Abstract
Introduction Optimal management of vestibular schwannoma (VS) demands involvement of an experienced multidisciplinary team. As the number of training programs in neurotology and skull base neurosurgery continues to rise, ensuring that trainees are capable of evidence-based decision-making and treatment, whether microsurgical or radiosurgical, is of paramount importance. The purpose of this study is to characterize the landscape of neurotologic and neurosurgical fellowship training programs in North America, with special reference to VS management.
Methods A 64-item web-based survey assessing VS practice trends was devised by members of the North American Skull Base Society (NASBS) Research Task Force and distributed electronically to NASBS membership via SurveyMonkey as a cross-sectional study. Participation was entirely voluntary and there was no remuneration for survey completion. The survey link was active from November 29 to December 14, 2016.
Results Of 719 members of the NASBS who were emailed a survey link, a total of 57 were returned (8%) completed surveys. Of all respondents, 51 (89%) claimed to have formal training in skull base neurosurgery or neurotology. Thirty-three respondents (65%) were skull base neurosurgeons while the remainder were neurotologists ( n = 18; 35%). Institutions with fellowship programs tended to have a higher surgical, radiosurgical, and overall case volume than those with a residency program alone. However, 20% of respondents at institutions with fellowship programs reported evaluating less than 50 new diagnoses of VS per year and 12% reported a surgical case volume of less than 10 cases per year.
Conclusion As the number of skull base training programs expands, it is our duty to ensure that trainees gain sufficient experience to enter independent practice with the ability to exercise informed decision-making and safely perform VS surgery and radiosurgery. In the current training climate, implementing multidisciplinary care models, formalized training requirements, and emerging surgical simulators will support the development of minimum proficiencies in VS care.
Keywords: vestibular schwannoma, acoustic neuroma, skull base surgery, cranial base surgery, microsurgery, radiosurgery
Introduction
Training in skull base surgery has evolved considerably over the past 100 years. Starting with the development of transtemporal approaches, a multidisciplinary approach to vestibular schwannoma (VS) and other skull base tumors increased in popularity. Also, the development and wide spread availability of stereotactic radiosurgery (SRS) in the late 1980s and 1990s in North America provided additional treatment options for many patients with skull base tumors. Coincidentally, the mortality rates associated with VS treatment declined, giving rise to the modern approach to VS which focuses on preservation of neurologic function and hearing. In parallel, the fields of otolaryngology and neurosurgery recognized the importance of postresidency training to meet the demands of the specialty and changing patient expectations. In 1995, the Accreditation Council for Graduate Medical Education (ACGME) developed a subspecialty certificate in neurotology and established minimum case numbers for graduating fellows. While not yet independently accredited by the ACGME, neurologic surgery fellowship training programs have started to incorporate discrete curricula for skull base training for interested residents and as part of postresidency fellowships.
Secondary to greater access to head MRI (magnetic resonance imaging) and widespread adoption of screening protocols for asymmetrical sensorineural hearing loss, today VS are diagnosed at a smaller tumor size in patients of older age and more subtle symptoms. While the overall incidence of VS has increased incrementally over the last several decades, the proportion of cases that receive nonsurgical treatment has risen remarkably. In 2004, 56% of VS in the U.S. underwent surgery, compared with only 46% in 2011. It is estimated that by 2026, the trend observed from 2004 to 2011 is anticipated to continue with at least half of tumors undergoing an initial period of active observation. 1 While microsurgery remains the treatment of choice for the more challenging variety of VS–large tumors (> 3 cm in size), macrocystic tumors, and post-SRS failure–the percentage of small and medium-sized VS that receive microsurgery is decreasing.
Paralleling these developments, the number of otolaryngology and neurosurgery residency graduates that perform skull base surgery is increasing. Within the field of neurotology, there has been more than a two-fold increase in the number of programs and positions filled between 2000 and 2018 ( Fig. 1 ). Since 2011, six new neurotology fellowship programs have become ACGME-accredited, offering 10 additional spots. Based on the North American Skull Base Society (NASBS) Fellowship Registry, at least nine training programs focused on skull base neurologic surgery were founded since 2011. 2 These figures likely underestimate the true number of additional trainees as many skull base neurosurgery fellowships may not be indexed by the NASBS and some nonaccredited otology fellowships offer training in lateral skull base surgery. These changes may have profound implications for fellowship training and acquisition of minimum surgical competency.
Fig. 1.
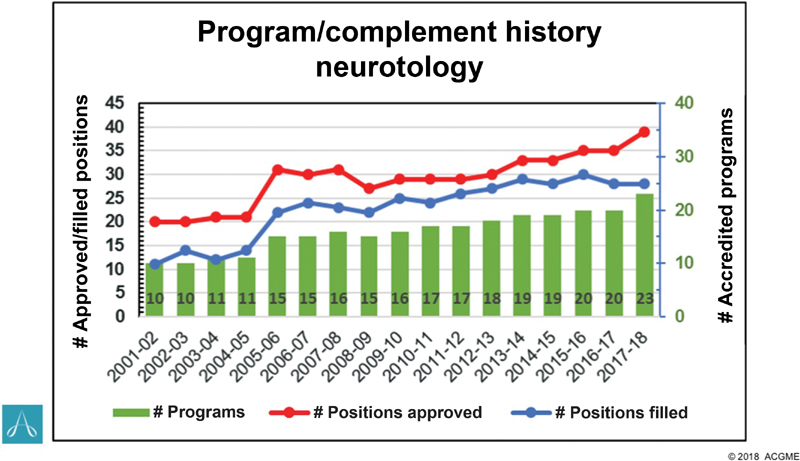
Number of neurotology programs and positions, including the number of accredited programs, positions, and filled positions from 2001–2018 (Accreditation Council for Graduate Medical Education copyright 2018).
Given these questions, recent attention has been paid to case volume among trainees entering anterior and lateral skull base surgery practices. Based on a survey of recent neurotology fellowship graduates, Dedmon et al found that 55% of respondents performed fewer than 20 skull base cases per year. 3 Similarly, a survey of members of the American Rhinologic Society (ARS) determined that over half of respondents reported performing 20 or fewer endoscopic skull base cases in a typical year. 4 When NASBS and ARS members were polled, 32% of respondents reported performing between 20 and 50 endoscopic skull base cases annually. 5
This study surveys the NASBS to gain insight into the current state of neurotologic and neurologic surgery fellowship training in North America, with particular reference to VS care.
Materials and Methods
A 64-item web-based survey assessing VS practice trends was devised by members of the NASBS Research Task Force and distributed to the NASBS membership via SurveyMonkey ( Appendix A ). Voluntary participation was solicited via e-mail with an attached electronic survey link available from November 29 to December 14, 2016. Prior to dissemination, the survey was distributed to several NASBS members to examine the survey for readability and content. Following initial contact, survey reminders were sent 1 week and 24 hours before survey closure.
Appendix A.
| Q. 1. Are you actively involved in vestibular schwannoma treatment at your center? (Please choose the single best answer) | ||
| Answer options | Response percent (%) | Response count |
| Yes | 90.8 | 79 |
| No | 9.2 | 8 |
| Q. 2. What is your age? | ||
| Answer options | Response percent (%) | Response count |
| < 30 y old | 0.0 | 0 |
| 30–39 y old | 17.5 | 10 |
| 40–49 y old | 26.3 | 15 |
| 50–59 y old | 40.4 | 23 |
| 60–69 y old | 14.0 | 8 |
| 70–79 y old | 1.8 | 1 |
| 80 y old or greater | 0.0 | 0 |
| Q. 3. What is your gender? | ||
| Answer options | Response percent (%) | Response count |
| Female | 0.0 | 0 |
| Male | 100.0 | 57 |
| Q. 4. Which race/ethnicity best describes you? (Please choose the single best answer) | ||
| Answer options | Response percent (%) | Response count |
| American Indian or Alaskan Native | 0.0 | 0 |
| Asian/Pacific Islander | 22.8 | 13 |
| Black or African American | 0.0 | 0 |
| Hispanic | 12.3 | 7 |
| White/Caucasian | 61.4 | 35 |
| Multiple ethnicity/other (please specify) | 3.5 | 2 |
| Q. 5A. In which country in North America do you primarily practice? (Please choose the single best answer, listed in alphabetical order) | ||
| Answer options | Response percent (%) | Response count |
| Antigua and Barbuda | 0.0 | 0 |
| Bahamas | 0.0 | 0 |
| Barbados | 0.0 | 0 |
| Belize | 0.0 | 0 |
| Canada | 3.5 | 2 |
| Costa Rica | 0.0 | 0 |
| Cuba | 0.0 | 0 |
| Dominica | 0.0 | 0 |
| Dominican Republic | 0.0 | 0 |
| El Salvador | 0.0 | 0 |
| Grenada | 0.0 | 0 |
| Guatemala | 0.0 | 0 |
| Haiti | 0.0 | 0 |
| Honduras | 0.0 | 0 |
| Jamaica | 0.0 | 0 |
| Mexico | 3.5 | 2 |
| Nicaragua | 0.0 | 0 |
| Panama | 0.0 | 0 |
| Saint Kitts and Nevis | 0.0 | 0 |
| Saint Lucia | 0.0 | 0 |
| Saint Vincent and the Grenadines | 0.0 | 0 |
| Trinidad and Tobago | 0.0 | 0 |
| The United States of America | 84.2 | 48 |
| I do not currently practice in North America | 8.8 | 5 |
| Q. 5B. In which state do you primarily practice? (Please choose the single best answer, listed in alphabetical order) | ||
| Answer options | Response percent (%) | Response count |
| Alabama | 0.0 | 0 |
| Alaska | 0.0 | 0 |
| Arizona | 8.3 | 4 |
| Arkansas | 2.1 | 1 |
| California | 12.5 | 6 |
| Colorado | 0.0 | 0 |
| Connecticut | 0.0 | 0 |
| Delaware | 0.0 | 0 |
| Florida | 8.3 | 4 |
| Georgia | 0.0 | 0 |
| Hawaii | 0.0 | 0 |
| Idaho | 0.0 | 0 |
| Illinois | 6.3 | 3 |
| Indiana | 2.1 | 1 |
| Iowa | 0.0 | 0 |
| Kansas | 2.1 | 1 |
| Kentucky | 2.1 | 1 |
| Louisiana | 2.1 | 1 |
| Maine | 0.0 | 0 |
| Maryland | 2.1 | 1 |
| Massachusetts | 6.3 | 3 |
| Michigan | 6.3 | 3 |
| Minnesota | 6.3 | 3 |
| Mississippi | 0.0 | 0 |
| Missouri | 2.1 | 1 |
| Montana | 0.0 | 0 |
| Nebraska | 0.0 | 0 |
| Nevada | 0.0 | 0 |
| New Hampshire | 0.0 | 0 |
| New Jersey | 0.0 | 0 |
| New Mexico | 2.1 | 1 |
| New York | 8.3 | 4 |
| North Carolina | 0.0 | 0 |
| North Dakota | 0.0 | 0 |
| Ohio | 6.3 | 3 |
| Oklahoma | 0.0 | 0 |
| Oregon | 0.0 | 0 |
| Pennsylvania | 4.2 | 2 |
| Rhode Island | 0.0 | 0 |
| South Carolina | 0.0 | 0 |
| South Dakota | 0.0 | 0 |
| Tennessee | 4.2 | 2 |
| Texas | 4.2 | 2 |
| Utah | 0.0 | 0 |
| Vermont | 0.0 | 0 |
| Virginia | 0.0 | 0 |
| Washington | 2.1 | 1 |
| West Virginia | 0.0 | 0 |
| Wisconsin | 0.0 | 0 |
| Wyoming | 0.0 | 0 |
| Q. 5C. Which province in Canada do you primarily practice in? (Please choose the single best answer) | ||
| Answer options | Response percent (%) | Response count |
| Alberta | 50.0 | 1 |
| British Columbia | 50.0 | 1 |
| Manitoba | 0.0 | 0 |
| New Brunswick | 0.0 | 0 |
| Newfoundland and Labrador | 0.0 | 0 |
| Nova Scotia | 0.0 | 0 |
| Northwest Territories | 0.0 | 0 |
| Nunavut | 0.0 | 0 |
| Ontario | 0.0 | 0 |
| Prince Edward Island | 0.0 | 0 |
| Quebec | 0.0 | 0 |
| Saskatchewan | 0.0 | 0 |
| Yukon | 0.0 | 0 |
| Q. 6. Which of the following best describes your training background? (Please choose the single best answer) | ||
| Answer options | Response percent (%) | Response count |
| General neurosurgery | 1.8 | 1 |
| Neurosurgery with specialization in skull base and cerebrovascular surgery | 57.9 | 33 |
| General otolaryngology | 0.0 | 0 |
| Otology without accredited fellowship in neurotology | 8.8 | 5 |
| Neurotology with accredited fellowship | 31.6 | 18 |
| No formal training in neurosurgery or otolaryngology | 0.0 | 0 |
| Q. 7. How long have you been in practice? (Please choose the single best answer) | ||
| Answer options | Response percent (%) | Response count |
| Still in training (residency or fellowship) | 1.8 | 1 |
| 1–5 y | 15.8 | 9 |
| 5–10 y | 8.8 | 5 |
| 11–15 y | 17.5 | 10 |
| 16–20 y | 14.0 | 8 |
| > 20 y | 42.1 | 24 |
| Q. 8. Which of the following best describes your clinical practice setting? (Please choose the single best answer) | ||
| Answer options | Response percent (%) | Response count |
| Solo private practice | 1.8 | 1 |
| Group private practice | 5.3 | 3 |
| Private practice with academic affiliation | 19.3 | 11 |
| Primarily academic practice | 73.7 | 42 |
| Q. 9. Do you evaluate vestibular schwannoma patients in clinic with a multidisciplinary team? (Please choose the single best answer) | ||
| Answer options | Response percent (%) | Response count |
| I am neurosurgeon and I evaluate and counsel vestibular schwannoma patients alone typically | 15.8 | 9 |
| I am a neurosurgeon and usually/always evaluate and counsel vestibular schwannoma patients with an otolaryngologist/neurotologist | 43.9 | 25 |
| I am an otolaryngologist and I evaluate and counsel vestibular schwannoma patients alone typically | 21.1 | 12 |
| I am an otolaryngologist and usually/always evaluate and counsel vestibular schwannoma patients with a neurosurgeon | 19.3 | 11 |
| I am not an otolaryngologist or neurosurgeon | 0.0 | 0 |
| Q. 10. Do you perform vestibular schwannoma surgery with a multidisciplinary team? (Please choose the single best answer) | ||
| Answer options | Response percent (%) | Response count (%) |
| I am neurosurgeon and I operate on vestibular schwannomas alone typically | 10.5 | 6 |
| I am a neurosurgeon and usually/always operate on vestibular schwannomas with otolaryngology | 49.1 | 28 |
| I am an otolaryngologist and I operate on vestibular schwannoma alone typically | 3.5 | 2 |
| I am an otolaryngologist and usually/always operate on vestibular schwannomas with a neurosurgeon | 36.8 | 21 |
| I am not an otolaryngologist or neurosurgeon | 0.0 | 0 |
| Q. 11. Approximately how many new cases of vestibular schwannomas are evaluated at your center annually? (Please choose the single best answer) | ||
| Answer options | Response percent (%) | Response count (%) |
| < 25 cases per y | 17.5 | 10 |
| 25–50 cases per y | 28.1 | 16 |
| 51–75 cases per y | 15.8 | 9 |
| 76–100 cases per y | 8.8 | 5 |
| 101–150 cases per y | 12.3 | 7 |
| 151–200 cases per y | 7.0 | 4 |
| > 200 cases per y | 10.5 | 6 |
| Q. 12. Approximately how many vestibular schwannomas does your center operate on annually? (Please choose the single best answer) | ||
| Answer options | Response percent (%) | Response count |
| None | 0.0 | 0 |
| 1–5 cases per y | 14.0 | 8 |
| 6–10 cases per y | 10.5 | 6 |
| 11–30 cases per y | 29.8 | 17 |
| 31–50 cases per y | 21.1 | 12 |
| 51–100 cases per y | 15.8 | 9 |
| > 100 cases per y | 8.8 | 5 |
| Q. 13. Approximately how many vestibular schwannomas does your center treat with radiosurgery (or fractionated stereotactic radiotherapy) annually? (Please choose the single best answer) | ||
| Answer options | Response percent (%) | Response count |
| None | 3.5 | 2 |
| 1–5 cases per y | 8.8 | 5 |
| 6–10 cases per y | 28.1 | 16 |
| 11–30 cases per y | 38.6 | 22 |
| 31–50 cases per y | 12.3 | 7 |
| 51–100 cases per y | 7.0 | 4 |
| > 100 cases per y | 1.8 | 1 |
| Q. 14. At your center, does a member of the surgical team regularly participate in radiation planning for patients that elect to undergo radiosurgery or fractionated stereotactic radiotherapy for vestibular schwannoma treatment? | ||
| Answer options | Response percent (%) | Response count |
| Yes | 89.5 | 51 |
| No | 10.5 | 6 |
| Q. 15. As a general rule, at what size cutoff do you believe stereotactic radiation therapy becomes a poor treatment option in an otherwise healthy patient with a vestibular schwannoma (excluding patients with substantial comorbidities or the extreme elderly)? (Please choose the single best answer) | ||
| Answer options | Response percent (%) | Response count |
| 1.5 cm | 5.3 | 3 |
| 2 cm | 24.6 | 14 |
| 2.5 cm | 43.9 | 25 |
| 3 cm | 22.8 | 13 |
| 3.5 cm | 1.8 | 1 |
| ≥ 4 cm | 1.8 | 1 |
| Q. 16. How do you typically counsel patients regarding the risk of malignant degeneration of vestibular schwannomas following radiation? (Please choose the single best answer) | ||
| Answer options | Response percent (%) | Response count |
| Should be a major consideration when deciding treatment | 0.0 | 0 |
| Is extremely rare and should only be a very minor consideration when deciding treatment | 89.5 | 51 |
| Do not usually discuss this issue unless brought up by the patient | 10.5 | 6 |
| Q. 17. Does your center currently have an affiliated radiation program where patients can be referred for vestibular schwannoma treatment? (Mark all that apply) | ||
| Answer options | Response percent (%) | Response count |
| Yes, Gamma Knife Unit | 63.2 | 36 |
| Yes, CyberKnife Unit | 24.6 | 14 |
| Yes, Novalis Linear Accelerator Unit | 24.6 | 14 |
| Yes, Proton Beam Unit | 15.8 | 9 |
| Yes, other | 7.0 | 4 |
| No, our center does not have an affiliated radiation program where patients can be referred for vestibular schwannoma treatment | 3.5 | 2 |
| Q. 18. At your center, how are most small (< 1.5cm) vestibular schwannomas initially managed? (Please choose the single best answer) | ||
| Answer options | Response percent (%) | Response count |
| Initial observation until growth demonstrated | 91.2 | 52 |
| Upfront microsurgery | 7.0 | 4 |
| Upfront radiosurgery | 1.8 | 1 |
| Upfront fractionated radiotherapy | 0.0 | 0 |
| Q. 19. What is your preferred surgical approach for hearing preservation surgery with vestibular schwannomas confined to the IAC or having only minimal involvement of the CPA? (Please choose the single best answer) | ||
| Answer options | Response percent | Response count |
| Middle cranial fossa | 43.9 | 25 |
| Retrosigmoid | 29.8 | 17 |
| Use both almost equally and decision depends upon fundal cap, tumor size, and anatomy | 15.8 | 9 |
| Rarely perform hearing preservation surgery | 10.5 | 6 |
| Q. 20. In your experience, what percentage of purely intracanalicular vestibular schwannomas grow over the first 5 years of observation? (Please choose the single best answer) | ||
| Answer options | Response percent (%) | Response count |
| Approximately 10% | 21.1 | 12 |
| Approximately 25% | 33.3 | 19 |
| Approximately 50% | 33.3 | 19 |
| Approximately 75% | 8.8 | 5 |
| Approximately 90% | 3.5 | 2 |
| Q. 21. In your experience, in a cisternal tumor with less than 1.5 cm of CPA extension, what is the risk of tumor growth over the first 5 years of observation? (Please choose the single best answer) | ||
| Answer options | Response percent | Response count |
| Approximately 10% | 8.8 | 5 |
| Approximately 25% | 17.5 | 10 |
| Approximately 50% | 49.1 | 28 |
| Approximately 75% | 21.1 | 12 |
| Approximately 90% | 3.5 | 2 |
| Q. 22. As a general rule, what treatment do you believe confers the best chance of retaining serviceable hearing at 10 years in patients with an intracanalicular vestibular schwannoma and 100% word recognition at diagnosis? (Please choose the single best answer) | ||
| Answer options | Response percent (%) | Response count |
| Microsurgery using the middle cranial fossa approach | 31.6 | 18 |
| Microsurgery using the retrosigmoid approach | 10.5 | 6 |
| Conservative observation with serial MRI | 50.9 | 29 |
| Radiosurgery (single fraction) with a marginal dose of 12 or 13 Gy | 7.0 | 4 |
| Fractionated stereotactic radiotherapy | 0.0 | 0 |
| Q. 23. In your experience, what is the chance of successful hearing preservation surgery (retaining serviceable hearing) in the above patient (MRI figure), with a 100% word-recognition score and 30 dB PTA (excellent hearing)? (Please choose the single best answer) | ||
| Answer options | Response percent (%) | Response count |
| 0% | 3.5 | 2 |
| 20% | 22.8 | 13 |
| 40% | 29.8 | 17 |
| 60% | 40.4 | 23 |
| 80% | 3.5 | 2 |
| 100% | 0.0 | 0 |
| Q. 24. Based on the same MRI figure, what is the likelihood of retaining serviceable hearing 10 years after stereotactic radiation therapy in a patient with a 100% word-recognition score and 30 dB PTA (excellent hearing) at diagnosis? (Please choose the single best answer) | ||
| Answer options | Response percent (%) | Response count |
| 0% | 8.8 | 5 |
| 20% | 35.1 | 20 |
| 40% | 42.1 | 24 |
| 60% | 10.5 | 6 |
| 80% | 3.5 | 2 |
| 100% | 0.0 | 0 |
| Q. 25. When hearing preservation is not a goal, what surgical approach do you favor for removal of vestibular schwannomas with CPA involvement? | ||
| Answer options | Response percent (%) | Response count |
| Translabyrinthine approach | 49.1 | 28 |
| Retrosigmoid approach | 24.6 | 14 |
| Balanced between translabyrinthine and retrosigmoid approaches | 26.3 | 15 |
| Q. 26. At what size do you typically recommend a translabyrinthine approach even in patients with serviceable hearing, understanding that hearing preservation is unlikely? (Please choose the single best answer) | ||
| Answer options | Response percent (%) | Response count |
| < 1 cm in the CPA | 0.0 | 0 |
| 1–1.5 cm in the CPA | 3.5 | 2 |
| 1.6–2 cm in the CPA | 12.3 | 7 |
| 2.1–2.5 cm in the CPA | 22.8 | 13 |
| 2.6–3 cm in the CPA | 17.5 | 10 |
| > 3 cm in the CPA | 7.0 | 4 |
| Generally always try to save serviceable hearing regardless of tumor size | 24.6 | 14 |
| Generally do not use the translabyrinthine approach in my practice | 12.3 | 7 |
| Q. 27. Rank the following items according to weight of impact on successful hearing preservation surgery: (1 = strongest predictor, 6 = weakest predictor) | |||||||
| Answer options | 1 | 2 | 3 | 4 | 5 | 6 | Rating average |
| Tumor size | 32 | 20 | 5 | 0 | 0 | 0 | 1.53 |
| Size of CSF fundal cap | 2 | 6 | 27 | 12 | 5 | 5 | 3.47 |
| Vestibular nerve of origin (i.e., superior or inferior vestibular nerve) | 1 | 0 | 5 | 22 | 22 | 7 | 4.49 |
| Preoperative hearing levels | 22 | 28 | 4 | 3 | 0 | 0 | 1.79 |
| Patient age | 0 | 1 | 9 | 12 | 20 | 15 | 4.68 |
| Widening of internal auditory canal | 0 | 2 | 7 | 8 | 10 | 30 | 5.04 |
| Q. 28. As a general rule, what level of hearing do you feel is worth attempting to preserve when tumor characteristics are at least reasonably favorable and the other ear has normal hearing? (Please choose the single best answer) | ||
| Answer options | Response percent (%) | Response count |
| Class A hearing only (PTA ≤ 30 dB and WRS ≥ 70%) | 26.3 | 15 |
| Class A or B hearing (serviceable hearing; PTA ≤ 50 dB and WRS ≥ 50%) | 63.2 | 36 |
| Class A, B, or C hearing (any PTA and WRS ≥ 50%) | 3.5 | 2 |
| Any detectable hearing | 7.0 | 4 |
| Q. 29. As a general rule, do you avoid the retrosigmoid approach due to the perceived risk of postoperative headaches? (Please choose the single best answer) | ||
| Answer options | Response percent (%) | Response count |
| Yes | 5.3 | 3 |
| No | 71.9 | 41 |
| Sometimes; decision may be influenced by patient factors including history of headaches | 22.8 | 13 |
| Q. 30. In your experience, how does the surgical removal of a vestibular schwannoma effect tinnitus? (Please choose the single best answer) | ||
| Answer options | Response percent (%) | Response count |
| Results in a reduction or resolution of tinnitus | 15.8 | 9 |
| Has no significant effect on tinnitus | 31.6 | 18 |
| Leads to worsening of tinnitus | 0.0 | 0 |
| Has an unpredictable effect on tinnitus | 52.6 | 30 |
| Q. 31. In your experience, how does surgical removal of a vestibular schwannoma affect long-term dizziness in patients reporting severe/frequent preoperative dizziness? (Please choose the single best answer) | ||
| Answer options | Response percent (%) | Response count |
| Results in a reduction or resolution of dizziness | 78.9 | 45 |
| Has no significant effect on dizziness | 3.5 | 2 |
| Leads to worsening dizziness | 3.5 | 2 |
| Has an unpredictable effect on dizziness | 14.0 | 8 |
| Q. 32. In your experience, what is the best treatment for a patient with a vestibular schwannoma and concomitant medically-refractory trigeminal neuralgia? (Please choose the single best answer) | ||
| Answer options | Response percent (%) | Response count |
| Radiosurgery or radiation therapy alone | 1.8% | 1 |
| Microsurgery to remove the tumor and indirectly decompress the 5th nerve | 54.4% | 31 |
| Surgery to remove the tumor and perform a microvascular decompression of the 5th nerve | 43.9% | 25 |
| Q. 33. When operating on a large or giant vestibular schwannoma (> 3cm) in someone under 60 years of age, which of the following best describes your approach to extent of resection? (Please choose the single best answer) | ||
| Answer options | Response percent (%) | Response count |
| Perform gross total resection, even at expense of facial nerve function | 1.8 | 1 |
| Attempt to achieve gross total resection, but willing to concede when tumor is severely adherent to reduce risk of facial nerve injury, generally resulting in aggressive subtotal or near-total resection in such cases | 93.0 | 53 |
| Enter the case planning subtotal resection to relieve brainstem compression and achieve a volume that can be effectively treated with stereotactic radiation therapy | 5.3 | 3 |
| Q. 34. How frequently do you perform subtotal resection for tumors greater than 3 cm in greatest CPA dimension? (Please choose the single best answer) | ||
| Answer options | Response percent (%) | Response count |
| Essentially never | 7.0 | 4 |
| Rarely (< 20%) | 21.1 | 12 |
| Sometimes (20–50%) | 49.1 | 28 |
| Frequently (51–80%) | 12.3 | 7 |
| Most of the time (81–99%) | 8.8 | 5 |
| Essentially always | 1.8 | 1 |
| Q. 35. Which of the following is the primary determinant used to decide when to stop tumor resection to preserve facial nerve functional integrity? (Mark all that apply) | ||
| Answer options | Response percent (%) | Response count |
| Severe seventh nerve splay and tumor adherence | 78.9 | 45 |
| Repeated or prolonged 7th nerve neurotonic firing | 49.1 | 28 |
| Increase in threshold of stimulation needed to elicit a response from the facial nerve at the brainstem (i.e., 0.2 mA goes to 0.5 mA to elicit a response) | 54.4 | 31 |
| Always remove all the tumor | 1.8 | 1 |
| Q. 36. As a general rule, what is your preferred surgical approach for resection of large (> 3cm) vestibular schwannomas? (Please choose the single best answer) | ||
| Answer options | Response percent (%) | Response count |
| Retrosigmoid approach (single stage) | 52.6 | 30 |
| Translabyrinthine approach (single stage) | 35.1 | 20 |
| Staged approach | 12.3 | 7 |
| Q. 37. Which surgical approach do you perceive to have the highest risk of postoperative CSF leak? (Please choose the single best answer) | ||
| Answer options | Response percent (%) | Response count |
| Retrosigmoid | 35.1 | 20 |
| Translabyrinthine | 57.9 | 33 |
| Middle cranial fossa | 7.0 | 4 |
| Q. 38. As a general rule, what is your preferred treatment strategy for managing residual disease following subtotal vestibular schwannoma resection? (Please choose the single best answer) | ||
| Answer options | Response percent (%) | Response count |
| Initially observe the tumor remnant and only treat with stereotactic radiation therapy if unequivocal growth is seen | 78.9 | 45 |
| Treat the remnant tumor volume with upfront (within the first 6 mo) stereotactic radiation therapy | 15.8 | 9 |
| Initially observe the tumor remnant and only treat with repeat microsurgery if unequivocal growth is seen | 5.3 | 3 |
Abbreviations: CPA, cerebellopontine angle; CSF, cerebrospinal fluid; IAC, internal auditory canal; MRI, magnetic resonance imaging; PTA, pure tone average; WRS, word recognition score.
Responses were requested from those who are engaged in VS treatment. As a secondary screening measure, the first question of the survey inquired, “Are you actively involved in vestibular schwannoma treatment at your center?,” and the survey episode was subsequently closed for respondents who selected “no.” All survey questions were multiple choice and in most cases, survey items required selection of the single best answer. Respondent data were collected and compiled anonymously. Data from this large survey was originally apportioned into two separate reports according to topic: (1) overall management trends in VS across North America 6 and (2) practice patterns of perioperative vestibular schwannoma care in North America. 7 An extension of this original research was conceived to examine the state of neurotology and skull base neurosurgery training in VS. Differences between programs with and without fellowship training programs and those with relatively high and low case volume were studied.
Descriptive statistical analysis was performed using Microsoft Excel (Redmond, WA). Comparisons between variables were assessed using Fisher's exact tests as appropriate. Statistical analyses were performed using version 13.1 of the JMP software package (SAS Institute, Cary, NC). All tests were two-sided and p -values < 0.05 were considered statistically significant. The Mayo Clinic Institutional Review Board deemed this study exempt from review.
Results
Population Demographic
A total of 719 members were initially emailed a survey link, comprising the entire membership of the NASBS as of October 2016. Of these, 87 opened the survey questionnaire. Eight reported they were not actively involved in VS treatment and an additional 22 did not finish the complete question set. In total, 57 (8%) completed surveys were analyzed from NASBS members who reported to have regular involvement in VS care. Of all respondents, 51 (89%) claimed to have formal training in skull base surgery or neurotology. Thirty-three respondents (65%) were skull base neurosurgeons while the remainder were neurotologists ( n = 18; 35%).
Case Volume
Surgical, radiosurgical, and overall case volume is summarized in Table 1 . Centers that evaluate 50 or fewer new cases of VS annually were considered “low volume” while those who evaluate greater than 50 new cases of VS annually were considered “high volume.” This level was utilized as it divided the respondents fairly evenly with 26 respondents (46%) in the low volume group and 31 respondents (54%) in the highvolume group. Of note, this does not equate to the number of patients operated or radiated; only the number evaluated or consulted determined the case volume.
Table 1. Responder reported case volume.
| Variable | All respondents % | Respondents with fellowship ( n = 25) | Respondents without fellowship ( n = 29) |
|---|---|---|---|
| Annual number of new VS evaluated at center | |||
| < 25 cases per y | 17.5% | 8% | 21% |
| 25–50 cases per y | 28.1% | 12% | 45% |
| 51–75 cases per y | 15.8% | 20% | 10% |
| 76–100 cases per y | 8.8% | 8% | 10% |
| 101–150 cases per y | 12.3% | 16% | 10% |
| 151–200 cases per y | 7.0% | 16% | 0% |
| Greater than 200 cases per y | 10.5% | 20% | 3% |
| Annual number of VS treated with surgery at center | |||
| None | 0.0% | 0% | 0% |
| 1–5 cases per y | 14.0% | 0% | 21% |
| 6–10 cases per y | 10.5% | 12% | 10% |
| 11–30 cases per y | 29.8% | 20% | 41% |
| 31–50 cases per y | 21.1% | 36% | 10% |
| 51–100 cases per y | 15.8% | 16% | 14% |
| > 100 cases per y | 8.8% | 16% | 3% |
| Annual number of VS treated with radiation at center | |||
| None | 3.5% | 0% | 3% |
| 1–5 cases per y | 8.8% | 4% | 7% |
| 6–10 cases per y | 28.1% | 20% | 38% |
| 11–30 cases per y | 38.6% | 48% | 34% |
| 31–50 cases per y | 12.3% | 16% | 10% |
| 51–100 cases per y | 7.0% | 12% | 3% |
| > 100 cases per y | 1.8% | 0% | 3% |
Abbreviation: VS, vestibular schwannoma.
Overall case volume was skewed toward institutions with fellowship programs compared with those with residency programs only ( Fig. 2 ). Of respondents representing institutions with fellowships in either skull base neurosurgery or neurotology, 80% reported evaluating over 50 new cases of VS annually (“high volume”), in contrast to 33% of respondents from institutions with residency programs in neurologic surgery or otolaryngology alone. This difference is more pronounced when considering higher volume centers. Fifty-two percent of respondents from centers with fellowship programs reported evaluating more than 100 cases of VS annually, compared with only 13% of respondents at institutions without skull base surgery fellowship programs.
Fig. 2.
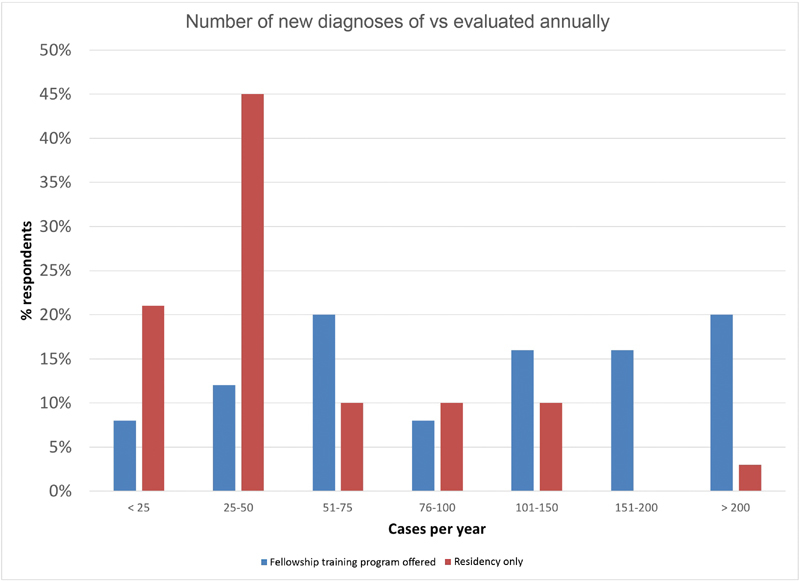
Number of new diagnoses of VS evaluated annually by institution volume. Respondents representing institutions with neurotologic or neurosurgical skull base training programs (blue) and those representing institutions with neurosurgery or otolaryngology training programs only (red) were separated. VS, vestibular schwannoma.
Microsurgical and radiosurgical case volume demonstrates a similar tendency ( Fig. 3 ). Twice as many respondents from institutions with fellowship training programs compared with those with residency programs alone reported treating over 50 VSs with microsurgery (32% vs. 17%) or radiation (12% vs. 6%) annually.
Fig. 3.
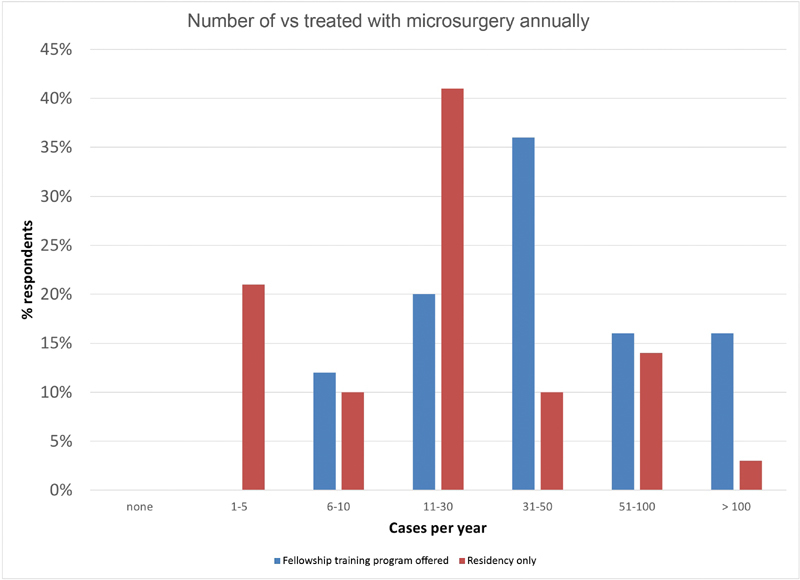
Number of VS treated with microsurgery annually by institution volume. Respondents representing institutions with neurotologic or neurosurgical skull base training programs (blue) and those representing institutions with neurosurgery or otolaryngology training programs only (red) were separated. VS, vestibular schwannoma.
Notably, several respondents from institutions with fellowship training programs reported a relatively low volume of new VS cases and operative VS cases. Of respondents at institutions with fellowship training programs, 12% reported an operative case volume of less than 10 cases per year. Of the same group, 20% reported evaluating fewer than 50 new diagnoses of VS per year.
Multidisciplinary Tumor Management
Overall, 36 of 57 (63%) respondents reported evaluating VS patients in clinic in a multidisciplinary team (i.e., a neurosurgeon with an otolaryngologist) as opposed to evaluating the patient “alone” (i.e., a single surgeon). Among low volume centers ( n = 26), 50% of respondents reported evaluating VS patients in a multidisciplinary fashion. Among high volume centers ( n = 31) in contrast, 74% of respondents reported multidisciplinary evaluation of VS patients ( p = 0.10). Respondents from programs with fellowship training programs reported evaluating VS patients in clinic as a multidisciplinary team roughly as often as those with residency programs alone (68 vs. 62%, respectively). A slightly higher proportion of respondents from institutions with fellowship training programs reported multidisciplinary VS microsurgery than those from institutions with residency programs alone (92 vs. 83%, respectively). Overall, however, very few respondents ( n = 8; 14%) reported performing VS microsurgery without a colleague from the counterpart specialty (e.g., a skull base neurosurgeon operating without a neurotologist).
Discussion
In this cross-sectional survey study analyzing VS management in North America, 57 physician members of the NASBS (8% of the total membership) who reported regular involvement in VS treatment returned a completed survey. This report utilizes relevant VS case volume and respondent characteristics to evaluate the landscape of lateral skull base training in North America, with particular reference to VS management.
In multiple surgical specialties, great attention has been paid to the concept of the learning curve in surgical education. In contrast to some areas of neurologic surgery or otolaryngology, anecdotal experience with VS management would suggest that the learning curve is not only steep but likely never truly plateaus–the field continues to evolve and no two tumors are the same. In addition to mastering basic surgical maneuvers, the greater challenges of knowing when to alter one's technique or how to optimally tailor extent of resection remain difficult even for the seasoned VS microsurgeon. In 1996, Moffat and colleagues examined facial nerve outcomes in a series of 300 patients who underwent microsurgical VS resection via either translabyrinthine (240 patients; 80%) or retrosigmoid (60 patients; 20%) craniotomy. Patients were divided in six chronologic groups of 50 patients to examine the effect of experience on outcome. All six groups included patients with similar mean tumor size and demographics. Facial nerve outcome by House–Brackmann grading scale was I and II in only 44% of the first group but increased to 76% of the sixth group. 8 A similar trend was noted by Buchman et al over a series of 96 VS patients. 9
There is likely a specific range or number of cases after which supervision may not be as critical. Both Moffat et al and Buchman et al attempted to utilize outcome data to estimate the minimum number of cases required to achieve a level of surgical proficiency appropriate for independent practice. In 2014, Moffat et al updated their initial study of outcomes relative to case volume and found the surgical learning curve to be steepest for the first 50 patients, similar to the 60 patient learning curve described by Buchman et al in 1996. 9 10 In contrast, however, a report by Welling et al described a learning curve of only 20 patients to achieve facial nerve outcomes similar to more experienced groups. 11 What is perhaps just as important, but not yet studied to our knowledge, is the influence of case concentration. Anecdotal experience suggests that 10 cases performed over a 2-month period is substantially more beneficial to surgical growth than 10 cases spaced over a 2-year interval. Another feature even more difficult to quantify is the influence and benefit of overlapping cases. Certainly, the added experience from microsurgical dissection of other cerebellopontine angle (CPA) tumors, or even middle ear disease improves surgical dexterity, technique, and overall proficiency.
As of April 1, 2018, the ACGME has accredited 23 programs in neurotology. The ACGME released updated requirements for case volume in specific procedure categories in March 2016 to be effective for all programs starting July 1, 2016 ( Table 2 ). 12 As a relevant example, the minimum case volume for neurotologic tumors (e.g., VS, facial nerve tumor, paraganglioma, etc.) is 20. Volume by diagnosis is 20 for VS, two for paraganglioma, and two for facial nerve tumors. The first graduating class of fellows expected to meet these minimums was the class of 2014 to 2015.
Table 2. Neurotologic procedures with ACGME-established minimum case numbers.
| Procedure and category | Minimum case volume |
|---|---|
| Approaches for skull base surgery | 25 |
| Middle fossa approach for removal of tumor | |
| Posterior fossa approach for removal of tumor (e.g., translabyrinthine, retrosigmoid/suboccipital) | |
| Repair encephalocele (posterior or middle fossa) | |
| Resection of neurotologic tumors | 20 |
| Paraganglioma (e.g., jugular, tympanic) | |
| Other skull base lesions (e.g., petrous apex, internal auditory canal, cavernous sinus, Kawase's triangle, facial nerve tumor, including retrosigmoid and translabyrinthine) | |
| Temporal bone resection | 2 |
| Reconstruction after resection of neurotologic tumors | 15 |
| Tissue graft (e.g., fat, fascia) | |
| Local or regional vascularized flap | |
| Vestibular surgery (e.g., endolymphatic sac surgery, labyrinthectomy, middle ear perfusion, semicircular canal dehiscence repair, vestibular nerve section) | 10 |
| Rehabilitative surgery (e.g., cochlear implantation, osseointegrated implant, stapedectomy, etc.) | 20 |
| Diagnoses | |
| Paraganglioma | 2 |
| Vestibular schwannoma | 20 |
| Facial nerve tumor | 2 |
| Vestibular disease | 15 |
Abbreviation: ACGME, Accreditation Council for Graduate Medical Education.
At minimum, this survey would suggest that 88% of institutions with fellowship programs have sufficient operative VS volume to satisfy these basic minimums. Considering that this estimate represents institutional volume and not necessary trainee surgical experience, it is possible that the actual number of fellowship training institutions that would meet ACGME neurotology criteria for graduating fellows (which, of course, is not applied to neurosurgical skull base fellowship training programs) may be lower.
Data from this survey shed light on several potential challenges and opportunities in skull base surgery training. The surgical treatment paradigm for skull base tumors such as VS is increasingly conservative. 1 Over 90% of respondents to this survey reported an active observation strategy for most VS under 1.5 cm. While the number of operative cases is decreasing, there is likely greater complexity of the microsurgical cases due to larger tumor size. With this shift, perhaps trainees will require a higher case volume prior to graduation to be able to perform safe microsurgical resection of larger VS. The number of VS treated with radiation therapy or radiosurgery has remained stable (roughly 25%) over the past 10 years. 1 As of 2016, over 100,000 VS had been treated with Gamma Knife radiosurgery (Elekta Instruments AB, Stockholm, Sweden). 13 Long-term data suggest that roughly 3 to 10% exhibit radiographic growth after treatment. 14 15 As the overall number of patients treated with SRS climbs, an increasingly relevant proportion may require salvage microsurgery if nonsurgical therapies fail. These are particularly challenging cases that the next generation of skull base surgeons will have to face more commonly than their more senior counterparts. Only 50% of programs with relatively low case volume (institutions that evaluate 50 or fewer new diagnoses of VS annually) evaluate patients in a multidisciplinary fashion. We should encourage institutions to adopt a more collaborative approach, as it not only benefits patients but also affords less experienced surgeons with the opportunity to reach out across specialty lines for advice and intraoperative assistance when indicated. Intuitively, it would seem advantageous for a younger skull base surgeon to pair with a more experienced counterpart to provide an opportunity to overcome the learning curve while minimizing patient morbidity. In addition, advancements in technology cannot be overlooked as we add three-dimensional surgical simulation to the repertoire of skull base surgery trainees. 16
The development of new skull base surgery training programs is inspired by several factors. Most institutions encourage departments to foster fellowship programs to bolster academic reputation. Patients tend to be drawn to institutions with training programs as it suggests that the staff possess the experience required to teach residents or fellows. Currently, there are no formal regulatory guidelines governing the number of new neurotology or skull base neurosurgery graduates per year. Anecdotally, multiple new positions are offered annually and the rate of attrition of current programs is very low. In opposition to this, the community of skull base surgeons tends to agree that centralization of care to VS “centers of excellence” is beneficial for patients. As mentioned above, the overall number of surgical cases is decreasing in favor of observation or radiosurgery. Logically, this raises the question of whether governing bodies should pay additional attention to training standards and minimums to ensure that trainees obtain adequate experience in VS care. Overall, however, the interplay between increased diagnosis of incidental VS, the growing number of training programs and the controversies in management remains a complex issue that will certainly be a focus of our leaders going forward.
There are several strengths and limitations of the present study that warrant discussion. As described in parts (1) and (2) of this series, this cross-sectional survey study incorporated a large detailed question set that was completed by members of the NASBS who reported regular involvement in VS treatment. The overall demographic makeup of the respondent group was broad and included representatives of institutions with neurosurgical and neurotologic training programs. However, roughly two-thirds of respondents were skull base neurosurgeons which may skew the generalizability of results to the experience gained by neurosurgical rather than neurotologic trainees. In addition, neurotologists are represented by other societies including the American Neurotology Society and American Otological Society. Therefore, results from neurotologists involved in skull base surgery who are not members of the NASBS would not be captured by this survey. Of the 719 members of the NASBS surveyed, only 57 (8%) returned a completed survey. Certainly, one reason may be that not all members are actively involved in the care of VS patients. However, undoubtedly, given the number of emails and requests that probably inundate the average member's ‘inbox,’ the request to participate may have been overlooked or ignored and our results may be somewhat skewed by this. Nevertheless, it is important to recognize that the denominators for analyses presented herein represent respondents to the survey and not the entire field of skull base surgery as represented by the NASBS. Individual fellow experience was not analyzed in this survey as questions regarding case volume were intended to capture institutional volume, not individual trainee or surgeon volume. As there are somewhere between 30 and 40 current trainees in skull base surgery, based on available data in the NASBS Fellowship Registry, it is possible that individual trainee volume may be different from institutional volume.
Several future directions are being considered. It may be beneficial to assess case volume over time to better characterize the evolution of VS management and whether the fraction of cases undergoing surgery is constant or changing. In addition, administration of this survey or a similar questionnaire to members of neurotologic, otologic, and other skull base surgery societies would aid in incorporating results from a wider range of respondents. Finally, based on geographic variation in the treatment of VS established by Carlson et al, 17 coupling a larger respondent cohort with data regarding specific respondent location and the number of nearby centers treating VS would help to evaluate whether institutional volume is dependent on these factors.
Conclusion
Results of this survey demonstrate that institutions with fellowship training programs evaluate and treat a greater number of VS than those with residency programs alone, with over 50% of fellow-training institutions evaluating over 100 new diagnoses of VS annually. However, still there are several institutions with an operative case volume of 10 cases per year or fewer. As skull base training programs expand to meet increasing patient need, attention should be paid to ensure that minimum case volume standards are maintained over time as treatment paradigms evolve. Institutions with a lower overall case volume may benefit from multidisciplinary care models, formalized training requirements, and emerging surgical simulation technologies to maintain a high level of patient care.
Institutional Review Board Approval
IRB exempt study.
Conflict(s) of Interest None.
Financial Material and Support
Internal departmental funding was utilized without commercial sponsorship or support.
References
- 1.Carlson M L, Habermann E B, Wagie A E et al. The changing landscape of vestibular schwannoma management in the United States--a shift toward conservatism. Otolaryngol Head Neck Surg. 2015;153(03):440–446. doi: 10.1177/0194599815590105. [DOI] [PubMed] [Google Scholar]
- 2.NASBS Skull Base Fellowship Registry. Available from:https://www.nasbs.org/nasbs-skull-base-fellowship-registry/. Accessed April 1, 2018
- 3.Dedmon M M, Locketz G D, Chambers K J, Naunheim M R, Lin D T, Gray S T. Skull base surgery training and practice patterns among recent otolaryngology fellowship graduates. J Neurol Surg B Skull Base. 2016;77(04):297–303. doi: 10.1055/s-0035-1567892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee J T, Kingdom T T, Smith T L, Setzen M, Brown S, Batra P S. Practice patterns in endoscopic skull base surgery: survey of the American Rhinologic Society. Int Forum Allergy Rhinol. 2014;4(02):124–131. doi: 10.1002/alr.21248. [DOI] [PubMed] [Google Scholar]
- 5.Batra P S, Lee J, Barnett S L, Senior B A, Setzen M, Kraus D H. Endoscopic skull base surgery practice patterns: survey of the North American Skull Base Society. Int Forum Allergy Rhinol. 2013;3(08):659–663. doi: 10.1002/alr.21151. [DOI] [PubMed] [Google Scholar]
- 6.Carlson M L, Van Gompel J J, Wiet R M et al. A cross-sectional survey of the North American Skull Base Society: current practice patterns of vestibular schwannoma evaluation and management in North America. J Neurol Surg B Skull Base. 2018;79(03):289–296. doi: 10.1055/s-0037-1607319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Gompel J J, Carlson M L, Wiet R M et al. A cross-sectional survey of the North American Skull Base Society on vestibular schwannoma, part 2: perioperative practice patterns of vestibular schwannoma in North America. J Neurol Surg B Skull Base. 2018;79(03):297–301. doi: 10.1055/s-0037-1607976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moffat D A, Hardy D G, Grey P L, Baguley D M. The operative learning curve and its effect on facial nerve outcome in vestibular schwannoma surgery. Am J Otol. 1996;17(04):643–647. [PubMed] [Google Scholar]
- 9.Buchman C A, Chen D A, Flannagan P, Wilberger J E, Maroon J C. The learning curve for acoustic tumor surgery. Laryngoscope. 1996;106(11):1406–1411. doi: 10.1097/00005537-199611000-00019. [DOI] [PubMed] [Google Scholar]
- 10.Moffat D A, Parker R A, Hardy D G, Macfarlane R. Factors affecting final facial nerve outcome following vestibular schwannoma surgery. J Laryngol Otol. 2014;128(05):406–415. doi: 10.1017/S0022215114000541. [DOI] [PubMed] [Google Scholar]
- 11.Welling D B, Slater P W, Thomas R D, McGregor J M, Goodman J E. The learning curve in vestibular schwannoma surgery. Am J Otol. 1999;20(05):644–648. [PubMed] [Google Scholar]
- 12.Neurotology minimum number requirements: review committee for otolaryngology.2016; available from:https://www.acgme.org/Portals/0/PFAssets/ProgramResources/Neurotology%20Case%20Log%20Minimums.pdf?ver=2016-03-29-094159-393. Accessed April 1, 2018
- 13.Leksell Gamma Knife Society: annual treatment statistics.2016; available from:https://www.lgksociety.com/library/annual-treatment-statistics/. Accessed May 23, 2018
- 14.Flickinger J C, Kondziolka D, Niranjan A, Lunsford L D. Results of acoustic neuroma radiosurgery: an analysis of 5 years' experience using current methods. J Neurosurg. 2001;94(01):1–6. doi: 10.3171/jns.2001.94.1.0001. [DOI] [PubMed] [Google Scholar]
- 15.Jacob J T, Pollock B E, Carlson M L, Driscoll C L, Link M J. Stereotactic radiosurgery in the management of vestibular schwannoma and glomus jugulare: indications, techniques, and results. Otolaryngol Clin North Am. 2015;48(03):515–526. doi: 10.1016/j.otc.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 16.Wiet G J, Stredney D, Kerwin Tet al. Virtual temporal bone dissection system: OSU virtual temporal bone system: development and testing Laryngoscope 2012122(Suppl 1)S1–S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carlson M L, Glasgow A E, Grossardt B R, Habermann E B, Link M J. Does where you live influence how your vestibular schwannoma is managed? Examining geographical differences in vestibular schwannoma treatment across the United States. J Neurooncol. 2016;129(02):269–279. doi: 10.1007/s11060-016-2170-5. [DOI] [PubMed] [Google Scholar]


