Abstract
Aim
To examine the distribution and association of sociodemographic, adherence and barriers-to-care factors in relation to glycaemic control within insulin regimens in US children with Type 1 diabetes in the SEARCH for Diabetes in Youth study.
Methods
Self- or parent-reported data from 1095 children with Type 1 diabetes aged 10–17 years were collected on insulin regimen, sociodemographics, diabetes self-management, diabetes-related family conflict and barriers to care. Multivariable logistic regression analysis identified poor glycaemic control correlates within each insulin regimen.
Results
Participants included 694 children on insulin pump therapy, 188 receiving basal–bolus injections, and 213 on a mixed insulin regimen. Of these, 28.5%, 45.2% and 51.2%, respectively, had poor glycaemic control [HbA1c ≥ 80 mmol/mol (9.5%)]. Family conflict between parent and child regarding diabetes management was the only factor significantly associated with poor glycaemic control in all insulin regimens (insulin pump, P≤ 0.0001; basal–bolus injections, P=0.0002; mixed insulin regimen, P=0.0103). For children on insulin pump, poor control was significantly associated with non-white race (P=0.0008), living in multiple households (P=0.0331), having Medicaid insurance (P=0.0090), and low insulin adherence (P<0.0001). For children on a mixed insulin regimen, living in multiple households (P=0.0256) and not spending enough time with healthcare provider (P=0.0058) correlated with poor control.
Conclusions
A high percentage of US children with Type 1 diabetes had poor glycaemic control, especially those not using an insulin pump. Early identification of children with risk factors associated with poor glycaemic control within insulin regimens and addressing diabetes-related family conflict may allow interventions to improve diabetes management.
Introduction
Achievement of optimal glycaemic control is a challenge for children with Type 1 diabetes. A significant proportion do not achieve the HbA1c target of < 58 mmol/mol (7.5%) recommended by the American Diabetes Association (ADA) [1–3]. The SEARCH for Diabetes in Youth (SEARCH) study reported that 56% of children with Type 1 diabetes overall and >70% of adolescents with Type 1 diabetes in the USA had HbA1c levels higher than the target recommended by the ADA [1,2]. In addition, 17% of these children had poor glycaemic control, defined as an HbA1c level ≥ 80 mmol/mol (9.5%) [1]. The T1D Exchange Clinic Network recently reported that only a small percentage of children (17–23%) meet the ADA HbA1c target [3].
Lower socio-economic status, adolescent age, ethnic minority group, single parent family structure, greater family conflict, lower parental involvement in diabetes care, access to care difficulties, and lack of a regular diabetes provider have all been linked to higher HbA1c levels [4,5]. In addition, the SEARCH study reported that sociodemographic characteristics were associated with type of insulin regimen used [2]. Insulin pump use was more likely in children of non-Hispanic white race/ethnicity, those from higher-income families, those with more highly educated parents, and those with private insurance [2]. Intensification of regimen to insulin pump therapy was also more likely in children with these sociodemographic characteristics [6] and the lowest HbA1c levels were reported in those on insulin pump therapy [2].
Although studies have examined glycaemic control in different insulin regimens, no study has examined glycaemic control within each insulin regimen group to determine the factors that distinguish those with poor from those with intermediate or good glycaemic control. Identifying these factors may lead to interventions that could improve glycaemic control within the insulin regimen deemed most clinically appropriate for a given patient. The objectives of the present study were (1) to examine the proportion of children with Type 1 diabetes from the SEARCH study with poor, intermediate and good glycaemic control within each insulin regimen group and (2) to examine the distribution and association of sociodemographic, adherence and barriers-to-care factors in relation to poor glycaemic control within insulin regimens.
Materials and Methods
The SEARCH study is an ongoing multicentre population-based study of prevalent and incident cases of physician-diagnosed diabetes mellitus in people aged <20 years. Participants were identified in geographically defined populations of Colorado, Ohio, South Carolina and Washington, and among health plan enrollees in California and Indian Health Service beneficiaries from American-Indian populations. A detailed description of the SEARCH study and methods has been published [7,8].
SEARCH study participants with incident diabetes in 2002–2006 and 2008 who had a baseline study visit near diabetes diagnosis and at least 5 years’ duration of diabetes were invited to participate in a follow up in-person study visit (cohort visit). Cohort visit participants with Type 1 diabetes who were aged 10–17 years at the time of their follow-up visit are included in the present analysis. Participants were excluded if they did not report taking insulin at the follow-up visit (n=7), or HbA1c data were missing (n=102).
Survey information on insulin regimen, medical history, sociodemographics, adherence and barriers to care was collected from the parent and child at the study visit. Clinical and sociodemographic factors are listed in Table 1. Adherence factors included diabetes-related family conflict, hypoglycaemia fear, eating problems, frequency of blood glucose monitoring, and frequency of missed insulin doses.
Table 1.
Characteristics of participants with Type 1 diabetes by insulin regimens
| Insulin regimen | |||||
|---|---|---|---|---|---|
| Totals | Insulin pump | Basal-bolus injections | Mixed insulin | P§ | |
| Number of participants | 1095 | 694 | 188 | 213 | |
| Mean HbA1c, | <0.0001 | ||||
| mmol/mol | 77 ± 19 | 74 ± 15 | 81 ± 20 | 85 ± 21 | |
| % | 9.2 ± 1.7 | 8.9 ± 1.4 | 9.6 ± 1.8 | 9.9 ± 1.9 | <0.0001 |
| Glycaemic control* | |||||
| Age at cohort study visit, years | 14.4 ± 2.2 | 14.1 ± 2.2 | 14.6 ± 2.2 | 15.1 ± 2.2 | <0.0001 |
| Diabetes duration, years | 7.5 ± 1.8 | 7.6 ± 1.8 | 7.5 ± 1.9 | 7.4 ± 1.7 | 0.3786 |
| Race/ethnicity, n (%) | <0.0001 | ||||
| White | 828 (75.6) | 593 (85.4) | 117 (62.2) | 118 (55.4) | |
| Black | 108 (9.9) | 36 (5.2) | 27 (14.4) | 45 (21.1) | |
| Sex, n (%) | 565 (51.6) | 365 (52.6) | 87 (46.3) | 113 (53.1) | 0.2743 |
| Female | 530 (48.4) | 329 (47.4) | 101 (53.7) | 100 (46.9) | |
| Male | |||||
| Household incom†, n (%) | <0.0001 | ||||
| <$25,000 | 156 (15.3) | 63 (9.5) | 33 (19.5) | 60 (31.6) | |
| $25,000–$49,000 | 189 (18.5) | 98 (14.8) | 50 (29.6) | 41 (21.6) | |
| $50,000–$74,000 | 182 (17.8) | 117 (17.7) | 29 (17.2) | 36 (18.9) | |
| >$75,000 | 493 (48.3) | 383 (57.9) | 57 (33.7) | 53 (27.9) | |
| Maximum parental education, n (%) | <0.0001 | ||||
| ≤High school graduate | 138 (12.6) | 49 (7.1) | 42 (22.3) | 47 (22.3) | |
| Some college to associates degree | 380 (34.8) | 214 (30.9) | 79 (42.0) | 87 (41.2) | |
| ≥Bachelors degree | 574 (52.6) | 430 (62.0) | 67 (35.6) | 77 (46.5) | |
| Health insurance‡, n (%) | <0.0001 | ||||
| Private | 805 (75.9) | 556 (83.1) | 127 (69.4) | 122 (58.7) | |
| Medicaid/Medicare | 255 (24.1) | 113 (16.9) | 56 (30.6) | 86 (41.3) | |
| Number of households lived in, n (%) | 0.0255 | ||||
| 1 | 925 (84.5) | 592 (85.3) | 147 (78.2) | 186 (87.3) | |
| 2+ | 170 (15.5) | 102 (14.7) | 41 (21.8) | 27 (12.7) | |
| Parent diabetes family conflict score¶ | 25.8 ± 5.5 | 25.3 ± 5.0 | 26.4 ± 5.9 | 26.7 ± 6.2 | 0.0012 |
Data are means ± SD unless otherwise indicated.
Poor: HbA1c ≥ 80 mmol/mol (≥ 9.5%); intermediate: HbA1c 58 to <80 mmol/mol (7.5% to <9.5%); good: HbA1c <58 mmol/mol (<7.5%).
Excluding participants who did not know or chose not to answer household income question.
Other/none category excluded.
P value evaluating differences across insulin regimen groups using chi-squared (categorical) or one-way ANOVA (continuous).
Score ranges from 19 to 57. Higher score indicates more conflict. Scored for complete surveys only (N=1056).
Family conflict was assessed with a parent and child version of the validated revised Diabetes Family Conflict Scale, consisting of 19 items [9]. This survey focused on conflict over diabetes care in the past month between parent and child. It includes the following items: remembering to give insulin; taking the appropriate amount of insulin; remembering to check blood sugars; results of blood sugar monitoring; type of meals and snacks eaten; carrying treatment for hypoglycaemia; rotating injection sites or infusion sets; and logging blood sugar results. Higher scores indicate greater family conflict (total score range 19–57).
Fear of hypoglycaemia was evaluated with the validated modified Hypoglycaemia Fear Survey which includes a parent and child version of a worry and behaviour subscale [10,11]. The worry subscale measures anxiety related to negative hypoglycaemia effects, and the behaviour subscale measures behaviours taken to avoid hypoglycaemia. Higher scores indicate greater hypoglycaemia fear. Eating problems were assessed with the validated revised Diabetes Eating Problem Survey and were completed by the child only [12]. This survey focused on disordered eating behaviours and attitudes in the past month, including insulin omission or underdosing. Higher scores indicate higher risk of disordered eating.
Barriers-to-care questions were derived from the National Longitudinal Study of Adolescent Health and Consumer Assessment of Healthcare Providers and Systems survey (CAHPS 3.0) and focused on access and process barriers in the past year according to parent report [13,14]. Access barrier factors included problems with access to care, lack of a regular provider, and cost of care. Process barrier factors included contextual care (receiving care that accounts for personal and family context), provider–family communication problems, and difficulty obtaining health information.
Blood was drawn during the study visit to measure HbA1c (analysed at Northwest Laboratories) and glycaemic control was categorized based on ADA guidelines as good [<58 mmol/mol (<7.5%), intermediate [58 to <80 mmol/mol (7.5 to <9.5%)] and poor [≥ 80 mmol/mol (≥ 9.5%)] [15]. All data were collected according to standardized study protocols by trained, certified staff.
Insulin regimens were classified into three categories: 1) basal–bolus with continuous subcutaneous infusion (insulin pump therapy); 2) basal–bolus injections with glargine or detemir plus rapid-acting insulin (insulin lispro, insulin aspart, or insulin glulisine); and 3) mixed insulin regimens consisting of (a) multiple daily injections (≥3 injections) with glargine or detemir insulin plus NPH insulin plus regular or rapid-acting insulin, (b) multiple daily injections (≥3 injections) with any insulin types excluding basal insulin (glargine or detemir), or (c) one to two injections per day, excluding insulin glargine or detemir.
Statistical analyses
Demographic and clinical characteristics were summarized within insulin regimens using mean ± SD for continuous variables and counts and percentages for categorical variables. Statistical associations of sociodemographic, adherence and barriers-to-care factors with poor glycaemic control were examined within each insulin regimen using multivariable logistic regression models. Initial models were adjusted for sex, race/ethnicity, age at cohort study visit, and diabetes duration. Forward stepwise model selection suggested additional adjustments, which varied across regimens. A final unified model was fitted to facilitate comparison and interpretation of results while adjusting for all other factors in the model. Statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC, USA).
Ethical approval
The institutional review boards for each site approved the study protocol and informed consent and assent were obtained from participants.
Results
Study population
The study sample included 1095 children with Type 1 diabetes aged 10–17 years with a mean diabetes duration of 7.5 ± 1.8 years (Table 1). Their mean age at the cohort study visit was 14.4 ± 2.2 years. There were 694 participants on insulin pump, 188 on basal–bolus injections, and 213 on a mixed insulin regimen.
Glycaemic control within insulin regimens
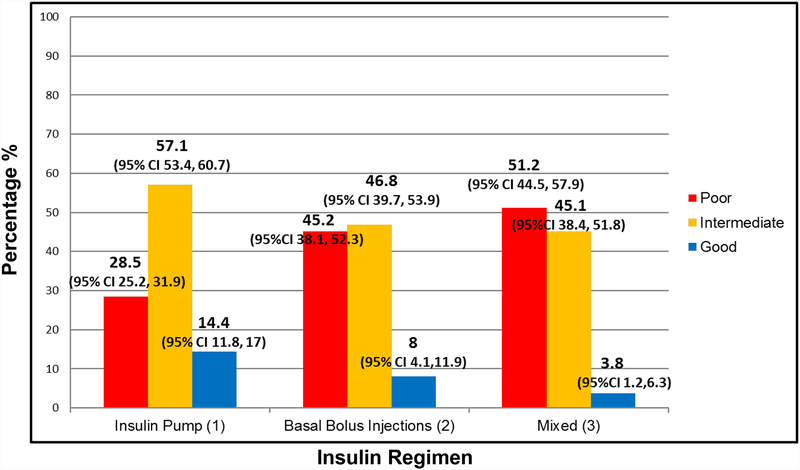
The overall mean HbA1c was 77 ± 19 mmol/mol (9.2 ± 1.7%), with 35.8% having poor glycaemic control, while only 11.2% had good glycaemic control. The percentage of children with poor glycaemic control was highest in the mixed insulin regimen group (regimen 3) and lowest in the insulin pump group (regimen 1). Of the participants using an insulin pump, 28.5% (95% CI 25.2–31.9) had poor glycaemic control, while 45.2% (95% CI 38.1–52.3) of those on basal–bolus injections and 51.2% (95% CI 44.5–57.9) of those on a mixed insulin regimen had poor glycaemic control. Only a small percentage of participants were within the ADA HbA1c target of <58 mmol/mol (<7.5%): 14.4% (95% CI 11.8–17.0) of those on insulin pump, 8.0% (95% CI 4.1–11.9) of those on basal–bolus injections, and 3.8% (95% CI 1.2–6.3) of those on mixed insulin (Table 1 and Fig. 1).
Figure 1. Glycaemic control within insulin regimen groups.
Poor: HbA1c ≥ 80 mmol/mol (≥ 9.5%); intermediate: HbA1c 58 to <80 mmol/mol (7.5% to <9.5%); good: HbA1c <58 mmol/mol (<7.5%).
Association of sociodemographic, adherence and barriers-to-care factors with poor glycaemic control within insulin regimens
Odds ratios (ORs) from the multivariable analysis examining the factors associated with poor glycaemic control within insulin regimens are shown in Table 2 (unadjusted) and Table 3 (adjusted for all variables including diabetes duration, race/ethnicity, age at cohort study visit, and sex). Unadjusted factors associated with higher odds of poor glycaemic control in all three insulin regimen groups were non-white race, living in multiple households, decreased adherence to insulin treatment, parent-reported diabetes-related family conflict, eating disorders, and not spending enough time with healthcare provider (Table 2). Having Medicaid insurance, longer diabetes duration, and older age at cohort study visit was associated with poor glycaemic control in those on insulin pump. Having Medicaid insurance, and being female were also associated with poor glycaemic control in children on a mixed insulin regimen.
Table 2.
Unadjusted odds ratios (95% CI) for logistic regression model predicting poor glycaemic control (HbA1c ≥ 80 mmol/mol, 9.5%) within insulin regimens in children with Type 1 diabetes
| Regimen | ||||||
|---|---|---|---|---|---|---|
| Insulin pump N=694 (198 events*) | Basal-bolus injections N=188 (85 events*) | Mixed insulin N= 213 (109 events*) | ||||
| Factor | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P |
| Diabetes duration, years† | 1.1 (1.01, 1.22) | 0.0265 | 1.1 (0.93, 1.26) | 0.3162 | 1.1 (0.96, 1.33) | 0.1321 |
| Non-white race/ethnicity (referent: non-Hispanic white) | 2.7 (1.74, 4.12) | <0.0001 | 1.9 (1.04, 3.42) | 0.0380 | 1.8 (1.02, 3.04) | 0.0424 |
| Female sex (referent: male) | 1.2 (0.85, 1.64) | 0.3237 | 0.8 (0.46, 1.46) | 0.4927 | 1.7 (1.00, 2.97) | 0.0495 |
| Age at cohort study visit† | 1.2 (1.07, 1.25) | 0.0003 | 1.1 (0.92, 1.20) | 0.4485 | 1.1 (0.99, 1.27) | 0.0668 |
| Number of households lived in ≥ 2 (referent: living in 1 household) | 2.4 (1.55, 3.66) | <0.0001 | 2.6 (1.26, 5.29) | 0.0093 | 3.9 (1.50, 10.10 | 0.0051 |
| Medicaid/Medicare Insurance (Referent: private insurance) | 2.6 (1.69, 3.88) | <0.0001 | 1.5 (0.80, 2.82) | 0.2087 | 2.2 (0.25, 3.87) | 0.0063 |
| Number of times missed insulin | <0.0001 | 0.0082 | 0.0011 | |||
| 1–5/week vs <1 week | 4.2 (2.75, 6.36) | <0.0001 | 2.8 (1.16, 6.80) | 0.0218 | 1.7 (0.75, 3.83) | 0.2034 |
| 1+/day vs <1 week | 3.5 (2.08, 5.96) | <0.0001 | 3.4 (1.23, 9.55) | 0.0185 | 9.8 (2.81, 33.91 | 0.0003 |
| Family conflict survey (parent report)‡ | 1.14 (1.10, 1.19) | <0.0001 | 1.2 (1.08, 1.23) | <0.0001 | 1.1 (1.06, 1.18) | <0.0001 |
| Low blood sugar survey (parent report)‡ | 0.99 (0.98, 1.00) | 0.0943 | 0.98 (0.96, 0.997) | 0.0269 | 1.00 (0.98, 1.02 | 0.8168 |
| Eating problem survey‡ | 1.06 (1.04, 1.07) | <0.0001 | 1.10 (1.05, 1.14) | <0.0001 | 1.06 (1.03, 1.08 | <0.0001 |
| Problems receiving care (access barrier; referent: no problem receiving care) | 1.1 (0.48, 2.38) | 0.8635 | 1.2 (0.35, 4.45) | 0.7375 | 0.6 (0.24, 1.55) | 0.2974 |
| Problem not spending enough time with provider (process barrier; referent: no problem spending enough time with provider) | 1.5 (1.06, 2.18) | 0.0235 | 2.0 (1.05, 3.72) | 0.0359 | 2.1 (1.13, 3.81) | 0.0183 |
OR, odds ratio.
Logistic regression model predicts odds of poor control, therefore events refers to number of participants with poor glycaemic control.
Per 1 year increase
Per 1 unit increase on survey.
Table 3.
Adjusted odds ratios (95% CI) for multivariable logistic regression model predicting poor glycaemic control [HbA1c ≥ 80 mmol/mol (9.5%)] within insulin regimens in children with Type 1 diabetes
| Insulin regimen | ||||||
|---|---|---|---|---|---|---|
| Insulin pump N=596 (171 events) | Basal-bolus injections N=160 (68 events) | Mixed insulin N=171 (90 events) | ||||
| Factor | Adjusted OR (95% CI)* | P | Adjusted OR (95% CI)* | P | Adjusted OR (95% CI)* | P |
| Diabetes duration (years)† | 1.1 (0.97, 1.22) | 0.1594 | 1.1 (0.89, 1.38) | 0.3581 | 1.1 (0.90, 1.47) | 0.2783 |
| Non-white race/ethnicity (referent: non-Hispanic white) | 2.7 (1.50, 4.72) | 0.0008 | 1.7 (0.64, 4.62) | 0.2848 | 1.7 (0.70, 4.22) | 0.2421 |
| Female sex (referent: male) | 1.1 (0.69, 1.66) | 0.7502 | 0.5 (0.21, 1.22) | 0.1280 | 1.4 (0.60, 3.10) | 0.4632 |
| Age at cohort study visit† | 1.1(0.99, 1.22) | 0.0634 | 0.9 (0.76, 1.12) | 0.4117 | 1.1 (0.89, 1.26) | 0.4888 |
| Number of households livet n > 2 (referent: living in 1 household) | 1.8 (1.05, 3.08) | 0.0331 | 2.2 (0.78, 5.95) | 0.1384 | 4.8 (1.21, 19.27) | 0.0256 |
| Medicaid/Medicare Insurance (referent: private insurance) | 2.0 (1.19, 3.38) | 0.0090 | 0.8 (0.29, 1.93) | 0.5565 | 2.2 (0.985, 5.17) | 0.0649 |
| Number of times missed insulin | <0.0001 | 0.1360 | 0.2130 | |||
| 1–5/week vs <1 week | 4.0 (2.40, 6.75 | <0.0001 | † | † | † | † |
| 1+/day vs <1 week | 2.4 (1.27, 4.73) | 0.0075 | † | † | † | † |
| Family conflict survey (parent report)‡ | 1.1 (1.05, 15) | <0.0001 | 1.2 (1.10, 1.35) | 0.0002 | 1.1 (1.03, 1.22) | 0.0103 |
| Low blood sugar survey (parent report)‡ | 0.97 (0.95, 0.99) | 0.0004 | 0.93 (0.90, 0.97) | 0.0001 | 1.0 (0.98,1.03) | 0.6998 |
| Eating problem survey‡ | 1.0 (0.99, 1.04) | 0.2609 | 1.1 (0.99, 1.12) | 0.0857 | 1.0 (0.97, 1.06) | 0.4114 |
| Problem not receiving care (access barrier; referent: no problem receiving care) | 0.9 (0.32, 2.28) | 0.7476 | 0.4 (0.06, 2.80) | 0.3716 | 0.1 (0.03, 0.51) | 0.0038 |
| Problem not spending enough time with provider (process barrier; referent: no problem spending enough time with provider) | 1.3 (0.85, 2.11) | 0.2136 | 1.4 (0.57, 3.48) | 0.4649 | 3.8 (1.47, 9.64) | 0.0058 |
OR, odds ratio.
Adjusted for all variables shown in the table, as well as clinical site.
Per 1-year increase.
Per 1-unit increase on survey.
Not displayed where overall covariate P value is >0.05.
After adjusting for all selected clinical, sociodemographic, adherence and barriers-to-care factors in the unified model, the variables significantly associated with poor glycaemic control in children on insulin pump therapy included: non-white race [OR 2.7 (95% CI 1.5–4.72)], living in multiple households [OR 1.8 (95% CI 1.05–3.08)], having Medicaid insurance [OR 2.0 (95% CI 1.19–3.38)], and low insulin treatment adherence [OR 4.0 (95% CI 2.40–6.75) for missing insulin one to five times per week vs less than once per week (Table 3)]. For children on a mixed insulin regimen, living in multiple households [OR 4.8 (95% CI 1.21–19.27)] and not spending enough time with healthcare provider [OR 3.8 (95% CI 1.47–9.64)] were correlates of poor control (Table 3). In the multivariable analysis after adjusting for all factors in the unified model, parent-reported diabetes-related family conflict was the only factor significantly associated with poor glycaemic control in all three insulin regimen groups. Child-reported diabetes-related family conflict was not significantly associated with poor glycaemic control. In children on insulin pump therapy with poor glycaemic control, the mean ± SD parent-reported diabetes family conflict score was 27.8 ±5.8, in children on basal-bolus injections the score was 28.8 ± 6, and for children on a mixed insulin regimen the score was 28.5±6.7. Each 1-point increase in the parent-reported family conflict score above the minimum score of 19 (score range 19–57, with a higher score indicating more conflict) was associated with increasing odds of poor glycaemic control [per 1-point increase: insulin pump therapy, OR 1.1 (95% CI 1.05–1.15); basal–bolus injections, OR 1.2 (95% CI 1.10, 1.35); mixed insulin regimen, OR 1.1 (95% CI 1.03, 1.22); Table 3].
Parental fear of hypoglycaemia was associated with slightly lower odds of poor glycaemic control in young people on insulin pump therapy [OR 0.97 (95% CI 0.95, 0.99)] and basal–bolus injections [OR 0.93 (95% CI 0.90, 0.97); Table 3].
Discussion
The results from this SEARCH study of 1095 US children with Type 1 diabetes show that a high proportion had poor glycaemic control regardless of insulin regimen, especially among those not on an insulin pump. Diabetes-related family conflict was a key factor associated with poor glycaemic control noted within all insulin regimen groups. The distribution of additional sociodemographic, adherence and barriers-to-care factors associated with poor, intermediate and good glycaemic control varied within insulin regimens. In the group with the best glycaemic control (those on insulin pump therapy), non-white race, having Medicaid insurance, living in multiple households, and low insulin adherence were notable factors associated with poor glycaemic control. In the most vulnerable group with the highest HbA1c (those on a mixed insulin regimen), significant correlates of poor glycaemic control were inadequate social and environmental support (living in multiple households, not spending enough time with the provider, and diabetes-related family conflict). This study demonstrates that a combination of sociodemographic, adherence and barriers-to-care factors are significantly related to glycaemic control and that these factors vary within insulin regimens. Addressing these factors within insulin regimens may improve care for children with Type 1 diabetes.
Parent-reported diabetes-related family conflict was the only factor significantly associated with poor glycaemic control in all insulin regimen groups after adjustment for other variables in the unified model. Higher diabetes-related family conflict in children is a strong predictor of poor glycaemic control [16,17], probably as a result of its impact on adherence to diabetes management. Anderson et al. [16] reported that child and parental report of higher diabetes-related family conflict was significantly associated with higher HbA1c levels. In another study of 145 adolescents with Type 1 diabetes and their parents over 1 year, higher diabetes-related family conflict scores predicted decreased adherence to blood glucose monitoring and poorer HbA1c [17]. Furthermore, higher diabetes-related family conflict has been reported as a significant predictor of decreased quality of life in children with Type 1 diabetes [18,19]. Increased diabetes-related family conflict is also linked with depression and anxiety in children with Type 1 diabetes [20,21]. The present study supports the findings of previous studies and highlights the significant association of diabetes-related family conflict with poor glycaemic control regardless of type of insulin regimen. It is important that the medical team identify this conflict so that it can be addressed. To our knowledge, the present study is the largest Type 1 diabetes cohort examination of diabetes-related family conflict.
Targeted behavioural interventions may be used to address diabetes-related family conflict and potentially improve diabetes management. Wysocki et al. [22] reported the effectiveness of Behavioural Family Systems Therapy for Diabetes (BFST-D) over 18 months, with improvement of HbA1c in adolescents with Type 1 diabetes. In that study, children with Type 1 diabetes and their families received training sessions focused on communication, problem-solving, cognitive restructuring of attitudes/viewpoints, and family function and structure [22]. Implementation of interventions to reduce diabetes-related family conflict is important given it is one of the potentially modifiable factors in the home environment that could significantly improve diabetes care management.
Sociodemographic and adherence factors have also been associated with poor glycaemic control in numerous studies of children with Type 1 diabetes [3,23]. Similarly to other studies, we found that minority race/ethnicity (non-white) was associated with higher HbA1c [24,25]; however, after adjustment for other sociodemographic and adherence factors, this factor remained significant only for children on insulin pump therapy. Non-Hispanic white children were more likely to be on insulin pumps compared to other insulin regimen groups, and within the insulin pump group, race/ethnicity was an important correlate of glycaemic control. The smaller sample size for those on basal–bolus injections and mixed insulin regimens compared to the insulin pump group may explain the nonsignificance of race/ethnicity after adjustment for basal–bolus injection regimen and mixed insulin regimen.
As in the present study, previous studies have also reported that public health insurance (Medicaid) is independently associated with poorer glycaemic control [26,27]. In the present study, this correlate of poor glycaemic control was significant only for children on insulin pump therapy. Children with private insurance were more likely to be on insulin pumps compared to other insulin regimens.
Family structure may also affect glycaemic control. Previous studies have reported worse glycaemic control in children whose parents were separated or divorced compared to children with married parents [28,29]. This supports our finding that participants living in two or more households were more likely to have poor glycaemic control in both the insulin pump therapy and the mixed insulin regimen groups.
Not surprisingly, decreased adherence to insulin administration was also associated with poor glycaemic control. This factor remained significant after adjustment for other sociodemographic and adherence factors in children on insulin pump therapy only. This may be attributable to inaccurate reporting of insulin adherence from children on basal–bolus injections and mixed insulin regimens.
Despite being on an insulin pump, children with lower socio-economic status and decreased environmental stability (living in multiple households) remained at high risk of poor glycaemic control. Awareness of the above factors may help diabetes providers in their management of children with Type 1 diabetes. Early identification of children with the risk factors associated with poor glycaemic control noted above may lead to increased and earlier interventions resulting in improved patient care.
Inadequate time with the diabetes care provider was the only barrier-to-care factor that remained a significant correlate of poor glycaemic control after adjustment for other sociodemographic, adherence and barriers-to-care factors. This was only noted for the group on a mixed insulin regimen, which contained the highest percentage of minority race children and Medicaid insured children of all regimen groups. The SEARCH study group has previously reported an association between barriers to care and poor glycaemic control [5]. Targeted efforts to improve diabetes care in this high-risk population may include increased time with the diabetes provider, social worker and the certified diabetes educator.
Limitations of the present study include self-report of adherence and barriers-to-care factors; participants may have biased estimations of their adherence (frequency of self-blood glucose monitoring, frequency of missed insulin injections, diabetes-related family conflict, hypoglycaemia fear, and eating problems) and may have under-reported or denied their barriers to care. The notably lower number of participants on basal–bolus injections and a mixed insulin regimen compared to those on insulin pump therapy may have limited the study’s statistical power to detect potential significant correlates of poor glycaemic control in those on a basal–bolus injection regimen or a mixed insulin regimen. Our results derive from participants who attended at least two research visits and therefore their access to care may be relatively high, with a bias towards lower report of barriers to care. Finally, as this was a cross-sectional study, causality cannot be determined between factors such as diabetes-related family conflict and poor glycaemic control.
The SEARCH study includes a large and racially diverse cohort in multiple centres across the USA. The present study is also the first study, to our knowledge, that examines the influence of sociodemographic, adherence and barriers-to-care factors within insulin regimens. Furthermore, the inclusion of additional social and psychological factors including diabetes-related family conflict in our analysis of poor glycaemic correlates builds on previous SEARCH studies.
In conclusion, the substantial proportion and numbers of US children with poor glycaemic control and HbA1c values over the ADA-recommended target is a critical and complex problem. Multiple sociodemographic, adherence and barriers-to-care factors are associated with poor glycaemic control, and the distribution of these factors varied within insulin regimens. Lower socio-economic status significantly increased the likelihood of poor control for children on insulin pump therapy, while reporting inadequate time with their diabetes provider was a notable correlate of poor control for those on a mixed insulin regimen. Home environmental instability was a significant poor glycaemic correlate for both children on insulin pump therapy and those on a mixed insulin regimen. The present study highlights the importance of increased support and education for children with Type 1 diabetes with these circumstances to overcome these social obstacles. In addition, the association between diabetes-related family conflict and poor glycaemic control regardless of insulin regimen supports the importance of screening for and addressing family conflict in all children with Type 1 diabetes. The identification and implementation of effective and practical strategies aimed at expanding social support for children with Type 1 diabetes and their families may result in improved glycaemic control.
What’s new?
Despite advances in the treatment of Type 1 diabetes, a significant proportion of children with Type 1 diabetes have poor glycaemic control.
This study in children with Type 1 diabetes identified multiple sociodemographic, adherence and barriers-to-care correlates of poor glycaemic control, which varied within each insulin regimen group.
This study provides the largest Type 1 diabetes cohort examination of diabetes-related family conflict, and found that family conflict regarding diabetes management was the only significant poor glycaemic correlate in all insulin regimens.
Routine screening for and addressing of correlates of poor glycaemic control, including diabetes-related family conflict, is critical in improving the care of children with Type 1 diabetes.
Acknowledgements
The SEARCH study is indebted to the many young people and their families, and their healthcare providers, whose participation made this study possible. SEARCH 3/4: The authors wish to acknowledge the involvement of the Kaiser Permanente Southern California’s Clinical Research Centre (funded by Kaiser Foundation Health Plan and supported in part by the Southern California Permanente Medical Group; the South Carolina Clinical and Translational Research Institute, at the Medical University of South Carolina, National Institute of Health (NIH)/National Centre for Advancing Translational Sciences (NCATS) grant number UL1 TR000062, UL1 Tr001450; Seattle Children’s Hospital and the University of Washington, NIH/NCATS grant number UL1 TR00423; University of Colorado Paediatric Clinical and Translational Research Centre, NIH/NCATS grant Number UL1 TR000154; the Barbara Davis Centre at the University of Colorado at Denver (DERC NIH grant number P30 DK57516); the University of Cincinnati, NIH/NCATS grant number UL1 TR000077, UL1 TR001425; and the Children with Medical Handicaps programme, managed by the Ohio Department of Health. This study includes data provided by the Ohio Department of Health, which should not be considered an endorsement of this study or its conclusions. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centres for Disease Control and Prevention or the National Institute of Diabetes and Digestive and Kidney Diseases.
Funding sources
Grant support SEARCH 3: The SEARCH for Diabetes in Youth study is funded by the Centres for Disease Control and Prevention (PA numbers 00097, DP-05–069, and DP-10–001) and supported by the National Institute of Diabetes and Digestive and Kidney Diseases. Grant support SEARCH 4: The SEARCH for Diabetes in Youth Cohort study (1UC4DK108173) is funded by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases and supported by the Centres for Disease Control and Prevention. The Population Based Registry of Diabetes in Youth Study (1U18DP006131, U18DP006133, U18DP006134, U18DP006136, U18DP006138, U18DP006139) is funded by the Centres for Disease Control and Prevention and supported by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. Sites SEARCH 3/4: Kaiser Permanente Southern California (U18DP006133, U48/CCU919219, U01 DP000246, and U18DP002714), University of Colorado Denver (U18DP006139, U48/CCU819241–3, U01 DP000247, and U18DP000247–06A1), Cincinnati’s Children’s Hospital Medical Centre (U18DP006134, U48/CCU519239, U01 DP000248, and 1U18DP002709), University of North Carolina at Chapel Hill (U18DP006138, U48/CCU419249, U01 DP000254, and U18DP002708), Seattle Children’s Hospital (U18DP006136, U58/CCU019235–4, U01 DP000244, and U18DP002710–01), Wake Forest University School of Medicine (U18DP006131, U48/CCU919219, U01 DP000250, and 200-2010-35171).
Footnotes
Competing interests
None declared.
References
- 1.Petitti DB, Klingensmith GJ, Bell RA, Andrews JS, Dabelea D, Imperatore G et al. Glycaemic control in youth with diabetes: the SEARCH for diabetes in Youth Study. J Pediatr 2009; 155:668–672, e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paris CA, Imperatore G, Klingensmith G, Petitti D, Rodriguez B, Anderson AM et al. Predictors of insulin regimens and impact on outcomes in youth with type 1 diabetes: the SEARCH for Diabetes in Youth study. J Pediatr 2009; 155:183–189. [DOI] [PubMed] [Google Scholar]
- 3.Miller KM, Foster NC, Beck RW, Bergenstal RM, DuBose SN, DiMeglio LA et al. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D exchange clinic registry. Diabetes Care 2015;38:971–978. [DOI] [PubMed] [Google Scholar]
- 4.Neylon OM, O’Connell MA, Skinner TC, Cameron FJ. Demographic and personal factors associated with metabolic control and self-care in youth with type 1 diabetes: a systematic review. Diabetes Metab Res Rev 2013; 29:257–272. [DOI] [PubMed] [Google Scholar]
- 5.Valenzuela JM, Seid M, Waitzfelder B, Anderson AM, Beavers DP, Dabelea DM et al. ; SEARCH for Diabetes in Youth Study Group. Prevalence of and disparities in barriers to care experienced by youth with type 1 diabetes. J Pediatr 2014;164:1369–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pihoker C, Badaru A, Anderson A, Morgan T, Dolan L, Dabelea D et al. Insulin regimens and clinical outcomes in a type 1 diabetes cohort: the SEARCH for Diabetes in Youth study. Diabetes Care 2013; 36: 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamman RF, Bell RA, Dabelea D, D’Agostino RB Jr, Dolan L, Imperatore G et al. The SEARCH for Diabetes in Youth study: rationale, findings, and future directions. Diabetes Care 2014; 37:3336–3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.SEARCH Study Group. SEARCH for Diabetes in Youth Study: a multicenter study of the prevalence, incidence and classification of diabetes mellitus in youth. Control Clin Trials 2004; 25: 458–471. [DOI] [PubMed] [Google Scholar]
- 9.Hood KK, Butler DA, Anderson BJ, Laffel LMB. Updated and revised Diabetes Family Conflict Scale. Diabetes Care 2007;30:1764–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green LB, Wysocki T, Reineck BM. Fear of hypoglycemia in children and adolescents with diabetes. J Pediatr Psychol 1990;15:633–641. [DOI] [PubMed] [Google Scholar]
- 11.Clarke WL, Gonder-Frederick A, Snyder AL, Cox DJ. Maternal fear of hypoglycemia in their children with insulin dependent diabetes mellitus. J Pediatr Endocrinol Metab 1998;11 (Suppl. 1):189–194. [DOI] [PubMed] [Google Scholar]
- 12.Markowitz JT, Butler DA, Volkening LK, Antisdel JE, Anderson BJ, Laffel LM. Brief screening tool for disordered eating in diabetes: internal consistency and external validity in a contemporary sample of pediatric patients with type 1 diabetes. Diabetes Care 2010;33:495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ford CA, Bearman PS, Moody J. Foregone Health Care Among Adolescents. JAMA. 1999;282:2227–2234. [DOI] [PubMed] [Google Scholar]
- 14.Consumer assessment of healthcare providers and systems (CAHPS 3.0) Supplemental Item Set for Children with Chronic Conditions questionnaires [Internet] Available at https://www.ahrq.gov/sites/default/files/wysiwyg/cahps/surveys-guidance/item-sets/children-chronic/102_Children_with_Chronic_Conditions_Set_2008.pdf. Last accessed.
- 15.American Diabetes Association: Standards of medical care in diabetes (position statement) Diabetes Care 2010;33(Suppl. 1):S11–S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson BJ, Vangsness L, Connell A, Butler D, Goebel-Fabbri A, Laffel LM. Family conflict, adherence, and glycaemic control in youth with short duration Type 1 diabetes. Diabet Med 2002; 19:635–642. [DOI] [PubMed] [Google Scholar]
- 17.Hilliard ME, Guilfoyle SM, Dolan LM, Hood KK. Prediction of adolescents’ glycaemic control 1 year after diabetes-specific family conflict: The mediating role of blood glucose monitoring adherence. Arch Pediatr Adolesc Med 2011;165:624–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laffel LM, Connell A, Vangsness L, Goebel-Fabbri A, Mansfield A, Anderson BJ. General quality of life in youth with type 1 diabetes: relationship to patient management and diabetes- specific family conflict. Diabetes Care 2003;26:3067–3073. [DOI] [PubMed] [Google Scholar]
- 19.Weissberg-Benchell J, Nansel T, Holmbeck G, Chen R, Anderson B, Wysocki T et al. Generic and Diabetes-specific Parent- Child Behaviors and Quality of Life Among Youth with Type 1 Diabetes. J Pediatr Psychol 2009;34:977–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams LB, Laffel LM, Hood KK. Diabetes-specific family conflict and psychological distress in paediatric Type 1 diabetes. Diabet Med 2009;26:908–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herzer M, Vesco A, Ingerski LM, Dolan LM, Hood KK. Explaining the family conflict-glycaemic control link through psychological variables in adolescents with type 1 diabetes. J Behav Med 2011; 34: 268–274. [DOI] [PubMed] [Google Scholar]
- 22.Wysocki T, Harris MA, Buckloh LM, Mertlich D, Lochrie AS, Mauras N et al. Randomized Trial of Behavioral Family Systems Therapy for Diabetes: maintenance of effects on diabetes outcomes in adolescents. Diabetes Care 2007; 30: 555–560. [DOI] [PubMed] [Google Scholar]
- 23.Campbell MS, Schatz DA, Chen V, Wong JC, Steck A, Tamborlane W. T1D Exchange Clinic Network A contrast between children and adolescents with excellent and poor control: The T1D Exchange Clinic Registry experience. Pediatr Diabetes 2014;15:110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gallegos-Macias AR, Macias SR, Kaufman E, Skipper B, Kalishman N. Relationship between glycaemic control, ethnicity and socioeconomic status in hispanic and white non-hispanic youths with type 1 diabetes mellitus. Pediatr Diabetes 2003; 4: 19–23. [DOI] [PubMed] [Google Scholar]
- 25.Redondo MJ, Connor CG, Ruedy KJ, Beck RW, Kollman C, Wood JR et al. Pediatric Diabetes Consortium Type 1 Diabetes (T1D) New Onset (NeOn) Study: Factors Associated with HbA1c Levels One Year after Diagnosis. Pediatr Diabetes 2014;15:294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wintergerst KA, Hinkle KM, Barnes CN, Omoruyi AO, Foster MB. The impact of health insurance coverage on pediatric diabetes management. Diabetes Res Clin Pract 2010;90:40–44. [DOI] [PubMed] [Google Scholar]
- 27.Beck JK, Lewis TV, Logan KJ, Harrison DL, Gardner AW, Copeland KC. Intensive vs conventional insulin management initiated at diagnosis in children with diabetes: Should payer source influence the choice of therapy? Pediatr Diabetes 2009;10:368–373. [DOI] [PubMed] [Google Scholar]
- 28.Cameron FJ, Skinner TC, de Beaufort CE, Hoey H, Swift PG, Aanstoot H et al. Are family factors universally related to metabolic outcomes in adolescents with Type 1 diabetes? Diabet Med 2008; 25: 463–468. [DOI] [PubMed] [Google Scholar]
- 29.Urbach SL, LaFranchi S, Lambert L, Lapidus JA, Daneman D, Becker TM. Predictors of glucose control in children and adolescents with Type 1 diabetes mellitus. Pediatr Diabetes 2005;6:69–74. [DOI] [PubMed] [Google Scholar]