Asparaginase-associated hepatotoxicity (AAH) is common in patients treated for acute lymphoblastic leukemia (ALL), usually manifested as hyperbilirubinemia, transaminitis, and steatosis, and prolonged prothrombin time [1]. It represents a significant cause of treatment delay and interruption, and limits the use of asparaginase in adults despite its benefit on treatment outcome [2, 3]. Currently, there are no standard guidelines for the management of AAH in ALL patients. In the past several years, the potential of L-carnitine as a solution for AAH has begun to emerge in literature, with over twenty reported cases attributing the resolution of AAH to its administration [4, 5, 6, 7, 8, 9]. L-carnitine is an endogenous quaternary ammonium compound which transports fatty acids into mitochondria under the catalyzation of carnitine palmitoyltransferases for β-oxidation, and has proved effective in the context of hepatotoxicity induced by valproate and anti-tuberculosis medications [10, 11]. However, the mechanism underlying the pathogenesis of AAH remains unclear, although the involvement of oxidative stress has been proposed [12]. Preclinical data on asparaginase hepatotoxicity and the effect of L-carnitine in well-established animal models are scarce.
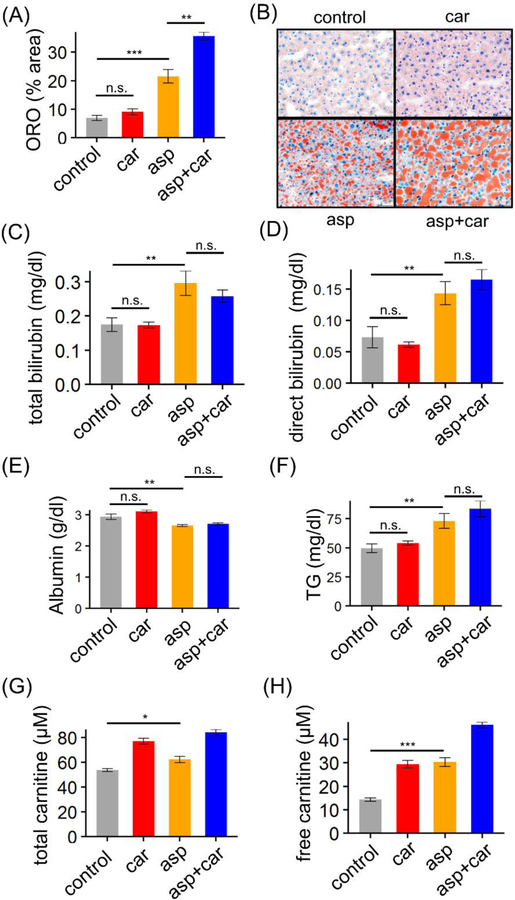
In the study, we used mid-aged (31–35 week old) female C57BL6 mice (Jackson Lab) as a model to elucidate what effects L-carnitine (Carnitor, Leadiant Biosciences) has on liver damage caused by intense treatment with pegaspargase (Oncaspar, Baxalta US Inc.), the most common formulation of asparaginase used in US and European ALL regimens. Mice were assigned to one of four treatment groups: control, carnitine, asparaginase, and asparaginase+carnitine (Figure 1). Dosages of pegaspargase and L-carnitine were 1200U/kg twice a week and 500mg/kg daily respectively, based on those used in previous publications [13, 14], and are clinically relevant. Mice were sacrificed and tissues collected after overnight fasting at the end of the 2 week regimens. Consistent with murine data [13] and ultrasound observations in patients [1], hepatic steatosis was induced by pegaspargase treatment alone (p = 5.9×10−4) (Figure 2(A–B)). One of the most common clinical signs of hepatotoxicity, hyperbilirubinemia, was also induced by pegaspargase treatment, indicated by both total bilirubin and direct bilirubin levels (p = 0.0076 for total bilirubin, and p = 0.0088 for direct bilirubin) (Figure 2(C–D)). When given alone, L-carnitine did not have any impact on liver fat accumulation (p = 0.21) (Figure 2(A–B)) or serum bilirubin levels (p = 0.94 for total bilirubin, and p = 0.40 for direct bilirubin) (Figure 2(C–D)). However, the addition of L-carnitine to the pegaspargase regimen worsened steatosis by over 60% (p = 0.0015) (Figure 2(A–B)), and had no impact on bilirubin levels (p = 0.35 for total bilirubin, and p = 0.96 for direct bilirubin) (Figure 2(C–D)). In line with clinical observations [15], we also saw lower albumin and higher triglyceride levels in serum from pegaspargase-treated mice (p = 0.0054 for albumin, and p = 0.0041 for triglyceride) (Figure 2(E–F)), neither of which were reversed by L-carnitine (p = 0.22 for albumin, and p = 0.07 for triglyceride) (Figure 2(E–F)). Although common in patients, transaminitis was not a symptom after pegaspargase or L-carnitine treatment in our mice (data not shown).
Figure 1.
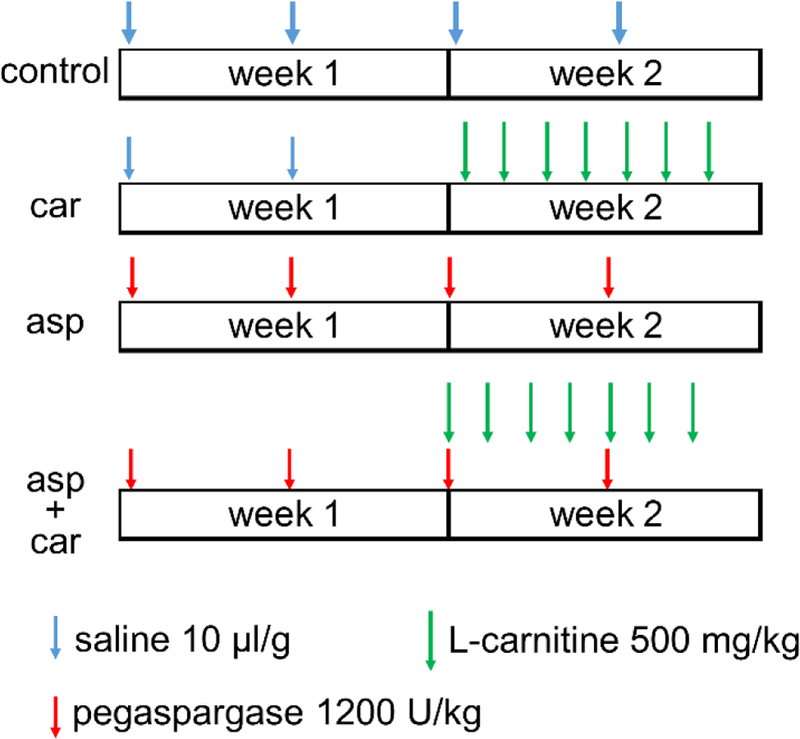
Treatment regimens. 15, 10, 10, and 5 C57BL6 female mice were assigned to one of four treatment groups, control, carnitine (car), asparaginase (asp) and asparaginase+carnitine (asp+car), respectively, and were given saline, L-carnitine, and pegaspargase intraperitoneally. L-carnitine and pegaspargase were diluted with saline to reach target concentrations. No replicates were conducted.
Figure 2.
Changes in liver lipid levels and blood chemistry following treatment regimens with vehicle control, carnitine (car), and asparaginase (asp) as depicted in Figure 1. (A) Hepatic lipid accumulation is shown as percentage of oil-red O (ORO) stained area on mouse liver sections. (B) ORO stain of mouse liver sections indicating levels of hepatic steatosis. Original magnification X40. (C-H) Serum levels of bilirubin, albumin, triglyceride and carnitine. Bars in (A) and (C-H) represent mean ± SEM. Significance was evaluated with pair-wise 2-tailed t test (*p < 0.05; ** p < 0.01; *** p < 0.001, n.s. not significant).
Carnitine deficiency can be induced by valproate [10], and was reported in up to 48% of tuberculosis patients [11]. Those facts may explain why hepatotoxicity caused by valproate and anti-tuberculosis can be ameliorated by L-carnitine repletion, a remedy not to be readily extrapolated to AAH. By contrast, in our study, carnitine deficiency was not implicated in the pathogenesis of AAH. In fact, pegaspargase increased both total and free carnitine levels in mouse serum (p = 0.036 for total carnitine, and p = 2.8×10−5 for free carnitine) (Figure 2(G–H)). Of note, published data characterizing carnitine levels among ALL patients or ALL animal models, including those treated with asparaginase, are lacking.
In summary, we showed for the first time, data from intact mice treated with pegaspargase and L-carnitine individually and in combination. Our pegaspargase-treated mice recapitulated some important features seen in ALL patients, including hepatic steatosis, and higher bilirubin and blood triglyceride. L-carnitine both failed to improve laboratory parameters indicative of hepatic injury and was histologically associated with exacerbation of hepatic steatosis. It should be noted that this study was designed to explore the therapeutic effects of L-carnitine in AAH rather than its potential role as a prophylactic intervention administered before the onset of AAH, which remains a potential area for further pre-clinical investigation. L-carnitine’s effects using other schedules of L-carnitine and pegaspargase are also not known. Our study does not reflect the impact of L-carnitine on the antileukemic effect of pegaspargase or other chemotherapeutics agents, nor the effect of leukemia burden on drug-induced steatosis. Further investigations using leukemia-bearing mice would be needed for that purpose. Despite the limitations, our findings raise the alert on both the safety and efficacy of L-carnitine for AAH previously described in case reports, suggesting the need for further investigation of AAH and carnitine prior to proceeding with large-scale clinical trials. Together with the paucity of data demonstrating a lack of effect on antileukemia effectiveness for L-carnitine, caution is advised before adding unproven interventions to ALL regimens.
Acknowledgements
This research was supported by R01 CA142665.
Potential conflict of interest
Dr. Relling receives investigator-initiated support from Shire Pharmaceuticals for clinical asparaginase studies.
References
- 1.Christ TN, Stock W, Knoebel RW. Incidence of asparaginase-related hepatotoxicity, pancreatitis, and thrombotic events in adults with acute lymphoblastic leukemia treated with a pediatric-inspired regimen. J Oncol Pharm Pract 2018. June;24(4):299–308. doi: 10.1177/1078155217701291. [DOI] [PubMed] [Google Scholar]
- 2.Patel B, Kirkwood AA, Dey A, et al. Pegylated-asparaginase during induction therapy for adult acute lymphoblastic leukaemia: toxicity data from the UKALL14 trial. Leukemia 2017. January;31(1):58–64. doi: 10.1038/leu.2016.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toft N, Birgens H, Abrahamsson J, et al. Toxicity profile and treatment delays in NOPHO ALL2008-comparing adults and children with Philadelphia chromosome-negative acute lymphoblastic leukemia. Eur J Haematol 2016. February;96(2):160–9. doi: 10.1111/ejh.12562. [DOI] [PubMed] [Google Scholar]
- 4.Alshiekh-Nasany R, Douer D. L-Carnitine for Treatment of Pegasparaginase-Induced Hepatotoxicity. Acta Haematol 2016;135(4):208–10. doi: 10.1159/000442342. [DOI] [PubMed] [Google Scholar]
- 5.Blackman A, Boutin A, Shimanovsky A, et al. Levocarnitine and vitamin B complex for the treatment of pegaspargase-induced hepatotoxicity: A case report and review of the literature. J Oncol Pharm Pract 2017. January 1: 10.1177/1078155217710714. [DOI] [PubMed] [Google Scholar]
- 6.Schulte RR, Madiwale MV, Flower A, et al. Levocarnitine for asparaginase-induced hepatic injury: a multi-institutional case series and review of the literature. Leuk Lymphoma 2018. February 12:1–9. doi: 10.1080/10428194.2018.1435873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wieduwilt MJ, Goodman A, Jonas BA, et al. L-carnitine for pegylated-l-asparaginase induced hepatotoxicity. Journal of Clinical Oncology 2017;35(15_suppl):e21626–e21626. doi: 10.1200/JCO.2017.35.15_suppl.e21626. [DOI] [Google Scholar]
- 8.Al-Nawakil C, Willems L, Mauprivez C, et al. Successful treatment of l-asparaginase-induced severe acute hepatotoxicity using mitochondrial cofactors. Leuk Lymphoma 2014. July;55(7):1670–4. doi: 10.3109/10428194.2013.845886. [DOI] [PubMed] [Google Scholar]
- 9.Lu G, Karur V, Herrington JD, et al. Successful treatment of pegaspargase-induced acute hepatotoxicity with vitamin B complex and L-carnitine. Proc (Bayl Univ Med Cent) 2016. January;29(1):46–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bohan TP, Helton E, McDonald I, et al. Effect of L-carnitine treatment for valproate-induced hepatotoxicity. Neurology 2001. May 22;56(10):1405–9. [DOI] [PubMed] [Google Scholar]
- 11.Hatamkhani S, Khalili H, Karimzadeh I, et al. Carnitine for prevention of antituberculosis drug-induced hepatotoxicity: a randomized, clinical trial. J Gastroenterol Hepatol 2014. May;29(5):997–1004. doi: 10.1111/jgh.12474. [DOI] [PubMed] [Google Scholar]
- 12.Alachkar H, Fulton N, Sanford B, et al. Expression and polymorphism (rs4880) of mitochondrial superoxide dismutase (SOD2) and asparaginase induced hepatotoxicity in adult patients with acute lymphoblastic leukemia. Pharmacogenomics J 2017. June;17(3):274–279. doi: 10.1038/tpj.2016.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y, Fernandez CA, Smith C, et al. Genome-Wide Study Links PNPLA3 Variant with Elevated Hepatic Transaminase After Acute Lymphoblastic Leukemia Therapy. Clin Pharmacol Ther 2017. January 16. doi: 10.1002/cpt.629. [DOI] [PMC free article] [PubMed]
- 14.Kaya I, Citil M, Sozmen M, et al. Investigation of protective effect of L-carnitine on L-asparaginase-induced acute pancreatic injury in male Balb/c mice. Dig Dis Sci 2015. May;60(5):1290–6. doi: 10.1007/s10620-014-3461-3. [DOI] [PubMed] [Google Scholar]
- 15.Hinson A, Newbern D, Linardic CM. Asparaginase-Induced Hypertriglyceridemia Presenting as Pseudohyponatremia during Leukemia Treatment. Case Rep Pediatr 2014;2014: 10.1155/2014/635740. [DOI] [PMC free article] [PubMed] [Google Scholar]