Abstract
Growing evidence points to persistent neurological injury in chronic HIV infection. It remains unclear whether chronically HIV-infected individuals on combined antiretroviral therapy (cART) develop progressive brain injury and impaired neurocognitive function despite successful viral suppression and immunological restoration. In a longitudinal neuroimaging study for the HIV Neuroimaging Consortium (HIVNC), we mapped the annual rate of change of regional brain volumes in 155 chronically infected and treated HIV+ participants (mean age 48.0 ± 8.9 yrs; 83.9% Male), using tensor-based morphometry between two time points (mean time interval: 1.0 ± 0.5 yrs). We tested for associations between rates of brain tissue loss and clinical measures of disease severity (nadir or baseline CD4+ cell count and baseline HIV plasma RNA concentration), HIV duration, cART CNS penetration-effectiveness scores, age, as well as change in AIDS Dementia Complex stage. We found significant brain tissue loss across HIV+ participants, including those neuro-asymptomatic with undetectable viral load, largely localized to subcortical regions. Measures of disease severity, age, and neurocognitive decline were associated with greater atrophy. Chronically HIV-infected and treated individuals may undergo progressive brain tissue despite stable and effective cART, which may contribute to neurocognitive decline. Understanding neurological complications of chronic infection, and identifying factors associated with atrophy may help inform strategies to maintain brain health in people living with HIV.
Keywords: HIV, ADC, MRI, Brain Volume, cART, TBM
INTRODUCTION
Combined antiretroviral therapy (cART) has dramatically improved life expectancies for HIV-infected individuals (Nakagawa et al. 2013). Today, acute HIV encephalitis and HIV-associated dementia are far less prevalent (Ances and Ellis 2007), but many HIV-infected adults still experience a range of neurocognitive impairments (NCI) known as HIV-associated neurocognitive disorders (HAND), or the AIDS dementia complex (ADC) (Navia et al. 1986a, Navia et al. 1986b, Antinori et al. 2007, Robertson et al. 2007, Heaton et al. 2011). It is not well understood whether neurologically asymptomatic, chronically HIV-infected adults on stable cART are at heightened risk for neurodegeneration and NCI as they age.
Brain imaging studies provide evidence of persistent brain decline and NCI in chronically HIV-infected individuals on cART. Reduced cortical gray matter, basal ganglia, and white matter volumes, as well as larger ventricular volumes, have been associated with duration of infection, cognitive impairment, brain metabolite disruption, and immunological markers of disease severity –particularly nadir CD4+ count (Cardenas et al. 2009, Cohen et al. 2010, Becker et al. 2011, Harezlak et al. 2011, Tate et al. 2011, Ances et al. 2012, Hua et al. 2013a, Harezlak et al. 2014). These trends have even been identified in neuro-asymptomatic individuals whose viral load is suppressed by cART (Cohen et al. 2010, Tate et al. 2011).
To date, most studies have been cross-sectional with few longitudinal studies assessing rates of brain atrophy. Whether viral suppression with stable treatment protects against premature decline is still unresolved (Heaton et al. 2015, Correa et al. 2016, Sacktor et al. 2016, Sanford et al. 2018). As part of the HIV Neuroimaging Consortium (HIVNC), we assessed chronically infected individuals on stable cART, including virologically suppressed individuals with minimal or no NCI, to determine whether these individuals continue to show patterns of progressive brain atrophy beyond that expected from aging. We used a longitudinal brain mapping technique, tensor-based morphometry (TBM), to generate maps of annual brain tissue loss. We hypothesized that 1) measures of disease severity at baseline would predict brain atrophy rates, and 2) greater atrophy would be associated with decline in neurocognitive function. Identifying the pattern of degeneration in treated asymptomatic individuals, along with factors associated with rates of decline, may provide targeted neurological bases for treatments and help identify pre-symptomatic individuals at heightened risk for NCI.
METHODS
HIVNC Participants
Between 2003 and 2009, 1.5 T T1-weighted MRI, clinical, and neuropsychological data were collected at two time points (mean time interval: 1.0 ± 0.5 yrs) from 155 chronically HIV-infected HIVNC participants (mean baseline age: 48.0 ± 8.9 yrs; 83.9% Male) on stable cART with a history of advanced disease (nadir CD4+ count < 200 cells/mm3) across seven sites. Baseline demographic and clinical characteristics are reported in Table 1. Inclusion criteria, clinical assessments, and MRI image acquisition parameters have been previously described (Harezlak et al. 2011, Gongvatana et al. 2013, Hua et al. 2013a) and are summarized in Supplementary Appendix 1.1. Procedures were approved by local institutional review boards. Participants gave written informed consent.
Table 1.
Clinical characteristics of 155 HIVNC study participants. Mean and standard deviation (SD) are listed for continuous variables. Percent and absolute number are noted for categorical variables.
| Continuous Variables | Mean (SD) |
|---|---|
| Age (yrs) | 48.0 (8.9) |
| HIV Duration (yrs) | 11.6 (6.9) |
| cART Duration (yrs) | 5.4 (4.5) |
| CPE Scorea | 8.2 (3.1) |
| Nadir CD4+ (cells/mm3) | 58.6 (58.2) |
| CD4+ (cells/mm3)a | 369.1 (203.0) |
| Inter-Scan Time Interval (yrs) | 1.0 (0.5) |
| Categorical Variables | % (N) |
| Sex (male) | 83.9% (130) |
| Education (≤ high school) | 43.9% (68) |
| Race/Ethnicity | |
| Caucasian | 69.7% (108) |
| African American | 27.8% (43) |
| Asians/Native Americans/American Indian | 2.6% (4) |
| Suppressed CD4+ (≤ 350 cells/mm3) a | 53.6% (82) |
| Detectable Plasma HIV RNA (≥400 copies/mL) a | 24.8% (38) |
| ADCa | |
| Stage 0: | 64.3% (92) |
| Stage 0.5: | 24.5% (35) |
| Stage 1: | 10.5 % (15) |
| Stage 2: | 0.01% (1) |
Measures only available in subset of participants: CPE score N=152; CD4+ and Suppressed CD4+ N=153; Detectable Plasma HIV RNA N=153; ADC Stage N=143.
NCI was assessed using AIDS Dementia Complex (ADC) staging, as previously described (Navia et al. 1986a, Navia et al. 1986b, Price and Brew 1988). Based on both clinical evaluations and neuropsychological tests, participants were classified at baseline and prospectively as follows: ADC stage 0-neurologically asymptomatic (NA) with no evidence of cognitive, functional or neuropsychological impairment; ADC stage 0.5-subclinical impairment, with evidence of neuropsychological impairment only, as defined above; ADC stage 1-mild neurocognitive impairment with evidence of definite cognitive and functional impairment on activities of daily living (ADL) but without loss of independent functioning; ADC stage 2-moderate impairment, requiring assistance on ADLs; or ADC stage 3-severe global cognitive and functional impairment (Price and Brew 1988). Of 143 participants with available baseline ADC stage, none were ADC stage 3, and only one was ADC stage 2. Follow-up ADC stage was available in 134 participants. Two years after the start of this study, a revised classification of HIV-associated cognitive impairment, referred to as the Frascati criteria, was proposed (Antinori et al. 2007), where in general, ADC stage 0.5 approximately corresponds to asymptomatic neurocognitive impairment (ANI), ADC stage 1 to mild neurocognitive disorder (MND), and ADC stage 2 or greater to HIV-associated dementia or HAD.
Image Processing and Tensor-Based Morphometry (TBM)
Tensor based morphometry (TBM), as described in (Hua et al. 2013a, Hua et al. 2013b, Hua et al. 2016), is a robust and sensitive technique to create three-dimensional maps that reflect the rates of change at each point (voxel) in the brain. Briefly, each subject’s pre-processed follow-up T1-weighted scan was registered to its baseline scan using a non-linear inverse-consistent elastic mutual information based registration algorithm (Leow et al. 2007). The resulting Jacobian determinant map, or change map, characterizes the local volume differences (expansion/contraction) between the two scans. For each subject, change maps were normalized by the respective time interval (in years) between scans to reflect the annualized rate of change in each voxel. To align maps across participants for statistical analysis, baseline T1-weighted scans were warped to a study specific template, and the resulting warp was applied to each subjects’ change map. The cerebellum was often partly outside of the field of view (FOV; i.e., cropped during image acquisition) and was therefore excluded from all analyses. For further details, see Supplementary Appendix 1.3.
Group Average Maps of Annual Rates of Brain Atrophy
We computed the mean annual rate of brain tissue loss (percent) in HIV+ participants, at each voxel. Mean maps were computed in HIV+ subgroups classified as either neurologically asymptomatic (NA; ADC=0; N=92) or NA with undetectable viral load (NA-uVL; plasma HIV RNA < 400 copies/mL; N=76). A two-tailed, one-sample T-test was used to find regions with significant change (change not equal to zero). We corrected for multiple comparisons across voxels using the searchlight false discovery rate (sFDR) method at q=0.05 (Langers et al. 2007).
For each group, voxel-wise annual change was averaged within 26 regions of interest (ROIs) spanning cortical and subcortical gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) compartments (Supplementary Table S3). To generate the ROIs, the template to which all Jacobian maps were spatially normalized was segmented with FreeSurfer (Fischl et al. 2004). A two-tailed, one-sample T-test was used to find ROIs with significant mean change across participants, at the multiple comparisons FDR corrected P-value (Benjamini and Hochberg 1995).
Statistical Associations with Annual Rates of Brain Atrophy
Random-effects multiple linear regressions were used to test associations between brain atrophy rates and variables of interest. We covaried for baseline age, sex, race/ethnicity, and inter-scan time-interval. To ensure baseline brain volumes did not confound our analyses of brain tissue change, we also covaried for baseline TBM maps representing volumetric differences from the template (i.e. the degree of volume differences already present at baseline). To account for possible scanner effects, scanning parameters and acquisition site were used as the grouping variable. All voxel-wise analyses were corrected for multiple comparisons using sFDR at q=0.05 (Langers et al. 2007).
We tested for group differences in annual brain volume changes between HIV+ neuro-asymptomatic (NA) and symptomatic (NS) participants, and between the subset of NA-uVL participants and the remaining population. Across HIV+ participants, we evaluated the effects of age as well as HIV-related clinical variables at baseline on annual volumetric change: nadir CD4+, immunologically reconstituted current CD4+ status —defined as a CD4+ > 350 cells/μl, the threshold below which cART is generally recommended (World Health Organization 2015), detectable HIV RNA viral load (dVL) in plasma (≥ 400 copies/mL). Secondary analyses evaluated duration of HIV infection, and baseline cART CNS penetration-effectiveness (CPE) score (Letendre et al. 2009).
Variables that showed a statistically significant association in the entire HIV+ population were then subsequently tested in two subsets: 1) all NA participants (the majority of the study population) and 2) a smaller NA-uVL subset. Finally, we assessed whether brain volume changes were associated with longitudinal changes in ADC stage. Details on variable definitions are available in Supplementary Appendix 1.4.
Uninfected Comparison Group - Parkinson’s Progression Markers Initiative (PPMI) Controls
Because healthy controls were not part of the original study design, healthy control 3T T1-weighted MRI scans were obtained from the publicly-available, multi-center Parkinson’s Progression Markers Initiative (PPMI; Supplementary Appendix 1.2) (Marek et al. 2011). Longitudinal MRIs from 63 controls (mean age: 59.3 ± 10.9 yrs; 61.9% male) were downloaded from the PPMI database (http://www.ppmi-info.org/). A subset of 48 controls were selected to match on age, sex, race/ethnicity, and education with a subset of 65 HIV+ HIVNC participants. This subset was representative of the full cohort for all HIV-related measures. Demographic and clinical characteristics for the full and matched PPMI and HIVNC groups are reported in Supplementary Tables S1-S2. As for HIVNC, baseline and follow-up T1-weighted images were pre-processed, and change maps generated and registered to the HIVNC template for an exploratory comparison with HIV+ individuals.
RESULTS
Clinical and Demographic Characteristics
Demographic and clinical factors were compared between groups using one-way ANOVA or chi-squared analyses. Within the full HIV+ cohort, compared to neuro-symptomatic participants (NS), the NA group was comprised of a marginally higher number of Caucasian participants (P=0.021) and fewer participants with detectable viral loads (P=0.011; Table 2). Compared to NA-uVL participants, those either with dVL or NS were significantly older (P=0.010) with longer HIV duration (P=0.004).
Table 2.
Comparison of demographic and clinical characteristics between neuro-asymptomatic subgroups and symptomatic participants using ANOVA or chi-squared tests. Key: NA: Neuro-asymptomatic (ADC=0); NS: Neuro-symptomatic (ADC>0); dVL: Detectable Viral Load (plasma HIV RNA ≥ 400 cp/mL); uVL: Undetectable Viral Load (plasma HIV RNA <400 cp/mL); SD: Standard Deviation.
| NS N=51 |
NA N=92 |
P-Value | NS or dVL aN=66 |
NA-uVL N=76 |
P-Value | |
|---|---|---|---|---|---|---|
| Continuous Variables | Mean (SD) | ANOVA | Mean (SD) | ANOVA | ||
| Age (yrs) | 50.0 (8.0) | 47.0 (9.6) | 0.066 | 50.2 (8.3) | 46.3 (9.5) | 0.011* |
| HIV Duration (yrs) | 12.9 (6.5) | 10.6 (7.2) | 0.068 | 13.1 (6.4) | 9.8 (7.1) | 0.004* |
| cART Duration (yrs) | 5.1 (4.9) | 4.7 (4.0) | 0.376 | 5.1 (4.52) | 4.7 (4.1) | 0.508 |
| CPE Score | 8.1 (3.3) bN=49 | 8.1 (2.7) | 0.977 | 7.8 (3.24) bN=64 | 8.3 (2.6) | 0.298 |
| Nadir CD4+ (cells/mm3) | 67.8 (62.5) | 57.1 (56.7) | 0.297 | 65.0 (60.3) | 57.6 (58.0) | 0.456 |
| CD4+ Count (cells/mm3) | 387.0 (205.8) | 370.2 (197.3) bN=91 | 0.632 | 372.8 (211.5) | 379.3 (190.6) | 0.848 |
| Inter-Scan Time Interval (yrs) | 1.1 (0.5) | 1.0 (0.6) | 0.613 | 1.0 (0.5) | 1.1 (0.5) | 0.855 |
| Categorical Variables | % (N) | χ2-Test | % (N) | χ2-Test | ||
| Sex (male) | 78.4% (40) | 84.8% (78) | 0.338 | 80.3% (53) | 84.2% (64) | 0.542 |
| Education (≤ high school) | 47.1% (24) | 41.3% (38) | 0.506 | 42.4% (28) | 44.7% (34) | 0.782 |
| Race/Ethnicity (Caucasian) | 54.9% (28) | 75.0% (69) | 0.014* | 53.0% (35) | 80.3% (61) | 0.001* |
| Suppressed CD4+ (≤ 350 cells/mm3) | 49.0% (25) | 53.9% (49) bN=91 | 0.581 | 53.0% (35) | 51.3% (39) | 0.838 |
| Detectable Plasma HIV RNA (≥400 copies/mL) | 35.3% (18) | 16.5% (15) bN=91 | 0.011* | 50.0% (33) | 0% (0) | 1.98×10−12* |
P ≤ 0.05
N = 18 are both NS with dVL
Total N with available data are noted
Annual Rates of Brain Volume Change
The full HIVNC cohort, NA participants, and only those NA with uVL all showed significant CSF expansion and widespread tissue atrophy (Figure 1a-c), especially within subcortical structures and WM (Pcorrected ≤ 0.05). Mean regional changes, summarized in Supplementary Table S3, reveal the greatest annual changes were consistently detected in the pallidum and thalamus (0.4–0.5% atrophy annually), and ventricles (up to 0.7% expansion), followed by the putamen and nucleus accumbens (0.3–0.4% atrophy).
Fig. 1.
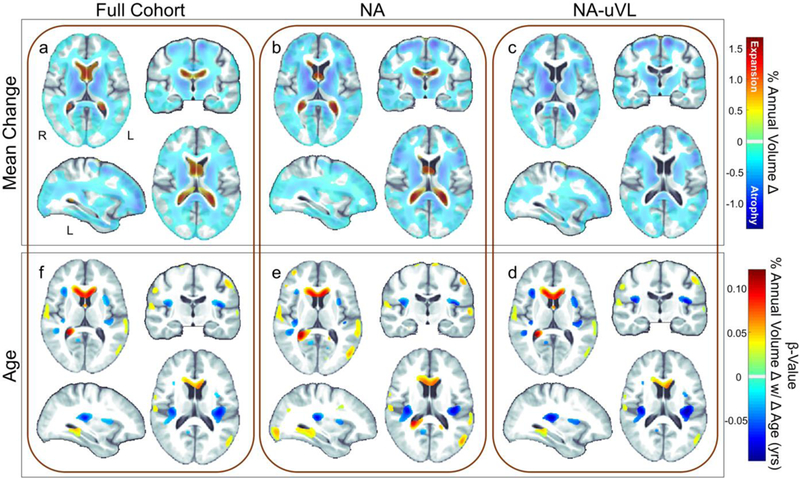
Atrophy rates in HIV+ individuals. Annual volumetric change (%) maps averaged across (a) the full HIVNC cohort (mean age: 48.01 ± 8.88 yrs; N=155) revealed significant ventricular expansion (red) and tissue atrophy (blue) throughout the brain (regions with no change are colored gray/white; one-sample T-test; Pcorrected ≤ 0.05). Maps of the (b) subset of neuro-asymptomatic individuals (NA; ADC Stage=0; N=92) and (c) those who are NA with undetectable viral load (NA-uVL; plasma HIV RNA < 400 copies/mL; N=76) showed a similar distribution and magnitude of change as the full cohort. (d-f) In the full cohort and the NA subsets, older age at baseline was significantly associated with greater annual ventricular and sulcal expansion and tissue atrophy (Pcorrected ≤ 0.05). However, the association between brain atrophy and age was relatively localized and did not account for the pervasive brain atrophy detected across participants over time
Rates of brain change were similar between HIV+ groups. There were no significant differences between NA participants and those with an ADC stage greater than 0, or between NA-uVL and the remainder of the population.
Age and HIV Infection Severity Predict Brain Tissue Loss Rates
We evaluated the independent effects of age on brain atrophy rates in the entire HIV+ cohort, and found that older age at baseline was associated with greater tissue loss (Pcorrected ≤ 0.05). For each year in age, HIV+ participants showed an average of 0.04% greater ventricular/CSF expansion and 0.04% greater tissue atrophy (Table 2a; Figure 1d). Similar patterns and rates were detected in the subsets of NA and NA-uVL participants (Figure 1e,f). The association between brain atrophy and age was relatively localized and did not account for the pervasive brain atrophy detected across participants over time. Age and duration of infection were not strongly correlated (Pearson’s r=0.30; P = 0.0001) and associations between age and atrophy rates remained significant when covarying for HIV duration.
When covarying for age, indices of HIV infection also predicted greater brain atrophy and CSF space expansion in the full HIV+ cohort. Participants with a lower current CD4+ cell count showed on average a 0.78% increase in ventricular/CSF expansion rates and 0.78% increase in atrophy rates in the WM, bilateral thalami, caudate, and putamen, and right globus pallidus and amygdala (Figure 2a; Table 3b; Pcorrected ≤ 0.05). On average, every 10 unit reduction in nadir CD4+ was associated with 0.06% faster sulcal CSF expansion and 0.06% increase in atrophy rates the left thalamus and internal capsule WM (Figure 2b; Table 2c). Compared to virologically suppressed participants, those with detectable plasma HIV RNA (dVL) showed 1.0% greater ventricular/CSF expansion, and 0.82% greater atrophy throughout the WM, thalamus, globus pallidus, and putamen as well as the left hippocampus and amygdala (Figure 2c; Table 2d). Atrophy rates were not associated with duration of infection, or CPE score after correcting for multiple comparisons. Ranking the significant baseline HIV-related predictors based on the spatial extent of their effects across the brain revealed the following order of relative importance on brain changes: dVL, CD4+ status at baseline, and nadir CD4+ cell count (Table 3).
Fig. 2.

Brain tissue loss rates are associated with measures of HIV infection severity. (a-c) In the full cohort and (d-f) the subset of neuro-asymptomatic (NA) participants (ADC=0), lower nadir CD4+ cell count, suppressed immune status at baseline (current CD4+ ≤350 cells/mm3), and detectable viral load (plasma HIV RNA ≥ 400 cp/mL) at baseline were significantly associated (Pcorrected ≤ 0.05) with a greater annual ventricular and sulcal expansion (red) and tissue atrophy (blue). Only (g) current CD4+ was associated with volume loss in NA participants with undetectable viral load (NA-uVL)
Table 3.
Summary of effects from Figures 1–3 are reported as (1) the spatial extent or percent of voxels (out of 1,625,341 voxels tested) that are significant after multiple comparisons correction (Pcorrected ≤ 0.05) and (2) the interquartile range (IQR) and mean percent change in those voxels with positive or negative associations with each measure. Key: NA: Neuro-asymptomatic; uVL: Undetectable Viral Load.
| Analysis | Significant Voxels | Positive/Expansion IQR (Mean) | Negative/Atrophy IQR (Mean) | |
|---|---|---|---|---|
| Baseline Clinical Predictors | ||||
| A. Age (per year older) | Full Cohort | 3.62% | 0.032–0.047% (0.043%) | 0.030–0.045% (0.038%) |
| NA | 6.69% | 0.036–0.052% (0.046%) | 0.033–0.048% (0.042%) | |
| NA-uVL | 3.88% | 0.037–0.053% (0.045%) | 0.036–0.055% (0.046%) | |
| B. Suppressed current CD4+(≤ 350 cells/mm3) | Full Cohort | 3.67% | 0.578–0.843% (0.778%) | 0.573–0.941% (0.777%) |
| NA | 1.85% | 0.641–0.832% (0.736%) | 0.839–0.988% (0.839%) | |
| NA-uVL | 0.85% | 0.685–0.922% (0.810%) | 0.708–1.013% (0.877%) | |
| C. Nadir CD4+(every 10 cells/μl lower) | Full Cohort | 1.45% | 0.049–0.066% (0.057%) | 0.047–0.078% (0.063%) |
| NA | 0.15% | 0.057–0.072% (0.069%) | 0.045–0.054% (0.049%) | |
| NA-uVL | 0 | -- | -- | |
| D. Detectable plRNA (≥ 400 copies/mL) | Full Cohort | 8.95% | 0.768–1.206% (1.034%) | 0.657–0.941% (0.820%) |
| NA | 1.13% | 1.619–2.621% (2.144%) | 0.930–1.221% (1.082%) | |
| Neurocognitive Impairment | ||||
| E. ADC Change | Decline a vs Stable/Improve | 0.48% | 1.423–2.669% (2.072%) | 0.752–1.110% (0.908%) |
| Decline a vs Improve | 2.12% | 1.963–4.052% (3.045%) | 1.030–1.789% (1.429%) | |
Decline: Neurocognitive decline (increase in ADC stage)
The subset of NA participants showed similar atrophy rates to the full HIV+ cohort (Table 3); greater ventricular/CSF expansion and tissue atrophy were associated with dVL and lower current or nadir CD4+ counts. However, findings were less widespread, perhaps due to a smaller sample size (Figure 2d-f). While atrophy in NA-uVL participants was not associated with nadir CD4+, significant associations were detected with current CD4+ (Figure 2g).
Changes in ADC Stage and Tissue Loss
134 HIVNC participants had ADC staging at both time points; 78.4% remained stable, 7.5% improved (ADC decrease), and 14.2% declined (10.4% went from ADC=0 to ADC ≥ 0.5; Figure 3a). Change in ADC status was not significantly associated with either change in viral load or CD4+ status (CD4+: Spearman’s r = −0.15, P = 0.096; VL: Spearman’s r = 0.10, P = 0.614; Supplementary Appendix 2.4). Compared to those who remained stable or improved (N=115), an increase in ADC stage (N=19), reflecting neurocognitive decline, was associated with on average 2.1% greater ventricular expansion and 0.91% greater atrophy sparsely distributed in temporal WM (Table 3e; Figure 3b). Greater differences were detected when an increase in ADC stage (N=19) was compared only to participants who improved (N=10): 3.1% greater ventricular expansion and 1.4% greater atrophy in the left frontal, temporal, occipital, and internal capsule WM, as well as the left putamen, pallidum, and thalamus (Figure 3c). No significant difference was detected between those who remained stable and those who improved.
Fig. 3.
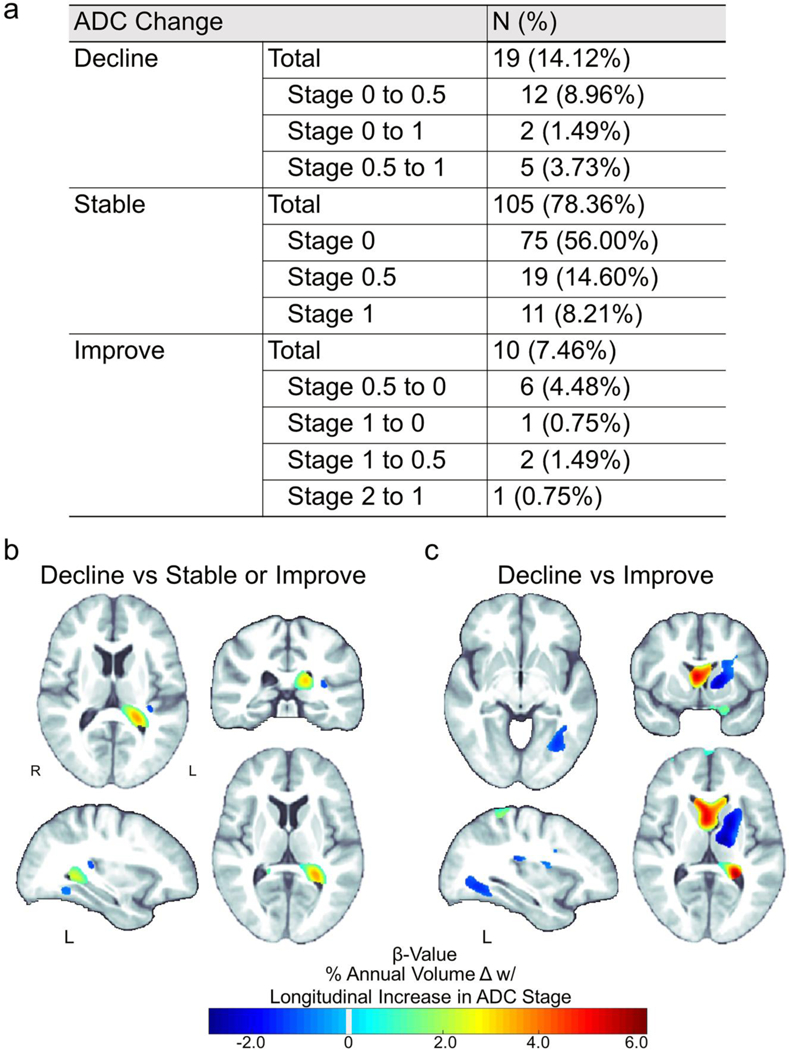
Brain tissue loss rates are associated with change in ADC stage. (a) Total number of participants (out of 134) for each type of change in ADC stage between baseline and follow-up assessments. Relative to participants with (b) stable or improved neurocognitive status (no change or decreased ADC stage; N=115) or (c) just improved neurocognitive status (decreased ADC stage; N=10) over time, a decline in neurocognitive status (an increase in ADC stage; N=19) was significantly associated (Pcorrected ≤ 0.05) with greater rates of tissue atrophy (blue) and ventricular expansion (green-red)
Annual Rates of Brain Volume Change in HIV+ Participants Compared to Controls
HIVNC HIV+ participants were significantly younger (P=1.16×10−13), less educated (P=0.0001), and were comprised of fewer females (P=0.0004) and Caucasians (P=0.0004) than the full PPMI control cohort (Supplementary Table S1). The subset of HIV+ participants selected to match the demographic characteristics of PPMI were not significantly different clinically to the full HIV+ group, however they were significantly older (P=2.47×10−6) and had fewer males (P=0.048) than the full group to match demographic characteristics of the PPMI cohort (Supplementary Table S2).
Voxel-wise analyses (Figure 4) revealed that compared to healthy controls (N=48; mean age: mean age: 55.6 ± 9.5 yrs), matched HIV+ participants (N=65; mean age: 54.2 ± 7.9 yrs) had significantly greater tissue atrophy rates in small regions bilaterally in the temporal lobes and in the left precuneus (Pcorrected≤0.05, Mean: 0.67%; IQR: 0.58–0.77%; Significant Voxels: 0.12%).
Fig. 4.
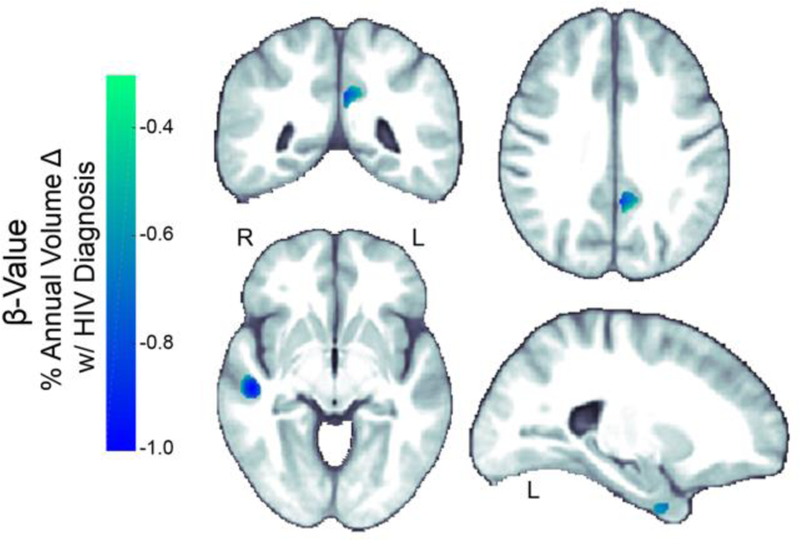
Atrophy rates in HIV+ individuals compared to healthy controls. Compared to a subset of age matched controls (N=48; mean age: 55.6 ± 9.5 yrs), HIV+ individuals (N=65; mean age: 54.2 ± 7.9 yrs) showed significantly (Pcorrected ≤ 0.05) greater rates of GM atrophy bilaterally in the temporal lobes and the left precuneus.
DISCUSSION
This study represents one of few published longitudinal studies to map patterns of ongoing regional brain volume loss in a large cohort of chronically HIV-infected middle-aged participants on cART, including neurologically asymptomatic and virologically suppressed individuals. While recent cross-sectional studies have identified disrupted brain processes in HIV-infected individuals with chronic, stable disease (Cohen et al. 2010, Tate et al. 2011, Harezlak et al. 2014), relatively few longitudinal studies have been conducted, and there is little consensus regarding the pattern and degree of ongoing brain change. Our study has two main findings: 1) Atrophy (CSF expansion and tissue loss) persists in HIV-infected individuals despite cART and 2) rates of atrophy in HIV are associated with measures of disease severity and neurocognitive decline.
As life expectancy of HIV-infected individuals has increased significantly in the setting of cART, it has been postulated that HIV-associated brain injury and NCI may accelerate with aging (Brew et al. 2009, Holt et al. 2012, Brew and Cysique 2017, Ding et al. 2017). Several cross-sectional studies have reported either independent or additive effects of age and HIV (Ances et al. 2012, Becker et al. 2012, Nir et al. 2014, Cohen et al. 2015) or HIV-by-age interactions (Harezlak et al. 2011, Cysique et al. 2013, Kuhn et al. 2017). The HIVNC study did not include seronegative individuals as part of the original study, necessary to directly compare HIV effects with healthy aging. However, the association between brain atrophy and age was relatively localized and did not account for a majority of the annual tissue loss detected throughout the brain of HIV+ participants. We found that markers of HIV severity and age were both independent, and sometimes overlapping, predictors of progressive brain atrophy in HIV+ participants, suggesting atrophy beyond that expected from healthy aging alone. To further confirm atrophy rates beyond that expected with healthy aging, a group of seronegative participants from the multi-site longitudinal PPMI study were used in an exploratory analysis. We found suggestive evidence that longitudinal atrophy rates in HIV+ individuals on stable cART were significantly higher than those in a group of age-matched healthy controls, in line with other longitudinal studies (Cardenas et al. 2009, Clifford et al. 2017). Compared to HIVNC T1-weighted brain scans, which were acquired with 1.5 T MRI, PPMI scans were acquired with 3 T MRI, which can result in improved tissue contrast, and affect volume estimates (Jovicich et al. 2009, Heinen et al. 2016, Lysandropoulos et al. 2016). The magnitude and extent of the differences between HIV+ and HIV-individuals presented in this paper should be interpreted with caution due to differences in participant inclusion criteria and MRI acquisition between studies. These preliminary findings motivate the need for additional longitudinal MRI studies with matched seronegative controls.
Within HIV+, atrophy was associated with baseline measures of infection severity: lower current CD4+ count, higher HIV RNA levels, and lower nadir CD4+. The subcortical pattern of atrophy associated with markers of disease severity in this study is consistent with early neuropathological and immunohistochemical studies of HIV encephalitis that show the presence of multinucleated giant cells and microglial nodules, as well as viral antigens, with a predilection for subcortical structures (Navia et al. 1986a, Navia et al. 1986b, Neuen-Jacob et al. 1993, Brew et al. 1995, Berger and Nath 1997, Morgello 2018), such as the putamen where decreased neuronal densities have been identified post mortem (Everall et al. 1995). Similarly, more recent in vivo neuroimaging studies suggest that HIV prominently affects the basal ganglia and WM (Tucker et al. 2004, Cohen et al. 2010, Jernigan et al. 2011, Ances et al. 2012, Heaps et al. 2015). HIVNC participants all had a history of advanced disease (nadir CD4+ count < 200 cells/mm3); nadir CD4+ count has been associated with brain volume deficits and cognitive impairment in several cross sectional studies, suggesting that severe immunosuppression may lead to persistent and potentially irreversible brain injury despite immune recovery in individuals on cART (Ellis et al. 2011, Jernigan et al. 2011, Tate et al. 2011, Hua et al. 2013a), and reinforcing the need for early intervention. However, we found that among baseline measures of disease severity, detectable plasma VL was a better predictor of progressive atrophy throughout the brain, followed by current CD4+ status, and then nadir CD4+. Studies of post mortem brain specimens in the antiretroviral era show correlations between elevated plasma HIV viral load and HIV brain-tissue viral load and pathology (Everall et al. 2009, Gelman et al. 2013). These results suggest that, against a background of a severe immunosuppression (nadir CD4+ count < 200 cells/mm3) and aging, ongoing HIV-related processes contribute to progressive brain change. Two smaller longitudinal studies have similarly shown accelerated loss of WM volume and regional cortical volume in individuals with lower current CD4+ count (Pfefferbaum et al. 2014) and detectable VL (Cardenas et al. 2009). These results support the importance of achieving viral suppression and adequate immunological restoration with effective cART.
Importantly, tissue loss throughout the brain was found in NA participants with uVL, and was not significantly different than that detected in NS participants or those with dVL, consistent with cross-sectional studies that suggest brain decline persists in these individuals (Harezlak et al. 2011, Tate et al. 2011). A prospective MRS study of the HIVNC cohort revealed significant decreases in neuronal and glial cell function in NA participants (Gongvatana et al. 2013). Together, these findings suggest that HIV-related brain injury may persist or worsen over time even in treated HIV-infected individuals with a history of advanced disease, who have successful viral suppression and stable disease. In contrast to the full HIV+ cohort, other than age, only current CD4+ count (i.e., not nadir CD4+) was significantly associated with these changes, and with modest effect sizes. We cannot, however, discount that ongoing low-grade viral replication, undetected by the older assay thresholds used in this study, may drive these brain changes. Sequestered HIV RNA has been found in postmortem brain tissue from cART patients with uVL (Lamers et al. 2016). Two smaller longitudinal studies (N ≤ 48) of similarly aged HIV+ individuals with VL < 50 copies/mL report similar changes in brain volume over time between HIV+ and uninfected participants (Correa et al. 2016, Sanford et al. 2018). Concordantly, participants in Correa et al. (2016) and Sanford et al. (2018), showed higher median current CD4+ counts than HIVNC participants (678 and 630 cells/mm3 respectively), and, in Sanford et al., higher median nadir CD4+ counts (190 cells/mm3, implying a history of severe immunosuppression), prompting further investigation to determine viral load, current and nadir CD4 count thresholds that mitigate atrophy. Although the underlying mechanisms remain unclear, pro-inflammatory factors including chemokines such as MCP-1 and sCD14, which were shown to contribute to subcortical and WM injury in the HIVNC cohort, may also play a role (Anderson et al. 2015). Similar to the mechanisms that underlie some of the systemic complications of chronic HIV infection, persistent immune activation also likely contributes to HIV neuropathogenesis (Deeks et al. 2013).
Building on evidence suggesting that neuro-asymptomatic HIV-infected individuals can develop progressive decline in cognitive function (Grant et al. 2014), our results show that such decline in NCI are significantly associated with atrophy rates in subcortical GM and WM regions. A recent HIVNC study found that reduced levels of the neuronal marker N-acetylaspartate in the basal ganglia was the most significant predictor of conversion to neurocognitive impairment, relative to the same clinical predictors evaluated here (Navia et al. In Review). Together, these results are consistent with a growing body of evidence pointing to the pathogenic role of these subcortical structures in HAND (Navia et al. 1986a, Navia et al. 1986b).
When viewed closely, maps of associations between greater indices of disease severity and CSF expansion may appear to extend into neighboring cortical GM (indicating slower rates of GM loss with increased disease severity), which could reflect compensatory hypermetabolic or inflammatory processes that occur at early stages of neurocognitive impairment (a majority of the cohort) (Rottenberg et al. 1987, Hinkin et al. 1995, von Giesen et al. 2000, Chang et al. 2004, Castelo et al. 2007), or ceiling effects at the parenchyma/CSF interface. TBM has been validated as a powerful and unbiased technique to map longitudinal brain change, especially in large multi-site studies (Hua et al. 2016). However, changes in cortical GM are difficult to measure with registration based methods, as the cortex is thin and prone to partial volume effects, with sulcal and morphological variability leading to subtle misalignments. TBM is arguably better powered to detect subcortical changes (e.g. WM and subcortical GM structures) (Hua et al. 2009, Hua et al. 2013a, Hua et al. 2013b, Hua et al. 2013c). Even so, voxel-wise studies allow for more regionally unbiased analyses compared to predetermined ROIs, which limit our power to map patterns of complex effects. Further validation of findings using other independent methods and cohorts may help evaluate these cortical GM effects.
Another potential limitation to the TBM method used here is that evaluating change between two time points assumes that changes are linear. Although estimating longitudinal brain changes in a cohort is commonly achieved with two time points (Cardenas et al., 2009; Clifford et al., 2017; Correa et al., 2016; Sanford et al., 2018), more informative rates and trajectories may be calculated with a greater number of time points.
The immediate clinical utility for neuroimaging in the setting chronic HIV infection and cART is limited given the small number of longitudinal studies published to date, but further evaluation of regional brain changes along with the contributing factors will be important to form a better understanding of disease progression in relation to cognitive decline and treatment intervention. Based on available literature in other fields, notably Alzheimer’s disease (Reiman and Jagust 2012, Hua et al. 2016, Veitch et al. 2018), imaging biomarkers have provided an important non-invasive, in vivo approach to identify subgroups at higher risk for cognitive decline and a marker to monitor the effects novel drug treatments in clinical trials that aim to slow or halt such decline (McArthur 2012, Chang and Shukla 2018). Identifying regions of disease-specific vulnerability in chronic HIV infection using noninvasive MRI techniques may be an important step towards determining brain regions that may be associated with cognitive decline which in turn may suggest targets for therapeutic intervention. In addition, future studies of associations between volumetric changes in chronically HIV infected patients as described in this manuscript and pro-inflammatory factors or cellular changes as detected by proton MRS, would further our understanding of processes contributing to HIV-related brain injury.
While standards in neuropsychological and viral load evaluation may have shifted from the time the HIVNC study was conducted, our findings add to a body of evidence that chronically HIV-infected individuals, even on cART, are at increased risk for future brain tissue loss and cognitive decline. The findings from this study support a critical unmet need to identify novel therapies to protect the CNS, even in the era of effective cART treatment.
Supplementary Material
Acknowledgments
The study was funded by NIH NINDS R01 NS080655 and U54 EB020403. Data used in the preparation of this article were obtained from the Parkinson’s Progression Markers Initiative (PPMI) database (www.ppmi-info.org/data). For up-to-date information on the study, visit www.ppmi-info.org. PPMI– a public-private partnership– is funded by the Michael J. Fox Foundation for Parkinson’s Research and funding partners, including AbbVie, Allergan, Avid Radiopharmaceuticals, Biogen, BioLegend, Bristol-Myers Squibb, Denali, GE Healthcare, Genentech, GlaxoSmithKline (GSK), Eli Lilly and Company, Lundbeck, Merck, Meso Scale Discovery (MSD), Pfizer, Piramal Imaging, Roche, Sanofi Genzyme, Servier, Takeda, Teva, and UCB (www.ppmi-info.org/fundingpartners).
Footnotes
Conflict of Interest
Jeffry R Alger owns NeuroSpectroScopics LLC. Thomas B Campbell is a consultant for Gilead Sciences and Theratechnologies Inc. Xue Hua now works for M3 Biotechnology; the work included in the manuscript was conducted during her appointment at USC, and she reports no disclosures. The remaining authors declare that they have no conflict of interest.
References
- Ances BM and Ellis RJ (2007). Dementia and neurocognitive disorders due to HIV-1 infection. Semin Neurol, 27(1): 86–92. [DOI] [PubMed] [Google Scholar]
- Ances BM, Ortega M, Vaida F, Heaps J and Paul R (2012). Independent effects of HIV, aging, and HAART on brain volumetric measures. J Acquir Immune Defic Syndr, 59(5): 469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AM, Harezlak J, Bharti A, Mi D, Taylor MJ, Daar ES, Schifitto G, Zhong J, Alger JR, Brown MS, Singer EJ, Campbell TB, McMahon DD, Buchthal S, Cohen R, Yiannoutsos C, Letendre SL, Navia BA and Consortium HIVN (2015). Plasma and Cerebrospinal Fluid Biomarkers Predict Cerebral Injury in HIV-Infected Individuals on Stable Combination Antiretroviral Therapy. J Acquir Immune Defic Syndr, 69(1): 29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V and Wojna VE (2007). Updated research nosology for HIV-associated neurocognitive disorders. Neurology, 69(18): 1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JT, Maruca V, Kingsley LA, Sanders JM, Alger JR, Barker PB, Goodkin K, Martin E, Miller EN, Ragin A, Sacktor N, Selnes O and Multicenter ACS (2012). Factors affecting brain structure in men with HIV disease in the post-HAART era. Neuroradiology, 54(2): 113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JT, Sanders J, Madsen SK, Ragin A, Kingsley L, Maruca V, Cohen B, Goodkin K, Martin E, Miller EN, Sacktor N, Alger JR, Barker PB, Saharan P, Carmichael OT, Thompson PM and Multicenter ACS (2011). Subcortical brain atrophy persists even in HAART-regulated HIV disease. Brain Imaging Behav, 5(2): 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y and Hochberg Y (1995). CONTROLLING THE FALSE DISCOVERY RATE - A PRACTICAL AND POWERFUL APPROACH TO MULTIPLE TESTING. Journal of the Royal Statistical Society Series B-Methodological, 57(1): 289–300. [Google Scholar]
- Berger JR and Nath A (1997). HIV dementia and the basal ganglia. Intervirology, 40(2–3): 122–131. [DOI] [PubMed] [Google Scholar]
- Brew BJ, Crowe SM, Landay A, Cysique LA and Guillemin G (2009). Neurodegeneration and ageing in the HAART era. J Neuroimmune Pharmacol, 4(2): 163–174. [DOI] [PubMed] [Google Scholar]
- Brew BJ and Cysique L (2017). Does HIV prematurely age the brain? Lancet HIV, 4(9): e380–e381. [DOI] [PubMed] [Google Scholar]
- Brew BJ, Rosenblum M, Cronin K and Price RW (1995). AIDS dementia complex and HIV-1 brain infection: clinical-virological correlations. Ann Neurol, 38(4): 563–570. [DOI] [PubMed] [Google Scholar]
- Cardenas VA, Meyerhoff DJ, Studholme C, Kornak J, Rothlind J, Lampiris H, Neuhaus J, Grant RM, Chao LL, Truran D and Weiner MW (2009). Evidence for ongoing brain injury in human immunodeficiency virus-positive patients treated with antiretroviral therapy. J Neurovirol, 15(4): 324–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelo JM, Courtney MG, Melrose RJ and Stern CE (2007). Putamen hypertrophy in nondemented patients with human immunodeficiency virus infection and cognitive compromise. Arch Neurol, 64(9): 1275–1280. [DOI] [PubMed] [Google Scholar]
- Chang L, Lee PL, Yiannoutsos CT, Ernst T, Marra CM, Richards T, Kolson D, Schifitto G, Jarvik JG, Miller EN, Lenkinski R, Gonzalez G, Navia BA and Consortium HM (2004). A multicenter in vivo proton-MRS study of HIV-associated dementia and its relationship to age. Neuroimage, 23(4): 1336–1347. [DOI] [PubMed] [Google Scholar]
- Chang L and Shukla DK (2018). Imaging studies of the HIV-infected brain. Handb Clin Neurol, 152: 229–264. [DOI] [PubMed] [Google Scholar]
- Clifford KM, Samboju V, Cobigo Y, Milanini B, Marx GA, Hellmuth JM, Rosen HJ, Kramer JH, Allen IE and Valcour VG (2017). Progressive Brain Atrophy Despite Persistent Viral Suppression in HIV Patients Older Than 60 Years. J Acquir Immune Defic Syndr, 76(3): 289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RA, Harezlak J, Schifitto G, Hana G, Clark U, Gongvatana A, Paul R, Taylor M, Thompson P, Alger J, Brown M, Zhong J, Campbell T, Singer E, Daar E, McMahon D, Tso Y, Yiannoutsos CT and Navia B (2010). Effects of nadir CD4 count and duration of human immunodeficiency virus infection on brain volumes in the highly active antiretroviral therapy era. J Neurovirol, 16(1): 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RA, Seider TR and Navia B (2015). HIV effects on age-associated neurocognitive dysfunction: premature cognitive aging or neurodegenerative disease? Alzheimer’s Research & Therapy, 7(1): 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa DG, Zimmermann N, Tukamoto G, Doring T, Ventura N, Leite SC, Cabral RF, Fonseca RP, Bahia PR and Gasparetto EL (2016). Longitudinal assessment of subcortical gray matter volume, cortical thickness, and white matter integrity in HIV-positive patients. J Magn Reson Imaging, 44(5): 1262–1269. [DOI] [PubMed] [Google Scholar]
- Cysique LA, Moffat K, Moore DM, Lane TA, Davies NWS, Carr A, Brew BJ and Rae C (2013). HIV, Vascular and Aging Injuries in the Brain of Clinically Stable HIV-Infected Adults: A (1)H MRS Study. PLoS ONE, 8(4): e61738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks SG, Tracy R and Douek DC (2013). Systemic effects of inflammation on health during chronic HIV infection. Immunity, 39(4): 633–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Lin H, Shen W, Wu Q, Gao M and He N (2017). Interaction Effects between HIV and Aging on Selective Neurocognitive Impairment. J Neuroimmune Pharmacol, 12(4): 661–669. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Badiee J, Vaida F, Letendre S, Heaton RK, Clifford D, Collier AC, Gelman B, McArthur J, Morgello S, McCutchan JA, Grant I and Group C (2011). CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS, 25(14): 1747–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everall I, Barnes H, Spargo E and Lantos P (1995). Assessment of neuronal density in the putamen in human immunodeficiency virus (HIV) infection. Application of stereology and spatial analysis of quadrats. J Neurovirol, 1(1): 126–129. [DOI] [PubMed] [Google Scholar]
- Everall I, Vaida F, Khanlou N, Lazzaretto D, Achim C, Letendre S, Moore D, Ellis R, Cherner M, Gelman B, Morgello S, Singer E, Grant I, Masliah E and National Neuro ATC (2009). Cliniconeuropathologic correlates of human immunodeficiency virus in the era of antiretroviral therapy. Journal of neurovirology, 15(5–6): 360–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B and Dale AM (2004). Automatically parcellating the human cerebral cortex. Cereb Cortex, 14(1): 11–22. [DOI] [PubMed] [Google Scholar]
- Gelman BB, Lisinicchia JG, Morgello S, Masliah E, Commins D, Achim CL, Fox HS, Kolson DL, Grant I, Singer E, Yiannoutsos CT, Sherman S, Gensler G, Moore DJ, Chen T and Soukup VM (2013). Neurovirological correlation with HIV-associated neurocognitive disorders and encephalitis in a HAART-era cohort. J Acquir Immune Defic Syndr, 62(5): 487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gongvatana A, Harezlak J, Buchthal S, Daar E, Schifitto G, Campbell T, Taylor M, Singer E, Algers J, Zhong J, Brown M, McMahon D, So YT, Mi D, Heaton R, Robertson K, Yiannoutsos C, Cohen RA, Navia B and Consortium HIVN (2013). Progressive cerebral injury in the setting of chronic HIV infection and antiretroviral therapy. J Neurovirol, 19(3): 209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant I, Franklin DR Jr., Deutsch R, Woods SP, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Collier AC, Marra CM, Clifford DB, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Smith DM, Heaton RK and Group C (2014). Asymptomatic HIV-associated neurocognitive impairment increases risk for symptomatic decline. Neurology, 82(23): 2055–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harezlak J, Buchthal S, Taylor M, Schifitto G, Zhong J, Daar E, Alger J, Singer E, Campbell T, Yiannoutsos C, Cohen R, Navia B and Consortium HIVN (2011). Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. AIDS, 25(5): 625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harezlak J, Cohen R, Gongvatana A, Taylor M, Buchthal S, Schifitto G, Zhong J, Daar ES, Alger JR, Brown M, Singer EJ, Campbell TB, McMahon D, So YT, Yiannoutsos CT, Navia BA and Consortium HIVN (2014). Predictors of CNS injury as measured by proton magnetic resonance spectroscopy in the setting of chronic HIV infection and CART. J Neurovirol, 20(3): 294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaps JM, Sithinamsuwan P, Paul R, Lerdlum S, Pothisri M, Clifford D, Tipsuk S, Catella S, Busovaca E, Fletcher JL, Raudabaugh B, Ratto-Kim S, Valcour V, Ananworanich J and groups Ss (2015). Association between brain volumes and HAND in cART-naive HIV+ individuals from Thailand. J Neurovirol, 21(2): 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, Group C and Group H (2011). HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol, 17(1): 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR Jr., Deutsch R, Letendre S, Ellis RJ, Casaletto K, Marquine MJ, Woods SP, Vaida F, Atkinson JH, Marcotte TD, McCutchan JA, Collier AC, Marra CM, Clifford DB, Gelman BB, Sacktor N, Morgello S, Simpson DM, Abramson I, Gamst AC, Fennema-Notestine C, Smith DM, Grant I and Group C (2015). Neurocognitive change in the era of HIV combination antiretroviral therapy: the longitudinal CHARTER study. Clin Infect Dis, 60(3): 473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinen R, Bouvy WH, Mendrik AM, Viergever MA, Biessels GJ and de Bresser J (2016). Robustness of Automated Methods for Brain Volume Measurements across Different MRI Field Strengths. PLoS One, 11(10): e0165719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkin CH, van Gorp WG, Mandelkern MA, Gee M, Satz P, Holston S, Marcotte TD, Evans G, Paz DH, Ropchan JR and et al. (1995). Cerebral metabolic change in patients with AIDS: report of a six-month follow-up using positron-emission tomography. J Neuropsychiatry Clin Neurosci, 7(2): 180–187. [DOI] [PubMed] [Google Scholar]
- Holt JL, Kraft-Terry SD and Chang L (2012). Neuroimaging studies of the aging HIV-1-infected brain. J Neurovirol, 18(4): 291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua X, Boyle CP, Harezlak J, Tate DF, Yiannoutsos CT, Cohen R, Schifitto G, Gongvatana A, Zhong J, Zhu T, Taylor MJ, Campbell TB, Daar ES, Alger JR, Singer E, Buchthal S, Toga AW, Navia B, Thompson PM and Consortium HIVN (2013a). Disrupted cerebral metabolite levels and lower nadir CD4 + counts are linked to brain volume deficits in 210 HIV-infected patients on stable treatment. Neuroimage Clin, 3: 132–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua X, Ching CR, Mezher A, Gutman BA, Hibar DP, Bhatt P, Leow AD, Jack CR Jr., Bernstein MA, Weiner MW, Thompson PM and Alzheimer’s Disease Neuroimaging I (2016). MRI-based brain atrophy rates in ADNI phase 2: acceleration and enrichment considerations for clinical trials. Neurobiol Aging, 37: 26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua X, Hibar DP, Ching CRK, Boyle CP, Rajagopalan P, Gutman BA, Leow AD, Toga AW, Jack CR Jr, Harvey D, Weiner MW and Thompson PM (2013b). Unbiased tensor-based morphometry: Improved robustness and sample size estimates for Alzheimer’s disease clinical trials. NeuroImage, 66: 648–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua X, Leow AD, Levitt JG, Caplan R, Thompson PM and Toga AW (2009). Detecting brain growth patterns in normal children using tensor-based morphometry. Human Brain Mapping, 30(1): 209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua X, Thompson PM, Leow AD, Madsen SK, Caplan R, Alger JR, O’Neill J, Joshi K, Smalley SL, Toga AW and Levitt JG (2013c). Brain growth rate abnormalities visualized in adolescents with autism. Human Brain Mapping, 34(2): 425–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Taylor MJ, Theilmann RJ, Julaton MD, Notestine RJ, Wolfson T, Letendre SL, Ellis RJ, Heaton RK, Gamst AC, Franklin DR Jr., Clifford DB, Collier AC, Gelman BB, Marra C, McArthur JC, McCutchan JA, Morgello S, Simpson DM, Grant I and Group C (2011). Clinical factors related to brain structure in HIV: the CHARTER study. J Neurovirol, 17(3): 248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Han X, Salat D, van der Kouwe A, Quinn B, Pacheco J, Albert M, Killiany R, Blacker D, Maguire P, Rosas D, Makris N, Gollub R, Dale A, Dickerson BC and Fischl B (2009). MRI-derived measurements of human subcortical, ventricular and intracranial brain volumes: Reliability effects of scan sessions, acquisition sequences, data analyses, scanner upgrade, scanner vendors and field strengths. Neuroimage, 46(1): 177–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn T, Schonfeld D, Sayegh P, Arentoft A, Jones JD, Hinkin CH, Bookheimer SY and Thames AD (2017). The effects of HIV and aging on subcortical shape alterations: A 3D morphometric study. Hum Brain Mapp, 38(2): 1025–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers SL, Rose R, Maidji E, Agsalda-Garcia M, Nolan DJ, Fogel GB, Salemi M, Garcia DL, Bracci P, Yong W, Commins D, Said J, Khanlou N, Hinkin CH, Sueiras MV, Mathisen G, Donovan S, Shiramizu B, Stoddart CA, McGrath MS and Singer EJ (2016). HIV DNA Is Frequently Present within Pathologic Tissues Evaluated at Autopsy from Combined Antiretroviral Therapy-Treated Patients with Undetectable Viral Loads. J Virol, 90(20): 8968–8983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langers DR, Jansen JF and Backes WH (2007). Enhanced signal detection in neuroimaging by means of regional control of the global false discovery rate. Neuroimage, 38(1): 43–56. [DOI] [PubMed] [Google Scholar]
- Leow AD, Yanovsky I, Chiang MC, Lee AD, Klunder AD, Lu A, Becker JT, Davis SW, Toga AW and Thompson PM (2007). Statistical properties of Jacobian maps and the realization of unbiased large-deformation nonlinear image registration. IEEE Trans Med Imaging, 26(6): 822–832. [DOI] [PubMed] [Google Scholar]
- Letendre S, Ellis RJ, Best B, Bhatt A, Marquie-Beck J, LeBlanc S, Rossi S, Capparelli E and McCutchan A (2009). Penetration and Effectiveness of Antiretroviral Therapy in the Central Nervous System. Anti-Inflammatory & Anti-Allergy Agents in Medicinal Chemistry, 8(2): 169–183. [Google Scholar]
- Lysandropoulos AP, Absil J, Metens T, Mavroudakis N, Guisset F, Van Vlierberghe E, Smeets D, David P, Maertens A and Van Hecke W (2016). Quantifying brain volumes for Multiple Sclerosis patients follow-up in clinical practice - comparison of 1.5 and 3 Tesla magnetic resonance imaging. Brain Behav, 6(2): e00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek K, Jennings D, Lasch S, Siderowf A, Tanner C, Simuni T, Coffey C, Kieburtz K, Flagg E, Chowdhury S, Poewe W, Mollenhauer B, Klinik P-E, Sherer T, Frasier M, Meunier C, Rudolph A, Casaceli C, Seibyl J, Mendick S, Schuff N, Zhang Y, Toga A, Crawford K, Ansbach A, De Blasio P, Piovella M, Trojanowski J, Shaw L, Singleton A, Hawkins K, Eberling J, Brooks D, Russell D, Leary L, Factor S, Sommerfeld B, Hogarth P, Pighetti E, Williams K, Standaert D, Guthrie S, Hauser R, Delgado H, Jankovic J, Hunter C, Stern M, Tran B, Leverenz J, Baca M, Frank S, Thomas C-A, Richard I, Deeley C, Rees L, Sprenger F, Lang E, Shill H, Obradov S, Fernandez H, Winters A, Berg D, Gauss K, Galasko D, Fontaine D, Mari Z, Gerstenhaber M, Brooks D, Malloy S, Barone P, Longo K, Comery T, Ravina B, Grachev I, Gallagher K, Collins M, Widnell KL, Ostrowizki S, Fontoura P, Ho T, Luthman J, Brug Mvd, Reith AD and Taylor P (2011). The Parkinson Progression Marker Initiative (PPMI). Progress in Neurobiology, 95(4): 629–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur RA (2012). Translational Neuroimaging: Tools for CNS Drug Discovery, Development and Treatment, Elsevier Science. [Google Scholar]
- Morgello S (2018). Chapter 2 - HIV neuropathology. Handbook of Clinical Neurology. B. J. Brew, Elsevier; 152: 3–19. [DOI] [PubMed] [Google Scholar]
- Nakagawa F, May M and Phillips A (2013). Life expectancy living with HIV: recent estimates and future implications. Curr Opin Infect Dis, 26(1): 17–25. [DOI] [PubMed] [Google Scholar]
- Navia B, Harezlak J, Schifitto G, Taylor MJ, Heaton RK, Robertson K, Daar ES, Campbell T, Singer E and Buchthal S (In Review). Predictors of HIV-associated Cognitive Decline in the Era of Combined Antiretroviral Therapy. Social Neuroscience and Health
- Navia BA, Cho ES, Petito CK and Price RW (1986a). The AIDS dementia complex: II. Neuropathology. Ann Neurol, 19(6): 525–535. [DOI] [PubMed] [Google Scholar]
- Navia BA, Jordan BD and Price RW (1986b). The AIDS dementia complex: I. Clinical features. Ann Neurol, 19(6): 517–524. [DOI] [PubMed] [Google Scholar]
- Neuen-Jacob E, Arendt G, Wendtland B, Jacob B, Schneeweis M and Wechsler W (1993). Frequency and topographical distribution of CD68-positive macrophages and HIV-1 core proteins in HIV-associated brain lesions. Clin Neuropathol, 12(6): 315–324. [PubMed] [Google Scholar]
- Nir TM, Jahanshad N, Busovaca E, Wendelken L, Nicolas K, Thompson PM and Valcour VG (2014). Mapping white matter integrity in elderly people with HIV. Hum Brain Mapp, 35(3): 975–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Rogosa DA, Rosenbloom MJ, Chu W, Sassoon SA, Kemper CA, Deresinski S, Rohlfing T, Zahr NM and Sullivan EV (2014). Accelerated aging of selective brain structures in human immunodeficiency virus infection: a controlled, longitudinal magnetic resonance imaging study. Neurobiol Aging, 35(7): 1755–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RW and Brew BJ (1988). The AIDS dementia complex. J Infect Dis, 158(5): 1079–1083. [DOI] [PubMed] [Google Scholar]
- Reiman EM and Jagust WJ (2012). Brain imaging in the study of Alzheimer’s disease. NeuroImage, 61(2): 505–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson KR, Smurzynski M, Parsons TD, Wu K, Bosch RJ, Wu J, McArthur JC, Collier AC, Evans SR and Ellis RJ (2007). The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS, 21(14): 1915–1921. [DOI] [PubMed] [Google Scholar]
- Rottenberg DA, Moeller JR, Strother SC, Sidtis JJ, Navia BA, Dhawan V, Ginos JZ and Price RW (1987). The metabolic pathology of the AIDS dementia complex. Ann Neurol, 22(6): 700–706. [DOI] [PubMed] [Google Scholar]
- Sacktor N, Skolasky RL, Seaberg E, Munro C, Becker JT, Martin E, Ragin A, Levine A and Miller E (2016). Prevalence of HIV-associated neurocognitive disorders in the Multicenter AIDS Cohort Study. Neurology, 86(4): 334–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford R, Fellows LK, Ances BM and Collins DL (2018). Association of Brain Structure Changes and Cognitive Function With Combination Antiretroviral Therapy in HIV-Positive Individuals. JAMA Neurol, 75(1): 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate DF, Sampat M, Harezlak J, Fiecas M, Hogan J, Dewey J, McCaffrey D, Branson D, Russell T, Conley J, Taylor M, Schifitto G, Zhong J, Daar ES, Alger J, Brown M, Singer E, Campbell T, McMahon D, Tso Y, Matesan J, Letendre S, Paulose S, Gaugh M, Tripoli C, Yiannoutsos C, Bigler ED, Cohen RA, Guttmann CR, Navia B and Consortium HIVN (2011). Regional areas and widths of the midsagittal corpus callosum among HIV-infected patients on stable antiretroviral therapies. J Neurovirol, 17(4): 368–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker KA, Robertson KR, Lin W, Smith JK, An H, Chen Y, Aylward SR and Hall CD (2004). Neuroimaging in human immunodeficiency virus infection. J Neuroimmunol, 157(1–2): 153–162. [DOI] [PubMed] [Google Scholar]
- Veitch DP, Weiner MW, Aisen PS, Beckett LA, Cairns NJ, Green RC, Harvey D, Jack CR Jr., Jagust W, Morris JC, Petersen RC, Saykin AJ, Shaw LM, Toga AW, Trojanowski JQ and Alzheimer’s Disease Neuroimaging I (2018). Understanding disease progression and improving Alzheimer’s disease clinical trials: Recent highlights from the Alzheimer’s Disease Neuroimaging Initiative. Alzheimers Dement [DOI] [PubMed]
- von Giesen HJ, Antke C, Hefter H, Wenserski F, Seitz RJ and Arendt G (2000). Potential time course of human immunodeficiency virus type 1-associated minor motor deficits: electrophysiologic and positron emission tomography findings. Arch Neurol, 57(11): 1601–1607. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2015) Guideline on when to start ART and on PrEP for HIV from http://apps.who.int/iris/bitstream/10665/186275/1/9789241509565_eng.pdf?ua=1.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


