Abstract
Objective
The aim of this study was to investigate the effects of physiologic ischemia training (PIT) on the proliferation of endothelial progenitor cells (EPCs) and the corresponding changes in the influencing factors in atherosclerotic rabbits, including vascular endothelial growth factor (VEGF) and nitric oxide (NO).
Materials and methods
Eighteen rabbits were assigned randomly to three groups: a high-fat diet (HD) group, an HD-with-training (HT) group, and a control group. Rabbits in the HD and HT groups were fed high-fat food and those in the HT group were administered PIT from the seventh week onward. Atherosclerotic plaques in the thoracic aorta were stained with Oil Red O and measured by Image-Pro Plus 6.0; VEGF expression was measured using an enzyme-linked immunosorbent assay and real-time PCR to determine both protein and mRNA levels. EPCs were counted using a fluorescence-activated cell sorter; NO in plasma was measured by the Griess reaction; and the levels of blood lipids were measured using a biochemical analyzer.
Results
More lipid-containing lesions were found in the HD group than in the HT group (P<0.01), whereas atherosclerotic plaques were not observed in the control group. In addition, the expression of VEGF, production of NO, and levels of blood lipids were consistent with the proportion of plaques. It is noteworthy that the proliferation of EPCs increased in the HT group throughout the 10 weeks, whereas those in the control and HD groups increased in the first 6 weeks and declined during the 10th week (P<0.01).
Conclusion
PIT may prevent the development of aortic atherosclerosis by promoting the proliferation of EPCs in atherosclerotic rabbits.
Keywords: atherosclerosis, endothelial progenitor cells, physiologic ischemia training, vascular endothelial growth factor
Introduction
Atherosclerosis is a chronic inflammatory disease that results in the development of plaques and progressive stenosis of the coronary arteries 1, leading to cardiovascular pathologies, such as myocardial infarction. It is also a leading cause of morbidity and mortality in developed countries and remains the most significant cause of death worldwide.
Physiologic ischemic training (PIT) is a form of isometric exercise training prompting normal skeletal muscles to trigger a process that can improve angiogenesis in remote areas of ischemia 2,3. Previous studies showed that PIT in normal limbs can facilitate collateral angiogenesis in corresponding ischemic limbs 4 and the remote myocardia of rabbits 3. A study carried out in 74 human patients with coronary artery disease found that myocardial perfusion and left ventricular ejection fraction were significantly improved in the PIT group that received isometric handgrip-induced training; these improvements appeared to result from an increase in the collateral circulation 5. Further study of myocardial ischemia in a rabbit model found that PIT might be a new treatment strategy for patients with coronary heart disease. That is, this study showed that stimulation by PIT led to the vascular endothelial growth factor (VEGF)-mediated mobilization of endothelial progenitor cells (EPCs) 6. Thus, the repair 7 or replacement 8 of endothelial cells and subsequent angiogenesis appear to have occurred 2.
It is known that myocardial ischemia is frequently caused by the formation of atherosclerotic plaques in the blood vessels. Before the occurrence of myocardial ischemia, atherosclerotic plaques have already formed. Although PIT might protect the myocardium when ischemia is developing, no research has reported that PIT can slow this process. There are, however, limited data on the manner in which PIT affects the development of ischemia. We propose that PIT slows the formation of atherosclerotic plaques by promoting the proliferation of EPCs, influencing the expression of VEGF, and increasing the production of nitric oxide (NO). In this study, atherosclerosis was established in rabbits to illustrate the effect and the fundamental mechanism of PIT in myocardial ischemia or coronary heart disease.
Materials and methods
Animal care and use
A total of 18 male adult New Zealand white rabbits (2.2–2.5 kg, 3 months of age) were purchased from the Laboratory Animal Center of the Agricultural Science Institute of Jiangsu Province. The animals were housed under conventional conditions with a 12-h light/dark cycle with food and water ad libitum. All animal experimental protocols were in accordance with the Guide for the Care and Use of Laboratory Animals (US National Institutes of Health) and approved by the ethics committee of Nanjing First Hospital of Nanjing Medical University.
Animal model of physiologic ischemia training
All rabbits were anesthetized with 3% (vol/vol) sodium pentobarbital (1 ml/kg body weight) into a marginal ear vein; supplemental anesthesia was provided as needed. Under sterile surgical conditions, a platinum filament electrode was implanted into the sciatic nerve of each rabbit to implement isometric contraction and PIT. The steps were as follows: the sciatic nerve of the left hind limb was exposed under anesthesia. A wire electrode was then inserted longitudinally into the epineurium of the nerve and sutured to the epineurium. The reference electrode was then implanted into the ipsilateral gluteus maximus. Wires from these electrodes were run under the skin to the nuchal region and connected to an external stimulator (SY-708A; Suyun Co. Ltd, Lianyungang, Jiangsu, China). Postoperatively, all rabbits were monitored closely and administered 4×105 units of penicillin intramuscularly daily for 3 days.
Experimental groups and feeding
Seven days after the operation, the rabbits were assigned randomly to three groups: a control group, a high-fat diet (HD) group, and a high-fat diet group with training (HT). Animals in the control group were given normal food, whereas those in HD and HT groups were given high-fat food comprising 6% lard, 1% cholesterol, and 2% egg yolk powder.
Training protocol
In the first 6 weeks, all rabbits remained inactive. In the HT group from week 7 onward, PIT training was provided by stimulating the left sciatic nerve to induce isometric contraction of muscles. PIT of the hind limb in rabbits was effected using cuff inflation or by electrical stimulation of the sciatic nerve 4. Cuff inflation was used to block the blood flow by pressure. An electrical stimulation (2.5 mA, 40 Hz for 1 ms) for the sciatic nerve can lead to an isometric contraction of hind limb muscles. As blood flow was blocked by isometric contraction of muscles, venous lactate levels became significantly (0.85±0.17 to 4.87±0.77 mmol/l, P<0.001) higher than the normal muscle contraction levels (0.81±0.17 to 1.20±0.21 mmol/l), which suggested that electrical stimulation can lead to ischemia and venous lactates cannot be carried away quickly enough 9. Before the rabbits in the HT group were trained by electrical stimulation of the sciatic nerve, a strain gauge was fixed to the distal hind limbs of the conscious animals to measure the force of muscle contraction during electrical stimulation. This was done by using a 2.5 mA, 1 ms square-wave pulse applied at a frequency of 40 Hz (i.e. 1 ms applied voltage, alternating with 24 ms at 0 voltage). This protocol was sufficient to cause the hind limb to produce 40% of its maximal force without pain 3,4. The stimulation protocol was 2 min of contraction and 5 min of relaxation, which was repeated three times in each session twice a day and 5 days a week for 4 weeks.
Measurement of atherosclerotic plaques
Aortas were collected and stored in 10% Faure Marin medium at the conclusion of the experiment. They were bathed in Oil Red O working solution (0.5%) for 30 min and then washed in distilled water to stain the aortic atherosclerotic plaques. The plaque area was finally measured using Image-Pro Plus 6.0.
Detection of cholesterol, triglyceride, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol
Blood samples from the marginal ear vein were taken at the first, sixth, and 10th weeks to monitor changes in the total cholesterol, triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C). Serum was separated by centrifugation (at 2000 g for 20 min at 4°C) and used for biochemical analysis. Serum cholesterol, TG levels, HDL-C, and LDL-C were measured using a biochemical analyzer (AU5800; Beckman Coulter, Brea, California, USA).
Flow cytometric analysis for endothelial progenitor cells
The number of EPCs was measured at the beginning, at the sixth week, and at the end of the experiment. A total of 65 μl peripheral blood from each rabbits was pretreated with lysate. The number of EPCs was analyzed by fluorescence-activated cell sorting (BD, Canton, Ohio, USA) with the following mouse antibodies (as rabbit antibodies were not available at the time of the study): allophycocyanin-conjugated mouse anti-CD3 (eBioscience, San Diego, California, USA) antibody, fluorescein-conjugated mouse anti-CD309 (eBioscience,) antibody, and phycoerythrin-conjugated mouse anti-CD34 (eBioscience) antibody.
Vascular endothelial growth factor protein expression by enzyme-linked immunosorbent assay
Plasma VEGF levels were detected by enzyme-linked immunosorbent assay using a VEGF-A rabbit enzyme-linked immunosorbent assay kit (KeyGEN BioTECH, Nanjing, China) by drawing 2 ml of arterial blood from each rabbit’s ear artery. This was placed into a heparin anticoagulation tube. This was done at the beginning, at the sixth week, and the end of the experiment. The results were determined as follows: (a) the absorbance value at 450 nm was calculated by correcting for the blank value; (b) using the absorbance value of the standard product, a standard curve was drawn on semilogarithmic paper; (c) the VEGF levels were then determined according to the absorbance value of the sample using the standard curve.
Vascular endothelial growth factor-mRNA expression by real-time PCR
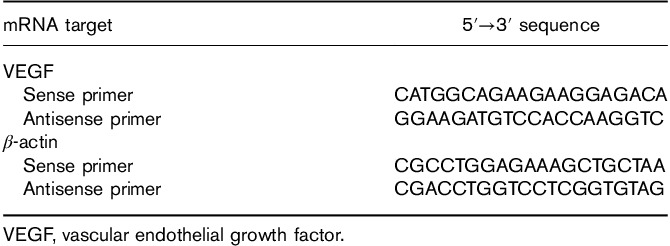
Total RNA was isolated from the plasma. The expression of plasma VEGF-mRNA was analyzed by real-time reverse-transcription PCR (KeyGEN BioTECH) with the specific primers listed in Table 1.
Table 1.
Primers for real-time reverse-transcription PCR assays
Nitric oxide metabolites determination
The concentration of total NO metabolites (nitrite and nitrate) in plasma was measured using NO assay kits (Beyotime, Shanghai, China) based on the Griess reaction and absorbance values at 540 nm. Nitrite content, indirectly reflecting NO content, was determined by optical density.
Statistical analysis
Data were expressed as means±SD. Data analysis and concentration–response curves were obtained with Prism (GraphPad Software). The comparison between groups was performed by one-way analysis of variance (SPSS Version 13.0, SPSS Inc., Chicago, Illinois, USA). Values of P less than 0.05 were considered significant. When statistical significance was observed, post-hoc analysis was carried out to identify pairwise differences. Pearson product–moment correlation analysis was used to determine the significance of the relationship between variables.
Results
Atherosclerotic plaques
As shown in Fig. 1, multiple lipid-containing lesions (43.3±13.3%) covering the vessel wall of the ascending aorta were observed on the rabbits in the HD group, whereas no atherosclerotic plaques were found in the control group. There were fewer lipid-containing lesions in the HT group (8.1±1.6%) than in the HD group (P<0.01).
Fig. 1.
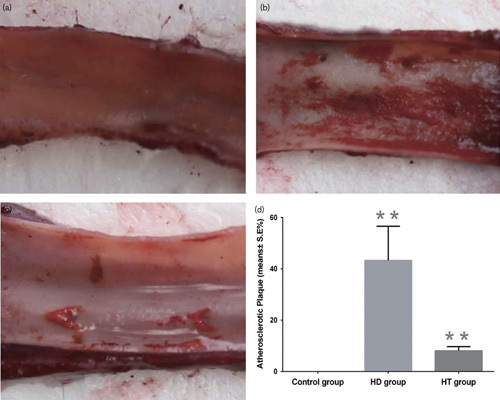
Oil Red O staining of the thoracic aorta in the three groups. (a) After Oil Red O staining, the thoracic aortas of the control group showed no atherosclerotic plaque. (b) Many atherosclerotic plaque were found in the thoracic aorta of the HD group. (c) Several atherosclerotic plaques were found in the thoracic aorta of the HT group. (d) The thoracic aortas of the HD group showed more lipid-containing lesions than those of the HT and control groups (P<0.01). The atherosclerotic plaque in the HT group was greater than that of the control group (P<0.01). **P<0.01. HD, high-fat diet group; HT, high-fat diet with training group.
Cholesterol, triglyceride, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol results
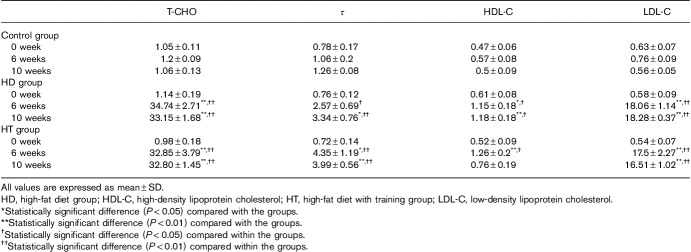
The levels of cholesterol, TG, HDL-C, and LDL-C in the control group did not change significantly between the first week and 10th weeks (P<0.05). In the HD group, these four indices increased significantly in the sixth and 10th weeks compared with the baseline at the first week (Pall<0.05 and Pall<0.05). Meanwhile, the level of cholesterol, TG, and LDL-C in the HT group increased significantly at the sixth and 10th weeks compared with the baseline at the first week (P<0.05 and P<0.05) and the level of HDL-C increased significantly at the sixth week (P<0.05).
On comparing the three groups, the levels of cholesterol and LDL-C in the HD and HT groups were significantly increased at the sixth and 10th weeks, respectively, compared with the control group (P<0.01). Similarly, compared with the control group, at the sixth week, the level of TG in the HT group had increased significantly (P<0.05); by the 10th week, the level of TG in HD (P<0.05) and HT groups (P<0.01) had increased significantly; and at the sixth week, the level of HDL-C in the HD (P<0.05) and HT groups (P<0.01) had increased significantly. However, the level of HDL-C in the HT group decreased at the 10th week; the level of HDL-C increased significantly (P<0.01) only in the HD group (Table 2).
Table 2.
Levels [mean±SE (mmol/l)] of cholesterol, triglycerides, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol in blood
Number of endothelial progenitor cells
The number of EPCs in the control and HD groups showed both an increase and a decline; there was a significant increase in the sixth week (P<0.01) and then a marked decline by the 10th week. The number of EPCs in the HT group increased over time, and the maximum number was observed in the 10th week, which was significantly higher than that in the first week (P<0.01) and sixth week (P<0.05) (Fig. 2a).
Fig. 2.
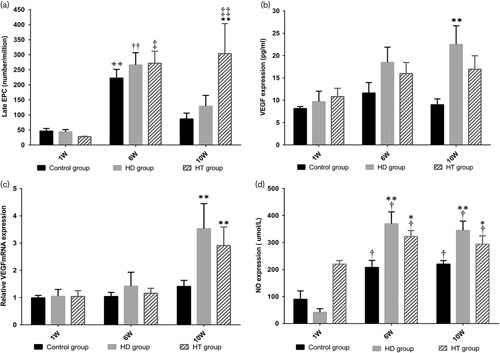
EPC number, VEGF expression, VEGF-mRNA expression, and NO content of the three groups in different weeks. (a) Initially there was no significant difference in the number of EPCs between groups. After 6 weeks of feeding, the number of EPCs in all three groups increased significantly (control group: ++P<0.01 vs. first week, HD group: ††P<0.01 vs. first week, HT group: ‡P<0.05 vs. first week). At the end of the experiment, the number of EPCs in the control and HD groups had decreased (control group: P<0.05 vs. first week, HD group: P<0.05 vs. first week), but they had increased in the HT group (‡P<0.01 vs. first week). By the 10th week, the EPCs in the HT group had increased significantly (**P<0.01). (b) Initially, there was no significant difference in the expression of VEGF among the groups, first week (P<0.05) and sixth week (P<0.05); however, a significant difference was observed among groups by the 10th week (P<0.05) and VEGF expression in the HD group was higher than that in the control group (P<0.01). (c) For VEGF-mRNA expression, there was initially no significant difference among the groups, first week (P<0.05) and sixth week (P<0.05), whereas a significant difference was found among the groups by the 10th week (P<0.01). Moreover, VEGF-mRNA expression in the HD (P<0.01) and HT groups (P<0.01) was significantly higher than that in the control group (**P<0.01), respectively. (d) Initially, there was no significant difference in the NO contents between the three groups. By the sixth week, the NO content in the three groups had increased significantly (control group: †P<0.05 vs. first week, HD group: †P<0.05 vs. first week, HT group: †P<0.05 vs. first week). A significant difference was found among the groups (HD group vs. control group: **P<0.01, HT group vs. control group: *P<0.05). By the 10th week, the NO content in all three groups had increased significantly (control group: †P<0.05 vs. first week, HD group: †P<0.05 vs. first week, HT group: †P<0.05 vs. first week). A significant difference was found among groups (HD group vs. control group: **P<0.01, HT group vs. control group: *P<0.05). EPC, endothelial progenitor cell; HD, high-fat diet group; HT, high-fat diet with training group; NO, nitric oxide; VEGF, vascular endothelial growth factor.
Expression of vascular endothelial growth factor and vascular endothelial growth factor mRNA
There was no significant difference in VEGF and VEGF-mRNA expression among the three groups from the first week to the sixth week. At the end point of the experiment, the difference in VEGF expression among groups was significant (P<0.05); there was also a significant difference between the control and HD groups (P<0.01) (Fig. 2b). There was a significant difference in VEGF-mRNA expression among the groups, showing that it had changed after PIT training (P<0.01) (Fig. 2c) between the control and HD groups (P<0.01), the control group, and the HT group (P<0.01), respectively.
Nitric oxide level
There was no statistically significant difference in the NO level among groups in the first week, whereas in the sixth and 10th weeks the plasma NO level in the HD (P<0.01) and HT groups (P<0.05) increased significantly compared with that of the control group. In each group, compared with the first week, the NO level increased significantly (Pall<0.01) in the sixth and 10th weeks (Fig. 2d).
Discussion
Angiogenesis and collateral circulation development associated with upregulation of VEGF expression were the result of 4 to 5 week’s PIT 4,9. The effect of PIT on myocardial ischemia has been researched previously. These animal studies have shown that PIT may offer a new treatment strategy for patients with coronary heart disease, and the mechanism might be VEGF 3 or VEGF-mediated mobilization of EPCs 6. Lin’s clinical study proved that isometric handgrip exercises, a kind of PIT, may promote remote collateral recruitment in patients with coronary artery disease; this was deduced to be beneficial because of the resultant increase in VEGF 5. However, whether this observation is related to the status of EPCs was not studied. In addition, it is well known that the process of atherosclerosis is preceded by vascular endothelial injury. Although PIT can increase the expression of VEGF and EPCs in animal models with myocardial ischemia 10, it remains unknown whether PIT can help to protect the vascular endothelium by increasing VEGF and EPCs during the process of atherosclerosis. Moreover, this pathologic process itself is not fully understood. Therefore, the present study, designed to mimic human atherosclerosis in an animal model, has served to explore the effect of PIT on atherosclerotic endothelium and to clarify the mechanism involved.
In the present study, the result of Oil Red O staining showed that PIT can effectively slow the development of atherosclerosis. Such a result has not been reported previously. In addition, the levels of cholesterol, TG, HDL-C, and LDL-C correlated with the formation of atherosclerotic plaques. This also suggested that PIT exercise can slow the pace of atherosclerotic plaque development.
EPCs have been reported to be recruited into blood circulation by stimuli such as drugs, ischemia, or exercise. Once circulating, EPCs home in on target organs and participate in the maintenance of the endothelial cell layer 11 by repairing 7 or replacing endothelial cells 8 and promoting myocardial neovascularization 6. Some research has suggested that the levels of EPCs may be surrogate biologic markers for vascular function and cumulative cardiovascular risk 12–14. In other words, increased circulating levels of EPCs may protect blood vessels 15 and reduce levels of circulating EPC, which may independently predict the progression of atherosclerotic disease 16–18. In this respect, this study observed that the number of EPCs in the HT group was higher than that in the HD group, and that there were fewer atherosclerotic plaques in the HT group than that in the HD group, which was consistent with the reported results. The mechanism of the phenomenon in the HT group may stem from the fact that PIT increased the proliferation and mobilization of EPCs as studies have shown that tissue ischemia is a necessary trigger for the release of circulating EPCs and their mobilization into the peripheral circulation 7,15,19–21. We concluded that the decrease in the number of EPCs in the control and HD groups may be related to their negative association with aging 18.
In previous studies, VEGF was proven to mobilize EPCs, thus establishing an angiogenic environment 22; moreover, NO has been reported to be related closely to endothelial dysfunction and atherosclerosis 23,24 and is also an important mediator of the mobilization of EPCs 25,26. Some evidence has shown that although the mobilization and reproduction of EPCs relies on an increase in VEGF 7 and NO 25, the number of EPCs has been shown to decrease with increasing atherosclerosis 16,18,27. Therefore, our results confirm other studies on VEGF, NO, EPCs, and the vascular endothelium. Our results also suggest that PIT can protect the vascular endothelium and slow the pace of atherosclerosis, which is consistent with the results of Yu Zheng’s research on PIT and myocardial ischemia 6,10.
This study may have several limitations. VEGF and NO are considered the two key molecules stimulating the proliferation of EPCs 25,28–31; however, the VEGF, VEGF-mRNA, and NO in the HT group in the 10th week were not in accord with the number of EPCs. Although studies have reported weak linear relations of VEGF to EPC numbers 10, a deeper mechanism should be sought. Actually, because it functions as a key regulator of EPCs 23,25, NO bioavailability affects endothelial function 24. Thus, in this study, the plasma NO level in the HD group was higher than that in the HT group, but the bioavailability of NO was largely unknown, which should be explored in a future study. Another limitation of this study is the use of mouse antibodies to measure the number of EPCs as rabbit antibodies are not currently available.
Conclusion
PIT can protect the vascular endothelium and slow the pace of atherosclerotic plaque development during the process of atherosclerosis. The proliferation of EPCs may be the fundamental mechanism underlying these protective effects, but it is not clearly correlated with the increased expression of VEGF and NO.
Acknowledgements
The authors would also like to express their heartfelt thanks to Bang-Shun He from the general clinical research center for the assistance during the experiment.
Aicui Lin takes responsibility for all aspects of the reliability and freedom from bias of the data presented and their interpretation.
A.C.L. designed the study; A.C.L. and M.Y.K. wrote the manuscript; and Y.Z. and A.D.C. conducted the experiment.
National Natural Science Foundation of China (Grant number: 81101456); Nanjing Medical Science and Technique Development Foundation (Grant number: QRX17065); The Natural Science Foundation of the Jiangsu Higher Education Institutions of China (Grant number:18KJA320002).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Mingya Kong and Yan Zhao contributed equally to the writing of this article.
References
- 1.Khan R, Spagnoli V, Tardif JC, L’Allier PL. Novel anti-inflammatory therapies for the treatment of atherosclerosis. Atherosclerosis 2015; 240:497–509. [DOI] [PubMed] [Google Scholar]
- 2.Shen M, Gao J, Li J, Su J. Effect of stimulation frequency on angiogenesis and gene expression in ischemic skeletal muscle of rabbit. Can J Physiol Pharmacol 2009; 87:396–401. [DOI] [PubMed] [Google Scholar]
- 3.Lin A, Li J, Zhao Y, Xiao M, Xiao B, Lu X, et al. Effect of physiologic ischemic training on protection of myocardial infarction in rabbits. Am J Phys Med Rehabil 2011; 90:97–105. [DOI] [PubMed] [Google Scholar]
- 4.Zhao Y, Li J, Lin A, Xiao M, Xiao B, Wan C. Improving angiogenesis and muscle performance in the ischemic limb model by physiological ischemic training in rabbits. Am J Phys Med Rehabil 2011; 90:1020–1029. [DOI] [PubMed] [Google Scholar]
- 5.Lin S, Chen Y, Li Y, Li J, Lu X. Physical ischaemia induced by isometric exercise facilitated collateral development in the remote ischaemic myocardium of humans. Clin Sci 2014; 127:581–588. [DOI] [PubMed] [Google Scholar]
- 6.Zheng Y, Lu X, Li J, Zhang Q, Reinhardt JD. Impact of remote physiological ischemic training on vascular endothelial growth factor, endothelial progenitor cells and coronary angiogenesis after myocardial ischemia. Int J Cardiol 2014; 177:894–901. [DOI] [PubMed] [Google Scholar]
- 7.Sandri M, Adams V, Gielen S, Linke A, Lenk K, Kränkel N, et al. Effects of exercise and ischemia on mobilization and functional activation of blood-derived progenitor cells in patients with ischemic syndromes: results of 3 randomized studies. Circulation 2005; 111:3391–3399. [DOI] [PubMed] [Google Scholar]
- 8.Kaushal S, Amiel GE, Guleserian KJ, Shapira OM, Perry T, Sutherland FW, et al. Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nat Med 2001; 7:1035–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen M, Gao J, Li J, Su J. Effect of ischaemic exercise straining of a normal limb on angiogenesis of a pathological ischaemic limb in rabbits. Clin Sci 2009; 117:201–208. [DOI] [PubMed] [Google Scholar]
- 10.Zheng Y, Xiao M, Li L, Li J, Reinhardt JD, Lu X. Remote physiological ischemic training promotes coronary angiogenesis via molecular and cellular mobilization after myocardial ischemia. Cardiovasc Ther 2017; 35:12257. [DOI] [PubMed] [Google Scholar]
- 11.Mobius-Winkler S, Hollriegel R, Schuler G, Adams V. Endothelial progenitor cells: Implications for cardiovascular disease. Cytometry A 2009; 75:13. [DOI] [PubMed] [Google Scholar]
- 12.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med 2003; 348:593–600. [DOI] [PubMed] [Google Scholar]
- 13.Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation 2005; 111:363–368. [DOI] [PubMed] [Google Scholar]
- 14.Werner N, Wassmann S, Ahlers P, Schiegl T, Kosiol S, Link A, et al. Endothelial progenitor cells correlate with endothelial function in patients with coronary artery disease. Basic Res Cardiol 2007; 102:565–571. [DOI] [PubMed] [Google Scholar]
- 15.Kimura M, Ueda K, Goto C, Jitsuiki D, Nishioka K, Umemura T, et al. Repetition of ischemic preconditioning augments endothelium-dependent vasodilation in humans: role of endothelium-derived nitric oxide and endothelial progenitor cells. Arterioscler Thromb Vasc Biol 2007; 27:1403–1410. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt-Lucke C, Rossig L, Fichtlscherer S, Vasa M, Britten M, Kämper U, et al. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation 2005; 111:2981–2987. [DOI] [PubMed] [Google Scholar]
- 17.Briguori C, Testa U, Riccioni R, Colombo A, Petrucci E, Condorelli G, et al. Correlations between progression of coronary artery disease and circulating endothelial progenitor cells. FASEB J 2010; 24:1981–1988. [DOI] [PubMed] [Google Scholar]
- 18.Chironi G, Walch L, Pernollet MG, Gariepy J, Levenson J, Rendu F, et al. Decreased number of circulating cd34+kdr+ cells in asymptomatic subjects with preclinical atherosclerosis. Atherosclerosis 2007; 191:115–120. [DOI] [PubMed] [Google Scholar]
- 19.Zhen X, Zheng Y, Hong X, Chen Y, Gu P, Tang J, et al. Physiological ischemic training promotes brain collateral formation and improves functions in patients with acute cerebral infarction. Front Neurol 2016; 7:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med 2003; 9:702–712. [DOI] [PubMed] [Google Scholar]
- 21.Adams V, Lenk K, Linke A, Lenz D, Erbs S, Sandri M, et al. Increase of circulating endothelial progenitor cells in patients with coronary artery disease after exercise-induced ischemia. Arterioscler Thromb Vasc Biol 2004; 24:684–690. [DOI] [PubMed] [Google Scholar]
- 22.Carmeliet P, Ferreira V, Breier G, Pollegeyt S, Kieckens L, Gertsenstein M, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 1996; 380:435–439. [DOI] [PubMed] [Google Scholar]
- 23.Ozuyaman B, Ebner P, Niesler U, Ziemann J, Kleinbongard P, Jax T, et al. Nitric oxide differentially regulates proliferation and mobilization of endothelial progenitor cells but not of hematopoietic stem cells. Thromb Haemost 2005; 94:770–772. [DOI] [PubMed] [Google Scholar]
- 24.Cavieres V, Valdes K, Moreno B, Moore-Carrasco R, Gonzalez DR. Vascular hypercontractility and endothelial dysfunction before development of atherosclerosis in moderate dyslipidemia: Role for nitric oxide and interleukin-6. Am J Cardiovasc Dis 2014; 4:114–122. [PMC free article] [PubMed] [Google Scholar]
- 25.Laufs U, Werner N, Link A, Endres M, Wassmann S, Jürgens K, et al. Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation 2004; 109:220–226. [DOI] [PubMed] [Google Scholar]
- 26.Xiao M, Lu X, Li J, Li L, Li Y. Physiologic ischaemic training induces endothelial progenitor cell mobilization and myocardial angiogenesis via endothelial nitric oxide synthase related pathway in rabbits. J Cardiovasc Med 2014; 15:280–287. [DOI] [PubMed] [Google Scholar]
- 27.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med 2005; 353:999–1007. [DOI] [PubMed] [Google Scholar]
- 28.Khakoo AY, Finkel T. Endothelial progenitor cells. Annu Rev Med 2005; 56:79–101. [DOI] [PubMed] [Google Scholar]
- 29.Namba T, Koike H, Murakami K, Aoki M, Makino H, Hashiya N, et al. Angiogenesis induced by endothelial nitric oxide synthase gene through vascular endothelial growth factor expression in a rat hindlimb ischemia model. Circulation 2003; 108:2250–2257. [DOI] [PubMed] [Google Scholar]
- 30.Inoue M, Itoh H, Ueda M, Naruko T, Kojima A, Komatsu R, et al. Vascular endothelial growth factor (VEGF) expression in human coronary atherosclerotic lesions: Possible pathophysiological significance of vegf in progression of atherosclerosis. Circulation 1998; 98:2108–2116. [DOI] [PubMed] [Google Scholar]
- 31.Zemani F, Silvestre JS, Fauvel-Lafeve F, Bruel A, Vilar J, Bieche I, et al. Ex vivo priming of endothelial progenitor cells with sdf-1 before transplantation could increase their proangiogenic potential. Arterioscler Thromb Vasc Biol 2008; 28:644–650. [DOI] [PubMed] [Google Scholar]