Abstract
Introduction Although numerous anatomical and operative atlases have been published, those that have focused on the skull base either have provided views that are quite difficult to achieve in the operating room to better depict surgical anatomy or are written at the level of an audience with considerable knowledge and experience.
Methods Five sides of three formalin-fixed latex-injected specimens were dissected under microscopic magnification. A posterior petrosectomy approach was performed by three neurosurgical residents at different training levels with limited previous experience in anatomical dissection mentored by the senior authors (C. L. W. D. and M. J. L.) and a clinical skull base fellow with additional anatomical dissection experience (M. P. C.). Anatomical dissections were performed until the expected level of dissection quality was achieved to demonstrate each important step of the surgical approach that would be understandable to all trainees of all levels. Following dissection education, representative case applications were reviewed.
Results The posterior petrosectomy (also known as presigmoid retrolabyrinthine approach) affords excellent access to cranial nerves III to XI and a diverse array of pathologies. Key steps include positioning and skin incision, scalp and muscle flaps, burr holes, craniotomy flap elevation, superficial mastoidectomy, otic capsule exposure and presigmoid dura decompression, primary presigmoid durotomy, inferior temporal durotomy, superior petrosal sinus ligation, tentorium sectioning, and final exposure.
Conclusion The posterior petrosectomy is a challenging approach; thorough operative-style laboratory dissection is essential to provide trainees with a suitable guide. We describe a comprehensive approach to learning this technique, intended to be understandable and usable by a resident audience.
Keywords: posterior petrosectomy, meningioma, brainstem, skull base, education
Introduction
The posterior petrosal approaches (also known as transmastoid or presigmoid approaches) include mastoid drilling and exposure of the presigmoid dura mater (Trautmann's triangle). These presigmoid approaches can be further classified into retrolabyrinthine, transcrural, translabyrinthine, and transcochlear, depending on the degree of inner ear drilling. This study focuses on the retrolabyrinthine posterior petrosal approach, with exposure of the presigmoid and temporal dura mater. This approach leaves the labyrinth fully intact and is thus intended to preserve cochlear and vestibular function. The superior petrosal sinus and tentorium are divided for wide exposure of the supra- and infratentorial spaces.
Several prominent atlases have described this family of approaches; however, they typically employ radical dissection techniques and nonoperative exposures and perspectives to demonstrate three-dimensional (3D) relationships (e.g., Rhoton et al) or are written for an experienced audience that is more familiar with skull base anatomy (e.g., Fukushima et al, Tew et al). 1 2 Correspondingly, the chief goal of our study was to develop an educational resource for junior residents to learn the posterior petrosectomy by step-by-step, easily understood, and operatively oriented dissection.
Methods
All aspects of this study were approved by our institutional review board and biospecimens committee, as required by standard protocols. Three specimens were formalin-fixed and latex-injected using a six-vessel technique by the study staff. Five sides were dissected out under microscopic magnification by three neurosurgery residents who, at the time of this study, had limited neuroanatomical dissection experience and were at training levels of PGY-2 (L. P. C.), PGY-4 (C. S. G.), and PGY-5 (A. P.). Dissections were supervised by the senior authors (C. L. W. D. and M. J. L.) and a clinical skull base fellow with advanced neuroanatomy experience (M. P. C.). Each dissection was carried forward or repeated until the expected quality level was achieved so that each critical approach step was clearly understandable to all three trainees, who would then document the exposure in stepwise 3D photographic images. Following dissection education and reproduction, representative case applications were reviewed.
Results: Step-by-Step Surgical Approach
Positioning and Skin Incision in the Operating Room
After the induction of general endotracheal anesthesia and placement of appropriate cranial nerve (CN) monitoring, the patient is placed in the lateral decubitus position with an axillary role to protect the contralateral brachial plexus. The bed is brought into approximately 30% of reverse Trendelenburg. It is very important to pad the down hip very well during a long anesthetic as well as to use a soft axillary roll to avoid any pressure palsies. Additionally, we use a well-padded footboard to ensure that the patient would not “slide” down the table while the head is fixed in pinions. The head is placed in three-point pinion fixation, in slight flexion, with the vertex tilted slightly toward the floor. The net effect is to bring the superior sagittal sinus roughly parallel to the floor while minimizing compression of the contralateral internal jugular vein (IJV) and opening up the angle between the ipsilateral shoulder and neck. If the head is too flexed, it is possible to compress both IJVs with the mandible and to artificially raise intracranial pressure, which may result in the need for excessive brain retraction later in the operation. We do not employ a lumbar drain for this operation, as it is relatively straightforward to release cerebrospinal fluid (CSF) from the cerebellopontine angle (CPA) upon opening the presigmoid dura, even in large tumors.
The location of the transverse sinus is approximated by connecting an imaginary line between the inion and a point just above the root of the ipsilateral zygoma ( Fig. 1A ). The sigmoid sinus is projected along the digastric groove extending from the mastoid tip up to the junction with the transverse sinus. Once the sinuses are estimated using surface landmarks, a curvilinear incision is planned originating from 1 cm anterior to the tragus over the root of the zygoma to 6 to 8 cm above the pinna and then posteriorly maintaining a distance of roughly 6 cm from the posterior aspect of the pinna until the inferior pole of the incision has passed the mastoid tip, usually in a preexisting skin crease of the upper neck. The incision needs to extend far enough posteriorly to allow a true retrosigmoid bony exposure as well as far enough inferiorly and anteriorly so that the entire mastoid can be exposed up to the external auditory canal (EAC). The scalp flap will maintain its blood supply from a combination of the posterior limb of the superficial temporal artery (STA), the posterior auricular artery, and the occipital artery; care should be taken during exposure to protect the arteries and their major branches.
Fig. 1.
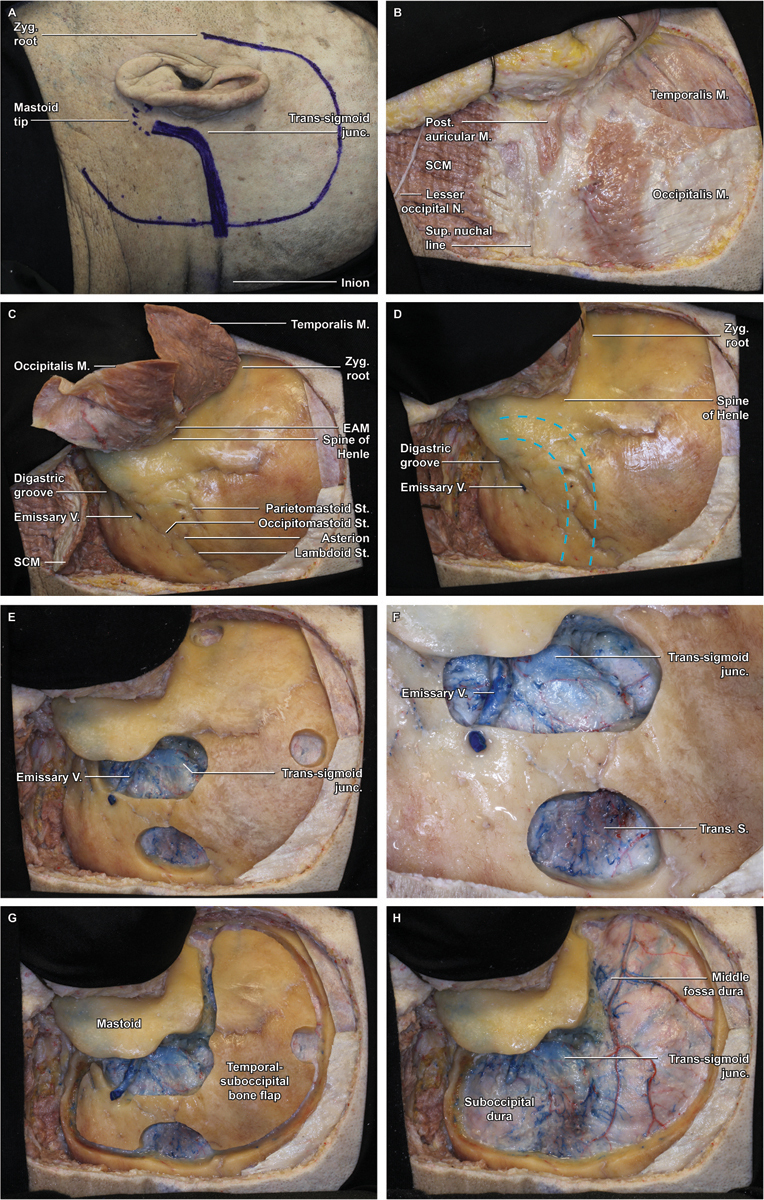
Step-by-step combined posterior petrosectomy approach in an anatomical specimen (left side). ( A ) Marked skin incision 1 cm anterior to the tragus, 6 to 8 cm superior to the pinna, and 6 cm posterior to the pinna. The transverse sinus is approximated by connecting an imaginary line between the inion and a point just above the root of the ipsilateral zygoma, whereas the sigmoid sinus is projected along the digastric groove. ( B ) The scalp flap is reflected anteriorly and secured in place with fishhooks, revealing the underlying musculature, including temporalis, occipitalis, posterior auricular, and sternocleidomastoid (SCM) muscles. ( C ) Overview demonstrating the myopericranial flaps: temporalis anteriorly, SCM and nuchal musculature inferiorly, and occipitalis laterally. ( D ). Overview of relationships between superficial bony landmarks and underlying dural venous sinuses (dashed blue lines), which are best approximated by a line from the zygomatic root to the inion for the transverse sinus and by the digastric groove for the sigmoid sinus. ( E ) Four burr holes are fashioned: at the zygomatic root, at the superior aspect of the exposure just below the superior temporal line, directly spanning the proximal transverse sinus, and overriding the transverse–sigmoid junction and sigmoid sinus. ( F ) Detail view of the inferior burr holes demonstrating the exposure of the transverse–sigmoid junction, as well as a large emissary vein. ( G ) The suprainfratentorial bone flap is seen in place following the craniotomy and prior to elevation. ( H ) Removal of the craniotomy flap widely exposes the underlying dura of the temporal lobe and posterior fossa.
Scalp and Muscle Flaps: Anatomical Landmarks for Craniotomy Planning
The skin is incised and dissected off the pericranium and temporalis fascia as a single flap, taking care to protect the STA anteriorly and the nuchal musculature inferiorly. Three fishhooks secure the flap and retract the auricle while maintaining a low profile and not obstructing the operative corridor ( Fig. 1B ).
The myopericranial layers are elevated in three segments ( Fig. 1C ):
The temporalis muscle is incised anteriorly and carried superiorly to approximately 1 cm below the superior temporal line (STL), leaving a cuff of muscle for closure, and posteriorly along the posteroinferior aspect of the STL. The muscle is dissected and retracted anteriorly and inferiorly.
Posteriorly, the nuchal musculature is incised superiorly at its attachment to the superior nuchal line, anteriorly parallel and bisecting the mastoid, and posteriorly at the limit of the scalp flap, paralleling the skin incision. The posterior flap is reflected inferiorly at its pedicle, exposing the mastoid and the occipital bone to just above the foramen magnum. The arch of C1 can usually be palpated but does not need to be exposed.
The remaining tissue interposed between the anterior and posterior muscle flaps is typically of poor quality for reconstruction but, where present, can be reflected toward the EAC with the scalp flap. Avoid detaching the periosteum from around the EAC, as this prevents excessive tension on the fragile EAC skin when the flap is retracted.
While elevating these muscle and pericranial flaps, it is very important to not violate the skin of the EAC, otherwise there will be a much increased risk of CSF leak and postoperative infection. It is also of paramount importance to have clear anatomical references to preserve the integrity of the venous sinuses before placing the burr holes. The transverse sinus can be estimated by a line traced from just above the root of the zygoma toward the inion. This line is located usually inferiorly or at the level of the superior nuchal line, where the nuchal muscles and the occipital belly of the occipitotemporalis muscle meet. The posterior edge of the sigmoid sinus can be estimated by a line following the mastoid notch (digastric groove) upward ( Fig. 1D ). This line is anterior to the occipitomastoid suture.
Burr Holes
A series of burr holes are sequentially fashioned using a high-speed drill to expose the critical venous structures and allow safe and efficient dissection of the dura and transverse sinus from the inner calvarium of the skull to facilitate a safe temporal–occipital craniotomy ( Fig. 1E ).
At the anterior limit of the exposure, a temporal burr hole is placed just above the root of the zygoma.
Posterosuperiorly, a second hole is placed superiorly in the temporal squamous bone just below the STL.
A third, rather oblong posteroinferior burr hole is placed directly over the transverse sinus at the posterior limit of the exposure. This burr hole exposes the dura mater superior and inferior to the transverse sinus from which the dura can safely be stripped superiorly and inferiorly ( Fig. 1F ).
The critical dural exposure prior to turning the craniotomy involves drilling a burr hole just below the junction of the transverse–sigmoid sinus and extending a trough along the posterior sigmoid sinus to further expose the posterior fossa dura. The transverse–sigmoid sinus is then carefully unroofed with a large diamond burr and copious irrigation. This drilling is continued superiorly until the middle fossa dura is uncovered. The angle between the superior aspect of the transverse–sigmoid junction and the middle fossa dura can be quite acute and therefore progressively smaller diamond burrs may need to be employed to safely uncover this aspect of the sinus.
It is critically important to make sure the sinus in this location is completely free of bone so that it is not torn when the bone flap is elevated. Careful exposure of the supratentorial and infratentorial dura adjacent to the transverse sinus is critical in achieving this goal. To further assist the safe elevation of the craniotomy flap, a trough is often drilled inferiorly from the fourth burr hole, exposing the posterior fossa dura and posterior aspect of the sigmoid sinus until it turns anteriorly to become the jugular bulb. The temporal and posterior fossa dura is then dissected free to facilitate the craniotomy.
It is also essential to dissect the transverse sinus from the inner table of the calvarium, which it is prone to tear; correspondingly, stripping is best performed working from the junction distally toward the more proximal transverse sinus. Great care must be taken when separating the transverse–sigmoid sinus junction from the bone at the anterior aspect of the dissection, as the sinus is almost always invested within a deep groove in the bone and is often joined at that location by a sizeable transosseous emissary vein that can bleed profusely during exposure. If such an emissary is encountered, it can be rapidly controlled with bone wax and then circumferentially exposed to allow for bipolar coagulation and ligation—a process that may need to be repeated several times if the vein is particularly robust and proximal to the junction. Additionally, the sinus itself is frequently noted to be fairly thin-walled at the transverse–sigmoid junction and therefore highly susceptible to tearing. Correspondingly, it may be very helpful to fashion large burr holes sizeable enough to allow a Penfield (Sklar) 3 dissector or similar instrument to aid in stripping the dura before turning the craniotomy flap. If the dura does not strip well, placing additional burr holes along the planned course of the craniotomy is recommended.
Craniotomy and Bone Flap Elevation
After careful stripping of the dura at each burr hole and away from the transverse–sigmoid sinuses, a spiral bit and footplate attachment are used to connect the burr holes and complete the craniotomy ( Fig. 1G ). Of note, with proper placement of the burr holes, none of the craniotomy cuts cross a venous sinus. While image guidance may be helpful to accurately localize the venous sinuses, we have not found this necessary and have always relied on the anatomical landmarks. The dura over the transverse sinus is dissected last, once the craniotomy has been performed. In this fashion, the bone flap can be quickly removed in case there is injury to the sinus during this maneuver.
Following final dural stripping, the bone flap is gently released and moved to the back table. The exposure comprises supratentorial and infratentorial dura mater ( Fig. 1H ). As mentioned previously, a transosseous emissary vein is often exposed and can be transgressed during bone flap elevation, and bleeding can be profuse. It is important to remember that this is indeed venous bleeding, which can be controlled with gel foam, a small cotton patty, and moderate pressure. A figure-of-eight 6–0 monofilament suture can then be placed to close the hole in the sinus. If there is a larger tear in the sinus, additional sutures may be necessary. Under such circumstances, it is helpful to increase the degree of reverse Trendelenburg and have an assistant place direct pressure over the proximal transverse sinus using a cotton patty, which may significantly decrease flow through the sinus and make suture placement much more efficient. Before proceeding with mastoid drilling, the dura is tacked at the superior and posterior margins of the craniotomy in a usual fashion, with sutures specifically placed just superior and inferior to the transverse sinus at the posterior limit of the craniotomy.
Mastoidectomy
Although the mastoidectomy can be completed prior to the craniotomy, we have always performed the temporal–occipital craniotomy first, as early identification of the sigmoid sinus and middle fossa dura expedites safe and efficient mastoidectomy and this workflow just logistically works best for our group. However, proceeding from elevation of the muscle flaps directly to the mastoidectomy is a reasonable alternative that may be better suited to certain practices; additionally, it has the benefit of providing the neuro-otologist with a more accommodating resting position for their hands.
The general principles of mastoid dissection include proceeding from lateral to medial and identifying peripheral anatomical landmarks early in the procedure so that larger quantities of bone can be safely and swiftly removed. Key boundaries include the sigmoid sinus and endolymphatic sac posteromedially, the middle fossa dura superiorly, the jugular bulb inferiorly, and the fallopian and EACs anterolaterally. The entire mastoidectomy can be completed using the operating microscope, although we typically prefer to work quickly through the preliminary steps, bringing in the microscope once the mastoid antrum is well exposed.
The first step in this process is removal of the superficial mastoid cortex, which is completed using a 6- or 7-mm cutting bit and copious irrigation ( Fig. 2A , B ). The mastoid is opened along its full superior–inferior extent and carried anteriorly to the EAC wall, as this should be an unambiguous landmark identifiable prior to any mastoid drilling. It is important to leave a thin shell of bone along the posterior margin of the EAC (e.g., a “canal wall up” mastoidectomy). If this bone is violated, the thin, adherent skin of the EAC is almost always also violated as well, significantly increasing the risks of CSF leak or wound infection.
Fig. 2.
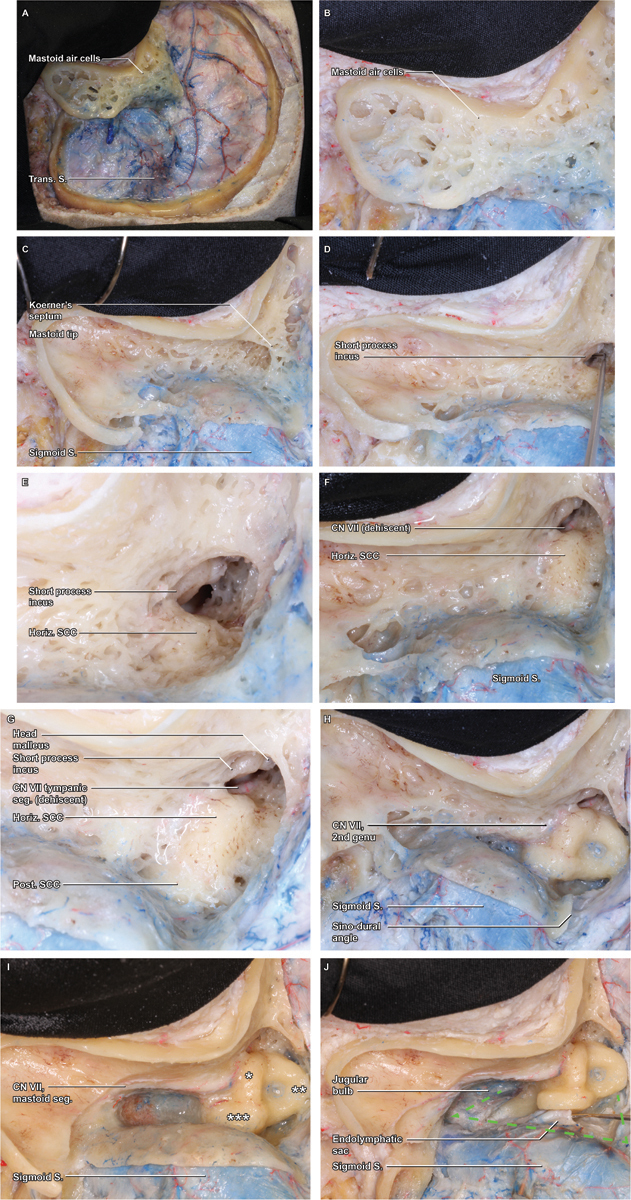
Mastoidectomy. ( A ) With the craniotomy flap removed, attention can be turned to the mastoidectomy, which is initiated by removal of the superficial cortex with a large cutting burr. ( B ) A detail view demonstrates the thin cortical rim circumscribing the cortex, which will be the boundary of the ensuing bone removal. ( C ) As the superficial mastoid air cells are removed, the broad saucer of the mastoid cortex is outlined, as is the deep limit of thin cortical bone overlying the sigmoid sinus. The mastoid air cells coalesce in the antrum, which is obscured by Koerner's septum, the last layer of medullary bone removed during the superficial mastoidectomy. ( D ) Within the antrum, the short process of the incus is visualized, a key landmark that points toward the mastoid genu of the facial nerve (cranial nerve VII). ( E ) As the floor of the antrum is exposed, a thin rim of dense, yellow otic capsule bone emerges, indicating the lateral curve of the horizontal semicircular canal (SCC). ( F ) Removal of the final mastoid trabeculae fully reveals the curvature of the horizontal SCC, beyond which the tympanic segment of the facial nerve may be appreciable, if the normal bony covering is dehiscent ( G ) As bone removal proceeds along the curve of the horizontal SCC, the otic capsule bone of the posterior SCC is revealed in a perpendicular orientation, medial and posterior. ( H ) Still further dissection along the superior arc of the posterior canal leads to the common crus, where the posterior and superior canals coalesce at the superomedial corner of the bony labyrinth. Maximizing bone removal by carefully skeletonizing this corner of the labyrinth is a critical means of optimizing the exposure. ( I ) Inferior to the horizontal SCC and lateral to the posterior SCC, the facial nerve (VII) within the fallopian canal is skeletonized and traced inferiorly through its mastoid segment. ( J ) With the course of CN VII clearly identified, completion of the bony dissection can be rapidly completed, exposing the jugular bulb, sigmoid sinus, and superior petrosal sinus—the anatomical boundaries of Trautman's dural triangle.
With the outer mastoid cortex removed, dissection proceeds from superficial to deep as the air cells are removed with wide, controlled sweeps, removing a thin layer of medullary bone with each pass and ensuring that the dissection is performed in layers without prematurely exposing the deeper structures in one single location. At the superomedial aspect of the mastoid, the air cells coalesce into the antrum, typically located 1.5 cm deep to the spine of Henle; this typically comes into view with a “pop” as the last layer of medullary bone, known as Kerner's septum, is removed ( Fig. 2C ). The degree of mastoid aeration can differ widely between patients, resulting in variable relationships between the sigmoid sinus, facial nerve, middle fossa dura, and other landmarks. Preoperative imaging is useful for anticipating these relationships.
Exposure of the Horizontal and Posterior Semicircular Canal
With the antrum exposed, the short process of the incus is visualized in the fossa incudis—a confirmatory marker for the antrum, and a key landmark for identifying the facial nerve, as the short process of the incus effectively points to the nerve's mastoid genu ( Fig. 2D ). Care should be taken to avoid disturbing the ossicular chain and thereby inducing a conductive hearing loss. Correspondingly, we recommend switching to a 3mm diamond bit at this stage of the operation, which allows for more precise bone removal around the incus and SCC. The floor of the antrum contains the horizontal SCC, which is easily distinguished from the surrounding medullary bone due to the high-density, yellow-tinged, otic capsule ( Fig. 2E–F ). Once the horizontal SCC has been identified, any residual overlying bone can be speedily removed, as the critical structures all lie deep to this canal. Careful skeletonization of horizontal SCC is paramount, as it is an important reference for many adjacent structures, including the middle fossa dura superomedially which can be exposed safely and quickly after the canal is identified, and the mastoid genu of the facial nerve inferomedially, which travels just beyond the inferior wall of the horizontal SCC, making this a critical barrier to identify and respect. As the curvature of the horizontal canal becomes fully defined, it should be followed posteriorly, where the posterior SCC will be identified in a perpendicular orientation; this should be carefully skeletonized along its inferior curvature to the middle fossa dura superiorly, and the posterior fossa dura medially ( Fig. 2G ). The mastoid segment of the facial nerve can be identified with a 5mm cutting burr or a 3–5mm diamond burr depending on access, visualization, and surgeon comfort level. Utilizing the largest burr that fits the space is most safe because small burrs will more easily penetrate (“puncture”) through structures. Copious irrigation should be used to help “see” through the bone, remove bone particles and prevent heating which can result in thermal injury to adjacent structures.
Skeletonization of the Superior SCC and Decompression of Presigmoid Dura (Trautmann's Triangle)
The superior SSC lies much deeper in the exposure, beyond the curvature of the horizontal canal, where it is encountered running orthogonally to the other SCC. With the facial canal and three SCC identified, final bony decompression of eggshell-like inner cortical bone overlying the presigmoid dura or the medial sigmoid sinus should be completed using an orbital rongeur or Lempert elevator ( Fig. 2I ). This reveals the presigmoid posterior fossa dura known as Trautman's triangle, bounded by jugular bulb inferiorly, the sigmoid sinus posteriorly, and the superior petrosal sinus (SPS) superiorly ( Fig. 2J ). Elevating the dura off the posterior face of the petrous bone will allow for the bone removal to continue in the retrolabyrinthine space and more medially in the petrous apex. Maximizing bone removal at the superomedial corner of the labyrinth at the junction of the posterior and superior SCC (e.g., the common crus) is among the most important steps of the exposure, and a 2mm diamond should be used to deeply drill out the sinodural angle and optimize the exposure ( Fig. 2H ). Residual bone in this area will significantly compromise the approach, particularly when approaching lesions extending along the petroclival junction. If one SSC is inadvertently opened during drilling, the opening should be occluded with bone wax immediately, and care must be taken not to suction endolymph, in the hopes that hearing and balance function may be preserved. Removing the bone around the endolymphatic sac and duct provides a few additional important millimeters improving the angle of view into the posterior fossa. Depending on the patient's unique anatomy it is possible to decompress some portion of the internal auditory canal.
Primary Presigmoid Dural Flap
The dura is opened in two stages. The primary dural flap is a rectangle pedicled anteriorly, fashioned using three cuts ( Fig. 3A–C ).
Fig. 3.
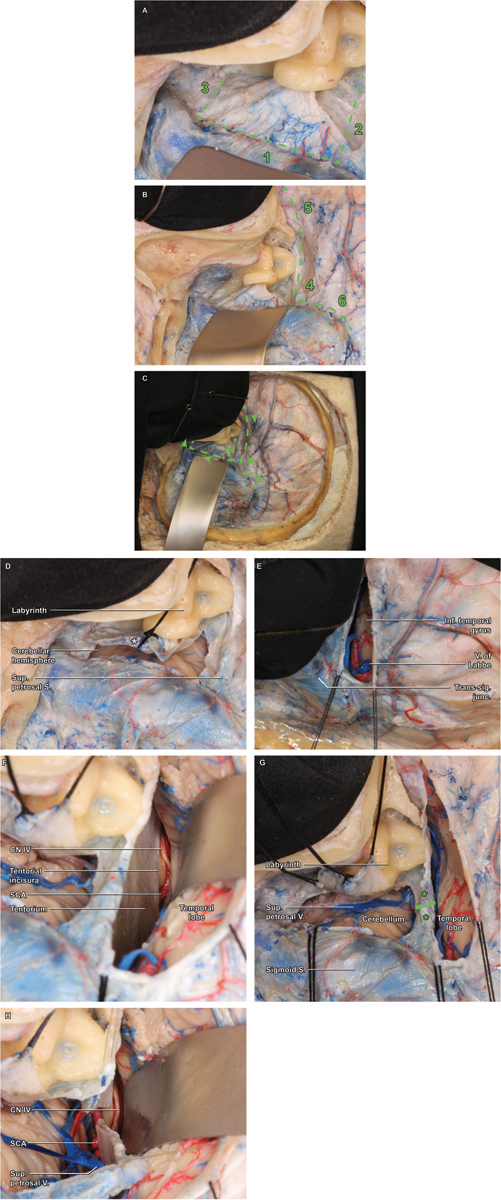
Dural opening. ( A ) Trautman's triangle constitutes the primary dural flap (dashed line), which is opened in three stepwise cuts, paralleling the sigmoid sinus superior-to-inferior (1), followed by the superior petrosal sinus (2) and jugular bulb (3) posterior-to-anterior. ( B ) The secondary dural flap is then opened along the course of the superior petrosal sinus and in parallel to the inferior temporal gyrus (dashed line), similarly fashioned using three stepwise cuts, starting with a posterior-to-anterior cut just above the superior petrosal sinus. The insertion of the vein of Labbe into the dural venous sinus system should be continuously searched for to avoid inadvertent injury to this critical venous structure. ( C ) Arrowheads demonstrate the recommended direction for each dural incision (dashed lines). ( D ) Detail of the completed primary durotomy highlights the placement of the first tack suture, used to ligate the aperture of the endolymphatic sac following the incision—a critical maneuver for hearing preservation. ( E ) Detail of the secondary durotomy emphasizes the anatomical limit of the posterior cut—the vein of Labbe, beyond which the cut should not be extended, to prevent excessive traction and risk of avulsion. ( F ) With both dural incisions completed and tacked, a broad subtemporal retractor is placed and the tentorial incisura is visualized, beyond which the trochlear nerve (cranial nerve IV) and superior cerebellar artery are encountered. Having confirmed the anterior attachment of CN IV along the tentorium, a safe trajectory for ligation of the superior petrosal sinus and tentorium itself can be planned, taking care to angle posteriorly enough to protect the nerve. ( G ) The superior petrosal sinus is clip ligated using two vascular hemostatic clips (*), and the vessel and underlying tentorium are bipolar coagulated and sharply divided (dashed line) with microscissors. ( H ) Once the tentorium has been divided, the full course of CN IV is better appreciated, circumnavigating the midbrain.
The first cut (posterior) is made approximately 2 mm anterior to the anterior margin of the sigmoid sinus and carried inferiorly from just below the SPS in parallel to the sigmoid sinus until it turns anteriorly to become the jugular bulb ( Fig. 3A ). During this cut, the endolymphatic sac will be divided. It is preferable to cut this as far distal from the duct as possible and avoid placing the suction over the opening. A 3–0 silk suture is placed in a figure-of-eight fashion to overclose this opening, simultaneously providing a retraction stitch for the dural flap, following completion of the remaining dural incisions ( Fig. 3D ).
The second cut (inferior) cut is made posterior to anterior, just above the apex of the jugular bulb. Completion of this cut provides ready access to the inferior CPA cistern for CSF drainage, which, in turn, facilitates a safer and more expedited final exposure.
The third cut (superior) cut is made along the inferior aspect of the SPS out to the petrous apex dura.
Secondary Dural Flap Adjacent to the Superior Petrosal Sinus
A longitudinal dural incision is made just superior to the SPS, along the inferior temporal dura mater ( Fig. 3B , E–F ). Initially, a small opening is made in the supratentorial dura, just superior to the transverse–sigmoid junction. The incision is then carried anteriorly a few millimeters superior and parallel with the SPS toward the anterior limit of the craniotomy. The surgeon should repeatedly inspect the supratentorial space to identify any temporal veins inserting early into the temporal dura, anterior to the transverse–sigmoid junction. Rarely, the vein of Labbe follows such a course, and care is required to minimize the risk of injury. Minor venous bleeding from bridging veins may be encountered simply due to the dural opening, which should be controlled with the application of gel foam and gentle pressure. Once the secondary dural flap is released, retention stitches are placed to retract the presigmoid flap anteriorly.
SPS Ligation and Sectioning of the SPS and Tentorium
The exposed temporal lobe and lateral cerebellum are covered with Surgicel (Ethicon) prior to retractor placement. Using the operating microscope, and after a generous amount of CSF has been drained from the posterior fossa, a broad retractor is inserted to gently elevate the temporal lobe, revealing the medial tentorial edge ( Fig. 3G ). When elevating the temporal lobe, dissection should begin at least 1.5cm anterior to the transverse–sigmoid junction, allowing again for careful inspection of any subtemporal draining veins that insert into the tentorium. These veins can typically be dissected off the tentorium and preserved. It is essential to definitively identify the tentorial edge to guide the sectioning, particularly given that some tumors have been observed to elevate the trochlear nerve (CN IV) superiorly. Once the medial tentorial edge is defined, the trajectory of the incision in the tentorium becomes apparent. If the tentorial edge is cut too far anteriorly, it will result in an inadvertent sacrifice of CN IV; correspondingly, it is helpful to directly visualize CN IV prior to sectioning the tentorium. Depending on the pathology, identification of CN IV may be obvious or quite challenging, and both the supra- and infratentorial trajectories may be useful to visualize it in various circumstances.
Prior to proceeding with the tentorial incision, the SPS is clip-ligated, coagulated, and divided, allowing the tentorial cut to proceed in parallel to the petrous ridge, carried medially toward the incisura ( Fig. 3F–H ). The superior petrosal vein is often sacrificed, especially when its drainage is rather anteromedial in the superior petrosal sinus and impedes division of the tentorium. In some cases, when the anatomy is favorable, it can be preserved, which is preferable. Incising the tentorium is always a very bloody undertaking and may require multiple applications of vascular clips and/or frequent bipolar coagulation. Occasionally, the subtemporal veins will drain into the tentorium and directly obstruct the operative trajectory. These veins may be safely coagulated and divided if it can be confirmed that they are draining only the inferior temporal lobe and are indeed not the vein of Labbe.
Final Presigmoid Retrolabyrinthine Exposure
Final exposure is achieved by the placement of two retractor blades that are positioned, one mobilizing the cerebellum and sigmoid sinus posteriorly and the other elevating the temporal lobe and posterior tentorial leaflet superiorly ( Fig. 4A–D ). Excellent visualization of CN III to XI is afforded by the approach, which provides a broad, lateral-to-medial trajectory for resecting lesions of the posterior fossa, in particular those seated at the petrous apex or lateral clivus.
Fig. 4.
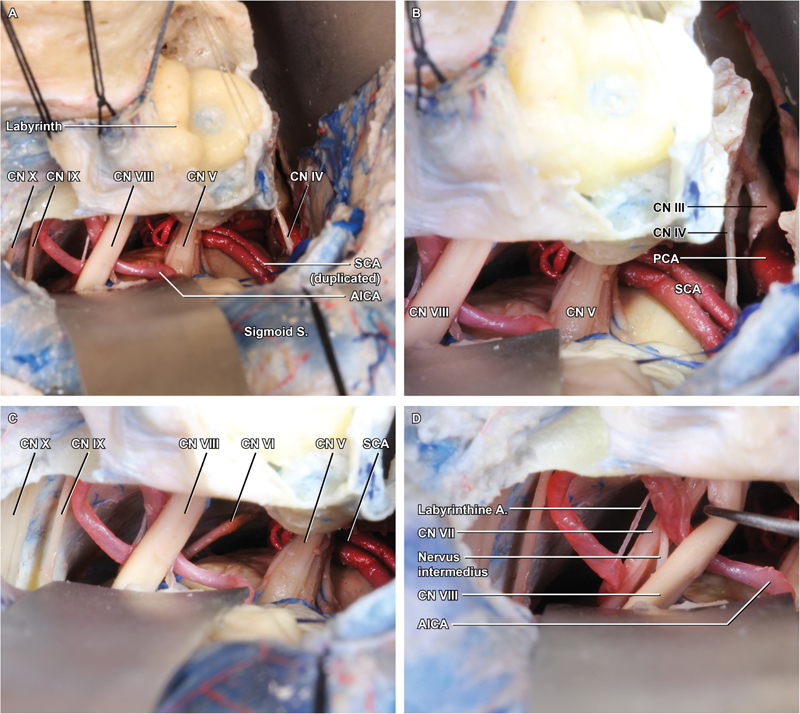
Intradural exposure. ( A ) The final exposure is achieved by positioning broad retractors along the interior temporal gyrus and sigmoid sinus/cerebellum. ( B ) Superiorly, the posterior petrosectomy allows visualization to the level of the oculomotor nerve (cranial nerve [CN] III), emerging from the medial midbrain between posterior cerebral artery and superior cerebellar artery. ( C ) Inferiorly, the lower CNs are well demonstrated, as is the abducens nerve (CN VI) deep to CN VII. ( D ) Gentle retraction of the vestibulocochlear nerve (CN VIII) reveals the full course of CN VII, with the nervus intermedius running between them, and the labyrinthine branch of AICA entering the IAC together with the CN VII/VIII complex.
Results: Representative Case Review
Extra-axial Case Illustration: Clival Chordoma
A healthy 75-year-old woman presented with 4 weeks of persistent left facial numbness punctuated by intermittent spells of neuralgic left facial pain involving V1 to V3. Over that same timeframe, she developed new headaches, light-headedness, imbalance, and bilateral tinnitus, left greater than right. Magnetic resonance imaging (MRI) of the brain identified a large, vividly enhancing, extra-axial, moderately heterogeneous mass with sellar, suprasellar, and posterior fossa extension, most consistent with chordoma ( Fig. 5A ). A short course of dexamethasone improved both the numbness and neuralgia, and a posterior petrosectomy was selected as the optimal operative approach, given the shortened working distance to the central skull base, and improved access to the suprasellar space and ventral surface of the basilar artery, all of which were extensively involved with the tumor. The patient was taken to surgery, and gross total resection of the all extra-sellar components of the lesion was successfully completed, after which she recovered very well with no new neurologic deficits including retained hearing ( Fig. 5B ).
Fig. 5.
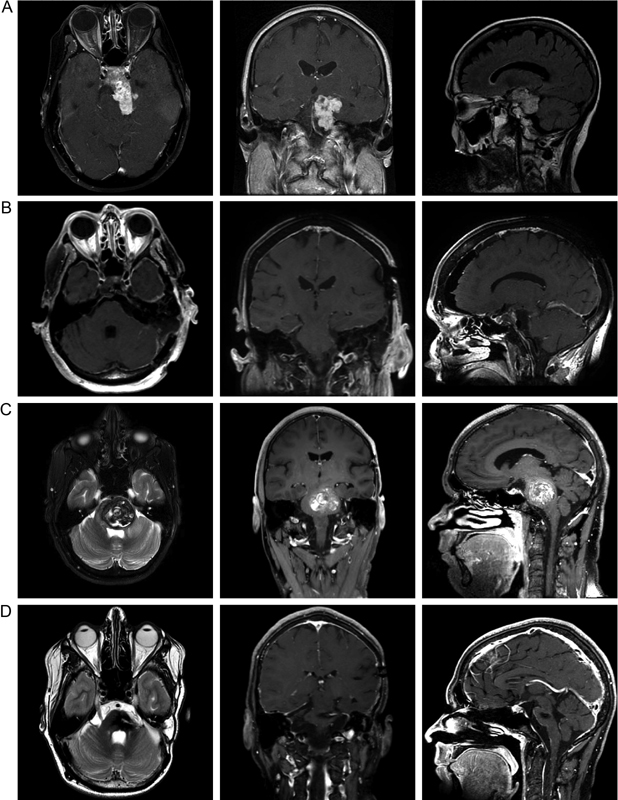
Illustrative cases. ( A ) Contrast-enhanced T1-weighted axial and coronal images are shown alongside a T2-weighted FLAIR (Fluid-attenuated inversion recovery) sagittal image from the preoperative magnetic resonance imaging (MRI), which demonstrates a vividly enhancing, extra-axial, moderately heterogeneous mass with sellar, suprasellar, and posterior fossa extension. ( B ) Postoperative contrast-enhanced T1-weighted MRI demonstrates in three planes gross total resection of all extrasellar tumors. ( C ) T2-weighted axial and T1-weighted contrast-enhanced coronal and sagittal MR images demonstrate a highly markedly heterogeneous, mixed-density, intra-axial lesion seated in the left midpons. ( D ) Comparable postoperative images demonstrated gross total resection of the lesion, with a modest region of edema and a minimal, collapsed, cerebrospinal fluid containing resection cavity, seated below a minimal corticotomy with extensive preservation of the surrounding normal brain parenchyma.
Intra-axial Case Illustration: Pontine Cavernous Malformation
A 20-year-old man with a history of left temporal fibrillary astrocytoma 8 years prior, status post near-total resection and adjuvant radiotherapy 5940 cGy, and temozolomide chemotherapy, presented with subacute right facial weakness, right upper and lower extremity weakness and incoordination, and headache. MRI demonstrated a large, hemorrhagic, markedly mixed-density, intraparenchymal lesion with heterogeneous appearance on T2 sequences, centered in the left-central pons, suggestive of a radiation-induced cavernous malformation ( Fig. 5C ). Surgical resection was recommended, shortly after which the patient was urgently admitted to the hospital for new diplopia, dysconjugate gaze, dysphagia, and worsening right hemibody weakness and ataxia; repeat imaging showed additional hemorrhage had occurred with enlargement of the lesion. The patient was taken to the operating room for a left posterior petrosectomy, with resection of the pontine lesion performed through a ¼” vertical corticotomy placed immediately inferior and posterior to the trigeminal nerve dorsal root entry zone. A gross total resection was completed, and the patient ultimately made an excellent neurologic recovery, with resolution of diplopia, dysphagia, and hemiparesis, preservation of ipsilateral hearing and facial nerve function, and persistent mild ipsilateral facial numbness without dysesthesias ( Fig. 5D ). The patient has remained neurologically stable and free of residual/recurrent disease over 3 years of follow-up.
Discussion
Anatomical Comparison between Presigmoid Approaches
As described in detail throughout this study, in the posterior petrosectomy (also known as presigmoid retrolabyrinthine approach) the presigmoid dura mater is exposed between the sigmoid sinus posteriorly, the labyrinth and the descending mastoid segment of the facial nerve anteriorly, the superior petrosal sinus, temporal dura, and temporal lobe superiorly, and the jugular bulb inferiorly. This affords an excellent view of the lateral brainstem and CN III to XI, without an excessively long working distance or a need to sacrifice functional hearing. With the tentorium sectioned and the sigmoid/cerebellar and temporal lobe retractors in place, a wide posterior corridor is opened, leaving the labyrinth as the key structure, limiting the surgical corridor of the posterior petrosectomy.
With this boundary in mind, subsequent presigmoid variations involve a stepwise removal of the otic capsule bone to further widen that aspect of the field of view. In the transcrural approach, most aspects of the exposure are identical to the retrolabyrinthine, with additional removal of the posterior and, rarely, the superior semicircular canals (SCCs). Theoretically, hearing may still be preserved in these approaches, provided that the openings created into the inner ear are immediately and carefully occluded with bone wax, preventing extrusion or suctioning of endolymph. Admittedly, we have only attempted this perhaps three times in the past 19 years and have not been successful in preserving useful hearing. Of course, it is not possible to fully know if hearing was lost due to opening an SCC or due to manipulation of the vestibulocochlear nerve in the posterior fossa during tumor resection. If the patient does not have useful hearing, or if the operation demands that hearing be sacrificed, the next presigmoid variation is a true translabyrinthine approach, which requires complete removal of the bony labyrinth, allowing for wide exposure of the internal auditory canal (IAC), whereas the descending mastoid segment of the facial nerve remains protected within the bony fallopian canal.
The most aggressive presigmoid exposures involve complete resection of the labyrinth, exposure of the IAC, sectioning of the greater superficial petrosal nerve, and mobilization of the facial nerve's meatal, labyrinthine, geniculate, tympanic, and descending mastoid segments, which allows for complete exposure and removal of the cochlea. With the facial nerve transposed posteriorly, the transcochlear exposure adds direct access to the clivus through an exceptionally wide operative corridor, allowing for maximum angulation and excellent light entry into the posterior fossa. Without exception, the transcochlear approach results in moderate-to-severe temporary facial weakness, frequently with some mild residual weakness appreciable in long-term follow-up of (e.g., House–Brackmann II–III). A modification of the transcochlear is the transotic approach, in which the facial nerve is circumferentially skeletonized within a bony fallopian bridge, allowing drilling of the cochlea without direct mobilization of CN VII. Although the corridor is more limited, the transotic almost never causes any facial weakness attributable to the approach itself and therefore provides a low morbidity extension of the translabyrinthine approach, extending access to the lateral clivus if desired. These latter two approaches are best accomplished after transecting and oversewing the external ear canal, removing the tympanic membrane and ossicles, and obliterating the Eustachian tube.
Clinical Pearls for Selection of a Presigmoid Approach
The illustrative cases reviewed previously demonstrate the versatility of the posterior petrosectomy as an important tool in the skull base armamentarium, with a broad range of applications to extra- and intra-axial lesions alike. In the first example, the lesion predominantly arose in the central skull base and was intimately related with the basilar artery. As compared with a retrosigmoid craniotomy, a presigmoid approach allowed for a more ventral angulation and a shortened working distance to the most treacherous region of the operative field, without the morbidity of unavoidable hearing loss that the translabyrinthine exposure involves. Furthermore, the more anterior positioning of the craniotomy and the sectioning of the tentorium allowed us access to the suprasellar tumor as well.
In the second case presented, the posterior petrosectomy allowed for a very perpendicular trajectory to the pons, which allowed us to work within the pons itself in an orthogonal rather than tangential orientation. This allowed for safe removal of a rather large pontine cavernous malformation while minimizing manipulation and therefore risk to the healthy adjacent brainstem tissue, whether by the excessive retraction or by larger corticotomy likely required from a retrosigmoid approach. Instead, we were able to work effectively through a narrow and precise window, fully visualize and inspect the walls of the hematoma cavity, and achieve a gross total resection with minimal morbidity to the patient, including preservation of normal hearing.
Extrapolating these lessons to more general circumstances, the posterior petrosectomy is an optimal approach for CPA and posterior fossa lesions with a significant central skull base component, particularly in the prepontine cistern or the pons itself or in the midline or ipsilateral suprasellar space. Coupled with the possibility of hearing preservation, this makes the posterior petrosectomy a compelling approach in several appropriately selected circumstances.
While the posterior petrosal approach was highly touted as an innovative and a very useful approach in the late 1980s and 1990s, it has become less commonly used particularly for petroclival tumors. 3 4 5 6 7 8 9 10 However, we feel it still has an important role in select cases. Most importantly, although there are several key steps in safely and effectively carrying out the exposure, we believe we have demonstrated that trainees of all levels can efficiently learn the technique and that it can be usefully employed in a busy clinical setting.
Conclusion
The posterior petrosectomy is a challenging approach to master, particularly given the intricate temporal bone anatomy that is often unfamiliar to neurosurgeons in general and junior residents in particular. However, given its clinical versatility, incorporating it into primary skull base education is essential. Based on our experience, we recommend achieving this educational goal through a supervised, structured, and operatively oriented dissection curriculum—the critical details of which we have outlined in this study, which we anticipate will helpfully inform the education of any residents and fellows aspiring to master the fundamental skull base approaches.
Funding Statement
Funding None.
Conflict of Interest None.
Co-first authors.
References
- 1.Wanibuchi M, Friedmann A H, Fukushima T. Stuttgart: Thieme Medical Publishers; 2009. Photo Atlas of Skull Base Dissection: Techniques and Operative Approaches; p. 432. [Google Scholar]
- 2.Tew J M, van Loveren H R, Keller J T. London: WB Saunders Co. Ltd; 2001. Atlas of Operative Microneurosurgery: Brain Tumors v. 2; p. 446. [Google Scholar]
- 3.Bambakidis N C, Kakarla U K, Kim L Jet al. Evolution of surgical approaches in the treatment of petroclival meningiomas: a retrospective review Neurosurgery 2007610502202–209., discussion 209–211 [DOI] [PubMed] [Google Scholar]
- 4.Daspit C P, Spetzler R F, Pappas C T. Combined approach for lesions involving the cerebellopontine angle and skull base: experience with 20 cases--preliminary report. Otolaryngol Head Neck Surg. 1991;105(06):788–796. doi: 10.1177/019459989110500604. [DOI] [PubMed] [Google Scholar]
- 5.Samii M, Ammirati M.The combined supra-infratentorial pre-sigmoid sinus avenue to the petro-clival region. Surgical technique and clinical applications Acta Neurochir (Wien) 198895(1-2):6–12. [DOI] [PubMed] [Google Scholar]
- 6.Samii M, Ammirati M, Mahran A, Bini W, Sepehrnia A. Surgery of petroclival meningiomas: report of 24 cases. Neurosurgery. 1989;24(01):12–17. doi: 10.1227/00006123-198901000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Samii M, Tatagiba M.Experience with 36 surgical cases of petroclival meningiomas Acta Neurochir (Wien) 1992118(1-2):27–32. [DOI] [PubMed] [Google Scholar]
- 8.Samii M, Tatagiba M, Carvalho G A. Resection of large petroclival meningiomas by the simple retrosigmoid route. J Clin Neurosci. 1999;6(01):27–30. doi: 10.1054/jocn.1997.0201. [DOI] [PubMed] [Google Scholar]
- 9.Spetzler R F, Daspit C P, Pappas C T. Combined approach for lesions involving the cerebellopontine angle and skull base: experience with 30 cases. Skull Base Surg. 1991;1(04):226–234. doi: 10.1055/s-2008-1057102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spetzler R F, Daspit C P, Pappas C T. The combined supra- and infratentorial approach for lesions of the petrous and clival regions: experience with 46 cases. J Neurosurg. 1992;76(04):588–599. doi: 10.3171/jns.1992.76.4.0588. [DOI] [PubMed] [Google Scholar]


