Abstract
Natural transmission of cytomegalovirus (CMV) has been difficult to observe. However, recent work using the mouse model of murine (M)CMV demonstrated that MCMV initially infects the nasal mucosa after transmission from mothers to pups. We found that intranasal (i.n.) inoculation of C57BL/6J mice resulted in reliable recovery of replicating virus from the nasal mucosa as assessed by plaque assay. After i.n. inoculation, CD8+ T cell priming occurred in the mandibular, deep-cervical and mediastinal lymph nodes within 3 days of infection. Although i.n. infection induced “memory inflation” of T cells specific for the M38316–323 epitope, there were no detectable CD8+ T cell responses against the late-appearing IE3416–423 epitope, which contrasts with intraperitoneal (i.p.) infection. MCMV-specific T cells migrated into the nasal mucosa where they developed a tissue-resident memory (TRM) phenotype and this could occur independently of local virus infection or antigen. Strikingly however, virus replication was poorly controlled in the nasal mucosa and MCMV was detectable by plaque assay for at least 4 months after primary infection, making the nasal mucosa a second site for MCMV persistence. Unlike in the salivary glands, the persistence of MCMV in the nasal mucosa was not modulated by IL-10. Taken together, our data characterize the development of local and systemic T cell responses after intranasal infection by MCMV and define the nasal mucosa, a natural site of viral entry, as a novel site of viral persistence.
Keywords: cytomegalovirus, nasal mucosa, intranasal infection, tissue resident memory T cells
Introduction
Human cytomegalovirus (HCMV) is a β-herpesvirus that establishes a persistent/latent infection in the majority of the worldwide population. In immune-competent individuals infected after birth, HCMV infection is generally asymptomatic and the virus persists for life without inducing obvious morbidity. However, HCMV infection or reactivation from latency causes severe complications in immune-compromised patients including organ damage, graft rejection and death [1]. In addition, HCMV causes a common congenital infection and transplacental transmission from mother to baby can result in disseminated HCMV disease in the baby as well as infection of the central nervous system resulting in long-term neurological sequelae including hearing loss, vision impairment and developmental delays [2]. Thus, an understanding of HCMV transmission and dissemination from the site(s) of entry is critical to develop treatments or vaccines that can interrupt HCMV pathogenesis.
HCMV is shed in saliva, urine and breast-milk for prolonged periods of time, even in immune competent hosts, and these are thought to be important sources of infectious virus for transmission to a new host [2, 3]. Moreover, HCMV can be reactivated from latency and infectious particles can be shed even long after the primary infection. However, defining the site(s) of entry into a new host has proven to be more difficult since primary HCMV infection in immune-competent people is usually clinically silent or produces only mild symptoms. Oral and nasal routes of entry have been proposed as natural routes of infection (e.g. [4–6]) but the evidence for either the oral mucosa or nasal mucosa as sites of entry is indirect in humans and no primary infection site has been clearly identified.
Due to the strict-species specificity of CMVs, HCMV cannot be studied in experimental animals. Fortunately, several related viruses have been studied in their natural hosts including Rhesus (Rh)CMV, guinea pig (GP)CMV, murine (M)CMV, and rat (R)CMV with MCMV being the most widely used [7]. Although these animal viruses are not identical to HCMV, their host/pathogen relationship is remarkably similar to that established by HCMV in humans and they have provided excellent models for understanding HCMV pathogenesis and immunobiology.
In animal models, oral infection has been demonstrated by gavage of neonatal (6 day old) mice with MCMV [8] or oral inoculation of infant macaques with RhCMV [9]. Interestingly however, infant macaques were more susceptible to oral inoculation than adults in this study and experimental gavage of adult mice was unable to produce infected cells in the gastrointestinal tract [10], or any immune response indicative of infection [11]. Thus, oral infection may be inefficient. Consistent with this, CMVs are sensitive to low pH such as would be found in the stomach [12]. It is possible that the oral mucosa could serve as a site of entry, although this has not been directly shown. Moreover, the oral cavity and nasal cavity are directly connected via the back of the mouth and both tissues could be exposed to any fluid in the mouth, which may be especially difficult to avoid in experimental settings in which neonates are inoculated with high doses of virus in solution. In contrast, intranasal inoculation of MCMV robustly establishes infection and olfactory neurons have been identified as primary sites of entry in the nasal mucosa [13, 14]. Most importantly, a recent report showed direct evidence for MCMV infection of the nasal mucosa after natural transmission from infected mothers to pups [13]. Collectively, these data suggest that the nasal mucosa is a major, and likely predominant site for natural CMV entry.
Over the past 6 decades, most research with MCMV has been done by introducing the virus via intraperitoneal (i.p.), intravenous (i.v.) or foot-pad (f.p.) inoculation. Each of these routes involves mechanically breaking the barrier tissue. Moreover, while i.p. and i.v. inoculation clearly introduce virus systemically, there is evidence that even f.p. inoculation allows some MCMV direct access to the spleen [15], suggesting that the break in barrier tissue allows the virus direct access to the circulation. In contrast, intranasal (i.n.) inoculation infects a natural site of viral entry without breaking barrier tissue. However, relatively few studies have utilized the i.n. route of inoculation and little is known about how immunity develops after infection of the nasal mucosa. Moreover, no previous studies have examined immunity and viral replication kinetics in the nasal mucosa itself.
We found that the nasal mucosa was reliably infected by MCMV after i.n. inoculation, but not i.p. or f.p. infection. Intranasal infection resulted in T cell priming in mandibular, deep-cervical and mediastinal lymph nodes. Circulating T cell responses were smaller after i.n. infection, consistent with previous work [11], and a greater proportion of responding CD8+ T cells were memory-phenotype T cells (KLRG1−/CD127+) after i.n. compared to i.p. infection. Interestingly, i.n. inoculation did not induce the late-appearing IE3-specific CD8+ T cell responses, in contrast to i.p. inoculation. Tissue-resident memory T cells (TRM) were formed in the nasal mucosa and we found that CD8+ TRM could develop in the nasal mucosa in an antigen-independent manner. Despite this local immunity, MCMV replication in the nasal mucosa was remarkably persistent. Interestingly however, IL-10 blockade was unable to reduce viral titers in the nasal mucosa, unlike the salivary gland, suggesting that different mechanisms may regulate MCMV persistence in different mucosal tissues. In summary, our data demonstrated the characteristics of intranasal infection by MCMV, which shed light on the processes that may be expected to occur upon natural infection.
Experimental model and subject details
Mice, viruses and experimental infection
Six to seven-week old female mice were used for all experiments. C57BL/6J mice were purchased from the Jackson Laboratory. OT-I transgenic mice (C57BL/6-Tg (TcraTcrb)1100Mjb/J) and CD45.1 congenic mice (B6.SJL-Ptprca Pepcb/BoyJ) were originally purchased from the Jackson Laboratory and crossed in-house to produce double positive (CD45.1+/CD45.2+) OT-I mice. Murine cytomegalovirus strains including WT-BAC (pSM3fr), K181-MCMV, and MCMV-Ova have been described previously [16–18]. All viruses were propagated in M2–10B4 cells as previously described [19]. Mice were either infected by the i.n. route with 106 PFU MCMV in 20 μL PBS, by the i.p. route with 106 PFU MCMV in 100 μL PBS, or by the f.p. route with 106 PFU MCMV in 30 μL PBS. All experiments were approved by the Thomas Jefferson University Institutional Animal Care and Use Committee.
Nasal mucosa collection
A detailed description of a method for recovery of nasal mucosa for histological analyses has been described[20]. We have modified this approach for faster recovery of nasal mucosa for homogenization. In brief, the mice were first euthanized by carbon dioxide. Skin on the head and nose was removed, followed by removal of the lower jaw. The upper jaw and nasal mucosa was separated from the back of the skull and brain by a transverse cut along the bregma of the skull, behind the eye-sockets and olfactory bulb. The nasal bone covering the nasal passages was removed with a scalpel and any remaining zygomatic and ethmoid bones were removed with scissors at this time. Olfactory bulbs, remaining brain tissue, connective tissue and muscles were then removed from the upper jaw. Teeth on the upper jaw were removed with scissors. Then, the soft and hard palates were removed with tweezers, which also removes the nasal associated lymphoid tissues (NALT). Finally, the maxilla was removed from each side of the nasal tissue. The resulting soft tissue was homogenized for virus titration or FACS analysis as described below.
Virus titration
Twenty-percent homogenates (w/v) of tissue were individually prepared from nasal mucosa, lungs and SG for virus titration. Briefly, each tissue was weighed and homogenized using a pestle with a small amount of sterile sand in an 1.5 ml centrifuge tube prior to suspension in RPMI-1640 medium with L-glutamine, supplemented with 10% FBS and 100 units/ml Penicillin, 100 μg/ml Streptomycin. Homogenates were spun (2400 xg for 10min) and supernatants were collected for plaque assay. Plaque assays were performed on M2–10B4 cells as previously described [19] except that the M2–10B4 cells were ~80%−100% confluent at the start of the assay, and the tissue homogenates at the highest dilution tested (1:10 from the 20% homogenate) were removed by vacuum suction after 1.5 hrs incubation and replaced with fresh media without washing. Homogenates diluted 1:100 or 1:1000 were not removed prior to the addition of the viscous overlay.
Adoptive transfer and in vitro activation of OT-I T cells
The indicated numbers of naïve OT-I, CFSE-labelled OT-I T cells, or in vitro activated OT-I T cells (generated as previously described [21]) were adoptively transferred in 100 μl PBS into a congenic recipient via retro-orbital sinus.
Intravenous antibodies injection, lymphocytes isolation, antibodies, tetramer staining and FACS analysis
In order to distinguish cells localized to the vasculature and parenchyma of the nasal mucosa and salivary gland, mice were intravenously injected with 3 μg fluorescently-labelled antibodies specific for CD8α (clone 53–6.7) or CD4 (clone RM4–4), 3 minutes before sacrifice. Animals were not perfused and lymphocytes were extracted from tissue as described below in the presence of 60 μg/ml unlabeled anti-CD8α (clone 53–6.7) to limit background staining from this antibody. Lymphocytes from the blood, spleen, SG and lungs were isolated as described [21, 22]. Lymphocytes from nasal mucosa were recovered using the same method as for the SG. For assessment of T cells in lymph nodes, the mediastinal, deep cervical and mandibular lymph nodes were homogenized with two needles in T cell medium (RPMI-1640 medium with L-glutamine, 10% FBS, 100 units/ml Penicillin, 100 μg/ml Streptomycin, and 5 × 10−5 M β- mercaptoethanol) prior to passage through a 70 μm cell strainer. To label all CD4+ and CD8+ T cells in the homogenate (vascular and parenchymal), cells were stained for CD8β (clone 53–5.8) and CD4 (clone GK1.5). OT-I cells were distinguished from recipient cells by CD45.1 (clone A20), CD45.2 (clone 104) and TCR Vα2 (clone B20.1). The remaining phenotypic analysis was performed with following antibodies: TCR-β (clone H57–597), CD11a (clone M17/4), KLRG1 (clone 2F1/KLRG1), CD127 (clone A7R34), CD69 (clone H1.2F3) and CD103 (clone 2E7). All antibodies were purchased from Biolegend. MHC-I-tetramers loaded with peptides derived from M38, M45 or IE3 were produced at the NIH tetramer core facility (http://tetramer.yerkes.emory.edu/) and used to identify MCMV-specific CD8+ T cells as previously described [17]. In all cases, samples were collected on BD Fortessa and analyzed with FlowJo software (TreeStar). Representative gating strategies for flow cytometry analyses are shown in Supplemental Figure 1.
In vivo IL-10R blockade
To block IL-10 signalling, mice were injected i.p. with 500 μg rat IgG1 (clone HRPN; BioxCell) or anti-CD210 (anti–IL-10R clone 1B1.3A; BioxCell) at 1 and 7 dpi.
Statistical analysis
Error bars represented standard error of the mean (SEM) unless specified otherwise. All data analysis was performed in Graphpad 5. 8Statistical analyses are described in the figure legends.
Results
MCMV infects the nasal mucosa of the immunocompetent mice via i.n. infection, but not i.p. or f.p. infection
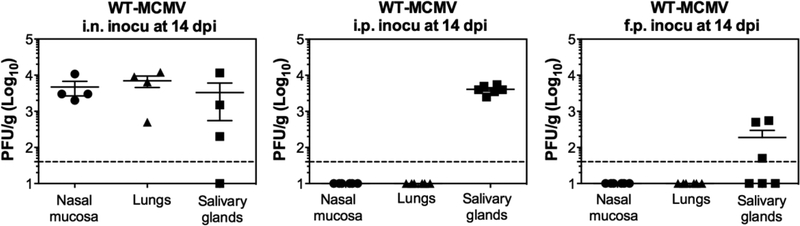
In order to test whether different routes of infection result in viral replication in the nasal mucosa, C57BL/6J mice were infected with MCMV via i.n., i.p. or f.p. routes. Each infection route caused virus replication in the SG, but only i.n. inoculation established infection in the nasal mucosa itself within 2 weeks, as assessed by plaque assay (Figure 1).
Figure 1. MCMV infects the nasal mucosa of the immunocompetent mice after i.n. infection, but not after i.p. or f.p. infection.
Virus titers in the nasal mucosa, lungs and SG at 14 dpi after i.n., i.p. or f.p. inoculation of 106 PFU WT-MCMV. Each symbol represents an individual mouse. The solid line shows the mean titer, and error bars represent the SEM. Dashed lines show the detection limit (50 PFU/g). One representative experiment out of at least three independent experiments is shown for i.n. inoculation. Data are combined from two independent experiments for i.p. and f.p. inoculation.
OT-I cells are primed first in mandibular, deep-cervical and mediastinal lymph nodes.
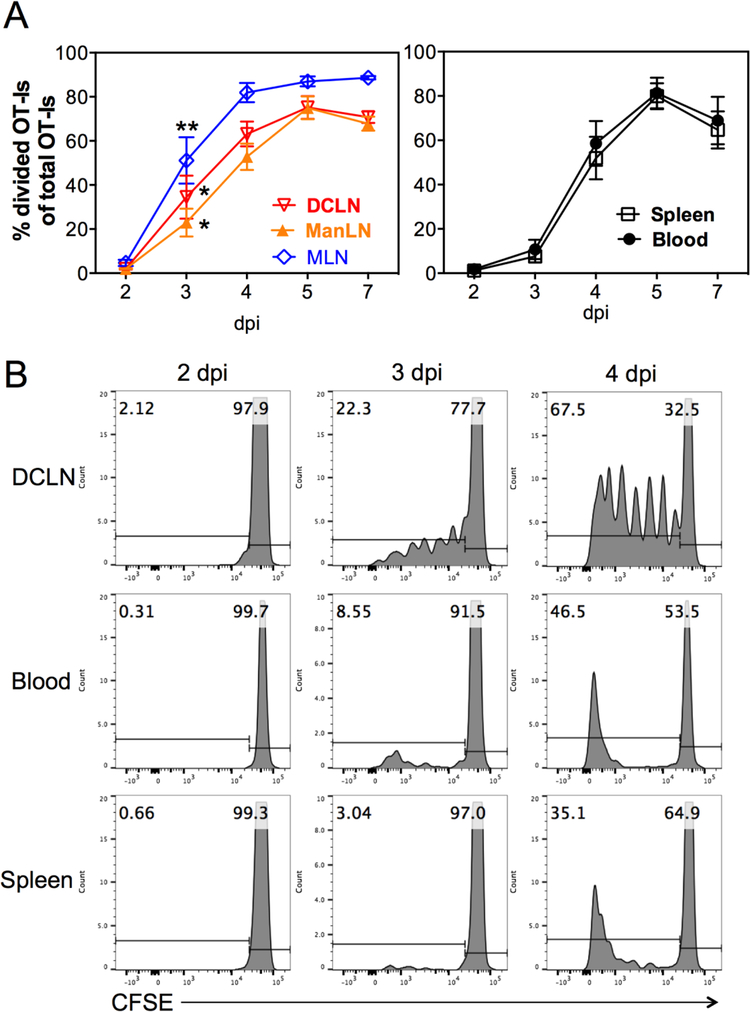
To define the site(s) of CD8+ T cell priming after i.n. infection, we adoptively transferred CFSE-labeled OT-I T cells to congenic recipients, which then were i.n. inoculated with 106 PFU MCMV-Ova on the following day. Dividing OT-Is that had gone through at least one round of division were measured in lymph nodes that drain the nasal mucosa and lungs, as well as in the spleen and blood. The numbers (not shown) and frequencies (Figure 2A) of dividing OT-I cells increased markedly in the mandibular, deep-cervical and mediastinal LNs between days 2 and 3 after infection and were significantly elevated on day 3 relative to their frequency in the spleen. In contrast, accumulation of dividing OT-Is was delayed until day 4 post infection, which paralleled the accumulation in the blood (Figure 2A). Moreover, while many cells with intermediate intensities of CFSE could be found in lymph nodes, dividing cells in the spleen and blood tended to be CFSE-negative by day 4, indicating that these cells had undergone multiple rounds of division before they became detectable (Figure 2B). Collectively, these data suggest that dividing cells in the spleen were arriving via the circulation, rather than undergoing activation in the spleen itself and that MCMV-specific CD8+ T cells are primed in the mandibular, deep-cervical and mediastinal LNs after i.n. infection.
Figure 2. OT-I cells are primed first in mandibular, deep-cervical and mediastinal lymph nodes.
A. 106 CFSE-labeled OT-I cells were injected into congenic recipients via the retroorbital sinus. The following day, recipients were i.n. infected with 106 PFU MCMV-Ova. The frequency of OT-I cells that had gone through at least one round of division was assessed in the mandibular (manLN), deep-cervical (DCLN) and mediastinal (MLN) lymph nodes, as well as the blood and spleen on the indicated days after infection. Shown are frequencies of dividing OT-Is pooled from 2 individual experiments (n=6 mice per time point at days 2, 3 and 4) and from 1 experiment (n=3 mice per time point) for days 5 and 7. The solid lines connect the mean frequency of the dividing OT-Is and error bars represent the SEM. Asterisks indicate statistical significance of differences between frequencies of divided cells in each lymph node versus the spleen at day 3 after infection as assessed by paired student’s t-tests (* = p<0.05, ** = p<0.01). B. Shown are representative FACS plots of the CFSE-dilution profile of OT-I T cells (CD45.1+, Vα2+) recovered from the DCLN, blood and spleen on days 2, 3 and 4 after i.n. infection with MCMV-Ova. Data are from one mouse that was part of the group shown in “A”.
Intranasal inoculation induces memory inflation with a lower frequency of effector-phenotype T cells compared with i.p. inoculation
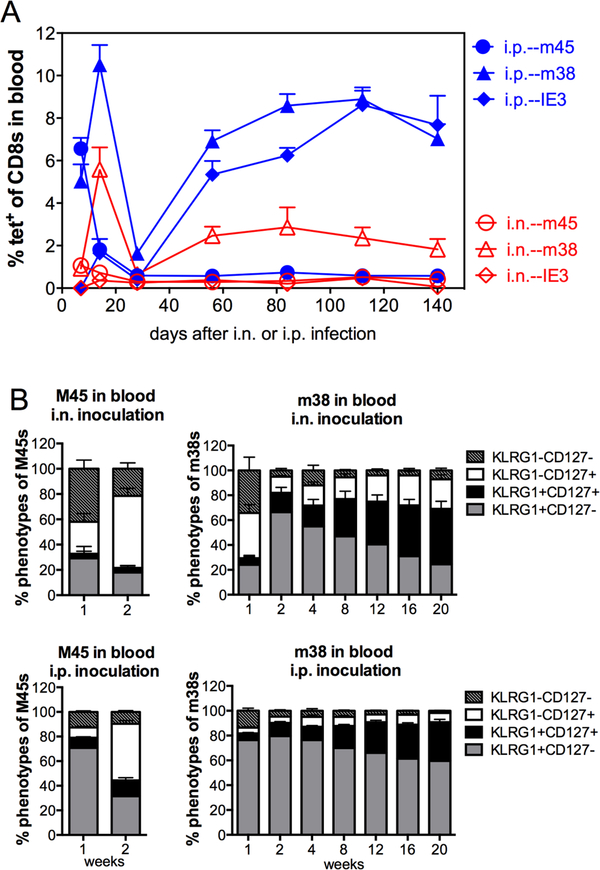
One of the hallmarks of the T cell response to MCMV infection after i.p. and i.v. infection is the process of “memory inflation”, a late accumulation of T cells specific for certain viral epitopes after the acute phase of infection is resolved. Previous work in BALB/c mice demonstrated that T cells underwent memory inflation with similar kinetics after i.n. and i.p. infection, but that inflationary populations were relatively reduced in frequency and number after i.n. infection compared to systemic i.p. infection [11]. In agreement with this, we found that M38-specific CD8+ T cells underwent memory inflation with similar kinetics after i.n. and i.p. infection of C57BL/6J mice, but were significantly reduced in frequency after i.n. infection (Figure 3A, p ≤ 0.0151 by paired t-tests at all time points). We also studied a second inflationary response elicited by an epitope from the IE3 protein. This response is unique in that IE3-specific T cells are undetectable during the acute phase of infection but become significant populations after several weeks of infection. Interestingly, while i.p. inoculation induced memory inflation of IE3-specific CD8+ T cells with the expected kinetics, i.n. inoculation failed to induce any detectable IE3-specific T cell responses over the course of the experiment. This was not a general failure to respond to epitopes expressed in the IE locus as Ova-specific T cells were robustly stimulated by i.n. infection when the Ova gene was expressed under control of the IE2 promoter (Figure 2 and not shown). Likewise, IE1-specific T cells underwent inflation in BALB/c mice [11]. As a comparison, we analysed M45-specific T cells, which are evident within the first week of infection, but do not undergo memory inflation in C57BL/6J mice. After i.n. and i.p. infection, M45-specific T cells expanded and contracted with similar kinetics (Figure 3A). Again however, the final frequencies of M45-specific T cells were slightly, but significantly reduced after i.n. infection (p ≤ 0.0373 at all time points by paired t-tests except 112 dpi, which did not reach significance).
Figure 3. Intranasal inoculation induces memory inflation with a lower frequency of effector-phenotype T cells compared with i.p. inoculation.
C57BL/6J mice were infected with 106 PFU WT-BAC MCMV (pSM3fr) via either i.n. or i.p. inoculation. A. Shown is the frequency of MCMV-specific CD8+ T cells in the blood, as measured by tetramer staining at the indicated time points after infection. Results are shown as the group means + SEM (n=5) at the indicated time points. B. Phenotypic analyses of non-inflationary (M45-specific) and inflationary (M38-specific) CD8+ T cells from the experiment shown in A. Data are from one experiment.
Phenotypic analyses showed that i.p. inoculation generated higher frequencies of effector-like phenotype CD8+ T cells (KLRG1+, CD127−) within both non-inflationary (M45-specific) and inflationary (M38-specific) populations (Figure 3B). In contrast, i.n. infection resulted in more memory phenotype T cells (KLRG1−, CD127+) and more double-positive T cells (KLRG1+, CD127+), which was consistent over time (Figure 3B). Previous work from our lab, the Oxenius lab and the Arens lab has indicated that cells retaining a memory phenotype have increased proliferative capacity and increased survival [23–26]. Thus, these data indicate that i.n. infection drives less expansion and effector differentiation of MCMV-specific CD8+ T cells in comparison with i.p. infection.
T cells become resident in the nasal mucosa independently of local infection.
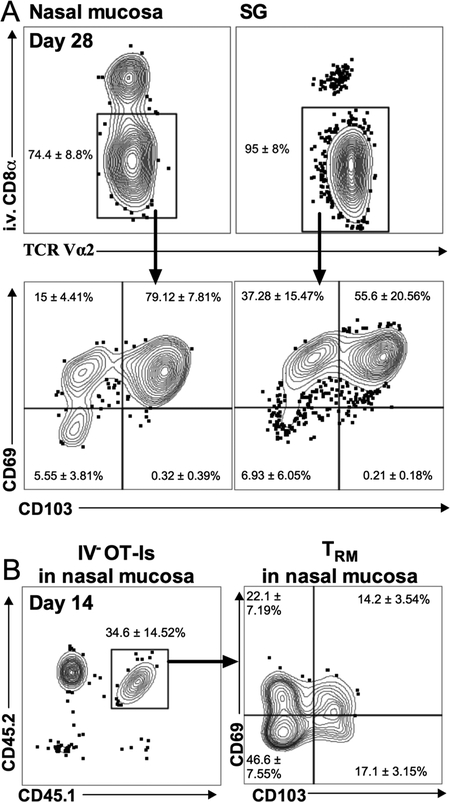
MCMV induces the development of TRM in multiple tissues in the body [22, 27]. However, no previous work has assessed the nasal mucosa. We transferred OT-I T cells and infected the adoptive recipients with MCMV-Ova via i.n. inoculation. Within 14 days OT-Is with a TRM phenotype (CD69+, CD103+) were recovered from the nasal mucosa and salivary gland (Figure 4A). Similarly, in multiple independent experiments with various strains of MCMV, we have found TRM phenotype M38-specific CD8+ T cells in the nasal mucosa after i.n infection using MHC-I tetramers (not shown). Previous data indicated that CD8+ TRM formation in the salivary gland can occur independently of antigen or infection of the salivary gland [21, 22, 27]. Moreover, i.n. infection did not seem to be necessary for TRM formation in the nasal mucosa: M38-specific TRM-phenotype cells could be recovered from the nasal mucosa even after i.p. infection with spread-defective (ΔgL) MCMV (not shown), which could only go through one round of infection in vivo[28, 29]. These data suggest that infection of the nasal mucosa itself is not needed to induce TRM formation. To formally test this, OT-Is were activated in vitro and adoptively transferred into naïve congenic recipients, as previously described [21]. Fourteen days later, TRM phenotype OT-I T cells were recovered from the nasal mucosa, indicating that activated T cells have access to the nasal mucosa and can become TRM without local infection or antigen (Figure 4B).
Figure 4. T cells become resident in the nasal mucosa independently of local infection.
A. Naïve OT-I cells expressing CD45.1 (5 × 103) were injected into C57BL/6J recipients via the retroorbital sinus. The following day, recipients were i.n. infected with 106 PFU MCMV-Ova. To distinguish cells that had migrated into the parenchyma of the nasal mucosa and salivary gland, animals were i.v. injected with anti-CD8α prior to sacrifice. Representative FACS plots show donor OT-I T cells (CD45.1+, Vα2+) that were i.v.-antibody negative in the nasal mucosa and salivary glands (top plots) and their expression of CD69 and CD103 (bottom plots) 14 days after infection. Values in the representative plots indicate the average for each gate or quadrant ± the standard deviation (n=5). Data are from one experiment and representative of two independent experiments (n=10 mice total). B. In vitro activated CD45.1+/CD45.2+ OT-Is (3 × 106) were adoptively transferred into naïve, congenic (CD45.2+) recipients. Nasal mucosa was collected 14 days after the transfer. Shown are representative FACS plots of CD69 and CD103 expression on donor OT-Is in the i.v. antibody-negative fraction of the nasal mucosa. Values in the representative plots indicate the average for each gate or quadrant ± the standard deviation (n=6). Data are from one experiment and representative of two independent experiments (n=9 mice total).
MCMV persistently replicates in the nasal mucosa
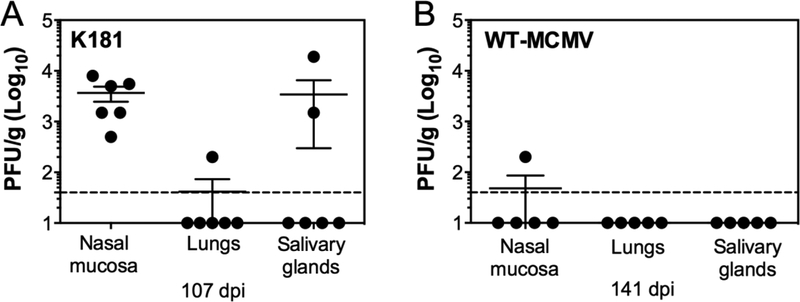
MCMV was originally discovered as a salivary gland virus that could be passed from mouse-to-mouse from salivary gland homogenates injected via subcutaneous, intraperitoneal, intracranial or intraglandular injections [30, 31]. Since these initial observations, research has indicated that the virus persistently replicates in the salivary gland for prolonged periods of time, presumably for horizontal transfer to other hosts. Other sites of persistent replication have not been discovered in the mouse. Surprisingly, we found that after i.n. inoculation, MCMV persistently replicated in the nasal mucosa. Indeed, the K181 strain of MCMV could be detected in all infected mice by plaque assay as late as 107 days after infection (Figure 5A). Even the attenuated BAC-derived MCMV strain (pSM3fr) could be detected in the nasal mucosa of 1 out of 5 mice, at 141 days after infection (Figure 5B). This virus contains a mutation in mck-2 that affects the viral titers in the salivary gland [32], likely as a result of impaired dissemination to the salivary gland [33–37]. These data show that the nasal mucosa and salivary gland can both support persistent MCMV replication after i.n. infection.
Figure 5. MCMV persists its replication in the nasal mucosa.
A. Shown are virus titers in the nasal mucosa, lungs and SG at 107 days after i.n. infection with 106 PFU K181-MCMV. Data are pooled from 2 independent experiments (n=6 mice total). B. Shown are virus titers in the nasal mucosa, lungs and SG at 141 days after infection with 106 PFU WT-BAC MCMV. Data are from one experiment (n=5 mice total).
Blockade of IL-10R does not limit MCMV replication in the nasal mucosa
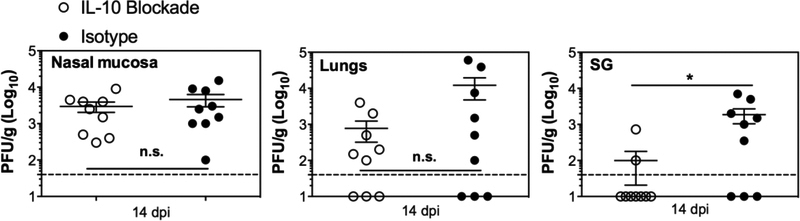
Previous work has shown that blockade of the IL-10R enables better control of MCMV in the salivary gland [38], thus we speculated that this would be true in the nasal mucosa as well. Surprisingly however, blockade of the IL-10R did not significantly decrease virus titers in the nasal mucosa, unlike its effect in the salivary gland (Figure 6). These data indicate that additional mechanisms must allow viral persistence in the nasal mucosa.
Figure 6. Blockade of IL-10R does not limit MCMV replication in the nasal mucosa.
C57BL/6J mice were i.n. infected with 106 PFU WT-BAC MCMV (pSM3fr) and treated with either anti-IL-10R blocking antibody or isotype control antibody on days 1 and 7 post infection. Shown are the titers of virus in the nasal mucosa, lungs and SG, 14 days after infection. Each dot represents an individual mouse and the group means ± SEM is shown. Dashed lines show the detection limit (50 PFU/g). Results represent data pooled from two individual experiments.
Discussion
Natural transmission of CMV has been difficult to observe. It has been proposed by the Mocarski laboratory that footpad inoculation of mice is a model for transmission of MCMV through biting, which might occur in nature [34]. In addition, previous work from the Puddington laboratory demonstrated that infectious MCMV could be found in mouse breast milk and naturally transmitted to pups through suckling [8]. However the site of initial viral infection in the pups was not defined until a recent study from the Stevenson lab provided direct evidence that the nasal mucosa is the primary site of infection after vertical transmission between mothers and pups [13]. These authors argued that the nasal mucosa, which is accessible to fluids in the mouth via the oropharynx and nasopharynx, is most likely the natural portal for CMV entry.
Although intranasal inoculation has been known for many years to allow MCMV infection and dissemination through the body [39], relatively few studies have utilized this route of inoculation. However, MCMV has been reported to target the olfactory epithelium in the nasal mucosa instead of the respiratory epithelium [13, 14]. The explanation for this could be the presence of heparan sulfate, which is expressed both apically and basolaterally on the olfactory epithelium, but only basolaterally on the respiratory epithelium [40]. Heparan sulfate has been reported to contribute to MCMV and HCMV binding and entry, and viruses unable to bind to heparan sulfate show poor host entry [41]. Likewise, this mechanism of infecting olfactory epithelium via binding to heparin sulfate has been reported for murid hepersvirus-4, a gamma herpesvirus [42], and Herpes Simplex Virus-1, an alpha herpesvirus [43]. Our data showed that i.n. inoculation, but not i.p. or f.p. routes of infection, caused sufficient infection of the nasal mucosa for virus to be detected by plaque assay. However, further investigation will be needed to determine whether MCMV reaches the nasal mucosa in reduced quantities after hematogenous dissemination or whether viral dissemination to the nasal mucosa is poor from other parts of the body.
It was surprising to discover that the nasal mucosa supported persistent MCMV replication after i.n. inoculation (Figure 5). It is well known that MCMV replicates persistently in the SG epithelium, in part by exploiting IL-10-mediated immune regulation and in part by evading CD8+ T cells [38, 44]. Therefore, we expected that IL-10 would regulate MCMV persistence in the nasal mucosa as well. However, we found that blockade of the IL-10R, which allowed accelerated control of MCMV in the SG, was unable to affect virus titers in the nasal mucosa (Figure 6). Therefore, other factors must be regulating the local immune response to MCMV in the nasal mucosa. It is currently unknown whether MCMV persists in the olfactory epithelium or whether it spreads to other cells for persistent replication. It also remains to be tested whether evasion of MHC-I antigen presentation and CD8+ T cells allows MCMV persistence in these tissues. MCMV encodes at least 3 genes for proteins that modulate MHC-I expression on infected cells (m04, m06 and m152) and deletion of these has been reported to limit MCMV persistence in the SG [44]. Thus, future work will need to test the persistence of MCMV expressing or lacking these evasion genes. The Stevenson group has recently reported that MCMV gene M78 encodes a protein that degrades MHC-II and that an M78-deleted virus is attenuated for dissemination to the salivary gland after i.n. infection [45]. It will be interesting to test whether M78-deleted virus also fails to persist in the nasal mucosa over time, especially since epithelial cells may express class II MHC under inflammatory conditions. It is also interesting to note that i.n. inoculation, but not i.p. or f.p. inoculation, resulted in prolonged viral replication in the lungs (Figure 1). This result is consistent with very recent work demonstrating that intratracheal infection of MCMV led to a prolonged virus replication in the lungs of immunocompetent mice [46]. It is tempting to speculate that i.n. infection results in prolonged replication in these sites due to the improved infection of epithelial cells. Again, further work will be needed to dissect this question.
After i.n. inoculation, we found that the earliest evidence of CD8+ T cell priming could be detected in the mandibular, deep-cervical and mediastinal draining lymph nodes (Figure 2). This result is consistent with a recent report of CD8+ T cell priming after i.n. infection with influenza virus [47]. We also noticed that intranasal inoculation induced a circulating T cell response with reduced magnitude compared to i.p. inoculation, which is consistent with a previous work in BALB/c mice [11]. Very recent data from the Arens lab also showed reduced T cell responses induced by i.n. infection and demonstrated that such responses are less effective at preventing tumor growth in a cancer vaccine model [48]. Phenotypic analysis of the responding T cells showed that i.n. inoculation resulted in more memory-like phenotype T cells (KLRG1− CD127+), while i.p. inoculation resulted in more effector-phenotype T cells (KLRG1+ CD127−), which is also consistent with the recent data from the Arens lab [48]. Just why the responses are less robust after i.n. infection is unclear however. The Arens lab had previously shown that the inoculum dose directly correlates with the magnitude of MCMV-specific memory inflation and the proportion of effector-phenotype T cells that emerged after i.p. infection [25]. Therefore, it is plausible that i.n. infection leads to a significantly reduced antigen burden that suboptimally drives T cell expansion and differentiation. This could be due to the fact that i.n. inoculation does not break the barrier tissue and it is likely that part of inoculum will be washed away by nasal secretions or swallowed into the stomach. Alternatively, it is possible that the altered phenotype is due to differences in the cells that are infected by each route of inoculation. Several reports have suggested that the endothelial cells of the liver and spleen may be a major site of latency and a source of antigen for driving memory inflation [23, 49–53]. Therefore, it is possible that i.n. infection poorly infects these sinusoidal cells, reducing the antigen burden on inflationary T cells. Indeed, the spleen seems to be poorly infected by MCMV after i.n. inoculation [14, 54]. In theory, such differences in the infected cells might also be responsible for our failure to detect T cell responses to the IE3416–423 epitope. Responses to this epitope develop only late after an i.p. infection [55], but were not detected in our experiments after an i.n. infection (Figure 3).
Finally, we observed tissue-localized (unlabelled by i.v. antibody) CD8+ T cells in the nasal mucosa which expressed CD103 and CD69, the markers of TRM (Figure 4). Such TRM cells likely reside in all tissues of the body and are thought to provide rapid and specific recall responses to any re-encounter with antigen [56]. In some tissues such as the skin, lungs, brain and vaginal mucosa, it has been reported that CD8+ TRM formation depends on local antigen or inflammation (e.g. [57–60]). In contrast, CD8+ TRM development in the other tissues such as the small intestine [61] and salivary gland [21, 22, 27, 62], was independent of antigen or inflammation, although the numbers of T cells that migrated into SG could be boosted by i.v. administration of poly(I:C) [63]. We found that in vitro activated T cells could migrate to the nasal mucosa and develop a TRM phenotype independently of local antigen or infection (Figure 4). Infection of the nasal mucosa was associated with more T cells that were recovered from this tissue (not shown), but more work will be needed to determine how much local inflammation or antigen modulates TRM formation. It is also important to note that we did not study the retention of TRM in the nasal mucosa. Thus, it remains to be determined whether these cells will persist for long periods of time and whether they can provide any protection against an MCMV infection of this tissue. Regardless, it is clear that they could not quench the ongoing MCMV replication in the nasal mucosa. An analogous situation exists in the salivary gland, where MCMV replicates persistently despite the presence of MCMV-specific TRM. In the salivary gland however, pre-existing TRM could control locally introduced MCMV [27]. Therefore, even though the development of MCMV-specific TRM did not correlate with a cessation of viral replication, it remains possible that pre-existing TRM in the nasal mucosa may aid in prevention of MCMV dissemination from this natural site of entry. Besides virus-specific CD8+ TRM residing in the nasal mucosa, we also observed tissue-localized CD4+ TRM (CD11a+, CD69+) in the nasal mucosa (not shown) after i.n. infection. Attempts to define the specificity of these CD4+ T cells with MHC-II tetramers failed, which is perhaps not surprising given the small percentages of T cells specific for any one viral antigen [64, 65] and the very low numbers of T cells recovered from the nasal mucosa of any given mouse (typically between 2×104 and 1×105 total T cells per mouse by day 14 of infection, with CD4+ and CD8+ T cells at an approximately 1:1 ratio). Therefore defining the specificity of the CD4+ TRM in the nasal mucosa requires further investigation.
In summary, our data show that the nasal mucosa is a second site that supports persistent MCMV replication. T cells were primed in respiratory draining lymph nodes and developed into TRM localized to the nasal mucosa. However, neither local MCMV-specific TRM cells nor blockade of IL-10 prevented MCMV persistence in this tissue. These data provide new details about the host/pathogen relationship and immune responses that develop after intranasal infection by MCMV and suggest that it may be difficult to control MCMV at the natural site of entry.
Supplementary Material
Supplemental Figure 1. Representative gating strategies. Representative gates show the analyses of dividing CD8+ T cells to identify the sites of priming (data shown in Figure 2), the identification of CD8+ TRM in the i.v. antibody-negative fraction of the nasal mucosa and salivary glands (data shown in Figure 4), and phenotypic analyses of circulating virus-specific CD8+ T cells (data shown in Figure 3).
Acknowledgments
This work was supported by grant AI106810 awarded to C.M.S.
Footnotes
Declaration of interests
The authors declare no competing interests.
References:
- 1.Styczynski J (2018) Who Is the Patient at Risk of CMV Recurrence: A Review of the Current Scientific Evidence with a Focus on Hematopoietic Cell Transplantation. Infect Dis Ther 7:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manicklal S, Emery VC, Lazzarotto T, Boppana SB, Gupta RK (2013) The “silent” global burden of congenital cytomegalovirus. Clin Microbiol Rev 26:86–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crough T, Khanna R (2009) Immunobiology of human cytomegalovirus: from bench to bedside. Clin Microbiol Rev 22:76–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mayer BT, Krantz EM, Swan D, Ferrenberg J, Simmons K, Selke S, Huang ML, Casper C, Corey L, Wald A, Schiffer JT, Gantt S (2017) Transient Oral Human Cytomegalovirus Infections Indicate Inefficient Viral Spread from Very Few Initially Infected Cells. J Virol 91:e00380–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schleiss MR (2006) Role of breast milk in acquisition of cytomegalovirus infection: recent advances. Curr Opin Pediatr 18:48–52 [DOI] [PubMed] [Google Scholar]
- 6.Dworsky M, Yow M, Stagno S, Pass RF, Alford C (1983) Cytomegalovirus infection of breast milk and transmission in infancy. Pediatrics 72:295–299 [PubMed] [Google Scholar]
- 7.Krmpotic A, Bubic I, Polic B, Lucin P, Jonjic S (2003) Pathogenesis of murine cytomegalovirus infection. Microbes Infect 5:1263–1277 [DOI] [PubMed] [Google Scholar]
- 8.Wu CA, Paveglio SA, Lingenheld EG, Zhu L, Lefrancois L, Puddington L (2011) Transmission of murine cytomegalovirus in breast milk: a model of natural infection in neonates. J Virol 85:5115–5124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.dela Pena MG, Strelow L, Barry PA, Abel K (2012) Use of specific-pathogen-free (SPF) rhesus macaques to better model oral pediatric cytomegalovirus infection. J Med Primatol 41:225–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stahl FR, Heller K, Halle S, Keyser KA, Busche A, Marquardt A, Wagner K, Boelter J, Bischoff Y, Kremmer E, Arens R, Messerle M, Forster R (2013) Nodular inflammatory foci are sites of T cell priming and control of murine cytomegalovirus infection in the neonatal lung. PLoS Pathog 9:e1003828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oduro JD, Redeker A, Lemmermann NA, Ebermann L, Marandu TF, Dekhtiarenko I, Holzki JK, Busch DH, Arens R, Čičin-Šain L (2016) Murine cytomegalovirus (CMV) infection via the intranasal route offers a robust model of immunity upon mucosal CMV infection. J Gen Virol 97:185–195 [DOI] [PubMed] [Google Scholar]
- 12.Davey A, Eastman L, Hansraj P, Hemmings DG (2011) Human cytomegalovirus is protected from inactivation by reversible binding to villous trophoblasts. Biol Reprod 85:198–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farrell HE, Lawler C, Tan CSE, MacDonald K, Bruce K, Mach M, Davis-Poynter N, Stevenson PG (2016) Murine Cytomegalovirus Exploits Olfaction To Enter New Hosts. mBio 7:e00251–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang S, Xiang J, Van Doorsselaere J, Nauwynck HJ (2015) Comparison of the pathogenesis of the highly passaged MCMV Smith strain with that of the low passaged MCMV HaNa1 isolate in BALB/c mice upon oronasal inoculation. Vet Res 46:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farrell HE, Davis-Poynter N, Bruce K, Lawler C, Dolken L, Mach M, Stevenson PG (2015) Lymph Node Macrophages Restrict Murine Cytomegalovirus Dissemination. J Virol 89:7147–7158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wagner M, Jonjic S, Koszinowski UH, Messerle M (1999) Systematic excision of vector sequences from the BAC-cloned herpesvirus genome during virus reconstitution. Journal of Virology 73:7056–7060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snyder CM, Cho KS, Bonnett EL, van Dommelen S, Shellam GR, Hill AB (2008) Memory inflation during chronic viral infection is maintained by continuous production of short-lived, functional T cells. Immunity 29:650–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turula H, Smith CJ, Grey F, Zurbach KA, Snyder CMD (2013) Competition between T cells maintains clonal dominance during memory inflation induced by MCMV. Eur J Immunol 43:1252–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zurbach KA, Moghbeli T, Snyder CM (2014) Resolving the titer of murine cytomegalovirus by plaque assay using the M2–10B4 cell line and a low viscosity overlay. Virol J 11:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dunston D, Ashby S, Krosnowski K, Ogura T, Lin W (2013) An effective manual deboning method to prepare intact mouse nasal tissue with preserved anatomical organization. J Vis Exp [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caldeira-Dantas S, Furmanak T, Smith C, Quinn M, Teos LY, Ertel A, Kurup D, Tandon M, Alevizos I, Snyder CM (2018) The Chemokine Receptor CXCR3 Promotes CD8+ T Cell Accumulation in Uninfected Salivary Glands but Is Not Necessary after Murine Cytomegalovirus Infection. J Immunol 200:1133–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith CJ, Caldeira-Dantas S, Turula H, Snyder CM (2015) Murine CMV Infection Induces the Continuous Production of Mucosal Resident T Cells. Cell Rep 13:1137–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torti N, Walton SM, Brocker T, Rulicke T, Oxenius A (2011) Non-Hematopoietic Cells in Lymph Nodes Drive Memory CD8 T Cell Inflation during Murine Cytomegalovirus Infection. PLoS Pathog 7:e1002313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baumann NS, Torti N, Welten SPM, Barnstorf I, Borsa M, Pallmer K, Oduro JD, Cicin-Sain L, Ikuta K, Ludewig B, Oxenius A (2018) Tissue maintenance of CMV-specific inflationary memory T cells by IL-15. PLoS Pathog 14:e1006993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Redeker A, Welten SP, Arens R (2014) Viral inoculum dose impacts memory T-cell inflation. Eur J Immunol 44:1046–1057 [DOI] [PubMed] [Google Scholar]
- 26.Quinn M, Turula H, Tandon M, Deslouches B, Moghbeli T, Snyder CM (2015) Memory T Cells Specific for Murine Cytomegalovirus Re-Emerge after Multiple Challenges and Recapitulate Immunity in Various Adoptive Transfer Scenarios. J Immunol 194:1726–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thom JT, Weber TC, Walton SM, Torti N, Oxenius A (2015) The Salivary Gland Acts as a Sink for Tissue-Resident Memory CD8(+) T Cells, Facilitating Protection from Local Cytomegalovirus Infection. Cell Rep 13:1125–1136 [DOI] [PubMed] [Google Scholar]
- 28.Snyder CM, Allan JE, Bonnett EL, Doom CM, Hill AB (2010) Cross-Presentation of a Spread-Defective MCMV Is Sufficient to Prime the Majority of Virus-Specific CD8+ T Cells. PLoS One 5:e9681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Snyder CM, Cho KS, Bonnett EL, Allan JE, Hill AB (2011) Sustained CD8+ T Cell Memory Inflation after Infection with a Single-Cycle Cytomegalovirus. PLoS Pathog 7:e1002295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuttner AG, Wang SH (1934) THE PROBLEM OF THE SIGNIFICANCE OF THE INCLUSION BODIES FOUND IN THE SALIVARY GLANDS OF INFANTS, AND THE OCCURRENCE OF INCLUSION BODIES IN THE SUBMAXILLARY GLANDS OF HAMSTERS, WHITE MICE, AND WILD RATS (PEIPING). J Exp Med 60:773–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCordock HA, Smith MG (1936) THE VISCERAL LESIONS PRODUCED IN MICE BY THE SALIVARY GLAND VIRUS OF MICE. J Exp Med 63:303–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jordan S, Krause J, Prager A, Mitrovic M, Jonjic S, Koszinowski UH, Adler B (2011) Virus Progeny of Murine Cytomegalovirus Bacterial Artificial Chromosome pSM3fr Show Reduced Growth in Salivary Glands due to a Fixed Mutation of MCK-2. J Virol 85:10346–10353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saederup N, Lin YC, Dairaghi DJ, Schall TJ, Mocarski ES (1999) Cytomegalovirus-encoded beta chemokine promotes monocyte-associated viremia in the host. Proc Natl Acad Sci U S A 96:10881–10886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saederup N, Aguirre SA, Sparer TE, Bouley DM, Mocarski ES (2001) Murine cytomegalovirus CC chemokine homolog MCK-2 (m131–129) is a determinant of dissemination that increases inflammation at initial sites of infection. J Virol 75:9966–9976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noda S, Aguirre SA, Bitmansour A, Brown JM, Sparer TE, Huang J, Mocarski ES (2006) Cytomegalovirus MCK-2 controls mobilization and recruitment of myeloid progenitor cells to facilitate dissemination. Blood 107:30–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daley-Bauer LP, Roback LJ, Wynn GM, Mocarski ES (2014) Cytomegalovirus hijacks CX3CR1(hi) patrolling monocytes as immune-privileged vehicles for dissemination in mice. Cell Host Microbe 15:351–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lemmermann NA, Krmpotic A, Podlech J, Brizic I, Prager A, Adler H, Karbach A, Wu Y, Jonjic S, Reddehase MJ, Adler B (2015) Non-redundant and redundant roles of cytomegalovirus gH/gL complexes in host organ entry and intra-tissue spread. PLoS Pathog 11:e1004640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Humphreys IR, de Trez C, Kinkade A, Benedict CA, Croft M, Ware CF (2007) Cytomegalovirus exploits IL-10-mediated immune regulation in the salivary glands. J Exp Med 204:1217–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jordan MC (1978) Interstitial pneumonia and subclinical infection after intranasal inoculation of murine cytomegalovirus. Infect Immun 21:275–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gillet L, Frederico B, Stevenson PG (2015) Host entry by gamma-herpesviruses--lessons from animal viruses. Curr Opin Virol 15:34–40 [DOI] [PubMed] [Google Scholar]
- 41.Price P, Allcock RJ, Coombe DR, Shellam GR, McCluskey J (1995) MHC proteins and heparan sulphate proteoglycans regulate murine cytomegalovirus infection. Immunol Cell Biol 73:308–315 [DOI] [PubMed] [Google Scholar]
- 42.Milho R, Frederico B, Efstathiou S, Stevenson PG (2012) A heparan-dependent herpesvirus targets the olfactory neuroepithelium for host entry. PLoS Pathog 8:e1002986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shivkumar M, Milho R, May JS, Nicoll MP, Efstathiou S, Stevenson PG (2013) Herpes simplex virus 1 targets the murine olfactory neuroepithelium for host entry. J Virol 87:10477–10488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walton SM, Mandaric S, Torti N, Zimmermann A, Hengel H, Oxenius A (2011) Absence of cross-presenting cells in the salivary gland and viral immune evasion confine cytomegalovirus immune control to effector CD4 T cells. PLoS Pathog 7:e1002214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yunis J, Farrell HE, Bruce K, Lawler C, Sidenius S, Wyer O, Davis-Poynter N, Stevenson PG (2018) Murine cytomegalovirus degrades MHC class II to colonize the salivary glands. PLoS Pathog 14:e1006905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reuter S, Lemmermann NAW, Maxeiner J, Podlech J, Beckert H, Freitag K, Teschner D, Ries F, Taube C, Buhl R, Reddehase MJ, Holtappels R (2019) Coincident airway exposure to low-potency allergen and cytomegalovirus sensitizes for allergic airway disease by viral activation of migratory dendritic cells. PLoS Pathog 15:e1007595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pizzolla A, Wang Z, Groom JR, Kedzierska K, Brooks AG, Reading PC, Wakim LM (2017) Nasal-associated lymphoid tissues (NALTs) support the recall but not priming of influenza virus-specific cytotoxic T cells. Proc Natl Acad Sci U S A 114:5225–5230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beyranvand Nejad E, Ratts RB, Panagioti E, Meyer C, Oduro JD, Cicin-Sain L, Früh K, van der Burg SH, Arens R (2019) Demarcated thresholds of tumor-specific CD8 T cells elicited by MCMV-based vaccine vectors provide robust correlates of protection. j immunotherapy cancer 7:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mercer JA, Wiley CA, Spector DH (1988) Pathogenesis of murine cytomegalovirus infection: identification of infected cells in the spleen during acute and latent infections. J Virol 62:987–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seckert CK, Renzaho A, Tervo HM, Krause C, Deegen P, Kuhnapfel B, Reddehase MJ, Grzimek NK (2009) Liver sinusoidal endothelial cells are a site of murine cytomegalovirus latency and reactivation. J Virol 83:8869–8884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dag F, Dolken L, Holzki J et al. (2014) Reversible silencing of cytomegalovirus genomes by type I interferon governs virus latency. PLoS Pathog 10:e1003962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith CJ, Turula H, Snyder CM (2014) Systemic hematogenous maintenance of memory inflation by MCMV infection. PLoS Pathog 10:e1004233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seckert CK, Schader SI, Ebert S, Thomas D, Freitag K, Renzaho A, Podlech J, Reddehase MJ, Holtappels R (2011) Antigen-presenting cells of haematopoietic origin prime cytomegalovirus-specific CD8 T-cells but are not sufficient for driving memory inflation during viral latency. J Gen Virol 92:1994–2005 [DOI] [PubMed] [Google Scholar]
- 54.Zhang S, Xiang J, Theuns S, Desmarets LM, Trus I, Nauwynck HJ (2016) MCMV exploits the spleen as a transfer hub for systemic dissemination upon oronasal inoculation. Virus Res 217:47–54 [DOI] [PubMed] [Google Scholar]
- 55.Munks MW, Cho KS, Pinto AK, Sierro S, Klenerman P, Hill AB (2006) Four distinct patterns of memory CD8 T cell responses to chronic murine cytomegalovirus infection. J Immunol 177:450–458 [DOI] [PubMed] [Google Scholar]
- 56.Schenkel JM, Masopust D (2014) Tissue-resident memory T cells. Immunity 41:886–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wakim LM, Woodward-Davis A, Bevan MJ (2010) Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc Natl Acad Sci U S A 107:17872–17879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee YT, Suarez-Ramirez JE, Wu T, Redman JM, Bouchard K, Hadley GA, Cauley LS (2011) Environmental and antigen receptor-derived signals support sustained surveillance of the lungs by pathogen-specific cytotoxic T lymphocytes. J Virol 85:4085–4094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mackay LK, Stock AT, Ma JZ, Jones CM, Kent SJ, Mueller SN, Heath WR, Carbone FR, Gebhardt T (2012) Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc Natl Acad Sci U S A 109:7037–7042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shin H, Iwasaki A (2012) A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature 491:463–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Casey KA, Fraser KA, Schenkel JM, Moran A, Abt MC, Beura LK, Lucas PJ, Artis D, Wherry EJ, Hogquist K, Vezys V, Masopust D (2012) Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J Immunol 188:4866–4875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hofmann M, Pircher H (2011) E-cadherin promotes accumulation of a unique memory CD8 T-cell population in murine salivary glands. Proc Natl Acad Sci U S A 108:16741–16746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Woyciechowski S, Hofmann M, Pircher H (2017) α4 β1 integrin promotes accumulation of tissue-resident memory CD8(+) T cells in salivary glands. Eur J Immunol 47:244–250 [DOI] [PubMed] [Google Scholar]
- 64.Arens R, Wang P, Sidney J, Loewendorf A, Sette A, Schoenberger SP, Peters B, Benedict CA (2008) Cutting edge: murine cytomegalovirus induces a polyfunctional CD4 T cell response. J Immunol 180:6472–6476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Walton SM, Wyrsch P, Munks MW, Zimmermann A, Hengel H, Hill AB, Oxenius A (2008) The Dynamics of Mouse Cytomegalovirus-Specific CD4 T Cell Responses during Acute and Latent Infection. J Immunol 181:1128–1134 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Representative gating strategies. Representative gates show the analyses of dividing CD8+ T cells to identify the sites of priming (data shown in Figure 2), the identification of CD8+ TRM in the i.v. antibody-negative fraction of the nasal mucosa and salivary glands (data shown in Figure 4), and phenotypic analyses of circulating virus-specific CD8+ T cells (data shown in Figure 3).