Abstract
Human immunodeficiency virus-1 (HIV) infection of the central nervous system damages synapses and promotes axonal injury, ultimately resulting in HIV-associated neurocognitive disorders (HAND). The mechanisms through which HIV causes damage to neurons are still under investigation. The cytoskeleton and associated proteins are fundamental for axonal and dendritic integrity. In this article, we review evidence that HIV proteins, such as the envelope protein gp120 and Transactivator of transcription (Tat), impair the structure and function of the neuronal cytoskeleton. Investigation into the effects of viral proteins on the neuronal cytoskeleton may provide a better understanding of HIV neurotoxicity and suggest new avenues for additional therapies.
Keywords: BDNF, gp120, neurodegeneration, actin, Tat, microtubules
Introduction
Human immunodeficiency virus (HIV), which depletes the cells of the immune system, also infects the central nervous system (CNS) and promotes direct neurotoxic pathology and neurocognitive impairments termed HIV-associated neurocognitive disorders (HAND). Symptoms of HAND can range in severity from asymptomatic neurocognitive impairment (ANI), to minor neurocognitive disorder (MND) and HIV-associated dementia (HAD) (Tan and McArthur, 2011). The HIV-positive population continues to age as combined antiretroviral therapy (cART) successfully reduces HIV-mediated immune dysfunction (Sandler and Sereti, 2014; Crowell et al., 2016). However, even with suppression of viral load in the periphery, this population still demonstrates cognitive impairments and memory loss (Tozzi et al., 2007; Heaton et al., 2010; Gates et al., 2016). Within this aging population (Mahy et al., 2014), the prevalence of cognitive alterations is increasing and over 50% of HIV-positive patients will develop neurocognitive symptoms (Sacktor et al., 2002; Clifford and Ances, 2013). HIV-positive individuals are seven-times more likely to experience a mild cognitive impairment than their age-matched HIV-negative counterparts, even when adhering to cART and having undetectable viral load in the periphery (Sheppard et al., 2015). In addition, there is evidence that the CNS serves as a reservoir for HIV replication (Fois and Brew, 2015), thereby limiting the prospect of complete viral eradication.
Before cART, the pathology of HAND included robust neuronal apoptosis, with multinucleated giant cells and astrogliosis in cortical and subcortical areas (Adle-Biassette et al., 1995; Petito and Roberts, 1995; Kaul et al., 2001). Clinically, these patients exhibited motor and cognitive impairments (Price et al., 1988; Heaton et al., 2011). However, with the introduction of cART, HAND pathology has shifted considerably. In fact, there is a reduction in widespread brain atrophy, which was commonly observed in the pre-cART era (Gongvatana et al., 2009). Notably, these patients lack robust neuronal cell loss but the gray matter of cART-treated HIV subjects contain neurons with damaged synapses and axons, as well as short dendrites, a neuropathological condition referred to as synaptic simplification, or “synaptopathy” (Masliah et al., 1997; Ellis et al., 2007). This neuropathological presentation may be reflected by the decrease in prevalence of the most severe category of HAND, HAD, which has declined from about 15–20% to 2–8% of the HIV-positive population, and increases in the two less-severe categories, MND and ANI, from 25–30% to 50–60% (Heaton et al., 2010; Saylor et al., 2016). Nevertheless, HAND subjects still exhibit thinning of several regions including the cerebral cortex (Nichols et al., 2018) as well as the subcortical regions of hippocampus and basal ganglia (McArthur, 2004; Alakkas et al., 2018).
The underlying causes of synaptic simplification observed in these subjects remain under investigation. These pathological features can be seen in the HAND population, even though HIV cannot productively infect neurons (Kanmogne et al., 2000). One of many challenges facing the neuroAIDS field is the integration of mechanisms of neuronal loss due to secondary or indirect effects, such as inflammation, with those pertaining to the direct neurotoxicity of viral proteins. A further understanding of the core pathophysiological mechanisms for HAND will eventually assist in the development of additional therapies. It is important to note that HIV encephalitis (HIVE), a neuro-inflammatory condition characterized by the presence of HIV infected microglial cells, microglial nodules, multinucleated giant cells, astrogliosis and myelin loss (Wiley and Achim, 1994; Anderson et al., 2002; Everall et al., 2005), does not entirely explain the mechanism of synapto-dendritic loss. Indeed, current cART, which prolongs the life span of HIV-positive subjects, may reduce HIVE but does not prevent the activation of microglia (Ginsberg et al., 2018; Rubin et al., 2018) or HAND (Gelman, 2015). Therefore, many recent studies have focused on the neurotoxic effects coordinated by HIV proteins, and their molecular and cellular mechanisms that lead to direct neuronal loss (Kanmogne et al., 2002; Nath, 2002). As the basic mechanisms activated by HIV proteins become better understood, novel strategies can be devised to prevent their neurotoxicity.
In this review, we summarize evidence indicating a mechanism of direct neurotoxicity by HIV proteins via alterations in the neuronal cytoskeleton structure and function. Because the cytoskeleton is important for determining the mechanical properties of synapses, impairments in the cytoskeleton can easily explain the synaptopathy seen in HAND. Thus, in addition to therapeutics aimed at reducing viral load, alternative drug therapies that reduce synaptopathy should be developed and applied.
Neuronal cytoskeleton and neurodegenerative diseases
The ability of neurons to receive and transmit signals and impulses depends on their polarized organization. The neuronal cytoskeleton is crucial for the establishment and maintenance of spatial organization and architectural support of axons and dendrites. In addition, the cytoskeleton is crucial to preserve the functionality of intracellular transport and dendritic spines. The neuronal cytoskeleton is made of microtubules, actin, neurofilaments, and their associated proteins.
Microtubules
Microtubules (MTs) are dynamic structures consisting of α- and β-tubulin heterodimers (Nogales et al., 1998), that have several functions within a cell, including formation of mitotic spindles for cell division. In neurons, MTs are found both in axons and dendrites and are primarily responsible for long-distance intracellular transport of cargo. As post-mitotic cells, neurons require localized, targeted movement of cargo between the cell soma and distal dendritic and axonal segments. MTs are intracellular highways and are assembled in a head-to-tail manner to form highly polarized filaments upon which cargo is directed in a controlled fashion. Impairments in the highly-regulated and dynamic process of MT growth and shrinkage inhibit the trafficking of essential organelles, including mitochondria and cargo-containing vesicles (Cartelli et al., 2010). Impairments in neuronal MTs and therefore the trafficking of cargo are detrimental to neuronal health (McMurray, 2000; Cartelli et al., 2010; Baird and Bennett, 2013). For example, neurons with inefficient MT-based transport of neurotrophic factors, which are essential for preserving the integrity of neurons, causes degeneration of dendrites and axons (Mariga et al., 2017). Thus, interruption of MT-based transport has been suggested to play a role in the development of neurodegenerative diseases including Huntington’s (HD) and Alzheimer’s diseases (AD) (Millecamps and Julien, 2013). As HIV-positive subjects age, there is much to learn by examining the mechanisms discovered for other diseases of aging to fully characterize similarities and differences between HAND and other neurodegenerative diseases.
Stability of MT
MTs are dynamic structures and undergo cycles of tubulin polymerization and depolymerization, which alter their functionality and length. This directed, dynamic instability is crucial for MT function and is regulated by interactions with other intracellular factors. These include MT-associated motor proteins of the kinesin (Verhey and Hammond, 2009) and dynein (Vallee et al., 2004) families, as well as non-motor MT associated proteins (MAPs), such as tau and MAP2.
The assembly of tubulin dimers into fully functional MTs and the properties of these formed MTs are also modified by post-translational modifications (PTMs) such as phosphorylation, acetylation, tyrosination, and others (Westermann and Weber, 2003). For instance, acetylation occurs after MT assembly, promotes stabilization, and consequently neuronal survival. Acetylation of tubulin is enriched in the axon when compared to dendrites (Fig. 1) and contributes to the successful transport of cargo along axons (Cambray-Deakin and Burgoyne, 1987). In fact, deacetylation of tubulin following activation of histone deacetylase (HDAC)-6 (Hubbert et al., 2002) reduces axonal regeneration and inhibition of this enzyme protects neurons after injury (Rivieccio et al., 2009). Tubulin detyrosination affects MT interactions with intermediate filaments, another component of the cytoskeleton. In addition, tyrosination, polyglutamination, and other PTMs affect the binding of motor and non-motor proteins to MTs (Song and Brady, 2015). Thus, PTMs on fully formed MTs modify many aspects of neuronal function.
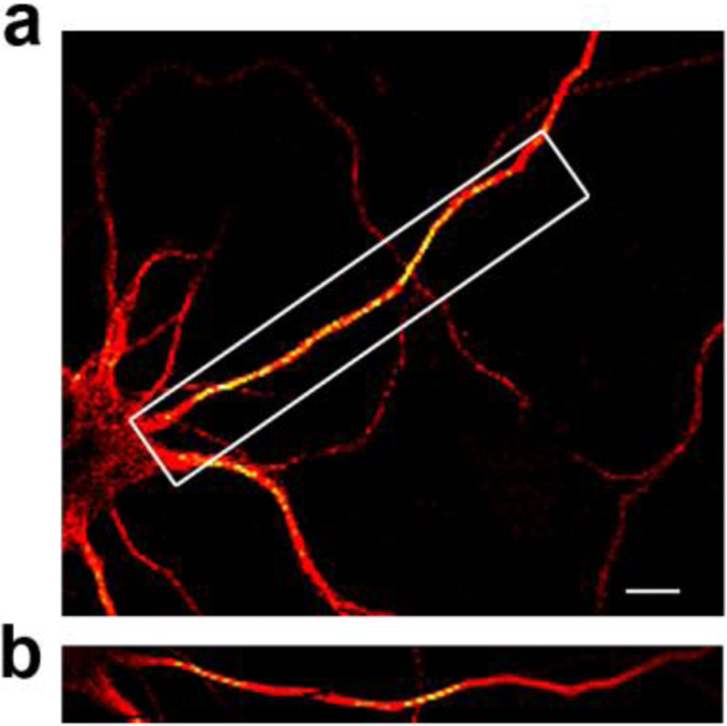
Figure 1. Acetylated tubulin is enriched in the axon.
a) Primary rat cortical neurons (7 days in vitro) were fixed, stained for acetylated tubulin (red), and imaged using a STEDYCON microscope (Abberior Instruments). Yellow, a coloring construct from the imaging system, indicates the enrichment of acetylated tubulin. b) Enlarged image of box in (a) showing that acetylated tubulin is enriched in the axon when compared to the rest of the cell. Scale bar: 5nm.
Non-motor proteins known to regulate MT stability and influence molecular motor transport include tau (Hirokawa et al., 1988), MAP2 (Lewis et al., 1988), and MAP4 (Olson et al., 1995). Within the CNS, tau and MAP2 are exclusively found in neurons (Dehmelt and Halpain, 2005). Tau, the main constituent of the neurofibrillary tangles observed in AD, is present mainly in axons, whereas MAP2 segregates in dendrites and cell bodies (Dehmelt and Halpain, 2005). Tau stabilizes axonal MTs (Binder et al., 1985) and, when phosphorylated, inhibits kinesin-1 motility (Dixit et al., 2008; Stern et al., 2017) most likely by disrupting MTs (Drewes et al., 1997). MAP2 binding to tubulin also favors stability of MTs, however, MAP2 phosphorylation may also impair MAP2 function and consequently, its ability to stabilize MTs (Sanchez et al., 2000). The emerging picture is indicates a complex, tightly-regulated process of MT dynamics that, when pathologically targeted, promotes neurodegeneration.
Actin
Actin is a highly conserved and abundant cytoskeleton protein that in neurons plays a role in axonal elongation, the formation and maintenance of growth cones, presynaptic terminals, dendritic spines, and synaptic homeostasis (Coles and Bradke, 2015). Actin exists as soluble monomers (G-actin) as well as actin filaments (F-actin). F-actin, together with MTs, is a key mediator of neuronal polarity and formation of dendritic structures (Pacheco and Gallo, 2016). F-actin is also an important component of dendritic spines, essential protrusions along dendrites that harbor synapses. In addition, F-actin forms patches, longitudinal fibers that traverse the lengths of dendrites (D’Este et al., 2015), and rings (Konietzny et al., 2017). The dynamics of F-actin, including stability and expression levels, is enhanced by long-term potentiation and impaired by long-term depression (Fukazawa et al., 2003; Szabo et al., 2016). Overall, actin plays a role in support of neurite shape, stabilization of the cytoskeleton (Qu et al., 2017), and synaptic plasticity (Bar et al., 2016). Because of these roles, accumulation of actin or alterations to the actin cytoskeleton have been shown to induce neurotoxicity that results in various neurological diseases such as Parkinson’s disease (PD) (Ordonez et al., 2018) and AD (Fulga et al., 2007; Bamburg et al., 2010).
Neurofilaments
Neurofilaments (NFs) are intermediate filament cytoskeleton proteins that interact with MTs in axons, provide structural support, maintain axon caliber, and affect the transmission of electrical impulses along axons (Yuan et al., 2012). NFs consist of several components that differ in molecular size: light (70–86kDa), medium/intermediate (145–160kDa) and heavy (200–220kDa) chains, as well as α-internexin (58–66kDa) (Liem and Messing, 2009; Khalil et al., 2018). Because of their abundance and the exclusive expression of NFs in neurons, cerebrospinal fluid (CSF) or blood serum levels of NF-light chain (NF-L) has been used as a biomarker to detect axonal damage/degeneration in acute and chronic neurological disorders (Khalil et al., 2018).
In HAND subjects, levels of NF-L have been used to predict neuronal injury. In fact, high levels of NF-L are found in both plasma and CSF plasma when compared to healthy controls. Importantly, NF-L levels correlate with higher viral loads (Gisslen et al., 2016) and neurocognitive impairments, and are reduced in subjects under cART (Jessen Krut et al., 2014). In addition, elevated NF-L levels in the CNS has been found in some patients up to two years before onset of HAD (Gisslen et al., 2007), suggesting that HIV promotes axonal injury even in the mildest form of HAND.
This loss of NF-L in neurons can be recapitulated in vitro. Prolonged (21 days) exposure of “neurospheres” derived from human neuroepithelial progenitor cells to either HIV or its envelope protein, gp120, reduces the expression of neuron-specific NF-L, without altering the expression of astrocytic intermediate filament protein, glial fibrillary acidic protein (GFAP) (McCarthy et al., 2006). Based on the specific roles of NFs in axons, the loss of NFs could explain the pathology of axonal degeneration seen in HAND subjects. However, whether the loss of NFs is a consequence or the cause of neurodegeneration observed in HAND subjects is not well established.
HIV proteins and Neurodegeneration
HIV proteins and neuronal MTs
In immune cells, the host cell’s cytoskeleton is crucial for the HIV life cycle. The virus uses its proteins and a variety of strategies to manipulate the cytoskeleton to enhance entry and stabilize the transport of genetic material to the cell nucleus. Manipulation of host cell’s cytoskeleton improves replication and infection, and ultimately the release of new viral particles (Ospina Stella and Turville, 2018). Contrastingly, in the CNS, HIV cannot infect neurons and instead utilizes its viral proteins to destabilize and damage neurons. Soluble viral proteins that induce neurodegeneration include the transactivator of transcription, Tat, and the envelope protein gp120. These proteins are produced in the brain of HIV-positive individuals (Keys et al., 1993; Di Stefano et al., 1996; Bachani et al., 2013; Johnson and Nath, 2016) and are released in the extracellular space from HIV-infected cells. Both proteins undergo uptake by neurons and can travel along axons to cause injury at distal regions (Bruce-Keller et al., 2003; Bachis et al., 2006). The degeneration caused by these proteins in animal models is similar to that observed in HAND. For instance, both Tat and gp120 have been shown to be neurotoxic to various neuronal populations in vivo (Sabatier et al., 1991; Bansal et al., 2000; Maragos et al., 2003; Acquas et al., 2004). Transgenic mice in which Tat can be inducibly expressed under the GFAP promoter show evidence of learning and memory deficits (Carey et al., 2012), associated thinning of the cortex (Carey et al., 2013), and synaptodendritic injury (Fitting et al., 2013). Likewise, transgenic mice expressing the envelope protein gp120 under the GFAP promoter exhibit neuronal loss and dendritic simplification (Toggas et al., 1994), altered long-term potentiation in the hippocampus (Krucker et al., 1998), reduced neurogenesis (Lee et al., 2013), and loss of dendritic spines in the hippocampus (Bachis et al., 2016). Importantly, the neurotoxic effects of viral proteins in animal models are also seen in HAND and the pathological features demonstrate good correlation with the severity of neurocognitive decline. These features include synaptodendritic injury (Masliah et al., 1992), mitochondrial damage (Haughey and Mattson, 2002; Langford et al., 2004; Fields et al., 2016) and loss of neurotrophic factors (Bachis et al., 2012). Thus, Tat and gp120 animals have gained increased attention as experimental models to study mechanisms of neurotoxicity and possible treatment targets.
More recently, a new line of investigation has proposed that Tat and gp120 are neurotoxic after their endocytosis into neurons. Tat enters neurons in a receptor-independent manner (Liu et al., 2000) and can bind to MTs to induce neuronal damage (Chen et al., 2002; Aprea et al., 2006) as demonstrated by the decreased expression and altered distribution of in MAP2 positive processes (Langford et al., 2018). Unlike Tat, gp120 is internalized by neurons primarily in a chemokine receptor/clathrin-dependent manner (Wenzel et al., 2017). Once endocytosed, gp120 is axonally transported both in vitro and in vivo (Bachis et al., 2006; Melli et al., 2006; Ahmed et al., 2009; Berth et al., 2015) to adjacent neurons. The axonal transport process requires gp120 to bind to MTs by forming a vesicular complex with mannose binding lectin (Teodorof et al., 2014), a carrier that facilitates glycoprotein trafficking (Nonaka et al., 2007). Alternatively, once inside neurons, gp120 can bind to the neuronal specific beta III tubulin (Avdoshina et al., 2016a). Intriguingly, this binding occurs through a conserved helix domain rather than the hypervariable region 3 (V3) of gp120, which is responsible for the phenotypic diversity of HIV. The direct interaction of Tat or gp120 with MTs impairs the formation/polymerization of MTs (Butler et al., 2011) and gp120 decreases the acetylation of tubulin (Avdoshina et al., 2017). Deacetylated MTs have a lower affinity for the motor proteins of kinesin-1 and dynein (Reed et al., 2006); thus, gp120 or Tat may impair MT-based, axonal transport by altering MAPs.
Viral proteins exhibit a direct effect on MTs, nevertheless, we cannot exclude that some of the neurotoxic effects of these proteins encompass other mechanisms. For instance, gp120 could also alter the neuronal cytoskeleton through signaling of chemokine co-receptors CXCR4 or CCR5. These receptors, which are expressed by several neuronal populations in vivo (Klein et al., 1999; Stumm et al., 2003; Maung et al., 2014), promote phosphorylation and inactivation of glycogen synthase kinase-3 beta (GSK3β) (Chalasani et al., 2003), a signaling molecule involved with MT assembly in axons (Zhou and Snider, 2005). Although controversy exists whether inactivation of GSK3β is beneficial or detrimental to neurons, it certainly alters MT dynamics and stability (Conde and Caceres, 2009). Thus, chemokine co-receptor signaling may also contribute to alterations of the neuronal cytoskeleton caused by viral proteins.
MT stability and axonal transport
There are several cellular events that could explain how binding of gp120 and Tat to MTs induces neurodegeneration. These include impaired axonal transport through altered MT stability, changes in neuronal morphology, and possibly decreased expression of NFs and axonal diameter (Hoffman et al., 1987).
One of the immediate consequences of viral protein impairment of MT structure and function may be the decreased axonal transport of mitochondria. These essential organelles are highly dynamic and control high-energy intermediates, including adenosine triphosphate (ATP). Neuronal function depends on ATP because these cells have a high energy demand and require ATP at distal areas including axonal and dendritic synapses (Dickey and Strack, 2011; Merrill et al., 2011; Berthet et al., 2014). Moreover, mitochondria play a role in spine maturation and neuronal survival. They regulate Ca2+ homeostasis, thus affecting neurotransmission and synaptic plasticity (Levy et al., 2003), as well as reduce the production of reactive oxygen species, which is crucial for neuronal survival (Gleichmann and Mattson, 2011). In order to maintain energy homeostasis and to continue essential activities, neurons must precisely establish an adequate distribution of mitochondria to axons and dendrites through the process of fusion and fission (Westermann, 2010; van der Bliek et al., 2013). Fusion is the process of exchanging mitochondrial DNA and other components, with the goal of repairing damaged mitochondria. The opposite process, fission, is required for mitochondrial transport. Both processes utilize various proteins such as mitofusins, and GTPase dynamin-related protein (DRP), located on the outer membrane of mitochondria, and optic atrophy protein 1, located in the inner membrane.
Mitochondrial fission and distribution are impaired in HAND brains (Fields et al., 2016). Further, the distribution and function of mitochondria is altered in many animal models of HAND including gp120 transgenic mice (Avdoshina et al., 2016b), Tat transgenic mice (Rozzi et al., 2018), and HIV transgenic rats (Villeneuve et al., 2016), which express gp120 and Tat as well as five other viral proteins. Gp120 brings mitochondrial movement to a halt (Avdoshina et al., 2016b) whereas Tat causes an accumulation of mitochondria at the cell soma (Rozzi et al., 2018). This suggests that HIV, though its viral proteins, alters the axonal transport of mitochondria. Further, those mitochondria that accumulate defective proteins or DNA must be either repaired by fusion with healthy mitochondria or cleared from the cell by selective process of mitophagy (Twig et al., 2008). For this processes, axonal transport is essential because mitochondria need to be retrogradely transported to the cell body by MTs to be repaired or degraded. Both gp120 and Tat impair mitophagy, creating an accumulation of damaged mitochondria in the cell (Teodorof-Diedrich and Spector, 2018). Furthermore, altered transport could interfere with the natural process of autophagy, which clears misfolded or foreign proteins from the cytoplasm. In neurons, autophagy is slightly different from that in other cells because autophagosomes are formed at the neurite tip and undergo dynein-mediated retrograde transport to their site of functionality, the soma (Maday et al., 2012). When this maturation is prevented, autophagy is impaired. This could be the mechanism through which HIV proteins Vpr (Dumas et al., 2015), Tat (Fields et al., 2015), and Nef (Kyei et al., 2009), interfere with autophagy and initiate neurodegeneration.
There are additional functional implications of impaired MT function that could explain how HIV proteins damage neurons. One is the loss of anterograde transport of neurotrophic factors, such as brain-derived neurotrophic factor (BDNF), and synaptic vesicle containing neurotransmitters. Neurons use these biomolecules at the presynaptic site to maintain synaptic function and plasticity. Likewise, neurotransmitter receptors earmarked for degradation or other biomolecules involved in retrograde signaling must be returned to the cell body by retrograde transport. Anterograde and retrograde transport involves kinesin-1 and dynein (Bhattacharyya et al., 2002; Chowdary et al., 2015; Sainath and Gallo, 2015). Thus, an effect on motor proteins could decrease neurotrophic support and change levels of neurotransmitters and their receptors. These alterations, which are suggested mechanisms for the atrophy of neurons in neurodegenerative diseases including HD and Amyotrophic lateral sclerosis (ALS) (Hirokawa et al., 2010; Hinckelmann et al., 2013; Millecamps and Julien, 2013), are now considered also to explain reduction in synaptic connectivity in HAND (Kumar et al., 2011; Fields et al., 2014; Mocchetti et al., 2014; Zhu et al., 2018). Nevertheless, neuronal atrophy induced by impaired BDNF axonal transport can be significantly limited by restoring BDNF transport (Zhao et al., 2016). Likewise, therapeutic compounds that increase neurotransmitter levels are neuroprotective against HIV-mediated neuronal injury (Schifitto et al., 2009; Steiner et al., 2015). Thus, drugs that can restore axonal transport of neurotrophic factors, cargo containing vesicles, and mitochondria have potential therapeutic significance for HAND.
Lastly, impairment in axonal transport, which is a direct mechanism of neurotoxicity by viral proteins, may be compounded with a pro-inflammatory environment. For instance, interleukin-1 β (IL-1β), a pro-inflammatory cytokine that is released from microglia or infiltrating monocytes in response to gp120 or Tat (Koka et al., 1995; Yang et al., 2010), decreases the retrograde transport of BDNF (Carlos et al., 2017). Additionally, after exposure to gp120 or Tat, astrocytes release reactive oxygen species. Impairment in axonal transport of mitochondria occurs quickly after exposure to exogenous H2O2, a mimetic for a redox-rich environment (Errea et al., 2015). Therefore the direct effects of viral proteins and a pro-inflammatory or redox-rich environment may be additive in impairing MT-based, axonal transport.
Viral proteins and dendritic spines
Investigation into how viral proteins regulate synapse loss through the cytoskeleton is crucial for the development of therapeutics to prevent this degeneration. In neurons, actin plays a large role in the formation of dendritic spines (Matus, 2000; Hotulainen and Hoogenraad, 2010). Dendritic spines are considered important postsynaptic contact sites for learning and memory. Consistent with this notion, suppression of actin dynamics inhibit long-term potentiation (LTP) (Krucker et al., 2000), which forms the basis for learning and memory. Dendritic spines are damaged and synaptic density is decreased in humans with HAND (Ellis et al., 2007) and in neurons exposed to gp120 or Tat in vitro (Viviani et al., 2006; Shin et al., 2012; Bertrand et al., 2014) or in vivo (Bachis et al., 2016; Raybuck et al., 2017). The decrease in dendritic spine density is a predictor of cognitive deficit (Yang et al., 2009). Complementary to these data, several investigators have established that Tat prevents LTP when injected intracerebroventricularly (icv) in mice (Li et al., 2004) or when applied to hippocampal slices (Behnisch et al., 2004). Similarly, HIV-derived gp120 impairs LTP both ex vivo (Dong and Xiong, 2006) and in rodents, including gp120 transgenic mice (Krucker et al., 1998) and rats injected icv with gp120 (Sanchez-Alavez et al., 2000). Although more studies are needed to reach a definitive conclusion about the mechanism whereby HIV reduces dendritic spines, a direct effect of viral proteins on the cytoskeleton dynamics could explain cognitive deficits seen in HAND.
Studies investigating other neurodegenerative diseases allow us to speculate on mechanisms that cause the alterations in dendritic spines in HAND. First, dysregulation of Rho-GTPase family signaling alters actin dynamics. This is seen in different brain areas of subjects with PD, HD, and AD (Eira et al., 2016). In T-cells, HIV, through its envelope protein binding to CD4 and chemokine co-receptors, activate Rho GTPases (Lucera et al., 2017); however, investigation into the effect of HIV proteins on this signaling in neurons is still in early stages. One of the canonical members of the Rho GTPase family that is activated by Tat is RhoA (Krogh et al., 2015). RhoA recruits its specific kinase (ROCK), which modulates MT dynamics and cell polarity, and remodeling of the actin cytoskeleton (Sit and Manser, 2011), leading to N-methyl-D-aspartate (NMDA) receptor dysfunction among other problems (Krogh et al., 2015). A defective NMDA receptor is particularly important in the context of HAND because Tat has been shown to induce neuronal apoptosis by binding to NMDA receptor and affecting its function (Haughey et al., 2001; Eugenin et al., 2007; Li et al., 2008). Activation of the NMDA receptor can change the dynamics of the cytoskeleton because it decreases the phosphorylation of MAP2 (Quinlan and Halpain, 1996). Phosphorylation of MAP2 is crucial for its association with MTs and their stability. Thus, the modulation of the phosphorylated state of MAP2 by NMDA-glutamate receptors may be implicated in dendritic plasticity. Furthermore, NMDA treatment induces ICAM-5 dependent alpha-actinin clustering (Tian et al., 2007), a cellular mechanism that could also affect the integrity of the actin cytoskeleton. Thus, Tat activation of NMDA might alter the properties of MAPs that could account for some of the negative effects of glutamate on postsynaptic neurons.
Additionally, this signaling cascade regulates actin-binding proteins (ABP), whose functions in the cytoplasm include actin cytoskeletal organization in repose to intracellular and extracellular stimuli (Rajakyla and Vartiainen, 2014). One key ABP is cofilin which coordinates the spatial organization of actin filament assembly/disassembly (Bamburg and Bernstein, 2010). When activity or the PTMs of cofilin is impaired, actin-based transport is inhibited resulting in protein aggregation and neuronal loss (Minamide et al., 2000). Although not yet examined in neurons, the activation of chemokine receptor signaling in HIV infected T-cells causes dephosphorylation of cofilin and decreased expression of F-actin (Cameron et al., 2010). Therefore, either chemokine receptor-mediated or Rho GTPase-mediated signaling may be a mechanism through which gp120 and Tat damage dendritic spines.
Conclusions
Intact cytoskeleton organization, efficient intracellular transport, and functional dendritic spines are key for neuronal health and survival. However, investigation into how HIV and HIV proteins impair the neuronal cytoskeleton should be further explored. There are several existing mechanisms through which HIV proteins may alter the neuronal cytoskeleton. First, direct binding of HIV proteins, including gp120 (Avdoshina et al., 2016a) and Tat (Chen et al., 2002; Aprea et al., 2006), could modify MT dynamics and stability. Further, gp120 binds to the terminal tail of beta III tubulin (Avdoshina et al., 2016a), which contains the binding site for kinesin-1 through three essential amino acids E410, D417, and E421 (Uchimura et al., 2006). Consequently, gp120 may prevent the recruitment of MAPs, including motor proteins, to MTs. Moreover, gp120 and Tat binding to MTs could reduce the anterograde transport of neurotrophic factors and therefore diminish their activity-dependent release, which ultimately, could impair the survival of postsynaptic neurons. Both Tat and gp120 impair neuronal MT polymerization (Butler et al., 2011; Avdoshina et al., 2017), which may be an additional mechanism whereby HIV proteins interfere with MT dynamics. MT impairment has detrimental consequences for many intracellular processes including transport of mitochondria, cargo-containing vesicles, neurotrophic factors, and autophagy/mitophagy, among others.
In addition to tubulin, neuronal health and functionality is regulated by actin. During development, the actin cytoskeleton supports the formation, extension and branching of neurites and dendritic spines. In the adult CNS, spines containing excitatory synapses need to be strengthened to support learning and memory. Viral proteins gp120 and Tat both cause decreased spines in animal models of HAND (Bachis et al., 2016; Raybuck et al., 2017) and primary neurons in vitro (Shin et al., 2012; Bertrand et al., 2014). The functionality of dendritic spines is also impaired with viral proteins. These alterations may occur due to signaling through Rho GTPases or chemokine receptors. However, signaling is not the only potential mechanism through which HIV proteins alter actin and actin dynamics. Similar to MTs, F-actin and its regulatory protein, cofilin, can undergo PTMs which regulates their functionality will cause dysregulation of the actin cytoskeleton (Terman and Kashina, 2013). NMDA receptor activation, a well-characterized mechanism of Tat toxicity (Haughey et al., 2001; Song et al., 2003) causes phosphorylation of cofilin and dephosphorylation of MAP2 (Quinlan and Halpain, 1996). Intriguinly, NMDA-mediated dephosphorylation of MAP2 occurs through the calcium/calmodulin-dependent protein phosphatase calcineurin (Quinlan and Halpain, 1996), which is also activated by Tat in vitro (Rozzi et al., 2018) or in infected patients (Hu, 2016), suggesting a commonality of signaling mechanism between Tat and NMDA in altering the cytoskeleton dynamic.
In conclusion, cytoskeletal proteins could play a role in long-term synaptic modifications occurring in infected patients. Binding to actin, tubulin, and intermediate filaments by the viral proteins gp120 and Tat could lead to altered function of these specialized cells and ultimately a decrease in their survival (Fig. 2). The impact of these viral proteins, as well as several other HIV proteins including Nef and Vpr, on the neuronal cytoskeleton is a new mechanism to explain the synaptopathy seen in HAND. The mechanisms though which these alterations occur- direct binding, signaling, or altered network dynamics- still need to be fully uncovered. Current and future studies will inform whether the neuronal cytoskeleton should be targeted therapeutically to mediate the cognitive deficits seen in HAND.
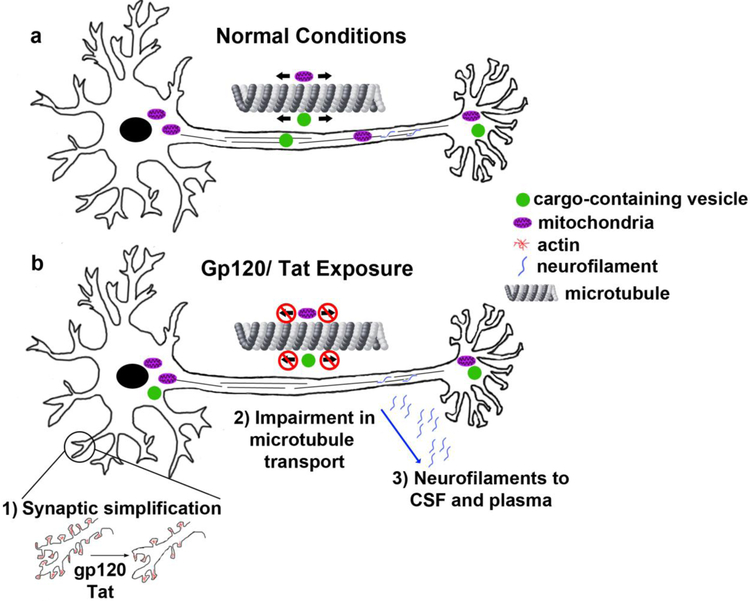
Figure 2. Schematic of the effect of HIV proteins on the neuronal cytoskeleton.
a) Under normal conditions, neurons are able to transport mitochondria and cargo-containing vesicles bidirectionally (arrows) along microtubules. b) Viral proteins gp120 and Tat cause several impairments to the neuronal cytoskeleton including: 1) Synaptic simplification due to alterations in actin and actin binding proteins; 2) Impairment in microtubule based transport including decreased motility of mitochondria and cargo-containing vesicles; 3) Loss of proper localization of neurofilaments and increased levels in the cerebral spinal fluid (CSF) and blood plasma.
Acknowledgement
Support was provided by HHS grants NINDS NS107106 to EW and NS079172 and NS104000 to IM.
Footnotes
The authors declare that they have no conflict of interest.
References
- Acquas E, Bachis A, Nosheny RL, Cernak I, Mocchetti I (2004) Human immunodeficiency virus type 1 protein gp120 causes neuronal cell death in the rat brain by activating caspases. Neurotox Res 5:605–615 [DOI] [PubMed] [Google Scholar]
- Adle-Biassette H, Levy Y, Colombel M, Poron F, Natchev S, Keohane C, Gray F (1995) Neuronal apoptosis in HIV infection in adults. Neuropathol Appl Neurobiol 21:218–227 [DOI] [PubMed] [Google Scholar]
- Ahmed F, MacArthur L, De Bernardi MA, Mocchetti I (2009) Retrograde and anterograde transport of HIV protein gp120 in the nervous system. Brain Behav Immun 23:355–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alakkas A, Ellis RJ, Watson CW, Umlauf A, Heaton RK, Letendre S, Collier A, Marra C, Clifford DB, Gelman B, Sacktor N, Morgello S, Simpson D, McCutchan JA, Kallianpur A, Gianella S, Marcotte T, Grant I, Fennema-Notestine C, Group C (2018) White matter damage, neuroinflammation, and neuronal integrity in HAND. J Neurovirol 10.1007/s13365-018-0682-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson E, Zink W, Xiong H, Gendelman HE (2002) HIV-1-associated dementia: a metabolic encephalopathy perpetrated by virus-infected and immune-competent mononuclear phagocytes. J Acquir Immune Defic Syndr 31 Suppl 2:S43–54 [DOI] [PubMed] [Google Scholar]
- Aprea S, Del Valle L, Mameli G, Sawaya BE, Khalili K, Peruzzi F (2006) Tubulin-mediated binding of human immunodeficiency virus-1 Tat to the cytoskeleton causes proteasomal-dependent degradation of microtubule-associated protein 2 and neuronal damage. J Neurosci 26:4054–4062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avdoshina V, Caragher SP, Wenzel ED, Taraballi F, Mocchetti I, Harry GJ (2017) The viral protein gp120 decreases the acetylation of neuronal tubulin: potential mechanism of neurotoxicity. J Neurochem 141:606–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avdoshina V, Taraballi F, Dedoni S, Corbo C, Paige M, Saygideger Kont Y, Uren A, Tasciotti E, Mocchetti I (2016a) Identification of a binding site of the human immunodeficiency virus envelope protein gp120 to neuronal-specific tubulin. J Neurochem 137:287–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avdoshina V, Fields JA, Castellano P, Dedoni S, Palchik G, Trejo M, Adame A, Rockenstein E, Eugenin E, Masliah E, Mocchetti I (2016b) The HIV protein gp120 alters mitochondrial dynamics in neurons Neurotox Res 29:583–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachani M, Sacktor N, McArthur JC, Nath A, Rumbaugh J (2013) Detection of anti-tat antibodies in CSF of individuals with HIV-associated neurocognitive disorders. J Neurovirol 19:82–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Aden SA, Nosheny RL, Andrews PM, Mocchetti I (2006) Axonal transport of human immunodeficiency virus type 1 envelope protein glycoprotein 120 is found in association with neuronal apoptosis. J Neurosci 26:6771–6780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Avdoshina V, Zecca L, Parsadanian M, Mocchetti I (2012) Human immunodeficiency virus type 1 alters brain-derived neurotrophic factor processing in neurons. J Neurosci 32:9477–9484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Wenzel E, Boelk A, Becker J, Mocchetti I (2016) The neurotrophin receptor p75 mediates gp120-induced loss of synaptic spines in aging mice. Neurobiol Aging 46:160–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird FJ, Bennett CL (2013) Microtubule defects & Neurodegeneration. J Genet Syndr Gene Ther 4:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamburg JR, Bernstein BW (2010) Roles of ADF/cofilin in actin polymerization and beyond. F1000 Biol Rep 2:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamburg JR, Bernstein BW, Davis RC, Flynn KC, Goldsbury C, Jensen JR, Maloney MT, Marsden IT, Minamide LS, Pak CW, Shaw AE, Whiteman I, Wiggan O (2010) ADF/Cofilin-actin rods in neurodegenerative diseases. Curr Alzheimer Res 7:241–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal AK, Mactutus CF, Nath A, Maragos W, Hauser KF, Booze RM (2000) Neurotoxicity of HIV-1 proteins gp120 and Tat in the rat striatum. Brain Res 879:42–49 [DOI] [PubMed] [Google Scholar]
- Bar J, Kobler O, van Bommel B, Mikhaylova M (2016) Periodic F-actin structures shape the neck of dendritic spines. Sci Rep 6:37136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnisch T, Francesconi W, Sanna PP (2004) HIV secreted protein Tat prevents long-term potentiation in the hippocampal CA1 region. Brain Res 1012:187–189 [DOI] [PubMed] [Google Scholar]
- Berth S, Caicedo HH, Sarma T, Morfini G, Brady ST (2015) Internalization and axonal transport of the HIV glycoprotein gp120. ASN Neuro 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthet A, Margolis EB, Zhang J, Hsieh I, Zhang J, Hnasko TS, Ahmad J, Edwards RH, Sesaki H, Huang EJ, Nakamura K (2014) Loss of mitochondrial fission depletes axonal mitochondria in midbrain dopamine neurons. J Neurosci 34:14304–14317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand SJ, Mactutus CF, Aksenova MV, Espensen-Sturges TD, Booze RM (2014) Synaptodendritic recovery following HIV Tat exposure: neurorestoration by phytoestrogens. J Neurochem 128:140–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya A, Watson FL, Pomeroy SL, Zhang YZ, Stiles CD, Segal RA (2002) High-resolution imaging demonstrates dynein-based vesicular transport of activated Trk receptors. J Neurobiol 51:302–312 [DOI] [PubMed] [Google Scholar]
- Binder LI, Frankfurter A, Rebhun LI (1985) The distribution of tau in the mammalian central nervous system. J Cell Biol 101:1371–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Chauhan A, Dimayuga FO, Gee J, Keller JN, Nath A (2003) Synaptic transport of human immunodeficiency virus-Tat protein causes neurotoxicity and gliosis in rat brain. J Neurosci 23:8417–8422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler TR, Smith KJ, Self RL, Braden BB, Prendergast MA (2011) Neurodegenerative effects of recombinant HIV-1 Tat(1–86) are associated with inhibition of microtubule formation and oxidative stress-related reductions in microtubule-associated protein-2(a,b). Neurochem Res 36:819–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambray-Deakin MA, Burgoyne RD (1987) Posttranslational modifications of alpha-tubulin: acetylated and detyrosinated forms in axons of rat cerebellum. J Cell Biol 104:1569–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron PU, Saleh S, Sallmann G, Solomon A, Wightman F, Evans VA, Boucher G, Haddad EK, Sekaly RP, Harman AN, Anderson JL, Jones KL, Mak J, Cunningham AL, Jaworowski A, Lewin SR (2010) Establishment of HIV-1 latency in resting CD4+ T cells depends on chemokine-induced changes in the actin cytoskeleton. Proc Natl Acad Sci U S A 107:16934–16939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey AN, Sypek EI, Singh HD, Kaufman MJ, McLaughlin JP (2012) Expression of HIV-Tat protein is associated with learning and memory deficits in the mouse. Behav Brain Res 229:48–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey AN, Liu X, Mintzopoulos D, Paris JJ, Muschamp JW, McLaughlin JP, Kaufman MJ (2013) Conditional Tat protein expression in the GT-tg bigenic mouse brain induces gray matter density reductions. Prog Neuropsychopharmacol Biol Psychiatry 43:49–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlos AJ, Tong L, Prieto GA, Cotman CW (2017) IL-1beta impairs retrograde flow of BDNF signaling by attenuating endosome trafficking. J Neuroinflammation 14:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartelli D, Ronchi C, Maggioni MG, Rodighiero S, Giavini E, Cappelletti G (2010) Microtubule dysfunction precedes transport impairment and mitochondria damage in MPP+ -induced neurodegeneration. J Neurochem 115:247–258 [DOI] [PubMed] [Google Scholar]
- Chalasani SH, Baribaud F, Coughlan CM, Sunshine MJ, Lee VM, Doms RW, Littman DR, Raper JA (2003) The chemokine stromal cell-derived factor-1 promotes the survival of embryonic retinal ganglion cells. J Neurosci 23:4601–4612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Wang M, Zhou S, Zhou Q (2002) HIV-1 Tat targets microtubules to induce apoptosis, a process promoted by the pro-apoptotic Bcl-2 relative Bim. EMBO J 21:6801–6810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdary PD, Che DL, Zhang K, Cui B (2015) Retrograde NGF axonal transport--motor coordination in the unidirectional motility regime. Biophys J 108:2691–2703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford DB, Ances BM (2013) HIV-associated neurocognitive disorder. Lancet Infect Dis 13:976–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles CH, Bradke F (2015) Coordinating neuronal actin-microtubule dynamics. Curr Biol 25:R677–691 [DOI] [PubMed] [Google Scholar]
- Conde C, Caceres A (2009) Microtubule assembly, organization and dynamics in axons and dendrites. Nat Rev Neurosci 10:319–332 [DOI] [PubMed] [Google Scholar]
- Crowell TA, Fletcher JL, Sereti I, Pinyakorn S, Dewar R, Krebs SJ, Chomchey N, Rerknimitr R, Schuetz A, Michael NL, Phanuphak N, Chomont N, Ananworanich J, Group RSS (2016) Initiation of antiretroviral therapy before detection of colonic infiltration by HIV reduces viral reservoirs, inflammation and immune activation. J Int AIDS Soc 19:21163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Este E, Kamin D, Gottfert F, El-Hady A, Hell SW (2015) STED nanoscopy reveals the ubiquity of subcortical cytoskeleton periodicity in living neurons. Cell Rep 10:1246–1251 [DOI] [PubMed] [Google Scholar]
- Dehmelt L, Halpain S (2005) The MAP2/Tau family of microtubule-associated proteins. Genome Biol 6:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Stefano M, Gray F, Leitner T, Chiodi F (1996) Analysis of ENV V3 sequences from HIV-1-infected brain indicates restrained virus expression throughout the disease. J Med Virol 49:41–48 [DOI] [PubMed] [Google Scholar]
- Dickey AS, Strack S (2011) PKA/AKAP1 and PP2A/Bbeta2 regulate neuronal morphogenesis via Drp1 phosphorylation and mitochondrial bioenergetics. J Neurosci 31:15716–15726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit R, Ross JL, Goldman YE, Holzbaur EL (2008) Differential regulation of dynein and kinesin motor proteins by tau. Science 319:1086–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Xiong H (2006) Human immunodeficiency virus type 1 gp120 inhibits long-term potentiation via chemokine receptor CXCR4 in rat hippocampal slices. J Neurosci Res 83:489–496 [DOI] [PubMed] [Google Scholar]
- Drewes G, Ebneth A, Preuss U, Mandelkow EM, Mandelkow E (1997) MARK, a novel family of protein kinases that phosphorylate microtubule-associated proteins and trigger microtubule disruption. Cell 89:297–308 [DOI] [PubMed] [Google Scholar]
- Dumas A, Le-Bury G, Marie-Anais F, Herit F, Mazzolini J, Guilbert T, Bourdoncle P, Russell DG, Benichou S, Zahraoui A, Niedergang F (2015) The HIV-1 protein Vpr impairs phagosome maturation by controlling microtubule-dependent trafficking. J Cell Biol 211:359–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eira J, Silva CS, Sousa MM, Liz MA (2016) The cytoskeleton as a novel therapeutic target for old neurodegenerative disorders. Prog Neurobiol 141:61–82 [DOI] [PubMed] [Google Scholar]
- Ellis R, Langford D, Masliah E (2007) HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci 8:33–44 [DOI] [PubMed] [Google Scholar]
- Errea O, Moreno B, Gonzalez-Franquesa A, Garcia-Roves PM, Villoslada P (2015) The disruption of mitochondrial axonal transport is an early event in neuroinflammation. J Neuroinflammation 12:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin EA, King JE, Nath A, Calderon TM, Zukin RS, Bennett MVL, Berman JW (2007) HIV-tat induces formation of an LRP-PSD-95-NMDAR-nNOS complex that promotes apoptosis in neurons and astrocytes. Proc Nat Acad Sci USA 104:3438–3443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everall IP, Hansen LA, Masliah E (2005) The shifting patterns of HIV encephalitis neuropathology. Neurotox Res 8:51–61 [DOI] [PubMed] [Google Scholar]
- Fields J, Dumaop W, Langford TD, Rockenstein E, Masliah E (2014) Role of neurotrophic factor alterations in the neurodegenerative process in HIV associated neurocognitive disorders. J Neuroimmune Pharmacol 9:102–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields J, Dumaop W, Eleuteri S, Campos S, Serger E, Trejo M, Kosberg K, Adame A, Spencer B, Rockenstein E, He JJ, Masliah E (2015) HIV-1 Tat alters neuronal autophagy by modulating autophagosome fusion to the lysosome: implications for HIV-associated neurocognitive disorders. J Neurosci 35:1921–1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields JA, Serger E, Campos S, Divakaruni AS, Kim C, Smith K, Trejo M, Adame A, Spencer B, Rockenstein E, Murphy AN, Ellis RJ, Letendre S, Grant I, Masliah E (2016) HIV alters neuronal mitochondrial fission/fusion in the brain during HIV-associated neurocognitive disorders. Neurobiol Dis 86:154–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Ignatowska-Jankowska BM, Bull C, Skoff RP, Lichtman AH, Wise LE, Fox MA, Su J, Medina AE, Krahe TE, Knapp PE, Guido W, Hauser KF (2013) Synaptic dysfunction in the hippocampus accompanies learning and memory deficits in human immunodeficiency virus type-1 Tat transgenic mice. Biol Psychiatry 73:443–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fois AF, Brew BJ (2015) The Potential of the CNS as a Reservoir for HIV-1 Infection: Implications for HIV Eradication. Curr HIV/AIDS Rep 12:299–303 [DOI] [PubMed] [Google Scholar]
- Fukazawa Y, Saitoh Y, Ozawa F, Ohta Y, Mizuno K, Inokuchi K (2003) Hippocampal LTP is accompanied by enhanced F-actin content within the dendritic spine that is essential for late LTP maintenance in vivo. Neuron 38:447–460 [DOI] [PubMed] [Google Scholar]
- Fulga TA, Elson-Schwab I, Khurana V, Steinhilb ML, Spires TL, Hyman BT, Feany MB (2007) Abnormal bundling and accumulation of F-actin mediates tau-induced neuronal degeneration in vivo. Nat Cell Biol 9:139–148 [DOI] [PubMed] [Google Scholar]
- Gates TM, Cysique LA, Siefried KJ, Chaganti J, Moffat KJ, Brew BJ (2016) Maraviroc-intensified combined antiretroviral therapy improves cognition in virally suppressed HIV-associated neurocognitive disorder. AIDS 30:591–600 [DOI] [PubMed] [Google Scholar]
- Gelman BB (2015) Neuropathology of HAND With Suppressive Antiretroviral Therapy: Encephalitis and Neurodegeneration Reconsidered. Curr HIV/AIDS Rep 12:272–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg SD, Alldred MJ, Gunnam SM, Schiroli C, Lee SH, Morgello S, Fischer T (2018) Expression profiling suggests microglial impairment in human immunodeficiency virus neuropathogenesis. Ann Neurol 83:406–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisslen M, Hagberg L, Brew BJ, Cinque P, Price RW, Rosengren L (2007) Elevated cerebrospinal fluid neurofilament light protein concentrations predict the development of AIDS dementia complex. J Infect Dis 195:1774–1778 [DOI] [PubMed] [Google Scholar]
- Gisslen M, Price RW, Andreasson U, Norgren N, Nilsson S, Hagberg L, Fuchs D, Spudich S, Blennow K, Zetterberg H (2016) Plasma Concentration of the Neurofilament Light Protein (NFL) is a Biomarker of CNS Injury in HIV Infection: A Cross-Sectional Study. EBioMedicine 3:135–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleichmann M, Mattson MP (2011) Neuronal Calcium Homeostasis and Dysregulation. Antioxidants & Redox Signaling 14:1261–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gongvatana A, Schweinsburg BC, Taylor MJ, Theilmann RJ, Letendre SL, Alhassoon OM, Jacobus J, Woods SP, Jernigan TL, Ellis RJ, Frank LR, Grant I, Charter G (2009) White matter tract injury and cognitive impairment in human immunodeficiency virus-infected individuals. J Neurovirol 15:187–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughey NJ, Mattson MP (2002) Calcium dysregulation and neuronal apoptosis by the HIV-1 proteins Tat and gp120. J Acquir Immune Defic Syndr 31 Suppl 2:S55–61 [DOI] [PubMed] [Google Scholar]
- Haughey NJ, Nath A, Mattson MP, Slevin JT, Geiger JD (2001) HIV-1 Tat through phosphorylation of NMDA receptors potentiates glutamate excitotoxicity. J Neurochem 78:457–467 [DOI] [PubMed] [Google Scholar]
- Heaton RK et al. (2010) HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 75:2087–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK et al. (2011) HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 17:3–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinckelmann MV, Zala D, Saudou F (2013) Releasing the brake: restoring fast axonal transport in neurodegenerative disorders. Trends Cell Biol 23:634–643 [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Shiomura Y, Okabe S (1988) Tau proteins: the molecular structure and mode of binding on microtubules. J Cell Biol 107:1449–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N, Niwa S, Tanaka Y (2010) Molecular motors in neurons: transport mechanisms and roles in brain function, development, and disease. Neuron 68:610–638 [DOI] [PubMed] [Google Scholar]
- Hoffman PN, Cleveland DW, Griffin JW, Landes PW, Cowan NJ, Price DL (1987) Neurofilament gene expression: a major determinant of axonal caliber. Proc Natl Acad Sci U S A 84:3472–3476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotulainen P, Hoogenraad CC (2010) Actin in dendritic spines: connecting dynamics to function. J Cell Biol 189:619–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XT (2016) HIV-1 Tat-Mediated Calcium Dysregulation and Neuronal Dysfunction in Vulnerable Brain Regions. Curr Drug Targets 17:4–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, Yoshida M, Wang XF, Yao TP (2002) HDAC6 is a microtubule-associated deacetylase. Nature 417:455–458 [DOI] [PubMed] [Google Scholar]
- Jessen Krut J, Mellberg T, Price RW, Hagberg L, Fuchs D, Rosengren L, Nilsson S, Zetterberg H, Gisslen M (2014) Biomarker evidence of axonal injury in neuroasymptomatic HIV-1 patients. PLoS One 9:e88591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TP, Nath A (2016) Protocol for Detection of HIV-Tat Protein in Cerebrospinal Fluid by a Sandwich Enzyme-Linked Immunosorbent Assay. Methods Mol Biol 1354:343–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanmogne G, Grammas P, Kennedy R (2000) Analysis of human endothelial cells and cortical neurons for susceptibility to HIV-1 infection and co-receptor expression. J Neurovirol 6:519–528 [DOI] [PubMed] [Google Scholar]
- Kanmogne GD, Kennedy RC, Grammas P (2002) HIV-1 gp120 Proteins and gp160 Peptides Are Toxic to Brain Endothelial Cells and Neurons: Possible Pathway for HIV Entry into the Brain and HIV-Associated Dementia. Journal of Neuropathology & Experimental Neurology 61:992–1000 [DOI] [PubMed] [Google Scholar]
- Kaul M, Garden G, Lipton S (2001) Pathways to neuronal injury and apotosis in HIV-associated dementia. Nature 410:988–994 [DOI] [PubMed] [Google Scholar]
- Keys B, Karis J, Fadeel B, Valentin A, Norkrans G, Hagberg L, Chiodi F (1993) V3 sequences of paired HIV-1 isolates from blood and cerebrospinal fluid cluster according to host and show variation related to the clinical stage of disease. Virology 196:475–483 [DOI] [PubMed] [Google Scholar]
- Khalil M, Teunissen CE, Otto M, Piehl F, Sormani MP, Gattringer T, Barro C, Kappos L, Comabella M, Fazekas F, Petzold A, Blennow K, Zetterberg H, Kuhle J (2018) Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol [DOI] [PubMed] [Google Scholar]
- Klein RS, Williams KC, Alvarez-Hernandez X, Westmoreland S, Force T, Lackner AA, Luster AD (1999) Chemokine receptor expression and signaling in macaque and human fetal neurons and astrocytes: implications for the neuropathogenesis of AIDS. J Immunol 163:1636–1646 [PubMed] [Google Scholar]
- Koka P, He K, Camerini D, Tran T, Yashar SS, Merrill JE (1995) The mapping of HIV-1 gp160 epitopes required for interleukin-1 and tumor necrosis factor alpha production in glial cells. J Neuroimmunol 57:179–191 [DOI] [PubMed] [Google Scholar]
- Konietzny A, Bar J, Mikhaylova M (2017) Dendritic Actin Cytoskeleton: Structure, Functions, and Regulations. Front Cell Neurosci 11:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogh KA, Lyddon E, Thayer SA (2015) HIV-1 Tat activates a RhoA signaling pathway to reduce NMDA-evoked calcium responses in hippocampal neurons via an actin-dependent mechanism. J Neurochem 132:354–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krucker T, Siggins GR, Halpain S (2000) Dynamic actin filaments are required for stable long-term potentiation (LTP) in area CA1 of the hippocampus. Proc Natl Acad Sci U S A 97:6856–6861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krucker T, Toggas SM, Mucke L, Siggins GR (1998) Transgenic mice with cerebral expression of human immunodeficiency virus type-1 coat protein gp120 show divergent changes in short- and long-term potentiation in CA1 hippocampus. Neuroscience 83:691–700 [DOI] [PubMed] [Google Scholar]
- Kumar AM, Ownby RL, Waldrop-Valverde D, Fernandez B, Kumar M (2011) Human immunodeficiency virus infection in the CNS and decreased dopamine availability: relationship with neuropsychological performance. J Neurovirol 17:26–40 [DOI] [PubMed] [Google Scholar]
- Kyei GB, Dinkins C, Davis AS, Roberts E, Singh SB, Dong C, Wu L, Kominami E, Ueno T, Yamamoto A, Federico M, Panganiban A, Vergne I, Deretic V (2009) Autophagy pathway intersects with HIV-1 biosynthesis and regulates viral yields in macrophages. J Cell Biol 186:255–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford D, Grigorian A, Hurford R, Adame A, Crews L, Masliah E (2004) The role of mitochondrial alterations in the combined toxic effects of human immunodeficiency virus Tat protein and methamphetamine on calbindin positive-neurons. J Neurovirol 10:327–337 [DOI] [PubMed] [Google Scholar]
- Langford D, Oh Kim B, Zou W, Fan Y, Rahimain P, Liu Y, He JJ (2018) Doxycycline-inducible and astrocyte-specific HIV-1 Tat transgenic mice (iTat) as an HIV/neuroAIDS model. J Neurovirol 24:168–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MH, Amin ND, Venkatesan A, Wang T, Tyagi R, Pant HC, Nath A (2013) Impaired neurogenesis and neurite outgrowth in an HIV-gp120 transgenic model is reversed by exercise via BDNF production and Cdk5 regulation. J Neurovirol 19:418–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy M, Faas GC, Saggau P, Craigen WJ, Sweatt JD (2003) Mitochondrial regulation of synaptic plasticity in the hippocampus. J Biol Chem 278:17727–17734 [DOI] [PubMed] [Google Scholar]
- Lewis SA, Wang DH, Cowan NJ (1988) Microtubule-associated protein MAP2 shares a microtubule binding motif with tau protein. Science 242:936–939 [DOI] [PubMed] [Google Scholar]
- Li ST, Matsushita M, Moriwaki A, Saheki Y, Lu YF, Tomizawa K, Wu HY, Terada H, Matsui H (2004) HIV-1 Tat inhibits long-term potentiation and attenuates spatial learning [corrected]. Ann Neurol 55:362–371 [DOI] [PubMed] [Google Scholar]
- Li W, Huang Y, Reid R, Steiner J, Malpica-Llanos T, Darden TA, Shankar SK, Mahadevan A, Satishchandra P, Nath A (2008) NMDA receptor activation by HIV-Tat protein is clade dependent. J Neurosci 28:12190–12198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem RK, Messing A (2009) Dysfunctions of neuronal and glial intermediate filaments in disease. J Clin Invest 119:1814–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Jones M, Hingtgen CM, Bu G, Laribee N, Tanzi RE, Moir RD, Nath A, He JJ (2000) Uptake of HIV-1 tat protein mediated by low-density lipoprotein receptor-related protein disrupts the neuronal metabolic balance of the receptor ligands. Nat Med 6:1380–1387 [DOI] [PubMed] [Google Scholar]
- Lucera MB, Fleissner Z, Tabler CO, Schlatzer DM, Troyer Z, Tilton JC (2017) HIV signaling through CD4 and CCR5 activates Rho family GTPases that are required for optimal infection of primary CD4+ T cells. Retrovirology 14:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maday S, Wallace KE, Holzbaur EL (2012) Autophagosomes initiate distally and mature during transport toward the cell soma in primary neurons. J Cell Biol 196:407–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahy M, Autenrieth CS, Stanecki K, Wynd S (2014) Increasing trends in HIV prevalence among people aged 50 years and older: evidence from estimates and survey data. AIDS 28 Suppl 4:S453–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maragos WF, Tillman P, Jones M, Bruce-Keller AJ, Roth S, Bell JE, Nath A (2003) Neuronal injury in hippocampus with human immunodeficiency virus transactivating protein, Tat. Neuroscience 117:43–53 [DOI] [PubMed] [Google Scholar]
- Mariga A, Mitre M, Chao MV (2017) Consequences of brain-derived neurotrophic factor withdrawal in CNS neurons and implications in disease. Neurobiol Dis 97:73–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Achim CL, Ge N, DeTeresa R, Terry RD, Wiley CA (1992) Spectrum of human immunodeficiency virus-associated neocortical damage. Ann Neurol 32:321–329 [DOI] [PubMed] [Google Scholar]
- Masliah E, Heaton RK, Marcotte TD, Ellis RJ, Wiley CA, Mallory M, Achim CL, McCutchan JA, Nelson JA, Atkinson JH, Grant I (1997) Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. HNRC Group. The HIV Neurobehavioral Research Center. Ann Neurol 42:963–972 [DOI] [PubMed] [Google Scholar]
- Matus A (2000) Actin-based plasticity in dendritic spines. Science 290:754–758 [DOI] [PubMed] [Google Scholar]
- Maung R, Hoefer MM, Sanchez AB, Sejbuk NE, Medders KE, Desai MK, Catalan IC, Dowling CC, de Rozieres CM, Garden GA, Russo R, Roberts AJ, Williams R, Kaul M (2014) CCR5 knockout prevents neuronal injury and behavioral impairment induced in a transgenic mouse model by a CXCR4-using HIV-1 glycoprotein 120. J Immunol 193:1895–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur JC (2004) HIV dementia: an evolving disease. J Neuroimmunol 157:3–10 [DOI] [PubMed] [Google Scholar]
- McCarthy M, Vidaurre I, Geffin R (2006) Maturing neurons are selectively sensitive to human immunodeficiency virus type 1 exposure in differentiating human neuroepithelial progenitor cell cultures. J Neurovirol 12:333–348 [DOI] [PubMed] [Google Scholar]
- McMurray C (2000) Nerurodegeration: diseases of the cytoskeleton? Cell Death and Differentiation 7:861–865 [DOI] [PubMed] [Google Scholar]
- Melli G, Keswani SC, Fischer A, Chen W, Hoke A (2006) Spatially distinct and functionally independent mechanisms of axonal degeneration in a model of HIV-associated sensory neuropathy. Brain 129:1330–1338 [DOI] [PubMed] [Google Scholar]
- Merrill RA, Dagda RK, Dickey AS, Cribbs JT, Green SH, Usachev YM, Strack S (2011) Mechanism of neuroprotective mitochondrial remodeling by PKA/AKAP1. PLoS Biol 9:e1000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millecamps S, Julien JP (2013) Axonal transport deficits and neurodegenerative diseases. Nat Rev Neurosci 14:161–176 [DOI] [PubMed] [Google Scholar]
- Minamide LS, Striegl AM, Boyle JA, Meberg PJ, Bamburg JR (2000) Neurodegenerative stimuli induce persistent ADF/cofilin-actin rods that disrupt distal neurite function. Nat Cell Biol 2:628–636 [DOI] [PubMed] [Google Scholar]
- Mocchetti I, Bachis A, Campbell LA, Avdoshina V (2014) Implementing neuronal plasticity in NeuroAIDS: the experience of brain-derived neurotrophic factor and other neurotrophic factors. J Neuroimmune Pharmacol 9:80–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A (2002) Human Immunodeficiency Virus (HIV) Proteins in Neuropathologenesis of HIV Dementia. J Infect Dis 186:S193–S198 [DOI] [PubMed] [Google Scholar]
- Nichols MJ, Gates TM, Soares JR, Moffat KJ, Rae CD, Brew BJ, Cysique LA (2018) Atrophic brain signatures of mild forms of neurocognitive impairment in virally suppressed HIV infection. AIDS 10.1097/QAD.0000000000002042 [DOI] [PubMed] [Google Scholar]
- Nogales E, Wolf SG, Downing KH (1998) Structure of the alpha beta tubulin dimer by electron crystallography. Nature 391:199–203 [DOI] [PubMed] [Google Scholar]
- Nonaka M, Ma BY, Ohtani M, Yamamoto A, Murata M, Totani K, Ito Y, Miwa K, Nogami W, Kawasaki N, Kawasaki T (2007) Subcellular localization and physiological significance of intracellular mannan-binding protein. J Biol Chem 282:17908–17920 [DOI] [PubMed] [Google Scholar]
- Olson KR, McIntosh JR, Olmsted JB (1995) Analysis of MAP 4 function in living cells using green fluorescent protein (GFP) chimeras. J Cell Biol 130:639–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordonez DG, Lee MK, Feany MB (2018) Alpha-synuclein Induces Mitochondrial Dysfunction through Spectrin and the Actin Cytoskeleton. Neuron 97:108–124 e106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ospina Stella A, Turville S (2018) All-Round Manipulation of the Actin Cytoskeleton by HIV. Viruses 10:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco A, Gallo G (2016) Actin filament-microtubule interactions in axon initiation and branching. Brain Res Bull 126:300–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petito C, Roberts B (1995) Evidence of apoptic cell death in HIV encephalitis. Am J Pathol 146:1121–1130 [PMC free article] [PubMed] [Google Scholar]
- Price RW, Brew B, Sidtis J, Rosenblum M, Scheck AC, Cleary P (1988) The brain in AIDS: central nervous system HIV-1 infection and AIDS dementia complex. Science 239:586–592 [DOI] [PubMed] [Google Scholar]
- Qu Y, Hahn I, Webb SE, Pearce SP, Prokop A (2017) Periodic actin structures in neuronal axons are required to maintain microtubules. Mol Biol Cell 28:296–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan EM, Halpain S (1996) Postsynaptic mechanisms for bidirectional control of MAP2 phosphorylation by glutamate receptors. Neuron 16:357–368 [DOI] [PubMed] [Google Scholar]
- Rajakyla EK, Vartiainen MK (2014) Rho, nuclear actin, and actin-binding proteins in the regulation of transcription and gene expression. Small GTPases 5:e27539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybuck JD, Hargus NJ, Thayer SA (2017) A GluN2B-Selective NMDAR Antagonist Reverses Synapse Loss and Cognitive Impairment Produced by the HIV-1 Protein Tat. J Neurosci 37:7837–7847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed NA, Cai D, Blasius TL, Jih GT, Meyhofer E, Gaertig J, Verhey KJ (2006) Microtubule acetylation promotes kinesin-1 binding and transport. Curr Biol 16:2166–2172 [DOI] [PubMed] [Google Scholar]
- Rivieccio MA, Brochier C, Willis DE, Walker BA, D’Annibale MA, McLaughlin K, Siddiq A, Kozikowski AP, Jaffrey SR, Twiss JL, Ratan RR, Langley B (2009) HDAC6 is a target for protection and regeneration following injury in the nervous system. Proc Natl Acad Sci U S A 106:19599–19604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozzi SJ, Avdoshina V, Fields JA, Mocchetti I (2018) Human immunodeficiency virus Tat impairs mitochondrial fission in neurons. Cell Death Discov 4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LH, Sacktor N, Creighton J, Du Y, Endres CJ, Pomper MG, Coughlin JM (2018) Microglial activation is inversely associated with cognition in individuals living with HIV on effective antiretroviral therapy. AIDS 32:1661–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier J, Vives E, Marbrouk K, Benjouad A, Rochat H, Duval A, Hue B, Bahraoui E (1991) Evidence for Neurotoxic Activity of tat from Human Immunodeficiency Virus Type 1. J Virol 65:961–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacktor N, McDermott MP, Marder K, Schifitto G, Selnes OA, McArthur JC, Stern Y, Albert S, Palumbo D, Kieburtz K, De Marcaida JA, Cohen B, Epstein L (2002) HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol 8:136–142 [DOI] [PubMed] [Google Scholar]
- Sainath R, Gallo G (2015) The dynein inhibitor Ciliobrevin D inhibits the bidirectional transport of organelles along sensory axons and impairs NGF-mediated regulation of growth cones and axon branches. Dev Neurobiol 75:757–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez C, Diaz-Nido J, Avila J (2000) Phosphorylation of microtubule-associated protein 2 (MAP2) and its relevance for the regulation of the neuronal cytoskeleton function. Prog Neurobiol 61:133–168 [DOI] [PubMed] [Google Scholar]
- Sanchez-Alavez M, Criado J, Gomez-Chavarin M, Jimenez-Anguiano A, Navarro L, Diaz-Ruiz O, Galicia O, Sanchez-Narvaez F, Murillo-Rodriguez E, Henriksen SJ, Elder JH, Prospero-Garcia O (2000) HIV- and FIV-derived gp120 alter spatial memory, LTP, and sleep in rats. Neurobiol Dis 7:384–394 [DOI] [PubMed] [Google Scholar]
- Sandler NG, Sereti I (2014) Can early therapy reduce inflammation? Curr Opin HIV AIDS 9:72–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M, Mankowski JL, Brown A, Volsky DJ, McArthur JC (2016) HIV-associated neurocognitive disorder--pathogenesis and prospects for treatment. Nat Rev Neurol 12:234–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schifitto G, Yiannoutsos CT, Ernst T, Navia BA, Nath A, Sacktor N, Anderson C, Marra CM, Clifford DB, Team A (2009) Selegiline and oxidative stress in HIV-associated cognitive impairment. Neurology 73:1975–1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard DP, Iudicello JE, Bondi MW, Doyle KL, Morgan EE, Massman PJ, Gilbert PE, Woods SP (2015) Elevated rates of mild cognitive impairment in HIV disease. J Neurovirol 21:576–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin AH, Kim HJ, Thayer SA (2012) Subtype selective NMDA receptor antagonists induce recovery of synapses lost following exposure to HIV-1 Tat. Br J Pharmacol 166:1002–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sit ST, Manser E (2011) Rho GTPases and their role in organizing the actin cytoskeleton. J Cell Sci 124:679–683 [DOI] [PubMed] [Google Scholar]
- Song L, Nath A, Geiger JD, Moore A, Hochman S (2003) Human immunodeficiency virus type 1 Tat protein directly activates neuronal N-methyl-D-aspartate receptors at an allosteric zinc-sensitive site. J Neurovirol 9:399–403 [DOI] [PubMed] [Google Scholar]
- Song Y, Brady ST (2015) Post-translational modifications of tubulin: pathways to functional diversity of microtubules. Trends Cell Biol 25:125–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner JP, Bachani M, Wolfson-Stofko B, Lee MH, Wang T, Li G, Li W, Strayer D, Haughey NJ, Nath A (2015) Interaction of paroxetine with mitochondrial proteins mediates neuroprotection. Neurotherapeutics 12:200–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JL, Lessard DV, Hoeprich GJ, Morfini GA, Berger CL (2017) Phosphoregulation of Tau modulates inhibition of kinesin-1 motility. Mol Biol Cell 28:1079–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumm RK, Zhou C, Ara T, Lazarini F, Dubois-Dalcq M, Nagasawa T, Hollt V, Schulz S (2003) CXCR4 regulates interneuron migration in the developing neocortex. J Neurosci 23:5123–5130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo EC, Manguinhas R, Fonseca R (2016) The interplay between neuronal activity and actin dynamics mimic the setting of an LTD synaptic tag. Sci Rep 6:33685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan IL, McArthur JC (2011) HIV-associated central nervous system diseases in the era of combination antiretroviral therapy. Eur J Neurol 18:371–372 [DOI] [PubMed] [Google Scholar]
- Teodorof C, Divakar S, Soontornniyomkij B, Achim CL, Kaul M, Singh KK (2014) Intracellular mannose binding lectin mediates subcellular trafficking of HIV-1 gp120 in neurons. Neurobiol Dis 69:54–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teodorof-Diedrich C, Spector SA (2018) Human Immunodeficiency Virus Type-1 gp120 and Tat Induce Mitochondrial Fragmentation and Incomplete Mitophagy in Human Neurons. J Virol 10.1128/JVI.00993–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terman JR, Kashina A (2013) Post-translational modification and regulation of actin. Curr Opin Cell Biol 25:30–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Stefanidakis M, Ning L, Van Lint P, Nyman-Huttunen H, Libert C, Itohara S, Mishina M, Rauvala H, Gahmberg CG (2007) Activation of NMDA receptors promotes dendritic spine development through MMP-mediated ICAM-5 cleavage. J Cell Biol 178:687–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toggas SM, Masliah E, Rockenstein EM, Rall GF, Abraham CR, Mucke L (1994) Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature 367:188–193 [DOI] [PubMed] [Google Scholar]
- Tozzi V, Balestra P, Bellagamba R, Corpolongo A, Salvatori MF, Visco-Comandini U, Vlassi C, Giulianelli M, Galgani S, Antinori A, Narciso P (2007) Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: prevalence and risk factors. J Acquir Immune Defic Syndr 45:174–182 [DOI] [PubMed] [Google Scholar]
- Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS (2008) Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J 27:433–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchimura S, Oguchi Y, Katsuki M, Usui T, Osada H, Nikawa J, Ishiwata S, Muto E (2006) Identification of a strong binding site for kinesin on the microtubule using mutant analysis of tubulin. EMBO J 25:5932–5941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee RB, Williams JC, Varma D, Barnhart LE (2004) Dynein: An ancient motor protein involved in multiple modes of transport. J Neurobiol 58:189–200 [DOI] [PubMed] [Google Scholar]
- van der Bliek AM, Shen Q, Kawajiri S (2013) Mechanisms of mitochondrial fission and fusion. Cold Spring Harb Perspect Biol 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhey KJ, Hammond JW (2009) Traffic control: regulation of kinesin motors. Nat Rev Mol Cell Biol 10:765–777 [DOI] [PubMed] [Google Scholar]
- Villeneuve LM, Purnell PR, Stauch KL, Callen SE, Buch SJ, Fox HS (2016) HIV-1 transgenic rats display mitochondrial abnormalities consistent with abnormal energy generation and distribution. J Neurovirol 22:564–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viviani B, Gardoni F, Bartesaghi S, Corsini E, Facchi A, Galli CL, Di Luca M, Marinovich M (2006) Interleukin-1 beta released by gp120 drives neural death through tyrosine phosphorylation and trafficking of NMDA receptors. J Biol Chem 281:30212–30222 [DOI] [PubMed] [Google Scholar]
- Wenzel ED, Bachis A, Avdoshina V, Taraballi F, Tasciotti E, Mocchetti I (2017) Endocytic Trafficking of HIV gp120 is Mediated by Dynamin and Plays a Role in gp120 Neurotoxicity. J Neuroimmune Pharmacol 12:492–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann B (2010) Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol 11:872–884 [DOI] [PubMed] [Google Scholar]
- Westermann S, Weber K (2003) Post-translational modifications regulate microtubule function. Nat Rev Mol Cell Biol 4:938–947 [DOI] [PubMed] [Google Scholar]
- Wiley CA, Achim C (1994) Human immunodeficiency virus encephalitis is the pathological correlate of dementia in acquired immunodeficiency syndrome. Ann Neurol 36:673–676 [DOI] [PubMed] [Google Scholar]
- Yang G, Pan F, Gan WB (2009) Stably maintained dendritic spines are associated with lifelong memories. Nature 462:920–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Wu J, Lu Y (2010) Mechanism of HIV-1-TAT induction of interleukin-1beta from human monocytes: Involvement of the phospholipase C/protein kinase C signaling cascade. J Med Virol 82:735–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan A, Rao MV, Veeranna Nixon RA (2012) Neurofilaments at a glance. J Cell Sci 125:3257–3263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Chen XQ, Han E, Hu Y, Paik P, Ding Z, Overman J, Lau AL, Shahmoradian SH, Chiu W, Thompson LM, Wu C, Mobley WC (2016) TRiC subunits enhance BDNF axonal transport and rescue striatal atrophy in Huntington’s disease. Proc Natl Acad Sci U S A 113:E5655–5664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FQ, Snider WD (2005) Cell biology. GSK-3beta and microtubule assembly in axons. Science 308:211–214 [DOI] [PubMed] [Google Scholar]
- Zhu J, Ananthan S, Zhan CG (2018) The role of human dopamine transporter in NeuroAIDS. Pharmacol Ther 183:78–89 [DOI] [PMC free article] [PubMed] [Google Scholar]