Abstract
Introduction:
Sarcomas of the mandible are extremely rare tumors, with Osteosarcoma being the most common, followed by Ewing’s sarcoma
Materials and Methods:
A retrospective review of the clinical records, imaging studies and pathology slides of patients with sarcoma of the mandible at a tertiary care cancer center between 1998–2014 was undertaken. The impact of neoadjuvant chemotherapy and postoperative radiotherapy with or without chemotherapy was studied, and factors impacting upon local control and disease specific survival were analyzed.
Results:
Twenty-two patients were treated over the study period, comprising of 15 males and 7 females. External swelling, intraoral growth or facial numbness were the presenting symptoms. Eighteen patients had osteosarcoma and four had Ewing’s sarcoma. Nine patients received neoadjuvant chemotherapy. All but one patient underwent surgery. Eleven had negative margins, with 90% recurrence free survival at 3 years, compared to 10 with positive or close margins, leading to 67% recurrence free survival. None of the patients receiving neoadjuvant chemotherapy developed recurrence and all were alive at 3 years. The impact of postoperative radiotherapy or adjuvant chemotherapy was not statistically significant.
Conclusions:
Wide surgical resection with negative margins remain the hall mark of surgical treatment.
Keywords: Sarcoma, Mandible tumors, Osteosarcoma, Ewing’s sarcoma
INTRODUCTION
Sarcomas of the craniofacial skeleton are extremely rare tumors comprising approximately 1% of all head and neck malignancies.1 Sarcomas of the mandible account for only 4–10%2 of all the sarcomas in head and neck. These rare tumors are of mesenchymal origin, with osteosarcoma and chondrosarcoma being the most common histological types; followed by Ewing sarcoma. Generally, osteosarcoma occurs in the metaphyseal region of the long bones in the extremities3 showing evidence of osteogenic differentiation,4 commonly affecting the pediatric adolescent population with a second peak in older adults. Chondrosarcoma on the other hand is a tumor of adults, showing hyaline cartilage differentiation which may arise within or on the surface of a bone.5,6 In the mandible these lesions may cause deviation and progressive occlusal changes.7 Ewing sarcoma arises in the medullary cavity in the diaphysis of long bones and was originally described as the ‘diffuse endothelioma of the bone’.8,9 All of these sarcomas arising in the cranio-facial skeleton are grouped together because of their rarity and somewhat similar clinical presentation but with a variable prognostic outcome .10 However, they are listed as separate entities based on their histological diagnosis and biological behavior
The treatment of choice for most bone sarcomas regardless of the histological subtype is complete surgical resection with wide margins with or without pre operative chemotherapy .11 While wide resection with clear margins is attainable in the extremities, it is challenging in the head and neck region due to the intricate anatomy and proximity of vital organs, often resulting in incomplete resection leading to local recurrence.12–17 Therefore surgical resection is often followed by adjuvant radiotherapy and/or chemotherapy to prevent recurrence. While the role of chemotherapy in osteosarcomas of the extremities in the pediatric population is well established, it remains controversial in the adult population and particularly in the head and neck region.18,19 However, there are some reports in the literature showing improved survival in select cases from 20% to 60–70% with the use of chemotherapy.20–22 Due to the rarity of mandibular sarcomas, there is limited data reporting on the factors influencing prognosis or survival in patients with these tumors.10 The purpose of this study is to report our experience with sarcomas of the mandible treated at a tertiary care cancer center over the last two decades.
MATERIAL AND METHODS
After approval from Institutional Review Board (IRB# 16–164), we conducted a retrospective review of all patients with the diagnosis of sarcoma of the mandible treated at Memorial Sloan Kettering Cancer Center between 1998 and 2014. Twenty-two patients were identified from the institutional database. Only patients who received their initial definitive treatment at our institution were included in the study. Because of the rarity of these tumors only a small number of patients were recorded over several years, and as a result there was no established protocol for the use of neo-adjuvant chemotherapy. The decision to administer neo-adjuvant chemotherapy was based on assessment by the treating physician. In general, neo-adjuvant chemotherapy was offered to patients with high-grade osteosarcoma and to the patients with tumors presenting at an advanced stage or deemed unresectable. All chemotherapy protocols employed during this period called for the administration of cisplatin (120mg/m2), high dose methotrexate (12g/m2), and doxorubicin (75 mg/m2) intravenously. In addition, a subset of more recently treated patients also received intravenous ifosfamide (9 g/m2) and etoposide (500mg/m2). All but one patient underwent surgery. The remaining patient had chemotherapy and radiation therapy. Eleven of the 22 patients received post-operative radiotherapy.
Archived histologic material available from 18 cases was retrieved and reviewed for confirmation of diagnosis and grading by two pathologists who were blinded to the patient’s outcomes. All 18 cases were osteosarcomas and were histologically typed as osteoblastic, chondroblastic and fibroblastic subtypes. Response to therapy and margin status were recorded from pathology reports. As per published literature,23 Grade 1 corresponds to tumors with tumor response in less than 50% of tumor, grade 2 to tumors that had areas of acellular tumor osteoid and necrotic/fibrotic material (50–90%), grade 3 to tumors with predominant areas of acellular tumor osteoid, necrosis, and/or fibrotic material with only scattered foci of histologically viable tumor cells (90–99%), and grade 4 showing no histological evidence of viable tumor (100%). Recurrence rates and follow up data for survivorship was collected in the usual fashion. Kaplan-Meier curves were created to report longitudinal outcomes of therapy.
RESULTS
A total of 22 patients were identified that met our inclusion criteria, aged between 6–78 years (5 patients were younger than 18 years). There were 15 (68%) males and 7 (32%) females. A mass or swelling (Fig.1) was the presenting symptom in most patients (n=15), of which 6 patients presented with a painful mass. Four of these patients also presented with intraoral findings presenting as an ulcerated submucosal mass or a fleshy nodular submucosal mass. without any active sign of bleeding was present in 3 patients. (Fig.2) Four patients presented with numbness of the skin of the chin. Of the 22 patients, 8 (36%) had undergone dental extractions and/or biopsy for a suspected dental problem before the diagnosis of sarcoma was established.
Figure. 1:
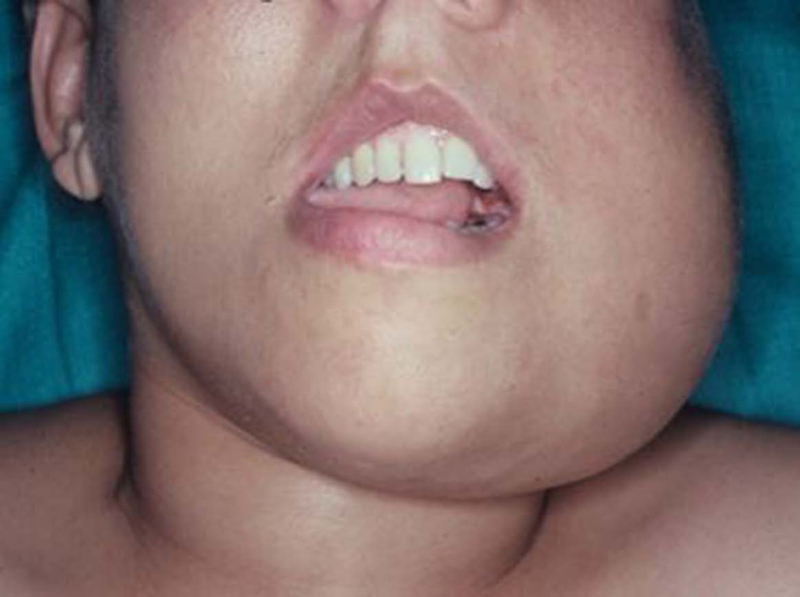
Extraoral clinical feature of osteosarcoma showing diffuse swelling.
Figure. 2:
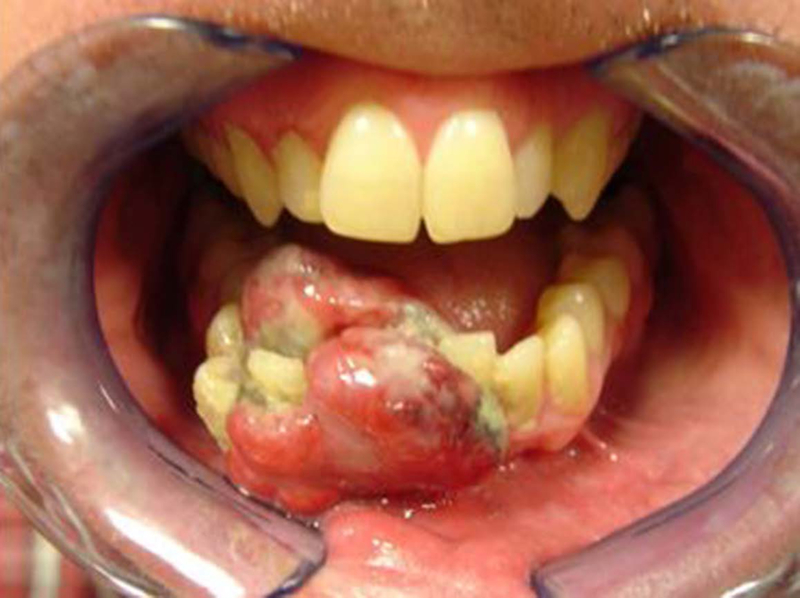
Intraoral presentation of osteosarcoma showing a fleshy nodular submucosal mass.
Radiological evaluation with a CT scan or an MRI, or both, were performed in all patients. The characteristic features on imaging studies of various sarcomas in general, but osteosarcoma in particular often predicts the biological behavior of the tumor. For instance, the presence of osteoid matrix formation is a characteristic feature of osteosarcoma. It also shows a variety of other features including, osteolytic to mixed and osteogenic patterns in the involved bone.24 The tumor invading the periosteum often produces a ‘sun-burst’ radiographical appearance showing many thin irregular spicules of new bone developing outward and perpendicular to the surface of lesion. Five patients exhibited this characteristic radiographic finding of osteosarcoma. (Fig.3 A and B) On the other hand, lesion of the other 6 patients remained confined to the bone, with only slight cortical expansion. (Fig.4 A and B)
Figure. 3:
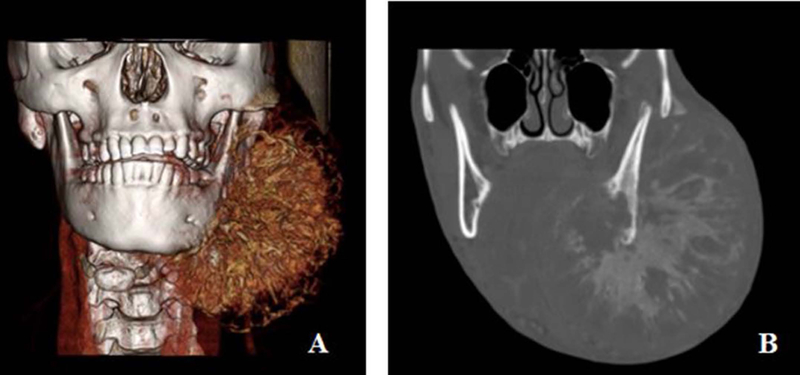
3 D reconstruction of the CT scan showing extensive soft tissue disease with new bone formation (A), and coronal of the CT scan with bone windows show the characteristic sun burst appearance of new bone formation in the tumor. (B).
Figure. 4:
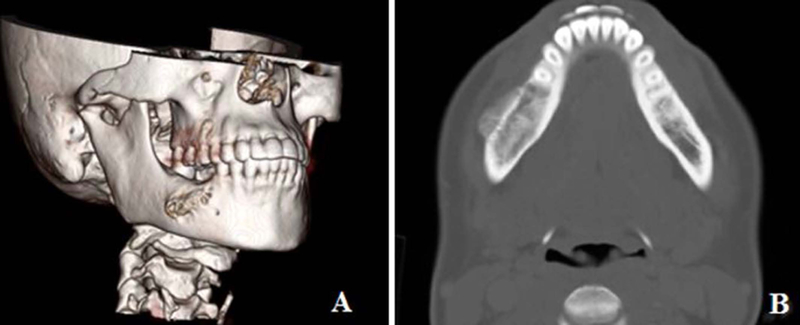
3 D reconstruction of the CT scan of the mandible showing only slight expansion of the lateral cortex of the madible (A), and axial view of the CT scan in bone window majority of the tumor being endosteal with slight expansion of the lateral cortex (B).
Clinical cohort included 18 patients with the diagnosis of osteosarcoma, and 4 patients with Ewing’s sarcoma. Surgery with gross tumor resection was completed for 21 patients. Microscopic soft tissue margins of resection were reported as negative in 11 patients (52%) and positive or close in 10 (48%). Only one patient had positive bone margins. The patient who did not have surgery was treated with chemotherapy and radiation therapy.
Overall, 13 patients (60 %) had high-grade sarcomas, and 3 (13%) had low-grade tumors. A reliable histological grade could not be assigned to the remaining 6 patients (26%). Neo-adjuvant chemotherapy was used in 9 of the 22 patients (40%) with high-grade sarcomas. The histologic response to neo-adjuvant chemotherapy was classified as “unfavorable” (Grade 1 or 2) in 7 of 9 patients (78%) and “favorable” (Grade 3 or 4) in only 1 of 9 patients (11%). (Fig. 5 A and B) No information regarding tumor response was available in the patient who did not undergo surgery. 11 patients (50%) received postoperative external beam radiation therapy. The overall survival of the patients who received neo-adjuvant chemotherapy (Fig.6), post-operative chemotherapy (Fig.7) and post-operative radiation therapy (Fig.8), is shown by Kaplan-Meier curves.
Figure. 5.
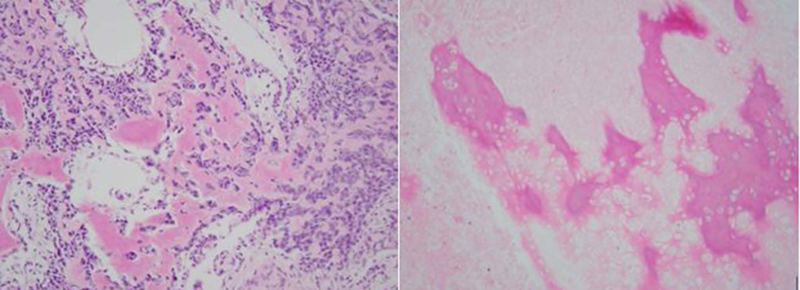
A: Histopathological response after induction chemotherapy showing viable tumor classified as grade 1 respone. B: Histopathological response after induction chemotherapy showing non-viable necrotic tumor classified as grade 4 response.
Figure. 6:
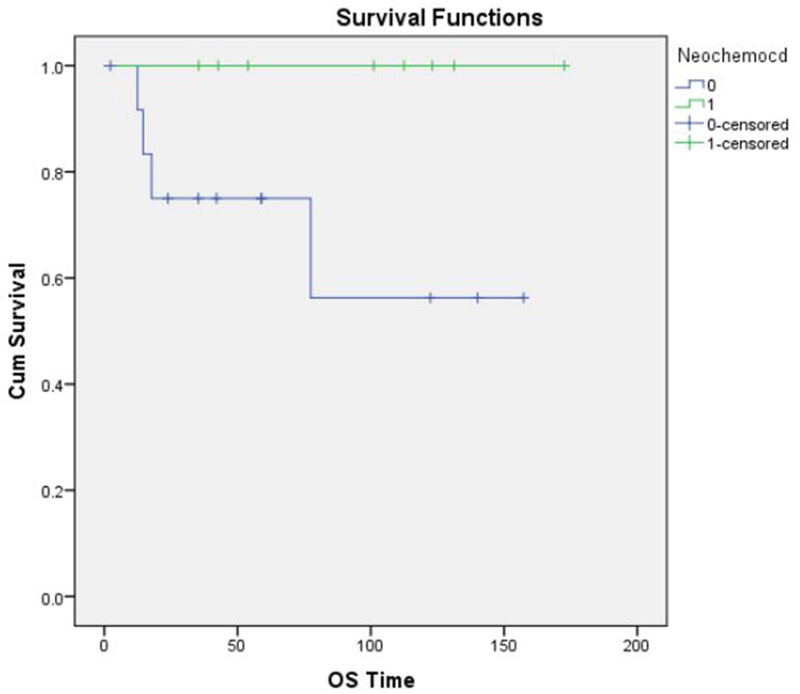
Kaplan-Meier curve showing overall survival for patients who received neoadjuvant chemotherapy.
Figure. 7:
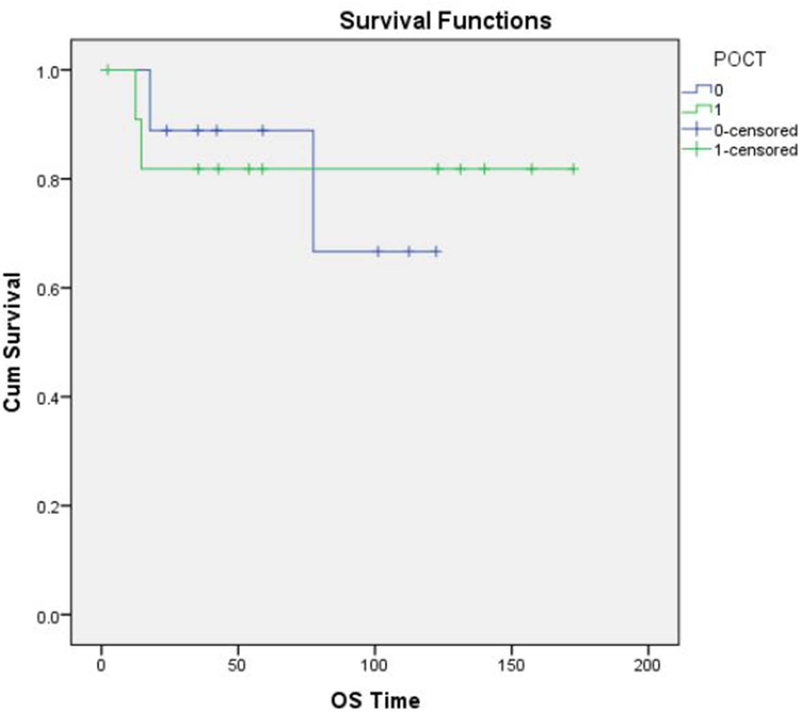
Kaplan-Meier curve showing overall survival for patients who received post-operative (adjuvant) chemotherapy.
Figure. 8:
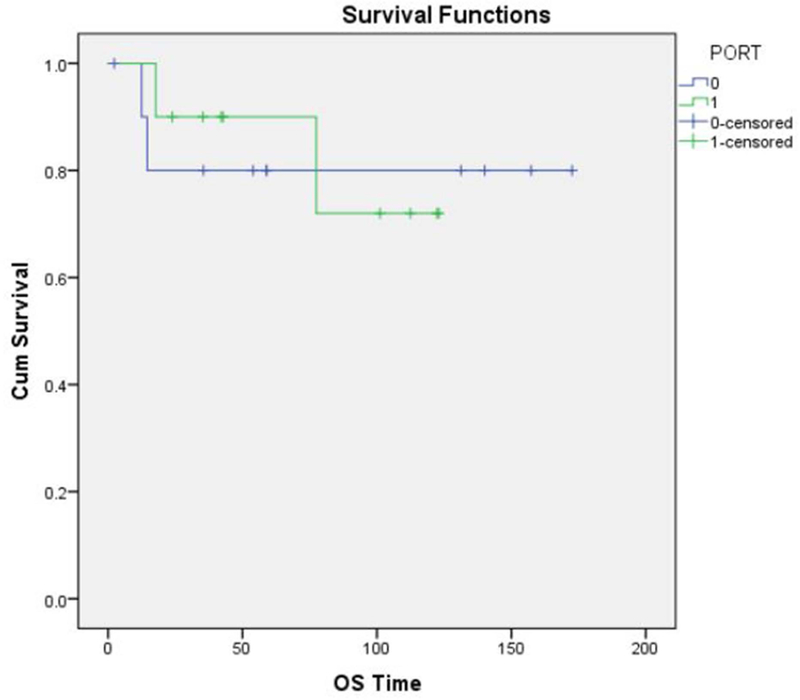
Kaplan-Meier curve showing overall survival for patients who received post-operative radiotherapy.
Six patients (27%) developed tumor recurrence after initial treatment with 3 (50%) having local recurrence and 3 (50%) with distant metastases. Ten patients had positive or close margins, and two of them developed local recurrence. On the other hand, one of the six patients with negative margins also developed local recurrence. One patient each, with and without positive margin developed distant failure. The impact of adjuvant therapy on the patients with or without positive margins and subsequent development of recurrence is shown in (Table 1). There were no cases of regional lymph node failure reported. Two of the 3 patients with local recurrence and one of three patients with distant metastasis had received post-operative radiotherapy. The recurrence free survival and overall survival of the patients in relation to adjuvant therapies administered is shown in (Table 2). No conclusions can be drawn from this small group of patients with regard to the impact of adjuvant therapies.
Table. 1:
Impact of adjuvant therapy on patients with and without positive margins.
| Osteosarcoma | Positive surgical margin N=6 | Close surgical margin N=4 | Negative surgical margin N=8 |
|---|---|---|---|
| Neo-adjuvant chemotherapy | 2 | 1 | 2 |
| Post-operative chemotherapy | 4 | 0 | 5 |
| Post-operative radiotherapy | 4 | 3 | 3 |
| Local recurrence | 1 | 1 | 1 |
| Distal recurrence | 1 | 0 | 1 |
| Ewing sarcoma | Positive surgical margin n=0 | Close surgical margin N=0 | Negative surgical margin N=3 |
| Neo-adjuvant chemotherapy | 3 | ||
| Post-operative chemotherapy | 3 | ||
| Post-operative radiotherapy | 0 | ||
| Local recurrence | 0 | ||
| Distal recurrence | 0 | ||
1 patient without surgery, received chemotherapy and radiotherapy; and had distant failure.
Table. 2:
The recurrence free and overall survival and the impact of adjuvant therapies.
| Recurrence free survival | Overall survival | |||||
|---|---|---|---|---|---|---|
| 3-year | P=value | 3-year | P=value | |||
| #Patients | n=21* | 78.3% | 85.0% | |||
| Gender | Male | 14 | 68.6% | 0.124 | 78.6% | 0.196 |
| Female | 7 | 100.0% | 100% | |||
| Margin | Negative | 11 | 90.0% | 0.293 | 100% | 0.156 |
| Positive/Close | 10 | 66.7% | 70.0% | |||
| Grade(osteosarcoma) | Low | 3 | 100.0 | 0.201 | 100% | 0.312 |
| High | 15 | 60.8% | 75.0% | |||
| Neo-adjuvant Chemo | No | 13 | 63.5% | 0.031 | 75.0% | 0.066 |
| Yes | 9 | 100.0% | 100% | |||
| Post-operative Chemo | No | 9 | 87.5% | 0.752 | 88.9% | 0.895 |
| Yes | 12 | 71.6% | 81.8% | |||
| Post-operative RT | No | 11 | 70.0% | 0.601 | 80.0% | 0.890 |
| Yes | 12 | 87.5% | 90.0% | |||
the patient with non-surgical treatment was not included.
DISCUSSION
This study presents the management and outcomes for sarcomas of mandible treated over a sixteen-year period at a tertiary care cancer center. The overall recurrence rate was 27%. The histological analysis of the excised tumors is the most reliable method to assess response to induction or neo adjuvant chemotherapy, signifying its importance to predict treatment efficacy and tumor control.25,26 On the other hand , the results of post-operative adjuvant chemotherapy alone in the treatment of head and neck sarcomas have been disappointing,27 There has been however renewed interest in using , combination treatment with chemotherapy and radiotherapy in recent years. Agents such as doxorubicin and ifosfamide have been used in combination with radiotherapy, however they produce severe side effects, and increased morbidity and long-term impact on improving disease specific survivorship is not yet reported. The overall response rate to chemotherapy is short lived and response rate of only 25% is reported in the literature.28,29
A fundamental prognostic factor in head and neck osteosarcoma is complete surgical resection of the tumor with negative margins.18,19,30–33 A confounding factor however in achieving this goal is the complexity of head and neck anatomy, which often limits the extent of resection. Hence, local recurrence is the most common site of treatment failure in osteosarcoma of the head and neck with a reported incidence of 17% to 70%.2,13 In our series the overall treatment failure rate was 27%. From these 13% had local recurrence and 13% had distant failure. Two of the three patients in our series with local recurrence had either positive or close surgical margin. In another study of 47 patients with sarcomas, a higher local recurrence rate was observed in patients with positive surgical margins, when compared to that in patients with close or negative surgical margins.34 Similar observations were reported in an European cooperative study published by Grimmer et al, where positive surgical margins were correlated with local recurrence.35 Hence, the importance of securing negative surgical margins remains crucial. Some authors recommend a bony margin of 2cm and soft tissue margin of 5cm to decrease the risk of local recurrence.36 However, this may not be achievable in many patients with sarcomas in the head and neck region.
In our small study we were unable to show convincingly the correlation between margin status and local recurrence. One out of six patients with positive surgical margins, and one out of four with close surgical margins had local recurrence. On the other hand, one out of six patients with negative margins also had local recurrence. Although, in this patient the margin was only 1.5 cm. Thus, the extent of margins, remains a topic of debate. Clearly, complete surgical resection with “adequate” surgical margin improves local control as well as disease specific survival. Clearly, further research is needed to confirm and correlate the relationship between local recurrence of the tumor and appropriate extent of resection for negative margins beyond the gross tumor.
The main limitation of this study is that it is a retrospective review, and the sample size is small over a long period of time. Clearly, a prospective randomized trial would be a more powerful study, but the feasibility of such a study from any single institution in a reasonable time frame is unlikely. Obviously, a collaborative or a national trial would be necessary to answer these questions. In the absence of such, we have to rely on retrospective analysis of larger cohorts of patients to draw any conclusions.10
Future possibilities may include review of clinical trials, reporting new treatment approaches and collection of ‘big data’ from national and international groups to draw any conclusions on treatment protocols. Multidisciplinary management of patients with head and neck sarcomas requires adherence to a detailed and tailored treatment plan for successful local-regional control.
CONCLUSION
Sarcomas of the mandible are rare clinical entities, and data on outcomes from any larger series is lacking. It is however apparent from our limited study and review of the literature, that wide surgical resection with negative margins followed by adjuvant radiotherapy with or without chemotherapy remains the hall mark for a successful outcome. Also, it can be assessed that positive or close surgical margins remain the most important cause of treatment failure, leading to local recurrence.
Synopsis for Table of Contents:
This study presents a 20-year experience of treating these tumors at a tertiary care cancer center, and emphasizes the critical role of adequate surgery to secure negative margins. The beneficial role of adjuvant radiotherapy or chemotherapy could not be shown conclusively. Similarly, neoadjuvant chemotherapy in mandible sarcomas in adults remains investigational.
Acknowledgments
Financial Disclosures: This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Data Availability Statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Shellenberger T, et al. Sarcomas of the head and neck region. Curr Oncol Rep 2009; 11:135–42 [DOI] [PubMed] [Google Scholar]
- 2.Ketabchi A, et al. Sarcomas of the head and neck: a 10-year retrospective of 25 patients to evaluate treatment modalities, function and survival. Br J Oral Maxillofac Surg 2011; 49:116–20 [DOI] [PubMed] [Google Scholar]
- 3.Levy ML, Jaffe N. Osteosarcoma in early childhood. Pediatrics 1982; 70:302–3 [PubMed] [Google Scholar]
- 4.Bennett JH, Thomas G, Evans AW, Speight PM. Osteosarcoma of the jaws: A 30-year retrospective review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2000; 90:323–32 [DOI] [PubMed] [Google Scholar]
- 5.Angiero F, Vinci R, Sidoni A, Stefani M. Mesenchymal chondrosarcoma of the left coronoid process: Report of a unique case with clinical, histopathological and immunohistochemical findings and a review of literature. Quintessence Int 2007; 38:349–55 [PubMed] [Google Scholar]
- 6.Randall RL, Hunt KJ. Chondrosarcoma of the bone. An ESUN article-Liddy Shriver Sarcoma Initiative 2006. February; V3N1
- 7.Kurita K, Ogi N, Echiverre NV, Yoshida K. Osteochondroma of the mandibular condyle. A case report. Int J Oral Maxillofac Surg 1999; 28:380–382 [PubMed] [Google Scholar]
- 8.Ewing J: Diffuse endothelioma of bone. New York Path Soc 21:17–24, 1921 [Google Scholar]
- 9.Potdar GG: Ewing’s tumors of the jaws. Surg 29:505–12, 1970 [DOI] [PubMed] [Google Scholar]
- 10.Mucke T, et al. Outcome in adult patients with head and neck sarcomas: a 10-year analysis. J Surg Oncol 2010; 102:170–4 [DOI] [PubMed] [Google Scholar]
- 11.Mendehall W, et al. Adult head and neck soft tissues sarcomas. Head Neck 2005; 10:916–9 [DOI] [PubMed] [Google Scholar]
- 12.De Bree R, et al. Management of adult softtissue sarcomas ofthe head and neck. Oral Oncol 2010;46:786–90 [DOI] [PubMed] [Google Scholar]
- 13.Clark JL, Unni KK, Dahlin DC, Devine KD. Osteosarcoma of the jaw. Cancer 1983; 51:2311–6 [DOI] [PubMed] [Google Scholar]
- 14.Delgado R, Maafs E, Alfeiran A, Mohar A, Barrera JL, Zinser J, et al. Osteosarcoma of the jaw. Head Neck 1994;16:246–52 [DOI] [PubMed] [Google Scholar]
- 15.Mark RJ, Sercarz JA, Tran L, Dodd LG, Selch M, Calcaterra TC. Osteogenic sarcoma of the head and neck. The UCLA experience. Arch Otolaryngol Head Neck Surg 1991;117:761–6 [DOI] [PubMed] [Google Scholar]
- 16.Forteza G, Colmenero B, López-Barea F. Osteogenic sarcoma of the maxilla and mandible. Oral Surg Oral Med Oral Pathol 1986;62:179–84 [DOI] [PubMed] [Google Scholar]
- 17.LiVolsi VA. Osteogenic sarcoma of the maxilla. Arch Otolaryngol 1977;103:485–8 [DOI] [PubMed] [Google Scholar]
- 18.Kassir RR, Rassekh CH, Kinsella JB, Segas J, Carrau RL, Hokanson JA. Osteosarcoma of the head and neck: Meta-analysis of nonrandomized studies. Laryngoscope 1997;107:56–61 [DOI] [PubMed] [Google Scholar]
- 19.Smeele LE, Kostense PJ, van der Waal I, Snow GB. Effect of chemotherapy on survival of craniofacial osteosarcoma: A systematic review of 201 patients. J Clin Oncol 1997;15:363–7 [DOI] [PubMed] [Google Scholar]
- 20.Glasser DB, Lane JM. Stage IIB osteogenic sarcoma. Clin Orthop 1991; 270:29–39 [PubMed] [Google Scholar]
- 21.Link MP, Goorin AM, Horowitz M, et al. Adjuvant chemotherapy of high‐grade osteosarcoma of the extremity: updated results of the multi‐institutional osteosarcoma study. Clin Orthop 1991;270:8–14 [PubMed] [Google Scholar]
- 22.Windle-Taylor PC. Osteosarcoma of the upper jaw. J Maxillofac Surg 1977;5:62–8 [DOI] [PubMed] [Google Scholar]
- 23.Huvos AG, Bone Tumors: Diagnosis, Treatment, and Prognosis, W.B. Saunders, Philadelphia, PA, USA, 2nd edition, 1990 [Google Scholar]
- 24.Chaudhary M, Chaudhary SD. Osteosarcoma of jaws. J Oral Maxillofac Pathol 2012;16:233–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, et al. (2002) Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol 20: 776–790 [DOI] [PubMed] [Google Scholar]
- 26.Thiele OC, Freier K, Bacon C, et al. : Interdisciplinary combined treatment of craniofacial osteosarcoma with neoadjuvant and adjuvant chemotherapy and excision of the tumour: A retrospective study. Br J Oral Maxillofac Surg 2008; 46:533–536 [DOI] [PubMed] [Google Scholar]
- 27.Bernier J, Vermorken JB, Koch WM. Adjuvant therapy in patients with resected poor-risk head and neck cancer. J Clin Oncol 2006; 24:2629–2635 [DOI] [PubMed] [Google Scholar]
- 28.Molin Y, Fayette J. Current chemotherapies for recurrent/metastatic head and neck cancer. Anticancer Drugs 2011; 22:621–5 [DOI] [PubMed] [Google Scholar]
- 29.Kearsley JH. Cytotoxic chemotherapy for common adult malignancies: “the emperor’s new clothes” revisited? Br Med J (Clin Res Ed) 1986. October 4;293(6551):871–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guadagnolo BA, Zagars GK, Raymond AK, Benjamin RS, Osteosarcoma of the jaw/craniofacial region: outcomes after multimodality treatment. Sturgis EM Cancer 2009. July 15; 115(14):3262–70 [DOI] [PubMed] [Google Scholar]
- 31.Van Es RJ, Keus RB, van der Waal I, Koole R, Vermey A. Osteosarcoma of the jaw bones. Long-term follow up of 48 cases. Int J Oral Maxillofac Surg 1997;26:191–7 [DOI] [PubMed] [Google Scholar]
- 32.August M, Magennis P, Dewitt D. Osteogenic sarcoma of the jaws: factors influencing prognosis. Int J Oral Maxillofac Surg 1997; 26:198–204. [DOI] [PubMed] [Google Scholar]
- 33.Granowski-LeCornu M, Chuang SK, Kaban LB, August M. Osteosarcoma of the jaws: factors influencing prognosis. J Oral Maxillofac Surg 2011;69:2368–75 [DOI] [PubMed] [Google Scholar]
- 34.Li Xin, Moretti Vincent M., Ashana Adedayo O., and Lackman Richard D.. Impact of close surgical margin on local recurrence and survival in osteosarcoma Int Orthop 2012. January; 36(1): 131–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grimer RJ, Taminiau AM, Cannon SR, Surgical outcomes in osteosarcoma. Surgical Subcommitte of the European Osteosarcoma Intergroup. Bone Joint Surg Br 2002. April; 84(3):395–400 [DOI] [PubMed] [Google Scholar]
- 36.Thariat J, Julieron M, Brouchet A, Italiano A, Schouman T, Marcy PY, Odin G, Lacout A, Dassonville O, Peyrottes-Birstwisles I, Miller R, Thyss A, Isambert N. Osteosarcomas of the mandible: are they different from other tumor sites. Crit Rev Oncol Hematol 2012. June;82(3):280–95 [DOI] [PubMed] [Google Scholar]


