Abstract
Many cognitive abilities decline with age even in the absence of detectable pathology. Recent evidence indicates that age-related neural dedifferentiation, operationalized in terms of the neural selectivity, may contribute to this decline. Here, we review work exploring the relationship between neural dedifferentiation, cognition, and age. The evidence for age effects on neural selectivity comes from both non-human animal and human research and is compelling. However, current data suggest that age does not moderate observed relationships between neural dedifferentiation and cognitive performance. We propose that functionally significant variance in measures of neural dedifferentiation reflects both age-dependent and age-independent factors. We further propose that the effects of age on neural dedifferentiation do not exclusively reflect detrimental consequences of aging.
Keywords: Differentiation, Cognitive Aging, Neural Selectivity, Individual Differences
Determinants of Cognitive Aging
Many cognitive abilities, including episodic memory, executive control and processing speed decline with increasing age, even in the absence of detectable pathology [1-3]. Given how quickly human populations are aging (the United Nations projects that the global population aged 80 years or older will rise from 137 million to 437 million between 2017 and 2050 [4]), understanding the causes of and factors moderating age-related cognitive decline are urgent goals. The use of functional neuroimaging – especially functional magnetic resonance imaging (fMRI) – to compare neural correlates of perceptual and cognitive processing in samples of healthy young and older adults plays an important role in this endeavor [5-7], and findings from some of these studies are discussed in the present paper.
Cognitive function in later life is influenced by multiple factors, including ones as diverse as childhood intelligence, rate of cortical thinning, and levels of physical activity and social engagement [6-8], to name only a few. In this review, we focus on the possible role of age-related neural dedifferentiation – the finding that neural representations of perceptual and, perhaps, conceptual information are less distinctive with increasing age. Neural dedifferentiation is thought to reflect an impairment in neural resource allocation that compromises the precision and fidelity of neural representations and processes, and to play a role in cognitive decline [9-12]. Here, we review studies of age-related neural dedifferentiation and its relationship to cognition. We propose that current evidence supports a view of neural dedifferentiation that includes both age-dependent and age-invariant factors.
Aging and Cognitive Dedifferentiation
The concept of age-related dedifferentiation pre-dates functional neuroimaging, and was developed in response to psychometric evidence that across-participant correlations between performance on different cognitive and sensory tasks strengthen over the adult lifespan [13-17]. The term dedifferentiation was used to contrast these findings from those indicating that cognitive abilities differentiate (i.e., become less strongly correlated) during childhood [17-20]. Evidence for age-related cognitive dedifferentiation (we use ‘cognitive’ to distinguish dedifferentiation of behavioral measures from neural dedifferentiation) served as motivation for an influential ‘common cause’ account of cognitive aging [14,15,21] (for evidence opposing common cause accounts, see, e.g., [22,23]) and inspired an early study of age-related neural dedifferentiation [24].
Ironically perhaps, despite its important role in the genesis of studies seeking evidence for age-related neural dedifferentiation, evidence for the existence of age-related cognitive dedifferentiation is decidedly mixed [25]. In conflict with the psychometric findings discussed above, other studies have found little or no evidence that correlations between different measures of cognition increase with age [26-35]. A similar lack of evidence was revealed in a recent meta-analysis of twenty-two longitudinal studies [36]. The meta-analysis yielded strong evidence for what the authors termed dynamic dedifferentiation, defined as an increase with baseline age in across-participant correlations of change over time in different cognitive measures [16,37], However, evidence of static dedifferentiation – age-dependent increases in correlations between the measures themselves – was lacking. It is this latter effect that corresponds to what we refer to here as cognitive dedifferentiation, and which helped motivate the search for evidence of neural dedifferentiation. Thus, while the empirical evidence for age-related neural differentiation is compelling (see Age-Related Neural Dedifferentiation), evidence for a putative functional counterpart – age-related cognitive dedifferentiation – is equivocal at best. Moreover, to our knowledge, a neural counterpart for dynamic dedifferentiation has yet to be proposed. Therefore, it seems worthwhile to consider accounts of age-related neural dedifferentiation that are not predicated on the concept of dedifferentiation at the psychometric level.
Neural Dedifferentiation as a Cause of Cognitive Aging
Before turning our attention to the neural dedifferentiation literature we first briefly discuss the influential computational model of Li and colleagues [9-12]. The model provides a neurobiological basis for age-related cognitive dedifferentiation (which, as noted above, is controversial) and cognitive aging more generally. The model proposes that cognitive aging and dedifferentiation both result from lowered neural efficiency caused by a reduction in the integrity of ascending neuromodulatory systems (for review, see [10,12,38,39]). According to this model, decreased neuromodulator availability (most importantly, dopamine) reduces signal-to-noise properties of neurons which, in turn, leads to a reduction in the fidelity of neural representations. Thus, whereas young brains will tend to form sparse representations of perceptual and other kinds of information, analogous representations in older brains will be distributed across overlapping neural populations and hence will be less distinct from one another. Simulations based on this model successfully capture several of the behavioral phenomena reported to accompany aging, including reductions in measures of ‘fluid’ abilities, such as lower working memory capacity, as well as associative memory deficits and increased susceptibility to mnemonic interference [9,10,40,41].
The above-mentioned model has several important strengths. Notably, it is parsimonious, proposing that the fidelity of neural representations is dependent on only a single age-varying parameter (the ‘gain’ of a neural activation function). Additionally, the model provides a ready explanation for relationships between neural differentiation and behavioral performance in terms of individual differences in neuromodulatory drive. Importantly, the model implies that such brain-behavior relationships need not be restricted to older adults. Although growing older is associated with a weakening of neuromodulation (for reviews, see [10,12,38,39]), individual differences in neuromodulation and, therefore, neural differentiation should be a determinant of cognitive performance throughout the lifespan. Thus, regardless of their age, individuals with low neural differentiation should have worse cognitive performance than similarly aged individuals with higher levels of differentiation. We return to this issue when we review studies that examined the relationship between measures of neural dedifferentiation and cognitive performance (see Relationship Between Neural Dedifferentiation and Behavior).
Age-Related Neural Dedifferentiation
Establishing Criteria for Neural Dedifferentiation
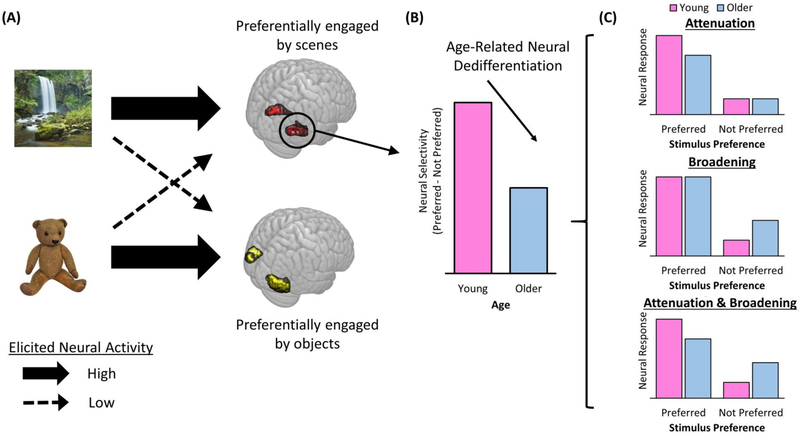
The notion of dedifferentiation has been invoked to account for a wide array of findings in the cognitive neuroscience of aging literature (e.g., [24,42-46]). In this section, we advance a definition of neural dedifferentiation and in this light consider the scope of relevant findings. Following others [24,47], we propose that neural dedifferentiation (or, reciprocally, differentiation) is ideally operationalized in terms of the selectivity of neural activity. This operationalization is rooted in the vast literature documenting that both single neurons and neural populations can exhibit preferential activity for certain stimuli or classes of stimuli (e.g., a 100Hz tone or an image of a scene) relative to other stimuli (e.g., a 500Hz tone or an image of an object; Fig. 1A). Age-related neural dedifferentiation takes the form of a smaller difference between the activity elicited by a neuron’s or a brain region’s preferred and less preferred stimuli (Fig. 1B). As is illustrated in Fig. 1C, this reduced selectivity can result from lower activity for a preferred stimulus (attenuation), increased activity for a non-preferred stimulus (broadening), or a mixture of attenuation and broadening [48].
Figure 1. Example of category selective age-related dedifferentiation as indexed by fMRI BOLD activity.
(A) Participants are presented with exemplars of different perceptual categories (here, scenes and objects) that elicit category-selective activity in different regions of occipito-temporal cortex. (B) Age-related neural dedifferentiation takes the form of reduced category-selectivity – the difference between a region’s response to a preferred relative to a not-preferred stimulus – in older relative to young participants. (C) Three possible response patterns in category-selective cortex to preferred and non-preferred stimuli that could underlie the age-related neural dedifferentiation in B. Neural dedifferentiation can be driven by a reduction in a region’s response to its preferred stimulus (Attenuation), an increase in a region’s response to a non-preferred stimulus (Broadening), or a mixture of the two [48].
The above definition of differentiation entails the use of experimental designs that permit a comparison of neural activity elicited in at least two experimental conditions, since only then can (de)differentiation be quantified (Box 1). From this perspective, the mere finding of more widespread neural activity during task engagement in older than in younger individuals (sometime referred to as age-related over-recruitment [49]) is insufficient to allow one to draw conclusions about the effects of age on neural differentiation (Box 2). Thus, we focus the review below on studies that have directly examined age differences in neural selectivity.
Box 1. Measuring Neural Differentiation.
Several approaches have been used to quantify differentiation according to differences in neural selectivity (see Figure 1).
Cellular Level
Receptive Field Mapping: This approach, thus far employed exclusively in non-human animals, measures neural differentiation at the cellular level by examining the tuning functions of neurons that respond to a particular stimulus dimension, such as the orientation or direction of a light bar [50] or to different auditory frequencies [58]. Neural dedifferentiation can result from a weaker response to a neuron’s preferred dimension, a stronger response to non-preferred dimensions, or both.
Population Level
fMRI BOLD Amplitude: A popular approach is to estimate the difference in amplitude of a region’s BOLD response to exemplars of preferred and non-preferred stimulus categories [24,48,77,79]. This approach provides a quantitative measure of neural differentiation and allows for a direct assessment of whether group differences in neural differentiation result from neural ‘attenuation’ or ‘broadening’. A limitation is that the approach is insensitive to trial-wise variability in BOLD signal, which might vary with age [112,113].
Differentiation Index: This approach is similar to BOLD amplitude, but takes account of potential age differences in the trial-wise variability of BOLD responses [47,80] (also see [114,115]). It requires that responses are estimated at the level of single trials. The index is computed as the difference in mean BOLD amplitude between a region’s preferred and nonpreferred stimulus category, scaled by the pooled inter-item variance. Importantly, it is insensitive to age differences in the gain of the hemodynamic response function (HRF) that mediates the relationship between neural and BOLD activity [116].
Multi-voxel Pattern Analysis (MVPA): MVPA quantifies the extent to which different experimental conditions or stimuli elicit similar profiles of BOLD activity across a population of voxels. Two important MVPA approaches to probe neural differentiation involve the use of linear classifiers to decode different stimulus categories [81,83] and ‘pattern similarity analysis’ (PSA) – correlational methods that measure the similarity of neural activity patterns elicited by within-vs. across-category stimulus pairs [80,84] (see also [71]). Like the differentiation index, MVPA is insensitive to individual differences in HRF gain.
Item-Level Representational Stability: PSA has also been used to measure neural differentiation for individual stimuli. This approach indexes differentiation by correlating neural patterns across repetitions of the same item relative to the similarity observed between an item and repetitions of other items [84,93]. The approach depends on the assumption that higher levels of relative similarity across repetitions of the same item reflect higher neural differentiation. The validity of this assumption is debatable, and, importantly, it does not consider the possibility that repetition differentially modulates neural representations as a function of age.
Repetition Suppression: Neural differentiation for individual items can also be indexed using fMRI adaptation [94], comparing adaptation (or ‘repetition suppression’) effects for items that differ in their level of similarity along one or more dimensions [95,97]. The underlying assumption is that neural differentiation is reflected in the specificity of adaptation effects.
Box 2. Age-Related Over-recruitment and Neural Dedifferentiation.
Among the most prominent and celebrated findings arising from functional neuroimaging studies of cognitive aging are ‘right-frontal over-recruitment’ (for review, see [117,118]), along with age-related ‘cortical over-recruitment’ more generally (e.g., [119,120]; for reviews, see [7,121]). In both cases, over-recruitment refers to the finding of more extensive task-related cortical engagement in older than in young individuals. Age-related over-recruitment has frequently been interpreted in terms of neural dedifferentiation, especially when it is found to covary negatively (or not at all) with task performance (e.g., [44,122,123]). This interpretation rests on the idea that cognitive dedifferentiation is a consequence of age-related decline in functional specialization (cf. [11,40]), such that brain regions specialized for a single cognitive function in younger individuals are co-opted in support of multiple functions in older adults (cf. [6,14,15,46]). The interpretation encounters three obstacles. First, it is predicated on the validity of age-related cognitive dedifferentiation, which, as discussed in the main text, is tenuous. Second, it must compete with other accounts of age-related over-recruitment; notably, that over-recruitment reflects the engagement of processes that adaptively compensate for the detrimental effects of aging in cortical regions sufficient to support task performance in younger participants [5,6,118,124] (also see [82]). Lastly, age-related over-recruitment for a single stimulus category or cognitive task does not meet the definition of neural dedifferentiation as a reduction in neural selectivity, at least as we have articulated it here (see Figure 1 and Box 1).
Studies in non-human animals
Research with animals has operationalized age-related neural dedifferentiation in terms of the selectivity of the receptive fields of single neurons. Importantly, this is the level of differentiation that is conceptualized in the model proposed by Li and colleagues [9-12]. Initial studies [50,51] focused on the orientation and directional selectivity of neurons in macaque striate cortex (V1) and reported reduced selectivity in senescent, relative to younger, animals. Subsequent research demonstrated analogous findings for other visual features in V1 [52-54], as well as age differences in receptive fields in extrastriate visual areas including V2 [55] and MT [52,53]. Similar findings have been reported in other species, including cats [56] and rats [57]. Wider tuning functions in aged animals have also been reported for frequency- [58-60] and spatially-responsive neurons in auditory cortex [61,62] (for review, see [62]), and for tactile stimulation in somatosensory cortex [63,64]. Together, these findings provide strong evidence for an age-dependent reduction in the selectivity of sensory neurons in animals. It remains to be seen whether analogous findings will emerge in neural populations encoding higher-level features of sensory inputs, such as those underlying face-selectivity (e.g., [65]). The extent to which the above findings generalize to humans is also currently unclear given the absence of relevant data. It is noteworthy, however, that behavioral studies in humans have found null effects of age on psychophysical measures of visual orientation and spatial frequency selectivity (e.g., [66]). There is, however, some evidence for age-related decreases in selectivity for specific visual features of items retained in working memory [67,68].
Category Selectivity in Human Studies
The great majority of studies of age-related neural dedifferentiation in humans have been conducted using fMRI, and therefore have examined neural activity at the population level. Building on well-established findings of category selectivity in ventral occipito-temporal cortex [69], most of these studies have examined the neural responses elicited by exemplars drawn from different visual categories (e.g., faces, scenes, and objects). Accordingly, our review places a heavy emphasis on findings pertaining to neural dedifferentiation in visually responsive cortical regions. It is important to note though that analogous findings have been reported in motor [70] and auditory [71] cortical systems. Thus, like the single neuron findings discussed above, age-related neural dedifferentiation at the population level is not confined to visually responsive cortical regions.
An early and influential study of category-selective neural activity in different age groups was reported by Park and colleagues [24]. These investigators examined age differences in the neural responses elicited during passive viewing of faces, scenes (houses), objects (chairs) and pseudowords, exemplars of stimulus categories associated with category-selective fMRI BOLD responses in the ‘fusiform face area’ (FFA) [72], ‘parahippocampal place area’ (PPA) [73], ‘lateral occipital complex’ (LOC) [74], and ‘visual word form area’ [75], respectively. The prediction was that age-related neural dedifferentiation would manifest as a decrease in the selectivity of neural responses in these regions. The results were in line with the prediction: for example, the findings showed age-related reductions in the PPA’s selectivity to houses – operationalized as the difference in recruitment of scene selective voxels for preferred (i.e., house) versus non-preferred (e.g., faces) images. A subsequent study employing a cross-sectional sample covering much of the adult lifespan reported a linear decrease in neural selectivity with chronological age [48] (at least for faces, the only stimulus category for which results were reported). This latter finding provides the only evidence to date that neural dedifferentiation might decline continuously across the lifespan.
The above findings of age-related neural dedifferentiation in ventral occipito-temporal cortex have been replicated in numerous cross-sectional studies that have adopted both univariate [47,48,76-80] and multivariate [70,71,81-85] analysis approaches (Box 1). Importantly, while the evidence for age-related decreases in the selectivity of neural responses is undeniably robust, the phenomenon is not consistently observed for all types of stimuli. For instance, one study reported no age differences in neural differentiation for color patches and familiar words in color- and word-selective extrastriate regions, respectively. However, the same study reported robust effects for scenes in the PPA and faces in the FFA [47]. Additionally, a more recent study reported no age differences in the accuracy of multivariate pattern analysis (MVPA) classifiers trained to discriminate between neural responses to visual words and objects [86,87].
The most consistent evidence for neural dedifferentiation arguably comes from studies that have examined neural selectivity for scene [24,47,80,84] and face [24,47,48] stimuli (although see [79] and [77] for failures to observe age effects for scenes and faces, respectively). Notably, the evidence for age-related differences in the selectivity of neural responses to visual objects in the LOC is highly inconsistent. In contrast to initial findings of age-related neural dedifferentiation in this region [24], three subsequent studies failed to find effects of age [76,80,84], In one recent study [80], for example, absent age effects on object selectivity in the LOC were accompanied by robust evidence for age-related neural dedifferentiation for scenes in the PPA. In contrast to these null findings, one other recent study did report evidence of age-related dedifferentiation for objects, albeit in perirhinal cortex [79], a region also implicated in object processing [88-90]. There are numerous factors that might have contributed to these inconsistent results, including differences in task demands (e.g., passive [24,47] vs. active [77,80,81] viewing; see also [76]), the nature of the stimuli (e.g., object exemplars drawn from one [24] versus multiple [80] categories) and differences in lifetime experience with the experimental materials (e.g., [76,91]). We enlarge on these issues below (see ‘Age-Related Neural Dedifferentiation and Lifetime Experience’ and Box 3). It is worth noting here, though, that findings of regional and material specificity in dedifferentiation rule out explanations of the phenomenon that appeal to generic age differences in such variables as the shape, signal-to-noise or variability of the BOLD signal.
Box 3. Factors Contributing to Age-Related Neural Dedifferentiation.
Neuromodulatory Drive. There are well documented age differences in the function of ascending neuromodulatory systems [10,12,39]. Given the role posited for these systems in enhancing signal-to-noise at the single neuron level [125,126] (for related work in humans, see [127-129]), it is plausible that age-related decline in these systems contributes to age differences in neural selectivity. The dopaminergic system receives a particularly heavy emphasis in Li and colleagues’ [9-12] computational model. Intriguingly, relative to older adults carrying other variants of the COMT gene, the correlation between scores on tests of spatial working memory and verbal episodic memory are higher in older adults carrying the Val/Val polymorphism, which is associated with relatively low levels of frontal dopamine [130]. This finding hints at a relationship between dopamine availability and age-related cognitive dedifferentiation (for analogous findings from simulations, see [9,10]). To our knowledge, there are no published data speaking to the relationship between dopamine availability and neural dedifferentiation as defined in this review.
GABAergic Neurotransmission. A decline in γ-aminobutyric acid (GABA) inhibitory neurotransmission [131] plays a role in the age-related reductions in single neuron selectivity reported in non-human animals [51,57,132]. For instance, administration of GABA agonists enhanced the orientation and directional selectivity of single V1 neurons in senescent macaques [51]. At present, evidence of a role for GABA in age-related neural dedifferentiation in humans is lacking. The application of magnetic resonance spectroscopy (MRS), which can be employed to assay regional brain concentrations of GABA in vivo, has the potential to shed light on this issue and help bridge the human and animal literatures (e.g., [133]). Of note, psychophysical evidence in humans supports an indirect link between age-related reductions in GABA-mediated inhibition and age differences in center-surround antagonism [134].
Passive versus Active Tasks. Task demands might play a role in human fMRI studies of age-related neural dedifferentiation. For instance, object-based attention modulates category-selective neural responses in ventral occipito-temporal cortex (e.g., [135-137]). If young and older adults tend to adopt different attentional ‘sets’ under passive viewing conditions, these findings raise the possibility that the distinction between passive and active viewing of category exemplars might be relevant to whether or not age-related dedifferentiation is observed. As one example, if older adults are more prone than young individuals to ‘tune out’ during passive viewing, one might expect to see an age-related reduction of category-selectivity under these conditions. Consistent with this expectation, age-related neural dedifferentiation has consistently been reported for face stimuli in the FFA during passive viewing tasks [24,47,48], but has proven harder to detect during tasks requiring active attention to the stimuli [77,81]. However, age differences in attentional strategies are very unlikely to provide a general account of age-related neural dedifferentiation: dedifferentiation has been reported for scene stimuli in the PPA under both passive [24,47] and active [80] viewing conditions (although see [79]). Nonetheless, the passive versus active distinction warrants future investigation.
Analogous to neural dedifferentiation at the cellular level [50,51], dedifferentiation at the population level can result from attenuation, broadening, or a mixture of the two [48] (Fig. 1). To date, only two fMRI studies have directly addressed this question [48,80]. Age-related dedifferentiation for faces in the FFA was reported to result from neural broadening [48]: while no age differences were observed for neural responses to face stimuli in the region, responses to house stimuli were enhanced in older relative to younger adults. By contrast, in the same study, face-selective regions in the ‘extended face network’ [92] showed a pattern consistent with neural attenuation (age-related reductions in responses to face stimuli). Responses in house-selective cortical regions such as the PPA were not reported, leaving open the question of the generality of the findings obtained in the FFA. In a recent study relevant to this issue it was reported that dedifferentiation in the PPA (operationalized by the contrast between responses to scenes and objects) was driven by neural attenuation [80] (for similar findings, see [76]). Together, these results raise the possibility that different mechanisms underlie age-related neural dedifferentiation in a region-dependent manner.
In summary, the existing data indicate that age-related neural dedifferentiation in the visual system, and possibly the motor and auditory systems also, is a robust phenomenon. This reduction in neural selectivity with increasing age appears to be driven by both neural attenuation and neural broadening in a region-dependent manner and is especially robust for unfamiliar faces and scenes in the FFA and PPA, respectively. Findings for other visual categories, most notably, visual objects, are less consistent, however. These inconsistencies prompt us to consider alternatives to the commonly-held view that age-related dedifferentiation necessarily reflects a detrimental consequence of aging (e.g., [12,24]).
Item-level Dedifferentiation in Humans
Several fMRI studies have examined age-related neural dedifferentiation at the level of individual items, using one of two different approaches (Box 1). Studies examining neural pattern similarity across successive presentations of the same item have yielded little evidence for age-related dedifferentiation. For example, in one recent study [84] that examined pattern similarity between repeated presentations of faces, scenes, and objects, no significant age effects were evident in occipito-temporal cortex after controlling for baseline (within-category) similarity. Somewhat surprisingly, an age-related increase in neural differentiation in frontoparietal cortex accompanied the null findings in occipito-temporal cortex. Null age effects were also reported for pattern similarity measures derived from repetitions of brief movie clips during a memory encoding task [93].
A second approach to examining item-level neural differentiation is to exploit the phenomenon of fMRI adaptation or ‘repetition suppression’ [94]. The first study to adopt this approach employed faces as the critical stimuli [95]. Both young and older adults showed similar levels of repetition suppression in the FFA to repetitions of the same face, and no evidence of suppression for different, dissimilar faces. Crucially, though, older adults showed greater suppression effects than young participants for faces morphed to be visually similar to those presented initially. Highly analogous findings have been reported in entorhinal cortex [96] and hippocampus [97] for object stimuli (e.g., exact repetitions of a rubber duck vs. presentations of two visually similar exemplars of a rubber duck; see [98] for discussion of the implications of these findings for age-related decline in hippocampal ‘pattern-separation’). Taken together, the findings from these three fMRI adaptation studies [95-97] are consistent with the proposal of Li and colleagues [9-12] that age-related dedifferentiation should be evident at the single item level, although more research is needed to establish the generality of the findings to other stimulus categories and cortical regions. Moreover, convergent evidence from other methods, such as MVPA, has yet to emerge. Thus, additional research is needed to establish whether evidence of item-level dedifferentiation is manifest in neural measures other than repetition suppression.
Relationship between Neural Differentiation and Behavior
Li and colleagues’ [9-12] computational model proposes that neural dedifferentiation is an important determinant of cognitive aging. Yet, only a handful of studies have examined whether measures of neural dedifferentiation correlate with cognitive performance, and even fewer have directly examined whether such correlations are moderated by age. Here, we briefly review studies that have examined this question, asking whether, as postulated by Li and colleagues, neural dedifferentiation predicts poorer performance on tasks tapping ‘fluid’ cognitive processes and, if so, whether age moderates the relationship. We consider data both from studies that focused on measures of categorical dedifferentiation [79-81] and dedifferentiation of individual items [71,97]. The studies and their most relevant findings are summarized in Table 1.
Table 1.
Overview of studies examining the relationship between neural differentiation and cognition.
| Study | Differentiation Measure |
Stimuli | Brain Region | Cognitive Measure | Young Adult r |
Older Adult r |
Age Moderation |
|---|---|---|---|---|---|---|---|
| Yassa et al. [97]A | Item | Objects | Hippocampus | Recognition Memory | - | .53 | - |
| Goh et al. [95]B,C | Item | Faces | Fusiform Face Area | Face Change Detection | .31 | .31 | No |
| Park et al. [81]B | Category | Faces, Houses, and Objects | Ventral Visual Cortex | Fluid cognition factor | .22 | .48 | No |
| Du et al. [71] | Category | Phonemes | Inferior Prefrontal Cortex | Phoneme Detection (in noise) | .53 | .71 | No |
| Berron et al.[79]B | Category | Objects and Scenes | Perirhinal Cortex | Recognition Memory | .05 | .38 | No |
| Koen et al. [80]D | Category | Objects and Scenes | Parahippocampal Place Area | Recognition Memory | .48 | .48 | No |
| Koen et al. [80]D | Category | Objects and Scenes | Parahippocampal Place Area | Fluency Factor | .35 | .35 | No |
Correlations are reported as positive to indicate that higher levels of neural differentiation are associated with better performance.
Study did not examine the brain-behavior relationship in young adults.
Study did not directly test for age moderation. Fisher-z test on reported correlations indicated no difference (p’s > .103).
Correlation reported collapsed across age group without controlling for age.
Partial correlations after controlling for age group are reported.
Turning first to item-level measures of differentiation, one study [97] reported a significant correlation between neural dedifferentiation in the hippocampus and memory performance in older adults, but did not report the outcome of this analysis in young participants. Another study [95] reported a seemingly age-invariant correlation (collapsing across older and young participants) in the right FFA between amount of fMRI adaptation for faces that were ‘moderately’ similar to the initial presentation and an out-of-scanner measure of face discrimination threshold. This finding might suggest that the ability to discriminate faces benefits from more highly differentiated face representations in the FFA irrespective of age.
For category-level measures of neural dedifferentiation, two studies [79,81] reported apparent age-dependent relationships with cognition based on the finding of a significant correlation in older participants only. In one of these studies [81], the finding of a significant correlation between fluid processing ability and neural differentiation in older adults was accompanied by null findings in both age groups for the correlation between differentiation and crystallized knowledge (vocabulary score). The other study [79] reported an analogous pattern of results for the correlation between neural differentiation and recognition memory performance.
Importantly, whereas the above findings support a relationship between neural differentiation and cognitive performance, they do not offer strong evidence that the relationship is age-dependent: none of the above-cited three studies [79,81,95] examining brain-behavior correlations in older and young participants reported statistical contrasts of the respective correlations. When we conducted these contrasts (Fisher z-tests) they revealed no evidence that the correlations differed significantly according to age group (see Table 1). Thus, the findings from these studies are not in conflict with the possibility that relationships between neural dedifferentiation and cognitive ability are age-invariant.
Findings from two other studies [71,80] provide further evidence for age invariance of relationships between category-level measures of neural differentiation and cognitive performance. In one of these studies [80] a significant age-invariant correlation was identified between neural differentiation in the PPA and two behavioral measures: performance on a later recognition memory test and scores on a ‘fluency’ factor derived from a neuropsychological test battery. The other study [71] reported an age-invariant relationship between an MVPA classifier-based index of neural differentiation for phonemes in inferior prefrontal cortex and the ability to identify the same phonemes when masked by auditory noise.
The foregoing findings indicate that neural dedifferentiation can predict performance both on experimental tasks (i.e., memory [79,80,97] and phoneme discrimination [71]), and on psychometric tests that depend on fluid cognitive abilities [80,81]. That is, neural dedifferentiation can predict performance both on tasks that involve the experimental stimuli employed to generate the differentiation indices, and on ‘off-line’ tests tapping broader aspects of cognitive ability. Importantly, the findings reviewed in this section also indicate that measures of neural dedifferentiation correlate with cognitive performance not only within samples of older individuals, but within samples of young participants also. Furthermore, the findings suggest that age does not moderate the strength of these correlations. Of course, the age-invariance of the correlations does not preclude the possibility that neural dedifferentiation is an important determinant of cognitive aging. Nonetheless, these age-invariant relationships are consistent with the model proposed by Li and colleagues [9-12], which predicts that lower levels of neural differentiation should be associated with lower cognitive performance regardless of age.
Age-Related Neural Dedifferentiation and Lifetime Experience
Explanations of age-related neural dedifferentiation will need to accommodate two aspects of the findings reported above. First, they will need to link the findings from single neuron studies in non-human animals with those from functional neuroimaging studies in humans. Second, they will need to account for findings suggesting that, in humans at least, age-related neural dedifferentiation is evident only for some stimulus categories. Undoubtedly any explanation will implicate multiple and, almost certainly, interacting causal factors (see Box 3). Below, we discuss how one putative factor, cumulative life experience, might influence age-related neural dedifferentiation (for discussion of the effects of life experience in other domains, see [99-101]).
The ‘lifetime experience hypothesis’ [80] of age-related neural dedifferentiation extends an idea first considered (and rejected) in one of the earliest reports of age differences in neural differentiation [24]. (Another example of a model that includes lifetime experience as an explanatory factor for cognitive aging, in this case, in the realm of recognition memory, can be found in [100]). An important motivation for the hypothesis is evidence that in contrast to many aspects of cognition (e.g., processing speed and episodic memory), there are some cognitive domains - semantic memory and vocabulary for instance (e.g., [30,102]) - where performance continues on a positive trajectory until well into later life.
The starting point for the lifetime experience hypothesis [80] is the prosaic idea that perceptual experience and knowledge accumulate over the lifespan because of an ever-increasing number of encounters with new exemplars of different perceptual categories. Thus, when confronted with a novel exemplar, older individuals will often be better able to assimilate it into a pre-existing representational structure (i.e., a ‘perceptual’ schema [103]) compared to young adults, in whom such schemas are less well developed. Consequently, with increasing age, processing of novel category exemplars will more closely resemble the processing afforded previously experienced exemplars. This proposal is consistent both with results from computational modeling [104], and with empirical studies in animals [105] and humans [103], which converge on the conclusion that new information is more rapidly assimilated into cortical representations when it is consistent with existing knowledge (i.e., when it is schema-congruent).
The lifetime experience hypothesis accounts for two important aspects of extant data. First, it is consistent with the findings that age-related dedifferentiation seems frequently to result from neural attenuation [48,76,106]. According to the hypothesis, the processing of novel exemplars of a visual category will more closely resemble the processing engaged by familiar exemplars in older than in younger adults. Thus, when first encountered, such stimuli might be expected to elicit smaller neural responses in older individuals, that is, to demonstrate an analog of ‘repetition suppression’ – the much-studied neural correlate of perceptual priming (e.g., [107-109]). Interestingly, it has been proposed that attenuation of neural responses to repeated items is a reflection of the reduction in ‘prediction error’ that accompanies perception of a recently experienced event [110]. Expanding on this account, age-related increases in lifetime experience might result in an age-related decrease in prediction error when a novel exemplar of a familiar category is experienced. In turn, this gives rise to an age-related reduction in the neural response elicited by the item in category-selective cortex, in other words, to evidence for neural dedifferentiation owing to neural attenuation.
Second, the lifetime experience hypothesis can account for the absence of age-related neural dedifferentiation for object responses in the LOC [76,106], as well as for its absence in word- and color-selective cortical regions in [47]. The hypothesis predicts that age differences in neural differentiation will be smaller for category exemplars that are similarly familiar, and hence similarly schema-congruent, in young and older individuals. It seems highly probable that many young adults would have previously experienced numerous exemplars of the canonical objects employed in prior work [76,80,84], resulting in a blunting of age-differences in neural differentiation for such stimuli. The hypothesis also explains the failure to identify age-related dedifferentiation for words [47], items highly familiar to both young and older individuals, despite the evidence for age-related dedifferentiation of neural responses to pseudo-words [24], items unlikely to have been encountered pre-experimentally by members of either age group.
In light of the above discussion, we consider it likely that lifetime experience plays a significant role in age-related neural dedifferentiation. However, much additional research is needed to confirm this role and identify the boundaries of its influence.
Concluding Remarks
When operationalized in terms of the selectivity of neural responses, evidence that neural differentiation decreases with increasing age is strong and consistent with long-standing ideas about the effects of age on neural distinctiveness at both the cellular and population level. In what might be a challenge to these ideas, however, age-related neural dedifferentiation appears to be evident for only some classes of perceptual input. Moreover, the existing data suggest that age does not strongly moderate the relationship between neural differentiation and cognitive performance. These relationships likely reflect general (and, we assert, important) principles of neural function and organization that operate across the adult lifespan [111]. It is likely that multiple factors contribute to age-related neural dedifferentiation (Box 3), including reductions in neuromodulatory drive and inhibitory neurotransmission, as well as age differences in response to task demands and lifetime experience. Importantly, the lifetime experience hypothesis raises the possibility that age-related neural dedifferentiation should not be viewed solely as a detrimental consequence of aging. Assessment of how these different factors, and others, contribute to neural dedifferentiation will benefit from research that makes more extensive use of longitudinal study designs, and employs larger and more diverse samples of participants than have typically been studied to date (see Outstanding Questions).
Outstanding Questions.
What does age-related neural dedifferentiation look like from a longitudinal rather than a cross-sectional perspective? Do changes in neural differentiation over time predict cognitive change? What are the neural correlates of dynamic cognitive dedifferentiation?
Does age modulate the relationship between neural differentiation and cognitive performance in large, diverse samples of participants, including individuals beyond their 8th decade of life? Do these brain-behavior relationships differ across cognitive domains, and does age moderate these relationships for some, but not other, domains?
Do age-related structural brain changes predict neural dedifferentiation?
Is neural dedifferentiation exaggerated in the earliest stages of Alzheimer’s or other neurodegenerative diseases?
What are the roles of different neuromodulators (e.g., dopamine) and neurotransmitters (e.g., GABA) in age-related neural dedifferentiation? How do these and other neurochemical factors interact with such factors as cumulative life experience to modulate neural differentiation?
Are the mechanisms underlying neural dedifferentiation similar across the brain, or do they vary across regions?
What is the role of lifetime experience in mediating age-related neural dedifferentiation? Are there situations where ‘age-reversed’ neural dedifferentiation might be observed, with younger adults showing greater neural dedifferentiation than older adults? This latter question provides a strong test of the ‘lifetime experience hypothesis’.
Does age-related neural differentiation extend beyond perceptual and low-level motor processing to include higher-level cognitive processes (e.g., different classes of cognitive judgments)?
How does age-related neural dedifferentiation in ‘perceptual’ regions such as extrastriate visual cortex affect neural processing in downstream regions such as the hippocampus and prefrontal cortex?
Can other neuroscientific methods, such as EEG and TMS, provide additional insights into the functional significance of age-related neural dedifferentiation?
How do lifestyle interventions, such as exercise regimes or social engagement, influence age-related neural dedifferentiation and the relationship between neural dedifferentiation and cognition?
Highlights.
Research over the past two decades has significantly improved our understanding of the plethora of factors contributing to cognitive aging.
We provide a selective review of studies investigating age differences in neural differentiation as defined by measures of neural selectivity or specificity.
The evidence indicates that neural differentiation decreases in healthy older adults and predicts performance across multiple cognitive domains in an age invariant manner.
Many factors likely contribute to age-related neural dedifferentiation, including age differences in neuromodulatory drive, efficacy of inhibitory neurotransmission, response to task demands and cumulative life experience.
The current evidence raises the possibility that neural dedifferentiation does not exclusively reflect detrimental consequences of brain aging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Harada CN et al. (2013) Normal Cognitive Aging. Clin. Geriatr. Med 29, 737–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salthouse TA (2010) Major Issues in Cognitive Aging, Oxford University Press. [Google Scholar]
- 3.Salthouse TA (2019) Trajectories of normal cognitive aging. Psychol. Aging 34, 17–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.United Nations, Department of Economic and Social Affairs, Population Division (2017) World Population Prospects: The 2017 Revision, Key Findings and Advance Tables, United Nations. [Google Scholar]
- 5.Cabeza R et al. (2018) Maintenance, reserve and compensation: the cognitive neuroscience of healthy ageing. Nat. Rev. Neurosci DOI: 10/gfc667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reuter-Lorenz PA and Park DC (2014) How Does it STAC Up? Revisiting the Scaffolding Theory of Aging and Cognition. Neuropsychol. Rev 24, 355–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grady C (2012) The cognitive neuroscience of ageing. Nat. Rev. Neurosci 13, 491–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nyberg L and Pudas S (2019) Successful Memory Aging. Annu. Rev. Psychol 70, 219–243 [DOI] [PubMed] [Google Scholar]
- 9.Li S-C and Lindenberger U (1999) Cross-level unification: a computational exploration of the link between deterioration of neurotransmitter systems and dedifferentiation of cognitive abilities in old age In Cognitive Neuroscience of Memory (Nilsson L-G and Markowitsch M, eds), pp. 104–146, Hogrege & Huber [Google Scholar]
- 10.Li S-C et al. (2000) Unifying cognitive aging: From neuromodulation to representation to cognition. Neurocomputing 32–33, 879–890 [Google Scholar]
- 11.Li S-C et al. (2001) Aging cognition: from neuromodulation to representation. Trends Cogn. Sci 5, 479–486 [DOI] [PubMed] [Google Scholar]
- 12.Li S-C and Rieckmann A (2014) Neuromodulation and aging: implications of aging neuronal gain control on cognition. Curr. Opin. Neurobiol 29, 148–158 [DOI] [PubMed] [Google Scholar]
- 13.Baltes PB et al. (1980) Integration Versus Differentiation of Fluid/Crystallized Intelligence in Old Age. Dev. Psychol 16, 625–635 [Google Scholar]
- 14.Baltes PB and Lindenberger U (1997) Emergence of a Powerful Connection Between Sensory and Cognitive Functions Across the Adult Life Span: A New Window to the Study of Cognitive Aging? Psychol. Aging 12, 12–21 [DOI] [PubMed] [Google Scholar]
- 15.Lindenberger U and Baltes PB (1997) Intellectual functioning in old and very old age: cross-sectional results from the Berlin Aging Study. Psychol. Aging 12, 410–432 [DOI] [PubMed] [Google Scholar]
- 16.de Frias CM et al. (2007) Revisiting the dedifferentiation hypothesis with longitudinal multicohort data. Intelligence 35, 381–392 [Google Scholar]
- 17.Li S-C et al. (2004) Transformations in the Couplings Among Intellectual Abilities and Constituent Cognitive Processes Across the Life Span. Psychol. Sci 15, 155–163 [DOI] [PubMed] [Google Scholar]
- 18.Balinsky B (1941) An analysis of the mental factors of various age groups from nine to sixty. Genet. Psychol. Monogr 23, 191–234 [Google Scholar]
- 19.Garrett HE (1946) A developmental theory of intelligence. Am. Psychol 1, 372–378 [DOI] [PubMed] [Google Scholar]
- 20.Burt C (1954) The differentiation of intellectual ability. Br. J. Educ. Psychol 24, 76–90 [Google Scholar]
- 21.Lindenberger U and Ghisletta P (2009) Cognitive and sensory declines in old age: Gauging the evidence for a common cause. Psychol. Aging 24, 1–16 [DOI] [PubMed] [Google Scholar]
- 22.Goh JO et al. (2012) Differential trajectories of age-related changes in components of executive and memory processes. Psychol. Aging 27, 707–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schaie KW (1993) The Seattle longitudinal studies of adult intelligence. Curr. Dir. Psychol. Sci 2, 171–175 [Google Scholar]
- 24.Park DC et al. (2004) Aging reduces neural specialization in ventral visual cortex. Proc. Natl. Acad. Sci 101, 13091–13095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.La Fleur C-G et al. (2018) Exploring dedifferentiation across the adult lifespan. Psychol. Aging 33, 855–870 [DOI] [PubMed] [Google Scholar]
- 26.Anstey KJ et al. (2003) Cross-sectional and longitudinal patterns of dedifferentiation in late-life cognitive and sensory function: The effects of age, ability, attrition, and occasion of measurement. J. Exp. Psychol. Gen 132, 470–487 [DOI] [PubMed] [Google Scholar]
- 27.Batterham PJ et al. (2011) Comparison of age and time-to-death in the dedifferentiation of late-life cognitive abilities. Psychol Aging 26, 844–851 [DOI] [PubMed] [Google Scholar]
- 28.Hartung J et al. (2018) Dedifferentiation and differentiation of intelligence in adults across age and years of education. Intelligence 69, 37–49 [Google Scholar]
- 29.Juan-Espinosa M et al. (2002) Age dedifferentiation hypothesis: Evidence from the WAIS III. Intelligence 30, 395–408 [Google Scholar]
- 30.Park DC et al. (2002) Models of visuospatial and verbal memory across the adult life span. Psychol. Aging 17, 299–320 [PubMed] [Google Scholar]
- 31.Salthouse TA (2012) Does the level at which cognitive change occurs change with age? Psychol. Sci 23, 18–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tucker-Drob EM (2009) Differentiation of cognitive abilities across the life span. Dev. Psychol 45, 1097–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tucker-Drob EM and Salthouse TA (2008) Adult age trends in the relations among cognitive abilities. Psychol. Aging 23, 453–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whitley E et al. (2016) Variations in cognitive abilities across the life course: Cross-section evidence from Understanding Society: The UK Household Longitudinal Study. Intelligence 59, 39–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zelinski EM and Lewis KL (2003) Adult Age Differences in Multiple Cognitive Functions: Differentiation, Dedifferentiation, or Process-Specific Change? Psychol. Aging 18, 727–745 [DOI] [PubMed] [Google Scholar]
- 36.Tucker-Drob EM et al. (2019) Coupled cognitive changes in adulthood: A meta-analysis. Psychol. Bull 145, 273–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baltes PB et al. (2007) Life Span Theory in Developmental Psychology In Handbook of Child Psychology (Damon W and Lerner RM, eds), John Wiley & Sons, Inc. [Google Scholar]
- 38.Li S-C (2013) Neuromodulation and developmental contextual influences on neural and cognitive plasticity across the lifespan. Neurosci. Blobehav. Rev 37, 2201–2208 [DOI] [PubMed] [Google Scholar]
- 39.Mather M and Harley CW (2016) The Locus Coeruleus: Essential for Maintaining Cognitive Function and the Aging Brain. Trends Cogn. Sci 20, 214–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li S-C and Sikström S (2002) Integrative neurocomputational perspectives on cognitive aging, neuromodulation, and representation. Neurosci. Blobehav. Rev 26, 795–808 [DOI] [PubMed] [Google Scholar]
- 41.Li S-C et al. (2005) Aging Neuromodulation Impairs Associative Binding: A Neurocomputational Account. Psychol. Sci 16, 445–450 [DOI] [PubMed] [Google Scholar]
- 42.Logan JM et al. (2002) Under-Recruitment and Nonselective Recruitment: Dissociable Neural Mechanisms Associated with Aging. Neuron 33, 827–840 [DOI] [PubMed] [Google Scholar]
- 43.Morcom AM et al. (2003) Age effects on the neural correlates of successful memory encoding. Brain 126, 213–229 [DOI] [PubMed] [Google Scholar]
- 44.Morcom AM et al. (2007) Age Effects on the Neural Correlates of Episodic Retrieval: Increased Cortical Recruitment with Matched Performance. Cereb. Cortex 17, 2491–2506 [DOI] [PubMed] [Google Scholar]
- 45.Grady CL et al. (1994) Age-related changes in cortical blood flow activation during visual processing of faces and location. J. Neurosci 14, 1450–1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dennis NA and Cabeza R (2011) Age-related dedifferentiation of learning systems: an fMRI study of implicit and explicit learning. Neurobiol. Aging 32, 2318.e17–2318.e30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Voss MW et al. (2008) Dedifferentiation in the visual cortex: An fMRI investigation of individual differences in older adults. Brain Res. 1244, 121–131 [DOI] [PubMed] [Google Scholar]
- 48.Park J et al. (2012) Neural Broadening or Neural Attenuation? Investigating Age-Related Dedifferentiation in the Face Network in a Large Lifespan Sample. J. Neurosci 32, 2154–2158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grady CL (2008) Cognitive Neuroscience of Aging. Ann. N. Y. Acad. Sci 1124, 127–144 [DOI] [PubMed] [Google Scholar]
- 50.Schmolesky MT et al. (2000) Degradation of stimulus selectivity of visual cortical cells in senescent rhesus monkeys. Nat. Neurosci 3, 384–390 [DOI] [PubMed] [Google Scholar]
- 51.Leventhal AG et al. (2003) GABA and Its Agonists Improved Visual Cortical Function in Senescent Monkeys. Science 300, 812–815 [DOI] [PubMed] [Google Scholar]
- 52.Liang Z et al. (2010) Aging affects the direction selectivity of MT cells in rhesus monkeys. Neurobiol. Aging 31, 863–873 [DOI] [PubMed] [Google Scholar]
- 53.Yang Y et al. (2008) Aging affects contrast response functions and adaptation of middle temporal visual area neurons in rhesus monkeys. Neuroscience 156, 748–757 [DOI] [PubMed] [Google Scholar]
- 54.Zhang J et al. (2008) Spatial and temporal sensitivity degradation of primary visual cortical cells in senescent rhesus monkeys: Effects of aging on visual system. Eur. J. Neurosci 28, 201–207 [DOI] [PubMed] [Google Scholar]
- 55.Yu S et al. (2006) Functional degradation of extrastriate visual cortex in senescent rhesus monkeys. Neuroscience 140, 1023–1029 [DOI] [PubMed] [Google Scholar]
- 56.Hua T et al. (2006) Functional degradation of visual cortical cells in old cats. Neurobiol. Aging 27, 155–162 [DOI] [PubMed] [Google Scholar]
- 57.Ding Y et al. (2017) Changes in GABAergic markers accompany degradation of neuronal function in the primary visual cortex of senescent rats. Sci. Rep 7, 14897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Turner JG et al. (2005) Affects of Aging on Receptive Fields in Rat Primary Auditory Cortex Layer V Neurons. J. Neurophysiol 94, 2738–2747 [DOI] [PubMed] [Google Scholar]
- 59.de Villers-Sidani E et al. (2010) Recovery of functional and structural age-related changes in the rat primary auditory cortex with operant training. Proc. Natl. Acad. Sci 107, 13900–13905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kamal B et al. (2013) Shaping the aging brain: role of auditory input patterns in the emergence of auditory cortical impairments. Front. Syst. Neurosci 7, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Engle JR and Recanzone GH (2013) Characterizing spatial tuning functions of neurons in the auditory cortex of young and aged monkeys: a new perspective on old data. Front. Aging Neurosci 4, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Costa M et al. (2016) Effects of aging on peripheral and central auditory processing in rats. Eur. J. Neurosci 44, 2084–2094 [DOI] [PubMed] [Google Scholar]
- 63.Spengler F et al. (1995) Effects of ageing on topographic organization of somatosensory cortex. NeuroReport 6, 469–473 [DOI] [PubMed] [Google Scholar]
- 64.David-Jürgens M et al. (2008) Differential Effects of Aging on Fore- and Hindpaw Maps of Rat Somatosensory Cortex. PLoS ONE 3, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chang L and Tsao DY (2017) The Code for Facial Identity in the Primate Brain. Cell 169, 1013–1028.e14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Govenlock SW et al. (2009) The effect of aging on the orientational selectivity of the human visual system. Vision Res. 49, 164–172 [DOI] [PubMed] [Google Scholar]
- 67.Noack H et al. (2012) Normal aging increases discriminal dispersion in visuospatial short-term memory. Psychol. Aging 27, 627–637 [DOI] [PubMed] [Google Scholar]
- 68.Peich M-C et al. (2013) Age-related decline of precision and binding in visual working memory. Psychol. Aging 28, 729–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grill-Spector K and Malach R (2004) The human visual cortex. Annu. Rev. Neurosci 27, 649–677 [DOI] [PubMed] [Google Scholar]
- 70.Carp J et al. (2011) Age-Related Neural Dedifferentiation in the Motor System. PLoS ONE 6, e29411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Du Y et al. (2016) Increased activity in frontal motor cortex compensates impaired speech perception in older adults. Nat. Commun 7, 12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kanwisher N and Yovel G (2006) The fusiform face area: a cortical region specialized for the perception of faces. Philos. Trans. R. Soc. B Biol Sci 361, 2109–2128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Epstein R and Kanwisher N (1998) A cortical representation of the local visual environment. Nature 392, 598–601 [DOI] [PubMed] [Google Scholar]
- 74.Grill-Spector K et al. (2001) The lateral occipital complex and its role in object recognition. Vision Res. 41, 1409–1422 [DOI] [PubMed] [Google Scholar]
- 75.McCandliss BD et al. (2003) The visual word form area: expertise for reading in the fusiform gyrus. Trends Cogn. Sci 7, 293–299 [DOI] [PubMed] [Google Scholar]
- 76.Chee MWL et al. (2006) Age-related Changes in Object Processing and Contextual Binding Revealed Using fMR Adaptation. J. Cogn. Neurosci 18, 495–507 [DOI] [PubMed] [Google Scholar]
- 77.Payer D et al. (2006) Decreased neural specialization in old adults on a working memory task. NeuroReport 17, 487–491 [DOI] [PubMed] [Google Scholar]
- 78.Burianová H et al. (2013) Age-related dedifferentiation and compensatory changes in the functional network underlying face processing. Neurobiol. Aging 34, 2759–2767 [DOI] [PubMed] [Google Scholar]
- 79.Berron D et al. (2018) Age-related functional changes in domain-specific medial temporal lobe pathways. Neurobiol. Aging 65, 86–97 [DOI] [PubMed] [Google Scholar]
- 80.Koen JD et al. (2019) The relationship between age, neural differentiation, and memory performance. J. Neurosci 39, 149–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Park J et al. (2010) Neural Specificity Predicts Fluid Processing Ability in Older Adults. J. Neurosci 30, 9253–9259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Carp J et al. (2010) Age Differences in the Neural Representation of Working Memory Revealed by Multi-Voxel Pattern Analysis. Front. Hum. Neurosci 4, 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Carp J et al. (2011) Age differences in neural distinctiveness revealed by multi-voxel pattern analysis. NeuroImage 56, 736–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zheng L et al. (2018) Reduced Fidelity of Neural Representation Underlies Episodic Memory Decline in Normal Aging. Cereb. Cortex 28, 2283–2296 [DOI] [PubMed] [Google Scholar]
- 85.Kleemeyer MM et al. (2017) Exercise-Induced Fitness Changes Correlate with Changes in Neural Specificity in Older Adults. Front. Hum. Neurosci 11, 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thakral PP et al. (2019) Effects of age on across-participant variability of cortical reinstatement effects. NeuroImage 191, 162–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang TH et al. (2016) The Effects of Age on the Neural Correlates of Recollection Success, Recollection-Related Cortical Reinstatement, and Post-Retrieval Monitoring. Cereb. Cortex 26, 1698–1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Murray EA and Richmond BJ (2001) Role of perirhinal cortex in object perception, memory, and associations. Curr. Opin. Neurobiol 11, 188–193 [DOI] [PubMed] [Google Scholar]
- 89.Ranganath C and Ritchey M (2012) Two cortical systems for memory-guided behaviour. Nat. Rev. Neurosci 13, 713–726 [DOI] [PubMed] [Google Scholar]
- 90.Barense MD et al. (2010) Medial temporal lobe activity during complex discrimination of faces, objects, and scenes: Effects of viewpoint. Hippocampus 20, 389–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Goh JO et al. (2007) Age and culture modulate object processing and object—scene binding in the ventral visual area. Cogn. Affect. Behav. Neurosci 7, 44–52 [DOI] [PubMed] [Google Scholar]
- 92.Ishai A Let’s face it: it’s a cortical network. NeuroImage 40, 415–419 [DOI] [PubMed] [Google Scholar]
- 93.St-Laurent M et al. (2014) Memory Reactivation in Healthy Aging: Evidence of Stimulus-Specific Dedifferentiation. J. Neurosci 34, 4175–4186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Grill-Spector K et al. (2006) Repetition and the brain: neural models of stimulus-specific effects. Trends Cogn. Sci 10, 14–23 [DOI] [PubMed] [Google Scholar]
- 95.Goh JO et al. (2010) Reduced neural selectivity increases fMRI adaptation with age during face discrimination. NeuroImage 51, 336–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Reagh ZM et al. (2018) Functional Imbalance of Anterolateral Entorhinal Cortex and Hippocampal Dentate/CA3 Underlies Age-Related Object Pattern Separation Deficits. Neuron 97, 1187–1198.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yassa MA et al. (2011) Age-related memory deficits linked to circuit-specific disruptions in the hippocampus. Proc. Natl. Acad. Sci 108, 8873–8878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Leal SL and Yassa MA (2018) Integrating new findings and examining clinical applications of pattern separation. Nat. Neurosci 21, 163–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brod G et al. (2017) Neural activation patterns during retrieval of schema-related memories: differences and commonalities between children and adults. Dev. Sci 20, e12475. [DOI] [PubMed] [Google Scholar]
- 100.Buchler NEG and Reder LM (2007) Modeling age-related memory deficits: A two-parameter solution. Psychol. Aging 22, 104–121 [DOI] [PubMed] [Google Scholar]
- 101.Werkle-Bergner M et al. (2006) Cortical EEG correlates of successful memory encoding Implications for lifespan comparisons. Neurosci. Biobehav. Rev 30, 839–854 [DOI] [PubMed] [Google Scholar]
- 102.Rönnlund M et al. (2005) Stability, Growth, and Decline in Adult Life Span Development of Declarative Memory: Cross-Sectional and Longitudinal Data From a Population-Based Study. Psychol. Aging 20, 3–18 [DOI] [PubMed] [Google Scholar]
- 103.Gilboa A and Marlatte H (2017) Neurobiology of Schemas and Schema-Mediated Memory. Trends Cogn. Sci 21, 618–631 [DOI] [PubMed] [Google Scholar]
- 104.McClelland JL (2013) Incorporating rapid neocortical learning of new schema-consistent information into complementary learning systems theory. J. Exp. Psychol. Gen 142, 1190–1210 [DOI] [PubMed] [Google Scholar]
- 105.Tse D et al. (2007) Schemas and Memory Consolidation. Science 316, 76–82 [DOI] [PubMed] [Google Scholar]
- 106.Koen JD et al. (in press) The relationship between age, neural differentiation, and memory performance. J. Neurosci [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Henson RNA and Rugg MD (2003) Neural response suppression, haemodynamic repetition effects, and behavioural priming. Neuropsychologia 41, 263–270 [DOI] [PubMed] [Google Scholar]
- 108.Gotts SJ et al. (2012) Repetition Priming and Repetition Suppression: A Case for Enhanced Efficiency Through Neural Synchronization. Cogn Neurosci 3, 227–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Barron HC et al. (2016) Repetition suppression: a means to index neural representations using BOLD? Philos. Trans. R. Soc. B Biol. Sci 371, 20150355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Henson RN (2016) Repetition suppression to faces in the fusiform face area: A personal and dynamic journey. Cortex 80, 174–184 [DOI] [PubMed] [Google Scholar]
- 111.Rugg MD (2017) Interpreting age-related differences in memory-related neural activity In Cognitive Neuroscience of Aging: Linking Cognitive and Cerebral Aging (2nd edn) (Cabeza R et al. , eds), pp. 183–206, Oxford University Press [Google Scholar]
- 112.Garrett DD et al. (2017) Age differences in brain signal variability are robust to multiple vascular controls. Sci. Rep 7, 10149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Guitart-Masip M et al. (2016) BOLD Variability is Related to Dopaminergic Neurotransmission and Cognitive Aging. Cereb. Cortex 26, 2074–2083 [DOI] [PubMed] [Google Scholar]
- 114.Afraz S et al. (2006) Microstimulation of inferotemporal cortex influences face categorization. Nature 442, 692–695 [DOI] [PubMed] [Google Scholar]
- 115.Grill-Spector K et al. (2007) Corrigendum: high-resolution imaging reveals highly selective nonface clusters in the fusiform face area. Nat. Neurosci 10, 133. [DOI] [PubMed] [Google Scholar]
- 116.Liu P et al. (2013) Age-related differences in memory-encoding fMRI responses after accounting for decline in vascular reactivity. NeuroImage 78, 415–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rajah MN and D’Esposito M (2005) Region-specific changes in prefrontal function with age: a review of PET and fMRI studies on working and episodic memory. Brain 128, 1964–1983 [DOI] [PubMed] [Google Scholar]
- 118.Cabeza R and Dennis NA (2013) Frontal lobes and aging: deterioration and compensation In Principles of frontal lobe function (2nd edn) (Stuss DT and Knight RT, eds), Oxford University Press [Google Scholar]
- 119.Cabeza R et al. (1997) Age-related differences in neural activity during memory encoding and retrieval: a positron-emission tomography study. J. Neurosci 17, 391–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Madden DJ et al. (1999) Adult age differences in the functional neuroanatomy of verbal recognition memory. Hum. Brain Mapp 7, 115–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Maillet D and Rajah MN (2014) Age-related differences in brain activity in the subsequent memory paradigm: A meta-analysis. Neurosci. Biobehav. Rev 45, 246–257 [DOI] [PubMed] [Google Scholar]
- 122.Stevens WD et al. (2008) A Neural Mechanism Underlying Memory Failure in Older Adults. J. Neurosci 28, 12820–12824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.de Chastelaine M et al. (2011) The Effects of Age, Memory Performance, and Callosal Integrity on the Neural Correlates of Successful Associative Encoding. Cereb. Cortex 21, 2166–2176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Morcom AM and Johnson W (2015) Neural reorganization and compensation in aging. J. Cogn. Neurosci 27, 1275–1285 [DOI] [PubMed] [Google Scholar]
- 125.Arnsten AFT (2011) Catecholamine Influences on Dorsolateral Prefrontal Cortical Networks. Biol. Psychiatry 69, e89–e99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jacob SN et al. (2013) Dopamine Regulates Two Classes of Primate Prefrontal Neurons That Represent Sensory Signals. J. Neurosci 33, 13724–13734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Müller U et al. (1998) D1-Versus D2-Receptor Modulation of Visuospatial Working Memory in Humans. J. Neurosci 18, 2720–2728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Noudoost B and Moore T (2011) Control of visual cortical signals by prefrontal dopamine. Nature 474, 372–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yousif N et al. (2016) Dopamine Activation Preserves Visual Motion Perception Despite Noise Interference of Human V5/MT. J. Neurosci 36, 9303–9312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Papenberg G et al. (2014) COMT polymorphism and memory dedifferentiation in old age. Psychol. Aging 29, 374–383 [DOI] [PubMed] [Google Scholar]
- 131.Rozycka A and Liguz-Lecznar M (2017) The space where aging acts: focus on the GABAergic synapse. Aging Cell 16, 634–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hua T et al. (2008) Decreased proportion of GABA neurons accompanies age-related degradation of neuronal function in cat striate cortex. Brain Res. Bull 75, 119–125 [DOI] [PubMed] [Google Scholar]
- 133.Cassady K et al. (2019) Sensorimotor network segregation declines with age and is linked to GABA and to sensorimotor performance. NeuroImage 186, 234–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Betts LR et al. (2005) Aging Reduces Center-Surround Antagonism in Visual Motion Processing. Neuron 45, 361–366 [DOI] [PubMed] [Google Scholar]
- 135.Baldauf D and Desimone R (2014) Neural Mechanisms of Object-Based Attention. Science 344, 424–427 [DOI] [PubMed] [Google Scholar]
- 136.Gazzaley A et al. (2005) Top-down Enhancement and Suppression of the Magnitude and Speed of Neural Activity. J. Cogn. Neurosci 17, 507–517 [DOI] [PubMed] [Google Scholar]
- 137.Zanto TP et al. (2010) Top-down modulation of visual feature processing: The role of the inferior frontal junction. NeuroImage 53, 736–745 [DOI] [PMC free article] [PubMed] [Google Scholar]