Abstract
Head and neck squamous cell carcinoma (HNSCC) accounts for more than 600,000 cases and 380,000 deaths annually worldwide. While human papilloma virus (HPV)-associated HNSCCs have better overall survival compared to HPV-negative HNSCC, loco-regional recurrence remains a significant cause of mortality and additional combinatorial strategies are needed to improve outcomes. The primary conventional therapies to treat HNSCC are surgery, radiation, and chemotherapies; however multiple other targeted systemic options are used and being tested including cetuximab, bevacizumab, mTOR inhibitors, and metformin. In 2016 the first checkpoint blockade immunotherapy was approved for recurrent or metastatic HNSCC refractory to platinum based chemotherapy. This immunotherapy approval confirmed the critical importance of the immune system and immuno-modulation in HNSCC pathogenesis, response to treatment, and disease control. However, while immuno-oncology agents are rapidly expanding, the role that the immune system plays in the mechanism of action and clinical efficacy of standard conventional therapies is likely underappreciated. In this article, we focus on how conventional and targeted therapies may directly modulate the immune system and the tumor microenvironment to better understand the effects and combinatorial potential of these therapies in the context and era of immunotherapy.
Keywords: head and neck cancer, immunomodulation, tumor microenvironment, chemotherapy, radiotherapy, radiation, PD-1, CTLA-4, immunotherapy, cetuximab, mTOR, metformin, checkpoint blockade
Introduction
Head and neck squamous cell carcinoma (HNSCC) accounts more than 600,000 cases and 380,000 deaths annually worldwide.(1) In the United States, HNSCC is the sixth most common cancer, and 63,000 patients are diagnosed and approximately 13,000 deaths occur from the disease every year.(2) In addition to the classical risk factors of tobacco and alcohol use, oropharyngeal squamous cell carcinoma (OPSCC) is currently the most common head and neck cancer in the United States due to infection with high-risk human papilloma virus (HPVs) strains including HPV 16, 18, 31, 33, and 45. Different from HPV-negative HNSCC, HPV-associated HNSCC mainly occurs in younger patients. Within the oropharynx the status of HPV infection is usually identified by the surrogate marker p16, which is upregulated by with HPV infection. However importantly, for sites outside of the oropharynx p16 status does not necessarily correlate with HPV positivity. Of note, p16, also known as p16INK4a or cyclin-dependent kinase inhibitor 2A, is a cell cycle regulator and endogenous tumor suppressor which is upregulated as a counter-regulatory mechanism to the loss of cell cycle control and inactivation of the retinoblastoma protein (pRb) by the HPV E7 protein. Fortunately, p16-positive OPSCCs are associated with longer survival and better treatment outcomes.(3) Indeed, p16-negative and p16-positive OPSCCs are considered as two distinct types of tumors in the 8th edition of TNM-classification and staging by American Joint Commission on Cancer (AJCC).
The primary curative therapeutic options for previously untreated HNSCC are surgery with or without adjuvant radiation or chemoradiation as indicated by pathology, definitive radiation alone, or definitive chemoradiation. Standard surveillance is to then obtain imaging at 12 weeks post-treatment to assess for response and then follow with routine physical exam, nasopharyngolaryngoscopy, and additional imaging as indicated. However, among all comers approximately 50% of patients will eventually develop a local or regional recurrence and despite advances in treatment, the five-year survival rate remains low(4,5). Moreover, treatment is associated with significant long-term toxicity and morbidity(4,5). Traditionally, systemic chemotherapies and cetuximab are used for relapsed refractory or metastatic disease with limited improvement in long term survival. Importantly, the anti-programmed cell death-1 (PD-1) antibodies pembrolizumab and nivolumab were FDA approved to treat platinum refractory recurrent or metastatic HNSCC in 2016 (6,7). Responses and activity of anti-PD-1 agents is seen in patients with HPV-positive tumors and HPV-negative tumors; however, objective response rates to checkpoint blockade immunotherapy (CBI) remain low on the order of 16–25% (6,7). Of note an anti-PD-1 agent as a first-line therapy was recently demonstrated to improve overall survival compared to cetuximab and chemotherapy in recurrent or metastatic HNSCC whose tumors overexpress PD-1(8). As immunotherapy is now FDA approved with demonstrated activity in metastatic HNSCC, there is a large national and international effort to understand the role of the immune system and immuno-modulation in head and neck cancer. The demonstrated activity of immunotherapy in HNSCC has prompted a re-evaluation of the mechanisms of action of conventional therapies and highlights the important role that the immune system may play in the clinical efficacy of conventional therapies. Here, we overview conventional and targeted therapies, including chemotherapies, radiotherapy, cetuximab, and others as they relate to immune modulation of HNSCC and the tumor microenvironment to better understand the immune-context of these therapies and develop strategies to improve outcomes for patients with HNSCC (Figure 1).
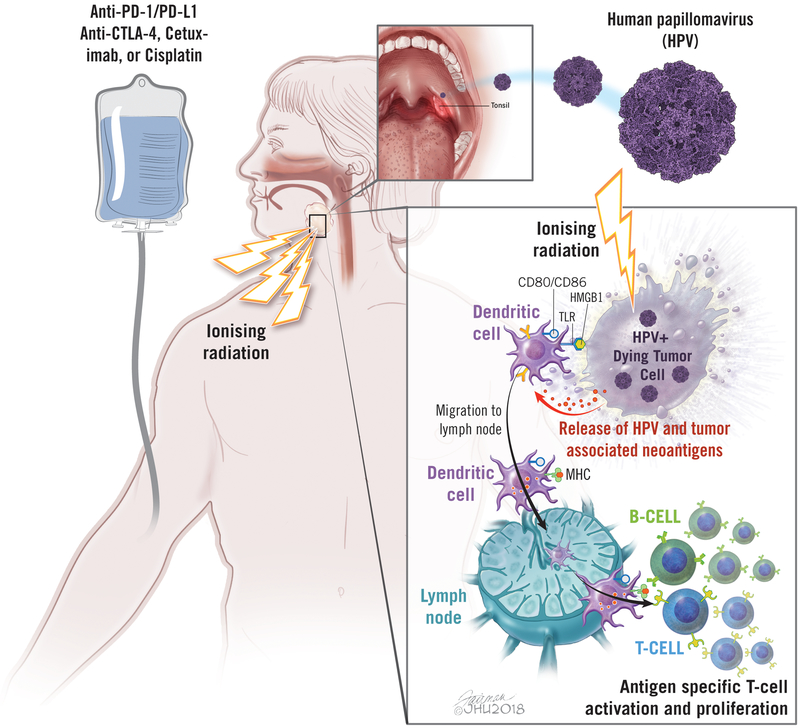
Figure 1. Radiation-induced immune responses in head and neck cancer.
Radiation induces 1) release of tumor antigens and damage-associated molecular pattern (e.g. HMGB1) via cell death, 2) activation and migration of dendritic cells to lymph node, 3) enhanced cross-presentation of tumor antigens via upregulation of MHC I, and 4) antigen-specific T cell activation and proliferation. Radiation therapy can be combined with immunotherapy (checkpoint blockade) or chemotherapy. TLR: toll-like receptor, HMGB1: high mobility group protein B1, MHC: major histocompatibility complex, PD-1: programmed cell death-1
1. Immunomodulatory Action of Chemotherapy in HNSCC
Immune Effects of Chemotherapy
Cytotoxic chemotherapies are frequently used in HNSCC in combination with radiation therapy for locally advanced disease and alone for recurrent or metastatic disease. Chemotherapies directly inhibit cell division or proliferation in a variety of ways, including interference with DNA replication, protein function, or microtubule formation. Because of myelosuppressive effects, chemotherapy is generally thought to be immunosuppressive, causing lymphopenia and neutropenia. Recent research suggests, however, that certain cytotoxic chemotherapies may also have important immunostimulatory effects.
Preclinical models suggest that chemotherapy is more effective in an immunocompetent host, with decreased efficacy of cisplatin and paclitaxel in immunodeficient mice.(9) Mechanistically certain chemotherapies can increase antigen presentation and can reduce expression of PD-L2, leading to increased T cell activation.(10,11) Additionally chemotherapies have been shown to increase the cytotoxic effects of CTLs and induce immunogenic cell death (ICD).(12–14) Specific chemotherapies certainly have differential effects on the immune system for example: platinums can increase T-cell activation by dendritic cells through downregulation by the STAT6 pathway, while docetaxel may decrease regulatory T cell populations to enhance anti-tumor immunity.(15,16) Moreover, taxanes, platinums, and 5-FU, all used frequently in HNSCC, have been shown in animal models to decrease myeloid derived suppressor cells (MDSCs), which can enhance anti-tumor immunity.(17–19) Interestingly, alterations observed in HNSCC patients could be used as potential biomarkers to guide the use of or avoidance of certain chemotherapy or chemo-immunotherapy combinations(20) such as: anthracyclines (e.g. doxorubicin) and TOP2A protein overexpression; Taxanes (e.g. paclitaxel) and TUBB3/TLE protein overexpression; fluoropyrimidines (e.g. 5-fluorouracil) and TS protein overexpression; platinum analogues (e.g. cisplatin) and ERCC1 protein overexpression; nucleoside analogues (e.g. gemcitabine) and RRMI protein overexpression; and alkylating agents (e.g. temozolomide) and MGMT protein overexpression. Given the ability of chemotherapy to decrease tumor burden while potentially modulating immune responses, combinations of chemotherapy and immunotherapy are under investigation in HNSCC.
Combinations of Chemotherapy and Immunotherapy
To date, most of the large trials combining chemotherapy and immunotherapy have been in non-small cell lung cancer (NSCLC). In a cohort of the CheckMate-012 trial, 56 patients with previously untreated NSCLC were treated with nivolumab in combination with one of three cytotoxic regimens (cisplatin/pemetrexed, cisplatin/gemcitabine, or carboplatin/paclitaxel). The combination was shown to be feasible, without unexpected toxicities. Two year overall survival in the patients receiving carboplatin/paclitaxel and nivolumab 5 mg/kg was promising at 62%.(21) Cohort G of the phase 2 KEYNOTE-021 study randomized 123 patients with non-squamous NSCLC to carboplatin and pemetrexed with or without pembrolizumab; improved response rates were seen with the pembrolizumab combination (55% vs 29%).(22) This led to accelerated approval of the combination by the FDA. The phase 3 KEYNOTE-189 trial confirmed these results, showing improved overall survival (HR 0.49, p < 0.001), progression free survival (HR 0.52, p < 0.001), and response rates (47.6% vs 18.9%) with carboplatin/pemetrexed/pembrolizumab compared to chemotherapy alone in patients with non-squamous NSCLC. Benefit was seen across all levels of PD-L1 expression.(23) More recently, the addition of pembrolizumab to carboplatin and paclitaxel or nab-paclitaxel in squamous cell carcinoma of the lung was shown to improve both progression free survival (HR 0.56, p < 0.001) and overall survival (HR 0.64, p < 0.001)(24); this regimen was FDA approved in October 2018. No large trials combining chemotherapy with immunotherapy have been published at this time HNSCC. Early results from the phase 3 KEYNOTE-048 trial (NCT02358031) were recently presented. In this trial, patients with recurrent/metastatic HNSCC who had not yet received systemic therapy for recurrent/metastatic disease were randomized between pembrolizumab, pembrolizumab in combination with cisplatin or carboplatin and 5-FU, and standard of care cetuximab/platinum/5-FU. Single agent pembrolizumab was found to improve overall survival compared to chemotherapy in patients with PD-L1 CPS ≥ 1; pembrolizumab combined with chemotherapy improved survival in the total population.(25) Another phase 3 trial in a similar setting is CheckMate 651 (NCT02741570) which is comparing the combination of two immunotherapy agents, nivolumab and ipilimumab, to standard therapy with cetuximab/platinum/5-FU. These trials will help define the use of chemo-immunotherapy in HNSCC.
2. Immunomodulatory Action of Radiation in HNSCC
Immunological Effects of Radiation on Tumor Microenvironment
Radiation therapy (RT) is given to approximately 50% of patients during the course of cancer treatment. It is known that radiation can induce DNA damage and ER stress via production of reactive oxygen species, leading to mitotic catastrophe and cell death. Radiation also induces cell death via intrinsic and extrinsic apoptotic pathways including upregulation of FAS expression on the cell surface.(26) Furthermore, radiation is able to induce immunogenic cell death (ICD) of cancer cells through damage-associated molecular patterns (DAMPs) – pattern recognition receptors. One such DAMP molecule is high mobility group protein B1 (HMGB1), a ligand for TLR4, which is released by radiation and successively activates the innate immune response and changes the cytokine profile towards an immune stimulatory phenotype in the tumor microenvironment.(27) More importantly, radiation can activate antigen-specific anti-tumor immune responses. One of the most important signatures induced by radiation is upregulation of major histocompatibility complex (MHC) I surface expression(28) which occurs in part via activation of the mTOR pathway.(29) Radiation-induced IFNs also contribute to increased MHC I expression.(30) This is a crucial step for enhancing tumor-specific immune responses as many tumors downregulate or lose MHC I expression to evade the endogenous immune response. Radiation also enhances activation and migration of DCs, improving antigen cross-presentation in the lymph node or secondary lymphoid organs.(31)
Moreover, radiation can increase the density and infiltration of TILs, including CTLs involved in lysing tumor cells, by altering the expression of cell adhesion molecules and chemokines. For example, the expression of cell adhesion molecules, such as intercellular adhesion molecule 1, vascular adhesion molecule 1, and E-selection, on the cell surface of endothelium are enhanced by radiation.(32–34) These cell adhesion molecule and chemokines induced by radiation can help with immune cell extravasation and infiltration into the tumor microenvironment.(35,36)
However, radiation can also increase Treg populations in the tumor microenvironment through increased TGF-β secretion, contributing to immunosuppression.(37,38) Additionally radiation can induce the expression of immune checkpoint ligands, including PD-L1, on tumor cells which could be a dynamic response to inflammation and induced anti-tumor immunity versus an inherent immunosuppressive effect of radiation therapy. Thus, it is critical to harness the immunogenic properties while blocking the immunosuppressive effects of radiation therapy.
Taken together, radiation can augment systemic antigen-specific anti-tumor immune responses by inducing; 1) release of tumor antigens via inflammatory cell death, 2) activation and migration of DCs, 3) enhanced cross-presentation of tumor antigens via upregulation of MHC I, and 4) increased density of TILs, leading tumor-specific T cell activation and proliferation (Figure 1).
In addition to total dose or biologically equivalent radiation dose, different fractions sizes or treatment schedules could alter immune responses. As each fraction of radiation induces a signaling cascade, the resultant effects on the immune system could certainly depend on whether hypofractionation with 1–5 fractions is delivered versus standard conventional fractionation in 30–35 fractions. With regard to tumor control, evidence suggests that alternative fractionation schedules may improve outcomes. RTOG 9003 (NCT00771641) randomly assigned stage III/IV HNSCC patients to: 1) Standard fractionation (SFX; 70 Gy/35 daily fractions/7 weeks), 2) Hyperfractionation (HFX; 81.6 Gy/68 twice-daily fractions/7 weeks), 3) Accelerated fractionation with split (AFX-S; 67.2 Gy/42 fractions/6 weeks with a 2-week rest after 38.4 Gy), 4) Continuous accelerated fractionation (AFX-C; 72 gy/42 fractions/6 weeks). At 5 years, only HFX improved local-regional control and overall survival without increasing long-term toxicity.(39) In the MARCH-meta analysis randomized trials comparing conventional RT with hyperfractionated or accelerated RT showed that altered fractionated RT is associated with improved overall survival and progression-free survival in patients with HNSCC.(40) An updated meta-analysis confirmed that hyperfractionated RT is a standard treatment for locally advanced HNSCC, along with concomitant chemoradiotherapy.(41) Given these findings it is certainly possible that optimal induction of immune responses depends not only on the radiation dose but radiation fractionation employed. Thus the role that radiation fractionation may play in differential modification of immune responses deserves further evaluation.
Combination of Radiation Therapy and Immunotherapy
Based on the diverse immunomodulatory effects of radiation, the combination of RT and immunotherapy is under intense investigation.(42,43) Phase 1/2/3 randomized trials of RT with concurrent and adjuvant anti-PD-1/PD-L1 immunotherapy with concurrent chemotherapy in patients with advanced/intermediate-risk HNSCC and numerous other clinical trials of RT combined with immunotherapy are underway (see Table 1). These clinical trials include combination therapies in the two different settings; definitive/locally advanced curative setting and metastatic/refractory setting, which will lead us to understand more effective combination strategies of radiation and immunotherapy for different stages of HNSCCs.
Table 1:
Clinical trials of combined radiation therapy and anti-PD-1/PD-L1 immunotherapy
| Study | Phase | Eligible Patients |
Arms | Enrollment | Main Outcome(s) |
Coordinating Institution |
Sponsor | Status |
|---|---|---|---|---|---|---|---|---|
| NCT03383094 | 2 | Locoregionally advanced HNSCC | RT + Pembrolizumab RT + Cisplatin |
122 (estimated) | PFS | UC San Diego Moores Cancer Center | Merck Sharp & Dohme Corp | Recruiting |
| NCT03317327 | 1,2 | Recurrent or new second primary HNSCC with prior RT | Radiation + Nivolumab | 20 (estimated) | Adverse Events | Oslo University Hospital | Bristol-Myers Squibb | Recruiting |
| NCT03546582 | 2 | Recurrent or new second primary HNSCC | SBRT + Pembrolizumab SBRT |
102 (estimated) | PFS | RTOG Foundation | Merck Sharp & Dohme Corp | Not yet recruiting |
| NCT02296684 | 2 | Locoregionally advanced HNSCC | Neoadjuvant Pembrolizumab +
Adjuvant Pembrolizumab + SOC Neoadjuvant Pembrolizumab + SOC |
66 (estimated) | Logoregional recurrence, distant failure rate, rate of major pathologic treatment effect | Washington University School of Medicine | Merck Sharp & Dohme Corp | Recruiting |
| NCT03051906 | 1,2 | Locoregionally advanced HNSCC | RT + cetuximab + durvalumab | 69 (estimated) | PFS | Azienda Ospedaliero-Universitaria Careggi | Azienda Ospedaliero-Universitaria Careggi | Not yet recruiting |
| NCT02999087 | 3 | Logoregionally advanced HNSCC | RT + Cisplatin RT + Cetuximab + Avelumab RT + Cetuximab |
688 (estimated) | PFS | Groupe Oncologie Radiotherapie Tete et Cou | Merck KGaA, Pfizer | Recruiting |
| NCT02764593 | 1 | Locoregionally advanced HNSCC | RT + Nivolumab + Cisplatin RT + Nivolumab + Cetuximab RT + Nivolumab |
40 (actual) | DLT | RTOG Foundation | Bristol-Myers Squibb | Active, not recruiting |
| NCT03247712 | 1,2 | Surgically resectable HNSCC | Neoadjuvant Nivolumab + RT + Surgery + Adjuvant Nivolumab | 18 (estimated) | Number of patients with unplanned delay to surgery | Providence Health & Services | Providence Cancer Center | Recruiting |
| NCT03673735 | 3 | Locoregionally advanced HPV-negative HNSCC | RT + Durvalumab + Cisplatin RT + Cisplatin + Placebo |
650 (estimated) | DFS | European Organisation for Research and Treatment of Cancer | None | Not yet recruiting |
| NCT03529422 | 1 | Locoregionally advanced HNSCC | RT + Durvalumab + Tremelimumab | 24 (estimated) | DLT, acute toxicities | UNC Lineberger Comprehensive Cancer Center | AstraZeneca | Recruiting |
| NCT03426657 | 2 | Logoregionally advanced HNSCC | RT + Durvalumab + Tremelimumab | 120 (estimated) | Feasibility, DLT, CD8+ T-cell Tumor Infiltration | University of Erlangen-Nürnberg Medical School | None | Not yet recruiting |
| NCT03509012 | 1 | Advanced HNSCC, NSCLC, SCLC | RT + Durvalumab + Cisplatin | 300 (estimated) | DLT, adverse events | Multiple | AstraZeneca | Recruiting |
| NCT03539198 | Recurrent logoregional or metastatic HNSCC | Proton SBRT + Nivolumab | 91 (estimated) | ORR | Mayo Clinic | Recruiting | ||
| NCT03085719 | 2 | Metastatic HNSCC | High-dose RT +
Pembrolizumab High-dose RT + low-dose RT + Pembrolizumab |
26 (estimated) | ORR | Dana Farber Cancer Institute | Merck Sharp & Dohme Corp | Recruiting |
| NCT03283605 | 1,2 | Metastatic HNSCC | SBRT + Durvalumab + Tremelimumab | 45 (estimated) | PFS, Acute Toxicities | Centre Hospitalier de l’Université de Montréal | AstraZeneca | Recruiting |
| NCT03313804 | 2 | Previously treated advanced or metastatic HNSCC or NSCLC | Immune checkpoint inhibitor + RT | 57 (estimated) | PFS | University of Kentucky Markey Cancer Center | None | Recruiting |
Abbreviations: DFS (disease-free survival), DLT (dose-limiting toxicity), HNSCC (head and neck squamous cell carcinoma), NSCLC (non-small cell lung cancer), ORR (objective response rate), PFS (progression-free survival), RT (radiotherapy), SOC (standard of care)
Regarding timing and sequencing, concurrent administration of radiotherapy and immunotherapy is commonly being tested. However, sequential therapy might be able to enhance treatment efficacy and reduce toxicities, particularly in the setting of concomitant chemotherapy. Both orders, radiotherapy prior to immunotherapy and immunotherapy prior to radiation, have potential to enhance the activity of each other. Further investigation is required to clarify the best timing and sequencing. An ongoing phase 2 randomized trial (NCT02777385) is currently evaluating the efficacy of concurrent versus sequential pembrolizumab, cisplatin and IMRT in stage III-IVb HNSCC.
The use of immunotherapy agents in the maintenance setting is not a current standard among patients treated with curative intent. This approach could keep a basal immune response against tumor higher, helping to eliminate residual tumor cells earlier and minimize the risk of recurrence. Several clinical trials are ongoing to check the efficacy of nivolumab (NCT02764593, NCT03349710), pembrolizumab (NCT02892201, NCT02841748, NCT03040999), avelumab (NCT02952586, NCT02999087), and atezolizumab (NCT03452137) in adjuvant/maintenance setting. In one of the ongoing trials RTOG3504 (NCT02764593), the feasibility of adjuvant nivolumab at 3–12 months post-RT was evaluated. An interim report showed that patients were able to tolerate continuing immunotherapy for up to a year, demonstrating that maintenance immunotherapy is feasible in this population.(44)
Development of loco-regional recurrence or a second primary tumor is unfortunately a relatively frequent event in patients with HNSCC. Treatment with a curative-intent surgical resection or re-irradiation are the primary options for these patients. Reirradiation in some cases with the addition of concurrent chemotherapy or cetuximab has been demonstrated to improve loco-regional control and may improve survival, although patients need to be selected appropriately(45). Given the relatively limited toxicity of immunotherapy, reirradiation with immunotherapy has a potential to improve the efficacy of reirradiation and clinical trials are ongoing to evaluate this in patients with recurrent HNSCC. In order to minimize toxicity from large field re-irradiation, stereotactic body radiation therapy may be quite useful in this setting. Indeed, the phase 2 randomized trial RTOG 3507 (NCT03546582) is evaluating whether the addition of pembrolizumab to stereotactic body radiation therapy (SBRT) reirradiation improves the progression-free survival for patients with recurrent or new second primary HNSCC.
Impact of HPV status on Radiation induced immuno-modulation in Head and Neck Cancer
HPV-status in HNSCC can strongly influence responses to therapy. Interestingly, HPV-positive HNSCC has been reported to be more radiosensitive in-vivo but not in-vitro when compared to HPV-negative disease(46). Thus, the status of HPV infection can be a biomarker for radiotherapy. Indeed, variations in HPV function within HPV-positive patient subsets was recently correlated with radiation sensitivity and associated with survival.(47,48) Gleber-Netto FO et al., recently analyzed and evaluated the expression pattern of 582 HPV-correlated genes from the 80 oropharyngeal squamous cell carcinomas from the cancer genome atlas (TCGA)(48). The authors identified two distinct expression profiles within HPV-positive tumors and a significant difference in 5-year OS between these two groups of HPV-positive tumors. Furthermore, alterations in HPV associated genes was found to translate to a differential sensitivity to radiation therapy when tested using in-vitro models(48). These findings demonstrate that HPV status can impact radiation sensitivity and that even within HPV positive tumors that subset likely exist with differential sensitivity to radiation therapy.
The underlying tumor microenvironment in HNSCC is dependent on the pathogenesis and mechanism of malignant transformation, namely alcohol, tobacco, or viral etiology. Thus HPV status can also impact the development of anti-tumor immune responses and presence or composition of tumor associated immune cells. Specifically there has been reported to be an increased immune infiltrate and inflammatory cytokines in the HPV-positive tumor microenvironment, which may contribute to the better tumor clearance after irradiation, although confirmation of these findings and mechanisms for this difference require further investigation.(49,50)
One common feature of locally advanced HNSCC is the occurrence of tumor hypoxia, which strongly attenuates the efficacy of radiotherapy and is a negative prognostic factor.(51) Radiation-induced DNA damage is decreased in the absence of oxygen due to lower production of reactive oxygen species, leading to radioresistance.(52) It has been shown that HPV-positive and HPV-negative tumors display a similar degree of hypoxia, and both HPV-positive and HPV-negative HNSCC cell lines demonstrate decreased radiosensitivity in hypoxic conditions.(53) Hypoxia modifiers, such as nimorazole, which can increase free radical formation, have been used to overcome radioresistance. It is effective for both HPV-positive and HPV-negative cell lines in vitro, but clinical studies showed that it was only effective on HPV-negative tumors in vivo.(54,55) Ultimately, differences in biochemical characteristics between HPV-positive and HPV-negative tumors suggest that distinct treatment strategies may be required for these two different types of tumors and this is reflected in the different AJCC staging systems used for these distinct disease entities.
3. Immunomodulatory Action of Cetuximab in HNSCC
The anti-tumor effects of cetuximab have primarily been attributed to the blockade of EGFR signaling resulting in single agent activity, activity in combination with chemotherapy, as well as enhancement of radiation-induced cytotoxicity.(56) However, recent studies have demonstrated that cetuximab also has robust immunomodulatory activities. The cetuximab antigen-binding site region (Fab) region binds EGFR on tumor cells while the constant region (Fc) binds to the CD16 receptor (i.e. FcγRIII) on myeloid cells and natural killer cells (NKCs). Antibodies themselves are designed to stimulate innate and adaptive immune systems, resulting in fixation and activation of the complement system, Fc receptor engagement, and antibody-dependent cell-mediated toxicity (ADCC)(57). Recruited myeloid cells can directly exert lytic effects on tumor cells, as well as modify the maturation, activation, and function of dendritic cells, B-cells and T-cells in the tumor microenvironment via cytokines including interleukin (IL)-10, transforming growth factor (TGF)-β, tumor necrosis factor (TNF)-α, IL-6 and interferon (IFN)-γ. In oropharynx SCC, crosstalk between dendritic cell (DC)-NKC is also modulated by stimulator of interferon genes (STING), an endoplasmic-reticulum associated adaptor protein. EGFR blockade with cetuximab and STING activation increased the maturation markers CD86, CD83, and HLA-DR and PD-1 ligand (PD-L1) on DC, when given alone and in combination(58).
Tumor antigens liberated by dying tumor cells are presented by macrophages and DCs to naïve cytotoxic T lymphocytes (CTLs) that can acquire EGFR-specificity(59), or specificity to other tumor associated antigens resulting in an anti-tumor adaptive immune response and epitope spreading. Release of perforin and granzyme B by CTLs induces membranolysis, activation of caspases, and subsequent apoptosis of tumor cells.(57) In a cetuximab neoadjuvant therapy trial, patients exhibited upregulated CD107a and CD137 on tumor-infiltrating NKCs and upregulated perforin and granzyme B on peripheral blood NKCs.(60) Furthermore, NKC surface expression of CD137 correlated with clinical response to neoadjuvant cetuximab.(60)
Cetuximab binding to EGFR-expressing cancer cells also results in complement-dependent cytotoxicity via C3b deposition, formation of C5b-C9 complex, and resultant osmotic lysis of the target cell(61,62). In support of these mechanisms, patients with HNSCC who exhibit higher baseline ADCC activity and EGFR expression are more likely to have a complete response with cetuximab and radiotherapy.(63)
However, the recently published RTOG 1016 (NCT01302834) provides us with considerable data regarding cetuximab combined with RT which may have important implications for combining radiation with other monoclonal antibodies. 849 patients with HPV-positive oropharyngeal cancer were randomly assigned to receive either cisplatin with RT or cetuximab with RT. Unexpectedly, overall survival on the cetuximab arm was significantly inferior to the cisplatin arm. Overall rates of serious adverse events (grade 3–5) were similar for patients in both groups although toxic side effects were different.(64) Importantly we must re-evaluate the direct mechanism of ‘radiosensitization’ between these drugs. Cisplatin impairs DNA repair and enhances DNA damage after irradiation by directly binding to DNA resulting in classical radiosensitization. On the other hand, cetuximab functions indirectly as a ‘radiosensitizer’, altering growth and cell signaling pathways to cause cell cycle dysregulation, apoptosis, or activate immune responses as described above. However, cetuximab does not directly increase DNA damage from radiation therapy and similarly checkpoint blockade immunotherapy does not directly enhance DNA damage from radiation therapy. Thus these monoclonal antibodies do not function as classical radiosensitizers and instead may enhance loco-regional control through alternative mechanisms in combination with radiation therapy. RTOG 1016 as well as similar trial reported at ESMO (Abstract LBA9_PR) highlight and confirm that the standard therapy for advanced HPV-positive oropharyngeal cancer remains concurrent cisplatin with RT. The results of these studies and associated differential mechanisms of radiosensitization raise important questions which need to be carefully addressed when using immunotherapy with concurrent radiotherapy in the definitive setting.
The Immunosuppressive Tumor Microenvironment and Resistance to Cetuximab
Tumor-infiltrating lymphocytes (TILs) are observed to have upregulated expression of immune checkpoint receptors including PD-1, cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), T-cell immunoglobulin and mucin domain 3 (TIM-3) and lymphocyte-activation gene 3 (LAG-3) which can paradoxically indicate activation as well as exhaustion, or anergy depending on the magnitude and chronicity of expression. Nonetheless, an EGFR-mediated immunosuppressive tumor microenvironment has been described where co-inhibitory signals are upregulated at the interface between tumor and T cells or antigen-presenting cells (APCs) and T cells.(57) In patients treated with cetuximab, CD8+ TILs expressed increased levels of PD-1 and TIM-3 over the course of cetuximab therapy.(65) PD-1 ligation by PD-L1 on tumor cells results in T cell receptor signaling inhibition, and TIM-3 stimulation results in T cell exhaustion.(65) Cetuximab-treated patients also exhibit an increase in circulating and intra-tumoral CD4+CD25+Foxp3high regulatory T cells (Treg) expressing CTLA-4. CTLA-4, when expressed by T cells, binds B7 expressed on antigen-presenting cells and induces a coinhibitory “signal 2” which destines the T cell to an anergic fate.(66) Increased circulating and intratumoral CTLA-4+ Treg correlate with worse oncologic outcome in HNSCC patients treated with cetuximab.(66) Of note, overexpression of PD-L1 is observed in a majority of patients with recurrent HNSCC. Seiwart et al. screened 104 patients with recurrent or metastatic HNSCC and identified PD-L1 positivity in 78%.(7) Ferris et al. found PD-L1 expression in 57% of patients with recurrent HNSCC.(67) Taken together, these data indicate that HNSCC recurrence involves hijacking of immunosuppressive pathways in order to evade immune-mediated cell death.(68)
Clinical Trials of Combined Immunomodulation and Cetuximab Therapy
In light of the immunomodulatory capabilities of cetuximab, there are multiple studies are actively investigating the safety and efficacy of cetuximab immunotherapy combinations (see Table 2). Targeting of immune checkpoint pathways (anti-CTLA-4, anti-PD-1, anti-PD-L1) as well as leveraging toll like receptor (TLR) 8 and 9, NKG2A/CD159 on NKCs, and IL-12 are all under investigation. Table 2 shows active, completed, and pending clinical trials of combined therapy of cetuximab plus a dedicated immunomodulating agent. Published results, if available, are included as well.(68–70)
Table 2:
Clinical trials of combined therapy using cetuximab and immunotherapy
| Study | Phase | Eligible Patients | Arms | Mechanism of Immunomodulator |
Enrollment | Main Outcome(s) |
Coordinating Institution |
Sponsor | Status |
|---|---|---|---|---|---|---|---|---|---|
| NCT01040832 | 2 | R/M HNSCC failing 1st line cytotoxic therapy | Cetuximab + EMD 1201081 Cetuximab alone | TLR-9 agonist | 107 (actual) | PFS | Multiple | EMD Serono | Completed. Ruzsa et al. |
| NCT01334177 | 1 | R/M HNSCC failing platinum or incurable with surgery or RT | Cetuximab + VTX-2337 | TLR-8 agonist | 13 (actual) | DLT, characterization of immunologic response | Fred Hutchinson Cancer Research Center/University of Washington Cancer Consortium | University of Washington | Completed. Dietsch et al. |
| NCT01360827 | 1 | R/M HNSCC not curable locally and not yet treated with systemic therapy or RT | EMD 1201081 + 5-FU + Cisplatin + Cetuximab | TLR-9 agonist | 13 (actual) | MTD, ORR | Clinical Research Unit and Pharmacology Lab EA 3035 Institut Claudius Regaud, Toulouse, France | Merck | Terminated due to safety concerns in combination with platinum-based therapy |
| NCT01468896 | 1, 2 | Unresectable R/M HNSCC | Cetuximab + recombinant IL-12 | IL-12 | 23 (actual) | DLT, ORR | MedStar Georgetown University Hospital | National Cancer Institute | Active. 2/23 DLT events. |
| NCT01836029 | 2 | R/M HNSCC not yet treated with systemic therapy | Cisplatin or carboplatin + 5-FU + cetuximab
+ VTX-2337 Cisplatin or carboplatin + 5-FU + cetuximab + placebo |
TLR-8 agonist | 175 (estimated) | PFS | Multiple | VentiRx Pharmaceuticals | Active |
| NCT01935921 | 1 | Locoregionally advanced HNSCC | Cetuximab + RT + ipilimumab | anti-CTLA4 | 19 (actual) | DLT, ORR | University of Pittsburgh Cancer Institute | National Cancer Institute | Completed. Ferris et al. |
| NCT02110082 | 1 | Advanced/metastatic CRC and incurable HNSCC | Cetuximab + urelumab | anti-CD 137 | 66 (actual) | Toxicities Objective response rate | Multiple | Bristol-Myers Squibb | Completed. Results pending. |
| NCT02124850 | 1 | Resectable primary HNSCC | Surgery + cetuximab + motolimod
Surgery + cetuximab + motolimod + nivolumab |
TLR-8 agonist (motolimod) anti-PD-1 Mab (nivolumab) | 24 (estimated) | Change in immune markers anti-tumor response | University of Pittsburgh Medical Center | VentiRx Pharmaceuticals | Recruiting |
| NCT02633800 | 2 | R/M HNSCC not previously treated with systemic therapy | Cetuximab + platinum +
patritumab Cetuximab + platinum + placebo |
anti-HER3 Mab | 87 (actual) | PFS | Multiple | Daiichi Sankyo, Inc. | Completed. Results submitted. |
| NCT02643550 | 1, 2 | Platinum-resistant R/M HNSCC | Cetuximab + monalizumab | anti-NKG2A Mab | 100 (estimated) | DLT, ORR | University of Pennsylvania | Innate Pharma | Recruiting |
| NCT02764593 | 1 | Locoregionally advanced HNSCC | Nivolumab + cisplatin Nivolumab + high dose cisplatin Nivolumab + cetuximab Nivolumab + IMRT |
anti-PD-1 Mab | 40 (actual) | DLT | Multiple | Radiation Therapy Oncology Group, Bristol-Myers Squibb | Active |
| NCT02938273 | 1 | New diagnosis locally advanced HNSCC | RT + cetuximab + avelumab | anti-PD-L1 Mab | 10 (estimated) | Grade 3-5 toxicity Overall response rate | The Netherlands Cancer Institute | Merck | Recruiting |
| NCT02999087 | 3 | Untreated locoregionally advanced HNSCC | RT + cisplatin RT + cetuximab + avelumab RT + cetuximab |
anti-PD-L1 Mab | 688 (estimated) | PFS | Centre Hospitalier Bretagne Sud, Lorient, France | Groupe Oncologie Radiotherapie Tete et Cou, Merck, Pfizer | Recruiting |
| NCT03051906 | 1, 2 | Locoregionally advanced HNSCC | RT + cetuximab + durvalumab | anti-PD-L1 Mab | 69 (estimated) | PFS | Azienda Ospedaliero-Universitaria Careggi | Azienda Ospedaliero-Universitaria Careggi | Not yet recruiting. Bonomo et al. |
| NCT03082534 | 2 | Incurable platinum-refratory or ineligibile HNSCC | Cetuximab + pembrolizumab | anti-PD-1 Mab | 83 (estimated) | ORR | UC San Diego Moores Cancer Center | Merck Sharp & Dohme Corp. | Recruiting |
| NCT03349710 | 3 | R/M HNSCC not curable locally and not yet treated with systemic therapy or RT | Cetuximab + nivolumab + RT Cetuximab + placebo + RT Nivolumab + cisplatin + RT Placebo + cisplatin + RT |
anti-PD-1 Mab | 1,046 (estimated) | PFS | Multiple | Bristol-Myers Squibb | Recruiting |
| NCT03370276 | 1, 2 | incurable R/M HNSCC | Cetuximab + nivolumab | anti-PD-1 Mab | 52 (estimated) | MTD, 1-year OS | H. Lee Moffitt Cancer Center and Research Institute | H. Lee Moffitt Cancer Center and Research Institute, Bristol-Myers Squibb, Eli Lilly and Company | Active |
| NCT03494322 | 2 | Incurable R/M HNSCC | Cetuximab + avelumab Avelumab alone |
anti-PD-L1 Mab | 130 (estimated) | DLT, ORR | University College, London | Merck | Recruiting |
| NCT03498378 | 1 | Incurable HNSCC | Cetuximab + avelumab + palbociclib | anti-PD-L1 Mab(avelumab) CDK4 and CDK6 inhibitor (palbociclib) | 24 (estimated) | MTD, ORR | UC San Diego Moores Cancer Center | Pfizer | Recruiting |
| NCT01860430 | 1 | Locoregionally advanced HNSCC | Cetuximab + IMRT + ipilimumab | anti-CTLA4 | 18 (estimated) | Dosing, ORR | University of Pittsburgh Cancer Institute | National Cancer Institute, Robert Ferris | Active |
Abbreviations: CRC (colorectal cancer), DLT (dose-limiting toxicities), HNSCC (head and neck squamous cell carcinoma), MTD (maximum tolerated dose), ORR (objective response rate), PFS (progression free survival), R/M (recurrent or metastatic), RT (radiotherapy)
A phase 1 study of motolimod, a toll-like receptor 8 agonist, by Dietsch et al. (NCT01334177) found that NK cells become more responsive to stimulation by NKG2D or FcγRIII following motolimod treatment. Ferris et al. (NCT01935921) reported on motolimod or placebo in combination with EXTREME (platinum, fluorouracil, cetuximab). In 195 patients, median PFS and OS was not significantly improved with motolimod combination (HR 0.99 [1 sided CI 0.00–1.22]; P=0.47 for PFS and HR 0.95 [1 sided CI 0.00–1.22; P=0.40). However, the authors noted significantly better PFS (7.8 vs 5.9 months; HR, 0.58; 1-sided 90% CI, 0.00–0.90; P = .046) and OS (15.2 vs 12.6 months; HR, 0.41; 1-sided 90% CI, 0.00–0.77; P = .03) in HPV-positive participants, and that patients with injection site reactions had longer PFS and OS (median PFS, 7.1 vs 5.9 months; HR, 0.69; 1-sided 90% CI, 0.00–0.93; P = .06; and median OS, 18.7 vs 12.6; HR, 0.56; 1-sided 90% CI, 0.00–0.81; P = .02), suggesting an immunological basis for these results.
A multi-institutional phase 2 study of pembrolizumab combined with cetuximab for treatment of recurrent/metastatic HNSCC is underway (NCT03082534). Eight-three patients are to be enrolled into one of four treatment arms: 1) PD-1/PD-L1 inhibitor-naïve and cetuximab-naïve patients treated with pembrolizumab + cetuximab; 2) PD-1/PD-L1 inhibitor-refractory and cetuximab-naïve patients treated with pembrolizumab + cetuximab; 3) PD-1/PD-L1 inhibitor-refractory and cetuximab-refractory patients treated with pembrolizumab + cetuximab; 4) Cutaneous HNSCC treated with pembrolizumab + cetuximab. Pembrolizumab (200 mg) is to be given every 3 weeks. Cetuximab (400 mg/m2) is to be given weekly. The main outcome measure will be overall response rate in six months from time of study enrollment.
Multiple other additional studies are active including: a multi-institutional phase 1 study of untreated, loco-regionally advanced HNSCC patients (NCT02764593) that will examine the safety of adding nivolumab to cisplatin, cetuximab, or radiation alone; a phase 2 randomized study which will examine biweekly avelumab alone vs. alternating biweekly avelumab plus biweekly cetuximab combination therapy (NCT03494322); and a study of nivolumab plus cetuximab combination therapy which will occur in 2 phases and seeks to enroll 52 patients with recurrent and/or metastatic HNSCC (NCT03370276).
Currently, over twenty clinical trials are underway or planned that will investigate cetuximab plus immunotherapies. Cetuximab already has established activity in HNSCC in combination with chemotherapy and radiation therapy. Given that it is a monoclonal antibody with intrinsic ability to recruit innate and adaptive immunity, cetuximab represents one of the best currently available targeted drugs to combine with immunotherapies and conventional therapies to modulate the tumor microenvironment in HNSCC.
4. Immunomodulation in HNSCC by mTOR and Metformin
Recent deep sequencing approaches, including a landmark study from The Cancer Genome Atlas (TCGA) Network (71), have recently revolutionized our understating of the HNSCC mutational landscape. We learned that HNSCC lesions harbor hundreds of genomic alterations, but surprisingly, the majority of them fall within a limited number molecular pathways whose dysregulation contribute to HNSCC initiation and progression (71,72). These include mutations resulting in persistent mitogenic signaling resulting in aberrant activation of the PI3K, MAPK and JAK/STAT pathways (73). Among them, the PI3K-mTOR pathway is mutated in the highest percentage of the cases, with multiple alterations converging in the activation of PI3K/AKT/mTOR pathway in most HNSCC lesions (72). This, and extensive experimental studies in mouse models provided a rationale for multiple efforts aimed at blocking mTOR for HNSCC treatment in the clinic (reviewed in (74)). mTOR is the target of immunosuppressive therapies, such as rapamycin (sirolimus), which has been used to prevent rejections in renal transplant patients for decades, most often together with cyclosporine and corticosteroids (75). Surprisingly, however, multiple trials using single-agent rapamycin and its analogs, referred to as rapalogs, have shown no evidence of increased immunosuppression in cancer patients (76–78). Paradoxically, mTOR inhibition with rapamycin has been recently shown to increase the immune responses in the clinic, and to potentiate the activity of Immuno-Oncology (IO) agents in cancer models (79–87). Thus, it is possible that mTOR blockade may increase rather than negate the anti-tumor activity of IO agents.
Multiple mechanisms can contribute to a potential beneficial effect of combining mTOR blockers with immune checkpoint inhibitors. mTOR inhibition in HNSCC can promote apoptotic tumor cell killing (88), which can expose multiple antigens thereby increasing cancer immunity. mTOR inhibition can also affect T cell differentiation programs, increasing the development of long-lived tumor specific memory T cells (89). Experimental studies in HNSCC suggest that simultaneous mTOR and PD-L1 inhibition reduces the tumor burden by increasing IFN-γ production in tumor-infiltrating CD8 T cells (87). On the other hand, the expression of immune suppressive cytokines secreted by Tregs and MDSCs, such as IL-10 and TGF-β, can be decreased by mTOR blockade (90–93), which can help to overcome cancer immune evasion. Thus, although counterintuitive, the use of mTOR inhibitors to suppress a key HNSCC driver pathway could be optimized to concomitantly enhance the anti-tumor immune response when combined with IO agents as a novel precision immune therapeutic strategy for HNSCC patients.
Due to the critical role of the PI3K-mTOR pathway in HNSCC initiation and progression, our team explored the possibility of targeting this signaling circuit for HNSCC prevention in patients with oral premalignant lesions (OPL). These efforts led to the discovery that metformin, the most widely used anti-diabetic agent, can potently block mTOR in OPL and halt their progression to HNSCC in experimental systems (94,95). Remarkably, two recent large retrospective population case-control cohort studies involving together more than 300,000 diabetic patients demonstrated a decreased HNSCC risk in patients on metformin (96,97). Based on these preclinical and epidemiological evidence, metformin is now under investigation for HNSCC prevention (NCT02581137). Of interest, recent findings also support that metformin can regulate proinflammatory cancer-promoting pathways in the tumor microenvironment. In pancreatic ductal adenocarcinoma (PDAC), metformin was shown to reduce the levels of tumor extracellular matrix (ECM) in overweight diabetic PDAC patients, which was recapitulated the exposure of pancreatic stellate cells (PSCs) to metformin in vitro (98). Furthermore, metformin exerts an anti-inflammatory activity by reducing the expression of inflammatory cytokines, including IL-1β, and by diminishing the polarization of macrophages to pro-tumorigenic M2 tumor associated macrophages (TAMs) in vivo and in vitro (98). Thus, by restricting the negative immune modulating role of M2-macrophages metformin may disrupt the establishment of an immune evasive pre-malignant microenvironment, thereby halting cancer progression.
In addition to this anti-inflammatory role, it was recently shown that metformin increases the number of CD8+ TILs, and that metformin can protect anti-tumoral CD8+ cytotoxic T cells from functional exhaustion in the tumor microenvironment (99). Remarkably, these resulted in increased cancer vaccine effectiveness by improving CD8+ TIL multifunctionality in response to metformin treatment (99).
Overall, the emerging data support that metformin may limit cancer progression at least in part by increasing the antitumor immune response by 1) preventing the M2 polarization of TAMs, 2) the secretion of pro-inflammatory and immune suppressive cytokines, 3) increasing cytotoxic CD8+ T cell function, and 4) preventing T cell exhaustion in the tumor microenvironment. This raises the exciting possibility of repurposing metformin, which is safely used by millions of type 2 diabetes patients, to boost the activity of immune checkpoint inhibitors (100).
5. Immunomodulatory Effects of Other Targeted Therapies
Bevacizumab, a monoclonal antibody against vascular endothelial growth factor, is FDA approved as a single agent or in combination with chemotherapy in multiple malignancies. There is evidence that VEGF inhibition can increase T-cell migration into tumors(101) and potentially improve efficacy of checkpoint inhibitors. There is also evidence of efficacy of bevacizumab in combination with atezeolizumab in renal cell carcinoma and hepatocellular carcinoma and in combination with chemotherapy and atezolizumab in non-squamous non-small cell lung cancer.(102–104) Concerns regarding the risk of hemorrhage with VEGF inhibition may limit the use of bevacizumab combinations in HNSCC, though there is an ongoing phase II trial enrolling patients with HPV or EBV associated HNSCC (NCT03074513).
There is also emerging evidence that cell cycle inhibition may be synergistic with checkpoint inhibitors. CDK4/6 inhibitors abemaciclib and palbociclib have been shown to increase antigen presentation in breast cancer cell lines, and these agents also appear to reduce regulatory T cells.(105) Based on this data, several trials are ongoing to study the combination of these agents with checkpoint inhibitors, including a phase I study combining PD-L1 inhibitor avelumab with palbociclib and cetuximab in HNSCC (NCT03498378).
In summary, the importance of the immune system in HNSCC responses to treatment and patient outcomes is now at the forefront. The approval and activity of checkpoint blockade immunotherapy in HNSCC was a pivotal event which opened entirely new opportunities and avenues for basic, translational, and clinical research. However, objective response rates to checkpoint blockade remain quite low and there is a tremendous amount of work and further investigation needed to better understand the role of the immune system in HNSCC. Here we highlighted some of the ways by which conventional therapies including chemotherapy, radiation, and cetuximab can modulate the immune system and tumor microenvironment in HNSCC. The incorporation of this knowledge and additional data from basic research, translational science, and ongoing clinical trials will hopefully elucidate mechanisms of action and the combinatorial strategies needed to improve outcomes for HNSCC patients in the era of immunotherapy.
Acknowledgements
This work was supported in part by National Institute of Health (1KL2TR001444) supporting AS.
Footnotes
Conflicts of Interest Statement:
KG reports research funding from Pharmacyclics, Molecular Partners, Pfizer, BerGenBio, Abbvie, and AstraZeneca, and consultant fees from AstraZeneca, Takeda, and Regeneron. J.S.G reports research funding from Kura Oncology and Mavupharma, and consultant fees from Oncoceutics Inc and Vividion Therapeutics. LM reports research funding from Merck and Astrazeneca and consulting fees and honoraria from Merck, Pfizer, and Varian Medical Systems. EC reports research funding from Pfizer, Merck, AstraZeneca, and Bristol-Myers Squibb outside the submitted work. AS reports research funding and honoraria from Pfizer and Varian Medical Systems, consultant fees from Astrazeneca, and other fees from Raysearch and Merck.
References
- 1.Global Burden of Disease Cancer C, Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 2017;3(4):524–48 doi 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67(1):7–30 doi 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 3.Weinberger PM, Yu Z, Haffty BG, Kowalski D, Harigopal M, Brandsma J, et al. Molecular classification identifies a subset of human papillomavirus--associated oropharyngeal cancers with favorable prognosis. J Clin Oncol 2006;24(5):736–47 doi 10.1200/JCO.2004.00.3335. [DOI] [PubMed] [Google Scholar]
- 4.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 2016;66(4):271–89 doi 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 5.Pulte D, Brenner H. Changes in survival in head and neck cancers in the late 20th and early 21st century: a period analysis. Oncologist 2010;15(9):994–1001 doi 10.1634/theoncologist.2009-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferris RL, Blumenschein G Jr., Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. The New England journal of medicine 2016. doi 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seiwert TY, Burtness B, Mehra R, Weiss J, Berger R, Eder JP, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol 2016;17(7):956–65 doi 10.1016/S1470-2045(16)30066-3. [DOI] [PubMed] [Google Scholar]
- 8. http://www.ascopost.com/News/59121.
- 9.Chang CL, Hsu YT, Wu CC, Lai YZ, Wang C, Yang YC, et al. Dose-dense chemotherapy improves mechanisms of antitumor immune response. Cancer Res 2013;73(1):119–27 doi 10.1158/0008-5472.CAN-12-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lesterhuis WJ, Punt CJ, Hato SV, Eleveld-Trancikova D, Jansen BJ, Nierkens S, et al. Platinum-based drugs disrupt STAT6-mediated suppression of immune responses against cancer in humans and mice. J Clin Invest 2011;121(8):3100–8 doi 10.1172/JCI43656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shurin GV, Tourkova IL, Kaneno R, Shurin MR. Chemotherapeutic agents in noncytotoxic concentrations increase antigen presentation by dendritic cells via an IL-12-dependent mechanism. J Immunol 2009;183(1):137–44 doi 10.4049/jimmunol.0900734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramakrishnan R, Assudani D, Nagaraj S, Hunter T, Cho HI, Antonia S, et al. Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice. J Clin Invest 2010;120(4):1111–24 doi 10.1172/JCI40269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tesniere A, Schlemmer F, Boige V, Kepp O, Martins I, Ghiringhelli F, et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene 2010;29(4):482–91 doi 10.1038/onc.2009.356. [DOI] [PubMed] [Google Scholar]
- 14.Zitvogel L, Kepp O, Senovilla L, Menger L, Chaput N, Kroemer G. Immunogenic tumor cell death for optimal anticancer therapy: the calreticulin exposure pathway. Clin Cancer Res 2010;16(12):3100–4 doi 10.1158/1078-0432.CCR-09-2891. [DOI] [PubMed] [Google Scholar]
- 15.Hato SV, Khong A, de Vries IJ, Lesterhuis WJ. Molecular pathways: the immunogenic effects of platinum-based chemotherapeutics. Clin Cancer Res 2014;20(11):2831–7 doi 10.1158/1078-0432.CCR-13-3141. [DOI] [PubMed] [Google Scholar]
- 16.Li JY, Duan XF, Wang LP, Xu YJ, Huang L, Zhang TF, et al. Selective depletion of regulatory T cell subsets by docetaxel treatment in patients with nonsmall cell lung cancer. J Immunol Res 2014;2014:286170 doi 10.1155/2014/286170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang X, Cui S, Shu Y. Cisplatin selectively downregulated the frequency and immunoinhibitory function of myeloid-derived suppressor cells in a murine B16 melanoma model. Immunol Res 2016;64(1):160–70 doi 10.1007/s12026-015-8734-1. [DOI] [PubMed] [Google Scholar]
- 18.Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res 2010;70(8):3052–61 doi 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 19.Kodumudi KN, Woan K, Gilvary DL, Sahakian E, Wei S, Djeu JY. A novel chemoimmunomodulating property of docetaxel: suppression of myeloid-derived suppressor cells in tumor bearers. Clin Cancer Res 2010;16(18):4583–94 doi 10.1158/1078-0432.CCR-10-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feldman R, Gatalica Z, Knezetic J, Reddy S, Nathan CA, Javadi N, et al. Molecular profiling of head and neck squamous cell carcinoma. Head Neck 2016;38 Suppl 1:E1625–38 doi 10.1002/hed.24290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rizvi NA, Hellmann MD, Brahmer JR, Juergens RA, Borghaei H, Gettinger S, et al. Nivolumab in Combination With Platinum-Based Doublet Chemotherapy for First-Line Treatment of Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34(25):2969–79 doi 10.1200/JCO.2016.66.9861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016;17(11):1497–508 doi 10.1016/S1470-2045(16)30498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378(22):2078–92 doi 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 24.Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gumus M, Mazieres J, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med 2018;379(21):2040–51 doi 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 25.Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, De Castro JG, et al. LBA8_PRKEYNOTE-048: Phase III study of first-line pembrolizumab (P) for recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC). Annals of Oncology 2018;29(suppl_8):mdy424.045–mdy424.045 doi 10.1093/annonc/mdy424.045. [DOI] [Google Scholar]
- 26.Chakraborty M, Abrams SI, Camphausen K, Liu K, Scott T, Coleman CN, et al. Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J Immunol 2003;170(12):6338–47. [DOI] [PubMed] [Google Scholar]
- 27.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med 2007;13(9):1050–9 doi 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 28.Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med 2006;203(5):1259–71 doi 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verbrugge I, Gasparini A, Haynes NM, Hagekyriakou J, Galli M, Stewart TJ, et al. The curative outcome of radioimmunotherapy in a mouse breast cancer model relies on mTOR signaling. Radiat Res 2014;182(2):219–29 doi 10.1667/RR13511.1. [DOI] [PubMed] [Google Scholar]
- 30.Wan S, Pestka S, Jubin RG, Lyu YL, Tsai YC, Liu LF. Chemotherapeutics and radiation stimulate MHC class I expression through elevated interferon-beta signaling in breast cancer cells. PLoS One 2012;7(3):e32542 doi 10.1371/journal.pone.0032542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta A, Probst HC, Vuong V, Landshammer A, Muth S, Yagita H, et al. Radiotherapy promotes tumor-specific effector CD8+ T cells via dendritic cell activation. J Immunol 2012;189(2):558–66 doi 10.4049/jimmunol.1200563. [DOI] [PubMed] [Google Scholar]
- 32.Hallahan D, Kuchibhotla J, Wyble C. Cell adhesion molecules mediate radiation-induced leukocyte adhesion to the vascular endothelium. Cancer Res 1996;56(22):5150–5. [PubMed] [Google Scholar]
- 33.Klug F, Prakash H, Huber PE, Seibel T, Bender N, Halama N, et al. Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell 2013;24(5):589–602 doi 10.1016/j.ccr.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 34.Mondini M, Nizard M, Tran T, Mauge L, Loi M, Clemenson C, et al. Synergy of Radiotherapy and a Cancer Vaccine for the Treatment of HPV-Associated Head and Neck Cancer. Mol Cancer Ther 2015;14(6):1336–45 doi 10.1158/1535-7163.MCT-14-1015. [DOI] [PubMed] [Google Scholar]
- 35.Matsumura S, Wang B, Kawashima N, Braunstein S, Badura M, Cameron TO, et al. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol 2008;181(5):3099–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoon MS, Pham CT, Phan MT, Shin DJ, Jang YY, Park MH, et al. Irradiation of breast cancer cells enhances CXCL16 ligand expression and induces the migration of natural killer cells expressing the CXCR6 receptor. Cytotherapy 2016;18(12):1532–42 doi 10.1016/j.jcyt.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 37.Sharabi AB, Nirschl CJ, Kochel CM, Nirschl TR, Francica BJ, Velarde E, et al. Stereotactic Radiation Therapy Augments Antigen-Specific PD-1-Mediated Antitumor Immune Responses via Cross-Presentation of Tumor Antigen. Cancer Immunol Res 2015;3(4):345–55 doi 10.1158/2326-6066.CIR-14-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wirsdorfer F, Cappuccini F, Niazman M, de Leve S, Westendorf AM, Ludemann L, et al. Thorax irradiation triggers a local and systemic accumulation of immunosuppressive CD4+ FoxP3+ regulatory T cells. Radiat Oncol 2014;9:98 doi 10.1186/1748-717X-9-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beitler JJ, Zhang Q, Fu KK, Trotti A, Spencer SA, Jones CU, et al. Final results of local-regional control and late toxicity of RTOG 9003: a randomized trial of altered fractionation radiation for locally advanced head and neck cancer. Int J Radiat Oncol Biol Phys 2014;89(1):13–20 doi 10.1016/j.ijrobp.2013.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bourhis J, Overgaard J, Audry H, Ang KK, Saunders M, Bernier J, et al. Hyperfractionated or accelerated radiotherapy in head and neck cancer: a meta-analysis. Lancet 2006;368(9538):843–54 doi 10.1016/S0140-6736(06)69121-6. [DOI] [PubMed] [Google Scholar]
- 41.Lacas B, Bourhis J, Overgaard J, Zhang Q, Gregoire V, Nankivell M, et al. Role of radiotherapy fractionation in head and neck cancers (MARCH): an updated meta-analysis. Lancet Oncol 2017;18(9):1221–37 doi 10.1016/S1470-2045(17)30458-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 2014;124(2):687–95 doi 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oweida A, Lennon S, Calame D, Korpela S, Bhatia S, Sharma J, et al. Ionizing radiation sensitizes tumors to PD-L1 immune checkpoint blockade in orthotopic murine head and neck squamous cell carcinoma. Oncoimmunology 2017;6(10):e1356153 doi 10.1080/2162402X.2017.1356153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gillison M, Ferris RL, Zhang Q, Colevas AD, Mell LK, Kirsch C, et al. Safety Evaluation of Nivolumab Concomitant With Platinum-Based Chemoradiation therapy for Intermediate and High-Risk Local-Regionally Advanced Head and Neck Squamous Cell Carcinoma: RTOG Foundation 3504. International Journal of Radiation Oncology • Biology • Physics 2018;100(5):1307–8 doi 10.1016/j.ijrobp.2017.12.022. [DOI] [Google Scholar]
- 45.Kim YS. Reirradiation of head and neck cancer in the era of intensity-modulated radiotherapy: patient selection, practical aspects, and current evidence. Radiat Oncol J 2017;35(1):1–15 doi 10.3857/roj.2017.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spanos WC, Nowicki P, Lee DW, Hoover A, Hostager B, Gupta A, et al. Immune response during therapy with cisplatin or radiation for human papillomavirus-related head and neck cancer. Arch Otolaryngol Head Neck Surg 2009;135(11):1137–46 doi 10.1001/archoto.2009.159. [DOI] [PubMed] [Google Scholar]
- 47.Ow TJ, Pitts CE, Kabarriti R, Garg MK. Effective Biomarkers and Radiation Treatment in Head and Neck Cancer. Arch Pathol Lab Med 2015;139(11):1379–88 doi 10.5858/arpa.2014-0574-RA. [DOI] [PubMed] [Google Scholar]
- 48.Gleber-Netto FO, Rao X, Guo T, Xi Y, Gao M, Shen L, et al. Variations in HPV function are associated with survival in squamous cell carcinoma. JCI Insight 2019;4(1) doi 10.1172/jci.insight.124762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Partlova S, Boucek J, Kloudova K, Lukesova E, Zabrodsky M, Grega M, et al. Distinct patterns of intratumoral immune cell infiltrates in patients with HPV-associated compared to non-virally induced head and neck squamous cell carcinoma. Oncoimmunology 2015;4(1):e965570 doi 10.4161/21624011.2014.965570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wood O, Woo J, Seumois G, Savelyeva N, McCann KJ, Singh D, et al. Gene expression analysis of TIL rich HPV-driven head and neck tumors reveals a distinct B-cell signature when compared to HPV independent tumors. Oncotarget 2016;7(35):56781–97 doi 10.18632/oncotarget.10788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nordsmark M, Bentzen SM, Rudat V, Brizel D, Lartigau E, Stadler P, et al. Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiother Oncol 2005;77(1):18–24 doi 10.1016/j.radonc.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 52.Bristow RG, Hill RP. Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat Rev Cancer 2008;8(3):180–92 doi 10.1038/nrc2344. [DOI] [PubMed] [Google Scholar]
- 53.Sorensen BS, Busk M, Olthof N, Speel EJ, Horsman MR, Alsner J, et al. Radiosensitivity and effect of hypoxia in HPV positive head and neck cancer cells. Radiother Oncol 2013;108(3):500–5 doi 10.1016/j.radonc.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 54.Lassen P, Eriksen JG, Hamilton-Dutoit S, Tramm T, Alsner J, Overgaard J, et al. HPV-associated p16-expression and response to hypoxic modification of radiotherapy in head and neck cancer. Radiother Oncol 2010;94(1):30–5 doi 10.1016/j.radonc.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 55.Toustrup K, Sorensen BS, Lassen P, Wiuf C, Alsner J, Overgaard J, et al. Gene expression classifier predicts for hypoxic modification of radiotherapy with nimorazole in squamous cell carcinomas of the head and neck. Radiother Oncol 2012;102(1):122–9 doi 10.1016/j.radonc.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 56.Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 2006;354(6):567–78 doi 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 57.Ferris RL, Lenz HJ, Trotta AM, Garcia-Foncillas J, Schulten J, Audhuy F, et al. Rationale for combination of therapeutic antibodies targeting tumor cells and immune checkpoint receptors: Harnessing innate and adaptive immunity through IgG1 isotype immune effector stimulation. Cancer Treat Rev 2018;63:48–60 doi 10.1016/j.ctrv.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu S, Concha-Benavente F, Shayan G, Srivastava RM, Gibson SP, Wang L, et al. STING activation enhances cetuximab-mediated NK cell activation and DC maturation and correlates with HPV(+) status in head and neck cancer. Oral Oncol 2018;78:186–93 doi 10.1016/j.oraloncology.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Srivastava RM, Lee SC, Andrade Filho PA, Lord CA, Jie HB, Davidson HC, et al. Cetuximab-activated natural killer and dendritic cells collaborate to trigger tumor antigen-specific T-cell immunity in head and neck cancer patients. Clin Cancer Res 2013;19(7):1858–72 doi 10.1158/1078-0432.CCR-12-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Srivastava RM, Trivedi S, Concha-Benavente F, Gibson SP, Reeder C, Ferrone S, et al. CD137 Stimulation Enhances Cetuximab-Induced Natural Killer: Dendritic Cell Priming of Antitumor T-Cell Immunity in Patients with Head and Neck Cancer. Clin Cancer Res 2017;23(3):707–16 doi 10.1158/1078-0432.CCR-16-0879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hsu YF, Ajona D, Corrales L, Lopez-Picazo JM, Gurpide A, Montuenga LM, et al. Complement activation mediates cetuximab inhibition of non-small cell lung cancer tumor growth in vivo. Mol Cancer 2010;9:139 doi 10.1186/1476-4598-9-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Holubec L, Polivka J Jr., Safanda M, Karas M, Liska V. The Role of Cetuximab in the Induction of Anticancer Immune Response in Colorectal Cancer Treatment. Anticancer Res 2016;36(9):4421–6 doi 10.21873/anticanres.10985. [DOI] [PubMed] [Google Scholar]
- 63.Lattanzio L, Denaro N, Vivenza D, Varamo C, Strola G, Fortunato M, et al. Elevated basal antibody-dependent cell-mediated cytotoxicity (ADCC) and high epidermal growth factor receptor (EGFR) expression predict favourable outcome in patients with locally advanced head and neck cancer treated with cetuximab and radiotherapy. Cancer Immunol Immunother 2017;66(5):573–9 doi 10.1007/s00262-017-1960-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gillison ML, Trotti AM, Harris J, Eisbruch A, Harari PM, Adelstein DJ, et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet 2018. doi 10.1016/S0140-6736(18)32779-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jie HB, Srivastava RM, Argiris A, Bauman JE, Kane LP, Ferris RL. Increased PD-1(+) and TIM-3(+) TILs during Cetuximab Therapy Inversely Correlate with Response in Head and Neck Cancer Patients. Cancer Immunol Res 2017;5(5):408–16 doi 10.1158/2326-6066.CIR-16-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jie HB, Schuler PJ, Lee SC, Srivastava RM, Argiris A, Ferrone S, et al. CTLA-4(+) Regulatory T Cells Increased in Cetuximab-Treated Head and Neck Cancer Patients Suppress NK Cell Cytotoxicity and Correlate with Poor Prognosis. Cancer Res 2015;75(11):2200–10 doi 10.1158/0008-5472.CAN-14-2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ferris RL, Blumenschein G Jr., Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med 2016;375(19):1856–67 doi 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bonomo P, Desideri I, Loi M, Mangoni M, Sottili M, Marrazzo L, et al. Anti PD-L1 DUrvalumab combined with Cetuximab and RadiOtherapy in locally advanced squamous cell carcinoma of the head and neck: A phase I/II study (DUCRO). Clin Transl Radiat Oncol 2018;9:42–7 doi 10.1016/j.ctro.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ruzsa A, Sen M, Evans M, Lee LW, Hideghety K, Rottey S, et al. Phase 2, open-label, 1:1 randomized controlled trial exploring the efficacy of EMD 1201081 in combination with cetuximab in second-line cetuximab-naive patients with recurrent or metastatic squamous cell carcinoma of the head and neck (R/M SCCHN). Invest New Drugs 2014;32(6):1278–84 doi 10.1007/s10637-014-0117-2. [DOI] [PubMed] [Google Scholar]
- 70.Dietsch GN, Lu H, Yang Y, Morishima C, Chow LQ, Disis ML, et al. Coordinated Activation of Toll-Like Receptor8 (TLR8) and NLRP3 by the TLR8 Agonist, VTX-2337, Ignites Tumoricidal Natural Killer Cell Activity. PLoS One 2016;11(2):e0148764 doi 10.1371/journal.pone.0148764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cancer Genome Atlas N. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015;517(7536):576–82 doi 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Iglesias-Bartolome R, Martin D, Gutkind JS. Exploiting the head and neck cancer oncogenome: widespread PI3K-mTOR pathway alterations and novel molecular targets. Cancer Discov 2013;3(7):722–5 doi 10.1158/2159-8290.CD-13-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lui VW, Hedberg ML, Li H, Vangara BS, Pendleton K, Zeng Y, et al. Frequent mutation of the PI3K pathway in head and neck cancer defines predictive biomarkers. Cancer Discov 2013;3(7):761–9 doi 10.1158/2159-8290.CD-13-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Z, Valera JC, Zhao X, Chen Q, Silvio Gutkind J. mTOR co-targeting strategies for head and neck cancer therapy. Cancer Metastasis Rev 2017;36(3):491–502 doi 10.1007/s10555-017-9688-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Haydar AA, Denton M, West A, Rees J, Goldsmith DJ. Sirolimus-induced pneumonitis: three cases and a review of the literature. Am J Transplant 2004;4(1):137–9. [DOI] [PubMed] [Google Scholar]
- 76.O’Donnell A, Faivre S, Burris HA 3rd, Rea D, Papadimitrakopoulou V, Shand N, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral mammalian target of rapamycin inhibitor everolimus in patients with advanced solid tumors. J Clin Oncol 2008;26(10):1588–95 doi 10.1200/JCO.2007.14.0988. [DOI] [PubMed] [Google Scholar]
- 77.Bissler JJ, McCormack FX, Young LR, Elwing JM, Chuck G, Leonard JM, et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med 2008;358(2):140–51 doi 10.1056/NEJMoa063564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hahnel PS, Thaler S, Antunes E, Huber C, Theobald M, Schuler M. Targeting AKT signaling sensitizes cancer to cellular immunotherapy. Cancer Res 2008;68(10):3899–906 doi 10.1158/0008-5472.CAN-07-6286. [DOI] [PubMed] [Google Scholar]
- 79.Dao V, Liu Y, Pandeswara S, Svatek RS, Gelfond JA, Liu A, et al. Immune-Stimulatory Effects of Rapamycin Are Mediated by Stimulation of Antitumor gammadelta T Cells. Cancer Res 2016;76(20):5970–82 doi 10.1158/0008-5472.CAN-16-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rao RR, Li Q, Odunsi K, Shrikant PA. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity 2010;32(1):67–78 doi 10.1016/j.immuni.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mannick JB, Del Giudice G, Lattanzi M, Valiante NM, Praestgaard J, Huang B, et al. mTOR inhibition improves immune function in the elderly. Sci Transl Med 2014;6(268):268ra179 doi 10.1126/scitranslmed.3009892. [DOI] [PubMed] [Google Scholar]
- 82.Pollizzi KN, Patel CH, Sun IH, Oh MH, Waickman AT, Wen J, et al. mTORC1 and mTORC2 selectively regulate CD8(+) T cell differentiation. J Clin Invest 2015;125(5):2090–108 doi 10.1172/JCI77746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jiang Q, Weiss JM, Back T, Chan T, Ortaldo JR, Guichard S, et al. mTOR kinase inhibitor AZD8055 enhances the immunotherapeutic activity of an agonist CD40 antibody in cancer treatment. Cancer Res 2011;71(12):4074–84 doi 10.1158/0008-5472.CAN-10-3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang Y, Wang XY, Subjeck JR, Shrikant PA, Kim HL. Temsirolimus, an mTOR inhibitor, enhances anti-tumour effects of heat shock protein cancer vaccines. Br J Cancer 2011;104(4):643–52 doi 10.1038/bjc.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li Q, Rao R, Vazzana J, Goedegebuure P, Odunsi K, Gillanders W, et al. Regulating mammalian target of rapamycin to tune vaccination-induced CD8(+) T cell responses for tumor immunity. J Immunol 2012;188(7):3080–7 doi 10.4049/jimmunol.1103365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lastwika KJ, Wilson W 3rd, Li QK, Norris J, Xu H, Ghazarian SR, et al. Control of PD-L1 Expression by Oncogenic Activation of the AKT-mTOR Pathway in Non-Small Cell Lung Cancer. Cancer Res 2016;76(2):227–38 doi 10.1158/0008-5472.CAN-14-3362. [DOI] [PubMed] [Google Scholar]
- 87.Moore EC, Cash HA, Caruso AM, Uppaluri R, Hodge JW, Van Waes C, et al. Enhanced Tumor Control with Combination mTOR and PD-L1 Inhibition in Syngeneic Oral Cavity Cancers. Cancer Immunol Res 2016;4(7):611–20 doi 10.1158/2326-6066.CIR-15-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dormond O, Madsen JC, Briscoe DM. The effects of mTOR-Akt interactions on anti-apoptotic signaling in vascular endothelial cells. J Biol Chem 2007;282(32):23679–86 doi 10.1074/jbc.M700563200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer 2012;12(4):237–51 doi 10.1038/nrc3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Weichhart T, Costantino G, Poglitsch M, Rosner M, Zeyda M, Stuhlmeier KM, et al. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity 2008;29(4):565–77 doi 10.1016/j.immuni.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 91.Song K, Wang H, Krebs TL, Danielpour D. Novel roles of Akt and mTOR in suppressing TGF-beta/ALK5-mediated Smad3 activation. EMBO J 2006;25(1):58–69 doi 10.1038/sj.emboj.7600917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang YE. Non-Smad pathways in TGF-beta signaling. Cell Res 2009;19(1):128–39 doi 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang YE. Non-Smad Signaling Pathways of the TGF-beta Family. Cold Spring Harb Perspect Biol 2017;9(2) doi 10.1101/cshperspect.a022129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vitale-Cross L, Molinolo AA, Martin D, Younis RH, Maruyama T, Patel V, et al. Metformin prevents the development of oral squamous cell carcinomas from carcinogen-induced premalignant lesions. Cancer Prev Res (Phila) 2012;5(4):562–73 doi 10.1158/1940-6207.CAPR-11-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Madera D, Vitale-Cross L, Martin D, Schneider A, Molinolo AA, Gangane N, et al. Prevention of tumor growth driven by PIK3CA and HPV oncogenes by targeting mTOR signaling with metformin in oral squamous carcinomas expressing OCT3. Cancer Prev Res (Phila) 2015;8(3):197–207 doi 10.1158/1940-6207.CAPR-14-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tseng CH. Metformin may reduce oral cancer risk in patients with type 2 diabetes. Oncotarget 2016;7(2):2000–8 doi 10.18632/oncotarget.6626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yen YC, Lin C, Lin SW, Lin YS, Weng SF. Effect of metformin on the incidence of head and neck cancer in diabetics. Head Neck 2015;37(9):1268–73 doi 10.1002/hed.23743. [DOI] [PubMed] [Google Scholar]
- 98.Incio J, Suboj P, Chin SM, Vardam-Kaur T, Liu H, Hato T, et al. Metformin Reduces Desmoplasia in Pancreatic Cancer by Reprogramming Stellate Cells and Tumor-Associated Macrophages. PLoS One 2015;10(12):e0141392 doi 10.1371/journal.pone.0141392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Eikawa S, Nishida M, Mizukami S, Yamazaki C, Nakayama E, Udono H. Immune-mediated antitumor effect by type 2 diabetes drug, metformin. Proc Natl Acad Sci U S A 2015;112(6):1809–14 doi 10.1073/pnas.1417636112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gutkind JS, Bui JD. The Next Frontier: Head and Neck Cancer Immunoprevention. Cancer Prev Res (Phila) 2017;10(12):681–3 doi 10.1158/1940-6207.CAPR-17-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wallin JJ, Bendell JC, Funke R, Sznol M, Korski K, Jones S, et al. Atezolizumab in combination with bevacizumab enhances antigen-specific T-cell migration in metastatic renal cell carcinoma. Nat Commun 2016;7:12624 doi 10.1038/ncomms12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Motzer RJ, Powles T, Atkins MB, Escudier B, McDermott DF, Suarez C, et al. IMmotion151: A Randomized Phase III Study of Atezolizumab Plus Bevacizumab vs Sunitinib in Untreated Metastatic Renal Cell Carcinoma (mRCC). Journal of Clinical Oncology 2018;36(6_suppl):578 doi 10.1200/JCO.2018.36.6_suppl.578. [DOI] [Google Scholar]
- 103.Stein S, Pishvaian MJ, Lee MS, Lee K-H, Hernandez S, Kwan A, et al. Safety and clinical activity of 1L atezolizumab + bevacizumab in a phase Ib study in hepatocellular carcinoma (HCC). Journal of Clinical Oncology 2018;36(15_suppl):4074 doi 10.1200/JCO.2018.36.15_suppl.4074. [DOI] [Google Scholar]
- 104.Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 2018;378(24):2288–301 doi 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 105.Goel S, DeCristo MJ, Watt AC, BrinJones H, Sceneay J, Li BB, et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature 2017;548(7668):471–5 doi 10.1038/nature23465. [DOI] [PMC free article] [PubMed] [Google Scholar]