1. Introduction
Glioblastoma multiforme (GBM) is the most common and aggressive primary brain tumor in adults. Over the last two decades many new multimodal therapeutic have been developed but the poor prognosis has remained largely unchanged. Recent research shows that immunosuppression plays a major role in GBM progression. While additional studies are needed to fully understand the immunosuppressive mechanisms that permit gliomas to evade immune surveillance, some key molecules have been identified that may hold therapeutic promise. We have investigated Fibrinogen-like Protein 2. The secreted form of this member of the fibrinogen family has unique immunosuppressive functions. Expression of FGL2 in the adult is primarily in T cells, endothelial and tumor cells. Its unique localization to these tissues gives it a prominent role in the microenvironment. In glioblastoma, it appears to facilitate immunosuppression which results in tumor progression.
2. Body
Recently, our laboratories have shown that gliomas that progress from low- to high-grade up regulate FGL2 expression. Additionally, RNA expression data from The Cancer Genome Atlas (TCGA) shows that low-grade gliomas (LGG) with high expression of FGL2 have significantly poorer overall survival relative to those with lower expression of FGL2 [1]**. Analysis of samples from GBM patients showed that low levels of FGL2 expression with concurrent high granulocyte-macrophage colony-stimulating factor (GM-CSF) expression, a known activator of dendritic cells (DC), is associated with higher cytotoxic T cell infiltration (CD8B expression) and longer survival [2].
In a murine glioma model, we showed that FGL2 overexpression increased CD4+ forkhead box P3 (FoxP3) Regulatory T cell (Treg), suggesting that immunosuppression may be the mechanism in FGL2 tumor progression [1]**. Furthermore, macrophages from these tumors were found to be skewed toward the immunosuppressive M2 phenotype. Additional studies have shown that exposure of immature DCs to soluble FGL2 inhibited the expression of CD80 and MHC class II molecules and markedly inhibited NF-κB nuclear translocation, thus inhibiting DC maturation and antigen presentation capabilities. Soluble FGL2-treated DC had an impaired ability to stimulate allogeneic T cell proliferation [3]**. Suppression of DC and B cell function by soluble FGL2 is thought to be mediated via its binding to FcγRIIB [1,4]. Studies in which FGL2 has been knocked out in immunocompetent murine models can reverse the immune dysfunction in dendritic cells (DCs) and induce CD103+ DC differentiation in both the brain and in tumor-draining lymph nodes [2]. Collectively, this data suggests that FGL2 exerts its immunosuppressive role through multiple mechanisms of tumor-mediated immune suppression. These properties make FGL2 an intriguing molecule to study for its potential as a therapeutic target including as an immune modulatory agent.
Most therapeutic approaches for GBM have focused on various combinations of surgery, radiation, chemotherapy. Recently immune checkpoint inhibition has demonstrated efficacy for the treatment of brain metastases [5]. However, recent trials with immune checkpoint inhibitors have largely been met with disappointment in the treatment of GBM [6]. Programmed cell death protein 1 (PD-1) immune checkpoint inhibitors exemplify the failure of this strategy in general for the treatment of GBM. More specifically, a phase III clinical trial along with single institutional studies have shown limited responses when compared to current standards of care (CheckMate 143 Study [NCT02017717]) [7,8]. PD-1 is commonly found on the cell surface of T cells and restrains immunological reactivity. PD-1 checkpoint inhibitors aim to block this interaction through the use of antibodies that bind to PD-1 or its ligand PD-L1. These findings raise an obvious yet perplexing question: Why does immunotherapy, a treatment which has revolutionized cancer treatment in some peripheral tumors, fail to exhibit the same efficacy in GBM? One proposed hypothesis for this shortcoming has centered around the dogma that the central nervous system (CNS) is an immune privileged environment. In the recent past, conventional wisdom held that immune responses in the CNS were limited because of the blood-brain-barrier (BBB), low levels of immune cells, and the absence of a conventional lymphatic drainage system [9,10]. These views have largely been refuted with a growing body of evidence indicating that the CNS actively communicates with the immune system [10,11]. Thus, the problem of therapeutic immunomodulation in GBM is not one of therapeutic penetrance, but rather pathway redundancy. While PD-1 checkpoint inhibition is one such biological mechanism through which GBM suppresses the immune system, other such mechanisms exist and may predominate like FGL2.
Unlike PD-1 and CTLA-4, which suppress T cell activation in response to inflammatory cues, FGL2 appears to target antigen presenting cells (APCs) primarily (Figure 1). Certain subtypes of APCs such as CD103+ DCs have been shown to play a crucial role in the priming CD8+ T cells via cross presentation of tumor cell-associated antigens [12]. Additionally, activated CD103+ DCs have been shown to play a vital role in the generation of memory CD8+ T-cells through the production of Th1-differentiating cytokines such as IL-12 [13]. Overexpression of FGL2 by glioma cells inhibits proper maturation of CD103+ cells and therefore attenuates the immune response in the tumor microenvironment [2]. FGL2 has also been shown to play a crucial role in the modulation of myeloid derived suppressor cells (MDSCs), which are known to be present in the glioma microenvironment and also play critical roles in immunosuppression through their inhibition of T cell proliferation [14]. Additionally, gliomas have been shown to skew macrophages and other APCs in the tumor microenvironment from the inflammatory (M1) or nascent (M0) phenotype towards the immunosuppressive (M2) phenotype.12 Overexpression of FGL2 has been shown to skew macrophages towards the M2 phenotype in a mouse glioma model, ultimately leading to diminished T cell priming capabilities.1,2 Our results, in genetically engineered murine glioma model, show that mice treated with anti-FGL2 antibody have reduced levels of CD39(+) Tregs, M2 macrophages, PD-1, and MDSCs.1 Similar effects were achieved through the ablation of the FGL2 receptor FCyRIIB in FCyRIIB −/− mice [15]**. FGL2 blockade with targeting resulted in 100% cures in preclinical murine models of glioblastoma [1]. These studies suggest that the mechanism behind FGL2 mediated immunosuppression may lie both upstream and downstream of PD-1 or CTLA-4 dependent pathways and provides additional insight into why PD-1 checkpoint inhibitors may not have been as efficacious as anticipated. Antigen presentation to T cells via APCs such as CD103+ DCs is a necessary step in T cell activation. FGL2 attenuates the APC maturation potentially mitigating this function. The development of FGL2 inhibitors may play a crucial role in therapeutic targeting of glioma-mediated immunosuppression. Its use in conjunction with checkpoint inhibitors may help to reverse immunosuppression within the tumor microenvironment.
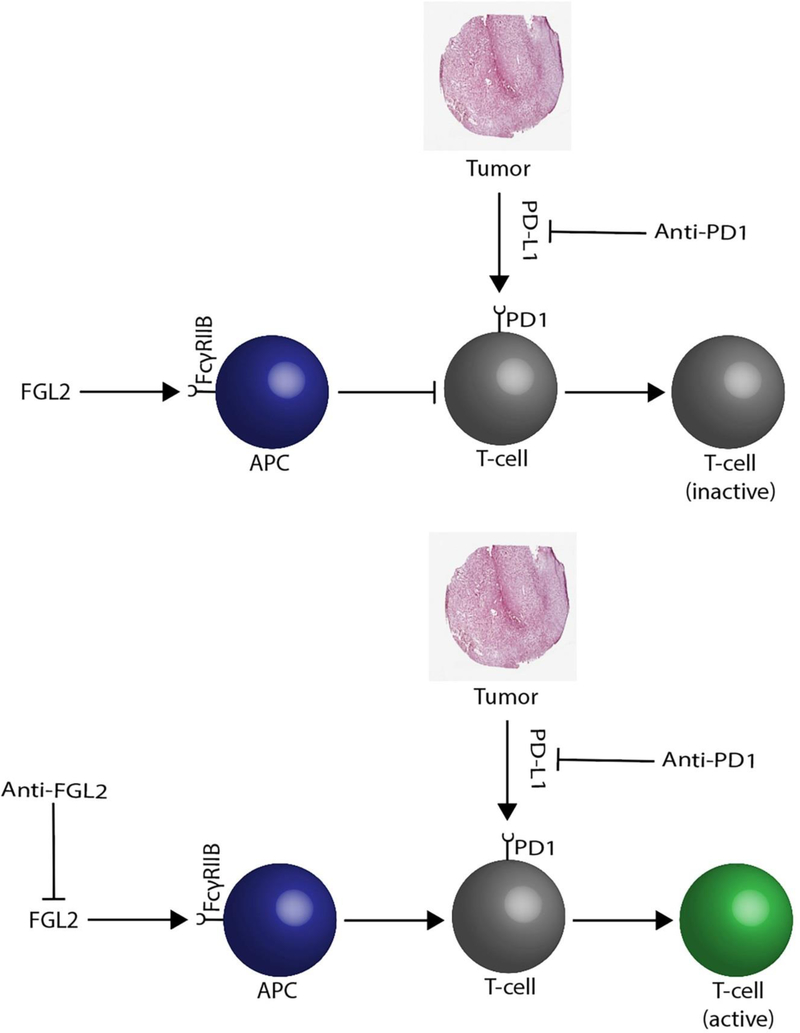
Figure 1:
Schematic of FGL2 function. FGL2 activates the FcγRIIB on antigen presenting cells (APC) which can override PD1 (programmed cell death) blockade, resulting in T-cell inactivation. FcγRIIB blockade will subsequently result in T-cell activation in conjunction with anti-PD1 antibody therapy.
Emerging evidence suggests that glioma-mediated immunosuppression may play a central role in GBM malignancy, allowing tumors to evade immune surveillance. This profound immunosuppression is unique to GBM and has been a key factor in the failure of immune checkpoint inhibition for the treatment of this cancer. As we have learned repeatedly in the treatment of GBM, targeting a single pathway or molecule is insufficient to significantly inhibit tumor growth and progression. Inhibiting multiple immunosuppressive pathways or those that are key nodes for many immune suppressive mechanisms such as FGL2 may provide sufficient immunomodulation to achieve a better therapeutic effect. Preliminary results of FGL2 inhibition in murine models have been promising. While it remains to be seen if anti-FGL2 therapies succumb to the same fate as their predecessors the dual ability of FGL2 inhibition to enhance the function of cell mediated and innate immunity provides a promising opportunity that should be explored.
3. Expert Opinion
Glioblastoma has remained incurable with survival rates that have remained largely unchanged for decades. Immunotherapy has revolutionized cancer care even for patients with disease metastatic to the brain, however, these remarkable results have not been observed in glioblastoma. This may be due to the immunosuppressive tumor microenvironment of glioblastoma which is hostile to various forms of immunotherapy. We have investigated a facilitator of this immunosuppression, Fibrinogen-like protein 2 (FGL2). FGL2 draws Tregs and M2-polarized macrophages into the microenvironment. We have been able to target FGL2 expression in vivo to reverse immunosuppression and improve survival in genetically engineered mouse models of glioma. This strategy of targeting the stroma to inhibit immunosuppression may be the key to unlocking the benefits of immunotherapy, such as checkpoint inhibition, for the treatment of glioblastoma.
Acknowledgments
Funding
The work of the authors is supported grants from U.S. Department of Health and Human Services, National Institutes of Health, National Institute of Neurological Disorders and Stroke (NS094615) and U.S. Department of Health and Human Services, National Institutes of Health (RO1 CA203493)
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers
- 1.Latha K, Yan J, Yang Y, et al. The Role of Fibrinogen-Like Protein 2 on Immunosuppression and Malignant Progression in Glioma. Journal of the National Cancer Institute. 2019;111(3):292–300.**Latha et al. and showed that FGL2 is a key molecule in the progression of low-grade glioma to high-grade glioma.
- 2.Yan J, Zhao Q, Gabrusiewicz K, et al. FGL2 promotes tumor progression in the CNS by suppressing CD103(+) dendritic cell differentiation. Nature communications. 2019;10(1):448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan CW, Kay LS, Khadaroo RG, et al. Soluble fibrinogen-like protein 2/fibroleukin exhibits immunosuppressive properties: suppressing T cell proliferation and inhibiting maturation of bone marrow-derived dendritic cells. Journal of immunology (Baltimore, Md : 1950). 2003;170(8):4036–4044.**Chan et al. reported that FGL2 exerts immunosuppressive effects on the proliferation of T-cells as well as the maturation of dendritic cells.
- 4.Luft O, Khattar R, Farrokhi K, et al. Inhibition of the Fibrinogen-Like Protein 2:FcgammaRIIB/RIII immunosuppressive pathway enhances antiviral T-cell and B-cell responses leading to clearance of lymphocytic choriomeningitis virus clone 13. Immunology. 2018;154(3):476–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tawbi HA, Forsyth PA, Algazi A, et al. Combined Nivolumab and Ipilimumab in Melanoma Metastatic to the Brain. 2018;379(8):722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Preusser M, Lim M, Hafler DA, Reardon DA, Sampson JH. Prospects of immune checkpoint modulators in the treatment of glioblastoma. Nature reviews Neurology. 2015;11(9):504–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurz SC, Cabrera LP, Hastie D, et al. PD-1 inhibition has only limited clinical benefit in patients with recurrent high-grade glioma. Neurology. 2018;91(14):e1355–e1359. [DOI] [PubMed] [Google Scholar]
- 8.Omuro A, Vlahovic G, Lim M, et al. Nivolumab with or without ipilimumab in patients with recurrent glioblastoma: results from exploratory phase I cohorts of CheckMate 143. Neuro-oncology. 2018;20(5):674–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson C, Ruzevick J, Phallen J, Belcaid Z, Lim M. Challenges in immunotherapy presented by the glioblastoma multiforme microenvironment. Clinical & developmental immunology. 2011;2011:732413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heimberger AB, Sampson JH. Immunotherapy coming of age: what will it take to make it standard of care for glioblastoma? Neuro-oncology. 2011;13(1):3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vauleon E, Avril T, Collet B, Mosser J, Quillien V. Overview of cellular immunotherapy for patients with glioblastoma. Clinical & developmental immunology. 2010;2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hussain SF, Yang D, Suki D, Aldape K, Grimm E, Heimberger AB. The role of human glioma-infiltrating microglia/macrophages in mediating antitumor immune responses. Neuro-oncology. 2006;8(3):261–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Broz ML, Binnewies M, Boldajipour B, et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer cell. 2014;26(5):638–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar R, de Mooij T, Peterson TE, et al. Modulating glioma-mediated myeloid-derived suppressor cell development with sulforaphane. PloS one. 2017;12(6):e0179012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan J, Kong LY, Hu J, et al. FGL2 as a Multimodality Regulator of Tumor-Mediated Immune Suppression and Therapeutic Target in Gliomas. Journal of the National Cancer Institute. 2015;107(8).**Yan et al. and showed that FGL2 mediates immunosuppression in glioblastoma multiforme