Abstract
Background:
Antifungal prophylaxis strategies for lung transplant recipients vary without consensus or standard of care. Our current study aims to identify antifungal prophylaxis practices in the United States.
Methods:
From November 29, 2018 to February 15, 2019, we emailed surveys to medical directors of adult lung transplant centers. An alternate physician representative was approached if continued non-response after three survey attempts. Descriptive statistics were used to report findings.
Results:
Forty-four of 62 (71.0%) eligible centers responded. All Organ Procurement and Transplantation Networks were represented. Only four (9.1%) centers used pre-transplant prophylaxis for prevention of tracheobronchitis (3 of 4) and invasive fungal disease (4 of 4). Thirty-nine of forty (97.5%) centers used post-transplant prophylaxis: 36 (90.0%) universal and 3 (7.5%) pre-emptive/ selective prophylaxis. Most centers used nebulized amphotericin with a systemic agent (26 of 36, 72.2%). Thirty-two of thirty-six (88.9%) centers continued universal prophylaxis beyond the hospital setting. Duration of prophylaxis ranged from the post-transplant hospitalization to lifelong with most centers (25 of 36, 69.4%) discontinuing prophylaxis six months or less post-transplant.
Conclusion:
Most United States’ lung transplant centers utilize a universal prophylaxis with nebulized amphotericin and a systemic triazole for six months or less post-transplant. Very few centers use pre-transplant antifungal prophylaxis.
Keywords: Lung transplant, Antifungal prophylaxis, Fungal Infections, Aspergillus, Azole
INTRODUCTION
Fungal infections are the second leading cause of death in lung transplant recipients and are associated with up to a three-fold increase in all-cause mortality1,2. Fungal airway colonization has also been associated with chronic lung allograft dysfunction3,4—the leading cause of post-transplant death1,2. Prevention of fungal disease with pharmacologic agents is a widely accepted practice in liver transplant and hematologic malignancies. While the morbidity and mortality from fungal disease is significant in lung transplant, no widely-accepted standard of care exists to direct preventative measures.
Several cohort studies have attempted to establish the efficacy of post-lung transplant antifungal prophylaxis with voriconazole5–8, itraconazole5,9,10, posaconazole 11,12, and nebulized amphotericin13–17. However, these studies utilized different prophylactic strategies and medication durations. While many of the risk factors for the development of fungal disease are dissimilar, much of the evidence in support of post-transplant antifungal prophylaxis is extrapolated from liver transplant and hematologic malignancy/ bone marrow transplant populations. We know from these populations that pharmacologic antifungal prophylaxis decreases the incidence of fungal infections11,18–20; however, the spectrum of activity of antifungal medications required for prophylaxis varies between these populations. For example, posaconazole is superior to itraconazole or fluconazole at preventing fungal disease in hematologic malignancies11, and posaconazole may decrease mortality related to some fungal infections compared to fluconazole in allogenic hematopoietic stem cell transplant recipients20. Fluconazole has similar efficacy to itraconazole or liposomal amphotericin in average risk liver transplant patients18,19. If we allow the evidence from these discrete populations to establish the efficacy of post-transplant antifungal prophylaxis, we still need to determine what medication for which lung transplant patients and for how long.
Unlike other solid organ transplant and bone marrow transplant patients, lung transplant recipients remain at increased risk for fungal infections even years after transplantation21. This is largely secondary to decreased muco-ciliary clearance and exposure of the grafted organ with the environment in the setting of ongoing immunosuppression. Prolonged exposure to antifungal medications, particularly voriconazole, introduces the risk of novel adverse effects such as hyperfluorosis, peri-ostitis, and skin cancer22,23. Moreover antifungal medications interact with anti-rejection medications such as calcineurin inhibitors and antimetabolite medications. Alterations of antifungal medications without careful monitoring can precipitate acute rejection episodes or calcineurin inhibitor toxicity. Without established standard of care in lung transplant, the onus to balance the presumed benefit of antifungal prophylaxis with the potential risks of side effects and drug interactions lies with the individual transplant centers.
Current lung transplant antifungal prophylactic practice in the United States is largely unknown. A United States based survey performed twenty years ago found that most transplant centers use pharmacologic agents to prevent post-lung transplant fungal disease, but the duration, agents, and prophylactic strategies were disparate amongst transplant centers24. A more recent survey performed in the United States in 2011 and 2013 with a focus on skin cancer risk and the availability of transplant dermatology also found wide practice variation25. Prior surveys have also found a difference in prophylactic strategies between transplant centers in the United States and Europe26,27. No survey has established the practice patterns in the United States since the advent of newer triazoles such as isavuconazole and posaconazole. It is important to understand current practice to move forward with comparative, multi-center research and establish a standard of care for antifungal prophylaxis in lung transplant patients.
To date, antifungal prophylaxis practices in lung transplant have not been clearly defined. In the current study, we surveyed physician representatives from lung transplant centers in the United States with the aim of determining current peri-transplant antifungal prophylaxis patterns and factors influencing prophylactic decisions. We specifically sought to determine practice in regards to the prophylactic strategy, pharmacologic agents, duration, and barriers to antifungal prophylaxis.
METHODS
This study was approved by the Mayo Clinic Institutional Review Board under IRB 18-008437 and adheres to the ethical standards and principles of the Declarations of Helsinki.
Study Participants
We surveyed physician representatives from all adult lung transplant centers in the United States. Any transplant center performing more than one adult lung transplant in 2017 based on the Scientific Registry of Transplant Recipients (SRTR) was eligible for inclusion28. Pediatric-based transplant centers were excluded.
Physician representatives were identified through each transplant center’s website and physician directory. The medical director was the preferred physician representative and first contact for each transplant center. In the event that the medical director did not respond to participation requests, an alternate physician representative was identified. Alternate physician representatives included transplant pulmonologists, transplant infectious diseases physicians, and surgical directors. If the medical director or alternate physician representative could not be identified with a web-based search, the transplant center was contacted via phone to provide the name(s) of the medical and/ or surgical directors.
Survey Instrument
We developed the web survey to specifically address the current practice of individual transplant centers in regards to the goals, type, duration, and barriers of antifungal prophylaxis in lung transplant candidates and recipients (Appendix 1). We used the Research Electronic Data Capture (REDCap) database29 as the web survey platform. Post-transplant outcomes and number of lung transplants performed in 2017-2018 were pulled from publicly available reports28 and not asked within the survey. The survey was divided into four sections: background information, prior to lung transplantation, following lung transplantation, and barriers to antifungal prophylaxis. If centers reported using fluconazole, isavuconazole, itraconazole, voriconazole, or posaconazole then they were asked if they dosed these medications using standard dosing, to serum detectable levels, or to specific serum trough level. Survey questions were both case-based and fact-based with multiple choice and multi-answer responses. Respondents had the option to provide more detailed written explanations for each question group.
We refined the survey through an iterative process and piloted it to pulmonary and infectious diseases physicians for format, clarity, and usability. The American College of Chest Physicians Transplant Network endorsed the final version of the survey.
Survey Administration
Surveys were administered by email in two rounds from November 29, 2018 through February 15, 2019. The first round of survey invitations were sent to medical directors only. When necessary a second round of survey invitations were sent to an alternate physician representative at non-responding centers. Each round consisted of an initial email invitation followed by another invitation 1-week later to non-responders. If no response two-weeks after the second email invitation, then the transplant center was contacted via telephone to encourage participation, and another email invitation was sent. If the medical director did not respond within two-weeks following the third email invitation, then the transplant center was considered a non-responding center, and second round physician representatives were contacted. The second round of surveys was administered in the same manner as the first round. During the survey administration phase, partial responders were treated the same as non-responders and received reminder emails to complete the survey. If a medical director responded late after a second round respondent for the same institution, we included the medical director’s response in the analysis and excluded the second round respondent.
We provided no remuneration for participation. Email invitations included an explanatory statement and a survey link unique to that participant. Responses were tracked via a participant list corresponding to the unique survey link. The survey responses were collected and stored with a corresponding code for each participant in a secured, web-based REDCap database29 hosted at Mayo Clinic.
Definitions
We defined pre-emptive prophylaxis as antifungal medications given to patients with evidence of fungal airway colonization and selective prophylaxis as antifungal medications given to patients with specific diagnoses that may place them at higher risk for fungal infections. We defined universal prophylaxis as prophylaxis given to everyone no matter colonization status or diagnoses. Examples of triazole and echinocandin antifungal medications were provided within the survey questions. Examples of triazoles included fluconazole, voriconazole, itraconazole, posaconazole, and isavuconazole, and examples of echinocandins included caspofungin, micafungin, and anidulafungin. We did not differentiate between nebulized amphotericin deoxycholate and nebulized liposomal amphotericin. We also did not differentiate different between formulations of systemic medications, i.e. intravenous versus liquid versus capsule versus tablet.
Data Analysis
Only one survey response per transplant center was included in the analysis. When more than one survey response was received from a transplant center, we analyzed completed surveys over partially completed surveys. If we received two responses of equivalent completion from a transplant center then we preferentially analyzed the medical director’s response over other respondents. We included partially completed surveys and surveys with omitted questions in the analysis if no physician representative from that transplant center completed all survey questions.
We used descriptive statistics to report the findings, and data were de-identified for analysis. Responses to individual questions are presented as a percentage with the denominator representing the number of centers that responded to that particular question, unless otherwise stated. A chi square test or Fisher’s exact test was used to compare the duration of post-transplant prophylaxis based on the use of pre-transplant prophylaxis, different triazole medications, and various demographic characteristics. Duration was dichotomized to six months or less and greater than six months for the analyses. P-values less than or equal to 0.05 were considered statistically significant. JMP statistical software (version 9.01, SAS, Cary, NC) was used for data analysis.
RESULTS
Respondent Demographics
Of the 62 eligible transplant centers, physician representatives from 44 (71.0%) transplant centers responded to the survey. Medical directors (22 of 44, 50.0%) and transplant pulmonologists (18 of 44, 40.9%) were the majority of respondents. Other respondents included transplant infectious diseases specialists (2 of 44, 4.5%) and surgical directors (2 of 44, 4.5%). All 11 OPTNs30 were represented, and the percentage of responses from each OPTN region ranged from 42.3% to 100.0%; three OPTN regions had a 100.0% response rate.
Per SRTR data, the median number of lung transplants performed from July 1, 2017-June 30, 2018 by the responding centers was 27 (IQR 18, 56) transplants and ranged from less than 10 to over 120 transplants28. Responding centers were distributed in regards to one year post-transplant outcomes (per SRTR): worse than expected (16 of 44, 36.3%), at expectation (10 of 44, 22.7%) and better than expected (18 of 44, 40.9%)28. Most of the responding centers have been performing lung transplants for more than 15 years (30 of 43, 69.8%). Only 3 of 44 (6.8%) centers have been performing lung transplants for less than 5 years. Most of the responding centers reported access to transplant infectious diseases specialists (37 of 43, 86.0%).
Most respondents defined antifungal prophylaxis as antifungal medications prescribed regardless of fungal colonization (36 of 43, 83.7%); all of these respondents reported using universal post-transplant prophylaxis. A minority of transplant centers defined antifungal prophylaxis as antifungal agents prescribed after identification of fungal colonization on sputum or bronchoscopic samples (6 of 43, 14.0%), defined as pre-emptive prophylaxis in our study.
Prior to lung transplant
Prior to lung transplant, 4 of 42 (9.5%) transplant centers routinely prescribed antifungal prophylaxis. The goal of pre-transplant antifungal medications was prevention of post-transplant invasive fungal disease for all four centers. Preventing post-transplant tracheobronchitis was an additional goal for three of four (75.0%) centers (Table 1).
Table 1.
Pre-transplant antifungal prophylactic regimens for each transplant center reporting routine pre-transplant prophylaxis. Each row represents a unique transplant center.
| Goal of prophylaxis | Prophylactic Strategy | Medications | Duration (mo) |
|---|---|---|---|
| Prevention of post-transplant TrB and IFD | Universal | Nebulized Amphotericin | <3 |
| Prevention of post-transplant TrB and IFD | Pre-emptive* | Nebulized Amphotericin Posaconazole |
6-12 3-6 |
| Prevention of post-transplant TrB and IFD | Pre-emptive/ Selective** | Based on diagnosis and organism | NR |
| Prevention of post-transplant IFD | Pre-emptive*** | Voriconazole | 3-6 |
Abbreviations: mo-months, TrB-tracheobronchitis, IFD-invasive fungal disease, NR-no response
Airway colonization with Aspergillus and non-Aspergillus molds
Airway colonization with Aspergillus and non-Aspergillus molds. Diagnosis of cystic fibrosis and non-cystic fibrosis bronchiectasis
Airway colonization with Aspergillus
Only 1 of 42 (2.4%) centers utilized universal pre-transplant prophylaxis (Table 1). This center reported using nebulized amphotericin for less than three months without a systemic antifungal medication. The remaining three centers, all located in the Midwest (OPTN 7, 8), utilized pre-emptive or selective prophylaxis rather than a universal prophylactic approach. The reasons for prescribing antifungal prophylaxis pre-transplant included: Aspergillus spp. airway colonization (3 of 3), non-Aspergillus mold airway colonization (2 of 3), diagnosis of cystic fibrosis (1 of 3), and diagnosis of non-cystic fibrosis bronchiectasis (1 of 3). The medication regimens varied between these three centers: one center preferred voriconazole for 3-6 months; one center preferred combination therapy with nebulized amphotericin for 6-12 months and posaconazole for 3-6 months; and the last center preferred to tailor the prophylactic regimen based on the organisms identified.
Following lung transplant, the centers that used routine pre-transplant prophylaxis did not differ from other centers in terms of duration of post-transplant prophylaxis (p=0.58). The majority of centers that did not routinely prescribe pre-transplant prophylaxis (24 of 34, 70.1%) continued post-transplant prophylaxis for six months or less, and half of the centers that used pre-transplant prophylaxis (2 of 4, 50.0%) continued post-transplant prophylaxis for six months or less.
While not a routine practice, 14 of 38 (36.8%) centers reported in hypothetical scenarios that they may give triazole medications for prophylaxis, prior to transplant. Those scenarios included Aspergillus spp. airway colonization (9 of 38, 23.7%), history of invasive Aspergillus spp. infection (9 of 38, 23.7%), history of invasive mold infection (other than Aspergillus spp.) (8 of 38, 21.1%), non-Aspergillus mold airway colonization (5 of 38, 13.2%), diagnosis of cystic fibrosis (3 of 38, 7.9%), Candida spp. airway colonization (3 of 38, 7.9%), history of Candida spp. infection (2 of 38, 5.3%), non-Candida yeast airway colonization (2 of 38, 5.3%), and history of non-Candida yeast infection (2 of 38, 5.3%).
Following lung transplant
Most transplant centers (39 of 40, 97.5%) reported using post-transplant antifungal prophylaxis, and most centers employ a universal prophylactic strategy (36 of 40, 90.0%) (Figure 1).
Figure 1.
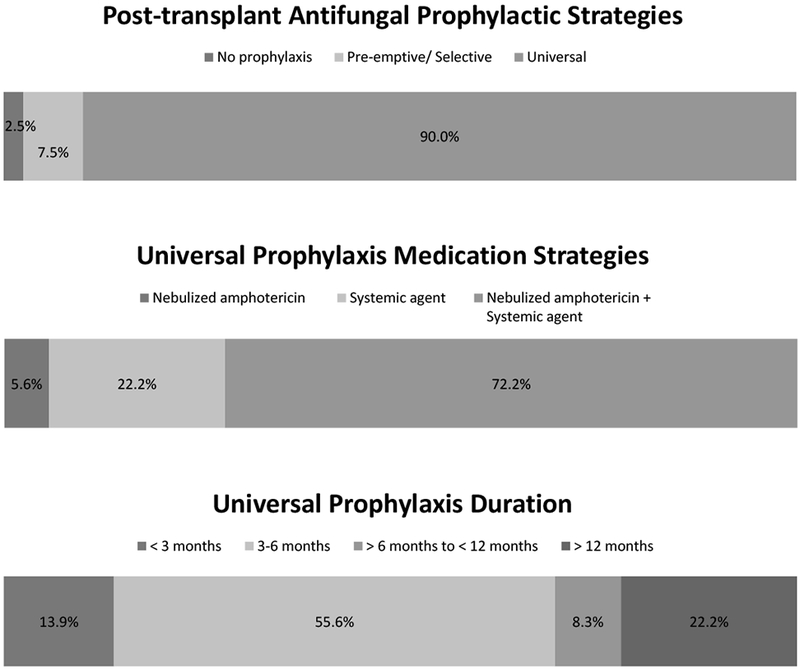
Post-lung transplant antifungal prophylaxis strategies with medications and duration of therapy for universal post-transplant prophylaxis.
Universal prophylaxis.
The goal of post-transplant universal prophylaxis was reported as prevention of invasive fungal disease (33 of 36, 91.7%), prevention of tracheobronchitis (22 of 36, 61.1%), and prevention of chronic lung allograft dysfunction (10 of 36, 27.8%) (Table 2). Universal prophylaxis was continued for 6 months or less by the majority of centers (25 of 36, 69.4%) (Figure 2).
Table 2.
The goals of post-transplant antifungal prophylaxis, as reported by transplant centers, and the medications used to achieve the prophylactic goal. Note that most transplant centers use more than 1 medication. The numbers in the columns reflect the number of transplant centers using that particular medication or medication class.
| Medications: |
Prophylactic Goals: Prevention of… |
||||
|---|---|---|---|---|---|
| TrB (2 centers) |
IFI (12 centers) |
TrB & IFI (11 centers) |
IFI & CLAD (1 center) |
TrB, IFI, & CLAD (9 centers) |
|
| Nebulized amphotericin | 5 | 10 | 1 | 8 | |
| Echinocandin class | 1 | 2 | |||
| Systemic amphotericin | 1 | ||||
| Fluconazole | 1 | 2 | |||
| Isavuconazole | 2 | 1 | |||
| Itraconazole | 2 | 4 | 6 | ||
| Posaconazole | 1 | 4 | 2 | ||
| Voriconazole | 1 | 6 | 1 | ||
Abbreviations: TrB-tracheobronchitis, IFI-invasive fungal infections, CLAD- Chronic Lung Allograft Dysfunction
Figure 2.
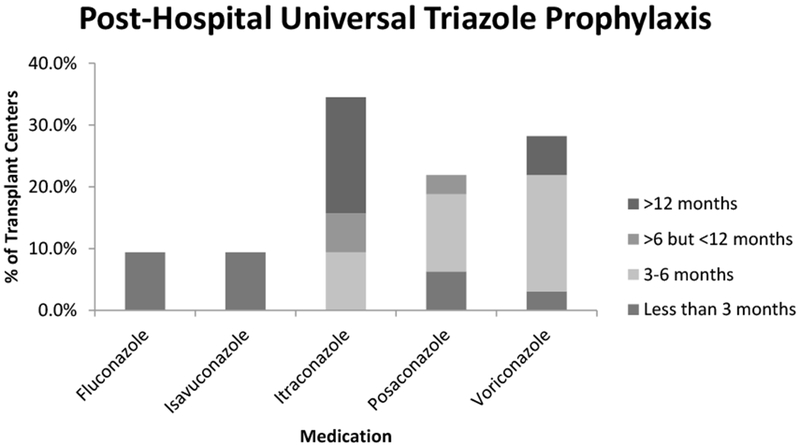
Post-lung transplant triazole medications and duration of use as universal prophylaxis beyond the post-transplant hospital setting. In total 32, centers reported continuing universal prophylaxis beyond the post-transplant hospital setting. All of these centers used a triazole medication. Itraconazole, when used, was continued significantly longer than other triazoles (p<0.0001).
The majority of centers using a universal prophylactic strategy preferred a combination of nebulized amphotericin with one or more systemic agents (26 of 36, 72.2%) (Figure 2). Only two centers (2 of 36, 5.6%) reported using nebulized amphotericin as monotherapy for antifungal prophylaxis. Both of those centers used nebulized amphotericin for less than three months post-transplant, but triazole (2 of 2) or echinocandin (1 of 2) agents may be given depending on airway colonization with Aspergillus spp. and non-Aspergillus molds. When nebulized amphotericin was given as a prophylactic agent, it tended to be given in-hospital post-transplant only (13 of 28, 46.4%) or for less than three months post-transplant (10 of 28, 35.7%). Only one center (1 of 28, 3.6%) reported giving nebulized amphotericin for longer than six months.
Of the centers using a systemic prophylactic agent, 1 of 34 (2.9%) and 2 of 34 (5.9%) centers used systemic amphotericin and micafungin, respectively, in the post-transplant hospital setting. An additional center reported using an echinocandin in the post-transplant hospital setting but did not specify an agent. The remaining 30 centers (30 of 34, 91.2%) that utilized systemic therapy used a triazole in the post-transplant hospital setting. Fluconazole (3 of 30, 10.0%), isavuconazole (3 of 30, 10.0%), posaconazole (7 of 30, 23.3%), itraconazole (8 of 30, 26.7%) and voriconazole (9 of 30, 30.0%) were all used as in-hospital systemic agents. Systemic agents were continued as universal prophylaxis beyond the hospital setting by 32 of 34 (94.2%) centers. One center that used micafungin as an in-hospital antifungal agent and the center that used an unspecified echinocandin did not continue universal systemic prophylaxis beyond the hospital setting.
All (32 of 32, 100.0%) centers that continued universal post-transplant prophylaxis beyond the post-transplant hospital setting used a triazole medication: itraconazole (11 of 32, 34.4%), voriconazole (8 of 32, 25.0%), posaconazole (7 of 32, 21.9%), fluconazole (3 of 32, 9.4%), and isavuconazole (3 of 32, 9.4%) (Figure 2).
Fluconazole and isavuconazole were continued for less than three months post-transplant by all centers (Figure 2). Centers that utilized itraconazole tended to continue therapy longer than those that utilized voriconazole or posaconazole (p<0.0001). Itraconazole was continued for 3 to 6 months by 3 of 11 (27.3%) centers, greater than 6 months but less than 12 months by 2 of 11 (18.2%) centers, 12 months but less than lifelong by 4 of 11 (36.4%) centers, and lifelong by 2 of 11 (18.2%) centers. In contrast, the longest duration of posaconazole was greater than 6 months but less than 12 months by 1 of 7 centers (14.3%). The remaining centers opted for less than three months (2 of 7, 28.6%) or three to six months (4 of 7, 57.1%) of posaconazole prophylaxis. Likewise, most centers opted for less than 3 months (1 of 8, 12.5%) or 3 to 6 months (6 of 8, 75.0%) of voriconazole prophylaxis. Only one center (1 of 8, 12.5%) continued voriconazole prophylaxis for more than 12 months.
Duration of post-transplant prophylaxis was not influenced by transplant volume (p=0.46), geographic region (p=0.80), years performing lung transplant (p=0.23), or presence of transplant infectious diseases specialists (p=0.63). Likewise, post-transplant 1-year outcomes was not influenced by duration of post-transplant prophylaxis (p=0.19).
Alterations in post-transplant prophylaxis.
Certain clinical criteria prompted a change to the prophylactic regimen at 23 of 36 (63.9%) centers employing universal prophylaxis. The centers reported changing the universal antifungal prophylactic regimen in the following scenarios: Aspergillus spp. airway colonization (18 of 36, 50.0%), non-Aspergillus mold airway colonization (13 of 36, 36.1%), non-Candida yeast airway colonization (4 of 36, 11.1%), increased immunosuppression (2 of 36, 5.6%), ischemic mucosal airway injury (2 of 36, 5.6%), pre-transplant diagnosis of cystic fibrosis (1 of 36, 2.8%), and pre-transplant diagnosis of non-cystic fibrosis bronchiectasis (1 of 36, 2.8%). Changes in the antifungal protocol included extending antifungal prophylaxis (12 of 36, 33.3%); altering the specific triazole agent (2 of 36, 5.6%); and adding an echinocandin (5 of 36, 13.9%), nebulized amphotericin (6 of 36, 16.7%), or triazole (5 of 36, 13.9%).
Selective/ Pre-emptive prophylaxis.
A selective or pre-emptive prophylactic strategy was reported by 3 of 40 centers (7.5%) (Figure 1). The reported goal of antifungal prophylaxis for these centers was to prevent post-transplant invasive fungal disease (2 of 3, 66.7%) and chronic lung allograft dysfunction (1 of 3, 33.3%). Pre and post-transplant airway colonization with Aspergillus spp. prompted antifungal prophylaxis for all (3 of 3, 100.0%) of the centers, and pre and post-transplant airway colonization with non-Aspergillus molds prompted antifungal prophylaxis at two of three (66.7%) centers. One center (1 of 3, 33.3%) reported using prophylaxis in patients with a history of Aspergillus spp. infection, and another center (1 of 3, 33.3%) reported using prophylaxis if the donor had a history of marijuana use or the recipient had signs of ischemic airways post-transplant.
Combination nebulized amphotericin and voriconazole were used by two of three (66.7%) centers. The duration of therapy varied between these two centers: one center reported using both nebulized amphotericin and voriconazole for three to six months post-transplant, and one center reported using nebulized amphotericin during the post-transplant hospitalization only and voriconazole for less than three months. The other center tailored prophylactic medications based on the reason for initiating post-transplant prophylaxis: nebulized amphotericin and a triazole were used for pre or post-transplant Aspergillus spp. colonization or history of pre-transplant Aspergillus spp. infection; an echinocandin may be added for post-transplant Aspergillus spp. colonization.
Triazole therapeutic drug monitoring
Triazole agents were routinely used as antifungal prophylaxis during the peri-transplant period by 32 of 44 (72.7%) responding centers. Dosing of triazole agents varied between centers and by agent used, but the majority of centers used therapeutic drug monitoring (16 of 32, 50.0%). Our survey did not include questions regarding center-specific trough levels. All centers that used fluconazole or isavuconazole for antifungal prophylaxis dosed using standard uniform dosing (fluconazole: 3 of 3, 100.0%; isavuconazole: 3 of 3, 100.0%) (Table 3). Dosing of posaconazole, itraconazole, and voriconazole varied between centers. Itraconazole was dosed using standard uniform dosing by five (5 of 11, 45.5%) centers, to serum trough detectable levels by three (3 of 11, 27.3%) centers, and to specific serum trough levels by three (3 of 11, 27.3%) centers. Most centers prescribing posaconazole for antifungal prophylaxis dosed to a specific serum trough level (6 of 7, 85.7%), and 1 (1 of 7, 14.3%) center used standard uniform dosing. Half of the centers using voriconazole for antifungal prophylaxis used standard dosing (4 of 8, 50.0%), while the other half dosed to a specific serum trough level (4 of 8, 50.0%).
Table 3.
Use of therapeutic drug monitoring by transplant centers for dosing triazole antifungal prophylaxis following lung transplant. No centers used therapeutic drug monitoring for fluconazole or Isavuconazole.
| Medication | Dosing Method | % for each dosing method |
|---|---|---|
| Itraconazole | Standard uniform dosing Serum ‘detectable’ level Specific serum trough level |
5/11 (45.5) 3/11 (27.3) 3/11 (27.3) |
| Posaconazole | Standard uniform dosing Specific serum trough level |
1/7 (14.3) 6/7 (85.7) |
| Voriconazole | Standard uniform dosing Specific serum trough level |
4/8 (50.0) 4/8 (50.0) |
Barriers
Insurance prior authorizations (27 of 43, 62.8%), cost of medications (23 of 43, 53.5%), insurance denial of coverage (22 of 43, 51.2%), side effects of antifungal agents (20 of 43, 46.5%), drug interactions (19 of 43, 44.2%), lack of formalized guidelines (10 of 43, 23.3%), and lack of strong quality evidence for antifungal prophylaxis (8 of 43, 18.6%) were cited as potential barriers or challenges for transplant centers prescribing antifungal prophylaxis in the peri-transplant period (Figure 3). For the one transplant center that did not use peri-transplant antifungal prophylaxis, they reported that cost, drug interactions, insurance prior authorizations/ coverage denial, medication side effects, patient pill burden, and lack of strong quality evidence were all barriers to prescribing antifungal prophylaxis. Reported barriers to patient adherence included cost of medications (31of 43, 72.1%), side effects of antifungal medications (26 of 43, 60.5%), insurance denial of coverage (16 of 43, 37.2%), insurance prior authorizations (13 of 43, 30.2%), drug interactions (9 of 43, 20.9%), pill burden (8 of 43, 18.6%), and lack of foreseen benefit (4 of 43, 9.3%).
Figure 3.
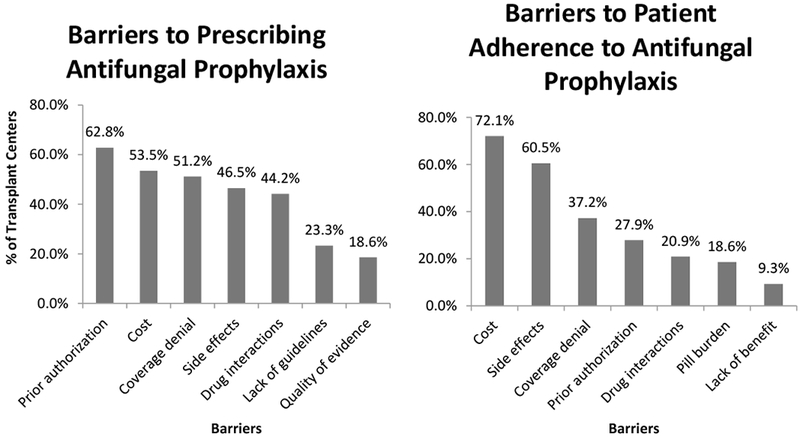
Barriers to prescribing and patient adherence to antifungal prophylaxis as reported by the 43 responding transplant centers that used peri-transplant prophylaxis.
DISCUSSION
In the current survey study, with responses from 71.0% of United States’ adult lung transplant centers, we found that almost all transplant centers employ post-transplant antifungal prophylaxis, and the vast majority use a universal prophylactic strategy. Medication choice and duration of prophylaxis were heterogeneous, but many centers preferred nebulized amphotericin in combination with a systemic agent and post-hospital triazole therapy. Very few centers routinely employed pre-transplant prophylaxis.
With almost all respondents using post-transplant antifungal prophylaxis, it is evident that transplant centers believe antifungal prophylaxis is warranted in lung transplant patients despite the lack of prospective, comparative efficacy trials. Prior surveys examining practice patterns in the United States found similar results24,31. In a survey of United States lung transplant centers conducted in 1999, all 37 responding transplant centers used antifungal prophylaxis following lung transplant24. While the tendency to use antifungal prophylaxis following lung transplant has not changed in United States over the last 20 years, the prophylactic strategy has shifted with more centers using a universal approach. In comparison to the current study, Dummer and colleagues found that 76% of centers opted for universal prophylaxis (compared to 90.0% in the current study) while 24% of centers utilized a selective/ pre-emptive approach (compared to 7.5% in the current study). Prior surveys conducted in 200232 and 200926 examining worldwide practice patterns found a similar distribution between universal and pre-emptive prophylactic strategies as Dummer and colleagues24. The reasons for this shift to a universal prophylactic strategy are likely secondary to increased antifungal medication options; the growing body of evidence supporting antifungal prophylaxis in other patient populations11,18–20; and observational studies indicating increased all-cause mortality in lung transplant patients diagnosed with fungal infections1,2.
While the need for post-transplant antifungal prophylaxis appears to be almost universally agreed upon by transplant centers, pre-transplant antifungal prophylaxis was less commonly used. We found that only one transplant center used universal pre-transplant prophylaxis, and three transplant centers used pre-emptive/ selective pre-transplant prophylaxis. Several transplant centers indicated that they would consider pre-transplant triazole therapy in hypothetical scenarios for reasons that included Aspergillus spp. colonization and non-Aspergillus mold colonization, but this was not a routine or protocol driven practice. Dummer and colleagues are the only prior survey that examined pre-transplant prophylaxis practices, and they found, in contrast to our study, that the majority of transplant centers used pre-emptive/ selective pre-transplant prophylaxis with nebulized amphotericin and/ or itraconazole prior to lung transplant24. Similar to our study, airway colonization with Aspergillus spp. prompted prophylaxis. In our study, the use pre-transplant prophylaxis did not affect post-transplant antifungal prophylaxis duration. While pre-transplant fungal airway colonization has been correlated with post-transplant fungal infections33, pre-transplant fungal airway colonization has not been definitively proven to be a risk factor for post-transplant fungal infections. Future studies should investigate whether pre-transplant antifungal prophylaxis affects post-transplant outcomes as it does not appear to affect the post-transplant prophylactic duration or strategy.
Similar to previous studies 27, nebulized amphotericin and triazoles were the most commonly used medications for antifungal prophylaxis. Echinocandins, when used, tended to be used in the immediate post-operative setting. Only one center used intravenous liposomal amphotericin for prophylaxis in our survey compared to 25% of transplant centers in a prior survey24. This is likely a reflection of the availability of more agents with mycelial activity, thereby replacing the more toxic amphotericin alternative. In contrast to prior survey studies26, we found that the majority of transplant centers used a combination of antifungal medications, most commonly nebulized amphotericin with a triazole. The type of triazole used for primary prophylaxis has also evolved since prior studies. Surveys conducted in 1999 and 2002 found that most centers used itraconazole followed by fluconazole31; whereas, a survey conducted in 2009 found that most centers preferred voriconazole followed by itraconazole, and virtually no centers used fluconazole26. The lack of activity of fluconazole against Aspergillus spp. and other molds is likely the reason this medication has fallen out of favor. We found a shift back to itraconazole as the preferred triazole agent followed by voriconazole and posaconazole. The shift back to itraconazole from voriconazole, although a number of centers continue to use voriconazole, is likely secondary to the unique side effects of voriconazole; long-term voriconazole has been associated with hyper-fluorosis, peri-ostitis, and squamous cell skin cancer23. These side-effects are likely the reason transplant centers using voriconazole continued prophylaxis for a shorter duration compared to itraconazole.
The introduction of more options for antifungal prophylaxis has made practice more disparate in regards medication used and spectrum of activity. In our study, the spectrum of activity of the antifungal agents used as prophylaxis has broadened compared to prior studies. Posaconazole and isavuconazole, which have activity against some agents of mucormycosis, were preferred triazole agents by several centers. Posaconazole was not widely used as prophylaxis in the most recent survey study conducted in 200926. Isavuconazole was not available during the prior survey studies. This shift to broader spectrum antifungal agents has not coincided with changes in the microbiology or epidemiology of fungal infections in lung transplant recipients34 and is more likely a reflection of market availability and ease of administration.
The duration of post-transplant prophylaxis has not significantly changed since prior studies26. Most centers utilize post-transplant prophylaxis for six months or less. The only factor, not inherent to specific patient cases, that influenced prophylaxis duration was the medication used. Transplant centers using itraconazole prophylaxis continued prophylaxis for longer than six months post-transplant. This is likely because itraconazole tends to be well-tolerated with few side effects compared to other agents, particularly voriconazole35. Several centers did report that fungal airway colonization may prolong prophylaxis.
Half of centers used therapeutic drug monitoring for triazole dosing. This represents a progression in practice since prior studies24,26,32. Itraconazole, posaconazole, and voriconazole were the medications where therapeutic drug monitoring was used. Because of their predictable bioavailability, fluconazole and isavuconazole were dosed without serum levels. Given drug interactions with anti-rejection medications and adverse effects from overexposure, therapeutic drug monitoring is recommended for solid organ transplant patients receiving itraconazole, posaconazole, or voriconazole36,37. With clear recommendations for therapeutic drug monitoring for itraconazole, posaconazole, and voriconazole, it is surprising that only half of transplant centers utilize therapeutic drug monitoring. Our survey did not explore reasons why transplant centers do not utilize serum drug levels. Information regarding center-specific serum drug levels was not obtained in the current study. No recommendations for serum levels or dosing in the setting of prophylaxis exist, and less than half of respondents indicated that drug interactions were barriers to antifungal prophylaxis.
Cost, insurance denial, and prior authorizations were the most commonly cited barriers or challenges to prescribing antifungal prophylaxis. Our survey did not explore whether or not these barriers prevented lung transplant recipients from receiving antifungal medications. While studies in hematologic malignancies have found antifungal prophylaxis cost-effective compared to treating fungal infections38,39, no such studies have been performed in lung transplant patients. Cost has previously been cited as an uncommon reason for voriconazole, posaconazole, or itraconazole discontinuation35. Further studies examining the impact of cost on antifungal prophylaxis in lung transplant patients are needed, especially with this trend toward prescribing a greater number of medications and prescribing newer, broader spectrum agents.
In comparison to prior studies, we saw an overall evolution in antifungal prophylaxis practice to a broader approach: more universal prophylaxis over pre-emptive/ selective, increase in number of medications prescribed, and use of newer, broader spectrum antifungal agents24. Several factors may be responsible for this change in practice including more options for antifungal prophylaxis and increased availability of therapeutic drug monitoring. Despite broader antifungal prophylaxis, post-lung transplant survival has not changed over the last 20 years40. Multi-center research examining the impact of antifungal prophylaxis on the development of post-transplant fungal infections and mortality in lung transplant are needed. Until these studies can be conducted, consensus based standard of care using available studies and expert opinions are merited to guide care. The trend for more and broader antifungal agents may ultimately have untoward effects, including increased patient financial burden and selecting for more resistant, difficult to treat fungal organisms.
Limitations
To maximize response rate, we limited the length of our survey. We, therefore, did not attempt to identify medication formulations, serum target trough levels, or perceived efficacy. We also asked respondents specifically about the presence of transplant infectious disease specialists at their center. Some transplant centers may have infectious disease specialists; so, our results should be interpreted specifically in the context of the presence of transplant infectious disease specialists.Data obtained in this survey were self-reported. While we asked respondents to reflect institutional practices and policies, responses may be a reflection of personal opinions.
CONCLUSION
Antifungal prophylaxis practice has evolved in lung transplant over the last 20 years with a tendency toward universal prophylaxis with a greater number of medications and broader spectrum of activity. Most United States’ lung transplant centers utilize a universal prophylactic approach with nebulized amphotericin and 1 or more systemic agents, and post-transplant prophylaxis tends to be continued for 6 months or less. Very few centers use pre-transplant antifungal prophylaxis.
Acknowledgments
This study was approved by the Mayo Clinic Institutional Review Board and approved under IRB 18-008437.
Financial Support: KMP is supported by the Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery, Mayo Clinic, Rochester, MN. CCK is supported by the NHLBI grant K23 HL128859 from the National Institutes of Health. The project utilized REDCap for data management. REDCap was supported by NIH/NCRR/NCATS CTSA Grant Number UL1 TR002377. The manuscript’s contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Abbreviations:
- OPTN
Organ Procurement and Transplantation Networks
- SRTR
Scientific Registry of Transplant Recipients
Appendix 1. Antifungal Prophylaxis in Lung Transplant
Please complete the survey below.
Thank you!
| Background Information |
|---|
| How many years has your transplant center been doing lung transplantation? |
| ○ Less than 5 years |
| ○ 5 to 15 years |
| ○ Greater than 15 years |
| Do you have Transplant Infectious Disease specialists at your center? |
| ○ Yes |
| ○ No |
| What is your definition of anti-fungal prophylaxis as it pertains to lung transplant candidates and recipients? |
| ○ Anti-fungal agents prescribed after identification of fungal colonization on sputum or bronchoscopic samples |
| ○ Anti-fungal agents prescribed regardless of fungal colonization |
| ○ Other |
| Please describe “Other” |
| Prior to Lung Transplant: The following questions refer to the pre-transplant period. |
|---|
| Does your center employ pre-transplant, pharmacologic anti-fungal prophylaxis? |
| ○ Yes |
| ○ No |
| ○ Unsure |
| What prompts pharmacologic anti-fungal prophylaxis prior to lung transplantation (Select all that apply)? |
| □ Airway colonization with Aspergillus sp. |
| □ Airway colonization with molds other than Aspergillus sp. |
| □ Airway colonization with non-Candida yeast |
| □ Airway colonization with Candida sp. |
| □ Diagnosis of Cystic Fibrosis |
| □ Patien’s occupation |
| □ Patien’s geographic location |
| □ Presence of bronchiectasis without diagnosis of Cystic Fibrosis |
| □ Our center uses universal pre-transplant anti-fungal prophylaxis |
| □ Other |
| Please describe “Other” |
| What is the goal of pre-transplant anti-fungal prophylaxis (Select all that apply)? |
| □ Prevention of post-transplantation tracheobronchitis |
| □ Prevention of post-transplantation invasive fungal infection |
| □ Other |
| Please describe “Other” |
| What anti-fungal agent(s) is preferred by your center for pre-transplant anti-fungal prophylaxis? (You may select up to 1 topical and 1 systemic agent) |
| Topical agents: |
| ○ Aerosolized amphotericin B |
| ○ Other |
| ○ No topical agent is used |
| Please describe “Other” |
| Systemic agents: |
| ○ Amphotericin |
| ○ Anidulafungin |
| ○ Caspofungin |
| ○ Fluconazole |
| ○ Micafungin |
| ○ Isavuconazole |
| ○ Itraconazole |
| ○ Posaconazole |
| ○ Voriconazole |
| ○ Other |
| ○ No systemic agent is used |
| Please describe “Other” |
| What is the average duration of pre-transplant topical (i.e. nebulized or aerosolized) anti-fungal prophylaxis? |
| ○ Less than 3 months |
| ○ 3 to 6 months |
| ○ Greater than 6 months but less than 12 months |
| ○ 12 months or greater |
| ○ Other |
| Please describe “Other” |
| What is the average duration of pre-transplant systemic anti-fungal prophylaxis? |
| ○ Less than 3 months |
| ○ 3 to 6 months |
| ○ Greater than 6 months but less than 12 months |
| ○ 12 months or greater |
| ○ Other |
| Please describe “Other” |
| What is the dosing method for your center’s preferred systemic anti-fungal? |
| ○ Standard uniform dosing regardless of serum anti-fungal levels |
| ○ Dosed to a serum “detectable” anti-fungal level |
| ○ Dosed to a specific target serum anti-fungal level |
| ○ Other |
| Please describe “Other” |
| Do patients listed for lung transplant receive inhaled amphotericin prior to lung transplantation for prophylaxis? |
| ○ All patients receive inhaled amphotericin prior to lung transplantation |
| ○ No patients receive inhaled amphotericin prior to lung transplantation |
| ○ Some patients receive inhaled amphotericin prior to lung transplantation |
| Which patients receive inhaled amphotericin prior to lung transplantation (select all that apply)? |
| □ Patients with a diagnosis of Cystic Fibrosis |
| □ Patients with bronchiectasis without a diagnosis of Cystic Fibrosis |
| □ Patients with Aspergillus sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with mold airway colonization (other than Aspergillus sp.) regardless of underlying pulmonary co-morbidities |
| □ Patients with non-Candida yeast airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with Candida sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with history of invasive Aspergillus sp. infection |
| □ Patients with history of invasive mold (other than Aspergillus sp.) infection |
| □ Patients with history of non-Candida yeast infection |
| □ Patients with history Candida sp. infection |
| □ Other |
| Please describe “Other” |
| Do patients listed for lung transplant receive systemic triazole therapy prior to lung transplantation for prophylaxis? Triazole therapy includes: fluconazole, voriconazole, itraconazole, posaconazole, isavuconazole. |
| ○ All patients receive systemic triazole prior to lung transplantation |
| ○ No patients receive systemic triazole prior to lung transplantation |
| ○ Some patients receive triazole therapy prior to lung transplantation |
| Which patients receive triazole therapy prior to lung transplantation (select all that apply)? |
| □ Patients with a diagnosis of Cystic Fibrosis |
| □ Patients with bronchiectasis without a diagnosis of Cystic Fibrosis |
| □ Patients with Aspergillus sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with mold airway colonization (other than Aspergillus sp.) regardless of underlying pulmonary co-morbidities |
| □ Patients with non-Candida yeast airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with Candida sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with history of invasive Aspergillus sp. Infection |
| □ Patients with history of invasive mold (other than Aspergillus sp.) infection |
| □ Patients with history of non-Candida yeast infection |
| □ Patients with history of Candida sp. infection |
| □ Other |
| Please describe “Other” |
| Do patients listed for lung transplant receive systemic echinocandin therapy prior to lung transplantation for prophylaxis? Echinocandin therapy includes: caspofungin, micafungin, anidulafungin. |
| ○ All patients receive systemic echinocandin prior to lung transplantation |
| ○ No patients receive systemic echinocandin prior to lung transplantation |
| ○ Some patients receive echinocandin therapy prior to lung transplantation |
| Which patients receive echinocandin therapy prior to lung transplantation (select all that apply)? |
| □ Patients with a diagnosis of Cystic Fibrosis |
| □ Patients with bronchiectasis without a diagnosis of Cystic Fibrosis |
| □ Patients with Aspergillus sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with mold airway colonization (other than Aspergillus sp.) regardless of underlying pulmonary co-morbidities |
| □ Patients with non-Candida yeast airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with Candida sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with history of invasive Aspergillus sp. infection |
| □ Patients with history of invasive mold (other than Aspergillus sp.) infection |
| □ Patients with history of non-Candida yeast infection |
| □ Patients with history of Candida sp. infection |
| □ Other |
| Please describe “Other” |
| Do patients listed for lung transplant receive systemic amphotericin therapy prior to lung transplantation for prophylaxis? |
| ○ All patients receive systemic amphotericin prior to lung transplantation |
| ○ No patients receive systemic amphotericin prior to lung transplantation |
| ○ Some patients receive systemic amphotericin therapy prior to lung transplantation |
| Which patients receive systemic amphotericin therapy prior to lung transplantation (select all that apply)? |
| □ Patients with a diagnosis of Cystic Fibrosis |
| □ Patients with bronchiectasis without a diagnosis of Cystic Fibrosis |
| □ Patients with Aspergillus sp. Airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with mold airway colonization (other than Aspergillus sp.) regardless of underlying pulmonary co-morbidities |
| □ Patients with non-Candida yeast airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with Candida sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with history of invasive Aspergillus sp. Infection |
| □ Patients with history of invasive mold (other than Aspergillus sp.) infection |
| □ Patients with history of non-Candida yeast infection |
| □ Patients with history of Candida sp. infection |
| □ Other |
| Please describe “Other” |
| Post Lung Transplant: The following questions pertain to the post-transplant period. |
|---|
| Does your center employ post-transplant, pharmacologic anti-fungal prophylaxis? |
| ○ Yes |
| ○ No |
| ○ Unsure |
| What prompts pharmacologic anti-fungal prophylaxis post-transplantation (Select all that apply)? |
| □ Our center uses universal post-transplant anti-fungal prophylaxis |
| □ Diagnosis of Cystic Fibrosis |
| □ Patient’s occupation |
| □ Patient’s geographic location |
| □ Pre-transplant airway colonization with Aspergillus sp. |
| □ Post-transplant airway colonization with Aspergillus sp. |
| □ Pre-transplant airway colonization with other molds |
| □ Post-transplant airway colonization with other molds |
| □ Pre-transplant airway colonization with non-Candida yeast |
| □ Pre-transplant airway colonization with Candida sp. |
| □ Post-transplant airway colonization with non-Candida yeast |
| □ Post-transplant airway colonization with Candida sp. |
| □ Presence of bronchiectasis without a diagnosis of Cystic Fibrosis |
| □ Other |
| Please describe “Other” |
| What is the goal of post-transplant anti-fungal prophylaxis (Select all that apply)? |
| □ Prevention of post-transplantation tracheobronchitis |
| □ Prevention of post-transplantation invasive fungal infection |
| □ Prevention of Bronchiolitis Obliterans Syndrome/ Chronic Lung Allograft Dysfunction |
| □ Other |
| Please describe “Other” |
| What anti-fungal agent(s) is preferred by your center for post-transplant anti-fungal prophylaxis? (You may select up to 1 topical,1 in-hospital systemic agent, and 1 post-hospital systemic agent) |
| Topical agents |
| ○ Aerosolized amphotericin B |
| ○ Other |
| ○ No topical agent is used |
| Please describe “Other” |
| In-hospital systemic agents |
| ○ Amphotericin |
| ○ Anidulafungin |
| ○ Caspofungin |
| ○ Fluconazole |
| ○ Micafungin |
| ○ Isavuconazole |
| ○ Itraconazole |
| ○ Posaconazole |
| ○ Voriconazole |
| ○ Other |
| ○ No in-hospital systemic agent is used |
| Please describe “Other” |
| Post-hospital systemic agents |
| ○ Amphotericin |
| ○ Anidulafungin |
| ○ Caspofungin |
| ○ Fluconazole |
| ○ Micafungin |
| ○ Isavuconazole |
| ○ Itraconazole |
| ○ Posaconazole |
| ○ Voriconazole |
| ○ Other |
| ○ No post-hospital systemic agent is used |
| Please describe “Other” |
| What is the average duration of post-transplant topical (i.e. nebulized or aerosolized) anti-fungal prophylaxis? |
| ○ In-hospital post-transplant surgery only |
| ○ Less than 3 months |
| ○ 3 to 6 months |
| ○ Greater than 6 months but less than 12 months |
| ○ 12 months but less than lifelong |
| ○ Lifelong |
| What is the average duration of post-transplant systemic antifungal prophylaxis? |
| ○ In-hospital post-transplant surgery only |
| ○ Less than 3 months |
| ○ 3 to 6 months |
| ○ Greater than 6 months but less than 12 months |
| ○ 12 months but less than lifelong |
| ○ Lifelong |
| What is the dosing method used for your center’s preferred post-transplant triazole therapy? Triazole therapy includes: fluconazole, voriconazole, itraconazole, posaconazole, isavuconazole. |
| ○ Standard uniform dosing regardless of serum anti-fungal levels |
| ○ Dosed to a serum “detectable” anti-fungal level |
| ○ Dosed to a specific targeted serum anti-fungal level |
| ○ Other |
| Please describe “Other” |
| Do patients less than 3 months post-lung transplant receive inhaled amphotericin for prophylaxis? |
| ○ All patients receive inhaled amphotericin during this post-transplant period |
| ○ No patients receive inhaled amphotericin during this post-transplant period |
| ○ Some patients receive inhaled amphotericin during this post-transplant period |
| Which patients receive inhaled amphotericin less than 3 months post-lung transplant (Select all that apply)? |
| □ Patients with a diagnosis of Cystic Fibrosis |
| □ Patients with bronchiectasis without a diagnosis of Cystic Fibrosis |
| □ Patients with pre-transplant Aspergillus sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with post-transplant Aspergillus sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with pre-transplant mold airway colonization (other than Aspergillus sp.) regardless of underlying pulmonary co-morbidities |
| □ Patients with post-transplant mold airway colonization (other than Aspergillus sp.) regardless of underlying pulmonary co-morbidities |
| □ Patients with pre-transplant non-Candida yeast airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with post-transplant non-Candida yeast airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with pre-transplant Candida sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with post-transplant Candida sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with pre-transplant invasive Aspergillus sp. Infection |
| □ Patients with pre-transplant invasive mold (other than Aspergillus sp.) infection |
| □ Patients with pre-transplant non-Candida yeast infection |
| □ Patients with pre-transplant Candida sp. infection |
| □ Other |
| Please describe “Other” |
| Do patients less than 3 months post-lung transplant receive systemic triazole(s) for prophylaxis? Triazole therapy includes: fluconazole, voriconazole, itraconazole, posaconazole, isavuconazole. |
| ○ All patients receive systemic triazole therapy during this post-transplant period |
| ○ No patients receive systemic triazole therapy during this post-transplant period |
| ○ Some patients receive triazole(s) during this post-transplant period |
| Which patients receive triazole(s) less than 3 months post-lung transplant (Select all that apply)? |
| □ Patients with a diagnosis of Cystic Fibrosis |
| □ Patients with bronchiectasis without a diagnosis of Cystic Fibrosis |
| □ Patients with pre-transplant Aspergillus sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with post-transplant Aspergillus sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with pre-transplant mold airway colonization (other than Aspergillus sp.) regardless of underlying pulmonary co-morbidities |
| □ Patients with post-transplant mold airway colonization (other than Aspergillus sp.) regardless of underlying pulmonary co-morbidities |
| □ Patients with pre-transplant non-Candida yeast airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with post-transplant non-Candida yeast airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with pre-transplant Candida sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with post-transplant Candida sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with pre-transplant invasive Aspergillus sp. Infection |
| □ Patients with pre-transplant invasive mold (other than Aspergillus sp.) infection |
| □ Patients with pre-transplant non-Candida yeast infection |
| □ Patients with pre-transplant Candida sp. infection |
| □ Other |
| Please describe “Other” |
| Do patients less than 3 months post-lung transplant receive systemic echinocandin(s) for prophylaxis? Echinocandin therapy includes: caspofungin, micafungin, anidulafungin. |
| ○ All patients receive systemic echinocandin during this post-transplant period |
| ○ No patients receive systemic echinocandin therapy during this post-transplant period |
| ○ Some patients receive echinocandin(s) during this post-transplant period |
| Which patients receive systemic echinocandin(s) less than 3 months post-lung transplant (Select all that apply)? |
| □ Patients with a diagnosis of Cystic Fibrosis |
| □ Patients with bronchiectasis without a diagnosis of Cystic Fibrosis |
| □ Patients with pre-transplant Aspergillus sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with post-transplant Aspergillus sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with pre-transplant mold airway colonization (other than Aspergillus sp.) regardless of underlying pulmonary co-morbidities |
| □ Patients with post-transplant mold airway colonization (other than Aspergillus sp.) regardless of underlying pulmonary co-morbidities |
| □ Patients with pre-transplant non-Candida yeast airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with post-transplant non-Candida yeast airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with pre-transplant Candida sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with post-transplant Candida sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with pre-transplant invasive Aspergillus sp. Infection |
| □ Patients with pre-transplant invasive mold (other than Aspergillus sp.) infection |
| □ Patients with pre-transplant non-Candida yeast infection |
| □ Patients with pre-transplant Candida sp. infection |
| □ Other |
| Please describe “Other” |
| Do patients less than 3 months post-lung transplant receive systemic amphotericin for prophylaxis? |
| ○ All patients receive systemic amphotericin during this post-transplant period |
| ○ No patients receive systemic amphotericin during this post-transplant period |
| ○ Some patients receive systemic amphotericin during this post-transplant period |
| Which patients receive systemic amphotericin less than 3 months post-lung transplant (Select all that apply)? |
| □ Patients with a diagnosis of Cystic Fibrosis |
| □ Patients with bronchiectasis without a diagnosis of Cystic Fibrosis |
| □ Patients with pre-transplant Aspergillus sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with post-transplant Aspergillus sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with pre-transplant mold airway colonization (other than Aspergillus sp.) regardless of underlying pulmonary co-morbidities |
| □ Patients with post-transplant mold airway colonization (other than Aspergillus sp.) regardless of underlying pulmonary co-morbidities |
| □ Patients with pre-transplant non-Candida yeast airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with post-transplant non-Candida yeast airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with pre-transplant Candida sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with post-transplant Candida sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with pre-transplant invasive Aspergillus sp. Infection |
| □ Patients with pre-transplant invasive mold (other than Aspergillus sp.) infection |
| □ Patients with pre-transplant non-Candida yeast infection |
| □ Patients with pre-transplant Candida sp. infection |
| □ Other |
| Please describe “Other” |
| Do patients 3 months but less than 12 months post-lung transplant receive inhaled amphotericin for prophylaxis? |
| ○ All patients receive inhaled amphotericin during this post-transplant period |
| ○ No patients receive inhaled amphotericin during this post-transplant period |
| ○ Some patients receive inhaled amphotericin during this post-transplant period |
| Which patients receive inhaled amphotericin 3 months but less than 12 months post-lung transplant (Select all that apply)? |
| □ Patients with a diagnosis of Cystic Fibrosis |
| □ Patients with bronchiectasis without a diagnosis of Cystic Fibrosis |
| □ Patients with pre-transplant Aspergillus sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with post-transplant Aspergillus sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with pre-transplant mold airway colonization (other than Aspergillus sp.) regardless of underlying pulmonary co-morbidities |
| □ Patients with post-transplant mold airway colonization (other than Aspergillus sp.) regardless of underlying pulmonary co-morbidities |
| □ Patients with pre-transplant non-Candida yeast airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with post-transplant non-Candida yeast airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with pre-transplant Candida sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with post-transplant Candida sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with pre-transplant invasive Aspergillus sp. infection |
| □ Patients with pre-transplant invasive mold (other than Aspergillus sp.) infection |
| □ Patients with pre-transplant non-Candida yeast infection |
| □ Patients with pre-transplant Candida sp. infection |
| □ Other |
| Please describe “Other” |
| Do patients 3 months but less than 12 months post-lung transplant receive systemic triazole(s) for prophylaxis? Triazole therapy includes: fluconazole, voriconazole, itraconazole, posaconazole, isavuconazole. |
| ○ All patients receive systemic triazole therapy during this post-transplant period |
| ○ No patients receive systemic triazole therapy during this post-transplant period |
| ○ Some patients receive triazole(s) during this post-transplant period |
| Which patients receive systemic triazole(s) 3 months but less than 12 months post-lung transplant (Select all that apply)? |
| □ Patients with a diagnosis of Cystic Fibrosis |
| □ Patients with bronchiectasis without a diagnosis of Cystic Fibrosis |
| □ Patients with pre-transplant Aspergillus spp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with post-transplant Aspergillus spp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with pre-transplant mold airway colonization (other than Aspergillus spp.) regardless of underlying pulmonary co-morbidities |
| □ Patients with post-transplant mold airway colonization (other than Aspergillus spp.) regardless of underlying pulmonary co-morbidities |
| □ Patients with pre-transplant non-Candida yeast airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with pre-transplant Candida sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with post-transplant non-Candida yeast airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with post-transplant Candida sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with pre-transplant invasive Aspergillus spp. infection |
| □ Patients with pre-transplant invasive mold (other than Aspergillus spp.) infection |
| □ Patients with pre-transplant non-Candida yeast infection |
| □ Patients with pre-transplant Candida sp. infection |
| □ Other |
| Please describe “Other” |
| Do patients 3 months but less than 12 months post-lung transplant receive systemic echinocandin(s) for prophylaxis? Echinocandin therapy includes: caspofungin, micafungin, anidulafungin. |
| ○ All patients receive systemic echinocandin during this post-transplant period |
| ○ No patients receive systemic echinocandin therapy during this post-transplant period |
| ○ Some patients receive echinocandin(s) during this post-transplant period |
| Which patients receive systemic echinocandin(s) 3 months but less than 12 months post-lung transplant (Select all that apply)? |
| □ Patients with a diagnosis of Cystic Fibrosis |
| □ Patients with bronchiectasis without a diagnosis of Cystic Fibrosis |
| □ Patients with pre-transplant Aspergillus sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with post-transplant Aspergillus sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with pre-transplant mold airway colonization (other than Aspergillus sp.) regardless of underlying pulmonary co-morbidities |
| □ Patients with post-transplant mold airway colonization (other than Aspergillus sp.) regardless of underlying pulmonary co-morbidities |
| □ Patients with pre-transplant non-Candida yeast airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with post-transplant non-Candida yeast airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with pre-transplant Candida sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with post-transplant Candida sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with pre-transplant invasive Aspergillus sp. infection |
| □ Patients with pre-transplant invasive mold (other than Aspergillus sp.) infection |
| □ Patients with pre-transplant non-Candida yeast infection |
| □ Patients with pre-transplant Candida sp. infection |
| □ Other |
| Please describe “Other” |
| Do patients 3 months but less than 12 months post-lung transplant receive systemic amphotericin for prophylaxis? |
| ○ All patients receive systemic amphotericin during this post-transplant period |
| ○ No patients receive systemic amphotericin during this post-transplant period |
| ○ Some patients receive systemic amphotericin during this post-transplant period |
| Which patients receive systemic amphotericin 3 months but less than 12 months post-lung transplant (Select all that apply)? |
| □ Patients with a diagnosis of Cystic Fibrosis |
| □ Patients with bronchiectasis without a diagnosis of Cystic Fibrosis |
| □ Patients with pre-transplant Aspergillus sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with post-transplant Aspergillus sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with pre-transplant mold airway colonization (other than Aspergillus sp.) regardless of underlying pulmonary co-morbidities |
| □ Patients with post-transplant mold airway colonization (other than Aspergillus sp.) regardless of underlying pulmonary co-morbidities |
| □ Patients with pre-transplant non-Candida yeast airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with post-transplant non-Candida yeast airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with pre-transplant Candida sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with post-transplant Candida sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with pre-transplant invasive Aspergillus sp. infection |
| □ Patients with pre-transplant invasive mold (other than Aspergillus sp.) infection |
| □ Patients with pre-transplant non-Candida yeast infection |
| □ Patients with pre-transplant Candida sp. infection |
| □ Other |
| Please describe “Other” |
| Do patients 12 months or greater post-lung transplant receive inhaled amphotericin for prophylaxis? |
| ○ All patients receive inhaled amphotericin during this post-transplant period |
| ○ No patients receive inhaled amphotericin during this post-transplant period |
| ○ Some patients receive inhaled amphotericin during this post-transplant period |
| Which patients receive inhaled amphotericin 12 months or greater post-lung transplant (Select all that apply)? |
| □ Patients with a diagnosis of Cystic Fibrosis |
| □ Patients with bronchiectasis without a diagnosis of Cystic Fibrosis |
| □ Patients with pre-transplant Aspergillus sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with post-transplant Aspergillus sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with pre-transplant mold airway colonization (other than Aspergillus sp.) regardless of underlying pulmonary co-morbidities |
| □ Patients with post-transplant mold airway colonization (other than Aspergillus sp.) regardless of underlying pulmonary co-morbidities |
| □ Patients with pre-transplant non-Candida yeast airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with post-transplant non-Candida yeast airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with pre-transplant Candida sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with post-transplant Candida sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with pre-transplant invasive Aspergillus sp. infection |
| □ Patients with pre-transplant invasive mold (other than Aspergillus sp.) infection |
| □ Patients with pre-transplant non-Candida yeast infection |
| □ Patients with pre-transplant Candida sp. infection |
| □ Other |
| Please describe “Other” |
| Do patients 12 months or greater post-lung transplant receive systemic triazole(s) for prophylaxis? Triazole therapy includes: fluconazole, voriconazole, itraconazole, posaconazole, isavuconazole. |
| ○ All patients receive systemic triazole therapy during this post-transplant period |
| ○ No patients receive systemic triazole therapy during this post-transplant period |
| ○ Some patients receive triazole(s) during this post-transplant period |
| Which patients receive systemic triazole 12 months or greater post-lung transplant (Select all that apply)? |
| □ Patients with a diagnosis of Cystic Fibrosis |
| □ Patients with bronchiectasis without a diagnosis of Cystic Fibrosis |
| □ Patients with pre-transplant Aspergillus sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with post-transplant Aspergillus sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with pre-transplant mold airway colonization (other than Aspergillus sp.) regardless of underlying pulmonary co-morbidities |
| □ Patients with post-transplant mold airway colonization (other than Aspergillus sp.) regardless of underlying pulmonary co-morbidities |
| □ Patients with pre-transplant non-Candida yeast airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with post-transplant non-Candida yeast airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with pre-transplant Candida sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with post-transplant Candida sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with pre-transplant invasive Aspergillus sp. infection |
| □ Patients with pre-transplant invasive mold (other than Aspergillus sp.) infection |
| □ Patients with pre-transplant non-Candida yeast infection |
| □ Patients with pre-transplant Candida sp. infection |
| □ Other |
| Please describe “Other” |
| Do patients 12 months or greater post-lung transplant receive systemic echinocandin(s) for prophylaxis? Echinocandin therapy includes: caspofungin, micafungin, anidulafungin. |
| ○ All patients receive systemic echinocandin during this period post-lung transplantation |
| ○ No patients receive systemic echinocandin therapy during this post-transplant period |
| ○ Some patients receive echinocandin(s) during this post-transplant period |
| Which patients receive systemic echinocandins 12 months or greater post-lung transplant (Select all that apply)? |
| □ Patients with a diagnosis of Cystic Fibrosis |
| □ Patients with bronchiectasis without a diagnosis of Cystic Fibrosis |
| □ Patients with pre-transplant Aspergillus sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with post-transplant Aspergillus sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with pre-transplant mold airway colonization (other than Aspergillus sp.) regardless of underlying pulmonary co-morbidities |
| □ Patients with post-transplant mold airway colonization (other than Aspergillus sp.) regardless of underlying pulmonary co-morbidities |
| □ Patients with pre-transplant non-Candida yeast airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with post-transplant non-Candida yeast airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with pre-transplant Candida sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with post-transplant Candida sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with pre-transplant invasive Aspergillus sp. infection |
| □ Patients with pre-transplant invasive mold (other than Aspergillus sp.) infection |
| □ Patients with pre-transplant non-Candida yeast infection |
| □ Patients with pre-transplant Candida sp. infection |
| □ Other |
| Please describe “Other” |
| Do patients 12 months or greater post-lung transplant receive systemic amphotericin for prophylaxis? |
| ○ All patients receive systemic amphotericin during this period post-lung transplantation |
| ○ No patients receive systemic amphotericin during this post-transplant period |
| ○ Some patients receive systemic amphotericin during this post-transplant period |
| Which patients receive systemic amphotericin 12 months or greater post-lung transplant (Select all that apply)? |
| □ Patients with a diagnosis of Cystic Fibrosis |
| □ Patients with bronchiectasis without a diagnosis of Cystic Fibrosis |
| □ Patients with pre-transplant Aspergillus sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with post-transplant Aspergillus sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with pre-transplant mold airway colonization (other than Aspergillus sp.) regardless of underlying pulmonary co-morbidities |
| □ Patients with post-transplant mold airway colonization (other than Aspergillus sp.) regardless of underlying pulmonary co-morbidities |
| □ Patients with pre-transplant non-Candida yeast airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with post-transplant non-Candida yeast airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with pre-transplant Candida sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with post-transplant Candida sp. airway colonization regardless of underlying pulmonary co-morbidities |
| □ Patients with pre-transplant invasive Aspergillus sp. infection |
| □ Patients with pre-transplant invasive mold (other than Aspergillus sp.) infection |
| □ Patients with pre-transplant non-Candida yeast infection |
| □ Patients with pre-transplant Candida sp. infection |
| □ Other |
| Please describe “Other” |
| Barriers to anti-fungal prophylaxis: Almost Done! |
|---|
| At your center, what are the barriers you and your colleagues experience to prescribing anti-fungal prophylaxis for lung transplant candidates or recipients (Select up to 3 answers)? |
| □ Cost of the medications |
| □ Drug interactions |
| □ Insurance prior authorizations |
| □ Insurance denial of coverage |
| □ Lack of formalized guidelines for anti-fungal prophylaxis |
| □ Lack of strong quality evidence for the use of anti-fungal prophylaxis |
| □ Lack of intravenous access |
| □ Pill burden |
| □ Side effects of anti-fungal agents |
| □ Other |
| Please describe “Other” |
| From a patient perspective, what do you believe are the barriers to adherence of anti-fungal prophylaxis (Select up to 3 answers)? |
| □ Cost of the medications |
| □ Drug interactions |
| □ Insurance prior authorizations |
| □ Insurance denial of coverage |
| □ Lack of foreseen benefit |
| □ Pill burden |
| □ Risk of malignancy |
| □ Side effects of anti-fungal agents |
| □ Other |
| Please describe “Other” |
Footnotes
Conflict of interest: None of the authors have any conflict of interest to disclose. All authors have reviewed and contributed to this manuscript.
References:
- 1.Arthurs SK, Eid AJ, Deziel PJ, et al. The impact of invasive fungal diseases on survival after lung transplantation. Clin Transplant. 2010;24(3):341–348. [DOI] [PubMed] [Google Scholar]
- 2.Chong PP, Kennedy CC, Hathcock MA, Kremers WK, Razonable RR. Epidemiology of invasive fungal infections in lung transplant recipients on long-term azole antifungal prophylaxis. Clin Transplant. 2015;29(4):311–318. [DOI] [PubMed] [Google Scholar]
- 3.Weigt SS, Copeland CA, Derhovanessian A, et al. Colonization with small conidia Aspergillus species is associated with bronchiolitis obliterans syndrome: a two-center validation study. Am J Transplant. 2013;13(4):919–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weigt SS, Elashoff RM, Huang C, et al. Aspergillus colonization of the lung allograft is a risk factor for bronchiolitis obliterans syndrome. Am J Transplant. 2009;9(8):1903–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cadena J, Levine DJ, Angel LF, et al. Antifungal prophylaxis with voriconazole or itraconazole in lung transplant recipients: hepatotoxicity and effectiveness. Am J Transplant. 2009;9(9):2085–2091. [DOI] [PubMed] [Google Scholar]
- 6.Husain S, Paterson DL, Studer S, et al. Voriconazole prophylaxis in lung transplant recipients. Am J Transplant. 2006;6(12):3008–3016. [DOI] [PubMed] [Google Scholar]
- 7.Neoh CF, Snell GI, Levvey B, et al. Preemptive treatment with voriconazole in lung transplant recipients. Transpl Infect Dis. 2013;15(4):344–353. [DOI] [PubMed] [Google Scholar]
- 8.Tofte N, Jensen C, Tvede M, Andersen CB, Carlsen J, Iversen M. Use of prophylactic voriconazole for three months after lung transplantation does not reduce infection with Aspergillus: a retrospective study of 147 patients. Scand J Infect Dis. 2012;44(11):835–841. [DOI] [PubMed] [Google Scholar]
- 9.Brett J, Chong O, Graham GG, et al. Antifungal use and therapeutic monitoring of plasma concentrations of itraconazole in heart and lung transplantation patients. Ther Drug Monit. 2013;35(1):133–136. [DOI] [PubMed] [Google Scholar]
- 10.Kato K, Nagao M, Nakano S, et al. Itraconazole prophylaxis for invasive Aspergillus infection in lung transplantation. Transpl Infect Dis. 2014;16(2):340–343. [DOI] [PubMed] [Google Scholar]
- 11.Cornely OA, Maertens J, Winston DJ, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med. 2007;356(4):348–359. [DOI] [PubMed] [Google Scholar]
- 12.Stelzer D, Weber A, Ihle F, et al. Posaconazole liquid vs tablet formulation in lung transplant recipients. Mycoses. 2018;61(3):186–194. [DOI] [PubMed] [Google Scholar]
- 13.Borro JM, Sole A, de la Torre M, et al. Efficiency and safety of inhaled amphotericin B lipid complex (Abelcet) in the prophylaxis of invasive fungal infections following lung transplantation. Transplant Proc. 2008;40(9):3090–3093. [DOI] [PubMed] [Google Scholar]
- 14.Lowry CM, Marty FM, Vargas SO, et al. Safety of aerosolized liposomal versus deoxycholate amphotericin B formulations for prevention of invasive fungal infections following lung transplantation: a retrospective study. Transpl Infect Dis. 2007;9(2):121–125. [DOI] [PubMed] [Google Scholar]
- 15.Monforte V, Roman A, Gavalda J, et al. Nebulized amphotericin B prophylaxis for Aspergillus infection in lung transplantation: study of risk factors. J Heart Lung Transplant. 2001;20(12):1274–1281. [DOI] [PubMed] [Google Scholar]
- 16.Monforte V, Ussetti P, Gavalda J, et al. Feasibility, tolerability, and outcomes of nebulized liposomal amphotericin B for Aspergillus infection prevention in lung transplantation. J Heart Lung Transplant. 2010;29(5):523–530. [DOI] [PubMed] [Google Scholar]
- 17.Palmer SM, Drew RH, Whitehouse JD, et al. Safety of aerosolized amphotericin B lipid complex in lung transplant recipients. Transplantation. 2001;72(3):545–548. [DOI] [PubMed] [Google Scholar]
- 18.Playford EG, Webster AC, Sorell TC, Craig JC. Antifungal agents for preventing fungal infections in solid organ transplant recipients. Cochrane Database Syst Rev. 2004(3):CD004291. [DOI] [PubMed] [Google Scholar]
- 19.Playford EG, Webster AC, Sorrell TC, Craig JC. Systematic review and meta-analysis of antifungal agents for preventing fungal infections in liver transplant recipients. Eur J Clin Microbiol Infect Dis. 2006;25(9):549–561. [DOI] [PubMed] [Google Scholar]
- 20.Ullmann AJ, Lipton JH, Vesole DH, et al. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med. 2007;356(4):335–347. [DOI] [PubMed] [Google Scholar]
- 21.Mitsani D, Nguyen MH, Shields RK, et al. Prospective, observational study of voriconazole therapeutic drug monitoring among lung transplant recipients receiving prophylaxis: factors impacting levels of and associations between serum troughs, efficacy, and toxicity. Antimicrob Agents Chemother. 2012;56(5):2371–2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feist A, Lee R, Osborne S, Lane J, Yung G. Increased incidence of cutaneous squamous cell carcinoma in lung transplant recipients taking long-term voriconazole. J Heart Lung Transplant. 2012;31(11):1177–1181. [DOI] [PubMed] [Google Scholar]
- 23.Wermers RA, Cooper K, Razonable RR, et al. Fluoride excess and periostitis in transplant patients receiving long-term voriconazole therapy. Clin Infect Dis. 2011;52(5):604–611. [DOI] [PubMed] [Google Scholar]
- 24.Dummer JS, Lazariashvilli N, Barnes J, Ninan M, Milstone AP. A survey of anti-fungal management in lung transplantation. J Heart Lung Transplant. 2004;23(12):1376–1381. [DOI] [PubMed] [Google Scholar]
- 25.He SY, Makhzoumi ZH, Singer JP, Chin-Hong PV, Arron ST. Practice variation in Aspergillus prophylaxis and treatment among lung transplant centers: a national survey. Transpl Infect Dis. 2015;17(1):14–20. [DOI] [PubMed] [Google Scholar]
- 26.Neoh CF, Snell GI, Kotsimbos T, et al. Antifungal prophylaxis in lung transplantation--a world-wide survey. Am J Transplant. 2011;11(2):361–366. [DOI] [PubMed] [Google Scholar]
- 27.Husain S, Singh N. Bronchiolitis obliterans and lung transplantation: evidence for an infectious etiology. Semin Respir Infect. 2002;17(4):310–314. [DOI] [PubMed] [Google Scholar]
- 28.Scientific Registry of Transplant Recipient. https://www.srtr.org/transplant-centers/?organ=lung&recipientType=adult&query= Accessed 11/1/2018.
- 29.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Organ Procurement and Transplantation Networks. https://optn.transplant.hrsa.gov/ Accessed 2/27/2019.
- 31.Levine SM, Transplant/Immunology Network of the American College of Chest P. A survey of clinical practice of lung transplantation in North America. Chest. 2004;125(4):1224–1238. [DOI] [PubMed] [Google Scholar]
- 32.Husain S, Zaldonis D, Kusne S, Kwak EJ, Paterson DL, McCurry KR. Variation in antifungal prophylaxis strategies in lung transplantation. Transpl Infect Dis. 2006;8(4):213–218. [DOI] [PubMed] [Google Scholar]
- 33.Sole A, Morant P, Salavert M, Peman J, Morales P, Valencia Lung Transplant G. Aspergillus infections in lung transplant recipients: risk factors and outcome. Clin Microbiol Infect. 2005;11(5):359–365. [DOI] [PubMed] [Google Scholar]
- 34.Baker AW, Maziarz EK, Arnold CJ, et al. Invasive Fungal Infection after Lung Transplantation: Epidemiology in the Setting of Antifungal Prophylaxis. Clin Infect Dis. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pennington KM, Razonable RR, Peters S, et al. Why Do Lung Transplant Patients Discontinue Triazole Prophylaxis? Transpl Infect Dis. 2019:e13067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laverdiere M, Bow EJ, Rotstein C, et al. Therapeutic drug monitoring for triazoles: A needs assessment review and recommendations from a Canadian perspective. Can J Infect Dis Med Microbiol. 2014;25(6):327–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Husain S, Sole A, Alexander BD, et al. The 2015 International Society for Heart and Lung Transplantation Guidelines for the management of fungal infections in mechanical circulatory support and cardiothoracic organ transplant recipients: Executive summary. J Heart Lung Transplant. 2016;35(3):261–282. [DOI] [PubMed] [Google Scholar]
- 38.Pechlivanoglou P, De Vries R, Daenen SM, Postma MJ. Cost benefit and cost effectiveness of antifungal prophylaxis in immunocompromised patients treated for haematological malignancies: reviewing the available evidence. Pharmacoeconomics. 2011;29(9):737–751. [DOI] [PubMed] [Google Scholar]
- 39.Sung AH, Marcella SW, Xie Y. An update to the cost-effectiveness of posaconazole vs fluconazole or itraconazole in the prevention of invasive fungal disease among neutropenic patients in the United States. J Med Econ. 2015;18(5):341–348. [DOI] [PubMed] [Google Scholar]
- 40.Chambers DC, Cherikh WS, Goldfarb SB, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-fifth adult lung and heart-lung transplant report-2018; Focus theme: Multiorgan Transplantation. J Heart Lung Transplant. 2018;37(10):1169–1183. [DOI] [PubMed] [Google Scholar]


