Abstract
Background and Objectives:
We sought to evaluate the impact of chemotherapy sequence on survival by comparing node-positive ILC patients who received neoadjuvant (NACT) and adjuvant (ACT) chemotherapy.
Methods:
cT1–4c, cN1–3 ILC patients in the National Cancer Data Base (2004–2013) who underwent surgery and chemotherapy were divided into NACT and ACT cohorts. Kaplan-Meier curves and Cox proportional hazards modeling were used to estimate unadjusted and adjusted overall survival (OS), respectively.
Results:
5,551 (35.6%) of 15,573 ILC patients treated with chemotherapy received NACT. NACT patients had similar rates of pT3/4 disease (26.6% vs 26.2%), nodal involvement (median 3 vs 4), and number of lymph nodes examined (median 13 vs 14) but higher rates of mastectomy (81.8% vs 74.5%, p<0.001) vs ACT patients. 3.4% of NACT patients experienced pathologic complete response (pCR). Unadjusted 10-year OS was worse for NACT vs ACT patients (65.1% vs 54.4%, log-rank p<0.001). After adjustment for known covariates, NACT continued to be associated with worse OS (HR 1.38, 95% CI 1.25–1.52).
Conclusions:
In node-positive ILC, NACT yielded low rates of pCR, was not associated with lower rates of mastectomy or less extensive axillary surgery, and was associated with worse survival vs ACT, suggesting limited benefit for these patients.
Keywords: chemotherapy, invasive lobular breast cancer, locally advanced breast cancer, neoadjuvant
INTRODUCTION
Invasive lobular carcinoma (ILC) is the second most common histological subtype of breast cancer and affects approximately 10–15% of patients with invasive disease. Several studies have demonstrated significant differences in tumor biology and response to treatment between patients with ILC and the most common subtype, invasive ductal carcinoma (IDC), but staging and treatment recommendations do not differentiate between these two types of breast histology.1,2
Patients with ILC have higher rates of lymph node metastasis than patients with IDC and tend to present with larger tumor size.3,4 As a result, neoadjuvant chemotherapy (NACT) is not infrequently recommended for patients with ILC. But ILC is also nearly always hormone receptor (HR)-positive, and previous studies have demonstrated that patients with low-grade, HR-positive (HR+) ILC have a poor response to neoadjuvant chemotherapy, experiencing lower rates of pathologic complete response (pCR) than patients with IDC.5–7 Concomitantly, NACT has historically been less successful in enabling breast conservation or omission of more extensive axillary surgery among ILC patients, who have higher rates of mastectomy and completion axillary lymph node dissection (ALND) than those with IDC.8–11 Nevertheless, similar criteria – including nodal involvement – are used to determine whether NACT should be administered in both histologic subtypes.12
Data from several clinical trials and population-based studies report an association between pCR and improved survival, though some studies have also demonstrated that the lower pCR rates seen in ILC patients do not necessarily correlate with worse outcomes.13–17 With conflicting evidence of clinical benefit and a greater likelihood of advanced stage at presentation, the delay to surgery that results from NACT administration in ILC patients may translate into a delay in administering locoregional (e.g., surgery, radiation) and systemic (e.g., endocrine) treatments that are potentially more beneficial for patients with ILC. Accordingly, we sought to determine the impact of chemotherapy sequence on survival among node-positive (cN+) ILC patients receiving chemotherapy. We hypothesized that in this subset of patients, a delay in receiving locoregional and endocrine therapy in order to administer NACT may result in worse survival.
MATERIALS AND METHODS
Patients diagnosed with clinical tumor stage (cT) 1–4c, clinical node stage (cN) 1–3, ILC between 2004 and 2014 were identified in the National Cancer Data Base (NCDB). Patients with metastatic disease; those who did not undergo chemotherapy, lumpectomy, or mastectomy; those who received neoadjuvant endocrine; and those with unknown or missing survival or treatment data were excluded. As required by NCDB guidelines, patients diagnosed in 2014 were excluded from survival analyses due to insufficient length of follow-up. Patients were classified as having received either NACT (which, in the NCDB, also includes some patients who received both neoadjuvant and adjuvant chemotherapy) or only adjuvant chemotherapy (ACT) based on whether their chemotherapy start date was before or after surgery, respectively.
Patient characteristics were summarized with N (%) and median (interquartile range) values for categorical and continuous variables, respectively. Chi-square and t-tests compared study groups’ categorical and continous variables as appropriate. Among patients who received NACT, response to treatment was categorized as: (1) overall pCR (ypT0N0, i.e., the absence of any residual invasive or noninvasive carcinoma in the breast or lymph nodes on pathologic review); (2) breast-only pCR (ypT0, cN=ypN); (3) node-only pCR (cT=ypT, ypN0); (4) no stage change (cTN = ypTN); (5) upstage (ypT>cT and ypN>cN, i.e., a change from lower cT and cN stage to higher ypT and ypN stage); or (6) discordant (i.e., breast was upstaged while axilla was downstaged or vice versa)/partial (i.e, breast or nodal downstaging without achieving pCR) response.
Overall survival (OS) was defined as the time from diagnosis to death or last follow-up. Unadjusted OS was estimated using the Kaplan-Meier method, and differences in unadjusted OS between ACT and NACT patients were tested using the log-rank test. The Cox proportional hazards model estimated the effect of chemotherapy timing on OS after adjustment for known covariates. After testing the proportional hazards assumption for the included variables, the model was modified to stratify by age at diagnosis, year of diagnosis (grouped into 2004–2009 and 2010–2013 to reflect the fact that the NCDB only began to collect HER2 receptor information in 2010), and HR status. HR+ patients were estrogen (ER) and/or progesterone receptor (PR)-positive, while HR-negative (HR-) patients were ER and PR-negative. To address the time-dependent effects of tumor and treatment characteristics on long-term survival,18 radiation (analyzed separately for patients undergoing lumpectomy and mastectomy) and endocrine therapy were allowed to be time-varying in the model. Stage-specific sensitivity analyses were performed to only include patients with stage II or III cancer as defined in the 7th edition of the American Joint Commission on Cancer (AJCC) Staging Manual. To account for the correlation of patients treated at the same facility, a robust sandwich covariance estimator was used for the adjusted model. We report hazard ratios (HRs) and 95% confidence intervals (CIs). Two-tailed p<0.05 was considered significant for all analyses.
Only patients with available data were utilized in each model, and effective sample sizes are included in all tables and figures. No adjustments were made for multiple comparisons. All statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC), with which figures were also created. Due to use of de-identified data, our institutional review board granted the study exempt status.
RESULTS
Patient, Tumor, and Treatment Characteristics
Of 20,295 operative patients with cT1–4c, cN+ ILC, 15,573 (76.7%) received chemotherapy and were included in our study cohort (Figure 1). 35.6% (N=5,551) underwent NACT. Median age among all patients was 56 (IQR 48–64). A higher proportion of patients with cT1/2 disease received ACT (77.5% vs NACT 48.4%, p<0.001) and a higher proportion of patients with cT3/4 disease received NACT (51.6% vs ACT 22.5%, p<0.001). Among HR+ patients, a majority received adjuvant endocrine therapy, and treatment rates between groups were not significantly different (NACT 74.3% vs ACT 79.7%, p=0.91). A majority of both NACT and ACT patients underwent mastectomy, but a higher proportion of NACT patients (81.8%) underwent mastectomy as compared to ACT patients (74.5%, p<0.001). Among patients who underwent mastectomy, most received post-mastectomy radiation (PMRT), but rates of PMRT were higher among NACT patients (76.9% vs ACT 67.0%, p<0.001). Among lumpectomy patients, rates of post-lumpectomy radiation were also high but did not differ between groups (NACT 89.0% vs ACT 87.8%, p=0.33). Likewise, the median number of lymph nodes examined (NACT 13 vs ACT 14) and that were positive (NACT 3 vs ACT 4) were similar (Table 1). NACT resulted in an overall pCR rate of 3.4%, and a higher rate of pCR in the lymph nodes (3.3%) vs the breast (0.9%).
Fig. 1.
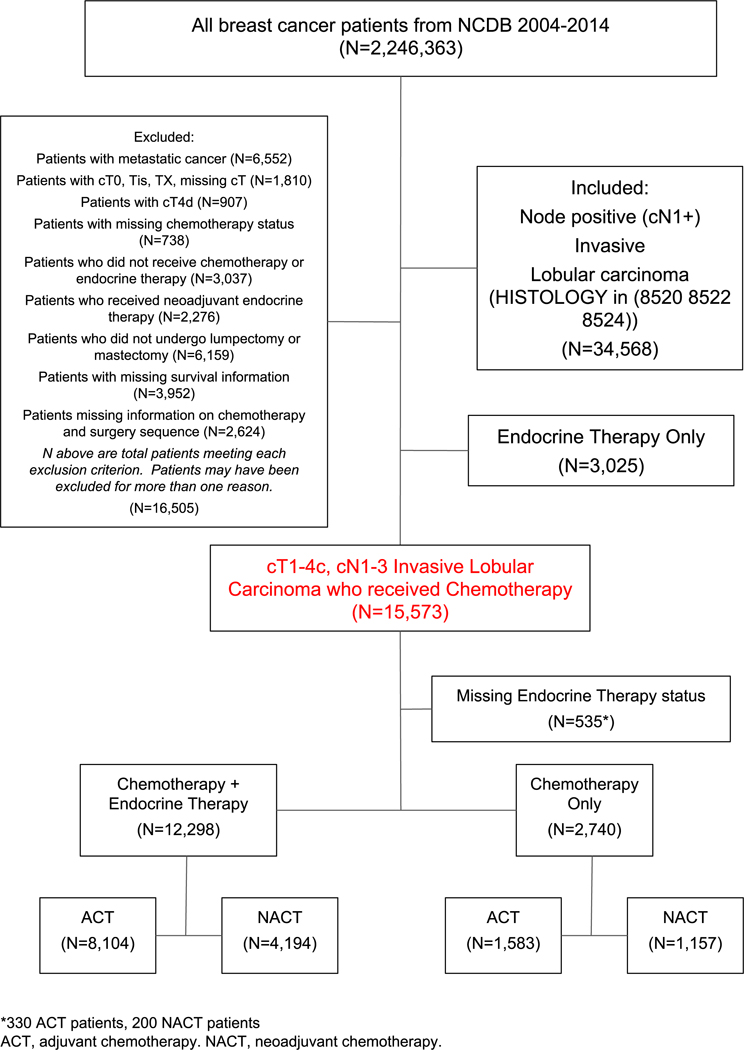
Study Cohort–Patients with cT1–4c, cN1–3 Invasive Lobular Carcinoma, National Cancer Data Base, 2004–2013 (N=15,573)
Table 1.
Patient Characteristics, Patients with cT1–4c, cN1–3 Invasive Lobular Carcinoma, National Cancer Data Base, 2004–2013 (N=15,573)
| All patients (N=15,573) n (%)a |
NACT (N=5551) n (%)a |
ACT (N=10,022) n (%)a |
P-Valuee | |
|---|---|---|---|---|
| Age (years) | <0.001 | |||
| ≥50 | 10,862 (69.7%) | 3450 (62.2%) | 7412 (74.0%) | |
| <50 | 4711 (30.3%) | 2101 (37.8%) | 2610 (26.0%) | |
| Median (IQR) | 56.0 (48.0–64.0) | 53.0 (46.0–61.0) | 58.0 (49.0–66.0) | <0.001 |
| Gender | 0.98 | |||
| Female | 15,497 (99.5%) | 5524 (99.5%) | 9973 (99.5%) | |
| Male | 76 (0.5%) | 27 (0.5%) | 49 (0.5%) | |
| Race | 0.03 | |||
| White | 13,159 (84.5%) | 4655 (83.9%) | 8504 (84.9%) | |
| Black | 1714 (11.0%) | 655 (11.8%) | 1059 (10.6%) | |
| Other | 533 (3.4%) | 176 (3.2%) | 357 (3.6%) | |
| Ethnicity | 0.009 | |||
| Hispanic | 942 (6.0%) | 374 (6.7%) | 568 (5.7%) | |
| Non-Hispanic | 13,773 (88.4%) | 4885 (88.0%) | 8888 (88.7%) | |
| Charlson/Deyo comorbidity score | <0.001 | |||
| 0 | 13,485 (86.6%) | 4937 (88.9%) | 8548 (85.3%) | |
| 1 | 1767 (11.3%) | 538 (9.7%) | 1229 (12.3%) | |
| ≥2 | 321 (2.1%) | 76 (1.4%) | 245 (2.4%) | |
| Grade | 0.01 | |||
| 1 | 2184 (14.0%) | 721 (13.0%) | 1463 (14.6%) | |
| 2 | 8243 (52.9%) | 2771 (49.9%) | 5472 (54.6%) | |
| 3 | 3325 (21.4%) | 1207 (21.7%) | 2118 (21.1%) | |
| ER status | <0.001 | |||
| ER+ | 14,079 (90.4%) | 4790 (86.3%) | 9289 (92.7%) | |
| ER- | 1233 (7.9%) | 664 (12.0%) | 569 (5.7%) | |
| PR status | <0.001 | |||
| PR+ | 12,085 (77.6%) | 4060 (73.1%) | 8025 (80.1%) | |
| PR- | 3146 (20.2%) | 1370 (24.7%) | 1776 (17.7%) | |
| HER2 statusb | <0.001 | |||
| HER2+ | 845 (10.9%) | 432 (14.9%) | 413 (8.5%) | |
| HER2- | 6620 (85.3%) | 2354 (81.5%) | 4266 (87.7%) | |
| Tumor size | <0.001 | |||
| <1 cm | 676 (4.3%) | 189 (3.4%) | 487 (4.9%) | |
| >1 to 2 cm | 2753 (17.7%) | 618 (11.1%) | 2135 (21.3%) | |
| >2 to 4 cm | 5597 (35.9%) | 1622 (29.2%) | 3975 (39.7%) | |
| >4 cm | 6191 (39.8%) | 2876 (51.8%) | 3315 (33.1%) | |
| Median (IQR) | 3.5 (2.2–5.5) | 4.5 (2.6–6.6) | 3.0 (2.0–5.0) | <0.001 |
| Clinical T stage | <0.001 | |||
| 1 | 3541 (22.7%) | 614 (11.1%) | 2927 (29.2%) | |
| 2 | 6911 (44.4%) | 2073 (37.3%) | 4838 (48.3%) | |
| 3 | 4313 (27.7%) | 2246 (40.5%) | 2067 (20.6%) | |
| 4 | 808 (5.2%) | 618 (11.1%) | 190 (1.9%) | |
| Pathological T stage | <0.001 | |||
| 0 | 281 (1.8%) | 269 (4.8%) | 12 (0.1%) | |
| 1 | 3756 (24.1%) | 1321 (23.8%) | 2435 (24.3%) | |
| 2 | 6353 (40.8%) | 1698 (30.6%) | 4655 (46.4%) | |
| 3 | 3657 (23.5%) | 1260 (22.7%) | 2397 (23.9%) | |
| 4 | 442 (2.8%) | 216 (3.9%) | 226 (2.3%) | |
| X | 823 (5.3%) | 609 (11.0%) | 214 (2.1%) | |
| Clinical N stage | <0.001 | |||
| 1 | 11,585 (74.4%) | 4265 (76.8%) | 7320 (73.0%) | |
| 2 | 2641 (17.0%) | 880 (15.9%) | 1761 (17.6%) | |
| 3 | 1347 (8.6%) | 406 (7.3%) | 941 (9.4%) | |
| Pathological N stage | <0.001 | |||
| 0 | 1102 (7.1%) | 874 (15.7%) | 228 (2.3%) | |
| 1 | 6182 (39.7%) | 1801 (32.4%) | 4381 (43.7%) | |
| 2 | 4140 (26.6%) | 1364 (24.6%) | 2776 (27.7%) | |
| 3 | 3244 (20.8%) | 964 (17.4%) | 2280 (22.7%) | |
| X | 724 (4.6%) | 445 (8.0%) | 279 (2.8%) | |
| Median no. of positive nodes (IQR) | 4.0 (1.0–9.0) | 3.0 (1.0–8.0) | 4.0 (2.0–9.0) | <0.001 |
| Median no. of nodes examined (IQR) | 14.0 (9.0–19.0) | 13.0 (8.0–18.0) | 14.0 (9.0–20.0) | <0.001 |
| Treated with endocrine therapy | ||||
| Out of all patients | 12,298 (79.0%) | 4194 (75.6%) | 8104 (80.9%) | <0.001 |
| Out of ER+ or PR+ patients | 12114 (77.8%) | 4127 (74.3%) | 7987 (79.7%) | 0.91 |
| Surgery type | <0.001 | |||
| Lumpectomy | 3561 (22.9%) | 1008 (18.2%) | 2553 (25.5%) | |
| Mastectomy | 12012 (77.1%) | 4543 (81.8%) | 7469 (74.5%) | |
| Radiation | ||||
| Post-lumpectomyc | 3139 (88.1%) | 897 (89.0%) | 2242 (87.8%) | 0.33 |
| Post-mastectomyd | 8496 (70.7%) | 3492 (76.9%) | 5004 (67.0%) | <0.001 |
Percentages are out of total population counts unless otherwise indicated, and may not add up to 100 due to rounding or missing values.
Percentages for HER2 status are out of patients diagnosed on or after 2010 (Overall N=7757, NACT N=2890, ACT N=4867).
Percentages represent rates of radiation receipt among patients receiving lumpectomy.
Percentages represent rates of radiation receipt among patients receiving mastectomy.
P-values for categorical variables are from chi-square tests. P-values from continuous variables are from pooled t-tests.
ACT: adjuvant chemotherapy. ER: estrogen receptor. HER2: human epidermal growth factor receptor 2. IQR: interquartile range. LN: lymph node. NACT: neoadjuvant chemotherapy. PR: progesterone receptor.
Unadjusted Survival Analyses
Unadjusted OS was higher for patients who received ACT compared to those who received NACT at 10 years (65.1% vs 54.4%, p<0.001). When stratified by HR status, patients with HR- ILC, who made up 9% (n=1404) of the entire cohort, had worse OS than patients with HR+ ILC, regardless of chemotherapy sequence; NACT patients had worse OS than adjuvant patients within both the HR+ and HR- cohorts. Among patients <50, those who received NACT had worse OS compared to those who received ACT at 10 years (61.5% vs 75.6%, log-rank p<0.001). Similarly among patients ≥50, NACT patients had worse OS compared to ACT patients at 10 years (49.6% vs 61.2%, p<0.001).
We compared patients with known endocrine therapy status (n=15,038) to node-positive ILC patients who only received endocrine therapy (n=3,025, Figure 2).We compared 5- and 10-year survival among 5 groups: (1) NACT alone, (2) ACT alone, (3) Endocrine therapy alone, (4) NACT + Adjuvant endocrine therapy, and (5) ACT + Adjuvant endocrine therapy. We found that patients treated with NACT alone had worse 10-year survival compared to all other groups including those who only received endocrine therapy (38.6% vs 45.4%, log-rank p<0.001). However, patients who received chemotherapy of any kind in combination with adjuvant endocrine therapy had the best overall survival compared to patients treated with only endocrine therapy or only chemotherapy. Among patients who received a combination of chemotherapy and adjuvant endocrine therapy, NACT + endocrine patients had worse survival compared to ACT + endocrine patients (58.4% vs 67.3%, log-rank p<0.001).
Fig. 2.
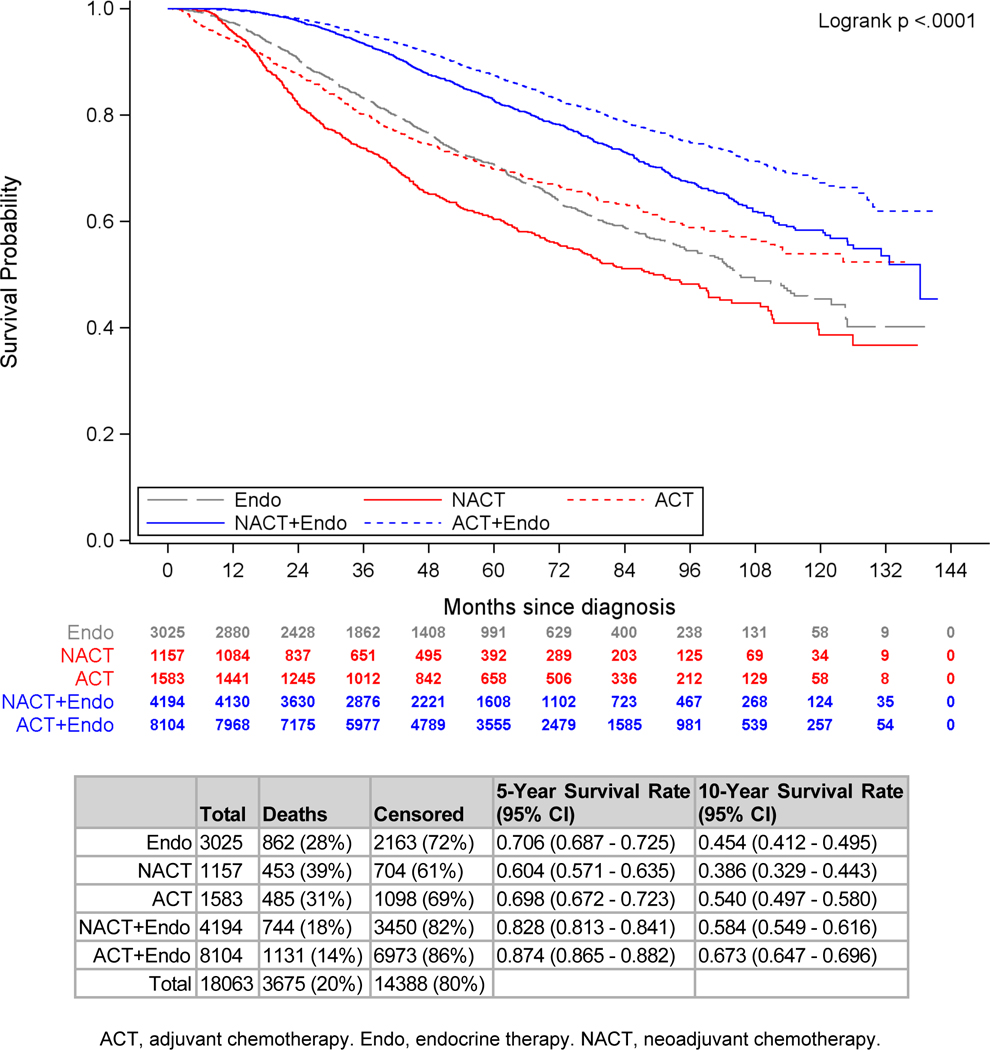
Unadjusted Overall Survival by Treatment Sequence, cT1–4c, cN1–3 Invasive Lobular Carcinoma Patients, National Cancer Data Base, 2004–2013 (N=18,063)
A majority of the 15,573 patients in the cohort had stage II (71.3%, N=11,100) disease. In stage-specific sensitivity analyses, 34.8% (N=3,865) of stage II patients received NACT, and 37.7% (N=1,686) of stage III patients received NACT. Similar to the overall cohort, NACT was associated with worse survival compared to ACT in both stage II and stage III patients.
Adjusted Survival Analysis
After adjustment for known covariates, patients who underwent NACT had worse OS than patients receiving ACT (HR 1.38, 95% CI 1.25–1.52, p<0.001, Table 2). Other factors associated with worse survival were black vs white race (HR 1.25, 95% CI 1.09–1.43); government vs private insurance (HR 1.55, 95% CI 1.42–1.69); and higher grade, cT stage, and cN stage (all p<0.001). Improved survival was associated with age <50 (HR 0.73, 95% CI 0.66–0.81), adjuvant endocrine therapy (HR 0.75, 95% CI 0.67–0.84), and post-lumpectomy radiation (HR 0.66, 95% CI 0.51–0.86, all p<0.01). In stage-specific sensitivity analyses, NACT continued to be associated with worse adjusted survival compared to ACT in stage II and III patients (Supplemental Tables 1–2).
Table 2.
Adjusted Overall Survival, cT1–4c, cN1–3 Invasive Lobular Carcinoma Patients, National Cancer Data Base, 2004–2013 (N=12,312)
| HR (95% CI) | P-Value | Overall P-Value | |
|---|---|---|---|
| Treatment group | <0.001 | ||
| ACT | REF | ||
| NACT | 1.38 (1.25 – 1.52) | <0.001 | |
| Age (years) | <0.001 | ||
| ≥50 | REF | ||
| <50 | 0.73 (0.66 – 0.81) | <0.001 | |
| Race | <0.001 | ||
| White | REF | ||
| Black | 1.25 (1.09 – 1.43) | <0.001 | |
| Other | 0.68 (0.51 – 0.93) | 0.01 | |
| Ethnicity | <0.001 | ||
| Hispanic | REF | ||
| Non-Hispanic | 1.43 (1.16 – 1.78) | <0.001 | |
| Charlson/Deyo comorbidity score | <0.001 | ||
| 0 | REF | ||
| 1 | 1.26 (1.13 – 1.42) | <0.001 | |
| ≥2 | 1.41 (1.10 – 1.81) | 0.006 | |
| Clinical T stage | <0.001 | ||
| 1 | REF | ||
| 2 | 1.29 (1.137 – 1.47) | <0.001 | |
| 3 | 1.55 (1.35 – 1.77) | <0.001 | |
| 4 | 2.26 (1.88 – 2.71) | <0.001 | |
| Clinical N stage | <0.001 | ||
| 1 | REF | ||
| 2 | 1.24 (1.11 – 1.38) | <0.001 | |
| 3 | 1.95 (1.72 – 2.21) | <0.001 | |
| Grade | <0.001 | ||
| 1 | REF | ||
| 2 | 1.24 (1.09 – 1.42) | 0.002 | |
| 3 | 1.69 (1.46 – 1.96) | <0.001 | |
| Treated with radiation post-lumpectomy | 0.002 | ||
| No | REF | ||
| Yes | 0.66 (0.51 – 0.86) | 0.002 | |
| Treated with endocrine therapy | <0.001 | ||
| No | REF | ||
| Yes | 0.75 (0.67 – 0.84) | <0.001 |
Hazard ratios (HRs), confidence intervals (CIs), and p-values are from a Cox proportional hazards model, stratified by year of diagnosis (grouped as 2004–2009 and 2010–2013) and hormone receptor status (positive/negative). A robust sandwich covariance estimator was used to account for correlation of patients treated at the same facility. Other covariates for which HRs are not shown include Surgery type, Treatment with radiation post-mastectomy, Income level, Education level, and Facility Type (all non-significant) as well as Facility location (p<0.001).
ACT: adjuvant chemotherapy. NACT: neoadjuvant chemotherapy.
DISCUSSION
In our analysis of node-positive ILC patients, receipt of NACT was associated with worse OS compared to ACT even among those with stage III disease. NSABP B-18 demonstrated comparable rates of overall and disease-free survival in patients receiving adjuvant and neoadjuvant chemotherapy as well as higher rates of breast conservation in NACT patients, in whom pCR rates of >20% were reported.19 However, our data support findings from additional studies that demonstrated low rates of overall and anatomically limited pCR in ILC,6,12,19 and, concomitantly, that patients with ILC may not derive the same clinical benefit from NACT as patients with IDC.
An important initial consideration is whether chemotherapy confers any additional benefit over endocrine therapy alone for ILC, the majority of which is low-grade and HR+. Truin et al published a study of patients selected from the Netherlands cancer registry evaluating adjuvant endocrine therapy only vs endocrine therapy and chemotherapy in a cohort of 19,609 postmenopausal patients, 3,865 of whom had a diagnosis of lobular cancer. They concluded that adjuvant chemotherapy did not confer an additional benefit in patients with lobular cancer.20 In addition, a more recent study by Marmor et al addressed the impact of adjuvant chemotherapy in the survival of patients with invasive lobular vs invasive ductal cancer within a California data registry.21 In this study, likewise, adjuvant chemotherapy did not improve outcomes in 4,638 patients with ER+ stage I/II invasive lobular cancer. Our study evaluated 15,573 patients with cN+ ILC who received chemotherapy, which is frequently recommended for cN+ patients regardless of histology. Indeed, this tendency was confirmed in our manuscript, which demonstrated that on a national level, a majority of node-positive lobular patients (76.7%) received chemotherapy. Among the groups of patients for whom endocrine therapy status was known, ACT patients who did not receive endocrine therapy outperformed NACT patients who did not receive endocrine therapy, and similarly, ACT patients who did receive endocrine therapy had improved survival as compared to NACT patients who also received endocrine therapy. Notably, the only group of patients to perform worse than those who only received endocrine therapy were those who only received NACT. Nevertheless, we can conclude that in cN+ ILC, there is a survival benefit conferred with the combination of chemotherapy and endocrine therapy and that sequence of chemotherapy does in fact matter, with worse outcomes seen among those who received NACT and adjuvant endocrine therapy as compared to those who received ACT and adjuvant endocrine therapy.
We sought to determine whether higher presenting stage among NACT patients contributed to the survival difference noted between NACT and ACT patients. Tsung et al. demonstrated that the 77 patients who received NACT had higher clinical stage at presentation and higher rate of mastectomy (84%) compared to ACT patients.22 In our study, 51.6% of NACT patients had clinical T3 or T4 disease, and despite achieving pathological T3 and T4 disease rates that were similar to those among ACT patients (NACT 26.6% vs ACT 26.2%), a greater proportion of NACT patients (81.8%) underwent mastectomy compared with patients who underwent surgery first (74.5%). Of the ILC patients in our cohort who underwent NACT, only 3.4% experienced pCR, with less than 1% experiencing pCR in the breast. The low rates of pCR among ILC patients in our study have been observed by other authors, including a pooled analysis of 6 clinical trials by Cristofanilli et al. in which 122 patients with ILC had lower rates of pCR as compared with IDC patients (3% v 15%).6 In a study examining the benefits of MRI in post-NACT surgical planning, patients with ILC had higher rates of mastectomy vs lumpectomy even after NACT.23 Our data is in keeping with previous studies that suggest that across a large population of ILC patients, clinicians may not observe sufficient clinical benefit in the form of tumor downstaging preoperatively to warrant routine use in patients with high clinical T stage even if on final pathology they are found to have some evidence of response.
Response to NACT in the nodal basins is also a potentially important intermediate outcome among ILC patients given high rates of node-positivity at diagnosis. In our study, only 3.3% of patients had nodal pCR. Interestingly, while 76.8% of the neoadjuvant patients in our study had cN1 disease, 74.4% of NACT patients were ypN1-N3 post-chemotherapy, indicating high rates of post-NACT residual disease and even upstage. These rates could also represent inadequate staging at diagnosis, in keeping with ILC’s tendency to be underestimated by conventional imaging.23 Overall and positive lymph node yields were similar (NACT 13 and 3 vs ACT 14 and 4), suggesting that NACT rarely obviated ALND or downstaged the axilla. Other authors have also demonstrated high rates of persistent nodal involvement in ILC patients that ultimately made ALND unavoidable. 11,24 In our study, 48.4% of patients who received NACT had T1 or T2 disease suggesting that in these cases, presentation with node-positive disease may have driven the decision to pursue NACT. With high rates of persistent nodal involvement, our data suggest that use of NACT to downstage the axilla in ILC patients may also be of limited utility.
Patients who underwent NACT had worse unadjusted survival compared to patients receiving ACT (77.8% vs 84.7%), and this finding persisted in the adjusted analysis, where NACT conferred a 38% increased risk of death. We recognize that NACT is typically administered for patients with higher stage disease, which may – as a reflection of as-yet undetected systemic disease – intrinsically contribute to worse OS. However, in stage-specific sensitivity analyses, worse survival outcomes persisted in patients who received NACT vs ACT regardless of stage.
In our subset analysis comparing HR- and HR+ ILC patients, HR- patients had worse OS compared to HR+ patients. Similarly, in a retrospective study using tumor registry data to evaluate disease-specific survival between stage-matched ILC and IDC patients, ILC was associated with worse survival outcomes in HR- patients.15 We also found that even among HR- patients, who had worse survival than HR+ patients, receipt of NACT yielded worse survival than ACT. These findings suggest that ILC has a distinct tumor biology that is potentially resistant to chemotherapy regardless of HR status, a conclusion further supported by studies demonstrating that ILC is typically low grade with a low to intermediate mitotic index, thus limiting the response to NACT.6
After adjusting for multiple covariates, adjuvant endocrine therapy was associated with improved survival among ILC patients in our study. Given this finding, there may be potential for improved survival outcomes with the use of aromatase inhibitors in the neoadjuvant setting. ACOSOG Z1031 demonstrated improved surgical outcomes in postmenopausal women with ER-rich, stage II/III breast cancer treated with neoadjuvant aromatase inhibitors.25 A tailored approach to treatment with neoadjuvant endocrine therapy may improve survival outcomes in ILC patients presenting with locally advanced disease.
In the NSABP B-18 and B-27 trials, there were no differences in disease-free or overall survival between NACT and ACT patients, with NACT resulting in pCR rates of 26.1%, a 175% increase in breast conservation, and a 37% increase in the incidence of pathologically negative nodes. However, these findings did not account for differences in histology.19 With very low rates of pCR, mastectomy rates and lymph nodes yields comparable to ACT as well as worse OS than ACT, NACT appears to offer no additional benefit to node-positive ILC patients and may even be detrimental.
Limitations
There were several limitations to our study. The NCDB does not report breast cancer-specific survival or recurrence rates so the only long-term outcome examined was survival. We could not account for patients with disease progression prompting earlier surgical intervention or for patients who did not complete recommended treatment. Among those patients who received NACT, selection bias may have contributed to decisions regarding initial treatment with chemotherapy though our survival analysis adjusted for several known pre-treatment variables including presenting stage. Furthermore, it is impossible to discern which patients might have chosen mastectomy after NACT even if lumpectomy were feasible. Our study specifically selected patients with cN+ disease, thus our results may not be applicable to node-negative patients with ILC who receive NACT for other indications (e.g., inflammatory carcinoma). Finally, in its NACT designation, the NCDB does not distinguish between patients who received only NACT or both NACT and ACT, a group who may have more extensive, chemoresistant disease and whose inclusion may have contributed to the worse survival observed in the NACT group.
CONCLUSIONS
Among node-positive ILC patients in our study, receipt of NACT rarely conferred pCR, was associated with worse survival compared with receipt of ACT, and did not decrease rates of mastectomy or extensive axillary surgery. Our findings suggest that the NACT-associated delay to locoregional and endocrine treatment in these patients may result in worse long-term outcomes. Patients with locally advanced ILC would benefit from prospective clinical trials to inform the development of evidence-based, histology-specific guidelines as to the optimal use and sequence of both chemotherapy and endocrine therapy.
Supplementary Material
Synopsis:
In node-positive invasive lobular breast cancer, NACT was not associated with lower rates of mastectomy or less extensive axillary surgery. NACT was associated with worse survival compared with adjuvant chemotherapy, suggesting NACT may be of limited benefit in node-positive ILC.
Acknowledgements:
Portions of this manuscript were presented at the 2018 American Society of Clinical Oncology Meeting (ASCO) on June 2, 2018. First author Dr. N. Tamirisa received an ASCO Conquer Cancer Merit Award for this work. None of the authors for this manuscript has any conflicts of interest, financial or otherwise, to disclose. The National Cancer Data Base (NCDB) is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The CoC’s NCDB and the hospitals participating in the CoC NCDB are the source of the de-identified data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
Funding: Dr. O. Fayanju is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) under Award Number 1KL2TR002554 (PI: Svetkey). Dr. R. Greenup is supported by the NIH BIRCWH K12HD043446 (PI: Andrews). This work is also supported by the Duke Cancer Institute through NIH grant P30CA014236 (PI: Kastan). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Data Availability Statement: Our data were sourced from the National Cancer Data Base (NCDB), a product of the American College of Surgeons. These data are available upon request, and instructions for data access can be found at https://www.facs.org/quality-programs/cancer/ncdb. The SAS code used to generate our analysis dataset (inv_lobular_data.sas) can be found on Github at https://github.com/thyslop/DCIBSR.git, under the account of co-author Dr. Terry Hyslop, Chair of Biostatistics at the Duke Cancer Institute.
ETHICAL APPROVAL: This article does not contain any studies with human participants or animals performed by any of the authors. The study used de-identified information and was given exempt status by our Institutional Review Board (IRB).
CONFLICT OF INTEREST: The authors declare that they have no conflicts of interest.
REFERENCES
- 1.Sikora MJ, Cooper KL, Bahreini A, et al. Invasive lobular carcinoma cell lines are characterized by unique estrogen-mediated gene expression patterns and altered tamoxifen response. Cancer research. 2014;74(5):1463–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ciriello G, Gatza ML, Beck AH, et al. Comprehensive Molecular Portraits of Invasive Lobular Breast Cancer. Cell. 2015;163(2):506–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernandez B, Paish EC, Green AR, et al. Lymph-node metastases in invasive lobular carcinoma are different from those in ductal carcinoma of the breast. Journal of clinical pathology. 2011;64(11):995–1000. [DOI] [PubMed] [Google Scholar]
- 4.Chen Z, Yang J, Li S, et al. Invasive lobular carcinoma of the breast: A special histological type compared with invasive ductal carcinoma. PloS one. 2017;12(9):e0182397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petruolo OA, Pilewskie M, Patil S, et al. Standard Pathologic Features Can Be Used to Identify a Subset of Estrogen Receptor-Positive, HER2 Negative Patients Likely to Benefit from Neoadjuvant Chemotherapy. Ann Surg Oncol. 2017;24(9):2556–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cristofanilli M, Gonzalez-Angulo A, Sneige N, et al. Invasive lobular carcinoma classic type: response to primary chemotherapy and survival outcomes. J Clin Oncol. 2005;23(1):41–48. [DOI] [PubMed] [Google Scholar]
- 7.Petrelli F, Barni S. Response to neoadjuvant chemotherapy in ductal compared to lobular carcinoma of the breast: a meta-analysis of published trials including 1,764 lobular breast cancer. Breast Cancer Res Treat. 2013;142(2):227–235. [DOI] [PubMed] [Google Scholar]
- 8.Delpech Y, Coutant C, Hsu L, et al. Clinical benefit from neoadjuvant chemotherapy in oestrogen receptor-positive invasive ductal and lobular carcinomas. British journal of cancer. 2013;108(2):285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Truin W, Vugts G, Roumen RM, et al. Differences in Response and Surgical Management with Neoadjuvant Chemotherapy in Invasive Lobular Versus Ductal Breast Cancer. Ann Surg Oncol. 2016;23(1):51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Straver ME, Rutgers EJ, Rodenhuis S, et al. The relevance of breast cancer subtypes in the outcome of neoadjuvant chemotherapy. Ann Surg Oncol. 2010;17(9):2411–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poodt IGM, Spronk PER, Vugts G, et al. Trends on Axillary Surgery in Nondistant Metastatic Breast Cancer Patients Treated Between 2011 and 2015: A Dutch Population-based Study in the ACOSOG-Z0011 and AMAROS Era. Ann Surg. 2017. [DOI] [PubMed] [Google Scholar]
- 12.Katz A, Saad ED, Porter P, Pusztai L. Primary systemic chemotherapy of invasive lobular carcinoma of the breast. Lancet Oncol. 2007;8(1):55–62. [DOI] [PubMed] [Google Scholar]
- 13.Loibl S, Volz C, Mau C, et al. Response and prognosis after neoadjuvant chemotherapy in 1,051 patients with infiltrating lobular breast carcinoma. Breast Cancer Res Treat. 2014;144(1):153–162. [DOI] [PubMed] [Google Scholar]
- 14.Farese SA, Aebi S. Infiltrating lobular carcinoma of the breast: systemic treatment. Breast disease. 2008;30:45–52. [DOI] [PubMed] [Google Scholar]
- 15.Wasif N, Maggard MA, Ko CY, Giuliano AE. Invasive lobular vs. ductal breast cancer: a stage-matched comparison of outcomes. Ann Surg Oncol. 2010;17(7):1862–1869. [DOI] [PubMed] [Google Scholar]
- 16.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–172. [DOI] [PubMed] [Google Scholar]
- 17.Fayanju OM, Ren Y, Thomas SM, et al. The Clinical Significance of Breast-only and Node-only Pathologic Complete Response (pCR) After Neoadjuvant Chemotherapy (NACT): A Review of 20,000 Breast Cancer Patients in the National Cancer Data Base (NCDB). Ann Surg. 2018;268(4):591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Natarajan L, Pu M, Parker BA, et al. Time-varying effects of prognostic factors associated with disease-free survival in breast cancer. American journal of epidemiology. 2009;169(12):1463–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26(5):778–785. [DOI] [PubMed] [Google Scholar]
- 20.Truin W, Voogd AC, Vreugdenhil G, van der Heiden-van der Loo M, Siesling S, Roumen RM. Effect of adjuvant chemotherapy in postmenopausal patients with invasive ductal versus lobular breast cancer. Annals of oncology : official journal of the European Society for Medical Oncology. 2012;23(11):2859–2865. [DOI] [PubMed] [Google Scholar]
- 21.Marmor S, Hui JYC, Huang JL, et al. Relative effectiveness of adjuvant chemotherapy for invasive lobular compared with invasive ductal carcinoma of the breast. Cancer. 2017;123(16):3015–3021. [DOI] [PubMed] [Google Scholar]
- 22.Tsung K, Grobmyer SR, Tu C, Abraham J, Budd GT, Valente SA. Neoadjuvant systemic therapy in invasive lobular breast cancer: Is it indicated? Am J Surg. 2018;215(3):509–512. [DOI] [PubMed] [Google Scholar]
- 23.Vriens IJH, Keymeulen K, Lobbes MBI, et al. Breast magnetic resonance imaging use in patients undergoing neoadjuvant chemotherapy is associated with less mastectomies in large ductal cancers but not in lobular cancers. Eur J Cancer. 2017;81:74–80. [DOI] [PubMed] [Google Scholar]
- 24.Adachi Y, Sawaki M, Hattori M, et al. Comparison of sentinel lymph node biopsy between invasive lobular carcinoma and invasive ductal carcinoma. Breast cancer (Tokyo, Japan). 2018. [DOI] [PubMed] [Google Scholar]
- 25.Ellis MJ, Suman VJ, Hoog J, et al. Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype--ACOSOG Z1031. J Clin Oncol. 2011;29(17):2342–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.[dataset] The National Cancer Data Base (NCDB). These data are available upon request, and instructions for data access can be found at https://www.facs.org/quality-programs/cancer/ncdb
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


