger et al. to account for urinary dilution [60].
2.3. Assessment of child’s behavior
Children’s behavior was assessed using the Behavioral Assessment System for Children, 2nd Edition (BASC-2) parent-rating scale for preschool children (PRS-P) [61]. The BASC-2 PRS-P is a parent-completed questionnaire of the parent’s perceptions of their child’s behavior, including both negative and positive behavioral qualities, which is valid for children ages 24 to 60 months [61]. Mothers completed the BASC-2 PRS-P questionnaire during the 36-month follow-up visit. Exposure status was unknown to mothers at time of completion of behavioral assessments.
To complete the BASC-2, mothers rated the frequency of their child’s behaviors on 134 questions using a four-point scale (Never, Sometimes, Often, Almost Always) and responses were used to score four composites and sixteen scales (Supplemental Table S1). The four BASC-2 composites include Externalizing Problems, Internalizing Problems, Behavioral Symptoms Index, and Adaptive Skills. Higher scores on the Externalizing Problems, Internalizing Problems, and Behavioral Symptoms Index composites (and constituent subscales) indicate more behavioral problems, while higher scores on the Adaptive Skills composite (and constituent subscales) indicate more favorable behavioral abilities. The BASC-2 includes indices of internal validity to identify assessments that may have been inappropriately completed; these include the “F-Index” that assesses the possibility that a child’s behavior was rated in an inordinately negative fashion, the “Response Pattern Index” that identifies inattentive reporting of behaviors (e.g., all “Never” or all “Almost Always”), and the “Consistency Index” that identifies inconsistent responses to questions that are usually answered similarly. We omitted assessments with internal consistency indices indicating “Caution” or “Extreme Caution” (n=13 (6.1%)). Raw scores on BASC-2 composites and scales were converted to age-specific T-scores of the BASC-2 standardized population (distributed with mean=50, SD=10) using the tables published in the manual.
2.4. Covariates
Throughout the PIN3, PIN Postpartum, and PIN Kids studies, women completed multiple interviews and questionnaires to provide information that characterized their health, nutrition, and life style [58]. PIN3 participants completed the Center for Epidemiologic Studies Depression Scale (CES-D) [62] to indicate the presence of depressive symptoms during pregnancy. PIN Kids staff administered a modification of the Home Observation for Measurement of the Environment (HOME) assessment to characterize physical and social influences on the child during the home visit at 3 years of age. While the HOME traditionally uses interview and observation to rate the several constructs that can impact the environment [63], we scored only the three HOME subscales that comprised mostly interview items (Learning, Language Stimulation, and Academic Stimulation) to create a modified HOME score for these constructs. Thus, other constructs of the HOME, such as modeling behaviors or parent responsivity, are not reflected.
We identified covariates to include in our analyses using a Directed Acyclic Graph and a review of the literature (Supplemental Figure 1). The Directed Acyclic Graph was used to model hypothesized unidirectional causal relations between variables and to identify variables to be included as covariates in order to block potential biasing pathways (e.g., confounding pathways) [64]. Our covariate set included the following variables, identified as potential confounders or predictors of the outcomes that would reduce residual variance without introducing bias: maternal age in years (quadratic), maternal pre-pregnancy Body Mass Index (quadratic), maternal education in years (linear), income as a percentage of the 2001 poverty level (linear), maternal race (non-Hispanic White/all other races), maternal CES-D score (<17/≥17), modified HOME Score (linear), and child’s sex (male/female).
2.5. Statistical analysis
In our primary analyses, we used linear regression to estimate the covariate-adjusted change in children’s scores on the BASC-2 composites and scales per tertile of SG-corrected OPE metabolite concentration measured in maternal urine during pregnancy. Because some participants’ OPE metabolite concentrations were below the method detection limit (MDL; ranging from 0 to 16% of samples, depending on metabolite), we used multiple imputation to impute OPE metabolite values for these participants, as well as missing covariate data. Our multiple imputation procedure used Monte Carlo methods to generate 50 imputations conditional on covariates and OPE metabolite concentrations; OPE metabolite concentrations were imputed using a truncated log-normal model conditional on covariates and other OPE metabolite concentrations [65]. Analyses were performed on each of the imputed datasets and summary estimates were derived using Rubin’s rules for multiple imputation [66].
We performed multiple supplementary analyses. First, sex-specific effects are often observed for endocrine disrupting compounds [67–69], and some studies have observed sex-specific effects of OPE exposures [46, 47, 49−51, 70]; therefore, we explored sex-specific effects by repeating our primary analyses in sex-stratified samples. Second, we repeated our primary analyses with OPE metabolite concentrations modeled as linear variables; specifically, we estimated the covariate-adjusted change in BASC-2 composites and scales per interquartile range (IQR) increase in SG-corrected log-10-transformed OPE metabolite concentrations. Third, we repeated our primary analyses with all OPE metabolites included in a single model. Fourth, we repeated our primary analyses with the omission of potentially influential variables, identified as variables with Cook’s distance >0.05.
3. Results
3.1. Study sample
In total, our study sample included 199 mother-child pairs who had both valid BASC-2 assessments and OPE metabolite measurements (Table 1). The median age of mothers in our study sample was 30 years (IQR: 27−33), and mothers were primarily White (82%) and highly educated (median years of education: 16; IQR: 15−18). Relative to the PIN Kids eligible cohort (n=577), mothers in our analysis sample were slightly older, more likely to be White, had more years of education, and were of higher income.
Table 1.
Characteristics of the PIN Kids eligible population and analysis sample.
| PIN Kids Eligible n = 577 |
Analysis Samplea n = 199 |
||
|---|---|---|---|
| Maternal Age at Child’s Birth (years) | Median (IQR) | 30 (26–33) | 30 (27–33) |
| Missing | 0 | 0 | |
| Maternal Race | White | 441 (77) | 164 (82) |
| Black | 86 (15) | 21 (11) | |
| American Indian | 2 (0) | 1 (1) | |
| Asian | 20 (3) | 2 (1) | |
| Other | 27 (5) | 11 (6) | |
| Missing | 1 | 0 | |
| Maternal Education (years) | Median (IQR) | 16 (14–18) | 16 (15–18) |
| Missing | 0 | 0 | |
| Income as Percentage of 2001 Poverty Index | Median (IQR) | 464 (232–596) | 473 (263–596) |
| Missing | 18 | 3 | |
| Body Mass Index (kg/m2) | Median (IQR) | 23 (21–27) | 23 (21–27) |
| Missing | 2 | 0 | |
| Modified HOME Score | Median (IQR) | 19 (17–20) | 19 (18–20) |
| Missing | 168 | 0 | |
| CES-D Score | 0–16 | 352 (72) | 138 (75) |
| ≥17 | 137 (28) | 46 (25) | |
| Missing | 88 | 15 | |
| Sex of the Child | Male | 308 (53) | 112 (56) |
| Female | 268 (47) | 87 (44) | |
| Missing | 1 | 0 | |
| Child’s Age at BASC-2 (Months) | Mean (SD) | 36 (36–38) | 36 (36–38) |
| Missing | 245 | 0 |
Abbreviations: BASC-2, Behavioral Assessment System for Children 2nd Edition; CES-D, Center for Epidemiologic Studies Depression Scale; PIN, Pregnancy, Infection, and Nutrition Study.
Participants with OPE metabolite measurements and valid BASC-2 assessments.
3.2. OPE metabolite concentrations
We detected DPHP, BDCIPP, ip-PPP, and BCIPHIPP in >80% of study participants (Table 2); tb-PPP and BCIPP were detected less frequently (2% and 51%, respectively), thus were omitted from bivariate analyses. Median concentrations were highest for ip-PPP (7.04 ng/ml), followed by BDCIPP (2.01 ng/ml), DPHP (1.38 ng/ml), and BCIPHIPP (0.45 ng/ml). The median concentrations specific gravity-uncorrected BDCIPP and DPHP in our study sample (1.15 ng/ml and 0.81 ng/ml, respectively) were similar to those more recently measured among females in the NHANES 2013−2014 cycle (0.89 ng/ml and 0.82 ng/ml, respectively) [35]. Correlations between the compounds ranged from −0.01 to 0.29. Descriptive statistics of SG-uncorrected OPE metabolite concentrations are located in Supplemental Table S2. Characteristics regarding OPE metabolite concentrations in PIN3 are detailed elsewhere [36].
Table 2.
Specific gravity-corrected OPE metabolite concentrations (ng/ml) measured in prenatal urine samples provided by the analysis sample (n = 199).
| Metabolite | % < MDL | Percentiles | Spearman Correlation Coefficients | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 25 | 50 | 75 | 95 | DPHP | BDCIPP | ip-PPP | BCIPHIPP | ||
| DPHP | 16 | < MDL | 0.80 | 1.38 | 2.37 | 9.55 | 1 | 0.29 | 0.19 | 0.19 |
| BDCIPP | 5 | < MDL | 0.93 | 2.01 | 3.75 | 11.92 | 1 | −0.01 | 0.23 | |
| ip-PPP | 0 | 1.93 | 4.35 | 7.04 | 10.63 | 22.34 | 1 | 0.11 | ||
| BCIPHIPP | 3 | 0.11 | 0.25 | 0.45 | 0.86 | 6.57 | 1 | |||
Abbreviations: BCIPHIPP, 1-hydroxy-2-propyl bis(1-chloro-2-propyl) phosphate; BDCIPP, bis(1,3-dichloro-2-propyl) phosphate; DPHP, diphenyl phosphate; ip-PPP, isopropyl-phenyl phenyl phosphate; MDL, method detection limit; OPE, organophosphate ester.
3.3. Assessment of child’s behavior
In general, the distributions of the age-standardized BASC-2 composite and scale scores were similar to BASC-2 scores among the BASC-2 standardization population [61]; i.e., the means of the age-standardized scores were approximately 50 and the standard deviations of these scores were approximately 10 (Table 3). However, the mean values of the BASC-2 Externalizing Problems, Internalizing Problems, and Behavioral Symptoms index were slightly lower than 50, whereas the mean value of the BASC-2 Adaptive Skills composite was slightly higher than 50, indicating slightly fewer behavioral problems and slightly better behavioral competencies in our study sample relative to the BASC-2 standardization population.
Table 3.
Age-standardized, sex-combined BASC-2 T-scores in the analysis sample (n = 199).
| Mean | SD | ||
|---|---|---|---|
| Composites | Externalizing Problems | 47 | 8.3 |
| Internalizing Problems | 48 | 8.6 | |
| Behavioral Symptoms Index | 48 | 8.1 | |
| Adaptive Skills | 52 | 8.0 | |
| Scales | Aggression | 47 | 8.7 |
| Hyperactivity | 48 | 8.7 | |
| Anxiety | 49 | 9.0 | |
| Depression | 49 | 8.0 | |
| Somatization | 46 | 8.5 | |
| Attention Problems | 50 | 8.9 | |
| Atypicality | 48 | 8.9 | |
| Withdrawal | 48 | 8.9 | |
| Activities of Daily Living | 50 | 9.2 | |
| Adaptability | 54 | 8.9 | |
| Functional Communication | 52 | 8.4 | |
| Social Skills | 55 | 8.0 |
Abbreviations: BASC-2, Behavioral Assessment System for Children 2nd Edition; SD, standard deviation.
3.4. Associations between OPE metabolite concentrations and child’s behavior
Higher concentrations of BDCIPP were associated with higher scores on the Behavioral Symptoms Index and Externalizing Problems composites (Table 4), which includes direct associations with scores on several specific scales, including Withdrawal, Attention Problems, Depression, Hyperactivity, and Aggression. These associations were generally monotonic, though the associations between BDCIPP and the Withdrawal and Attention Problems scales indicated a stronger association in the second tertile than the third tertile. Similar associations were observed for DPHP, though they were not as strong as those for BDCIPP. Higher concentrations of ip-PPP were associated with lower scores on the Internalizing Problems and Behavioral Symptoms Index composites, which were primarily driven by inverse associations between ip-PPP and the Anxiety, Atypicality, Somatization, and Depression scales. BCIPHIPP concentrations were not strongly associated with any of the BASC-2 composites or scales.
Table 4.
Estimated change in age-standardized, sex-combined BASC-2 T-scores per tertile of specific gravity-corrected OPE metabolite concentration, adjusted for covariates.
| DPHP | BDCIPP | ip-PPP | BCIPHIPP | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2nd
Tertile (0.99–1.89 ng/ml) |
3rd
Tertile (1.93–111.56 ng/ml) |
2nd
Tertile (1.19–3.09 ng/ml) |
3rd
Tertile (3.10–139.60 ng/ml) |
2nd
Tertile (5.01–8.99 ng/ml) |
3rd
Tertile (8.99–69.00 ng/ml) |
2nd
Tertile (0.31–0.68 ng/ml) |
3rd
Tertile (0.68–97.96 ng/ml) |
||||||||||
| Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | ||
| Composites | Extednalizing Pdoblems | 0.26 | [−2.40, 2.92] | 1.44 | [−1.22, 4.09] | 0.34 | [−2.23, 2.91] | 2.49 | [−0.12, 5.10] | −0.99 | [−3.64, 1.66] | −1.75 | [−4.51, 1.00] | −0.85 | [−3.58, 1.88] | −0.64 | [−3.28, 2.00] |
| Internalizing Problems | −0.28 | [−3.23, 2.66] | 0.21 | [−2.73, 3.15] | 1.03 | [−1.84, 3.89] | 1.33 | [−1.58, 4.24] | −0.99 | [−3.89, 1.90] | −3.74 | [−6.75, −0.74] | 0.97 | [−2.02, 3.96] | −1.48 | [−4.38, 1.42] | |
| Behavioral Symptoms Index | 1.07 | [−1.61, 3.75] | 1.98 | [−0.70, 4.66] | 1.87 | [−0.72, 4.47] | 3.03 | [0.40, 5.67] | −1.12 | [−3.80, 1.56] | −2.06 | [−4.84, 0.72] | −0.66 | [−3.42, 2.09] | −1.30 | [−3.97, 1.36] | |
| Adaptive Skills | −1.11 | −3.77, 1.54] | −0.53 | [−3.18, 2.12] | −0.32 | [−2.90, 2.27] | −1.42 | [−4.05, 1.21] | 0.91 | [−1.75, 3.56] | −0.06 | [−2.82, 2.69] | 0.24 | [−2.48, 2.97] | 0.40 | [−2.24, 3.03] | |
| Scales | Aggression | 0.14 | [−2.73, 3.01] | 1.40 | [−1.46, 4.26] | −1.08 | [v3.85, 1.68] | 2.09 | [v0.72, 4.90] | −0.56 | [−3.42, 2.31] | −1.59 | [−4.57, 1.38] | −2.03 | [−4.96, 0.90] | −1.43 | [−4.26, 1.41] |
| Hyperactivity | 0.18 | [−2.67, 3.04] | 1.14 | [−1.71, 3.98] | 1.60 | [−1.16, 4.37] | 2.32 | [−0.49, 5.12] | −1.33 | [−4.18, 1.51] | −1.66 | [−4.61, 1.29] | 0.42 | [−2.50, 3.35] | 0.14 | − 2.69, 2.97] | |
| Anxiety | 0.22 | [−2.82, 3.25] | 0.57 | [−2.47, 3.60] | 1.05 | [−1.90, 4.00] | −0.72 | [−3.72, 2.29] | −1.33 | [−4.33, 1.67] | −3.60 | [−6.71, −0.48] | 0.36 | [−2.74, 3.45] | −1.74 | [−4.74, 1.26] | |
| Depression | −0.63 | [−3.40, 2.15] | −1.20 | [−3.98, 1.57] | 1.66 | [−1.03, 4.35] | 2.43 | [−0.30, 5.17] | −1.53 | [−4.30, 1.23] | −2.27 | [−5.13, 0.60] | 0.65 | [−2.18, 3.49] | −1.08 | [−3.83, 1.67] | |
| Somatization | −0.35 | [−3.22, 2.52] | 1.16 | [−1.70, 4.03] | −0.65 | [−3.44, 2.14] | 1.15 | [−1.69,3. 99] | 0.49 | [−2.35, 3.32] | −2.64 | [−5.58, 0.31] | 1.26 | [−1.67, 4.19] | −0.44 | [−3.28, 2.40] | |
| Attention Problems Atypicality | 0.31 | [−2.52, 3.14] | 2.39 | [−0.44, 5.22] | 2.86 | [0.11, 5.61] | 2.36 | [−0.43, 5.16] | −1.08 | [−3.93, 1.77] | −1.12 | [−4.08, 1.83] | −1.10 | [−4.02, 1.81] | −1.70 | [−4.52, 1.12] | |
| 2.72 | [−0.19, 5.62] | 1.96 | [−0.94, 4.86] | 0.42 | [−2.43, 3.27] | 1.44 | [−1.45, 4.34] | −2.07 | [−4.96, 0.82] | −3.45 | [−6.45, −0.45] | −1.36 | [−4.35, 1.63] | −1.29 | [−4.19, 1.61] | ||
| Withdrawal | 1.95 | [−1.08, 4.98] | 2.99 | [−0.03, 6.02] | 3.09 | [0.15, 6.04] | 2.92 | [−0.08, 5.91] | 1.89 | [−1.16, 4.93] | 0.87 | [−2.30, 4.03] | 0.12 | [−3.02, 3.25] | −0.42 | [−3.45, 2.61] | |
| Activities of Daily Living Adaptability |
−0.77 | [−3.80, 2.25] | 0.53 | [−2.49, 3.55] | 0.82 | [−2.11, 3.75] | −1.74 | [−4.72, 1.24] | 2.19 | [−0.82, 5.20] | 0.17 | [−2.96, 3.29] | 0.62 | [−2.48, 3.73] | 0.67 | [−2.33, 3.67] | |
| −0.94 | [−3.89, 2.00] | −0.76 | [−3.70, 2.19] | −1.65 | [−4.51, 1.21] | 0.31 | [−2.59, 3.22] | −0.54 | [−3.48, 2.40] | 0.27 | [−2.79, 3.33] | −0.90 | [−3.89, 2.10] | 1.69 | [−1.21, 4.59] | ||
| Functional Communication Social Skills | −1.19 | [−3.91, 1.53] | −1.10 | [−3.82, 1.62] | 0.06 | [−2.58, 2.71] | −1.38 | [−4.07, 1.31] | 0.29 | [−2.42, 3.00] | −1.50 | [−4.32, 1.31] | 0.71 | [−2.07, 3.49] | −1.16 | [−3.85, 1.52] | |
| −0.49 | [−3.13, 2.15] | −0.10 | [−2.74, 2.54] | −0.51 | [−3.07, 2.05] | −1.79 | [−4.40 0.81] |
0.98 | [−1.66, 3.61] | 1.00 | [−1.73, 3.74] | 0.31 | [−2.40, 3.01] | −0.03 | [−2.65, 2.58] | ||
Notes: Adjusted for maternal age (quadratic), maternal BMI (quadratic), income as percentage of 2001 poverty index (linear), maternal education (linear), maternal race (White non-Hispanic/Other), maternal CES-D score (< 17/≥17), HOME Score (linear), and sex (male/female).
3.5. Supplementary analyses
Our results were robust to several supplementary analyses. First, sex-specific associations between OPE metabolite concentrations and BASC-2 scores were generally consistent with sex-combined associations (Supplemental Table S3, Supplemental Figure 2). As a general trend, adverse associations appeared stronger among females than males, particularly for BDCIPP and DPHP. However, the results of these sex-specific analyses were highly imprecise and must be interpreted cautiously. Second, specifying OPE metabolite concentrations as linear terms produced results similar to our primary analyses specifying exposure in tertiles, although associations with DPHP were notably attenuated (Supplemental Table S4). Third, when all OPE metabolites were included in a single model, many of the strongest associations we observed in the primary analyses persisted and the directionality of most associations was unchanged relative to the primary analyses (Supplemental Table S5), though precision decreased, and some estimates were attenuated. Finally, when influential observations were removed from analyses, we similarly observed that most of the associations present in our primary analyses were unchanged, indicating that the primary analyses were not unduly influenced by a small number of influential observations (Supplemental Table S6).
4. Discussion
In this prospective birth cohort study, we observed that concentrations of certain OPE metabolites measured in maternal urine during pregnancy were associated with offspring’s scores on the BASC-2, as reported by their mothers, at approximately three years of age. BDCIPP concentrations were positively associated with a variety of adverse behavioral symptoms, including more withdrawal, attention problems, depression, and hyperactivity. DPHP concentrations were also associated with higher behavioral symptom scores, including withdrawal, attention problems, and atypicality, though to a lesser degree than BDCIPP. Conversely, ip-PPP concentrations were generally associated with fewer behavioral symptoms, particularly internalizing behaviors such as anxiety, depression, and somatization, as well as atypicality. BCIPHIPP was consistently not associated with behavioral symptoms in this study.
Our results contribute to the growing body of epidemiologic evidence relating prenatal and early life OPE exposure to early-life behavioral development. Lipscomb et al. performed a cross-sectional study among preschool-aged children (ages 3−5 years, n=72) and reported that greater environmental ∑OPE exposure (assessed by passive silicone samplers) was associated with fewer responsible behaviors and more externalizing behaviors, as assessed by teacher report using the Social Skills Improvement Rating Scale [56]. The investigators did not estimate associations with individual OPE compounds, but their summed exposure metric included parent compounds of several of the metabolites investigated in our study, including tris (1,3-dichloro-2-propyl) phosphate (TDCIPP, a parent compound of BDCIPP), triphenyl phosphate (TPHP, a parent compound of DPHP), tris(1-chloro-2-propyl) phosphate (TCIPP, a parent compound of BCIPHIPP), and tris(2-chloroethyl) phosphate (TCEP). More recently, Castorina et al. reported that higher concentrations of BDCIPP measured in maternal prenatal urine were associated with higher scores on the Attention Problems subscale of the BASC-2 (teacher report, n=247), and also that higher concentrations of ip-PPP measured in maternal prenatal urine were associated with higher scores on the Hyperactivity subscale of the BASC-2 among children at 7 years (maternal report, n=281) [57].
In our study, we observed similar associations between BDCIPP and increased externalizing behaviors and other behavioral symptoms, such as attention problems and hyperactivity; we did not, however, observe that ip-PPP concentrations were associated with more externalizing behavioral symptoms, and conversely observed that ip-PPP concentrations were associated with fewer internalizing behaviors. Thus, some, but not all, associations have been somewhat consistently observed across the small number of available studies. Some discrepancies in results among these studies may be attributable to differences in exposure levels among the study populations, methods of exposure assessment, and children’s ages at behavioral assessments. First, while both our study and Castorina et al. [57] measured OPE metabolite concentrations in maternal urine sampled during pregnancy to assess prenatal exposure, the median concentration of ip-PPP in our study population was approximately twenty times that measured in Castorina et al.’s study population (7.04 ng/ml vs. 0.34 ng/ml), and median DPHP and BDCIPP concentrations were approximately two to three times the median concentrations of these metabolites in Castorina et al.’s study population, using the same analytic technique. Direct comparison of results to Lipscomb et al.’s study is more challenging because the investigators assessed postnatal exposure among children at three to five years of age and used passive silicone samplers to measure environmental concentrations of OPEs. Although measurements of OPEs in silicone wristbands correlate with urinary metabolites of these compounds [71], such differences in methods of exposure assessment may have contributed to differences in observed associations. Another potential contributing factor to inconsistent study findings is differences in children’s ages at behavioral assessments among the three studies; children in our study were assessed at approximately three years of age, whereas Lipscomb et al. assessed children at three to five years and Castorina et al. assessed children at approximately seven years. While each of these studies used age-appropriate behavioral assessments completed by persons familiar with the children’s behavior (e.g., parents or teachers), the norms around behavior do change with age and scores may also be impacted by perceptions and reporting. Additionally, some behavioral aspects and developmental abnormalities may only become apparent at later ages, which would not be picked up in our study of young children. Altogether, despite some discrepant findings, the available evidence across observational studies indicates that early life exposure to certain OPEs, particularly TDCIPP, are potentially associated with adverse behavioral effects, particularly externalizing behaviors.
The epidemiologic evidence of behavioral effects of early life OPE exposures is supported by a growing body of observational and experimental evidence linking OPE exposure to physiological processes related to behavioral development. Human behavior and behavioral development are largely governed by the endocrine and neurological systems [72−75], and these physiological systems develop rapidly in early life, particularly during the prenatal period, and are highly sensitive to perturbations caused by exogenous pollutants [76−79]. Available evidence indicates that OPEs may exert endocrine-disrupting and neurotoxic effects, which may affect behavioral development through both direct exposure and maternally-mediated effects that occur as a result of maternal exposure to OPEs during the prenatal period. For example, observational studies have reported associations between thyroid hormone concentrations and TDCIPP and TPHP concentrations in house dust [45] and also DPHP concentrations in urine [46]; experimental studies have similarly reported associations between OPE exposures and altered thyroid function [48, 80−82], including altered thyroid hormone concentrations and altered thyroid-related gene and protein expression. Maternal thyroid function during pregnancy and offspring’s thyroid function during early life are critical to both the development of anatomical structures and the government of physiological processes related to behavior [68, 83–86]; further, mounting evidence suggests that maternal thyroid dysfunction during pregnancy is associated with ADHD-like behaviors in offspring [87−91]. As such, maternal exposures to OPEs, including TDCIPP, during pregnancy may result in maternal thyroid dysregulation that leads to thyroid-mediated developmental effects on the fetus, which manifest as adverse behavioral symptoms among children that are consistent with ADHD (e.g., externalizing-like behaviors). In a similar manner, sex steroid exposure during the prenatal period and early life is important to behavioral development [92], and available evidence indicates OPE exposures can influence sex steroid expression and function [49, 50, 70, 93−95], which can lead to sex steroid-mediated behavioral effects of OPE exposure. Experimental studies have found associations between OPE exposure and sex steroid concentrations in exposed animals [49−51, 70] and sex steroid mRNA and protein expression in in vitro settings [49, 51, 70]. Further still, experimental evidence indicates OPE exposure may cause potential neurotoxic effects, such as cytotoxicity to neuronal cells [53, 96−98] and alteration of neurotransmitter levels [47, 54]. Of particular concern are neurotoxic effects that follow direct exposure to the fetus during the prenatal period as a result of maternal-fetal transmission of exposure; such maternal-fetal transmission of exposure is supported by observational studies that have detected OPEs at the maternal-fetal interface (e.g., placental tissue, chronic villi and deciduae) [43, 44]. In summary, available evidence suggests that early life exposure to certain OPEs, including TDCIPP and DPHP, may be associated with behavioral development, particularly externalizing behaviors, through both endocrine- and neurologically-mediated pathways.
As a contribution to the evidence of behavioral effects of early life OPE exposures, our study is noteworthy for its prospective design, assessment of exposures during the sensitive prenatal period, and investigation of a broad array of behavioral outcomes. Yet, certain study characteristics bear upon our results and interpretation. Assessment of exposure during the prenatal period is valuable, as the prenatal period is a uniquely sensitive period for development, including development of anatomical structures related to behavioral development [76–79]. We measured concentrations of OPE metabolites in a single spot urine sample collected from mothers during the 25th to 29th weeks of pregnancy, which would not capture potential variability in exposure throughout different sensitive windows of fetal development across pregnancy. Although this may result in some exposure misclassification, previous studies have characterized variability in OPE metabolite concentrations throughout pregnancy and observed moderate to good consistency [33, 34]. Still, future investigations will likely benefit from exposure assessments that occur at multiple points during pregnancy across the sensitive prenatal and early childhood periods.
Measured OPE metabolite concentrations in urine are imperfectly sensitive and specific markers of OPE parent compound exposure. For instance, multiple metabolites may result from a single parent compound, such that measurement of a single metabolite may not fully characterize an individual’s exposure to that parent compound [21, 28, 40]; conversely, some metabolites, such as DPHP, may result from multiple compounds [99, 100], and may even be used in products in their own right [101, 102]. Such limitations in sensitivity and specificity may be reduced by identifying and measuring additional OPE metabolites.
Detection of OPE metabolites has varied over time [39]. We detected four OPE metabolites with high frequency (>80%) from urine samples collected between 2002 to 2005 from the PIN Study. The median concentrations of specific-gravity uncorrected BDCIPP and DPHP were 1.15 ng/ml and 0.81 ng/ml (respectively), which is similar to those more recently measured among females in the NHANES 2013–2014 cycle (0.89 ng/ml and 0.82 ng/ml, respectively) [35]. However, ip-PPP concentrations in our sample were higher than those reported by other observational studies [38, 42], which may increase our likelihood of detecting effects of exposures at higher levels, but also may limit the generalizability of our results. The sources of high ip-PPP exposure in our study sample are unclear. Data to characterize and compare TCIPP exposure in the population, and its most frequently detected metabolite BCIPHIPP, are limited [40, 103]. For the four metabolites reported here, non-detect rates were low, still we used multiple imputation to handle values below the limit of detection, which is superior to alternative approaches, such as single value replacement or single imputation [65].
Our assessment of children’s behavior also possessed strengths and limitations. The BASC-2 has demonstrated validity and correlates well with other behavioral assessments [61]. In our study, we used a well validated parent-rating scale, the BASC-2. Assessments that rely on parent report may be partial to a parent’s perceptions about typical child behavior that may be influenced by a variety of factors, including their own behaviors and the behaviors of other children. In our analyses, we included mothers’ scores on the Center for Epidemiologic Studies Depression Scale (CES-D) as a covariate, which may have reduced reporting biases related to maternal behavior. However, future studies should consider including direct neurobehavioral assessments. Additionally, the behavioral assessment scores produced by the BASC-2 may not be as immediately significant as a clinical diagnosis of a behavioral disorder, though among young children they can have predictive utility for future behavioral disorders and subclinical differences [61]. Even subclinical effects can greatly influence children’s behavior over the course of their lifetimes and may be more relevant for studies of environmental toxicants, where effects on behavior are likely to be modest. Future investigations of behavioral effects of OPE exposure will benefit from continued use of these instruments and other similar instruments to assess subclinical differences, but clinically relevant assessments may also be valuable. A final limitation of our behavioral assessments is that we administered them at approximately three years of age, which may have limited our ability to accurately assess certain behavioral dimensions that become more pronounced with age. The body of evidence relating prenatal and early life OPE exposures to behavioral development would benefit from behavioral assessments administered at multiple ages.
The PIN studies provided an efficient setting to explore our research question, including OPE metabolite concentrations, developmental assessments, and considerable data on covariates important for assessment of OPEs and behavior. The PIN Kids protocol did not begin until the final years of the PIN3 and PIN Postpartum studies, which limited the sample size; but the characteristics of the mother-child pairs in our sample were generally similar to the baseline cohort. We adjusted for maternal age, race, and education in our analyses to reduce any potential bias resulting from selectivity by these factors. To investigate potential selection bias resulting from differences in OPE metabolite concentrations between the PIN3 participants with OPE metabolite concentrations and our analysis sample, we compared OPE metabolite concentrations between these samples (Supplemental Table S7) and observed only modest differences between the larger sample with OPE metabolite concentrations and our analysis sample. We similarly compared BASC-2 scores between the larger sample that had these scores and our analysis sample (Supplemental Table S8) and similarly only observed modest differences in BASC-2 scores between these two populations. These differences did not persist after covariate adjustment for variables included in our analyses (Supplemental Table S9). Therefore, although the mothers in our analysis sample differed somewhat from the larger PIN3 population, which potentially limits the generalizability of our results, it does not seem that the exposure or outcomes differed substantially between these samples in a manner that would have substantially biased our findings. Nonetheless, future studies would benefit from larger study populations to allow for greater generalizability and greater statistical power, particularly to assess associations among boys and girls separately.
In our study, we performed an exploratory analysis of sex-specific associations between prenatal OPE exposure and measures of behavioral development. Because our sex-stratified analysis samples were small, sex-specific associations were imprecise and we advise caution in interpreting these results. Still, we observed that prenatal BDCIPP concentrations appeared to be more strongly associated with adverse behavioral development among females than males. In particular, we observed that BDCIPP was associated with lower scores on the Adaptive Skills Composite and constituent scales among females, but not among males (with the exception of a weak inverse association with the Social Skills scale). This finding is interesting because in a recent investigation of prenatal OPE exposure in relation to cognitive development in this same cohort [104] we observed stronger adverse associations between BDCIPP and performance on the Mullen Scales of Early Learning (a measure of early life cognitive development) among females than males. With consideration of the cautions noted above, further investigation of these findings suggesting potentially greater developmental toxicity of BDCIPP among females than males is warranted in future studies, especially given the endocrine disrupting compounds [67–69].
Our study contributes to a growing body of evidence that OPEs may adversely affect behavioral development. Pervasive exposure among women of reproductive age and young children to an environmental toxicant potentially capable of producing adverse behavioral effects is of immediate public health significance. Early life, particularly the prenatal period, is a uniquely sensitive period for human development, and even subtle perturbations in developmental trajectories can amount to significant effects throughout the lifecourse [76–79]. Behavioral disorders, such as ADHD, autism, and other behavioral conditions, incur steep costs to individuals, their families, and society, and even subclinical impairments to behavioral development can impact an individual’s ability to thrive. As such, the identification of intervenable risk factors for suboptimal behavioral development is of chief importance to public health. OPEs are promoted as replacements for brominated flame retardants (e.g., PBDEs), which were phased-out amid concerns of their toxicity, particularly their neurodevelopmental toxicity, and environmental persistence. The available evidence suggests that OPEs should be highly scrutinized as a suitable alternative. Further investigation of developmental effects of OPEs, including observational studies with greater statistical power and improved exposure assessment, will aid in the characterization of the risks involved with such pervasive exposure to these compounds, and will inform decisions regarding their continued usage.
5. Conclusions
In a prospective birth cohort study, we observed that higher concentrations of BDCIPP in prenatal maternal urine were associated with more adverse behaviors in offspring, including Withdrawal, Attention Problems, and others. We observed similar associations with DPHP concentrations, although associations were not as strong as those observed for BDCIPP. Conversely, ip-PPP was associated with fewer behavioral symptoms, particularly those associated with internalizing behaviors. BCIPHIPP was not apparently associated with behavioral symptoms in this study. Our study contributes to the growing body of evidence pertaining to the behavioral effects of early life exposure to OPEs that indicates that OPEs may adversely affect early childhood development. However, further research is needed to better characterize the toxicity of specific OPE compounds and identify developmental endpoints most sensitive to such toxicity. The results of this study can be used in combination with other data on these chemicals to inform decision making regarding the usage of OPEs.
Supplementary Material
Highlights.
We examined prenatal exposure to four OPEs in relation to behavioral development.
BDCIPP was associated with more adverse behavioral symptoms.
DPHP was associated with more behavioral symptoms, though not as strongly as BDCIPP.
ip-PPP was associated with fewer internalizing behavioral symptoms.
BCIPHIPP was not associated with behavioral development.
Funding sources
This research was supported in part by grants from the National Institute of Environmental Health Sciences (R21 ES023904 and P30ES10126) and the U.S. Environmental Protection Agency (RD832736). KH was supported in part by a training grant from the National Institute of Environmental Health Sciences (T32 ES007018). BTD was supported in part by a training grant from the National Institute of Environmental Health Sciences [T32 ES007018] and a training grant from the National Institute of Child Health and Development [T32 HD52468].
Abbreviations
- ADHD
Attention Deficit Hyperactivity Disorder
- BASC-2
Behavior Assessment System for Children 2nd Edition
- BCIPHIPP
1-hydroxy-2-propyl bis(1-chloro-2-propyl) phosphate
- BCIPP
bis(1-chloro-2-propyl) phosphate
- BDCIPP
bis(1,3-dichloro-2-propyl) phosphate
- CI
confidence interval
- DPHP
diphenyl phosphate
- ip-PPP
isopropyl-phenyl phenyl phosphate
- IQR
interquartile range
- MDL
method limit of detection
- NHANES
National Health and Nutrition Examination Survey
- OPE
Organophosphate Ester
- PBDEs
polybrominated diphenyl ethers
- PIN
Pregnancy Infection and Nutrition
- SD
standard deviation
- SG
specific gravity
- tb-PPP
tert-butyl phenyl phenyl phosphate
- TCEP
tris(2-chloroethyl) phosphate
- TCIPP
tris(1-chloro-2-propyl) phosphate
- TPHP
triphenyl phosphate
- TDCIPP
tris(1,3-dichloro-2-propyl) phosphate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest
None.
Submission declaration
All of the authors have read and approved the paper, and it has not been published previously nor is it currently being considered by any other peer-reviewed journal.
References
- 1.ATSDR, Toxicological Profile for Phosphate Ester Flame Retardants. U.S. DHHS 2012. [PubMed]
- 2.van der Veen I and de Boer J, Phosphorus flame retardants: properties, production, environmental occurrence, toxicity and analysis. Chemosphere, 2012. 88(10): p. 1119–53. [DOI] [PubMed] [Google Scholar]
- 3.Wei GL, et al. , Organophosphorus flame retardants and plasticizers: sources, occurrence, toxicity and human exposure. Environ Pollut, 2015. 196: p. 29–46. [DOI] [PubMed] [Google Scholar]
- 4.Stapleton HM, et al. , Novel and high volume use flame retardants in US couches reflective of the 2005 PentaBDE phase out. Environ Sci Technol, 2012. 46(24): p. 13432–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper EM, et al. , Results from Screening Polyurethane Foam Based Consumer Products for Flame Retardant Chemicals: Assessing Impacts on the Change in the Furniture Flammability Standards. Environ Sci Technol, 2016. 50(19): p. 10653–10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stapleton HM, et al. , Detection of organophosphate flame retardants in furniture foam and U.S. house dust. Environ Sci Technol, 2009. 43(19): p. 7490–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marklund A, Andersson B, and Haglund P, Screening of organophosphorus compounds and their distribution in various indoor environments. Chemosphere, 2003. 53(9): p. 1137–46. [DOI] [PubMed] [Google Scholar]
- 8.Zheng X, et al. , Flame retardants on the surface of phones and personal computers. Sci Total Environ, 2017. 609: p. 541–545. [DOI] [PubMed] [Google Scholar]
- 9.Hoffman K, et al. , High Exposure to Organophosphate Flame Retardants in Infants: Associations with Baby Products. Environ Sci Technol, 2015. 49(24): p. 14554–9. [DOI] [PubMed] [Google Scholar]
- 10.Stapleton HM, et al. , Identification of flame retardants in polyurethane foam collected from baby products. Environ Sci Technol, 2011. 45(12): p. 5323–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mendelsohn E, et al. , Nail polish as a source of exposure to triphenyl phosphate. Environ Int, 2016. 86: p. 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keller AS, et al. , Flame Retardant Applications in Camping Tents and Potential Exposure. Environ Sci Technol Lett, 2014. 1(2): p. 152–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomes G, et al. , Characterizing Flame Retardant Applications and Potential Human Exposure in Backpacking Tents. Environ Sci Technol, 2016. 50(10): p. 5338–45. [DOI] [PubMed] [Google Scholar]
- 14.Carignan CC, et al. , Urinary biomarkers of flame retardant exposure among collegiate U.S. gymnasts. Environ Int, 2016. 94: p. 362–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergh C, et al. , Organophosphate and phthalate esters in air and settled dust - a multi-location indoor study. Indoor Air, 2011. 21(1): p. 67–76. [DOI] [PubMed] [Google Scholar]
- 16.He R, et al. , Organophosphorus flame retardants and phthalate esters in indoor dust from different microenvironments: Bioaccessibility and risk assessment. Chemosphere, 2016. 150: p. 528–35. [DOI] [PubMed] [Google Scholar]
- 17.Reemtsma T, et al. , Organophosphorus flame retardants and plasticizers in water and air occurrence and fate. Trends in Analytical Chemistry, 2008. 27(9): p. 727–737. [Google Scholar]
- 18.Wu M, et al. , Characterization and human exposure assessment of organophosphate flame retardants in indoor dust from several microenvironments of Beijing, China. Chemosphere, 2016. 150: p. 465–71. [DOI] [PubMed] [Google Scholar]
- 19.Zhou L, et al. , Organophosphate flame retardants (OPFRs) in indoor and outdoor air in the Rhine/Main area, Germany: comparison of concentrations and distribution profiles in different microenvironments. Environ Sci Pollut Res Int, 2017. 24(12): p. 10992–11005. [DOI] [PubMed] [Google Scholar]
- 20.Meeker JD, et al. , Urinary metabolites of organophosphate flame retardants: temporal variability and correlations with house dust concentrations. Environ Health Perspect, 2013. 121(5): p. 580–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hou R, Xu Y, and Wang Z, Review of OPFRs in animals and humans: Absorption, bioaccumulation, metabolism, and internal exposure research. Chemosphere, 2016. 153: p. 78–90. [DOI] [PubMed] [Google Scholar]
- 22.Cequier E, et al. , Human exposure pathways to organophosphate triesters - a biomonitoring study of mother-child pairs. Environ Int, 2015. 75: p. 159–65. [DOI] [PubMed] [Google Scholar]
- 23.Dodson RE, et al. , Urinary biomonitoring of phosphate flame retardants: levels in California adults and recommendations for future studies. Environ Sci Technol, 2014. 48(23): p. 13625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu X, et al. , Occurrence of organophosphorus flame retardants on skin wipes: Insight into human exposure from dermal absorption. Environ Int, 2017. 98: p. 113–119. [DOI] [PubMed] [Google Scholar]
- 25.Makinen MS, et al. , Respiratory and dermal exposure to organophosphorus flame retardants and tetrabromobisphenol A at five work environments. Environ Sci Technol, 2009. 43(3): p. 941–7. [DOI] [PubMed] [Google Scholar]
- 26.Li J, et al. , Occurrence of organophosphate flame retardants in drinking water from China. Water Res, 2014. 54: p. 53–61. [DOI] [PubMed] [Google Scholar]
- 27.Cooper EM, et al. , Analysis of the flame retardant metabolites bis(1,3-dichloro-2-propyl) phosphate (BDCPP) and diphenyl phosphate (DPP) in urine using liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem, 2011. 401(7): p. 2123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van den Eede N, et al. , First insights in the metabolism of phosphate flame retardants and plasticizers using human liver fractions. Toxicol Lett, 2013. 223(1): p. 9–15. [DOI] [PubMed] [Google Scholar]
- 29.Lynn RK, et al. , Disposition of the flame retardant, tris(1,3-dichloro-2-propyl) phosphate, in the rat. Drug Metab Dispos, 1981. 9(5): p. 434–41. [PubMed] [Google Scholar]
- 30.Nomeir AA, Kato S, and Matthews HB, The metabolism and disposition of tris(1,3-dichloro-2-propyl) phosphate (Fyrol FR-2) in the rat. Toxicol Appl Pharmacol, 1981. 57(3): p. 401–13. [DOI] [PubMed] [Google Scholar]
- 31.Minegishi K, et al. , Comparative studies on absorption, distribution, and excretion of flame retardants halogenated alkyl phosphate in rats. Eisei Kagaku, 1988. 34(2): p. 102–114. [Google Scholar]
- 32.Sasaki K, Takeda M, and Uchiyama M, Toxicity, absorption and elimination of phosphoric acid triesters by killifish and goldfish. Bull Environ Contam Toxicol, 1981. 27(6): p. 775–82. [DOI] [PubMed] [Google Scholar]
- 33.Hoffman K, Daniels JL, and Stapleton HM, Urinary metabolites of organophosphate flame retardants and their variability in pregnant women. Environ Int, 2014. 63: p. 169–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romano ME, et al. , Variability and predictors of urinary concentrations of organophosphate flame retardant metabolites among pregnant women in Rhode Island. Environ Health, 2017. 16(1): p. 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ospina M, et al. , Exposure to organophosphate flame retardant chemicals in the U.S. general population: Data from the 2013–2014 National Health and Nutrition Examination Survey. Environ Int, 2018. 110: p. 32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoffman K, et al. , Predictors of urinary flame retardant concentration among pregnant women. Environ Int, 2017. 98: p. 96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castorina R, et al. , Flame retardants and their metabolites in the homes and urine of pregnant women residing in California (the CHAMACOS cohort). Chemosphere, 2017. 179: p. 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Butt CM, et al. , Regional comparison of organophosphate flame retardant (PFR) urinary metabolites and tetrabromobenzoic acid (TBBA) in mother-toddler pairs from California and New Jersey. Environ Int, 2016. 94: p. 627–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoffman K, et al. , Temporal Trends in Exposure to Organophosphate Flame Retardants in the United States. Environ Sci Technol Lett, 2017. 4(3): p. 112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van den Eede N, et al. , Age as a determinant of phosphate flame retardant exposure of the Australian population and identification of novel urinary PFR metabolites. Environ Int, 2015. 74: p. 1–8. [DOI] [PubMed] [Google Scholar]
- 41.Feng L, et al. , Levels of Urinary Metabolites of Organophosphate Flame Retardants, TDCIPP, and TPHP, in Pregnant Women in Shanghai. J Environ Public Health, 2016. 2016: p. 9416054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carignan CC, et al. , Urinary Concentrations of Organophosphate Flame Retardant Metabolites and Pregnancy Outcomes among Women Undergoing in Vitro Fertilization. Environ Health Perspect, 2017. 125(8): p. 087018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ding J, et al. , Organophosphate ester flame retardants and plasticizers in human placenta in Eastern China. Sci Total Environ, 2016. 554–555: p. 211–7. [DOI] [PubMed] [Google Scholar]
- 44.Zhao F, et al. , Organophosphorus Flame Retardants in Pregnant Women and Their Transfer to Chorionic Villi. Environ Sci Technol, 2017. 51(11): p. 6489–6497. [DOI] [PubMed] [Google Scholar]
- 45.Meeker JD and Stapleton HM, House dust concentrations of organophosphate flame retardants in relation to hormone levels and semen quality parameters. Environ Health Perspect, 2010. 118(3): p. 318–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Preston EV, et al. , Associations between urinary diphenyl phosphate and thyroid function. Environ Int, 2017. 101: p. 158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Q, et al. , Bioconcentration and transfer of the organophorous flame retardant 1,3-dichloro-2-propyl phosphate causes thyroid endocrine disruption and developmental neurotoxicity in zebrafish larvae. Environ Sci Technol, 2015. 49(8): p. 5123–32. [DOI] [PubMed] [Google Scholar]
- 48.Wang Q, et al. , Exposure of zebrafish embryos/larvae to TDCPP alters concentrations of thyroid hormones and transcriptions of genes involved in the hypothalamic-pituitary-thyroid axis. Aquat Toxicol, 2013. 126: p. 207–13. [DOI] [PubMed] [Google Scholar]
- 49.Wang Q, et al. , Developmental exposure to the organophosphorus flame retardant tris(1,3-dichloro-2-propyl) phosphate: estrogenic activity, endocrine disruption and reproductive effects on zebrafish. Aquat Toxicol, 2015. 160: p. 163–71. [DOI] [PubMed] [Google Scholar]
- 50.Liu X, Ji K, and Choi K, Endocrine disruption potentials of organophosphate flame retardants and related mechanisms in H295R and MVLN cell lines and in zebrafish. Aquat Toxicol, 2012. 114–115: p. 173–81. [DOI] [PubMed] [Google Scholar]
- 51.Liu X, et al. , Effects of TDCPP or TPP on gene transcriptions and hormones of HPG axis, and their consequences on reproduction in adult zebrafish (Danio rerio). Aquat Toxicol, 2013. 134–135: p. 104–11. [DOI] [PubMed] [Google Scholar]
- 52.Dishaw LV, et al. , Is the PentaBDE replacement, tris (1,3-dichloro-2-propyl) phosphate (TDCPP), a developmental neurotoxicant? Studies in PC12 cells. Toxicol Appl Pharmacol, 2011. 256(3): p. 281–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li R, et al. , Tris (1,3-dichloro-2-propyl) phosphate-induced apoptotic signaling pathways in SH-SY5Y neuroblastoma cells. Neurotoxicology, 2017. 58: p. 1–10. [DOI] [PubMed] [Google Scholar]
- 54.Wang Q, et al. , Bioconcentration, metabolism and neurotoxicity of the organophorous flame retardant 1,3-dichloro 2-propyl phosphate (TDCPP) to zebrafish. Aquat Toxicol, 2015. 158: p. 108–15. [DOI] [PubMed] [Google Scholar]
- 55.Yuan L, et al. , Targeting neurotrophic factors and their receptors, but not cholinesterase or neurotransmitter, in the neurotoxicity of TDCPP in Chinese rare minnow adults (Gobiocypris rarus). Environ Pollut, 2016. 208(Pt B): p. 670–7. [DOI] [PubMed] [Google Scholar]
- 56.Lipscomb ST, et al. , Cross-sectional study of social behaviors in preschool children and exposure to flame retardants. Environ Health, 2017. 16(1): p. 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Castorina R, et al. , Current-use flame retardants: Maternal exposure and neurodevelopment in children of the CHAMACOS cohort. Chemosphere, 2017. 189: p. 574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.PIN — Pregnancy Infection, and Study Nutrition [cited 2018; Available from: http://www.cpc.unc.edu/projects/pin. [Google Scholar]
- 59.Van den Eede N, et al. , Analysis of organophosphate flame retardant diester metabolites in human urine by liquid chromatography electrospray ionisation tandem mass spectrometry. J Chromatogr A, 2013. 1303: p. 48–53. [DOI] [PubMed] [Google Scholar]
- 60.Boeniger MF, Lowry LK, and Rosenberg J, Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. Am Ind Hyg Assoc J, 1993. 54(10): p. 615–27. [DOI] [PubMed] [Google Scholar]
- 61.Reynolds CR and Kamphaus RW, BASC-2: Behavior Assessment System for Children. 2 2004, Bloomington, MN: Pearson. [Google Scholar]
- 62.Radloff LS, The CES-D scale: a self-report depression scale for research in the general population. . Appl Psychol Measure, 1977. 1: p. 385–401. [Google Scholar]
- 63.Bradley RH and Caldwell BM, Home observation for measurement of the environment: a revision of the preschool scale. American journal of mental deficiency, 1979. [PubMed] [Google Scholar]
- 64.Greenland S, Pearl J, and Robins JM, Causal diagrams for epidemiologic research. Epidemiology, 1999: p. 37–48. [PubMed] [Google Scholar]
- 65.Lubin JH, et al. , Epidemiologic evaluation of measurement data in the presence of detection limits. Environ Health Perspect, 2004. 112(17): p. 1691–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rubin DB, Multiple imputation for nonresponse in surveys. Vol. 81 2004: John Wiley & Sons. [Google Scholar]
- 67.Frye CA, et al. , Endocrine disrupters: a review of some sources, effects, and mechanisms of actions on behaviour and neuroendocrine systems. J Neuroendocrinol, 2012. 24(1): p. 144–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gore AC, et al. , EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr Rev, 2015. 36(6): p. E1–e150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Venerosi A, et al. , Sex dimorphic behaviors as markers of neuroendocrine disruption by environmental chemicals: the case of chlorpyrifos. Neurotoxicology, 2012. 33(6): p. 1420–6. [DOI] [PubMed] [Google Scholar]
- 70.Liu X, et al. , Long-term exposure to triphenylphosphate alters hormone balance and HPG, HPI, and HPT gene expression in zebrafish (Danio rerio). Environ Toxicol Chem, 2016. 35(9): p. 2288–96. [DOI] [PubMed] [Google Scholar]
- 71.Hammel SC, et al. , Measuring Personal Exposure to Organophosphate Flame Retardants Using Silicone Wristbands and Hand Wipes. Environ Sci Technol, 2016. 50(8): p. 4483–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Clark DL, Boutros NN, and Mendez MF, The brain and behavior: an introduction to behavioral neuroanatomy. 2010: Cambridge University Press. [Google Scholar]
- 73.Filley C, Neurobehavioral Anatomy. 1995: University of Colorado. [Google Scholar]
- 74.Fink G, Pfaff D, and Levine J, Handbook of Neuroendocrinology. 2011: Academic Press. [Google Scholar]
- 75.Wilkinson M and Brown RE, An Introduction to Neuroendocrinology. 2015: Cambridge University Press. [Google Scholar]
- 76.Bondy SC and Campbell A, Developmental neurotoxicology. J Neurosci Res, 2005. 81(5): p. 605–12. [DOI] [PubMed] [Google Scholar]
- 77.Grandjean P and Landrigan PJ, Neurobehavioural effects of developmental toxicity. Lancet Neurol, 2014. 13(3): p. 330–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rice D and Barone S Jr., Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect, 2000. 108 Suppl 3: p. 511–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Miodovnik A, Environmental neurotoxicants and developing brain. Mt Sinai J Med, 2011. 78(1): p. 58–77. [DOI] [PubMed] [Google Scholar]
- 80.Farhat A, et al. , In Ovo effects of two organophosphate flame retardants--TCPP and TDCPP--on pipping success, development, mRNA expression, and thyroid hormone levels in chicken embryos. Toxicol Sci, 2013. 134(1): p. 92–102. [DOI] [PubMed] [Google Scholar]
- 81.Kim S, et al. , Thyroid disruption by triphenyl phosphate, an organophosphate flame retardant, in zebrafish (Danio rerio) embryos/larvae, and in GH3 and FRTL-5 cell lines. Aquat Toxicol, 2015. 160: p. 188–96. [DOI] [PubMed] [Google Scholar]
- 82.Xu T, et al. , Bioconcentration, metabolism and alterations of thyroid hormones of Tris(1,3-dichloro-2-propyl) phosphate (TDCPP) in Zebrafish. Environ Toxicol Pharmacol, 2015. 40(2): p. 581–6. [DOI] [PubMed] [Google Scholar]
- 83.Ghassabian A, Henrichs J, and Tiemeier H, Impact of mild thyroid hormone deficiency in pregnancy on cognitive function in children: lessons from the Generation R Study. Best Pract Res Clin Endocrinol Metab, 2014. 28(2): p. 221–32. [DOI] [PubMed] [Google Scholar]
- 84.de Escobar GM, Obregon MJ, and del Rey FE, Maternal thyroid hormones early in pregnancy and fetal brain development. Best Pract Res Clin Endocrinol Metab, 2004. 18(2): p. 225–48. [DOI] [PubMed] [Google Scholar]
- 85.Haddow JE, et al. , Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med, 1999. 341(8): p. 549–55. [DOI] [PubMed] [Google Scholar]
- 86.Williams GR, Neurodevelopmental and neurophysiological actions of thyroid hormone. J Neuroendocrinol, 2008. 20(6): p. 784–94. [DOI] [PubMed] [Google Scholar]
- 87.Modesto T, et al. , Maternal Mild Thyroid Hormone Insufficiency in Early Pregnancy and Attention-Deficit/Hyperactivity Disorder Symptoms in Children. JAMA Pediatr, 2015. 169(9): p. 838–45. [DOI] [PubMed] [Google Scholar]
- 88.Ghassabian A, et al. , Maternal thyroid autoimmunity during pregnancy and the risk of attention deficit/hyperactivity problems in children: the Generation R Study. Thyroid, 2012. 22(2): p. 178–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Andersen SL, et al. , Attention deficit hyperactivity disorder and autism spectrum disorder in children born to mothers with thyroid dysfunction: a Danish nationwide cohort study. Bjog, 2014. 121(11): p. 1365–74. [DOI] [PubMed] [Google Scholar]
- 90.Vermiglio F, et al. , Attention deficit and hyperactivity disorders in the offspring of mothers exposed to mild-moderate iodine deficiency: a possible novel iodine deficiency disorder in developed countries. J Clin Endocrinol Metab, 2004. 89(12): p. 6054–60. [DOI] [PubMed] [Google Scholar]
- 91.Pakkila F, et al. , The impact of gestational thyroid hormone concentrations on ADHD symptoms of the child. J Clin Endocrinol Metab, 2014. 99(1): p. E1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gore AC, et al. , Implications of prenatal steroid perturbations for neurodevelopment, behavior, and autism. Endocr Rev, 2014. 35(6): p. 961–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu C, et al. , Effects of tris(1,3-dichloro-2-propyl) phosphate and triphenyl phosphate on receptor-associated mRNA expression in zebrafish embryos/larvae. Aquat Toxicol, 2013. 128–129: p. 147–57. [DOI] [PubMed] [Google Scholar]
- 94.Zhang Q, et al. , Potential estrogenic effects of phosphorus-containing flame retardants. Environ Sci Technol, 2014. 48(12): p. 6995–7001. [DOI] [PubMed] [Google Scholar]
- 95.Krivoshiev BV, et al. , Assessing in-vitro estrogenic effects of currently-used flame retardants. Toxicol In Vitro, 2016. 33: p. 153–62. [DOI] [PubMed] [Google Scholar]
- 96.Pei Y, et al. , Comparative neurotoxicity screening in human iPSC-derived neural stem cells, neurons and astrocytes. Brain Res, 2016. 1638(Pt A): p. 57–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Crump D, Chiu S, and Kennedy SW, Effects of tris(1,3-dichloro-2-propyl) phosphate and tris(1-chloropropyl) phosphate on cytotoxicity and mRNA expression in primary cultures of avian hepatocytes and neuronal cells. Toxicol Sci, 2012. 126(1): p. 140–8. [DOI] [PubMed] [Google Scholar]
- 98.Ta N, et al. , Toxicity of TDCPP and TCEP on PC12 cell: changes in CAMKII, GAP43, tubulin and NFH gene and protein levels. Toxicol Lett, 2014. 227(3): p. 164–71. [DOI] [PubMed] [Google Scholar]
- 99.Nishimaki-Mogami T, et al. , Isolation and identification of metabolites of 2-ethylhexyl diphenyl phosphate in rats. Arch Toxicol, 1988. 61(4): p. 259–64. [DOI] [PubMed] [Google Scholar]
- 100.Ballesteros-Gomez A, Van den Eede N, and Covaci A, In vitro human metabolism of the flame retardant resorcinol bis(diphenylphosphate) (RDP). Environ Sci Technol, 2015. 49(6): p. 3897–904. [DOI] [PubMed] [Google Scholar]
- 101.Makiguchi K, et al. , Diphenyl phosphate as an efficient acidic organocatalyst for controlled/living ring-opening polymerization of trimethylene carbonates leading to block, end-functionalized, and macrocyclic polycarbonates. Macromolecules, 2013. 46(5): p. 1772–1782. [Google Scholar]
- 102.Makiguchi K, Satoh T, and Kakuchi T, Diphenyl phosphate as an efficient cationic organocatalyst for controlled/living ring-opening polymerization of δ-valerolactone and ε-caprolactone. Macromolecules, 2011. 44(7): p. 1999–2005. [Google Scholar]
- 103.Schindler BK, Forster K, and Angerer J, Quantification of two urinary metabolites of organophosphorus flame retardants by solid-phase extraction and gas chromatography-tandem mass spectrometry. Anal Bioanal Chem, 2009. 395(4): p. 1167–71. [DOI] [PubMed] [Google Scholar]
- 104.Doherty BT, et al. , Prenatal Exposure to Organophosphate Esters and Cognitive Development in Young Children in the Pregnancy, Infection, and Nutrition Study. Environmental Research, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.