Abstract
Background:
Although liver transplantation may potentially cure hepatocellular carcinoma (HCC), the risk of HCC recurrence is 8–20% at five years post-transplant. Pre-transplant alpha fetoprotein (AFP) is a predictor of HCC recurrence, but it is unknown if pre-transplant AFP also predicts survival in patients with recurrence.
Methods:
We performed a retrospective cohort study using the United Network for Organ Sharing (UNOS) database between 2002 and 2016. We identified adult transplant recipients with HCC recurrence after liver transplantation for HCC, and used Cox regression to compare patient survival among different maximum pre-transplant AFP levels.
Results:
The cohort (N=1,164) was primarily male, white, and with hepatitis C liver disease. The median time to HCC recurrence was 11.6 months (interquartile range 6.1–26.3). In Cox regression analysis, increasing pre-transplant AFP was associated with poorer survival when adjusting for age, pre-transplant model for end-stage liver disease (MELD), and time to HCC recurrence. For example, patients with pre-transplant AFP ≥500ng/mL had a 1.6-fold higher risk of death versus those with AFP ≤20ng/mL (p<0.001).
Conclusion:
Pre-transplant AFP is independently associated with survival in patients with HCC recurrence. These findings further contextualize the importance of pre-transplant AFP in liver transplantation, and may improve prognostication for patients with HCC recurrence.
Keywords: survival analysis, risk stratification, United Network for Organ Sharing (UNOS), national registry data, liver transplantation
Introduction
Hepatocellular carcinoma (HCC) is a primary liver malignancy occurring most often in the setting of cirrhosis.1 Liver transplantation (LT) is potentially curative, and in properly selected patients offers better long-term survival as compared to surgical resection.2,3 However, the incidence of HCC recurrence after transplantation is high, up to 8–20% at five years.4–6 Prior literature has identified numerous risk factors for patients at risk of HCC recurrence, including pre-transplant alpha fetoprotein (AFP), shorter time on the waiting list, etiology of liver disease, and pathology characteristics such as microvascular invasion.7–13 However, there are limited data on survival among patients who experience HCC recurrence, as well as associated risk factors for mortality. In particular, although AFP at the time of recurrence predicts patient survival,14 it is unknown if pre-transplant AFP similarly predicts survival among patients with HCC recurrence.
There is biological plausibility for such a claim. It has been shown that AFP levels correlate with mortality in patients with HCC in the non-transplant setting, across all etiologies, and specifically in patients with chronic hepatitis C virus infection.15–17 This occurs even after adjusting for tumor size and stage, and is therefore incorporated into one of the major prognostic scoring systems, the Cancer of the Liver Italian Program.18 We also know that AFP levels positively correlate with HCC tumor burden as well as HCC recurrence risk.19 Finally, AFP secretion corresponds to tumor proliferation, dedifferentiation, and anti-apoptosis.20–22 Because HCC recurrence is thought to occur as a result of circulating tumor cells that are not cleared with transplant,23,24 it stands to reason that increased pre-transplant AFP may predict increased aggressiveness of HCC tumors when and if they recur. To test this hypothesis, we used national transplant registry data to determine if pre-transplant AFP levels are associated with survival among patients with post-transplant HCC recurrence, adjusting for established risk factors. In doing so, we also sought to characterize the national burden of HCC recurrence cases, as well as trends in HCC recurrence mortality.
Materials and Methods
Study Design and Cohort Creation
We performed a retrospective cohort study using United Network for Organ Sharing (UNOS) data from 2/2002 to 9/2016. Our group has previously described the creation of a cohort of patients aged ≥18 who underwent LT for HCC during this period.9 For this study, we created an analytic subcohort of patients who experienced HCC recurrence. We identified these events using a validated algorithm that included report of (1) post-transplant recurrence of pre-transplant malignancy, or (2) post-transplant death caused by HCC or metastatic malignancy. In the UNOS database these fields are updated annually after LT. Importantly, although there is no dedicated field in the UNOS post-transplant database that indicates HCC recurrence, this algorithm has been previously validated and subsequently used in several studies, including a recent validation of the RETREAT score.9,25,26 Finally, for the primary analysis we excluded patients who had simultaneous coding of HCC recurrence and death, as these patients would automatically be excluded from subsequent survival analysis.
Variable Collection
From the UNOS dataset we obtained data on demographics (age at LT, age at HCC recurrence, sex, race), body mass index (BMI) at transplant, pre-LT model for end-stage liver disease (MELD), adherence to Milan criteria, last pre-LT imaging tumor characteristics (diameter, number of tumors), pre-LT locoregional therapy (embolization or ablation), downstaging prior to transplant, pre-LT surgical tumor resection, cold ischemia time, and UNOS transplant region. We coded etiology of liver disease as hepatitis C (HCV), hepatitis B (HBV), alcoholic (EtOH), non-alcoholic fatty liver (NAFLD), autoimmune, or other (including hemochromatosis, sarcoidosis, and inborn metabolic diseases, among other rarer etiologies). For additional analyses, we also categorized etiology of liver disease as viral or non-viral. Maximum pre-transplant AFP was binned into a four-level categorical variable, with levels adapted from prior studies (≤20ng/mL, 21–99ng/mL, 100–499ng/mL, and ≥500ng/mL).7,8,27,28 We also obtained AFP levels immediately prior to LT for a sensitivity analysis (detailed below), although studies suggest that maximum and immediate pre-LT AFP perform similarly in HCC recurrence models.9,29 This was defined as the most recent AFP level reported to UNOS prior to LT. Finally, survival time was computed for all patients from the time of HCC recurrence. This was done to avoid issues of immortal time bias, which could impact results if follow-up time was measured from the time of LT.30
Patient Characteristics and HCC Recurrence Trends
Standard descriptive statistics were used to summarize the analytic cohort, including medians and interquartile ranges (IQRs) for continuous variables. The time to HCC recurrence was computed for each patient, and these values were compared among maximum pre-transplant AFP categories using box plots and the Kruskal-Wallis test. We repeated this analysis with cohorts restricted to HCC recurrence less than 60 months, and less than 36 months, as limited literature suggests that “recurrence” may in fact be de novo disease beyond these timepoints.31,32 In order to visualize trends of HCC cases and mortality rates over time, we collapsed the dataset around computations of one-year mortality rates and total number of cases as a function of calendar year of recurrence. We also computed yearly HCC recurrence rates, and graphed these data using overlaid connected scatterplots.
Primary Analysis
For the primary analysis, the exposure of interest was maximum pre-transplant AFP category, as defined above. The outcome of interest was time to death after HCC recurrence, using a 36-month maximum follow-up interval. The Kaplan-Meier estimator was used to create unadjusted survival curves, stratified by maximum pre-transplant AFP category. The log-rank test was performed to compare these curves, using with an alpha = 0.05 threshold for statistical significance. Univariate Cox regression analysis was then performed to identify candidate predictors of interest using an alpha = 0.15 threshold for testing in subsequent multivariable models. Variables tested are summarized in Supplemental Table 1. Multivariable Cox regression was performed through backward and forward stepwise selection techniques, using alpha = 0.05 as a threshold for variable retention. The Cox proportional hazards assumption was tested using log-log plots and the Score test using Schoenfeld residuals. Time-varying covariates were considered based on this data, and Cox-adjusted survival curves were then plotted, stratified by pre-transplant maximum AFP category.
Secondary Analysis
In order to evaluate the relationship between maximum pre-LT AFP and post-LT HCC survival in patients where the diagnosis of recurrence versus de novo HCC was potentially unclear (i.e., those with HCC >36 months after LT), we performed a subgroup analysis of patients with post-LT HCC in the 36 to 60 month window, and an additional analysis of patients with HCC >60 months after LT. For each of these groups, we repeated the Kaplan-Meier and Cox regression analyses above.
Sensitivity Analyses
First, although prior literature suggests that pre-transplant maximum AFP performs similarly to immediate pre-transplant AFP in post-transplant HCC models,9,29 as noted above, we opted to perform a sensitivity analysis using immediate pre-transplant AFP. For these analyses, we replicated the Kaplan-Meier and Cox regression methods, as detailed above, using immediate pre-transplant AFP rather than pre-transplant maximum AFP. Second, in order to explore possible changes in HCC recurrence survival dynamics resulting from changes in HCC transplantation policy over time, we performed an additional stratified Cox regression analysis by era. Prior to 2006, patients with HCC were overprioritized relative to non-HCC LT indications, receiving up to 29 exception points for T2 lesions (in 2002), and exception points for T1 lesions (prior to 2004).33,34 During the 2005 calendar year, the policy was amended to award 22 MELD exception points for T2 lesions alone. As such, we defined pre- and post-2006 era for stratified analyses. Third, given the possibility that observed associations could be due to LT performed outside of standard indications, we excluded patients outside Milan criteria (n=76) or with maximum pre-LT AFP >1,000 (n=88), and repeated the analysis. Fourth, because some post-LT HCC cases diagnosed long after more LT may represent de novo HCC rather than true recurrence, we excluded patients with HCC diagnosed >60 months post-LT (n=128) in an additional analysis. Fifth, to address possible bias produced by excluding patients with simultaneous report of HCC recurrence and death (n=320), we arbitrarily imputed an HCC recurrence diagnosis date one half year prior to reported death for these patients, and reintroduced them into the cohort for Cox regression analysis. In a final sensitivity analysis, we excluded patients who were waitlisted for <6 months, noting 2015 UNOS policy changes mandating a 6-month waiting period prior to awarding of HCC exception points.35
Results
Patient Characteristics and HCC Recurrence Characteristics
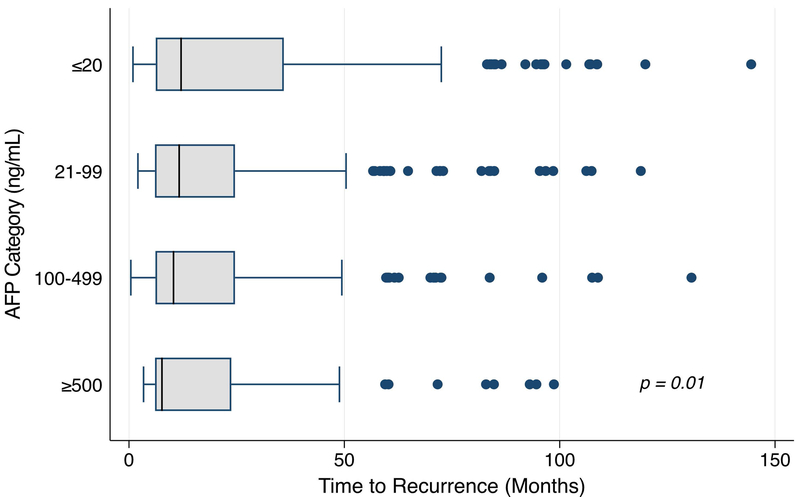
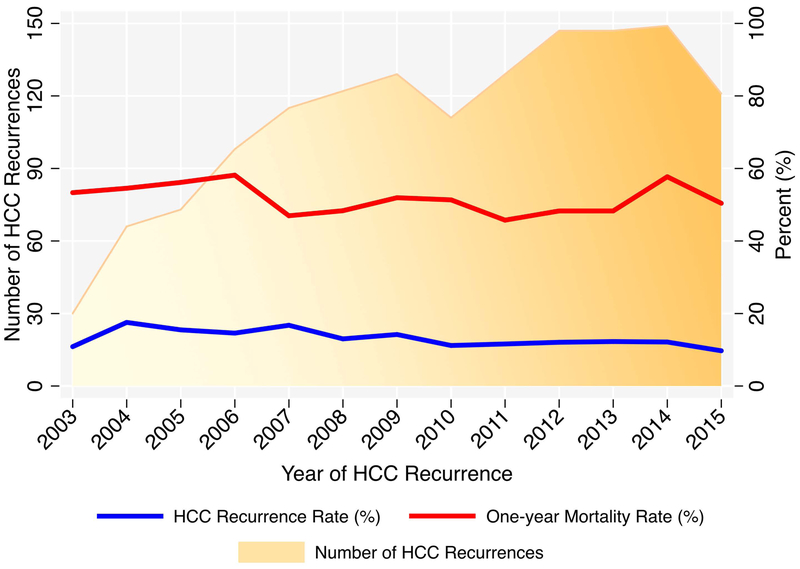
We identified a total 1,484 patients with HCC recurrence during the study window, of which 1,164 were included in the primary analytic cohort (Supplemental Figure 1). The cohort was predominantly male, white, with median age 58 years, and primarily reflected hepatitis C-related liver disease (Table 1). The overall median time to HCC recurrence was 11.6 months (IQR 6.1–26.3), with shorter time to recurrence with increasing maximum pre-transplant AFP category (Figure 1; p=0.01). This trend was also observed with immediate pre-transplant AFP (data not shown; p=0.03). When restricting the cohort to 60-month recurrence, the trend was similar but no longer statistically significant (p=0.06), and the trend was abolished when restricted to 36-month recurrence (p=0.95). The absolute annual number of HCC recurrence cases increased overall since 2003, however the one-year mortality rate remained generally unchanged (Figure 2). The annual rates of HCC recurrence fluctuated from 9.7% to 17.6%, with a modest decrease in rates over time.
Table 1 -.
Patient Characteristics (N = 1164)
| Variable | Value |
|---|---|
| Age at Transplant (years), median (IQR) | 58.0 (54.0, 63.0) |
| Age at HCC Recurrence, median (IQR) | 59.0 (55.0, 65.0) |
| Female sex | 221 (19.0%) |
| Race | |
| Diagnosis | |
| MELD at Listing, median (IQR) | 11.0 (8.0, 15.0) |
| BMI at Listing, median (IQR) | 27.9 (25.0, 31.5) |
| Months on Waiting List, median (IQR) | 4.2 (1.5, 9.4) |
| Immediate pre-LT AFP Category | |
| Maximum pre-LT AFP Category | |
| Number of Viable Tumors on Last pre-LT Imaging | |
| Largest Tumor on Last pre-LT Imaging (cm), median (IQR) | 2.5 (1.6, 3.3) |
| Within Milan Criteria on Last pre-LT Imaging | 1088 (93.5%) |
| Locoregional Therapy Prior to Transplant | 855 (73.5%) |
| Downstaged Prior to Transplant | 26 (2.2%) |
| Prior Surgical Tumor Resection | 18 (1.5%) |
| Cold Ischemia Time (Hours), median (IQR) | 6.4 (5.0, 8.1) |
| Time to HCC Recurrence (months), median (IQR) | 11.6 (6.1, 26.3) |
| HCC Recurrence Survival (months), median (IQR) | 13.2 (6.1, 29.7) |
Abbreviations: IQR, interquartile range; HCC, hepatocellular carcinoma; MELD, model for end-stage liver disease; BMI, body mass index; LT, liver transplantation; AFP, alpha fetoprotein
Figure 1 -.
Time to HCC Recurrence by Maximum Pre-transplant AFP Category (ng/mL)
Figure 2 -.
Trends in HCC Recurrence Cases and Mortality over Time
Kaplan-Meier Survival Analysis
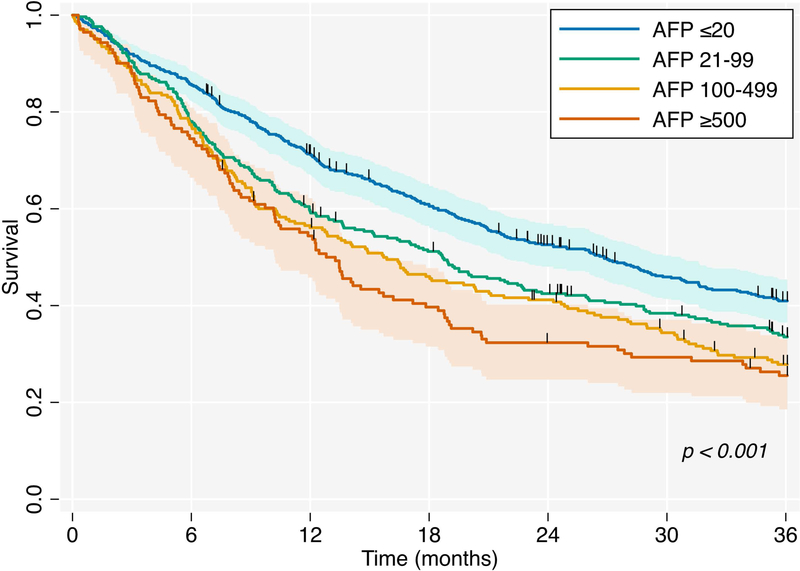
The overall median survival time after HCC recurrence was 13.2 months (IQR 6.1–29.7). Median survival was significantly shorter with increasing maximum pre-transplant AFP levels (Figure 3, p<0.001). As an example, median survival for patients with pre-transplant AFP ≥500 was 12.8 months (95% confidence interval [CI] 10.2–15.7), in contrast to 26.8 months (95% CI 21.8–30.7) for those with pre-transplant AFP ≤20. Similar results were obtained using immediate pre-transplant AFP categories (Supplemental Figure 2, p<0.001).
Figure 3 -.
Kaplan-Meier Survival Curves by Maximum Pre-transplant AFP Category (ng/mL)
Cox Regression Analysis
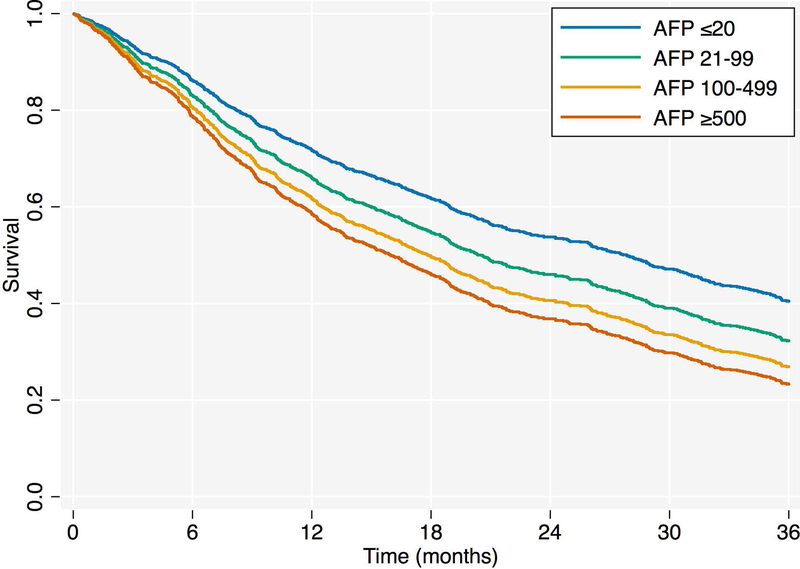
After univariate Cox regression analysis, the preliminary multivariable Cox regression model by both selection strategies included age at transplant, pre-transplant MELD, time to HCC recurrence, and maximum pre-transplant AFP category. This model, however, violated the proportional hazards assumption (Score test global p=0.03, time to HCC recurrence p-value<0.01). As such, time to HCC recurrence was modeled as a time-varying covariate. The final multivariable Cox regression model incorporating this time interaction variable satisfied the proportional hazards assumption (Table 2; Score test global p=0.61). Increasing maximum pre-transplant AFP category was associated with an increasing hazard of death (Figure 4; p<0.01), with a 1.6-fold higher risk of death for patients with pre-transplant AFP ≥500ng/mL relative to those with AFP ≤20ng/mL. Similar results were obtained using immediate pre-transplant AFP (Supplemental Table 2, Supplemental Figure 3). In all models, we did not find etiology of liver disease, or a stratification of viral versus non-viral liver disease, to be a significant predictor of survival after HCC recurrence. When stratifying the Cox regression models by transplantation policy era, increasing maximum pre-transplant AFP was significantly associated with poorer survival in both the pre- and post-2006 eras, adjusting for associated risk factors as before (Supplemental Table 3). The observed association between increasing pre-LT AFP and HCC recurrence survival was also similar when excluding patients outside of Milan criteria or with maximum pre-LT AFP >1,000 (Supplemental Table 4), and when isolating patients with post-LT HCC diagnosed within 60 months of transplant (Supplemental Table 5). Reintroduction of the 320 excluded HCC recurrence patients with imputation of recurrence dates modestly attenuated the point estimates of the association between maximum pre-transplant AFP and survival, relative to the primary analysis, but the trend was unchanged (Supplemental Table 6). Finally, when restricting the analysis cohort to patients on the waiting list for at least 6 months (n=455), we found that elevated pre-transplant AFP was still associated with poorer survival among patients with HCC recurrence (Supplemental Table 7; p=0.01). However, the hazard ratio for the 100–499ng/mL category was numerically higher than the ≥500ng/mL category.
Table 2 -.
Multivariable Cox Regression Model Predicting HCC Recurrence Survival
| Variable | Hazard Ratio | 95% Confidence Interval | P value |
|---|---|---|---|
| Age at transplant (per 5 years) | 1.06 | (1.01 – 1.11) | 0.03* |
| MELD score (per 5 points) | 1.07 | (1.02 – 1.13) | <0.01* |
| Maximum pre-transplant AFP category (ng/mL; ref ≤20) | |||
| 21–99 | 1.25 | (1.04 – 1.50) | 0.02* |
| 100–499 | 1.45 | (1.20 – 1.76) | <0.001* |
| ≥500 | 1.61 | (1.28 – 2.02) | <0.001* |
| Time to HCC recurrence (per month) | 0.99 | (0.99 – 1.00) | 0.12 |
| Time to HCC recurrence (TVC) | 1.00 | (1.00 – 1.00) | <0.01* |
MELD = model for end-stage liver disease, AFP = alpha fetoprotein, HCC = hepatocellular carcinoma, TVC = time-varying covariate
p < 0.05
Sex and transplant region were significant on univariate analysis but not retained in multivariable models
Figure 4 -.
Cox Regression-Adjusted Survival Curves by Maximum Pre-transplant AFP Category (ng/mL)
Discussion
In this large retrospective study of national transplant registry data, we reached several important conclusions regarding patients with post-transplant HCC recurrence. First, we report poor overall survival for patients with post-transplant HCC recurrence. Second, we found that increasing pre-transplant AFP levels corresponded to shorter time to HCC recurrence. Finally, and most importantly, we identified pre-transplant AFP as an independent risk factor for survival among patients with HCC recurrence, adjusting for associated risk factors. Importantly, this novel finding is not only biologically plausible, but also has clinical implications, as will be discussed herein.
To our knowledge, this is the largest study of post-transplant HCC recurrence survival and associated risk factors. Our estimates of survival in this cohort (median 13.2 months) are in agreement with the existing literature, although it is admittedly sparse. In a single-center study of 106 patients with post-transplant HCC recurrence, Bodzin et al reported a median survival of 10.6 months.36 Another single-center study of 57 patients found a median survival of 12.2 months.37 The largest study prior to ours was a meta-analysis that included 1,021 patients with HCC recurrence, reporting a median survival of 12.97 months.38 An added finding in our study is that from 2003 to 2015, the one-year mortality rate for HCC recurrence has been essentially unchanged with similar rates of HCC recurrence. This implies that advances in treatment options for patients with HCC recurrence have not meaningfully impacted patient survival thus far, or that higher risk patients are being transplanted over time.
The second major finding in this study is that pre-transplant AFP level is associated with earlier time to HCC recurrence, possibly related to the previously described association between increased AFP and features of tumor aggressiveness.19–22 Although numerous prior studies have found pre-transplant AFP to be a positive risk factor for HCC recurrence,7–10 no studies have explicitly evaluated the time at which those recurrences occur as a function of AFP. Interestingly, restricting the cohort to recurrences within 60 or 36 months attenuated the association, indicating that elevated pre-transplant AFP confers increased risk of HCC recurrence even at longer timepoints. This suggests that HCC beyond 36–60 months post-transplant may in fact represent recurrence rather than de novo disease, as is often stated in the literature. These insights have relevance for the primary novel finding in this study, that pre-transplant AFP (using either maximum or immediate pre-transplant values) is independently associated with survival after post-transplant HCC recurrence. Importantly, this finding is adjusted for time to HCC recurrence, which we identified as a time-varying covariate. These findings strongly suggest that post-transplant recurrence of pre-transplant HCC may inherit features of aggressiveness that are present and evaluable at the time of transplant listing. This is an important statement regarding the biology of HCC recurrence that has been missing from the literature, and which helps to explain the reason that pre-transplant AFP is strongly associated with post-transplant survival. Our results were also consistent when stratifying by transplantation policy era, implying that the association between pre-transplant AFP level and post-recurrence survival is biologically based as opposed to an artifact of policy change.
The clinical implications of our findings, in aggregate with the existing literature surrounding pre-transplant AFP, are significant in several ways. First, in patients with HCC who are borderline candidates for LT, a high AFP signals not only increased risk for HCC recurrence, but shorter time to recurrence as well as poorer survival if recurrence does develop. This may foster improved patient selection for LT. Indeed, our data support and strengthen the case for the recent changes in UNOS policy that mandate that patients with T2 HCC are eligible for a standard MELD exemption only with an AFP level ≤1,000ng/ml.39 Second, noting that there are currently no guideline-based protocols for post-LT HCC recurrence surveillance,40,41 our data suggest that patients with high pre-transplant AFP may warrant closer surveillance than those with lower pre-transplant AFP. Third, in patients who have experienced post-transplant HCC recurrence, this study provides an added data point with which to deliver prognostic information. Fourth, our data suggest that while the 2015 UNOS-mandated 6-month waiting period may have mitigated some degree of poor prognosis in HCC recurrence for high pre-transplant AFP patients, an alternative explanation is that the risk has simply been shifted to lower AFP groups. Indeed, in our final sensitivity analysis we found a notably higher risk of death in the 100–499ng/mL pre-transplant AFP category relative to the ≥500ng/mL category. This may reflect locoregional therapies and downstaging during the waiting period that serve to decrease AFP, but do not change the phenotypic aggressiveness of the tumor. Finally, given mounting evidence that significant reduction in high pre-transplant AFP improves post-transplant outcomes,42 one might speculate that HCC recurrence with high pre-transplant AFP may warrant more aggressive therapy up front.
There are several limitations to discuss in this work. First, as a national registry database study, there is likely some degree of misclassification of outcomes. Although we used a validated algorithm to identify patients with HCC recurrence, it is likely that some events were missed and thus we expect to have slightly underestimated the number of HCC recurrences in this study. We would not expect this to systematically impact the primary conclusions of the study, as the possible misclassification in the outcome is unlikely to be related to pre-transplant AFP. Second, we did not incorporate known predictors of patient survival that are determined at the time of HCC recurrence. In particular, bony metastasis at the time of recurrence is a poor prognostic factor.36,37 This granularity of data (i.e., imaging characteristics of recurrence, AFP at time of recurrence, subsequent treatment, etc.), is not present in the UNOS dataset, and as such this risk factor was omitted. However, we would expect that pre-transplant AFP would precede bony metastasis in a causal model, as patients are presumably transplanted without known metastasis. Third, explant pathology was not available throughout the study period as UNOS mandated this beginning in April 2012. Therefore, it is unclear if patients with elevated AFP had pathology characteristics that might contribute to differences in survival. Finally, there is likely some degree of selection bias imposed by our exclusion of patients with simultaneous reported HCC recurrence and post-transplant death. This is another limitation of the UNOS dataset, as transplant centers are only required to submit updated post-transplant information on an annual basis. As such, many patients with HCC recurrence are reported as recurring and dying at the same time, and thus not meaningfully contributing to survival data. Although it is difficult to predict the direction of this bias, we would expect to exclude patients who recurred and died within the same year. Thus, we may be slightly overestimating HCC recurrence survival in this study. This could explain the small differences from other literature cited above, although the magnitude of the bias appears to be small. Indeed, when imputing the expected value of HCC diagnosis date for excluded patients in a sensitivity analysis, the primary results were unchanged. Furthermore, as before, this limitation reflects an artifact of the data collection mechanism, rather than a true association between pre-transplant AFP category and likelihood of study exclusion.
In conclusion, we have found that HCC recurrence mortality remains extremely high, and has been unchanged over a 13-year period. Pre-transplant AFP is independently associated with post-transplant HCC recurrence survival, suggesting that elevated levels reflect increased tumor aggressiveness that is present even with recurrent disease. Although further research is needed to validate these findings prospectively and improve our understanding of the full implications and mechanisms behind AFP elevation and their significance in predicting HCC trajectories, our results may facilitate improved LT selection, as well as more accurate prognostication for patients who experience HCC recurrence.
Supplementary Material
Supplemental Figure 1 - Patient Flow Diagram
Supplemental Figure 2 - Kaplan-Meier Survival Curves by Immediate Pre-transplant AFP Category (ng/mL)
Supplemental Figure 3 - Cox Regression-Adjusted Survival Curves by Immediate Pre-transplant AFP Category (ng/mL)
Funding:
Nadim Mahmud is supported by a National Institutes of Health T32 grant (2-T32-DK007740–21A1).
Abbreviations:
- AFP
alpha fetoprotein
- BMI
body mass index
- EtOH
alcoholic liver disease
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- IQR
interquartile range
- LT
liver transplantation
- MELD
model for end-stage liver disease
- NAFLD
non-alcoholic fatty liver disease
- UNOS
United Network for Organ Sharing
Footnotes
Disclosures:
The authors declare no conflicts of interest related to this manuscript.
References
- 1.Lafaro KJ, Demirjian AN, Pawlik TM. Epidemiology of hepatocellular carcinoma. Surgical Oncology Clinics 2015;24:1–17. [DOI] [PubMed] [Google Scholar]
- 2.Figueras J, Jaurrieta E, Valls C, et al. Resection or transplantation for hepatocellular carcinoma in cirrhotic patients: outcomes based on indicated treatment strategy1. Journal of the American College of Surgeons 2000;190:580–7. [DOI] [PubMed] [Google Scholar]
- 3.Chapman WC, Klintmalm G, Hemming A, et al. Surgical treatment of hepatocellular carcinoma in North America: can hepatic resection still be justified? Journal of the American College of Surgeons 2015;220:628–37. [DOI] [PubMed] [Google Scholar]
- 4.Pomfret EA, Washburn K, Wald C, et al. Report of a national conference on liver allocation in patients with hepatocellular carcinoma in the United States. Liver Transplantation 2010;16:262–78. [DOI] [PubMed] [Google Scholar]
- 5.Yao FY, Mehta N, Flemming J, et al. Downstaging of hepatocellular cancer before liver transplant: long‐term outcome compared to tumors within Milan criteria. Hepatology 2015;61:1968–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schlansky B, Chen Y, Scott DL, Austin D, Naugler WE. Waiting time predicts survival after liver transplantation for hepatocellular carcinoma: a cohort study using the United Network for Organ Sharing registry. Liver Transplantation 2014;20:1045–56. [DOI] [PubMed] [Google Scholar]
- 7.Serper M, Taddei TH, Mehta R, et al. Association of provider specialty and multidisciplinary care with hepatocellular carcinoma treatment and mortality. Gastroenterology 2017;152:1954–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piñero F, Marciano S, Anders M, et al. Identifying patients at higher risk of hepatocellular carcinoma recurrence after liver transplantation in a multicenter cohort study from Argentina. European journal of gastroenterology & hepatology 2016;28:421–7. [DOI] [PubMed] [Google Scholar]
- 9.Mahmud N, Shaked A, Olthoff KM, Goldberg DS. Differences in Post‐Transplant Hepatocellular Carcinoma Recurrence by Etiology of Liver Disease. Liver Transplantation 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kashkoush S, El Moghazy W, Kawahara T, Gala‐Lopez B, Toso C, Kneteman NM. Three‐dimensional tumor volume and serum alpha‐fetoprotein are predictors of hepatocellular carcinoma recurrence after liver transplantation: refined selection criteria. Clinical transplantation 2014;28:728–36. [DOI] [PubMed] [Google Scholar]
- 11.Grąt M, Stypułkowski J, Morawski M, et al. Shadows Behind Using Simple Risk Models in Selection of Hepatocellular Carcinoma Patients for Liver Transplantation. Annals of surgery 2018. [DOI] [PubMed] [Google Scholar]
- 12.Duvoux C, Roudot–Thoraval F, Decaens T, et al. Liver transplantation for hepatocellular carcinoma: a model including α-fetoprotein improves the performance of Milan criteria. Gastroenterology 2012;143:986–94. e3. [DOI] [PubMed] [Google Scholar]
- 13.Mazzaferro V, Sposito C, Zhou J, et al. Metroticket 2.0 model for analysis of competing risks of death after liver transplantation for hepatocellular carcinoma. Gastroenterology 2018;154:128–39. [DOI] [PubMed] [Google Scholar]
- 14.Goldaracena N, Mehta N, Scalera I, et al. Multicenter validation of a score to predict prognosis after the development of HCC recurrence following liver transplantation. HPB 2018. [DOI] [PubMed] [Google Scholar]
- 15.Peng SY, Chen WJ, Lai PL, Jeng YM, Sheu JC, Hsu HC. High α‐fetoprotein level correlates with high stage, early recurrence and poor prognosis of hepatocellular carcinoma: significance of hepatitis virus infection, age, p53 and β‐catenin mutations. International journal of cancer 2004;112:44–50. [DOI] [PubMed] [Google Scholar]
- 16.Tyson GL, Duan Z, Kramer JR, Davila JA, Richardson PA, El–Serag HB. Level of α-Fetoprotein Predicts Mortality Among Patients With Hepatitis C–Related Hepatocellular Carcinoma. Clinical Gastroenterology and Hepatology 2011;9:989–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nomura F, Ohnishi K, Tanabe Y. Clinical features and prognosis of hepatocellular carcinoma with reference to serum alpha‐fetoprotein levels. Analysis of 606 patients. Cancer 1989;64:1700–7. [DOI] [PubMed] [Google Scholar]
- 18.Investigators CotLIP. A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients. Hepatology 1998;28:751–5. [DOI] [PubMed] [Google Scholar]
- 19.Tangkijvanich P, Anukulkarnkusol N, Suwangool P, et al. Clinical characteristics and prognosis of hepatocellular carcinoma: analysis based on serum alpha-fetoprotein levels. Journal of clinical gastroenterology 2000;31:302–8. [DOI] [PubMed] [Google Scholar]
- 20.Ho HK, Pok S, Streit S, et al. Fibroblast growth factor receptor 4 regulates proliferation, anti-apoptosis and alpha-fetoprotein secretion during hepatocellular carcinoma progression and represents a potential target for therapeutic intervention. Journal of hepatology 2009;50:118–27. [DOI] [PubMed] [Google Scholar]
- 21.Abelev G, Eraiser T. Cellular aspects of alpha-fetoprotein reexpression in tumors Seminars in cancer biology; 1999: Elsevier; p. 95–107. [DOI] [PubMed] [Google Scholar]
- 22.Johnson P, Williams R. Serum alpha-fetoprotein estimations and doubling time in hepatocellular carcinoma: influence of therapy and possible value in early detection. JNCI: Journal of the National Cancer Institute 1980;64:1329–32. [DOI] [PubMed] [Google Scholar]
- 23.Toso C, Mentha G, Majno P. Liver transplantation for hepatocellular carcinoma: five steps to prevent recurrence. American journal of transplantation 2011;11:2031–5. [DOI] [PubMed] [Google Scholar]
- 24.Schulze K, Gasch C, Staufer K, et al. Presence of EpCAM‐positive circulating tumor cells as biomarker for systemic disease strongly correlates to survival in patients with hepatocellular carcinoma. International journal of cancer 2013;133:2165–71. [DOI] [PubMed] [Google Scholar]
- 25.Samoylova ML, Dodge JL, Vittinghoff E, Yao FY, Roberts JP. Validating posttransplant hepatocellular carcinoma recurrence data in the United Network for Organ Sharing database. Liver Transplantation 2013;19:1318–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehta N, Dodge JL, Roberts JP, Yao FY. Validation of the prognostic power of the RETREAT score for hepatocellular carcinoma recurrence using the UNOS database. American Journal of Transplantation 2018;18:1206–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ioannou GN, Perkins JD, Carithers RL Jr. Liver transplantation for hepatocellular carcinoma: impact of the MELD allocation system and predictors of survival. Gastroenterology 2008;134:1342–51. [DOI] [PubMed] [Google Scholar]
- 28.Merani S, Majno P, Kneteman NM, et al. The impact of waiting list alpha-fetoprotein changes on the outcome of liver transplant for hepatocellular carcinoma. Journal of hepatology 2011;55:814–9. [DOI] [PubMed] [Google Scholar]
- 29.Berry K, Ioannou GN. Serum alpha‐fetoprotein level independently predicts posttransplant survival in patients with hepatocellular carcinoma. Liver Transplantation 2013;19:634–45. [DOI] [PubMed] [Google Scholar]
- 30.Liu J, Weinhandl ED, Gilbertson DT, Collins AJ, St Peter WL. Issues regarding ‘immortal time’in the analysis of the treatment effects in observational studies. Kidney international 2012;81:341–50. [DOI] [PubMed] [Google Scholar]
- 31.Welker MW, Bechstein WO, Zeuzem S, Trojan J. Recurrent hepatocellular carcinoma after liver transplantation–an emerging clinical challenge. Transplant International 2013;26:109–18. [DOI] [PubMed] [Google Scholar]
- 32.Saxena R, Ming QY, Emre S, Klion F, Nalesnik MA, Thung SN. De novo hepatocellular carcinoma in a hepatic allograft with recurrent hepatitis C cirrhosis. Liver Transplantation and Surgery 1999;5:81–2. [DOI] [PubMed] [Google Scholar]
- 33.Parikh ND, Singal AG. Model for end‐stage liver disease exception points for treatment‐responsive hepatocellular carcinoma. Clinical Liver Disease 2016;7:97–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldberg DS, Olthoff KM. Standardizing MELD exceptions: current challenges and future directions. Current transplantation reports 2014;1:232–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Revised liver policy regarding HCC exception scores. Organ Procurement and Transplantation Network, 2015. (Accessed May 31, 2019, at https://optn.transplant.hrsa.gov/news/revised-liver-policy-regarding-hcc-exception-scores/.)
- 36.Bodzin AS, Lunsford KE, Markovic D, Harlander-Locke MP, Busuttil RW, Agopian VG. Predicting mortality in patients developing recurrent hepatocellular carcinoma after liver transplantation. Annals of surgery 2017;266:118–25. [DOI] [PubMed] [Google Scholar]
- 37.Roayaie S, Schwartz JD, Sung MW, et al. Recurrence of hepatocellular carcinoma after liver transplant: patterns and prognosis. Liver Transplantation 2004;10:534–40. [DOI] [PubMed] [Google Scholar]
- 38.de’Angelis N, Landi F, Carra MC, Azoulay D. Managements of recurrent hepatocellular carcinoma after liver transplantation: a systematic review. World Journal of Gastroenterology: WJG 2015;21:11185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.OPTN/UNOS Policy Notice Clarification to Alpha-Fetoprotein (AFP) Levels for Liver Candidate Eligibility for Standardized HCC Exceptions. United Network for Organ Sharing, 2017. (Accessed January 14, 2019, at https://optn.transplant.hrsa.gov/media/2345/executive_policynotice_afp_201712.pdf.)
- 40.Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358–80. [DOI] [PubMed] [Google Scholar]
- 41.Benson III AB, D’Angelica MI, Abbott DE, et al. Hepatobiliary Cancers, Version 1.2017: Featured Updates to the NCCN Guidelines. Journal of the National Comprehensive Cancer Network: JNCCN 2017;15:563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mehta N, Dodge JL, Roberts JP, Hirose R, Yao FY. Alpha‐fetoprotein Decrease from> 1000 to< 500 ng/ml in Patients with Hepatocellular Carcinoma Leads to Improved Post‐Transplant Outcomes. Hepatology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 - Patient Flow Diagram
Supplemental Figure 2 - Kaplan-Meier Survival Curves by Immediate Pre-transplant AFP Category (ng/mL)
Supplemental Figure 3 - Cox Regression-Adjusted Survival Curves by Immediate Pre-transplant AFP Category (ng/mL)