Abstract
Background:
The Patient-Reported Outcomes Measurement Information System (PROMIS) Global-10 was recently developed to assess physical and mental health and provide an estimated EuroQol-5 Dimension (EQ-5D) score. This instrument needs to be validated for specific patient cohorts such as those with rotator cuff pathology.
Hypothesis:
There is moderate to high correlation between the PROMIS Global-10 and legacy patient-reported outcome measures; PROMIS Global-10 will not show ceiling effects; and estimated EQ-5D scores will show good correlation and low variance with actual EQ-5D scores.
Study Design:
Cohort study (diagnosis); Level of evidence, 2.
Methods:
A total of 323 patients with rotator cuff disease were prospectively enrolled before treatment. Each patient completed the PROMIS Global-10, EQ-5D, American Shoulder and Elbow Surgeons (ASES) shoulder assessment form, and Single Assessment Numeric Evaluation (SANE), and those with known rotator cuff tears completed the Western Ontario Rotator Cuff Index (WORC). Spearman correlations were calculated. Bland-Altman agreement tests were conducted between estimated EQ-5D scores from the PROMIS and actual EQ-5D scores. Ceiling and floor effects were assessed, defined as ≥15% respondents with highest or lowest possible score.
Results:
Correlation between the PROMIS Global-10 and EQ-5D was excellent (0.70, P < .0001). Correlation of the PROMIS physical scores was excellent-good with the ASES (0.62, P < .0001), good with the WORC (0.47, P < .0001), and good with the SANE (0.41, P < .0005). Correlation between the PROMIS mental scores was poor with the ASES (0.34, P < .0001), the WORC (0.32, P = .0016), and the SANE (0.24, P < .0001). No floor or ceiling effects were found. Agreement analysis showed substantial variance in individual scores, despite the overall similarity in mean scores between the estimated and actual EQ-5D scores, indicating poor agreement. Bland-Altman 95% limits of agreement for estimated EQ-5D scores ranged from 34% below to 31% above actual EQ-5D scores.
Conclusion:
Physical function scores of the PROMIS Global-10 show high correlation with legacy patient-reported outcome instruments, suggesting that it is a reliable tool for outcome assessment in a population with rotator cuff pathology. The large variability in 95% limit of agreement suggested that the estimated EQ-5D scores from the PROMIS Global-10 cannot replace traditional EQ-5D scores.
Keywords: shoulder, rotator cuff, shoulder, general, clinical assessment/grading scales, economic and decision analysis
Patient-reported outcome (PRO) instruments allow patients to evaluate their health and measure change after surgical intervention based on their interpretation of the symptoms, distress, and function.14-16,37 Since a primary treatment goal of orthopaedic surgery is to improve patients’ quality of life, PROs have become essential tools in assessing patient’s postoperative function and, arguably, the success and efficacy of orthopaedic interventions.2,16,17,37
Several well-validated PRO measures are used in orthopaedics and shoulder surgery. The 36-Item Short Form Health Survey (SF-36)5 and EuroQol-5 Dimension (EQ-5D)17 are broadly used in medicine to assess general health and quality of life, particularly for chronic conditions, and are not exclusive to orthopaedics.30 The EQ-5D is often used to calculate quality-adjusted life years (QALYs): a combination of the utility value (range, 1 = full health to 0 = death) of a particular health state and time spent in that state.36 QALYs are often used in health economic evaluations and for cost-utility analysis.10,25 Limb- and disease-specific PROs have also been validated, including the American Shoulder and Elbow Surgeons (ASES) shoulder assessment form,19,22 the Western Ontario Rotator Cuff Index (WORC),20 and the Western Ontario Shoulder Instability Index (WOSI).21,32
There has been a recent emphasis to decrease patient response burden and improve compliance while improving measurement precision, owing to the often lengthy process of completing PROs.18 The Patient-Reported Outcomes Measurement Information System (PROMIS) was created in 2004 by the National Institutes of Health (NIH) to develop and evaluate a set of publicly available efficient PROs for patients with various diseases and chronic conditions.6 The PROMIS databank includes question sets covering multiple health domains. The questions were designed to utilize item response theory, which examines individual question responses and their relationships within question sets.6 Recent studies validated the PROMIS physical function computer adaptive testing (PF CAT) and upper extremity instruments in comparison with traditional PROs for patients with meniscal injury and shoulder instability and those undergoing primary total shoulder arthroplasty.1,9,12 These studies found the PROMIS PF CAT and upper extremity instruments to be comparable in accuracy to traditional PROs while providing low patient burden, high reliability, and no ceiling effects.
While the performance of these extremity-specific PROs was assessed in several orthopaedic cross-sectional studies, the performance of the PROMIS global health PRO (Appendix 1, available in the online version of this article) has not yet been studied for specific diseases and patient populations. The NIH PROMIS Global-10 is a PRO utilizing 10 items to measure overall physical function, fatigue, pain, emotional distress, and social health, instead of physical function isolated to a specific extremity.2,14,29,32 This allows for respondents to receive both a physical health score and a mental health score upon completion of the PROMIS Global-10, as well as an estimated EQ-5D score.14,27,29,32 The PROMIS Global-10 includes fewer questions (n = 10) than the PROMIS upper extremity (n = 16) and WORC (n = 21) and, if reliable, could eliminate the need for patients to complete multiple legacy PROs during their office visit, resulting in decreased question burden, improved clinic flow, and possibly improved compliance.2,14,29,32 Unlike extremity-specific PROs, the PROMIS Global-10 is broadly applicable and can be implemented to assess patient outcomes for various orthopaedic disorders as well as in nonorthopaedic specialties.
One such disorder that is commonly seen in upper extremity and general orthopaedic practices is rotator cuff disease and tears. In the United States, rotator cuff disease accounts for >4.5 million physician visits alone,26 with degenerative rotator cuff tears being the most common upper limb condition among people >50 years old and with traumatic tears being common sequelae of acute and acute-on-chronic shoulder injuries.7,26 Despite the high prevalence of rotator cuff disease and potentially debilitating consequences, the performance of the PROMIS Global-10 has not yet been studied among patients with rotator cuff disease.1,9
The purpose of this study is to validate the PROMIS Global-10 for rotator cuff disease to legacy general health, limb-, and disease-specific PROs. We hypothesize the following: (1) There is moderate to high correlation between the PROMIS Global-10 and the legacy PROs (ASES, EQ-5D, and WORC). (2) PROMIS instruments will not show ceiling effects. (3) PROMIS Global-10 will show a decreased question burden when compared with other PROs. (4) Estimated EQ-5D scores from the PROMIS Global-10 will show good correlation and low variance with actual EQ-5D scores.
METHODS
This investigation was approved by the Yale University Human Investigation Committee (protocol 1510016580) and was compliant with standard protocols of the Health Insurance Portability and Accountability Act. From January 2015 to September 2017, 323 new patients with rotator cuff disease had their PROs prospectively collected in the office of 2 fellowship-trained sports medicine surgeons (T.A.B., D.K.) before they received continued medical or surgical treatment. Patients were included in the study if they were clinically diagnosed as having shoulder impingement or a partial- or full-thickness tear of the posterosuperior rotator cuff, with all clinical diagnoses confirmed by magnetic resonance imaging findings.
Other inclusion criteria were defined as ≥18 years of age, English speaking, and having the ability to provide informed consent. Previous rotator cuff surgery was an exclusion factor. Patients with multiple rotator cuff pathologies were classified by the most severe pathology level. For instance, patients with both impingement and a partialthickness rotator cuff tear would be considered to have a partial-thickness rotator cuff tear for the purposes of analysis.
Patients prospectively completed the PROMIS Global-10, EQ-5D, Single Assessment Numeric Evaluation (SANE), ASES, and WORC instruments on a tablet computer during their office visit. Clinical examination and advanced imaging findings were used to confirm that patients enrolled in the study who had completed the WORC had a diagnosed rotator cuff disorder. Patient’s demographic information, such as age, sex, hand dominance, and operative side, were also prospectively collected with the tablet. Smoking status, medical comorbidities, and insurance status were obtained from chart reviews. Medical comorbidities were defined as having a documented diagnosis of diabetes, hypertension, depression, heart disease requiring previous heart surgery, chronic obstructive pulmonary disease, body mass index >35 kg/m2, rheumatoid arthritis, or peripheral vascular disease. Study data were collected and managed with REDCap (Research Electronic Data Capture) hosted at our institution.13
PROMIS Global-10 results were used to calculate each patient’s physical and mental health raw scores, T scores, and estimated EQ-5D scores. T scores are standardized and derived from raw scores such that a 50 represents the average (mean) for the US general population and the SD around that mean is 10 points. Estimated EQ-5D scores were generated from a linear combination of 8 Global-10 items as described by Revicki et al.27 To assess construct validity, the correlation of PROMIS Global-10 physical and mental health scores with EQ-5D, SANE, ASES, and WORC instruments was calculated. While the EQ-5D, ASES, and WORC instruments are all legacy PROs that were validated for orthopaedic patients, the SANE has not yet been validated, and no minimal clinically significant difference has been reported.32 Normality of variables was assessed with histograms and the Kolmogorov-Smirnov test.
The associations between the PROMIS Global-10 and the legacy PROs were defined with Spearman correlation coefficients. Correlations were defined as excellent (>0.7), excellent-good (0.61-0.70), good (0.4-0.6), or poor (<0.4).31 Subgroup analysis of PRO scores and correlations were conducted for patients with a full-thickness rotator cuff tear, excluding those with a diagnosis of impingement or partial-thickness rotator cuff tear. A Bland-Altman analysis was conducted to assess the agreement between estimated and actual EQ-5D scores.3,4 Simple linear regression analysis was used to assess the correlation between demographics and PROMIS physical or mental score.
The presence of floor and ceiling effects was assessed by calculating the percentage of participants with the highest and lowest possible scores. If >15% of participants scored in either extreme, a floor or ceiling effect was considered present.23,33 Subgroup analysis for floor and ceiling effects was conducted for patients aged <40 years.
All statistical analyses were performed with SAS (v 9.4; SAS Institute). Statistical significance was set as P < .05 (2-sided).
RESULTS
A total of 323 patients met the eligibility criteria and were enrolled. Patient characteristics are summarized in Table 1. The median age of our patient population was 59 years (range, 18-89 years; 53.9% male, 46.1% female). Most patients had a diagnosis of full-thickness rotator cuff tear (44.6%) or impingement (43.7%), with only a small portion with a diagnosed partial tear (11.8%). Most patients had private insurance (54.4%), had never smoked (49.8%), and had no medical comorbidities (61.3%). Patients with bilateral pathology tended to have bilateral shoulder impingement.
TABLE 1.
Patient Characteristics
| Mean ± SD or n (%) | |
|---|---|
| Age, y | 57.7 ± 13.8 |
| Body mass index, kg/m2 | 29.4 ± 6.1 |
| Sex | |
| Male | 174 (53.9) |
| Female | 149 (46.1) |
| Diagnosis | |
| Full-thickness tear | 144 (44.6) |
| Impingement | 141 (43.7) |
| Partial tear | 38 (11.8) |
| Hand dominance | |
| Right | 271 (83.9) |
| Left | 37 (11.5) |
| Ambidextrous | 15 (4.6) |
| Affected side | |
| Right | 186 (57.6) |
| Left | 128 (39.6) |
| Both | 9 (2.8) |
| Comorbidity | |
| No | 198 (61.3) |
| Yes | 125 (38.7) |
| Insurance | |
| Private | 176 (54.4) |
| Medicare | 91 (28.2) |
| Medicaid | 53 (16.4) |
| Workers’ compensation | 3 (1.0) |
| Smoking status | |
| Current smoker | 49 (15.2) |
| Previous smoker | 113 (35.0) |
| Never | 161 (49.8) |
Mean scores for PRO instruments and PROMIS physical and mental health scores are shown in Table 2. The PROMIS mental T score was similar to that of the US general population (49.5 vs 50). The PROMIS physical T score was significantly lower than that of the US general population (42 vs 50, P < .0001), indicating that this study cohort with rotator cuff pathology has mental health similar to that of the general US population but a significantly lower physical function level.
TABLE 2.
PRO Scores for Rotator Cuff Populationa
| Instrument | Scale | Mean ± SD | Median (Range) |
|---|---|---|---|
| PROMIS Global-10 | |||
| Physical health | 4-20 | 12.7 ± 2.7 | 13.0 (6.0-18.0) |
| Mental health | 4-20 | 14.3 ± 3.5 | 14.0 (4.0-20.0) |
| Physical T score | 0-100 | 42.0 ± 7.3 | 42.3 (23.5-57.5) |
| Mental T score | 0-100 | 49.5 ± 7.3 | 48.3 (21.2-67.6) |
| EQ-5D | |||
| Actual | 0-1 | 0.6 ± 0.2 | 0.8 (0.1-1.0) |
| PROMIS estimated | 0-1 | 0.6 ± 0.1 | 0.7 (0.4-0.8) |
| ASES | 0-100 | 43.8 ± 20.7 | 45.0 (0.0-100) |
| WORC | 0-100 | 36.5 ± 9.2 | 13.0 (5.1-96.1) |
| SANE | 0-100 | 43.9 ± 23.6 | 13.0 (0.0-96.0) |
ASES, American Shoulder and Elbow Surgeons; EQ-5D, Euro-Qol-5 Dimension; PRO, patient-reported outcome; PROMIS, Patient-Reported Outcomes Measurement Information System; SANE, Single Assessment Numeric Evaluation; SF-36, 36-Item Short Form Health Survey; WORC, Western Ontario Rotator Cuff Index.
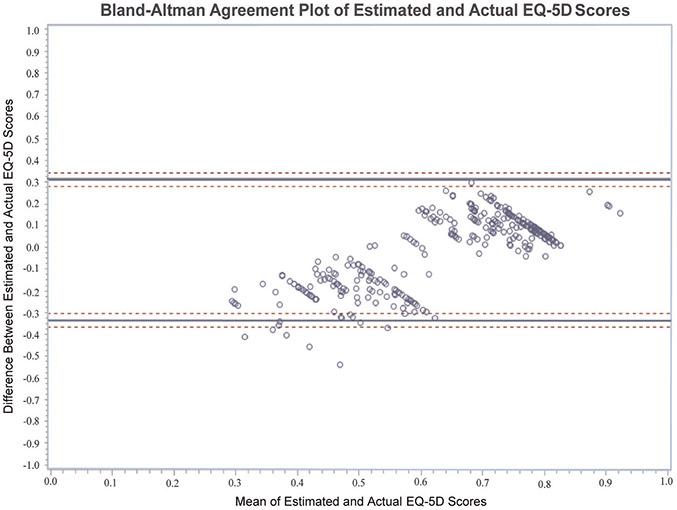
Correlation between the PROMIS Global-10 and the EQ-5D was excellent, with a Spearman correlation of 0.70 (P < .0001). The mean difference between estimated EQ-5D scores from the PROMIS Global-10 and actual EQ-5D scores was −0.0128 (95% CI, −0.03 to 0.01; P = .16) on a scale of 0 to 1, indicating that the scores were similar overall. A Bland-Altman plot was created (Figure 1) to examine the difference between estimated and actual EQ-5D scores. Agreement analysis showed substantial variance in individual scores, despite the overall similarity in mean scores between the estimated and actual EQ-5D indicating poor agreement. On a scoring scale of 0 to 1, any individual estimated score could range from −0.34 below to 0.31 above the actual EQ-5D score, respectively. No consistent bias was identified for the estimated EQ-5D factors.
Figure 1.
Plot demonstrating the difference between the actual EQ-5D score and the estimated EQ-5D score from the PROMIS (vertical axis) and the average of the actual EQ-5D score and the estimated EQ-5D score (horizontal axis). Each dot represents 1 respondent. The solid lines are the lower and upper 95% limits of agreement, and the dashed lines are the lower and upper 95% CI for the lower and upper limits. EQ-5D, EuroQol-5 Dimension; PROMIS, Patient-Reported Outcomes Measurement Information System.
Correlations of the PROMIS physical and mental scores with the legacy PRO instruments are shown in Table 3 for the overall population and in Table 4 for the subgroups of full-thickness rotator cuff tears, partial-thickness rotator cuff tears, and impingement. Correlation coefficients between the PROMIS physical instruments and all legacy PROs were >0.4, indicating good correlation. Similar correlation results were found for the patient subsets diagnosed with full-thickness rotator cuff tears, partialthickness tears, and impingement as compared with the entire cohort and each other. Correlation coefficients between the PROMIS mental instruments and the other physical function PROs were all <0.4, indicating poor correlation.
TABLE 3.
Correlation Coefficients Between the PROMIS Global-10 and Legacy Instruments: Entire Patient Cohort (N = 323)a
| Instrument | rs | P Value | Correlation Strength |
|---|---|---|---|
| PROMIS Global-10 | |||
| EQ-5D | 0.70b | <.0001 | Excellent |
| Global-10 physical health | |||
| ASES | 0.62 | <.0001 | Excellent-good |
| WORC | 0.47 | <.0001 | Good |
| SANE | 0.41 | <.0005 | Good |
| Global-10 mental health | |||
| ASES | 0.34 | <.0001 | Poor |
| WORC | 0.32 | .0016 | Poor |
| SANE | 0.24 | <.0001 | Poor |
ASES, American Shoulder and Elbow Surgeons; EQ-5D, Euro-Qol-5 Dimension; PROMIS, Patient-Reported Outcomes Measurement Information System; SANE, Single Assessment Numeric Evaluation; WORC, Western Ontario Rotator Cuff Index.
rs = 0.704.
TABLE 4.
Rotator Cuff Disease Severity and Correlation Strength Between the PROMIS Global-10 and Legacy Instrumentsa
| Subgroup: Instrument | rs | P Value | Correlation Strength |
|---|---|---|---|
| Full-thickness tear (n = 144) | |||
| EQ-5D | 0.70b | <.0001 | Excellent |
| Global-10 physical health | |||
| ASES | 0.60 | <.0001 | Good |
| WORC | 0.46 | .0003 | Good |
| SANE | 0.36 | <.0001 | Poor |
| Global-10 mental health | |||
| ASES | 0.27 | .0013 | Poor |
| WORC | 0.39 | .0031 | Poor |
| SANE | 0.14 | .0864 | Poor |
| Impingement (n = 141) | |||
| EQ-5D | 0.71 | <.0001 | Excellent |
| Global-10 physical health | |||
| ASES | 0.62 | <.0001 | Excellent-good |
| WORC | 0.49 | .005 | Good |
| SANE | 0.38 | <.0001 | Poor |
| Global-10 mental health | |||
| ASES | 0.33 | .0013 | Poor |
| WORC | 0.09 | .623 | Poor |
| SANE | 0.28 | .001 | Poor |
| Partial-thickness tear (n = 38) | |||
| EQ-5D | 0.67 | <.0001 | Excellent-good |
| Global-10 physical health | |||
| ASES | 0.53 | .001 | Good |
| WORC | 0.00 | ≥.999 | Poor |
| SANE | 0.53 | .001 | Good |
| Global-10 mental health | |||
| ASES | 0.39 | .019 | Poor |
| WORC | 0.10 | .870 | Poor |
| SANE | 0.33 | .042 | Poor |
ASES, American Shoulder and Elbow Surgeons; EQ-5D, Euro-Qol-5 Dimension; PROMIS, Patient-Reported Outcomes Measurement Information System; SANE, Single Assessment Numeric Evaluation; WORC, Western Ontario Rotator Cuff Index.
rs = 0.703.
Simple linear regressions were performed for patient demographics and the PROMIS physical and mental scores (Table 5). Age had a significant negative effect on PROMIS physical scores (P = .001) but not on PROMIS mental scores. Patients with impingement had significantly higher PROMIS physical health scores than patients with fullthickness rotator cuff tears (P = .001) and significantly higher mental scores (P = .01). Subgroup analysis of the correlation of the PROMIS Global-10 with the ASES, WORC, and SANE was performed for patients aged <40 years (n = 34) and >40 years, with similar correlation coefficients and results (Appendix 2, available online).
TABLE 5.
Simple Linear Regression for PROMIS Global-10 Physical and Mental Scoresa
| PROMIS Global-10 Physical Score |
PROMIS Global-10 Mental Score |
|||
|---|---|---|---|---|
| Variable | Regression Coefficient (95% CI)b | P Value | Regression Coefficient (95% CI) b | P Value |
| Age | −0.10 (−0.16 to −0.05) | .001 | −0.05 (−0.12 to 0.03) | .222 |
| Sex | ||||
| Male | 42.72 (41.64 to 43.80) | .053 | 49.42 (48.04 to 50.79) | .852 |
| Female | 41.15 (39.98 to 42.31) | 49.61 (48.12 to 51.10) | ||
| Affected side | ||||
| Left | 41.57 (40.30 to 42.84) | 49.08 (47.49 to 50.67) | ||
| Right | 42.28 (41.23 to 43.34) | .0696 | 49.42 (48.10 to 50.74) | .036 |
| Ambidextrous | 41.97 (37.18 to 46.75) | 57.24 (51.24 to 63.25) | ||
| Hand dominance | ||||
| Left | 41.19 (38.84 to 43.55) | 49.07 (46.08 to 52.06) | ||
| Right | 42.17 (41.30 to 43.05) | .575 | 49.58 (48.47 to 50.68) | .950 |
| Ambidextrous | 40.67 (36.96 to 44.37) | 49.33 (44.64 to 54.03) | ||
| Diagnosis | ||||
| Full tear | 40.81 (39.64 to 41.98) | 48.29 (46.79 to 49.78) | ||
| Partial tear | 40.20 (37.92 to 43.68) | .001 | 47.77 (44.86 to 50.68) | .013 |
| Impingement | 43.68 (42.50 to 44.87) | 51.22 (49.70 to 52.73) | ||
PROMIS, Patient-Reported Outcomes Measurement Information System.
Regression coefficient for continuous variable or least squares means for categorical variables.
No floor or ceiling effects were found for the study cohort in terms of PROMIS physical or mental health scores (Table 6). Only 16 patients (5%) had the highest PROMIS mental score. Subgroup analysis of patients aged <40 years did not reveal floor or ceiling effects.
TABLE 6.
Analysis of Floor and Ceiling Effects of the PROMIS Global-10: Patients Reaching Minimum or Maximum Scorea
| Minimum Score |
Maximum Score |
|
|---|---|---|
| Entire patient cohort (N = 323) | ||
| Global-10 physical health | 0 (0) | 0 (0) |
| Global-10 mental health | 1 (0.3) | 16 (5.0) |
| Age <40 y (n = 34) | ||
| Global-10 physical health | 0 (0) | 0 (0) |
| Global-10 mental health | 0 (0) | 2 (5.9) |
Data are given as No. (%) of patients. PROMIS, Patient-Reported Outcomes Measurement Information System.
Average time to complete each PRO was recorded (Table 7). The ASES took the most time (1.84 minutes), whereas the EQ-5D took the least (0.47 minutes).
TABLE 7.
Average Time to Complete Each PRO Instrumenta
| Minutes, Mean ± SD | |
|---|---|
| PROMIS Global-10 | 1.72 ± 1.13 |
| EQ-5D | 0.47 ± 0.55 |
| ASES | 1.84 ± 1.34 |
| SANE | 1.59 ± 0.84 |
ASES, American Shoulder and Elbow Surgeons; EQ-5D, Euro-Qol-5 Dimension; PRO, patient-reported outcome; PROMIS, Patient-Reported Outcomes Measurement Information System; SANE, Single Assessment Numeric Evaluation.
DISCUSSION
This study validated the PROMIS Global-10 among patients with rotator cuff disease. We found the following: (1) There was excellent correlation between the PROMIS Global-10 and EQ-5D, good to excellent correlation of the physical health score with legacy PROs (ASES, EQ-5D, and WORC), and poor correlation of the mental health score with legacy PROs. (2) PROMIS instruments did not show ceiling effects. (3) PROMIS Global-10 showed decreased question burden as compared with the WORC, similar question burden to the ASES, and more question burden than the EQ-5D and SANE. (4) Estimated EQ-5D scores from the PROMIS Global-10 had good correlation with actual EQ-5D scores but high variance. Considering the high prevalence of rotator cuff pathology found in orthopaedic practice, we believe a validated low-burden PRO tool such as the PROMIS Global-10 would be a significant advancement in how physicians can measure the success of their interventions in this patient population.
Beckmann et al2 examined the PROMIS PF CAT among presurgical patients (N = 187) with rotator cuff disease and found moderate correlation with the ASES (0.581, P< .001) and the Simple Shoulder Test (0.635, P < .001). The Pearson product-moment coefficient between the PROMIS PF CAT and ASES was similar to the coefficient that we found for the PROMIS Global-10 physical T score to the ASES (0.61, P < .0001).
We learned that the PROMIS Global-10 had excellent correlation with the EQ-5D. However, when comparing the individual PROMIS-generated EQ-5D scores and the actual EQ-5D scores, we found that although the mean difference was small, Bland-Altman plots showed significant variance between the individual actual scores and estimated scores. The 95% limits of agreement for difference in scores ranged from –0.34 below to 0.31 above an EQ-5D score on a scale of 0 to 1. This is somewhat higher than the Bland-Altman 95% limits of agreement for estimated versus actual EQ-5D scores reported by Revicki et al27 of –0.2 to 0.2. Thus, despite the excellent correlation between the PROMIS Global-10 items and the EQ-5D, the PROMIS-generated EQ-5D score may not be a sufficient substitute for the actual EQ-5D score of patients with rotator cuff pathology. This also indicates that PROMIS-estimated EQ-5D scores may not be an adequate substitute for EQ-5D-derived QALYs for economic and cost-effectiveness evaluations.
Although the PROMIS Global-10 is a general health PRO, it shows good correlation with legacy general wellness and extremity- and disease-specific PROs for assessment of rotator cuff pathology. The PROMIS Global-10 is similar in design to the 12-Item Short Form Health Survey (SF-12), another general health PRO measure that has been widely used in assessing patients with rotator cuff pathology and their response to treatment.8,11,24,28,38 The SF-12 is a short-form version of the SF-36 and is similar to the PROMIS Global-10 in that it has both physical and mental component summaries, with high scores indicating better function and with a mean score of 50 for the US population (range, 0-100).34,35 Ferreira et al11 found significantly lower SF-12 physical component scores for patients with rotator cuff arthropathy as compared with matched controls and significantly poorer functional results according to the ASES. Corpus et al8 used the SF-12 to measure response to treatment among patients with massive rotator cuff tears treated with an all-arthroscopic modified rotator interval slide, finding a significant improvement in both postoperative ASES scores and SF-12 physical component scores. Similar to these 2 studies, our data showed decreased PROMIS Global-10 physical function scores among patients with rotator cuff pathology, whereas the PROMIS Global-10 mental function scores were similar to the US population average.
Although there are various general health PROs from which to choose, such as the SF-12, EQ-5D, and SF-36, the PROMIS instruments, including the PROMIS Global-10, are being increasingly adopted by medical institutions and need to be validated for specific pathologies. The PROMIS instruments were created by the NIH and, as such, are likely to remain in clinical practice until thoroughly vetted for specific disease pathologies, as done in this study. In fact, Wylie et al38 conducted a review of PRO measurements after shoulder surgery, commenting that PROMIS PROs are likely to be increasingly reported in studies evaluating shoulder outcomes. At our institution, the PROMIS Global-10 is being adopted across orthopaedic subspecialties, including spine, shoulder, elbow, and sports medicine, as well as hip and knee joint arthroplasty, with plans on implementation and use for the entire department.
Traditional PROs can be both lengthy and timeconsuming, requiring completion of the entire questionnaire to ensure instrument validity. The high patient burden and time requirement can result in suboptimal office flow and lower patient compliance. We found that the PROMIS Global-10 has improved efficiency when compared with some other PRO measures. Only 10 questions must be completed as compared with the PROMIS upper extremity (16 questions), ASES (10 questions), and WORC (21 questions). The PROMIS PF CAT can range from 4 to 12 questions. The PROMIS Global-10 is, however, longer than the EQ-5D, which requires answering 6 questions. The ability to identify the same information with fewer questions can reduce patient burden and improve patient response rates and office flow.
Last, we found no floor or ceiling effects in our study cohort. This ensures that extremes of function can be measured, and it allows for continued measurements in response to treatment. Other recent articles validating various PROMIS instruments in shoulder instability, meniscal tears, and primary total shoulder arthroplasty also indicated no ceiling effects.1,12 A number of articles cited a ceiling effect for the PROMIS upper extremity instrument, with 1 article recommending that it not be used among patients <21 years old.1,15
Limitations of this study include that we cannot compare the PROMIS Global-10 with the PROMIS PF CAT score. This study also required patients to complete multiple PRO tools, which may have introduced responder fatigue. This could be addressed by randomization of test administration order. Serial PRO measurements before and after surgery would be useful for validation of PROMIS responsiveness to treatment.
Given its correlation with general health PROs and legacy upper extremity PROs, our results suggest that the PROMIS Global-10 is valid for assessing patients with rotator cuff disease and may be a useful alternative. Given this broad applicability, the PROMIS Global-10 can potentially be utilized by physicians in multispecialty groups. More important, while the PROMIS Global-10 provides estimated EQ-5D scores, we found that they may not be directly comparable with traditional EQ-5D scores, potentially limiting its ability to derive QALYs for economic and cost-effectiveness evaluations.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Xin Hu, MSPH, and Fangyong Li, MPH, for providing statistical support for this investigation.
One or more of the authors has declared the following potential conflict of interest or source of funding: T.A.B. owns stock or stock options in Catalyst Orthoscience; is a paid presenter or speaker for Tornier, Zimmer Biomet, and Arthrex; serves as a paid consultant to Tornier, Zimmer Biomet, Cartiva, and Wright Medical Technology; has received travel payments from Cartiva, Arthrex, Gotham Surgical Solutions & Device, Wright Medical Technology, Zimmer Biomet, and Tornier. D.K. has received education and hospitality payments from Arthrex, Depuy Synthes, and Tornier and has received honoraria from Fidia Pharma. Funding for this study provided by NIH National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) K08AR072092. The content is solely the responsibility of the authors. Funding was provided by the Yale Shoulder, Elbow and Sports Medicine Research Foundation. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
REFERENCES
- 1.Anthony CA, Glass NA, Hancock K, Bollier M, Wolf BR, Hettrich CM. Performance of PROMIS instruments in patients with shoulder instability. Am J Sports Med. 2017;45(2):449–453. [DOI] [PubMed] [Google Scholar]
- 2.Beckmann JT, Hung M, Bounsanga J, Wylie JD, Granger EK, Tashjian RZ. Psychometric evaluation of the PROMIS Physical Function Computerized Adaptive Test in comparison to the American Shoulder and Elbow Surgeons score and Simple Shoulder Test in patients with rotator cuff disease. J Shoulder Elbow Surg. 2015;24(12):1961–1967. [DOI] [PubMed] [Google Scholar]
- 3.Bland JM, Altman D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;327(8476):307–310. [PubMed] [Google Scholar]
- 4.Bland JM, Altman DG. Comparing methods of measurement: why plotting difference against standard method is misleading. Lancet. 1995;346(8982):1085–1087. [DOI] [PubMed] [Google Scholar]
- 5.Bohannon RW, DePasquale L. Physical functioning scale of the Short-Form (SF) 36: Internal consistency and validity with older adults. J Geriatric Phys Ther. 2010;33(1):16–18. [PubMed] [Google Scholar]
- 6.Cella D, Yount S, Rothrock N, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care. 2007;45(5)(suppl 1)S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chakravarty K, Webley M. Shoulder joint movement and its relationship to disability in the elderly. J Rheumatol. 1993;20(8):1359–1361. [PubMed] [Google Scholar]
- 8.Corpus KT, Taylor SA, O’Brien SJ, Gulotta LV. All-arthroscopic modified rotator interval slide for massive anterosuperior cuff tears using the subdeltoid space: Surgical technique and early results. HSS J. 2016;12(3):200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dowdle SB, Glass N, Anthony CA, Hettrich CM. Use of PROMIS for patients undergoing primary total shoulder arthroplasty. Orthop J Sports Med. 2017;5(9):2325967117726044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford, UK: Oxford University Press; 2015. [Google Scholar]
- 11.Ferreira AA, Malavolta EA, Assunção JH, Gracitelli MEC, Ocampos GP, Trindade EM. Quality of life in patients with rotator cuff arthropathy. Acta Ortop Bras. 2017;25(6):275–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hancock KJ, Glass N, Anthony CA, et al. Performance of PROMIS for healthy patients undergoing meniscal surgery. J Bone Joint Surg Am. 2017;99(11):954–958. [DOI] [PubMed] [Google Scholar]
- 13.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hays RD, Bjorner JB, Revicki DA, Spritzer KL, Cella D. Development of physical and mental health summary scores from the Patient-Reported Outcomes Measurement Information System (PROMIS) global items. Qual Life Res. 2009;18(7):873–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hung M, Clegg DO, Greene T, Saltzman CL. Evaluation of the PROMIS physical function item bank in orthopaedic patients. J Orthop Res. 2011;29(6):947–953. [DOI] [PubMed] [Google Scholar]
- 16.Hurwitz SR, Slawson D, Shaunessy A. Orthopaedic information mastery: applying evidence-based information tools to improve patient outcomes while saving orthopaedist’s time. J Bone Joint Surg Am. 2000;82(6):888. [DOI] [PubMed] [Google Scholar]
- 17.Jansson K-Å, Granath F. Health-related quality of life (EQ-5D) before and after orthopedic surgery. Acta Orthop. 2011;82(1):82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jepson C, Asch DA, Hershey JC, Ubel PA. In a mailed physician survey, questionnaire length had a threshold effect on response rate. J Clin Epidemiol. 2005;58(1):103–105. [DOI] [PubMed] [Google Scholar]
- 19.King GJ, Richards RR, Zuckerman JD, et al. A standardized method for assessment of elbow function. J Shoulder Elbow Surg. 1999;8(4):351–354. [DOI] [PubMed] [Google Scholar]
- 20.Kirkley A, Alvarez C, Griffin S. The development and evaluation of a disease-specific quality-of-life questionnaire for disorders of the rotator cuff: The Western Ontario Rotator Cuff Index. Clin J Sport Med. 2003;13(2):84–92. [DOI] [PubMed] [Google Scholar]
- 21.Kirkley A, Griffin S, McLintock H, Ng L. The development and evaluation of a disease-specific quality of life measurement tool for shoulder instability. Am J Sports Med. 1998;26(6):764–772. [DOI] [PubMed] [Google Scholar]
- 22.Kocher MS, Horan MP, Briggs KK, Richardson TR, O’Holleran J, Hawkins RJ. Reliability, validity, and responsiveness of the American Shoulder and Elbow Surgeons subjective shoulder scale in patients with shoulder instability, rotator cuff disease, and glenohumeral arthritis. J Bone Joint Surg Am. 2005;87(9):2006–2011. [DOI] [PubMed] [Google Scholar]
- 23.McHorney CA, Tarlov AR. Individual-patient monitoring in clinical practice: are available health status surveys adequate? Qual Life Res. 1995;4(4):293–307. [DOI] [PubMed] [Google Scholar]
- 24.Millett PJ, Espinoza C, Horan MP, et al. Predictors of outcomes after arthroscopic transosseous equivalent rotator cuff repair in 155 cases: a propensity score weighted analysis of knotted and knotless selfreinforcing repair techniques at a minimum of 2 years. Arch Orthop Trauma Surg. 2017;137(10):1399–1408. [DOI] [PubMed] [Google Scholar]
- 25.Obradovic M, Lal A, Liedgens H. Validity and responsiveness of EuroQol-5 dimension (EQ-5D) versus Short Form-6 dimension (SF-6D) questionnaire in chronic pain. Health Qual Life Outcomes. 2013;11(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oliva F, Osti L, Padulo J, Maffulli N. Epidemiology of the rotator cuff tears: a new incidence related to thyroid disease. Muscles Ligaments Tendons J. 2014;4(3):309. [PMC free article] [PubMed] [Google Scholar]
- 27.Revicki DA, Kawata AK, Harnam N, Chen W-H, Hays RD, Cella D. Predicting EuroQol (EQ-5D) scores from the Patient-Reported Outcomes Measurement Information System (PROMIS) global items and domain item banks in a United States sample. Qual Life Res. 2009;18(6):783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reynolds SB, Dugas JR, Cain EL, McMichael CS, Andrews JR. Débridement of small partial-thickness rotator cuff tears in elite overhead throwers. Clin Orthop Relat Res. 2008;466(3):614–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riley WT, Rothrock N, Bruce B, et al. Patient-Reported Outcomes Measurement Information System (PROMIS) domain names and definitions revisions: further evaluation of content validity in IRT-derived item banks. Qual Life Res. 2010;19(9):1311–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saarni SI, Härkänen T, Sintonen H, et al. The impact of 29 chronic conditions on health-related quality of life: a general population survey in Finland using 15D and EQ-5D. Qual Life Res. 2006;15(8):1403–1414. [DOI] [PubMed] [Google Scholar]
- 31.Shoukri MM, Cihon C. Statistical Methods for Health Sciences. Boca Raton, FL: CRC Press; 1998. [Google Scholar]
- 32.Smith MV, Calfee RP, Baumgarten KM, Brophy RH, Wright RW. Upper extremity-specific measures of disability and outcomes in orthopaedic surgery. J Bone Joint Surg Am. 2012;94(3):277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Terwee CB, Bot SD, de Boer MR, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2007;60(1):34–42. [DOI] [PubMed] [Google Scholar]
- 34.Ware JE, Keller SD, Kosinski M. SF-12: How to Score the SF-12 Physical and Mental Health Summary Scales. Boston, MA: Health Institute, New England Medical Center; 1995. [Google Scholar]
- 35.Ware JE Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. [DOI] [PubMed] [Google Scholar]
- 36.Wu J, Han Y, Zhao F-L, Zhou J, Chen Z, Sun H. Validation and comparison of EuroQoL-5 dimension (EQ-5D) and Short Form-6 dimension (SF-6D) among stable angina patients. Health Qual Life Outcomes. 2014;12(1):156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wyer PC, Silva SA. Where is the wisdom? I-a conceptual history of evidence-based medicine. J Eval Clin Pract. 2009;15(6):891–898. [DOI] [PubMed] [Google Scholar]
- 38.Wylie JD, Beckmann JT, Granger E, Tashjian RZ. Functional outcomes assessment in shoulder surgery. World J Orthop. 2014; 5(5):623–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.