Abstract
A role for matrix metalloproteinases (MMPs) in plasticity-dependent learning has been established. MMPs degrade the extracellular matrix (ECM) when synaptic reorganization is warranted. Previously, we showed that escalation of alcohol self-administration is a learned plasticity-dependent process that requires an intact MMP system. To identify the MMP subtypes within specific brain regions that are associated with plasticity underlying the negative reinforcing effects of alcohol (as measured by escalated alcohol self-administration) during acute withdrawal in alcohol dependence, male Wistar rats were trained to self-administer alcohol in an operant paradigm, subjected to one month of intermittent alcohol vapor exposure to induce alcohol dependence, and then allowed to self-administer alcohol during repeated acute withdrawal self-administration sessions. Subsequently, rat brains were extracted after initial or stable escalated alcohol self-administration phases of acute withdrawal and analyzed by immunoblot to detect MMP-2, −3 and −9 levels in the anterior cingulate cortex (ACC), bed nucleus of the stria terminalis, central amygdala (CeA), hippocampus and nucleus accumbens (NAc). The results showed that MMP-9 expression in the CeA and NAc of alcohol-dependent rats was increased, however, MMP-9 expression in the ACC was decreased during negative reinforcement learning. Subsequently, the importance of plasticity mediated by MMP-9 in escalated alcohol self-administration during acute withdrawal was functionally assessed through site-specific intra-CeA MMP-9 inhibition during repeated acute withdrawal self-administration sessions. MMP-9 inhibition prevented acute withdrawal-induced escalation of alcohol self-administration in a manner that was not confounded by locomotor effects or a permanent inability to learn about the negative reinforcing effects of alcohol.
Introduction
Alcohol dependence-induced neuroadaptations result in the emergence of aversive negative emotional / affective states, such as depressive-like and anxiety-like behavior, during withdrawal (Williams et al. 2012) that can be ameliorated by alcohol consumption making alcohol a negative reinforcer (i.e., the removal of the aversive withdrawal symptoms is reinforcing). This ‘self-medication’ can perpetuate alcohol use disorders (Markou et al. 1998) as an organism learns that negative affective states can be removed through escalation of alcohol consumption (Koob 2003). The negative reinforcing effects of alcohol become a significant motivational factor during the transition to alcohol dependence (Roberts et al. 2000; Walker 2012). Since reinforcement is a learned response and learning is a plasticity-dependent process, the negative reinforcing properties of alcohol in dependence (i.e., escalation of alcohol consumption during acute withdrawal) were hypothesized to be a plasticity-dependent process. Indeed, we showed for the first time that escalation of alcohol self-administration was a plasticity-dependent behavior (Smith et al. 2011) with an acquisition pattern that exhibits a standard learning curve evidenced by a progressive increase in alcohol self-administration during repeated acute withdrawal test sessions until escalated alcohol self-administration reaches a plateau and stabilizes (Smith et al. 2011).
Matrix metalloproteinases (MMPs) are a family of proteases that degrade components of the extracellular matrix (ECM) to allow for central nervous system reorganization and are important regulators of synaptic plasticity required for multiple forms of hippocampal long-term potentiation (LTP)-mediated learning (Wright and Harding 2009; Huntley 2012). In our initial demonstration of MMP involvement in negative reinforcement learning, we showed that broad-spectrum central MMP inhibition suppressed escalated alcohol self-administration during acute withdrawal in alcohol-dependent rats during the initial stage of escalation when negative reinforcement-related neuroplasticity was hypothesized to be necessary. In contrast, in alcohol-dependent animals displaying stable escalated responding indicative of completed learning and neuroplasticity, MMP inhibition had no effect (Smith et al. 2011; Walker 2012); confirming that once operant alcohol self-administration stabilizes, the plasticity-related MMP-mediated period of learning had concluded.
Of the more than 20 MMP subtypes (Huntley 2012), the MMP-2, −3, and −9 subtypes have been implicated in LTP-dependent dorsal hippocampal-based learning tasks including spatial and associative learning (Wright and Harding 2009), as well as plasticity associated with cocaine extinction and reinstatement (Smith et al. 2014). MMP-2 and MMP-9 belong to gelatinase subfamily of MMPs (Sternlicht and Werb 2001). Although MMP-2/−3 have been associated with MMP-9 activation (Ogata et al. 1992; Fridman et al. 1995; Toth et al. 2003), such associations are not consistently observed (Wright et al. 2003; Meighan et al. 2006). In addition, although MMP-2, −3 and −9 have been implicated in dorsal hippocampal-based learning tasks (Wright and Harding 2009), it could be that selective MMP subtypes are involved with different forms of learning, such as negative reinforcement learning. Specific to the hypothesis under evaluation, MMP-9 has been implicated in appetitive, but not aversive, learning-related plasticity in the central nucleus of the amygdala (CeA) of mice (Knapska et al. 2013) and impairments in alcohol seeking in MMP-9 KO mice during alcohol withdrawal were normalized by overexpression of exogenous MMP-9 in CeA (Stefaniuk et al. 2017). In human alcoholics, a C/T polymorphism of the MMP-9 gene (that can produce higher MMP-9 gene transcriptional activity) is associated with alcohol dependence (Samochowiec et al. 2010) and enhanced motivation to drink alcohol (Stefaniuk et al. 2017). Therefore, MMP-mediated plasticity has been identified as an emerging important contributor to factors related to alcohol use disorders (AUDs) and the converging evidence suggests that MMP-9 activity in the CeA could be important for understanding negative reinforcement learning in alcohol dependence.
The CeA, in conjunction with the nucleus accumbens shell (NAc shell) and bed nucleus of the stria terminalis (BNST), form a functionally-interconnected network that comprise the extended amygdala (Alheid and Heimer 1988) that is important for integrating emotion and motivation and has been championed as a critical network involved in alcohol abuse and dependence (Koob 2013). Nuclei within the extended amygdala have been heavily implicated in the positive and negative reinforcing properties of alcohol (Koob and Volkow 2010) and other limbic nuclei, such as the anterior cingulate cortex (ACC) and dorsal hippocampus (HPC), have been implicated in aversive and avoidance learning (Johansen and Fields 2004; Nagy et al. 2007), have AUD-related importance (Rezayof et al. 2008; Gremel et al. 2011), and are sites of potential neuroplasticity associated with negative reinforcement in alcohol dependence (Sirohi et al. 2012).
To identify specific MMPs subtypes in distinct brain regions that are required for negative reinforcement learning during acute withdrawal in alcohol-dependent rats (as evidenced by escalated alcohol self-administration), the current experiments examined the role of MMP-2, −3 and −9 expression in the ACC, BNST, CeA, HPC, and NAc in combination with site-specific MMP inhibition in a manner that dissociates the acquisition of escalated alcohol self-administration from stable escalated alcohol self-administration during acute withdrawal in alcohol-dependent Wistar rats.
METHODS
Animals
Male Wistar rats (N=56; PND 70) housed 2–3/cage in a temperature- and humidity-controlled vivarium on a reversed light-dark cycle (lights off at 6:00AM) had food and water ad libitum. Rats were handled daily 1-wk prior to training. All procedures followed the National Research Council Guide for the Care and Use of Laboratory Animals (National Research Council et al. 2011) and were approved by the Washington State University Institutional Animal Care and Use Committee.
Pre-dependence alcohol self-administration training
Rats were trained to self-administer a 10% alcohol (w/v) solution (0.1 ml / lever-press) on a continuous ratio schedule of reinforcement during 30-min limited-access sessions (5 days/week) in standard operant chambers (Med Associates, St. Albans, VT) containing custom drinking wells (Behavioral Pharma, La Jolla, CA) using a sweetener-fade procedure (Walker and Koob 2007). A sweetened fluid (0.125% saccharin and 3% glucose) that is preferred to sucrose (Valenstein et al. 1967) was used as the reinforcer for acquisition of operant self-administration. Then, 10% alcohol (w/v) was added and the sweeteners were removed from the solution over 3 weeks until only 10% alcohol remained. The number of lever-presses were converted to alcohol consumption (g/kg). Once responding for alcohol stabilized (three consecutive sessions with <10% deviation), rats progressed to the next phase of the experiments.
Alcohol dependence induction via intermittent alcohol vapor exposure
Following self-administration training, rats were subjected to one-month of intermittent alcohol vapor exposure (14h-on, 10h-off per day) which induces alcohol dependence (O’Dell et al. 2004) as evidenced by dependence-like behavioral phenotypes (O’Dell et al. 2004; Walker and Koob 2007; Williams et al. 2012) when animals are tested 6–8 h into acute withdrawal. Blood ethanol concentrations (BECs) were measured twice-weekly by tail blood collection prior to daily termination of the alcohol vapor exposure and analyzed using the Analox AM1 (Analox Instruments, Lunenberg, MA). Target BECs of 175–225 mg% were maintained throughout the experiments and confirmed prior to termination of daily alcohol vapor exposure on every acute withdrawal self-administration test day. The 6–8 h acute withdrawal timepoint used during each of the repeated acute withdrawal tests was first adopted (Walker and Koob 2007) because the BECs of alcohol-dependent rats maintained at ~225 mg% drop to zero within 6-h (Gilpin et al. 2009) and therefore avoids the confound of alcohol presence at the time of acute withdrawal testing.
Experiment 1:
Post-dependence self-administration during acute withdrawal
Following vapor exposure, rats (N=32) were separated into four groups (n=8/grp) matched for 30-min limited-access baseline alcohol consumption (g/kg): 1) initial escalation (IE; represents the first statistically escalated self-administration session during acute withdrawal, 2) initial escalation pharmacological control (IEC), 3) stable escalation (SE; after escalated alcohol self-administration had stabilized), and 4) stable escalation pharmacological control (SEC). Only the IE and SE groups self-administered alcohol following dependence induction, whereas the IEC and SEC groups did not self-administer alcohol following the dependence induction phase but were instead injected intraperitoneally (IP) with equivalent alcohol (g/kg) to that consumed by the IE and SE groups, respectively. The IEC and SEC groups served as pharmacological controls (essentially yoked-controls) since acute alcohol administration can alter MMP expression (Wright et al. 2003). After each test, rats were returned to vapor chambers to re-expose them to alcohol vapor between acute withdrawal self-administration tests so that animals could be repeatedly tested under identical conditions of acute withdrawal.
Immunoblotting
Briefly (see Supplementary Materials for detailed procedures), brains were extracted 3-hr after either initial or stable escalation for the IE and SE groups, respectively, or following equivalent IP injections of alcohol (g/kg) for the IEC and SEC groups. The 3-hr extraction timepoint was based on previous research showing that the 3-hr timepoint could capture both pro and active MMP expression (Meighan et al. 2006). Micropunches of the ACC, BNST, CeA, HPC, and NAc were sonicated and lysed with RIPA lysis buffer (Millipore:20–188) containing Halt protease and phosphatase inhibitor cocktail (Thermo:78441) and microcystin-LR (Enzo, ALX-350–431-C010). After centrifugation at 10,000g for 20 min at 4°C, supernatant was removed and tested for protein concentration using Pierce BCA protein assay kit (Thermo:23225). 15 μg of protein was utilized for separation by SDS-polyacrylamide gel electrophoresis (Bio-rad:5671025) and transferred to nitrocellulose membranes. To ensure equal loading and appropriate electrophoresis and transfer, as well as serving as a transfer control between membranes, the nitrocellulose membranes were stained with MemCode (Thermo:24580), a reversible total protein stain procedure (Fig. S1). Given that most MMPs are synthesized and secreted as an inactive pro-form and are activated following the removal of the pro-domain (Fujioka et al. 2012; Huntley 2012), we selected primary antibodies (Ab) that could detect both pro (P)- and active (A)-forms of MMP-2 (P72- and A67-kD), MMP-3 (P55- and A21-kD) and MMP-9 (P98-, A92- and A68-kD). Following MemCode stain removal, membranes were incubated overnight at 4°C with primary Ab against MMP-2 (1:150; Millipore: AB19167), MMP-3 (1:50; Fitzgerald:70R-11887), and MMP-9 (1:125; Millipore:AB19016). Following 1hr of incubation with HRP-conjugated secondary antibody (Millipore:12–348) at RT, the membrane was soaked in an enhanced ECL (Thermo:34080). Band images were captured with the Bio-rad ChemiDoc XRS+ imaging system and optical density of bands were measured using Image Lab software. The target protein to MemCode ratio was utilized to provide a standardized value for all bands and the percent pharmacological control was calculated.
Experiment 2:
Surgery
Following the establishment of stable 30-min limited-access alcohol self-administration and prior to intermittent alcohol vapor exposure (as described above), rats (N = 24) were anesthetized with isoflurane gas (5% for induction / 2% for maintenance). Bilateral guide cannulae (22 gauge; Plastics One, Roanoke) were implanted 2mm above the CeA (AP −2.3, ML ± 4.2, DV −6.2 from bregma; (Paxinos and Watson 2007), secured in place using dental acrylic cement anchored to four stainless-steel machine screws and obturators maintained cannulae patency. Rats received 5-d of post-operative care with an analgesic (flunixin, 2.5 mg/kg), antibiotic (Baytril, 5 mg/kg), 0.9% saline administered subcutaneously and allowed 2-wks of recovery prior to intermittent alcohol vapor exposure (described above).
Post-dependence self-administration during acute withdrawal
Following alcohol dependence induction (see above), rats (N = 24) were divided into two groups (n = 12/grp) matched for pre-dependence 30-min limited-access alcohol consumption as well as BECs on the initial post-dependence induction test day that occurred during acute withdrawal. Rats were repeatedly tested twice weekly during acute withdrawal (6–8 h into withdrawal) for 30-min limited-access operant alcohol self-administration. After each acute withdrawal test, rats were returned to alcohol vapor chambers to re-expose them to alcohol vapor until the next acute withdrawal 30-min limited-access alcohol self-administration test.
Assessment of Intra-CeA MMP-9 Inhibition: To test the hypothesis that CeA MMP-9 activity was necessary for acute withdrawal-induced escalation of alcohol self-administration, a MMP-9 inhibitor (MMP-9i; 0.1 nmol/side; Millipore:444278) or vehicle (0.6% DMSO in aCSF) were infused (0.5ul/side over 2 min) 15-min prior to, and 45-min following, post-dependence acute withdrawal alcohol self-administration sessions via bilateral 28-gauge injectors (extending 2-mm below the guide cannulae; Plastics One, Roanoke) targeting the CeA. Testing during acute withdrawal was repeated for six sessions until the vehicle-treated group displayed statistically significant escalation of alcohol self-administration. The timing and dosing of MMP-9i infusions were selected based on previous studies (Smith et al. 2011; Smith et al. 2014). Control Conditions: Two control conditions were included in this experiment to confirm the primary hypothesis under evaluation, as well as guard against potential confounds: 1) To address the possibility that intra-CeA MMP-9i administration might induce a permanent loss of an organism’s capacity to acquire escalated alcohol self-administration, once the MMP-9i infusions were terminated, the animals then received vehicle infusions under the same timing conditions as the original MMP-9i treatments to evaluate whether the animals would show normal negative reinforcement learning (i.e., escalated alcohol self-administration). 2) Implied in the hypothesis that MMP activity is necessary for the acquisition of escalated alcohol self-administration is that once stability of escalated alcohol self-administration occurs, MMPs are no longer required because learning/plasticity has concluded. Therefore, the prediction would be that in alcohol-dependent animals demonstrating stable escalated alcohol self-administration during acute withdrawal, intra-CeA MMP-9i would have no effect on their self-administration performance. In addition, because the MMP-9i was, in part, administered prior to the self-administration sessions, the MMP-9i pretreatment could have rate-decreasing effects and a failure to observe escalated alcohol self-administration could be a result of non-specific MMP-9i effects. To rule out this possibility, once the animals originally receiving vehicle treatments displayed stable escalated alcohol self-administration, an acute intra-CeA MMP-9i infusion was administered 15-min prior to a final self-administration session during acute withdrawal.
Histology
After the completion of Experiment 2 (19/24 animals successfully completed the dependence induction period and subsequent testing), rat brains were removed, sectioned and cannula placement was verified.
Statistics
Experiment 1
Baseline alcohol self-administration and dependence-induced escalation: A univariate analysis of variance (ANOVA) compared baseline alcohol consumption (g/kg) for the four conditions (IE, IEC, SE and SEC). Baseline and escalated alcohol consumption for the IE and SE conditions were compared using a two-way mixed-model ANOVA with condition as the between-group factor and session as the within-subject factor. Post-hoc repeated-measures tests were conducted if main effects or interactions were identified. Lastly, a univariate ANOVA was used to compare escalated alcohol consumption for the IE and SE groups prior to immunoblotting. Immunoblotting: Within the five brain regions and three different MMP subtypes analyzed (that included both pro and active MMPs), two-way ANOVAs were utilized to assess protein to MemCode ratios with escalation group (initial vs stable) and condition (self-administering vs pharmacological control) as the between-group factors. If significant interactions were identified, post-hoc comparisons were conducted to identify the specific group differences for each MMP pro and active form(s) in each of the brain regions.
Experiment 2
Baseline alcohol self-administration: A univariate ANOVA compared baseline alcohol consumption between the vehicle and MMP-9i groups. Intra-CeA MMP-9i effects on dependence-induced escalation: A two-way mixed-model ANOVA compared vehicle- and MMP-9i treatments during acute withdrawal with treatment (vehicle or MMP-9i) as the between-group factor and session (initial vs escalated) as the within-subject factor. If main effects or interactions were identified, the appropriate post-hoc comparisons (i.e., between-group and/or repeated-measures tests) were conducted. Control Conditions: In those animals that initially received intra-CeA MMP-9i infusions, a repeated-measures ANOVA compared the effects of intra-CeA vehicle infusions over a final three acute withdrawal self-administration sessions, with post-hoc comparisons conducted if a main effect was found. During acute withdrawal in the group of animals that were originally vehicle-treated and subsequently received acute MMP-9i in the CeA, a repeated-measures ANOVA was used to compare alcohol consumption between their final vehicle-treated stable escalated consumption session and the subsequent acute MMP-9i infusion session.
Results
Experiment 1
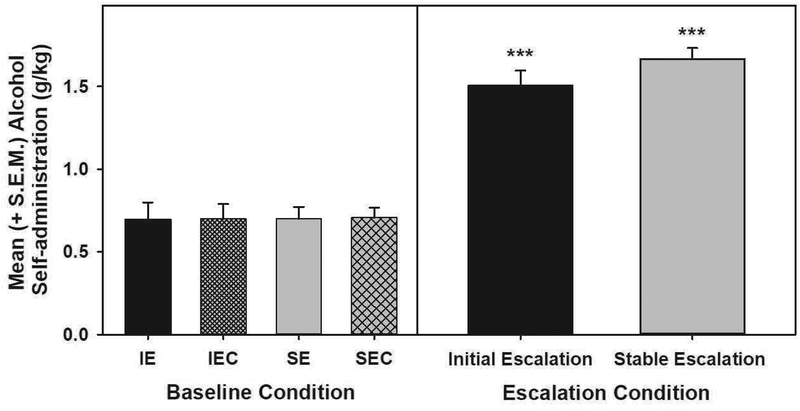
Baseline alcohol self-administration and dependence-induced escalation (Fig. 1): The univariate ANOVA confirmed that there were no differences in baseline alcohol consumption for the IE, IEC, SE and SEC groups (F(1,28)=0.002, p>0.05). The two-way mixed-model ANOVA conducted on baseline and escalated alcohol consumption for the IE and SE conditions identified a main effect of session (F(1,14)=175.929, p<0.001). Post-hoc comparisons showed that both the IE and SE group’s alcohol consumption was escalated compared to baseline consumption (F(1,7)=44.114, p<0.001 and F(1,7)=295.77, p<0.001). Lastly, the univariate ANOVA comparing post-dependent escalated alcohol consumption for the IE and SE groups showed that there were no differences (F(1,14)=2.089, p>0.05) between the escalated IE and SE group consumption levels.
Figure 1. Alcohol consumption during pre-vapor baseline and post-vapor dependence-induced escalation sessions.
Data shown as mean + SEM. Baseline alcohol consumption was similar between groups (n=8/grp). Alcohol consumption for the IE and SE groups was significantly escalated when compared to baseline (***p<0.001 vs baseline of the same group), but there were no differences in consumption levels between IE and SE when escalated. IE= Initial escalation; IEC= Initial escalation control; SE=stable escalation; SEC=stable escalation control.
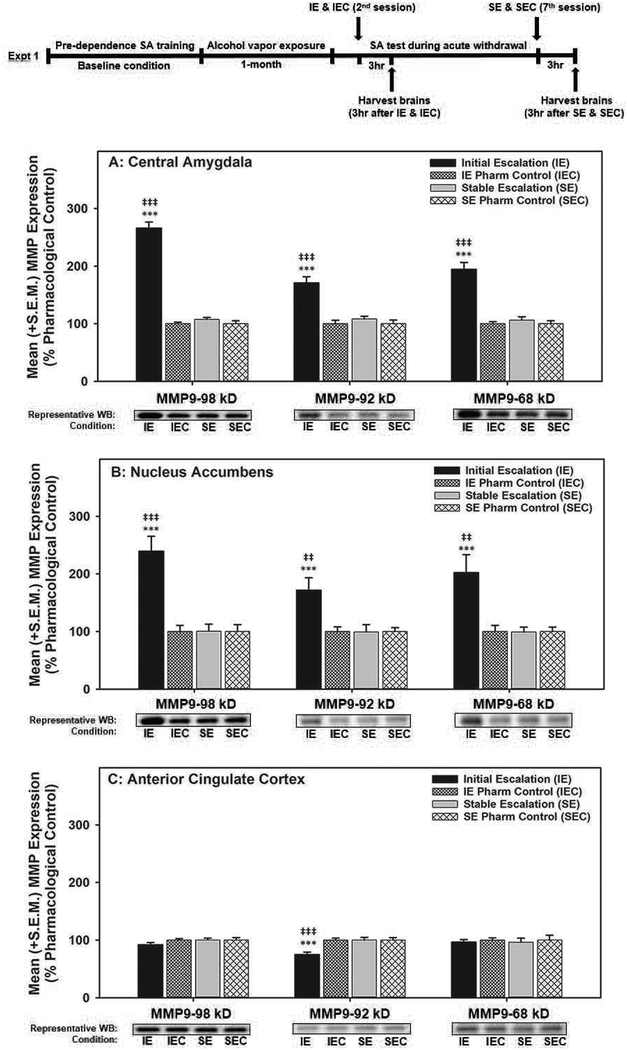
Immunoblotting (Fig. 2): In the CeA and NAc, the two-way ANOVAs conducted on the P98-, A92- and A68-kD forms of MMP-9 identified main effects for Group (CeA: F(1,28)=169.958, p<0.001; F(1,28)=18.529, p<0.001 and F(1,28)=36.166, p<0.001, respectively; NAc: F(1,28)=17.924, p<0.001; F(1,28)=7.161, p≤0.01 and F(1,28)=8.790, p<0.01, respectively) and Condition (CeA: F(1,28)=202.861, p<0.001; F(1,28)=29.345, p<0.001 and F(1,28)=47.215, p<0.001, respectively; NAc: F(1,28)=18.285, p<0.001; F(1,28)=6.809, p≤0.01 and F(1,28)=8.497, p<0.01, respectively), as well as Group x Condition interactions (CeA: F(1,28)=169.958, p<0.001; F(1,28)=18.529, p<0.001 and F(1,28)=36.166, p<0.001, respectively; NAc: F(1,28)=17.924, p<0.001; F(1,28)=7.161, p≤0.01 and F(1,28)=8.790, p<0.01, respectively). Alternatively, in the ACC, only A92-kD MMP-9 showed a main effect of Group (F(1,28)=8.927, p<0.01), Condition (F(1,28)=9.104, p<0.01) and a Group x Condition interaction (F(1,28)=8.927, p<0.01). Post-hoc comparisons indicated that in the CeA and NAc, the P98-, A92- and A68-kD forms of MMP-9 were elevated in the IE group when compared to IEC (CeA: t(14)=15.662, p<0.001; t(14) =5.781, p<0.001 and t(14)=7.579, p<0.001, respectively; NAc: t(14)=4.961, p<0.001; t(14) 3.128, p<0.01 and t(14)=3.110, p<0.01, respectively) and SE (CeA: t(14)=14.645, p<0.001; t(14)=5.325, p<0.001 and t(14)=6.621, p<0.001, respectively; NAc: t(14)=4.855, p<0.001; t(14)=2.896, p≤0.01 and t(14)=3.201, p<0.01, respectively). Conversely, in the ACC, the A92-kD MMP-9 was selectively reduced in the IE group when compared to IEC (t(14)=−4.623, p<0.001) and SE (t(14)=−3.906, p<0.01). See Figure S2 for ACC, CeA and NAc MMP-9 western blot images. No changes were identified for MMP-2 or MMP-3 expression in the CeA, NAc and ACC or MMP-2, −3 or −9 expression in the BNST or HPC (see Figure S3 for western blot images).
Figure 2. Alcohol dependence-induced MMP-9 immunoreactivity in the central amygdala, nucleus accumbens, and anterior cingulate cortex during acute withdrawal.
Data shown as mean + SEM (n=8/grp). Top Panel: Timeline for Experiment 1. Panel A: In the CeA, the IE group showed increased expression of pro and active (92- and 68-kD) MMP-9 than the IEC group (‡‡‡ = p < 0.001 when compared to IEC) and SE group (*** = p < 0.001). Panel B: Similarly, in the NAc, the IE group showed elevated pro and active (92- and 68-kD) MMP-9 expression compared to the IEC group (‡‡ = p < 0.01 and ‡‡‡ = p < 0.001) and the SE group (*** = p < 0.001). Panel C: In contrast to the CeA and NAc, the IE group in ACC showed a selective decrease in expression of active 92-kD MMP-9 compared to IEC and SE (‡‡‡ = p < 0.001 and *** = p < 0.001, respectively). IE = Initial escalation; IEC = Initial escalation pharmacological control; SA = self-administration; SE = stable escalation; SEC = stable escalation pharmacological control; WB = western blot.
Experiment 2
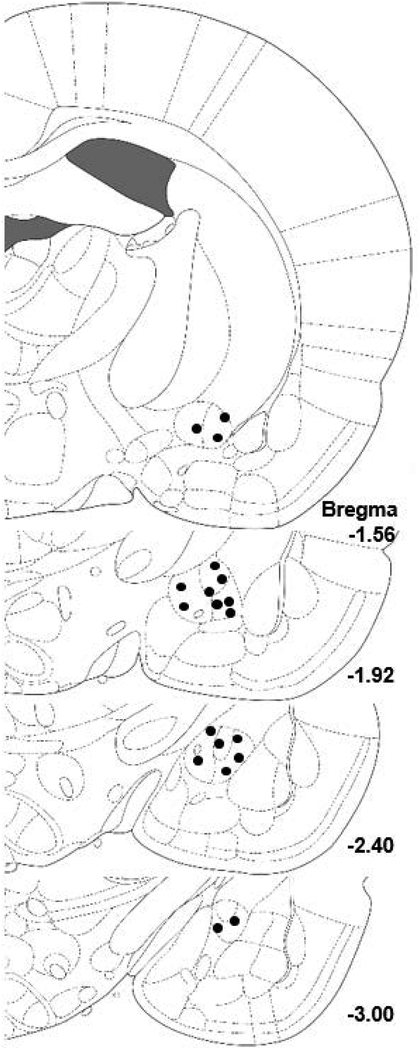
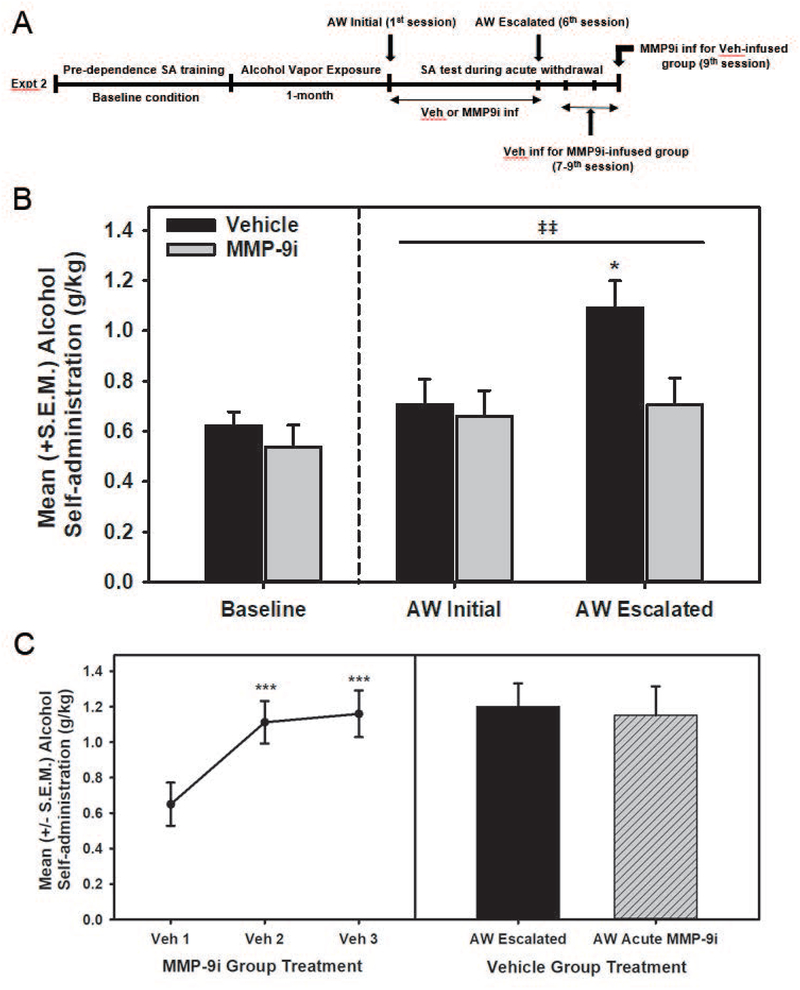
Histology: Only animals with verified CeA MMP-9i infusion sites were included in the statistical analysis (Fig. 3). The final number of animals included in the Experiment 2 analysis was n=10 for the MMP-9i group and n=9 for the vehicle-treated group. Baseline alcohol self-administration (Fig. 4B): The univariate ANOVA confirmed no differences in baseline alcohol consumption between the treatment (vehicle or MMP-9i) groups (F(1,17)=0.635, p>0.05). Intra-CeA MMP-9i effects on dependence-induced escalation (Fig. 4B): The two-way mixed-model ANOVA identified a main effect of session (F(1,17)=22.579, p<0.001) and a treatment x session interaction (F(1,17)=14.090, p<0.01), with post-hoc comparisons identifying that MMP-9i treatment group had significantly reduced alcohol consumption when compared to the vehicle-treated condition (t(17)=2.554, p<0.05). Control Conditions (Fig. 4C): In animals that were originally treated with intra-CeA MMP-9i and did not escalate, a repeated-measures ANOVA identified that the animals showed a significant escalation of alcohol consumption over three sessions when vehicle-treated (F(2,18)=42.596, p<0.001) with post-hoc comparisons identifying that both the 2nd and 3rd acute withdrawal alcohol self-administration were significantly escalated when compared to the 1st session (p<0.001 in both cases). Lastly, a repeated-measures ANOVA confirmed that there were no differences between the vehicle-treated group’s escalated responding when compared to the acute intra-CeA MMP-9i treatment condition (F(1,8)=0.944, p>0.05).
Figure 3: Schematic representation of CeA infusion sites.
Black circles indicate cannulae placement within the central amygdala and reflect bilateral infusion sites. Adapted from Paxinos and Watson, 2007.
Figure 4.
Panel A: Timeline for Experiment 2. Panel B: MMP-9 inhibition in the CeA prevents acute withdrawal-induced escalation of alcohol self-administration in alcohol dependence. Data shown as mean +/− SEM. Baseline alcohol consumption between the treatment groups (vehicle or MMP-9i) was comparable. The MMP-9 inhibitor (n=10) or vehicle (n=9) were infused into the CeA 15-min prior to and 45-min after acute withdrawal self-administration sessions following the one-month dependence induction phase (denoted by the vertical dotted line). The two treatment conditions displayed differential escalation of alcohol self-administration behavior (as evidenced by a significant treatment x session interaction, ‡‡ = p < 0.01) with the vehicle-treated group significantly escalated compared to the MMP-9i group (* = p < 0.05). Panel C: Control conditions for interpreting intra-CeA MMP-9 inhibition of escalated alcohol self-administration during acute withdrawal. Data shown as mean +/− SEM. Left Panel: The MMP-9 inhibitor-treated rats (n=10) that did not show escalated alcohol self-administration received subsequent vehicle infusions in the CeA 15-min prior to and 45-min after acute withdrawal self-administration sessions and displayed escalated alcohol self-administration over three sessions (*** = p < 0.001 when compared to Veh 1). Right Panel: The vehicle-treated rats (n=9) that displayed escalated alcohol self-administration during acute withdrawal were acutely administered the MMP-9 inhibitor in the CeA 15-min prior to a final post-dependence self-administration which did not alter their escalated alcohol self-administration profile. AW = acute withdrawal; inf = infusion; MMP-9i = MMP-9 inhibitor; SA = self-administration; Veh = Vehicle.
Discussion
The current experiments tested the hypothesis that MMP-mediated plasticity within limbic circuitry is required for negative reinforcement-related learning in alcohol-dependent rats during acute withdrawal. The results of the first experiment showed that in alcohol-dependent rats, the initial escalation phase of alcohol self-administration during acute withdrawal was specifically associated with increased P98-, A92- and A68-kD MMP-9 (but not MMP-2 or MMP-3) in the CeA and NAc (Fig. 1), with no changes in MMP-2, −3 or −9 expression in the BNST or HPC. Once escalated alcohol self-administration had stabilized, when learning / plasticity is posited to have completed, MMP-9 expression returned to pharmacological control levels in CeA and NAc (Fig. 2). Intriguingly, concomitant with increased MMP-9 expression in the CeA and NAc, a selective decrease in A92-kD MMP-9 expression was observed in the ACC (Fig. 2). Given the previously-observed relationships between MMP-2/3 and MMP-9 (Ogata et al. 1992; Fridman et al. 1995; Toth et al. 2003), an important caveat in relation to the timing of brain extractions is that MMP-2 or MMP-3 expression and activity could occur before MMP-9 expression. Therefore, it could be possible that MMP-2/3 expression was altered before we harvested brain tissue for analysis and would not have been detectable, but it should be noted that previous research has observed alterations in MMP-3 and MMP-9 under learning-related conditions using the same brain extraction timing (Meighan et al. 2006). In rat dorsal hippocampus, 92- and 68-kD MMP-9 have been identified as active forms of MMP-9 (Meighan et al. 2006), although a 86-kD form of MMP-9 detected in human serum has also been posited as active (Demestre et al. 2005) with these differences potentially attributable to species and substrate variation. The primary MMP-9 Ab used in the present study was able to consistently detect P98-, A92- and A68-kD MMP-9 in all brain regions assessed, whereas an 86-kD band was inconsistently observed and therefore, not included in our assessment (see Figure S2 and S3 for MMP-9 blots).
Alterations in MMP-9 expression in the present study are consistent with results showing that MMP-9 activity in the CeA is elevated in appetitive, but not aversive, learning and is localized to postsynaptic glutamatergic synapses (Knapska et al. 2013). Also consistent with these results, MMP-9 knock-out mice displayed disrupted LTP in the basal and central amygdala (Gorkiewicz et al. 2015) and disruptions in alcohol-seeking were ameliorated once MMP levels were normalized through exogenous overexpression of MMP-9 in the CeA (Stefaniuk et al. 2017). Given that the CeA results in the present study were accompanied by a parallel increase in MMP-9 expression in the NAc, but not in the BNST, is interesting given the functional interconnectivity of these extended amygdala regions and evidence implicating these regions in the motivational and reinforcing effects of alcohol (Koob et al. 1993; Koob 2013; Roberto and Varodayan 2017; Vranjkovic et al. 2017). The concomitant increase in NAc and CeA MMP-9 expression suggests that plasticity in both the NAc and CeA contribute to the negative reinforcing effects of alcohol during acute withdrawal. Neuroadaptations in both the NAc and CeA have been shown to mediate escalated operant alcohol self-administration during acute withdrawal in alcohol-dependence (e.g., Nealey et al. 2011; Kissler et al. 2014) and in the CeA, MMP-9 KO mice did not show silent synapse formation consistent with the plasticity-related changes associated with alcohol withdrawal (Stefaniuk et al. 2017). Within the NAc, plasticity-related adaptations such as silent synapse formation have been implicated in incubation of craving and an amygdala / accumbens circuit, consistent with the present results, has been shown to be important in drug relapse (Lee et al. 2013). Although we did not observe alterations in BNST MMP expression under the conditions tested, it should be noted that BNST dysregulation does contribute to escalated alcohol self-administration during acute withdrawal (Erikson et al. 2018). Future research could delineate BNST contributions to the formation or removal of negative affective states that appear to be required for escalated alcohol self-administration in dependence (Walker 2012). Although the dorsal HPC has been repeatedly implicated in MMP-mediated learning and plasticity in a variety of learning paradigms (Wright and Harding 2009; Huntley 2012), dorsal HPC MMP-2, −3 or −9 expression was not altered during the initial phase of escalation when compared to stable escalation or when compared to the alcohol pharmacological control during acute withdrawal in alcohol-dependent rats.
In contrast to increased MMP-9 in the CeA and NAc, the ACC showed a selective reduction in the A92-kD MMP-9 during the initial phase of escalation when compared to stable escalation and when compared to the alcohol pharmacological control (Fig. 2). Since the ACC is important for avoidance learning (Johansen and Fields 2004) and the CeA/NAc important for reinforcement (Koob 1992; Koob and Le Moal 2001), the disparity in MMP-9 activity between these brain regions could be indicative of the coordinated actions that underlie progressive escalation of alcohol self-administration during acute withdrawal. Given that the up-regulation of MMP-9 in the CeA/NAc of the IE group comprised both pro- and active-forms of MMP-9, whereas there was a specific reduction in A92-kD MMP-9, but not the pro-form, in the ACC suggests that this decrease in ACC MMP-9 activity occurs in a manner distinct from the traditional MMP synthetic pathway. The selective reduction in the A92-kD MMP-9 expression in the ACC could be mediated by increased expression and/or activity of tissue inhibitors of metalloproteinases (TIMPs), endogenous inhibitors of matrix metalloproteinases that can terminate MMP activity (Huntley 2012; Smith et al. 2015), with TIMP-1 identified as a potent inhibitor of MMP-9 (Okulski et al. 2007).
Based on the results of Experiment 1 showing specific increases in MMP-9 expression in the CeA and NAc as well as the existing literature (Knapska et al. 2013; Stefaniuk et al. 2017) related MMP-9 in the CeA, Experiment 2 directly evaluated the role of CeA MMP-9 in escalated alcohol self-administration during acute withdrawal, using our previously validated model for investigating the negative reinforcement learning in alcohol dependence and withdrawal (Smith et al. 2011; Walker 2012) in conjunction with site-specific intra-CeA MMP-9i infusions. In Experiment 2, we identified that inhibition of MMP-9 in the CeA during acute withdrawal suppressed the development of dependence-induced escalation of alcohol self-administration (Fig. 4), confirming that MMP-9 activity in the CeA is necessary for acute withdrawal-induced escalation of alcohol self-administration in alcohol dependence. The possibility that the intra-CeA MMP-9i infusions could spread to nuclei adjacent to the CeA and influence withdrawal-induced escalation should be noted, however we have shown that micro-injections of ligands in the CeA specifically impact escalated alcohol self-administration, while off-target infusions do not (Kissler and Walker 2016). These results are consistent with our previously published data using a broad spectrum MMP-inhibitor that prevented the development of dependence-induced escalation of alcohol self-administration (Smith et al. 2011). It is important to recognize that in those animals receiving the MMP-9i in the CeA, MMP-9 inhibition did not alter alcohol self-administration levels from baseline levels that are putatively indicative of the positive reinforcing effects of alcohol (Walker 2012). Indeed, MMP-9 inhibition specifically prevented the acquisition of escalated alcohol self-administration but did not impact stable escalated alcohol self-administration. Collectively, these data are indicative of the fact that once stable responding for alcohol has been achieved, MMP activity in the CeA is no longer relevant since learning-related plasticity has concluded.
The control conditions in the second experiment confirmed that the escalation-preventing effects of MMP-9 inhibition in the CeA were not related to learning deficits or response inhibition following intra-CeA MMP-9i infusions (Fig. 5). Namely, animals originally treated with the MMP-9i in the CeA that did not display escalated alcohol self-administration, showed normal escalation of alcohol self-administration during acute withdrawal once intra-CeA MMP-9i infusions were replaced with intra-CeA vehicle infusions showing that the MMP-9i infusion-mediated prevention of escalated alcohol self-administration was not permanent. Once dependence-induced escalation of alcohol self-administration stabilized, subsequent infusion of the MMP-9i had no effect on dependence-induced escalation of alcohol self-administration during acute withdrawal, suggesting that MMP-9 activity in the CeA was no longer relevant once synaptic plasticity associated with negative reinforcement learning had occurred. Furthermore, the lack of an acute MMP-9i effect on stable escalated alcohol self-administration showed that acute intra-CeA MMP-9i had no rate-decreasing effects. The MMP-9i used in the present study is reported to be a reversible ligand that has selectivity for the hypothesis under test. Although the MMP-9i used in the present study has been shown to inhibit MMP subtypes other than MMP-9, namely MMP-1 and MMP-13 (MMP-9, Ki = 5 nM; MMP-1, Ki = 1.05 mM; MMP-13, Ki = 113 nM; Levin et al. 2001), neither MMP-1 or MMP-13 have been implicated in learning-related neuronal plasticity (Huntley 2012) and therefore the specificity of the MMP-9i was assured when evaluating the plasticity associated with escalated alcohol self-administration during acute withdrawal in alcohol-dependence.
MMP-9 polymorphisms (i.e., a C/T polymorphism of the MMP-9 gene that can induce higher MMP-9 gene transcriptional activity) have been associated with alcohol dependence in humans (Samochowiec et al. 2010) and the serum MMP-9 concentration in alcoholics is higher compared to controls. Furthermore, MMP-9 knockout (KO) mice exhibit impaired synaptic plasticity in CeA following chronic alcohol exposure and display reduced alcohol-seeking that was normalized in the KO mice by exogenous expression of MMP-9 in CeA (Stefaniuk et al. 2017). Additionally, in humans, the same C/T MMP-9 gene polymorphisms observed by Samochowiec et al. 2010 have been shown to correlate with enhanced motivation to drink in alcoholic patients (Stefaniuk et al. 2017).
In addition to a role for MMPs in AUDs, research has also implicated MMPs in other substance use disorders (SUDs). MMP-9 protein levels in medial PFC of rats are upregulated following cocaine-induced reinstatement of cocaine-induced CPP (Brown et al. 2008) and MMP-9 activity is reduced in the hippocampus of cocaine abusers (Mash et al. 2007). Importantly, MMP-9 activity is transiently up-regulated in NAc following cue-induced cocaine reinstatement and MMP-9 inhibition in NAc suppressed cue-mediated reinstatement of cocaine seeking in rats (Smith et al. 2014). Similarly, repeated, but not acute, METH treatment increased MMP-9 activity in the rat frontal cortex and NAc (Mizoguchi et al. 2007b) and MMP-9 inhibition in the rat frontal cortex decreased repeated METH-induced behavioral sensitization as well as METH-induced increase in extracellular dopamine levels in the NAc (Mizoguchi et al. 2007a).
In summary, the present experiments identified increased MMP-9 expression in an amydaloaccumbal circuit concomitant with reduced MMP-9 in the anterior cingulate cortex during the initial phase of acute withdrawal-induced escalated alcohol self-administration that is hypothesized to correspond to negative reinforcement-mediated learning and plasticity in alcohol dependence. Importantly, the necessity of MMP-9 activity was functionally assessed through site-specific intra-CeA MMP-9 inhibition with results demonstrating that MMP-9 activity in the CeA is necessary for escalated alcohol self-administration during acute withdrawal. The current results are consistent with investigations into the role of MMPs in multiple forms of learning, as well as AUDs and SUDs. Collectively, the data presented herein, as well as that in the literature, support the emerging conceptual shift away from the importance of the tripartite synapse (i.e., pre- and post-synaptic neurons in addition to glial regulation) towards a tetrapartite synapse that incorporates the extracellular matrix domain as a critical component of synaptic plasticity (Dityatev and Rusakov 2011; Smith et al. 2015) that could be targeted by future therapeutic development efforts.
Supplementary Material
Acknowledgements
The authors would like to thank Dr. Joseph Harding and Dr. Leen Kawas for their expert technical advice and the WSU Psychology and Integrative Physiology and Neuroscience Departments for assistance with the completion of this project. The authors thank Cole Ziegler and members of the Laboratory of Alcoholism and Addiction Neuroscience and the WSU Veterinary and Biomedical Research Building vivarium staff for their continued support.
Funding and Declaration of Interest
The authors declare no conflict of interest. Support for this research was provided in part by R01AA020394 from the National Institute on Alcohol Abuse and Alcoholism and the WSU Alcohol and Drug Abuse Research Program according to the State of Washington Initiative Measure No. 171. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Alcohol Abuse and Alcoholism, the National Institutes of Health or the State of Washington.
References
- Alheid GF and Heimer L (1988) New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience 27: 1–39. [DOI] [PubMed] [Google Scholar]
- Brown TE, Forquer MR, Harding JW, Wright JW, Sorg BA (2008) Increase in matrix metalloproteinase-9 levels in the rat medial prefrontal cortex after cocaine reinstatement of conditioned place preference. Synapse 62: 886–889. [DOI] [PubMed] [Google Scholar]
- Demestre M, Parkin-Smith G, Petzold A, Pullen AH (2005) The pro and the active form of matrix metalloproteinase-9 is increased in serum of patients with amyotrophic lateral sclerosis. J. Neuroimmunol 159: 146–154. [DOI] [PubMed] [Google Scholar]
- Dityatev A and Rusakov DA (2011) Molecular signals of plasticity at the tetrapartite synapse. Curr. Opin. Neurobiol 21: 353–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson CM, Wei G, Walker BM (2018) Maladaptive behavioral regulation in alcohol dependence: Role of kappa-opioid receptors in the bed nucleus of the stria terminalis. Neuropharmacology 140: 162–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman R, Toth M, Pena D, Mobashery S (1995) Activation of progelatinase B (MMP-9) by gelatinase A (MMP-2). Cancer Res 55: 2548–2555. [PubMed] [Google Scholar]
- Fujioka H, Dairyo Y, Yasunaga K, Emoto K (2012) Neural functions of matrix metalloproteinases: plasticity, neurogenesis, and disease. Biochem. Res. Int 2012: 789083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Smith AD, Cole M, Weiss F, Koob GF, Richardson HN (2009) Operant Behavior and Alcohol Levels in Blood and Brain of Alcohol-Dependent Rats. Alcohol Clin. Exp. Res 33: 2113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorkiewicz T, Balcerzyk M, Kaczmarek L, Knapska E (2015) Matrix metalloproteinase 9 (MMP-9) is indispensable for long term potentiation in the central and basal but not in the lateral nucleus of the amygdala. Front Cell Neurosci 9: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremel CM, Young EA, Cunningham CL (2011) Blockade of opioid receptors in anterior cingulate cortex disrupts ethanol-seeking behavior in mice. Behav. Brain Res 219: 358–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntley GW (2012) Synaptic circuit remodelling by matrix metalloproteinases in health and disease. Nat. Rev. Neurosci 13: 743–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JP and Fields HL (2004) Glutamatergic activation of anterior cingulate cortex produces an aversive teaching signal. Nat. Neurosci 7: 398–403. [DOI] [PubMed] [Google Scholar]
- Kissler JL, Sirohi S, Reis DJ, Jansen HT, Quock RM, Smith DG, Walker BM (2014) The one-two punch of alcoholism: role of central amygdala dynorphins/kappa-opioid receptors. Biol Psychiatry 75: 774–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissler JL and Walker BM (2016) Dissociating Motivational From Physiological Withdrawal in Alcohol Dependence: Role of Central Amygdala kappa-Opioid Receptors. Neuropsychopharmacology 41: 560–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapska E, Lioudyno V, Kiryk A, Mikosz M, Gorkiewicz T, Michaluk P, Gawlak M, Chaturvedi M, Mochol G, Balcerzyk M, Wojcik DK, Wilczynski GM, Kaczmarek L (2013) Reward learning requires activity of matrix metalloproteinase-9 in the central amygdala. J. Neurosci 33: 14591–14600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF (1992) Neural mechanisms of drug reinforcement. Ann. N. Y. Acad. Sci 654: 171–191. [DOI] [PubMed] [Google Scholar]
- Koob GF (2003) Alcoholism: allostasis and beyond. Alcohol Clin. Exp. Res 27: 232–243. [DOI] [PubMed] [Google Scholar]
- Koob GF (2013) Theoretical frameworks and mechanistic aspects of alcohol addiction: alcohol addiction as a reward deficit disorder. Curr. Top. Behav. Neurosci 13: 3–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF and Le Moal M (2001) Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 24: 97–129. [DOI] [PubMed] [Google Scholar]
- Koob GF, Robledo P, Markou A and Caine S (1993) The mesocorticallimbic circuit in drug dependence and reward - a role for the extended amygdala, in Limbic motor circuits and neuropsychiatry, (Kalivas PW and Barnes CD, eds), pp. 289–309. CRC Press, Boca Raton. [Google Scholar]
- Koob GF and Volkow ND (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35: 217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BR, Ma YY, Huang YH, Wang X, Otaka M, Ishikawa M, Neumann PA, Graziane NM, Brown TE, Suska A, Guo C, Lobo MK, Sesack SR, Wolf ME, Nestler EJ, Shaham Y, Schluter OM, Dong Y (2013) Maturation of silent synapses in amygdala-accumbens projection contributes to incubation of cocaine craving. Nat. Neurosci 16: 1644–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin JI, Chen J, Du M, Hogan M, Kincaid S, Nelson FC, Venkatesan AM, Wehr T, Zask A, DiJoseph J, Killar LM, Skala S, Sung A, Sharr M, Roth C, Jin G, Cowling R, Mohler KM, Black RA, March CJ, Skotnicki JS (2001) The discovery of anthranilic acid-based MMP inhibitors. Part 2: SAR of the 5-position and P1(1) groups. Bioorg. Med. Chem. Lett 11: 2189–2192. [DOI] [PubMed] [Google Scholar]
- Markou A, Kosten TR, Koob GF (1998) Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology 18: 135–174. [DOI] [PubMed] [Google Scholar]
- Mash DC, ffrench-Mullen J, Adi N, Qin Y, Buck A, Pablo J (2007) Gene expression in human hippocampus from cocaine abusers identifies genes which regulate extracellular matrix remodeling. PLoS. One 2: e1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meighan SE, Meighan PC, Choudhury P, Davis CJ, Olson ML, Zornes PA, Wright JW, Harding JW (2006) Effects of extracellular matrix-degrading proteases matrix metalloproteinases 3 and 9 on spatial learning and synaptic plasticity. J. Neurochem 96: 1227–1241. [DOI] [PubMed] [Google Scholar]
- Mizoguchi H, Yamada K, Mouri A, Niwa M, Mizuno T, Noda Y, Nitta A, Itohara S, Banno Y, Nabeshima T (2007a) Role of matrix metalloproteinase and tissue inhibitor of MMP in methamphetamine-induced behavioral sensitization and reward: implications for dopamine receptor down-regulation and dopamine release. J. Neurochem 102: 1548–1560. [DOI] [PubMed] [Google Scholar]
- Mizoguchi H, Yamada K, Niwa M, Mouri A, Mizuno T, Noda Y, Nitta A, Itohara S, Banno Y, Nabeshima T (2007b) Reduction of methamphetamine-induced sensitization and reward in matrix metalloproteinase-2 and −9-deficient mice. J. Neurochem 100: 1579–1588. [DOI] [PubMed] [Google Scholar]
- Nagy V, Bozdagi O, Huntley GW (2007) The extracellular protease matrix metalloproteinase-9 is activated by inhibitory avoidance learning and required for long-term memory. Learn. Mem 14: 655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council, Committee for the Update of the Guide for the Care and Use of Laboratory Animals, Institute for Laboratory Animal Research and National Academies Press (2011) Guide for the care and use of laboratory animals National Academies Press, Washington, D.C. [Google Scholar]
- Nealey KA, Smith AW, Davis SM, Smith DG, Walker BM (2011) kappa-opioid receptors are implicated in the increased potency of intra-accumbens nalmefene in ethanol-dependent rats. Neuropharmacology 61: 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE, Roberts AJ, Smith RT, Koob GF (2004) Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin. Exp. Res 28: 1676–1682. [DOI] [PubMed] [Google Scholar]
- Ogata Y, Enghild JJ, Nagase H (1992) Matrix metalloproteinase 3 (stromelysin) activates the precursor for the human matrix metalloproteinase 9. J. Biol. Chem 267: 3581–3584. [PubMed] [Google Scholar]
- Okulski P, Jay TM, Jaworski J, Duniec K, Dzwonek J, Konopacki FA, Wilczynski GM, Sanchez-Capelo A, Mallet J, Kaczmarek L (2007) TIMP-1 abolishes MMP-9-dependent long-lasting long-term potentiation in the prefrontal cortex. Biol. Psychiatry 62: 359–362. [DOI] [PubMed] [Google Scholar]
- Paxinos G and Watson C (2007) The rat brain in stereotaxic coordinates Elsevier, Academic Press, San Diego. [Google Scholar]
- Rezayof A, Alijanpour S, Zarrindast MR, Rassouli Y (2008) Ethanol state-dependent memory: involvement of dorsal hippocampal muscarinic and nicotinic receptors. Neurobiol. Learn. Mem 89: 441–447. [DOI] [PubMed] [Google Scholar]
- Roberto M and Varodayan FP (2017) Synaptic targets: Chronic alcohol actions. Neuropharmacology 122: 85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF (2000) Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacology 22: 581–594. [DOI] [PubMed] [Google Scholar]
- Samochowiec A, Grzywacz A, Kaczmarek L, Bienkowski P, Samochowiec J, Mierzejewski P, Preuss UW, Grochans E, Ciechanowicz A (2010) Functional polymorphism of matrix metalloproteinase-9 (MMP-9) gene in alcohol dependence: family and case control study. Brain Res 1327: 103–106. [DOI] [PubMed] [Google Scholar]
- Sirohi S, Bakalkin G, Walker BM (2012) Alcohol-induced plasticity in the dynorphin/kappa-opioid receptor system. Front Mol. Neurosci 5: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AW, Kupchik YM, Scofield MD, Gipson CD, Wiggins A, Thomas CA, Kalivas PW (2014) Synaptic plasticity mediating cocaine relapse requires matrix metalloproteinases. Nat. Neurosci 17: 1655–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AW, Nealey KA, Wright JW, Walker BM (2011) Plasticity associated with escalated operant ethanol self-administration during acute withdrawal in ethanol-dependent rats requires intact matrix metalloproteinase systems. Neurobiol. Learn. Mem 96: 199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AW, Scofield MD, Kalivas PW (2015) The tetrapartite synapse: Extracellular matrix remodeling contributes to corticoaccumbens plasticity underlying drug addiction. Brain Res 1628: 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefaniuk M, Beroun A, Lebitko T, Markina O, Leski S, Meyza K, Grzywacz A, Samochowiec J, Samochowiec A, Radwanska K, Kaczmarek L (2017) Matrix Metalloproteinase-9 and Synaptic Plasticity in the Central Amygdala in Control of Alcohol-Seeking Behavior. Biol. Psychiatry 81: 907–917. [DOI] [PubMed] [Google Scholar]
- Sternlicht MD and Werb Z (2001) How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol 17: 463–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth M, Chvyrkova I, Bernardo MM, Hernandez-Barrantes S, Fridman R (2003) Pro-MMP-9 activation by the MT1-MMP/MMP-2 axis and MMP-3: role of TIMP-2 and plasma membranes. Biochem. Biophys. Res. Commun 308: 386–395. [DOI] [PubMed] [Google Scholar]
- Valenstein ES, Cox VC, Kakolewski JW (1967) Polydipsia elicited by the synergistic action of a saccharin and glucose solution. Science 157: 552–554. [DOI] [PubMed] [Google Scholar]
- Vranjkovic O, Pina M, Kash TL, Winder DG (2017) The bed nucleus of the stria terminalis in drug-associated behavior and affect: A circuit-based perspective. Neuropharmacology 122: 100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM (2012) Conceptualizing withdrawal-induced escalation of alcohol self-administration as a learned, plasticity-dependent process. Alcohol 46: 339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM and Koob GF (2007) The gamma-aminobutyric acid-B receptor agonist baclofen attenuates responding for ethanol in ethanol-dependent rats. Alcohol Clin. Exp. Res 31: 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AM, Reis DJ, Powell AS, Neira LJ, Nealey KA, Ziegler CE, Kloss ND, Bilimoria JL, Smith CE, Walker BM (2012) The effect of intermittent alcohol vapor or pulsatile heroin on somatic and negative affective indices during spontaneous withdrawal in Wistar rats. Psychopharmacology (Berl) 223: 75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JW and Harding JW (2009) Contributions of matrix metalloproteinases to neural plasticity, habituation, associative learning and drug addiction. Neural Plast 2009: 579382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JW, Masino AJ, Reichert JR, Turner GD, Meighan SE, Meighan PC, Harding JW (2003) Ethanol-induced impairment of spatial memory and brain matrix metalloproteinases. Brain Res 963: 252–261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.