Abstract
Objectives
To assess the prognostic value of a new, comprehensive coronary computed tomography angiography (CTA) score compared with the stenosis severity component of the Coronary Artery Disease – Reporting and Data System (CAD-RAD S).
Background
Current risk assessment with coronary CTA is mainly focused on maximal stenosis severity. Integration of plaque extent, location and composition in a comprehensive model may improve risk stratification.
Methods
A total of 2,134 patients with suspected but without known CAD were included. The predictive value of the comprehensive CTA score (ranging from 0–42 and divided into three groups: 0–5, 6–20, and >20) was compared with the CAD-RADS combined into 3 groups (0 – 30%, 30 – 70% and ≥70% stenosis). Its predictive performance was internally and externally validated (using the 5-year follow-up dataset of the Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry (CONFIRM) registry, n = 1,971).
Results
Patients mean age was 55±13 years, mean follow-up 3.6 ± 2.8 years and 130 events (myocardial infarction or death) occurred. The new, comprehensive CTA score demonstrated strong and independent predictive value using Cox proportional hazard analysis. A model including clinical variables + comprehensive CTA score showed better discrimination of events compared with a model consisting of clinical variables + CAD-RADS (0.768 vs 0.742, P=0.001). Also, the comprehensive CTA score correctly reclassified a significant proportion of patients compared with conventional approach (net reclassification improvement 12.4%, P<0.001). Good predictive accuracy was reproduced in the external validation cohort.
Conclusions
The new, comprehensive CTA score provides better discrimination and reclassification of events compared with the CAD-RADS score based on stenosis severity only. The score retained similar prognostic accuracy when externally validated. Anatomic risk scores can be improved with the addition of extent, location and compositional measures of atherosclerotic plaque. Comprehensive CTA risk score calculator is available at: http://18.224.14.19/calcApp/
Keywords: Coronary CTA, risk stratification, stable coronary artery disease
Introduction
Coronary computed tomography angiography (CTA) provides direct non-invasive anatomical assessment of the coronary arteries and has a high diagnostic accuracy for detection and exclusion of obstructive coronary artery disease (CAD) (≥50% stenosis) compared with invasive coronary angiography (1). Coronary CTA also provides prognostic information for prediction of future cardiovascular events (2,3). Several studies have shown that obstructive CAD on coronary CTA is associated with worse outcomes compared to non-obstructive or no CAD (4,5,6). Current coronary CTA reading is guided by the Coronary Artery Disease – Reporting and Data system (CAD-RADS), which is mainly based on maximal stenosis severity. However, other coronary plaque characteristics including plaque extent, location and composition carry prognostic value (2,7,8). The location of coronary plaque (proximal versus distal), the number of plaques, and plaque composition (non-calcified or mixed versus calcified lesions) have all been associated with clinical outcomes in cohort studies (7,9,10). The integration of this complex information into a risk score may further optimize risk stratification and enable maximum use of information derived from coronary CTA. The purpose of the current study was to determine whether a new, comprehensive risk score may provide incremental prognostic value over the stenosis severity component of the CAD-RADS score.
Methods
Study population
Derivation cohort from Leiden, The Netherlands
The primary study cohort to derive the novel risk score included a consecutive series of 2,809 stable patients with suspected or known CAD who were clinically referred for coronary CTA at the Leiden University Medical Center (LUMC), The Netherlands, between 2005 and 2015. Exclusion criteria for coronary CTA were cardiac arrhythmias, known hypersensitivity to iodine contrast media, or pregnancy. Patients with an uninterpretable CTA examination (n = 125), previous percutaneous intervention, coronary artery bypass surgery or myocardial infarction (MI, n = 148), coronary CTA in the setting of suspected acute coronary syndrome (n = 144), missing plaque composition data (n = 65) or missing follow-up (n = 193) were excluded, leaving 2,134 patients in the derivation cohort. Cardiovascular risk factors consisted of diabetes mellitus (defined as a fasting glucose ≥ 126 mg/dl or the use of insulin/oral hypoglycemic agents), hypertension (systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg or the use of antihypertensive medication), hypercholesterolemia (serum total cholesterol ≥230 mg/dl or serum triglycerides ≥200 mg/dl or treatment with lipid-lowering drugs), family history of CAD (presence of CAD in first-degree family members at <55 years of age in men and <65 years of age in women) and currently smoking. Chest pain typicality was categorized as non-anginal, atypical and typical chest pain.
Demographic and clinical data were prospectively collected from the departmental electronic information system (EPD- Vision©, Leiden University Medical Center, The Netherlands). The LUMC Institutional Review Board approved this evaluation of clinically-acquired data and waived the need for patient written informed consent.
External validation cohort, CONFIRM Registry
The comprehensive CTA score was tested in an external validation cohort (details described below) using the CONFIRM (Coronary CT angiography Evaluation for Clinical Outcomes: An International Multicenter) registry; a dynamic, international, multicenter, observational cohort study that prospectively collected clinical and follow-up data of patients undergoing ≥64-slice coronary CTA; the rationale and design of CONFIRM have been previously described (11). In brief, this cohort comprised 12,086 patients with 5-year follow-up data among 17 centers in 9 countries between 2002 and 2009 (12). Patients with missing coronary system dominance or plaque composition data (n = 5,553), missing follow-up data regarding myocardial infarction (MI, n = 3,763) and previous percutaneous intervention, coronary artery bypass surgery or MI (n = 799) were excluded. In total, 1,971 patients were included in the CONFIRM external validation cohort. Institutional review board approval was received for each study site and each patient provided written informed consent.
CTA acquisition and image analysis
For the derivation cohort (Leiden, The Netherlands), patients were scanned using a 64-slice CT scanner (Aquillion64, Toshiba Medical Systems, Japan) or a 320-slice CT scanner (Toshiba Multi-slice Aquilion ONE system, Toshiba Medical Systems, Japan). Before the examination, the patient’s heart rate and blood pressure were monitored. In the absence of contraindications, patients with a heart rate exceeding 60 beats per minute were administered beta-blocking medication (50–150 mg oral metoprolol, with an additional intravenous dose up to 15 mg if needed). Furthermore, sublingual nitro-glycerine (0.4 mg) was administered before scanning. All scan parameters have been previously published (13). Post-processing of the coronary CTA examinations was performed with dedicated software (Vitrea2 and VitreaFx, Vital Images, USA). Coronary anatomy was assessed using a 17-segment model according to a modified American Heart Association (AHA) classification (14). Stenosis severity was visually assessed for each coronary plaque and categorized as: normal, <30%, 30 – 50%, 50 – 70%, 70 – 99% and occluded (7). In addition, plaque composition was determined in all diseased segments and graded as non-calcified plaque (plaques having lower density compared with the contrast-enhanced lumen), calcified plaque (plaques with high density), and mixed plaque (containing elements of both non-calcified and calcified plaque). The CTA examinations were interpreted by two physicians highly experienced in CTA reading as previously described (13). Image analysis from the external validation cohort was uniformly performed at each site in accordance with the CT guidelines, as previously described (11).
CAD-RADS
The CAD-RADS categories are based on the highest grade coronary stenosis per patient and are defined as follows: CAD-RADS 0 = no coronary plaque, CAD-RADS 1 = 1–24% stenosis or present coronary plaque without stenosis, CAD-RADS 2 = 25–49% stenosis, CAD-RADS 3 = 50–69% stenosis, CAD-RADS 4a = 70–99% stenosis in 1 or 2 coronary arteries, CAD-RADS 4b = 70–99% stenosis in 3 coronary arteries or ≥50% stenosis in the left main, CAD-RADS 5 = occlusion. According to these definitions, patients in the present analysis were categorized in their appropriate CAD-RADS group, where a stenosis <30% was considered equal to 1–24%, 30–49% equal to 25–49%, 50–69%, 70–99% and occlusion equal to their exact similar CAD-RADS stenosis severity groups. The CAD-RADS classification also includes the presence of vulnerable high-risk plaque.
However, this information was not included in the present study because the high-risk plaque features were not systematically assessed. To allow for comparisons with the comprehensive CTA score, the several CAD-RADS categories were merged into three groups: 1 = CAD-RADS 0 or 1 (no to minimal CAD), 2 = CAD-RADS 2 or 3 (moderate CAD), 3 = CAD-RADS 4 or 5 (severe CAD).
Comprehensive CTA score
A comprehensive CTA score incorporating the presence, extent, severity, location and composition of CAD was constructed based on the following:
A 17-segment model of the coronary artery tree based on AHA criteria (14).
Previous literature describing the individual predictive value of plaque extent, severity and composition variables as observed on coronary CTA (2,3,7,9).
The Leaman score which provides weight factors for plaque location (15).
Regarding the presence and extent of CAD on coronary CTA, several studies have shown that the number of segments with CAD is associated with increased risk for events (2,3,5,6). When stratifying the diseased segments according to plaque composition, van Werkhoven etal. observed a HR of 1.1 for segments with calcified plaque, 1.2 for segments with non-calcified plaque, and 1.3 for segments with mixed plaques (7). Based on these findings the weight factor for the presence, extent and composition of plaque in the score are 1.1, 1.2 or 1.3, respectively, for calcified, non-calcified or mixed plaque. In addition to plaque presence, extent and composition, stenosis severity is also an important predictor for future events. In the comprehensive CTA score the weight factor for stenosis severity was based on the previously observed HR of 1.4 (1.2–1.6) for the number of segments with obstructive stenosis (7). Finally, lesions in more proximal coronary artery segments are known to convey a higher risk for cardiovascular events, possibly due to the larger volume of affected myocardium in case of a coronary occlusion (5). As a result, plaque location was integrated into the comprehensive CTA score using the Leaman score, which places weights on each segment’s relative contribution to the total left ventricular blood flow (15).
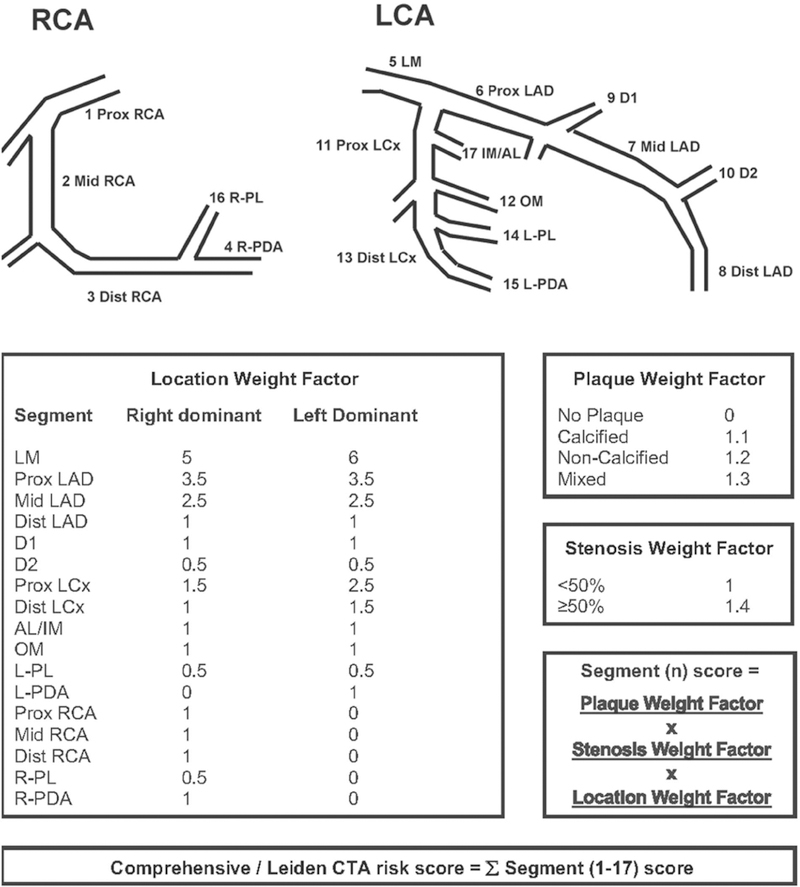
Altogether, the comprehensive CTA score is calculated using the following approach. First, the presence of CAD is determined in each segment. When plaque is absent the score is 0. When plaque is present a score of 1.1, 1.2 or 1.3 is given according to plaque composition (calcified, non-calcified and mixed plaque respectively). Subsequently, this score is multiplied by a weight factor for the location of the segment in the coronary artery tree (0.5 through 6 according to vessel, proximal location and system dominance), and multiplied by a weight factor for stenosis severity (1.4 for ≥50% stenosis and 1.0 for stenosis <50%). The final score (range 0–42) is calculated by addition of the individual segment scores (Figure 1). An online calculator is available at http://18.224.14.19/calcApp/ (16).
Figure 1. Comprehensive CTA score calculation.
The new, comprehensive CTA score is calculated by addition of the individual segment scores, which are obtained by multiplication of the plaque weight factor, the stenosis weight factor and the location weight factor. For example, a patient with a right dominant system with a non-calcified plaque with >50% stenosis in the middle RCA, and a mixed plaque with <50% stenosis in the proximal LAD has the following score: Segment 2 score (1.2 X 1.4 X 1= 1.68) + Segment 6 score (1.3 X 1 X 3.5 = 4.55) + other segments score (0) = 6.23.
Abbreviations: AL: anterolateral segment; D1: diagonal 1; D2: diagonal 2; IM: intermediate segment; LAD: left anterior descending coronary artery; LCA: left coronary artery; LCx: left circumflex coronary artery; LM: left main segment; L-PDA: left posterior descending artery; L-PL: left posterolateral segment; OM: obtuse marginal segment; RCA: right coronary artery; R-PDA: right posterior descending artery; R-PL: right posterolateral segment.
Follow-up and study endpoints
For the derivation cohort (Leiden, The Netherlands), mortality data were retrieved from the municipal civil registry of the Netherlands; and MI was assessed by clinical visit report review or standardized telephone interviews with confirmation from medical file data. The average follow-up time was 3.6 ± 2.8 years. For the external validation cohort (the CONFIRM registry), death was ascertained by a query of the national death index for Unites States (US) sites and by direct interview or telephone contact with the patient’s family, primary physician or review of the medical charts for non-US sites; and MI was ascertained by direct interview, telephone contact (and confirmed from the medical files) or medical record review. The primary endpoint was all-cause mortality or non-fatal MI (defined according to the standard definitions) (17,18). Patients were followed for a mean of 5.2 ± 1.7 years.
Statistical analysis
Continuous variables were presented as mean ± standard deviation (SD) or median with 25–75% interquartile range (IQR), according to the distribution. Categorical variables were presented as a number and percent. Event-free survival was estimated using the Kaplan-Meier method and the log-rank test was used to compare the event-free survival distributions of the groups within each score. The 2 scores were available for all patients; less than 1.5% of data was missing regarding cardiovascular risk factors or medication use. The uni- and multivariable hazard ratios (HR) with 95% confidence intervals (CI’s) were generated by Cox proportional hazard regression analysis. In each case, the proportional hazards assumption was met. Model overfitting was avoided by limiting multivariable models to 1 variable for every 10 clinical outcomes. Two multivariable models were created including clinical characteristics (age, sex, hypertension, hypercholesterolemia, diabetes mellitus, smoking, family history of CAD) together with the CAD-RADS (Model 1) or the comprehensive CTA score (Model 2). The discriminatory ability of several models was assessed using receiver operating characteristics (ROC) curve analysis and compared with the DeLong method. (19) The incremental value of the comprehensive CTA score compared with the CAD-RADS was assessed using the net reclassification improvement (NRI) statistic based on the methods developed by Pencina et al.(20) The 5-year predicted risk categories were defined as 0–3%, 3– 10% and >10%. These specific risk-thresholds were previously described by Polonsky et al.(21) The use of different cut-off values had minimal effect on the NRI (<0.5% change). The three comprehensive CTA score groups were defined using scores of: 0–5, 6–20, and >20; as these values revealed the best discriminatory value. For modeling of the comprehensive CTA score, internal validation was performed using bootstrapping analysis using 1,000 replicates and using a 70:30 random split of the derivation cohort for the training and validation cohorts, respectively. Then, this model was externally validated using data from the independent CONFIRM registry. All statistical analyses were 2-sided and a P-value <0.05 was considered statistically significant. The analyses were performed using SAS (version 9.4, Cary, NC) and SPSS (version 24, Armonk, NY).
Results
Conventional and new comprehensive CTA score
In total, 1150 (53.9%) patients had CAD-RADS 0–1, 867 (40.6%) patients had CAD-RADS 2–3 and 117 patients (5.5%) CAD-RADS 4–5 in the derivation cohort. Only 18 (2%) patients in the CAD-RADS 0–1 group had >2 segments with plaque. According to the comprehensive CTA score, 1274 (59.7%) patients had the lowest score (0–5), 725 (34.0%) patients had a score of 6–20 and 135 (6.3%) had the highest risk score category (>20). A mean score of 6.37 ± 3.85 was observed, ranging from 0 to 36.4. The primary endpoint occurred in 130 patients of the derivation cohort. Events occurred in 22 patients with CAD-RADS 0–1 (2.5%), in 93 patients with CAD-RADS 2–3 (8.1%) and in 15 patients with CAD-RADS 4–5 (12.8%).
Events occurred in 33 patients with score 0–5 (2.6%), in 67 with score 6–20 (9.2%) and in 30 with score >20 (22.2%).
Baseline characteristics according to risk score categories
Table 1 presents the baseline characteristics of the derivation cohort compared with the external validation cohort across the three comprehensive CTA score categories (0–5, 6–20, >20). The mean patient’s age was consistently lower in the derivation cohort compared with the external validation cohort. Moreover, in the derivation cohort fewer patients were male, and the prevalence of hypertension, hypercholesterolemia, or smoking was lower; conversely, diabetes mellitus was more prevalent in the derivation cohort.
Table 1.
Patient characteristics
| Comprehensive CTA score 0–5 | Comprehensive CTA score 5–20 | Comprehensive CTA score >20 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Derivation cohort N = 1274 |
External cohort N = 1096 |
P-value | Derivation cohort N = 725 |
External cohort N = 746 |
P-value | Derivation cohort N = 135 |
External cohort N = 129 |
P-value | |
| Age, years | 50.9 ± 12.4 | 58.2 ± 12.2 | <0.001 | 59.9 ± 10 .5 | 64.6 ± 10.0 | <0.001 | 63.0 ± 9.8 | 67.0 ± 8.0 | <0.001 |
| BMI, kg/m2 | 26.6 ± 5.0 | 26.8 ± 4.4 | 0.287 | 26.9 ± 4.4 | 27.0 ± 4.1 | 0.836 | 27.2 ± 4.9 | 27.2 ± 3.7 | 0.950 |
| Male | 559 (44) | 603 (55) | <0.001 | 392 (54) | 548 (74) | <0.001 | 95 (70) | 102 (79) | 0.104 |
| Chest pain symptoms | <0.001 | <0.001 | 0.071 | ||||||
| Asymptomatic | 504 (40) | 466 (44) | 304 (42) | 319 (44) | 61 (45) | 41 (32) | |||
| Non-cardiac | 214 (17) | 177 (17) | 92 (13) | 96 (13) | 13 (10) | 16 (13) | |||
| Atypical angina | 466 (37) | 306 (29) | 248 (34) | 178 (25) | 38 (28) | 35 (27) | |||
| Typical angina | 89 (7) | 119 (11) | 81 (11) | 133 (18) | 23 (17) | 36 (28) | |||
| Cardiovascular risk factors | |||||||||
| Diabetes mellitus | 270 (21) | 88 (8) | <0.001 | 235 (32) | 112 (15) | <0.001 | 64 (47) | 43 (33) | 0.020 |
| Hypertension | 441 (35) | 572 (52) | <0.001 | 398 (55) | 504 (68) | <0.001 | 90 (67) | 96 (75) | 0.138 |
| Hypercholesterolemia | 296 (23) | 507 (46) | <0.001 | 261 (36) | 477 (64) | <0.001 | 66 (49) | 92 (72) | <0.001 |
| Family history of CAD |
508 (40) | 343 (32) | <0.001 | 292 (40) | 263 (35) | 0.049 | 53 (39) | 44 (34) | 0.412 |
| Currently smoking | 206 (16) | 241 (22) | <0.001 | 120 (17) | 223 (30) | <0.001 | 42 (31) | 47 (37) | 0.337 |
| Cardiovascular medication | |||||||||
| Beta-blocker | 359 (29) | 181 (17) | <0.001 | 282 (39) | 179 (24) | <0.001 | 46 (35) | 28 (22) | 0.028 |
| ACE-I | 181 (15) | 155 (14) | 0.868 | 190 (27) | 163 (22) | 0.053 | 49 (37) | 34 (27) | 0.074 |
| Statin | 313 (25) | 260 (24) | 0.486 | 305 (43) | 329 (45) | 0.432 | 71 (53) | 76 (60) | 0.294 |
| Calcium antagonist | 104 (8) | 63 (7) | 0.110 | 90 (13) | 68 (11) | 0.413 | 25 (19) | 15 (14) | 0.339 |
| Aspirin | 237 (19) | 198 (18) | 0.626 | 191 (27) | 237 (32) | 0.022 | 50 (38) | 40 (32) | 0.302 |
Values are mean ± SD or n (%).
ACE-I, angiotensin converting enzyme-inhibitor; BMI, Body Mass Index; CAD, coronary artery disease
Prognostic performance of the novel comprehensive CTA score
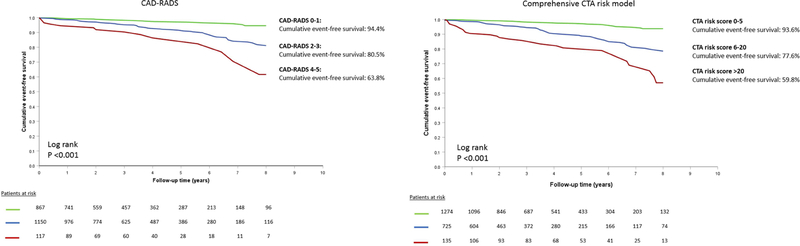
Table 2 shows the uni- and multivariable clinical and CTA Cox regression models. For the CAD-RADS, univariable HR for CAD-RADS 2–3 was: 3.19 (95% CI: 2.00–5.07, P<0.001) and for CAD-RADS 4–5: 6.28 (95% CI: 3.26–12.11, P<0.001), with CAD-RADS 0–1 as reference group. A strong association with events was also observed using the comprehensive CTA score categories: the HR of a score of 6–20 was 3.71 (2.44–5.62, P<0.001) and the HR of a score >20 was 8.00 (4.88–13.13, P<0.001) with a score of 0–5 as the reference group. A similar pattern was observed after adjusting for clinical characteristics (Table 2). The event-free survival curves are presented in Figure 2. In both approaches, a dose-dependent relationship is observed between the degree of CAD and worse event-free survival. For the CAD-RADS, event-free survival rates ranged from 94.4% for CAD-RADS 0–1, 80.5% for CAD-RADS 2–3 and 63.8% for CAD-RADS 4–5 (P<0.001). By comparison, the survival rate for a comprehensive CTA score of 0–5 was 93.6%, 77.6% for a score of 6–20 and 59.8% for a score >20 (P<0.001).
Table 2.
Uni- and multivariable Cox regression on the derivation cohort.
| Univariable | Multivariable Model 1 | Multivariable Model 2 | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p−value | HR (95% CI) | p−value | HR (95% CI) | p–value | |
| Age, years | 1.07 (1.05–1.08) | <0.001 | 1.06 (1.04–1.08) | <0.001 | 1.05 (1.03–1.07) | <0.001 |
| BMI, kg/m2 | 1.01 (0.97–1.05) | 0.734 | ||||
| Male | 1.17 (0.83–1.65) | 0.369 | 1.09 (0.76–1.57) | 0.622 | 0.98 (0.68–1.40) | 0.902 |
| Chest pain symptoms* | 0.134 | |||||
| Non–cardiac | 0.48 (0.56–0.90) | – | ||||
| Atypical | 0.89 (0.60–1.31) | – | ||||
| Typical | 1.04 (0.60–1.79) | – | ||||
| Cardiovascular risk factors | ||||||
| Diabetes mellitus | 1.44 (1.01–2.06) | 0.046 | 1.39 (0.85–2.03) | 0.086 | 1.27 (0.88–1.88) | 0.193 |
| Hypertension | 1.46 (1.03–2.06) | 0.033 | 0.98 (0.68–1.42) | 0.928 | 0.94 (0.65–1.35) | 0.722 |
| Hypercholesterolemia | 0.80 (0.55–1.18) | 0.263 | 0.62 (0.41–0.92) | 0.019 | 0.59 (0.39–0.89) | 0.011 |
| Family history of CAD | 0.55 (0.38–0.81) | 0.002 | 0.78 (0.46–1.01) | 0.054 | 0.66 (0.45–0.98) | 0.038 |
| Currently smoking | 1.70 (1.15–2.50) | 0.008 | 2.01 (1.39–3.13) | <0.001 | 1.90 (1.26–2.86) | 0.002 |
| CAD–RADS† | ||||||
| CAD–RADS 2–3 | 3.19 (2.00–5.07) | <0.001 | 1.95 (1.19–3.20) | 0.008 | – | |
| CAD–RADS 4–5 | 6.28 (3.26–12.11) | <0.001 | 2.68 (1.30–5.53) | 0.007 | – | |
| Comprehensive CTA score‡ | ||||||
| Score 6–20 | 3.71 (2.44–5.62 | <0.001 | – | 2.69 (1.72–4.22) | <0.001 | |
| Score >20 | 8.00 (4.88–13.13) | <0.001 | – | 4.64 (2.63–8.16) | <0.001 | |
Asymptomatic is the reference
CAD-RADS 0–1 is the reference
Comprehensive CTA score 0–5 is the reference
CAD-RADS, Coronary Artery Disease – Reporting and D ata System
Figure 2. Event-Free survival for the CAD-RADS and the new, comprehensive CTA score.
Both classifications (0–5, 6–20, >20) were associated with increased risk for events (death and myocardial infarction) over time.
CAD: coronary artery disease; CTA: computed tomography angiography.
The c-index of a model containing clinical variables (age, sex, hypertension, hypercholesterolemia, diabetes mellitus, smoking, family history of CAD) was 0.727. Adding the CAD-RADS increased the c-statistic to 0.742. A model consisting of clinical variables + the comprehensive CTA score performed significantly better (c-statistic 0.768 [95% CI 0.725–0.811], P=0.001) compared with a model including clinical variables + CAD-RADS, as shown in Supplemental Figure 1. Moreover, the model with the comprehensive CTA score significantly correctly reclassified patients, using risk thresholds of <3%, 3–10% and >10%, as demonstrated by a NRI of 12.4% (95% CI: 5.7% - 19.1%, P<0.001). Reclassification data for patients with and without events are included in Supplemental Tables 1a and 1b.
Internal and external validation of the comprehensive CTA score
In the external validation cohort, 1,096 (55.6%) patients had a score of 0 – 5, whereas 746 (37.8%) patients had a score of 6 – 20 and 129 (6.6%) patients had a score >20. The primary endpoint occurred in 254 patients. Supplemental Figure 2 shows the ROC curves for the internal validation (training sample: 70% of the patients, and validation sample: 30% of the patients) of the derivation cohort (Leiden, The Netherlands) and the external validation of the comprehensive CTA score within the external validation cohort (CONFIRM registry). The c-index of the training sample (derivation cohort), using a model containing clinical characteristics and the comprehensive CTA score was 0.749; the c-index of the validation sample was 0.789. In the external validation cohort, the c-index of this model was 0.718 (95% CI 0.682–0.744), significantly higher than the clinical model (0.689, P<0.001).
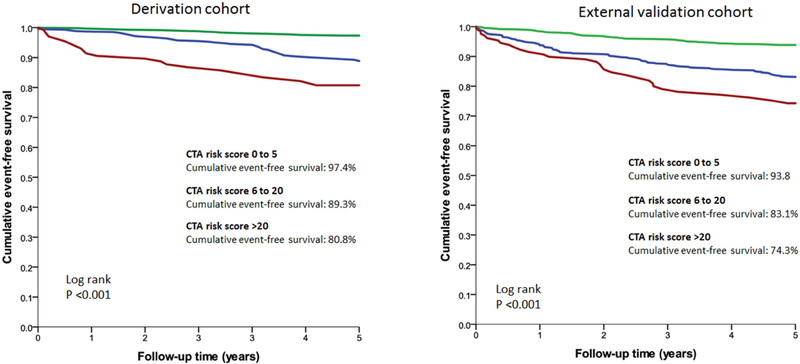
Figure 3 depicts the 5-year event-free survival curves of the derivation cohort and the external validation cohort; showing a similar discriminatory ability of the comprehensive CTA score in both cohorts. The 5-year event-free survival for patients with the comprehensive CTA score of 0–5 was 97.4%; 89.3% for a score 6–20 and 80.8% for the highest score category (>20) in the derivation cohort. In the external validation cohort, event-free survival was 93.8% for a score 0–5, 83.1% for a score 6 to 20 and 74.3% for a score >20.
Figure 3. External prognostic validation (CONFIRM registry) of the new, comprehensive CTA score.
Comparison of 5-years cumulative event free survival among the derivation and external validation cohort of the comprehensive CTA score showing similar discriminatory ability of the score.
Abbreviations: CAD: coronary artery disease; CTA: computed tomography angiography.
Discussion
The current study demonstrated the improved prognostic significance of a comprehensive CTA score incorporating multiple aspects of plaque detected by coronary CTA (plaque extent, severity, location and composition) to predict major clinical outcomes. Compared with the CAD-RADS, our new, comprehensive score provided improved prediction of outcomes and reclassification of risk for future events. We further evaluated the significance of this comprehensive CTA score by establishing its ability to accurately stratify risk in an external validation cohort. Often risk scores perform suboptimal when externally validated. However, the current validation findings support the added prognostication with varying plaque characteristics to improve classification of major clinical outcomes.
Prognostic value of plaque extent, location and composition
The CAD-RADS provides the current recommendations for coronary CTA reading (22). The majority of studies assessing the prognostic value of coronary CTA have used a stenosis severity-focused approach, which is the major component of the CAD-RADS. Patients without CAD have the lowest rate of major cardiovascular events with increasing clinical risk-adjusted hazard ratios for non-obstructive CAD (ranging from 1.2–1.6) and obstructive CAD (ranging from 2.3–2.6) (23,24). The importance of non-obstructive CAD on coronary CTA has been addressed recently, since the majority of patients who will experience have <50% stenosis (25). Although this approach permits risk stratification, it does not take full advantage of all information on coronary atherosclerosis that can be derived from coronary CTA. As a result, this method may considerably over- or underestimate the risk of events in both patients with obstructive and non-obstructive CAD, indicating the need for a more detailed, patient tailored approach (fitting the new concept of “precision medicine”). Prognostic value of several plaque measures has been reported in individual studies (2,4,5,7,9), including number of segments with obstructive CAD (7,12), plaque composition (7) and the location of plaque in the coronary tree (5). Since all parameters have prognostic value, the current study aimed to bring all these CTA parameters together and integrate them into a comprehensive risk score.
Comprehensive CTA score
The comprehensive CTA score categories of 0–5, 6–20, and >20 provided better discrimination and correct reclassification compared with risk groups based on stenosis severity only; and importantly, event rates in the lowest category of both scores were similarly low. However, the 3 groups of both scores include different CAD extent. For instance, a patient with 2 obstructive calcified lesions in the first diagonal and mid-RCA would have been classified in the intermediate/highest risk group using the CAD-RADS but lowest according to the new, comprehensive score. These findings support the hypothesis that a comprehensive approach to grade the severity of coronary atherosclerosis, instead of the classification based on the highest-grade stenosis, may improve risk stratification. This corresponds to previous observations that stenosis severity only plays a minor role in predicting plaque rupture and a significant proportion of acute MI’s occur at sites with mild stenosis (25,26). Using coronary CTA, previous studies demonstrated that integration of several plaque measures increase risk prediction. The CONFIRM score incorporated clinical risk parameters and the presence of non-obstructive proximal mixed or calcified plaques and proximal obstructive stenosis which increased predictive value over clinical scores (27). Mushtaq et al showed that the CT-Leaman score, integrating stenosis severity with the number and location of stenoses, was more strongly predictive of the segment involvement score (the total number of segments with plaque) or the segment stenosis score (obtained by grading the stenosis severity of each segment with plaque) (8). The current study adds further to the existing literature by separating three risk groups which showed similar good discrimination of events in an external validation cohort, indicating its robustness. To be used in clinical practice, a risk score must be easy to use, include a limited number of variables and be accurate. The current score fits this definition, and is based on location, composition and stenosis severity in the classical 17-segment model. Previously, prognostic angiographic risk scores have been developed in patients who underwent invasive coronary angiography, such as the Leaman score (15). The CAD prognostic index was described by Mark et al, which integrates information on lesion location, severity and number of coronary arteries involved (28). These scores were obtained in patients undergoing clinically indicated invasive coronary angiography, and are derived from higher risk cohorts, and may not be optimal for the lower risk patients undergoing coronary CTA.
Clinical implications
It is currently not clear which extent of coronary atherosclerosis warrants the initiation or intensification of lipid lowering therapy and the need for using aspirin. No randomized controlled trials have been performed that evaluated the benefit of treatment of coronary atherosclerosis based on coronary CTA findings. But previous observations have shown that the detection of atherosclerosis increased the prescription of medical therapy. In a study by Cheezum et al, statin therapy was started or intensified in 46% of patients after the detection of non-obstructive or obstructive CAD, which was associated with significant reductions in plasma cholesterol levels (29). Furthermore, blood pressure therapy was intensified in patients with non-obstructive and obstructive CAD in 21% and 24% of patients respectively; likewise, aspirin was started in 29% and 40% of patients respectively. The CAD-RADS significantly improves risk prediction over clinical variables and permits risk assessment. However, this scoring system does not perfectly “phenotype” the i ndividual patient with respect to the total coronary atherosclerotic burden in terms of plaque extent, location, and composition. The new score may be used to tailor medical treatment to the individual patient by maximizing therapy for patients in the highest risk group: targeting of very low cholesterol levels and optimizing blood pressure, and possibly reduce therapy for patients in the lowest risk group to minimize side effects of medication. Future studies should investigate whether clinical outcomes can be improved by the clinical application of this approach of personalizing risk stratification.
Limitations
The observational design of the study is a limitation; lifestyle changes, medical therapy and revascularization after coronary CTA might have influenced outcome in the current cohort, but this limitation relates to all large registries. A direct comparison between the performances of the new comprehensive CTA risk score and the original CAD-RADS (including high risk plaque features) could not be performed (since high risk plaque features were not systematically assessed) and remains to be evaluated. Patients in the derivation and validation cohort did not have similar cardiovascular risk profiles: patients in the external validation cohort were older and had more risk factors. This may clarify the higher event rates across the three risk categories for the validation cohort. Generalizability of the current study may be reduced by the lack of an independent core laboratory analysis or clinical event committee. Also, calculation of the new, comprehensive score is more complex than the CAD-RADS; however, automated score calculation is feasible. The new, comprehensive CTA score does not incorporate functional stenosis information, which can be derived with fractional flow reserve – CT. Future research shoul d investigate the potential added value of this technique. Finally, a large number of patients in the external validation cohort were excluded which may have introduced selection bias.
Conclusion
The CTA risk score incorporating coronary plaque extent, location, severity and composition improved prediction of events compared with the CAD-RADS based on stenosis severity. Moreover, the model retained good prognostic accuracy in an external validation cohort. The proposed model allows precise prediction of future events and may help further guide risk stratification.
Supplementary Material
Perspectives.
Competency in medical knowledge
A novel comprehensive CTA score based on the extent, severity, location and composition of CAD incorporates all aspects of coronary atherosclerosis into one per patient score and provides superior risk stratification then a score based on stenosis severity only.
Translational outlook
A holistic approach to classify CAD improves the estimation of a patient’s risk for future cardiovascular events which may translate into more accurate post-CTA medical care and improved cardiovascular outcome.
Acknowledgments
We thank Mohit Pandey M.S. for creating the online score calculator.
Disclosures
Alexander R. van Rosendael is supported by a research grant from the Netherlands Heart Institute (Utrecht, The Netherlands). Arthur J. Scholte received consulting fees from Toshiba Medical Systems and GE Healthcare. The Department of Cardiology of the LUMC received research grants from Biotronik, Medtronic, Boston Scientific Corporation and Edwards Lifesciences.
Funding
The research reported in this publication was funded, in part, by the National Institute of Health (Bethesda, MD, USA) under award number R01 HL115150, and also supported, in part, by the Dalio Institute of Cardiovascular Imaging (New York, NY, USA) and the Michael Wolk Foundation (New York, NY, USA).
Abbreviations
- AHA
American Heart Association
- BMI
Body mass index
- CAD
Coronary artery disease
- CAD-RADS
Coronary Artery Disease – Reporting and Data System
- CI
Confidence interval
- CTA
Computed tomography angiography
- HR
Hazard ratio
- NRI
Net reclassification improvement
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
None
References
- 1.Meijboom WB, Meijs MF, Schuijf JD, et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol 2008;52:2135–44. [DOI] [PubMed] [Google Scholar]
- 2.Min JK, Shaw LJ, Devereux RB, et al. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol 2007;50:1161–70. [DOI] [PubMed] [Google Scholar]
- 3.Carrigan TP, Nair D, Schoenhagen P, et al. Prognostic utility of 64-slice computed tomography in patients with suspected but no documented coronary artery disease. Eur Heart J 2009;30:362–71. [DOI] [PubMed] [Google Scholar]
- 4.Bittencourt MS, Hulten E, Ghoshhajra B, et al. Prognostic value of nonobstructive and obstructive coronary artery disease detected by coronary computed tomography angiography to identify cardiovascular events. Circ Cardiovasc Imaging 2014;7:282–91. [DOI] [PubMed] [Google Scholar]
- 5.Hadamitzky M, Achenbach S, Al-Mallah M, et al. Optimized prognostic score for coronary computed tomographic angiography: results from the CONFIRM registry (COronary CT Angiography EvaluatioN For Clinical Outcomes: An InteRnational Multicenter Registry). J Am Coll Cardiol 2013;62:468–76. [DOI] [PubMed] [Google Scholar]
- 6.Min JK, Berman DS, Dunning A, et al. All-cause mortality benefit of coronary revascularization vs. medical therapy in patients without known coronary artery disease undergoing coronary computed tomographic angiography: results from CONFIRM (COronary CT Angiography EvaluatioN For Clinical Outcomes: An InteRnational Multicenter Registry). Eur Heart J 2012;33:3088–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Werkhoven JM, Schuijf JD, Gaemperli O, et al. Incremental prognostic value of multi-slice computed tomography coronary angiography over coronary artery calcium scoring in patients with suspected coronary artery disease. Eur Heart J 2009;30:2622–9. [DOI] [PubMed] [Google Scholar]
- 8.Mushtaq S, De Araujo Goncalves P, Garcia-Garcia HM, et al. Long-term prognostic effect of coronary atherosclerotic burden: validation of the computed tomography-Leaman score. Circ Cardiovasc Imaging 2015;8:e002332. [DOI] [PubMed] [Google Scholar]
- 9.Pundziute G, Schuijf JD, Jukema JW, et al. Prognostic value of multislice computed tomography coronary angiography in patients with known or suspected coronary artery disease. J Am Coll Cardiol 2007;49:62–70. [DOI] [PubMed] [Google Scholar]
- 10.Motoyama S, Ito H, Sarai M, et al. Plaque Characterization by Coronary Computed Tomography Angiography and the Likelihood of Acute Coronary Events in Mid-Term Follow-Up. J Am Coll Cardiol 2015;66:337–46. [DOI] [PubMed] [Google Scholar]
- 11.Min JK, Dunning A, Lin FY, et al. Rationale and design of the CONFIRM (COronary CT Angiography EvaluatioN For Clinical Outcomes: An InteRnational Multicenter) Registry. J Cardiovasc Comput Tomogr 2011;5:84–92. [DOI] [PubMed] [Google Scholar]
- 12.Schulman-Marcus J, O Hartaigh B, Gransar H, et al. Sex-Specific Associations Between Coronary Artery Plaque Extent and Risk of Major Adverse Cardiovascular Events: The CONFIRM Long-Term Registry. JACC Cardiovasc Imaging 2016;9:364–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Graaf FR, Schuijf JD, van Velzen JE, et al. Diagnostic accuracy of 320-row multidetector computed tomography coronary angiography in the non-invasive evaluation of significant coronary artery disease. Eur Heart J 2010;31:1908–15. [DOI] [PubMed] [Google Scholar]
- 14.Austen WG, Edwards JE, Frye RL, et al. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation 1975;51:5–40. [DOI] [PubMed] [Google Scholar]
- 15.Leaman DM, Brower RW, Meester GT, Serruys P, van den Brand M. Coronary artery atherosclerosis: severity of the disease, severity of angina pectoris and compromised left ventricular function. Circulation 1981;63:285–99. [DOI] [PubMed] [Google Scholar]
- 16.Comprehensive CTA risk score calculator. Available at: http://18.224.14.19/calcApp/.
- 17.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Eur Heart J 2012;33:2551–67. [DOI] [PubMed] [Google Scholar]
- 18.Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37:267–315. [DOI] [PubMed] [Google Scholar]
- 19.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–45. [PubMed] [Google Scholar]
- 20.Pencina MJ, D’Agostino RB Sr., D’Agostino RB Jr., Vasan RS Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 2008;27:157–72; discussion 207–12. [DOI] [PubMed] [Google Scholar]
- 21.Polonsky TS, McClelland RL, Jorgensen NW, et al. Coronary artery calcium score and risk classification for coronary heart disease prediction. Jama 2010;303:1610–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cury RC, Abbara S, Achenbach S, et al. Coronary Artery Disease - Reporting and Data System (CAD-RADS): An Expert Consensus Document of SCCT, ACR and NASCI: Endorsed by the ACC. JACC Cardiovasc Imaging 2016;9:1099–113. [DOI] [PubMed] [Google Scholar]
- 23.Min JK, Dunning A, Lin FY, et al. Age- and sex-related differences in all-cause mortality risk based on coronary computed tomography angiography findings results from the International Multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry) of 23,854 patients without known coronary artery disease. J Am Coll Cardiol 2011;58:849–60. [DOI] [PubMed] [Google Scholar]
- 24.Nielsen LH, Botker HE, Sorensen HT, et al. Prognostic assessment of stable coronary artery disease as determined by coronary computed tomography angiography: a Danish multicentre cohort study. Eur Heart J 2017;38:413–21. [DOI] [PubMed] [Google Scholar]
- 25.Hoffmann U, Ferencik M, Udelson JE, et al. Prognostic Value of Noninvasive Cardiovascular Testing in Patients With Stable Chest Pain: Insights From the PROMISE Trial (Prospective Multicenter Imaging Study for Evaluation of Chest Pain). Circulation 2017;135:2320–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giroud D, Li JM, Urban P, Meier B, Rutishauer W. Relation of the site of acute myocardial infarction to the most severe coronary arterial stenosis at prior angiography. Am J Cardiol 1992;69:729–32. [DOI] [PubMed] [Google Scholar]
- 27.Deseive S, Shaw LJ, Min JK, et al. Improved 5-year prediction of all-cause mortality by coronary CT angiography applying the CONFIRM score. Eur Heart J Cardiovasc Imaging 2017;18:286–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mark DB, Nelson CL, Califf RM, et al. Continuing evolution of therapy for coronary artery disease. Initial results from the era of coronary angioplasty. Circulation 1994;89:20–15 [DOI] [PubMed] [Google Scholar]
- 29.Cheezum MK, Hulten EA, Smith RM, et al. Changes in preventive medical therapies and CV risk factors after CT angiography. JACC Cardiovasc Imaging 2013;6:574–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.