Abstract
The cortisol awakening response (CAR) has been shown to prospectively predict depression, but it remains unresolved whether a greater CAR predicts risk independently of subsequent acute stress, or whether greater CAR indicates increased vulnerability to subsequent acute stress. Further, no prior work has evaluated whether the CAR increases vulnerability to certain types of acute stress, but not others, in predicting depression. To address these gaps, we investigated whether the CAR predicted depressive symptoms alone and in interaction with acute interpersonal stress in a one-year longitudinal study of 86 early adolescent girls with no history of diagnosable depression. To index the CAR, adolescents collected saliva at waking and 30-minutes past waking for 3 days; compliance with the sampling protocol was electronically monitored. Diagnostic and objective contextual stress interviews were used to quantify acute stress in the 2-months prior to worst depressive symptom onset during the follow-up. Supporting hypotheses, results indicated that greater CAR predicted greater depressive symptoms, and interacted with acute interpersonal stress in predicting depressive symptoms. Further, the CAR interacted with acute dependent (i.e., at least partially arising from the person’s behavior) interpersonal stress in predicting depressive symptoms. In contrast, the CAR did not interact with acute non-interpersonal stress nor acute interpersonal independent (i.e., fateful) stress in predicting depressive symptoms. These results further refine circumstances in which the CAR is predictive of depressive symptoms among early adolescent girls, and highlight the importance of focusing on etiologically relevant stress when testing interactions between physiological stress indicators and environmental stress.
Keywords: Cortisol awakening response, Depressive symptoms, Interpersonal stress, Dependent stress, HPA axis
1. Introduction
Substantial cross-sectional evidence suggests that youth with depression exhibit alterations in hypothalamic pituitary adrenal (HPA) axis activity (for a review see, Lopez-Duran et al., 2009). Considerably fewer studies have examined whether indicators of the HPA diurnal pattern prospectively predict depressive symptoms and disorders in younger healthy populations. Recently, one diurnal indicator—the Cortisol Awakening Response (CAR)—has been implicated in depression’s etiology (e.g., Adam et al., 2010; Vrshek-Schallhorn et al., 2013). The CAR represents the substantial cortisol increase that occurs in response to waking (Wilhelm et al., 2007), with cortisol levels peaking approximately 30–40 min after waking (Clow et al., 2010). However, questions remain about the CAR’s role in the prediction of depression because it is unclear whether the CAR predicts risk independently of subsequent acute stress, or whether greater CAR indicates increased vulnerability to subsequent acute stress (Vrshek-Schallhorn et al., 2013). Further, it remains to be investigated whether the CAR increases vulnerability to certain types of acute stress, but not others, in predicting depression. Here we sought to address these gaps in a one-year longitudinal study of early adolescent girls. Girls experience greater depressive symptoms than boys (e.g., Oldehinkel et al., 1999), which may in part be due to gender differences in HPA axis functioning (e.g., Gunnar et al., 2009) and in stress exposure and sensitivity (e.g., Rudolph and Hammen, 1999). Further, mid-adolescence marks a developmental period in which clinically significant major depressive episodes emerge at high rates, particularly for girls (e.g., Rohde et al., 2009). Thus, further understanding of the roles of the CAR and acute stressors in predicting increases in depressive symptoms among early adolescent girls may be particularly informative for preventing first onsets as well as for elucidating the developmental course of such predictors.
1.1. The cortisol awakening response, acute stress, and depressive symptoms
Recent interest in the CAR was sparked by research demonstrating that a greater CAR prospectively predicted depression risk up to 2.5 years later (Adam et al., 2010; Vrshek-Schallhorn et al., 2013). Although CAR elevations may serve an adaptive function in the short-term by mobilizing the body’s resources (via influencing metabolic processes) to help meet perceived daily demands (e.g., Adam et al., 2006), persistent CAR elevations may indicate depression vulnerability, perhaps capturing engaged struggle (Vrshek-Schallhorn et al., 2013)—meaning effortfully coping with perceived internal and external demands more so than behaviorally and emotionally withdrawing. Moreover, elevations may actively heighten depression risk by triggering changes in the density, sensitivity, or ratio of glucocorticoid receptors (e.g., de Kloet, 2014). However, only one of the four subsequently-published prospective studies (Carnegie et al., 2014; Hardeveld et al., 2014; LeMoult et al., 2015; Schuler et al., 2017) has supported the CAR-depression association (Hardeveld et al., 2014), leaving questions about whether the CAR prospectively predicts depression.
Methodological features of prior work could have contributed to the mixed findings on whether the CAR prospectively predicts depression. Specifically, among studies that did not support the CAR-depression link, most examined self-reported depressive symptoms (e.g., in the past 2 weeks), which would overlook symptoms occurring in the months following CAR measurement when the CAR’s predictive effect is strongest (for an exception, see LeMoult et al., 2015). Similarly, because the CAR is thought to serve as a time-specific risk factor (Vrshek-Schallhorn et al., 2013), using long follow-up periods (i.e., greater than 2.5 years) would not be expected to produce significant associations with current symptoms. Further, among studies that did not support the CAR-depression link, none accounted for state covariates (i.e., momentary or daily factors that impact cortisol levels, such as past hour caffeine use; e.g., Doane and Adam, 2010) which may downwardly bias estimates of CAR’s effect (Kudielka et al., 2003). Finally, most did not electronically monitor sampling protocol compliance for the entire sample (for an exception, see Schuler et al., 2017) despite evidence that compliance helps to ensure accurate CAR estimates (e.g., Stalder et al., 2016).
Moreover, it remains unresolved whether greater CAR predicts risk independently of subsequent acute stress, or whether greater CAR indicates increased vulnerability to subsequent acute stress (Vrshek-Schallhorn et al., 2013). If CAR is a time-specific marker of engaged struggle, then diathesis-stress conceptualizations of depression (e.g., Monroe and Simons, 1991) would predict that subsequent life stress might interact with CAR-indicated vulnerability to predict increases in depressive symptoms. However, only one (Schuler et al., 2017) of three studies (Vrshek-Schallhorn et al., 2013; LeMoult et al., 2015) testing the CAR-acute stress interaction found a significant effect. Further, few of these prior studies have used gold-standard contextual stress interviews with blinded severity coding (Harkness and Monroe, 2016), which helps to disentangle stress exposure from psychological stress responses. Similarly, few have dated events to ensure temporal precedence of stress to depression and to isolate the period of time that events significantly increase depression risk—within 1–3 months of the event (e.g., Brown and Harris, 1978). However, to investigate whether the CAR prospectively predicts depression, it is critical to focus on proximal acute stress that occurs prior to symptom onsets (e.g., Harkness and Monroe, 2016).
Further, no prior work has evaluated whether the CAR increases vulnerability to certain types of acute stress, but not others, in predicting depression. Acute stress varies on several dimensions (e.g., severity, interpersonal nature), some of which influence its etiological significance for depression (e.g., Vrshek-Schallhorn et al., 2015a). However, existing research has examined whether the CAR interacts with a single index reflecting all types of acute stress. A focus on acute interpersonal stress is supported by interpersonal theories of depression (e.g., Hammen, 1991), and evidence that acute interpersonal stress uniquely contributes to risk for Major Depressive Disorder (MDD) onset (Vrshek-Schallhorn et al., 2015a) and is more potent in predicting MDD onsets than non-interpersonal acute stress (Stroud et al., 2011). Moreover, prior work examining cortisol reactivity to acute stress in laboratory settings (e.g., Dickerson and Kemeny, 2004), as well as the relationship between acute stress and trait cortisol (Stroud et al., 2016), suggests that the HPA axis may be particularly sensitive to acute interpersonal stress.
1.2. The present study
Here we examined whether the CAR alone and in interaction with subsequent acute stress predicted increases in depressive symptoms in a one-year study of early adolescent girls with no prior diagnosable depression. Given prior work, we expected that greater CAR would predict greater depressive symptoms, and interact with acute interpersonal, but not non-interpersonal, stress in predicting depressive symptoms. Moreover, we expected that CAR’s interaction with acute interpersonal stress would be significantly greater in magnitude than its interaction with non-interpersonal stress. Further, we conducted a follow-up analysis in which acute interpersonal stress was further stratified by event independence (i.e., the degree to which individuals contribute to the occurrence of events) to determine whether the CAR differentially interacted with independent (i.e., fateful) versus dependent (i.e., at least partially controllable) forms of acute interpersonal stress. Because some research suggests that each type of interpersonal stress contributes unique variance to depression risk (Vrshek-Schallhorn et al., 2015a), whereas other work suggests that one form may be more etiologically relevant (e.g., Stroud et al., 2011), this analysis was exploratory.
2. Methods
2.1. Participants
Participants were early adolescent girls who completed the saliva sampling portion of a larger study examining predictors of emotional disorders. Participants and their primary female caregivers (herein called mothers) were recruited from two predominately rural counties in New England using multiple methods, including advertisements or flyers (10.3%), word-of-mouth (13.1%), and local schools (76.6%). Of the 122 participants who completed the saliva sampling portion, 91 (74.59%) used a MEMS 6TM track cap container (a screw top bottle that provides a date and time stamp each time it is opened; Aardex Group, Richmond, VA), a device used to assess compliance with the sampling protocol. Because adherence to the sampling protocol is critical in accurately indexing the CAR (Stalder et al., 2016), only participants who used the track cap were included. On average, participants provided 8.74 (SD = .78) of the expected 9 samples. Of the 91 participants who used the track cap, 5 had a history of diagnosable depression and none had current diagnosable depression. Because prior diagnosable depression can influence diurnal cortisol indicators (e.g., Doane et al., 2013) and the CAR-depression association (e.g., Vrshek-Schallhorn et al., 2013), those 5 participants were excluded (Analytic N = 86).1
Participant characteristics are presented in Table 1. Eighty-one of the 86 (94.19%) completed the one-year follow-up (T2). There were not significant differences between: a) those who did and did not complete the cortisol assessment; b) those who did and did not use the track cap; and c) those who did and did not participate in T2 on any of the T1 variables (ps > .05), except those who did not complete the cortisol assessment had higher T1 past year acute stress and those who did not use the track cap had higher past year chronic stress (ps < .05).
Table 1.
Characteristics of Adolescent Participants and Descriptive Statistics.
| Participant Characteristics | n | % | M | SD | Range |
|---|---|---|---|---|---|
| T1 Age (years) | 86 | - | 12.31 | .75 | 10.83 – 13.91 |
| T1 Pubertal Status | 82 | - | 2.64 | .61 | 1.20–3.80 |
| Race/Ethnicity | |||||
| White | 75 | 87.2% | |||
| Black | 2 | 2.3% | |||
| Asian | 4 | 4.7% | |||
| Latina/Hispanic | 3 | 3.5% | |||
| Native American | 2 | 2.3% | |||
| Bi-/Multi Racial | 2 | 2.3% | |||
| Other | 10 | 11.7% | |||
| T1 Income | |||||
| < $40,000 | 12 | 14.0% | |||
| $41,000-$60,000 | 17 | 19.8% | |||
| $61,000-$100,000 | 22 | 25.6% | |||
| > $100,000 | 35 | 40.7% | |||
| T1 Current Depressive Symptoms | |||||
| 0 (no symptoms) | 82 | 95.3 | |||
| 1 (mild symptoms) | 2 | 2.3 | |||
| 2 (moderate, sub-threshold symptoms) | 2 | 2.3 | |||
| 3 (diagnosable, DSM-IV criteria) | 0 | 0 | |||
| T1 Past Depressive Symptoms | |||||
| 0 (no symptoms) | 68 | 79.1 | |||
| 1 (mild symptoms) | 9 | 10.5 | |||
| 2 (moderate, sub-threshold symptoms) | 9 | 10.5 | |||
| 3 (diagnosable, DSM-IV criteria) | 0 | 0 | |||
| T2 Depressive Symptoms | |||||
| 0 (no symptoms) | 67 | 77.9 | |||
| 1 (mild symptoms) | 5 | 5.8 | |||
| 2 (moderate, sub-threshold symptoms) | 7 | 8.1 | |||
| 3 (diagnosable, DSM-IV criteria) | 2 | 2.3 | |||
| Missing (due to attrition) | 5 | 5.8 | |||
| Other Study Variables | |||||
| CAR | 86 | .020 | .090 | –.71 – .15 | |
| Acute Interpersonal Stress | 81 | 1.611 | 1.901 | 0–8 | |
| Acute Non-Interpersonal Stress | 81 | .790 | 1.447 | 0–7 | |
| Acute Dependent Interpersonal stress | 81 | .716 | 1.381 | 0–8 | |
| Acute Independent Interpersonal stress | 81 | .895 | 1.380 | 0–6 | |
| T2 Depressive Symptoms | 86 | .301 | .736 | 0–3 | |
| Caffeine use | 86 | .048 | .159 | 0–.67 | |
| Waking time | 85 | 7.270 | 1.418 | 5.08 – 12.01 | |
| Non-compliance | 86 | .888 | .209 | 0–1 | |
| Negative affect | 85 | .241 | .268 | 0–1.63 | |
| Positive affect | 85 | .700 | .601 | 0 – 2.72 | |
| Perceived Stress | 78 | 2.502 | .667 | 1–4.33 | |
| T1 Past Year Acute Stress | 86 | 11.104 | 7.533 | 0 – 32 | |
| T1 Past Year Chronic Stress | 86 | 1.883 | .414 | 1.25–3.07 | |
| Days | 81 | 213.69 | 91.423 | 53 – 425 | |
Notes. Ns vary due to missing data and attrition at T2. For race/ethnicity, participants could select more than one category; thus, the percentages total greater than 100%. T1 = Time 1. T2 = Time 2. CAR = cortisol awakening response. Cortisol values were transformed using the natural log function. Stress variables were computed by summing the severity ratings of each type of event in the 2 months prior to symptom onsets or a randomly selected 2-month period if no symptom onset. Days = the number of days between the first day of the saliva collection and the first day of the symptomatic period (for those with symptom onset) or the number of days between the first day of the saliva collection and the first day after the 2-month randomly chosen period in which stress was examined (for those without symptom onset).
2.2. Measures
2.2.1. Cortisol
Samples were assayed in duplicate, using a solid phase time-resolved fluorescence immunoassay with fluorometric endpoint detection (DELFIA; Dressendorfer et al., 1992). The intra-assay coefficients of variation were 4.0%–6.7%, and the inter-assay coefficients of variation were 7.1%–9.0%. For the waking sample, M = .23μg/dl, SD = .10, range: .07–.63. For the 30min past-waking sample, M = .23μg/dl, SD = .17, range: .05–1.02. Outliers were winsorized to 50 μg/dl following recommendations (Nicolson, 2008). The CAR was calculated using the formula for area under the curve with respect to increase using the waking and 30min past-waking samples (Pruessner et al., 2003) after natural log transformation to address skew. The mean CAR was used (n = 81 had values for 3 days [94.19%]; n = 5 had values for 2 days [5.81%]).
2.2.2. Depressive symptoms
At T1 and T2, adolescents were interviewed with the Schedule for Affective Disorders and Schizophrenia for School-Aged Children-Present and Lifetime version (Kaufman et al., 1997). Interviews were conducted by a clinical psychologist, a licensed social worker, or a training student under close supervision. Symptoms were rated: 0 = none; 1 = mild; 2 = moderate; 3 = DSM-IV criteria. T1 ratings reflect lifetime history and current (past month) symptoms. T2 ratings reflect symptoms since T1. For T2 depressive symptoms, the worst period of symptoms (coded 1–3) between T1 and T2 was used in analyses (e.g., Vrshek-Schallhorn et al., 2015b). Symptom onsets and offsets were dated to establish temporal relationships to life events. A T1 past and current depressive symptoms composite variable (computed by taking the maximum of the T1 past and current depressive ratings) was examined as a potential covariate (see Table 1). Inter-rater reliability was assessed by rerating approximately 20% interviews using audio-recordings (ICCs = .97–1.00).
Given that the time period between the CAR assessment and T2 depressive symptoms varied for each participant as the worst period of symptoms between T1 and T2 was used, we calculated the number of days between the first day of the saliva collection and the first day of the symptomatic period (i.e., the first day after the 2-month period in which stress was examined). For those who did not have a symptomatic period, we calculated the number of days between the first day of the saliva collection and the first day after the 2-month randomly chosen period in which stress was examined. This difference (herein called days) was examined as a potential covariate.
2.2.3. Life stress
A modified version of the UCLA Life Stress Interview (LSI; adapted from Rudolph and Hammen, 1999; Rudolph et al., 2000) was used to assess adolescents’ past year acute (i.e., events with a brief onset and relatively short duration) and chronic (i.e., ongoing circumstances) life stress. At T1, the interview assessed the prior year; at T2, the interview assessed the time since T1. Mothers and daughters completed separate interviews with the same interviewer, and interviewers were blind to other data.
2.2.4. Acute stress
For each event, participants provided information about its surrounding context (e.g., circumstances and resources to cope with it, predictability, and prior experience with similar events), duration, and consequences to obtain the degree of impact for a typical individual given the context (i.e., objective impact). Interviewers prepared narrative accounts of each event (detailing the context, but excluding participants’ subjective reactions) that were presented to an independent rating team, comprising trained and reliable interviewers who were blind to all other data. Consistent with prior work (e.g., Rudolph et al., 2000), when mothers and daughters reported the same event, information from mothers and adolescents was combined into a single narrative. If only one reported the event, the narrative reflected only her report.
Consistent with prior work (e.g., Rudolph et al., 2000), for each event, the team rated: a) objective impact (1 [no negative impact] to 5 [extremely severe negative impact]; half-points permitted); b) interpersonal status (coded 1/0; rated interpersonal when the primary context involved relations with others or affected the participants’ relations); and c) independence (degree to which the event resulted from the participant’s behavior; 1 [fully independent of the person’s behavior] to 5 [fully dependent on the person’s behavior]; half-points permitted). Events rated as 3 or higher were dependent, and those 2.5 or lower were independent (e.g., Stroud et al., 2011). A second team, blind to the original ratings, rerated a set of events (n = 132) on objective impact (ICC = .92), interpersonal status (ICC = .98), and independence (ICC = .99).
Following prior work (e.g., Vrshek-Schallhorn et al., 2015b), acute stress composites were created by summing the severity ratings of all events occurring in the 2 months before the worst depressive symptom onset between T1 and T2. This approach is consistent with research indicating that triggered onsets occur most often within one month of an event, and almost always within 2 months of an event (e.g., Brown and Harris, 1978). For participants with no symptoms, a 2-month period was randomly selected. Events were conservatively excluded when the temporal precedence of event to the depressive symptom onset was indeterminate. Four composites were created: 1) acute interpersonal stress (interpersonal events; e.g., break-up, conflict); 2) acute non-interpersonal stress (non-interpersonal events; e.g., academic failure; extracurricular disappointment); 3) acute independent interpersonal stress (independent interpersonal events; e.g., parental job loss, death); and 4) acute dependent interpersonal stress (dependent interpersonal events; e.g., conflict, end of friendship). The frequencies for each type of event are provided in Supplemental Table 1.
A T1 past year acute stress variable was formed by summing the severity ratings of events that had occurred during the past year (e.g., Vrshek-Schallhorn et al., 2013).
2.2.5. Chronic stress
T1 past year chronic stress was examined as a potential covariate (e.g., Vrshek-Schallhorn et al., 2013). During the LSI, probes elicited behavioral descriptions of adolescents’ ongoing objective stress in several domains (academics, academic behavior, parent-child relationship, close friendships, peer social life, romantic relationships/dating, parents’ marital [or cohabiting] romantic relationship [if applicable]). Using behavioral indicators, interviewers rated adolescents’ chronic stress level in each domain (1-excellent/optimal circumstances to 5-very bad circumstances, half-points permitted). To asses inter-rater reliability, independent coders (blind to original ratings) rerated a set of interviews using audio-recordings: ICCs: M = .81 (.70–.91). The mean of the domain ratings was computed. Means derived from mothers and daughter interviews were correlated (r = .81, ps < .001) and thus, were combined by taking the mean of the mothers’ and daughters’ ratings for each domain (e.g., Stroud et al., 2016).
2.2.6. Demographic and health measures
Additional variables measured as potential covariates were: a) time of waking (mean); b) race/ethnicity (White = 1; non-White = 0); c) family income; d) oral contraceptive use; e) caffeine use (i.e., in the hour prior to each cortisol sample; mean); f) nicotine use (i.e., in the hour prior to each cortisol sample; mean); g) perceived stress (i.e., in the hour prior to each cortisol sample; rated: 1 [not at all] – 5 [very much]; mean); h) negative affect (mean); i) positive affect (mean); and j) pubertal status. To compute positive and negative affect, adolescents reported on 10 positive (e.g., excited) and 10 negative (e.g., upset) emotions in the hour before cortisol sampling (0-not at all; 4-extremely) using the Positive and Negative Affect Schedule (Watson et al., 1988).
At the T1 laboratory visit, adolescents completed the 5-item Pubertal Development Scale (PDS; Petersen et al., 1988); the mean was used (α = .70). Due to limited frequency, oral contraceptive (1.8%)2 and nicotine (0%) use were not examined.
2.3. Procedure
The college Institutional Review Board approved all procedures. During the Time 1 (T1) laboratory visit, adolescents and their mothers completed assent and consent forms, respectively; were invited to ask questions to ensure adequate understanding of study procedures; and each completed questionnaires and interviews. On average, approximately one week after the visit and in all but 1 case within 1 month (M = 7.14 days; SD = 7.24; range: 1–39 days), adolescents completed a 3-day cortisol collection (consecutive weekdays, avoiding atypical days), collecting whole saliva by passive drool at waking, 30 min post-waking, and bedtime. Participants were instructed to reschedule the collection in the case of fever and illness, and to avoid eating, drinking (other than water), and brushing their teeth prior to providing samples. For each sample, adolescents recorded the time and completed a diary, which included questions assessing time of waking as well as affect, perceived stress, caffeine use, and nicotine use in the hour preceding sampling. To assess compliance with the sampling protocol, straws necessary to expel saliva into the sampling tubes were stored in a track cap container which provided a date and time stamp each time it was opened. Samples were returned via mail; stored at –20 °C; and sent on dry ice over three days to the Biochemisches Labor at the University of Trier, Germany to be assayed.
2.4. Analytic strategy
In the primary analyses, path analyses were conducted in Mplus 8 (Muthen and Muthen, 1998–2017; Muthen and Muthen, 1998) using maximum likelihood estimation. Use of this approach, as opposed to simple regression, permitted estimation of missing data using full information maximum likelihood (e.g., Enders, 2013). Little’s MCAR test indicated that data were missing completely at random (χ2[65] = 53.392; p = .848). Model fit was assessed with the chisquare test (a p-value > .05 suggests good fit), the Comparative Fit Index (CFI; > .90 indicates good fit) and the Root Mean Square Error of Approximation (RMSEA; < .05 indicates good fit; Hu and Bentler, 1998).
Model 1 examined whether the CAR, each form of acute stress (i.e., acute interpersonal and non-interpersonal stress), and the interactions between each form of acute stress and the CAR predicted T2 depressive symptoms. In Model 2, we further stratified acute interpersonal stress by independence. Models included paths: a) from the CAR, each form of acute stress, and the interactions between CAR and the acute stress variables, to T2 depressive symptoms; and b) between each covariate and the main variables (i.e., each form of acute stress, the CAR, the 2 interactions, T2 depressive symptoms). Non-significant covariate paths were trimmed, except for paths between non-compliance and each of the main variables (i.e., each form of acute stress, the CAR, the 2 interactions, T2 depressive symptoms) which were retained regardless of significance. Predictor variables (i.e., CAR, acute stress) were standardized. Interaction variables were computed by multiplying the standardized predictor variables with each other. Covariances were included between the disturbances of: 1) predictor variables; 2) each of the predictor variables and the interaction variables; and 3) covariates.
Significant interactions were probed using simple slopes and the Johnson-Neyman procedure, which identified the values of acute stress at which the CAR significantly predicted depressive symptoms. A Wald Test of Parameter Constraints tested whether the unique variance contributed by the interactions included in each model were significantly different.
Prior to conducting the primary analyses, compliance and potential covariates were examined in preliminary analyses. The waking sample was considered compliant if the track cap-detected-time was within 15 min of self-reported waking time (e.g., Doane et al., 2015). The 30 min post-waking sample was considered compliant if the self-reported time difference between the waking and 30 min post-waking samples was between 23 and 37 min according to track cap data (e.g., Doane et al., 2015). For each collection day, a dummy variable was created to reflect non-compliance with the sampling protocol (1 = one or both samples non-compliant; 0 = both samples compliant). The mean of the non-compliance dummy variables was used as a covariate. Correlations between potential covariates (e.g., pubertal status) and the main variables included in each model (i.e., CAR, each type of stress, the 2 interactions, T2 depressive symptoms) were examined. For each model, potential covariates that were significantly (p < .05) associated with at least one of the main variables were included.
3. Results
3.1. Preliminary analyses
Based upon correlation analyses, negative affect, caffeine use, past and current T1 depressive symptoms, time of waking, T1 past year acute stress, and T1 past year chronic stress were initially included as covariates in Model 1 (see Table 2). Non-compliance was also included a priori. However, paths between T1 past year chronic stress and T1 past year acute stress and the main study variables (each type of stress, CAR, 2 interactions, T2 depressive symptoms) were not significant, and thus, were trimmed.
Table 2.
Intercorrelations Among Study Variables.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. CAR | - | |||||||||||||||||
| 2. Acute IP stress | .20 | - | ||||||||||||||||
| 3. Acute Non-IP stress | .00 | .11 | - | |||||||||||||||
| 4. Acute Dep. IP stress | .19 | .66*** | .12 | - | ||||||||||||||
| 5. Acute Ind. IP stress | .09 | .69*** | .03 | −.09 | - | |||||||||||||
| 6. T2 Depressive sx | .13 | .18 | −.07 | .33*** | −.08 | - | ||||||||||||
| 7. Caffeine use | .10 | .31** | −.16 | .20 | .22 | −.06 | - | |||||||||||
| 8. Waking time | −.12 | −.06 | −.11 | −.04 | −.04 | .24* | .15 | - | ||||||||||
| 9. Non-compliance | −.13 | .23* | .17 | .17 | .14 | .05 | .05 | .08 | - | |||||||||
| 10. Negative affect | .10 | .26* | −.14 | .40*** | −.04 | .24* | .22* | .09 | −.01 | - | ||||||||
| 11. Positive affect | .02 | .01 | −.08 | .12 | −.11 | -.05 | .14 | .08 | −.04 | .30** | - | |||||||
| 12. Perceived stress | −.06 | .10 | −.01 | −.01 | .14 | .10 | .18 | .19 | .06 | .21 | −.01 | - | ||||||
| 13. Past and current T1 depressive sx | .03 | .11 | −.10 | .13 | .01 | .38*** | .20 | .19 | .05 | .31** | .20 | .24* | - | |||||
| 14. Pubertal status | −.03 | .10 | −.16 | .13 | .01 | .22 | .02 | .05 | −.07 | .08 | .07 | .19 | .09 | - | ||||
| 15. Family Income | −.07 | −.19 | .07 | −.14 | −.11 | −.19 | −.04 | .05 | .10 | −.21 | −.26* | −.10 | −.06 | −.21* | - | |||
| 16. White | −.02 | −.01 | −.06 | −.02 | .01 | −.10 | .00 | −.32** | .07 | .16 | .01 | −.05 | −.07 | −.01 | .06 | - | ||
| 17. T1 past year acute stress | .10 | .23* | −.01 | .08 | .23* | −.13 | .12 | .10 | −.02 | .02 | .02 | .05 | .01 | .09 | −.20 | −.06 | - | |
| 18. T1 past year chronic stress | .17 | .27* | .09 | .31** | .06 | .13 | −.02 | .10 | −.03 | .29** | .03 | .02 | .21 | .13 | −.28** | −.07 | .30** | - |
| 19. Days | .00 | .13 | .20 | .23* | −.05 | −.02 | .07 | −.09 | .10 | .09 | .04 | .13 | −.20 | .13 | −.02 | .08 | −.11 | −.15 |
Notes.
p < .05.
p < .01.
p < .001.
CAR = cortisol awakening response. Cortisol values were transformed using the natural log function. IP = interpersonal. Stress variables were computed by summing the severity ratings of each type of event in the 2 months prior to symptom onsets or a randomly selected 2-month period if no symptom onset. sx = symptoms. T1 = Time 1. T2 = Time 2. Depressive symptoms were rated: 0 = no symptoms; 1 = mild symptoms; 2 = moderate, sub-threshold symptoms; 3 = DSM-IV criteria. Family income ranges from 2 ($10,000 - $20,000) to 6 (over $100,000/year). 1 = White race/ethnicity; 0 = Non-White race/ethnicity. Non-compliant = 1; 0 = Compliant. Days = the number of days between the first day of the saliva collection and the first day of the symptomatic period (for those with symptom onset) or the number of days between the first day of the saliva collection and the first day after the 2-month randomly chosen period in which stress was examined (for those without symptom onset). Ns vary due to missing questionnaire data and attrition.
3.2. Does the CAR predict T2 depressive symptoms alone and in interaction with acute interpersonal and non-interpersonal stress?
Model 1 fit indices were adequate (see Table 3). As hypothesized, greater CAR predicted greater T2 depressive symptoms (see Fig. 1). Additionally, the interaction between acute interpersonal stress and the CAR was significant: CAR predicted T2 depressive symptoms at higher (one SD above the mean; b = .472 [95% CI: .105, .838]; SE = .120; p = .012) and moderate (mean; b = .177 [95% CI: .001, .353]; SE = .090; p = .049), but not lower (one SD below the mean; b = −.118 [95% CI: −.353, .118]; SE = .120; p = .328) levels of acute interpersonal stress (see Fig. 2). The CAR significantly predicted T2 depressive symptoms at standardized values of acute interpersonal stress equal to and greater than .00 (raw = 1.611; 41.86% of the sample). The unique variance contributed by the interaction between the CAR and acute interpersonal stress was significantly greater than that contributed by the interaction between the CAR and acute non-interpersonal stress (χ2 [1] = 4.220, p = .040), which was not significant.
Table 3.
The CAR, Acute Stress, and their Interactions Predicting T2 Depressive Symptoms.
| Model 1: Acute IP and Non-IP Stress |
b (95% CI) |
SE | β | p | Model 2: Acute Dep. and Ind. IP Stress |
b (95% CI) |
SE | β | p |
|---|---|---|---|---|---|---|---|---|---|
| CAR | .177 (.001, .353) | .090 | .223 | .049 | CAR | .217 (.027, .407) | .097 | .275 | .025 |
| Acute IP stress | .083 (-.098, .264) | .092 | .099 | .370 | Acute Dep. IP stress | .064 (-.141, .268) | .104 | .075 | .541 |
| CAR x Acute IP stress | .295 (.042, .547) | .129 | .290 | .014 | CAR x Acute Dep. IP stress | .378 (.094, .661) | .145 | .357 | .009 |
| Acute non-IP stress | −.023 (-.164, .119) | .072 | −.031 | .751 | Acute Ind. IP stress | −.056 (-.191, .079) | .069 | −.077 | .417 |
| CAR x Acute non-IP stress | −.167 (-.432, .098) | .135 | −.138 | .216 | CAR x Acute Ind. IP stress | −.012 (-.202, .179) | .097 | −.011 | .905 |
| Caffeine use | −.158 (-.300, -.017) | .072 | −.217 | .029 | - | - | - | - | - |
| Waking time | .176 (.040, .313) | .070 | .241 | .011 | Waking time | .157 (.020, .283) | .067 | .209 | .024 |
| Non-compliance | −.011 (-.154, .131) | .073 | −.015 | .878 | Non-compliance | .008 (-.132, .147) | .071 | .010 | .916 |
| Past and current T1 depressive sx | .349 (.161, .538) | .096 | .349 | < .001 | Past and current T1 depressive sx | .290 (.106, .475) | .094 | .292 | .002 |
| Model fit indices | Model fit indices | ||||||||
| χ2 (df) | 14.510 (19) | χ2 (df) | 12.363 (19) | ||||||
| p value for χ2 test | .753 | p value for χ2 test | .870 | ||||||
| CFI | 1.000 | CFI | 1.000 | ||||||
| RMSEA (90% CI) | .000 (.000, .068) | RMSEA (90% CI) | .000 (.000, .050) | ||||||
| Total R2 | Total R2 | ||||||||
| Full model | 32.0 | P < | .001 | Full model | 34.1 | p< | .001 | ||
| Full model with CAR x acute interpersonal stress interaction constrained to 0 | 26.2 | p = .001 | Full model with CAR x acute interpersonal stress interaction constrained to 0 | 27.9 | p< | .001 | |||
Notes. CAR = cortisol awakening response. IP = interpersonal. Only paths between study variables and T2 depressive symptoms are shown in this table. In model 1, caffeine use, waking time, non-compliance, and past and current T1 depressive symptoms were included as covariates. In model 2, all covariates were the same, expect that caffeine use was not included. Non-significant paths between covariates and main variables were trimmed, and thus, covariates not significantly related to T2 depressive symptoms are not included in this table. Stress variables were computed by summing the severity ratings of each type of event in the 2 months prior to symptom onsets or a randomly selected 2-month period if no symptom onset. sx = symptoms. T1 = Time 1. T2 = Time 2. b = unstandardized coefficient. CI = confidence intervals. SE = standard error. ß = standardized regression coefficient. N = 86. Total R2 = total variance in T2 depressive symptoms explained by the model. Total R2 was computed for the full model, and for the full model when the significant interaction path was constrained to 0 to evaluate the variance explained by the interaction.
Fig. 1.
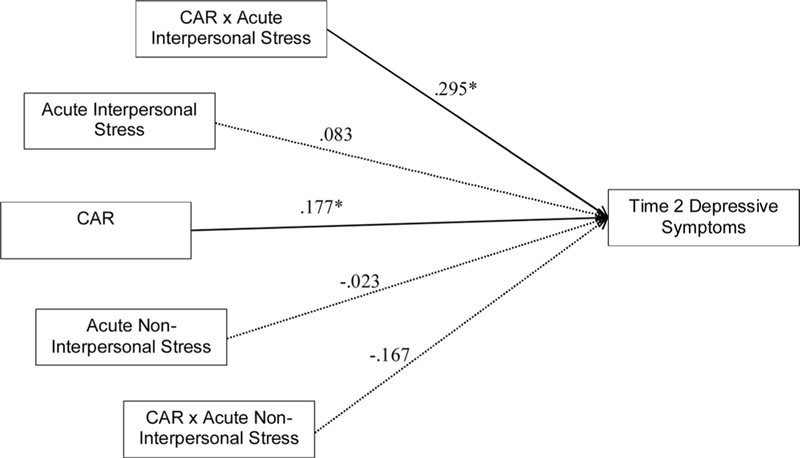
Path Model Testing the Predictive Effect of the CAR, and Its Interaction with Acute Interpersonal and Non-Interpersonal Stress (Model 1). Standardized coefficients presented. χ2 (19) = 14.510. CFI = 1.000. RMSEA = .000 (.000, .068). CAR = Cortisol Awakening Response. Caffeine use, waking time, non-compliance, and past and current T1 depressive symptoms were included as covariates. Non-significant paths between covariates and main variables were trimmed. For ease of presentation, covariates, disturbances, and covariances are not shown. * p < .05.
Fig. 2.
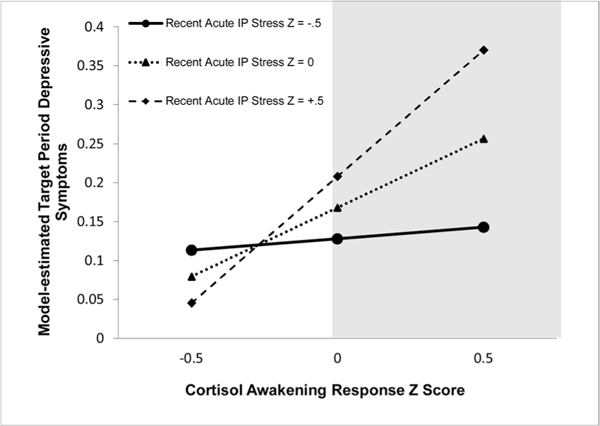
The CAR significantly predicts T2 depressive symptoms for standardized values of acute interpersonal stress ≥ .00 (raw score = 1.611; shaded region, 41.86% of the sample). IP = interpersonal. Z = standardized score.
3.3. Does the CAR interact with acute dependent and independent interpersonal stress in predicting T2 depressive symptoms?
Model 2 included the CAR, acute dependent interpersonal stress, acute independent interpersonal stress, and their interactions. The same covariates were used, with 2 exceptions: 1) caffeine use was not included because it was not significantly related to any of the main variables in this model (each type of stress, CAR, 2 interactions, T2 depressive symptoms); and 2) days between cortisol collection and onset prediction was initially included, but paths between this variable and the main study variables were not significant, and thus, thus, were trimmed.
Model 2 fit indices were adequate (Table 3). Greater CAR significantly predicted greater T2 depressive symptoms. Further, the interaction between acute dependent interpersonal stress and the CAR was significant: CAR predicted T2 depressive symptoms at higher (b = .595 [95% CI: .167, 1.023]; SE = .218; p = .006) and moderate (b = .217 [95% CI: .027, .407]; SE = .097; p = .025), but not lower (b = −.160 [95% CI: −.384, .063]; SE = .114; p = .160) levels of acute dependent interpersonal stress. The CAR significantly predicted T2 depressive symptoms at standardized values of interpersonal stress equal to and greater than −.12 (raw = .55; 34.88% of the sample). The unique variance contributed by the interaction between the CAR and acute dependent interpersonal stress was significantly greater than that contributed by the interaction between the CAR and acute independent interpersonal stress (χ2 [1] = 5.094, p = .024), which was not significant3,4 .
4. Discussion
The present findings indicate that greater CAR predicted subsequent higher depressive symptoms among early adolescent girls with no prior history of major depression. Furthermore, we provide novel evidence that the CAR interacts with acute interpersonal, but not non-interpersonal, stress in predicting subsequent depressive symptoms. Finally, we provide the first evidence that the CAR may interact with acute dependent, but not independent, stress in conferring risk. These findings highlight the role of the CAR in the development of depressive symptoms among early adolescent girls, and refine the circumstances under which the CAR confers risk during adolescence, a sensitive period for the development of depression.
4.1. The role of the CAR in the development of depressive symptoms
The present study demonstrated that greater CAR predicted subsequent higher depressive symptoms, accounting for the effects of both lifetime history and T1 current depressive symptoms, and in a sample of adolescent girls without a history of diagnosable depression, thereby ruling out the possibility that the findings reflect a concomitant of current depression, or a consequence of prior depression (Doane et al., 2013). Notably, focusing on the worst symptom onset between T1 and T2, rather than using a uniform time period for each participant, maximized the number of prospective symptom onsets captured. If we had used a uniform time point for T2, onsets that occurred between T1 and T2, but that had also remitted by T2 would have been “missed” in analyses, thereby increasing false-negative prediction by elevated CAR, and reducing power to detect the CAR-depression link
Other prospective studies have not supported the CAR-depression association (Carnegie et al., 2014; LeMoult et al., 2015; Schuler et al., 2017) , raising the question of what may account for the mixed findings. Several possibilities exist. First, the effect of the CAR on depression risk is strongest most proximal to the CAR assessment, decaying over time (Vrshek-Schallhorn et al., 2013); consequently, the use of longer follow-up periods (e.g., >2.5 years), and/or reliance on self-report measures of current depressive symptoms (rather than depression over the full follow up) may have contributed to a failure to capture depression and/ or the effect may have decayed beyond statistical detection. Thus, to catch the window during which CAR is relevant, future research should continue to use diagnostic interviews that assess the full assessment period or use shorter and/or repeated follow-ups, and focus on the most severe symptom manifestation. Second, failing to account for state variables, such as mood, waking time, and consumption of nicotine and caffeine (among others; Doane and Adam, 2010; Kudielka et al., 2003), as well as non-compliance with the sampling protocol, can bias CAR assessments (e.g., Kudielka et al., 2003; Stalder et al., 2016). Thus, not accounting for such factors, as well as not monitoring or accounting for compliance, could have contributed to previous null findings and, as such, future examinations of the CAR-depression link should follow expert recommendations (e.g., Stalder et al., 2016). Finally, the relevance of the CAR may depend on other physiological or contextual factors, which may have contributed to prior null findings. Investigating factors shown to influence the CAR, such as circadian processes (e.g., sleep; Clow et al., 2010), and perceptions of parental support (Doane et al., 2018a) may be fruitful pursuits. Given that individual or contextual moderators of neurobiological processes may vary over time and developmental stage (Doane et al., 2018b), research investigating how such moderators shape the CAR-depression link will likely yield substantial insights into when and for whom intervention efforts will be most fruitful.
The present findings suggest that one factor that affects the CAR-depression link is acute interpersonal stress: greater CAR predicted subsequent depressive symptoms only among girls experiencing moderate to high levels of acute interpersonal stress in the 2 months prior to symptom onset (approximately 40% of the sample). Importantly, the labels “moderate” and “high” are indices of levels of acute interpersonal stress relative to other girls in this community sample of adolescents. Results illustrated that among girls experiencing at least one minor (i.e., non-severe) acute interpersonal event (i.e., rated 2.0 out of 5.0), CAR predicted subsequent increases in depressive symptoms. This suggests that at least among early adolescent girls, greater CAR may confer risk for depressive symptoms even under relatively minor levels of acute interpersonal stress.
4.2. Role of interpersonal events
That the CAR interacted with interpersonal, but not non-interpersonal, acute stress, fits with prior laboratory research indicating that the HPA axis may be particularly sensitive to interpersonal stress (e.g., Dickerson and Kemeny, 2004), and extends it to naturally-occurring interpersonal events and to indicators of the diurnal patterns as opposed to cortisol reactivity. Moreover, this result helps clarify prior findings indicating that acute stress and the CAR did not interact in predicting depressive symptoms. In the only prior study to test this question using a gold-standard stress measure, the presence of major events did not interact with the CAR in predicting major depressive onsets (Vrshek-Schallhorn et al., 2013). Although methodological or developmental (i.e., late adolescents versus early adolescents) differences could have contributed to this discrepancy, it may also be that an interaction effect “washed out” due to using all major events instead of only those that are most etiologically salient for depression (e.g., acute interpersonal events). That the interaction between the CAR and acute interpersonal stress contributed significantly greater unique variance than that contributed by the interaction between the CAR and acute non-interpersonal stress interaction provides support for this explanation.
Findings also add to literature demonstrating that several stresssensitive biological systems—
such as the serotonin system (e.g., Vrshek-Schallhorn et al., 2014), the inflammatory/immune responses (e.g., Slavich et al., 2010), and the oxytocin and vasopressin systems (Tabak et al., 2016)—may be particularly susceptible to interpersonal stress. Collectively, these findings help explain why certain individuals may be particularly susceptible to acute interpersonal stress (Vrshek-Schallhorn et al., 2015a), and underscore the importance of focusing on etiologically-relevant stress when investigating interactions between environmental stress and stress-sensitive biological systems (e.g., Harkness and Monroe, 2016). Future research should examine how these systems interact to confer susceptibility. For example, serotonergic genetic variation moderates the CAR-depression link (Li-Tempel et al., 2016), and the effect of major interpersonal, but not non-interpersonal, events on depression (e.g., Vrshek-Schallhorn et al., 2014), suggesting that exploring the interplay of the CAR, serotonergic genetic variation, and acute interpersonal stress on depression risk may be fruitful.
The novel finding that the CAR interacted with acute dependent, but not independent, interpersonal stress to predict depressive symptoms further underscores the need to investigate specific types of stress when seeking to understand the interaction between a stress-sensitive biological system, like the HPA axis, and environmental stress. Replication of this finding is important given prior work is equivocal regarding whether independent or dependent interpersonal events are more relevant to depression (e.g., Kendler et al.,1999; Stroud et al., 2011) or whether their relevance is equivalent (Vrshek-Schallhorn et al., 2015a). Moreover, little prior work has evaluated whether typical or diurnal patterns of cortisol are particularly sensitive to independent as opposed to dependent stress with some evidence suggesting that past year acute independent, but not dependent, acute stress is associated with trait cortisol in the present sample (Stroud et al., 2016). If these findings are replicated, and with existing evidence that vulnerable adolescents including those with current or prior depressive symptoms generate dependent interpersonal events (Hammen, 1991; Liu and Alloy, 2010), one hypothesis is that certain adolescents may become entangled in a cycle of increasing stress and depression, that may further exacerbate HPA axis dysregulation (e.g., Doane et al., 2013; Stroud et al., 2016). Future research should investigate whether the CAR also influences risk for stress generation, and/or moderates stress generation effects, which would further escalate this cycle.
Future research is also needed to elucidate why the CAR interacted exclusively with acute dependent interpersonal stress. One possibility is that this finding was influenced by the early adolescent developmental stage of participants. As in prior adolescent samples, many of the independent interpersonal events faced by adolescents in the present sample were focused on their parents (e.g., parental job loss), and likely would have been coded as dependent in an adult sample (e.g., Harkness et al., 2006). In contrast, most of dependent interpersonal events were focused on the adolescents’ relationships with friends, classmates, and romantic partners (e.g., conflict with a friend, break-up). Thus, it may be that dependent, but not independent, interpersonal events interacted with the CAR because the dependent interpersonal events more often threatened adolescents’ social self (i.e., social evaluative threat; e.g., rejection), and often occurred in domains in which preserving their social status is becoming increasingly important (e.g., Steinberg, 1987). Although speculative, this assertation is consistent with both the social self-preservation model (e.g., Dickerson et al., 2004) and evidence for the potency of social evaluative threats for HPA axis reactivity (Dickerson and Kemeny, 2004). Future work should address whether these patterns replicate across developmental periods.
4.3. Future directions
The biological mechanisms through which the CAR confers risk for depressive symptoms merit research attention. Biologically-focused accounts of HPA-axis dysregulation in the pathway to disorder implicate a cycle between initial emotion distress, subsequent biological changes, and enhanced vulnerability to stressors (de Kloet, 2014). Evidence from mouse models indicates that high affinity miner-alocorticoid receptors (MR), which are differentially occupied under basal conditions when cortisol is relatively low, are activated during initial stress leading to short-acting upregulation in hippocampal activation (Karst et al., 2005) and longer lasting activation in basolateral amygdala (Karst et al., 2010). These changes may heighten threat appraisals, leading to further cortisol elevations, which in turn cause heightened activation of the low affinity glucocorticoid receptor (GR). Upon GR activation, the activating effect of cortisol may become inhibitory (Karst et al., 2010), provoking further alterations in cortico-limbic circuits and resulting in insufficient MR relative to GR density (Qi et al., 2013). Such biological changes have been linked to depression and anxiety (e.g., de Kloet, 2014; Herbert, 2013), raising the possibility that the underlying mechanisms are shared across anxiety and depressive disorders in adolescence.
Supporting this, prior work suggests that greater CAR prospectively predicts greater risk of anxiety disorder onsets (Adam et al., 2014) and growth in internalizing symptoms (Saridjan et al., 2014). Thus, although most prospective CAR data have examined depression, the CAR may serve as a transdiagnostic indicator of engaged struggle that marks a prodromal period prior to internalizing disorder onset, or possibly even broader forms of psychopathology. Furthermore, future research should evaluate the role of early adversity in associations among the CAR, acute interpersonal stress, and depressive symptoms. Indeed, research suggests that early adversity predicts alterations in HPA axis activity (e.g., Harkness et al., 2011), including the CAR (e.g., Gonzalez et al., 2009). Thus, it will be important to investigate whether the observed associations persist even after accounting for the effect of early adversity.
4.4. Limitations
Several limitations merit note. First, generalizability may be limited. The sample was self-selected and comprised mostly White early adolescent girls. Research in adolescent samples indicates gender differences in: a) the diurnal cortisol rhythm (e.g., Gunnar et al., 2009); b) the HPA axis regulation-depression link (e.g., Gunnar et al., 2009); and c) exposure and sensitivity to acute interpersonal stress (e.g., Rudolph and Hammen, 1999). Thus, findings may not replicate in boys. Generalizability of the findings to diagnosable depression is also unknown. Second, because of participants’ age, to increase feasibility, we used 2 samples to index the CAR, which may have biased estimates (Stalder et al., 2016). Third, we did not use objective (e.g., actigraphic) measures of waking which may have led to less precise measures of the CAR. Fourth, although non-compliance was statistically controlled for in the analyses, on average, participants were non-compliant with the sampling protocol, which may have biased CAR estimates. Thus, replication with enhanced compliance is needed. Fifth, although the frequency of eating within one hour prior to the morning samples was low (Day 1: n = 14; 15.7%; Day 2: n = 13; 14.6%; Day 3: n = 17; 19.1%), and participants were instructed to rinse their mouths if they consumed foods (e.g., Stalder et al., 2016), eating may have affected CAR estimates. Sixth, replication in a high-risk sample of adolescents facing higher levels of stress is needed. Seventh, although gold standard contextual stress interviews were used to assess acute stress (Harkness and Monroe, 2016), interviewers were completed by both mothers and daughters, and events were precisely dated to ensure temporal precedence of events to depression, the interviews were retrospective, which may have introduced recall bias. Finally, we cannot ascertain whether elevated CAR causally increases vulnerability to depression in response to acute interpersonal stress or whether CAR is a marker of depression vulnerability. However, our ability to generate flexible follow up periods to identify the most severe period of depressive symptoms in the study window is a methodological contribution to the study of the CAR and depressive symptoms, which allows for greater accuracy and sensitivity.
4.5. Conclusion
The present findings highlight the CAR’s relevance in the development of depressive symptoms among early adolescent girls with no prior history of depression. Moreover, results provide novel evidence that the CAR may interact with certain types of proximal acute stress—interpersonal, and specifically, dependent interpersonal—but not others (i.e., non-interpersonal and independent non-interpersonal) in conferring risk. Thus, researchers should persist in working to understand the CAR’s role in prospectively predicting increases in depression across developmental periods and study populations, and should emphasize interpersonal stress in doing so.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge the families who participated in this study and the staff of the Williams College Youth Emotion Center. In addition, we thank Andrea Gierens at Biochemisches Labor at the University of Trier for technical assistance with the salivary assays. This research was supported by institutional funds from Williams College (C.B.S., Principal Investigator). S.V.S. was supported by institutional funds from the University of North Carolina at Greensboro. L.D.D. was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under Award Number R01HD079520 and a William T. Grant Foundation Early Scholar Award. Portions of this paper were presented at the annual conference of the Association of the Association for Behavioral and Cognitive Therapies, Chicago, IL.
Role of funding source
This research was supported by institutional funds from Williams College to C.B.S. (Principal Investigator). Williams College had no role in the study design, data collection, analysis and interpretation of the data, in the writing of the manuscript or the decision to submit the article for publication.
Footnotes
Conflicts of interest
The authors do not have any conflicts of interest to declare with respect to this manuscript.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.psyneuen.2019.04.017.
Two siblings of participants participated. However, all results remained the same when these individuals were excluded from the analyses.
Results of models 1 and 2 were consistent with and without the inclusion of the 2 participants who were using oral contraceptives.
Analyses were conducted with the sample of individuals who used the track cap, consistent with best practice recommendations (Stalder et al., 2016). To probe the generalizability of findings to the full sample who provided cortisol data (n =122), we repeated Models 1 and 2, including potential covariates that were significantly related to one or more main variables in each model (i.e., each type of stress, CAR, 2 interactions, T2 depressive symptoms), and trimmed non-significant covariate paths. Non-compliance and T1 chronic stress were included a priori, the latter of which was included because of significant differences between those who did and did not use the track cap on T1 chronic stress (p<.05). Model fit indices were adequate. In Model 1, the main effect of the CAR remained significant (β = .244; p = .006), and the interaction between the CAR and acute interpersonal stress approached significance (β = .170; p = .078). In Model 2, the main effect of the CAR (β = .298; p = .001), and the interaction between the CAR and acute dependent interpersonal stress (β = .241; p = .022) remained significant. Full results available upon request.
Results of models 1 and 2 were consistent with and without the inclusion of T1 past and current anxiety and externalizing symptoms.
References
- Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT, 2006. Day-to-day dynamics of experience—cortisol associations in a population-based sample of older adults. Proc. Natl. Acad. Sci. U. S. A. 103, 17058–17063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam EK, Doane LD, Zinbarg RE, Mineka S, Craske MG, Griffith JW, 2010. Prospective prediction of major depressive disorder from cortisol awakening responses in adolescence. Psychoneuroendocrinology 35, 921–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam EK, Vrshek-Schallhorn S, Kendall AD, Mineka S, Zinbarg RE, Craske MG, 2014. Prospective associations between the cortisol awakening response and first onsets of anxiety disorders over a six-year follow-up. Psychoneuroendocrinology 44, 47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GW, Harris TO, 1978. Social Origins of Depression: A Study of Psychiatric Disorder in Women. Free Press, New York. [Google Scholar]
- Carnegie R, Araya R, Ben-Shlomo Y, Glover V, O’Connor TG, O’Donnell KJ, Lewis G, 2014. Cortisol awakening response and subsequent depression: prospective longitudinal study. Br. J. Psychiatry 204, 137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clow A, Hucklebridge F, Stalder T, Evans P, Thorn L, 2010. The cortisol awakening response: more than a measure of HPA axis function. Neurosci. Biobehav. Rev. 35, 97–103. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, 2014. From receptor balance to rational glucocorticoid therapy. Endocrinology 155, 2754–2769. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME, 2004. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol. Bull. 130, 355–391. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Gruenewald TL, Kemeny ME, 2004. When the social self is threatened: shame, physiology, and health. J. Pers. 72 (6), 1191–1216. [DOI] [PubMed] [Google Scholar]
- Doane LD, Adam EK, 2010. Loneliness and cortisol: momentary, day-to-day, and trait associations. Psychoneuroendocrinology 35, 430–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doane LD, Mineka S, Zinbarg RE, Craske M, Griffith JW, Adam EK, 2013. Are flatter diurnal cortisol rhythms associated with major depression and anxiety disorders in late adolescence? The role of life stress and daily negative emotion. Dev. Psychopathol. 25, 629–642. [DOI] [PubMed] [Google Scholar]
- Doane LD, Chen FR, Sladek MR, Van Lenten SA, Granger DA, 2015. Latent trait cortisol (LTC) levels: reliability, validity, and stability. Psychoneuroendocrinology 55, 21–35. [DOI] [PubMed] [Google Scholar]
- Doane LD, Sladek MR, Breitenstein RS, Park H, Castro S, Kennedy J, 2018a. Cultural neurobiology and the family: evidence from the daily lives of Latino adolescents. Dev. Psychopathol. 30, 1779–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doane LD, Sladek MR, Adam EK, 2018b. Introduction to the neurobiology of cultural experiences. In: Causadias JM, Telzer EH, Gonzales NA (Eds.), The Handbook of Culture and Biology: Bridging Evolutionary Adaptation and Development.. [Google Scholar]
- Dressendörfer RA, Kirschbaum C, Rohde W, Stahl F, Strasburger CJ, 1992. Synthesis of a cortisol-biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. J. Steroid Biochem. 43, 683–692. [DOI] [PubMed] [Google Scholar]
- Enders CK, 2013. Dealing with missing data in developmental research. Child Dev. Perspect. 7, 27–31. 10.1111/cdep.12008. [DOI] [Google Scholar]
- Gonzalez A, Jenkins JM, Steiner M, Fleming AS, 2009. The relation between early life adversity, cortisol awakening response and diurnal salivary cortisol levels in postpartum women. Psychoneuroendocrinology 34, 76–86. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C, 2009. Developmental changes in hypothalamus-pituitary-adrenal activity over the transition to adolescence: normative changes and associations with puberty. Dev. Psychopathol. 21, 69–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C, 1991. Generation of stress in the course of unipolar depression. J. Abnorm. Psychol. 100, 555–561. [DOI] [PubMed] [Google Scholar]
- Hardeveld F, Spijker J, Vreeburg SA, De Graaf R, Hendriks SM, Licht CMM, Beekman ATF, 2014. Increased cortisol awakening response was associated with time to recurrence of major depressive disorder. Psychoneuroendocrinology 50, 62–71. [DOI] [PubMed] [Google Scholar]
- Harkness KL, Monroe SM, 2016. The assessment and measurement of adult life stress: basic premises, operational principles, and design requirements. J. Abnorm. Psychol. 125 (5), 727–745. [DOI] [PubMed] [Google Scholar]
- Harkness KL, Bruce AE, Lumley MN, 2006. The role of childhood abuse and neglect in the sensitization to stressful life events in adolescent depression. J. Abnorm. Psychol. 115 (4), 730–741. [DOI] [PubMed] [Google Scholar]
- Harkness K, Stewart JG, Wynne-Edwards KE, 2011. Cortisol reactivity to social stress in adolescents: role of depression severity and child maltreatment. Psychoneuroendocrinology 3, 173–181. [DOI] [PubMed] [Google Scholar]
- Herbert J, 2013. Cortisol and depression: three questions for psychiatry. Psychol. Med. 43 (3), 449–469. [DOI] [PubMed] [Google Scholar]
- Hu L, Bentler PM, 1998. Fit indices in covariance structure modeling: sensitivity to underparameterized model misspecification. Psychol. Methods 3, 424–453. [Google Scholar]
- Karst H, Berger S, Turiault M, Tronche F, Schütz G, Joëls M, 2005. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc. Natl. Acad. Sci. U. S. A. 102, 19204–19207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karst H, Berger S, Erdmann G, Schütz G, Joëls M, 2010. Metaplasticity of amyg-dalar responses to the stress hormone corticosterone. Proc. Natl. Acad. Sci. U. S. A. 107, 14449–14454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rau U, Flynn C, Moreci P, Ryan N, 1997. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime version (K-SADS-PL): initial reliability and validity data. J. Am. Acad. Child Adolesc. Psychiatry 36, 980–987. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA, 1999. Causal relationship between stressful life events and the onset of major depression. Am. J. Psychiatry 156, 837–841. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Broderick JE, Kirschbaum C, 2003. Compliance with saliva sampling protocols: electronic monitoring reveals invalid cortisol profiles in noncompliant subjects. Psychosom. Med. 65, 313–319. [DOI] [PubMed] [Google Scholar]
- LeMoult J, Ordaz SJ, Kircanski K, Singh MK, Gotlib IH, 2015. Predicting first onset of depression in young girls: interaction of diurnal cortisol and negative life events. J. Abnorm. Psychol. 124, 850–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li-Tempel T, Larra MF, Winnikes U, Tempel T, DeRijk RH, Schulz A, Schote AB, 2016. Polymorphisms of genes related to the hypothalamic-pituitary-adrenal axis influence the cortisol awakening response as well as self-perceived stress. Biol. Psychol. 119, 112–121. [DOI] [PubMed] [Google Scholar]
- Liu RT, Alloy LB, 2010. Stress generation in depression: a systematic review of the empirical literature and recommendations for future study. Clin. Psych. Rev. 30, 582–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Duran NL, Kovacs M, George CJ, 2009. Hypothalamic-pituitary-adrenal axis dysregulation in depressed children and adolescents: a meta-analysis. Psychoneuroendocrinology 34, 1272–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe SM, Simons AD, 1991. Diathesis-stress theories in the context of life stress research: implications for the depressive disorders. Psychol. Bull. 110, 406–425. [DOI] [PubMed] [Google Scholar]
- Muthen LK, Muthen BO, 1998-2017. Mplus User’s Guide (seventh ed.). Los Angeles, CA: Muthen & Muthen. [Google Scholar]
- Nicolson NA, 2008. Measurement of cortisol In: Luecken LJ, Gallo LC (Eds.), Handbook of Physiological Research Methods in Health Psychology. NY: Sage Publications, New York, pp. 37–74. [Google Scholar]
- Oldehinkel AJ, Wittchen HU, Schuster P, 1999. Prevalence, 20-month incidence and outcome of unipolar depressive disorders in a community sample of adolescents. Psychol. Med. 29 (3), 655–668. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A, 1988. A self-report measure of pubertal status: reliability, validity, and initial norms. J. Youth Adolesc. 17,117–133. [DOI] [PubMed] [Google Scholar]
- Pruessner M, Hellhammer DH, Pruessner JC, Lupien SJ, 2003. Self-reported depressive symptoms and stress levels in healthy young men: associations with the cortisol response to awakening. Psychosom. Med. 65, 92–99. [DOI] [PubMed] [Google Scholar]
- Qi XR, Kamphuis W, Wang S, Wang Q, Lucassen PJ, Zhou J-N, Swaab DF, 2013. Aberrant stress hormone receptor balance in the human prefrontal cortex and hypothalamic paraventricular nucleus of depressed patients. Psychoneuroendocrinology 38, 863–870. [DOI] [PubMed] [Google Scholar]
- Rohde P, Beevers CG, Stice E, O’Neil K, 2009. Major and minor depression in female adolescents: onset, course, symptom presentation, and demographic associations. J. Clin. Psychol. 65, 1339–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KD, Hammen C, 1999. Age and gender as determinants of stress exposure, generation, and reactions in youngsters: a transactional perspective. Child Dev. 70, 660–677. [DOI] [PubMed] [Google Scholar]
- Rudolph KD, Hammen C, Burge D, Lindberg N, Herzberg DS, Daley SE, 2000. Toward an interpersonal life-stress model of depression: the developmental context of stress generation. Dev. Psychopathol. 12, 215–234. [DOI] [PubMed] [Google Scholar]
- Saridjan NS, Velders FP, Jaddoe VWV, Hofman A, Verhulst FC, Tiemeier H, 2014. The longitudinal association of the diurnal cortisol rhythm with internalizing and externalizing problems in pre-schoolers. The Generation R Study. Psychoneuroendocrinology 50, 118–129. [DOI] [PubMed] [Google Scholar]
- Schuler KL, Ruggero CJ, Goldstein BL, Perlman G, Klein DN, Kotov R, 2017. Diurnal cortisol interacts with stressful events to prospectively predict depressive symptoms in adolescent girls. J. Adolesc. Health. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, O’Donovan A, Epel ES, Kemeny ME, 2010. Black sheep get the blues: a psychobiological model of social rejection and depression. Neurosci. Biobehav. Rev. 35, 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalder T, Kirschbaum C, Kudielka BM, Adam EK, Pruessner JC, Wüst S, Clow A, 2016. Assessment of the cortisol awakening response: expert consensus guidelines. Psychoneuroendocrinology 63, 414–432. [DOI] [PubMed] [Google Scholar]
- Steinberg L, 1987. Impact of puberty on family relations: effects of pubertal status and pubertal timing. Dev. Psychol. 23, 451–460. [Google Scholar]
- Stroud CB, Davila J, Hammen C, Vrshek-Schallhorn S, 2011. Severe and nonsevere events in first onsets versus recurrences of depression: evidence for stress sensitization. J. Abnorm. Psychol. 120, 142–154. [DOI] [PubMed] [Google Scholar]
- Stroud CB, Chen FR, Doane LD, Granger DA, 2016. Individual differences in early adolescents’ latent trait cortisol (LTC): relation to recent acute and chronic stress. Psychoneuroendocrinology 70, 38–46. [DOI] [PubMed] [Google Scholar]
- Tabak BA, Vrshek-Schallhorn S, Zinbarg RE, Prenoveau JM, Mineka S, Redei EE, Craske MG, 2016. Interaction of CD38 variant and chronic interpersonal stress prospectively predicts social anxiety and depression symptoms over 6 years. Clin. Psychol. Sci. 4, 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrshek-Schallhorn S, Doane LD, Mineka S, Zinbarg RE, Craske MG, Adam EK, 2013. The cortisol awakening response predicts major depression: predictive stability over a 4-year follow-up and effect of depression history. Psychol. Med. 43, 483–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrshek-Schallhorn S, Mineka S, Zinbarg RE, Craske MG, Griffith JW, Sutton J, Adam EK, 2014. Refining the candidate environment: interpersonal stress, the serotonin transporter polymorphism, and gene-environment interactions in major depression. Clin. Psychol. Sci. 2, 235–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrshek-Schallhorn S, Stroud CB, Mineka S, Hammen C, Zinbarg RE, Wolitzky-Taylor K, Craske MG, 2015a. Chronic and episodic interpersonal stress as statistically unique predictors of depression in two samples of emerging adults. J. Abnorm. Psychol. 124, 918–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrshek-Schallhorn S, Stroud CB, Mineka S, Zinbarg RE, Adam EK, Redei EE, Craske MG, 2015b. Additive genetic risk from five serotonin system polymorphisms interacts with interpersonal stress to predict depression. J. Abnorm. Psychol. 124 (4), 776–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A, 1988. Development and validation of brief measures of positive and negative affect: the PANAS scales. J. Pers. Soc. Psychol. 54, 1063–1070. [DOI] [PubMed] [Google Scholar]
- Wilhelm I, Born J, Kudielka BM, Schlotz W, Wüst S, 2007. Is the cortisol awakening rise a response to awakening? Psychoneuroendocrinology 32, 358–366. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


